-
近年来,世界各国将开发新能源汽车作为一项极为重要的工作。中国科技部“863”计划中启动电动汽车专项这一措施推动了电动汽车产业的快速发展,与此同时,也促进了动力电池产业的发展[1]。预计到2020年,我国的纯电动汽车年产量将会超过2×106 辆,产销量累计将达到5×106 辆[2]。这预示着报废的动力锂电池的数量必将大幅度增长。预测到2020年,中国的报废电池量将达到5×105 t[3]。废旧动力电池含有贵重金属和电解液有机溶剂等,若随意丢弃或不妥当处理,不仅对周围环境造成严重的破坏,同时也会造成废旧电池中钴、锂和镍等有价金属的流失,可见废旧电池的回收在资源回收的同时也减少了对环境的污染[4]。
目前,锂电池回收的方法主要有酸浸取工艺、电化学工艺和生物浸取工艺等。电化学工艺浸出时,对电解液组成成分要求比较高且耗电量较大,生物浸取工艺中,在较高的金属浓度下细菌容易中毒失活,导致浸出效率受到了限制,同时,培养菌类要求条件苛刻且菌种易受到污染,最终导致浸出效率低[5]。酸浸取工艺一般分为有机酸和无机酸,无机酸一般采用H2SO4[6]、HCl[7]和HNO3[8]配合H2O2作为还原剂,无机酸在浸取的过程中会产生硫氧化物、氮氧化物和氯化物等有毒气体,对环境造成二次污染,同时H2SO4、HCl和HNO3都是强酸,具有强腐蚀性,对设备要求较高,因此,近年来人们探索采用有机酸来代替无机酸,如苹果酸(C6H4O5)[9]、草酸(H2C2O4)[10]、琥珀酸(C4H6O4)[11]、柠檬酸(C6H8O7)[12-13]和抗坏血酸(C6H8O6)[14]等。
蔡乐等[15]采用1 mol·L−1的稀H2SO4与质量分数为30%的H2O2浸出体系,在90 ℃条件下,反应1 h,然后在浸出液中加入K2S2O8,继续反应3 h,从而制得α型MnO2颗粒。实现三元锂电池的正极材料中Ni、Co和Li的浸出及Mn的回收。有研究[16]利用C6H8O7、dl-C6H4O5、H2C2O4、C2H4O2等不同有机酸从废锂离子电池中回收Co和Li的环保工艺。采用响应面法(RSM)对固液比、温度、酸浓度、有机酸类型、H2O2浓度等浸出参数进行了优化。根据优化过程得到的结果,温度被认为是影响最大的参数。LI等[17]采用1.25 mol·L−1 C6H8O7和10% H2O2,固液比为20 g·L−1,90 ℃条件下反应1 h,,H2O2添加时间间隔为30 min,从废旧锂电池中提取了近100%的Li和90%以上的Co,实现锂电池正极材料中Co和Li的浸出。CHEN等[18]用4 mol·L−1 H2SO4和10% H2O2体系85 ℃条件下反应2 h,固液比为1∶10,Co和Li的浸出效率分别为95%和96%。通过调节pH,析出浸出液中Fe(Ⅲ)、Cu(Ⅱ)、Mn(Ⅱ)杂质离子。 然后用皂化P507(2-乙基己基膦酸单-2-乙基己基酯)从纯化水相中选择性地提取Co(Ⅱ),并在条状液中以H2C2O4的形式进行化学沉积制备CoC2O4,CoC2O4的产率为93%。MENG等[19]将废旧LiCoO2分散于20 mL的1.25 mol·L−1 C6H4O5和0.3 mol·L−1 C6H12O6混合溶液中,温度为80 ℃,浸出时间为180 min。最终Co和Li的浸出效率分别达到99.87%和100%。紧接着向浸出液中添加摩尔比为1.15的(NH4)2C2O4,调节pH为2.0,在55 ℃下反应40 min,可分离出98%的Co。为了消除强酸在浸出反应过程中的二次污染问题,我们在不牺牲高浸出效率的前提下,寻找了强酸的替代品。C6H8O6是一种廉价易得的有机酸[20],为乙烯基羧酸以及温和的还原性有机酸,在浸出过程中,反应比较温和[21]。C6H8O6的工业生产已有80多年的历史。大多数商业生产的C6H8O6是通过传统的七步赖希斯坦法合成的[22]。近年来,C6H8O6的生产技术得到了进一步发展,这不仅提高了C6H8O6的生产效率,也降低了生产成本[23-24]。
针对三元锂电池的回收提出以C6H8O6为浸出剂和还原剂,直接浸出废旧三元锂电池正极材料中的Co、Mn、Li和Ni有价金属,同时添加H2SO4和KMnO4简便快速地制备β-CoC2O4·2H2O颗粒的方法。溶液中过量的C6H8O6在强氧化剂(KMnO4)的作用下生成脱水抗坏血酸,进而生成二酮古洛糖酸,二酮古洛糖酸继续被氧化,最终氧化成草酸[25],草酸参与反应回收Co[26]。由于剩余的抗坏血酸在溶液中被氧化消耗,即减少了废液中的有机酸含量。实现正极材料中有价金属的有效浸出和Co的回收。
全文HTML
-
实验所涉及的废旧三元锂电池正极材料由湖南杉杉能源科技股份有限公司拆解后提供。实验所用试剂为抗坏血酸(C6H8O6),硝酸(HNO3),盐酸(HCl),浓硫酸(H2SO4),高锰酸钾(KMnO4),以上试剂均为分析纯。
-
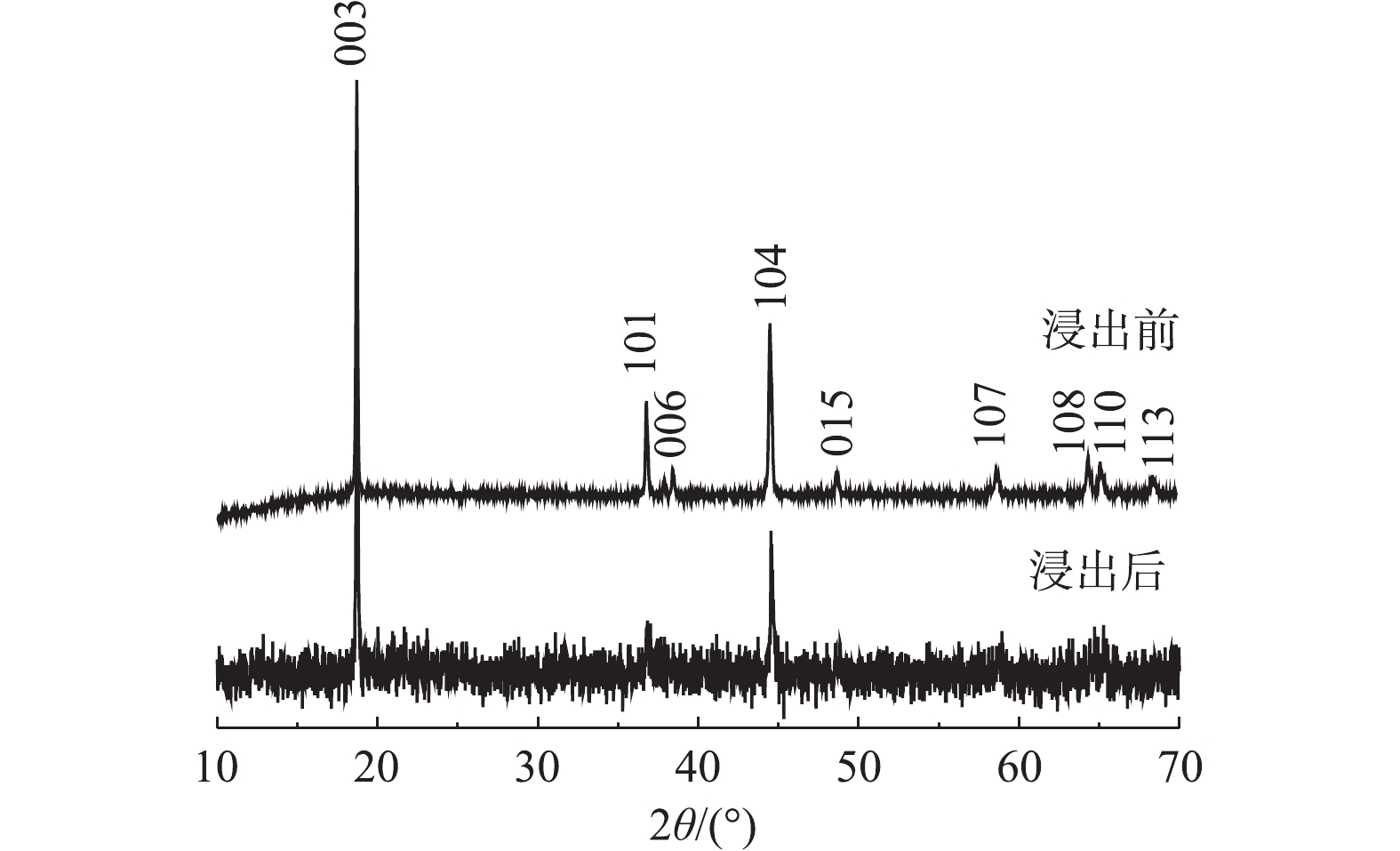
对三元动力锂电池正极材料进行XRD分析,初步确定正极活性材料中的金属种类,以便消解后进行ICP测定。通过与标准卡片对比分析,确定该正极活性材料为镍钴锰酸锂的衍射峰,见图1。
对原材料中有价金属Co、Mn、Li和Ni的质量分数进行实际分析,采用王水(HNO3∶HCl =1∶3)消解,将配置好的王水置于通风橱中3 min,之后称量三元锂电池正极材料,置于王水中反应12 h,稀释待测液1 000倍,取15 mL左右的稀释待测液于试管中备用,本研究采用ICP法测定滤液中的金属组分及其浓度,测试误差不大于5%。结果表明,正极材料中Co、Mn、Li和Ni的质量百分数分别为22.24%、18.17%、5.74%、17.83%。
-
实验采用C6H8O6、H2SO4配合KMnO4对三元锂电池正极材料进行浸出,同时分离Co制备β-CoC2O4·2H2O颗粒。
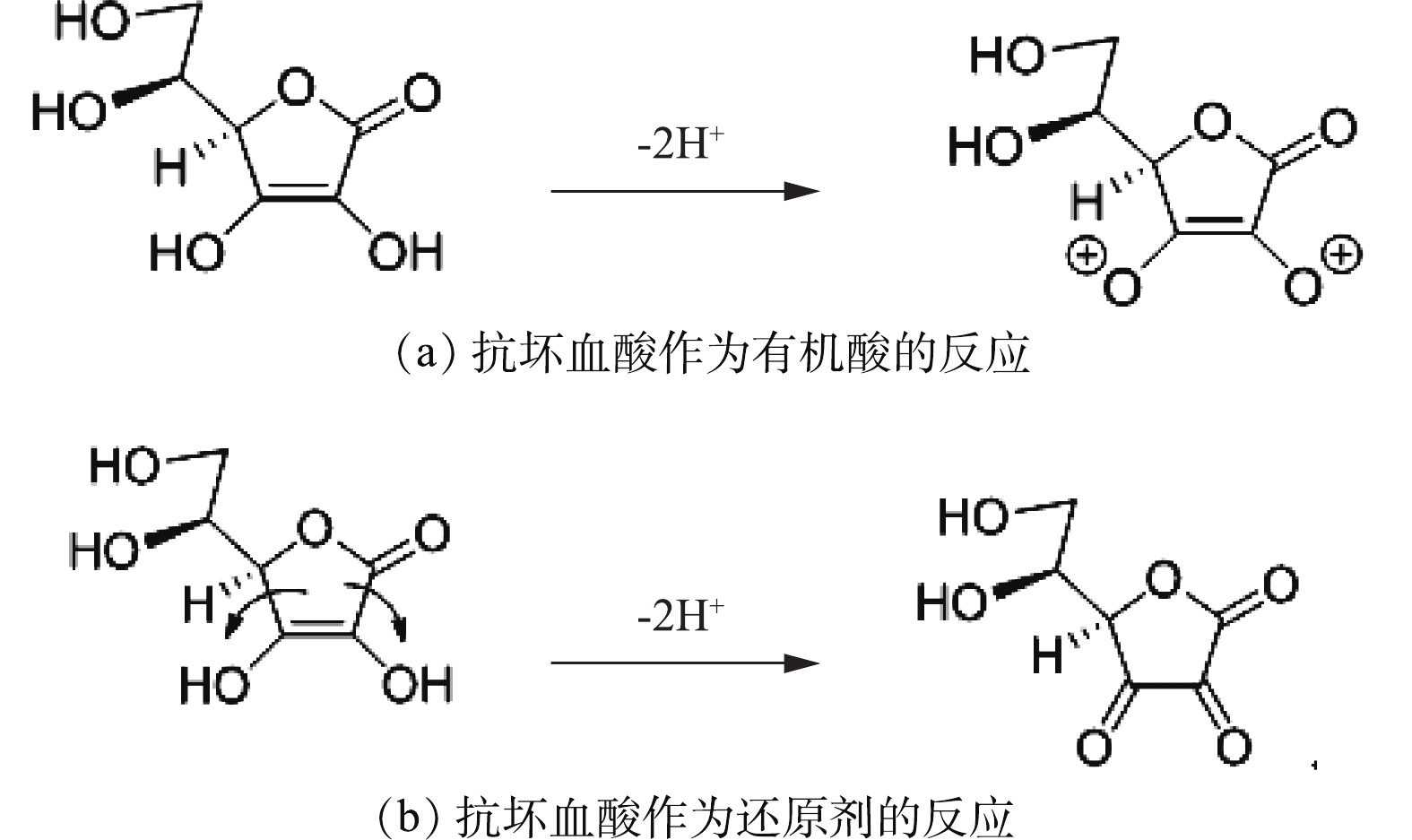
C6H8O6又称维生素C,是一种温和的有机还原酸,它可以被一个电子氧化成自由基,也可以被双重氧化成稳定的脱氢抗坏血酸(C6H6O6),抗坏血酸作为有机酸和还原剂的反应如图2所示。
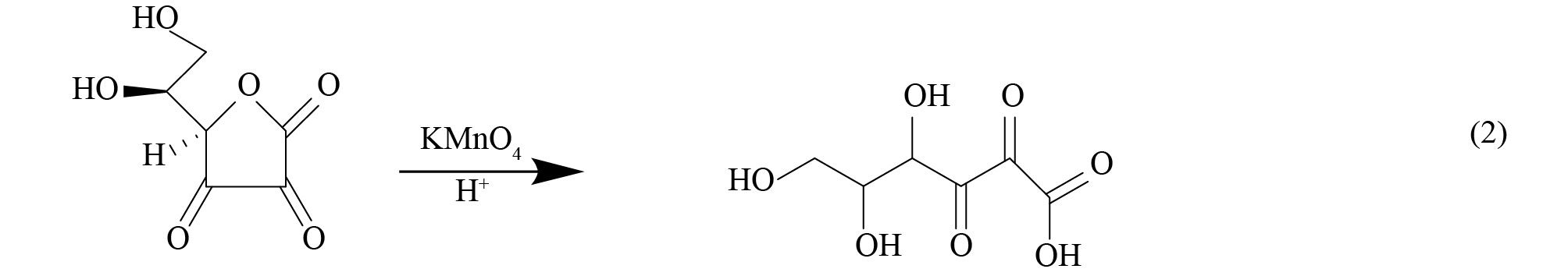
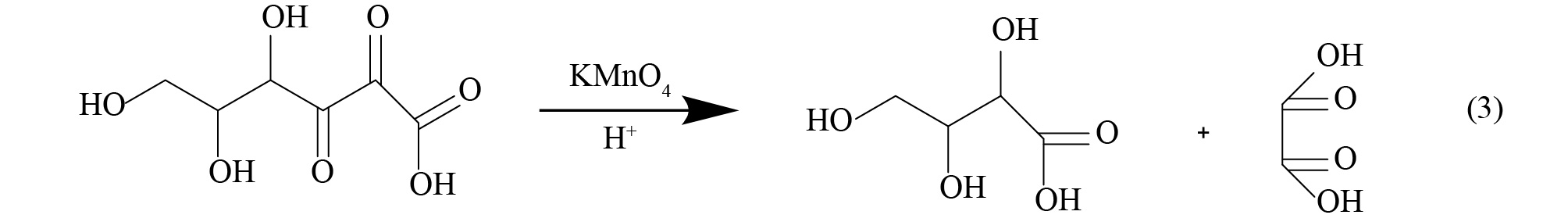
实验中所涉及的主要反应方程如式(1)~式(4)所示。
由式(1)可知,抗坏血酸可将正极材料中的金属盐浸出,由式(4)可知,溶液中存在的Co2+可反应生成β-CoC2O4·2H2O,降低溶液中C6H6O6Co含量,同时生成C6H8O6,促使反应式(1)加速向右进行。
实验采用湿法冶金技术对三元动力锂电池进行浸出反应,分别采用C6H8O6作为浸出体系,探究了反应温度、C6H8O6浓度、固液比对浸出的影响;C6H8O6浸出10 min后加H2SO4,而后加入KMnO4从浸出液中提取并制备量β-CoC2O4·2H2O颗粒,探究了反应温度、H2SO4添加量、KMnO4添加量及KMnO4添加时间对β-CoC2O4·2H2O回收率的影响,使用不同转速条件下制备的β-CoC2O4·2H2O颗粒,分离洗涤干燥,对其进行XRD、SEM等表征,探究反应转速对制备β-CoC2O4·2H2O形貌及粒径的影响。其回收技术方案路线如图3所示。
-
液相中金属元素的检测采用日本日立公司生产的S-4800型电感耦合等离子体光谱仪;实验中涉及CoC2O4颗粒的表征设备有X射线衍射仪(D8 ADVANCE),场发射扫描电子显微镜SEM (S-4800,扫描电压为10 kV),激光粒度仪(MS2000);实验中涉及的称量仪器采用上海恒平科学仪器有限公司生产的FA2004型电子天平;浸出实验加热设备采用郑州长城仪器有限公司生产的HWCL-5型集热式恒温磁力搅拌油浴锅。
1.1. 实验材料
1.2. 三元锂电池正极材料分析
1.3. 实验原理及方法
1.4. 仪器设备
-
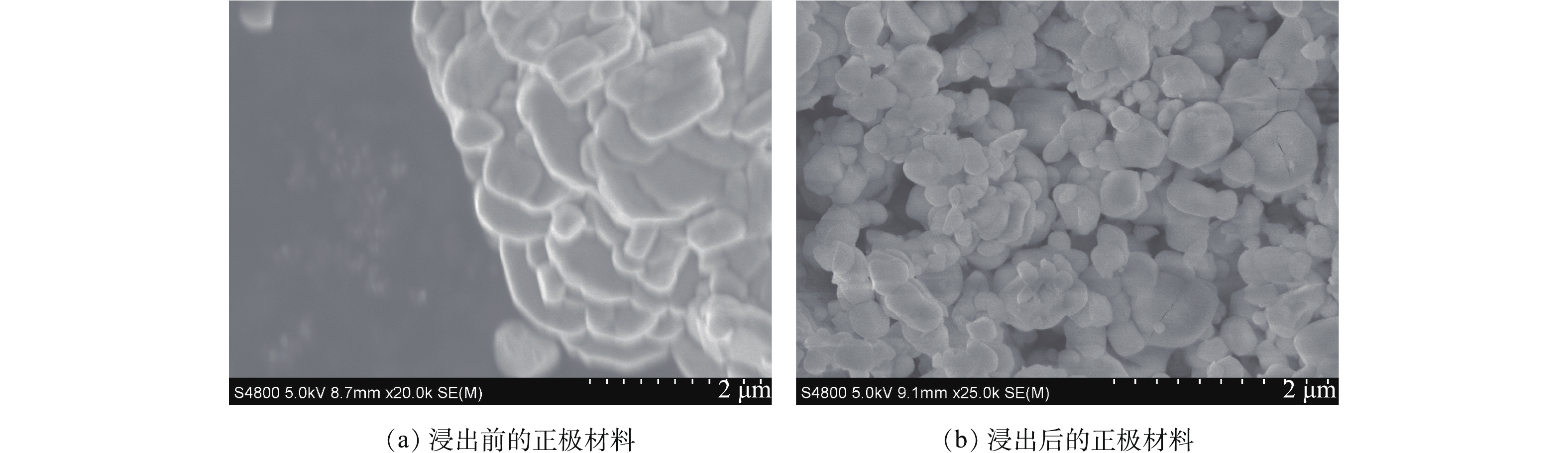
用扫描电镜观察正极材料粉末及其浸出后残渣的形貌,如图4所示。图4(a)为反应前正极材料的电镜图,图4(b)为反应后正极材料的电镜图,电池正极材料主要为Li(Ni1/3Co1/3Mn1/3)O2。由图4(a)可以看出,正极材料形态不规则,含有较大的二次粒子,粒径分布较广,与浸出前相比,浸出后的浸出渣形态不规则,粒度分布较窄,粒度减小。根据浸出前后形态和尺寸的分析,表明C6H8O6存在优良的还原性能和浸出性能,可以对废旧正极材料进行有效浸出。
-
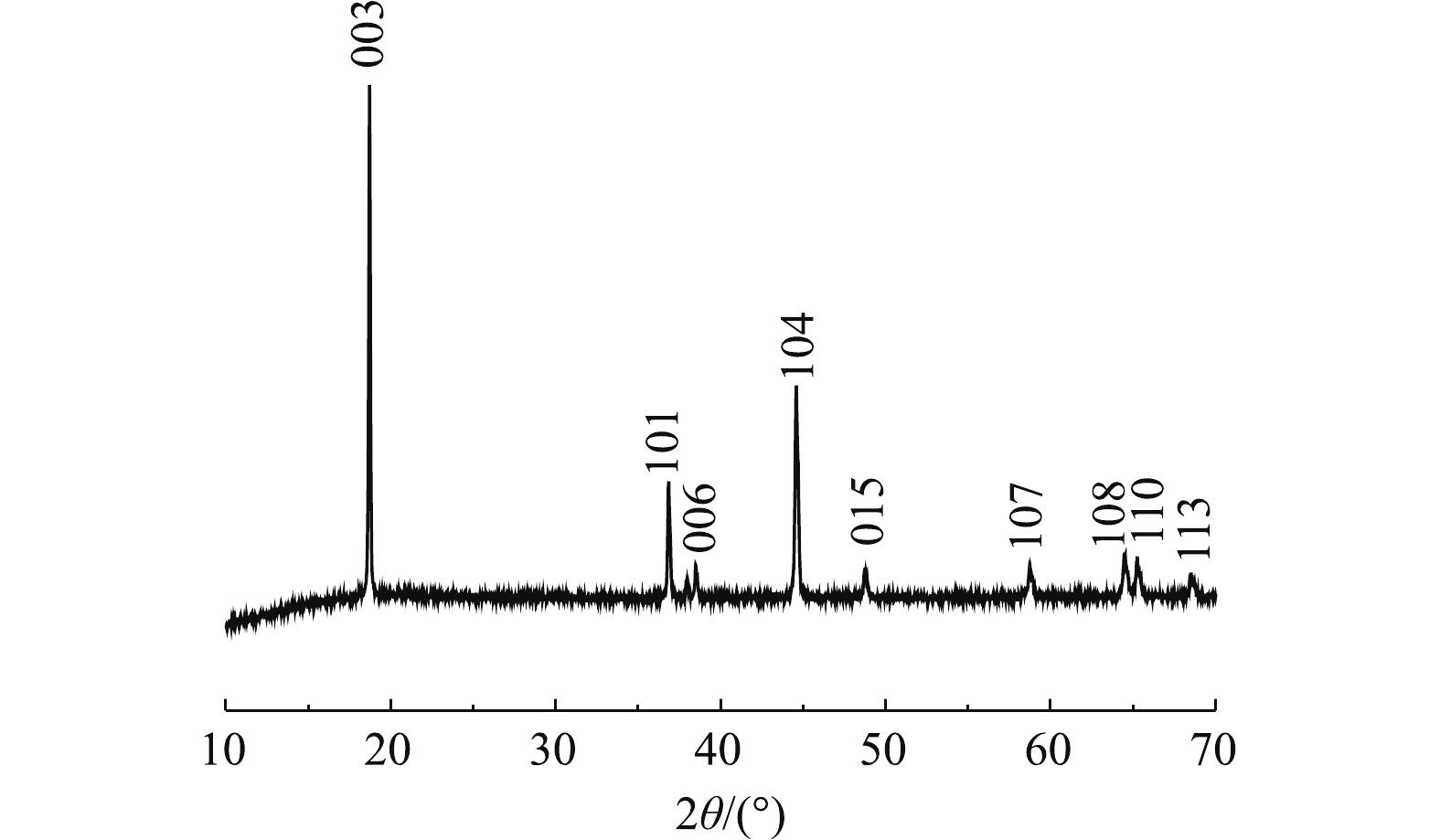
图5为浸出前后正极材料的XRD图谱。由图5可以看出,浸出后其中部分峰发生了变化,浸出后的正极活性材料X射线衍射强度变低,材料结构有序度减弱。杂峰变多,101、006、015、107、108、110和113峰被杂峰覆盖,仅剩下003峰和104峰。这可能由于反应浸液残留导致杂峰变多,从而掩盖材料的部分特征峰。部分峰强度发生了变化是由于浸出过程中部分金属浸出导致峰强度降低,这说明C6H8O6具有优良的还原性能和浸出性能,可以有效地浸出废正极材料。
-
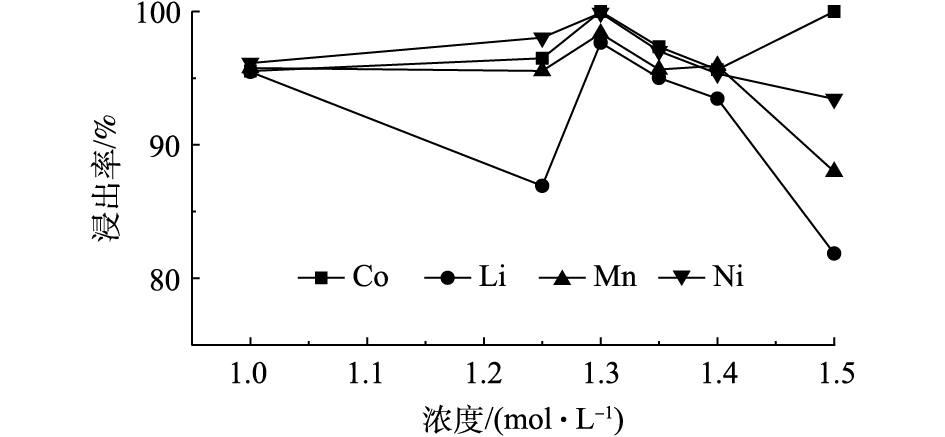
图6为70 ℃恒温条件下,磁力搅拌正极材料进行反应20 min,固液比为25 g·L−1,不同浓度的C6H8O6对Ni、Co、Mn和Li浸出率的影响结果。由图6可知,随着C6H8O6浓度的增加,Ni、Mn和Li的浸出率逐渐增加,Co在C6H8O6为1.3 mol·L−1时,其浸出率达到100%,可见C6H8O6浓度对Co浸出的影响较大,当C6H8O6的浓度大于1.4 mol·L−1时,Ni、Mn和Li浸出率会有所降低,有机酸到达一定浓度后,由于溶液浓度导致抗坏血酸黏度也变大,不利于金属的浸出。以上结果表明,在C6H8O6的浓度小于1.3 mol·L−1时,其浓度与有价金属元素的浸出率成正比,其中C6H8O6浓度对Co的浸出率影响最大。
-
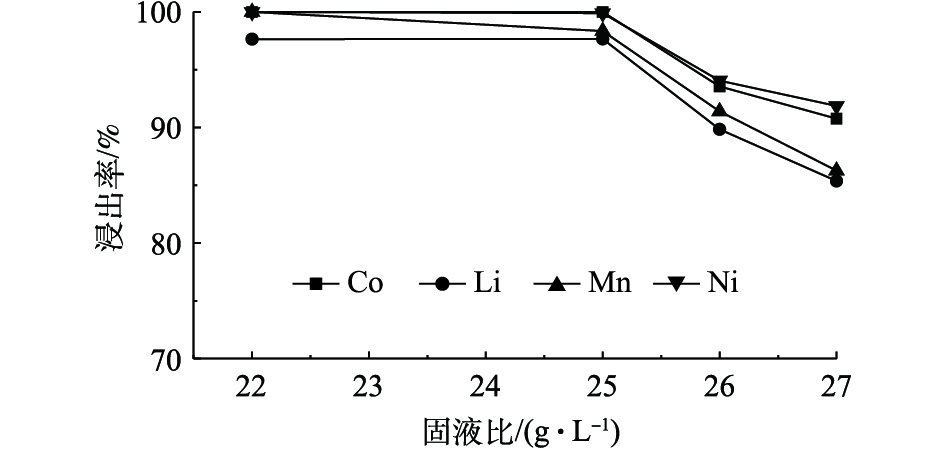
图7为70 ℃恒温条件下磁力搅拌正极材料进行反应20 min,C8H8O6浓度为1.3 mol·L−1,不同固液比对Ni、Co、Mn和Li浸出率的影响。由图7可知,随着固液比的升高Ni、Co、Mn和Li的浸出率逐渐降低,这说明固液比与有价金属元素的浸出率成反比关系,当固液比为22 g·L−1和25 g·L−1时,Co可以达到完全浸出,Ni、Co、Mn在固液比为22 g·L−1时可以达到完全浸出,同时Li的浸出率可以达到97.64%。综合考虑,反应最佳固液比确定为22 g·L−1。
-
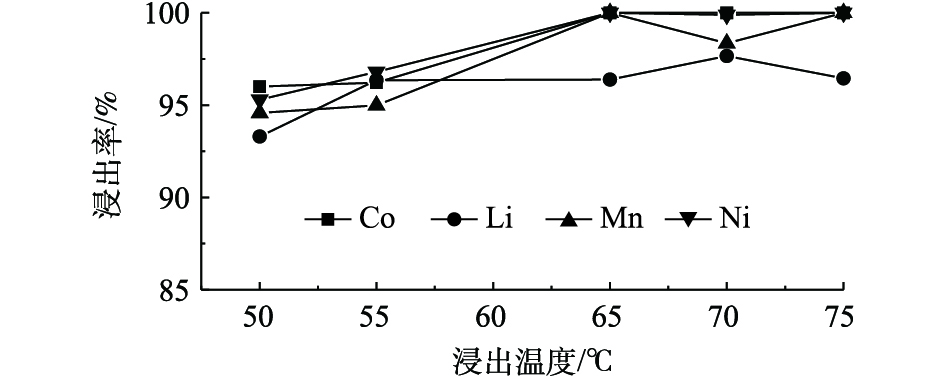
图8为C6H8O6浓度为1.3 mol·L−1,固液比为25 g·L−1,恒温磁力搅拌正极材料进行反应20 min,不同反应温度对Ni、Co、Mn和Li浸出率的影响结果。由图8可知,随着温度的升高,Ni、Co、Mn和Li的浸出率基本呈上升的趋势,其中Co和Mn浸出率得到明显提升,说明Co和Mn的浸出率受温度的影响较大,Ni、Co和Mn在65 ℃条件下能够实现完全浸出,当反应温度为70 ℃和75 ℃时,Mn和Li的浸出率有所降低,这可能是因为此温度下C6H8O6部分分解导致酸的浓度及还原性降低,从而导致Mn和Li浸出率下降,说明Mn和Li的浸出率受抗坏血酸浓度的影响较大。由图8可知,与其他元素相比,Li浸出率受温度影响最小。综合考虑,确定反应最佳温度为65 ℃。
-
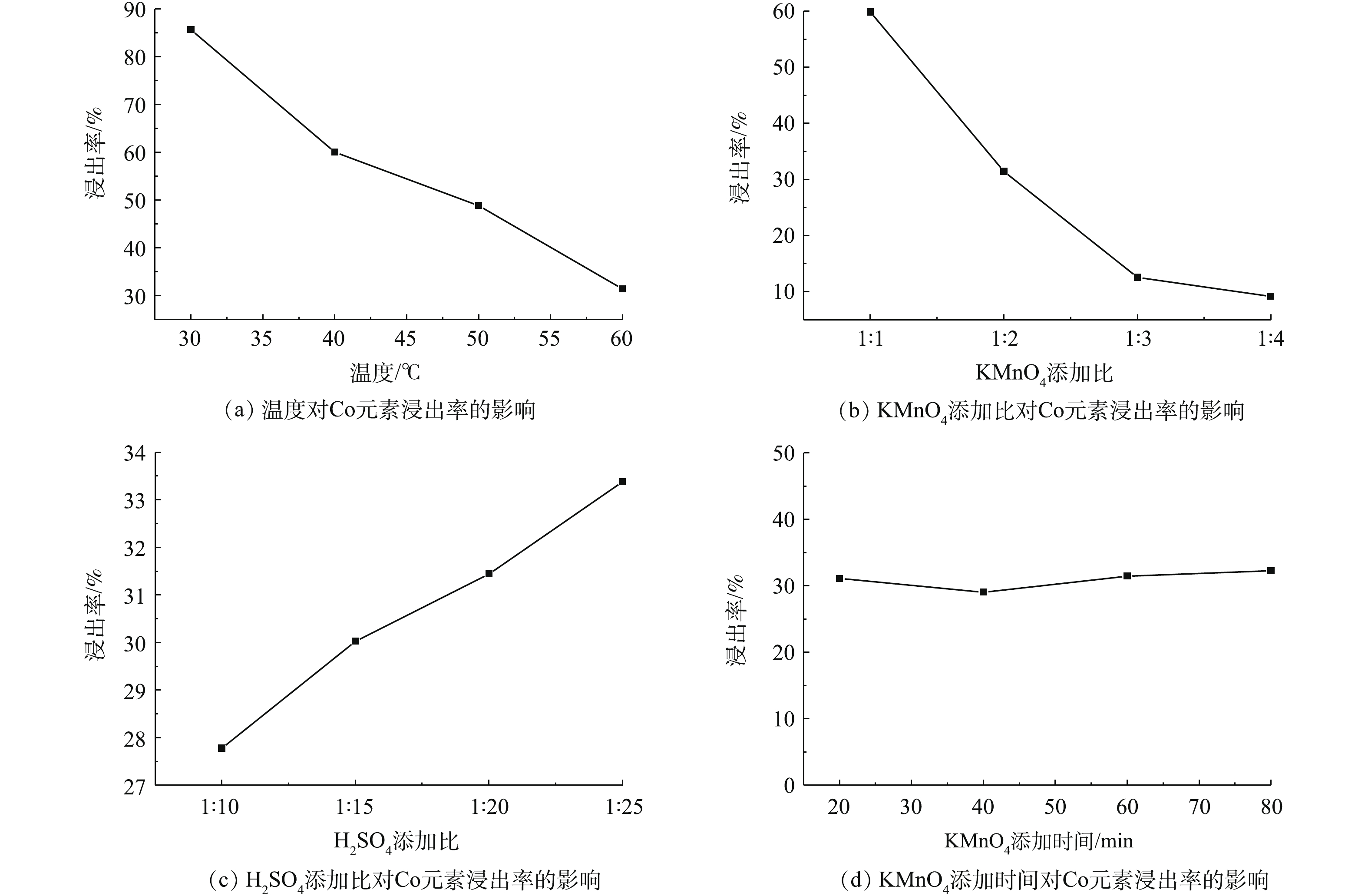
图9为不同实验条件对钴浸出率的影响结果。由图9(a)和图9(b)可知,随着温度的不断升高和KMnO4添加比不断增加,Co的浸出率不断降低,即β-CoC2O4·2H2O的回收率在不断地增加,KMnO4量的增加促使C6H8O6氧化产生H2C2O4,从而沉淀Co(Ⅱ)生成β-CoC2O4·2H2O。由图9(c)可知,随着H2SO4添加比的不断增加,Co的浸出率不断地增加,即β-CoC2O4·2H2O的回收率在不断地降低,说明H2SO4的增加导致溶液的pH减小,pH过低不利于β-CoC2O4·2H2O的沉淀,过量H2SO4的加入使得溶液pH不利于β-CoC2O4·2H2O的沉淀。由图9(d)可知,KMnO4的添加时间对Co的浸出率基本上没有影响。
-
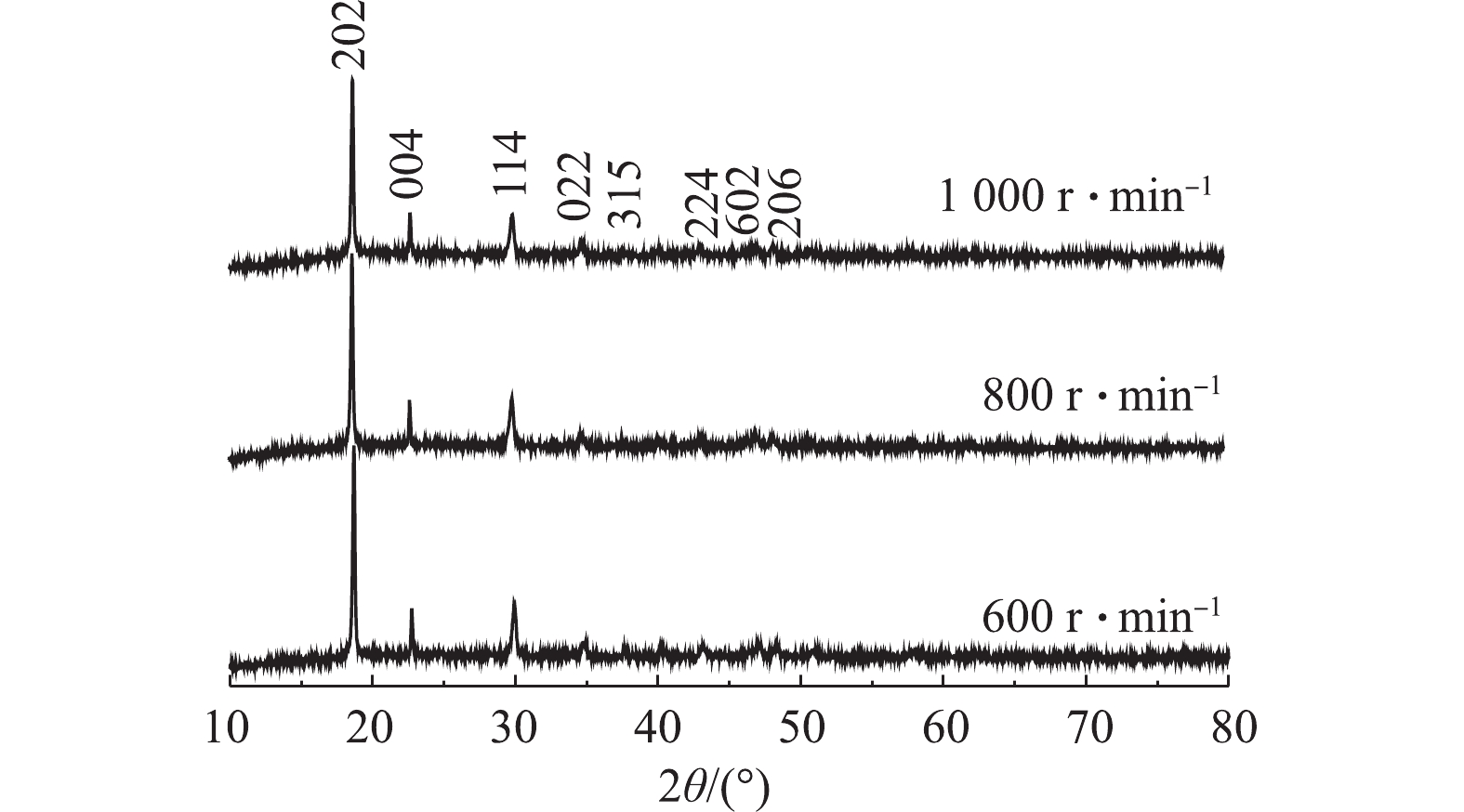
图10为反应温度为60 ℃、C6H8O6为1.3 mol·L−1、正极材料质量用量为25 g·L−1,正极材料/KMnO4添加质量比为1∶4,当反应进行到第20分钟时添加KMnO4,搅拌转速分别为600、800、1 000 r·min−1条件下制备所得的β-CoC2O4·2H2O对应的XRD图谱。样品的衍射峰值与报道的CoC2O4(β-CoC2O4·2H2O,JCPDSNO.25-0250)基本一致。主特征峰在18.6°、22.7°和29.9°,图谱中没有杂峰的存在,结晶良好且没有其他的杂相。与β-CoC2O4·2H2O晶体的特征峰吻合,基本可判断该工艺制备所得为β-CoC2O4·2H2O。由图10可知,当转速为1 000 r·min−1时,其对应的XRD峰宽更宽,响应强度更高,这说明该条件下制备出的β-CoC2O4·2H2O晶体晶粒更小,600 r·min−1时峰型尖锐,说明此时制备出的β-CoC2O4·2H2O的结晶度更好。
-
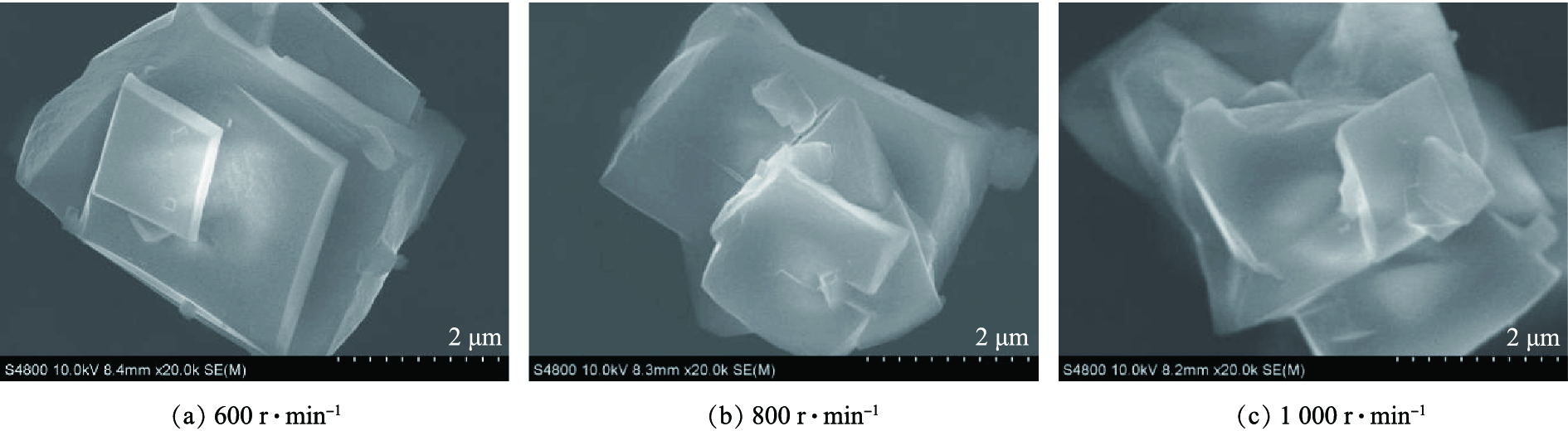
图11为不同转速下制备的β-CoC2O4·2H2O颗粒的扫描电镜图。可以看出,不同转速条件下所制得的β-CoC2O4·2H2O颗粒均为层状构成的立方体,对比图11(a)、图11(b)、图11(c)可知,转速为600 r·min−1下所制得的β-CoC2O4·2H2O颗粒比较规则,而在转速为800 r·min−1和1 000 r·min−1下所制得的β-CoC2O4·2H2O颗粒表面的层状结构略有破损。
-
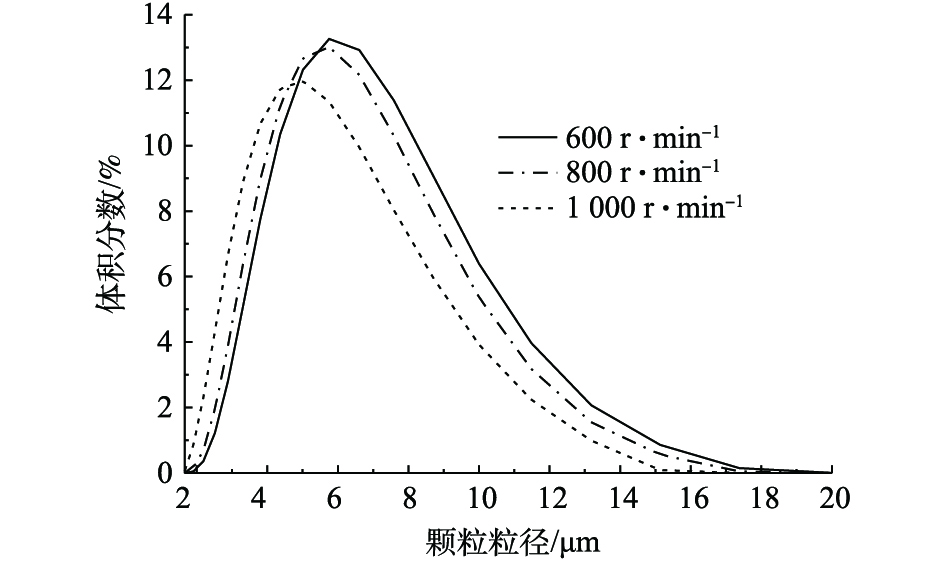
图12为采用马尔文2000激光粒度仪,分散介质为水,对3种不同转速条件下制备的β-CoC2O4·2H2O颗粒进行粒径分布测试结果。实验结果表明,600、800和1 000 r·min−1条件下制备的β-CoC2O4·2H2O平均粒径分别为6.391、6.007和5.276 μm。由图12可知,600 r·min−1条件下制备的β-CoC2O4·2H2O颗粒粒径跨度最大,粒径分布较集中;1 000 r·min−1条件下制备的β-CoC2O4·2H2O颗粒较其他转速条件下制备的β-CoC2O4·2H2O颗粒粒径跨度较小。这说明转数越高,颗粒粒径分布越均匀,制备的β-CoC2O4·2H2O颗粒粒径越小,粒径分布相对比较不集中。
2.1. 浸出前后正极材料相貌分析
2.2. 浸出前后正极材料物相结构分析
2.3. C6H8O6浓度对浸出率的影响
2.4. 固液比对浸出率的影响
2.5. 温度对各元素浸出率的影响
2.6. 不同条件下草酸钴的制备
2.7. 不同转速对CoC2O4物相结构的影响
2.8. 不同转速对CoC2O4形貌结构的影响
2.9. 不同转速对CoC2O4粒径分布的影响
-
1)实验采用1.3 mol·L−1的C6H8O6,正极材料质量/C6H8O6用量为1 : 25(g : mL),65 ℃下反应20 min,能够实现Ni、Co和Mn的完全浸出,Li的浸出率达到96.4%。
2) C8H8O6浸出夜中添加H2SO4,20 min后添加KMnO4反应1 h,正极材料质量/H2SO4用量为1 : 10(g : mL)、正极材料质量/ KMnO4添加量为1 : 4(g : g),在60 ℃条件下,能使正极材料中的Co生成β-CoC2O4·2H2O颗粒,Co的回收率达到91%。
3)采用600 r·min−1作为反应的搅拌速度,能生成层状结构完整、颗粒比较规则的β-CoC2O4·2H2O颗粒。



 下载:
下载: