-
核能作为一种清洁能源,提供了世界约9.7%的能源[1],但核电站泄露事故亦会给人类带来危害,长期暴露其中可对人体产生急、慢性损伤[2]。在核电站事故中,铯(Cs)以气溶胶或灰尘为载体扩散并最终大面积沉降并累积在土壤环境中[3]。据报道[4],日本福岛核事故导致距离其核电站24 km处土壤中核素134Cs和137Cs比活度达到7.8×105 Bq·kg−1。137Cs半衰期长达30 a,其环境健康风险长期存在[5-6]。
放射性污染土壤常用的处理方法主要有铲土法、深翻客土法、植物修复、土壤化学淋洗等[7]。铲土法、深翻客土法快速有效,但会造成土壤肥力损失;植物修复法周期长且含放射性植物的处理成本高,有二次污染的风险。土壤化学淋洗是一种简单、高效的土壤修复方法,淋洗剂的选择及淋洗参数的确定直接决定淋洗效果的好坏。常用淋洗剂有螯合剂、强酸强碱、天然有机酸[8]和强酸弱碱盐[9]等。国内外学者利用淋洗剂对重金属及放射性核素污染土壤进行了大量研究。李婷等[10]研究发现,当FeCl3浓度为10.0 mmol·L−1、液土比为10∶1、淋洗时间为1 440 min时,Pb的去除率可达到96.77%;徐辉[11]研究发现,盐酸、硝酸和柠檬酸对污染土壤中钚的去除率均可达90%左右。但强酸强碱对土壤结构破坏较大,而人工螯合剂价格昂贵且难以在土壤中降解,会长期残留于土壤。相比而言,天然有机酸[8,12]和强酸弱碱盐[9,13](如草酸、硫酸铵)因具备淋洗效果较好和对环境友好等优点已被广泛用于污染土壤淋洗修复。沈威等[14]采用浓度为1.0 mol·L−1草酸淋洗去除土壤中的铀,在40 ℃条件下淋洗8 h后去除率为83.78%。除淋洗剂外,还有研究发现,土壤理化性质、淋洗时间及淋洗液的液固比、老化时间也是影响土壤中Cs去除的重要因素[15]。土壤淋洗过程中会产生大量废液,然而,针对这些具有放射性的废液回收处理却少有研究。日本政府曾针对福岛地区污染农田排水,就地取材使用对Cs吸附能力较强的农田土壤作为吸附剂[7]。另外,天然黏土矿物因其来源广泛、成本低廉、层间阳离子交换能力强及比表面积大等特性[16-17],也常作为吸附剂处理废水。有研究发现,Cs不可逆地与高岭石类及长石类的土壤成分结合[18],且蒙脱石对Cs的吸附性能优于其他矿物[19]。
尽管我国核电站安全系数很高,但不可控因素如地震等导致的核泄漏对周边农田污染风险依然存在。目前,鲜有研究针对我国重要核电站周边农田土壤Cs污染开展淋洗技术探索。本研究旨在筛选出适宜放射性核素Cs污染土壤的优良淋洗剂的同时对淋洗液进行回收处理。比较硫酸铵、草酸和柠檬酸3种淋洗剂对Cs污染土壤的淋洗效果;同时,以蒙脱石和实地土壤为吸附剂,探究其对含Cs淋洗液回收效果的影响,提出针对我国重要核电站周边放射性核素污染土壤的淋洗剂和淋洗工艺参数,为实地土壤脱污提供参考。
全文HTML
-
红沿河核电站位于辽宁省大连市瓦房店市红沿河镇,现已建成4 台百万千瓦级核电机组,年发电量约2.9×1011 J。秦山核电站地处浙江省嘉兴市海盐县,是一座30.0×104 J压水堆核电站。供试土壤采集自大连市红沿河及嘉兴市秦山核电站周边不同利用类型的表层土壤,采样深度为0~5 cm。土壤样品取回后于室内自然风干,剔除大石块、植物根系及可见碎屑,过20 目筛后混合均匀,储存备用。采集的供试土壤理化性质如表1所示。
称取7种处理后的典型土壤各2 kg加入30 mg的氯化铯,加入超纯水不断搅拌,充分混匀后静置,风干后研磨过2目筛。老化14和140 d,在4 ℃条件下冰箱储存备用。测得土壤中Cs浓度为15.0 mg·kg−1。经前期培养,Cs的可迁移性相对稳定、组分分布相对均匀[20]。
-
1)淋洗剂筛选。选取老化14 d的7种Cs污染土壤样品,各准确称量1.0 g样品于50 mL离心管,分别加入10 mL 0.5 mol·L−1硫酸铵、草酸和柠檬酸,随后置于恒温振荡器(SHA-2,常州菲普实验仪器厂)中以185 r·min−1的转速在(25±1) ℃下振荡120 min,取样过0.45 μm滤膜后测Cs浓度[21],评估不同淋洗剂对Cs污染土壤的淋洗效果,筛选出最佳淋洗剂。
2)淋洗条件优化实验。根据最佳淋洗剂筛选实验的结果,选取淋洗效果最优的硫酸铵作为淋洗药剂,采用代表北方红沿河电厂周边土壤的2号土样和代表南方秦山电厂周边土壤的7号土样。分别称取老化14和140 d的2号和7号土壤样品各1.0 g,置于50 mL离心管中,分别加入10 mL 0.5 mol·L−1硫酸铵淋洗剂,在(25±1) ℃下恒温振荡(185 r·min−1)120 min后取样。为考察淋洗时间和液固比对淋洗效果的影响,分别设置淋洗时间为20、60、120和240 min,液固比分别为5∶1、10∶1和20∶1。每个处理均设置3个重复。
-
称取0.05 g蒙脱石、0.10 g 3号与7号土壤移入离心管中,加入6 mL含Cs浓度分别为1、2、3、4和5 mol·L−1淋洗液,在(25±1) ℃下恒温振荡(185 r·min−1)24 h,过0.45 μm滤膜后测Cs浓度。为考察硫酸铵浓度对蒙脱石吸附淋洗液中Cs的影响,设置硫酸铵浓度分别为0、0.0010、0.0025和0.0100 mol·L−1,每个处理均设置3个重复。
-
为研究不同吸附剂对含Cs淋洗剂吸附能力,运用Freundlich等温吸附方程进行线性拟合,表征其表面吸附量和介质中溶质平衡浓度之间的关系,探究吸附剂的吸附能力,以R2作为判断标准。Freundlich吸附等温方程如式(1)所示。
式中:Q为最大吸附容量,mg·g−1;KF为Freundlic吸附容量,mL·g−1;n为Freundlic吸附强度。
1.1. 供试土壤采集与制备
1.2. 化学淋洗实验
1.3. 淋洗液中Cs吸附实验
1.4. 数据处理方法
-
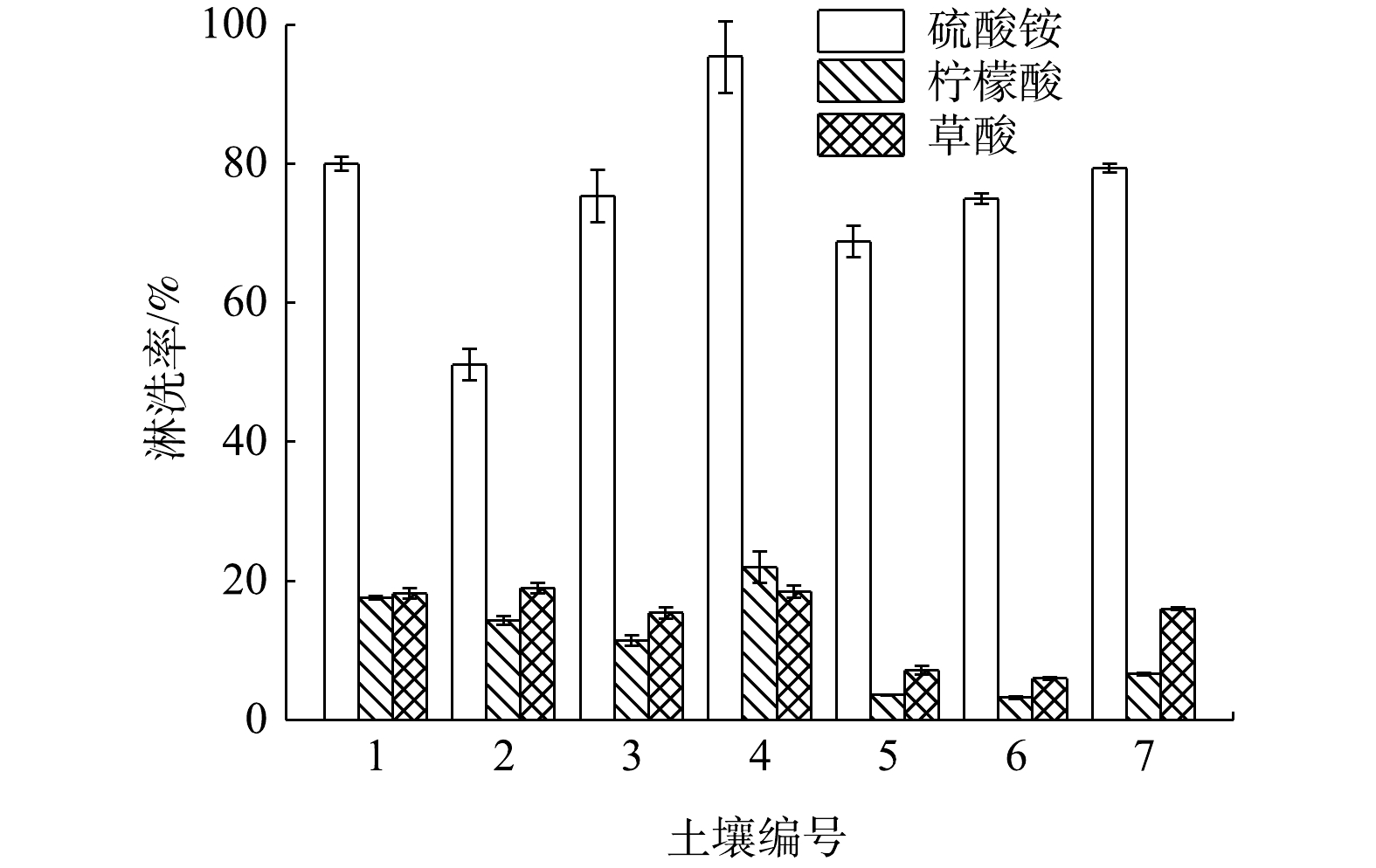
1)淋洗剂的影响。不同淋洗剂对老化14 d的Cs污染土壤淋洗效果如图1所示。采用硫酸铵、柠檬酸和草酸淋洗120 min后,Cs污染土壤的淋洗效率分别为45%~93%、8%~26%和4%~22%,添加硫酸铵的淋洗剂对Cs污染土壤的淋洗效果明显优于柠檬酸和草酸。KIM等[13]对韩国某核电站反应堆周围放射性核素污染土壤进行淋洗实验发现,由于草酸易被生物分解,形成稳定的金属络合物,所以其对Co及Cs的淋洗效果优于其他种类,这与本研究结果不同。其主要原因可能是:一方面Cs污染土壤通过静电作用吸附大量的
NH+4 ,强化了NH+4 与土壤中Cs的交换,以增强硫酸铵对Cs污染土壤的淋洗效果[22];另一方面SO2−4 的存在抑制了Ca2+等与NH+4 的交换,进一步提升了Cs污染土壤的淋洗效果[23]。同时,由于本研究分别选取我国北方及南方某电厂周边土壤,与韩国某核电站反应堆周边污染土壤相比,其两者土壤基本理化性质可能有所差异,我国南北方电厂周边土壤中Cs以可交换态存在的占比可能高于韩国反应堆周边土壤,使得NH+4 与土壤中Cs的交换反应增多,导致本研究结果为硫酸铵作为淋洗剂效果优于草酸。通过硫酸铵对不同Cs污染土壤样品的淋洗效果发现,2号和6号土壤的淋洗率较低,分别为51.1%和48.0%;1和5号土壤的淋洗效率最佳,分别为80.0%和95.4%。经分析土壤的理化性质发现,导致不同Cs污染土壤淋洗效果差异显著的原因可能与土壤中的有机质含量和阳离子交换量有关。例如,王瑞祥等[24]发现,土壤有机质通过含氧官能团进行络合或螯合反应专性吸附Pb2+,且吸附能力及吸附量随有机质含量的增加而增大。这可能导致有机质含量高的土壤淋洗效果较差,也是硫酸铵对2和6号Cs污染土壤的淋洗效果差的原因。
2)老化时间的影响。选取硫酸铵作为淋洗剂,研究不同老化时间对Cs污染土壤淋洗效果的影响,结果如图2所示。结果显示,在相同浓度硫酸铵的条件下,随着老化时间的延长,土壤中Cs的淋洗率显著降低。2号Cs污染土壤的淋洗率从51.1%降低至25.0%,8号Cs污染土壤的淋洗率从79.4%降低至40.0%,2种Cs土壤的淋洗率均降低了1倍。老化时间增长,表明Cs离子与土壤胶体相互作用时间也随之增加,并通过物理吸附、离子交换、微孔扩散及微沉淀等方式聚集在土壤中,使得其可浸提性、可交换性逐渐降低,从而降低淋洗效率。这与林瑞聪等[25]的研究结果一致,即随着老化时间延长,放射性核素Cs与土壤的结合强度不断增加,总分配系数不断降低,导致Cd(Ⅱ)和Cr(Ⅲ)的淋洗效率降低。
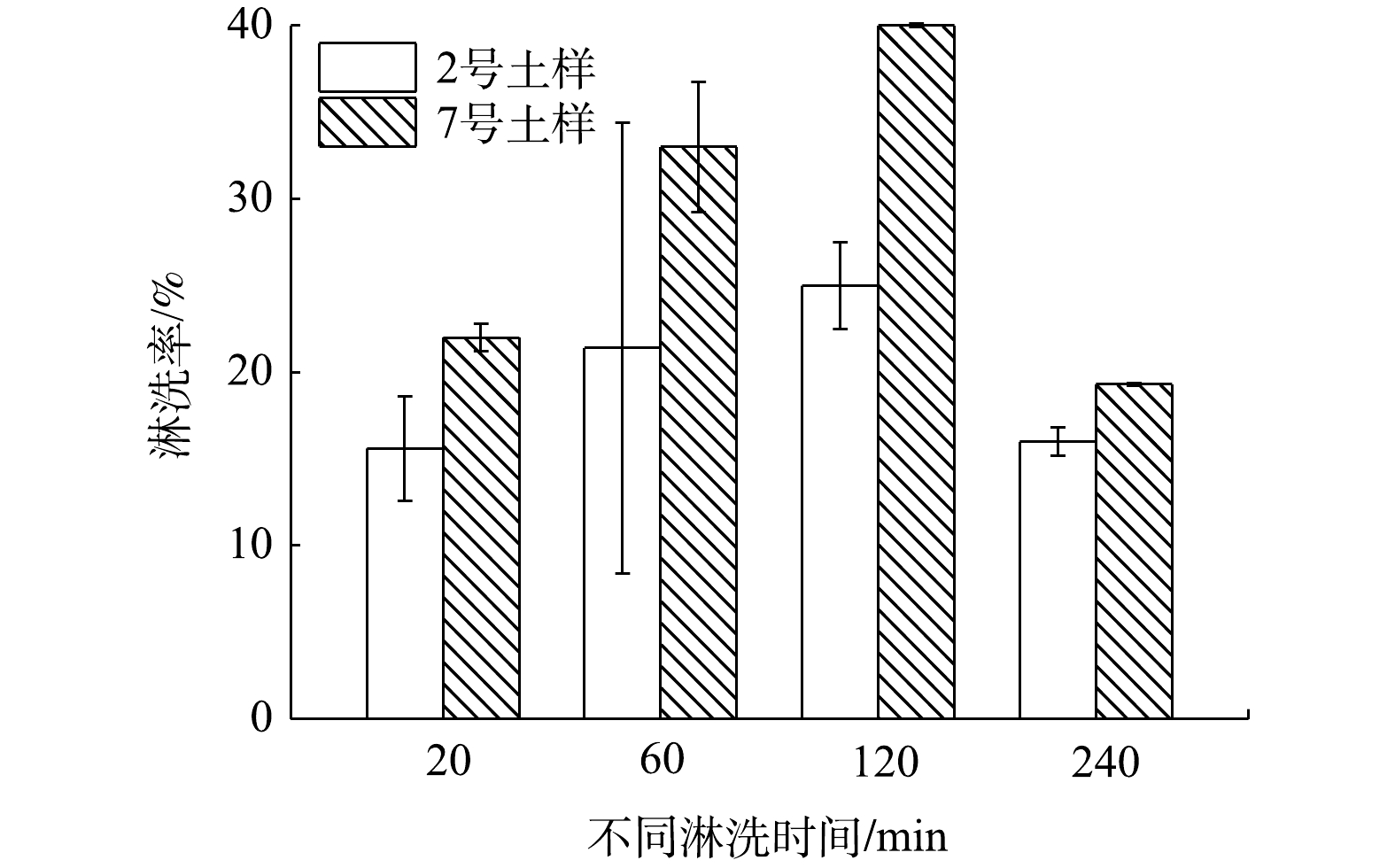
3)淋洗时间的影响。淋洗是固液体系中典型的非催化非均相反应[26],淋洗时间会影响土壤中重金属的淋洗效率。硫酸铵浓度为0.5 mol·L−1时,不同淋洗时间下土壤中Cs的淋洗效率如图3所示。如图所示,随着淋洗时间的延长,2种土壤中Cs的淋洗效率呈先上升后下降趋势;淋洗率在120 min达到最大,分别为25.0%和40.0%。当淋洗时间为240 min时,2种土壤中Cs的淋洗率分别降低了9.0%和20.7%,这可能是因为淋洗后期淋洗液形成乳化液,发生了反吸附过程,从而导致淋洗效率显著降低。
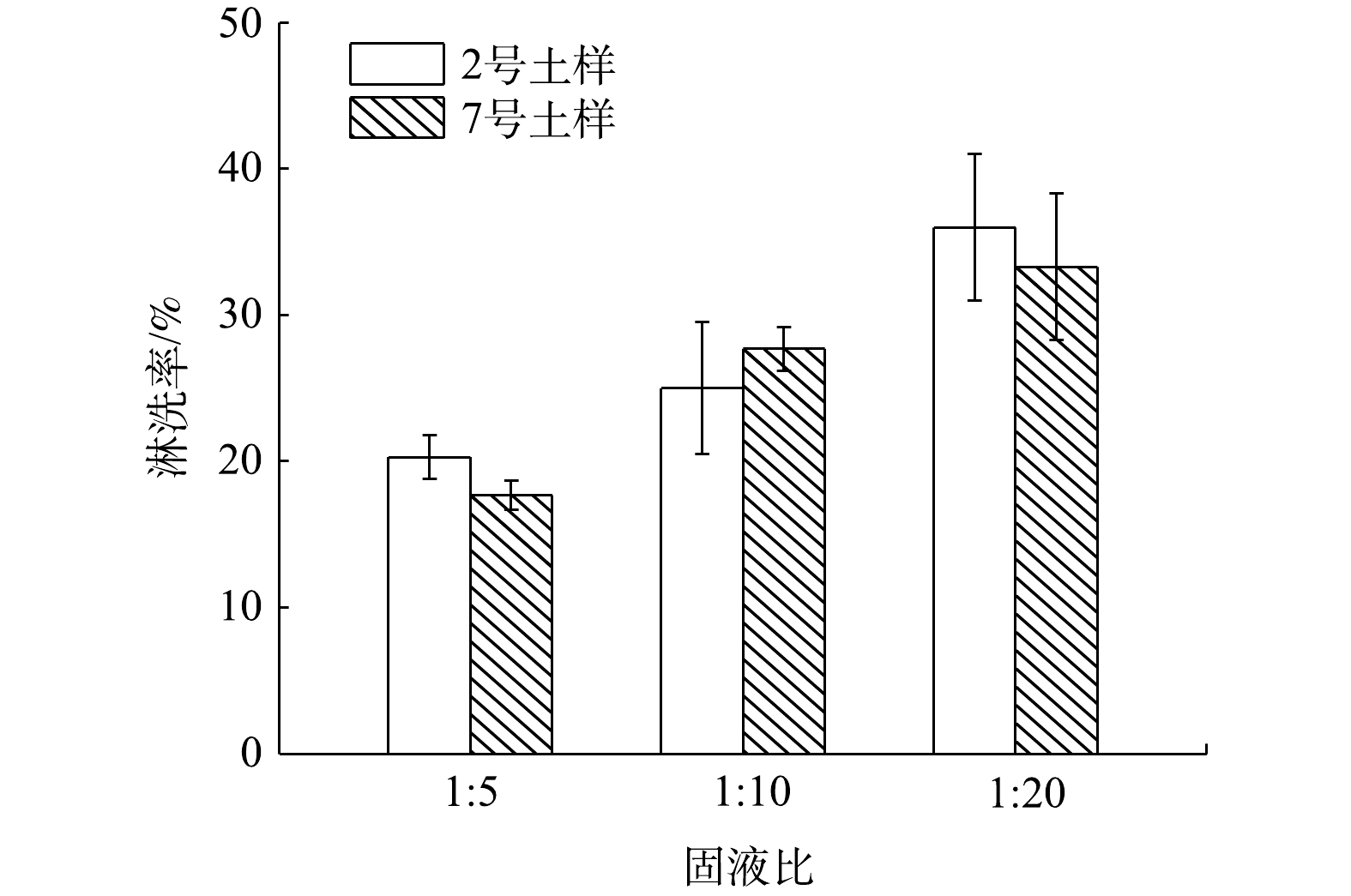
4)液固比的影响。不同液固比对Cs污染土壤淋洗效率的影响如图4所示。当液固比为5∶1时,2号和7号土壤中Cs的淋洗率最低,分别为20.1%和17.5%。随着液固比从5∶1升至20∶1,2和7号土的淋洗率都呈现上升的趋势,这符合土壤盐分淋洗规律[27]。最终,当液固比为20∶1时,3号土的淋洗效率最高达35%,7号土的淋洗效率最高达32%。这可能是因为,当液固比较低时,淋洗液与土壤未能均匀混合,从而影响淋洗剂对Cs污染土壤的淋洗效率。王东辉等[28]的研究结果也得出相似规律,即随着液固比的升高,淋洗剂与土壤中放射性核素Cs的结合位点也相应增多,交换与络合能力不断增强,从而导致淋洗效率也随之提高。
-
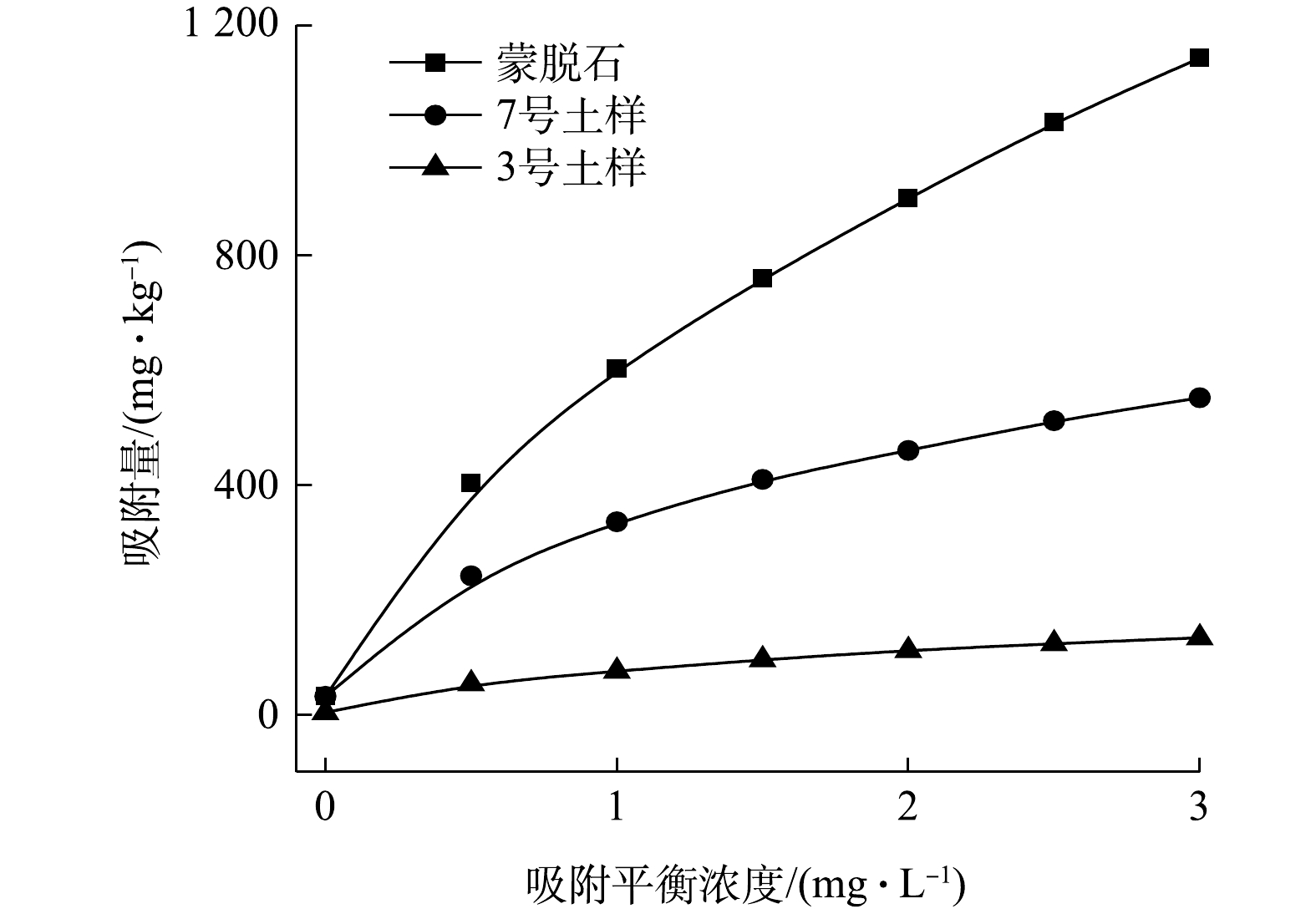
1)不同吸附剂对Cs污染土壤淋洗液吸附能力影响。吸附剂的种类是影响淋洗液中重金属回收的关键影响因素[29]。本研究选取蒙脱石、3号土壤和7号土壤作为吸附剂,分析蒙脱石和土壤对污染土壤中Cs的回收效率,结果如图5所示。由图5可见,随着平衡浓度的增加,蒙脱石、3号和7号土壤对Cs的吸附量的变化一致,均呈现逐渐增加的趋势。采用Freundlich方程拟合实验数据能更好地描述Cs在不同吸附质上的吸附行为(表2),R2>0.99,表明蒙脱石和土壤对淋洗液中的Cs均符合Freundlic非线性等温吸附方程。通过计算,蒙脱石、7号土壤和3号土壤的lnKF分别为6.40、5.75和4.35;n分别为0.58、0.54和0.46。由于Freundlich等温吸附常数KF反映的是吸附剂对Cs吸附能力的大小,KF越大,表明吸附剂对Cs的吸附能力越强。因此,3种不同吸附剂对污染土壤中Cs的吸附能力的大小顺序分别为:蒙脱石最佳、7号土壤次之、3号土壤最差。蒙脱石对Cs的吸附能力明显高于3号和7号土壤样品,这主要是因为蒙脱石由Si-O四面体和Al-(O,OH)或Mg-(O,OH)八面体构成的[30],晶层间结合力较弱,具有较好的阳离子交换性以及较大的比表面积,因此,其对Cs有很强的吸附性。这与智伟迪[31]的研究结果一致。此外,由于Cs在环境中的迁移与土壤中的粘土矿物种类、含量离子强度和天然有机物有关[32],从而导致3号与7号土壤对含Cs淋洗液的吸附能力有明显差异。
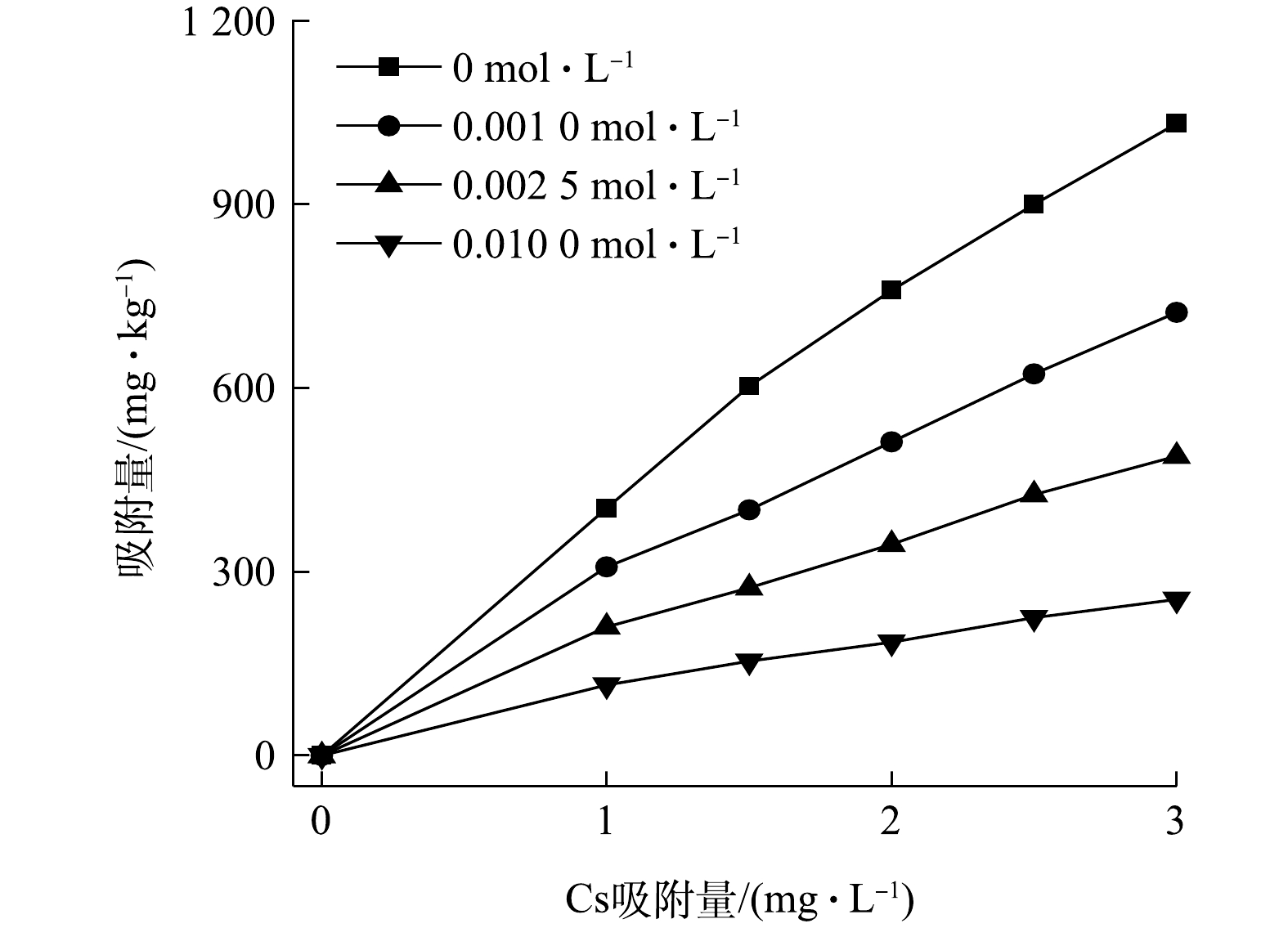
2)不同浓度硫酸铵对淋洗液中Cs吸附的影响。由图6可见,随着硫酸铵浓度升高,蒙脱石对淋洗液中Cs的吸附效率都明显降低,当硫酸铵浓度分别为0、0.001 0、0.002 5和0.010 0 mol·L−1时,吸附24 h后,蒙脱石对Cs的吸附量分别为1032.0、723.6、489.0和264.5 mg·kg−1。采用Freundlich等温吸附方程对不同条件下Cs的吸附数据进行拟合,发现lnKF分别为6.40、5.71、5.32和4.74,n分别为0.85、0.79、0.78和0.73,表明随着硫酸铵浓度的增加,蒙脱石对淋洗液中Cs的吸附能力和吸附强度均降低。这主要是因为硫酸铵浓度升高,淋洗液中含有大量的
NH+4 ,与Cs产生竞争作用,降低了蒙脱石对Cs的吸附能力,从而导致淋洗液中Cs的吸附效率逐渐降低。PIRI等[33]的研究结果与本研究相似,该研究以黏土作为吸附剂吸附水中的Zn,结果表明,柠檬酸显著抑制了黏土矿物对Zn的吸附,其原因可能是柠檬酸与Zn反应生成的可溶性复合物或柠檬酸与金属竞争土壤表面的吸附位点所致。
2.1. 不同条件下Cs污染土壤淋洗修复效果
2.2. 不同条件下Cs污染土壤淋洗修复效果研究
-
1)相比草酸及柠檬酸,添加硫酸铵的淋洗剂对Cs污染土壤的淋洗效果最佳;其淋洗效率随老化时间及液固比增加而升高,淋洗120 min时淋洗效率最高。
2)通过吸附回收淋洗液Cs实验发现,蒙脱石对Cs的吸附能力高于核电站周边土壤。淋洗液中残留硫酸铵对蒙脱石吸附Cs的抑制效果显著。
3)建议硫酸铵淋洗液循环使用,以使得残留的
NH+4 浓度降低至低于0.001 mol·L−1。





 DownLoad:
DownLoad: