-
氯霉素是一种用于治疗人类和动物细菌感染的典型广谱抗生素类药物[1]。尽管氯霉素在1994年就被一些场合禁止过,但由于其易获得和成本低,在我国很多养殖业中仍然被广泛使用,使得其在污水处理厂及地表水中频繁被检测到。研究表明在水环境中即使是痕量水平的氯霉素都可能会威胁到生态系统和人类健康[2]。因此,实现氯霉素的有效去除对于维护生态环境和人类身体健康具有十分重要的现实意义。
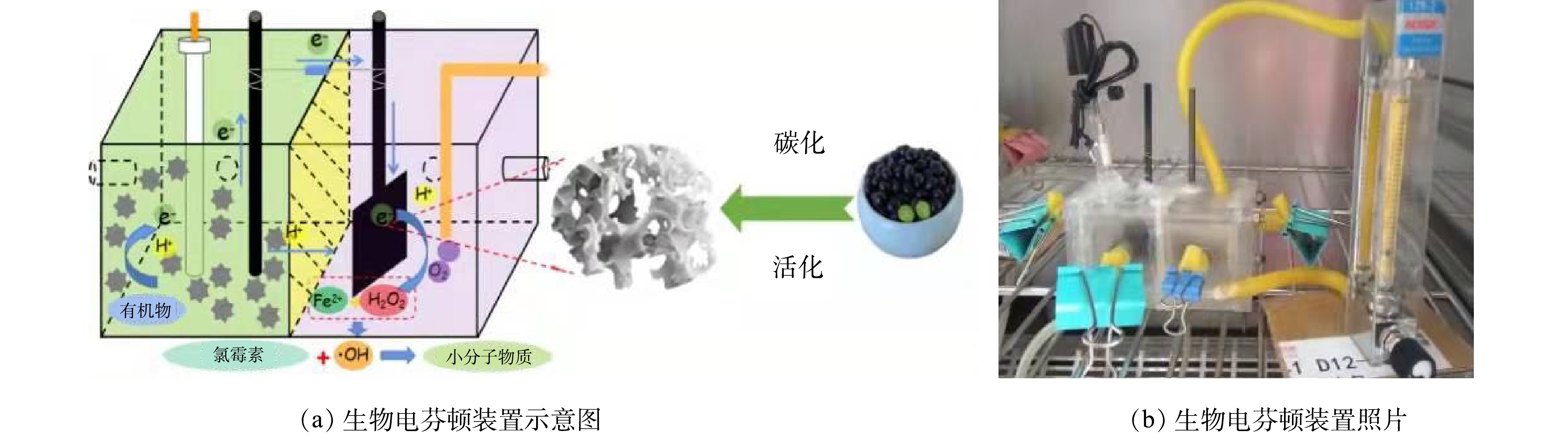
利用电芬顿技术可以对氯霉素进行高效地降解,但是这种工艺要求施加恒定电压,从而使得运行成本偏高[3]。生物电芬顿技术就是为解决这一问题而开发的,其为一种安全环保的新型生物发电技术[4]。生物电芬顿体系通常包括阳极室、阴极室和阳离子交换膜,产电微生物在阳极溶液中将化学能转换为电能,从而产生质子和电子(式(1)),随后通过阳离子交换膜和外部电路传输到阴极室,同时在阴极室曝入空气,使氧气获得电子和质子生成H2O2(式(2)),然后H2O2与Fe2+发生芬顿反应生成羟基自由基(·OH) (式(3)),而·OH作为强氧化性物种氧化甚至矿化有机物(式(4)~(5)),同时芬顿试剂Fe2+被氧化为Fe3+,随后又被从外电路传到阴极室的电子还原为Fe2+(式(6)),从而实现了Fe2+的循环使用,提高了芬顿试剂的利用率[5-6]。
生物电芬顿的阴极催化材料会影响其产电性能及降解污染物的性能。由于生物炭具有丰富的孔隙和较大的比表面积,可为氧的还原反应提供丰富的活性位点,缩短氧的还原-扩散路径[7],此外,有研究[8-9]表明,掺杂N、P、S、O和B等杂原子的碳材料可以作为电荷载体提供具有高电子亲合度的电子,并加速O2的吸收和还原。
本研究以黑豆为前驱体,制备了氮氧自掺杂型生物炭催化材料,考察了其负载于生物电芬顿阴极后的产电性能以及对氯霉素的降解效果。由于生物电芬顿体系存在氧供应不足、电压不稳定等问题,因此,认为生物电芬顿对氯霉素的降解作用有限,可以用于降解地表水中的低浓度氯霉素,故将其用于1 mg·L−1和50 mg·L−1的氯霉素降解。本研究以期为生物炭在生物电芬顿体系中的应用提供参考。
-
试剂主要包括磷酸二氢钠(NaH2PO4)、醋酸钠(CH3COONa)、钼酸钠(Na2MoO4)、二水氯化钙(CaCl2· 2H2O)、一水硫酸锰(MnSO4·H2O)、六水氯化钴(CoCl·6HO)、二水氯化铜(CuCl2·2H2O)、十二水合硫酸铝钾(KAl(SO4)2·12H2O)、六水硫酸钴(CoSO4·6H2O)、硼酸(H3BO3)、七水合硫酸亚铁(FeSO4·7H2O)、六水氯化镁(MgCl2·6H2O)、草酸钛钾(K2TiO(C2O4)2·2H2O)、四水氯化亚铁(FeCl2·4H2O)、氯霉素(C11H12Cl2N2O5)、甲醇(CH3OH)。
仪器主要包括ESJ120-4B型电子天平、S1020型紫外可见分光光度计、PB-10型pH计、CHI660E型电化学工作站、2695型高效液相色谱仪、HN1006B型超声清洗仪。
-
该反应器为含有容量为28 mL的双室型微生物燃料电池。阳极室与阴极室中间用阳离子交换膜分隔开,采用石墨毡作为阳极,采用2 cm×2 cm含有NOPC催化材料的碳布作为阴极电极,阴极溶液为Na2SO4溶液,以2 cm×2 cm的纯碳布作为对照,其反应装置如图1所示。
-
功率密度曲线通过测量梯度变化的外电阻值获得。测试前,先把新鲜的营养液换至生物电芬顿阳极室,待电压开路电压达到最大稳定值后,使用变阻箱对外部电流进行控制,使外部电路的电阻由2 000 Ω降低到20 Ω,每一次更换R值后需稳定5 min,再使用万用表测量电压,并将3个电压值的平均值作为外电阻阻值下的电压。根据对功率、电流密度的计算可以得出功率密度和极化曲线。
循环伏安曲线通过电化学工作站上的三电极体系对生物电芬顿进行循环伏安测试获得。在电化学工作站中施加循环电压,将起始电压以恒定的速度加到最终电势,然后以同样的速度恢复到初始电势,并将其作为周期记录下来。以甘汞电极作为参考电极,扫描频率为-0.8~0.8 V,扫描速度为10 mV·s−1,温度为25 ℃左右。
-
将预先制备的NOPC负载到生物电芬顿阴极的碳布上,以阴极碳布未负载NOPC的生物电芬顿作为对照。在阴极室的pH=3,1 mmol·L−1 Fe2+的Na2SO4溶液中,设置氯霉素的初始质量浓度为50 mg·L−1和1 mg·L−1,使用生物电芬顿体系对氯霉素进行降解。
氯霉素浓度的测定:使用高效液相色谱法(high-performance liquid chromatography,HPLC))测定溶液中氯霉素的浓度,其去除率采用式(7)进行计算。
式中:A为氯霉素去除率,%;C0和C分别为氯霉素的初始和t时刻的质量浓度,mg·L−1。
-
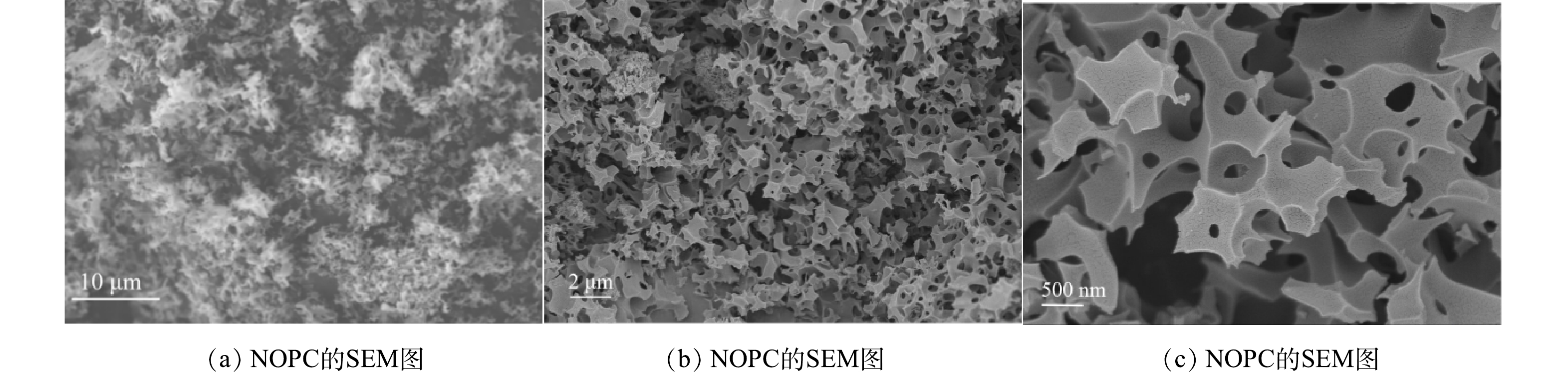
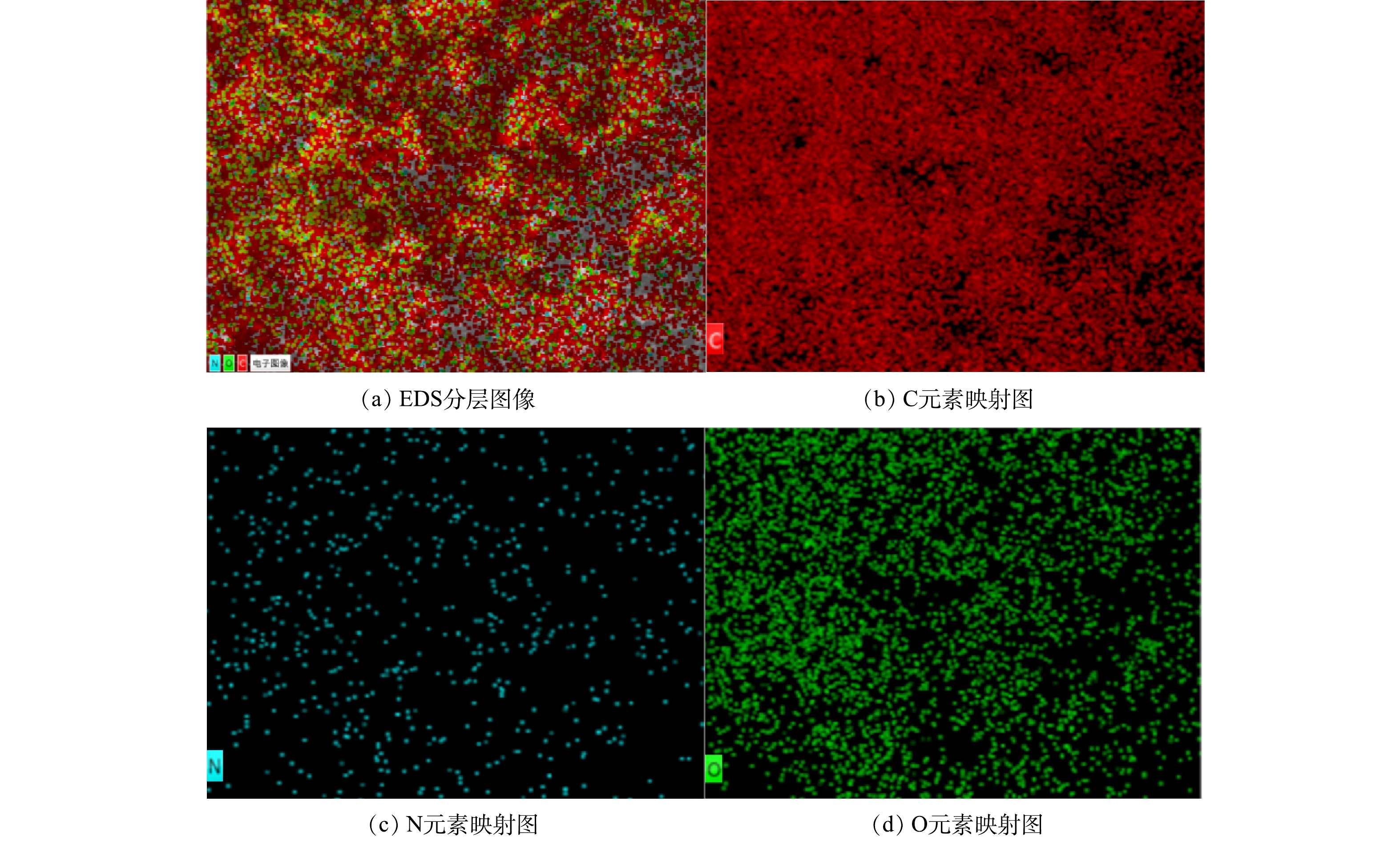
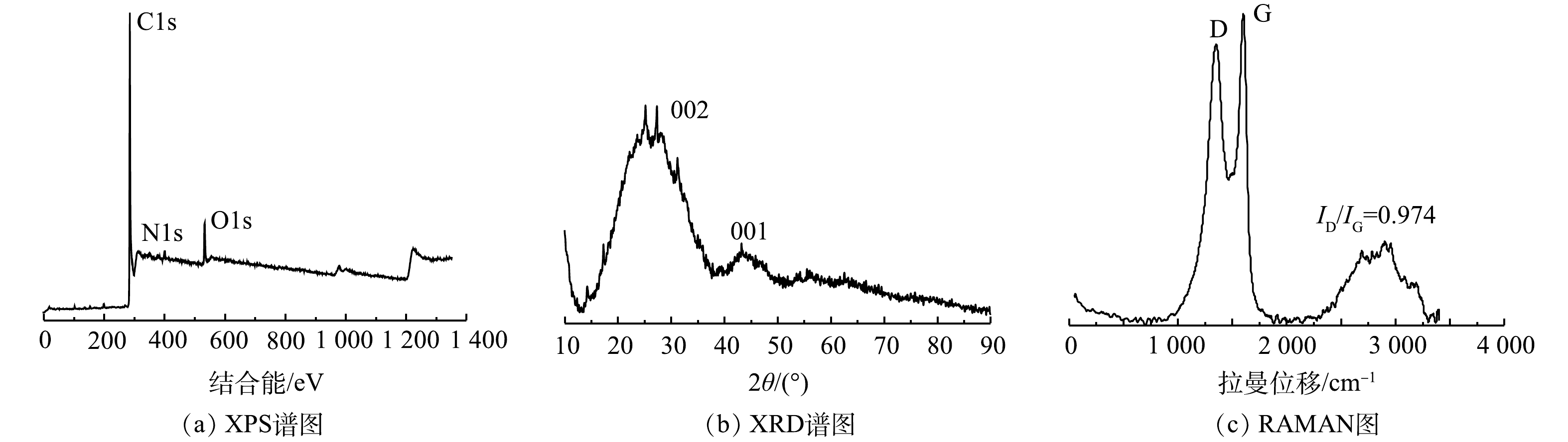
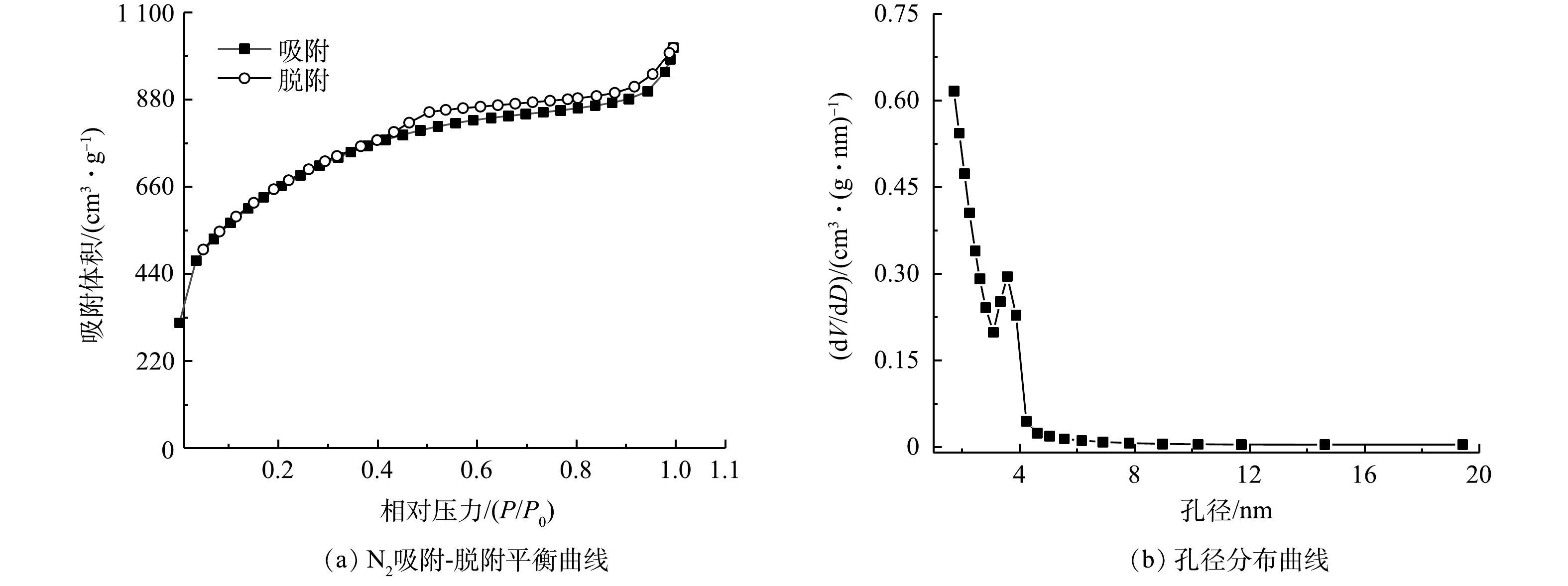
图2(a)~(c)反映了 NOPC在不同倍率下的SEM图像。结果显示其具有多孔结构的特征。由图3中EDS扫描结果可以看出,NOPC中的主要成分是碳、氮、氧,且可以看出该材料具有氮和氧的自掺杂,2种元素均匀地分布在材料当中。另外,XPS全谱图检测到C、N、O特征峰,也相应证实了该项结果。由图4(b)可见,通过比对XRD卡片发现其分别对应石墨碳的002和101晶格平面。说明实验所采用的材料完全被碳化,为了证实这个结论,对NOPC材料进行拉曼测试。由图4(c)中可以得到,ID/IG=0.974近似等于1。说明NOPC催化材料的sp3-C和缺陷比sp2-C更多,但两者几乎没有区别。通过BET对催化材料进行了比表面及孔隙尺寸的分析。这种吸附类型为 IV型吸附等温线(图5(a)),在P/P0>0.1时很清楚地看见出现滞后环。图5(b)为NOPC的孔径分布图。可见,NOPC材料为微孔-介孔类材料,其孔径约为3 nm。
-
1) 启动生物电芬顿系统。使用稳定运行0.5 a的微生物燃料电池系统,并用PBS溶液、微量金属元素溶液、醋酸钠溶液作为阳极室产电细菌的营养液,Na2SO4溶液作为阴极室电解液,以氧气作为阴极室的电子受体。在启动时,外部电路维持1 000 Ω的外部电阻,并在启动时使用数据采集卡(北京启创墨菲电子技术有限公司MPS-010602)持续地获取输出电压,输出电压见图6。从图中可以看出,在接种后的生物电芬顿系统的电压持续上升40 h后,可以得到260 mV的最大输出电压,证明成功地启动了生物电芬顿系统。
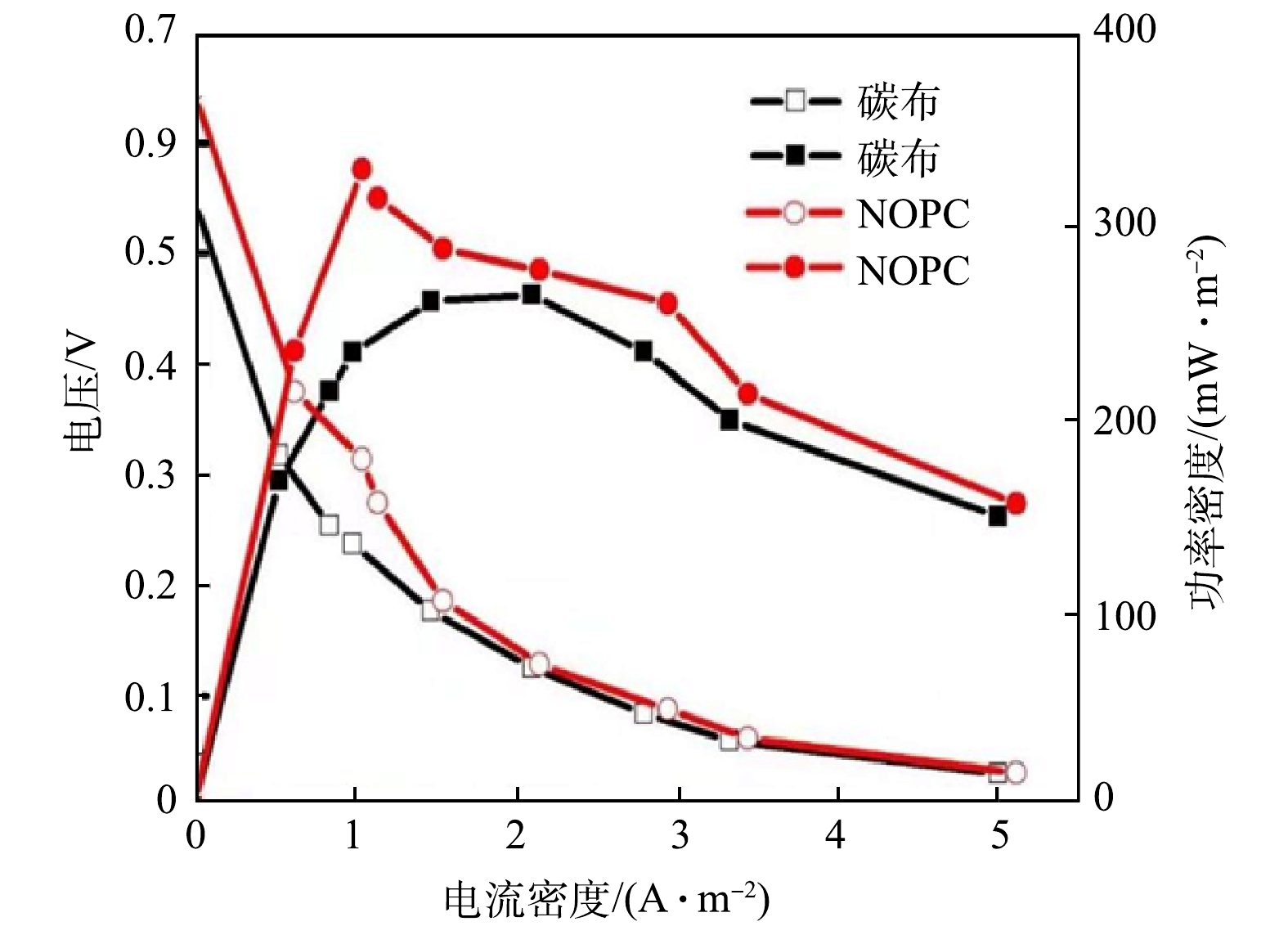
2)极化及功率密度曲线。将负载有NOPC催化材料的碳布作为生物电芬顿体系的阴极电极,以未负载NOPC催化材料的生物电芬顿体系作为对照。在此基础上,将外部电阻值由2 000 Ω降低至20 Ω,得出负载和未负载 NOPC催化材料的生物电芬顿体系的功率密度和极化曲线。通过测定得出未负载NOPC的生物电芬顿体系内阻为202.53 Ω,而负载NOPC的生物电芬顿体系内阻为147.27 Ω,比对照内阻降低了27.28%。有研究[10-11]表明,内阻会影响生物电芬顿体系的产电性能,内阻越小,输出电压和功率密度就越大。
由图7可得出,负载NOPC阴极的生物电芬顿系统最大功率密度为329.35 mW·m−2,相较于对照,最大功率密度提高了68.27 mW·m−2。这是由于 NOPC具有多孔结构和较大的比表面积,使其具有良好的电子转移性能,从而减少了生物电芬顿系统的内部电阻[12],此外,生物炭材料中加入了氮原子和氧原子,使其具有较大的活化位点和电负性,有利于氧的吸收,进而提高反应速度,进一步促进内阻的降低使得功率密度提高 [12-13]。
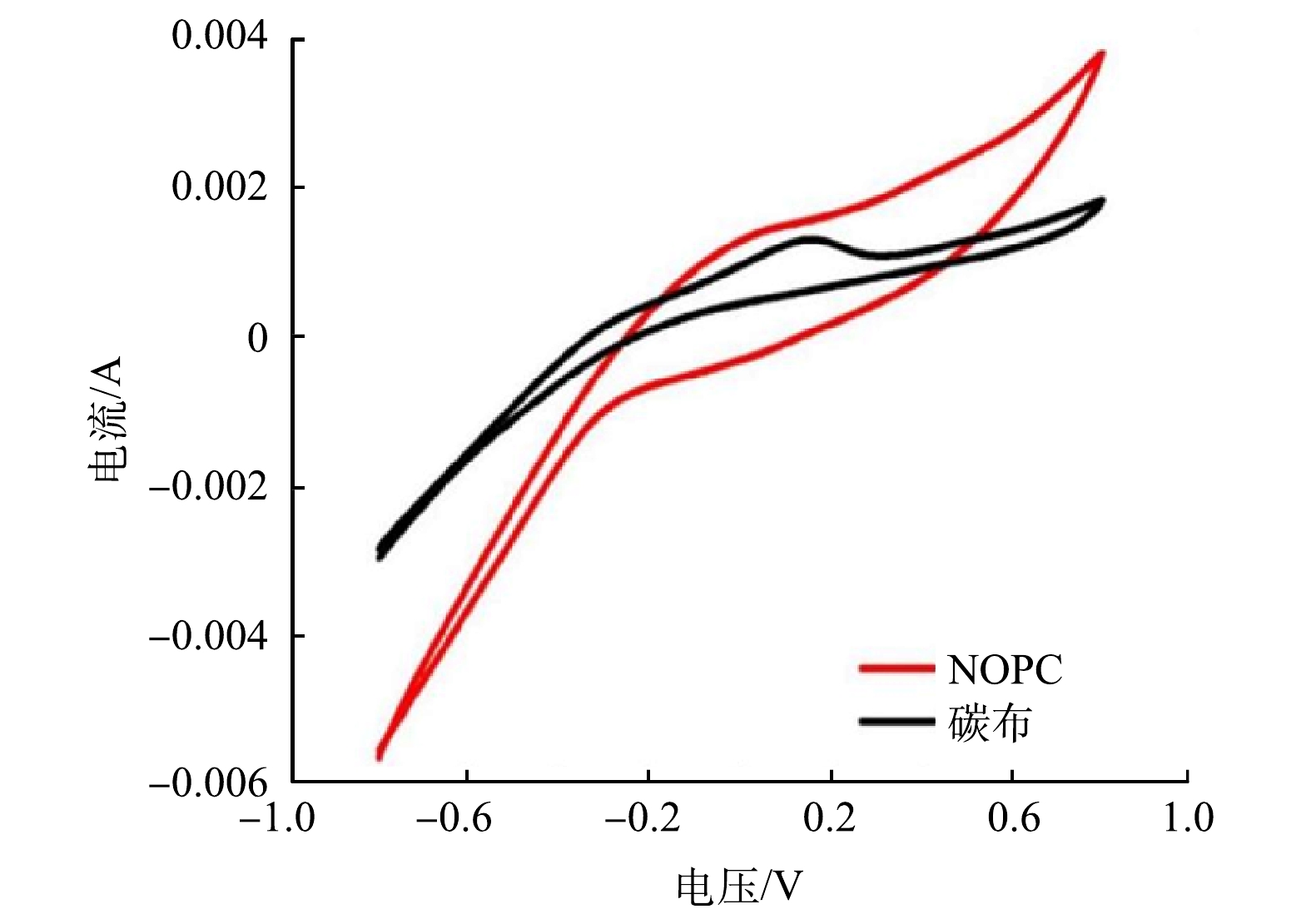
3)循环伏安曲线。循环伏安曲线可以对催化剂进行氧还原能力评价。氧化还原的主要影响因素是氧化还原电压和电流密度[14],同时,氧还原峰电流的绝对值也相应地提高了其氧还原活性[15]。图8为纯碳布和NOPC负载阴极的生物电芬顿体系的循环伏安图。可以看出,在−0.8~0.8 V内,NOPC负载阴极的生物电芬顿氧化还原电压有明显的正方向变化,而且峰值电流的绝对值也高于纯碳布电极的生物电芬顿体系。这表明 NOPC催化材料能有效地减小氧化还原过电势,提高阴极反应电流。
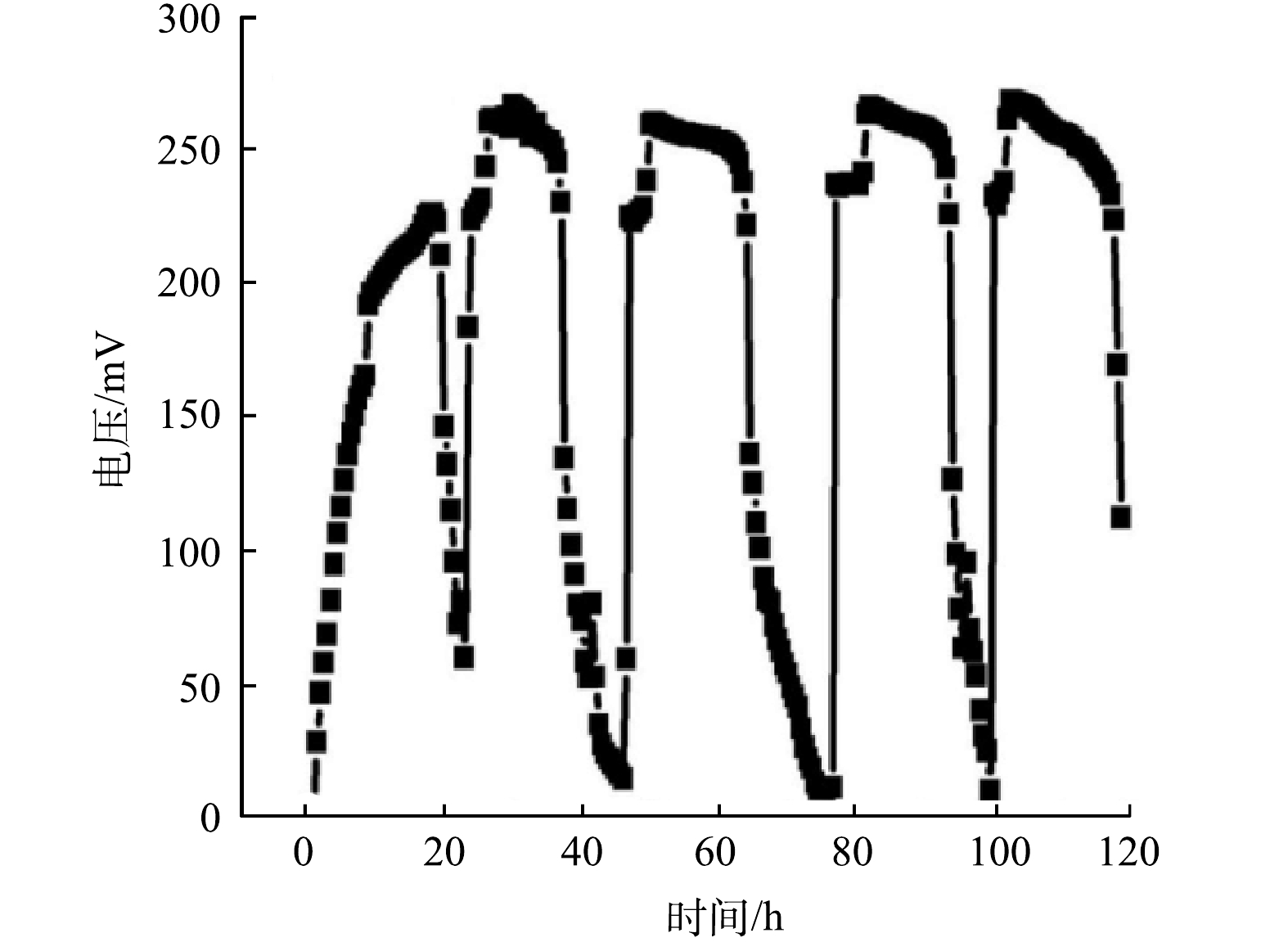
4)生物电芬顿系统的电压变化。在不同的生物电芬顿体系中的电压输出情况如图9所示。由图9可以看出,负载NOPC的生物电芬顿系统的电压比未负载NOPC的高,且其电压基本保持不变,纯碳布作为阴极的生物电芬顿电压持续降低。表1为电压输出的特定数值,与纯碳布电极相比,负载NOPC碳布阴极的生物电芬顿的电压输出的平均值、最大值和最小值均大于纯碳布阴极的生物电芬顿体系。
-
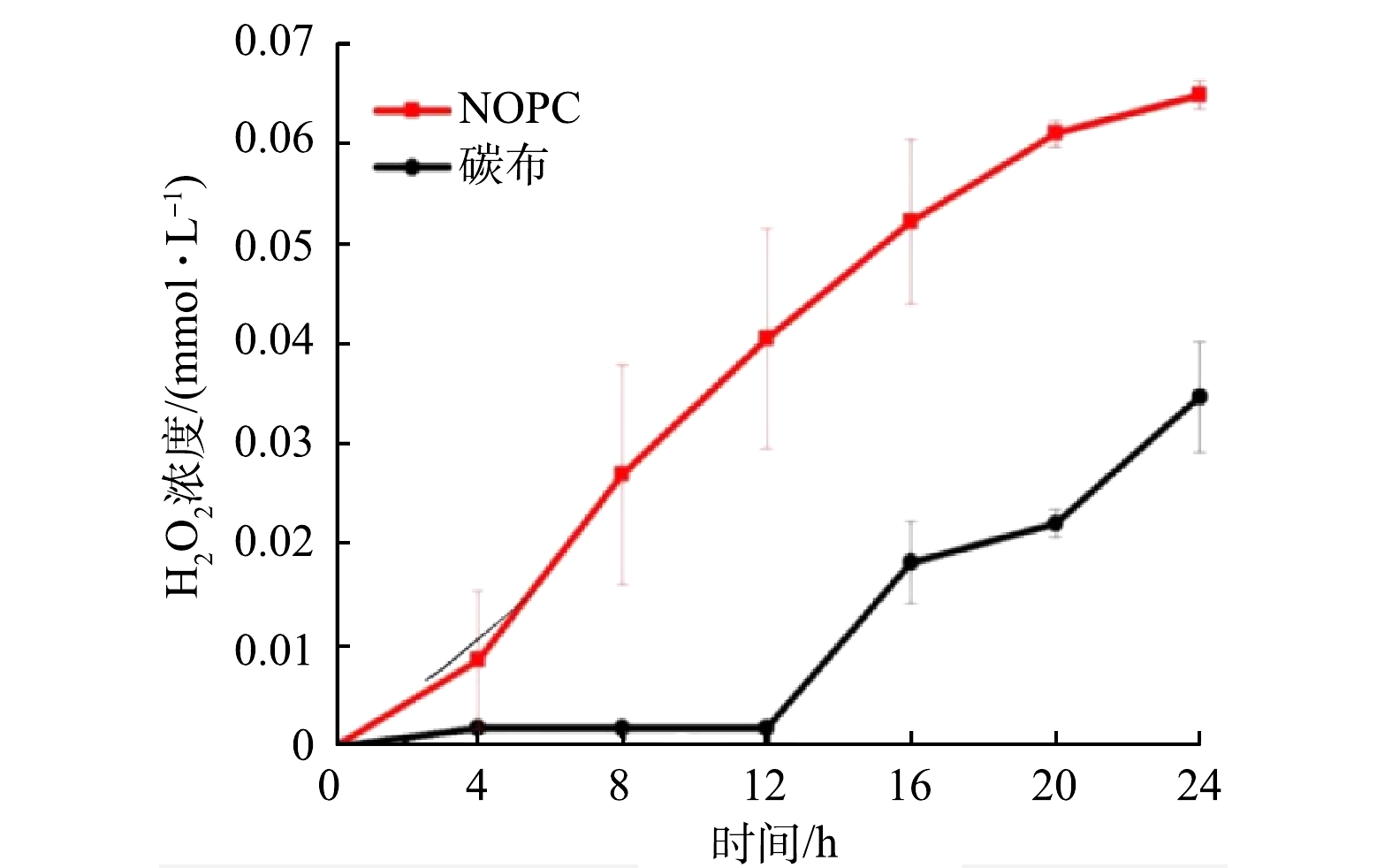
在pH=3的阴极电解液(Na2SO4溶液)中,24 h内2种生物电芬顿体系中H2O2的产生量如图10所示。 负载NOPC阴极和纯碳布阴极的生物电芬顿体系中H2O2浓度均随着时间的推移有所增加,在24 h内,负载NOPC阴极的生物电芬顿系统中H2O2的浓度最大值为0.065 mmol· L−1,而纯碳布阴极体系中仅为0.035 mmol· L−1。这是因为NOPC是一种比表面积大的微孔-介孔类材料,在含N和O杂原子的碳材料中,由于其电子亲合度较高,故其H2O2的产量是纯碳布体系的1倍左右。尽管如此,生物电芬顿体系整体H2O2产量仍低于电解池,在相同的pH和电压下,电解池中H2O2的产量可达到1 mmol· L−1 [7]。可能是由于生物电芬顿系统的电压不稳定,而电解池则是由电化学工作站提供的稳定恒压[7]。此外,由于生物电芬顿系统的电子受体氧气来源是空气,而电解池的氧气来源是纯氧,从而使其H2O2的产量大幅度降低。
-
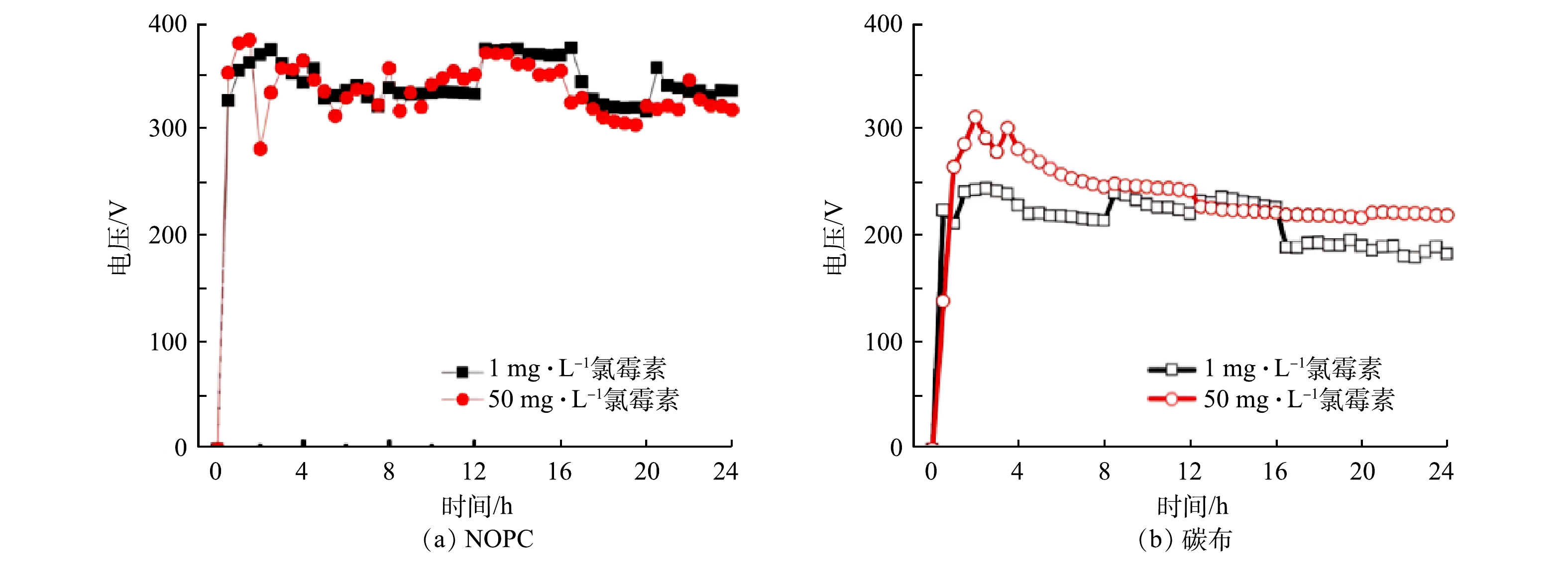
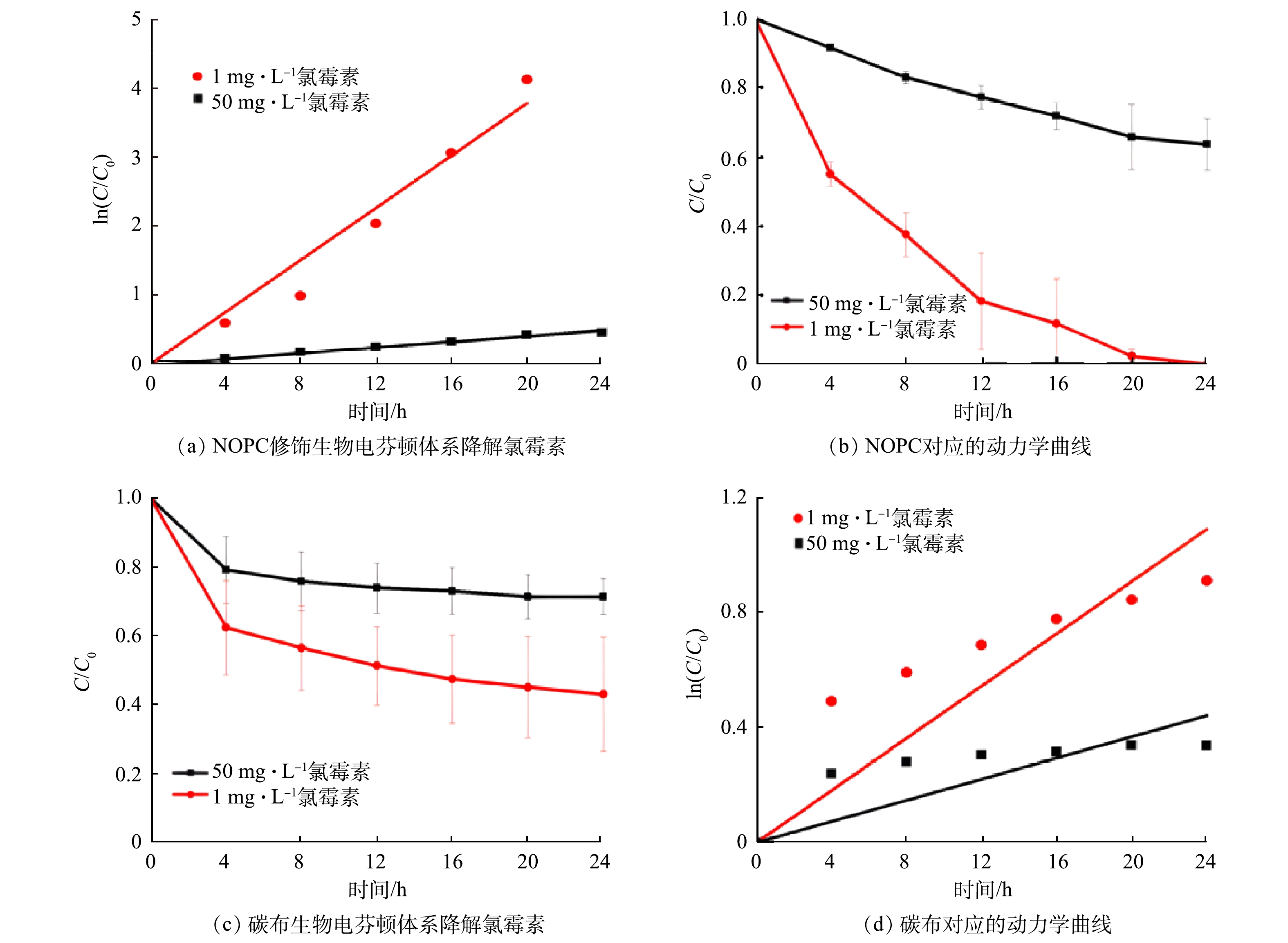
生物电芬顿体系是通过产电微生物将化学能转换为电能,所产生的e−及质子经外电路及阳离子交换膜进入阴极室,与阴极室中的O2进行氧化还原,并在原位生成H2O2后与Fe2+反应,生成羟基自由基用于降解有机物。因此,系统中H2O2的产率会影响有机物的降解效率。鉴于生物电芬顿体系中H2O2产率较电解池低,推测对于高浓度氯霉素的降解有限,故分别采用高浓度和低浓度的氯霉素进行降解实验。结果表明(图11(a)),在初始质量浓度为50 mg·L−1(高浓度)的氯霉素下,在24 h内负载NOPC阴极的生物电芬顿中氯霉素降解率为36.38%;而对初始浓度为1 mg·L−1的氯霉素降解率达到100%,由图11 (b)可知,该降解氯霉素反应遵循一级动力学。由表2可见,当氯霉素质量浓度为1 mg·L−1时,负载NOPC的生物电芬顿系统中Kapp值最大,说明氯霉素的降解速率最快。在图11 (c)中,用纯碳布阴极生物电芬顿系统对氯霉素进行降解实验,在24 h内,初始浓度为50 mg·L−1的氯霉素降解效率为28.45%,而1 mg·L−1氯霉素24 h的降解率为56.63%。与纯碳布阴极相比,负载NOPC阴极的生物电芬顿体系对于1 mg·L−1的氯霉素降解率较纯碳布的体系提高了4倍,对于氯霉素的降解主要靠生物电芬顿反应产生强氧化自由基(·OH)的氧化作用,因此H2O2的产量越高其对氯霉素的降解效果越好;由于负载NOPC阴极的生物电芬顿体系H2O2产率相对于纯碳布提高了近一倍,因此,氯霉素的降解效果优于纯碳布阴极体系,但是由于生物电芬顿系统产电不稳定,导致H2O2产率不如电解池,因此对于低浓度氯霉素的降解效果较好,而对于高浓度的氯霉素降解效果有限。从上述研究结果可以看出, NOPC对生物电芬顿体系的产电和氯霉素降解有很大的促进作用,生物电芬顿体系对低浓度污染物的降解效果更好。
-
1)负载NOPC阴极的生物电芬顿系统中内阻比纯碳布作为阴极的低、最大功率密度更高,分别是147.27 Ω和261.08 mW· m−2,同时负载NOPC阴极的生物电芬顿的最大、最小和平均输出电压均大于纯碳布阴极的生物电芬顿。
2)负载NOPC阴极的生物电芬顿体系中H2O2的产生量经24 h后为0.065 mmol· L−1 ,而相同条件下纯碳布阴极的生物电芬顿系统产H2O2的生成量仅为0.035 mmol· L−1 。
3)负载NOPC阴极的生物电芬顿体系中,在24 h内50 mg·L−1和1 mg·L−1的氯霉素降解率分别为36.38%和100%;而纯碳布电极体系中的降解率仅为28.45%和56.63%。
氮氧自掺杂生物质多孔炭修饰阴极的生物电芬顿产电及其对氯霉素的降解性能
Performance of electricity generation and chloramphenicol degradation by a bioelectro-Fenton cathode modified by N and O self-doped biomass porous carbon
-
摘要: 以黑豆为前驱体制备出具有多孔形貌的氮氧自掺杂生物炭(NOPC)材料,利用SEM、XPS、XRD、BET等手段对NOPC进行了表征和分析,并将NOPC负载于生物电芬顿体系的阴极碳布上,考察了生物电芬顿的产电性能及其对氯霉素的降解性能。结果表明:负载NOPC的生物电芬顿体系内阻明显降低,最大功率密度有所升高。与纯碳布电极相比,负载NOPC的生物电芬顿体系在24 h内H2O2的生成量增加了46.15%,且对50 mg·L−1和1 mg·L−1的氯霉素在24 h的降解率分别为36.38%和100%;而纯碳布的生物电芬顿体系在24 h内的降解率仅有28.45%和56.63%,负载NOPC的生物电芬顿体系对1 mg·L−1氯霉素的降解率提高了43.37%。以上结果表明NOPC不仅可以提高生物电芬顿体系的产电性能,还能提高氯霉素的降解效率,同时也表明生物电芬顿体系更适用于含有低浓度氯霉素水体的处理。Abstract: N and O self-doped biomass porous carbon (NOPC) was prepared using the black soya beans as precursor, and it was characterized and analyzed by SEM, XPS, XRD and BET. Then NOPC was loaded to onto the carbon cloth as bioelectric-Fenton cathode, the electrical production performance and chloramphenicol degradation effect were investigated. The result shows that the internal resistance of bioelectric-Fenton system decreased significantly and its maximum power density increased. Compared with carbon cloth cathode, the H2O2 production of bioelectro-Fenton with NOPC-loaded cathode increased by 46.15% after 24 h, for 50 mg·L−1 and 1 mg·L−1 chloramphenicol, the degradation rates were 36.38% and 100% at 24 h, respectively, while the chloramphenicol degradation rates were only 28.45% and 56.63% in the bioelectro-Fenton with carbon cloth at 24 h, respectively. The chloramphenicol degradation rate at the initial content of 1 mg·L−1 increased by 43.37% in bioelectro-Fenton with NOPC loaded cathode. The results indicate that NOPC could not only improve the performance of electricity generation , but also improve the chloramphenicol degradation rate in the bioelectro-Fenton system, and bioelectro-Fenton is more suitable for treating water with chloramphenicol of low initial concentration.
-

-
表 1 氯霉素降解过程中生物电芬顿电压输出对比
Table 1. Voltage output of bioelectro-Fenton during chloramphenicol degradation
阴极
材料氯霉素初始
质量浓度/(mg·L-1)平均输出
电压/mV最大输出
电压/mV最小输出
电压/mVNOPC 1 343.465 4 374.983 316.017 50 336.782 382.622 280.676 碳布 1 214.107 2 243.282 179.148 50 238.296 4 309.735 138.512 表 2 不同阴极材料在不同氯霉素初始浓度下的表观速率常数
Table 2. Apparent rate constants of different cathode at different initial chloramphenicol concentrations
阴极材料 氯霉素初始
质量浓度/(mg·L−1)Kapp/h−1 R2 NOPC 1 0.188 9 0.982 6 50 0.020 4 0.995 9 碳布 1 0.045 4 0.924 6 50 0.018 4 0.864 7 -
[1] LIANG B, CHENG H Y, KONG D Y, et al. Accelerated reduction of chlorinated nitroaromatic antibiotic chloramphenicol by biocathode[J]. Environmental Science & Technology, 2013, 47(10): 5353-5361. [2] 罗义, 周启星. 抗生素抗性基因(ARGs)——一种新型环境污染物[J]. 环境科学学报, 2008, 28(8): 13-19. doi: 10.3321/j.issn:0253-2468.2008.08.002 [3] HU X, DENG Y, ZHOU J T, et al. N- and O self-doped biomass porous carbon cathode in an electro-Fenton system for chloramphenicol degradation[J]. Separation and Purification Technology, 2020, 251: 117376. doi: 10.1016/j.seppur.2020.117376 [4] SATHE S M, CHAKRABORTY I, DUBEY B K, et al. Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: Applications and challenges[J]. Environmental Research, 2022, 204: 112135. doi: 10.1016/j.envres.2021.112135 [5] ZHU X P, Ni J R. Simultaneous processes of electricity generation and p-nitrophenol degradation in a microbial fuel cell[J]. Electrochemistry Communications, 2009, 11(2): 274-277. doi: 10.1016/j.elecom.2008.11.023 [6] FENG C H, LI F B, MAI H J, et al. Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment[J]. Environmental Science and Technology, 2010, 44(5): 1875-1880. doi: 10.1021/es9032925 [7] LI Y, FU Z Y, SU B L. Hierarchically structured porous materials for energy conversion and storage[J]. Advanced Functional Materials, 2012, 22: 4634-4667. doi: 10.1002/adfm.201200591 [8] GONG K P, DU F, XIA Z H, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009, 323: 760-764. doi: 10.1126/science.1168049 [9] ZHANG J T, DAI L M. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction[J]. ACS Catalysis, 2015, 5(12): 7244-7253. doi: 10.1021/acscatal.5b01563 [10] CHENG S, LIU H, LOGAN B E. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing[J]. Environmental Science & Technology, 2006, 40(7): 2426-32. [11] HE Z, WAGNER N, MINTEER S D, et al. An upflow microbial fuel cell with an interior cathode: Assessment of the internal resistance by impedance spectroscopy[J]. Environmental Science & Technology, 2006, 40(17): 5212-5217. [12] CHEN S, DUAN J J, JARONIEC M, et al. Nitrogen and oxygen dual-doped carbon hydrogel film as a substrate-free electrode for highly efficient oxygen evolution reaction[J]. Advanced Materials, 2014, 26: 2925-2930. doi: 10.1002/adma.201305608 [13] ZHU H, YIN J, WANG X L, et al. Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors[J]. Advanced Functional Materials, 2013, 23: 1305-1312. doi: 10.1002/adfm.201201643 [14] LIU Y, CHEN S, QUAN X, et al. Efficient mineralization of perfluorooctanoate by electro-Fenton with H2O2 electro-generated on hierarchically porous carbon[J]. Environmental Science & Technology, 2015, 49(22): 13528-13533. [15] 周汉. 聚卟啉修饰电极催化氧还原反应的研究[D]. 重庆: 重庆大学, 2015. -



 下载:
下载: