-
近年来,随着药物与个人护理用品 (pharmaceuticals and personal care products, PPCPs)大量使用,水环境中PPCPs及其代谢产物对淡水生态系统构成潜在威胁。布洛芬作为一种非甾体抗炎药,广泛应用于高烧、炎症和风湿病等疾病的治疗,是世界上消耗最多的PPCPs之一[1]。成人摄入的布洛芬往往只有20%被机体利用,其余则通过汗液或排泄物进入水环境[2-3]。制药行业生产废水布洛芬质量浓度甚至高达200 mg·L−1,而传统的污水处理工艺无法有效的去除水中布洛芬,河流和湖泊等地表水体中布洛芬检出浓度越来越高[4-5]。生物长期暴露于布洛芬的环境,其中枢神经系统[6]、心血管[7]、肾脏[8]等会受到损伤。因此,研究水中布洛芬有效去除方法具有一定的迫切性。
微生物燃料电池(microbial fuel cells, MFCs)通过阳极微生物代谢,将污染物蕴含的化学能转化为电能,实现废水资源化,具有成本低,无二次污染等特点[9]。严伟富等[10]研究表明,在132 h内MFCs对水中10 mg·L−1的氧四环素去除率可达99.0%。邓经惠[11]发现沉积物MFCs对沉积物中磺胺甲恶唑去除率高达80%以上。李峰等[12]研究表明,MFCs闭路运行模式下盐酸环丙沙星与磺胺甲恶唑的去除率均显著高于开路运行模式(p < 0.05)。目前,对于水中布洛芬的去除更多集中在厌氧生物法[13]、光催化法[14]和高级氧化技术等[15]。使用MFCs处理含布洛芬废水能够拓展布洛芬废水处理方法,对布洛芬废水资源化具有重要意义。本研究构建了双室MFCs处理含布洛芬废水,探讨了MFCs以布洛芬作为底物的产电性能,分析了阳极生物膜微生物的群落结构及功能基因组成,为微生物电化学法处理布洛芬废水提供参考。
-
布洛芬(分析纯,纯度99%)购自阿拉丁上海生化科技股份有限公司,色谱纯甲醇购自美国Fisher公司。
-
双室MFCs反应器由阳极室和阳极室构成,两室通过质子交换膜隔开;阳极和阴极均为碳毡(2.5 cm×2.5 cm×0.5 cm),外接1 000 Ω电阻。阳极室接种活性污泥,阳极液为含乙酸钠的人工废水,阴极液为含50 mmol·L−1K3Fe(CN)6的磷酸盐缓冲液(pH=7)[16]。MFCs以0.82 g·L−1乙酸钠为底物启动后,换成10 mg·L−1 布洛芬为底物,运行温度为28 ℃。设置开路MFCs为对照组,实验组和对照组运行条件一致。数据采集器(PS-DAQ,北京恩迈科华技术有限公司)每5 min记录一次MFCs输出电压。
-
待MFCs以布洛芬为底物稳定产电,阳极换液后每12 h使用岛津高效液相色谱(L-200,日立公司,日本)在波长223 nm波长下检测阳极液中布洛芬浓度。流动相由甲醇和水(pH=2,磷酸调节)按80∶20配制,流速为1 mL·min−1 [17]。
-
MFCs以布洛芬为底物运行3个月后,采用美国MP生物医疗公司土壤微生物DNA提取试剂盒提取阳极生物膜微生物基因组DNA,定量分析后,送样至安诺优达基因科技有限公司宏基因组测序。
-
非冗余基因集序列使用Diamond软件处理后与NCBI的NR数据库进行比对,而获得MFCs阳极生物膜微生物群落结构以及功能基因信息。直系同源蛋白分组比对数据库(evolutionary genealogy of genes: non-supervised orthologous groups, eggNOG)分析同源序列聚类(clusters of orthologous group, COG),京都基因和基因组数据库(Kyoto encyclopedia of genes and genomes, KEGG)分析代谢通路。
-
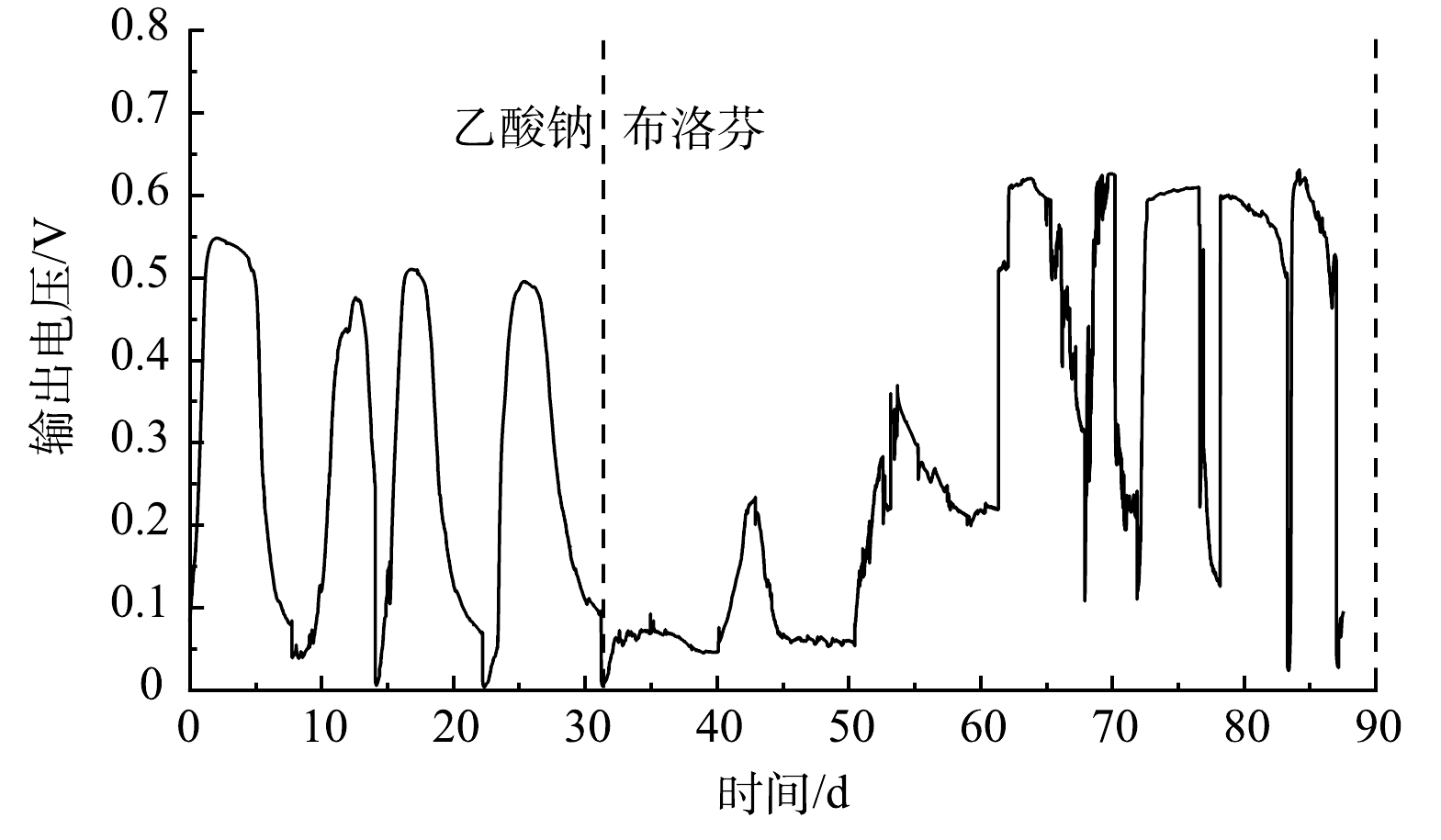
以乙酸钠与布洛芬为燃料的MFCs输出电压变化如图1所示。以乙酸钠为底物时,MFCs电压峰值为0.54 V;当底物更换为布洛芬时,输出电压急剧下降至0.1 V以下。布洛芬的化学结构相对复杂,不易被阳极微生物代谢,且布洛芬对部分阳极微生物具有毒性,布洛芬的加入抑制了阳极微生物的产电性能。经过约30 d的驯化,阳极碳毡上形成了肉眼可见的生物膜,MFCs以布洛芬为底物输出电压持续增加,且对阳极室布洛芬的加入响应灵敏,最大输出电压稳定在0.60 V左右。MFCs以布洛芬为底物时最大输出电压高于乙酸为底物,原因可能是阳极布洛芬降解过程生成了电子中介体,促进了电子传递;也可能是布洛芬降解产物促进了阳极产电微生物的代谢,提高了MFCs产电性能。LI等[18]发现MFCs以酚类化合物为底物的输出电压高于葡萄糖为底物。ZENG等[19]研究表明酚类化合物的降解产物促进了阳极电化学活性细菌的生长。
-
MFCs处理组和传统微生物厌氧处理对照组布洛芬去除率变化如图2(a)所示。MFCs处理组108 h的布洛芬去除率为85.33%,而对照组仅为46.11%。一级动力学拟合结果如图2(b)所示,对照组与MFCs处理组拟合方程R2分别为0.966和0.991,表明ln(Ct/C0)与处理时间相关性较好。MFCs能够强化微生物厌氧去除水中布洛芬,对照组中布洛芬半衰期达115.9 h,MFCs处理组中半衰期仅为36.4 h。陶玥彤等[20]研究表明,生物电化学系统促进了沉积物中大环内酯类和四环素的去除。
-
MFCs阳极生物膜的电镜照片如图3所示,MFCs在以布洛芬为燃料启动后,阳极碳毡表面聚集大量微生物,微生物通过胞外聚合物成团包裹碳丝,形成松散的生物膜。生物膜中存在大量孔隙,增加了生物膜的表面积,有利于对布洛芬的吸附和降解。
-
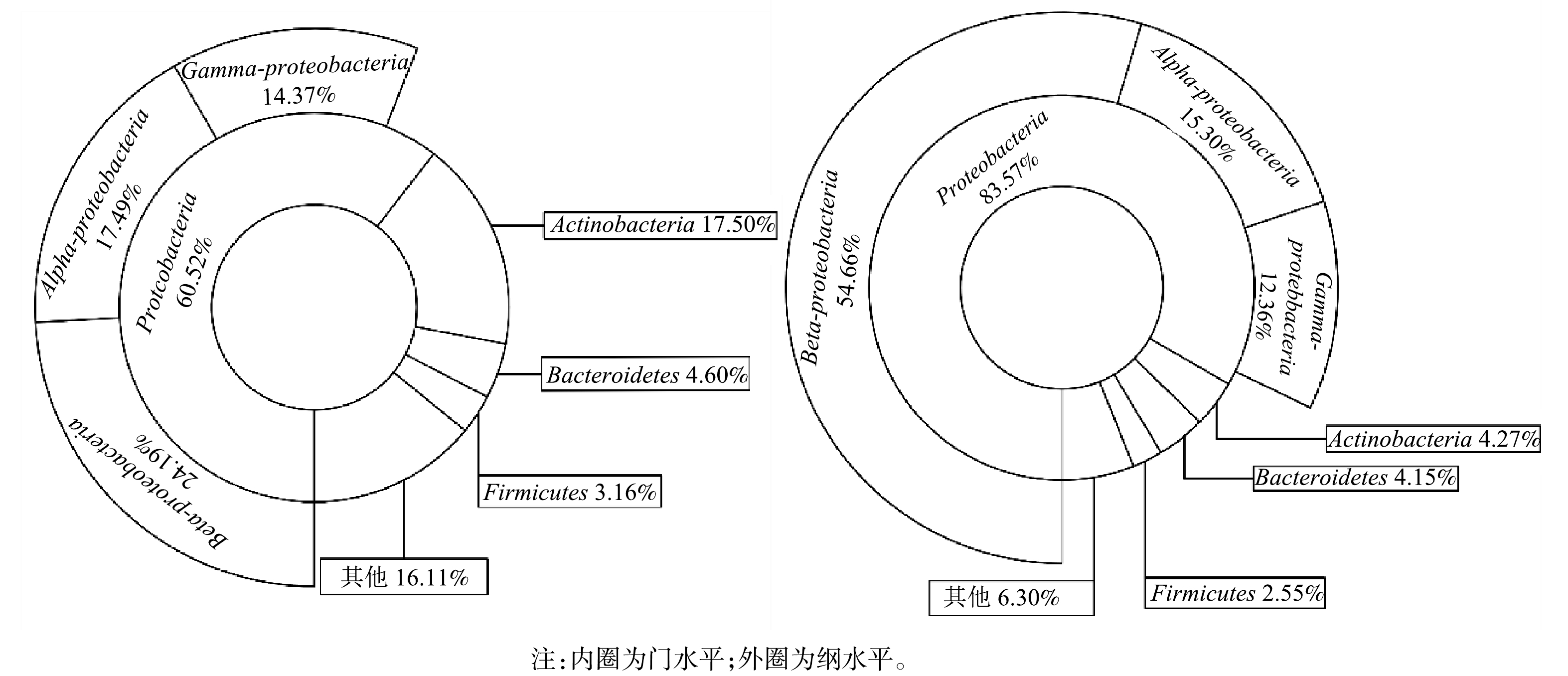
1)门和纲水平上对微生物群落结构的分析。膜生物反应器常用于水中布洛芬的去除,随着反应器去除布洛芬性能的稳定,生物膜常常含有较多Proteobacteria。ZHANG等[21]在降解含布洛芬的废水生物反应器中发现Proteobacteria为最优菌门。NAVROZIDOU等[22]使用固定化生物反应器处理布洛芬,Proteobacteria同样为优势菌。李峰等[12]等采用人工湿地型MFCs去除磺胺甲恶唑与盐酸环丙沙星,发现Proteobacteria在阳极生物膜中含量最高。由图4可见,Proteobacteria 在2个样品中含量最为丰富,在MFCs阳极生物膜中相对丰度为83.58%,是对照组的1.38倍。在纲水平,Beta-proteobacteria在阳极生物膜的相对丰度达到54.66%,高于对照组的24.19%。芳香族化合物降解性能电化学活性微生物在Beta-proteobacteria广为分布,阳极生物膜富集的Beta-proteobacteria有助于MFCs对水中布洛芬的去除[23]。HASSAN等[24]研究也表明,MFCs在污染物降解过程定向富集微生物,从而维持有利于污染物降解与产电的微生物群落结构。
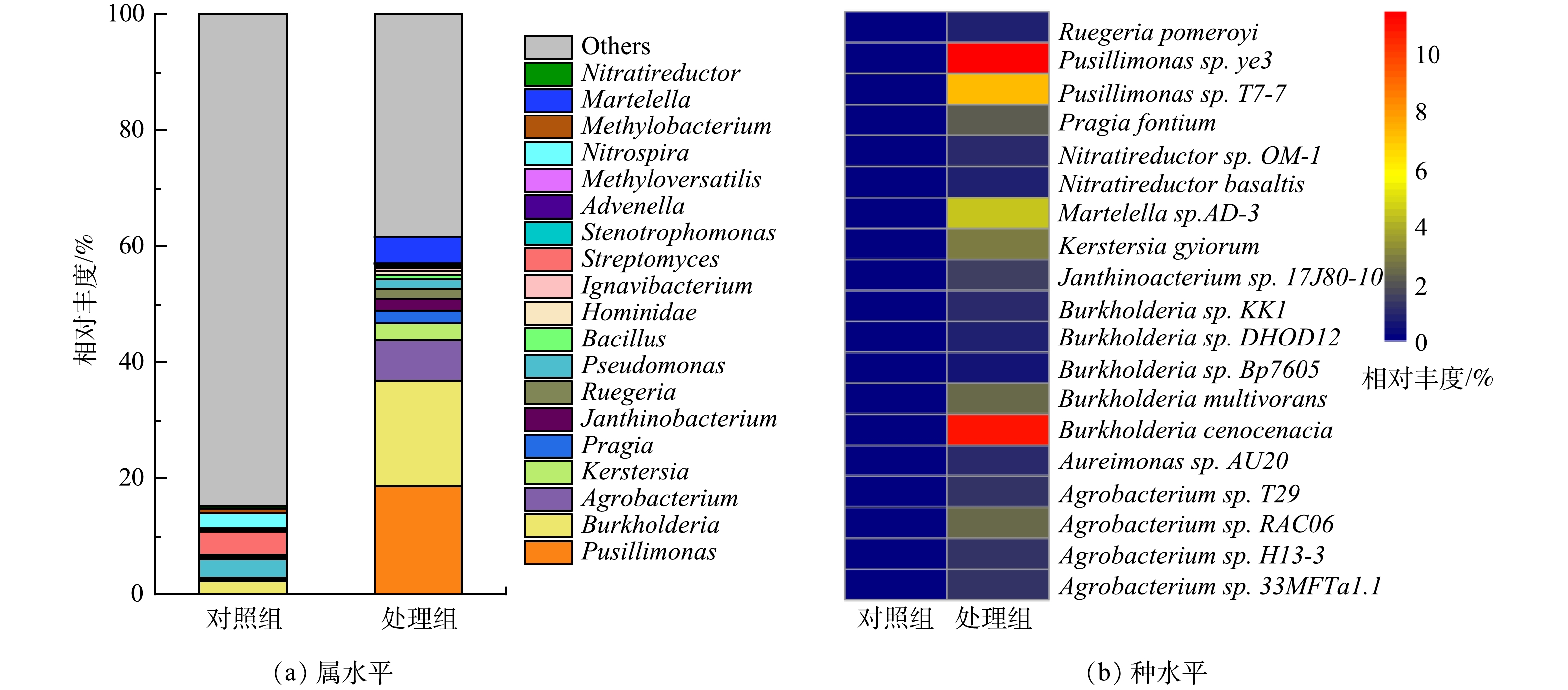
2)属与种水平微生物群落分析。电极生物膜和对照组细菌在属与种水平的含量差异如图5所示。由图5(a)可见,Pusillimonas在MFCs阳极生物膜中含量最高,相对丰度为18.64%,远高于对照组(0.02%)。Pusillimonas能以羟基吡啶、3-氰基吡啶以及5-羟基吡啶甲酸等杂环化合物作为唯一碳源进行代谢,常用于杂环有机污染物去除[25-26]。Burkholderia作为第2优势菌,在阳极生物膜中相对丰度为18.18%,高于对照组中的2.24%。Burkholderia可降解苯、甲苯和二甲苯等单环芳烃,生成的吩嗪作为电子中介体,强化了微生物对布洛芬的去除[27-28]。此外,芳香族化合物降解过程发挥重要作用的微生物如Agrobacterium(7.04%)[29]、Martelella(4.56%)[30]和Ruegeria(1.74%)[31]等在阳极生物膜的相对丰度也显著高于对照组。
生物膜在种水平的相对丰度统计结果如图5(b)所示。可见,Pusillimonas sp.ye3(11.49%)为相对丰度最高的微生物,其次为Burkholderia cenocepacia(11.02%)。Pusillimonas sp. T7-7(7.14%) 和Martelella sp. AD-3(4.56%) 在MFCs阳极生物膜中的含量也远高于对照组。其中,Burkholderia cenocepacia能以芳香烃作为碳源生长[32];Martelella sp. AD-3能降解蒽[33]和菲[34]等多环芳烃。这些能以芳香烃类化合物为碳源生长的微生物在阳极生物膜相对丰度高达34.21%,说明MFCs以布洛芬为底物,对芳香烃降解性能微生物有较好的富集。
-
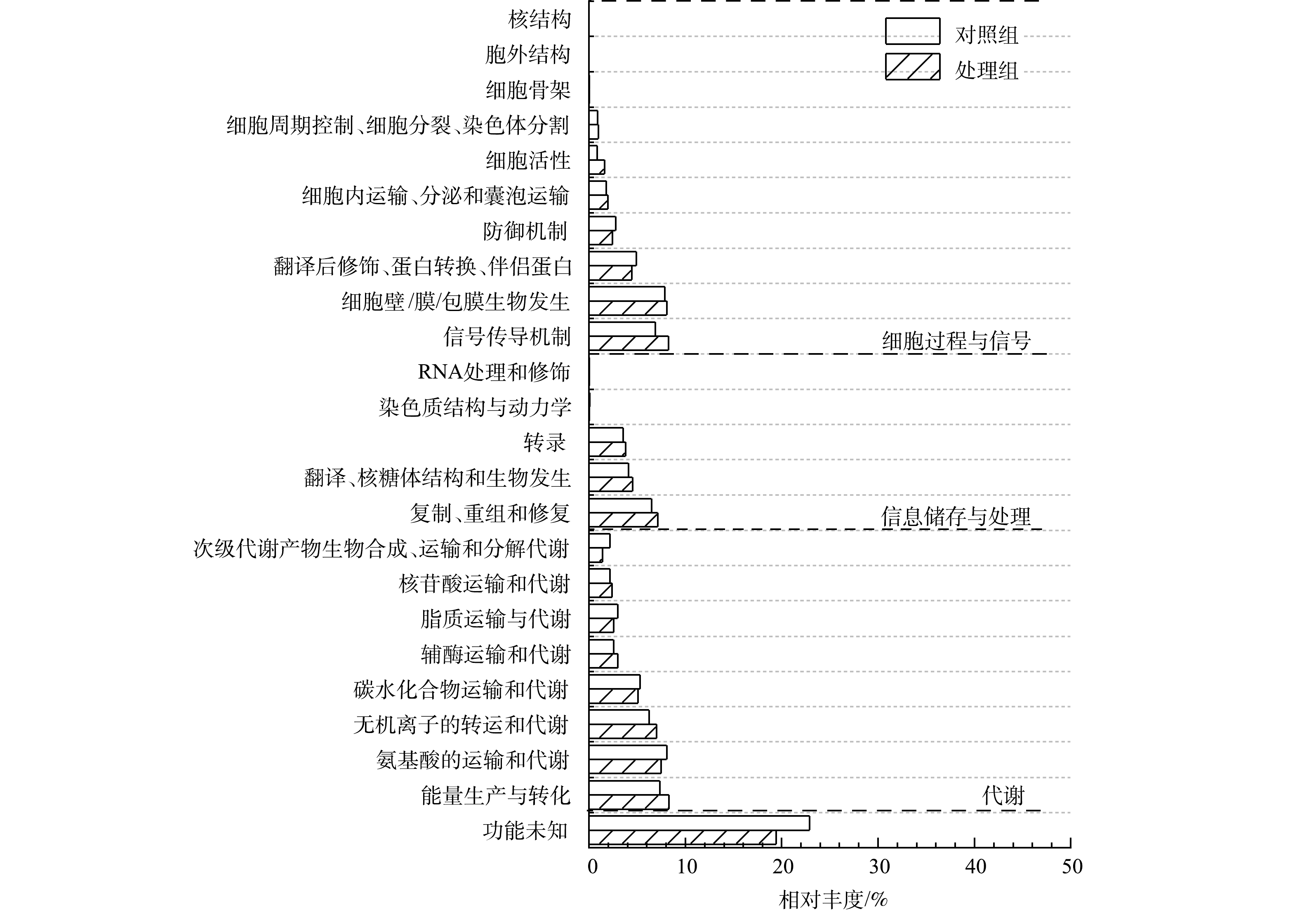
1) COG注释与分析。功能基因与eggNOG数据库比对后统计样本COG功能分类(图6)。代谢基因在2个样品中相对丰度最高,其次为细胞过程与信号基因和信息储存与处理基因,且这些基因丰度在MFCs阳极生物膜均高于对照组。在COG二级分类目录中,能量产生与转化基因、信号传导机制及无机离子转运与代谢等基因丰度在阳极生物膜含量高于对照组。另外,未知功能基因在2个样品中相对丰度也较高,表明微生物电化学法和传统厌氧生物法去除水中布洛芬涉及大量未知代谢途径。
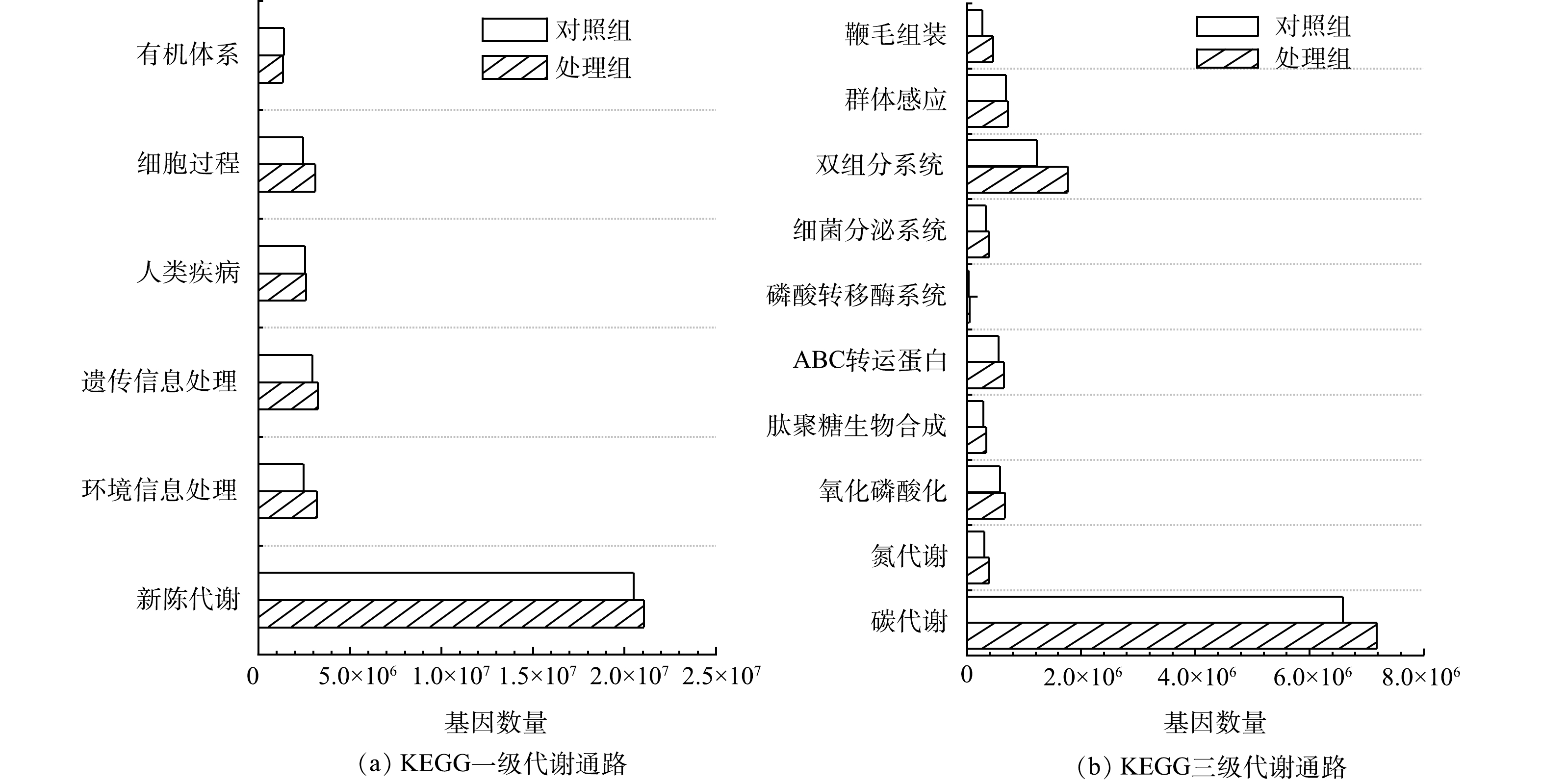
2) KEGG功能注释与分析。KEGG注释统计如表1所示。尽管MFCs阳极生物膜代谢通路、模块类别和酶种类均低于对照组,但基因数量却比对照组高11.22%,说明在MFCs去除水中布洛芬的过程中,阳极生物膜菌群功能向着专一化和特定化演化。严伟富等[10]研究也表明,随着MFCs以四环素为底物持续产电,阳极微生物向特定群落进化,从而可强化氧四环素的降解。
KEGG一级功能分类如图7(a)所示。除有机体系外,其他代谢类型基因数量在阳极生物膜微生物中均高于对照组,其中环境信息处理基因丰度为对照组的1.29倍。KEGG代谢通路的进一步分析结果如图7(b)所示。阳极生物膜微生物的碳代谢和氮代谢的功能基因数量分别是对照组的1.09倍和1.29倍,表明MFCs以布洛芬作为底物,促进了微生物的碳、氮代谢,更多的氧化磷酸化关联基因也为碳氮代谢提供了能量保障[35]。此外,双组分系统、细菌分泌系统、磷酸转移酶系统、ABC转运蛋白、鞭毛组装、群体感应以及肽聚糖生物合成等涉及微生物在应对环境逆境的信号传导及调控代谢相关基因在MFCs阳极微生物中也有所增加,表明MFCs处理组中生物膜微生物的抗逆性增强。
-
1) MFCs强化了微生物对水中布洛芬的去除性能,初始质量浓度为10 mg·L−1的布洛芬处理108 h,去除率达85.33%。
2)以布洛芬为唯一底物的MFCs阳极生物膜中,Pusillimonas、Burkholderia、Agrobacterium、Martelella和Ruegeria等与芳香族化合物降解相关微生物丰度显著高于传统厌氧生物法对照组。
3)抗逆代谢通路、碳代谢通路和氮代谢通路在MFCs处理组阳极微生物中得到了强化。
MFCs降解布洛芬性能及电极生物膜宏基因组分析
Ibuprofen degradation in MFCs and metagenomics analysis of electrode biofilms
-
摘要: 布洛芬作为一种高性价比消炎药被大量使用,环境中布洛芬浓度升高易引起抗生素抗性基因污染等环境问题。本研究构建了双室微生物燃料电池(MFCs)以去除水中布洛芬,分析了阳极生物膜微生物群落结构并注释了基因功能。结果表明,MFCs阳极微生物以10 mg·L-1布洛芬作为唯一碳源,外接1 000 Ω电阻,输出电压约为0.60 V,运行至108 h时布洛芬去除率达85.33%,是对照厌氧生物处理的3.18倍。微生物群落结构分析结果表明,MFCs阳极生物膜微生物群落结构与传统厌氧生物膜差异显著,Proteobacteria在阳极生物膜相对丰度高达83.57%,而对照组中仅为60.52%,Pusillimonas、Burkholderia、Agrobacterium、Martelella及Ruegeria在属水平相对丰度也高于对照组。代谢通路分析结果表明,环境信息处理通路在MFCs阳极微生物显著增强,其碳代谢及氮代谢基因数量分别高于对照组9.02%和28.58%。Abstract: Ibuprofen is a widely used non-steroidal anti-inflammatory drug with high performance and low price. The increase of ibuprofen concentration in the environment is easy to cause environmental problems such as resistance gene contamination. In this study, dual-chamber microbial fuel cells (MFCs) were constructed to remove ibuprofen from water, and metagenomic sequencing was used to analyze the microbial community structures and annotate functional genes of anodic biofilm. The results showed that the maximum voltage was about 0.60 V generated from MFCs when anodic microbes took ibuprofen as the sole carbon source and the external resistance was 1 000 Ω, and ibuprofen removal efficiency reached to 85.33% at 108h of MFCs running, which was 3.18 times of the anaerobic control group. Compared with the traditional anaerobic method, the composition of the microbial community in the anodic biofilm also changed significantly. At phyla level, the relative abundance of Proteobacteria was 83.57% in anodic biofilm, which was just 60.52% in control group. At genus level, the relative abundance of Pusillimonas, Burkholderia, Agrobacterium, Martelella and Ruegeria was also higher than that in control group. Metabolic pathway analysis showed that the environmental information processing pathway was significantly enhanced in anodic biofilm microorganisms, and the genes related to carbon and nitrogen metabolism were 9.02% and 28.58% higher than those of control group, respectively.
-
Key words:
- ibuprofen /
- microbial fuel cells /
- metagenomics /
- microbial community /
- functional genes
-

-
表 1 KEGG注释统计
Table 1. Annotation summary of KEGG
实验组 基因数量 代谢通路 KEGG模块 KO 酶 对照组 24 988 439 447 361 7 115 3 783 MFCs组 27 792 521 443 346 6 174 3 226 -
[1] RAINSFORD K D. Ibuprofen: Pharmacology, efficacy and safety[J]. Inflammopharmacology, 2009, 17(6): 275-342. doi: 10.1007/s10787-009-0016-x [2] MURDOCH R W, HAY A G. The biotransformation of ibuprofen to trihydroxyibuprofen in activated sludge and by Variovorax Ibu-1[J]. Biodegradation, 2015, 26(2): 105-113. doi: 10.1007/s10532-015-9719-4 [3] BUSER H R, POIGER T, MÜLLER M D. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater[J]. Environmental Science & Technology, 1999, 33(15): 2529-2535. [4] BROZINSKI J M, LAHTI M, MEIERJOHANN A, et al. The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant[J]. Environmental Science & Technology, 2013, 47(1): 342-348. [5] JALLOULI N, PASTRANA-MARTÍNEZ L M, RIBEIRO A R, et al. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system[J]. Chemical Engineering Journal, 2017, 334: 976-984. [6] JAMES S, REGAN M. Cognitive dysfunction associated with naproxen and ibuprofen in the elderly[J]. Arthritis & Rheumatology, 1982, 25(8): 1013-1015. [7] TALHAR S S, SONTAKKE B R, WAGHMARE J E, et al. Effects of ibuprofen on mice heart tissue: A histological study[J]. Journal of the Anatomical Society of India, 2017, 66: S55. [8] YUE Z, JIANG P, HE S, et al. Association between an excess risk of acute kidney injury and concomitant use of ibuprofen and acetaminophen in children, retrospective analysis of a spontaneous reporting system[J]. European Journal of Clinical Pharmacology, 2014, 70(4): 479-482. doi: 10.1007/s00228-014-1643-8 [9] XIN W, FENG Y, REN N, et al. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential[J]. Electrochimica Acta, 2009, 54(3): 1109-1114. doi: 10.1016/j.electacta.2008.07.085 [10] 严伟富, 肖勇, 王淑华, 等. 氧四环素的微生物燃料电池处理及微生物群落[J]. 环境科学, 2018, 39(3): 1379-1385. doi: 10.13227/j.hjkx.201708189 [11] 邓经惠. 沉积物微生物燃料电池对磺胺甲噁唑的降解及微生物群落结构变化的影响研究[D]. 天津: 天津大学, 2018. [12] 李峰, 杨宝山, 王惠, 等. 开闭路运行模式下微生物燃料电池型人工湿地处理抗生素废水的效果及微生物群落响应[J]. 环境工程学报, 2021, 15(9): 3035-3045. doi: 10.12030/j.cjee.202105073 [13] 李泽华, 程珂珂, 腾晓, 等. 布洛芬降解菌降解谱研究[J]. 环境科学学报, 2015, 35(1): 177-183. doi: 10.13671/j.hjkxxb.2014.0824 [14] 苏海英, 陈平, 王盈霏, 等. g-C3N4/TiO2复合材料光催化降解布洛芬的机制[J]. 中国环境科学, 2017, 37(1): 195-202. doi: 10.3969/j.issn.1000-6923.2017.01.025 [15] 王毅博, 王少坡, 王哲, 等. UV/O3高级氧化法对水中布洛芬降解效果及其动力学[J]. 工业水处理, 2020, 40(9): 40-43. [16] WU Y C, WANG Z J, ZHENG Y, et al. Light intensity affects the performance of photo microbial fuel cells with Desmodesmus sp. A8 as cathodic microorganism[J]. Applied Energy, 2014, 116: 86-90. doi: 10.1016/j.apenergy.2013.11.066 [17] FEI P A, RAN Y C, YL A, et al. Kinetics and mechanisms of enhanced degradation of ibuprofen by piezo-catalytic activation of persulfate[J]. Chemical Engineering Journal, 2020, 392: 123818. doi: 10.1016/j.cej.2019.123818 [18] LI B, LIU X N, TANG C, et al. Degradation of phenolic compounds with simultaneous bioelectricity generation in microbial fuel cells: Influence of the dynamic shift in anode microbial community[J]. Bioresource Technology, 2019, 291: 121862. doi: 10.1016/j.biortech.2019.121862 [19] ZENG X, BOROLE A P, PAVLOSTATHIS S G. Biotransformation of furanic and phenolic compounds with hydrogen gas production in a microbial electrolysis cell[J]. Environmental Science & Technology, 2015, 49(22): 13667-13675. [20] 陶玥彤, 李茹莹. 生物电化学系统对河道沉积物中抗生素的强化去除[J]. 环境科学学报, 2021, 41(4): 1383-1392. doi: 10.13671/j.hjkxxb.2020.0333 [21] ZHANG S, COURTOIS S, GITUNGO S, et al. Microbial community analysis in biologically active filters exhibiting efficient removal of emerging contaminants and impact of operational conditions[J]. Science of the Total Environment, 2018, 640(1): 1455-1464. [22] NAVROZIDOU E, MELIDIS P, NTOUGIAS S. Biodegradation aspects of ibuprofen and identification of ibuprofen-degrading microbiota in an immobilized cell bioreactor[J]. Environmental Science and Pollution Research, 2019, 26(14): 14238-14249. doi: 10.1007/s11356-019-04771-5 [23] CRAMPON M, BODILIS J, PORTET-KOLTALO F. Linking initial soil bacterial diversity and polycyclic aromatic hydrocarbons (PAHs) degradation potential[J]. Journal of Hazardous Materials, 2018, 359(5): 500-509. [24] HUZAIRY HASSAN, BO JIN, ERICA DONNER, et al. Microbial community and bioelectrochemical activities in MFC for degrading phenol and producing electricity: Microbial consortia could make differences[J]. Chemical Engineering Journal, 2018, 332: 647-657. doi: 10.1016/j.cej.2017.09.114 [25] KARVELIS L, GASPARAVIIŪT R, KLIMAVIIUS A, et al. Pusillimonas sp. 5HP degrading 5-hydroxypicolinic acid[J]. Biodegradation, 2013, 25(1): 11-19. [26] XIONG W, LU Z, PENG J. Development of an amendment recipe and identification of benzene degraders for anaerobic benzene bioremediation[J]. Water Air & Soil Pollution, 2018, 229(1): 7. [27] SANG-YEOP L, GUN-HWA K, YUN S H, et al. Proteogenomic characterization of monocyclic aromatic hydrocarbon degradation pathways in the aniline-degrading bacterium Burkholderia sp. K24[J]. Plos One, 2016, 11(4): e0154233. doi: 10.1371/journal.pone.0154233 [28] HENDRY S, STEINKE S, WITTSTEIN K, et al. Functional analysis of phenazine biosynthesis genes in Burkholderia spp.[J]. Applied and Environmental Microbiology, 2021, 87(11): e02348-20. [29] GU Q, WU Q, ZHANG J, et al. Community analysis and recovery of phenol-degrading bacteria from drinking water biofilters[J]. Frontiers in Microbiology, 2016, 7(14): 495. [30] JAMAL M T, PUGAZHENDI A. Isolation and characterization of halophilic bacterial consortium from seagrass, Jeddah coast, for the degradation of petroleum hydrocarbons and treatment of hydrocarbons-contaminated boat fuel station wastewater[J]. Clean Technologies and Environmental Policy, 2021, 23(1): 77-88. doi: 10.1007/s10098-020-01957-1 [31] KUMAR A G, RAJAN N N, KIRUBAGARAN R, et al. Biodegradation of crude oil using self-immobilized hydrocarbonoclastic deep sea bacterial consortium[J]. Marine Pollution Bulletin, 2019, 146: 741-750. doi: 10.1016/j.marpolbul.2019.07.006 [32] CAUDURO G P, LEAL A L, MARMITT M, et al. New benzo(a)pyrene-degrading strains of the Burkholderia cepacia complex prospected from activated sludge in a petrochemical wastewater treatment plant[J]. Environmental Monitoring and Assessment, 2021, 193(4): 1-12. [33] CUI C, MA L, SHI J, et al. Metabolic pathway for degradation of anthracene by halophilic Martelella sp. AD-3[J]. International Biodeterioration & Biodegradation, 2014, 89: 67-73. [34] FENG T C, CUI C Z, DONG F, et al. Phenanthrene biodegradation by halophilic Martelella sp. AD-3[J]. Journal of Applied Microbiology, 2012, 113(4): 779-789. doi: 10.1111/j.1365-2672.2012.05386.x [35] 向音波, 杨永刚, 孙国萍, 等. 微生物燃料电池对污染物的强化降解及其机理综述[J]. 微生物学通报, 2014, 41(2): 344-351. doi: 10.13344/j.microbiol.china.130135 -



 下载:
下载: