-
近些年,随着我国工业固废产生量的逐渐上升,工业固废减量化、无害化和资源化问题受到越来越多的关注[1-2]。其中,水泥窑协同处置工业固废和危废的方式和比例逐年上升,利用工业固废与传统硅酸盐水泥原料化学成分相近的特点替代天然矿物生产水泥,有助于解决大宗工业固废的消纳与资源化的问题[3]。例如,新疆天业水泥厂以煤化工电石渣作为钙质原料,粉煤灰和炉渣作为硅质原料,煤矸石作为铝制原料,硫酸渣、铜渣和铁质尾矿作为铁质原料,柠檬酸渣作为水泥缓凝原料,粉煤灰、煤矸石等作为拌合材原料,制备出符合国家标准的硅酸盐水泥[3-4]。在水泥生产的过程中,煤化工电石渣的氯元素质量分数较高[5],远高于传统硅酸盐水泥原料,不仅影响水泥熟料的性能[6],还会对水泥窑况产生着一定影响[7-8]。
有不少学者对氯元素在水泥窑中的行为规律进行了研究。崔育东等[9]开展了外掺CaCl2的水泥烧制实验,结果表明,生料中适量的氯离子可在水泥烧成反应中充当矿化剂的作用,生成低熔点含氯矿物相(C11A7·CaCl2)。施慧生等[10]认为,在含有CaCl2的CaO-SiO2-Al2O3-Fe2O3-MgO体系中,还可能发生CaO+SiO2+CaCl2+C12A7+MgO→Alinite的反应,生成化学组成为Ca10Mg1-x/2Vx/2[(SiO4)3+x(AlO4)1-x]O2Cl(V:晶格空位,0.35<x<0.45)的Alinite相。WANG[11]等的研究发现,随着生料中氯质量分数的上升,水泥窑中生成的低熔点含氯组分会随烟气循环富集,致使分解炉和回转窑窑尾处形成结皮堵塞。ZHU等[12]从整体工艺层面分析传统水泥生产过程中氯元素的流动途径和质量分配,对水泥品质的保证和窑设备的维护具有一定指导作用。就天然原料制备硅酸盐水泥工艺而言,已知氯质量分数低于0.03%时对熟料的形成有积极影响,但对氯质量分数为0.04%或更高的高氯原料的研究意义不大[13]。KWON等[14]在研究以污泥为水泥原料时指出,即使生料中的氯化物质量分数最高为0.04%,但窑炉中的氯化物质量分数仍可达1.5%,继而开展了掺氯量为0~2%的生料掺杂NH4Cl水泥烧制实验,并得出熟料的主要矿物相C3S、C2S、C3A和C4AF的质量分数变化规律,但仅从实验角度给出这一结论,并未继续监测水泥强度性能变化。
目前,有关水泥中氯元素行为规律的研究主要集中在低氯质量分数生料的传统硅酸盐水泥工艺上,且涉及到水泥强度性能方面的实验研究较少。为了探究电石渣中高质量分数的氯元素对熟料物相组成和水泥性能的影响规律,本研究以某水泥厂全废渣生料为原料,借助热力学计算方法模拟不同掺氯量CaO-Al2O3-SiO2-Fe2O3-MgO-Cl体系的高温煅烧反应相图[15],揭示不同氯质量分数下该体系高温煅烧过程中的物相转化,并结合不同掺氯质量分数水泥烧制实验,综合分析氯元素对矿物相组成变化规律和由此引起的水泥物理性能变化,最后对完整工艺中氯元素的物质流进行计算分析。本研究结果可为全废渣水泥生产生料中氯质量分数的控制提供参考。
-
实验试剂。氯元素标准溶液,硝酸(HNO3,65%~68%),氟化钙(CaF2),氯化钙(CaCl2),三乙醇胺(C6H15NO3),二水硫酸钙(CaSO4·2H2O)等,均为分析纯;纯水为实验室自制。
实验设备。实验电炉(HTF-1600型,武汉亚华);离子色谱仪(DX-120型,美国DIONEX);XRD衍射仪(XRD-7000型,日本Shimadzu);行星式球磨机(QM-3SP4型,南京南大);水泥砼恒温恒湿标准养护箱(HBY-40B型,上海康路);微机伺服水泥抗折抗压试验机(DYE-300B型,无锡新建)。
-
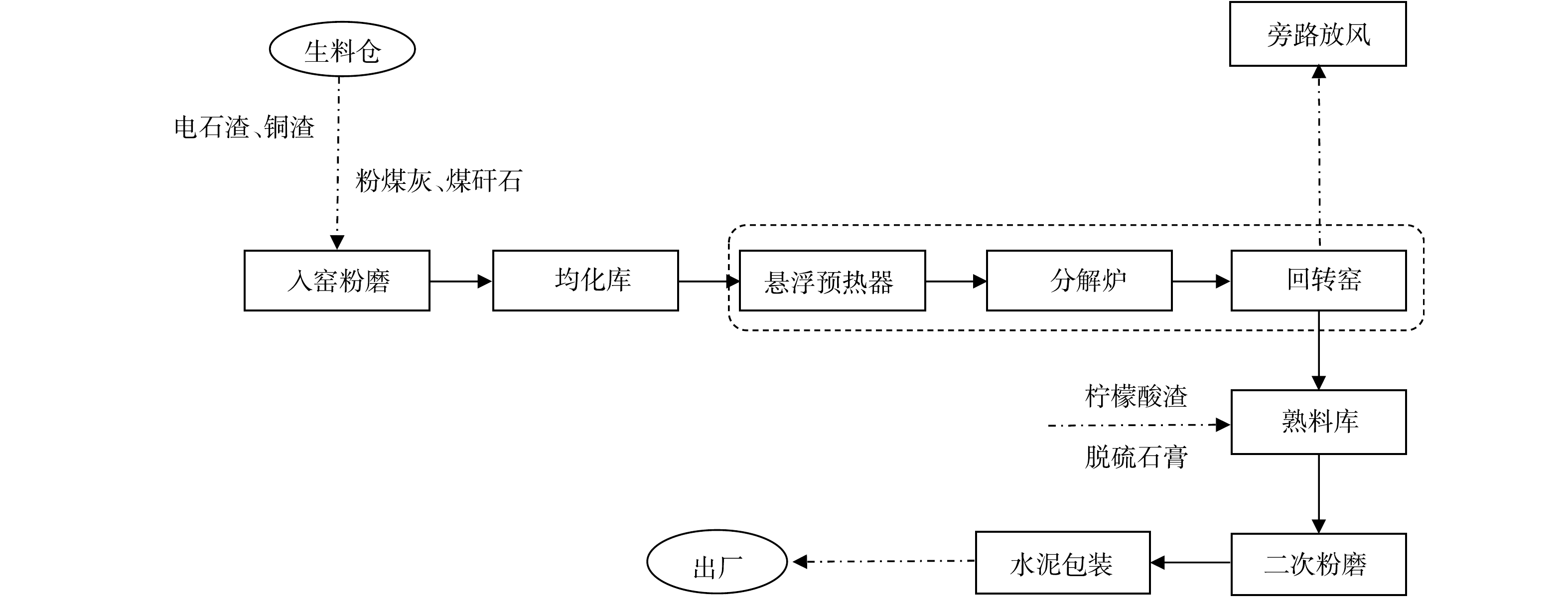
原料取自新疆天业水泥厂(以下简称T水泥厂)其中1条规模为4 000 t·d–1的水泥熟料生产线。其生产工艺流程如图1所示,采用电石渣、钢渣、粉煤灰和硅粉等10余种固废为生产水泥的原料,在经过原料预处理和生料配伍计算后,按照硅酸盐水泥正常生产流程生产符合国家标准要求的水泥产品。本研究所取原料为T水泥厂生料均化库中的生料。其中,生料的烧失量为13.99%;各组分的质量分数比例为,54.90%CaO、18.60%SiO2、4.45%Al2O3、3.33%Fe2O3、2.56%MgO、0.10%Cl−。熟料三率值,即石灰饱和系数(Lime Saturation Factor,LSF或KH)、硅率(Silica Modulus,SM)和铝率(Alumina Modulus,IM),分别为KH=0.84、SM=2.53、IM=1.31。
-
鉴于生料中氯元素基本上以Cl−形式存在,参考相关文献[16-18],拟用CaCl2作为掺氯剂,利用T水泥厂生料均化库生料,开展氯(Cl−)质量分数为0.1%~0.5%的生料煅烧实验制备熟料和水泥,并以熟料矿物相组分的质量分数和水泥3和28 d强度性能作为分析指标。外掺CaCl2水泥烧制实验设计如表1所示,S-1~S-5组生料的化学组分除了氯质量分数外,其余(氧化物组分和三率值)视为不变。
-
1)生料制备。根据实验设计,精准称量好不同掺氯质量分数的CaCl2粉末和生料,放置在橡胶球磨罐中,用球磨机混合均匀后过190目筛至筛余率为12%~14%。准确称取筛下生料粉末450.00 g,加入50 mL纯水,用电液式压力试验机压制成Φ50 mm×20 mm的生料饼,放置在105 ℃的烘箱内12 h至恒重。
2)熟料烧制。烘干的生料饼盛放在石墨坩埚中,移至高温电炉中进行煅烧。高温电炉的升温程序设置依次为:0~300 ℃,升温速率6 ℃·min–1,升温时间50 min;300~900 ℃,升温速率10 ℃·min–1,升温时间60 min;900 ℃,保温50 min;900~1 450 ℃,升温速率10 ℃·min–1,升温时间110 min;1 450 ℃,保温时间90 min。将烧制好的熟料块从炉中取出急冷至室温,准确称量熟料块质量并记录。
3)水泥制备。依照GB 175-2007《通用硅酸盐水泥》标准[19],在熟料块粉磨过程中加入质量分数为0.3%的三乙醇胺试剂,粉磨后过80目筛至筛余率20%以下,制得熟料粉末,取5.00 g粉末留样(XRD表征备用)。按照m(剩余熟料)∶m(CaSO4·2H2O)=95∶5的比例掺入CaSO4·2H2O粉末,放置在橡胶球磨罐中,用球磨机混合均匀后过190目筛至筛余率20%以下制得水泥。
4)净浆实验。取适量质量的水泥粉末,按照0.30的水灰质量比加入对应质量的纯水,放在水泥净浆搅拌机上低速搅拌30 s,高速搅拌90 s。搅拌均匀的水泥浆体放入20 mm×20 mm×20 mm的水泥模具内(每个配方6个平行样),并放置在振动台上震动(120±5) s,用水平刮尺将模具表面的多余浆体刮掉,保持试块与模具表面的高度齐平,置于标准养护箱内养护24 h后脱模,制得抗压强度测定用的水泥净浆试块。脱模后的试件在养护箱中继续养护至指定龄期,等待3和28 d强度测定[20]。同上述实验步骤,将搅拌均匀的净浆水泥浆体也另外放入20 mm×40 mm×80 mm的水泥模具内(每个配方3个平行样)制备抗折强度测定用的水泥净浆试块,后续养护步骤同上。
-
1)熟料XRD表征。采用XRD衍射仪定性分析水泥熟料矿物相组成;按照m(熟料)∶m(CaF2)=9∶1的比例掺入CaF2粉末作为内标物质,利用Rietveld全谱拟合分析法对熟料各矿物相的质量分数进行半定量分析[21]。XRD仪器采用CuKα靶,步长2θ=0.02(°)·step-1,扫描速度10 (°)·min–1(定量分析时采用4 (°)·min–1,电压30 kV,电流40 mA,石墨单色器。
2)热力学相图模拟。采用FactSage软件的Equilib模块模拟生料在800~1 500 ℃高温煅烧的物相转化过程[21]。输入T水泥厂生料均化库中的生料化学组分(质量分数),并将氯的质量分数设置在0.1%~0.5%的梯度内,继而模拟不同氯质量分数下,CaO-Al2O3-SiO2-Fe2O3-MgO-Cl体系的高温反应相变化历程。
-
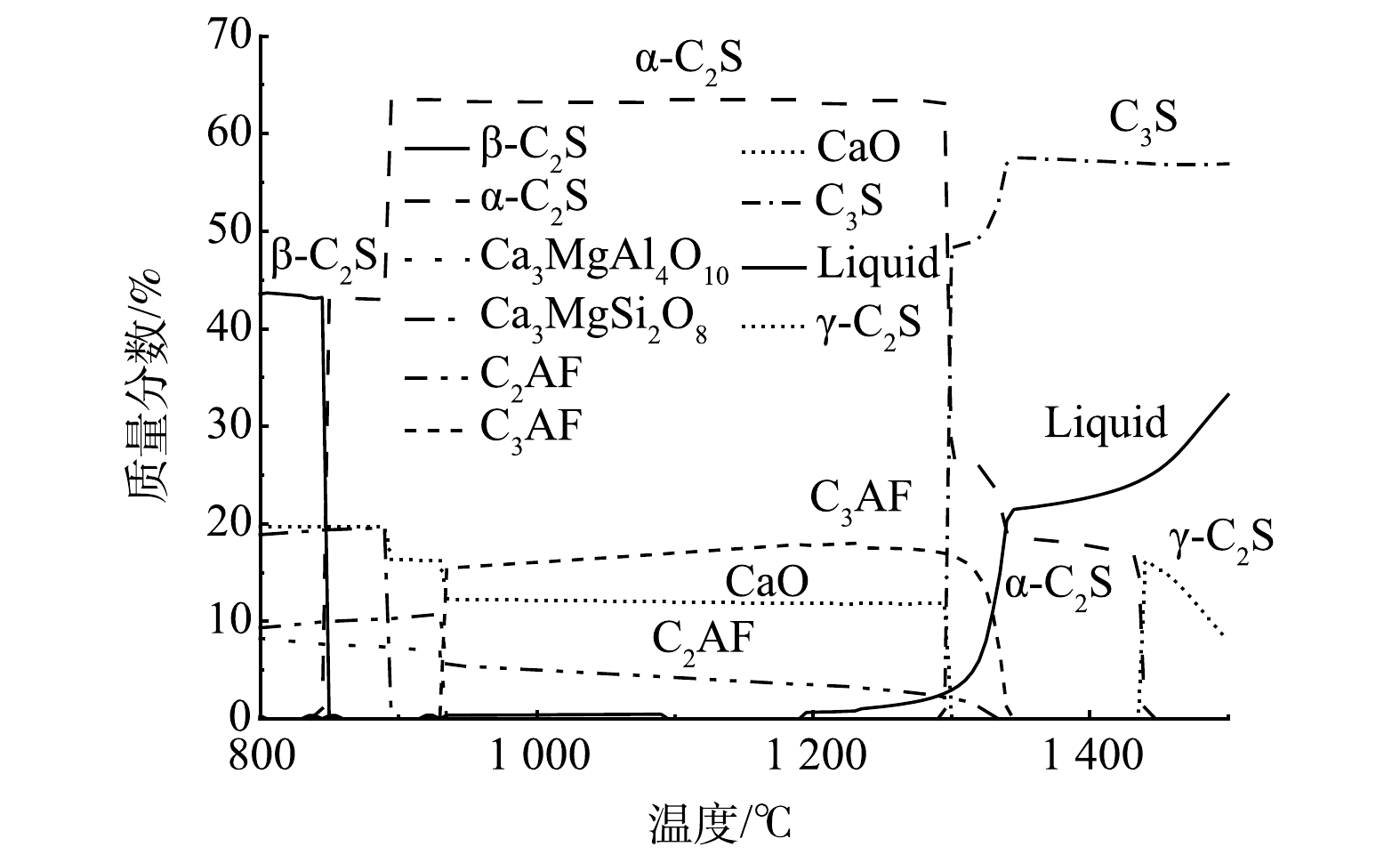
1)全废渣生料反应相图模拟。利用FactSage热力学软件,按照表1中第一行的生料各氧化物的质量分数比54.90%CaO∶18.60%SiO2∶4.45%Al2O3∶3.33%Fe2O3∶2.56%MgO∶0.10%Cl−模拟计算800~1 500 ℃温度区间内体系中各氧化物的反应历程,并绘制反应相图如图2所示。由图2可知,体系中的β-C2S在800 ℃前就已大量存在,伴随着铁相固溶体C2AF、钙镁铝盐中间矿物相Ca3MgSi2O8和Ca3MgAl4O10的生成。850 ℃后C2S由β型向α型转化,这与C2S在高温下的主要存在类型为α型的规律[22]是一致的。900~940 ℃,中间矿物Ca3MgSi2O8分解,进一步消耗体系中的CaO生成α-C2S,使得C2S质量分数骤增,并在900 ℃时达到最大值63%;同时,C2AF质量分数也有缓慢上升。940~1 300 ℃,Ca3MgAl4O10中间相全部分解,各矿物相相互转化,质量分数基本保持稳定,铁相固溶体中C2AF质量分数不断下降,C3AF组分占得优势[23];1 200 ℃后,体系中开始生成液相,为α-C2S与CaO结合生成C3S创造有利环境。1 300 ℃时,C2S大量转化为C3S,CaO的质量分数骤减为0。据此推测,熟料烧成实际温度对应热力学反应相图的1 300 ℃。1 300~1 340 ℃,热力学模拟的反应体系中除C3S以外的矿物如C3AF,倾向于分解成CaO、Al2O3、Fe2O3并熔融成液相,体系中的液相量持续上升,并进一步与C2S结合生成C3S。1 340 ℃后,C3S的生成量已达到稳定并呈轻微下降趋势。此时,C2S倾向于熔融生成液相,直至1 400 ℃后体系中的C2S由β型开始向γ型转化。热力学模拟相图图2反映出,体系中的C3S在1 300 ℃瞬时生成,f-CaO也在1 300 ℃时瞬间全部消耗。由此推断,热力学计算得到的CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系熟料烧成温度为1 300 ℃。
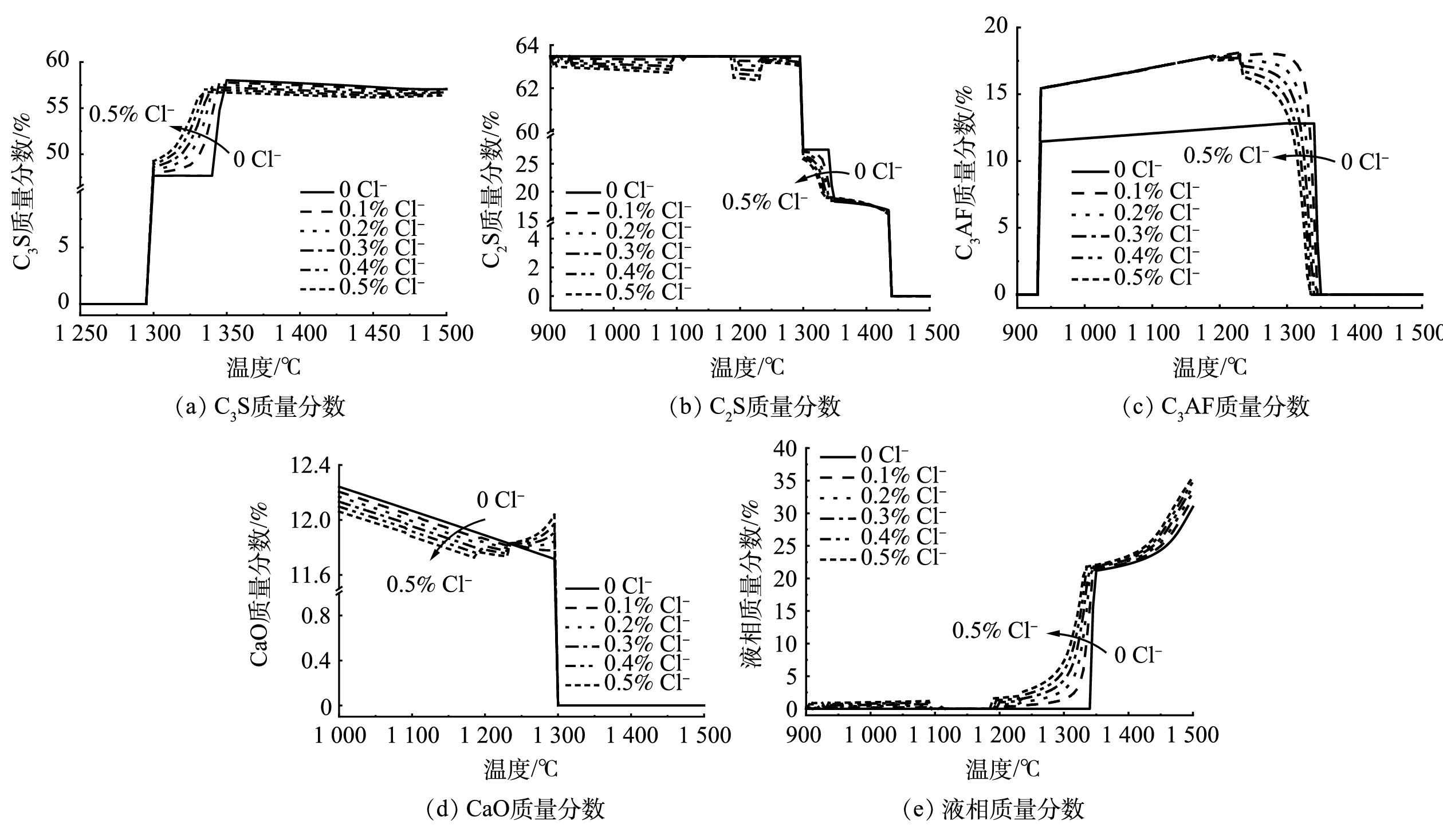
2)不同氯质量分数体系热力学相图模拟。进一步从热力学计算角度探索氯(Cl−)质量分数在0.1%~0.5%的范围内,CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系高温煅烧反应过程中的液相及各矿物相质量分数的生成变化规律,并与不含氯体系进行对比,着重关注1 300 ℃时各物相及液相的质量分数变化趋势。绘制不同氯质量分数下C3S、C2S、C3AF、CaO、液相的质量分数随温度变化图,如图3所示。由图3(a)可发现,随着氯质量分数的上升,1 300 ℃时体系中的C3S质量分数逐渐增加,氯质量分数从0.1%上升至0.5%时,C3S的质量分数由48%增加至49.5%。对照图3(b)和图3(d)可知,1 300 ℃时随着氯质量分数的上升,体系中的C2S质量分数呈现出不断下降的趋势,当氯质量分数由0.1%上升至0.5%时,C3S的质量分数从27.5%增加至25.5%;同时,体系中CaO的消耗量也随着氯质量分数上升不断增大。C3S、C2S和CaO的质量分数变化趋势与C3S的生成规律是一致的,即氯元素可以促进C2S结合CaO生成C3S的反应速率,并增加C3S生成量[17]。熟料中的铁相固溶体组成形式包括C2AF~C6AF[23],C3AF是其中的1种。由图3(c)可知,氯质量分数由0.1%上升至0.5%时,体系中C3AF的质量分数在1 300 ℃时从18%下降至13%,但均高于不含氯的体系。可见,氯元素的升高对于C3AF的生成起到了抑制作用。结合图3(e)可发现,液相量在1 300 ℃时随着氯质量分数的上升逐渐增加,但不含氯体系中液相量为0。因此,氯元素的存在促进了液相量的生成[6]。液相的形成主要依靠Al2O3、Fe2O3等氧化物组分[22]。可见,在热力学模拟的1 300 ℃时氯质量分数较高的体系中,Al2O3、Fe2O3等氧化物组分倾向于熔融成液相,因此C3AF的生成量逐渐降低。
结合热力学模拟CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系反应相图可知,熟料烧成温度为1 300 ℃。不同氯质量分数体系反应相图中各物相和液相量在1 300 ℃处的生成转化规律表明,氯元素可以促进C2S消耗CaO生成C3S,并促进液相量的生成。随着氯质量分数由0.1%上升至0.5%时,1 300 ℃时体系中的C3S质量分数从48%上升至49.5%;C2S质量分数由27.5%下降至25.5%,C3AF的质量分数从18%下降至13%。在不考虑动力学反应速率和时间限制的条件下,热力学相图模拟结果很好地反映了0.1%~0.5%氯质量分数生料体系高温煅烧反应过程中,主要矿物相C3S、C2S、C3AF及CaO和液相质量分数的变化趋势,这对水泥生产尤其是高氯质量分数的水泥生产工艺具有一定的理论指导价值。然而,热力学软件模拟有其局限性。一是未能考虑动力学方面的影响。这是因为,在实际生产中不可能达到理想的传质条件和无限的反应时间,如各物相的生成均在某温度处瞬时完成。二是FactSage软件数据库不含C3A组分,无法准确反映C3A的质量分数生成规律。因此,相图模拟的不同氯质量分数体系中各物相的质量分数变化规律,还有待开展不同的掺氯生料水泥烧制实验来进一步验证。
-
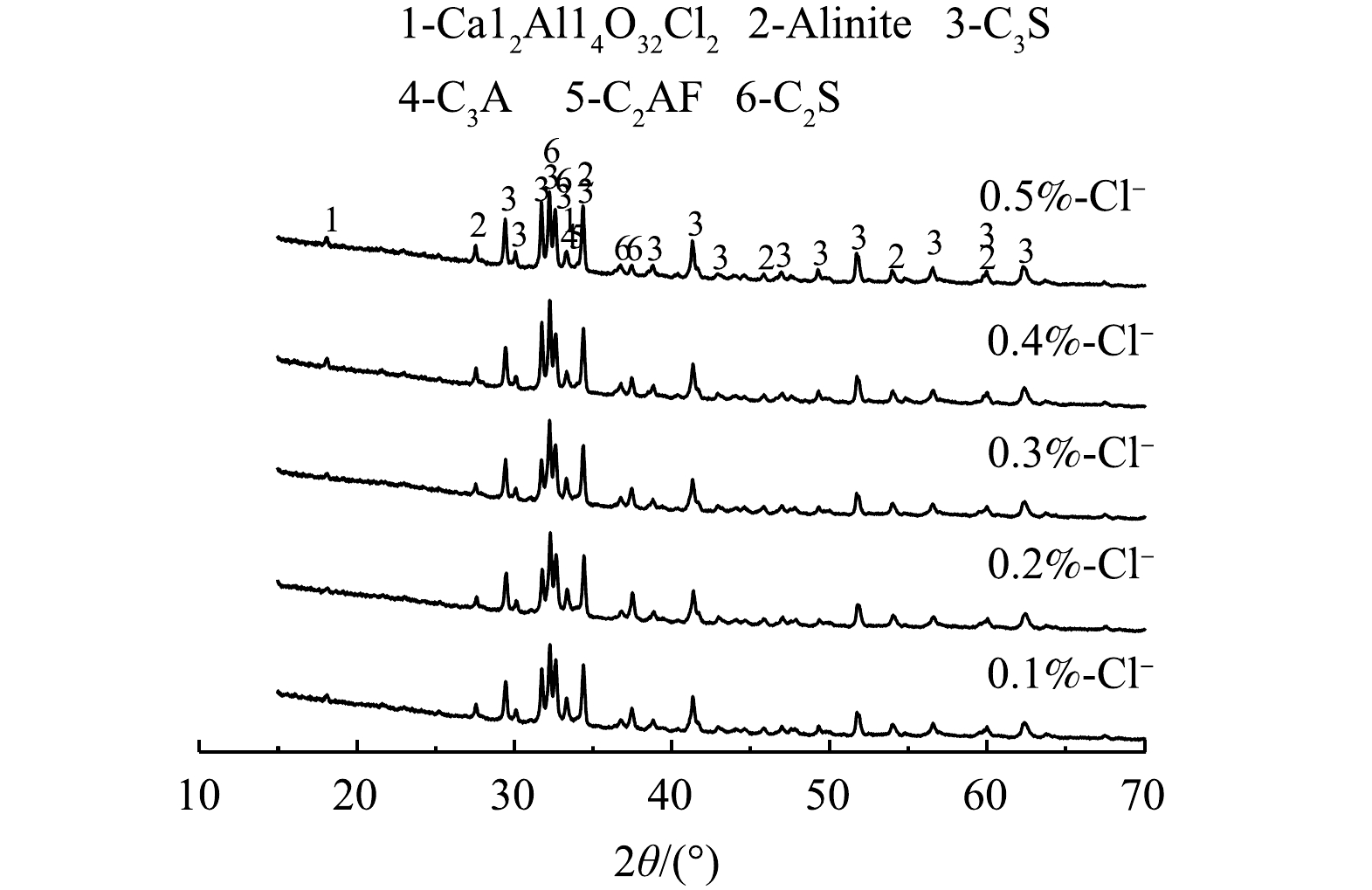
对熟料进行XRD表征分析,结果如图4所示。全废渣水泥熟料中除了常见的C3S、C2S、C2AF、C3A矿物相之外,还发现了Alinite相和Ca12Al14O32Cl2相2个新相[10]。采用Rietveld全谱拟合分析法并借助MDI Jade 6.0软件对内掺CaF2熟料XRD图谱中各物相的质量分数进行精修[24]。精修过程中发现,由于Alinite相是通式为Ca10Mg1-x/2Vx/2[(SiO4)3+x(AlO4)1-x]O2Cl(V:晶格空位,0.35<x<0.45)[25-27]的一类混合物,难以得到其准确分子式并进行精修,但由于其结构与C3S类似且质量分数较少,将Alinite相质量分数纳入到C3S相中计算。主要对C3S、C2S、C3A、C2AF、Ca12Al14O32Cl2相、f-CaO和无定型相(Amorphous)的质量分数进行半定量分析,结果如表2所示。主要矿物相组分C3S、C2S、C3A、C2AF和Ca12Al14O32Cl2的质量分数变化如图5(a)和图5(b)所示。由表2中的精修参数可知,S-1~S-5组内掺CaF2熟料XRD图谱的精修结果均满足E≤R≤2E。这表明,精修结果在置信区间内。熟料中存在一部分无定型态物质。这可能是因为,冷却过程中液相来不及结晶形成玻璃体相,其中含有少量氧化物如Fe2O3、MgO等[22]。由氯质量分数为0.1%时Ca12Al14O32Cl2的质量分数为2.44%计算得到氯的质量分数为0.12%,此结果超过了生料中氯的质量分数。这说明,精修结果存在着一定的误差。但在相同的工艺条件和精修参数背景下,仍可借助XRD精修结果对熟料中主要矿物相质量分数的变化趋势进行半定量分析。
表2反映出,熟料的主要矿物相为C3S和C2S。随着氯质量分数的上升,熟料中f-CaO的质量分数逐渐减少。这表明,氯元素增强了熟料的易烧性。由图5(a)可观察到,氯质量分数由0.1%上升至0.5%时,C3S质量分数由48.44%增加至65.02%,其中包含着少量Alinite相;C2S质量分数则由34.67%下降至19.36%,且在氯质量分数为0.4%时达到最低值,当氯质量分数上升至0.5%后,C2S的质量分数有所增加。熟料中C3S、C2S和f-CaO质量分数的变化趋势表明,氯元素促进了体系中的C2S相向C3S相的转化,但在氯质量分数超过0.4%后,促进作用减弱。图5(b)表明,熟料中的C3A和C2AF组分受氯质量分数影响较大,当生料掺氯量上升至0.2%后,C3A和C2AF质量分数均呈下降趋势。其中,氯质量分数从0.2%上升至0.5%的过程中,C3A和C2AF的质量分数分别由5.52%下降至2.45%和5.74%下降至2.13%;同时,体系中的Ca12Al14O32Cl2相作为产生C3A的中间相,其质量分数不断上升,直至超过C3A和C2AF组分。从Al元素的质量守恒角度来说,部分Al元素还可能参与了Alinite相的形成。这表明,在氯质量分数较高的高温煅烧反应过程中,较多的Al2O3倾向于与CaO、SiO2、MgO和含氯组分等结合生成Ca12Al14O32Cl2相和Alinite相。由图5可以发现,氯质量分数由0.4%上升至0.5%时,体系中的C2S质量分数由19.36%上升至20.89%;同时,Ca12Al14O32Cl2的质量分数由5.74%下降至4.37%,而C3S质量分数仍增长了1.32%。结合Ca、Al、Si和Cl元素的质量守恒和Alinite相的化学组成可知,氯质量分数为0.5%时C3S质量分数的微弱增长可能主要来源于Alinite相。此时,氯元素促进C2S向C3S转化的作用较弱;相对地,体系中Alinite相的生成速率变大。
不同掺氯量熟料的各矿物相质量分数变化趋势表明,氯元素可增加高温体系中CaO的消耗量,促进C2S向C3S的转化,并显著增加C3S的生成量。同时,在氯质量分数较高的高温反应体系中,CaO、SiO2和Al2O3倾向于与MgO和含氯物质等结合生成Alinite相和Ca12Al14O32Cl2相;相应地,熟料中的C2S、C3A和C2AF相质量分数随着氯质量分数的上升均呈现明显下降趋势,这与上文热力学模拟不同氯质量分数体系C3S、C2S和C3AF的质量分数变化规律是符合的。值得注意的是,当生料中的氯质量分数超过0.4%后,体系中的C2S组分质量分数有所增加。可推测,此时氯元素促进C2S相向C3S相转化的作用变弱,而Alinite相的增长速率变大。
-
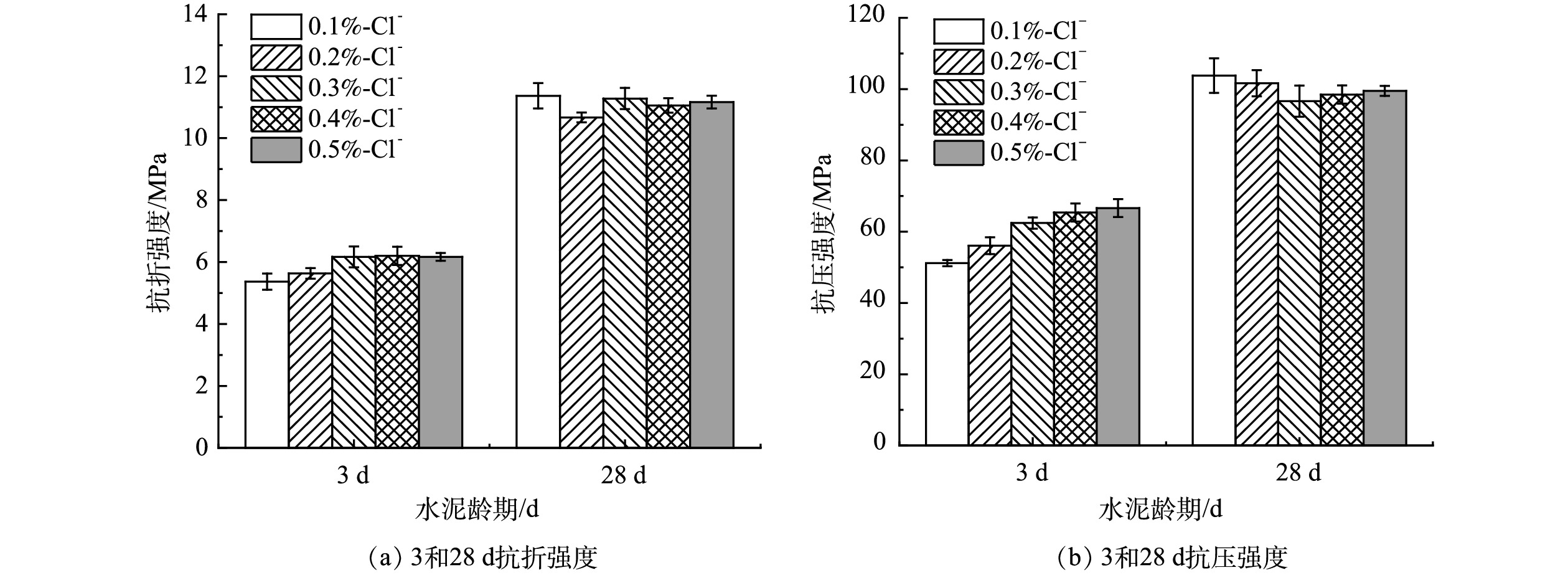
水泥3和28 d抗折、抗压强度数值如图6所示,结合上文熟料矿物相XRD精修半定量分析结果表2可知,影响水泥早期强度的C3S相在熟料中占比较大,可达50%~65%,其中包含的少量Alinite相也对3 d强度的增长有利,且随着氯质量分数的上升,C3S质量分数占比逐步上升,因此水泥3 d强度增长速率较快。使用SPSS软件进行相关性分析可知,氯离子质量分数与28 d抗折强度皮尔逊相关系数为0.972,表现为强正相关;氯离子质量分数与28天抗压强度皮尔逊相关系数为−0.666,表现为负相关。其中,氯质量分数为0.3%时,28 d抗压强度达96.6 MPa,相比氯质量分数为0.1%时的28 d抗压强度103.8 MPa降低了7%。实际上,水泥28 d强度受到多种矿物的综合影响,但主要是C3S和C2S质量分数的影响。熟料XRD矿物相质量分数精修结果表2表明,当生料掺氯质量分数上升时,熟料中C3S相质量分数不断上升的同时,C2S和C4AF质量分数却呈现下降趋势,C3S和C2S质量分数随掺氯量的变化趋势使得2者对水泥28 d强度的增长产生拮抗作用。因此,28 d抗压强度的增长空间不如3 d抗压强度,且总体呈现轻微下降的趋势。
对于附有旁路除氯系统的高氯原料水泥生产工艺而言,熟料中含有部分Alinite组分可使水泥具有易磨的特性,有利于水泥粉磨环节节省能耗[28-29]。结合S-1组~S-5组熟料XRD表征和水泥强度性能测定结果,为了极大程度地促进熟料中C3S相的生成,同时保证熟料中适宜的C2S质量分数和水泥优良的3 d和28 d性能,将生料氯元素质量分数控制在0.4%以下是较好的选择。
-
取流水线上某24 h工作周期内水泥烧制物料投入量和输出量数据,并根据GB/T 176-2017《水泥化学分析方法》[30]离子色谱法测定各样品中的氯元素质量分数,结果如表3所示。
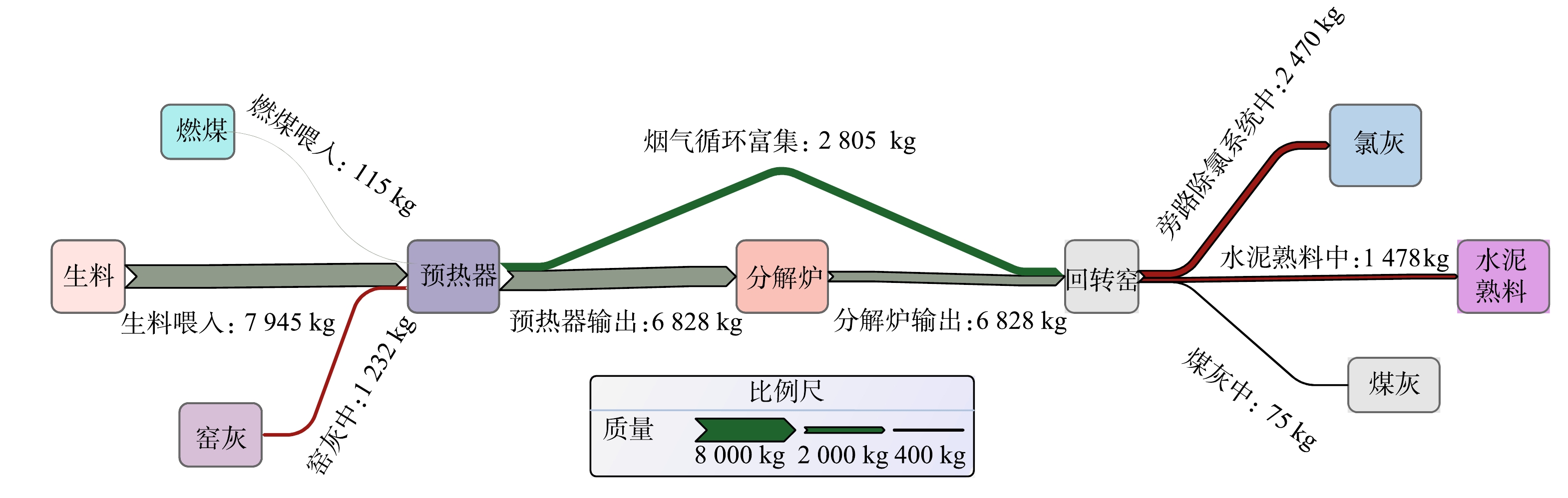
结合图1的T水泥厂全废渣水泥工艺流程得知,整体工艺中的氯元素来源于电石渣、粉煤灰等工业废渣原料,在生料均化环节其质量分数得到均化。随着生料进料,熟料煅烧至1 100 ℃时,氯元素与生料中其他碱性金属组分如钠、钾形成低熔点的氯盐挥发到预热器、分解炉和回转窑中。当窑中煅烧温度进一步上升至1 300 ℃后,体系中的氯组分已大部分挥发至烟气中,只有小部分掺杂在熟料矿物相中被带出。一部分烟气中的挥发性氯组分随烟气至窑头处被新进的生料裹挟,随后在回转窑中循环富集;另一部分随烟气被抽取至旁路除氯系统中,被迅速冷凝除尘作为捕集的氯灰进行离线处理。随着窑尾处的烟气回到预热器后经过降温与除尘器收集到的窑灰被回喂进生料系统中,部分窑尾处烟气在温度适宜区的预热器和分解炉处富集形成结皮堵塞。根据图1工艺流程和表3的数据对氯元素的物质流动进行质量平衡计算。氯元素有2个来源,通过计算得到,来自生料的氯为7.945 t,来自燃料携带的氯为0.115 t,总量为8.060 t。氯元素有4个去向,其中,进入熟料中的氯的质量为1.478 t,进入旁路除氯系统收尘灰中的氯为2.470 t,去往窑灰中的氯为1.232 t,进入煤灰中的氯为0.075 t。根据质量守恒的原则进行计算得到随窑尾结皮、随烟气循环富集中的氯的质量为2.805 t。因此,系统旁路除氯的效率为47%。
由氯元素的质量平衡分析计算和工艺流程图1绘制氯元素的物质流分析Sankey图,如图7所示。氯元素的总量达8.060 t,其中随烟气循环富集的量为2.805 t,占氯元素总量的34.80%;熟料中氯元素的质量分数达1.478 t,占氯元素总质量分数的18.34%。可见,回转窑工艺下高氯全废渣生料在高温煅烧过程中氯元素的循环富集情况较为普遍,应特别注重水泥氯元素质量分数的指标。全废渣工艺的旁路除氯系统效率达47.00%,而普通水泥窑的旁路除氯效率可达70%以上[31],与普通硅酸盐水泥工艺相比,该除氯系统设备的效率仍有很大提升潜力。
-
1)掺氯质量分数为0.1%~0.5%的CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系热力学相图计算和水泥烧制实验均反映出,氯元素可促进C2S结合f-CaO转化生成C3S,并使得熟料中C3S质量分数上升,C2S、C3A和C4AF质量分数下降。
2)在氯质量分数较高的高温反应体系中,CaO、SiO2和Al2O3倾向于与MgO和含氯物质结合生成Alinite相和Ca12Al14O32Cl2相。当氯质量分数超过0.4%后,促进C2S结合CaO转化生成C3S的作用变弱,此时Alinite相的增长速率变大。
3)氯质量分数由0.1%上升至0.5%时,水泥3 d抗压强度从51.2 MPa上升至66.6 Mpa,呈上升趋势;C3S和C2S质量分数随氯质量分数变化趋势表明2者对水泥28 d强度的增长产生了拮抗作用,28 d强度增长空间较小。
4)在附有旁路除氯系统的高氯原料水泥生产实际中,建议生料中氯质量分数控制在0.4%以下,可极大程度地促进熟料中C3S的生成,并保证适宜的C2S质量分数和较为优良的3 d和28 d强度性能。
氯元素对全废渣水泥性能的影响
Influence of chlorine on property of cement produced solely from industrial slag and material flow analysis
-
摘要: 针对以煤化工电石渣为主要原料的全废渣水泥生料中氯质量分数较高的问题,通过开展掺氯质量分数为0.1%~0.5%的CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系热力学相图计算和水泥烧制实验,结合XRD表征、水泥强度测定和物质流分析等方法,探索了生料中质量分数高的氯元素对熟料物相和水泥性能的影响规律。结果表明,氯元素可促进f-CaO结合C2S生成C3S,并使得熟料中C2S、C3A和C4AF质量分数降低。氯质量分数由0.1%上升至0.5%时,C3S质量分数增加了16.58%,其中包含着少量Alinite相,C2S质量分数在氯质量分数为0.4%时达最低值19.36%。当氯质量分数超过0.4%后,其促进C2S向C3S转化作用变弱,而Alinite相增长速率变大,在氯质量分数较高的体系中CaO、SiO2和Al2O3倾向于与MgO和含氯物质结合生成Alinite相和Ca12Al14O32Cl2相。氯元素对熟料物相的影响规律映射到水泥强度上表现为,氯质量分数由0.1%上升至0.5%时,水泥3 d强度增长了约30%,但28 d强度上升空间不大。本研究结果可为附有旁路除氯系统的高氯水泥生产工艺生料氯质量分数的控制提供参考。Abstract: To solve the problem of high chlorine content in the raw meal of all-waste cement with carbide slag of the coal chemical industry as the primary raw material, the thermodynamic phase diagram calculations and cement calcination experiments of the CaO-SiO2-Al2O3-Fe2O3-MgO-Cl system with 0.1%-0.5% chlorine content were carried out. Combined with XRD characterization, cement strength measurement and material flow analysis, the influence law and material flow of high-quality chlorine content in raw meal on clinker phase and cement performance were explored. The results showed that chlorine promoted f-CaO to combine with C2S to generate C3S, and caused a decrease in the mass fractions of C2S, C3A and C4AF in the clinker. When the chlorine content increased from 0.1% to 0.5%, the C3S content increased by 16.58%, including a small amount of the Alinite phase, and the C2S content reached the lowest value of 19.36% when the chlorine content was 0.4%. When the chlorine mass fraction is more than 0.4%, the promotion of C2S to C3S conversion becomes weaker, while the growth rate of the Alinite phase becomes higher. In the system with a higher chlorine mass fraction, CaO, SiO2 and Al2O3 tend to combine with MgO and chlorine-containing substances to form the Alinite phase and Ca12Al14O32Cl2 phase. The influence of chlorine on the clinker phase was mapped to the strength of the cement as follows: when the chlorine content increased from 0.1% to 0.5%, the 3 d strength of the cement increased by approximately 30%, but the 28 d strength had little room to increase. The results of this study can provide a reference for the control of the raw material chlorine mass fraction in the production process of high chlorine cement with a bypass dechlorination system.
-
Key words:
- industrial solid waste /
- cement properties /
- chlorine /
- cement strength /
- thermodynamic simulation /
- mass flow analysis
-
近些年,随着我国工业固废产生量的逐渐上升,工业固废减量化、无害化和资源化问题受到越来越多的关注[1-2]。其中,水泥窑协同处置工业固废和危废的方式和比例逐年上升,利用工业固废与传统硅酸盐水泥原料化学成分相近的特点替代天然矿物生产水泥,有助于解决大宗工业固废的消纳与资源化的问题[3]。例如,新疆天业水泥厂以煤化工电石渣作为钙质原料,粉煤灰和炉渣作为硅质原料,煤矸石作为铝制原料,硫酸渣、铜渣和铁质尾矿作为铁质原料,柠檬酸渣作为水泥缓凝原料,粉煤灰、煤矸石等作为拌合材原料,制备出符合国家标准的硅酸盐水泥[3-4]。在水泥生产的过程中,煤化工电石渣的氯元素质量分数较高[5],远高于传统硅酸盐水泥原料,不仅影响水泥熟料的性能[6],还会对水泥窑况产生着一定影响[7-8]。
有不少学者对氯元素在水泥窑中的行为规律进行了研究。崔育东等[9]开展了外掺CaCl2的水泥烧制实验,结果表明,生料中适量的氯离子可在水泥烧成反应中充当矿化剂的作用,生成低熔点含氯矿物相(C11A7·CaCl2)。施慧生等[10]认为,在含有CaCl2的CaO-SiO2-Al2O3-Fe2O3-MgO体系中,还可能发生CaO+SiO2+CaCl2+C12A7+MgO→Alinite的反应,生成化学组成为Ca10Mg1-x/2Vx/2[(SiO4)3+x(AlO4)1-x]O2Cl(V:晶格空位,0.35<x<0.45)的Alinite相。WANG[11]等的研究发现,随着生料中氯质量分数的上升,水泥窑中生成的低熔点含氯组分会随烟气循环富集,致使分解炉和回转窑窑尾处形成结皮堵塞。ZHU等[12]从整体工艺层面分析传统水泥生产过程中氯元素的流动途径和质量分配,对水泥品质的保证和窑设备的维护具有一定指导作用。就天然原料制备硅酸盐水泥工艺而言,已知氯质量分数低于0.03%时对熟料的形成有积极影响,但对氯质量分数为0.04%或更高的高氯原料的研究意义不大[13]。KWON等[14]在研究以污泥为水泥原料时指出,即使生料中的氯化物质量分数最高为0.04%,但窑炉中的氯化物质量分数仍可达1.5%,继而开展了掺氯量为0~2%的生料掺杂NH4Cl水泥烧制实验,并得出熟料的主要矿物相C3S、C2S、C3A和C4AF的质量分数变化规律,但仅从实验角度给出这一结论,并未继续监测水泥强度性能变化。
目前,有关水泥中氯元素行为规律的研究主要集中在低氯质量分数生料的传统硅酸盐水泥工艺上,且涉及到水泥强度性能方面的实验研究较少。为了探究电石渣中高质量分数的氯元素对熟料物相组成和水泥性能的影响规律,本研究以某水泥厂全废渣生料为原料,借助热力学计算方法模拟不同掺氯量CaO-Al2O3-SiO2-Fe2O3-MgO-Cl体系的高温煅烧反应相图[15],揭示不同氯质量分数下该体系高温煅烧过程中的物相转化,并结合不同掺氯质量分数水泥烧制实验,综合分析氯元素对矿物相组成变化规律和由此引起的水泥物理性能变化,最后对完整工艺中氯元素的物质流进行计算分析。本研究结果可为全废渣水泥生产生料中氯质量分数的控制提供参考。
1. 材料与方法
1.1 实验试剂及设备
实验试剂。氯元素标准溶液,硝酸(HNO3,65%~68%),氟化钙(CaF2),氯化钙(CaCl2),三乙醇胺(C6H15NO3),二水硫酸钙(CaSO4·2H2O)等,均为分析纯;纯水为实验室自制。
实验设备。实验电炉(HTF-1600型,武汉亚华);离子色谱仪(DX-120型,美国DIONEX);XRD衍射仪(XRD-7000型,日本Shimadzu);行星式球磨机(QM-3SP4型,南京南大);水泥砼恒温恒湿标准养护箱(HBY-40B型,上海康路);微机伺服水泥抗折抗压试验机(DYE-300B型,无锡新建)。
1.2 实验原料
原料取自新疆天业水泥厂(以下简称T水泥厂)其中1条规模为4 000 t·d–1的水泥熟料生产线。其生产工艺流程如图1所示,采用电石渣、钢渣、粉煤灰和硅粉等10余种固废为生产水泥的原料,在经过原料预处理和生料配伍计算后,按照硅酸盐水泥正常生产流程生产符合国家标准要求的水泥产品。本研究所取原料为T水泥厂生料均化库中的生料。其中,生料的烧失量为13.99%;各组分的质量分数比例为,54.90%CaO、18.60%SiO2、4.45%Al2O3、3.33%Fe2O3、2.56%MgO、0.10%Cl−。熟料三率值,即石灰饱和系数(Lime Saturation Factor,LSF或KH)、硅率(Silica Modulus,SM)和铝率(Alumina Modulus,IM),分别为KH=0.84、SM=2.53、IM=1.31。
1.3 实验设计
鉴于生料中氯元素基本上以Cl−形式存在,参考相关文献[16-18],拟用CaCl2作为掺氯剂,利用T水泥厂生料均化库生料,开展氯(Cl−)质量分数为0.1%~0.5%的生料煅烧实验制备熟料和水泥,并以熟料矿物相组分的质量分数和水泥3和28 d强度性能作为分析指标。外掺CaCl2水泥烧制实验设计如表1所示,S-1~S-5组生料的化学组分除了氯质量分数外,其余(氧化物组分和三率值)视为不变。
表 1 外掺CaCl2水泥烧制实验设计表Table 1. Design of experimental table for calcination of CaCl2 containing cement实验编号 氯(Cl−)掺量/% 氯(Cl−)质量分数/% CaCl2掺量/g 生料原料/g 总生料/g S-1 0 0.1 0 1 350.00 1 350.00 S-2 0.1 0.2 2.11 1 347.89 1 350.00 S-3 0.2 0.3 4.22 1 345.78 1 350.00 S-4 0.3 0.4 6.33 1 343.67 1 350.00 S-5 0.4 0.5 8.44 1 341.56 1 350.00 1.4 实验方法
1)生料制备。根据实验设计,精准称量好不同掺氯质量分数的CaCl2粉末和生料,放置在橡胶球磨罐中,用球磨机混合均匀后过190目筛至筛余率为12%~14%。准确称取筛下生料粉末450.00 g,加入50 mL纯水,用电液式压力试验机压制成Φ50 mm×20 mm的生料饼,放置在105 ℃的烘箱内12 h至恒重。
2)熟料烧制。烘干的生料饼盛放在石墨坩埚中,移至高温电炉中进行煅烧。高温电炉的升温程序设置依次为:0~300 ℃,升温速率6 ℃·min–1,升温时间50 min;300~900 ℃,升温速率10 ℃·min–1,升温时间60 min;900 ℃,保温50 min;900~1 450 ℃,升温速率10 ℃·min–1,升温时间110 min;1 450 ℃,保温时间90 min。将烧制好的熟料块从炉中取出急冷至室温,准确称量熟料块质量并记录。
3)水泥制备。依照GB 175-2007《通用硅酸盐水泥》标准[19],在熟料块粉磨过程中加入质量分数为0.3%的三乙醇胺试剂,粉磨后过80目筛至筛余率20%以下,制得熟料粉末,取5.00 g粉末留样(XRD表征备用)。按照m(剩余熟料)∶m(CaSO4·2H2O)=95∶5的比例掺入CaSO4·2H2O粉末,放置在橡胶球磨罐中,用球磨机混合均匀后过190目筛至筛余率20%以下制得水泥。
4)净浆实验。取适量质量的水泥粉末,按照0.30的水灰质量比加入对应质量的纯水,放在水泥净浆搅拌机上低速搅拌30 s,高速搅拌90 s。搅拌均匀的水泥浆体放入20 mm×20 mm×20 mm的水泥模具内(每个配方6个平行样),并放置在振动台上震动(120±5) s,用水平刮尺将模具表面的多余浆体刮掉,保持试块与模具表面的高度齐平,置于标准养护箱内养护24 h后脱模,制得抗压强度测定用的水泥净浆试块。脱模后的试件在养护箱中继续养护至指定龄期,等待3和28 d强度测定[20]。同上述实验步骤,将搅拌均匀的净浆水泥浆体也另外放入20 mm×40 mm×80 mm的水泥模具内(每个配方3个平行样)制备抗折强度测定用的水泥净浆试块,后续养护步骤同上。
1.5 分析方法
1)熟料XRD表征。采用XRD衍射仪定性分析水泥熟料矿物相组成;按照m(熟料)∶m(CaF2)=9∶1的比例掺入CaF2粉末作为内标物质,利用Rietveld全谱拟合分析法对熟料各矿物相的质量分数进行半定量分析[21]。XRD仪器采用CuKα靶,步长2θ=0.02(°)·step-1,扫描速度10 (°)·min–1(定量分析时采用4 (°)·min–1,电压30 kV,电流40 mA,石墨单色器。
2)热力学相图模拟。采用FactSage软件的Equilib模块模拟生料在800~1 500 ℃高温煅烧的物相转化过程[21]。输入T水泥厂生料均化库中的生料化学组分(质量分数),并将氯的质量分数设置在0.1%~0.5%的梯度内,继而模拟不同氯质量分数下,CaO-Al2O3-SiO2-Fe2O3-MgO-Cl体系的高温反应相变化历程。
2. 结果与讨论
2.1 热力学模拟分析
1)全废渣生料反应相图模拟。利用FactSage热力学软件,按照表1中第一行的生料各氧化物的质量分数比54.90%CaO∶18.60%SiO2∶4.45%Al2O3∶3.33%Fe2O3∶2.56%MgO∶0.10%Cl−模拟计算800~1 500 ℃温度区间内体系中各氧化物的反应历程,并绘制反应相图如图2所示。由图2可知,体系中的β-C2S在800 ℃前就已大量存在,伴随着铁相固溶体C2AF、钙镁铝盐中间矿物相Ca3MgSi2O8和Ca3MgAl4O10的生成。850 ℃后C2S由β型向α型转化,这与C2S在高温下的主要存在类型为α型的规律[22]是一致的。900~940 ℃,中间矿物Ca3MgSi2O8分解,进一步消耗体系中的CaO生成α-C2S,使得C2S质量分数骤增,并在900 ℃时达到最大值63%;同时,C2AF质量分数也有缓慢上升。940~1 300 ℃,Ca3MgAl4O10中间相全部分解,各矿物相相互转化,质量分数基本保持稳定,铁相固溶体中C2AF质量分数不断下降,C3AF组分占得优势[23];1 200 ℃后,体系中开始生成液相,为α-C2S与CaO结合生成C3S创造有利环境。1 300 ℃时,C2S大量转化为C3S,CaO的质量分数骤减为0。据此推测,熟料烧成实际温度对应热力学反应相图的1 300 ℃。1 300~1 340 ℃,热力学模拟的反应体系中除C3S以外的矿物如C3AF,倾向于分解成CaO、Al2O3、Fe2O3并熔融成液相,体系中的液相量持续上升,并进一步与C2S结合生成C3S。1 340 ℃后,C3S的生成量已达到稳定并呈轻微下降趋势。此时,C2S倾向于熔融生成液相,直至1 400 ℃后体系中的C2S由β型开始向γ型转化。热力学模拟相图图2反映出,体系中的C3S在1 300 ℃瞬时生成,f-CaO也在1 300 ℃时瞬间全部消耗。由此推断,热力学计算得到的CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系熟料烧成温度为1 300 ℃。
2)不同氯质量分数体系热力学相图模拟。进一步从热力学计算角度探索氯(Cl−)质量分数在0.1%~0.5%的范围内,CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系高温煅烧反应过程中的液相及各矿物相质量分数的生成变化规律,并与不含氯体系进行对比,着重关注1 300 ℃时各物相及液相的质量分数变化趋势。绘制不同氯质量分数下C3S、C2S、C3AF、CaO、液相的质量分数随温度变化图,如图3所示。由图3(a)可发现,随着氯质量分数的上升,1 300 ℃时体系中的C3S质量分数逐渐增加,氯质量分数从0.1%上升至0.5%时,C3S的质量分数由48%增加至49.5%。对照图3(b)和图3(d)可知,1 300 ℃时随着氯质量分数的上升,体系中的C2S质量分数呈现出不断下降的趋势,当氯质量分数由0.1%上升至0.5%时,C3S的质量分数从27.5%增加至25.5%;同时,体系中CaO的消耗量也随着氯质量分数上升不断增大。C3S、C2S和CaO的质量分数变化趋势与C3S的生成规律是一致的,即氯元素可以促进C2S结合CaO生成C3S的反应速率,并增加C3S生成量[17]。熟料中的铁相固溶体组成形式包括C2AF~C6AF[23],C3AF是其中的1种。由图3(c)可知,氯质量分数由0.1%上升至0.5%时,体系中C3AF的质量分数在1 300 ℃时从18%下降至13%,但均高于不含氯的体系。可见,氯元素的升高对于C3AF的生成起到了抑制作用。结合图3(e)可发现,液相量在1 300 ℃时随着氯质量分数的上升逐渐增加,但不含氯体系中液相量为0。因此,氯元素的存在促进了液相量的生成[6]。液相的形成主要依靠Al2O3、Fe2O3等氧化物组分[22]。可见,在热力学模拟的1 300 ℃时氯质量分数较高的体系中,Al2O3、Fe2O3等氧化物组分倾向于熔融成液相,因此C3AF的生成量逐渐降低。
结合热力学模拟CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系反应相图可知,熟料烧成温度为1 300 ℃。不同氯质量分数体系反应相图中各物相和液相量在1 300 ℃处的生成转化规律表明,氯元素可以促进C2S消耗CaO生成C3S,并促进液相量的生成。随着氯质量分数由0.1%上升至0.5%时,1 300 ℃时体系中的C3S质量分数从48%上升至49.5%;C2S质量分数由27.5%下降至25.5%,C3AF的质量分数从18%下降至13%。在不考虑动力学反应速率和时间限制的条件下,热力学相图模拟结果很好地反映了0.1%~0.5%氯质量分数生料体系高温煅烧反应过程中,主要矿物相C3S、C2S、C3AF及CaO和液相质量分数的变化趋势,这对水泥生产尤其是高氯质量分数的水泥生产工艺具有一定的理论指导价值。然而,热力学软件模拟有其局限性。一是未能考虑动力学方面的影响。这是因为,在实际生产中不可能达到理想的传质条件和无限的反应时间,如各物相的生成均在某温度处瞬时完成。二是FactSage软件数据库不含C3A组分,无法准确反映C3A的质量分数生成规律。因此,相图模拟的不同氯质量分数体系中各物相的质量分数变化规律,还有待开展不同的掺氯生料水泥烧制实验来进一步验证。
2.2 熟料XRD分析
对熟料进行XRD表征分析,结果如图4所示。全废渣水泥熟料中除了常见的C3S、C2S、C2AF、C3A矿物相之外,还发现了Alinite相和Ca12Al14O32Cl2相2个新相[10]。采用Rietveld全谱拟合分析法并借助MDI Jade 6.0软件对内掺CaF2熟料XRD图谱中各物相的质量分数进行精修[24]。精修过程中发现,由于Alinite相是通式为Ca10Mg1-x/2Vx/2[(SiO4)3+x(AlO4)1-x]O2Cl(V:晶格空位,0.35<x<0.45)[25-27]的一类混合物,难以得到其准确分子式并进行精修,但由于其结构与C3S类似且质量分数较少,将Alinite相质量分数纳入到C3S相中计算。主要对C3S、C2S、C3A、C2AF、Ca12Al14O32Cl2相、f-CaO和无定型相(Amorphous)的质量分数进行半定量分析,结果如表2所示。主要矿物相组分C3S、C2S、C3A、C2AF和Ca12Al14O32Cl2的质量分数变化如图5(a)和图5(b)所示。由表2中的精修参数可知,S-1~S-5组内掺CaF2熟料XRD图谱的精修结果均满足E≤R≤2E。这表明,精修结果在置信区间内。熟料中存在一部分无定型态物质。这可能是因为,冷却过程中液相来不及结晶形成玻璃体相,其中含有少量氧化物如Fe2O3、MgO等[22]。由氯质量分数为0.1%时Ca12Al14O32Cl2的质量分数为2.44%计算得到氯的质量分数为0.12%,此结果超过了生料中氯的质量分数。这说明,精修结果存在着一定的误差。但在相同的工艺条件和精修参数背景下,仍可借助XRD精修结果对熟料中主要矿物相质量分数的变化趋势进行半定量分析。
表 2 S-1~S-5组内掺CaF2熟料物相组分精修质量分数表Table 2. Phase content of S-1~S-5 clinker after Rietveld refinement with addition of CaF2 within group编号 矿物相组分质量分数/% 精修参数 C3S C2S C3A C2AF Ca12Al14O32Cl2 f-CaO Amorphous R/% E/% S-1 48.44 34.67 4.94 5.28 2.44 4.22 0.00 2.18 1.62 S-2 55.74 26.38 5.52 5.74 1.77 3.86 1.00 2.43 1.69 S-3 59.49 22.51 4.39 4.18 2.36 2.79 4.29 2.31 1.68 S-4 63.70 19.36 3.08 2.87 5.74 1.84 3.40 2.12 1.70 S-5 65.02 20.89 2.45 2.13 4.37 1.20 3.94 2.34 1.68 表2反映出,熟料的主要矿物相为C3S和C2S。随着氯质量分数的上升,熟料中f-CaO的质量分数逐渐减少。这表明,氯元素增强了熟料的易烧性。由图5(a)可观察到,氯质量分数由0.1%上升至0.5%时,C3S质量分数由48.44%增加至65.02%,其中包含着少量Alinite相;C2S质量分数则由34.67%下降至19.36%,且在氯质量分数为0.4%时达到最低值,当氯质量分数上升至0.5%后,C2S的质量分数有所增加。熟料中C3S、C2S和f-CaO质量分数的变化趋势表明,氯元素促进了体系中的C2S相向C3S相的转化,但在氯质量分数超过0.4%后,促进作用减弱。图5(b)表明,熟料中的C3A和C2AF组分受氯质量分数影响较大,当生料掺氯量上升至0.2%后,C3A和C2AF质量分数均呈下降趋势。其中,氯质量分数从0.2%上升至0.5%的过程中,C3A和C2AF的质量分数分别由5.52%下降至2.45%和5.74%下降至2.13%;同时,体系中的Ca12Al14O32Cl2相作为产生C3A的中间相,其质量分数不断上升,直至超过C3A和C2AF组分。从Al元素的质量守恒角度来说,部分Al元素还可能参与了Alinite相的形成。这表明,在氯质量分数较高的高温煅烧反应过程中,较多的Al2O3倾向于与CaO、SiO2、MgO和含氯组分等结合生成Ca12Al14O32Cl2相和Alinite相。由图5可以发现,氯质量分数由0.4%上升至0.5%时,体系中的C2S质量分数由19.36%上升至20.89%;同时,Ca12Al14O32Cl2的质量分数由5.74%下降至4.37%,而C3S质量分数仍增长了1.32%。结合Ca、Al、Si和Cl元素的质量守恒和Alinite相的化学组成可知,氯质量分数为0.5%时C3S质量分数的微弱增长可能主要来源于Alinite相。此时,氯元素促进C2S向C3S转化的作用较弱;相对地,体系中Alinite相的生成速率变大。
不同掺氯量熟料的各矿物相质量分数变化趋势表明,氯元素可增加高温体系中CaO的消耗量,促进C2S向C3S的转化,并显著增加C3S的生成量。同时,在氯质量分数较高的高温反应体系中,CaO、SiO2和Al2O3倾向于与MgO和含氯物质等结合生成Alinite相和Ca12Al14O32Cl2相;相应地,熟料中的C2S、C3A和C2AF相质量分数随着氯质量分数的上升均呈现明显下降趋势,这与上文热力学模拟不同氯质量分数体系C3S、C2S和C3AF的质量分数变化规律是符合的。值得注意的是,当生料中的氯质量分数超过0.4%后,体系中的C2S组分质量分数有所增加。可推测,此时氯元素促进C2S相向C3S相转化的作用变弱,而Alinite相的增长速率变大。
2.3 水泥强度性能测定
水泥3和28 d抗折、抗压强度数值如图6所示,结合上文熟料矿物相XRD精修半定量分析结果表2可知,影响水泥早期强度的C3S相在熟料中占比较大,可达50%~65%,其中包含的少量Alinite相也对3 d强度的增长有利,且随着氯质量分数的上升,C3S质量分数占比逐步上升,因此水泥3 d强度增长速率较快。使用SPSS软件进行相关性分析可知,氯离子质量分数与28 d抗折强度皮尔逊相关系数为0.972,表现为强正相关;氯离子质量分数与28天抗压强度皮尔逊相关系数为−0.666,表现为负相关。其中,氯质量分数为0.3%时,28 d抗压强度达96.6 MPa,相比氯质量分数为0.1%时的28 d抗压强度103.8 MPa降低了7%。实际上,水泥28 d强度受到多种矿物的综合影响,但主要是C3S和C2S质量分数的影响。熟料XRD矿物相质量分数精修结果表2表明,当生料掺氯质量分数上升时,熟料中C3S相质量分数不断上升的同时,C2S和C4AF质量分数却呈现下降趋势,C3S和C2S质量分数随掺氯量的变化趋势使得2者对水泥28 d强度的增长产生拮抗作用。因此,28 d抗压强度的增长空间不如3 d抗压强度,且总体呈现轻微下降的趋势。
对于附有旁路除氯系统的高氯原料水泥生产工艺而言,熟料中含有部分Alinite组分可使水泥具有易磨的特性,有利于水泥粉磨环节节省能耗[28-29]。结合S-1组~S-5组熟料XRD表征和水泥强度性能测定结果,为了极大程度地促进熟料中C3S相的生成,同时保证熟料中适宜的C2S质量分数和水泥优良的3 d和28 d性能,将生料氯元素质量分数控制在0.4%以下是较好的选择。
2.4 氯元素物质流分析
取流水线上某24 h工作周期内水泥烧制物料投入量和输出量数据,并根据GB/T 176-2017《水泥化学分析方法》[30]离子色谱法测定各样品中的氯元素质量分数,结果如表3所示。
表 3 某24 h工作周期内各物料氯元素质量分数及物料喂入量和输出量数据Table 3. Chlorine content of each material as well as material feeding and output data in 24 h含氯物料 氯质量分数/% 物料消耗量/(t·d–1) 生料 0.112 7 094.000 燃煤 0.022 522.000 熟料 0.025 5 912.000 旁路除氯氯灰 21.900 11.280 窑灰 0.196 628.760 煤灰 0.084 88.840 结合图1的T水泥厂全废渣水泥工艺流程得知,整体工艺中的氯元素来源于电石渣、粉煤灰等工业废渣原料,在生料均化环节其质量分数得到均化。随着生料进料,熟料煅烧至1 100 ℃时,氯元素与生料中其他碱性金属组分如钠、钾形成低熔点的氯盐挥发到预热器、分解炉和回转窑中。当窑中煅烧温度进一步上升至1 300 ℃后,体系中的氯组分已大部分挥发至烟气中,只有小部分掺杂在熟料矿物相中被带出。一部分烟气中的挥发性氯组分随烟气至窑头处被新进的生料裹挟,随后在回转窑中循环富集;另一部分随烟气被抽取至旁路除氯系统中,被迅速冷凝除尘作为捕集的氯灰进行离线处理。随着窑尾处的烟气回到预热器后经过降温与除尘器收集到的窑灰被回喂进生料系统中,部分窑尾处烟气在温度适宜区的预热器和分解炉处富集形成结皮堵塞。根据图1工艺流程和表3的数据对氯元素的物质流动进行质量平衡计算。氯元素有2个来源,通过计算得到,来自生料的氯为7.945 t,来自燃料携带的氯为0.115 t,总量为8.060 t。氯元素有4个去向,其中,进入熟料中的氯的质量为1.478 t,进入旁路除氯系统收尘灰中的氯为2.470 t,去往窑灰中的氯为1.232 t,进入煤灰中的氯为0.075 t。根据质量守恒的原则进行计算得到随窑尾结皮、随烟气循环富集中的氯的质量为2.805 t。因此,系统旁路除氯的效率为47%。
由氯元素的质量平衡分析计算和工艺流程图1绘制氯元素的物质流分析Sankey图,如图7所示。氯元素的总量达8.060 t,其中随烟气循环富集的量为2.805 t,占氯元素总量的34.80%;熟料中氯元素的质量分数达1.478 t,占氯元素总质量分数的18.34%。可见,回转窑工艺下高氯全废渣生料在高温煅烧过程中氯元素的循环富集情况较为普遍,应特别注重水泥氯元素质量分数的指标。全废渣工艺的旁路除氯系统效率达47.00%,而普通水泥窑的旁路除氯效率可达70%以上[31],与普通硅酸盐水泥工艺相比,该除氯系统设备的效率仍有很大提升潜力。
3. 结论
1)掺氯质量分数为0.1%~0.5%的CaO-SiO2-Al2O3-Fe2O3-MgO-Cl体系热力学相图计算和水泥烧制实验均反映出,氯元素可促进C2S结合f-CaO转化生成C3S,并使得熟料中C3S质量分数上升,C2S、C3A和C4AF质量分数下降。
2)在氯质量分数较高的高温反应体系中,CaO、SiO2和Al2O3倾向于与MgO和含氯物质结合生成Alinite相和Ca12Al14O32Cl2相。当氯质量分数超过0.4%后,促进C2S结合CaO转化生成C3S的作用变弱,此时Alinite相的增长速率变大。
3)氯质量分数由0.1%上升至0.5%时,水泥3 d抗压强度从51.2 MPa上升至66.6 Mpa,呈上升趋势;C3S和C2S质量分数随氯质量分数变化趋势表明2者对水泥28 d强度的增长产生了拮抗作用,28 d强度增长空间较小。
4)在附有旁路除氯系统的高氯原料水泥生产实际中,建议生料中氯质量分数控制在0.4%以下,可极大程度地促进熟料中C3S的生成,并保证适宜的C2S质量分数和较为优良的3 d和28 d强度性能。
-
表 1 外掺CaCl2水泥烧制实验设计表
Table 1. Design of experimental table for calcination of CaCl2 containing cement
实验编号 氯(Cl−)掺量/% 氯(Cl−)质量分数/% CaCl2掺量/g 生料原料/g 总生料/g S-1 0 0.1 0 1 350.00 1 350.00 S-2 0.1 0.2 2.11 1 347.89 1 350.00 S-3 0.2 0.3 4.22 1 345.78 1 350.00 S-4 0.3 0.4 6.33 1 343.67 1 350.00 S-5 0.4 0.5 8.44 1 341.56 1 350.00 表 2 S-1~S-5组内掺CaF2熟料物相组分精修质量分数表
Table 2. Phase content of S-1~S-5 clinker after Rietveld refinement with addition of CaF2 within group
编号 矿物相组分质量分数/% 精修参数 C3S C2S C3A C2AF Ca12Al14O32Cl2 f-CaO Amorphous R/% E/% S-1 48.44 34.67 4.94 5.28 2.44 4.22 0.00 2.18 1.62 S-2 55.74 26.38 5.52 5.74 1.77 3.86 1.00 2.43 1.69 S-3 59.49 22.51 4.39 4.18 2.36 2.79 4.29 2.31 1.68 S-4 63.70 19.36 3.08 2.87 5.74 1.84 3.40 2.12 1.70 S-5 65.02 20.89 2.45 2.13 4.37 1.20 3.94 2.34 1.68 表 3 某24 h工作周期内各物料氯元素质量分数及物料喂入量和输出量数据
Table 3. Chlorine content of each material as well as material feeding and output data in 24 h
含氯物料 氯质量分数/% 物料消耗量/(t·d–1) 生料 0.112 7 094.000 燃煤 0.022 522.000 熟料 0.025 5 912.000 旁路除氯氯灰 21.900 11.280 窑灰 0.196 628.760 煤灰 0.084 88.840 -
[1] PABLOS J M, SICHIERI E P, DE A Z, et al. Study for utilization of industrial solid waste, generated by the discharge of casting sand agglomeration with clay and of blast furnace slag, in concrete composition[J]. Key Engineering Materials, 2012, 517: 611-616. doi: 10.4028/www.scientific.net/KEM.517.611 [2] 黄东升. 城市一般工业固废现状及对策研究[J]. 资源节约与环保, 2018(9): 44+49. doi: 10.16317/j.cnki.12-1377/x.2018.09.033 [3] REN C Z, WANG W L, LI G L. Preparation of high-performance cementitious materials from industrial solid waste[J]. Construction and Building Materials, 2017, 152: 39-47. doi: 10.1016/j.conbuildmat.2017.06.124 [4] 郭随华, 翁端, 陈益民. 我国水泥工业“生态化”的研究现状和发展趋势[J]. 硅酸盐学报, 2001(2): 172-177. doi: 10.3321/j.issn:0454-5648.2001.02.016 [5] 吴莉娜, 吴融权. 我国氯碱行业节能减排技术分析[J]. 氯碱工业, 2013, 49(5): 39-45. doi: 10.3969/j.issn.1008-133X.2013.05.014 [6] 王昕, 刘晨, 颜碧兰, 等. 国内外水泥窑协同处置城市固体废弃物现状与应用[J]. 硅酸盐通报, 2014, 33(8): 1989-1995. doi: 10.16552/j.cnki.issn1001-1625.2014.08.028 [7] SILVA R V, BRITO J D, LYNN C J, et al. Environmental impacts of the use of bottom ashes from municipal solid waste incineration: A review[J]. Resources Conservation and Recycling, 2019, 140: 23-35. doi: 10.1016/j.resconrec.2018.09.011 [8] MURAT M, SORRENTINO F. Effect of large additions of Cd, Pb, Cr, Zn, to cement raw meal on the composition and the properties of the clinker and the cement[J]. Cement and Concrete Research, 1996, 26: 377-385. doi: 10.1016/0008-8846(96)00010-5 [9] 崔育东. 钾钠氯硫挥发特性及对熟料煅烧的影响[D]. 浙江: 浙江大学, 2011 [10] 施惠生, 吴凯, 郭晓潞. 垃圾焚烧飞灰煅烧阿利尼特水泥熟料的形成过程及其水化性能研究[J]. 水泥技术, 2010(6): 23-27. doi: 10.3969/j.issn.1001-6171.2010.06.002 [11] WANG Y F, ZHU H M, JIANG X G, et al. Study on the evolution and transformation of Cl during Co-incineration of a mixture of rectification residue and raw meal of a cement kiln[J]. Waste Management, 2019, 84: 112-118. doi: 10.1016/j.wasman.2018.11.036 [12] ZHU H M, WANG Y F, JING N J, et al. Study on the evolution and transformation of chlorine during co-processing of hazardous waste incineration residue in a cement kiln[J]. Waste Management & Research, 2019, 37: 495-501. [13] CHOI J H, OH S C. Removal of chlorine from municipal solid waste fly ash by using acid and alkaline solution[J]. Journal of Korea Society of Waste Management, 2019, 36(5): 471-479. doi: 10.9786/kswm.2019.36.5.471 [14] KWON W T, KIM Y H, CHU Y S. Effect of P2O5 and chloride on clinkering reaction[J]. Advances in Technology of Materials Materials Processing Journal, 2005, 7(1): 63-66. [15] TAZUDDIN, AIYER H N, CHATTERJEE A. Phase equilibria studies of CaO-SiO2-Al2O3-Fe2O3-MgO system using CALPHAD[J]. Calphad-computer Coupling of Phase Diagrams and Thermochemistry, 2018, 60: 116-125. doi: 10.1016/j.calphad.2017.12.003 [16] KOLOVOS K, TSIVILIS S, KAKALI G. The effect of foreign ions on the reactivity of the CaO–SiO2–Al2O3–Fe2O3 system: Part II: Cations[J]. Cement Concrete Research, 2002, 32(3): 463-469. doi: 10.1016/S0008-8846(01)00705-0 [17] 陈明源, 陈韬, 邱树恒, 等. 用氯化钙作矿化剂制成的水泥的15年钢筋锈蚀试验[J]. 水泥, 2003(12): 7-9. doi: 10.3969/j.issn.1002-9877.2003.12.003 [18] KIM T Y, KIM N I, CHU Y S. Burnability and mineral properties of clinker added chlorine[J]. Resources Recycling, 2020, 29(6): 48-56. [19] 中华人民共和国国家市场监督管理总局, 中国国家标准化管理委员会. 通用硅酸盐水泥标准: GB/T 21372-2008[S]. 北京: 中国环境科学出版社, 2008 [20] 李贵强. 掺杂SrO和SrSO4对高阿利特水泥合成和性能的影响[D]. 济南: 济南大学, 2011 [21] GHERIBIA E, ROBELIN C, DIGABEL S L, et al. Calculating all local minima on liquidus surfaces using the FactSage software and databases and the Mesh Adaptive Direct Search algorithm[J]. Journal of Chemical Thermodynamics, 2011, 43(9): 1323-1330. doi: 10.1016/j.jct.2011.03.021 [22] 沈威. 水泥工艺学[J]. 武汉:武汉理工大学出版社, 1991: 5-32. [23] SONER İ. Utilization of fluidized bed combustion ashes as raw material in the production of a special cement[J]. Middle East Technical University, 2009: 32-44. [24] 常钧, 崔凯. 基于Rietveld方法的硫铝酸盐水泥熟料及其水化产物定量相分析[J]. 建筑材料学报, 2020, 23(2): 438-446. [25] ILYUKHIN V V, NEVSKY N N, BICKBAU M J, et al. Crystal structure of alinite[J]. Nature, 1977, 269(5627): 397-398. doi: 10.1038/269397a0 [26] LAMPE F V, HILMER W, JOST K H, et al. Synthesis, structure and thermal decomposition of alinite[J]. Cement Concrete Research, 1986, 16(4): 505-510. doi: 10.1016/0008-8846(86)90088-8 [27] NEUBAUER J, POLLMANN H. Alinite—Chemical composition, solid solution and hydration behaviour[J]. Cement Concrete Research, 1994, 24(8): 1413-1422. doi: 10.1016/0008-8846(94)90154-6 [28] BIKBAOU M Y. Crystal chemistry and properties of alinite and jasmundite[J]. 8th International Congress on the Chemistry of Cement, 1986, 2: 352-357. [29] KOSTOGLOUDIS G C, KALOGRIDIS D, FTIKOS C, et al. Comparative investigation of corrosion resistance of steel reinforcement in alinite and Portland cement mortars[J]. Cement Concrete Research, 1998, 28(7): 995-1010. doi: 10.1016/S0008-8846(98)00072-6 [30] 中华人民共和国国家市场监督管理总局, 中国国家标准化管理委员会. 水泥化学分析方法: GB/T 176-2017[S]. 北京: 中国环境科学出版社, 2017 [31] WANG F, WANG H F, XU J, et al. Determination of fluorine and chlorine in coal by high temperature combustion hydrolyzing-ion chromatography[J]. Chemical Analysis and Meterage, 2016, 25: 27-30. -






 下载:
下载: