-
近年来,土壤地下水重金属污染问题受到了广泛关注。由于重金属污染物通过土壤运移扩散至地下水参与循环,对人类健康造成威胁,故需阻滞污染物在土壤中的运移,以修复土壤从而缓解地下水污染防治的压力[1-4]。纳米零价铁 (nZVI) 是一种粒径小于100 nm的零价铁,其具有比表面积大、反应活性高、无二次污染残留等优点而广泛应用于固化重金属污染物从而修复土壤[5-8]。然而,在nZVI-土水污染混合体系中,土壤广泛存在的矿物胶体可能影响重金属、nZVI的迁移,从而影响其固化修复的效果。杜晓丽[9]通过下渗柱实验研究胶体对重金属运移的影响,结果表明径流胶体能够促进重金属离子的下渗迁移。王凯丽等[10]使用饱和沙壤土柱研究了SiO2胶体对Cd迁移的影响,发现SiO2胶体促进土壤对Cd的吸附,抑制了Cd在土柱中的迁移。同时,多种重金属往往共存于土壤,其在迁移过程中存在竞争吸附作用[11],进一步增加固化修复过程的不确定性。目前,矿物胶体、竞争吸附对nZVI修复土壤重金属的影响尚未报道。因此,有必要在通过实验验证重金属对土壤的污染行为及nZVI对其修复过程的同时,进一步研究矿物胶体对nZVI修复土壤重金属单一或复合污染的影响及作用机制。
判断重金属对土壤的污染程度及修复剂的效果,或深入探究胶体等影响因素作用修复行为的机制,一般将定量的吸附剂与土壤经过长时间的充分接触使其达到平衡 (静态吸附试验) ,或使吸附剂呈持续流动状态穿透土壤 (动态土柱实验) 。程景[12]开展静态吸附批实验探究不同溶解态腐殖质对Cr(Ⅵ)在土壤上吸附解吸行为的作用,并揭示其作用机理。付雪[13]同样采用静态吸附实验研究时间和矿物胶体种类对nZVI修复固定土壤Pb、U重金属效果的影响。但在实际情况下,重金属对土壤的污染及其修复是动态的过程,需要研究测试重金属、修复剂及影响组分在土壤中的运移、扩散速度,静态吸附实验不易准确模拟,所以需要进行动态土柱实验。李志亮[14]通过土柱实验探究新型桥连氯磷灰石对复合污染酸性土壤中重金属Pb2+、Zn2+、Cu2+、Cd2+的固定机制。李平[15]通过柱实验研究nZVI体系对山地、河滩、农田及煤矿地区土壤中Cr (Ⅵ) 的去除和滤出特性的影响。土柱实验由于可近似刻画物质在土壤中运移的动态过程,由实验绘制的穿透曲线能较为客观反映实验过程及结果,所以得到广泛应用[16-20],基于上述优点,采用动态土柱实验进行本研究。
随着深圳经济的发展,化工、电气及核工业排放的铅 (Pb) 和铀 (U) 重金属对土壤、地下水环境及人类健康造成一定影响,因此选择Pb、U作为待修复污染物[21-22]。刘军[23]通过相关研究表明高岭土胶体对铀存在表面吸附作用,吸附容量为2.51 mg·g−1。孙慧敏[24]研究指出高岭土胶体在水中对铅的吸附容量为 6.06 mg·g−1。由于高岭土胶体在土壤中相对含量较高,成本低廉,且对铀和铅均有一定的吸附效果,所以选择高岭土胶体作为nZVI固化Pb、U重金属的影响因素[25]。
本研究以自然界中广泛存在,具有环境友好性的硅藻土作为运移介质,拟研究高岭土矿物胶体对nZVI修复Pb、U重金属单一或复合污染土壤的影响机制[26]。研究成果可为综合整治土壤地下水重金属污染提供理论依据和数据支持。
-
实验所用试剂主要有硝酸铀酰 (UO2(NO3)2·6H2O) 、硝酸铅 (Pb(NO3)2) 、nZVI (粒径50 nm) 、硅藻土、高岭土。以上试剂均为购买所得,纯度AR (分析纯) ,所有溶液均采用去离子水配制。
-
将硝酸铀酰与硝酸铅试剂分别溶于去离子水制备质量浓度为1 000 mg·L−1的U与Pb母液,进一步稀释至10 mg·L−1的U溶液与Pb溶液,并将上述U与Pb母液混合稀释,制得各组分质量浓度均为10 mg·L−1的U+Pb混合溶液,冷藏保存。将1 g高岭土粉末悬浮在1 L去离子水中,将悬浮液静置24 h,回收上部液体从而制得高岭土原料悬浮液,使用干燥-称重法得到质量浓度10 mg·L−1的高岭土胶体悬浮液。重金属与高岭土胶体、nZVI混合溶液的制法则是将上述重金属溶液制法的去离子水替换为相应的nZVI、高岭土胶体悬浮液。
硅藻土作为运移介质,需要进一步降低其在实验中的不确定影响因素,因此将硅藻土熟化处理。具体制备方法为土水质量比2∶1混合搅拌均匀,静置1周使土壤达到均匀状态。
-
土柱选取长12 cm,内径2 cm的透明丙烯酸柱,内壁较为粗糙,防止液体沿内测管壁流动产生优先流。柱子的底部和顶部装有两层尼龙筛网以密封硅藻土。填充过程中需轻轻捣压以确保紧密填充,测定土柱有效高度为10 cm。填充后先由去离子水由上至下冲洗去除杂质,随后相同方向通入重金属、nZVI和胶体溶液,其中,含有nZVI或胶体的溶液临实验前进行超声处理以避免团聚。使用蠕动泵以1.5 mL·min−1速度通液体,出流液通入自动接样器,以确保每3 min取样,测定出流液铁、铀、铅及高岭土胶体的质量浓度,并绘制穿透曲线。随后对实验前后的土样进行微观结构表征分析与物相分析,并对流出液进行铁、铀、铅及高岭土胶体质量浓度的测定,得到实验期间各时间点的质量浓度与初始质量浓度之比 (C/C0) ,从而以时间为横坐标,C/C0为纵坐标绘制各指标在硅藻土柱中的穿透曲线。各实验内容如表1所示。
-
本实验通过电感耦合等离子体质谱仪 (ICP-MS) (7700X,美国Thermo Fisher仪器公司) 测定出流液中铅、铀及铁元素的质量浓度,使用紫外分光光度计 (Multiskan SkyHigh,美国Thermo Fisher仪器公司) 测定高岭土胶体质量浓度,具体方法为配制各质量浓度梯度的高岭土胶体,利用胶体的吸光度与质量浓度的线性关系绘制标准曲线,再通过测定样品的吸光度对应标准曲线得到高岭土胶体的质量浓度。为了验证nZVI对土壤Pb、U重金属的修复效果及机制,提取nZVI及重金属参与的实验前后硅藻土样,将土样的表面微观结构使用环境扫描电子显微镜 (SEM) (Quattro S,美国Thermo Fisher仪器公司) 测定,采用X射线衍射仪 (XRD) (Smartlab 9 kw,日本Rigaku仪器公司) 对样品进行物相分析。
为了定量探究矿物胶体对nZVI修复效果的影响,在穿透曲线基础上,需对实验中各组分初始 (曲线上升) 及收尾 (曲线下降) 阶段迁移平均速率进行探究。
定义各实验初始阶段为待测组分进入土柱至连续5次出流液C/C0均保持0.9以上的第1个时间点的时间段,收尾阶段为80 min时间点至C/C0稳定低于0.01的第1个时间点的时间段。定义溶质 (组分) 初始迁移平均速率如式(1)所示。
式中:t升表示初始阶段,min;m升为初始阶段出流液检出的溶质总质量,mg;v升为初始阶段单位时间的溶质质量,即初始阶段溶质迁移平均速率,mg·min−1。
由于各实验土柱条件及淋溶液速率保持一致,在进入土柱前各组分与溶剂运移速率无明显差异,且已测定去离子水2 min由试剂瓶进入土柱,可推知各实验溶质均在20 min进入土柱,因此具备计算运移速率的条件。v升反映了溶质与硅藻土之间达到吸附饱和的速率。
同理可进行收尾阶段迁移平均速率计算,时间段取80 min时间点至C/C0稳定低于0.01的第1个时间点,计算收尾平均速率v降如式(2)所示。v降表示收尾阶段溶质迁移平均速率,mg·min−1。其反映了溶质与硅藻土之间解吸的速率。
-
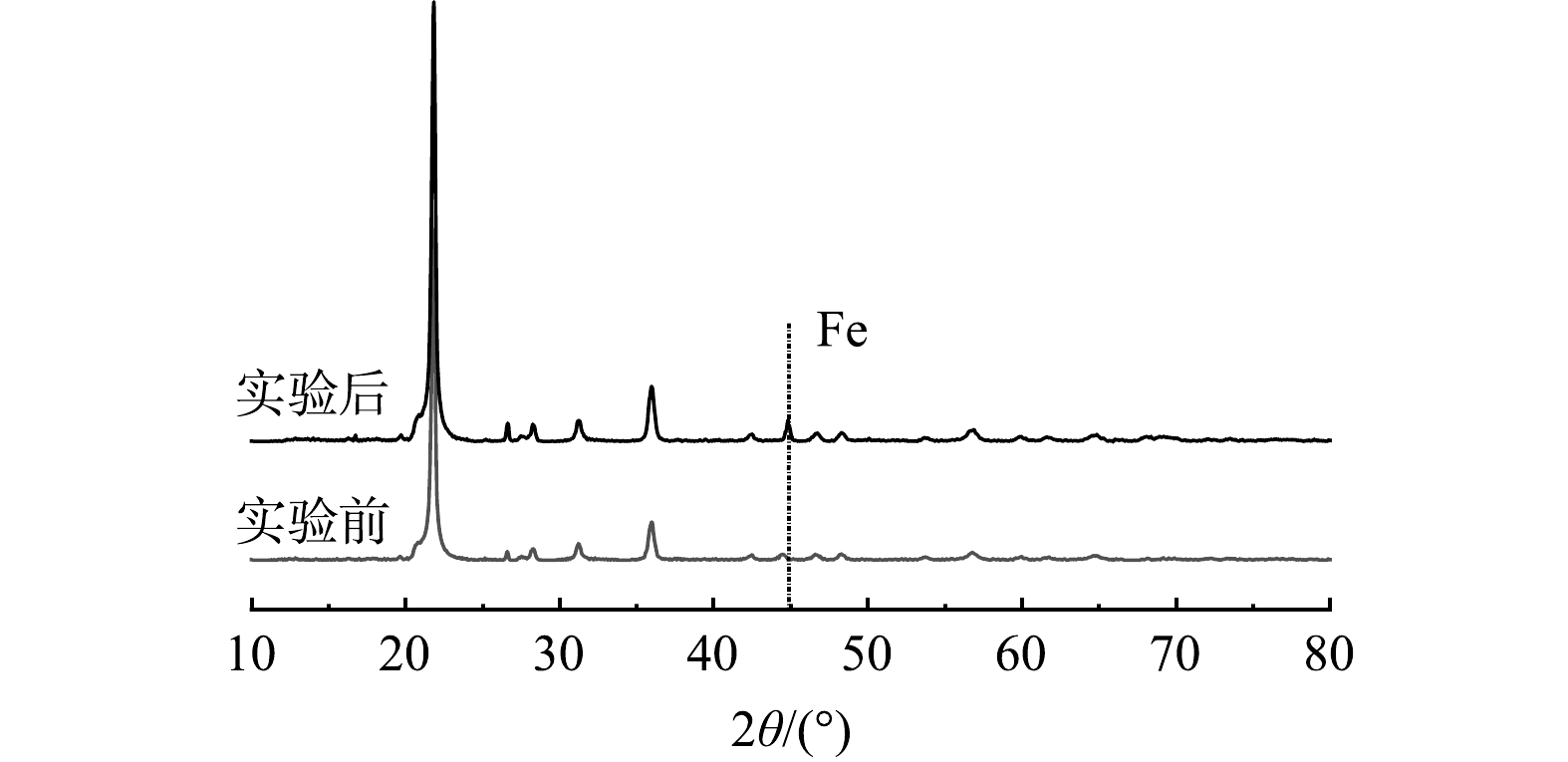
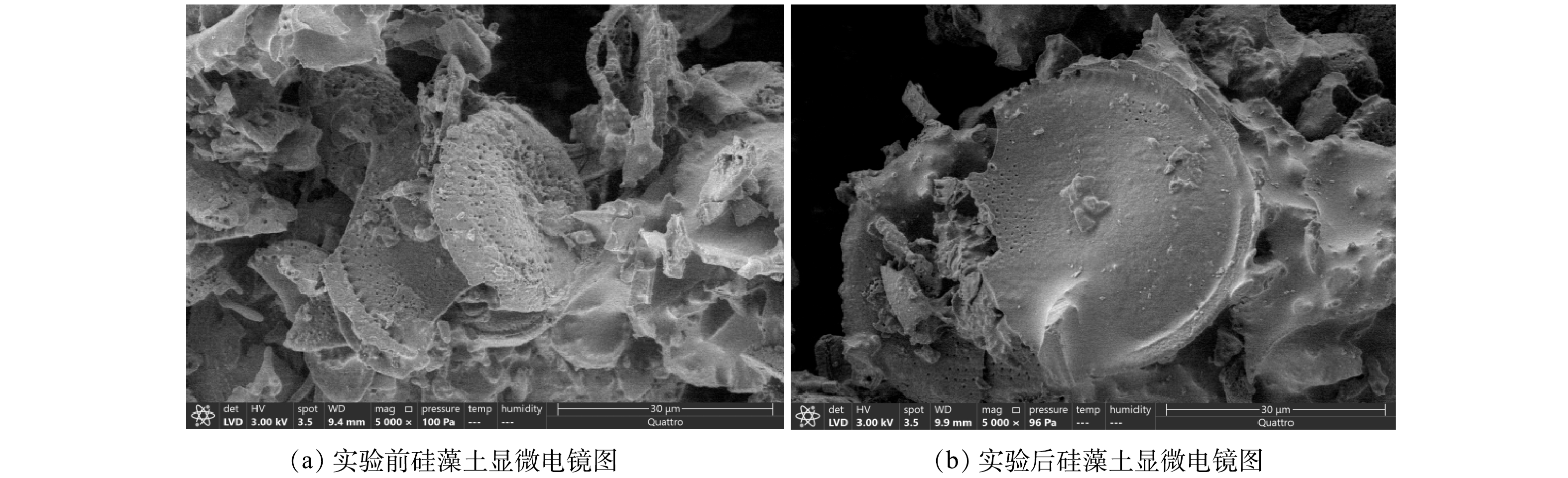
作为运移介质,硅藻土自身的微观结构与化学组成可能影响柱实验各组分的运移与相互作用,因此对添加nZVI与Pb、U重金属实验前后的硅藻土进行SEM分析,分别得出结果见图1,并进行XRD分析得出图2。由图1可知,硅藻土以圆盘状为主,且均为多孔结构,孔洞分布均匀。实验后硅藻土质地相对光滑,孔径变细,说明硅藻土的孔洞对nZVI具有一定的吸附作用。由图2可知,硅藻土衍射角度在2θ为21.840°出现最大衍射峰, 表明本实验所用硅藻土物相组成以SiO2及硅羟基为主。实验后硅藻土在44.84°观测到最大衍射峰,说明nZVI已被硅藻土吸附。SEM与XRD分析结果表明,硅藻土化学组成较为单一,仅硅羟基可能参与各组分反应,可最大程度降低实验中的不确定性,以获得较为准确的结论,且自身所含的大量孔洞为溶质各组分的吸附、相互反应提供了条件[27]。
-
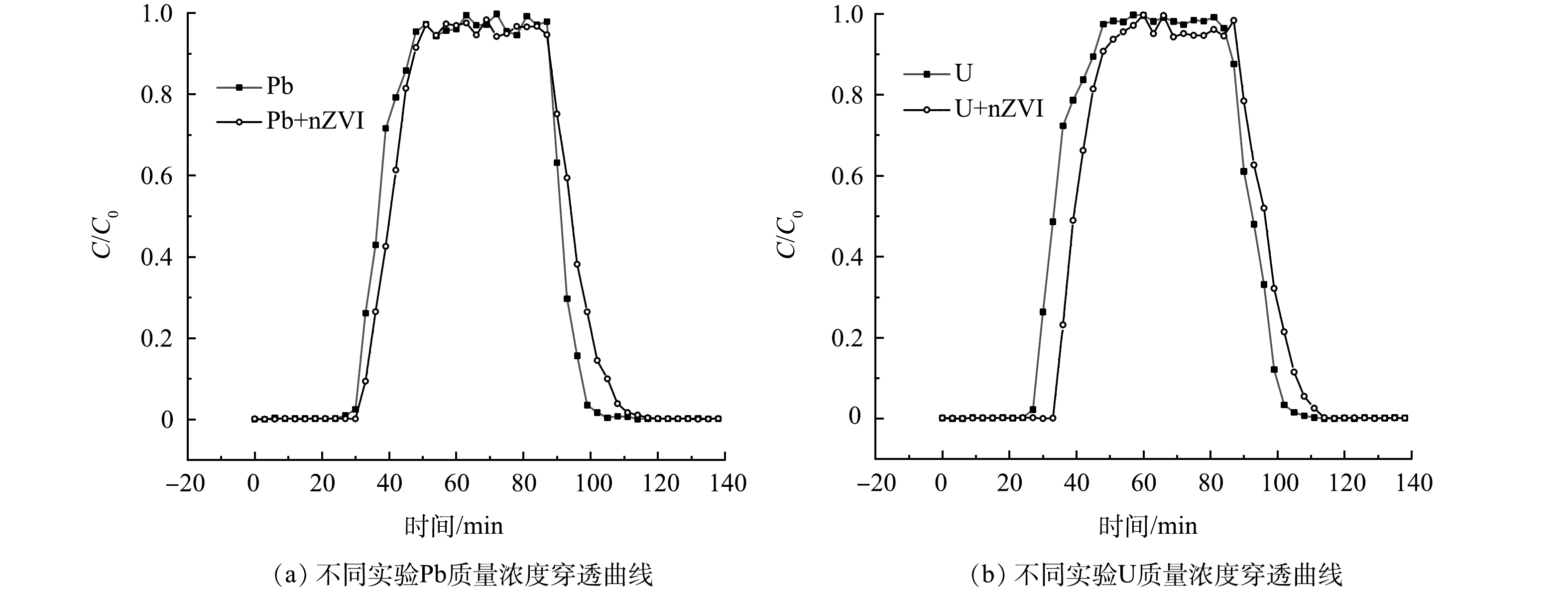
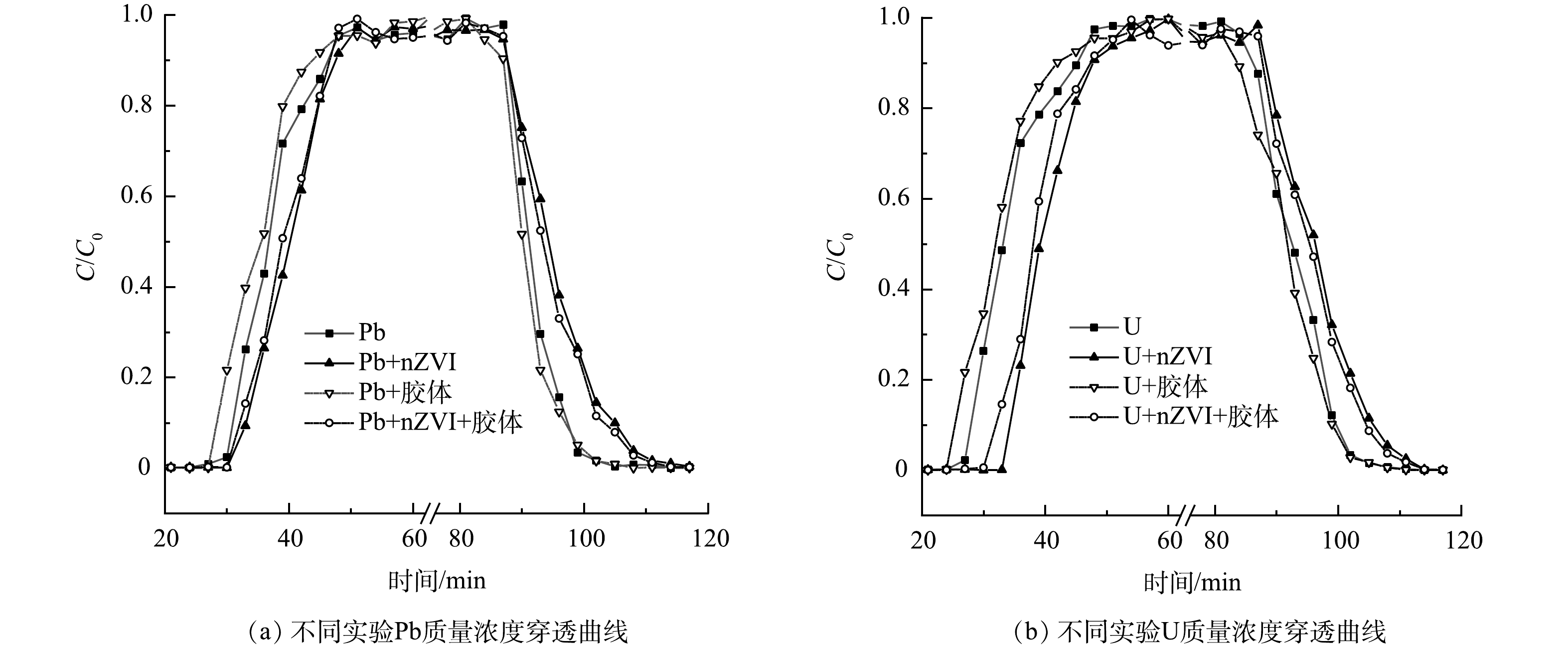
研究高岭土胶体对nZVI修复Pb、U重金属污染硅藻土的影响,首先需探明nZVI对重金属在土壤中运移的影响。为此分别进行表1中所示实验1~4,各土柱测定出流液中Pb、U的穿透曲线如图3所示。图3表明,若nZVI参与运移,则流出液Pb、U通过土柱及C/C0达到峰值的时间相对滞后,可知nZVI阻滞Pb、U在硅藻土中的运移。nZVI对Pb、U重金属存在吸附、固化作用,其固化作用的具体反应如式(3)至式(6)所示,最终Pb、U元素附着在nZVI及其腐蚀产物表面被固定[28-31]。
结合2.1节SEM与XRD分析,可知吸附Pb、U重金属的nZVI占据硅藻土的吸附位点及孔隙中。且硅藻土含有一定量的硅羟基(Si-OH),在溶液流动下,硅羟基所含的氢游离出来,从而表面带负电荷,可吸附由于溶于水而表面带正电的nZVI[32-33]。所以硅藻土表面微观结构与物相组成促使其对携带Pb、U重金属的nZVI具有更强的阻滞、吸附作用。 同理,穿透曲线下降阶段,nZVI参与的实验Pb、U的C/C0下降及达到最低点的时间同样相对滞后,出现明显的拖尾现象,表明携带重金属的nZVI与硅藻土的结合更稳固。所以去离子水冲刷下,nZVI与硅藻土的解吸需要较长时间。综合上述结果可知,nZVI可阻滞Pb、U在硅藻土中的运移,达到修复Pb、U污染硅藻土的目的。
-
为了进一步探究高岭土胶体对污染土壤修复的影响,设置如表1所示实验5~10,在高岭土胶体、nZVI、重金属、硅藻土混合条件下,分别测定土柱流出液中Fe、高岭土胶体、Pb和U的穿透曲线,并计算各成分的v升和v降,以分析高岭土胶体的影响机制(图4~图7)。
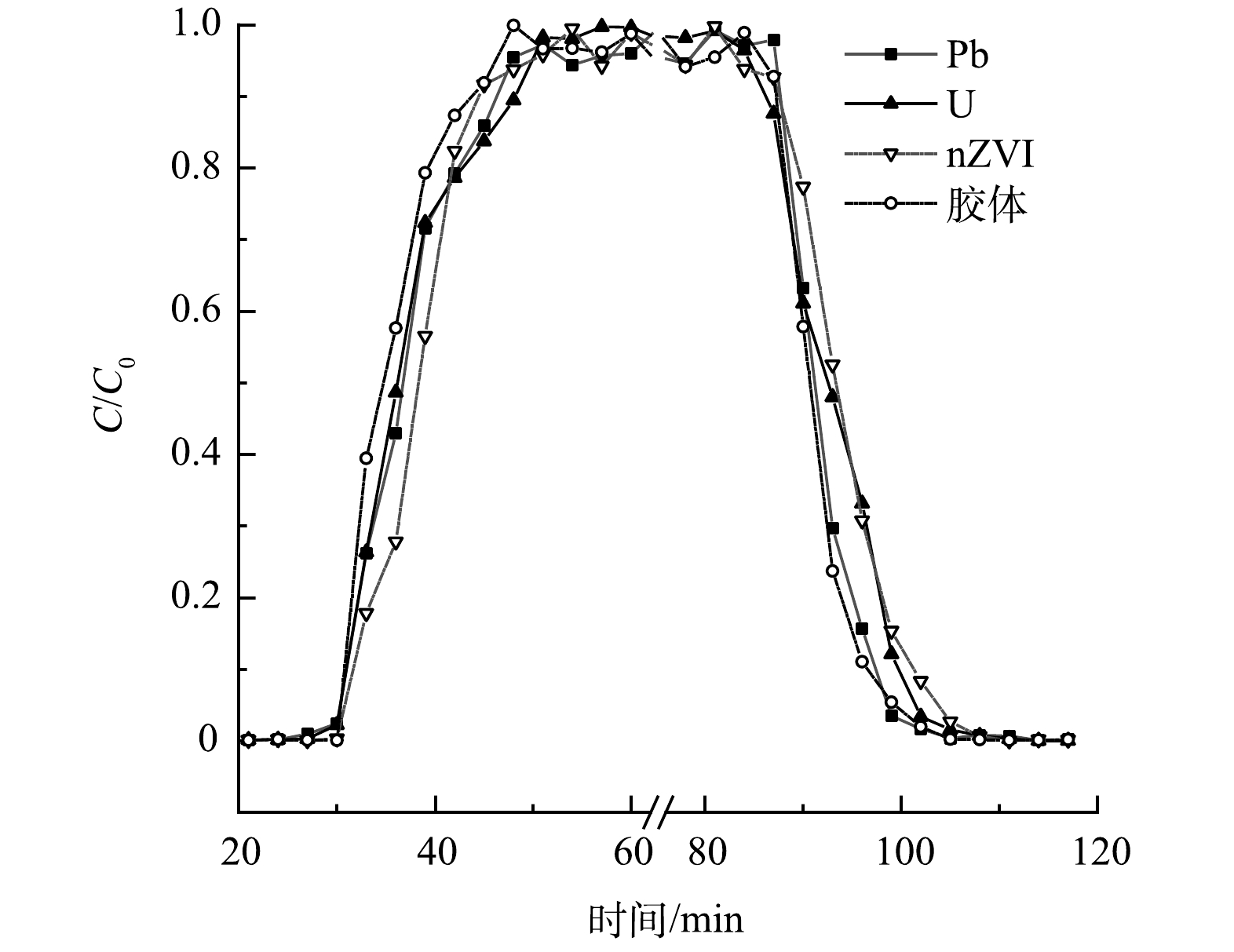
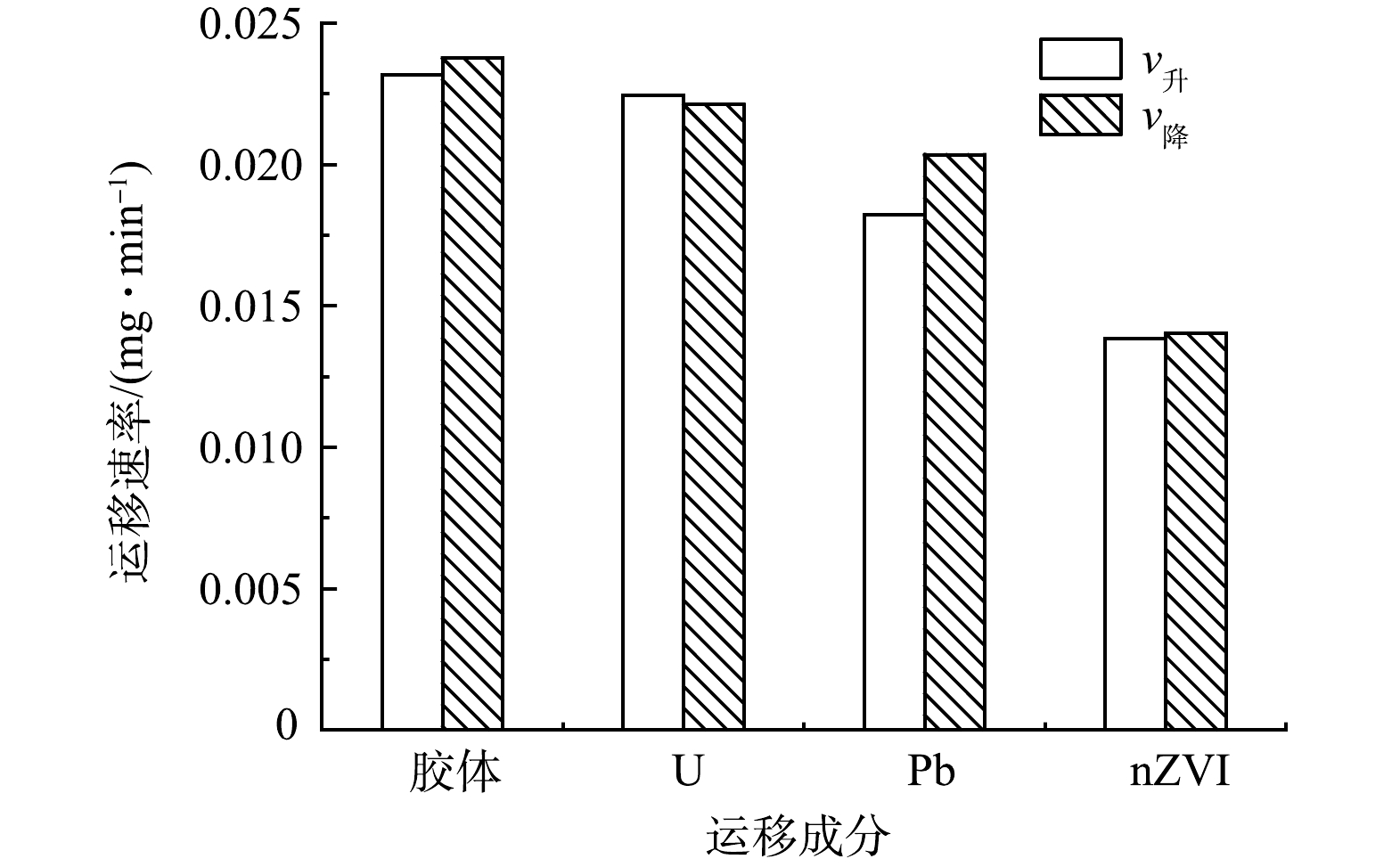
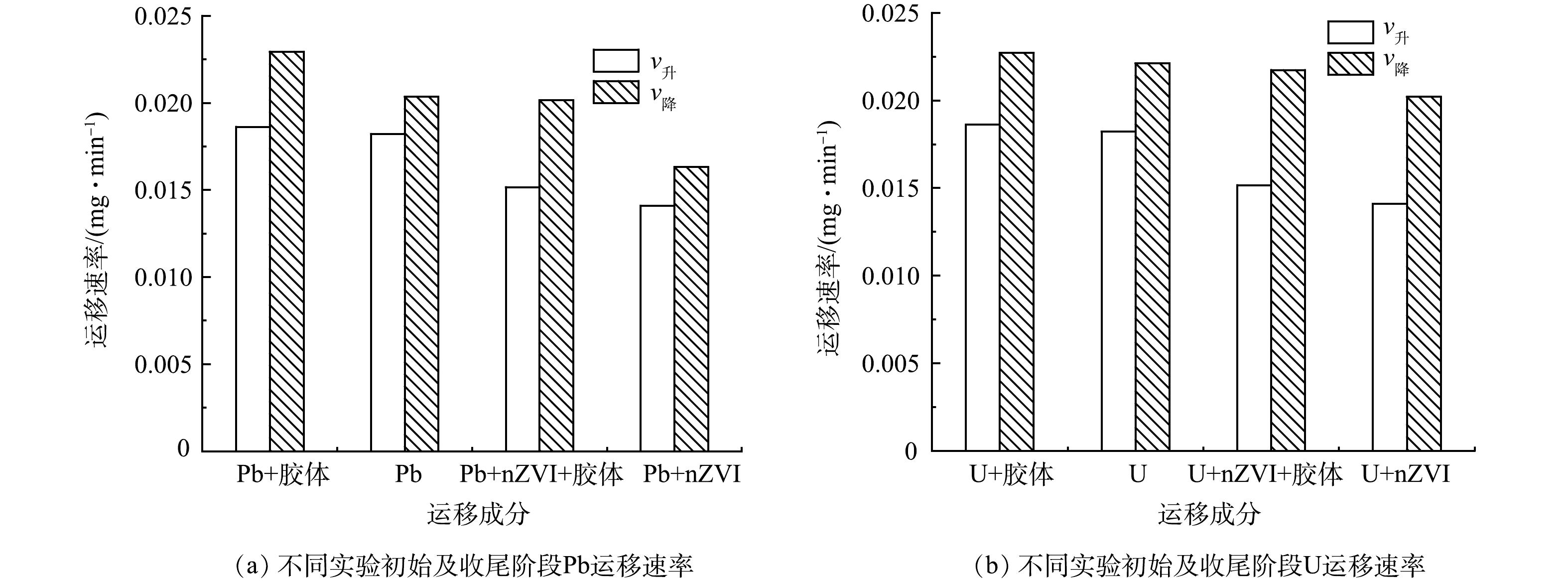
图4为Pb、U、nZVI以及高岭土胶体分别在土柱中单独运移的穿透曲线。结果表明,高岭土胶体通过土柱、达到峰值、完全解吸的时间均快于Pb、U及nZVI,说明在4种成分中,硅藻土对高岭土胶体的吸附能力最弱,Pb、U其次,对nZVI的吸附能力最强。从带电性分析,硅藻土表面带负电荷,而nZVI,Pb和U溶于水则表面带正电,高岭土胶体表面带负电,因此高岭土胶体能够较快运移出硅藻土柱[34-39]。图5中对各成分v升、v降的计算结果表明,高岭土胶体的运移速率最快,U、Pb、nZVI依次降低,进一步证实了上述认识。
图6为高岭土胶体及nZVI影响下Pb、U的穿透曲线,结果显示,仅加入高岭土胶体参与Pb、U土柱运移时,Pb、U穿透曲线C/C0开始上升及达到峰值的时间相对Pb、U单独运移较早,表明高岭土胶体由于表面带负电荷,可携带正电荷的Pb、U重金属离子较快运移穿过土柱,且高岭土胶体与土壤基质的结合可掩蔽土壤表面的吸附位点,抑制土壤对重金属的吸附作用,从而促进重金属的迁移。高岭土胶体加入到Pb、U和nZVI的混合体系中,Pb、U的穿透曲线上升及到达峰值时间早于无胶体的情况,说明即使在存在nZVI固化Pb和U,高岭土胶体仍可提高Pb、U的迁移速度,降低nZVI对Pb和U运移的阻滞效果。原因可能是高岭土胶体不但可直接吸附Pb、U,也可携带nZVI,其中部分nZVI吸附Pb、U,从而共同运移出土壤环境[40]。
同等高岭土、nZVI质量浓度条件下,高岭土胶体可加速修复体系中的Pb、U迁移速率,但仍达不到Pb和U的单独运移速率,说明该实验条件下高岭土胶体对nZVI修复硅藻土的影响有限。原因是nZVI与重金属的结合包括了吸附、固化作用,且nZVI可被硅藻土所含的硅羟基吸附,结合较为紧密。与之相比,矿物胶体与重金属或nZVI的结合相对松散。
图7为高岭土胶体及nZVI影响下Pb、U的v升、v降大小关系。结果表明,2种重金属随高岭土胶体迁移速度最快,单独运移次之,nZVI和重金属体系运移最慢,进一步证实了前述研究结果。
-
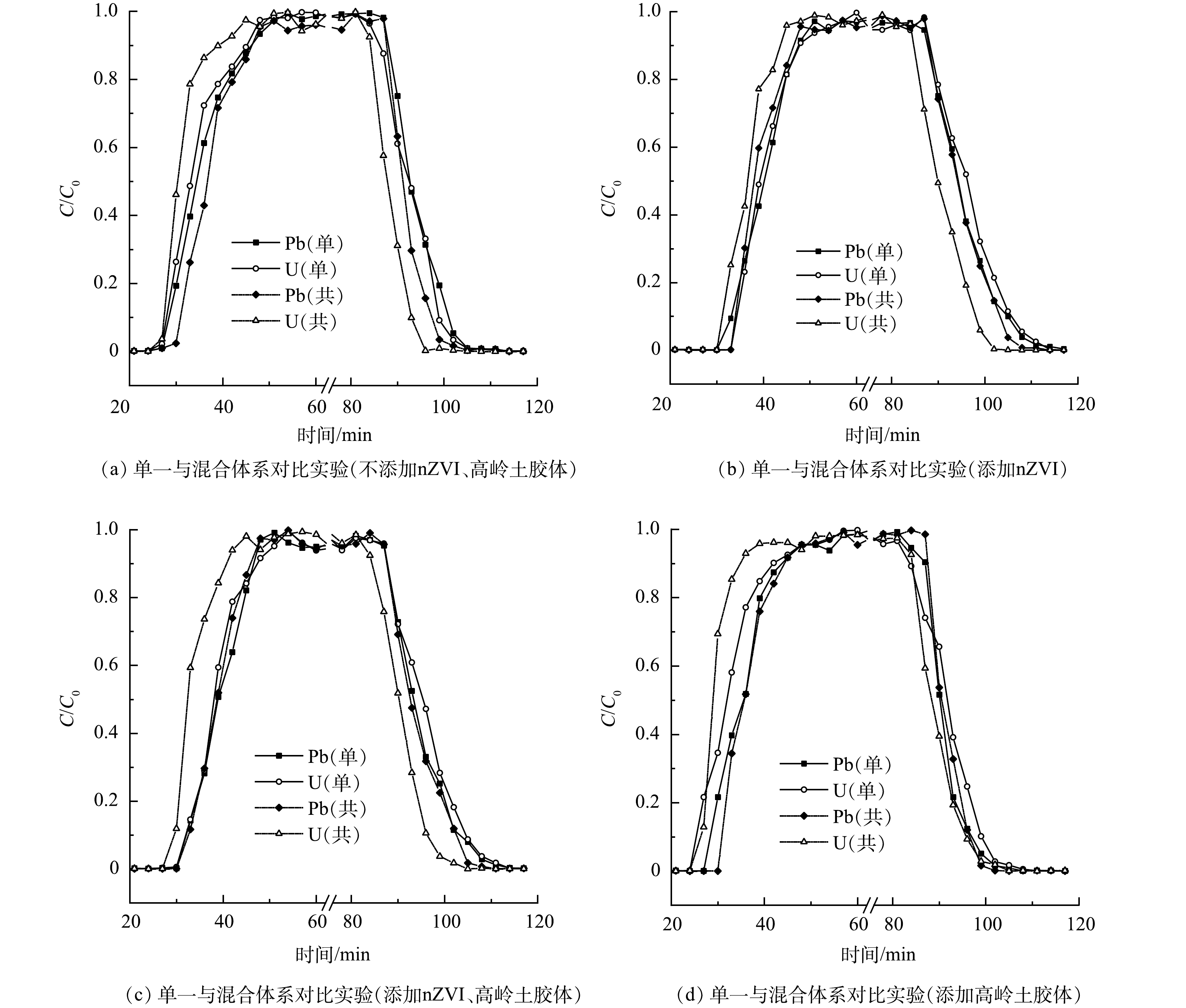
在实际修复过程中,不同重金属往往处于共存状态,nZVI对各重金属运移的阻滞可能存在差异,高岭土胶体的作用更不容忽视。为此进行了Pb、U、nZVI和高岭土胶体混合体系的对比土柱实验 (表1中实验11~14) 。图8描述了不同条件下Pb、U重金属复合运移体系的穿透曲线,并与前述单金属运移体系进行对比。图9显示了混合体系中Pb、U 的v升、v降的大小关系。图8(a)显示,在Pb、U复合运移体系中,穿透曲线均表现出竞争吸附的特性。且相较于单一运移体系,U在混合体系中的运移有所加速,表明混合体系中Pb更易被硅藻土吸附,滞留于土柱的吸附位点,从而迫使U加速运移出土柱。Pb在竞争吸附中相对占据优势的原因可能是Pb的离子半径、电负性均大于U,更易与吸附位点形成化学键,因此更易与吸附位点结合[41]。上述竞争吸附也影响nZVI对Pb、U重金属运移的阻滞作用,图8(b)显示,在Pb、U体系中加入nZVI之后,U通过土柱与达到峰值时间仍早于Pb,说明Pb更易与nZVI结合,nZVI携带Pb滞留于硅藻土的吸附位点中,从而降低了nZVI对U运移的阻滞程度。
图8(c)表明,Pb、U共存且添加同等质量浓度的nZVI及高岭土胶体时,nZVI对Pb的阻滞效果较强。原因是nZVI-重金属之间的吸附作用力大于高岭土胶体-重金属[42],重金属优先被nZVI吸附,所以相对更多U被高岭土胶体携带运移出土柱,因此该条件U更易被高岭土胶体影响从而加速运移。图8(d)描述了在Pb、U混合运移体系中添加高岭土胶体的穿透曲线,图像表明U的运移速率快于Pb,高岭土胶体对U的影响更大。原因是溶液中的Pb优先被硅藻土吸附,从而游离的U随高岭土胶体运移出土柱。由上述分析可知,U比Pb更易被高岭土胶体影响从而加速运移。
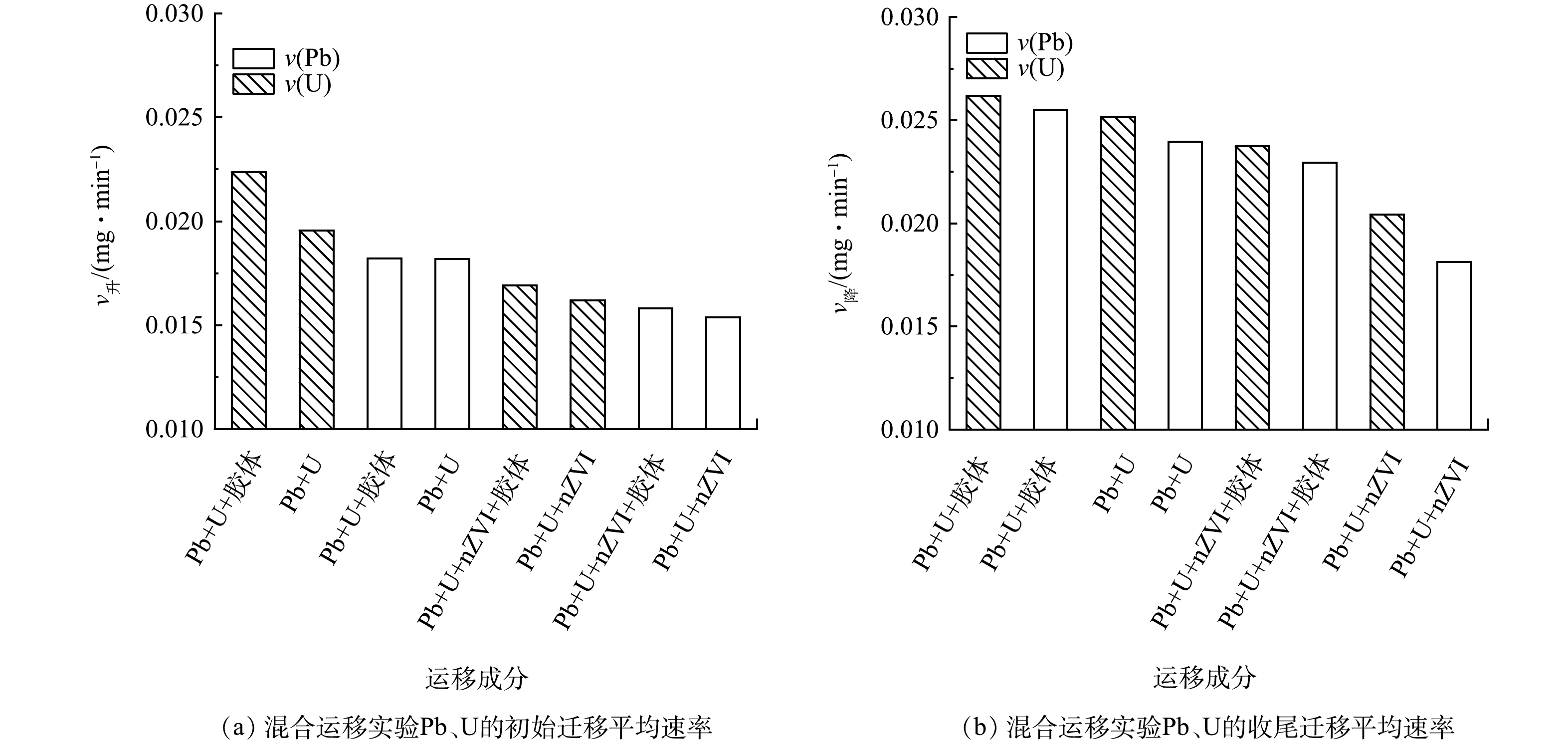
图9为高岭土胶体及nZVI影响下Pb、U混合迁移体系的v升、v降大小关系。结果表明,Pb在硅藻土中的运移速率相对U较缓,nZVI对Pb的运移有较强的阻滞效果,而相对Pb,高岭土胶体更促进U的运移,进一步证实前述结果。
-
1) 在Pb、U和nZVI浓度均为10 mg·L−1的重金属单一运移体系中,nZVI可阻滞Pb、U在硅藻土中的运移扩散,以修复受Pb、U污染的土壤。
2) 高岭土胶体可制约nZVI对重金属运移的阻滞效果,然而高岭土胶体与nZVI同等质量浓度时,高岭土胶体对nZVI修复效果的影响有限。
3) 在Pb、U与硅藻土的复合体系中,由于竞争吸附作用,相对于U,Pb的运移速率较慢且更易被nZVI修复,而相对Pb,高岭土胶体对U运移扩散的促进作用更强,导致Pb与U修复效果的差异。所以在Pb、U复合污染体系的nZVI修复工作中,应相对重视U的修复效果。
高岭土胶体对nZVI修复Pb、U污染硅藻土的影响及机制
Influence and mechanism of kaolin colloid on remediation of Pb, U contaminated diatomaceous earth by nZVI
-
摘要: 纳米零价铁 (nZVI) 大量应用于土壤重金属污染的修复,但由于土壤广泛存在的胶体可协同污染物的迁移与扩散,影响nZVI对土壤的修复效果。针对这一问题,通过硅藻土柱实验和重金属运移速率分析,并采用微观结构表征分析 (SEM) 与物相分析 (XRD) 方法,探究高岭土胶体对nZVI修复铅 (Pb) 、铀 (U) 重金属污染土壤的影响机制。结果表明,nZVI可固化Pb和U,阻滞其运移以修复土壤;高岭土胶体的加入可削弱nZVI对重金属的阻滞效果,增强Pb和U的运移能力;但在高岭土胶体与nZVI同等质量浓度下,高岭土胶体对阻滞效果的影响有限;另外,在Pb、U复合体系中,存在竞争吸附现象,nZVI对Pb的阻滞效果优于U,U的运移更易受到高岭土胶体的影响。本研究结果可为土水重金属污染防治提供参考。Abstract: Nano-zero-valent iron (nZVI) is widely applied in soil remediation of heavy metal contamination. Nevertheless, the presence of natural colloids in soils may coordinate the migration and diffusion of the contaminates, which reduce the effectiveness of the soil remediation by nZVI. To tackle this matter, the influence mechanism of kaolin colloids on nZVI restoring the soil contaminated by heavy mental Pb, U were explored through diatomite column experiments, heavy metal transport rate analysis, SEM and XRD. The results demonstrated that nZVI was capable of restoring soil by solidifying Pb and U to retarding the migration in soil. However, the presence of kaolin colloids can weaken the retarding effect of nZVI and enhancing the migration ability of Pb and U. Nevertheless, the impact of kaolin colloids on retarding effect was limited at the same mass concentration of the kaolin colloids and nZVI. In addition, a competitive adsorption phenomenon was observed in the mixed system of Pb and U, which leads a better retarding effect of nZVI on Pb than that of U. The migration of U wwa shown to be more susceptible to the influence of kaolin colloids. The findings of this study have theoretical significance for the prevention and control of heavy metal pollution in soil and water systems.
-

-
表 1 高岭土胶体对nZVI修复硅藻土的影响实验
Table 1. The experiments on the effect on diatomaceous earth remediation with nZVI by kaolin colloids
实验
编号运移成分 各成分质量浓度/
(mg·L−1)测定成分 1 Pb 10 Pb 2 Pb+nZVI 10 Pb 3 U 10 U 4 U+nZVI 10 U 5 nZVI 10 Fe 6 高岭土胶体 10 高岭土胶体 7 Pb+nZVI+高岭土胶体 10 Pb 8 Pb+高岭土胶体 10 Pb 9 U+nZVI+高岭土胶体 10 U 10 U+高岭土胶体 10 U 11 Pb+U 10 Pb、U 12 Pb+U+nZVI 10 Pb、U 13 Pb+U+nZVI+高岭土胶体 10 Pb、U 14 Pb+U+高岭土胶体 10 Pb、U -
[1] KONG S F, TANG J, FAN O Y, et al. Research on the treatment of heavy metal pollution in urban soil based on biochar technology[J]. Environmental Technology & Innovation, 2021, 23: 101670. [2] 王浩铭. 尾矿库浅层土壤中铀的浸出特性与数值模拟研究[D]. 南华大学, 2018. [3] GUO L J, PENG L Q, LI J H, et al. Simultaneously efficient adsorption and highly selective separation of U(Ⅵ) and Th(Ⅳ) by surface-functionalized lignin nanoparticles: A novel pH-dependent process[J]. Journal of Hazardous Materials, 2022, 4: 130123. [4] 韩丰磊, 李婷, 孙慧, 等. 废弃石化用地土壤和地下水污染调查与评估[J]. 土壤通报, 2020, 51(5): 1238-1245. [5] 孟繁健, 朱宇恩, 李华, 等. 改性生物炭负载nZVI对土壤Cr(Ⅵ)的修复差异研究[J]. 环境科学学报, 2017, 37(12): 4715-4723. [6] EKRAMI E, POURESMAIELI M, HASHEMIYOON E, et al. Nanotechnology: A sustainable solution for heavy metals remediation[J]. Environmental Nanotechnology, Monitoring & Management, 2022, 18: 100718. [7] ZHAO K Q, YANG Y, ZHANG L H, et al. Silicon-based additive on heavy metal remediation in soils: Toxicological effects, remediation techniques, and perspectives[J]. Environmental Research, 2022, 205: 112244. doi: 10.1016/j.envres.2021.112244 [8] 刘诗婷, 刘静, 刘爱荣, 等. 纳米零价铁基材料用于地下水修复研究进展[J]. 环境科学与技术, 2022, 45(9): 181-193. [9] 杜晓丽, 刘殿威, 崔申申. 径流入渗时土壤胶体释放对重金属截留的影响[J]. 中国环境科学, 2022, 42(3): 1278-1286. doi: 10.3969/j.issn.1000-6923.2022.03.033 [10] 王凯丽, 徐绍辉, 杨永亮, 等. 胶体存在时不同质地土壤对锌镉的吸附试验研究[J]. 土壤, 2011, 43(2): 239-246. doi: 10.13758/j.cnki.tr.2011.02.021 [11] 文吉昌. 黔西南汞铊矿废弃物中典型多金属迁移转化及生态调控机制研究[D]. 贵州大学, 2021. [12] 程景. 不同溶解态腐殖质对Cr(Ⅵ)在土壤上吸附行为的作用[D]. 中国地质大学(北京), 2018. [13] 付雪. 矿物胶体对纳米零价铁固定土壤中铀和铅的影响机理研究[D]. 南方科技大学, 2021. [14] 李志亮. 新型桥连的氯磷灰石对土壤中重金属的固定机制研究[D]. 华南理工大学, 2021. [15] 李平. 纳米零价铁体系对土壤中Cr(Ⅵ)的去除和滤出特性影响及转化机理研究[D]. 太原理工大学, 2019. [16] BHAWNA, ACHARYA A D, KAUR S. Rapid reductive degradation of dye contaminated water by using a core-shell nano zerovalent iron (nZVI)[J]. Journal of the Indian Chemical Society, 2022, 99: 100598. doi: 10.1016/j.jics.2022.100598 [17] 李志萍, 李欣益, 马燕, 等. 地下水中砷去除机理实验研究[J]. 河南理工大学学报(自然科学版), 2014, 33(2): 248-252. [18] 刘瑜, 王雨, 李银, 等. 铬在含水介质中的迁移及释放规律[J]. 土壤通报, 2017, 48(2): 313-318. doi: 10.19336/j.cnki.trtb.2017.02.09 [19] WANG B, XIONG M Y, SHI B, et al. Treatment of shale gas flowback water by adsorption on carbon- nanotube-nested diatomite adsorbent[J]. Journal of Water Process Engineering, 2021, 42: 102074. doi: 10.1016/j.jwpe.2021.102074 [20] 郭蕾蕾, 许模, 王璐, 等. 定流量条件下壤土夹层对苯和Br-穿透包气带的影响[J]. 环境工程学报, 2017, 11(12): 6524-6531. [21] QIN L Y, SUN X Y, YU L, et al. Ecological risk threshold for Pb in Chinese soils[J]. Journal of Hazardous Materials, 2023, 444: 130418. doi: 10.1016/j.jhazmat.2022.130418 [22] ZHAUNG S T, GHENG R, KANG M, et al. Kinetic and equilibrium of U(Ⅵ) adsorption onto magnetic amidoxime-functionalized chitosan beads[J]. Journal of Cleaner Production, 2018, 188: 655-661. doi: 10.1016/j.jclepro.2018.04.047 [23] 刘军. 铀-碳酸/钙-铀-碳酸络合物对红土及纳米零价铁吸附铀性能的影响[D]. 东华理工大学, 2015. [24] 孙慧敏. 粘土矿物胶体对铅的环境行为影响研究[D]. 西北农林科技大学, 2011. [25] 杨德湧. 中国土壤胶体研究——Ⅸ. 广东两对黄壤和红壤的粘粒矿物比较[J]. 土壤学报, 1985, 22(1): 36-46. [26] 张义, 朱吉颖, 张聪, 等. 硅藻土在环境领域的研究和应用[J]. 生态环境学报, 2022, 31(12): 2441-2448. [27] 马书翠. 硅藻土表面性质及对Pb(Ⅱ)吸附机理的研究[D]. 长春理工大学, 2015. [28] 杨晓丹. 纳米零价铁的制备、改性及去除水中典型重金属和有机物的效能与机理[D]. 陕西师范大学, 2020. [29] 洪彦峰. 磷酸化纳米零价铁去除水中Cd(Ⅱ)和Cu(Ⅱ)的研究[D]. 华中师范大学, 2020. [30] GOPAL G, SANKAR H, NATARAJAN C, et al. Tetracycline removal using green synthesized bimetallic nZVI-Cu and bentonite supported green nZVI-Cu nanocomposite: A comparative study[J]. Journal of Environmental Management, 2020, 254: 109812. doi: 10.1016/j.jenvman.2019.109812 [31] 谢武明, 毕小林, 黄子峻, 等. 纳米活性氧化铝负载磁性纳米零价铁对不同重金属的吸附机理[J]. 环境科学学报, 2020, 40(8): 2732-2740. [32] YOUNG J O, HOCHEOL S, WON S S, et al. Effect of amorphous silica and silica sand on removal of chromium(Ⅵ) by zero-valent iron[J]. Chemosphere, 2007, 66(5): 858-865. doi: 10.1016/j.chemosphere.2006.06.034 [33] LI C Q, YANG S S, BIAN R Z, et al. Efficient catalytic degradation of bisphenol A coordinated with peroxymonosulfate via anchoring monodispersed zero-valent iron on natural kaolinite[J]. Chemical Engineering Journal, 2022, 448: 137746. doi: 10.1016/j.cej.2022.137746 [34] 杨勤桃, 解庆林, 陈南春. 壳聚糖改性硅藻土对水中As(Ⅴ)的吸附特性[J]. 非金属矿, 2021, 44(5): 103-106. [35] 左国强, 苏小莉, 刘改云, 等. 纳米零价铁吸附法去除废水中的Pb2+[J]. 化学试剂, 2022, 44(6): 828-834. [36] SHEN T M, XU H, MIAO Y, et al. Study on the adsorption process of Cd(Ⅱ) by Mn-diatomite modified adsorbent[J]. Materials Letters, 2021, 300: 130087. doi: 10.1016/j.matlet.2021.130087 [37] 孙亚楼. 高岭土胶体与Eu(Ⅲ)/Am(Ⅲ)的相互作用研究[D]. 兰州大学, 2020. [38] 陈娟娟. 磷在高岭土、腐殖酸及海洋沉积物上的吸附行为研究[D]. 中国海洋大学, 2014. [39] ZHENG H L, REN X M, ZHANG X D, et al. Mutual effect of U(Ⅵ) and phosphate on the reactivity of nanoscale zero-valent iron (nZVI) for their co-removal[J]. Journal of Molecular Liquids, 2020, 297: 111853. doi: 10.1016/j.molliq.2019.111853 [40] XI X L, DING D J, ZHOU H L, et al. Interactions of pristine and aged nanoplastics with heavy metals: Enhanced adsorption and transport in saturated porous media[J]. Journal of Hazardous Materials, 2022, 437: 129311. doi: 10.1016/j.jhazmat.2022.129311 [41] HUANG X Y, NIU X J, ZHANG D Q, et al. Fate and mechanistic insights into nanoscale zerovalent iron (nZVI) activation of sludge derived biochar reacted with Cr(Ⅵ)[J]. Journal of Environmental Management, 2022, 319: 115771. doi: 10.1016/j.jenvman.2022.115771 [42] YANG Y M, NASEER M, ZHU Y, et al. Dual effects of nZVI on maize growth and water use are positively mediated by arbuscular mycorrhizal fungi via rhizosphere interactions[J]. Environmental Pollution, 2022, 308: 119661. doi: 10.1016/j.envpol.2022.119661 -



 下载:
下载: