-
焦炉煤气净化过程产生大量脱硫废液,其COD、氨氮、硫化物依次高于100 000、20 000、2 000 mg·L−1,副盐(主要为硫氰酸盐、硫代硫酸盐和硫酸盐)约为200 g·L−1[1-2]。高浓度副盐,尤其硫氰酸盐市场需求重大,提盐工艺能够回收副盐并实现脱硫废液循环利用[3]。然而,硫氰酸盐和硫代硫酸盐溶解度差异小,导致提取的硫氰酸盐产品纯度低,故去除硫代硫酸盐成为提取高品质硫氰酸盐的关键[4]。目前,工业上多采用强氧化剂浓硫酸氧化硫代硫酸盐,其对管道和设备材质要求苛刻且反应剧烈和运行风险大。近年来,研究人员采用光催化[5-7]、电催化[8]、催化H2O2氧化技术[9]将硫代硫酸盐氧化为硫酸盐。由于脱硫废液中副盐浓度高、腐蚀性极强,故光催化和电催化局限性较大而催化湿式氧化更具可行性,但催化空气氧化硫代硫酸盐研究却鲜有报道。
碳纳米管及其衍生物因出色机械性能、电子性能及热导性而常作催化湿式氧化催化剂或载体。过渡金属元素在催化湿式氧化技术中表现出优秀催化性能。N掺杂不仅能够提高碳基催化剂活性,而且可为催化剂中金属原子配位耦合提供限域锚定位点以改善其稳定性[10]。因此,N掺杂碳纳米管作为重要碳基功能材料,已引起催化、吸附、电容器等领域广泛关注[11]。生物质取材广泛、碳含量高,成为制备N掺杂碳纳米管的良好碳源[12]。本研究以马铃薯薯渣(简称薯渣)为碳源,通过热处理技术制备铁原子限域的N掺杂碳纳米管催化剂,研究其催化空气氧化脱硫废液中硫代硫酸盐性能,以期实现薯渣高附加值资源化利用并为脱硫废液中硫代硫酸盐氧化提供参考。
-
主要试剂:硫代硫酸钠(Na2S2O3·5H2O)、硝酸铁(Fe(NO3)3·9H2O)、硝酸锰(Mn(NO3)2、硝酸铜(Cu(NO3)2·3H2O)、硝酸钴(Co(NO3)2·6H2O)、硝酸镍(Ni(NO3)2·6H2O)、硫酸(H2SO4)、氢氧化钾(KOH)、氯化亚铁(FeCl2·4H2O)、双氰胺(C2H4N4)均为分析纯;高纯氮气(N2,99.999%)。薯渣取自甘肃某马铃薯淀粉加工厂;焦化脱硫废液取自河北、辽宁某两家采用HPF(hydroquinone, phthalocyanine, ferrous sulfate)脱硫工艺的焦化企业,实验用焦化脱硫废液为采用粉末活性炭对其脱色后的脱硫废液,其成分见表1。
主要仪器:pH计(PHS-3C,上海仪电科学仪器股份有限公司);电子分析天平(ATX124,日本岛津公司);高温箱式气氛炉(SG-QF1700,上海识捷电炉有限公司);恒温加热磁力搅拌器(DF-101S,上海力辰邦西仪器科技有限公司);真空干燥箱(DFZ型,北京市永光明医疗仪器厂);电热恒温干燥箱(DHL-1003,山东省龙口市先科仪器公司);超纯水机(AXLC1820-2,重庆阿修罗科技发展有限公司);超声清洗机(F-020S,苏州迈弘电器有限公司);扫描电子显微镜(Supra-55,Zeiss);N2物理吸附仪(Quadrasorb SI,Quantachrome);X射线光电子能谱仪(ESCA Lab250,Thermo Fisher Scientific);电感耦合等离子体发射光谱仪(NexION 300X,PerkinElmer)。
-
活性组分筛选:配制10 g·L−1硫代硫酸钠溶液并取200 mL于烧瓶中,依次加入0.01 mol Fe、Mn、Cu、Co、Ni的硝酸盐并混匀,通入空气,85 ℃下反应2 h。实验装置及具体步骤参见1.3节。
催化剂制备:取50 g薯渣于800 ℃、200 mL·min−1 N2保护条件下焙烧2 h,得到薯渣碳(PC)。取3 g PC与3 g KOH和5 mL去离子水混匀后密封于带盖塑料瓶中,置于80 ℃烘箱中24 h;取出用去离子水清洗至中性后干燥,向干燥后样品中加入5 mL质量浓度234 g·L−1的FeCl2·4H2O溶液,超声约1 min,混匀后于55 ℃真空干燥(真空度约0.05 MPa),样品干燥后与10 g双氰胺充分混匀;再在200 mL·min−1 N2条件下于400 ℃焙烧2 h获得还原态Fe,其后分别在700、800、900 ℃焙烧3 h获得Fe-NCNT超结构;用1.0 mol·L−1 H2SO4清洗5 h以去除纳米管表面铁纳米颗粒,用250 mL 1.0 mol·L−1 H2SO4于120 ℃、1.3 MPa N2条件下处理6 h以进一步去除残留铁纳米颗粒或团簇;用去离子水洗涤至中性;最后将样品在50 ℃、-0.08 MPa下干燥6 h获得超结构催化剂,分别记为Fe-NCNT/PC-700、Fe-NCNT/PC-800、Fe-NCNT/PC-900。
催化剂表征:采用N2物理吸附仪测定比表面积和孔道结构:真空条件下300 ℃处理3 h,77 K恒温吸附;扫描电子显微镜(SEM)观察催化剂表面形貌:粉末样品粘在铜座表面导电胶上测定。X射线光电子能谱仪(XPS)分析催化剂中元素价态:单色化Al Kα源,结合能通过C1s(284.6 eV)校正;电感耦合等离子体发射光谱仪(ICP-MS)测定催化剂中活性组分铁溶出量。
-
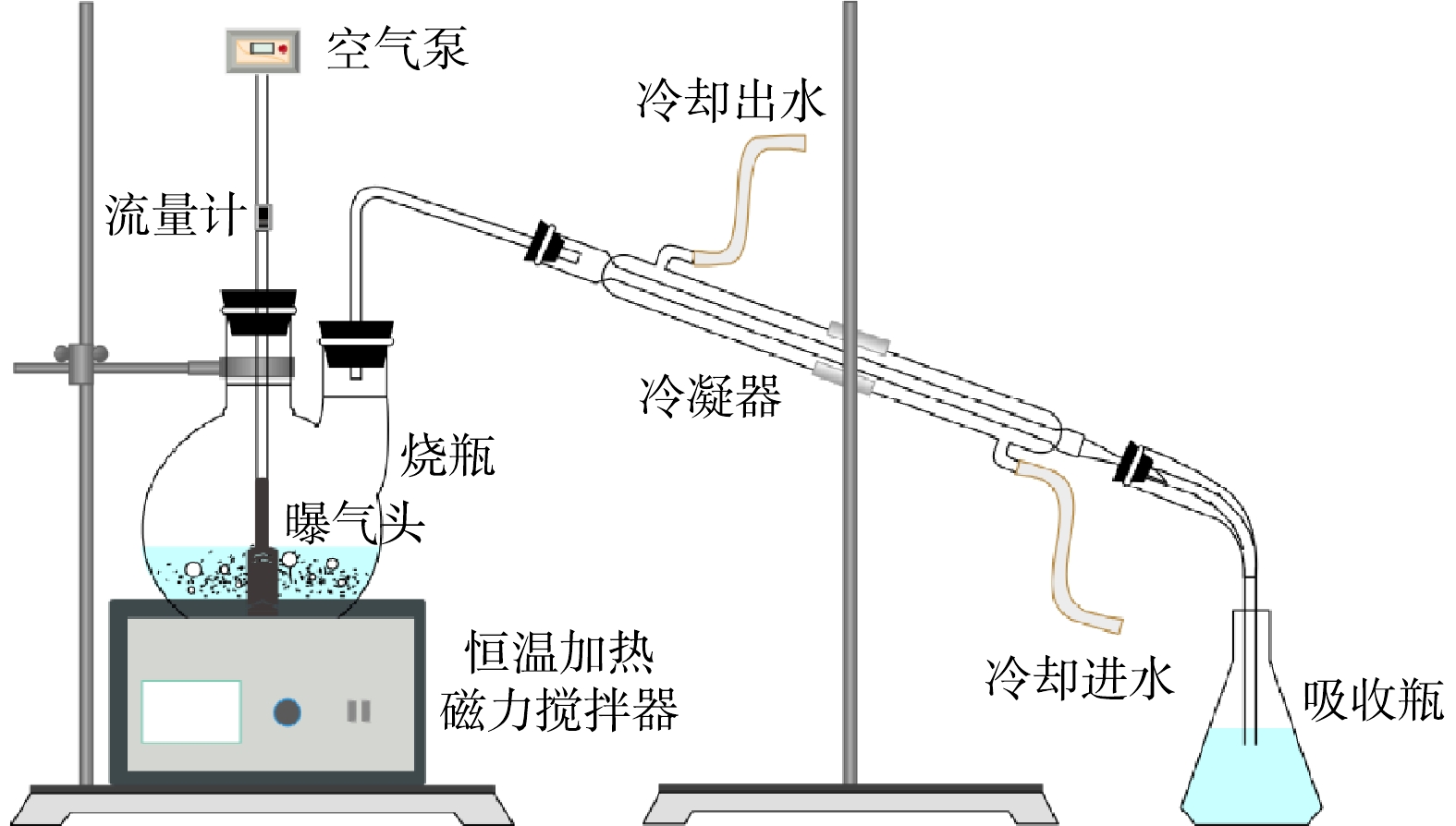
脱硫废液中硫代硫酸盐氧化实验装置如图1所示,主要由恒温加热磁力搅拌器、烧瓶、钛材质曝气头、空气流量计、空气泵、冷凝器和吸收瓶组成。
催化剂焙烧温度考察实验。取250 mL实验用HB、LN 2种脱硫废液,投入1.0 g催化剂于烧瓶中并混合均匀,置于恒温加热磁力搅拌器中加热至85 ℃,采用空气泵鼓入空气(作氧化剂并兼具搅拌作用)并通过流量计控制空气流量为2 L·min−1,开启循环冷却水并用稀硫酸吸收氧化反应过程中尾气,控制氧化反应持续至2 h。氧化反应结束后,静置并过滤脱硫废液。采用碘量法测定脱硫废液中硫代硫酸盐质量浓度,按式(1)计算硫代硫酸盐去除率。
式中:α为硫代硫酸盐去除率,%;C0为氧化前脱硫废液中硫代硫酸盐质量浓度,g·L−1;C为氧化后脱硫废液中硫代硫酸盐质量浓度,g·L−1。
催化剂用量考察实验:采用Fe-NCNT/PC-700催化剂,催化剂用量0~2.0 g,其他条件同催化剂焙烧温度考察实验。反应时间考察实验:采用1.5 g Fe-NCNT/PC-700催化剂,反应时间1~7 h,其他条件同焙烧温度考察实验。氧化过程中pH考察实验:将实验装置中两口烧瓶换为三口烧瓶(其中一口插入pH计),采用1.5 g Fe-NCNT/PC-700催化剂,反应时间1~7 h,间隔0.5 h测定脱硫废液pH,其他条件同焙烧温度考察实验。催化剂稳定性考察实验:采用250 mL稀硫酸水溶液(pH 3.5)代替脱硫废液,控制Fe-NCNT/PC-700催化剂用量1.5 g、反应时间6 h,其他条件同焙烧温度考察实验;实验结束后测定水溶液铁溶出量。
-
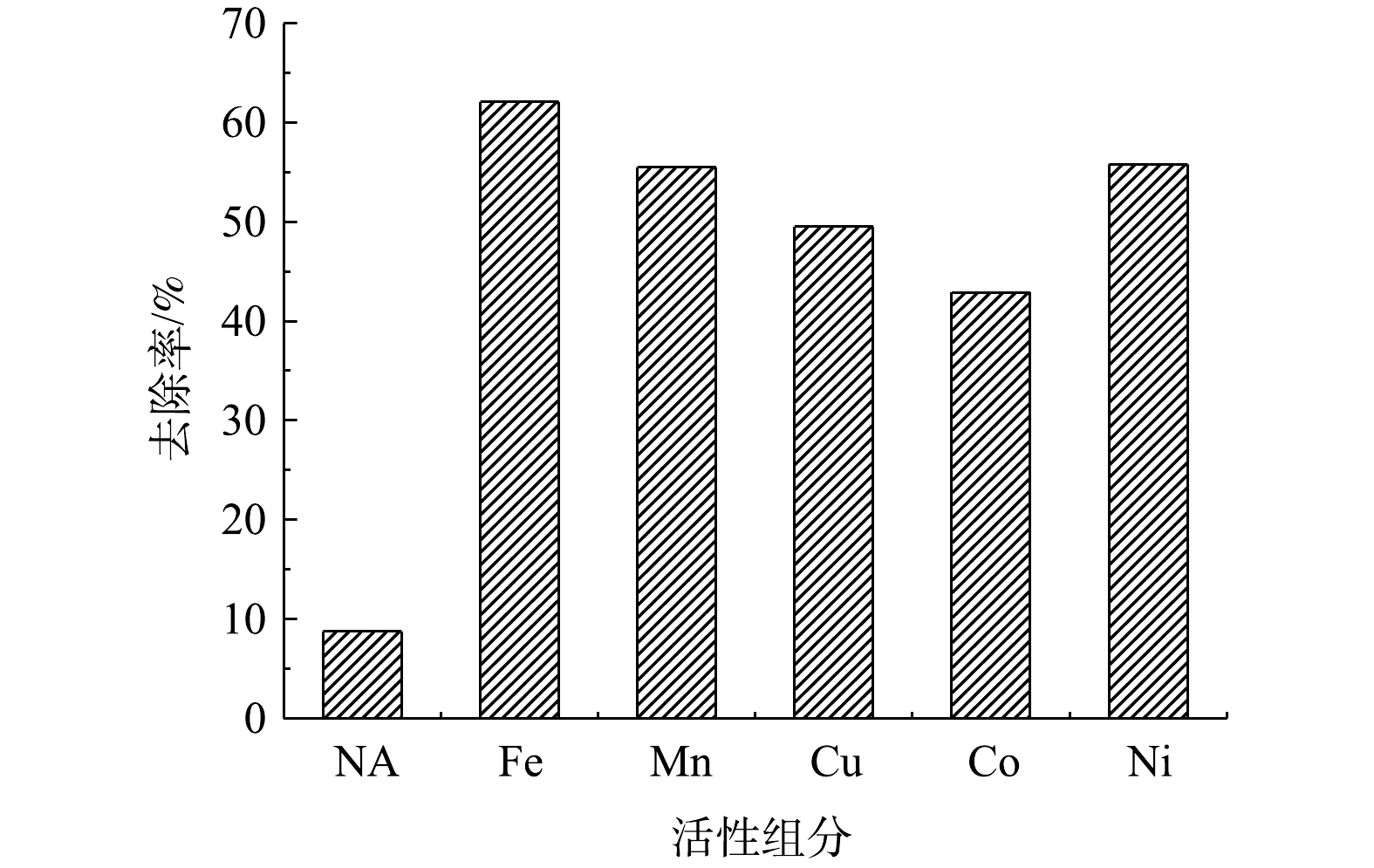
1)活性组分对硫代硫酸盐去除的影响。不同活性组分对硫代硫酸盐去除率影响结果见图2。由图2可见,无活性组分(NA)时,硫代硫酸盐去除率约9%;相比之下,几种过渡金属活性组分对硫代硫酸盐去除率均逾42%,影响顺序为Fe>Ni≈Mn>Cu>Co,其中Fe对硫代硫酸盐去除率高达62%,表明Fe对空气氧化硫代硫酸盐的催化活性最佳。XU等[13-14]亦发现Fe能够显著催化空气氧化硫代硫酸盐,可能与其电子结构及对溶液中含硫化合物的强亲和性有关。因此,选择Fe作为催化剂活性组分。
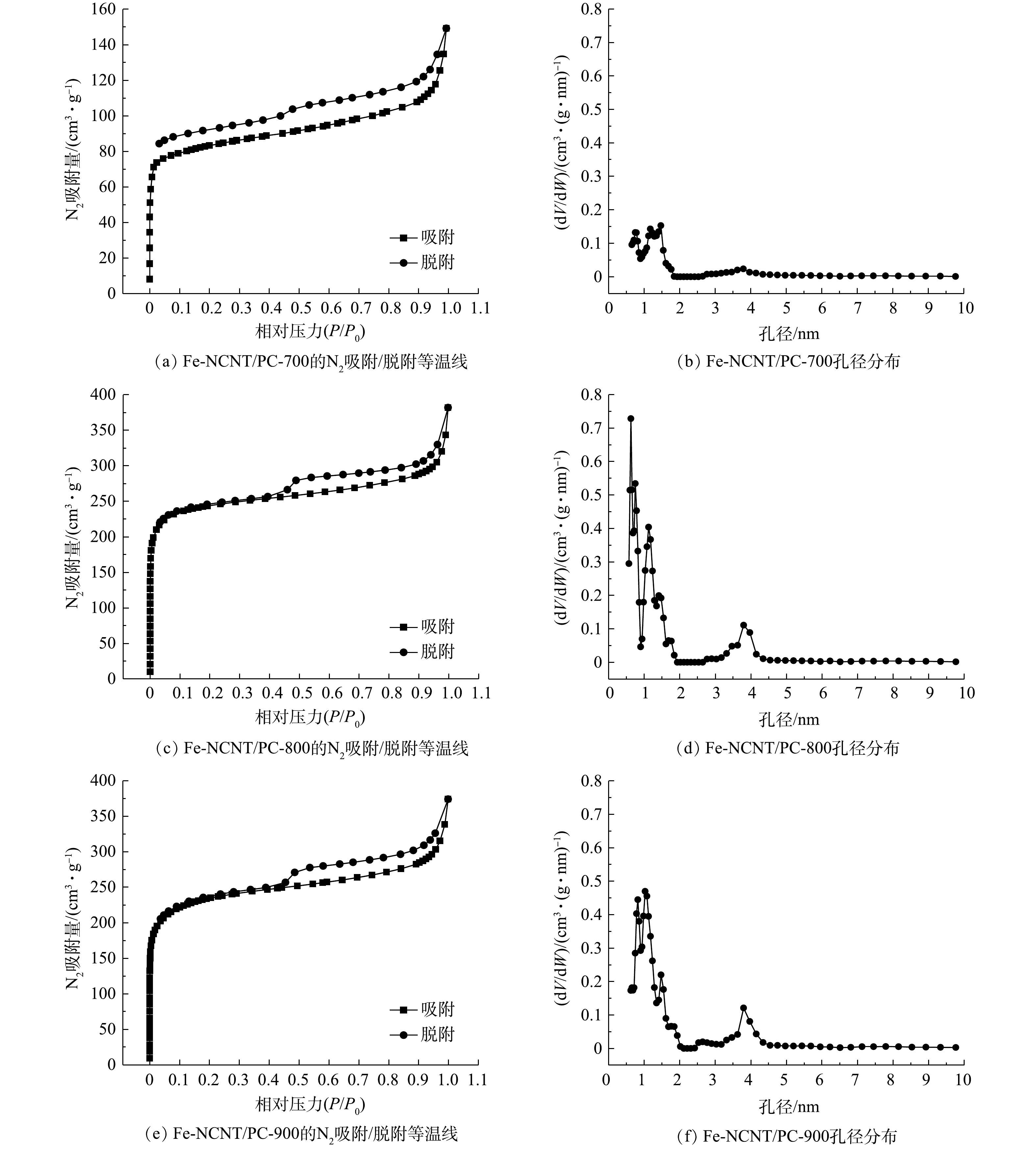
2)比表面积与孔结构。催化剂比表面积和孔道结构影响其性能。Fe-NCNT/PC催化剂的N2吸附/脱附等温线见图3。根据IUPAC吸附等温线分类,图3所示吸附等温线兼具I型和IV型特征:吸附量在相对低压(P/P0 = 0~0.01)区呈垂线式陡增(I型),而在相对高压(P/P0 = 0.45~0.99)时再次较快增大且出现H4型迟滞环(IV型)[15];同时孔径分布集中于0~2 nm和3.5~4.5 nm,表明催化剂属于微-介孔材料。此外,微孔可为催化剂活性位点均匀分散提供相应场所,介孔则可为离子和电解质溶液等介质传质提供相应孔道,故二者协同更有利于提高催化剂活性[16]。
Fe-NCNT/PC催化剂比表面积及孔容见表2。由表2可见,Fe-NCNT/PC-700、Fe-NCNT/PC-800、Fe-NCNT/PC-900比表面积依次为319.971、939.804、886.910 m2·g−1,呈先骤增后缓减趋势。其中,当焙烧温度由700 ℃升至800 ℃时,比表面积、微孔孔容和总孔容显著增大,可能是PC中疏松碳被催化热解参与表面纳米管生长而形成大量微-介孔结构;当焙烧温度进一步升至900 ℃时,比表面积、微孔孔容和总孔容减小,可能是高温环境使微孔结构烧结[4]或转化为介孔结构。
3)形貌表征。Fe-NCNT/PC催化剂SEM表征结果见图4。由图4(a)可见,薯渣高温碳化后表面形成许多疏松的无定型淀粉碳球,为碳纳米管生长提供良好碳源[17]。由图4(b)~(d)可见,PC经铁盐浸渍和高温热解后,淀粉碳球基本消失,表面形成管径为10~100 nm碳纳米管;Fe-NCNT/PC-700表面碳纳米管管径(10~50 nm)较为均一;随焙烧温度的升高,管径越来越不均一且呈现两极分化(大管径>100 nm,小管径<10 nm),推断纳米管管径变化与催化金属颗粒铁烧结有关。首先,分布于PC上的铁盐在双氰胺作用下还原为铁单质并在高温环境中形成粒径均一的铁纳米颗粒,但焙烧温度达到碳纳米管最佳生长温度后,气氛中C、N源不断沉积至铁纳米颗粒表面并在其催化作用下生长成纳米管结构;其次,焙烧温度升高后,高温打破铁纳米颗粒间表面能平衡,在奥斯特瓦尔熟化作用下,较大铁纳米颗粒不断长大且较小铁纳米颗粒不断缩小,从而使催化纳米管生长的纳米颗粒“种子”出现两极分化,最终导致制备的纳米管管径发生两极分化。因此,推断700 ℃较利于保持铁纳米颗粒间表面能平衡和获得碳纳米管管径均一的催化剂,由此预测Fe-NCNT/PC-700催化性能更佳。
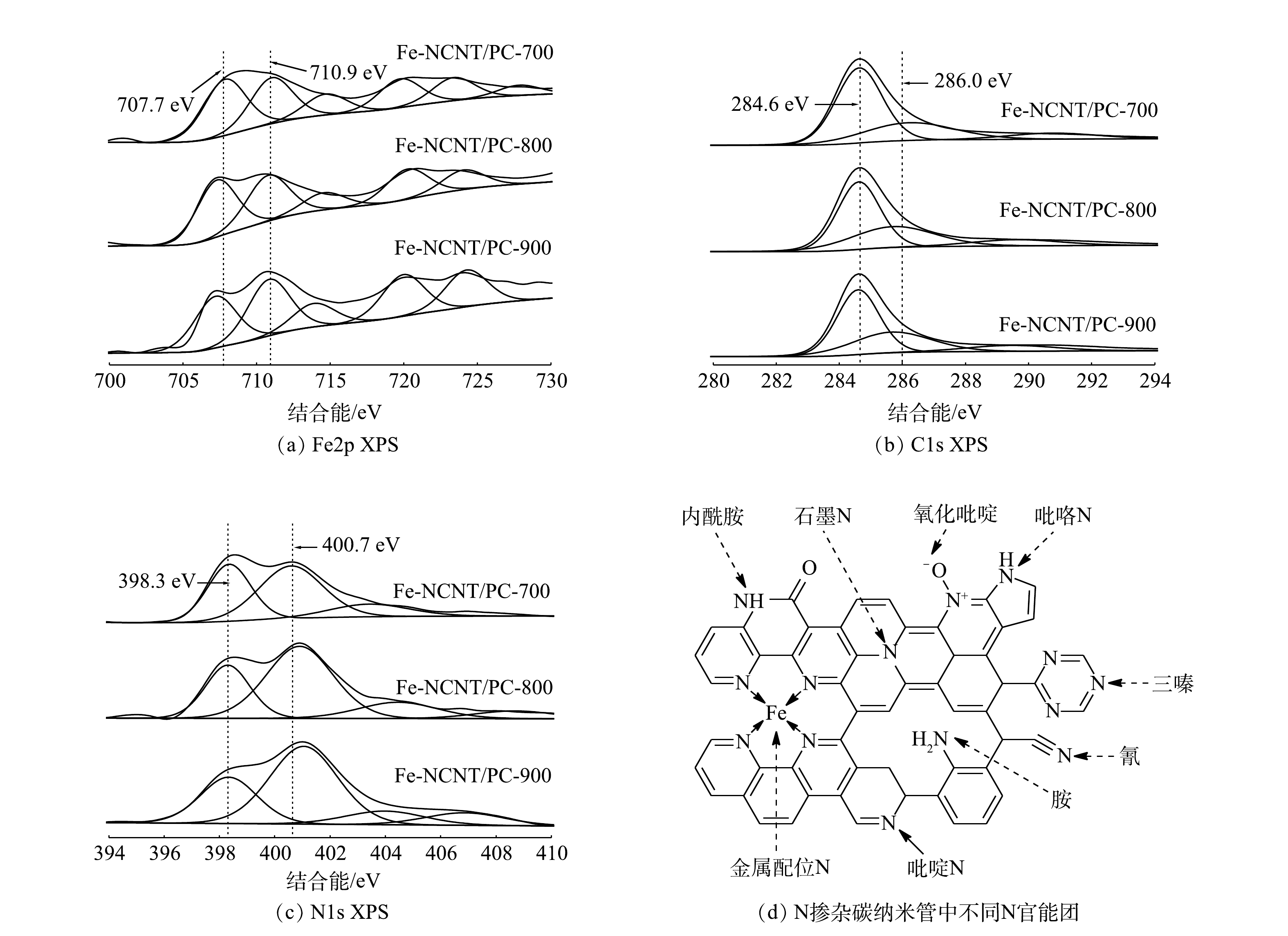
4) XPS分析。催化剂表面化学元素及其价态影响其性能。Fe-NCNT/PC催化剂XPS能谱图见图5。由图5(a)~(c)可知,Fe-NCNT/PC催化剂XPS能谱图出现Fe2p、C1s、N1s信号,表明Fe、N均掺入碳纳米管结构。由图5(a)可见,Fe2p XPS图谱分峰拟合后主要在707.7 eV和710.9 eV处出现明显高斯峰,分别对应FeNx和N-Fe-O[18]。催化剂在焙烧后均经高温酸处理洗除表面铁及其氧化物,FeNx作为一种限域结构能够有效抵御酸腐蚀并具有良好的催化活性[17-18],而铁氧化物可被酸溶解去除,故710.9 eV处高斯峰应为FeNx结构中铁吸附环境中氧而形成的N-Fe-O结构。由于该结构中Fe活性位点被O封死而达到配位饱和状态,故Fe不再发挥催化作用。由图5(b)可见,C1s XPS图谱分峰拟合后在284.6 eV和286.0 eV处出现明显高斯峰,分别对应石墨碳和C-N结构;N掺杂引起C峰显著迁移,说明N以较高比例掺入碳纳米管结构[11]。N掺杂碳材料中N官能团及含量同样对催化剂性能有重要影响[15]。由图5(c)可见,N1s XPS图谱分峰拟合后在398.3 eV和400.7 eV处出现吡啶氮和石墨氮[11-12]。N掺杂碳纳米管中不同N官能团[19]见图5(d)。其中,吡啶氮有利于吸附氧气且其孤对电子易与铁形成FeNx而提高催化剂活性[16];石墨氮能够形成大π键并降低键能,促进氧化过程中电子转移[18]。同时,随着焙烧温度升高,吡啶氮与石墨氮峰强之比不断降低,而吡啶氮是锚定铁原子的配位元素,表明FeNx结构在高温焙烧条件下不稳定,故催化剂活性提升主要来自其表面具有限域铁原子结构的纳米管结构增长。综上所述,焙烧温度升高不利于形成FeNx结构和提高催化剂活性,由此预测Fe-NCNT/PC-700催化剂活性最佳。
-
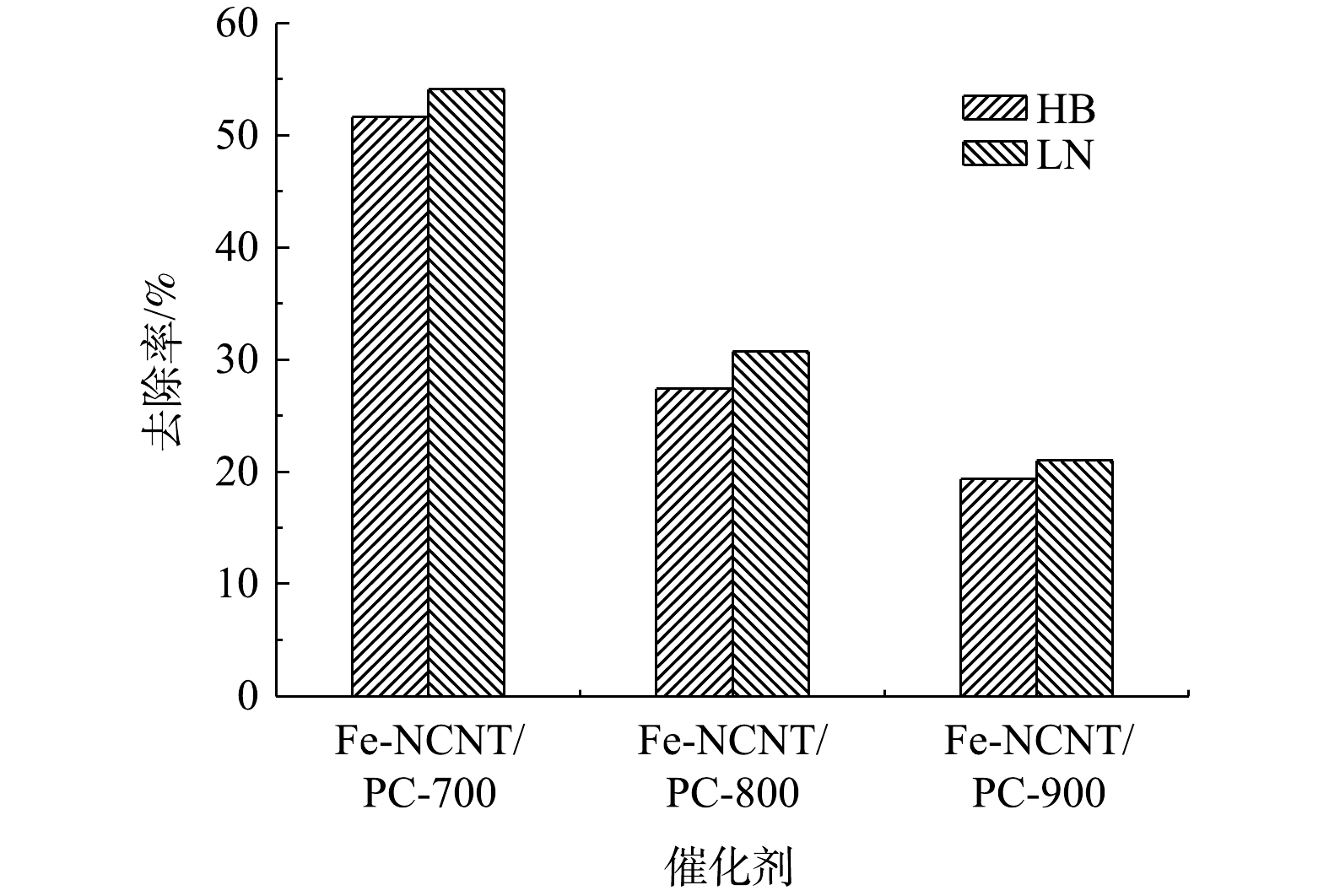
1)催化剂焙烧温度影响。催化剂焙烧温度对脱硫废液中硫代硫酸盐去除率影响如图6所示。由图6可知,随着催化剂焙烧温度的升高,其对HB、LN 2种脱硫废液中硫代硫酸盐去除率依次降低,且Fe-NCNT/PC-700显著大于Fe-NCNT/PC-800、Fe-NCNT/PC-900。以上结果表明催化剂焙烧温度升高会降低催化剂对硫代硫酸盐催化氧化性能,与上述催化剂的SEM和XPS分析表征结果一致。主要原因是焙烧温度升高导致催化剂表面缺陷及铁原子限域结构减少[17]。此外,Fe-NCNT/PC-700、Fe-NCNT/PC-800、Fe-NCNT/PC-900对LN脱硫废液中硫代硫酸盐去除率均较HB脱硫废液略高,可能是前者初始pH较后者低而有利于硫代硫酸盐氧化反应。GONZÁLEZ-LARA等[20]亦研究发现,硫代硫酸盐溶液初始pH越低,空气氧化相同条件下去除率越高;这是因为初始pH越低,硫代硫酸盐对空气中氧消耗越快,从而硫代硫酸盐氧化速率越快[21-22]。
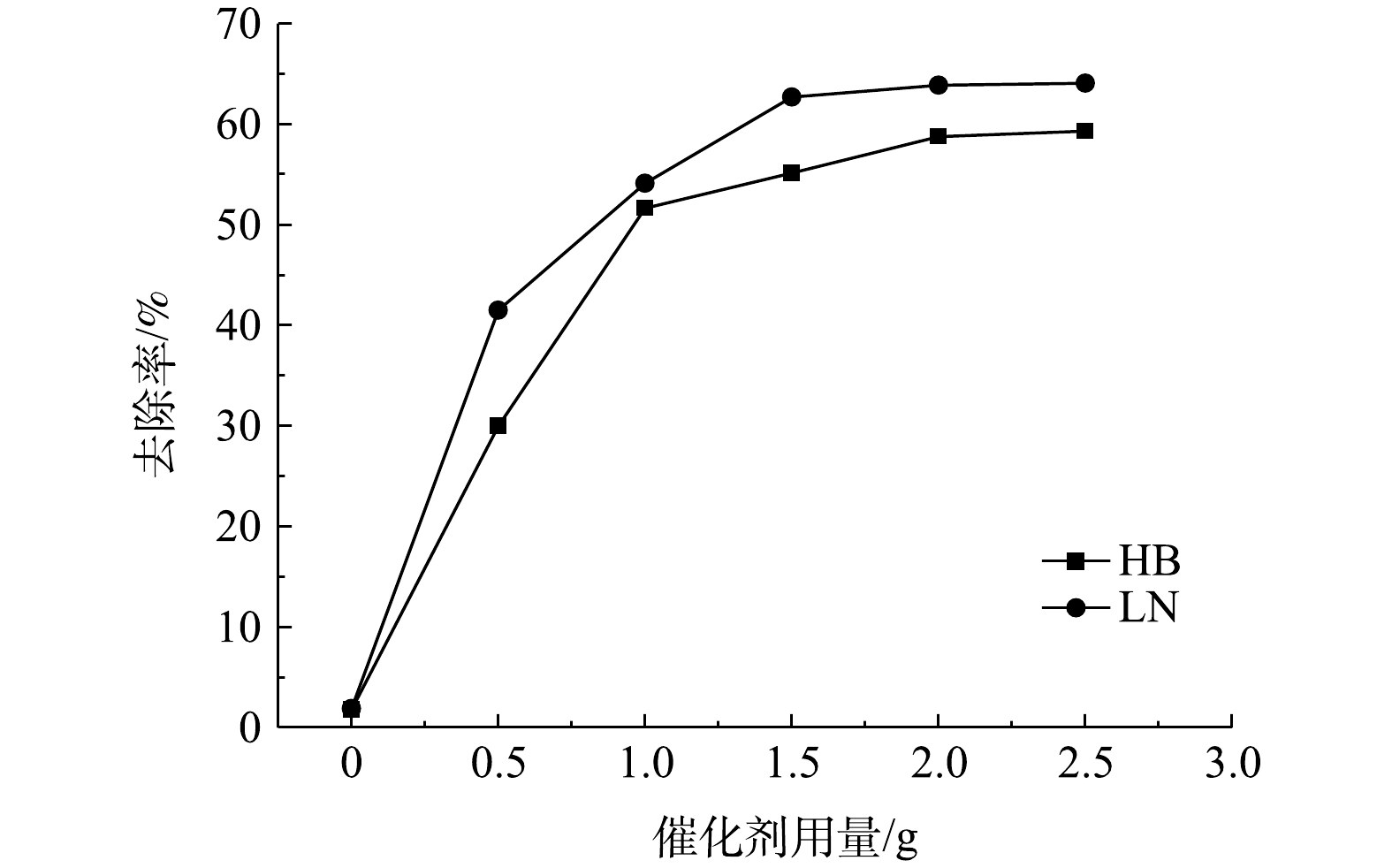
2)催化剂用量影响。Fe-NCNT/PC-700催化剂用量对脱硫废液中硫代硫酸盐去除率影响如图7所示。由图7可知,空气氧化(即无催化剂)对2种脱硫废液中硫代硫酸盐去除率极低;当催化剂用量由0.5 g增至1.5 g时,2种脱硫废液中硫代硫酸盐去除率均显著升高,这是因为催化剂能够提供的活性点位随其用量增加而快速增加,从而促进硫代硫酸盐快速氧化;当催化剂用量由1.5 g增至2.5 g时,2种脱硫废液中硫代硫酸盐去除率的升高相对缓慢,说明此时催化剂用量已逐渐成为催化空气氧化反应的非限速步骤。值得一提的是,LN脱硫废液中硫代硫酸盐去除率始终较HB脱硫废液上升快,其原因或在于:在催化剂用量相同条件下,催化活性点位数量一定,而LN脱硫废液中硫代硫酸盐质量浓度较HB脱硫废液中的低,故其催化空气氧化反应速率较快。鉴于催化剂通常在脱硫废液中硫代硫酸盐氧化后会进入副产物硫磺,为降低对硫磺品质影响及催化空气氧化运行成本,综合考虑催化剂用量选择1.5 g。
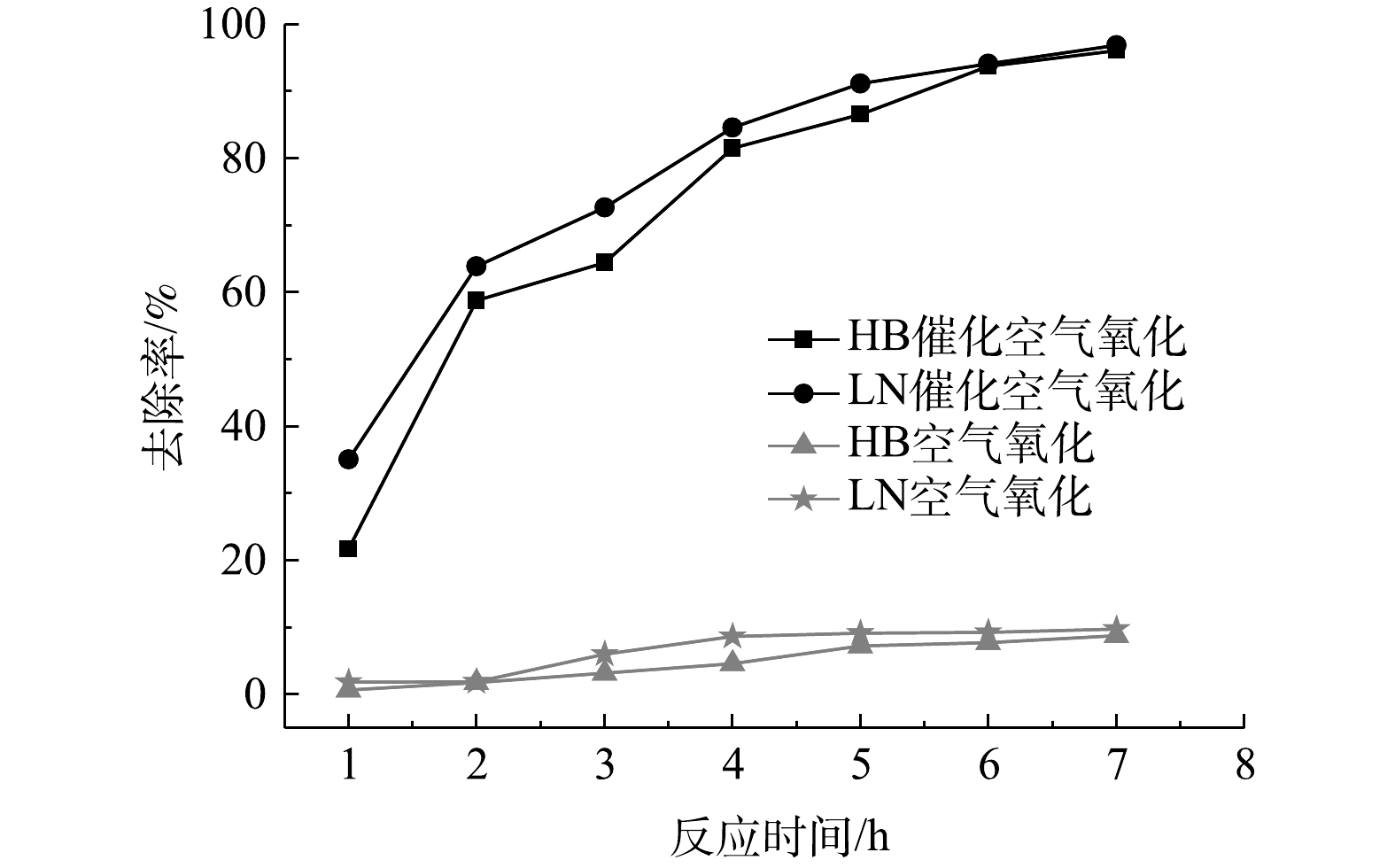
3)反应时间影响。氧化反应时间对脱硫废液中硫代硫酸盐去除率影响如图8所示。由图8可知,无论是空气氧化还是催化空气氧化,HB、LN 2种脱硫废液中硫代硫酸盐去除率均随反应时间延长而呈先升高后稳定趋势,但相同时间下催化空气氧化对硫代硫酸盐去除率显著高于空气氧化,而空气氧化对硫代硫酸盐去除率低于10%。GONZÁLEZ-LARA等[20]利用空气鼓泡氧化硫代硫酸盐,发现在常压、空气流量1 L·min−1、60 ℃下反应5 d转化率最高为30%,同样证明空气氧化硫代硫酸盐较困难[23]。当反应时间由1 h延长至5 h时,催化空气氧化对硫代硫酸盐去除率快速升高;当反应时间由5 h延长至7 h时,硫代硫酸盐去除率略有升高而后趋于稳定,说明硫代硫酸盐逐渐去除完全,符合硫代硫酸盐氧化2个阶段特征[20];第1阶段主要为硫代硫酸盐快速转化为SO32−、SO42−和亚稳态S4O62-[23],脱硫废液pH显著降至酸性;第2阶段主要为剩余硫代硫酸盐发生酸分解和SO32−、S4O62−继续缓慢氧化为硫酸盐[22]。因此,催化空气氧化反应时间宜控制在6 h,此时HB、LN 2种脱硫废液中硫代硫酸盐去除率约为95%。
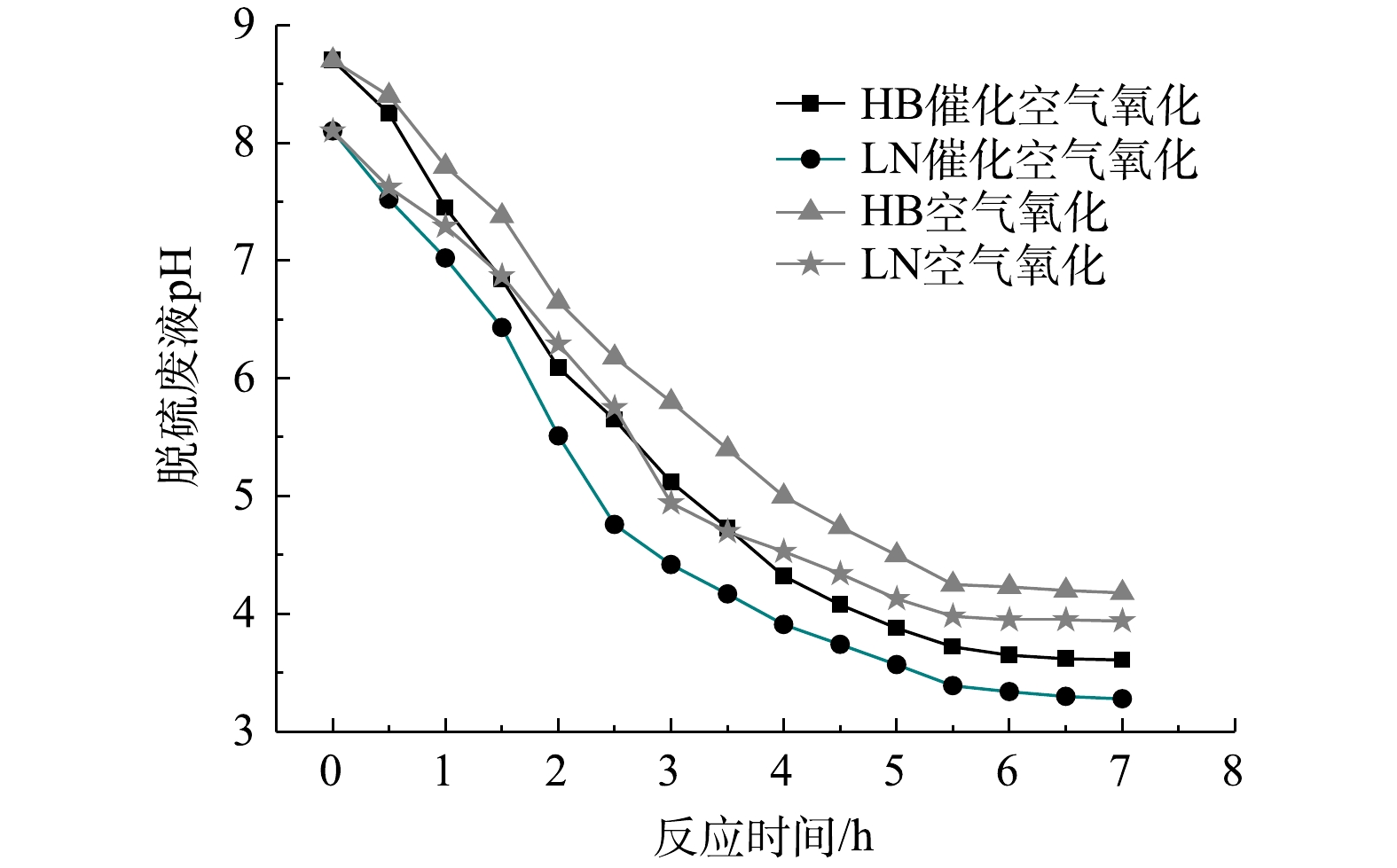
4)氧化反应过程中pH变化与催化剂稳定性。随着氧化反应时间延长,脱硫废液pH发生显著变化,可能会导致催化剂活性组分流失。因此,氧化过程中测定脱硫废液pH,其随反应时间变化如图9所示。由图9可知,HB、LN 2种脱硫废液的初始pH均呈碱性;随着氧化反应时间延长,脱硫废液pH逐渐降至酸性;在HB、LN 2种脱硫废液催化氧化过程中pH降低更快且最终pH更低,依次为3.6、3.3,空气氧化最终pH均约4.0,而硫代硫酸盐在pH小于4.0时不稳定易发生酸分解[20]。结合氧化实验现象,分析认为脱硫废液中硫代硫酸盐氧化过程中pH下降主要原因是溶解性氨逸出、氧化过程中产生HSO3−和SO2等酸性物质[20]以及高浓度有机物氧化产生乙酸等小分子酸[24]。
基于对HB、LN 2种脱硫废液催化氧化最终pH,利用pH=3.5的稀硫酸水溶液代替脱硫废液评价催化剂中活性组分稳定性。实验结束后测得水溶液中铁溶出量为0.010 2 mg·L−1。显然,Fe-NCNT/PC-700催化剂中活性组分铁溶出量极低,再次证明FeNx限域结构能够锚定催化剂中活性金属铁,防止催化剂中活性组分铁流失,进而有利于后续提取高品质盐产品。
-
1)过渡金属元素(Fe、Mn、Cu、Co、Ni)对硫代硫酸盐催化空气氧化活性顺序为Fe>Ni≈Mn>Cu>Co。
2) Fe-NCNT/PC催化剂具有微-介孔结构和FeNx限域结构,升高焙烧温度有利于改善催化剂比表面积和孔道结构,但使催化剂表面碳纳米管尺寸出现两极分化和FeNx限域结构不稳定,从而降低催化剂活性和稳定性,最适焙烧温度为700 ℃。
3) Fe-NCNT/PC-700催化剂能够催化空气氧化HB、LN 2种脱硫废液中的硫代硫酸盐,最佳催化剂用量1.5 g、氧化时间6 h,去除率大于95%;脱硫废液初始pH越低,硫代硫酸铵氧化速率越快。
4)在HB、LN 2种脱硫废液中的硫代硫酸盐氧化过程中,脱硫废液pH由碱性显著降至酸性;Fe-NCNT/PC-700催化剂的FeNx限域结构能够防止其在酸性环境中活性组分铁流失。
基于氮掺杂碳负载铁催化剂的硫代硫酸盐催化空气氧化
Catalytic air oxidation of thiosulfate by nitrogen-doped carbon supported iron catalyst
-
摘要: 焦化脱硫废液中硫代硫酸盐严重制约高品质硫氰酸盐产品提取。利用马铃薯薯渣为碳源,通过热处理技术制备氮掺杂碳负载铁催化剂(Fe-NCNT/PC),采用N2物理吸附仪、扫描电子显微镜、X射线光电子能谱对催化剂的表面积、微观形貌和表面结构特征进行了表征,考察了其对HB、LN 2种脱硫废液中硫代硫酸盐的催化空气氧化性能。结果表明,Fe-NCNT/PC具有微-介孔结构和FeNx限域结构,焙烧温度的升高会降低催化剂活性和稳定性,最适焙烧温度为700 ℃;当Fe-NCNT/PC-700用量1.5 g、氧化时间6 h时,HB、LN脱硫废液中硫代硫酸盐的去除率可达95%;脱硫废液氧化后pH显著降至酸性,Fe-NCNT/PC-700的限域结构能够防止活性组分铁流失。此外,Fe较其他过渡金属(Mn、Cu、Co、Ni)对硫代硫酸盐催化空气氧化活性更佳。Abstract: Thiosulfate in coking desulfurization waste liquid (CDWL) seriously restricts extraction of high-quality thiocyanate products. In this study, potato residue was taken as carbon source, nitrogen-doped carbon supported iron catalyst (Fe-NCNT/PC) was prepared by heat treatment technology. The specific surface area, morphology and surface structure of the catalyst were characterized by N2 physical adsorption instrument, scanning electron microscope and X-ray photoelectron spectroscopy. The catalytic air oxidation of thiosulfate in two kinds of CDWL: HB and LN, was investigated. The results showed that Fe-NCNT/PC had micro-mesoporous structure and FeNx confined structure, the activity and stability of the catalyst decreased with the increase of calcination temperature, and the optimum calcination temperature was 700 ℃. When the dosage of Fe-NCNT/PC-700 was 1.5 g and the oxidation time was 6 h, the removal rates of thiosulfate in the above two kinds of CDWL could reach 95%. After oxidation, the pH of CDWL decreased significantly to acidity, and the confined structure of Fe-NCNT/PC-700 could prevent the loss of active component iron. Besides, Fe showed a better catalytic activity for air oxidation of thiosulfate than other transition metals (Mn, Cu, Co, Ni).
-

-
表 1 实验用焦化脱硫废液组成
Table 1. Composition of coking desulfurization waste liquid used in experiment
脱硫废液来源 NH4SCN/
(g·L−1)(NH4)2S2O3/
(g·L−1)(NH4)2SO4/
(g·L−1)pH 河北(HB) 112.16 44.17 34.24 8.7 辽宁(LN) 98.37 32.60 30.18 8.1 表 2 Fe-NCNT/PC催化剂BET比表面及孔容
Table 2. BET surface area and pore volume of catalyst Fe-NCNT/PC
催化剂 比表面积/
(m2·g−1)总孔容/
(cm3·g−1)微孔孔容/
(cm3·g−1)Fe-NCNT/PC-700 319.971 0.231 0.100 Fe-NCNT/PC-800 939.804 0.591 0.327 Fe-NCNT/PC-900 886.910 0.579 0.302 -
[1] HUANG J, HOU B, GUO N, et al. Solid-liquid phase equilibria of ternary system Na2S2O3-Na2SO4-H2O in a wide range of temperatures: Measurement and application[J]. The Journal of Chemical Thermodynamics, 2018, 125: 1-10. doi: 10.1016/j.jct.2018.05.014 [2] LI C, LI G, ZHANG S, et al. Study on the pyrolysis treatment of HPF desulfurization wastewater using high-temperature waste heat from the raw gas from a coke oven riser[J]. RSC Advances, 2018, 8(54): 30652-30660. doi: 10.1039/C8RA06099A [3] 张亚峰, 刘硕, 裴振, 等. 焦化脱硫废液提盐工程实例[J]. 工业水处理, 2021, 41(1): 136-141. [4] 陈红萍, 齐雪, 王超, 等. 选择性吸附氧化硫代硫酸盐催化剂的研究[J]. 环境污染与防治, 2017, 39(4): 418-421. doi: 10.15985/j.cnki.1001-3865.2017.04.015 [5] 崔玉民, 孙文中. 固体超强酸光催化氧化硫代硫酸钠[J]. 河南科技大学学报:自然科学版, 2006, 27(5): 97-100. [6] ILIEV V, PRAHOV L, BILYARSKA L, et al. Oxidation and photooxidation of sulfide and thiosulfate ions catalyzed by transition metal chalcogenides and phthalocyanine complexes[J]. Journal of Molecular Catalysis A:Chemical, 2000, 151: 161-169. doi: 10.1016/S1381-1169(99)00263-0 [7] 刘凤玉, 凌开成. 漂浮型TiO2/EP光催化剂催化氧化硫代硫酸钠[J]. 工业催化, 2010, 18(7): 66-70. doi: 10.3969/j.issn.1008-1143.2010.07.015 [8] SABZI R E, HASSANZADEH A, HERAVI P, et al. Al electrode modified by Au atoms as a novel electrode for electrocatalytic oxidation of thiosulfate[J]. Journal of the Chinese Chemical Society, 2007, 54: 977-982. doi: 10.1002/jccs.200700140 [9] CHATTERJEE D, SHOME S, JAISWAL N, et al. Mechanism of the oxidation of thiosulfate with hydrogen peroxide catalyzed by aqua-ethylenediaminetetraacetatoruthenium(III)[J]. Journal of Molecular Catalysis A:Chemical, 2014, 386: 1-4. doi: 10.1016/j.molcata.2014.01.029 [10] 孙金龙, 张宇, 刘福跃, 等. 基于碳基催化剂活化过二硫酸盐降解有机污染物的研究进展[J]. 化工进展, 2021, 40(3): 1653-1666. doi: 10.16085/j.issn.1000-6613.2020-0883 [11] GAO L, LI R, SUI X, et al. Conversion of chicken feather waste to N-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol[J]. Environmental Science & Technology, 2014, 48(17): 10191-10197. [12] LIAN F, CUI G, LIU Z, et al. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity[J]. Journal of Environmental Management, 2016, 176: 61-68. doi: 10.1016/j.jenvman.2016.03.043 [13] XU Y, SCHOONEN M A A. The stability of thiosulfate in the presence of pyrite in low-temperature aqueous solutions[J]. Geochimica et Cosmochimica Acta, 1995, 59(22): 4605-4622. doi: 10.1016/0016-7037(95)00331-2 [14] XU Y, SCHOONEN M A A, STRONGIN D R. Thiosulfate oxidation: Catalysis of synthetic sphalerite doped with transition metals[J]. Geochimica et Cosmochimica Acta, 1996, 60(23): 4701-4710. doi: 10.1016/S0016-7037(96)00279-7 [15] HUANG Y, LIU K, KAN S, et al. Highly dispersed Fe-Nx active sites on Graphitic-N dominated porous carbon for synergetic catalysis of oxygen reduction reaction[J]. Carbon, 2021, 171: 1-9. doi: 10.1016/j.carbon.2020.09.010 [16] 姚智子, 冯欢欢, 董森, 等. 铁氮掺杂生物质/聚苯胺衍碳氧还原催化剂研究[J]. 新疆大学学报(自然科学版)(中英文), 2022, 39(3): 314-322. [17] 安路阳, 张立涛, 卫皇曌, 等. 超结构碳催化剂催化过硫酸盐降解间甲酚性能研究[J]. 环境污染与防治, 2022, 44(11): 1462-1466. [18] 杨婷婷, 朱能武, 芦昱, 等. 铁氮掺杂碳纳米管/纤维复合物制备及其催化氧还原的效果[J]. 环境科学, 2016, 37(1): 350-338. doi: 10.13227/j.hjkx.2016.01.045 [19] LI M, XU F, LI H, et al. Nitrogen-doped porous carbon materials: Promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation[J]. Catalysis Science & Technology, 2016, 6(11): 3670-3693. [20] GONZÁLEZ-LARA J M, ROCA A, CRUELLS M, et al. The oxidation of thiosulfates with copper sulfate. Application to an industrial fixing bath[J]. Hydrometallurgy, 2009, 95(1/2): 8-14. [21] CHANDA M, REMPEL G L. Catalysed air oxidation of thiosulfate and tetrathionate for pollution abatement[J]. Applied Catalysis, 1985, 19(1): 33-48. doi: 10.1016/S0166-9834(00)82668-8 [22] CHANDA M, REMPEL G L. Cuprous oxide catalyzed air oxidation of thiosulfate and tetrathionate[J]. Applied Catalysis, 1986, 23(1): 101-110. doi: 10.1016/S0166-9834(00)81455-4 [23] BREUER P L, JEFFREY M I. Copper catalysed oxidation of thiosulfate by oxygen in gold leach solutions[J]. Minerals Engineering, 2003, 16(1): 21-30. doi: 10.1016/S0892-6875(02)00287-X [24] JAGUSHTE M V, MAHAJANI V V. Insight into spent caustic treatment-on wet oxidation of thiosulfate to sulfate[J]. Journal of Chemical Technology and Biotechnology, 1999, 74: 437-444. doi: 10.1002/(SICI)1097-4660(199905)74:5<437::AID-JCTB63>3.0.CO;2-1 -



 下载:
下载: