-
电氧化技术通过生成的强氧化物质将有机物氧化为二氧化碳和水,无二次污染,反应条件温和,常温常压下就能进行,因此电氧化技术被称为环境友好技术。目前,电氧化技术已在污水净化、垃圾渗滤液、制革废水、印染废水、医药废水和焦化废水等治理领域具有广泛的应用研究。垃圾渗滤液中含有较高浓度的氯离子,经过电氧化处理后,产水余氯浓度较高,使产水带有刺激性气味,不适合直接排放,需要对余氯进行去除。目前,水中余氯去除的主要方法有四价含硫化合物法、活性炭法、KDF(Kinetic Degradation Fluxion)介质过滤法、紫外光照法、过氧化氢法和铁及其化合物法等[1]。活性炭法具有去除效率高、不产生二次污染、能同时去除有机污染物等特点,因此常用于大规模去除余氯的工艺中。活性炭可以依靠吸附作用去除水中的余氯[2],还可以依靠活性炭表面化学性质的作用,基于活性炭表面的氧化还原反应[3-5]。活性炭与水中余氯接触初期,去除余氯以吸附作用为主,达到吸附平衡后,余氯浓度继续下降则是由于化学反应的作用[6]。
影响活性炭吸附性能的因素主要有活性炭的性质、被吸附污染物的性质和其他因素(反应时间、温度、溶液pH、无机离子成分等),其中活性炭的性质(粒径、种类、表面官能团等)是影响活性炭吸附性能最重要的因素[7-9]。粉末活性炭接触面积大,吸附速率快、效果好。本文通过小试试验,探究反应时间、溶液pH、活性炭材质、活性炭投加量、余氯初始浓度等对活性炭去除余氯的影响。在小试的基础上,采用“粉末活性炭+真空转盘过滤”去除电氧化处理填埋场渗滤液后的产水余氯,考察该工艺对余氯的去除效果,分析该工艺用于去除水中余氯的可行性。
-
本试验中电氧化处理对象为江苏某垃圾填埋场渗滤液的纳滤浓缩液,该填埋场渗滤液处理工艺为“预处理+两级A/O+MBR+NF+RO”,纳滤浓缩液的处理工艺为“Fenton+电氧化”。本试验中试验水样为电氧化产水,纳滤浓缩液和电氧化产水的主要水质指标见表1。
-
本试验采用电氧化处理垃圾渗滤液的产水作为试验水样,采用“Fenton+电氧化”工艺处理渗滤液纳滤浓缩液,利用钛涂层钌依电极在电流密度为600 A/m2时,电解后的水样作为试验水样。试验前制取10 L电氧化产水水样,混合均匀后放在冰箱中保存。
小试试验采用烧杯作为去除余氯的反应器,利用六联磁力搅拌器作为烧杯搅拌装置,试验时取1 L水样于每个烧杯中,用电子天平准确称量粉末活性炭,倒入烧杯中,将转速调至720 r/min,搅拌均匀后,开始计时。测定水样的余氯前,先用0.45 µm的滤膜过滤水样。配制(1+35)的H2SO4和2%的NaOH溶液用于调节水样的pH。具体操作步骤:取500 mL电氧化产水,放入1 000 ml烧杯中,用酸溶液或碱溶液调节水样初始pH,向水样中分别加入粉末活性炭,并放置于磁力搅拌器上以720 r/min进行充分搅拌,反应结束后,连续取样用0.45 µm滤膜过滤后测定余氯。
中试试验中,采用“粉末活性炭反应器+真空转盘过滤”去除电氧化产水中的余氯,试验期间,调节水样的pH为7.0~7.5,粉末活性炭的运行浓度为10 kg/m3,粉末活性炭停留时间为5 d,每天运行6 h,连续运行30 d,试验水样为电氧化处理4 h后的产水。运行期间连续进水和产水,每2 h取1次水样测定产水中的余氯。
试验药剂:6种国产商品粉末活性炭:1种椰壳活性炭,2种木质活性炭(木质活性炭Ⅰ、木质活性炭Ⅱ),3种煤质活性炭(煤质活性炭Ⅰ、煤质活性炭Ⅱ、煤质活性炭Ⅲ)。浓硫酸(98%)分析纯,氢氧化钠(分析纯)。
-
中试试验采用自主设计的试验装置,余氯去除工艺为“粉末活性炭反应器+真空转盘过滤”,试验装置主要包括反应器、活性炭投加装置、鼓风机、真空转盘过滤机、产水泵、进水泵、循环泵、排水泵、控制箱等组成,试验装置由PLC控制。试验工艺流程见图1。
粉末活性炭反应器主要包括活性炭投加装置、搅拌机、进水管、循环管、曝气管、排空管、支架等。反应器由内外筒组成,搅拌机在内筒中,搅拌机功率0.37 kW,内筒有效容积为100 L,外筒有效容积为400 L,反应器设计水力停留时间为60 min,设计处理能力为0.5 m3/h。进水管和循环管出口在内筒中,活性炭、进水和循环水在内筒中混合搅拌,外筒采用曝气搅拌,外筒上设置溢流出口管,外筒的出水从溢流管流入真空转盘过滤机的进水箱,通过循环泵回流至内筒中,保持活性炭浓度的稳定,循环泵的流量为1~2 m3/h。
真空转盘过滤机主要由转盘水箱、转盘、中心产水管、进水管、冲洗管、曝气管、减速电机、排空管、溢流管、进水泵、进水箱、鼓风机、产水泵和产水箱等组成,进水箱和产水箱中有液位计,通过液位控制真空转盘过滤机的运行。转盘上有过滤层,过滤层下面有支撑层,过滤层采用滤布,本试验采用的滤布材质为加厚型涤纶,透气率为2。转盘的尺寸为500 mm,转盘数量为3个,有效过滤面积为1 m2,设计转盘的过滤通量为0.5~1 m3/(m2·h)。
-
试验过程中,对处理前和处理后的余氯进行测定,采用《水和废水分析监测方法(第四版)》中规定的方法。余氯的测定方法为碘量法和DPD(N,N-二乙基-1,4-苯二胺)分光光度法。
-
活性炭的颗粒大小对余氯的去除有很大影响[7],活性炭的颗粒越小,过滤面积就越大,去除效果越好,活性炭的孔径分布以及粒度对扩散会有很大的影响[8],椰壳活性炭具有很好的去除余氯效果[9]。本试验采用的6种活性炭的性质表2。
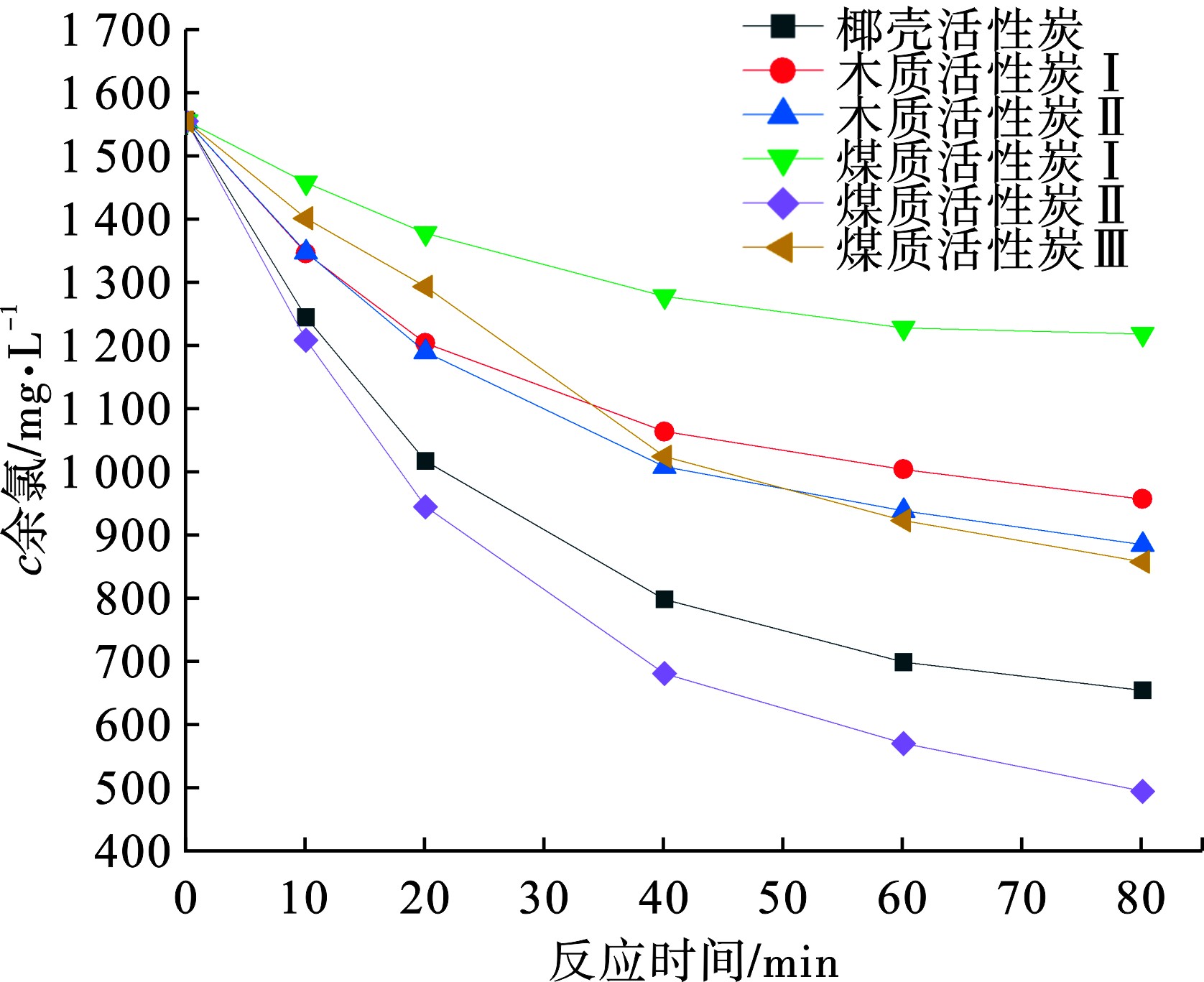
水样初始pH为7.5,粉末活性炭投加量为1 g/L,反应时间为80 min,不同粉末活性炭对余氯的去除效果见图2。
图2可知,不同性质的粉末活性炭对余氯的差异性较大,活性炭去除余氯的性能除了和吸附碘值有关,还和活性炭材质有关。研究表明余氯去除速率为煤质活性炭>椰壳活性炭>木质活性炭>果壳活性炭[3]。本试验中,余氯去除速率为煤质活性炭Ⅱ>椰壳活性炭>煤质活性炭Ⅲ>木质活性炭Ⅱ>木质活性炭Ⅰ>煤质活性炭Ⅰ,一般认为,余氯主要与活性炭表面的还原性基团反应得以去除[10],动力学拟合结果表明反应前期余氯的去除速率显著高于后期,这主要是由于反应初期依靠吸附作用,吸附平衡后主要依靠还原性基团的反应。不同性质的活性炭,表面的官能团差异较大。采用Boehm滴定法测定活性炭表面官能团,不同性质的活性炭表面官能团性质见表3。煤质活性炭I的表面官能团含量最少,表现出对余氯的去除性能最差,煤质活性炭Ⅱ的表面官能团较多,余氯去除能力较强。综合分析,本试验中粉末活性炭对余氯去除速率主要受到吸附碘值、表面官能团、活性炭材质的影响。
-
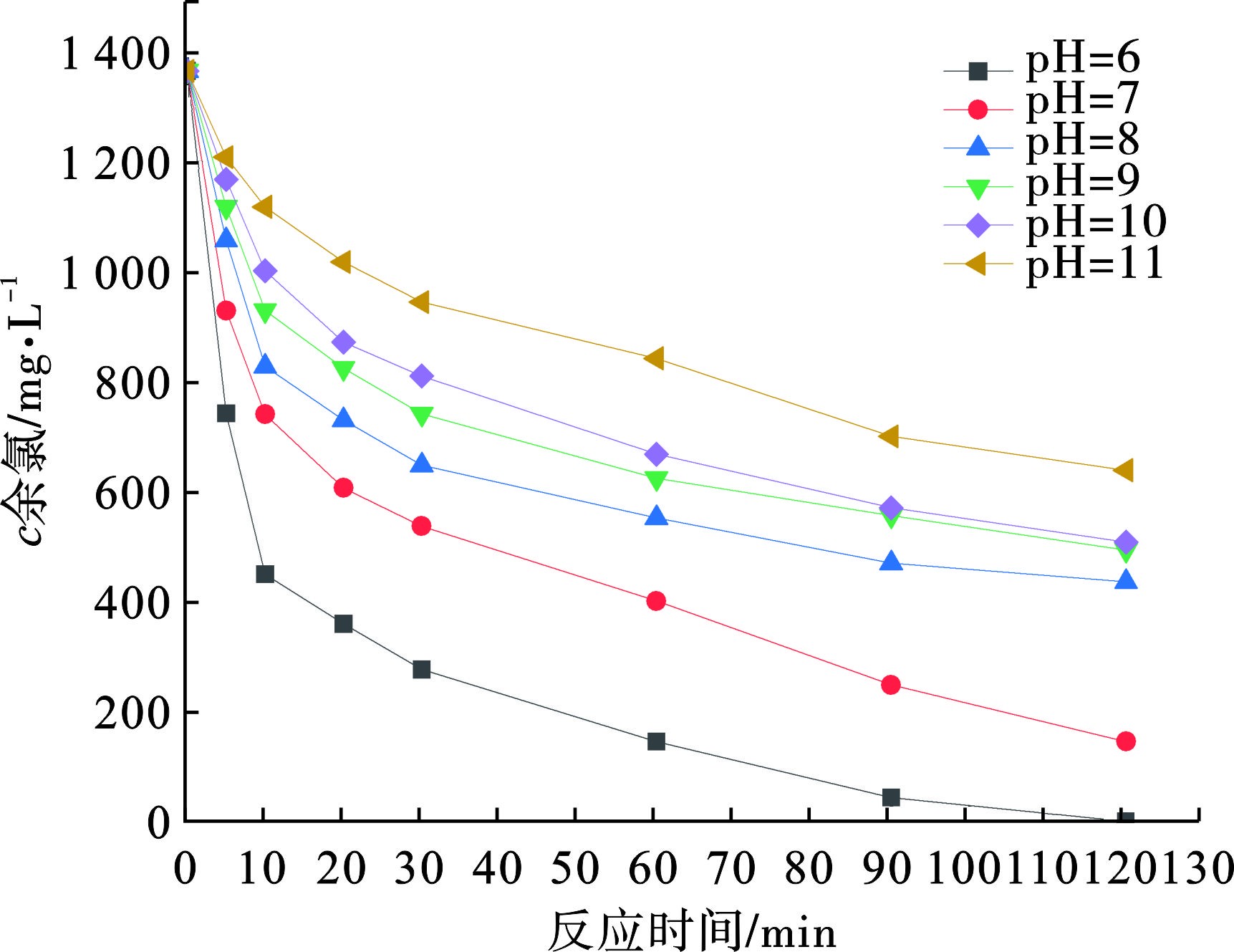
pH会影响活性炭对余氯的去除效果,一般酸性条件有利于活性炭与余氯的反应,主要原因是碱性条件下电氧化产水中的余氯多以ClO-的形式存在,HClO的氧化能力要强于ClO-,HClO更容易与活性炭表面官能团反应[11]。选取性能适中的粉末活性炭(煤质活性炭Ⅲ)去除余氯,粉末活性炭的投加量为1 g/L,反应时间为2 h,不同pH条件下,粉末活性炭对余氯的去除效果见图3。
图3可知,溶液初始pH对余氯的去除速率影响很大,初始pH从6到11,余氯去除速率不断降低,在初始pH为6时,反应2 h后,水样中余氯被完全去除,随着反应时间的增加,余氯的去除速率也会随之降低。实际废水中pH一般为6~9,在用活性炭去除余氯时可以适当投加酸以降低溶液pH,有助于提高活性炭对余氯的去除效果。
-
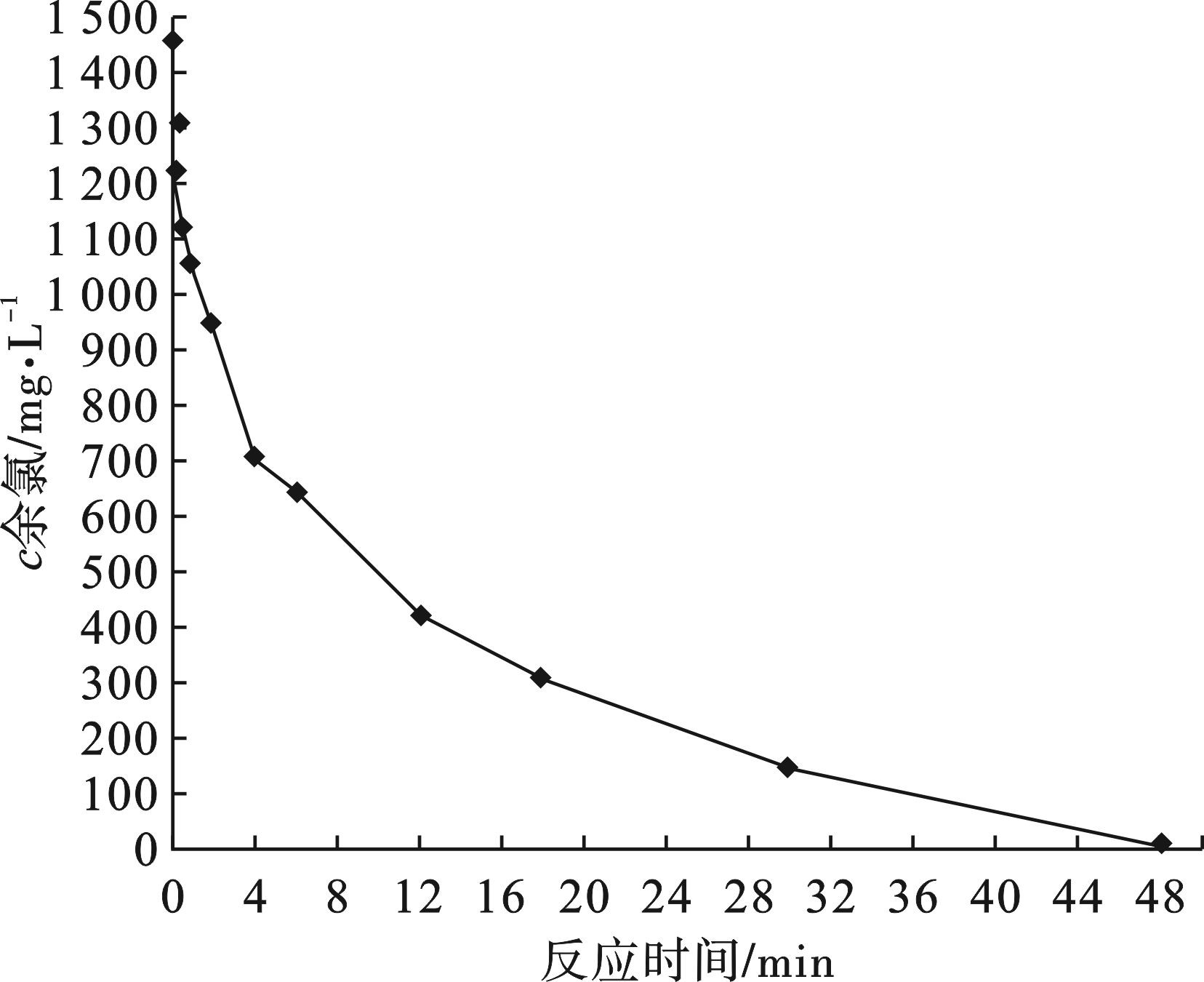
溶液初始的pH对活性炭去除余氯有明显的影响,为了提高余氯去除效果,还可以通过延长时间来提高余氯去除量。本试验中,控制水样的初始pH为11,采用煤质活性炭Ⅲ去除余氯,活性炭的投加量为0.5 g/L,虽然余氯的去除速率较慢,在延长反应时间为48 h
后,水样中的余氯仍然可以被去除,试验结果见图4。
图4可知,虽然水样中的余氯被去除,但是活性炭被过度氧化,水样的颜色变成棕褐色,色度大于500倍。主要原因为活性炭去除余氯是吸附作用和氧化还原反应的共同结果,当活性炭被氧化时,活性炭上的含碳物质向液相转移,活性炭在碱性条件下被氧化时结构断裂产生的溶解性碳质物质进入到溶液中,这类物质主要多为环芳香类物质[12],通常被认为是富里酸(FAs)。
对比图3和图3可以看出,溶液初始pH对余氯去除的影响比反应时间更明显,pH会严重影响余氯的去除速率,需要更长的时间才能将余氯去除,而且在较长时间在碱性条件下反应,还容易使活性炭上的碳物质转移到液相中导致水样颜色变成深棕色,而且反应时间越长,颜色越深,色度可以达到1 000倍以上。
-
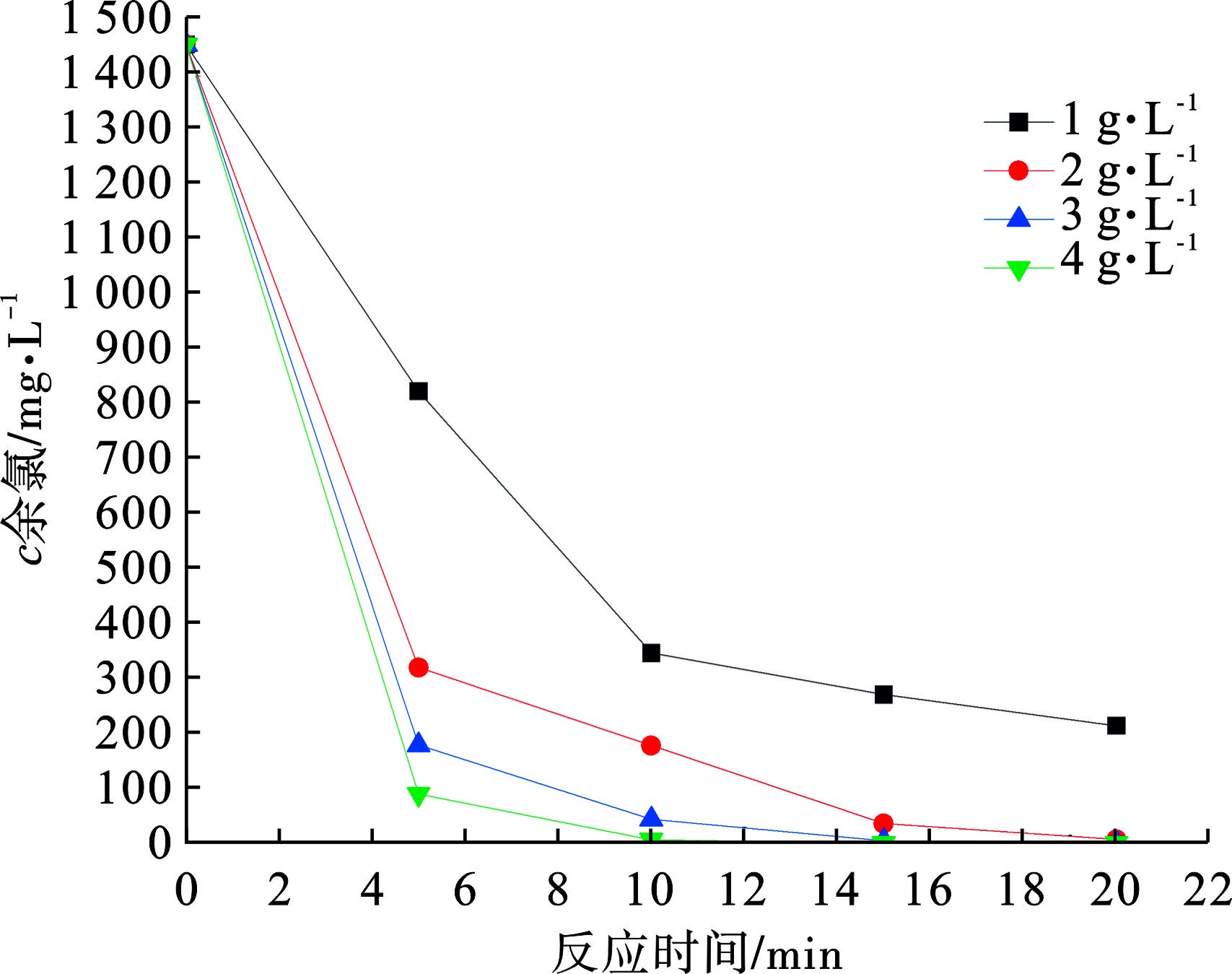
活性炭去除余氯初期主要以吸附作用为主,后续主要依靠化学反应[6],随着反应时间的延长,活性炭对余氯的去除量也随之增加,0.5 g煤质活性炭Ⅲ至少可以去除1 461 mg的余氯,远远超过活性炭的吸附容量。用煤质活性炭Ⅲ进行试验,不同的活性炭投加量去除余氯的结果见图5。增加活性炭的投加量可以明显加快余氯去除速率,反应初期余氯去除速率较快,主要依靠了活性炭的吸附作用,反应后期则依靠活性炭表面的氧化还原作用去除余氯,随着余氯浓度的降低,反应速率逐渐降低。
-
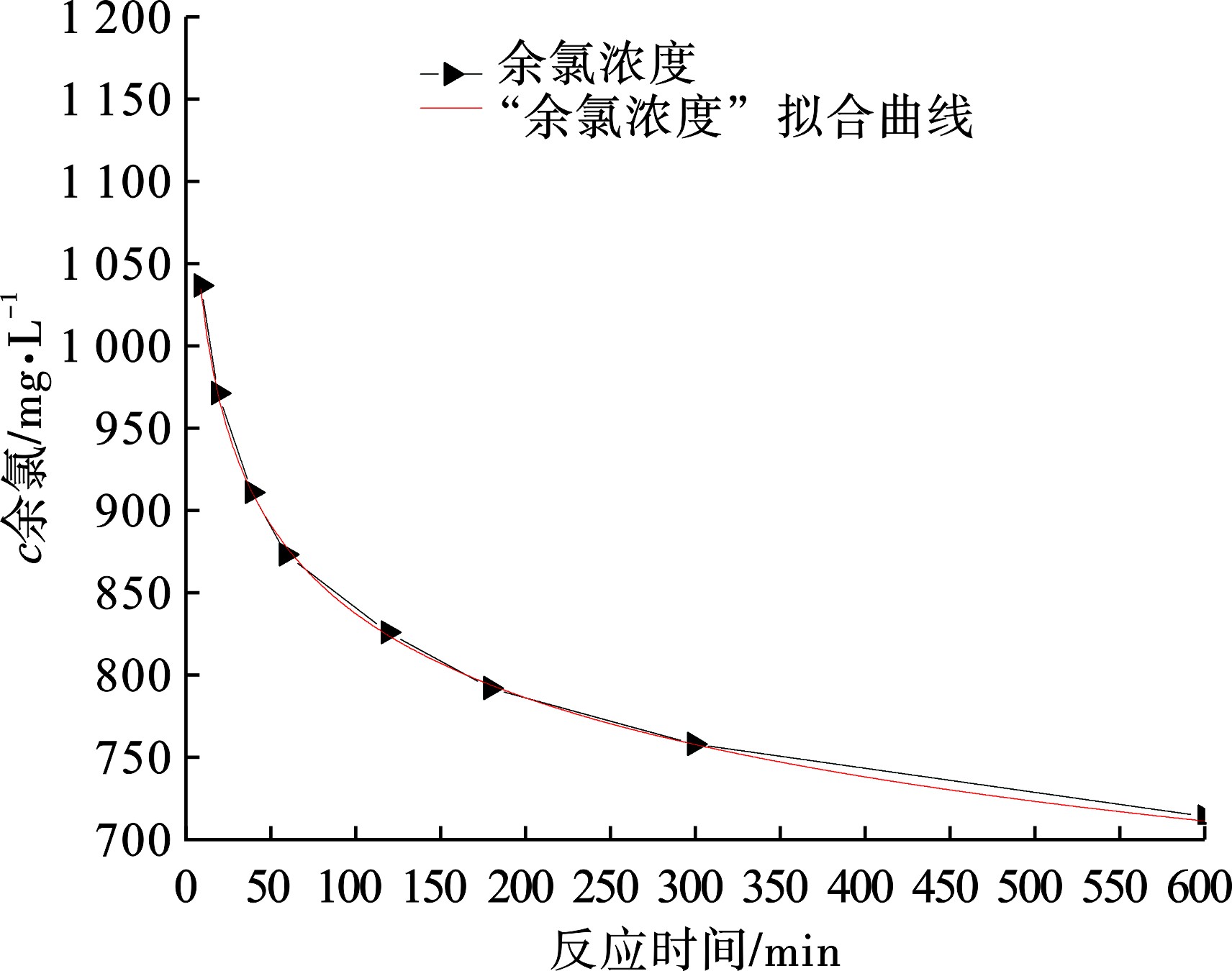
为了验证粉末活性炭对余氯的最大去除能力,试验中选取性能最好的粉末活性炭(煤质活性炭Ⅱ)去除余氯。由于粉末活性炭去除余氯能力较强,本试验中溶液初始余氯浓度在1 000~2 000 mg/L,粉末活性炭投加量不宜过大,因此选取活性炭的投加量为0.1 g/L,同时为了缩短试验反应时间,选取初始pH为5.0提高反应速率。试验数据经过拟合曲线的结果见图6。
图6可知,随着反应时间的延长,活性炭去除余氯的速率也逐渐降低,反应负荷符合一级反应动力学。根据拟合方程计算活性炭去除余氯的最大值为824.84 mg/L,考虑到实际应用中,反应装置的水力停留时间不可能无限大,以水力停留时间120 h计算,可以估算出余氯的浓度为569.06 mg/L,得到活性炭的去除能力为3 006.5 mg/g。
为了验证拟合曲线的准确度,延长反应试验为48 h后,实际的余氯浓度为453.015 mg/L,说明实际的余氯去除能力比拟合曲线计算值大,计算得到活性炭的去除能力为3 586.725 mg/g,但是活性炭被过度氧化,溶液会变成淡黄色,溶液色度超过32倍,因此活性炭的停留时间不宜过长。
为了验证余氯去除后长时间浸泡不会导致余氯的释放,设置反应条件为:活性炭投加量为2 g/L、初始pH=7.0、反应时间10 d、反应结束时余氯浓度<1 mg/L,试验结果表明,活性炭在中性条件下去除余氯后,长时间浸泡不会导致余氯的释放,溶液颜色也不会加深。
根据文献[13],活性炭去除余氯发生反应见式(1)和式(2):
式中:C*为活性炭自由基;CO*代表活性炭的表面氧化炭,是活性炭表面官能团被氧化后的形态;CO*可能释放到水溶液中,从而产生新的反应位点[14]。本试验中,在初始余氯浓度为1 344 mg/L,活性炭投加量为1 g/L时,当余氯去除量为724.199 mg/L时,溶液中氯离子增量为658.281 mg/L,可以证明余氯的去除是吸附和氧化反应的共同结果。根据氧化还原反应过程中电子守恒计算,假设余氯都为HClO,当活性炭氧化产物为CO时,反应式见式(3):
计算得到1 g活性炭的最大余氯去除量为1×35.45/12×1 000=2 954.17 mg;
当活性炭氧化产物为CO2时,反应式见式(4):
计算得到1 g活性炭的最大余氯去除量为1×(35.45×2)/12×1 000=5 908.33 mg;
因此可以推断,根据此理论活性炭的氧化产物可能为CO2,实际上反应过程中余氯的去除难以达到理论最大值。
综合考虑,为了保证活性炭对余氯有高效的去除效果,同时防止活性炭被过度氧化导致水样颜色变深以及活性炭表面的碳物质转移到水中对水样造成污染,活性炭的设计去除能力不宜过高。
-
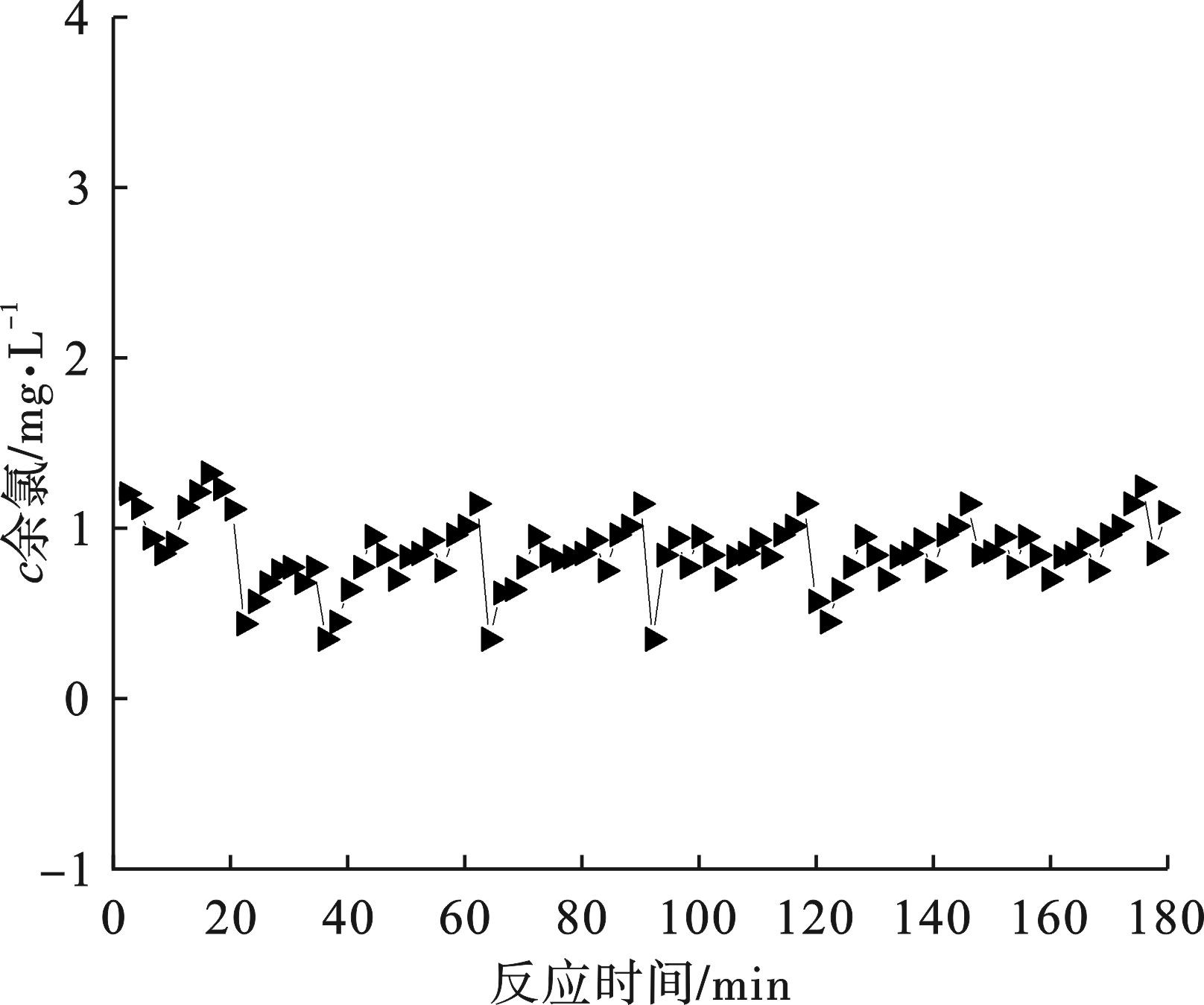
本试验中,采用煤质活性炭Ⅱ进行试验,进水的余氯浓度在1 000~2 000 mg/L之间,运行初期产水中的余氯基本被完全去除,去除效果较好,并且产水色度较低,无明显气味,稳定运行后180 h内的运行数据见图7。
图7可知,活性炭对余氯的去除效果比较稳定,产水余氯维持在1 mg/L左右,本试验中,活性炭的停留时间可以控制在5 d左右,根据活性炭的理论最大去除能力,同时保证活性炭的有效利用,设计活性炭的余氯去除能力为3 000 mg/g左右。R2
试验期间,真空转盘过滤机的转盘转速为50~100 r/min,进水流量为1 m3/h,设备运行稳定,出水中SS浓度<10 mg/L,在运行中,每2 h停机1次,先排空转盘水箱,再启动冲洗程序,利用高压水流对转盘的盘片进行冲洗,可以有效地防止活性炭在转盘过滤层上积累。连续运行1个月,每天运行6 h,运行过程中的过滤通量、产水负压的情况见图8。
图8可知,在真空转盘过滤机的连续运行过程中,过滤通量一直稳定在0.5 m3/(m2·h)左右,产水负压在16 kPa以内,真空转盘过滤机运行稳定,说明采用真空滤布过滤粉末活性炭是可行的。
-
本文通过小试试验,探究了粉末活性炭去除余氯的工艺条件,并设计了一种用于粉末活性炭去除余氯的工艺,以电氧化处理填埋场渗滤液后的产水为研究对象,采用“粉末活性炭反应器+真空转盘过滤”试验装置进行中试试验,考察该工艺的运行情况和对余氯的去除效果。
(1)溶液初始pH、活性炭性质、活性炭投加量等对余氯去除的影响很大。降低pH有利于提高活性炭对余氯去除速率,活性炭的吸附容量和表面官能团都对去除余氯有明显的影响。活性炭在使用过程中表面官能团会被氧化而逐渐降低活性,因此需要连续更换活性炭以保证余氯去除效果,本试验中设计活性炭的最大处理能力约3 000 mg/g。
(2)采用“粉末活性炭反应器+真空转盘过滤”工艺去除电氧化产水中的余氯,在余氯浓度1 000~2 000 mg/L,活性炭浓度10 g/L,水力停留时间为120 min时,粉末活性炭对余氯的去除率接近100%,连续运行1个月,试验装置运行稳定,出水SS<10 mg/L。
本试验中,每天需要排除部分活性炭混合液,因此实际工程应用需要考虑活性炭的固液分离,建议采用板框压滤机对活性炭的混合液进行脱水。
粉末活性炭去除电氧化产水余氯的试验研究
Experimental study on removal of residual chlorine from water produced by electrooxidation with powder activated carbon
-
摘要: 针对电氧化工艺处理废水后存在产水余氯的问题,该研究以电氧化处理垃圾渗滤液后的产水为研究对象,采用粉末活性炭去除水中的余氯,考察了反应时间、溶液初始pH、活性炭性质、活性炭投加量等对活性炭去除余氯的影响,并进行了粉末活性炭去除余氯的工艺试验。结果表明,降低溶液初始pH有利于余氯的去除,不同性质的粉末活性炭对余氯的去除效果存在明显差异,试验中所用的粉末活性炭对余氯的最大去除能力为3 000 mg/g,经过“粉末活性炭+真空转盘过滤”工艺处理后的电氧化产水余氯稳定在1mg/L左右。Abstract: In view of the problem of residual chlorine after wastewater treatment by electrooxidation process,this experiment takes the water production after electrooxidation treatment of landfill leachate as the research object., uses the powder activated carbon to remove the residual chlorine in the water.The effects of reaction time, initial pH of solution, properties of activated carbon and dosage of activated carbon on the removal of residual chlorine by activated carbon were investigated.The results showed that reducing the initial pH of solution was beneficial to the removal of residual chlorine, and there were significant differences in the removal effects of different properties of powdered activated carbon on residual chlorine. The maximum removal capacity of powdered activated carbon for residual chlorine was 3000mg·g-1, and the residual chlorine of electrooxidation water was stabilized at about 1mg·L-1 after the process of "powdered activated carbon + vacuum rotary plate filtration".
-
Key words:
- electrical oxidation /
- leachate /
- residual chlorine /
- powder activated carbon
-

-
表 1 试验水质指标
Table 1. Test water quality index
实验水样 电导率/ms·cm1 COD/mg·L−1 pH 氯离子/mg·L−1 余氯/mg·L−1 纳滤浓缩液 18.5~20.4 2 200~3 600 7.5~8.5 5 500~6 500 0 电氧化产水 16.5~19.6 20~100 7.3~8.5 4 500~5 000 1 000~2 000 表 2 活性炭性质
Table 2. Properties of activated carbon
活性炭 碘值/mg·g−1 元素分析/% C H N 椰壳活性炭 820 73.46 1.68 0.11 木质活性炭I 800 66.78 2.12 0.10 木质活性炭Ⅱ 820 73.60 2.86 0.25 煤质活性炭I 600 76.71 1.35 0.14 煤质活性炭Ⅱ 1 000 68.91 1.98 0.21 煤质活性炭Ⅲ 820 73.24 2.04 0.15 表 3 不同活性炭的表面官能团性质
Table 3. Surface functional group properties of differrent activated carbons
样品 表面官能团/mmol·g−1 —OH —COOR —COOH 酸性官
能团碱性官
能团椰壳活性炭 1.37 0.22 1.75 3.34 0.72 木质活性炭I 0.12 0.07 0.04 0.23 0.22 木质活性炭Ⅱ 0.16 0.06 0.23 0.46 0.45 煤质活性炭I 0.06 0.02 0.04 0.12 0.09 煤质活性炭Ⅱ 1.12 1.21 2.34 4.68 0.84 -
[1] 刘柳君, 刘晓艳, 熊鹰, 等. 水中余氯去除技术及材料研究进展[J]. 广州化工, 2020, 48(19): 29 − 32. doi: 10.3969/j.issn.1001-9677.2020.19.009 [2] 邹萍, 隋贤栋, 黄肖容. 铜锌改性活性炭的制备及对水中余氯的去除效果[J]. 材料开发与应用, 2009, 24(4): 48 − 50. doi: 10.3969/j.issn.1003-1545.2009.04.012 [3] 王丽萍, 徐斌, 钱灏. 净水用颗粒活性炭对水中余氯去除的动力学原理效能[J]. 净水技术, 2018, 37(1): 47 − 52. doi: 10.15890/j.cnki.jsjs.2018.01.008 [4] BAUER R C, SNOEYINK V L. Reactions of Chloramines with Active Carbon[J]. Journal Water Pollution Control Federation, 1973, 45(11): 2290 − 2301. [5] MARTIN R J, SHACKLETON R C. Comparison of two partially activated carbon fabrics for the removal of chlorine and other impurities from water[J]. Water Research, 1990, 24(2): 474 − 484. [6] 张怀旭, 刘婉冬, 李冰璟, 等. 活性炭去除水中余氯的研究[J]. 环境污染与防治, 2008, 30(5): 63 − 68. doi: 10.3969/j.issn.1001-3865.2008.05.018 [7] 朱建华, 王时雄. 不同活性炭滤芯去除饮用水中余氯性能的研究[J]. 绿色化工, 2017, 3: 62 − 64. [8] SKIBINSKI B, GOTZE C, WORCH E, et al. Pore diffusion limits removal of monochloramine in treatment of swimming pool water using granular activated carbon[J]. Water Research, 2018, 132: 270 − 281. doi: 10.1016/j.watres.2017.12.060 [9] JAGUARIBE E F, MEDEIROS L L, BARRETO M C S, et al. The performance of activated carbons from sugarcane bagasse, babassu, and coconut shells in removing residual chlorine[J]. Brazilian Journal of Chemical Engineering, 2005, 22(1): 41 − 47. doi: 10.1590/S0104-66322005000100005 [10] SUIDAN M T. Reduction of aqueous free chlorine with granular activated carbon-pH and temperature effects[J]. Environmental Science&Technology, 1977, 8(11): 785 − 789. [11] MENG F K, Li G P, ZHANG B B, et al. Chemical kinetics and particle size effects of activated carbon for free chlorine removal from drinking water[J]. Water Practice and Technology, 2018, 14: 19 − 26. [12] PERRARD A, RETAILLEAU L, BEERJOAN R, et al. Liquid phase oxidation kinetics of an ex-cellulose activated carbon cloth by NaOCl[J]. Carbon, 2012, 50(6): 2226 − 2234. doi: 10.1016/j.carbon.2012.01.039 [13] BAUER R C, SNOEYINK V L. Reactions of Chloramines with Active Carbon[J]. Journal Water Pollution Control Federation, 1973, 45(11): 2290 − 2301. [14] MARTIN R J, SHACKLETON R C. Comparison of two partially activated carbon fabrics for the removal of chlorine and other impurities from water[J]. Water Research, 1990, 24(2): 474 − 484. -




 下载:
下载: