-
从2004年“微塑料”被首次定义(小于5 mm的塑料颗粒)以来[1],有关微塑料的话题便逐步受到各国政府和学者的关注。微塑料污染普遍存在于淡水和海洋生态系统中,近年来研究人员在全球各个湖泊、河流、大洋、大洲、甚至极地地区都检测到了微塑料的存在[2-7]。据估计,每年约有800多万吨塑料残骸被排放到全球海洋环境中,这些塑料垃圾包括大块的塑料残骸以及直接排入环境的微塑料颗粒(比如化妆品中的塑料微珠)[8-9]。大塑料残骸作为微塑料的前驱体,在自然环境中经过长期的物理化学作用,也将逐渐形成微塑料颗粒。
微塑料被认为是水体环境污染物的重要组成部分[10],尽管其环境丰度与其它水体介质(如纤维素、溶解性有机质等)相比较小,但其对生物体可能产生的潜在风险却不容小觑。一则,微塑料粒径较小而易被生物摄入并在生物体内累积,对生物造成机械损伤或影响其生长发育,甚至造成死亡[11-14]。二则,微塑料可以吸附环境中的污染物质(特别是疏水性有机污染物)或自身携带添加剂,这些污染物在脱附作用下,会在生物体内累积、迁移和转化,并对生物产生毒性效应[15-18]。因此,研究微塑料与有机污染物的相互作用的强度和机理,对全面评估二者的环境风险,深入探索微塑料致毒机制十分重要。
由于微塑料本身的聚合物类型、粒径、比表面积和老化等情况复杂多样,水体环境中的有机污染物也具有不同的理化性质,这就导致两者之间的吸附作用机理复杂,并可能受到多种环境因素的影响。本文将从微塑料的基本特性、吸附有机污染物的作用机理、环境因素的影响以及吸附行为对有机污染物生物有效性的影响等方面展开详细阐述,并提出有机污染物在微塑料上吸附研究的未来发展方向。
-
塑料是典型的有机合成高分子聚合物,具有较高的疏水性能。由于生产的原材料不同,它们的密度多变(0.9—2.3 g·cm−3),而其中加入的各式添加剂又使之呈现出不同颜色和功能,此外环境的破碎、老化等作用还导致其形状各异[4]。纳米塑料和微塑料颗粒除了具有普通塑料制品的上述特性外,由于它们的粒径更小,比表面积更大,有效吸附位点更多,因而吸附有机污染物的能力更强。例如,Mattsson等[19]计算发现,一个普通的塑料袋(0.2 m2)完全分解成平均直径为40 nm的塑料颗粒后,其比表面积增大13000倍至2600 m2。通常粒径越小的塑料颗粒对有机污染物的吸附能力越强,在Wang等[20]的研究中比较了PE、PS、PVC等3种微塑料的比表面积,比表面积的大小对于其吸附芘的贡献非常大。比表面积的大小是PE>PS>PVC,与之相对应的是对于芘的吸附能力也随着比表面积的增大而增大。但并不是所有情况下微塑料的吸附能力都会随着粒径的减小而增大,Wang等[21]的研究报道了更小的尺寸,特别是纳米尺度时粒子的聚集性能增强,反而降低了粒子的比表面积和吸附能力,例如他们在研究中发现纳米级的PS对菲的吸附量明显低于微米级的,这可能就是由于纳米塑料的团聚,导致了有效吸附位点的减少。
塑料性质会影响其对有机污染物的吸附能力。表1列举了几种常见的影响吸附能力的理化特性。(1)接触角:这是衡量液体在塑料表面润湿性能的重要参数。通常接触角越小,塑料的表面越亲水,对有机污染物的吸附能力越弱;反之,接触角越大,塑料表面越疏水,对有机污染物的吸附能力越强。(2)结晶度:该指标可以提示聚合物中结晶区域所占的比例。通常微塑料的结晶度越高,分子排列越规则,可用于发生吸附反应的自由体积和有效位点就越少,吸附能力越低。而在无定形区,分子链无序缠绕形成大量的孔,该区域是吸附污染物的有效区域[22]。(3)玻璃化转变温度:此数值是指塑料无定形区从玻璃态转变为高弹态所对应的温度。在此温度以上塑料处于高弹态,此时塑料中的孔隙更多,可以对有机污染物进行孔隙截留,能有效吸附有机污染物;在此温度以下塑料的状态为玻璃态,分子受到外界束缚,排列齐整、流动性较低,对有机污染物的吸附能力较弱[23]。(4)原子比指标:例如((N+O)/C)原子比可以反映塑料的极性高低,而(H/C)原子比可以反映芳香性的强弱。这两个指标会随着塑料在环境中暴露时间的延长,发生老化和裂解等作用后,发生改变[24-25]。另外,破碎、降解、风化等作用可使微塑料表面积增大。上述(微)塑料的特性都与其对有机污染物的吸附能力密切相关。通过对这些基本特性的认知,也有助于理解和探索有机污染物在微塑料上的吸附行为及作用机理。
-
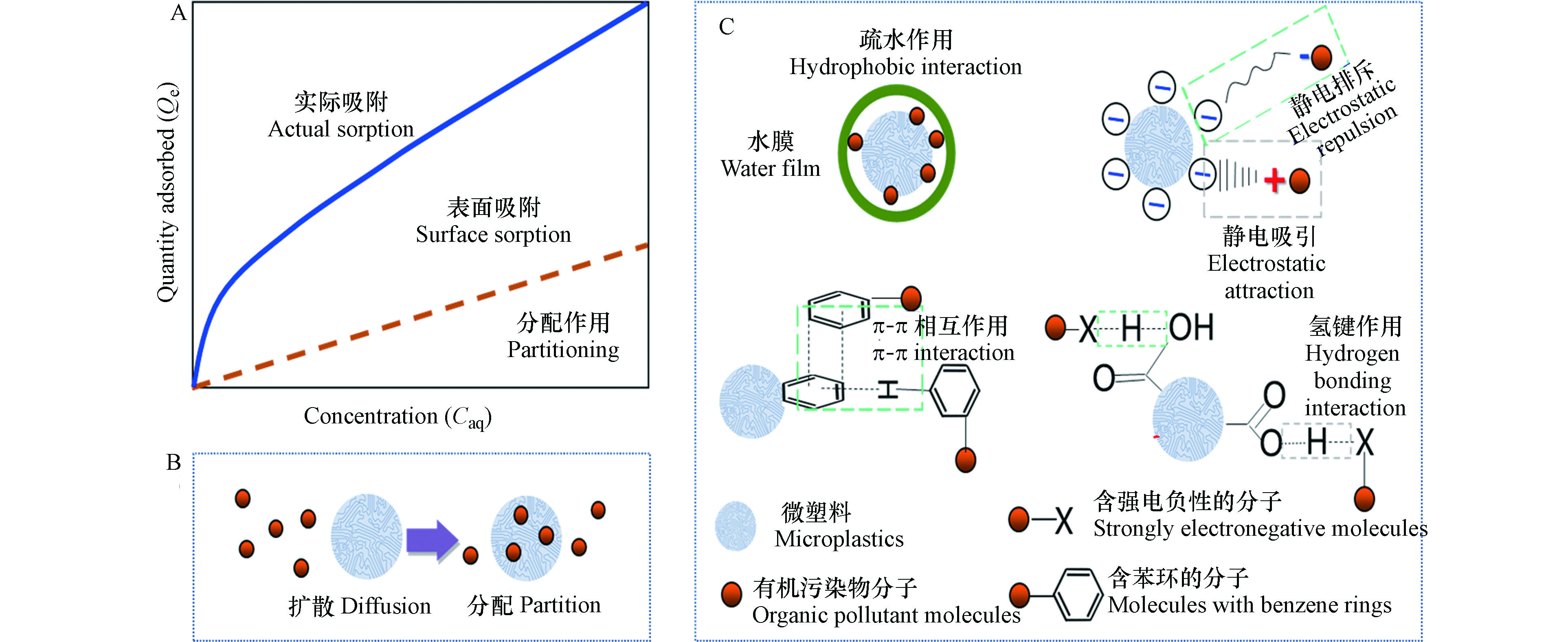
大量的研究发现,环境中的微塑料上往往负载着各类有机污染物,例如多环芳烃(PAHs)、多氯联苯(PCBs)、多溴联苯醚(PBDEs) 、内分泌干扰物(EDCs)、药品和个人护理用品(PPCPs)等[30-37]。这些有机污染物既包括塑料生产和使用过程中使用的添加剂或残留的中间体和降解产物,也包括从周围环境迁移进入微塑料的污染物。有机污染物在微塑料上的吸附往往受到分配作用、表面吸附、交互作用和其他微观机制的共同驱动,其中分配作用和表面吸附是两种最主要的作用机制(图1)。
-
分配作用是有机物在亲水相和疏水相之间的一种分配过程[38]。研究者认为分配作用是有机污染物根据“相似相溶”的原理在亲水相和疏水相之间达到一种平衡状态[39], 该过程主要取决于固体颗粒中疏水相的含量, 而与颗粒表面的吸附位点数量无关[36,40]。在微塑料-水体系中,分配作用主要指非离子型的有机污染物可通过溶解作用分配到微塑料中,并经过一定时间达到分配平衡,其吸附等温线为直线。而有机污染物在水相与微塑料相之间的平衡分配系数
$ \left({K}_{\mathrm{d}}\right) $ ,则是评价微塑料对有机污染物吸附能力的重要参数[39]。线性分配模型,即
$ {Q}_{\mathrm{e}}={K}_{\mathrm{d}}\times {C}_{\mathrm{e}} $ 常用于拟合有机污染物在微塑料上的分配吸附[41]。其中$ {Q}_{\mathrm{e}} $ 为有机污染物在微塑料上的平衡吸附量,$ {C}_{\mathrm{e}} $ 为平衡时有机污染物的水相浓度。分配作用的强弱,受到多方面因素的影响。当微塑料颗粒与有机污染物的极性匹配程度较高时,主要发生分配吸附[42]。当微塑料中的无定形区比例较高,或水相有机污染物的浓度较低时,也以非竞争性的分配作用为主。此外,有机污染物在微塑料上的分配吸附也与微塑料的芳香性和极性紧密相关。
$ {K}_{\mathrm{d}} $ 与(N+O)/C原子比往往呈负相关[39]。当塑料聚合物的(N+O)/C值越小,则其极性越弱,更易吸附非极性的有机污染物,因为非极性的有机污染物结合到塑料聚合物上时更易吸附和扩散到微塑料的基体中[3,11]。例如, PE、PP和PS的(N+O)/C原子比都为0,大多数疏水性有机污染物在这几种微塑料上都有较高的分配吸附量。$ {K}_{\mathrm{d}} $ 与H/C通常呈正相关[39]。H/C值越大,微塑料的芳香性越弱,有机污染物在其上的分配作用越强烈[43]。微塑料的老化、裂解往往伴随着脱氢和脱氧反应,这将导致碳元素含量增加,从而使微塑料的芳香性和非极性都增强,改变其对有机污染物的分配吸附强度[44]。这是由于芳香碳不利于有机污染物的有效“溶解”,高度芳香性的微塑料由于体系能量低、性质稳定,表面不易发生加成和氧化反应,从而降低分配吸附作用[45]。例如PE和PP的H/C数值均为2,远大于其他塑料的H/C比值,它们对17β-雌二醇(E2)也的确有更高的吸附量,说明低芳香性的微塑料可以更好地“溶解”并分配吸附有机污染物[26]。 -
表面吸附作用指的是固体表面有吸附水相中溶解性有机污染物或胶体物质的能力[46-48]。通常,比表面积大的微塑料往往具有更强的吸附能力。对于表面吸附,Langmuir等温线常用于描述有限吸附位点和吸附单层有机污染物分子的情况[49];而Freundlich经验公式,更适合描述非均匀固相表面对有机污染物的吸附[50]。
式中,
$ {Q}_{\mathrm{e}} $ 为微塑料上的平衡吸附量;$ {C}_{\mathrm{e}} $ 为水相中有机污染物的浓度;$ {Q}_{0} $ 是在平衡状态下微塑料上有机污染物的浓度;b是与低浓度条件下结合强度和等温线斜率有关的常数。其中,n为表面非均匀性因子;
$ {K}_{\mathrm{F}} $ 为Freundlich常数;$ {C}_{\mathrm{e}} $ 为水相中有机污染物的浓度。表面吸附可分为物理吸附和化学吸附。如果吸附剂与吸附质之间是通过分子间相互作用力产生吸附,称为物理吸附;如果吸附剂与吸附质之间产生化学作用,生成化学键而引起吸附,则称为化学吸附。环境有机污染物分子在微塑料上的表面吸附以物理吸附为主,其中疏水作用和静电相互作用是主要的作用机制[30,47,50-52]。微塑料的疏水性越强,其对有机污染物的疏水作用也越强。例如,Liu等[26]发现E2在PE、PP、PS、PET、PC、PMMA以及PVC上的吸附分配系数与微塑料的表面疏水性呈现出显著的正相关关系。静电相互作用是由于带相反电荷的分子相互吸引或带相同电荷的分子相互排斥而引起的,是有机污染物与微塑料表面含氧官能团之间的一种弱相互作用。通常,微塑料表面的零电荷点(pHpzc)值低于环境pH值而带负电[36,52-53],与带正电荷的吸附质发生静电吸引的相互作用[39]。
此外,氢键和π-π相互作用也是影响微塑料吸附有机污染物的重要作用力。氢键是一种特殊的分子间作用力[54-55],它也会显著影响微塑料对有机污染物的吸附强度[56]。π-π相互作用是一种芳香环之间的弱相互作用,当芳香环以面对面和面对边的构象存在时易于发生[57]。Hüffer等[58]发现由于π-π相互作用的存在,PS对芳香族化合物的平衡吸附量比PE、PA和PVC高出了1个数量级。
化学吸附会伴随化学键的形成或强烈的分子间相互作用的发生,较少见于微塑料对有机污染物的吸附。但在某些条件下,例如有机污染物在碱性环境中发生去质子化,与表面带有羧基官能团(—COOH)的物质也可形成化学键[59]。 因此将微塑料酸化处理后,可能有利于其对有机污染物的表面吸附能力。一方面,是由于表面酸性含氧官能团(羧基、羟基等)增加,可增进微塑料与极性有机物之间发生离子交换,形成化学键从而增强吸附能力;另一方面,酸化增强了溶液对微塑料的“溶解”,暴露出更多的有效吸附位点,也可促进表面吸附[59]。
总体而言,微塑料的疏水性、表面电负性,以及环境中溶液的酸碱性都可以影响微塑料与有机污染物的表面相互作用。此外,微塑料的含氧官能团和有机污染物的极性强弱等也会影响微塑料对有机污染物的表面吸附能力。
-
微塑料对有机污染物的吸附作用机理因其自身理化性质、有机物特性和吸附条件等的差异而不同[60],吸附是微塑料和吸附质的各种性质共同作用的结果[36]。很多研究发现仅采用单一的分配作用、表面吸附或孔隙截留作用来描绘微塑料对有机污染物的吸附现象,存在一定的局限性。在实际吸附过程中,通常以某种吸附作用为主,多种吸附作用共存。特别是分配和表面吸附作用一般会同时发生,很难将两个过程区分开[46,61]。比如,Weber等[62]提出了多元反应模型,认为大多数物质都是高度不均匀的吸附剂,对有机污染物的宏观吸附是由一系列线性的和非线性的微观吸附过程组合而成,线性部分的吸附服从分配机理,而非线性部分则与表面吸附有关[63]。通常表面吸附在微塑料对较低浓度的有机污染物的吸附中起主要作用;而当有机污染物的浓度较高时,由于微塑料表面吸附的位点有限,分配作用将占据主导地位[36,48]。
-
微塑料对有机污染物的吸附作用受到多种吸附机理的共同影响,同时也随着环境因素的变化而改变。本节就不同环境因素对微塑料吸附有机污染物的行为影响展开具体讨论(表2)。
-
pH是极其重要的环境参数,它对吸附过程的影响主要体现在对微塑料表面电荷及有机污染物存在形式的影响,究竟发生静电引力还是静电斥力,取决于环境介质的pH值、塑料的pHz值(Zeta电位)和有机污染物的pKa值(酸性解离常数)[57]。一般情况下,当pH<pHz时,微塑料表面带正电;当pH>pHz时,微塑料表面带负电。当pH=pKa时,有机污染物以分子态存在;当pH<pKa时,有机污染物带正电;当pH>pKa时,有机污染物带负电。因此,究竟发生静电引力还是静电斥力作用,取决于介质的pH值、聚合物塑料的pHpzc(零电荷点)和有机污染物的pKa等因素的综合效应[57]。
Wang等[51]研究了pH对全氟辛烷磺酸(PFOS)在PE、PS 和 PVC上的吸附行为的影响,结果表明在3.0—7.0的pH值范围内PFOS与微塑料之间以静电斥力为主。这是由于PFOS的pKa<3,微塑料和PFOS在此pH范围内均带负电,随着 pH 的增加,静电斥力增强而导致两者之间的吸附作用逐渐降低。类似地,有研究发现当pH值从3.0逐渐增加到7.0时,PS和PVC对泰乐菌素(TYL)的吸附量也逐渐减小[50]。这是因为当pH<7.1时,TYL(pKa=7.1)带正电,微塑料带负电,TYL与微塑料之间产生静电吸引。随着溶液pH的上升,TYL+在溶液中的比例逐渐减小,导致吸附量逐渐下降。
-
温度的变化可以改变微塑料的晶体结构组成,也可以改变有机污染物的溶解度和流动性,从而影响两者之间的分子间相互作用力。一般来说,当温度较低时,微塑料对有机污染物的吸附能力较弱;处于常见环境温度时,吸附能力通常随着温度的升高而增强;但当温度过高时,吸附能力又会随温度的升高而减弱。Dong等[11]通过研究发现PE和PVC微塑料对4种麝香的吸附能力随着温度变化有明显改变:当温度较低时(<15 ℃),温度的升高可增加麝香在溶液中的流动性和溶解度,导致麝香的吸附量下降。当温度处于环境温度(15—25 ℃)范围时,微塑料对麝香的吸附量随温度的升高而增加,这是由于吸附是一个吸热过程,温度的升高可增加系统的熵值,从而促进吸附。当温度较高(>25 ℃)时,吸附量会再次随着温度的升高而减小,这是由于高温有利于解吸的发生,而导致净吸附量下降。类似地,Zhang等[64]关于PP微塑料对合成麝香的吸附行为的研究也证实了上述观点:在15—25 ℃内,由于吸附过程吸热使系统熵增加,所以随着温度的升高微塑料对麝香吸附量逐渐增加;当温度大于25 ℃时,由于解吸加剧而导致吸附量下降。但也有例外,Li等[65]发现在15—45 ℃区间,三氯生(TCS)在PS微塑料上的平衡分配系数与温度之间并无显著关系。因此在考察温度对吸附作用的影响时,需要细致分析不同温度范围,同时关注微塑料和有机污染物各自的理化性质。
-
微塑料对有机污染物的吸附也受到盐度因子的影响,盐度可以改变吸附过程中静电相互作用强弱以及离子交换的程度。盐度升高,在多数情况下对有机污染物在微塑料上的吸附有促进作用。此类现象称为盐析效应,即水中无机盐浓度的增加会降低有机污染物的溶解度,从而增强其在微塑料表面的吸附[51,66-67]。例如,PE和PVC微塑料对麝香的吸附量随盐度的增大而增大,当盐度为14%时吸附量达到最大值[11]。同样地,微塑料对E2和双酚A等有机污染物的吸附作用,也由于盐度的增加导致这些有机污染物在溶液中的溶解度下降,而在微塑料上的吸附量上升[26, 55]。但也有例外,比如高盐度会抑制微塑料对磺胺甲噁唑(SMX)和DDT的吸附[68-69],其主要原因可能为Na+浓度随盐度的增加而增加,带正电荷的Na+容易通过静电吸引作用吸附在表面带负电的微塑料上,吸附能力的降低可能是由于无机可交换阳离子Na+可以取代酸性基团的氢离子,从而抑制氢键的形成。这种阳离子交换打破了电荷平衡,中和了吸附剂表面电荷,阻碍了静电相互作用,使得吸附量下降[36, 70]。总体而言,盐度对有机污染物在微塑料上的吸附作用的影响程度有限,其影响可能随污染物种类改变而改变,并且受多种吸附机制驱动[65,71-72]。
-
溶解性有机质(DOM)广泛存在于天然水体中,腐殖酸是其中的典型代表。腐殖酸是一种多相大分子,具有许多与有机污染物结合的位点,因此可以直接影响有机污染物在微塑料上的吸附行为[55]。多项研究表明在腐殖酸存在的情况下,微塑料对E2[26]、四环素[52]、PBDEs[72]等有机污染物的吸附能力下降,其主要原因可能是由于有机污染物和腐殖酸之间形成了复合物,从而促进有机小分子的溶解,导致有机污染物在微塑料上吸附量的下降[55]。但也存在不同现象和观点,例如Zhang等[53]研究结果显示腐殖酸可以促进微塑料对土霉素的吸附,其原因主要是由于微塑料和土霉素分子间形成了π-π共轭而增强了表面吸附作用。值得注意的是,腐殖质的降解程度也会影响微塑料对有机污染物的吸附能力。在降解程度较低的腐殖质形态中,持久性有机污染物在微塑料上的吸附量比在降解程度较高的腐殖质中更高,即腐殖质降解程度越低越有利于吸附[73]。此外,天然水体环境中还存在着广泛的食源性颗粒,这些食源性颗粒中的有机质含量通常较高(蛋白质+脂质≥50.0%)。因此在自然环境中,食源性颗粒与微塑料对有机污染物的竞争也是不可避免的[74]。有机污染物的亲脂性与其在微塑料或食源性颗粒上的吸附呈正相关[75],因此在实际环境中食源性颗粒的存在,也可在一定程度上竞争抑制有机污染物在微塑料上的吸附。
-
本文中老化的概念主要指微塑料在环境中,随着停留时间的增加而导致的自然氧化行为和紫外线辐射所导致的紫外老化行为。老化会导致微塑料的理化性质相对于原始颗粒发生改变,因此老化后的微塑料对有机污染物的吸附能力也将发生变化。
随着老化程度的增加,微塑料的表面逐渐产生裂纹,尺寸不断减小,使其比表面积不断增大。并且老化常导致微塑料表面的含氧基团增加,从而对某些有机污染物产生更强的吸附能力。Hüffer和Hofmann[58]认为老化会降低塑料的疏水性,从而增加了它们对亲水污染物的吸附能力。例如,研究表明紫外线加速老化后的PS和PVC微塑料相比原始颗粒,对环丙沙星具有更高的吸附能力[76]。这主要是因为老化作用在塑料表面引入了许多含氧官能团(如羧基、羟基、醛基、酮基等)[77],导致塑料表面的亲水性、极性和表面电荷增加,从而增强了其对极性较强的有机污染物的吸附能力。
对于疏水性有机污染物(HOC)而言,塑料老化的影响是双重的。老化既可以导致微塑料比表面积增加,从而促进微塑料对HOC的吸附;老化也可以引入含氧官能团,减少HOC在微塑料表面的吸附。例如,Müller和Hüffer等研究者发现老化后的PS微塑料对PAHs的吸附量下降,其原因就是老化使得微塑料表面引入许多含氧官能团,形成的氧化膜抑制了微塑料对PAHs的吸附[78-79]。此外,环境老化过程会破坏微塑料的无定形区,改变聚合物的分子链结构,使得微塑料的结晶度增加,从而使微塑料对疏水性有机污染物的吸附能力降低。但也有研究者发现了不同的现象,例如Endo等[80]认为老化对微塑料吸附 PCBs的能力无显著影响。因此,老化对微塑料吸附能力的最终影响,取决于哪方面因素占主导,要具体分析微塑料在不同条件下的环境行为和老化过程[81]。
-
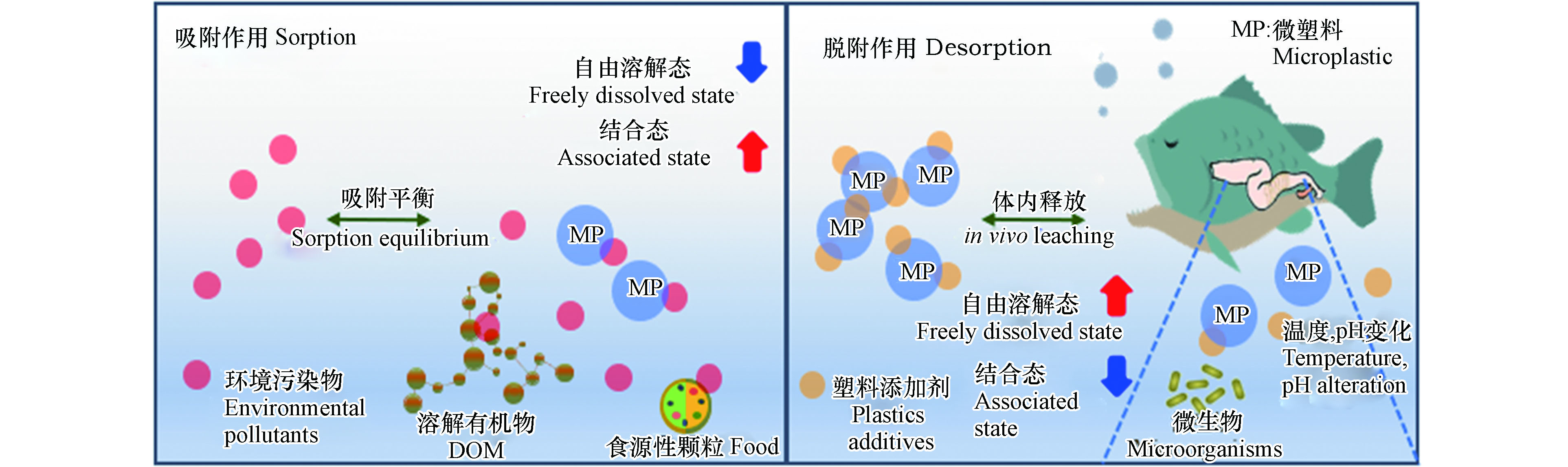
生物有效性可以用来表征污染物被生物吸收利用的程度及潜在的毒性,评价生物有效性最直接的方法是采用生物富集或毒性测试实验[84]。微塑料对有机污染物生物有效性的影响主要体现在对其浓度的影响上,图2描绘了微塑料通过对有机污染物的吸附和释放,改变有机污染物的自由溶解态浓度和生物有效性的示意图。
在自然环境中,微塑料可以通过分配和表面吸附等作用机制吸附周围环境中的各类有机污染物,降低局部环境中的有机污染物的水相自由溶解态浓度,从而改变这些有机污染物对水生生物的生物有效性[85]。例如,Yang等[86]的研究发现在微塑料和壬基酚对蛋白核小球藻的复合毒性实验中,由于微塑料对壬基酚具有较强的吸附能力,两者共存时壬基酚的自由溶解态浓度显著下降,因而两者对小球藻的联合毒性呈现拮抗作用。Zhu等[87]研究同样发现了在与PE、PS或PVC微塑料共存时,TCS对微藻的毒性明显下降。其主要原因是微塑料对TCS有较强的吸附能力,使得TCS的自由溶解态浓度显著下降所致。此外,环境中的其他介质,例如DOM和食源性颗粒也会与有机污染物发生结合和吸附作用,来影响有机污染物对水生生物的有效性。
微塑料从环境中吸附有机污染物的同时,也会在某些条件下向环境再释放出这些污染物,亦或是向周边环境释放出塑料自身携带的添加剂成分。任何不利于吸附的条件,例如减压、加热、改变pH,以及生物体的消化作用等,都有利于有机污染物从微塑料上脱附下来[88-89]。
脱附作用一方面会导致水体环境中有机污染物的自由溶解态浓度增加,增加水生生物的暴露风险;另一方面当负载有机污染物的微塑料经由摄食作用进入生物体消化道后, 在消化道特殊的pH和温度等条件下,被吸附的有机污染物更易从微塑料上释放出来,这些释放的有机污染物将被生物体吸收、累积、转化和迁移,产生一系列的生态毒理学效应[90]。例如,Chen等[91]在研究中发现,当塑料暴露于鱼类模拟胃液中时,其上的各类添加剂组分会大量释放出来,造成了日本青鳉幼鱼孵化率下降等急性毒性,并产生了内分泌干扰效应。Coffin等[92]也发现,经海鸟和鱼肠道模拟消化液处理后的微塑料,能释放出大量的雌激素类污染物质,双酚A和邻苯二甲酸酯类物质显著高于对照组。
-
负载有机污染物是微塑料的重要特性,微塑料对有机污染物有较强的吸附作用,其吸附机理以分配作用和表面吸附为主,并受到多种其他机制的共同调控。在真实环境水体中,水化学因子pH、温度、盐度、DOM和老化等也会在一定程度上改变微塑料对有机污染物吸附的强弱。最后,有机污染物在微塑料上的吸附和脱附,都将改变其水相或生物体内的溶解态浓度,从而降低或增强其生物有效性和毒性。特别值得引起我们注意的是,由于塑料制品在生产过程中往往会添加大量的阻燃剂、塑化剂、颜料等助剂[93],因此环境微塑料上负载的添加剂浓度往往没有和周围环境达到分配平衡,存在逸度扩散梯度。一旦这些微塑料被生物体摄入,加之消化道的脱附促进作用,微塑料上负载的添加剂的生物有效性将增强,微塑料像“源”一般向生物体释放有机污染物。
在今后的研究中,以下几方面值得展开进一步深入的探索。(1)微塑料吸附有机污染物的机理研究多数还处于定性描述阶段,阐明微塑料吸附过程中不同作用机制对吸附的贡献率,以及微塑料的结构或表面化学基团对吸附贡献的构效关系将是未来的一个研究重点。(2)由于不同微塑料的性质迥异,它们对有机污染物的吸附能力与特点也不尽相同,在今后的研究中应当进一步细致分析各环境因子对微塑料的吸附能力的影响,为环境特征微塑料对有机污染物的吸附行为提供更多的理论支撑。(3)塑料残骸及微塑料中添加剂释放的风险需要得到进一步的重视。由于添加剂的浓度往往高于环境本底值,因此当塑料添加剂成分随着微塑料的迁移而逐渐释放出来,会增加局部环境中污染物的自由溶解态浓度,并可能引发一系列的生态毒理学效应。
微塑料吸附有机污染物的研究进展
Research progress on sorption of organic pollutants by microplastics
-
摘要: 微塑料作为一种新型环境污染物在全球环境介质中普遍存在,其存在可能会影响传统有机污染物的分布、迁移和环境归趋。微塑料本身具有强疏水特性和较大的比表面积,使其能够有效地吸附有机污染物并将其输送到生物体内,从而改变微塑料潜在的环境风险。微塑料与有机污染物之间的相互作用机制主要受二者自身的理化性质,及溶液pH、温度、盐度、溶解性有机质和老化作用等环境因素的影响。本文从微塑料的基本特性、与有机污染物的作用机制、环境影响因素,以及二者复合对有机污染物生物有效性的影响等方面进行了综述,并提出微塑料与有机污染物相互作用研究中亟需解决的问题和未来的研究方向。Abstract: As a new type of environmental pollutant, microplastics are ubiquitous in global environmental media. Their presence can change the distribution, transportation and environmental fate of traditional organic pollutants. Microplastics have strong hydrophobic properties and large specific surface areas, which enables them to effectively sorb organic pollutants and later transport into organisms, thereby altering the potential environmental risks of both microplastics and the associated organic pollutants. The interaction mechanisms between microplastics and organic pollutants are mainly affected by their physical and chemical properties, as well as environmental influential factors such as pH, temperature, salinity, dissolved organic matter, and aging time. This paper reviews the basic characteristics of microplastics, the interaction mechanisms between microplastics and organic pollutants, effects of environmental influential factors, and the bioavailability of organic pollutants with the presence of microplastics, and finally puts forward the issues related to the interactions between microplastics and organic pollutants that needed for future studies.
-
Key words:
- microplastics /
- sorption /
- organic pollutants /
- interaction mechanism
-

-
表 1 几种常见塑料的理化特性
Table 1. The physical and chemical properties of several common plastics
塑料类型
Plastic type密度/ (g·cm−3)
Density接触角/(°)
Contact angle结晶度/%
Crystallinity玻璃化转变温度/℃
Glass transition temperature(N+O)/C
原子比
Atomic ratioH/C
原子比
Atomic ratio低密度聚乙烯
(LDPE)0.91—0.93 95.6±3.2[26] 55—65 −125 — 2.0 高密度聚乙烯(HDPE) 0.92—0.97 85.8±3.8[26] 80—95 −125 — 2.0 聚丙烯(PP) 0.90—0.91 106.3±2.3[26] 38.4 −10 — 2.0 聚苯乙烯(PS) 1.04—1.07 95.7±1.4[26] 3.7 100 0.02 1.0 聚碳酸酯(PC) 1.20 74.4±2.0[26] 16.2 145—150 0.13 1.1 聚酰胺(PA) 1.04—1.14 70.9±6.6[26] 30—40 55 0.33 1.8 聚氯乙烯(PVC) 1.35—1.45 73.32[27] 5—10 75—85 — 1.50 聚甲基丙烯酸甲酯(PMMA) 1.17—1.20 80.1±1.9[26] 6.1 104 0.40 1.60 聚对苯二甲酸乙二醇酯(PET) 1.37—1.38 74.03[27] 0.5 80—120 0.40 0.80 聚氨酯(PU) 1.045 96±2.2[28] — −17.1 0.384 2.01 聚丁二酸丁二醇酯(PBS) 1.26 119.4[29] 30—45 −33.8 0.444 1.75 注:表中数据来自PubChem数据库、摩贝化学平台、《高分子材料概论》及标注的文献.
Note: The data in the table are taken from PubChem database, Mobei chemistry platform, Introduction to Polymer Materials Book, and annotated literature.表 2 不同环境因子对有机污染物在微塑料上的吸附的影响及作用机理
Table 2. The effects and mechanisms of different environmental factors on the sorption of organic pollutants on microplastics
环境
因子
Environmental factors微塑料
Microplastics有机污染物
Organic pollutants主要吸附
机理
Main sorption mechanism影响结果
Results类型(粒径)
Type( Particle size)类型(浓度)
Type(Concentration)pH PE (150 μm)
PS (250 µm)
PVC (230 μm)PFOS、FOSA
(5—50 µg·L−1)
分配作用pH:3.0—7.0,PFOS的吸附量随pH下降逐渐上升,对FOSA无影响[51] PE (150 μm) SMX(0.2—5 mg·L−1) 分配作用
范德华力pH:2.0—12.0,pH对吸附无明显
影响[71]PE (150 μm)
PP、PS (<280 mm)TC(0.2—5 mg·L−1) 静电作用 pH:2.0—12.0,吸附量先上升后下降,6.0时达到峰值[52] PE、PP、PS、PVC
(<75 μm)TYL (1—30 mg·L−1) 静电作用
疏水作用pH:3.0—7.0,吸附量随pH升高逐渐下降[50] PS (2、10、100 μm) HEX、MYC、TRI
(100 µg·L−1)静电作用
疏水作用pH:3.0—11.0,吸附量随pH升高逐渐上升[70] PE、PP、PS、PC、PMMA、PA、PVC
(>250 μm或<75 μm)E2 (500 ng·L−1—
500 µg·L−1)疏水作用 吸附量与微塑料的疏水性呈正相
关[26]PE、PS、PVC
(<75 μm)DEP、DBP
(0.4—10 mg·L−1)疏水作用 pH:5.5—8.5,pH对吸附无明显
影响[82]PE、PET、PS、PP、PA、PVC、PU、PMMA (NA) BPA (100 µg·L−1) 静电作用 pH:2.8—11.0,吸附量随pH升高逐渐上升[55] PE、PVC
(200—250 μm)DDT、PHE、DEHP
(0.6—6.1 µg·L−1)疏水作用 pH:7.5—8.4,吸附量随pH升高逐渐
上升[68]PE、PP、PS、PA
(4 mm)PBDEs
(0.2—1 µg·L−1)静电作用 pH:1.0—13.0,pH对吸附无明显
影响[72]PE、PP、PET、PS、PVC、PA
(100—150 μm)SMX (0—12 mg·L−1) 静电作用 pH:3.0—9.0,吸附量随pH升高逐渐下降[69] 温度 PE、PVC (≤0.15 mm) SM (1 mg·L−1) 疏水作用 温度:5—30 ℃,吸附量随温度的升高先下降后上升再下降[11] PS (75.4—214.6 μm) TCS (1—12 mg·L−1) 疏水作用
静电作用温度:15—45 ℃,温度对吸附无明显影响影响[65] PE、PVC (200—250 μm) DDT、PHE、DEHP
(0.6—6.1 µg·L−1)疏水作用 温度:18—38 ℃,高温条件抑制吸附[68] PP (0.45—0.85 mm) SM (5 µg·L−1) 范德华力 温度:5—30 ℃,高温条件抑制吸
附[64]PP (0.18—5.0 mm) PCB (0.2 mg·L−1) 离子交换 温度:4—32 ℃,高温条件抑制吸
附[41]PP (0.45—0.85 mm) TBC (10 µg·L−1)
HBCDs (2 µg·L−1)离子交换 温度:5—45 ℃,随温度升高,吸附量先上升后下降[83] 盐度 PE、PP、PS、PA、PVC
(75—180 µm)SDZ、AMX、TC、CIP、TMP
(0.5—15 mg·L−1)静电作用
疏水作用
范德华力
氢键高离子强度抑制吸附[36] PE (150 μm)、
PP、PS (<280 mm)TC (0.2—5.0 mg·L−1) 静电作用 盐度:0.05%—3.5%,盐度对吸附无明显影响[52] PS (2、10、100 μm) HEX、MYC、TRI
(100 µg·L−1)静电作用
疏水作用盐度:0—50 mmol·L−1,高盐度促进吸附[70] PE、PP、PS、PVC
(<75 μm)TYL (1—30 mg·L−1) 静电作用
疏水作用盐度:0—0.1 mol·L−1,随着盐度升高,吸附量先上升后下降[50] PS (75.4—214.6 μm) TCS (1—12 mg·L−1) 静电作用
疏水作用盐度:0.001 mol·L−1—0.1 mol·L−1,盐度对吸附无明显影响[65] 盐度 PE、PVC (≤0.15 mm) SM (1 mg·L−1) 疏水作用 盐度:1%—21%,随着盐度升高,吸附量先上升后下降再上升[11] PE、PP、PS、PC、PMMA、
PA、PVC (>250 μm、<75 μm)E2 (500 ng·L−1—500 µg·L−1) 疏水作用 盐度:0.05%—3.5%,高盐度促进吸附[26] PE、PS、PVC
(<75 μm)DEP、DBP
(0.4—10 mg·L−1)疏水作用 盐度:0—0.3 mol·L−1,高盐度促进吸附[82] PE (150 μm)、
PS (250 μm)、
PVC (230 μm)PFOS、FOSA
(5—50 µg·L−1)疏水作用 盐度:0—1 mol·L−1,高盐度促进对PFOS的吸附,对FOSA无影响[51] PP (0.45—
0.85 mm)SM (5 µg·L−1) 离子交换 盐度:1%—21%,盐度对吸附无明显影响[64] PE、PP、PS、PA
(4 mm)PBDEs
(0.2—1 µg·L−1)静电作用 盐度:0—3.5%,盐度对吸附无明显影响[72] PEc (2.0—3.3 mm)、PEv
(400 µm)、PS (250 μm)、
PBAT (1.9—2.8 μm)PHE(178.4—
623.7 µg·L−1)疏水作用 盐度:0—35‰,高盐度促进吸
附[67]PE (150 μm) SMX (0.2—5 mg·L−1) 分配作用
范德华力盐度:0.05%—3.5%,盐度对吸附无明显影响[71] PE、PET、PS、PP、PA、PVC、PU、PMMA (NA) BPA (10 µg·L−1) 氢键 盐度:12‰—35‰,高盐度促进吸附[55] PE、PP、PET、PA、PET、PVC
(100—150 μm)SMX (0—12 mg·L−1) 静电作用 盐度:0—35‰,高盐度抑制吸
附[69]PP (0.45—0.85 mm) TBC (10 µg·L−1)
HBCDs (2 µg·L−1)离子交换 盐度:0—24.5%,随盐度升高,吸附量先上升后下降[83] 溶解性有机质(DOM) PE、PP、PS、PC、PMMA、PA、PVC
(>250 μm或<75 μm)E2 (500 ng·L−1—500 µg·L−1) 疏水作用 高DOM含量抑制吸附[26] PE (150 μm)、
PP、PS (<280 mm)TC (0.2—5 mg·L−1) 疏水作用
静电作用DOM抑制吸附[52] PE、PS、PVC
(<75 μm)DEP、DBP
(0.4—10 mg·L−1)疏水作用 DOM对吸附无显著影响[82] PEc (2.0—3.3 mm)、PEv
(400 μm)、PS (250 μm)、
PBAT (1.9—2.8 μm)PHE(178.4—
623.7 µg·L−1)疏水作用 高DOM含量抑制吸附[67] PE (150 μm) SMX(0.2—5 mg·L−1) 分配作用
范德华力DOM对吸附无显著影响[71] PE、PET、PS、PP、PA、PVC、PU、PMMA (NA) BPA
(100—200 µg·L−1)静电作用 BPA/DOM复合物的形成促进BPA的溶解,吸附能力降低[55] PE、PP、PS、PA (4 mm) PBDEs(0.2—1 µg·L−1) 静电作用 DOM对吸附有轻微抑制[72] PE (250—280 μm) EE2、TCS(100 µg·L−1) 疏水作用 DOM抑制吸附[66] 注:表格中的缩写:PE:聚乙烯;PP:聚丙烯;PS:聚苯乙烯; PVC:聚氯乙烯; PA:聚酰胺; PU:聚氨酯; PET:聚对苯二甲酸乙二醇酯; PMMA:聚甲基丙烯酸甲酯; PBAT:对苯二甲酸丁二酯; PEc:可降解聚乙烯; PEv:聚乙烯粉; DOM:溶解性有机质; TC:四环素; TYL:泰乐菌素; HEX:乙唑醇; MYC:腈菌唑; TRI:三唑醇; E2:17β-雌二醇; PFOS:全氟辛烷磺酸盐; FOSA:全氟辛烷磺酰胺; DEP:邻苯二甲酸二乙酯; DBP:邻苯二甲酸二丁酯; BPA:双酚A; DDT:有机氯农药; PHE:菲; DEHP:邻苯二甲酸酯; PBDEs:多溴联苯醚; SMX: 磺胺甲噁唑; SM:合成麝香; TCS:三氯生; PCB:多氯联苯; TBC:三(2,3-二溴丙基)异氰脲酸盐; HBCDs:六溴环十二烷; SDZ:磺胺嘧啶; AMX:阿莫西林; CIP:环丙沙星; TMP:甲氧苄啶; EE2:17α-乙炔雌二醇;NA:无法获得.
Note:Abbreviations in the table: PE: polyethylene; PP: polypropylene; PS: polystyrene; PVC: polyvinyl chloride; PA: polyamide; PU: polyurethane; PET: polyethylene terephthalate; PMMA: Polymethyl methacrylate; PBAT: Butylene terephthalate; PEc: degradable polyethylene; PEv: polyethylene powder; DOM: soluble organic matter; TC: tetracycline; TYL: tylosin; HEX: B Conazole; MYC: Myclobutanol; TRI: Triadimenol; E2: 17β-estradiol; PFOS: Perfluorooctane Sulfonate; FOSA: Perfluorooctane Sulfonamide; DEP: Diethyl Phthalate Esters; DBP: dibutyl phthalate; BPA: bisphenol A; DDT: organochlorine pesticides; PHE: phenanthrene; DEHP: phthalates; PBDEs: polybrominated diphenyl ethers; SMX: sulfamethoxazole; SM : Synthetic musk; TCS: triclosan; PCB: polychlorinated biphenyls; TBC: tris(2,3-dibromopropyl) isocyanurate; HBCDs: hexabromocyclododecane; SDZ: sulfadiazine; AMX: Amoxicillin; CIP: Ciprofloxacin; TMP: Trimethoprim; EE2: 17α-ethinyl estradiol; NA: not available. -
[1] RICHARD C T, YLVA O, RICHARD P M, et al. Lost at sea: Where is all the plastic? [J]. Science, 2004, 304: 838. doi: 10.1126/science.1094559 [2] YU Q, HU X J, YANG B, et al. Distribution, abundance and risks of microplastics in the environment [J]. Chemosphere, 2020, 249: 126059. doi: 10.1016/j.chemosphere.2020.126059 [3] AUTA H S, EMENIKE C U, FAUZIAH S H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions [J]. Environment International, 2017, 102: 165-176. doi: 10.1016/j.envint.2017.02.013 [4] CHEN Q Q, REISSER J, CUNSOLO S, et al. Pollutants in plastics within the North Pacific Subtropical Gyre [J]. Environmental Science & Technology, 2017, 52(2): 446-456. [5] LEBRETON L, SLAT B, FERRARI F F, et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic [J]. Scientific Reports, 2018, 8(1): 4666. doi: 10.1038/s41598-018-22939-w [6] BERGMANN M, SOPHIA M, PRIMPKE S, et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic [J]. Science Advances, 2019, 5: 1157. doi: 10.1126/sciadv.aax1157 [7] KOELMANS A A, NOR N H M, HERMSEN E, et al. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality [J]. Water Research, 2019, 155: 410-422. doi: 10.1016/j.watres.2019.02.054 [8] JAMBECK J R, GEYER R, WILCOX C, et al. Plastic waste inputs from land into the ocean [J]. Science, 2015, 347(6223): 768-771. doi: 10.1126/science.1260352 [9] ERNI-CASSOLA G, ZADJELOVIC V, GIBSON M I, et al. Distribution of plastic polymer types in the marine environment; A meta-analysis [J]. Journal of Hazardous Materials, 2019, 369: 691-698. doi: 10.1016/j.jhazmat.2019.02.067 [10] ARTHUR C, BAKER J, BAMFORD H, et al. Summary of the international research workshop on the occurrence, effects, and fate of microplastic marine debris [R]. Department of Commerce: National Oceanic and Atmospheric Administration. Technocal Memorandum NOS-OR&R-30. 2009. [11] DONG X F, ZHENG M G, QU L Y, et al. Sorption of tonalide, musk xylene, galaxolide, and musk ketone by microplastics of polyethylene and polyvinyl chloride [J]. Marine Pollution Bulletin, 2019, 144: 129-133. doi: 10.1016/j.marpolbul.2019.04.046 [12] WRIGHT S L, ROWE D, THOMPSON R C, et al. Microplastic ingestion decreases energy reserves in marine worms [J]. Current Biology, 2013, 23(23): R1031-R1033. doi: 10.1016/j.cub.2013.10.068 [13] BROWNE M A, NIVEN S J, GALLOWAY T S, et al. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity [J]. Current Biology, 2013, 23(23): 2388-2392. doi: 10.1016/j.cub.2013.10.012 [14] BIHANIC F L, CLÉRANDEAU C, CORMIER B, et al. Organic contaminants sorbed to microplastics affect marine medaka fish early life stages development [J]. Marine Pollution Bulletin, 2020, 154: 111059. doi: 10.1016/j.marpolbul.2020.111059 [15] PEREZ-LOBATO R, MUSTIELES V, CALVENTE I, et al. Exposure to bisphenol A and behavior in school-age children [J]. NeuroToxicology, 2016, 53: 12-19. doi: 10.1016/j.neuro.2015.12.001 [16] HAL N V D, YERUHAM E, SHUKIS D, et al. Uptake and incorporation of PCBs by eastern Mediterranean rabbitfish that consumed microplastics [J]. Marine Pollution Bulletin, 2020, 150: 110697. doi: 10.1016/j.marpolbul.2019.110697 [17] PAULINE P, JEROME C, CHRISTELLE C, et al. Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: Part Ⅰ-adverse effects on fish cell line [J]. Environmental Pollution, 2019, 248: 1088-1097. doi: 10.1016/j.envpol.2018.12.091 [18] XIA B, ZHANG J, ZHAO X G, et al. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri [J]. Environmental Pollution, 2020, 258: 113657. doi: 10.1016/j.envpol.2019.113657 [19] MATTSSON K, HANSSON L A, CEDERVALL T. Nano-plastics in the aquatic environment [J]. Environmental Science Processes & Impacts, 2015, 17: 1712-1721. [20] WANG W F, WANG J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics [J]. Chemosphere, 2018, 193: 567-573. doi: 10.1016/j.chemosphere.2017.11.078 [21] WANG J, LIU X H, LIU G N, et al. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene [J]. Ecotoxicology and Environmental Safety, 2019, 173: 331-338. doi: 10.1016/j.ecoenv.2019.02.037 [22] HALE S E, CORNELISSEN G, ARP H P H. Comment on "partition coefficients of organic contaminants with carbohydrates" [J]. Environmental Science & Technology, 2011, 45(3): 1158. [23] ROCHMAN C M, HOH E, HENTSCHEL B T, et al. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris [J]. Environmental Science & Technology, 2013, 47(3): 1646-1654. [24] TERZYK A P, GAUDEN P A, FURMANIAK S, et al. Molecular dynamics simulation insight into the mechanism of phenol adsorption at low coverages from aqueous solutions on microporous carbons [J]. Physical Chemistry Chemical Physics, 2010, 12(4): 812-817. doi: 10.1039/B919794J [25] ROCHMAN C M, MANZANO C, HENTSCHEL B T, et al. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment [J]. Environmental Science & Technology, 2013, 47(24): 13976-13984. [26] LIU X M, XU J, ZHAO Y P, et al. Hydrophobic sorption behaviors of 17β-Estradiol on environmental microplastics [J]. Chemosphere, 2019, 226: 726-735. doi: 10.1016/j.chemosphere.2019.03.162 [27] 彭盼盼, 杨建军, 吴庆云, 等. 硅基和氨酯基协同改性水性丙烯酸树脂的制备与性能 [J]. 精细化工, 2020, 37: 1710-1715. PENG P P, YANG J J, WU Q Y, et al. Preparation and properties of waterborne acrylic resin synergistic modification by silicon and polyurethane groups [J]. Fine Chemicals, 2020, 37: 1710-1715(in Chinese).
[28] RAZANAJATOVO R M, DING J N, ZHANG S S, et al. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics [J]. Marine Pollution Bulletin, 2018, 136: 516-523. doi: 10.1016/j.marpolbul.2018.09.048 [29] 王晖, 顾帼华, 邱冠周. 接触角法测量高分子材料的表面能 [J]. 中南大学学报, 2006, 37(5): 942-947. WANG H, GU G H, QIU G Z. Evaluation of surface free energy of polymers by contact angle goniometry [J]. Journal of Central South University, 2006, 37(5): 942-947(in Chinese).
[30] YUKIE M, TOMOHIKO I, HIDESHIGE T, et al. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment [J]. Environmental Science & Technology, 2001, 35(2): 318-324. [31] JENNY W, MOHAMMED B, TOMAS R, et al. Additives and other hazardous compounds in electronic products and their waste [M]. 2011. The Handbook of Environmental Chemistry, vol 18: Global Risk-Based Management of Chemical Additives I: Production, Usage and Environmental Occurrence. [32] ROCHMAN C M, HOH E, KUROBE T, et al. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress [J]. Scientific Reports, 2013, 3(1): 3263. doi: 10.1038/srep03263 [33] RIOS M L M, R J P. Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre [J]. Environmental Chemistry, 2015, 12(5): 611-617. doi: 10.1071/EN14236 [34] BRENNECKE D, DUARTE B, PAIVA F, et al. Microplastics as vector for heavy metal contamination from the marine environment [J]. Estuarine, Coastal and Shelf Science, 2016, 178: 189-195. doi: 10.1016/j.ecss.2015.12.003 [35] WANG W F, WANG J. Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: Microplastics in comparison to natural sediment [J]. Ecotoxicology and Environmental Safety, 2017, 147: 648-655. [36] ZHANG J, HUA K N. Adsorption of antibiotics on microplastics [J]. Environmental Pollution, 2018, 237: 460-467. doi: 10.1016/j.envpol.2018.02.050 [37] WANG Z, CHEN M L, ZHANG L W. Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China [J]. Science of the Total Environment, 2018, 628–629: 1617-1626. [38] CHIOU C T, FREED V H, SCHMEDDING D W, et al. Partition coefficient and bioaccumulation of selected organic chemicals [J]. Environmental Science & Technology, 1977, 11(5): 475-478. [39] 李晓娜, 宋洋, 贾明云, 等. 生物质炭对有机污染物的吸附及机理研究进展 [J]. 土壤学报, 2017, 54(6): 1313-1325. LI X N, SONG Y, JIA M Y, et al. A review of researches on biochar adsorbing organic contaminants and its mechanism [J]. Acta Pedologica Sinica, 2017, 54(6): 1313-1325(in Chinese).
[40] HWANG L, JOON S W, HWAN K J. Sorption capacity of plastic debris for hydrophobic organic chemicals [J]. Science of the Total Environment, 2014, 470-471: 1545-1552. doi: 10.1016/j.scitotenv.2013.08.023 [41] ZHAN Z W, WANG J D, PENG J P, et al. Sorption of 3,3',4,4'-tetrachlorobiphenyl by microplastics: A case study of polypropylene [J]. Marine Pollution Bulletin, 2016, 110(1): 559-563. doi: 10.1016/j.marpolbul.2016.05.036 [42] HUANG W H, CHEN B L. Interaction mechanisms of organic contaminants with burned straw ash charcoal [J]. Journal of Environmental Sciences, 2010, 22(10): 1586-1594. doi: 10.1016/S1001-0742(09)60293-X [43] ZHANG P, HUANG P, SUN H W, et al. The structure of agricultural microplastics (PT, PU and UF) and their sorption capacities for PAHs and PHE derivates under various salinity and oxidation treatments [J]. Environmental Pollution, 2020, 257: 113525. doi: 10.1016/j.envpol.2019.113525 [44] 王菲, 孙红文. 生物炭对极性与非极性有机污染物的吸附机理 [J]. 环境化学, 2016, 35(6): 1134-1141. doi: 10.7524/j.issn.0254-6108.2016.06.2015122404 WANG F, SUN H W. Sorption mechanisms of polar and apolar organic contaminants onto biochars [J]. Environmental Chemistry, 2016, 35(6): 1134-1141(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.06.2015122404
[45] LI Z W, HU X L, QIN L X, et al. Evaluating the effect of different modified microplastics on the availability of polycyclic aromatic hydrocarbons [J]. Water Research, 2019, 170: 115290. [46] DABROWSKI A. Adsorption-from theory to practice [J]. Advances in Colloid and Interface Science, 2001, 93(1-3): 135-224. doi: 10.1016/S0001-8686(00)00082-8 [47] WU P F, CAI Z W, JIN H B, et al. Adsorption mechanisms of five bisphenol analogues on PVC microplastics [J]. Science of the Total Environment, 2018, 650: 671-678. [48] VELEZ J F M, SHASHOU Y, SYBERG K, et al. Considerations on the use of equilibrium models for the characterisation of HOC-microplastic interactions in vector studies [J]. Chemosphere, 2018, 210: 359-365. doi: 10.1016/j.chemosphere.2018.07.020 [49] BAKIR A, ROWLAND S J, THOMPSON R C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment [J]. Marine Pollution Bulletin, 2012, 64(12): 2782-2789. doi: 10.1016/j.marpolbul.2012.09.010 [50] GUO X T, PANG J W, CHEN S Y, et al. Sorption properties of tylosin on four different microplastics [J]. Chemosphere, 2018, 209: 240-245. doi: 10.1016/j.chemosphere.2018.06.100 [51] WANG F, SHIH K M, LI X Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics [J]. Chemosphere, 2015, 119: 841-847. doi: 10.1016/j.chemosphere.2014.08.047 [52] XU B L, LIU F, BROOKES P C, et al. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter [J]. Environmental Pollution, 2018, 240: 87-94. doi: 10.1016/j.envpol.2018.04.113 [53] ZHANG H B, WANG J Q, ZHOU B Y, et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors [J]. Environmental Pollution, 2018, 243: 1550-1557. doi: 10.1016/j.envpol.2018.09.122 [54] NG H Y, ELIMELECH M. Influence of colloidal fouling on rejection of trace organic contaminants by reverse osmosis [J]. Journal of Membrane Science, 2004, 244(1-2): 215-226. doi: 10.1016/j.memsci.2004.06.054 [55] LIU X M, SHI H H, XIE B, et al. Microplastics as both a sink and a source of Bisphenol A in the marine environment [J]. Environmental Science & Technology, 2019, 53(17): 10188-10196. [56] ENDO S, DROGE S T J, GOSS K U. Polyparameter linear free energy models for polyacrylate fiber−water partition coefficients to evaluate the efficiency of solid-phase microextraction [J]. Analytical Chemistry, 2011, 83(4): 1394-1400. doi: 10.1021/ac102868e [57] LUDMILLA A, CLAIRE M, JOCELYNE M-B, et al. Interactions of oxytetracycline with a smectite clay: A spectroscopic study with molecular simulations [J]. Environmental Science & Technology, 2010, 44(20): 7839-7845. [58] HÜFFER T, HOFMANN T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution [J]. Environmental Pollution, 2016, 214: 194-201. doi: 10.1016/j.envpol.2016.04.018 [59] MARC T, J P J, L B J, et al. Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar) [J]. Environmental Science & Technology, 2011, 45(23): 10020-10027. [60] KARANFIL T, KILDUFF J E. Role of granular activated carbon surface chemistry on the adsorption of organic compounds. 1. Priority Pollutants [J]. Environmental Science & Technology, 1999, 33(18): 3217-3224. [61] SATOSHI E, PETER G, C S T. Absorption or adsorption? Insights from molecular probes n-alkanes and cycloalkanes into modes of sorption by environmental solid matrices [J]. Environmental Science & Technology, 2008, 42(11): 3989-3995. [62] WEBER W J, HUANG W. A distributed reactivity model for sorption by soils and sediments. 4. Intraparticle heterogeneity and phase-distribution relationships under nonequilibrium conditions - Response [J]. Environmental Science & Technology, 1996, 30(10): 3130-3131. [63] 王宁, 侯艳伟, 彭静静, 等. 生物炭吸附有机污染物的研究进展 [J]. 环境化学, 2012, 31(3): 287-295. WANG N, HOU Y W, PENG J J, et al. Research progess on sorption of orgnic contaminants to biochar [J]. Environmental Chemistry, 2012, 31(3): 287-295(in Chinese).
[64] ZHANG X J, ZHENG M G, WANG L, et al. Sorption of three synthetic musks by microplastics [J]. Marine Pollution Bulletin, 2017, 126: 606-609. [65] LI Y D, LI M, LI Z, et al. Effects of particle size and solution chemistry on Triclosan sorption on polystyrene microplastic [J]. Chemosphere, 2019, 231: 308-314. doi: 10.1016/j.chemosphere.2019.05.116 [66] WU C X, ZHANG K, HUANG X L, et al. Sorption of pharmaceuticals and personal care products to polyethylene debris [J]. Environmental Science and Pollution Research, 2016, 23: 1-8. doi: 10.1007/s11356-015-5714-x [67] ZUO L Z, LI H X, LIN L, et al. Sorption and desorption of phenanthrene on biodegradable poly(butylene adipate co-terephtalate) microplastics [J]. Chemosphere, 2018, 215: 25-32. [68] ADIL B, J R S, C T R. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions [J]. Environmental Pollution, 2014, 185: 16-23. doi: 10.1016/j.envpol.2013.10.007 [69] GUO X, CHEN C, WANG J L. Sorption of sulfamethoxazole onto six types of microplastics [J]. Chemosphere, 2019, 228: 300-308. doi: 10.1016/j.chemosphere.2019.04.155 [70] FANG S, YU W S, LI C L, et al. Adsorption behavior of three triazole fungicides on polystyrene microplastics [J]. Science of the Total Environment, 2019, 691: 1119-1126. doi: 10.1016/j.scitotenv.2019.07.176 [71] XU B L, LIU F, BROOKES P C, et al. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics [J]. Marine Pollution Bulletin, 2018, 131: 191-196. doi: 10.1016/j.marpolbul.2018.04.027 [72] XU P C, GE W, CHAI C, et al. Sorption of polybrominated diphenyl ethers by microplastics [J]. Marine Pollution Bulletin, 2019, 145: 260-269. doi: 10.1016/j.marpolbul.2019.05.050 [73] PANDELOVA M, HENKELMANN B, BUSSIAN B M, et al. Results of the second national forest soil inventory in Germany - Interpretation of level and stock profiles for PCDD/F and PCB in terms of vegetation and humus type [J]. Science of the Total Environment, 2018, 610-611: 1-9. doi: 10.1016/j.scitotenv.2017.07.246 [74] 于艳新, 李奇, 王慧, 等. 食物中典型持久性有机污染物(POPs)的生物可给性研究综述 [J]. 生态环境学报, 2015, 24(8): 1406-1414. YU Y X, LI Q, WANG H, et al. The bioaccessibility of typical persistent organic pollutants (POPs) in food matrix: A review [J]. Ecology and Environment, 2015, 24(8): 1406-1414(in Chinese).
[75] HEINRICH P, BRAUNBECK T. Bioavailability of microplastic-bound pollutants in vitro: The role of adsorbate lipophilicity and surfactants [J]. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology, 2019, 221: 59-67. [76] LIU G Z, ZHU Z L, YANG Y X, et al. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater [J]. Environmental Pollution, 2018, 246: 26-33. [77] LIU P, QIAN L, WANG H Y, et al. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes [J]. Environmental Science & Technology, 2019, 53(7): 3579-3588. [78] HÜFFER T, WENIGER A K, HOFMANN T. Sorption of organic compounds by aged polystyrene microplastic particles [J]. Environmental Pollution, 2018, 236: 218-225. doi: 10.1016/j.envpol.2018.01.022 [79] MÜLLER A, BECKER R, DORGERLOH U, et al. The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics [J]. Environmental Pollution, 2018, 240: 639-646. doi: 10.1016/j.envpol.2018.04.127 [80] ENDO S, TAKIZAWA R, OKUDA K, et al. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences [J]. Marine Pollution Bulletin, 2005, 50(10): 1103-1114. doi: 10.1016/j.marpolbul.2005.04.030 [81] DING L, MAO R F, MA S R, et al. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants [J]. Water Research, 2020, 174: 115634. doi: 10.1016/j.watres.2020.115634 [82] LIU F F, LIU G Z, ZHU Z L, et al. Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry [J]. Chemosphere, 2018, 214: 688-694. [83] LIU X W, ZHENG M G, WANG L, et al. Sorption behaviors of tris-(2, 3-dibromopropyl) isocyanurate and hexabromocyclododecanes on polypropylene microplastics [J]. Marine Pollution Bulletin, 2018, 135: 581-586. doi: 10.1016/j.marpolbul.2018.07.061 [84] 潘伟健, 康园. 环境介质中有机污染物生物有效性研究综述 [J]. 广东化工, 2020, 47(6): 147-148,134. doi: 10.3969/j.issn.1007-1865.2020.06.063 PAN W J, KANG Y. Review on the bioavailability of organic pollutants in environmental media [J]. Guangdong Chemical Industry, 2020, 47(6): 147-148,134(in Chinese). doi: 10.3969/j.issn.1007-1865.2020.06.063
[85] 张凯, 孙红文. (可降解)微塑料颗粒吸附有机污染物及对其生物有效性的影响 [J]. 环境化学, 2018, 37(3): 375-382. doi: 10.7524/j.issn.0254-6108.2018020509 ZHANG K, SUN H W. Adsorption of organic pollutants on (degradable) microplastics and the influences on their bioavailability [J]. Environmental Chemistry, 2018, 37(3): 375-382(in Chinese). doi: 10.7524/j.issn.0254-6108.2018020509
[86] YANG W F, GAO X X, WU Y X, et al. The combined toxicity influence of microplastics and nonylphenol on microalgae Chlorella pyrenoidosa [J]. Ecotoxicology and Environmental Safety, 2020, 195: 110484. doi: 10.1016/j.ecoenv.2020.110484 [87] ZHU Z L, WANG S C, ZHAO F F, et al. Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum [J]. Environmental Pollution, 2018, 246: 509-517. [88] GUO X Y, WANG X L, ZHOU X Z, et al. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition [J]. Environmental Science & Technology, 2012, 46(13): 7252-7259. [89] SUHRHOFF T J, SCHOLZ-BöTTCHER B M. Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics - A lab experiment [J]. Marine Pollution Bulletin, 2016, 102(1): 84-94. doi: 10.1016/j.marpolbul.2015.11.054 [90] 杨婧婧, 徐笠, 陆安祥, 等. 环境中微(纳米)塑料的来源及毒理学研究进展 [J]. 环境化学, 2018, 37(3): 383-396. YANG J J, XU L, LU A Y, et al. Research progress on the sources and toxicology of micro (nano) plastics in environment [J]. Environmental Chemistry, 2018, 37(3): 383-396(in Chinese).
[91] CHEN Q Q, SANTOS M M D, TANABE P, et al. Bioassay guided analysis coupled with non-target chemical Screening in polyethylene plastic shopping bag fragments after exposure to simulated gastric juice of fish [J]. Journal of Hazardous Materials, 2020, 401: 123421. [92] COFFIN S, HUANG G Y, LEE I, et al. Fish and seabird gut conditions enhance desorption of estrogenic chemicals from commonly-ingested plastic items [J]. Environmental Science & Technology, 2019, 53: 4588-4599. [93] CORCORAN P L, NORRIS T, CECCANESE T, et al. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record [J]. Environmental Pollution, 2015, 204: 17-25. doi: 10.1016/j.envpol.2015.04.009 -



 下载:
下载: