-
混凝剂是指能和水溶液中的胶体或者悬浮颗粒产生絮状物沉淀的水处理药剂,按化学成分可分为有机混凝剂、无机混凝剂和复合混凝剂三大类,主要机理包括压缩双电子层、电中和、吸附架桥和网捕卷扫作用等[1-2]。目前我国使用的无机高分子混凝剂占据80%以上,这一类混凝剂是在传统铝盐和铁盐的基础上发展起来的一类混凝剂,水解后大多呈络合离子形态[3]。其中,聚合氯化铝[PAC,Alm(OH)n(H2O)x]是发展多年的一种无机高分子混凝剂,也是目前我国水处理和饮用水净化的主导铝盐产品。结果显示,PAC的水解产物呈现较多正电荷、较强的电中和能力和较优异的吸附能力,具有絮体成型快、沉淀性能好、污泥产生量少等优点[4-5]。但是PAC的水解反应受环境条件的影响较大,它在水中的残余铝也具有生物毒性;此外相比有机混凝剂,PAC的吸附架桥能力相对较弱且污泥产量仍较高[6-7]。因此近年来学者开展了关于PAC与高分子有机混凝剂复合应用的相关研究[8-9],结果表明改性或复合混凝剂能够兼具强阳离子基团和大分子长链结构的特征,因此能够减少铝盐用量、提高吸附架桥作用、增强絮凝能力、拓展PAC的适用范围[10]。
近年来湖库水体富营养化程度不断提高,它影响了社会的可持续发展并始终是研究热点[11]。有害水华暴发是富营养化最重要的表现形式和最直接的后果,其中蓝藻由于繁殖迅速且危害较大而成为学者研究最多的种属[12-13]。多年来大量学者开展关于含藻水体应急的相关研究,其主要目的在于迅速去除或转移水体中的蓝藻细胞,其中混凝除藻是一种常见的化学除藻方法。它是指在混凝剂的作用下,使水体中处于相对稳定状态的藻细胞脱稳凝聚,进而形成较大尺寸的絮体,最终实现絮体沉淀和固液分离[14]。但是,这些研究大多仅聚焦于去除率或仅关注单一因子的作用效果,它们对混凝内在机理的研究相对缺乏,对多种环境条件耦合影响下改性或复合混凝剂对蓝藻处理效果的论述也不多见。此外,关于不同生长阶段蓝藻的代谢物含量等生理特征对混凝效果影响的研究较少,因此开展相关研究可为混凝法除藻工艺的深入研究和推广应用提供科学依据和理论基础。
本研究选择典型蓝藻为研究对象,开展自制的PAC−改性淀粉复合混凝剂对蓝藻处理效果的相关研究,系统研究了不同环境条件耦合下蓝藻去除率的变化,具体包括:(1)不同pH条件下混凝剂的投加量对蓝藻去除效果的影响;(2)蓝藻胞外多糖(EPS)对混凝去除效果的影响;(3)主要操作工况对蓝藻去除效果的影响。
-
实验所用蓝藻(铜绿微囊藻 FACHB−905)购自中国科学院水生生物研究所,并使用标准的无菌BG−11培养基在500 mL锥形瓶中进行批量式培养[15]。观测不同时段铜绿微囊藻的生长状况,并根据藻细胞密度绘制生长曲线。
-
本研究使用的混凝剂为自主改进的PAC−阳离子型淀粉复合混凝剂,它能够克服无机混凝剂分子链短小和卷扫小颗粒不充分等方面的缺点,并且能增加混凝剂携带的正电荷量[16],其具体制作方法和步骤如下。第一步:将天然淀粉阳离子化:取20 g玉米淀粉溶解,加入氢氧化钠作为催化剂,加入5 g阳离子单体2,3−环氧丙基三甲基氯化铵(GTA),随后加入2 mL异丙醇溶液作为分散剂,搅拌混合均匀后在65 ℃条件下反应2 h,冷却后用乙醇溶液重复清洗、抽滤,在烘箱中干燥至松散颗粒状[17-18]。第二步:将阳离子型淀粉与PAC复合:将5 g PAC([Al2(OH)nCl6−n]m,n = 3.6—5.0,m < 10,Al2O3含量 ≥ 28%)溶于1 L超纯水,置于磁力搅拌器缓慢搅拌,同时加入5 g阳离子型淀粉并继续搅拌至溶解,定容后可得质量配比为1∶1的PAC−改性淀粉复合混凝剂。随后,对常规PAC和复合混凝剂进行红外光谱分析。
-
混凝实验采用烧杯搅拌法并在六联电动搅拌仪(常州国华电器有限公司,搅拌桨直径7 cm)上进行,通过加药小管投加混凝剂,混凝处理步骤分为快搅拌、慢搅拌和静置沉淀的3个主要阶段:每次量取500—800 mL不同细胞密度的含藻水样,投加不同剂量的混凝剂后,先快速搅拌,再慢速搅拌,避光静置沉淀一段时间,最后取样并计算蓝藻去除率。
所有实验装置经超纯水洗涤后在120℃和150 kPa条件下灭菌30 min;实验溶液均用Milli−Q超纯水进行配置。
-
选择含对数生长期蓝藻的水样,稀释调节铜绿微囊藻初始细胞密度为2.0×106 cells·mL−1(680 nm处吸光度值约为0.05),在自然水体中此浓度属于高浓度含藻水[15]。量取稀释后藻液,调节系统初始pH(4.5、5.5、7.5和9.5),并投加终浓度为0—20 mg·L−1的复合混凝剂,本实验所选pH能大致涵盖我国富营养化湖库水体的pH值。投加混凝剂后先在200 r·min−1条件下快速搅拌30 s,再在50 r·min−1条件下慢速搅拌15 min,避光静置沉淀30 min后取样分析蓝藻去除率。
-
选择铜绿微囊藻的不同生长时段,通过离心浓缩延滞期的含藻水样和稀释调节对数期、平稳期和衰亡期的含藻水样,调节铜绿微囊藻的初始细胞密度为2.0×106 cells·mL−1(680 nm处吸光度值约为0.05)。随后开展混凝实验:复合混凝剂终浓度为10 mg·L−1,调节初始pH值为7.5,其他操作步骤与1.2.1节相同。
此外,选择含对数生长期蓝藻的水样,调节系统pH值为10.0后采取不同时间的离心处理并去除上清液(转速为10500 r·min−1,时间分别为0、5、15 min)。接着,将离心后藻团使用磷酸盐缓冲液(0.05 mol·L−1,pH=7.8)冲洗3次后重新悬浮、稀释,稀释后的初始藻细胞密度为2.0×106 cells·mL−1(680 nm处吸光度值约为0.05)。随后开展混凝实验:复合混凝剂终浓度为10 mg·L−1,调节初始pH值为7.5,其他操作步骤与1.2.1节相同。前人研究表明碱性条件下不同时间的离心处理可以不同程度地去除蓝藻细胞EPS含量(包括主要进入水体中的溶解性EPS和主要附着在藻细胞上的固着性EPS)[19-20]。本次研究不同条件下铜绿微囊藻细胞EPS含量结果如表1所示,并根据公式(1)计算总EPS含量降低百分比,其中TEPS0和TEPScen分别是未离心和不同时间离心后藻细胞总EPS含量。
-
选择含对数生长期蓝藻的水样并开展混凝实验,初始pH、搅拌等操作步骤与上相同,避光静置沉淀30—120 min后取样分析混凝剂对蓝藻去除的去除率,确定最佳静置时间。
选择含对数生长期蓝藻的水样,稀释调节不同的初始藻细胞密度,量取藻液并调节系统初始pH值为7.5。随后投加不同剂量的复合混凝剂并开展混凝实验:其他操作步骤与1.2.1节相同。
此外,选择含对数生长期蓝藻的水样,稀释调节初始细胞密度为2.0×106 cells·mL−1(680 nm处吸光度值约为0.05),随后开展混凝实验,期间调节快搅拌和慢搅拌的速率和时间:复合混凝剂终浓度为10 mg·L−1,调节初始pH值为7.5,其他操作步骤与1.2.1节相同。
-
铜绿微囊藻的细胞密度使用光学显微镜直接计数,蓝藻生长曲线如图1a所示。选择对数生长期的蓝藻,稀释后于200—900 nm波长下进行扫描(图1b)。结果显示,可见光波段内680 nm波长附近出现了明显吸收峰,该波长处不同密度藻细胞吸收光照的灵敏度较高,因此选用680 nm处吸光度(OD680)来衡量铜绿微囊藻的生物量。拟合结果也表明,OD680与藻细胞密度和藻液浊度均呈现显著的相关性(图1c和图1d)。
-
铜绿微囊藻的光合活性使用浮游植物荧光仪(PHYTO−PAM II,德国Walz公司)测定,具体方法为:先将藻细胞样品置于黑暗条件下暗适应10 min,再通过公式Fv/Fm =(Fm−F0)/ Fm计算藻细胞的最大光化学量子产量,并以此衡量铜绿微囊藻的光合活性。其中Fm是暗适应之后在饱和脉冲下的最大荧光产率,F0是暗适应之后的最小荧光产率。
-
混凝前后分别取10 mL藻液(混凝前直接吸取,混凝后采用虹吸方法在静置沉淀后取液面下5 cm处取样),分光光度法测定铜绿微囊藻OD680变化,计算蓝藻去除率(以%表示)。
-
Zeta电位变化是混凝过程的重要控制环节,通过分析混凝过程絮体Zeta电位的变化能够分析比较混凝剂的电中和能力[21]。本次研究中铜绿微囊藻细胞、混凝剂和混凝絮体的Zeta电位使用Zetasizer Nano S90电位仪直接进行测定,其中混凝剂表面电荷电位为零所对应的pH值被定义为等电点。
-
为直接观察藻细胞产生EPS情况,取对数生长期藻液并加入Alcian blue 8GX(Sigma−Aldrich)进行染色,该染色剂可以与藻细胞产生的EPS形成络合物。
蓝藻细胞EPS的提取和测定方法改进自Yang等[19]和Gao等[22],包括藻细胞产生的溶解性EPS和固着性EPS,并根据藻密度计算单细胞产生EPS含量,具体操作为:
①10 mL蓝藻培养液置于离心管进行快速超声处理(100 W,30 s),这可以将藻类聚集体分散以提高分离效果;②取超声后藻液直接在10500 r·min−1转速下离心15 min,取上清液用于测定藻细胞产生的溶解性EPS;③将离心后藻细胞重新加入超纯水,调节pH值为10.0后水浴震荡4 h(45℃,60 r· min−1),随后在10500 r·min−1转速下离心15 min,取上清液用于测定藻细胞产生的固着性EPS。滤液中的多聚糖含量采用蒽酮法进行测定。
-
混凝处理前后,藻液的三维荧光发射光谱(3D−EEMs)由荧光光谱仪(F−7000,日本日立公司)进行测定,3D−EEMs的激发和发射光谱范围分别设定为200—450 nm(间隔 5 nm)和250—600 nm(间隔1 nm),激发和发射光谱测定时狭缝宽度均设为5 nm,扫描速度设定为1200 nm· min−1。使用Milli−Q超纯水作为空白样品用于消除水拉曼效应造成的荧光光谱拉曼峰。
混凝后絮体的扫描电镜图(SEM images)使用扫描电子显微镜(S−4800,日本日立公司)进行观测,其预处理方法参考阮玲玲[23]和Wang等[24]的研究:①混凝实验结束并充分静置沉淀后,利用虹吸法去除烧杯上清液;②使用2.5%戊二醛固定烧杯底部絮体24 h,③不同浓度的乙醇进行系列脱水(30%、50%、70%、90%、无水乙醇各1次,每次15 min),随后使用乙酸异戊酯置换乙醇2次(每次15 min),并在二氧化碳临界点干燥,从而更加完整的保持絮体的表面形态;④离子溅射镀金后在显微镜下观察并拍照分析。为了更好地分析混凝剂的作用,对混凝处理前处于对数生长期的铜绿微囊藻也进行扫描电镜观测,具体步骤与上相同。
-
本研究所有结果取3次测量平均值,使用方差分析(ANOVA)比较分析pH、混凝剂投加量、操作条件等因素的影响,P < 0.05表示差异性显著。数据在Excel 2010中统计计算后,利用SigmaPlot 12.5和Origin 18.0作图,利用SPSS 18.0对数据进行相关性分析、回归分析或方差分析。
-
常规PAC和复合混凝剂的红外光谱如图2所示,其中3428 cm−1处的较宽吸收峰是PAC中与铝离子相连的—OH基团和吸附水分子中的—OH基团发生伸缩振动产生的吸收峰;1630 cm−1处的吸收峰是水分子边角振动产生的,它是PAC的特征吸收峰。与改性淀粉复合后,波数在1155 cm−1和994 cm−1处的吸收峰为淀粉中C—O—C振动耦合产生的,而它在1480 cm−1和1560 cm−1处的较弱吸收峰为季铵盐基团碳氮键的吸收峰,这表明改性淀粉携带了更多的阳离子基团。与常规PAC相比,复合混凝剂在吸收峰型和峰面积发生了较大变化,此外复合混凝剂在2411 cm−1处多了一个吸收峰,可初步确定PAC与改性淀粉发生了化学键作用而非单纯的物理混合[25]。
-
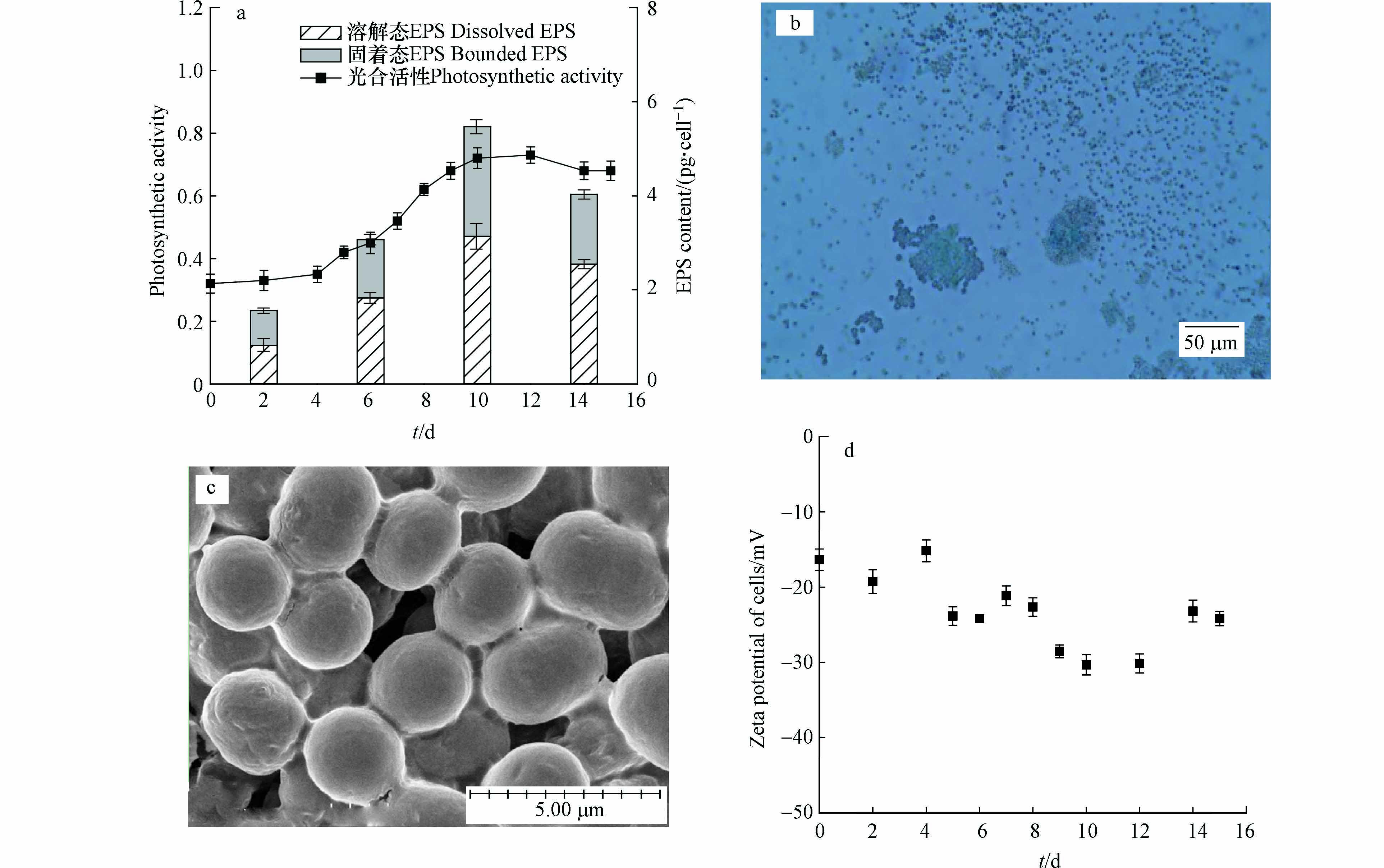
光合活性高低能反映蓝藻新陈代谢、抵抗胁迫等生理活动状态,本研究不同生长期铜绿微囊藻的光合活性变化如图3a所示。结果显示铜绿微囊藻的光合活性与其生长态势密切相关,它在对数生长期逐渐达到最高并在进入衰亡期的初始阶段下降。与此同时,蓝藻能够合成EPS并释放到细胞外及周围环境中,这是其为适应复杂环境而进化出的一种适应性机制[26]。本次研究不同生长期藻细胞EPS含量结果显示:培养期间藻细胞溶解态EPS和固着态EPS含量相近,它们在延滞期相对较低、在对数期逐渐增加并在稳定期达到最高。EPS具有黏性并会包裹新分裂细胞[27],因此对数期、稳定期和衰亡期均能观察到铜绿微囊藻以一定尺寸的聚积体存在。以对数期为例,光学显微镜下微囊藻聚积体周围充斥着染色的绿色絮状物,这是藻细胞EPS与染色剂形成的络合物[28](图3b);扫描电镜下藻细胞圆润饱满、边缘清晰,EPS主要以胶鞘或黏液层的形式围绕在细胞或群体周围(图3c)。
近年来Zeta电位在水处理领域得到了大量应用,其基本原理为:Zeta电位绝对值较高的体系中颗粒间静电斥力较大、颗粒不易发生聚集沉降并处于相对稳定状态;Zeta电位绝对值较低的体系中颗粒间静电斥力较小、颗粒易发生聚沉并处于失稳状态[16]。本研究培养期间铜绿微囊藻细胞Zeta电位如图3d所示,结果表明,4个时期藻细胞Zeta电位波动较大但均呈现明显的负值(−30.32 mV到−15.18 mV),因此培养期间藻细胞能静电相斥、呈现明显悬浮状态。藻细胞的电负性主要来自于包裹在藻细胞表面的代谢物[29-30],包括蛋白质、多糖、脂肪酸和小分子物质等,Zeta电位波动主要源自胞外有机物组分和含量的变化[16]。对比不同时期,平稳期藻细胞Zeta电位绝对值较大,此阶段蓝藻细胞活力旺盛且稳定繁殖;延滞期藻细胞Zeta电位绝对值相对较小,这些结果也与蓝藻EPS含量基本保持一致。根据絮凝理论,若投加混凝剂使藻细胞的表面电位绝对值降低,从而减小静电斥力、增大范德华引力,这必定能使蓝藻细胞发生有效的沉降。如Sharp等[31]指出混凝剂投加后絮体Zeta电位在−10 mV和+3 mV之间时混凝效果较好,此时水体浊度也呈现稳定低值。
-
pH是影响水处理混凝效果的重要指标,它会直接导致胶体颗粒表面电荷和稳定性的差异,而不同pH条件下混凝剂的水解平衡和产物形态的变化也较大。另一方面,混凝剂投加量是评价其性能的重要指标,在达到相同处理效果的前提下降低混凝剂投加量可以大幅度降低生产成本,本研究不同pH条件下复合混凝剂的投加量对蓝藻去除率的影响图4a所示。
结果显示复合混凝剂对铜绿微囊藻的去除率和最佳投加量受到水体初始pH的显著影响(P < 0.05)。所选pH范围内,复合混凝剂的蓝藻去除效果在碱性条件下(pH=9.5)较差,最高去除率仅为38.8%,但是混凝剂在弱酸性和中性条件下效果较好(最高去除率能达到82.3%和95.1%)。上述现象主要是由于PAC水解后在不同pH区间内会同时存在若干不同形态的羟基络合铝离子,它们的单独混凝效果不同且在水解平衡后所占比例也不同,此外改性天然淀粉引进了大量阳离子季铵盐基团且具有大分子长链结构特征,因此复合混凝剂在不同pH条件下的主要作用机理会发生明显变化。pH=9.5条件下PAC水解产物多为负离子,它们可能会与带负电荷的藻细胞产生排斥作用而不利于蓝藻的去除,但是改性淀粉会增加混凝剂携带的正电荷;此外聚合羟基络合铝离子和淀粉也会发挥吸附架桥和网捕卷扫作用,因此实验中蓝藻也能被明显地去除。pH=5.5和pH=7.5的条件下PAC则主要以带正电荷的高聚合度羟基络合铝离子以及难溶氢氧化物存在,这些物质会高效率中和蓝藻表面的负电荷以促进细胞聚集,而吸附架桥和网捕卷扫等作用会进一步促进蓝藻的絮凝去除效果[32]。pH=4.5条件下PAC的主要组成为
$\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{n}^{3+} $ ,这类铝单体络合离子的吸附絮凝能力相对较弱,学者们的众多研究也证实Al13等高聚合羟基络合铝离子和难溶氢氧化物是PAC中的最佳絮凝成分[33-34]。考虑到长江中下游湖库水体多为弱碱性水体,本研究中的复合混凝剂对于天然含藻水体的处理具有一定应用前景。另一方面,不同pH条件下,随着复合混凝剂投加量的增加蓝藻去除率均呈现“先升高后下降”的变化趋势,上述结果可能与混凝机理也有着密切联系:相比初始呈现的负电性,混凝剂投加过量后蓝藻细胞会吸附大量反离子、藻细胞表面逐渐带正电荷,这会再次导致蓝藻细胞的互斥和稳定现象,因此蓝藻去除率会呈现下降的变化趋势;此外pH=9.5条件下混凝剂对蓝藻的去除主要以吸附架桥和网捕卷扫为主,混凝剂投加过多后可能会形成过多的高分子链并将独立的微囊藻细胞或小尺寸藻团包裹,从而难以聚集形成稳定大尺寸的絮体[25]。 -
本研究在投加不同剂量的复合混凝剂后,藻细胞表面Zeta电位和反应体系pH值如图4b所示(初始pH=7.5)。所加浓度范围内,混凝反应后上清液pH值变化不大,但是细胞表面Zeta电位均明显升高,而且混凝剂投加量越大藻细胞带有越多的正电荷。其中,混凝剂投加量为10 mg·L−1时藻细胞表面Zeta电位近乎为零,此时细胞表面的负电荷可能被混凝剂水解产物携带的正电荷中和、藻细胞间存在较低的静电斥力并伴有较大的范德华引力。此外,聚合羟基络合铝离子、难溶氢氧化物和淀粉也会发挥吸附架桥和网捕卷扫作用[25],因此pH=7.5条件下投加10 mg·L−1复合混凝剂后会观测到最高的蓝藻去除率。但混凝剂投加量为15 mg·L−1时藻细胞表面Zeta电位变为+16.43 mV,藻细胞之间会再次产生较明显的排斥力,因此蓝藻去除率发生下降。上述结果证实了电中和作用是本研究中复合混凝剂对蓝藻的重要去除机理。
为进一步验证混凝机理,测定藻细胞表面带电情况和不同pH条件下混凝剂Zeta电位(图4c)。测定结果显示,pH=4—10范围内藻细胞表面均呈现负电荷(Zeta电位为−38.24 mV到−18.43 mV),随着pH增大藻细胞电负性进一步增强。但是随着pH由碱性转为酸性,复合混凝剂所带电荷逐渐由负电荷变为正电荷、它的等电点在7—8之间,这是pH处于酸性和中性条件下复合混凝剂有较好效果的重要原因。相比之下,常规PAC等电点为6左右,这表明阳离子型淀粉复合确实增加了混凝剂所带的正电荷量,这主要来自于淀粉经改性后与GTA发生醚化和接枝共聚反应从而引进大量阳离子季铵盐基团,因此混凝剂能通过更强的电中和作用达到更高的除藻效率(图4d)。相比pH 5.5和pH 7.5,pH 4.5时复合混凝剂带有更高的正电荷,但是pH 4.5时蓝藻去除率却有所下降,这说明混凝剂水解产物的吸附架桥和网捕卷扫作用可能也起到了重要作用。其他学者研究也表明,随着pH升高单体络合铝离子的聚合程度会提高[35],这类物质在去除蓝藻的过程中会发挥吸附架桥作用,形成“藻细胞−高分子−藻细胞”的凝聚体。该观点通过混凝前后扫描电镜图得到进一步证实:初始pH 7.5条件下絮体电镜图表明,混凝剂投加后藻细胞结构完整且圆润,被絮凝沉淀的藻细胞之间会有一些明显的丝状物质(图4e),而混凝前藻细胞间并无丝状物质存在(图3c)。
-
一般而言,藻细胞代谢物会导致藻细胞在生长过程中Zeta电位呈现负电荷,其中多糖约占代谢物总量的70%—80%,因此很多时候使用蓝藻EPS含量来表示它们的代谢物含量[36]。4个生长阶段铜绿微囊藻产生的EPS含量差别较大,而不同剂量的复合混凝剂对蓝藻的去除效果如5a所示。
结果显示,复合混凝剂对不同时期藻细胞都能达到高于90%的去除效果,但是对应的最佳投加量差别较大:延滞期的蓝藻只需2 mg·L−1混凝剂便能够达到最佳去除效果,对数期和衰亡期的蓝藻需要10—15 mg·L−1混凝剂能达最佳去除效果,稳定生长期的蓝藻需要20 mg·L−1混凝剂能达到最佳去除效果。对比本研究不同时期蓝藻细胞EPS含量和表面Zeta电位变化(稳定期绝对值最高,延滞期绝对值最低,对数期和稳定期相似),藻细胞代谢物可能决定了其表面电荷和藻液稳定性,进而影响复合混凝剂的作用效果。Zhang等[37]研究铝盐对不同生长期小球藻的混凝效果,结果显示对数期比稳定期和衰亡期需要更多的混凝剂,其原因在于实验所用小球藻在对数期产生更多代谢物、细胞的电负性也会相对更高。
-
为进一步验证上述猜想,我们取不同前处理藻液进行混凝实验,前处理方法能够不同程度地去除藻细胞EPS(表1),最终的蓝藻去除结果如图5b所示。正常情况下铜绿微囊藻产生的代谢物成分复杂(图6a),三维荧光分析显示蓝藻EPS包括可溶性类蛋白物质(T1峰)、芳香性蛋白物质(T2峰)、富里酸类物质(A峰)和腐殖酸类物质(C峰)。经复合混凝剂处理后,四个荧光峰均发生了明显减弱,表明混凝产物对蓝藻EPS中蛋白类、腐殖酸和富里酸类物质均有所去除(图6b)。经离心5 min并去除上清液处理后,藻液中蓝藻产生的EPS含量明显减少(58.0%),三维荧光分析显示此时减少的主要是富里酸类物质、腐殖酸类物质和一部分可溶性类蛋白物质,因此对应荧光峰发生明显减弱(图6c)。但此时复合混凝剂对蓝藻的去除效果却发生提高,投加量为5 mg·L−1时蓝藻去除率达到最高(93.1%)。该结果与一些学者的研究一致[38-39],他们认为藻类产生的胞外物质会增加细胞电负性,而且这些物质溶解并进入水体后会与混凝剂及其水解产物结合,因此相同藻细胞去除效果下混凝剂的消耗量会增加。钱爱娟[40]指出随着蓝藻EPS被去除后,细胞与混凝剂结合的接触面积和碰撞概率也会增加,因此她也认为去除藻细胞的EPS有助于强化混凝去除蓝藻。
经离心15 min并去除上清液处理后,藻液中蓝藻产生的EPS含量进一步降低,三维荧光分析显示藻液中仅剩余部分富里酸类物质(图6d),但是此时复合混凝剂对蓝藻的混凝去除效果却明显下降,不同投加量条件下蓝藻的最高去除率仅有68.3%(图5b)。该结果的主要原因为,一定浓度的EPS能够辅助复合混凝剂发挥吸附架桥和网捕卷扫的作用[40-42]。PAC水解后会产生一些带正电荷的羟基铝络合离子,这些羟基铝络合离子会聚合形成线性、面状和立体化结构,并通过正负电荷引力、范德华力等作用将藻细胞和悬浮胶粒等联合起来形成絮凝体,从而起到吸附架桥的作用。在这个过程中羟基铝络合离子被铜绿微囊藻细胞产生的EPS吸附,藻细胞就像这些架桥的联结点。与此同时,阳离子化淀粉携带大量阳离子季铵盐基团并具有大分子长链结构特征,这有助于混凝剂发挥连接架桥作用;此外复合混凝剂会生成难溶氢氧化物絮状物,而蓝藻产生的溶解性EPS可以与这些氢氧化物沉淀结合,从而更好地捕获和卷扫藻细胞和水中的细微悬浮物质。
-
混凝操作条件的对蓝藻去除效果的影响如图7所示。结果表明,静置沉降时间会明显影响蓝藻去除率(图7a):以pH=7.5条件为例,沉降时间低于30 min时蓝藻去除率随着时间的增加而增加,而沉降时间高于30 min后蓝藻去除率基本稳定。可能原因为:微囊藻细胞比重小且内部含有伪空胞,当藻细胞或小尺寸藻团与混凝剂发生反应后,絮体需要结合密实程度较高且达到一定尺寸或重量后才能稳定沉降[40],30 min内上清液中藻细胞密度尚处于缓慢降低阶段,此时混凝剂和藻细胞结合形成的絮体可能尚未完全稳定,因此本研究选择30 min的静置沉降时间能够较好地衡量不同条件下复合混凝剂对蓝藻的去除效果。
如图7b所示,蓝藻的藻细胞密度也会显著影响混凝剂的处理效果。在所选范围内(根据自然水体蓝藻水华暴发过程设定),随着初始藻细胞密度的逐步升高,混凝剂对蓝藻的去除效率发生明显下降,如初始藻密度为1.0×105 cells·mL−1时1 mg·L−1的混凝剂投加量便呈现最佳去除率(90.3%),而初始藻密度为1.5×106 cells·mL−1时10 mg·L−1的混凝剂投加量才达到最佳去除率(95.1%)。该现象在其他混凝剂的相关研究中也有涉及,Divakaran等[43]发现使用壳聚糖对藻类进行絮凝去除时,壳聚糖的最佳投加量与水体浊度和藻类生物量密切相关;Vandamme等[44]发现混凝去除小球藻时阳离子型淀粉的用量与藻的初始生物量成较好的线性关系。随着本研究中混凝剂的投加,絮体Zeta电位逐渐从负值增加之后接近零或达到正值(图7c);而随着初始藻密度增加,相同剂量的混凝剂对絮体Zeta电位的提升效果逐渐下降。根据前面的研究,这可能是复合混凝剂对蓝藻的去除是先依靠它对藻细胞EPS等代谢物的去除,继而与藻细胞通过吸附电中和作用而絮凝沉降下来,因此更高的藻密度必然会增加混凝剂的消耗量[45-46]。结果还显示,复合混凝剂为最佳投加量时絮体对应Zeta电位在−10 mV到+2 mV之间,该范围与Henderson等[47]的研究结果类似。他们认为可以通过测定藻絮体的Zeta电位来快速评价混凝效果,并通过调节Zeta电位来达到混凝除藻的最佳药物投加量。
-
搅拌是混凝除藻的重要过程,选择pH=7.5和混凝剂投加量为10 mg·L−1条件下进行混凝试验,不同搅拌速率和搅拌时间对蓝藻去除效果的影响如图7d-g所示。结果表明,快搅拌速率在300 r·min−1时蓝藻去除效果最好,此时蓝藻的去除率能够达到95.1%,若继续增大快搅拌速率则会导致去除率的下降,快搅拌速率为600 r·min−1时蓝藻去除率仅为35.2%。由于快速搅拌是为了使混凝剂及其水解产物和藻细胞充分地混匀,因此快搅拌速率过低可能无法将混凝剂均匀分散于藻液体系中;快搅拌速率过高则会导致较大水流剪切力,会影响混凝剂的电中和作用和桥接作用以及絮体的结合牢固程度[48]。选择快搅拌速率为300 r·min−1时,只需40 s便能达到最高的蓝藻去除率,随后继续延长时间则对去除效果的影响较小,此时混凝剂和蓝藻的混合已达到均匀。
经过40 s的300 r·min−1快速搅拌后,结果显示过低和过高的慢搅拌速率也均不利于蓝藻的去除效果,过长或过短的慢搅拌时间也会造成蓝藻去除率的下降,结果表明慢搅拌速率为40 r·min−1且搅拌时间为20 min时蓝藻去除率最高。其可能原因为慢搅拌是为了使藻细胞与混凝剂的水解产物逐渐结合,从而形成尺寸较大且结构稳定的絮体,因此搅拌速率过低或时间过短可能会不利于絮体形成或使形成的絮体尺寸偏小,而搅拌速率过高或搅拌时间过长容易将已经形成的絮体打散[21]。与此同时,无论是快搅拌或是慢搅拌,搅拌强度高低或时间的长短对絮体Zeta电位的影响较小。
-
(1)复合混凝剂对蓝藻细胞的电中和作用是它对蓝藻重要的去除机理,而混凝剂水解产物和改性淀粉的吸附架桥和网捕卷扫作用也起到了重要作用,本研究中混凝剂对蓝藻的去除率受到水体初始pH的显著影响,它在弱酸性和中性条件下的去除效果较好。不同pH条件下复合混凝剂对蓝藻的去除均存在最佳投加量,随着投加量增加藻细胞表面所带电荷会出现反转,因此蓝藻去除率也会发生下降。
(2)复合混凝剂对不同生长阶段的蓝藻都能达到高于90%的去除率,但是对应的最佳投加量差别较大:处理延滞期蓝藻时混凝剂的最佳投加量较低(2 mg·L−1),处理稳定生长期蓝藻时混凝量的最佳投加量较高(20 mg·L−1)。蓝藻细胞产生的EPS会明显增加复合混凝剂的消耗量,但是一定量的EPS也会有助于混凝剂对蓝藻的去除效果。
(3)随着初始藻细胞密度的升高,相同剂量复合混凝剂对蓝藻的去除效果也会发生降低。过高或过低的快搅拌速率均不利于混凝剂的处理效果,快搅拌速率在300 r·min−1时蓝藻去除率最高;慢搅拌速率和搅拌时间也会影响混凝剂对蓝藻的去除效果,慢搅拌速率为40 r·min−1且搅拌时间为20 min时蓝藻去除率最高。
PAC−改性淀粉复合混凝剂对铜绿微囊藻的去除
Effects of composite coagulant with modified starch and PAC on the removal of Microcystis aeruginosa
-
摘要: 利用阳离子型淀粉复合聚合氯化铝(PAC)制得的混凝剂对铜绿微囊藻进行处理,并进行多因素影响下的比较分析。Zeta电位、扫描电镜等结果表明,相比传统PAC,复合混凝剂所带正电荷量升高,它对藻细胞的电中和作用是对蓝藻重要的去除机理,而PAC水解产物和改性淀粉的吸附架桥和网捕卷扫作用也有重要作用。在所选范围内,复合混凝剂在弱酸性(pH=5.5)和中性条件下(pH=7.5)的去除效果较好,最佳去除率能超过80%。不同pH下复合混凝剂对蓝藻的去除存在最佳投加量,投加量过高时它对蓝藻的去除率发生下降。当达到最佳去除率时,延滞期蓝藻所需复合混凝剂投加量最低,稳定生长期蓝藻需要的投加量最高,蓝藻生长过程中产生的胞外物质是导致该结果的重要原因,具体表现为:蓝藻的胞外多糖(EPS)会增加混凝剂的消耗量,但一定量EPS也有助于混凝剂的作用效果。随着初始藻密度提高(1×105—5×106 cells·mL−1),复合混凝剂对蓝藻的处理效果逐渐发生下降。由于搅拌是为了使混凝剂与藻细胞充分混匀并形成尺寸较大且结构稳定的絮体,因此快搅拌速率不宜过高或过低,快搅拌速率在300 r·min−1时蓝藻的去除效果更好;慢搅拌速率为40 r·min−1且搅拌时间为20 min时蓝藻去除率最高。Abstract: The composite coagulant with modified starch and polymeric aluminium chloride (PAC) was used to deal with Microcystis aeruginosa under different conditions. Confirmed by results such as Zeta potential and scanning electron microscope (SEM) images, the amount of positive charges carried by composite coagulant increased compared with that of original PAC, and charge neutralization of cells played an important role on the removal of M. aeruginosa. Besides, the adsorption bridging and sweeping by the hydrolysate of PAC and modified starch were also involved in the removal process. Within the selected pH range, the removal rate of M. aeruginosa was higher under weak acidity conditions (pH=5.5) and neutral conditions (pH=7.5) by the composite coagulant, when more than 80% of M. aeruginosa could be removed. Moreover, there was an optimal dosage for the composite coagulant at different pH, and the removal rate decreased when coagulant dosage was too high. At the best removal rate, the required dosage of composite coagulant was lowest for M. aeruginosa at the lag phase and highest for M. aeruginosa at stationary growth, which could be mainly caused by the extracellular substances produced by algae. The extracellular polysaccharide (EPS) produced by M. aeruginosa increased the consumption of coagulant, while certain amount of EPS could also contribute to the coagulation performance. For the initial densities of M. aeruginosa ranging from 1×105—5×106 cells·mL−1, the removal efficiency of composite coagulant decreased with increasing algal densities. Since agitation was used to thoroughly mix the coagulant and cells and to form the large size flocs with stable structure, the speed of fast-stirring should not be too high nor too low. The removal rate of M. aeruginosa was better at 300 r·min−1 of fast−stirring. Meanwhile, the removal rate of M. aeruginosa was highest at 40 r·min−1 of slow−stirring for 20 min.
-
Key words:
- composite coagulant /
- cyanobacteria /
- removal rate /
- Zeta potential /
- extracellular polysaccharide (EPS)
-

-
图 3 (a)不同时期铜绿微囊藻光合活性及EPS含量,(b)对数生长期经Alcian blue 8GX染色的铜绿微囊藻光学显微镜观测图,(c)混凝前对数期铜绿微囊藻的扫描电镜图,(d)培养期间藻细胞Zeta电位
Figure 3. (a) Photosynthetic activity and EPS content, (b) microscopic observation of M. aeruginosa stained with Alcian blue 8GX, (c) SEM images M. aeruginosa before flocculation, and (d) Zeta potential of M. aeruginosa cells
图 4 (a)不同pH条件下混凝剂投加量的影响;(b)不同混凝剂投加量下藻细胞表面Zeta电位和系统pH变化(初始pH = 7.5);(c)不同pH条件下藻细胞和混凝剂Zeta电位;(d)不同pH条件下10 mg L−1复合混凝剂和常规PAC对铜绿微囊藻去除率的比较,(e)使用复合混凝剂处理后絮体电镜图(投加量为10 mg L−1,初始pH = 7.5)
Figure 4. (a) Effects of different dosages of coagulant on the removal of M. aeruginosa at different pH, (b) Zeta potential of M. aeruginosa and pH of samples with different dosages of coagulant (initial pH = 7.5), (c) Zeta potential of M. aeruginosa and coagulant at different pH, (d) the removal of M. aeruginosa by composite coagulant and normal PAC at different pH with the dosage of 10 mg L−1, (e) SEM images of floc with composite coagulant (initial pH = 7.5 with the dosage of 10 mg L−1)
表 1 不同离心处理后蓝藻细胞EPS含量(pg·cell−1)
Table 1. EPS content of cyanobacterial cells after different centrifugation treatments
溶解性
Dissolved EPS固着性
Bounded EPS总含量
Total content总含量降低百分比
Percentage reduction of total EPS未离心
Without centrifugation1.83 1.24 3.07 — 离心5 min
After 5 min of centrifugation0.35 0.94 1.29 58.0% 离心15 min
After 15 min of centrifugation0.11 0.18 0.29 90.6% -
[1] ZHU G C, ZHENG H L, CHEN W Y, et al. Preparation of a composite coagulant: Polymeric aluminum ferric sulfate (PAFS) for wastewater treatment [J]. Desalination, 2012, 285: 315-323. doi: 10.1016/j.desal.2011.10.019 [2] BARRADO-MORENO M M, BELTRÁN-HEREDIA J, MARTÍN-GALLARDO J. Removal of Oocystis algae from freshwater by means of tannin-based coagulant [J]. Journal of Applied Phycology, 2016, 28(3): 1589-1595. doi: 10.1007/s10811-015-0718-y [3] JANČULA D, MARŠÁLEK B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms [J]. Chemosphere, 2011, 85(9): 1415-1422. doi: 10.1016/j.chemosphere.2011.08.036 [4] HUANG J, YANG Z H, ZENG G M, et al. Influence of composite flocculant of PAC and MBFGA1 on residual aluminum species distribution [J]. Chemical Engineering Journal, 2012, 191: 269-277. doi: 10.1016/j.cej.2012.03.015 [5] 张文艺, 范培成, 李秋艳, 等. 聚合氯化铝-壳聚糖复合絮凝剂的合成及在蓝藻沼液预处理中的应用 [J]. 环境化学, 2012, 31(7): 1057-1062. ZHANG W Y, FAN P C, LI Q Y, et al. Synthesis of PACl-CTS composite coagulant and application in the pre-treatment of blue algae biogas slurry [J]. Environmental Chemistry, 2012, 31(7): 1057-1062(in Chinese).
[6] YANG Z L, GAO B Y, YUE Q Y, et al. Effect of pH on the coagulation performance of Al-based coagulants and residual aluminum speciation during the treatment of humic acid-Kaolin synthetic water [J]. Journal of Hazardous Materials, 2010, 178(1/2/3): 596-603. [7] SUN F, PEI H Y, HU W R, et al. The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes [J]. Chemical Engineering Journal, 2012, 193-194: 196-202. doi: 10.1016/j.cej.2012.04.043 [8] 周庆, 杨小杰, 韩士群. PAC改性粘土处理蓝藻水华对水环境的影响 [J]. 湖泊科学, 2017, 29(2): 343-350. doi: 10.18307/2017.0210 ZHOU Q, YANG X J, HAN S Q. Impacts of PAC modified clay applied in the control of cyanobacteria bloom and left in water on water environment [J]. Journal of Lake Sciences, 2017, 29(2): 343-350(in Chinese). doi: 10.18307/2017.0210
[9] 杜晴, 宋荻, 唐宇农, 等. 淀粉改性絮凝剂与PAC复合絮凝发制品废水性能研究: 小试和中试 [J]. 环境化学, 2019, 38(9): 2081-2092. doi: 10.7524/j.issn.0254-6108.2018111101 DU Q, SONG D, TANG Y N, et al. Flocculation of hairwork wastewater using starch-based flocculants combined with PAC: Laboratory and pilot scale [J]. Environmental Chemistry, 2019, 38(9): 2081-2092(in Chinese). doi: 10.7524/j.issn.0254-6108.2018111101
[10] DU Q, WEI H, LI A M, et al. Evaluation of the starch-based flocculants on flocculation of hairwork wastewater [J]. The Science of the Total Environment, 2017, 601/602: 1628-1637. doi: 10.1016/j.scitotenv.2017.06.029 [11] GLIBERT P M, BURKHOLDER J M. Harmful algal blooms and eutrophication: “strategies” for nutrient uptake and growth outside the Redfield comfort zone [J]. Chinese Journal of Oceanology and Limnology, 2011, 29(4): 724-738. doi: 10.1007/s00343-011-0502-z [12] PAERL H W, XU H, MCCARTHY M J, et al. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy [J]. Water Research, 2011, 45(5): 1973-1983. doi: 10.1016/j.watres.2010.09.018 [13] 秦伯强, 杨桂军, 马健荣, 等. 太湖蓝藻水华“暴发”的动态特征及其机制 [J]. 科学通报, 2016, 61(7): 759-770. doi: 10.1360/N972015-00400 QIN B Q, YANG G J, MA J R, et al. Dynamics of variability and mechanism of harmful cyanobacteria bloom in Lake Taihu, China [J]. Chinese Science Bulletin, 2016, 61(7): 759-770(in Chinese). doi: 10.1360/N972015-00400
[14] 张龙, 乔俊莲, 雷青. 高锰酸钾预氧化强化混凝去除绿藻的研究 [J]. 环境科学学报, 2013, 33(1): 73-78. ZHANG L, QIAO J L, LEI Q. The study of green algae removal by potassium permanganate pre-oxidation enhanced coagulation [J]. Acta Scientiae Circumstantiae, 2013, 33(1): 73-78(in Chinese).
[15] REN L X, WANG P F, WANG C, et al. Algal growth and utilization of phosphorus studied by combined mono-culture and co-culture experiments[J]. Environmental Pollution, 2017, 220(Pt A): 274-285. [16] WANG L, LIANG W Y, YU J, et al. Flocculation of Microcystis aeruginosa using modified larch tannin [J]. Environmental Science & Technology, 2013, 47(11): 5771-5777. [17] 马亚锋, 王玉琪, 郑岚, 等. 阳离子淀粉絮凝剂合成及处理煤矿井废水性能研究 [J]. 工业用水与废水, 2013, 44(1): 58-62. doi: 10.3969/j.issn.1009-2455.2013.01.016 MA Y F, WANG Y Q, ZHENG L, et al. Synthesis of cationic starch flocculant and its performance when treating coal mine wastewater [J]. Industrial Water & Wastewater, 2013, 44(1): 58-62(in Chinese). doi: 10.3969/j.issn.1009-2455.2013.01.016
[18] KHALIL M I, FARAG S, HASHEM A. Preparation and characterization of some cationic starches [J]. Starch - Strke, 2010, 45(6): 226-231. [19] YANG Z, KONG F X, SHI X L, et al. Changes in the morphology and polysaccharide content of Microcystis aeruginosa (cyanobacteria) during flagellate grazing(1) [J]. Journal of Phycology, 2008, 44(3): 716-720. doi: 10.1111/j.1529-8817.2008.00502.x [20] 郭丽丽, 朱伟, 李明. 水中主要阳离子对铜绿微囊藻生长及多糖的影响 [J]. 生态环境学报, 2013, 22(8): 1358-1364. doi: 10.3969/j.issn.1674-5906.2013.08.014 GUO L L, ZHU W, LI M. Effect of major cations in water on the growth and polysaccharide contents of Microcystis aeruginosa [J]. Ecology and Environment Sciences, 2013, 22(8): 1358-1364(in Chinese). doi: 10.3969/j.issn.1674-5906.2013.08.014
[21] HOU J, YANG Z J, WANG P F, et al. Changes in Microcystis aeruginosa cell integrity and variation in microcystin-LR and proteins during Tanfloc flocculation and floc storage [J]. The Science of the Total Environment, 2018, 626: 264-273. doi: 10.1016/j.scitotenv.2018.01.074 [22] GAO L, PAN X L, ZHANG D Y, et al. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa [J]. Water Research, 2015, 69: 51-58. doi: 10.1016/j.watres.2014.10.060 [23] 阮铃铃. 植物多酚抑藻效能与其作用下藻细胞生理特征的研究[D]. 北京: 北京林业大学, 2011. RUAN L L. Study on the algal inhibition effect of plant polyphenols and algal cell physiological characteristics treated by them[D]. Beijing: Beijing Forestry University, 2011 (in Chinese).
[24] WANG X, WANG P F, WANG C, et al. Microcystin biosynthesis in Microcystis aeruginosa: Indirect regulation by iron variation [J]. Ecotoxicology & Environmental Safety, 2018, 148: 942-952. [25] 尤俊杰. PAC-改性淀粉复合絮凝剂的制备及性能研究[D]. 荆州: 长江大学, 2019. YOU J J. Study on the preparation and flocculating performance of the PAC-modified starch composite coagulant[D]. Jingzhou: Yangtze University, 2019.
[26] 吴挺峰, 秦伯强, 马健荣, 等. 浅水富营养化湖泊中蓝藻群体运动研究述评 [J]. 科学通报, 2019, 64(36): 3833-3843. WU T F, QIN B Q, MA J R, et al. Movement of cyanobacterial colonies in a large, shallow and eutrophic lake: A review [J]. Chinese Science Bulletin, 2019, 64(36): 3833-3843(in Chinese).
[27] LI M, ZHU W, GAO L, et al. Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates [J]. Journal of Applied Phycology, 2013, 25(4): 1023-1030. doi: 10.1007/s10811-012-9937-7 [28] YANG Y Y, HOU J, WANG P F, et al. Influence of extracellular polymeric substances on cell-NPs heteroaggregation process and toxicity of cerium dioxide NPs to Microcystis aeruginosa [J]. Environmental Pollution, 2018, 242(Nov.Pt.B): 1206-1216. [29] 乔俊莲, 董磊, 徐冉, 等. 胞外分泌物对铜绿微囊藻混凝去除的影响 [J]. 同济大学学报(自然科学版), 2011, 39(6): 879-883. doi: 10.3969/j.issn.0253-374x.2011.06.017 QIAO J L, DONG L, XU R, et al. Effect of extracellular organic matter on Microcystis aeruginosa coagulation removal [J]. Journal of Tongji University (Natural Science), 2011, 39(6): 879-883(in Chinese). doi: 10.3969/j.issn.0253-374x.2011.06.017
[30] HENDERSON R K, PARSONS S A, JEFFERSON B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae [J]. Water Research, 2010, 44(12): 3617-3624. doi: 10.1016/j.watres.2010.04.016 [31] SHARP E L, PARSONS S A, JEFFERSON B. The impact of seasonal variations in DOC arising from a moorland peat catchment on coagulation with iron and aluminium salts [J]. Environmental Pollution, 2006, 140(3): 436-443. doi: 10.1016/j.envpol.2005.08.001 [32] 周庆, 韩士群, 严少华. 聚合氯化铝与黏土的改性对富营养水体磷和蓝藻的同步去除 [J]. 环境化学, 2015, 34(11): 2059-2066. doi: 10.7524/j.issn.0254-6108.2015.11.2015041401 ZHOU Q, HAN S Q, YAN S H. Simultaneous removal of phosphorus and algae in eutrophic waters by modified complexes of aluminium polychlorid and clay [J]. Environmental Chemistry, 2015, 34(11): 2059-2066(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.11.2015041401
[33] PI K W, GAO L X, LI Z, et al. PAC with high content of Al13 polymer prepared by electrolysis with periodical reversal of electrodes [J]. Colloids & Surfaces:A Physicochemical & Engineering Aspects, 2011, 387(1-3): 113-117. [34] 张大为, 徐慧, 王希, 等. 藻形态及混凝剂组成对混凝-超滤过程的影响 [J]. 环境科学, 2017, 38(8): 3281-3289. ZHANG D W, XU H, WANG X, et al. Effects of algal morphology and Al species distribution on the coagulation-ultrafiltration process [J]. Environmental Science, 2017, 38(8): 3281-3289(in Chinese).
[35] 杨忠莲. 铝盐混凝剂在给水处理中残留铝含量、组分及影响机制研究[D]. 济南: 山东大学, 2013. YANG Z L. Content, speciation and influencing mechanism of residual Al during drinking water treatment using Al-based coagulants[D]. Jinan: Shandong University, 2013(in Chinese).
[36] PANNARD A, PÉDRONO J, BORMANS M, et al. Production of exopolymers (EPS) by cyanobacteria: Impact on the carbon-to-nutrient ratio of the particulate organic matter [J]. Aquatic Ecology, 2016, 50(1): 29-44. doi: 10.1007/s10452-015-9550-3 [37] ZHANG X Z, AMENDOLA P, HEWSON J C, et al. Influence of growth phase on harvesting of Chlorella zofingiensis by dissolved air flotation [J]. Bioresource Technology, 2012, 116: 477-484. doi: 10.1016/j.biortech.2012.04.002 [38] CHOW J S, LEE C, ENGEL A. The influence of extracellular polysaccharides, growth rate, and free coccoliths on the coagulation efficiency of Emiliania huxleyi [J]. Marine Chemistry, 2015, 175: 5-17. doi: 10.1016/j.marchem.2015.04.010 [39] 曹西华, 宋秀贤, 俞志明. 改性黏土除藻的絮凝形态学特征初步研究 [J]. 海洋学报(中文版), 2017, 39(6): 33-42. CAO X H, SONG X X, YU Z M. Morphological attributes of modified clays coagulated with red tide algae [J]. Acta Oceanologica Sinica, 2017, 39(6): 33-42(in Chinese).
[40] 钱爱娟. 蓝藻胞外聚合物对混凝工艺的影响与调控研究[D]. 扬州: 扬州大学, 2018. QIAN A J. Study on the effect and regulation of extracellular polymers of cyanobacteria on coagulation process[D]. Yangzhou: Yangzhou University, 2018(in Chinese).
[41] 王林. PAC与硅藻土强化混凝处理水中铜绿微囊藻影响因素的研究[D]. 广州: 华南理工大学, 2014. WANG L. Study on influencing factors of Microcystis aeruginosa removal in water by enhanced coagulation with PAC combining diatomite[D]. Guangzhou: South China University of Technology, 2014(in Chinese).
[42] WEI J C, GAO B Y, YUE Q Y, et al. Performance and mechanism of polyferric-quaternary ammonium salt composite flocculants in treating high organic matter and high alkalinity surface water [J]. Journal of Hazardous Materials, 2009, 165(1-3): 789-795. doi: 10.1016/j.jhazmat.2008.10.069 [43] DIVAKARAN R, PILLAI V N S. Flocculation of algae using chitosan [J]. Journal of Applied Phycology, 2002, 14(5): 419-422. doi: 10.1023/A:1022137023257 [44] VANDAMME D, FOUBERT I, MEESSCHAERT B, et al. Flocculation of microalgae using cationic starch [J]. Journal of Applied Phycology, 2010, 22(4): 525-530. doi: 10.1007/s10811-009-9488-8 [45] TAKAARA T, SANO D, KONNO H, et al. Cellular proteins of Microcystis aeruginosa inhibiting coagulation with polyaluminum chloride [J]. Water Research, 2007, 41(8): 1653-1658. doi: 10.1016/j.watres.2007.01.035 [46] 方艳娟. 藻类对混凝过程影响机制的研究[D]. 重庆: 重庆大学, 2018. FANG Y J. Study on the influence mechanism of algae on coagulation process[D]. Chongqing: Chongqing University, 2018(in Chinese).
[47] HENDERSON R K, PARSONS S A, JEFFERSON B. Successful removal of algae through the control of Zeta potential [J]. Separation Science and Technology, 2008, 43(7): 1653-1666. doi: 10.1080/01496390801973771 [48] AHMAD A L, YASIN N H M, DEREK C J C, et al. Optimization of microalgae coagulation process using chitosan [J]. Chemical Engineering Journal, 2011, 173(3): 879-882. doi: 10.1016/j.cej.2011.07.070 -




 下载:
下载: