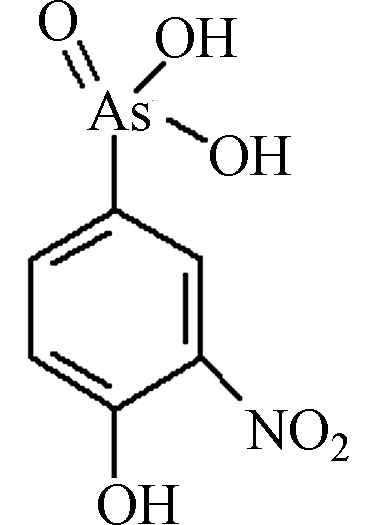
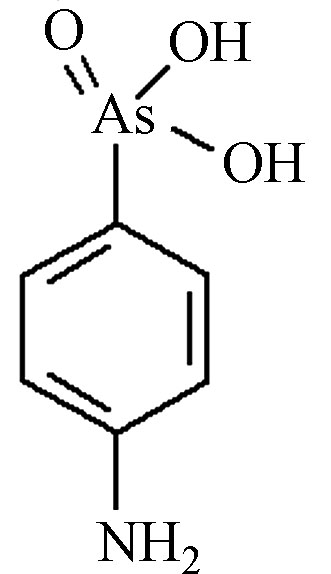
-
砷(As)是一种天然物质,可以多种形态存在于自然环境中。其氧化物对白血病、哮喘等疾病有很好的疗效,医用历史已超过2400年。然而,砷的使用在近一百多年来急剧下降,主要原因是其长期持续使用会导致砷中毒并引发血管疾病及多种癌症。因此,WHO和EPA的饮用水标准中限制均将砷浓度在10 μg·L−1 以下。然而,我国乃至全球范围内的砷污染仍十分严重,2020年Science报道,地下水砷污染已成为全球危机[1]。
砷的毒性与存在形态有关。其中,无机砷会通过与蛋白巯基反应造成毒性,还会破坏改变人类细胞及遗传物质的完整性。有机砷相对于无机砷来说,毒性较低。由于一些有机砷类化合物有促进畜禽生长、提高饲料利用率、杀菌和抑菌的效果,因此在养殖业中得到较为广泛的使用[2-3]。畜禽饲料添加剂中的有机砷主要为芳香族有机砷化合物(aromatic organoarsenic compounds, AOCs),用量主要在20—200 mg·kg−1[4-5]。AOCs被动物食用后在体内被吸收的很少,90%以上会以原形态通过代谢排出体外[6],从而使养殖废弃物中有大量的砷残留。在养殖业动物粪便的总砷中,有70%—75%为可溶性砷[7],说明大部分动物粪便中的有机砷易溶于养殖废水中。这些有机砷以直接或施肥的方式进入水域、土壤中,通过化学和生物降解等方式转化为对环境毒性更大的无机砷,并在环境中经生物富集作用对人体产生潜在危害,从而引起一系列砷污染的环境效应[8]。
鉴于AOCs对环境的潜在危害,欧盟、美国和中国均已禁止了其在动物饲料添加剂中的使用。然而,在许多肉类生产及出口大国,如墨西哥、阿根廷、巴西、越南等,AOCs类饲料添加剂仍被允许使用,可能导致砷在环境中的大量堆积[9-10]。因此,研究AOCs在环境中的存在和迁移转化,探索其去除方法及影响去除效果的关键机制,对于中国乃至全球亟需解决的砷污染防治问题具有重要的理论与现实意义。目前,虽然无机砷在环境(如土壤、沉积物、地表水和地下水)中的迁移转化和去除已被广泛报道和总结,然而对有机砷的研究还很有限。因此,本综述首先整理了环境中常见AOCs的种类、性质和污染现状;进而系统总结国内外去除废水及环境中AOCs的方法、效果及机制;在此基础上,探讨目前AOCs去除领域的研究空白和需求。
-
AOCs类物质具有广谱抗菌性,可杀死动物肠道中多种致病菌和有害菌,有利于动物生长。此外,AOCs还具有提高饲料利用率,促进动物生长,防治硒中毒及促进畜色素沉积等作用,因此被广泛应用于畜禽养殖业的动物饲料添加剂中[7]。常用的AOCs包括洛克沙胂(4-羟基-3-硝基-苯胂酸,Roxarsone,ROX)、阿散酸(对氨基苯胂酸,p-Arsanilic Acid,p-ASA)、硝苯胂酸(4-硝基硝苯胂酸,Nitarsone,NIT)等,其中又以洛克沙胂和阿散酸最为常见,其主要结构、性质及用量见表1。
可以看出,AOCs类物质主要为苯环上带有不同官能团(如羟基、氨基、硝基等)的苯胂酸衍生物。美国药品与食品管理局(FDA)于1964年首先允许洛克沙胂应用于鸡饲料,并于1983年正式批准AOCs制剂作为猪、鸡的促生长剂[9]。我国农业部于1993年批准阿散酸用于养猪和养鸡业,于1996年批准了洛克沙胂在饲料中的使用。2010年,美国饲养的90亿只食用鸡中,约有88%饲喂了洛克沙胂[14]。2019年,中国养殖业中阿散酸和洛克沙胂在饲料中的添加量分别为100 mg·kg−1和50 mg·kg−1。
-
AOCs类饲料添加剂的使用,促进了养殖业的发展,取得了较好的社会经济效益。AOCs本身没有毒性,但其在动物体内残留很少,大多以原形态随排泄物排出动物体外。有研究表明,珠江三角洲使用AOC类饲料添加剂的养殖场附近的表层土壤中可检测出浓度为771 μg·kg−1的p-ASA[15]。同时,各个猪场的污水中均有较高的砷含量,其平均值为0.55 mg·L−1,表明猪场污水虽经过3级处理,但仍具有较高的砷含量[16]。这些AOCs类物质虽然本身毒性较低,但其在水和土壤中微生物的作用下能转化为无机砷,引起有机砷和无机砷之间的相互转化,因此将直接导致或加重周边环境的无机砷污染。有研究发现含AOCs类物质的有机肥经灌溉进入土壤后,一部分AOCs经淋溶迁移进入水域环境,而土壤和水系中的苯胂酸可通过生物的或非生物的作用(微生物降解、光降解、光氧化等)转变为迁移能力更强、毒性更大的三价和五价无机砷化合物,并在一定程度上蓄积于土壤中[17]。Wang等[18]研究表明,p-ASA在遇到酸性红壤中的一种常见矿物δ-MnO2时,会从溶液中被快速地吸附到δ-MnO2表面,随后转化为As(Ⅲ),As(Ⅲ)再继续氧化为As(Ⅴ),然后从矿物表面解吸到溶液中,从而对周围环境造成无机砷污染。Angelo等[19]发现,在堆肥或者进行农业灌溉时,肥料中的ROX较容易被雨水淋洗进入土壤-水体环境中。王克俭等[20]检测猪场附近水域中的砷含量,发现其砷超标率为66%,这可能与猪饲料中AOCs制剂的使用和猪场粪便的处理方式有关。同时,养殖场富集水体的鱼类中全部都有砷检出,其中超标率高达67%,表明水域环境中砷已经开始向生物机体富集。
另外,AOCs类物质随有机肥进入土壤后,在土壤微生物等的作用下,可逐渐降解为小分子的有机砷和无机砷,并通过吸附-沉淀、离子交换、络合、氧化还原反应等理化作用滞留在土壤中,并大量蓄积。畜禽养殖中有机砷在动物排泄物中的残留对养殖场附近土壤、水质均有影响,长期使用畜禽粪便作为有机肥的农田土壤中砷含量明显增加。有研究资料表明,ROX在土壤环境中的移动性较强,可同时吸附于土壤中并迁移至其它环境中[21]。同时,将使用过AOCs添加剂的猪场排泄物当做有机肥施用于1000亩的土地上,4年内可使土壤中砷含量人为增加4.6 mg·kg−1,同时也会导致水域环境中砷含量的增加,并最终经过食物链危害人体健康[22]。因此,在AOCs被转换成有毒的无机砷前将其除去,是维持水和土壤环境安全的重要手段。
-
近年来,养殖废水及环境中AOCs污染物的去除越来越受到国内外科研工作者的关注。AOCs在实际环境中的浓度很低(0.5—5000 μg·L−1)[23],属于微污染物的一种,且受大量共存杂离子的影响,对其深度去除造成了一定困难。从表1中可以看出,AOCs结构上同时含有有机基团和重金属部分,是一种有机-无机复合型污染物。其中,有机基团则以苯环为主,以及其上的氨基、硝基等取代基,而含砷的重金属部分性质上跟无机的砷酸根十分类似。由于AOCs具有这种有机-无机共存结构特点,目前对其的去除方法主要有降解、吸附、降解-吸附联合及微生物法几种。
-
由于AOCs结构上具有芳香族有机污染物的特点,因此通过氧化降解的方法将AOCs降解为无毒的有机物和无机砷是其去除的重要方法之一。降解机制大多是通过强氧化性物质(如自由基等)攻击AOCs结构中苯环和砷酸根连接的As—C键,使AOCs降解为无机的As(Ⅲ)/As(Ⅴ)和其它芳香族化合物。具体方法包括光降解、Fenton降解等。
-
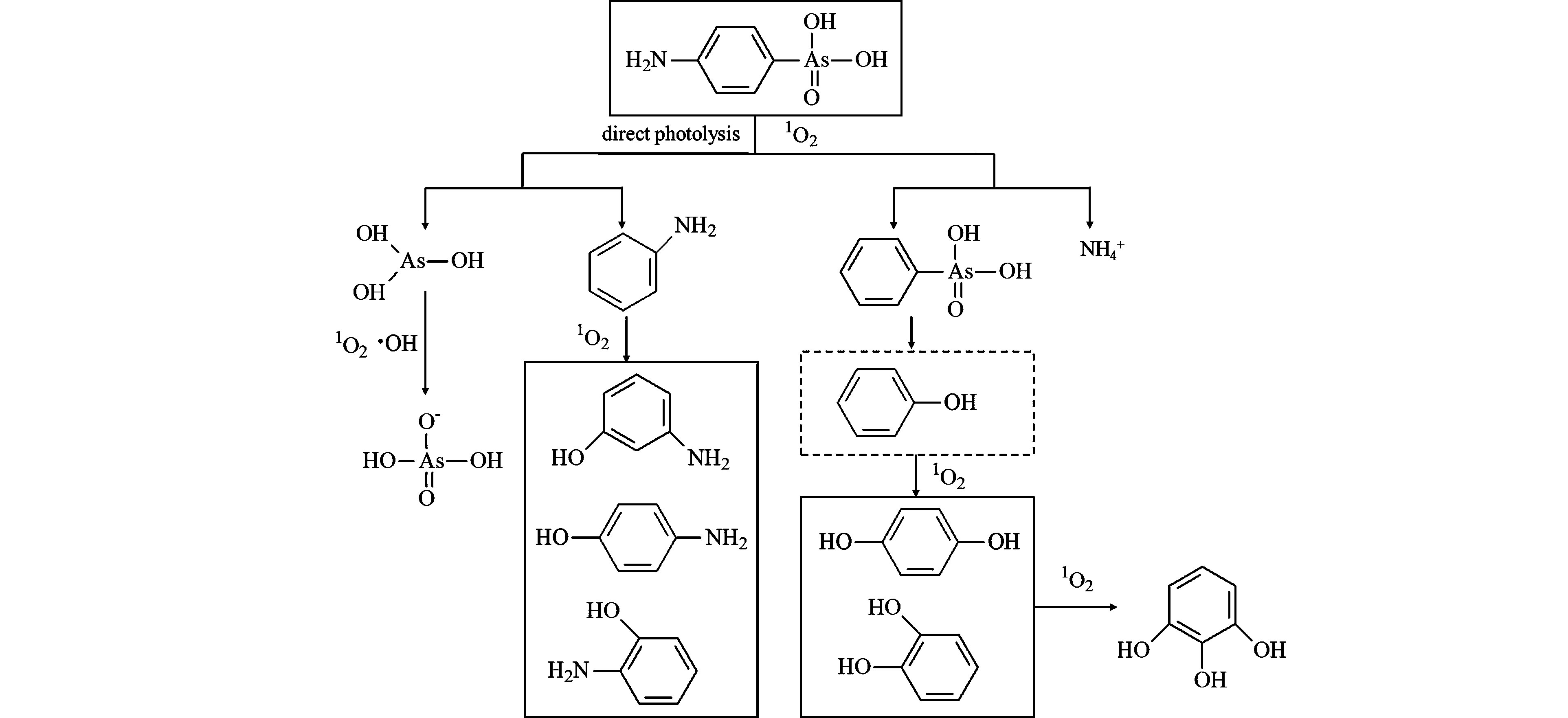
Bednar等在2003年首先报道了家禽垃圾渗滤液中的ROX可在紫外光下被降解为苯酚、As(Ⅲ)和As(Ⅴ)。迄今为止,AOCs在紫外/可见光作用下的降解主要用于研究AOCs在环境中的迁移转化。但是,对于这一过程中AOCs降解速率、产物及影响因素的全面认识,对于AOCs的降解去除同样具有十分重要的意义。光降解AOCs的过程主要是光引发产生活性氧自由基(1O2、·OH等),自由基进一步引发AOCs结构中的As—C键断裂,从而分解产生无机砷和芳香族有机物(图1)[24-26]。Zhu等[24]研究发现,p-ASA在紫外光下处理180 min后,90%以上会转化为无机砷。这一过程是由光引发产生的1O2主导的。p-ASA结构中的As—C键被1O2氧化断裂后,含苯环的有机部分转化为NH4+、氨基苯酚和羟基苯酚。苯环上的砷酸根脱落后,转化为As(Ⅲ),进一步被氧化为As(Ⅴ)。Li等[25]的研究发现,p-ASA在UV-Vis作用下会降解为苯胺、对氨基苯酚、偶氮苯衍生物和As(Ⅴ)。不同的是,他们认为降解过程有49%是受1O2控制,19%受·OH控制,32%是直接光分解的贡献。Xie等[26]研究发现,p-ASA和4-羟基苯胂酸在模拟太阳光下也可快速降解释放As(Ⅲ)和As(Ⅴ),其降解半衰期分别为(11.82 ± 0.19)min和(20.06 ± 0.10)min。这同样是一个由1O2主导的氧化过程。同时,AOCs的降解受溶液物化性质的影响很大。其中,溶解氧(DO)对p-ASA的降解有双重作用[24]。一方面,DO会猝灭氧化过程产生的激发三线态分子(*3ASA),从而抑制p-ASA的降解。另一方面,DO也会在反应过程中,转化1O2而促进p-ASA的降解。从p-ASA的最终降解效果来看,DO的抑制作用大于促进作用。高浓度的天然有机物(DOM >10 mg·L−1)和氯离子(Cl− >1000 mmol·L−1)会猝灭反应过程中产生的活性氧自由基,从而抑制AOCs的光降解[26-27].
除紫外/可见光降解外,外加氧化剂的光氧化反应,也是降解AOCs的有效方法,常用的氧化剂有过氧化氢(H2O2)、臭氧(O3)、过硫酸盐(PMS)、过二硫酸盐(PDS)等。光氧化降解AOCs的过程和产物与紫外/可见光降解过程类似,但由于氧化剂的加入,其降解速率一般要高于紫外/可见光降解。其中,以H2O2为氧化剂的降解效率最高。同时,降解速率及产物受溶液pH影响较大。Czaplicka等的研究表明,在pH < 2.5时,p-ASA的降解速率UV/H2O2 > UV/O3 > H2O2 > O3 > UV[28]。其中,UV/H2O2条件下,p-ASA降解率在160 min内可达100%。同时,50%的砷以As(Ⅲ)的羟基氧化物As3O5(OH)的形式生成沉淀而被去除。相同条件下,UV/O3体系中,降解后的砷均以As(Ⅴ)的形式溶解在水中,没有沉淀生成。而在pH = 7.0时p-ASA的降解速率UV/O3 > O3 > UV/H2O2 > H2O2 > UV[29]。这可能是由于在中性条件下,O3主要是通过生成强氧化性的活性氧自由基降解p-ASA,而在酸性条件下,则主要是以较低活性的分子态O3直接氧化降解p-ASA。除p-ASA外,ROX和NIT也可通过UV和UV/H2O2实现降解,其中UV/H2O2的降解速率更快[14]。这一过程中,ROX中91%的砷转化为As(Ⅴ),因此为了进一步去除这些无机砷,需要在降解后通过固定床对砷进行吸附。另外,Chen等[30]发现,UV/PMS、UV/PDS体系也可通过产生硫酸根自由基(SO4·-)来降解ROX。其降解速率低于UV/H2O2,但UV/PDS在降低TOC方面效果最好。ROX中的砷降解后同样转化为As(Ⅴ)。
以TiO2为催化剂的光催化氧化法也是一种降解AOCs的有效方法[31-34]。降解机理同样是TiO2催化产生的·OH引发AOCs结构中As—C键的断裂,降解后砷全部转化为As(Ⅴ)[35]。氧气在·OH形成过程中起了重要作用,因此AOCs在不同氧浓度下的降解速率为饱和氧 > 空气 > 氩气。同时,TiO2不仅作为光催化剂,在一定程度上还可吸附降解产生的As(Ⅴ)[36],极大减弱了AOCs降解产生的无机砷对环境的危害。因此光催化氧化单纯的光降解和光氧化法更适用于AOCs的去除。近年来,有研究使用ZnO和维生素B2衍生物(RTA)代替TiO2作为催化剂降解AOCs,发现虽然ZnO对AOCs的降解效果优于TiO2,但由于ZnO对砷没有吸附作用,其溶液中残余的As(Ⅴ)浓度远高于TiO2[36]。同时,在模拟太阳光和自然光下条件下,RTA可在90 min和50 min内降ROX先降解为As(Ⅲ),再进一步转变为As(Ⅴ),酸性及碱性条件会抑制降解,中性条件有利于降解[37]。与ZnO类似,RTA对As(Ⅴ)没有固定效果,致使降解后溶液中总砷浓度仍然较高。总体而言,光降解AOCs后均会产生大量无机砷,这一方面目前仍主要用于研究AOCs在环境中的迁移转化上。在作为一种AOCs去除方法使用时,则仍需要后续加入吸附、沉淀等方法进一步去除产生的无机砷。
-
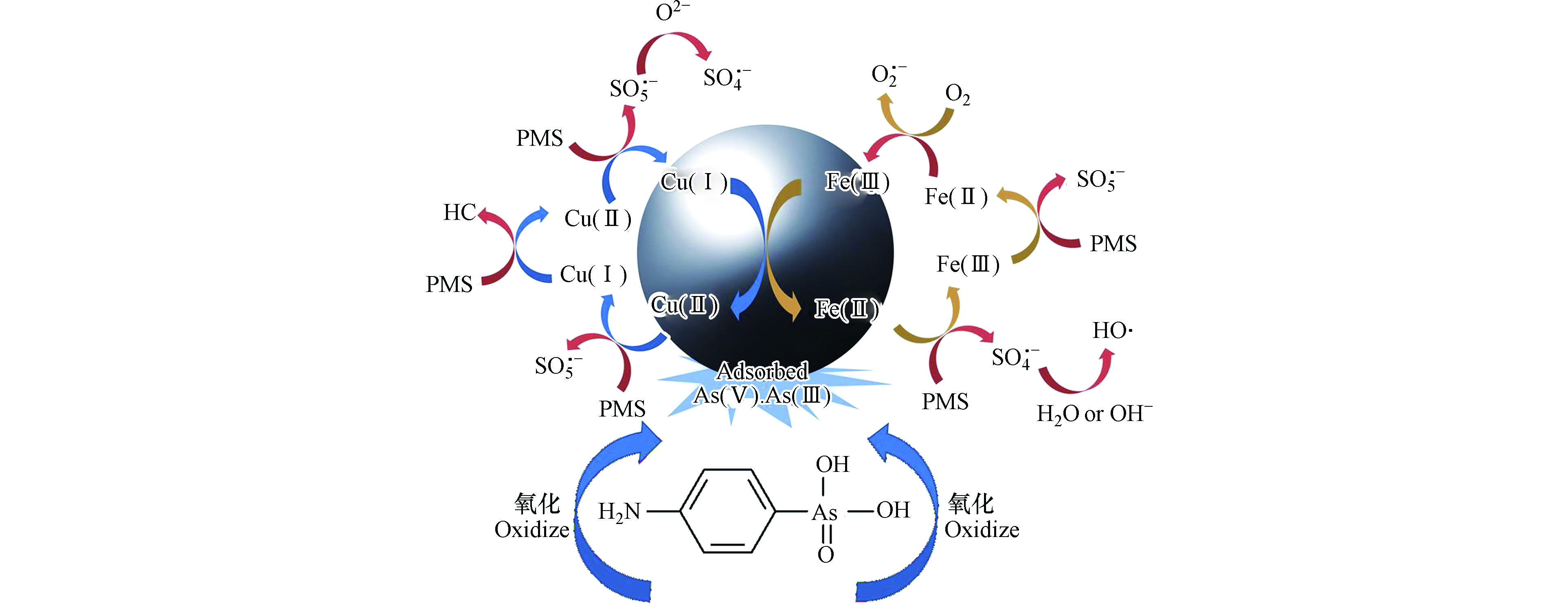
Qin等在2016年发现,在含ROX的土壤中加入Fenton试剂,土壤中会检测出砷酸盐存在,说明Fenton法能将AOCs降解为无机砷[38]。自此,以Fenton法为代表的高级氧化,在AOCs去除领域越来越受到关注。与光降解类似,Fenton法是通过Fe(Ⅱ)催化产生的·OH使AOCs结构中As—C键氧化断裂,产生无机砷和芳香族有机物,有机物中间体可进一步矿化[39]。然而,AOCs降解产生的无机砷毒性更强,目前光降解法较少考虑到对这部分无机砷的后续固定。与之相比,Fenton法中使用的铁系材料对无机砷有天然的固定优势,水铁矿、针铁矿(α-FeOOH)、赤铁矿(α-Fe2O3)及磁铁矿(Fe3O4)等都是对无机砷具有较强吸附能力的铁基材料。因此,在均相芬顿体系中,Fe(Ⅱ)在反应过程中被氧化为Fe(Ⅲ),Fe(Ⅲ)可进一步通过调整体系pH形成氢氧化物沉淀,在此过程中与AOCs降解产生的无机砷,特别是As(Ⅴ),可与Fe(Ⅲ)形成共沉淀或通过表面吸附去除。Xie等发现,初始浓度为10 mg·L−1的p-ASA可通过Fenton氧化在30 min完全降解为As(Ⅴ)[39],在此过程中调节体系pH=4.0,产生的As(Ⅴ)可通过铁砷共沉淀作用进一步去除,纯水和天然水体中砷的残余浓度分别为1.1 μg·L−1和40.3 μg·L−1,低于砷在灌溉用回用水中的浓度标准。而腐植酸的存在会抑制Fenton体系·OH的产生,从而抑制p-ASA的氧化,同时会与As(Ⅴ)在铁沉淀表面发生竞争吸附,从而抑制砷的固定。Liu等用易拉罐中提取的零价铝(AIBCs)与Fenton体系协同去除水中的p-ASA,发现强酸和好氧的条件有利于p-ASA的降解[40]。当溶液pH < 2.0时,AIBCs/Fe(Ⅱ)体系能在180 min内将p-ASA完全降解为As(Ⅴ)。但是在无氧条件下,p-ASA降解缓慢,因为无氧条件限制了体系中H2O2的产生。在AIBCs/Fe(Ⅱ)体系中,Fe(Ⅱ)不仅作为Fenton的催化剂将产生的H2O2转化为·OH,同时还可与AIBCs氧化生成的Al(Ⅲ)发生共沉淀。因此,可通过调节体系至pH 6.0,使降解产生的As(Ⅴ)与铁铝形成共沉淀而从水中进一步去除。除了以Fe(Ⅱ)为催化剂的均相芬顿反应外,以过硫酸盐活化的类Fenton高级氧化技术也是去除水中AOCs的主要方法,其对AOCs的降解机制与Fenton体系类似。而与Fenton体系以·OH为主要活性氧自由基不同,类Fenton体系可产生SO4−、·OH和O2·−等多种自由基,因此其对溶液pH的适应范围更广,可在pH=3—11的范围内将AOCs有效降解。降解产生的As(Ⅴ)同样需要后续处理进一步去除,通常是使用对无机砷有较好吸附效果的材料,如还原氧化石墨烯、Co3O4-Y2O3、CuFe2O4纳米颗粒等[30, 41-42](图2)。
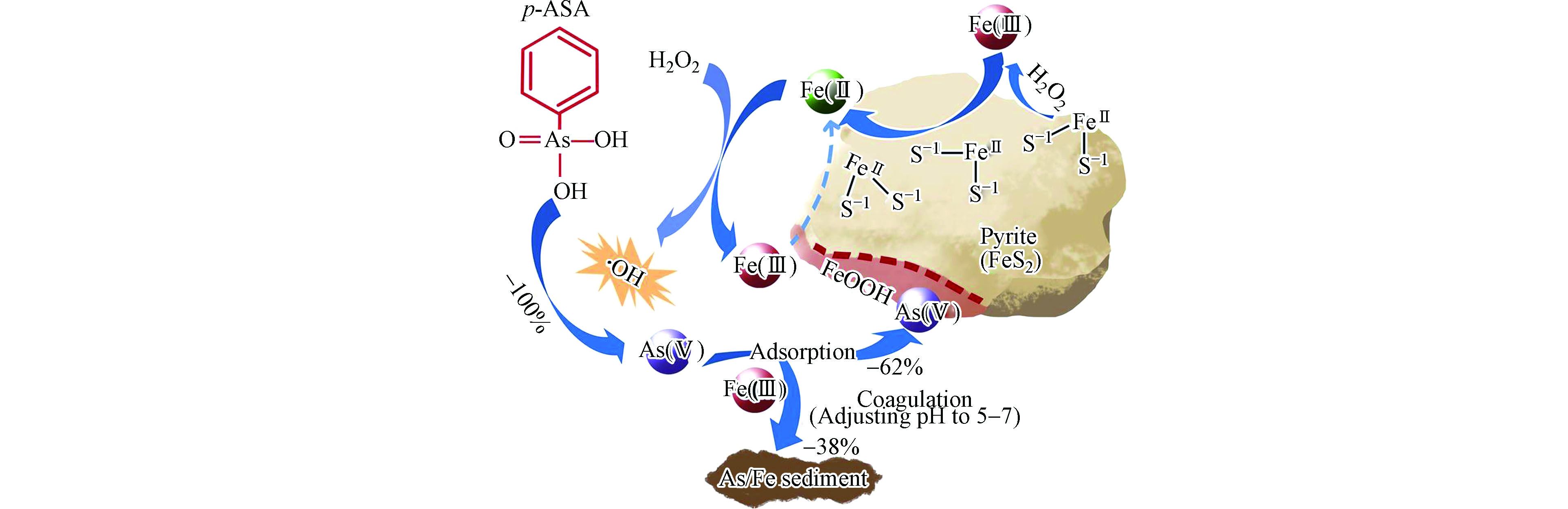
从以上总结可以看出,在均相Fenton体系中,AOCs降解产生的无机砷往往要通过沉淀、吸附等后续处理方法进一步去除,增加了处理流程。与之相比,以铁氧化物及硫化物催化的非均相Fenton体系,可充分利用铁系纳米材料自身对砷的强吸附效果,实现对AOCs的一步性降解-吸附联合去除。Zhao等发现FeS2/H2O2非均相Fenton体系可有效去除水中的p-ASA[43]。体系产生的·OH将p-ASA降解为As(Ⅲ),最终转化为As(Ⅴ),被FeS2表面新生成的铁氧化物吸附(图3)。FeS2/H2O2在溶液pH= 3—7时,可在2 h内去除92%—100%的p-ASA。这是因为反应过程释放大量H+,使pH值稳定在3—4,有利于降解和吸附反应的进行。Li等也发现,污泥基生物炭负载的纳米零价铁(nZVI/SBC)可实现对水中ROX的降解-吸附一步去除[44]。这一过程中,ROX首先被nZVI和生物炭的含氧官能团吸附到材料表面,随后在材料被nZVI催化的非均相Fenton体系氧化降解,产生的As(Ⅴ)被随即被nZVI表面腐蚀产生的铁氧化物同步吸附,从而实现了ROX的高效降解和无机砷的同步去除。因此,Fenton氧化降解实际上是一种降解-吸附联合去除AOCs的方法。
-
除Fe(Ⅱ)可通过引发Fenton反应来实现对AOCs的氧化降解外,部分高价态的铁,如Fe(Ⅵ),也可通过自身的强氧化性对AOCs进行氧化降解,其中比较具有代表性的是高铁酸盐氧化。与产生强氧化性自由基的Fenton反应不同,高铁酸盐氧化降解主要是通过氧转移过程进行的[45]。氧原子从Fe(Ⅵ)转移到目标污染物,形成羟基化降解产物,Fe(Ⅵ) 被同步氧化物为Fe(Ⅲ),并进一步形成铁氢/氧化物,如Fe2O3、FeOOH及无定型水合铁氧化物等。这些原位形成的铁基纳米颗粒分散性高,且表面含大量羟基官能团,有利于进一步通过氢键等作用吸附降解产物。鉴于铁基纳米颗粒自身对无机砷的强吸附性,利用高铁酸盐的强氧化作用和其原位形成的铁氧化物的吸附作用可实现对AOCs的一步高效去除。当高铁酸盐用量较高时(高铁酸盐/AOCs > 3.5),p-ASA的降解产物为As(Ⅴ)、氨基酚及硝苯胂酸,ROX降解产物为As(Ⅴ)和1,4-硝基苯醌[45-46]。98%—99.2%的As(Ⅴ)和85%的硝苯胂酸被同步生成的三价铁氧化物吸附去除,同时可去除40%的TOC,是O3及HClO氧化去除TOC效率的1.6—38倍。当高铁酸盐用量进一步提升(高铁酸盐/AOCs = 20),可在10 min内去除95%以上的砷。同时,再进一步加大高铁酸盐的用量,可将初始砷浓度10 mg·L−1的含AOCs实际养殖废水中总砷去除99%以上。溶液中常见的共存杂离子,如K+/Ca2+/Na+/Mg2+/
${\rm{SO}}_4^{2-} $ /${\rm{NO}}_3^{-} $ /Cl−等对AOCs的高铁酸盐氧化降解基本没有影响。${\rm{PO}}_4^{3-} $ 和${\rm{NH}}_4^{+} $ 会促进AOCs的降解,但是${\rm{PO}}_4^{3-} $ 会跟降解产生的As(Ⅴ)竞争铁氧化物表面的吸附位点,从而抑制体系中总砷的去除[47]。高锰酸钾也有跟高铁酸酸盐类似的氧化性质。Xie等用KMnO4-Fe(Ⅲ)联合降解AOCs,最佳条件下,p-ASA和ROX可分别在35 min和240 min内被降解99%以上,高锰酸钾转化为二氧化锰沉淀[48]。降解产生的As(Ⅴ)被Fe(Ⅲ)转化生成的Fe(OH)3吸附沉淀,溶液中残余砷浓度低80 μg·L−1。实际废水中存在的腐植酸和有机质明显抑制降解过程,但加大KMnO4-Fe(Ⅲ)用量仍可保持较高的AOCs去除率。与Fenton体系相比,高铁酸盐氧化体系的材料用量明显减少,且可以一步实现AOCs的降解-吸附联合去除。 -
降解法对AOCs的去除率虽然很高(部分可达99%以上),但在处理过程中会产生大量高毒的无机砷,需要通过沉淀、絮凝、吸附等进行后续处理,使处理过程复杂化,并且仍有向环境中排放无机砷而造成二次污染的潜在危险(表2)。因此,与之相比,能在不改变AOCs结构的基础上将其直接提取出来的吸附法,更适合这类污染物的去除。由于AOCs结构中同时含有苯环、氨基、硝基、羟基等有机基团,以及类砷酸根的无机基团,同时,大部分AOCs为极性分子,在溶液中会发生电离而使分子表面带电,因此可能通过静电、π-π、氢键、重金属配位等多种作用力被吸附。每种作用力强弱不同,对AOCs的吸附效果也相差较大。一般来说,可表示材料对一定浓度范围的污染物的吸附效果的参数主要有最大吸附容量(qm)和吸附亲和力两个参数。其中,吸附亲和力的平均值可用Langmuir模型的平衡常数kL表示。Yang等的研究表明,qm主要与材料中可及的活性位点数量(比表面积)及其与污染物间的物理作用力(静电、疏水作用等)有关,而亲和力则主要与材料与污染物之间的化学作用力相关[49]。同时,对于高浓度污染物,qm高的材料效果较好,而对于低浓度污染物,kL高的材料效果好[50]。因此,在吸附过程中其主导作用的吸附作用力不同,其对不同浓度的AOCs去除效果也会存在很大差异。
-
从表1中可以看出,代表性AOCs在pH > 3.5的条件下基本带负电,因此在早期工作中,经常对材料表面进行电荷改性,使其与AOCs见产生静电引力或取向力,而实现对AOCs的吸附。Hu等使用多壁碳纳米管(MWCNTs)吸附ROX,在ROX初始浓度为40 mg·L−1范围内,qm为10 mg·g−1,此过程中其主要作用的是MWCNTs与ROX之间的取向力[51]。Poon等使用交联壳聚糖材料(CG)吸附ROX,qm可达490 mg·g−1,亲和力为24 L·g−1。主要吸附作用力是材料表面带正电的氨基与ROX带负电的砷酸根之间的静电引力[52]。Jung等使用沸石基MOF材料ZIF-8去除水中的p-ASA。在原始的ZIF-8中引入介孔,大幅提高了p-ASA的吸附速率和吸附容量。在p-ASA初始浓度为3—350 mg·L−1范围内,qm高达790 mg·g−1,亲和力为32 L·g−1。吸附机理主要是ZIF-8表面大量的正电荷与p-ASA砷酸根电离出的负电荷之间的静电引力[53]。除静电作用外,由多孔材料大比表面积和多孔结构导致的孔沉积作用,也可有效吸附AOCs。Zhu等通过生物质水热法将碳化合物、氯化锌和氯化铁同时碳化得到磁性碳基复合物,微孔比表面积高达1392 m2·g−1。高孔隙率使复合材料对ROX表现出较好的孔吸附作用,初始浓度50 —500 mg·L−1范围内,qm为588 mg·g−1[54],亲和力为63 L·g−1。
因此,以静电和孔沉积为代表的物理作用,对高浓度的AOCs均具有较高的吸附容量。然而,物理吸附是一种相对较弱的作用力,对低浓度污染物吸附效果要明显低于高浓度污染物,因此很难对实际环境中低浓度的AOCs达到较好的去除效果。同时,这类物理作用力受环境条件影响很大。材料表面电荷和AOCs电离程度会随溶液pH的变化而改变,当pH大于材料等电点时,其表面的负电荷会与AOCs阴离子间产生静电斥力,不仅抑制材料的静电吸附作用,也会对其它化学吸附作用产生很大影响,导致整体吸附容量下降。因此,以静电作用为主的物理吸附作用往往是在酸性条件下对AOCs吸附效果较好,随着溶液pH的升高,吸附容量会急剧下降[52-53]。
-
AOCs分子中基本都带有苯环结构,因此以碳材料为代表的含大π结构材料可与AOCs的苯环间产生π-π堆积作用,从而吸附AOCs。同时,AOCs的苯环上通常带有氨基、硝基、羟基等可作为氢键位点的取代基,因此在也可能会通过与材料中的含氧官能团或其它基团间产生氢键作用而被吸附。Sarker等使用羟基改性的MIL-101(OH)3吸附初始浓度25—100 mg·L−1的p-ASA和苯胂酸(PAA),qm分别为238 mg·g−1和139 mg·g−1,亲和力为52 L·g−1和53 L·g−1[55]。吸附的主要作用力是MIL-101(OH)3表面羟基和AOCs砷酸根中的氧及苯环上的-NH2之间形成的氢键作用。由于p-ASA苯环上比PAA多带一个氨基,更加有利于氢键的形成,因此MIL-101(OH)3对p-ASA的吸附效果比PAA好。同时,MIL-101(OH)3对p-ASA的吸附效果也随材料表面羟基含量的增加而增加。Liu等使用氨基改性的纳米纤维素膜吸附5—100 mg·L−1的p-ASA、ROX和PAA,发现引入氨基有利于材料与AOCs之间氢键的形成[56]。同时,由于ROX结构中的—NO2作为一种强电子受体,其与材料的氨基间形成的氢键要强于p-ASA、PAA结构中的—NH2、—OH等与材料氨基之间形成的氢键,因此该材料对ROX的吸附容量是p-ASA和PAA的3倍左右,亲和力也高达366 L·g−1,是其它两种AOCs的5倍以上。目前通过氢键作用吸附AOCs的材料主要包括碳材料和MOFs两类,这两类材料的结构特点导致吸附过程中除了氢键作用外,往往还同时存在π—π作用。Lv等使用氨基改性的铟基MOF NH2-MIL-68(In)吸附水中的p-ASA,初始浓度5—400 mg·L−1范围内,最大吸附容量为402 mg·g−1,亲和力55 L·g−1[57]。吸附过程中,MOFs配体中的苯环和引入的氨基分别作为单独的吸附位点,通过π—π和氢键的共同作用吸附p-ASA。
吸附材料与AOCs间氢键和π—π作用的强度可比物理作用强2—10倍,但又比形成共价键的化学作用要弱。同时,易受环境中大量存在的DOM的影响。DOM表面有大量含氧/氮/的官能团,且结构中含有大量苯环,因此能与AOCs的有机基团产生多种作用,从而影响材料对AOCs的吸附。一方面,DOM中的—COOH、—OH、—NH2等基团会与AOCs结构中的有机基团竞争材料表面的氢键位点,从而抑制吸附过程中的氢键作用。另一方面,DOM中的苯环也会与AOCs分子产生π—π作用,在一定程度上强化对AOCs的吸附[58]。但总体来说,环境中DOM对吸附有机砷的抑制作用要大于促进作用,且这种抑制作用与DOM的浓度成正比。
-
AOCs结构中的含砷部分与无机砷酸根的性质十分相似,因此对As(Ⅴ)具有强结合力的重金属配位作用,也会对AOCs产生极好的吸附效果,如自然界中普遍存在的铁系矿物。Chen等使用针铁矿(FeOOH)和铝氧土(Al2O3)吸附p-ASA和ROX,发现两种AOCs在针铁矿表面的吸附效率是铝氧土的3倍[58]。这一过程中,AOCs结构中的砷酸根与针铁矿之间形成的As—O—Fe单齿单核配位,是主要的吸附作用力[59]。Cao等通过EXAFS、ATR-FTIR、DFT等手段联合研究了赤铁矿不同晶面对PAA的配位模式和吸附效果[60]。结果表明,赤铁矿的{012}晶面更倾向与PAA形成As-O-Fe双齿双核配位,而{001}晶面则更倾向于形成As—O—Fe单齿单核配位。同时,双齿双核配位模式下的吸附能比单齿单核配位低了2.7 eV,因此赤铁矿的{012}晶面更有利于AOCs的吸附。除天然矿物外,对砷有强配位作为的Fe、Al、Zr等,也常作为材料的配位中心被用于吸附去除AOCs,其中比较常见的是Fe/Zr基氧化物纳米颗粒及MOFs材料。以铁系尖晶石为代表的铁氧化物纳米颗粒,由于具有跟铁矿物相似的结构,被广泛用于AOCs的去除研究。Hu等研究了介孔MnFe2O4纳米材料对AOCs的吸附,发现在初始浓度10—400 mg·L−1范围内,不同AOCs的分子结构会对吸附效果产生影响,对p-ASA和ROX的最大吸附容量分别为59和51 mg·g−1[61]。主要吸附作用力是MnFe2O4与AOCs间的As—O—Fe及As—O—Mn配位作用。Liu等合成了花状CoFe2O4材料并将其用于5种AOCs的吸附去除,在浓度5—30 mg·L−1范围内,最大qm值为46 mg·g−1[62]。同时,由于As—O—Fe双齿双核强配位作用,使MnFe2O4对p-ASA的亲和力高达1.64 × 103 L·g−1。与铁氧化物类似,水合锆氧化物纳米材料(HZO)也可通过As-O-Zr配位作用吸附AOCs[63]。与含微孔的阴离子交换树脂(HCA)复合后,HZO对100 mg·L−1的p-ASA吸附容量45 mg·g−1,亲和力41 L·g−1。同时,由于HCA的微孔结构具有尺寸阻排作用,极大降低了DOM对吸附过程的影响,使材料在高DOM浓度下仍对p-ASA有较好的吸附效果。
从以上工作可以看出,Fe/Zr基氧化物纳米材料对较低浓度反应的AOCs(< 30 mg·L−1)具有极高的亲和力,这主要是因为重金属配位作为一种强化学作用力,更加有利于提高材料亲和力,从而促进对低浓度AOCs的吸附。然而,受到纳米颗粒本身易团聚等问题导致的活性位点暴露不足的限制,其对高浓度AOCs的吸附效果明显下降。因此,有研究开始使用大表面积、多孔且结构可调性强的Fe/Zr基MOFs材料,以期在能充分利用重金属配位作用的同时,提高吸附位点的可及性,进一步提高材料对高浓度AOCs的吸附容量。Jun等使用Fe、Cr、Al核心的MIL-100系列MOFs材料对AOCs进行吸附,发现在AOCs初始浓度12—200 mg·L−1范围内,3种材料的吸附效果MIL-100-Fe » MIL-100-Al ≡ MIL-100-Cr,主要是因为Fe对As的配位强度远强于Al和Cr,且Fe金属簇与有机配位形成了不饱和配位结构,为与As形成重金属配位提供了足够的活性位点[64]。同时,吸附效果受溶液pH影响较小,说明静电和π—π作用均不是主要的吸附作用力。Pang等合成了一种Fe基MOF材料MIL-88A(Fe),将其用于1—150 mg·L−1浓度的p-ASA和ROX的吸附,最大吸附容量为261 mg·g−1和428 mg·g−1[65]。对于Zr基MOFs材料,由于其Zr金属簇往往是与有机配位通过饱和配位连接的,因此只能AOCs形成较弱的As-O-Zr单齿配位,对吸附亲和力的提升有限。为了解决这一问题,有研究通过在UiO-66的Zr金属核心簇中制造缺陷,形成不饱和配位位点,从而促进其与AOCs之间形成更稳定的As—O—Zr双齿配位,增强配位吸附作用[66]。对初始浓度10—200 mg·L−1的ROX吸附容量高达730 mg·g−1,比无缺陷的UiO-66提高了4倍,同时亲和力也可达113 L·g−1。
由于重金属配位作用是通过材料与含氧阴离子间的配体交换形成,因此其吸附效果受溶液中共存离子的影响较大。其中,由于P是跟As同主族的元素,其化学性质与As很相似,且活性比As更强[4],所以溶液中共存的PO43-会跟AOCs竞争材料中的配位活性位点,从而抑制AOCs的吸附,甚至可以用于AOCs的解吸[14, 67]。相比之下,SO42-和NO3−等常见阴离子通常在材料表面形成外球配位,不会占据AOCs的配位位点,因此不会对AOCs的吸附产生影响[58]。同时,溶液中的DOM由于带有大量的负电基团,也会因为静电斥力的作用抑制AOCs的吸附。
-
在吸附AOCs的三类典型作用力中,由强到弱依次为重金属配位(化学作用)> 氢键/π—π作用 > 物理吸附作用。表3列举了不同作用力吸附AOCs的浓度范围、最大吸附容量及亲和力。可以看出,即使是作用力最强的重金属配位作用,也无法保证材料对AOCs同时具有高吸附容量和高亲和力。同时,目前对AOCs的吸附研究仍多集中如何提高材料在高浓度(> 100 mg·L−1)范围内的吸附容量上,往往忽视了对亲和力的强化,因此很难使低浓度的AOCs被富集到材料表面。鉴于实际水环境中AOCs的浓度很低(0.5—5000 μg·L−1),且受大量共存杂离子的影响,因此对材料的亲和力提出了更高的要求。然而,以单一作用力作为主导来吸附AOCs的方法,对吸附亲和力的强化效果有限。而基于AOCs同时具有有机-无机基团的结构特点,在材料结构中同时设计可形成以上两种或多种作用的官能团,通过与AOCs之间的有机-无机协同作用,可在保证材料吸附容量的前提下,大幅提高亲和力。
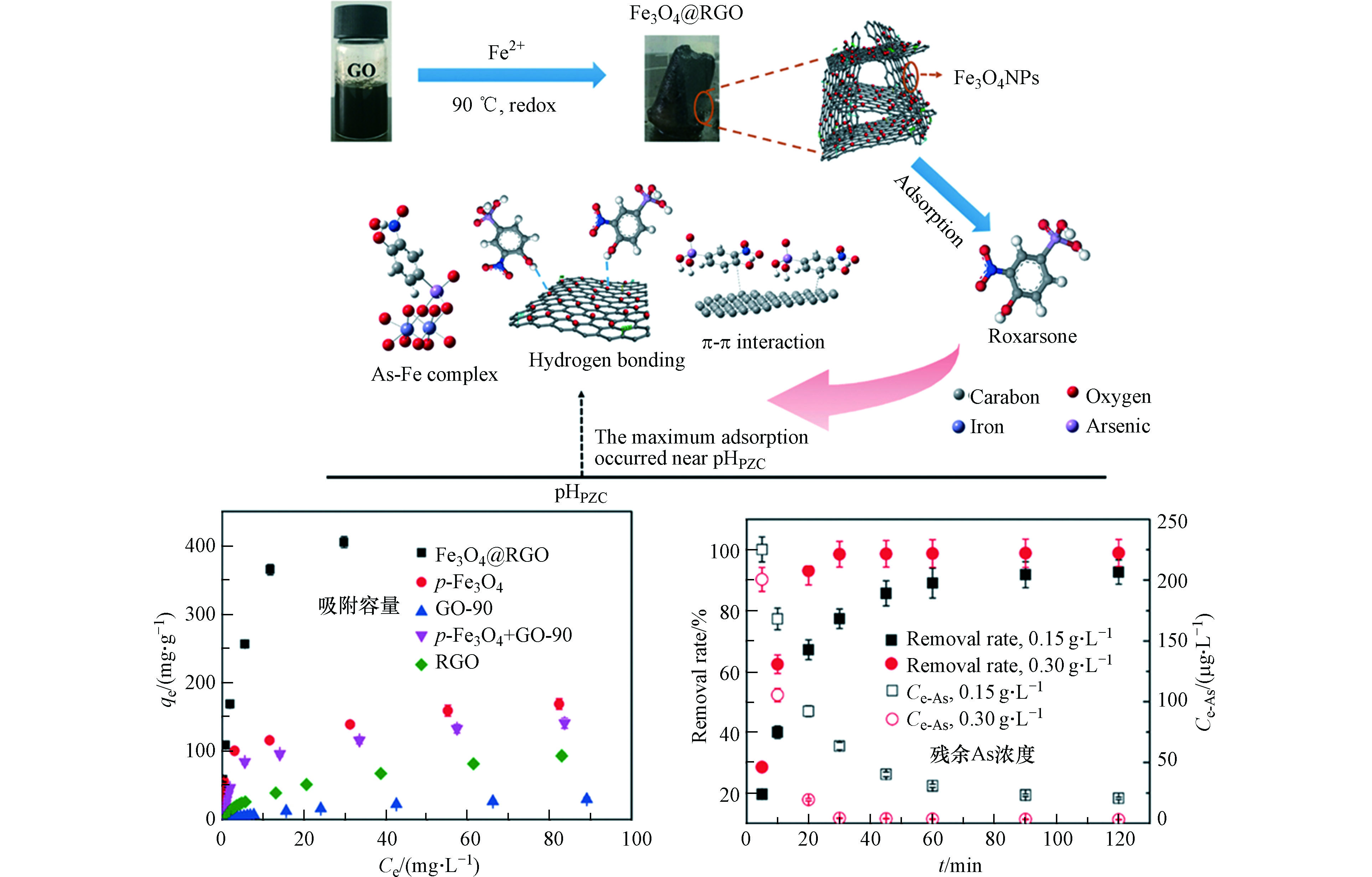
这种协同作用的设计包括两种思路。一种是多种作用力同时作为吸附位点吸附AOCs,并且相互强化,从而达到1+1 > 2的目的[65, 68-69]。基于以上想法,本团队针对ROX的结构特点设计了一种Fe3O4@RGO三维复合材料,利用Fe3O4对ROX的As—O—Fe配位作用,协同RGO对ROX的π—π作用,同步强化材料对ROX的吸附容量和亲和力[70]。结果表明,两种材料的复合不仅同时引入了两种吸附作用力,还对Fe3O4配位ROX的优势晶面进行了调控,并且大量增加了表面羟基的含量,一方面形成了氢键作为新的吸附作用力,另一方面极大强化了As—O—Fe配位作用,使复合材料对1—100 mg·L−1的ROX吸附容量为454 mg·g−1,亲和力780 L·g−1。与四氧化三铁和石墨烯的混合物相比,吸附容量和亲和力分别提高了4倍和11倍,并且可以在90 min内将实际含ROX废水中的As浓度降至50 μg·L−1以下(图4)。
同时,本团队还将类似的协同机制用于 p-ASA的强化吸附,制备了一系列表面含氨基的Zr基MOF材料UiO-67-(NH2)2,利用其Zr核心与 p-ASA砷酸根间的As—O—Zr配位作用,配体与 p-ASA有机基团间的π-π和氢键作用,协同强化对 p-ASA的吸附效果(图5)[71]。结果表明,在p-ASA浓度1—100 mg·L−1范围内,吸附容量是未改性的UiO-67的1.5倍,同时亲和力提高了3倍。结合EXAFS、XPS分析和DFT计算表明,UiO-67-(NH2)2中引入的氨基,可在吸附p-ASA过程中与其它功能团间产生协同作用,一方面增强了MOF与p-ASA间的As—O—Zr配位和π—π作用,另一方面通过氢键形成了新的吸附位点。这种协同作用显著提高了UiO-67-(NH2)2对p-ASA的亲和力,可将实际废水中不同浓度的p-ASA有效降至中国地表水标准(50 μg·L−1)及WHO饮用水标准中(10 μg·L−1)的砷浓度以下,同时用量仅为仅为活性炭的1/40。
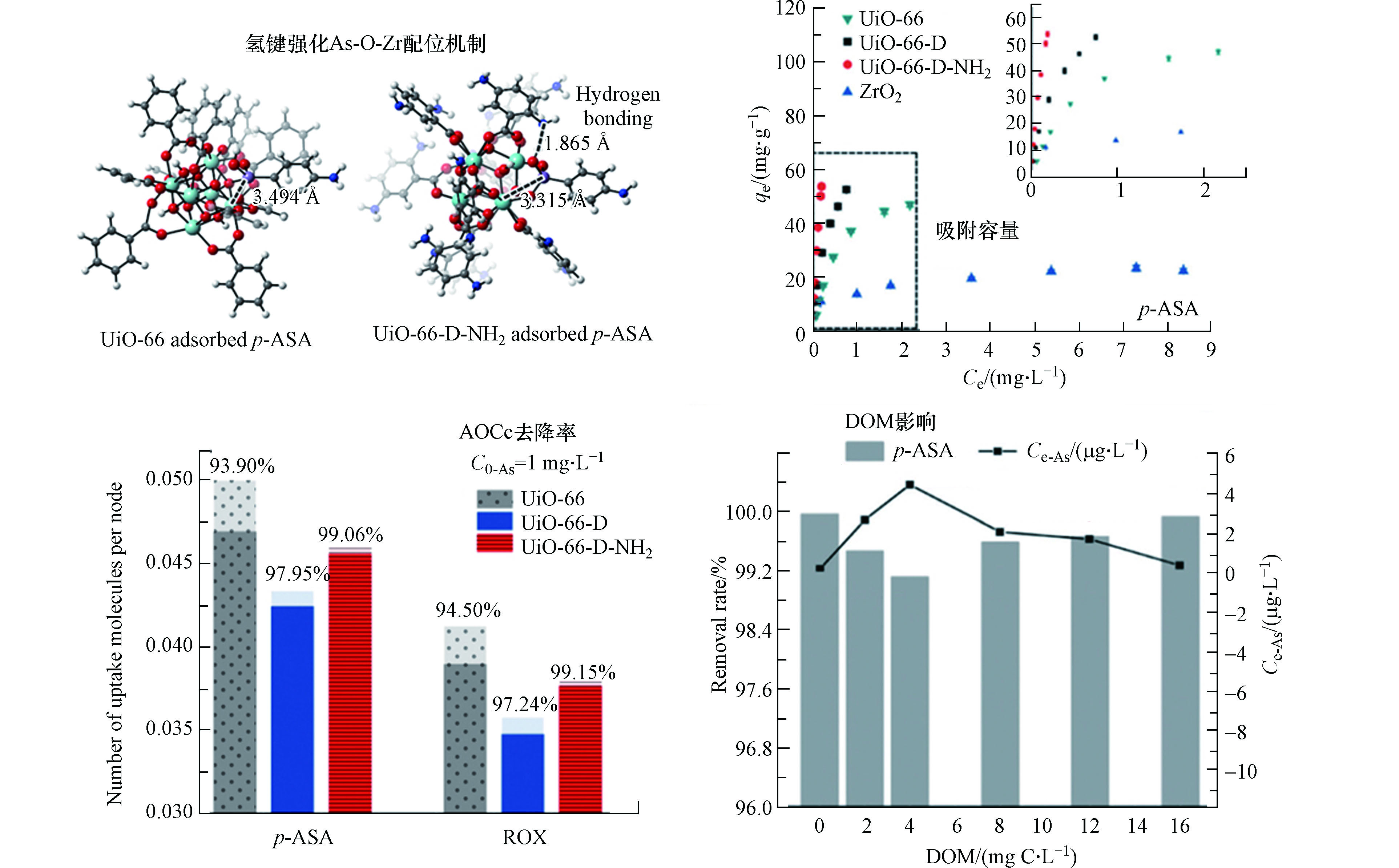
除了同步强化多种吸附作用力外,对于实际环境中的低浓度有机砷,另一种协同思路是集中强化核心作用力,并降低弱作用力的影响,即以最强的吸附作用力为核心,其它作用力不作为单独的吸附位点,而是作为辅助作用力对核心作用力进行强化。这种集中强化作用对吸附容量的提高有限,但可使亲和力得到极大提升,非常有利于低浓度污染物的去除。鉴于重金属配位是吸附AOCs的最强作用力之一,本团队在Zr基MOFs材料UiO-66的金属簇表面制造缺陷来增加As-O-Zr配位作用的位点,同时在其配体上引入氨基,通过缺陷协同氢键强化MOF对低浓度AOCs的重金属配位作用,从而进一步提高亲和力(图6)[72]。结果表明,改性后的UiO-66-D-NH2对1—10 mg·L−1的AOCs亲和力高达7.33 × 103 L·g−1,对初始浓度10 mg·L−1和1 mg·L−1的AOCs去除率分别高于98%和99%。机理研究表明,UiO-66-D-NH2的表面缺陷使其暴露更多的Zr-OH位点,促进了其对AOCs的配位模式从As—O—Zr单齿单核配位向更稳定的双齿双核配位转变。氨基改性后,UiO-66-D-NH2配体中的氨基可与AOCs的砷酸根中的羟基间形成0.186 nm的短程氢键,使两者整体的结合能下降了19 kJ·mol−1,进一步强化了双齿配位络合物的稳定性。这种集中强化核心作用力的协同机制使UiO-66-D-NH2对低浓度AOCs的亲和力和去除效果均得到极大提高,并极大减弱了环境杂质对吸附效果的影响,在DOM浓度为16 mg·L−1C时,对AOCs的去除效果仍高达99%以上,可将实际含AOCs废水中的砷浓度降至2.5 μg·L−1以下。
-
微生物降解(包括有氧及无氧降解)也是去除AOCs的有效方法之一。其中,厌氧条件下的微生物降解研究更为深入[73]。Han等报道了产电细菌(EEB)介导的厌氧条件下的ROX生物还原降解。发现在ROX降解过程中,胞内和胞外降解同时发生,As(Ⅲ)是主要的降解产物[74]。加入蒽醌和2,6-二磺酸盐可有效促进ROX的还原。许多环境因素,如温度、水分、有机质及电子给体等,均会影响AOCs在厌氧环境下的降解。当污泥在15 ℃或20 ℃下保持30 h时,其中大约10%的ROX会转化为无机砷、DMA和其它未鉴别出形态的砷化合物。同时,降解速率随温度的上升而提高。有机质会给微生物提供营养,从而加速AOCs的生物转化。Stolz等通过DFT计算了ROX在厌氧条件下的电子结构,发现ROX的降解更倾向于—NO2基团被攻击,而不是砷酸根被攻击,因此降解过程中无机砷的释放可能不是来源于As—C键的断裂,而是涉及到苯环的裂解[75]。同时,AOCs的有氧生物降解在近年来也有所研究。Guzma’n-Fierro等从ROX污染的土壤中分离出了甲型变形菌和厚壁菌,并将这类好氧菌用于ROX的去除[76]。发现81.04%的ROX可在一周的孵化内被去除。虽然好氧菌及厌氧菌均可降解AOCs,但AOCs更倾向与在厌氧环境下发生降解[77]。然而,厌氧降解比好氧降解更容易造成后续的砷污染,因此AOCs在好氧及厌氧条件下的微生物降解机制及影响因素仍需进一步深入研究。
-
动物饲料添加剂中AOCs的使用,不可避免地导致其中的砷向环境中释放,造成砷污染的潜在危害。虽然中国、美国、欧盟都已禁止了AOCs在饲料添加剂中的使用,但在许多其它国家的使用仍然相当广泛。因此,了解AOCs在环境中的迁移转化,并快速高效地进行去除,对降低环境砷污染风险具有重要意义。本综述总结了现有关于AOCs迁移转化、环境影响及去除方法的研究,包括降解、吸附、微生物法等。可以看出,由于AOCs结构相对复杂,具有有机-无机官能团共存的特点,且降解后会产生毒性更高的无机砷,因此其转化过程和去除方法与传统的有机污染物和砷污染物相比,均存在较大差异。现有研究虽然取得的了部分成果,但与无机砷的研究范围及深度相比,仍相距甚远。可以说,对AOCs的研究尚处在初始阶段,仍有许多有待完善之处。基于本文综述,以下问题或将是未来研究的重点:
(1)对AOCs及其降解产物的定性、定量检测手段尚不完善。AOCs在废水及环境中的浓度都很低,尤其是在环境中,往往是μg·L−1级别甚至更低。且降解过程中的中间产物复杂。而现有的分析检测手段,如HPLC-ICP-MS只能简单的分类定量As(Ⅴ)、As(Ⅲ)、p-ASA、ROX等常见的As物种,且精度仅能达到1 μg·L−1左右,使AOCs降解过程中很多含砷中间产物,无法准确定性及定量,使AOCs降解途径及环境影响的准确分析仍然十分困难。因此,发展各种形态的AOCs的定性定量分析方法,进一步鉴别AOCs降解过程中的中间产物,对于准确评估AOCs的环境风险具有重要意义。
(2)目前通过降解法(生物/非生物降解)去除AOCs,过程中会产生毒性更大的无机砷。虽然已有通过沉淀、絮凝、吸附等方法对无机砷进行后续去除,但去除过程往往需要外加药剂或调节pH,造成处理工艺复杂化,且仍有向环境中释放无机砷的风险。因此,一步降解-固定AOCs的处理方法及机制仍需深入研究,如使用微生物-铁基纳米材料复合体系、非均相Fenton体系等。在简化处理工艺的同时,强化对降解产生的无机砷的深度固定,进一步降低其向环境中排放的风险。
(3)吸附法虽然能在不改变AOCs结构的基础上将其直接去除,但AOCs极低的浓度、大量的共存杂离子对吸附材料的亲和力提出了很高的要求。然而,目前对AOCs吸附材料的研究还多集中在如何提高材料比表面积,增加活性位点,从而提高对AOCs的吸附容量方面,对亲和力的关注甚少。同时,目前AOCs的吸附还多在高浓度范围内,最高达到500 mg·L−1,即使相对低浓度也有10 mg·L−1左右,远远高于实际废水和环境中的AOCs浓度,很难准确评估材料对实际环境中AOCs的吸附效果。因此,如何通过强化吸附过程中的化学作用力来提高材料对AOCs的亲和力,同时研究材料在低AOCs浓度下的吸附效果,可能是今后吸附法的研究重点。
(4)目前对AOCs的迁移转化及去除研究多集中在水体系中,土壤中的研究还相对较少。事实上,水和土壤是环境中两个连通且相互影响的介质,且土壤中本身也存在多种对砷有固定及降解作用的矿物(如Fe、Mn)等。因此,加强AOCs在土壤中的迁移转化及相应的修复方法的研究,进一步进行AOCs在多环境介质中的联合转化研究,可为AOCs在环境中的全生命周期分析风险评估提供理论依据。
新型芳香族有机砷污染物的环境行为及去除研究进展
An emerging pollutant of aromatic organoarsenic compounds: Environmental transfer and remediation methods
-
摘要: 砷作为一种剧毒的重金属元素,其环境行为和去除方法一直受到国内外的广泛关注。目前,无机砷在全球范围内的迁移转化、毒性和去除方法已经进行了深入的研究并取得了一系列成果。与之相比,芳香族有机砷化合物(AOCs)作为一种新型的有机-无机复合型砷污染物,其相关研究仍然较少。AOCs作为动物饲料添加剂的一种,在使用过程中有向环境中释放无机砷的风险,因此逐渐成为砷污染领域的新关注点之一。本文综述了AOCs类污染物的来源、环境行为及去除方法的研究进展,对不同去除方法的效果及机制进行了对比分析,并针对现有研究中存在的不足和问题,提出了对AOCs污染物未来研究趋势的展望,对环境中的砷污染防治具有重要意义。Abstract: Arsenic is a highly toxic heavy metal element. Its environmental transfer and remediation method have been a major global concern for a long time. At present, the migration, toxicity and removal of inorganic arsenic have been studied in depth, which have made a series of achievements. However, as a new type of organic-inorganic arsenic contaminant, the environmental transfer and removal of aromatic organoarsenic compounds (AOCs) are relatively less studied. As a kind of animal feed additive, AOCs have the risk of releasing inorganic arsenic into environment during their use, and thus has become one of the emerging concerns for of arsenic remediation. This paper reviewed the research progress in the origin, transfer and removal of AOCs pollutants. The removal effect and mechanism of different remediation methods were summarized and discussed by contrast. In view of the shortcomings and problems existing in current research, the prospect and research trend of AOCs pollutants in the future were also proposed. The results and discussion of this paper is of great significance to the prevention and control of arsenic pollution in environment.
-
Key words:
- organoarsenic /
- migration and transformation /
- degradation /
- adsorption /
- organic-inorganic synergism
-

-
图 1 p-ASA的光降解过程[24]
Figure 1. Proposed photodegradation pathway of p-ASA
图 2 CuFe2O4活化过硫酸盐对p-ASA去除机制(CuFe2O4活化PMS产生SO4−、·OH和O2·−等多种自由基,将p-ASA氧化为As(Ⅲ)和As(Ⅴ),产生的无机砷被直接吸附在CuFe2O4表面)[41]
Figure 2. Removal mechanism of p-ASA by CuFe2O4 activated peroxymonosulfate process
图 3 非均相Fenton体系对p-ASA去除机制(FeS2与H2O2反应产生·OH将p-ASA降解为As(Ⅴ),一部分As(Ⅴ)被FeS2氧化产生的FeOOH直接吸附,一部分As(Ⅴ)通过调节pH与Fe(Ⅲ)共沉淀)[41]
Figure 3. Removal mechanism of p-ASA by heterogeneous Fenton system
图 4 Fe3O4@RGO三维复合材料对ROX有机-无机协同吸附机制,吸附容量及残余砷浓度[70]
Figure 4. Organic-inorganic synergetic mechanism of ROX adsorption on Fe3O4@RGO and the corresponding adsorption capacity and residual As contents.
图 5 UiO-67-(NH2)2对p-ASA的协同吸附机制,吸附容量及残余砷浓度[71]
Figure 5. Synergetic mechanism of p-ASA adsorption on UiO-67-(NH2)2 and the corresponding adsorption capacity and residual As contents.
图 6 缺陷协同氢键强化重金属配位机制,对低浓度p-ASA的吸附容量,去除率及在不同DOM浓度下砷的残余浓度[72]
Figure 6. Synergetic mechanism of defects and hydrogen bonds on As—O—Zr coordination for p-ASA adsorption by UiO-66-D-NH2 and the corresponding adsorption capacity, removal rate, and the residual As contents with different DOM concentrations
表 1 AOC类饲料添加剂的物化性质及用量
Table 1. Physicochemical property and dosage of AOC feed additives.
续表1 添加剂种类
Additivies分子式
Molecular formula化学结构
Chemical structure电离常数
pKa用量/(mg·kg−1)
Dosage参考文献
References硝苯胂酸
NITC6H6AsNO5 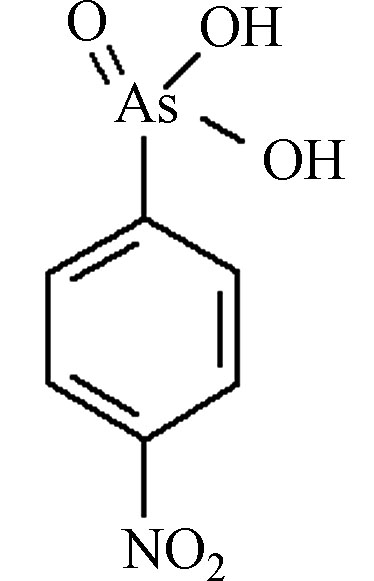
pKa1 = 2.20
pKa2 = 7.78375 [13] 表 2 降解法对AOCs的去除效果
Table 2. Degradation effects of AOCs via various methods.
降解方法
Degradation method去除原理
Degradation mechanismAOCs降解率
Degradation rate优势及不足 紫外/可见光降解 光引发产生活性氧自由基(1O2、·OH等)攻击AOCs结构中的As-C键,降解产生无机砷和芳香族有机物 >90% 可用于研究AOCs在环境中的降解过程;产生的无机砷毒性更强 氧化光降解 H2O2、O3、PMS、PDS等强氧化剂在紫外/可见光下产生活性氧自由基,攻击AOCs结构中的As-C键 可达100% 降解率高于紫外/可见光降解;需要沉淀、吸附等方式进一步去除产生的无机砷 光催化氧化 TiO2等光催化剂催化产生的·OH引发AOCs结构中As-C键的断裂 可达100% 降解率高,可在一定程度上同步吸附产生的无机砷;不同的催化剂吸附效果相差较大 均相Feton降解 Fe(II)催化产生的·OH使AOCs结构中As-C键氧化断裂,产生无机砷和芳香族有机物 可达100% 降解率高;pH适用范围范围较窄,后续需通过调控pH进一步去除无机砷 类Feton降解 过硫酸盐等催化剂催化产生SO4-、·OH和O2·-等多种自由基降解AOCs 90%—100% pH适用范围广;后续需通过吸附进一步去除无机砷 非均相Feton降解 铁氧化物及硫化物催化产生·OH降解AOCs >98% 可实现AOCs的高效降解和无机砷的同步去除;pH适用范围范围较窄 高铁酸盐氧化 氧原子从Fe(VI)转移到AOCs,降解产生无机砷和羟基化有机物 >99% 材料用量少,可实现AOCs的降解-吸附同步去除;受磷酸盐等阴离子影响较大 表 3 各种吸附作用力对AOCs的吸附效果
Table 3. Adsorption effects of AOCs via various adsorption interactions.
吸附作用力
Interactions吸附材料
Adsorption materialsAOCs种类
AOC types浓度/(mg·L−1)
Concentrationsqm/
(mg·g−1)kL/
(L·g−1)参考文献 取向力 多壁碳纳米管(MWCNTs) ROX 40 10 — [51] 静电引力 交联壳聚糖材料(CG) ROX — 490 24 [52] 静电引力 ZIF-8 p-ASA 3—350 790 32 [53] 孔沉积 磁性碳基复合物 5—500 588 63 [54] 氢键 MIL-101(OH)3 p-ASA, PAA 25—100 238
13952
53[55] 氢键 氨基纳米纤维素 ROX 5—100 366 [56] 氢键 NH2-MIL-68(In) p-ASA 5—400 402 55 [57] As-O-Fe/Mn配位 介孔MnFe2O4 p-ASA, ROX 10—400 59
51— [61] As-O-Fe配位 花状CoFe2O4 p-ASA 5—30 46 1640 [62] As-O-Zr配位 水合锆氧化物 p-ASA 0—100 45 41 [63] As-O-Fe配位 MIL-88A(Fe) p-ASA, ROX 1—150 262
428— [65] As-O-Zr配位 缺陷UiO-66 ROX 10—200 780 113 [66] As-O-Zn配位+氢键 BUC-10 p-ASA, ROX 10—300 937
738— [68] As-O-Fe配位+氢键+静电+π-π MIL-88A(Fe) p-ASA, ROX 1—150 147 19 [69] As-O-Fe配位+氢键+π-π Fe3O4@RGO ROX 1—100 454 780 [70] As-O-Zr配位+氢键+π-π UiO-67-(NH2)2 p-ASA 1—100 167 830 [71] As-O-Zr配位+氢键 UiO-66-D-NH2 p-ASA, ROX 1—10 98
916380
7330[72] -
[1] PODGORSKI J, BERG M. Global threat of arsenic in groundwater [J]. Science, 2020, 368(6493): 845. doi: 10.1126/science.aba1510 [2] JONES F T. A broad view of arsenic [J]. Poultry Science, 2007, 86(1): 2-14. doi: 10.1093/ps/86.1.2 [3] 李雪霞. 对饲料中大量添加有机砷制剂的思考 [J]. 云南畜牧兽医, 2008(6): 35-36. doi: 10.3969/j.issn.1005-1341.2008.06.029 LI X X. Thinking about the large amount of organic arsenic preparation added to feed [J]. Yunnan Journal of Animal Science and Veterinary Medicine, 2008(6): 35-36(in Chinese). doi: 10.3969/j.issn.1005-1341.2008.06.029
[4] P MANGALGIRI K, ADAK A, BLANEY L. Organoarsenicals in poultry litter:Detection, fate, and toxicity [J]. Environment International, 2015, 75: 68-80. doi: 10.1016/j.envint.2014.10.022 [5] SILBERGELD E K, NACHMAN K. The environmental and public health risks associated with arsenical use in animal feeds [J]. Annals of the New York Academy of Sciences, 2008, 1140: 346-357. doi: 10.1196/annals.1454.049 [6] GUPTA S K, LE X C, KACHANOSKY G, et al. Transfer of arsenic from poultry feed to poultry litter:A mass balance study [J]. Science of the Total Environment, 2018, 630: 302-307. doi: 10.1016/j.scitotenv.2018.02.123 [7] GARBARINO J R, BEDNAR A J, RUTHERFORD D W, et al. Environmental fate of roxarsone in poultry litter.I.Degradation of roxarsone during composting [J]. Environmental Science & Technology, 2003, 37(8): 1509-1514. [8] BROWN B L, SLAUGHTER A D, SCHREIBER M E. Controls on roxarsone transport in agricultural watersheds [J]. Applied Geochemistry, 2005, 20(1): 123-133. doi: 10.1016/j.apgeochem.2004.06.001 [9] FISHER D J, YONKOS L T, STAVER K W. Environmental concerns of roxarsone in broiler poultry feed and litter in Maryland, USA [J]. Environmental Science & Technology, 2015, 49(4): 1999-2012. [10] HU Y, CHENG H, TAO S, et al. China's ban on phenylarsonic feed additives, A major step toward reducing the human and ecosystem health risk from arsenic [J]. Environmental Science & Technology, 2019, 53(21): 12177-12187. [11] QIANG ZM, ADAMS C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics [J]. Water Research, 2004, 38(12): 2874-2890. doi: 10.1016/j.watres.2004.03.017 [12] JOSHI T P, ZHANG G, JEFFERSON W A, et al. Adsorption of aromatic organoarsenic compounds by ferric and manganese binary oxide and description of the associated mechanism [J]. Chemical Engineering Journal, 2017, 309: 577-587. doi: 10.1016/j.cej.2016.10.084 [13] ADAK A, MANGALGIRI K P, LEE J, et al. UV irradiation and UV-H2O2 advanced oxidation of the roxarsone and nitarsone organoarsenicals [J]. Water Research, 2015, 70: 74-85. doi: 10.1016/j.watres.2014.11.025 [14] NACHMAN K E, BARON P A, RABER G, et al. Roxarsone, inorganic arsenic, and other arsenic species in chicken:A US-based market basket sample [J]. Environmental Health Perspectives, 2013, 121(7): 818-824. doi: 10.1289/ehp.1206245 [15] LIU X, ZHANG W, HU Y, et al. Extraction and detection of organoarsenic feed additives and common arsenic species in environmental matrices by HPLC-ICP-MS [J]. Microchemical Journal, 2013, 108: 38-45. doi: 10.1016/j.microc.2012.12.005 [16] 孙永学, 陈杖榴. 有机胂添加剂的毒性、代谢及环境行为研究进展 [J]. 动物毒物学, 2004(1): 7-10. SUN Y X, CHEN Z L. Research Progress on toxicity, metabolism and environmental behavior of organic arsenic additives [J]. Journal of animal toxicology, 2004(1): 7-10(in Chinese).
[17] ARROYO-ABAD U, MATTUSCH J, MOEDER M, et al. Identification of roxarsone metabolites produced in the system:Soil-chlorinated water-light by using HPLC-ICP-MS/ESI-MS, HPLC-ESI-MS/MS and High Resolution Mass Spectrometry (ESI-TOF-MS) [J]. Journal of Analytical Atomic Spectrometry, 2011, 26(1): 171-177. doi: 10.1039/C0JA00105H [18] WANG L, CHENG H. Birnessite (δ-MnO2) mediated degradation of organoarsenic feed additive p-Arsanilic acid [J]. Environmental Science & Technology, 2015, 49(6): 3473-3481. [19] D'ANGELO E, ZEIGLER G, BECK E G, et al. Arsenic species in broiler (Gallus gallus domesticus) litter, soils, maize (Zea mays L.), and groundwater from litter-amended fields [J]. Science of the Total Environment, 2012, 438: 286-292. doi: 10.1016/j.scitotenv.2012.08.078 [20] 王克俭, 廖新俤. 猪场周围环境中砷的分布及迁移规律研究 [J]. 家畜生态学报, 2005, 26(2): 29-32. doi: 10.3969/j.issn.1673-1182.2005.02.007 WANG K J, LIAO X D. Study on the distribution and migrating disciplinavian of arsenic around the pig farm [J]. Acta Ecologiae Animalis Domastici, 2005, 26(2): 29-32(in Chinese). doi: 10.3969/j.issn.1673-1182.2005.02.007
[21] SIERRA-ALVAREZ R, CORTINAS I, FIELD J A. Methanogenic inhibition by roxarsone (4-hydroxy-3-nitrophenylarsonic acid) and related aromatic arsenic compounds [J]. Journal of Hazardous Materials, 2010, 175(1): 352-358. [22] 奚功芳. 典型有机胂在土壤-蔬菜系统中的迁移残留规律研究[D]. 芜湖: 安徽师范大学, 2014. XI G F. Study on the migration and residue of typical organoarsenic in soil vegetable system[D].Wuhu: Anhui Normal University, 2014(in Chinese).
[23] HU Y, ZHANG W, CHENG H, et al. Public health risk of arsenic species in chicken tissues from live poultry markets of Guangdong Province, China [J]. Environmental Science & Technology, 2017, 51(6): 3508-3517. [24] ZHU X, WANG Y, LIU C, et al. Kinetics, intermediates and acute toxicity of arsanilic acid photolysis [J]. Chemosphere, 2014, 107: 274-281. doi: 10.1016/j.chemosphere.2013.12.060 [25] LI S, XU J, CHEN W, et al. Multiple transformation pathways of p-arsanilic acid to inorganic arsenic species in water during UV disinfection [J]. Journal of Environmental Sciences, 2016, 47: 39-48. doi: 10.1016/j.jes.2016.01.017 [26] XIE X, HU Y, CHENG H. Mechanism, kinetics, and pathways of self-sensitized sunlight photodegradation of phenylarsonic compounds [J]. Water Research, 2016, 96: 136-147. doi: 10.1016/j.watres.2016.03.053 [27] LIU X, ZHANG W, HU Y, et al. Arsenic pollution of agricultural soils by concentrated animal feeding operations (CAFOs) [J]. Chemosphere, 2015, 119: 273-281. doi: 10.1016/j.chemosphere.2014.06.067 [28] CZAPLICKA M, BRATEK A, JAWOREK K, et al. Photo-oxidation of p-arsanilic acid in acidic solutions: Kinetics and the identification of by-products and reaction pathways [J]. Chemical Engineering Journal, 2014, 243: 364-371. doi: 10.1016/j.cej.2014.01.016 [29] CZAPLICKA M, JAWOREK K, BĄK M. Study of photodegradation and photooxidation of p-arsanilic acid in water solutions at pH = 7:Kinetics and by-products [J]. Environmental Science and Pollution Research, 2015, 22(21): 16927-16935. doi: 10.1007/s11356-015-4890-z [30] CHEN L, LI H, QIAN J. Degradation of roxarsone in UV-based advanced oxidation processes: A comparative study [J]. Journal of Hazardous Materials, 2020: 124558. [31] XU T, KAMAT P V, JOSHI S, et al. Hydroxyl radical mediated degradation of phenylarsonic acid [J]. The Journal of Physical Chemistry A, 2007, 111(32): 7819-7824. doi: 10.1021/jp072135y [32] ZHENG S, CAI Y, O'SHEA K E. TiO2 photocatalytic degradation of phenylarsonic acid [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2010, 210(1): 61-68. doi: 10.1016/j.jphotochem.2009.12.004 [33] DENG Y, TANG L, ZENG G, et al. Enhanced visible light photocatalytic performance of polyaniline modified mesoporous single crystal TiO2 microsphere [J]. Applied Surface Science, 2016, 387: 882-893. doi: 10.1016/j.apsusc.2016.07.026 [34] XIAO R, GAO L, WEI Z., et al Mechanistic insight into degradation of endocrine disrupting chemical by hydroxyl radical:An experimental and theoretical approach [J]. Environmental Pollution, 2017, 231: 1446-1452. doi: 10.1016/j.envpol.2017.09.006 [35] LU D, JI F, WANG W, et al. Adsorption and photocatalytic decomposition of roxarsone by TiO2 and its mechanism [J]. Environmental Science and Pollution Research, 2014, 21(13): 8025-8035. doi: 10.1007/s11356-014-2729-7 [36] MIRANDA C, SANTANDER P, MATSCHULLAT J, et al. Degradation of organoarsenicals by heterogeneous photocatalysis using ZnO, TiO2 and UVA [J]. Journal of Advanced Oxidation Technologies, 2016, 19(2): 276-283. [37] MENG J, XU F, YUAN S, et al. Photocatalytic oxidation of roxarsone using riboflavin-derivative as a photosensitizer [J]. Chemical Engineering Journal, 2019, 355: 130-136. doi: 10.1016/j.cej.2018.08.127 [38] QIN J, LI H, LIN C. Fenton process-affected transformation of roxarsone in paddy rice soils:Effects on plant growth and arsenic accumulation in rice grain [J]. Ecotoxicology and Environmental Safety, 2016, 130: 4-10. doi: 10.1016/j.ecoenv.2016.03.047 [39] XIE X, HU Y, CHENG H. Rapid degradation of p-arsanilic acid with simultaneous arsenic removal from aqueous solution using Fenton process [J]. Water Research, 2016, 89: 59-67. doi: 10.1016/j.watres.2015.11.037 [40] LIU Y, HU P, ZHENG J, et al. Utilization of spent aluminum for p-arsanilic acid degradation and arsenic immobilization mediated by Fe(Ⅱ) under aerobic condition [J]. Chemical Engineering Journal, 2016, 297: 45-54. doi: 10.1016/j.cej.2016.03.092 [41] CHEN S, DENG J, YE C, et al. Simultaneous removal of Para-arsanilic acid and the released inorganic arsenic species by CuFe2O4 activated peroxymonosulfate process [J]. Science of the Total Environment, 2020, 742: 140587. doi: 10.1016/j.scitotenv.2020.140587 [42] CHEN C, LIU L, LI Y, et al. Efficient degradation of roxarsone and simultaneous in-situ adsorption of secondary inorganic arsenic by a combination of Co3O4-Y2O3 and peroxymonosulfate [J]. Journal of Hazardous Materials, 2021, 407: 124559. doi: 10.1016/j.jhazmat.2020.124559 [43] ZHAO Z, PAN S, YE Y, et al. FeS2/H2O2 mediated water decontamination from p-arsanilic acid via coupling oxidation, adsorption and coagulation: Performance and mechanism [J]. Chemical Engineering Journal, 2020, 381: 122667. doi: 10.1016/j.cej.2019.122667 [44] LI B, WEI D, LI Z., et al Mechanistic insights into the enhanced removal of roxsarsone and its metabolites by a sludge-based, biochar supported zerovalent iron nanocomposite:Adsorption and redox transformation [J]. Journal of Hazardous Materials, 2020, 389: 122091. doi: 10.1016/j.jhazmat.2020.122091 [45] YANG T, WANG L, LIU Y., et al Removal of organoarsenic with ferrate and ferrate resultant nanoparticles:Oxidation and adsorption [J]. Environmental Science & Technology, 2018, 52(22): 13325-13335. [46] YANG T, LIU Y, WANG L, et al. Highly effective oxidation of roxarsone by ferrate and simultaneous arsenic removal with in situ formed ferric nanoparticles [J]. Water Research, 2018, 147: 321-330. doi: 10.1016/j.watres.2018.10.012 [47] XIE X, CHENG H. A simple treatment method for phenylarsenic compounds: Oxidation by ferrate (VI) and simultaneous removal of the arsenate released with in situ formed Fe(Ⅲ) oxide-hydroxide [J]. Environment International, 2019, 127: 730-741. doi: 10.1016/j.envint.2019.03.059 [48] XIE X, ZHAO W, HU Y, et al. Permanganate oxidation and ferric ion precipitation (KMnO4-Fe(Ⅲ)) process for treating phenylarsenic compounds [J]. Chemical Engineering Journal, 2019, 357: 600-610. doi: 10.1016/j.cej.2018.09.194 [49] YANG K, XING B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase:Polanyi theory and its application [J]. Chemical Reviews, 2010, 110(10): 5989-6008. doi: 10.1021/cr100059s [50] CAO Q, HUANG F, ZHUANG Z, et al. A study of the potential application of nano-Mg(OH)2 in adsorbing low concentrations of uranyl tricarbonate from water [J]. Nanoscale, 2012, 4(7): 2423-2430. doi: 10.1039/c2nr11993e [51] HU J, TONG Z, HU Z., et al Adsorption of roxarsone from aqueous solution by multi-walled carbon nanotubes [J]. Journal of Colloid and Interface Science, 2012, 377(1): 355-361. doi: 10.1016/j.jcis.2012.03.064 [52] POON L, YOUNUS S, WILSON L D. Adsorption study of an organo-arsenical with chitosan-based sorbents [J]. Journal of Colloid and Interface Science, 2014, 420: 136-144. doi: 10.1016/j.jcis.2014.01.003 [53] JUNG B K, JUN J W, HASAN Z, et al. Adsorptive removal of p-arsanilic acid from water using mesoporous zeolitic imidazolate framework-8 [J]. Chemical Engineering Journal, 2015, 267: 9-15. doi: 10.1016/j.cej.2014.12.093 [54] ZHU X, QIAN F, LIU Y, et al. Environmental performances of hydrochar-derived magnetic carbon composite affected by its carbonaceous precursor [J]. RSC Advances, 2015, 5(75): 60713-60722. doi: 10.1039/C5RA07339A [55] SARKER M, SONG J Y, JHUNG S H. Adsorption of organic arsenic acids from water over functionalized metal-organic frameworks [J]. Journal of Hazardous Materials, 2017, 335: 162-169. doi: 10.1016/j.jhazmat.2017.04.044 [56] LIU K, HUANG Z, DAI J, et al. Fabrication of amino-modified electrospun nanofibrous cellulose membrane and adsorption for typical organoarsenic contaminants: Behavior and mechanism [J]. Chemical Engineering Journal, 2020, 382: 122775. doi: 10.1016/j.cej.2019.122775 [57] LV Y, ZHANG R, ZENG S, et al. Removal of p-arsanilic acid by an amino-functionalized indium-based metal-organic framework: Adsorption behavior and synergetic mechanism [J]. Chemical Engineering Journal, 2018, 339: 359-368. doi: 10.1016/j.cej.2018.01.139 [58] CHEN W, HUANG C. Surface adsorption of organoarsenic roxarsone and arsanilic acid on iron and aluminum oxides [J]. Journal of Hazardous Materials, 2012, 227-228: 378-385. doi: 10.1016/j.jhazmat.2012.05.078 [59] MITCHELL W, GOLDBERG S, AL-ABADLEH H A. In situ ATR-FTIR and surface complexation modeling studies on the adsorption of dimethylarsinic acid and p-arsanilic acid on iron-(oxyhydr)oxides [J]. Journal of Colloid and Interface Science, 2011, 358(2): 534-540. doi: 10.1016/j.jcis.2011.02.040 [60] CAO S, ZHANG X, HUANG X, et al. Insights into the facet-dependent adsorption of phenylarsonic acid on hematite nanocrystals [J]. Environmental Science: Nano, 2019, 6(11): 3280-3291. doi: 10.1039/C9EN00879A [61] HU Q, LIU Y, GU X, et al. Adsorption behavior and mechanism of different arsenic species on mesoporous MnFe2O4 magnetic nanoparticles [J]. Chemosphere, 2017, 181: 328-336. doi: 10.1016/j.chemosphere.2017.04.049 [62] LIU J, LI B, WANG G, et al. Facile synthesis of flower-like CoFe2O4 particles for efficient sorption of aromatic organoarsenicals from aqueous solution [J]. Journal of Colloid and Interface Science, 2020, 568: 63-75. doi: 10.1016/j.jcis.2020.02.004 [63] ZHAO Z, WU P, FANG Z, et al. Selective sequestration of p-arsanilic acid from water by using nano-hydrated zirconium oxide encapsulated inside hyper-cross-linked anion exchanger [J]. Chemical Engineering Journal, 2020, 391: 123624. doi: 10.1016/j.cej.2019.123624 [64] JUN J, TONG M, JUNG B K, et al. Effect of Central Metal Ions of Analogous Metal-Organic Frameworks on Adsorption of Organoarsenic Compounds from Water:Plausible Mechanism of Adsorption and Water Purification [J]. Chemistry-A European Journal, 2015, 21(1): 347-354. doi: 10.1002/chem.201404658 [65] PANG D, WANG C, WANG P, et al. Superior removal of inorganic and organic arsenic pollutants from water with MIL-88A(Fe) decorated on cotton fibers [J]. Chemosphere, 2020, 254: 126829. doi: 10.1016/j.chemosphere.2020.126829 [66] LI B, ZHU X, HU K, et al. Defect creation in metal-organic frameworks for rapid and controllable decontamination of roxarsone from aqueous solution [J]. Journal of Hazardous Materials, 2016, 302: 57-64. doi: 10.1016/j.jhazmat.2015.09.040 [67] ARTS D, ABDUS SABUR M, AL-ABADLEH H A. Surface interactions of aromatic organoarsenical compounds with hematite nanoparticles using ATR-FTIR:Kinetic studies [J]. The Journal of Physical Chemistry A, 2013, 117(10): 2195-2204. doi: 10.1021/jp311569m [68] WANG C, ZHANG X, WANG J, et al. A new one-dimensional coordination polymer synthesized from zinc and guanazole: Superior capture of organic arsenics [J]. Applied Organometallic Chemistry, 2020, 34(6): e5637. [69] LIU B, LIU Z, WU H, et al. Effective and simultaneous removal of organic/inorganic arsenic using polymer-based hydrated iron oxide adsorbent:Capacity evaluation and mechanism [J]. Science of the Total Environment, 2020, 742: 140508. doi: 10.1016/j.scitotenv.2020.140508 [70] TIAN C, ZHAO J, ZHANG J, et al. Enhanced removal of roxarsone by Fe3O4@3D graphene nanocomposites: Synergistic adsorption and mechanism [J]. Environmental Science: Nano, 2017, 4(11): 2134-2143. doi: 10.1039/C7EN00758B [71] TIAN C, ZHAO J, OU X, et al. Enhanced adsorption of p-arsanilic acid from water by amine-modified UiO-67 as examined using extended X-ray absorption fine structure, X-ray photoelectron spectroscopy, and density functional theory calculations [J]. Environmental Science & Technology, 2018, 52(6): 3466-3475. [72] XU Y, LV J, SONG Y, et al. Efficient removal of low-concentration organoarsenic by Zr-based metal-organic frameworks:Cooperation of defects and hydrogen bonds [J]. Environmental Science:Nano, 2019, 6(12): 3590-3600. doi: 10.1039/C9EN00923J [73] FISHER E, DAWSON A M, POLSHYNA G, et al. Transformation of inorganic and organic arsenic by alkaliphilus oremlandiisp. Nov. Strain OhILAs [J]. Annals of the New York Academy of Sciences, 2008, 1125(1): 230-241. doi: 10.1196/annals.1419.006 [74] HAN J, ZHANG F, CHENG L, et al. Rapid release of arsenite from roxarsone bioreduction by exoelectrogenic bacteria [J]. Environmental Science & Technology Letters, 2017, 4(8): 350-355. [75] STOLZ J F, PERERA E, KILONZO B, et al. Biotransformation of 3-nitro-4-hydroxybenzene arsonic acid (roxarsone) and release of inorganic arsenic by Clostridium species [J]. Environmental Science & Technology, 2007, 41(3): 818-823. [76] GUZMÁN-FIERRO V G, MORAGA R, LEÓN C G, et al. Isolation and characterization of an aerobic bacterial consortium able to degrade roxarsone [J]. International Journal of Environmental Science and Technology, 2015, 12(4): 1353-1362. doi: 10.1007/s13762-014-0512-4 [77] FU Q L, LIU C, ACHAL V, et al. Aromatic arsenical additives (AAAs) in the soil environment: Detection, environmental behaviors, toxicities, and remediation [J]. Advances in Agronomy, 2016, 140: 1-41. -



 下载:
下载: