-
人类社会快速发展的同时也带来了严重的环境污染,特别是印染造成的水污染,如何消除这些污染物是行业可持续发展面临的一个关键问题[1]。光催化降解过程是非均相的高级氧化过程,它吸收自然光产生的光能发生催化作用,将周围的氧分子和水分子激发成具有强氧化性的游离阴离子,而不会产生有害的物质[2-3]。然而诸如二氧化钛( TiO2 )锐钛矿型的Eg>3.2eV,只能吸收紫外光,这严重限制了其在催化领域的应用。相对于 TiO2,硫化镉( CdS )则具有较窄的带隙( Eg=2.4 eV ),因此更容易被可见光所激发,常作为可见光催化剂应用于降解水和空气中的污染物[4-5]。然而,CdS 纳米粒子 (CdS-NPs) 相对不稳定,易发生团簇,这将导致其表面积减少,引起光致电子-空穴对复合率的增高,因此阻碍了其光催化的应用。
金属有机骨架(MOFs) 是一类由无机金属中心(金属离子或金属簇)和悬索连接的有机配体构成的多孔晶体材料,在吸附[6]、催化[7]、传感[8]、储气[9]等领域引起了广泛关注。作为 MOFs 的一种,ZIF-8 具有类沸石的高度多孔拓扑结构以及开放的大通道,这使得该材料容易产生较多的活性位点,并且作为传统催化剂的有效替代品也一直受到很大关注[10-11]。值得注意的是,MOFs 的金属中心和有机配体在光催化反应中起着至关重要的作用,在光的照射下,有机配体吸收光子,过量的电子被激发并通过有机配体运输到金属中心,这样金属中心作为电子受体,具有还原性,而有机配体作为电子给体具有氧化性,进而在表面发生氧化还原反应[12-14]。如今,许多半导体/MOF 复合材料已经开发并应用于增强半导体可见光催化[15-16]。目前常见的有半导体在 MOF 上的原位生长[17];MOFs 在半导体上的异质沉积[18];半导体@MOF核壳复合材料的合成等策略[19]。使用 MOFs 作为半导体载体的优越性体现在 MOFs 的高比表面积非常有利于半导体颗粒的分散防止其发生团聚;MOFs 的高比表面积可以产生更多的催化位点,这将大大增强光激发电子-空穴对的分离;MOFs 的多孔性质也可以为光激发电子的迁移提供额外的途径,从而促进电荷载流子的分离[20]。
本次研究通过在 CdS-NPs 上原位异质沉积 ZIF-8 以获得 ZIF-8 包裹的 ZIF-8/CdS 纳米复合材料用来除去亚甲基蓝( MB ),首先利用溶剂热法制备出分散性较好的 CdS-NPs,之后采用原位异质沉积法在 CdS-NPs 表面生成 ZIF-8,并探究 ZIF-8 的引入对 CdS 的光催化性能带来的影响。
-
在本次研究中,所有化学品和试剂均采购自国药集团化学试剂有限公司(中国上海),未经进一步处理。四水乙酸镉 Cd(CH3COO)2·4H2O、硫脲 (CH4N2S)、六水硝酸锌 (Zn(NO3)2·6H2O) 2-甲基咪唑 (HMeIM) 和聚乙烯吡咯烷酮 (PVP,K-30) (C6H9NO, K = 30) 从阿拉丁(中国上海)获得。 甲醇 (AR)、乙醇 (AR) 和丙酮 (AR) 购自 Macklin(中国上海)。
-
和现有报道的类似,在传统的溶剂热法中加入表面活性剂 PVP-K30 制备了相对分散的 CdS-NPs [21]。将 1.066 g (4 mmol) Cd(CH3COO)2·4H2O、0.3045 g (4 mmol) 硫脲、0.050 g (0.449 mmol) PVP-K30 和乙二醇 (60 mL) 混合并超声处理 40 min直至完全溶解,将溶液在 100 mL 衬有 PTFE 的不锈钢高压釜中加热至 140 ℃,并保持该温度 8 h。黄色产物用一定量的丙酮提纯并用甲醇和乙醇充分洗涤,最后在60 ℃的真空烘箱中干燥。
-
通过原位异质沉积在 CdS-NPs 表面合成 ZIF-8。0.2974 g (1 mmol) Zn(NO3)2·6H2O 和 0.3284 g (4 mmol) HMeIM 分别溶解在 25 mL 甲醇中并超声处理以获得均匀的溶液。将预先分散在 2 mL 甲醇中的 CdS-NPs 溶液缓慢加入到 HMeIM 溶液中,同时磁力搅拌 2 h 得到均匀的淡黄色溶液。之后将盛有 Zn(NO3)2 的甲醇溶液加入到该混合溶液中,磁力搅拌 1 h。将最终的溶液以 8000 r·min−1 离心收集,并用甲醇和去离子水各洗涤3次,再真空冷冻干燥 24 h获得最终产物记为ZC-X。通过改变 CdS-NPs 用量 40、80 mg 制得不同的 ZIF-8/CdS 复合材料记为 ZC-40、ZC-80,同时保持其他条件不变,制得纯的 ZIF-8。
-
采用日本理学 Rigaku Ultima IV X 射线粉末衍射仪对样品的晶体结构进行表征,工作电压和工作电流分别是 45kv,40mA,扫描范围是 5°—80°。采用 Zeiss Sigma 300 扫描电镜(SEM)和蔡司 libra 200透射电镜( TEM )观察样品的形貌与表面组成,采用Thermo Scientific X 射线光电子分析仪确定用于确定复合材料的结晶相,采用全自动比表面及孔隙度分析仪(BET,Quantachrome Autosorb iQ-MP)来确定样品的比表面积和孔隙度,CHI660E 电化学工作站进行电化学性能测试,光电流相应的测试时间为 390 s,每次光照 30 s,避光 30 s,使用配有 420 nm 截止滤光片的 300 W氙灯作为光源,电解质均为 0.5 mol·L−1 的 Na2SO4 溶液,电阻抗则全程在暗环境下进行。同时采用用日立 F-4700 荧光分光光度计和岛津 UV-3600荧光光谱仪测定样品的 UV-vis 漫反射光谱 (DRS)和荧光发射光谱(PL)。
-
选用 500W 的氙灯作为可见光源,将 50 mg 的 ZIF-8/CdS 复合材料加入 100 mL 初始浓度为 15 mg·L−1 的 MB 溶液中,在黑暗条件下匀速搅拌吸附 30 min 后,在打开光源下进行降解测试.并在同等条件下将所有的样品进行对照实验和空白对照,采用日本岛津UV-2600 紫外可见分光光度计测定不同降解时间的 MB 溶液吸光度,分析各个样品的降解率和降解速率,确定出最佳的样品。
-
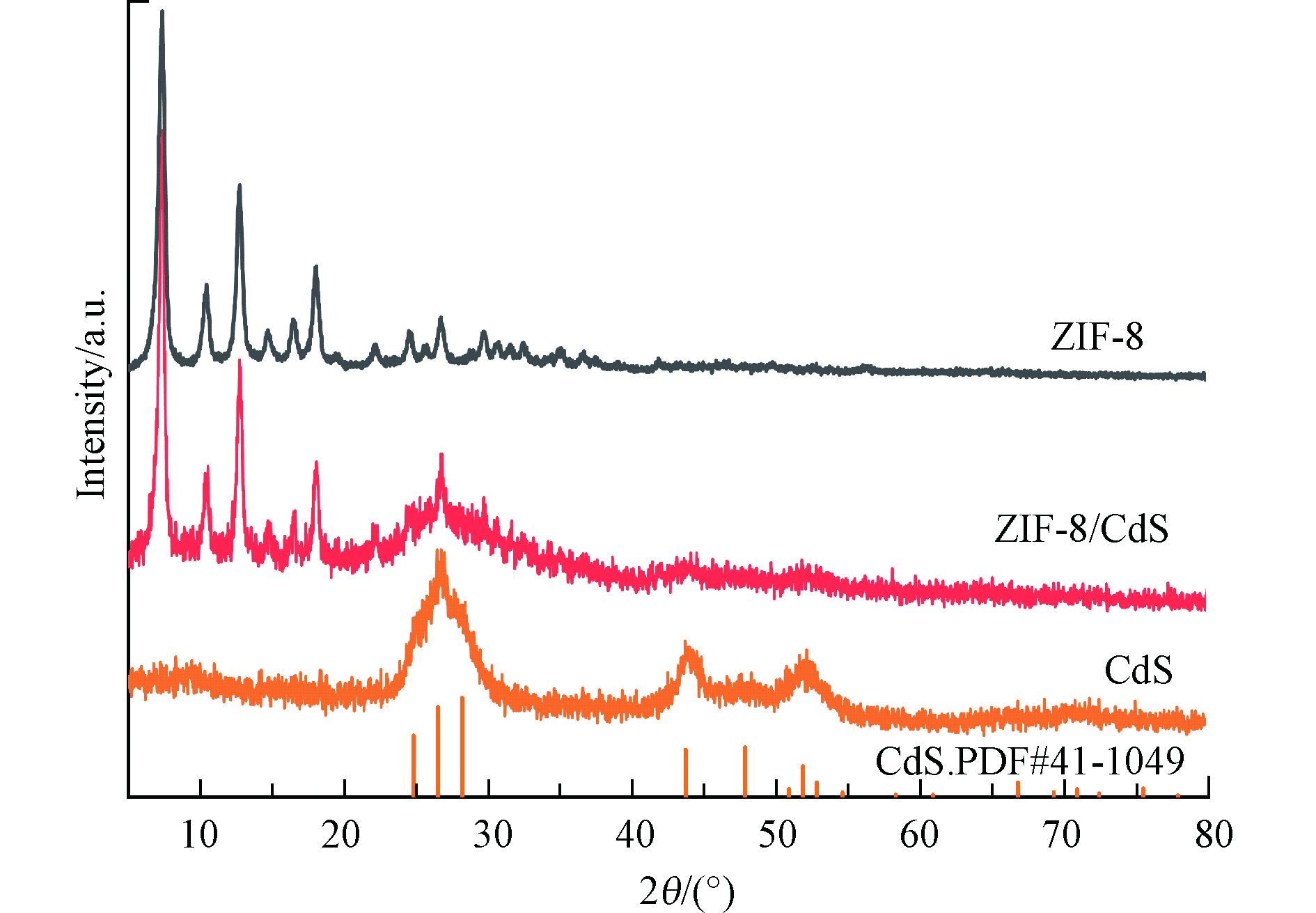
通过粉末 X 射线衍射 (XRD) 分析所制备样品的晶体结构。如图1所示, ZIF-8/CdS光催化剂的 XRD 图谱在2 θ =26.5°、43.8°、51.9°处显示出多个衍射峰,分别对应 CdS的不同晶面,这和CdS的标准卡一致。此外,2 θ= 7.4°、10.4°、12.8°、14.7°、16.5°和18.1°处的衍射峰对应的是具有高结晶度的 ZIF-8 的晶面。这与现有文献的记录相符合[22],证明 ZIF-8/CdS 复合材料的成功合成。
-
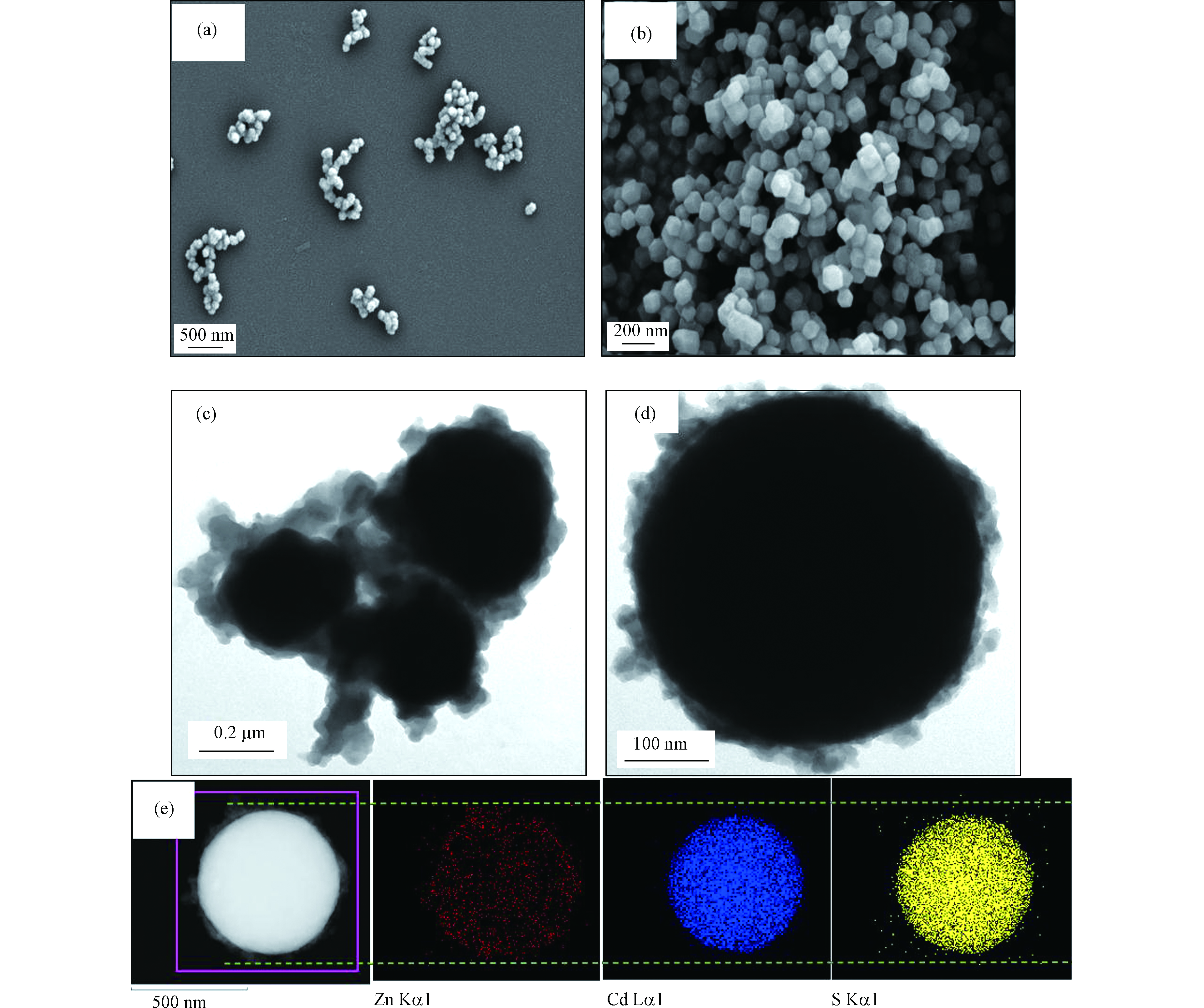
通过 SEM 和 TEM 测量 CdS-NPs 和 ZIF-8/CdS 复合材料的形态和结构。图2(a、b)反映了 CdS-NPs 与 ZIF-8 晶体的形貌特征,图2(a)表明单个的 CdS-NPs 平均粒径为 60 nm 左右,但是由于比表面能等因素,这些颗粒仍表现出局部聚集的形态,由于 PVP 的加入,这种现象得到了一定程度的缓解。图2(b)中均匀分布着 100 nm 左右的ZIF-8晶体,颗粒成长良好,呈十二面体结构。图2(c、d)可以直观的看出, CdS 颗粒的周围充满了规则的 ZIF-8 小颗粒,表明 ZIF-8 纳米晶体在 CdS 表面成功生长。同时,本次研究还表征了 CdS-NPs 在甲醇溶液中的Zeta电位用于辅助实验分析,纯 CdS-NPs 在甲醇溶液中带负电荷(−12.5 mV),因此,在 ZIF-8/CdS 复合材料的制备过程中,Zn2+首先在 CdS 悬浮液中混合并吸引在 CdS 表面,这可以促进 ZIF-8 纳米晶体在 CdS 表面的快速生长。通过比较可以看出 CdS-NPs 的引入缩小了 ZIF-8 的平均粒径,晶粒尺寸在 20—40 nm 之间。图2e显示了单个 ZIF-8/CdS 颗粒的元素映射图像并表明 Cd、S、Zn元素均匀分布在纳米粒子上,Zn 元素的轮廓要更大,进一步验证了 ZIF-8/CdS 复合材料的结构。
-
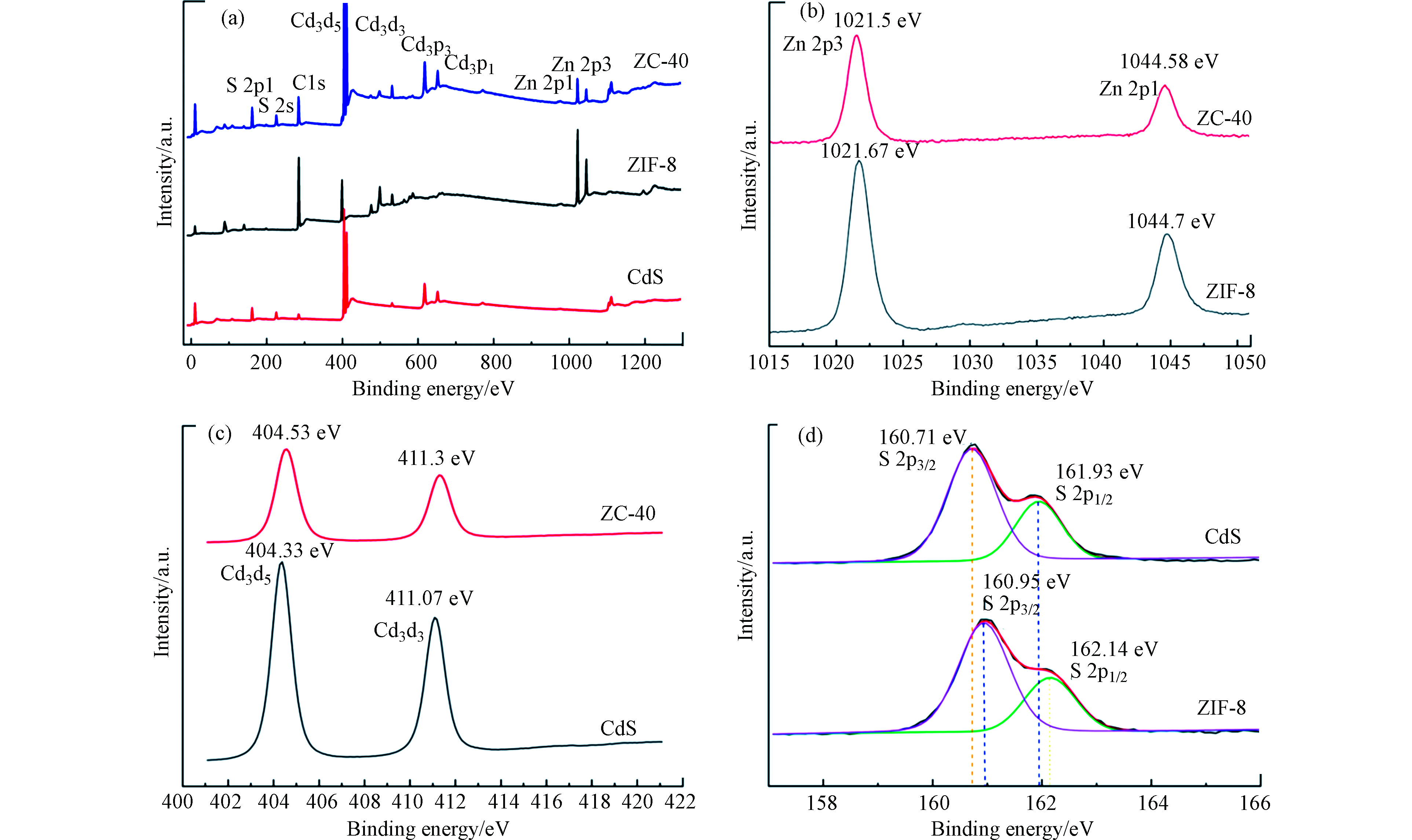
通过 XPS 分析样品的表面化学状态。图3(a)显示了 ZIF-8、CdS、ZIF-8/CdS 完整的 XPS电子能谱,这表明复合物中有 Zn、N、O、C、S和Cd 元素的存在。尽管大多数CdS-NPs都被包裹,但不可避免地存在一些暴露的 CdS-NPs,导致复合材料的 XPS中出现 S 和 Cd 的信号。比较图3(a)中3个XPS特征峰后表明, ZIF-8/CdS 复合材料的峰是 CdS 和 ZIF-8 的组合。图3(b—d)分别反映了 Zn 2p 、Cd 3d 与 S 2p 的XPS 能谱,Zn 2p 的XPS 能谱显示出两个以1021.67 eV 和 1044.7 eV 为中心的峰,分别表示Zn 2p3/2和 Zn 2p1/2的结合能。结合能为 404.33 eV 和 411.07 eV 的两个特征峰分别对应于 Cd 3d5/2和 Cd 3d3/2。这两个峰的出现归因于Cd 3d 轨道的自旋轨道分裂,其自旋轨道间距为6.74 eV,从而能表明 Cd 的化学状态为Cd2+[22-23]。S 2p 的XPS能谱可以拟合成结合能为 160.95 eV 和 162.14 eV 的两个峰,它们分别归属于S 2p3/2 和 S 2p1/2,表明 S2− 的存在。同时观察发现ZIF-8/CdS 中Cd 3d 与S 2p 的结合能要高于 CdS,同时Zn 2p 的结合能相比于ZIF-8要稍低一些,这可能是由于 CdS 和 ZIF-8 之间形成了Zn-S的影响,这种电子的相互作用通常被认为有利于光催化过程中的电子-空穴的分离[23]。
-
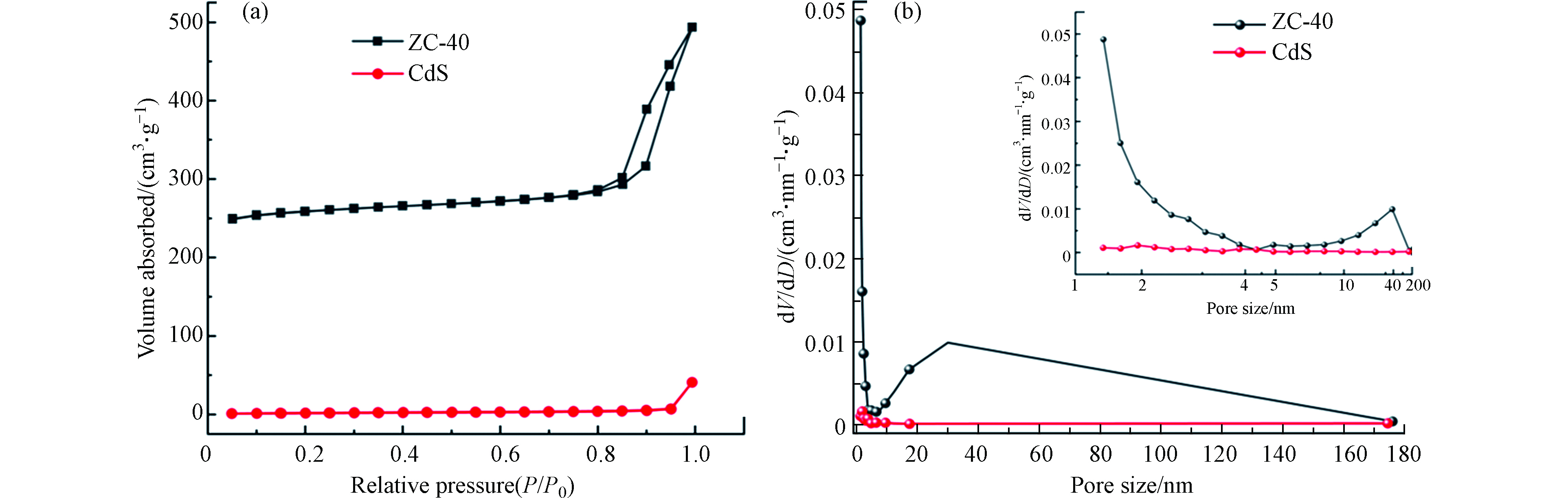
通过 N2 吸附/解吸测量来研究3个样品的比表面积。ZIF-8 是一种典型的 MOFs 材料,具有高孔隙率、低密度和高比表面积的特点。N2吸附/解吸通常用于探索孔隙特征和性质,包括比表面积和孔径分布。图4(a)显示了纯 CdS-NPs 和 ZIF-8/CdS 的 N2 吸附/解吸等温线。在整个范围内,ZIF-8/CdS 复合材料的 N2 吸附比纯 CdS 高得多,这主要是因为 ZIF-8 晶体孔隙率高。BET结果表明,纯CdSNPs的BET比表面积为5.675 m2·g−1,而 ZIF-8/CdS 的BET比表面积高达 784.79 m2·g−1。此外,根据 BDDT(Brunauer-Deming-Demin-Teller) 分类,还可以从图4(b)的ZIF-8/CdS的 N2 吸附/解吸等温线中看出其属于 Ⅳ 型等温线,表明存在丰富的介孔框架,孔径分布主要集中在 3.9 nm以内。此外,ZIF-8 还具有协同捕光能力,大表面积和丰富的大通道不仅有利于反应物与 ZIF-8/CdS 复合光催化剂之间的充分接触,而且还可以为目标污染物的降解提供更多的活性位点。
-
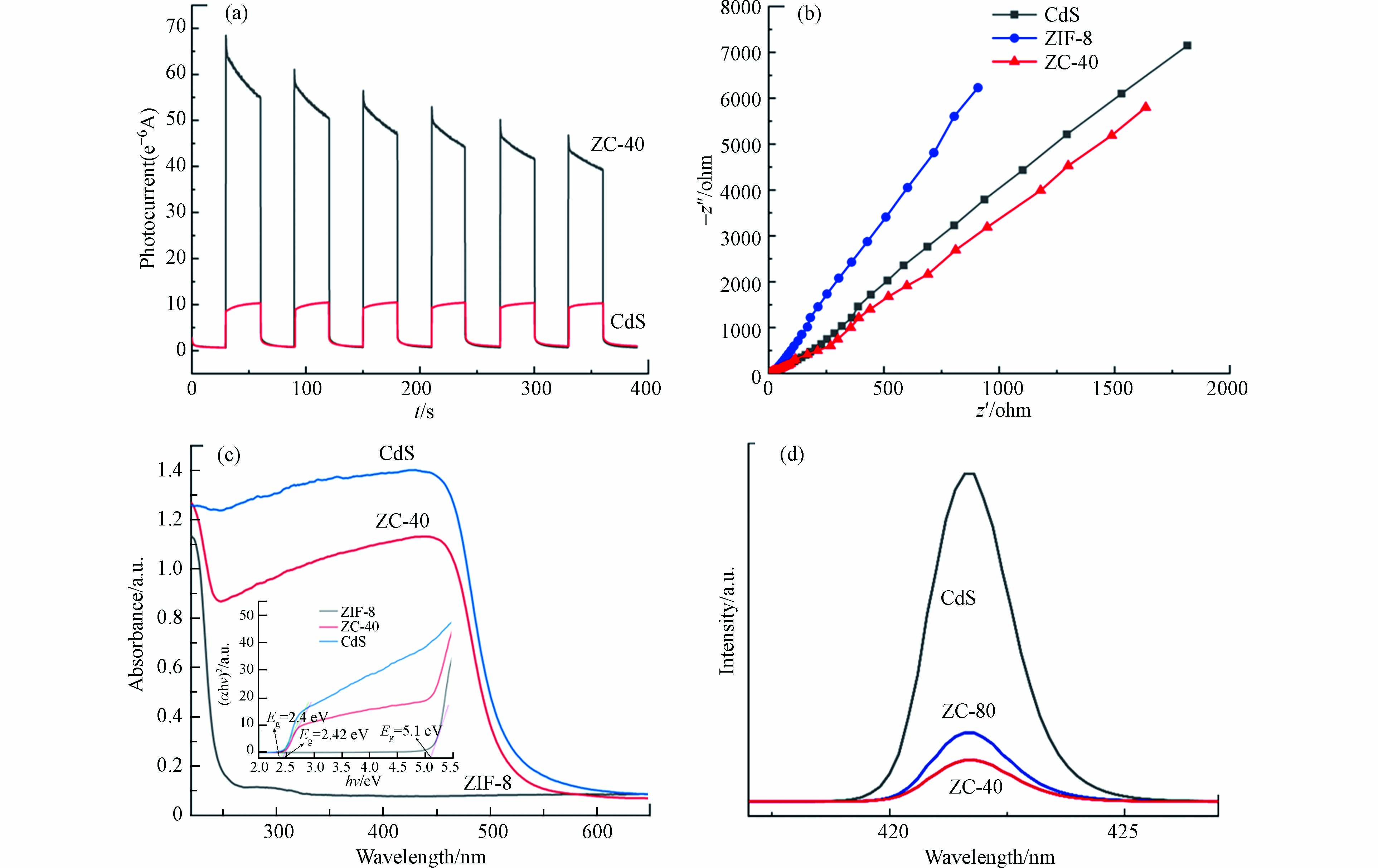
光生电子-空穴对的有效分离直接影响催化剂的光催化能力,可以通过可见光照射下的瞬态光电流响应来进行研究。三电极系统用于测量 CdS 和 ZIF-8/CdS 复合材料的瞬态光电流响应,将样品浸涂到氧化氟锡 (FTO) 玻璃上制成工作电极,配100 mL 0.5 mol·L−1 Na2SO4 水溶液作为电解质溶液,使用配有 420 nm 截止滤光片的 300 W氙灯作为光源。图5(a)显示了 ZIF-8/CdS 与纯 CdS 修饰的电极的光电流响应,显然,虽然存在光腐蚀现象,但是稳定时的 ZIF-8/CdS 仍表现出比纯 CdS(10 μA·cm−2) 更高的光电流密度 (50 μA·cm−2)。并且还可以看出,ZIF-8/CdS 的光电流在开灯期间显著增加,而在关灯时光电流急剧下降,并在开关循环中重复响应。该现象反映了 ZIF-8/CdS 光催化剂具有极强的活性,在可见光照射下可以产生连续高效的光电流。电化学阻抗谱 (EIS) 反映了电子或电荷转移电阻,可以用来研究光电极的各个样品的电荷分离过程和转移电子的特性,以进一步了解光电效率。图5(b)显示了不同光电极的 EIS,其中 ZIF-8/CdS 的曲线显然要低于纯 CdS 和 ZIF-8,这说明 ZIF-8/CdS 在可见光照射下具有较小的电子转移阻力和快速的电子转移能力。以上的光电化学结果可以反映出 ZIF-8/CdS 强烈的可见光吸收和优异的光诱导电荷转移性能,这表明 CdS-NPs 表面沉积的 ZIF-8 可以有效的改善光催化剂中光生电子-空穴对的分离。
-
半导体材料的带宽和光生电荷的有效分离反映了光催化剂的催化效率。图5(c、d)采用紫外-可见漫反射光谱 (UV-vis DRS) 和荧光发射光谱 (PL)来研究 ZIF-8/CdS 光催化剂的光学性质,并分析 ZIF-8 对 CdS-NPs 表面的影响。UV-vis DRS 用于反映合成催化剂的光吸收特性。纯 CdS 在 UV-vis 区域有很强的吸收,主要吸收波长小于 550 nm 的光,这与相关文献的表述一致[18]。ZIF-8晶体的吸收边缘约为250 nm,主要吸收紫外区的光,由X轴上的截距得到的Tauc 图的切线确定其带隙为5.1 eV,因此没有明显的可见光吸收。尽管 ZIF-8/CdS 比纯 CdS 表现出较短的吸收带,从较短波长的一侧出现到约 530 nm,光捕获能力只有轻微的下降,而且 ZIF-8/CdS 呈现出稍宽的吸收带隙值为 2.42 eV,大于纯 CdS-NPs (2.4 eV),可以降低光生电子-空穴对的复合概率,增强光催化效率。因此,ZIF-8 和 CdS-NPs 之间异质结的形成为其提供了良好的界面接触,可协同提高光催化剂在可见光区的吸光能力。为了进一步分析光生电子-空穴对的分离和转移,图5(d)测量了光催化剂样品的荧光发射光谱(PL),PL反映了光催化剂的电荷分离能力。显然,ZIF-8 显著降低了 CdS-NPs 的荧光强度,而且,在 CdS 的添加量在40 mg时其峰值最低,说明在本次实验的各组分浓度下,ZC-40 的电荷分离能力最好。PL分析表明由于 CdS-NPs 和 ZIF-8 之间的强相互作用,光生电子可以通过半导体 ZIF-8 到达外层,ZIF-8 可以促进异质结中的电荷转移,从而限制电子-空穴对的复合。
-
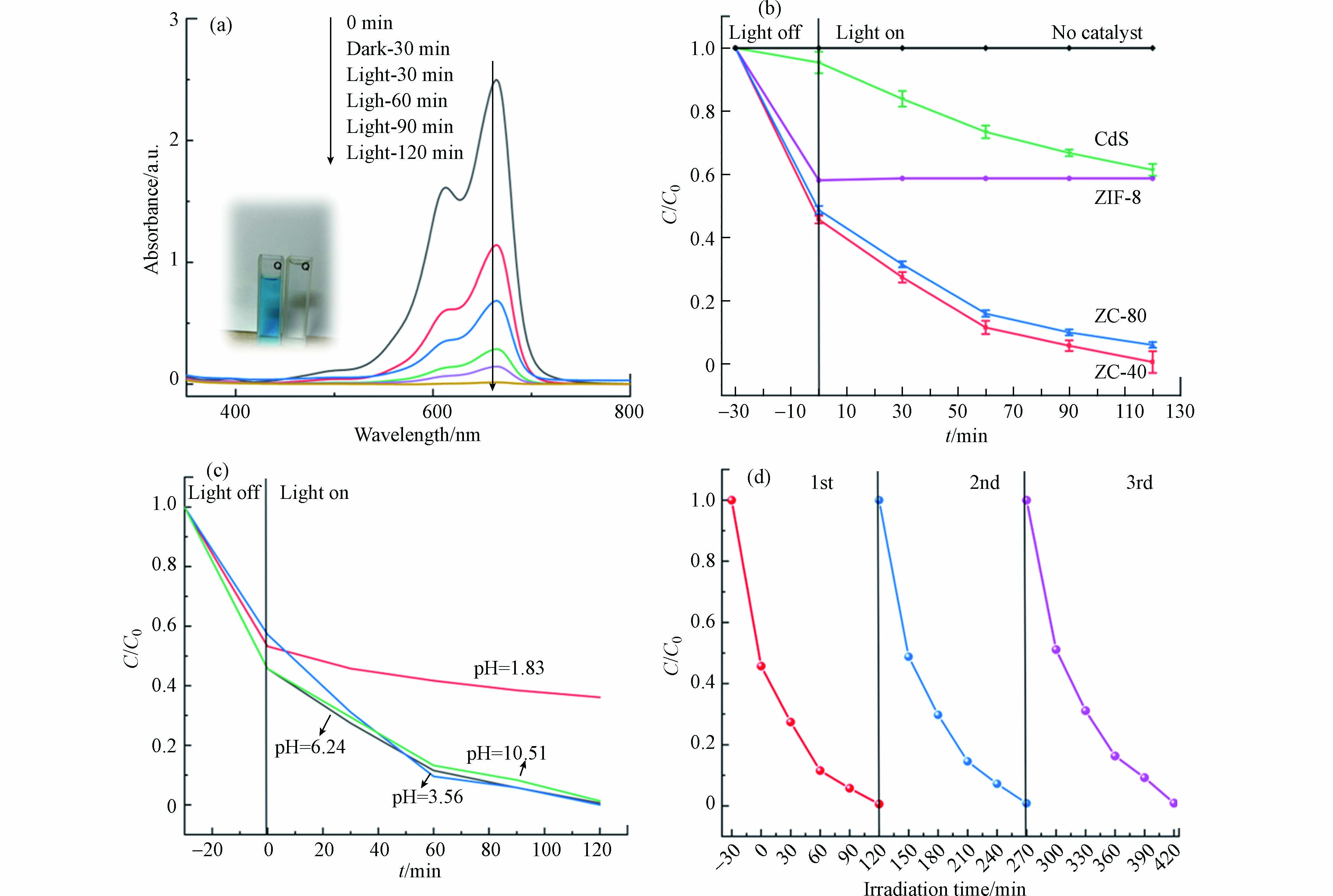
MB是一种常见的阳离子染料,在此次实验中被用作催化降解的目标污染物,以评估光催化剂的催化效率。将 50 mg 的 ZIF-8、CdS-NPs、ZC-40、ZC-80 分别放入盛有 15 mg·L−1 的 100 mL MB 溶液中,首先在光反应发生器中进行暗反应,如图6(b)所示,样品在暗反应的过程中对 MB 有很明显的吸附效果,而 ZIF-8/CdS 表现出比 ZIF-8 和CdS 更好的吸附能力,这是由于 ZIF-8 的高孔隙率造成的影响,而单纯的 ZIF-8 疏水性较强,在水中容易聚集成团进而降低了与染液的接触,使得吸附量比复合物的吸附量低[24]。各组分的光催化过程是在550 W的氙灯照射下进行的,如图6(b)所示,装有 ZIF-8 的样品染液浓度几乎没有再降低,说明在可见光照射下,达到吸附平衡的 ZIF-8 几乎不对 MB 染料有降解效果。在持续 90 min 的光照下 ZIF-8/CdS 显示出较高的催化活性,如图6(a、b)所示, ZC-40 的催化活性最强,几乎完全的去除了 MB,远高于纯 CdS (37.2%),催化速率也明显快于 ZC-80。由Langmuir-Hinshelwood(L-H)一级动力学反应方程算得光催化表观速率常数为 0.0481 min−1,是纯 CdS(0.00489 min−1)的9.836倍。同时对光催化剂的适用性和稳定性进行了探究,图6(c)显示了在不同的pH环境下 ZC-40 的催化降解能力,初始MB溶液的pH为6.24,使用醋酸和氨水调整pH,可以看出在pH=3.56的溶液环境下,ZC-40 的降解速度最快。当溶液的pH较低时,ZC-40 的降解能力明显降低,这或许是因为CdS 在强酸性条件下不稳定导致的。而碱性条件下对 ZC-40 的性能影响并不明显。图6(d)显示了3个重复循环中,除了吸附平衡值略有降低外,其催化降解能力几乎没有损失。结合TEM图像可以认为是ZIF-8的涂层可以通过稳定核内的硫化物和 Cd2+ 有效保护 CdS 免受光腐蚀, 其稳定性得到有效的维持。
-
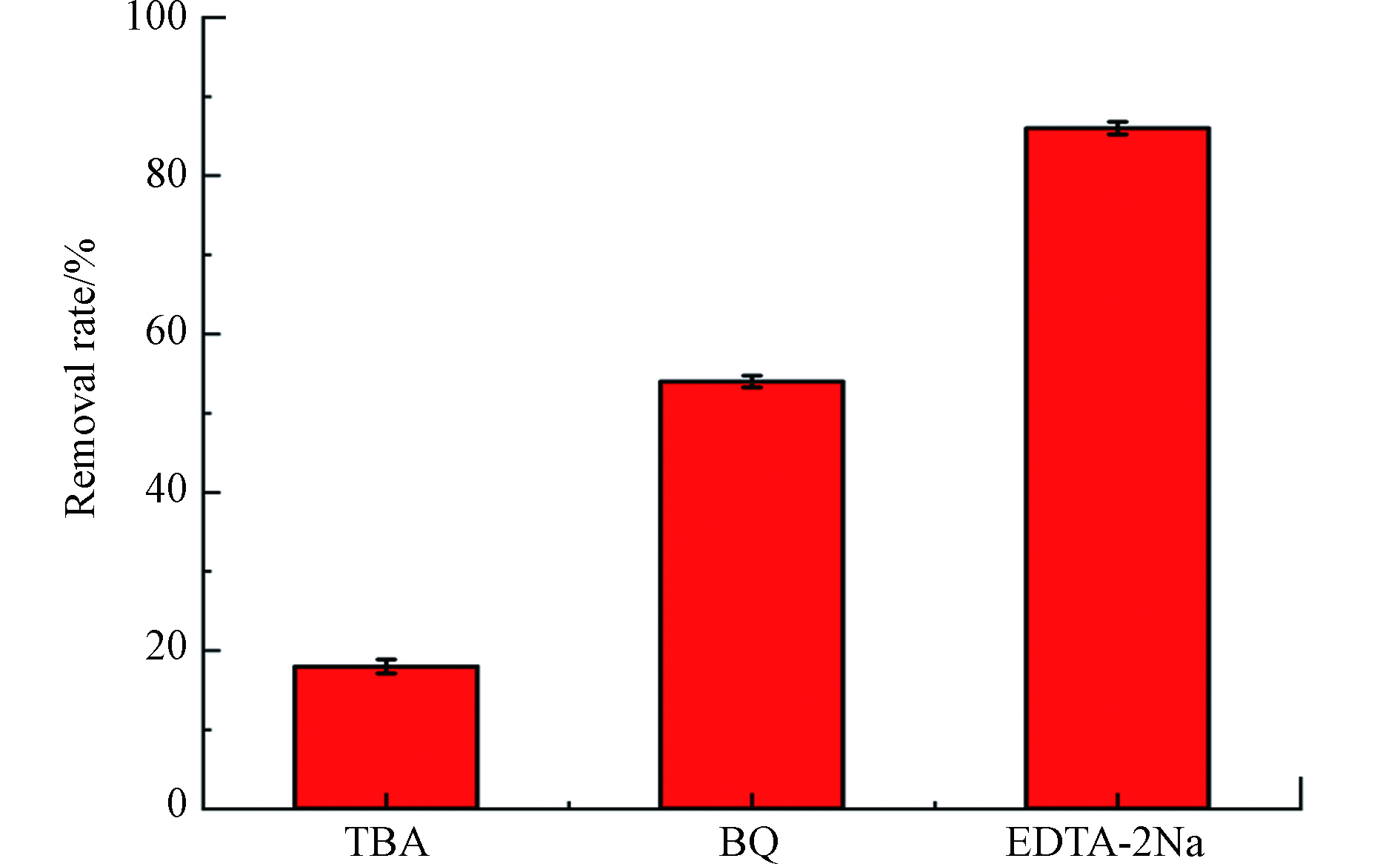
CdS 因为它的带隙在可见光区,所以被认为是一种具有可见光驱动的半导体光催化剂。因此,电子在可见光的照射下被激发跃迁,导致其解离为导带(CB)中的自由电子和价带(VB)中的空穴。电子将氧分子 (O2) 还原为超氧化物 (O2·−),而附着在催化剂表面的水分子或羟基被超氧化物 (O2·−) 氧化成高活性羟基自由基 (·OH),具有很强的将有机污染物催化为无毒无害的无机分子的能力[25]。为了进一步了解 ZIF-8/CdS 的光催化机理,进行了反应性自由基猝灭研究,通过添加不同的清除剂进行了一系列活性自由基的清除实验。在反应混合物中加入叔丁醇 (TBA)、对苯醌 (BQ) 和乙二胺四乙酸二钠 (EDTA-2Na) 以淬灭羟基自由基 (·OH)、超氧自由基 (O2·−) 和空穴 ( h+)。结果如图7所示,在加入TBA时,MB的降解得到了显著的降低,仅为18%左右,因此 ·OH 对MB的降解起主导作用。在加入BQ与EDTA-2Na时,其对MB的降解都起一定的抑制作用,其中EDTA-2Na的引入MB仍有88%左右的降解率,而BQ的引入则让MB的降解率降到了56%左右。这些结果表明3个反应性自由基(·OH、h+ 和 O2·−)的产生都证明了光催化过程中发生了有效的电荷分离。在反应性自由基中,h+是在 ZIF-8/CdS 催化降解 MB 中最不占优势的物种。
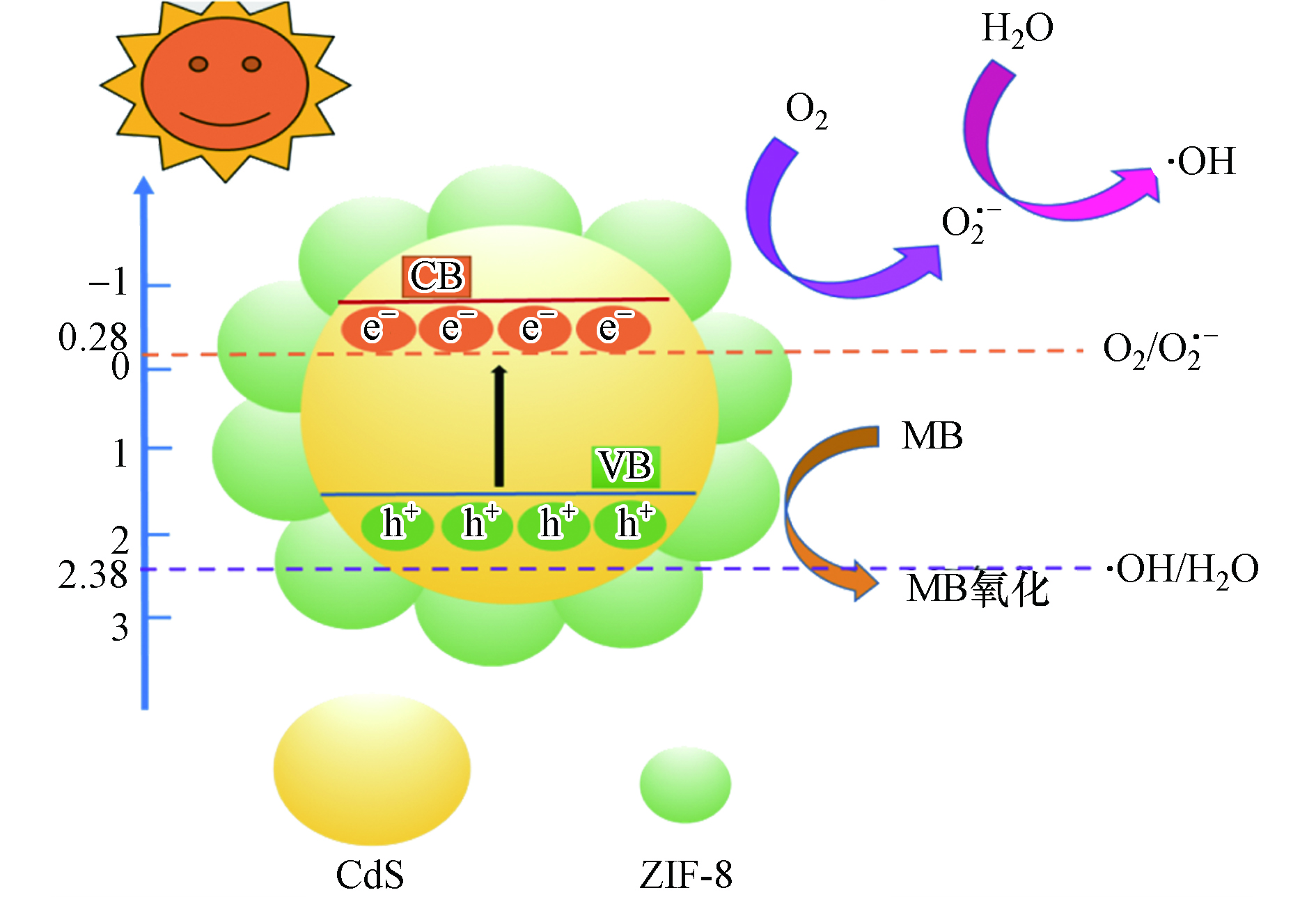
基于 PL 和 UV-vis DRS 等以上研究结果,提出可能的光催化过程,如图8所示.首先,ZIF-8/CdS 的带隙相对较窄 (2.42eV),在可见光的激励下,ZIF-8/CdS 的电子从价带 (VB) 跃迁到导带 (CB),在在价带中留下空穴(h+)。
其次,ZIF-8/CdS 的导带电位负于标准氧化还原电位 O2/ O2·− (-0.28V,vs. NHE),说明光致电子具有较强的还原能力,理论上可以将ZIF-8表面的 O2 还原成 O2·−,同时形成其他的光催化基团:
然而,CdS 的价带电位大约为1.6V[20],这明显高于 ·OH/OH- (2.38 V, vs. NHE),因此可以氧化 OH- 而产生 ·OH 自由基,同时h+也能直接氧化吸附在 ZIF-8/CdS 表面的 MB 分子,从而使得在可见光激励下使 MB 分子被有效降解:
从单一的半导体来看,ZIF-8 的价带电位要低于 CdS 的价带电位,这就使得ZIF-8能更有效地接受来自 CdS 导带的光致电子,从而提高电子的转移效率,降低光致电子的复合率。除此之外,由于 ZIF-8 的大的比较面积和孔隙结构的存在,能够为 MB 分子的吸附和催化降解提供更多的活性位点,从而增强了整个体系去除污染物的能力。
-
本文制备了 ZIF-8/CdS 复合材料并应用于光催化降解 MB。ZIF-8 的引入在防止小尺寸 CdS-NPs 聚集方面起重要作用, ZIF-8生长在 CdS-NPs 周围,提高 MB 的降解效率,增强了系统的光电转换效率和材料的抗光腐蚀性能。除颗粒的分散性提高和比表面积的增加这些因素外,ZIF-8 和 CdS-NPs 在界面上还形成了异质结,这使得光生电子-空穴对有效的分离。与 CdS 相比,ZIF-8/CdS 在光催化 MB 方面表现出更高的效率和更好的选择性,通过光电流测量和循环稳定性实验验证了 ZIF-8/CdS 的光稳定性,ZIF-8 的包裹可以提高对 MB 的吸附能力和光稳定性,同时不影响其光收集能力。本次研究提供了一种MOFs/半导体复合材料的构建方法,可能为光催化降解 MB 提供更多思路和解决方案,该复合材料可以结合两者的优点,提高半导体在催化、能量转换的效率和稳定性。
ZIF-8/CdS复合材料对亚甲基蓝的光催化降解
Photocatalytic degradation of methylene blue by ZIF-8/CdS composites
-
摘要: 为了改善 CdS 的光腐蚀,提高利用性,通过在 CdS 表面构建 2-甲基咪唑配体,成功的合成了一种可见光驱动的 ZIF-8 包裹CdS 的复合光催化剂。结构和形貌分析表明,通过在 CdS 周围原位沉积 ZIF-8,形成了具有丰富孔隙率和较高比表面积的复合光催化剂。光催化实验结果表明,ZIF-8 的形成不仅可以提高 CdS 的光稳定性,还可以增强对亚甲基蓝 (MB) 吸附能力,同时 ZIF-8 与 CdS 形成的异质结构对 MB 的降解有明显的促进作用,对 15 mg·L−1 的 MB 的去除率为 99%,在3个循环过程中光催化能力几乎没有损失。Abstract: In order to improve the photocorrosion and utilization of CdS, a composite photocatalyst driven by visible light-driven ZIF-8 wrapped in CdS was successfully synthesized by constructing a 2-methylimidazole ligand on the surface of CdS. Structural and morphological analysis show that a composite photocatalyst with abundant porosity and a higher specific surface area has been formed by depositing ZIF-8 in situ around CdS. Photocatalytic experiments show that the formation of ZIF-8 can not only improve the optical stability of CdS, but also enhance its MB absorption capacity. At the same time, the heterogeneous structure formed by ZIF-8 and CdS has a significant effect on the degradation of MB. The removal rate of 15 mg·L−1 MB is 99%, and there is virtually no loss of photocatalysis capacity during the three cycles.
-
Key words:
- CdS /
- ZIF-8 /
- photocatalysis /
- methylene blue
-

-
图 5 (a) CdS和 ZIF-8/CdS 复合材料在可见光照射下 (λ > 420 nm) 的瞬态光电流响应,(b)不同修饰电极的电化学阻抗谱,(c) 各组分的UV-vis DRS曲线,(d) 各组分的PL强度曲线
Figure 5. (a) Photocurrent responses of CdS and ZIF-8/CdS composite under visible light irradiation (λ > 420 nm), (b) the electrochemical impedance spectra of different-modified electrodes, (c) UV-vis DRS curves for each component, (d) PL strength curves of each component.
图 6 (a) 存在 ZC-40 (50 mg)的 MB 溶液 (15 mg L−1) 的 UV-vis 吸收光谱图,(b) CdS、ZIF-8、ZC-80、和 ZC-40 的降解曲线(所有样品质量:50 mg;MB 溶液浓度:15 mg L−1),(c)MB溶液的pH 环境对ZC-40降解能力的影响,(d) ZC-40在3次循环中的可回收能力
Figure 6. (a) UV-vis absorption spectra of ZC-40 (50 mg) in MB solution (15 mg L−1), (b) Degradation curves of CdS, ZIF-8, ZC-80 and ZC-40 (All samples quality: 50 mg; MB solution concentration: 15 mg L−1), (c)Effect of pH environment of MB solution on ZC-40 degradation ability,(d) The recyclability ability of ZC-40 in three cycles.
-
[1] AKERDI A G, BAHRAMI S H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review [J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103283. doi: 10.1016/j.jece.2019.103283 [2] CHOWDHURY P, ELKAMEL A, RAY A K. Photocatalytic processes for the removal of dye[M]//Green Chemistry for Dyes Removal from Wastewater. Hoboken, NJ, USA: John Wiley & Sons, Inc. , 2015: 119-137. [3] ZHANG T, LIN W B. Metal-organic frameworks for artificial photosynthesis and photocatalysis [J]. Chemical Society Reviews, 2014, 43(16): 5982-5993. doi: 10.1039/C4CS00103F [4] ZHANG N, ZHANG Y H, PAN X Y, et al. Assembly of CdS nanoparticles on the two-dimensional graphene scaffold as visible-light-driven photocatalyst for selective organic transformation under ambient conditions [J]. The Journal of Physical Chemistry C, 2011, 115(47): 23501-23511. doi: 10.1021/jp208661n [5] JANG J S, HAM D J, LAKSHMINARASIMHAN N, et al. Role of platinum-like tungsten carbide as cocatalyst of CdS photocatalyst for hydrogen production under visible light irradiation [J]. Applied Catalysis A:General, 2008, 346(1/2): 149-154. [6] JIANG D N, CHEN M, WANG H, et al. The application of different typological and structural MOFs-based materials for the dyes adsorption [J]. Coordination Chemistry Reviews, 2019, 380: 471-483. doi: 10.1016/j.ccr.2018.11.002 [7] FARRUSSENG D, AGUADO S, PINEL C. Metal-organic frameworks: Opportunities for catalysis [J]. Angewandte Chemie International Edition, 2009, 48(41): 7502-7513. doi: 10.1002/anie.200806063 [8] DOLGOPOLOVA E A, RICE A M, MARTIN C R, et al. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements [J]. Chemical Society Reviews, 2018, 47(13): 4710-4728. doi: 10.1039/C7CS00861A [9] LI H, WANG K C, SUN Y J, et al. Recent advances in gas storage and separation using metal-organic frameworks [J]. Materials Today, 2018, 21(2): 108-121. doi: 10.1016/j.mattod.2017.07.006 [10] DHAKSHINAMOORTHY A, ALVARO M, GARCIA H. Commercial metal–organic frameworks as heterogeneous catalysts [J]. Chemical Communications, 2012, 48(92): 11275. doi: 10.1039/c2cc34329k [11] FANG Z L, BUEKEN B, DE VOS D E, et al. Defect-engineered metal-organic frameworks [J]. Angewandte Chemie International Edition, 2015, 54(25): 7234-7254. doi: 10.1002/anie.201411540 [12] DHAKSHINAMOORTHY A, ASIRI A M, GARCÍA H. Metal-organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production [J]. Angewandte Chemie (International Ed. in English), 2016, 55(18): 5414-5445. doi: 10.1002/anie.201505581 [13] DHAKSHINAMOORTHY A, LI Z H, GARCIA H. Catalysis and photocatalysis by metal organic frameworks [J]. Chemical Society Reviews, 2018, 47(22): 8134-8172. doi: 10.1039/C8CS00256H [14] WEN M C, MORI K, KUWAHARA Y, et al. Design and architecture of metal organic frameworks for visible light enhanced hydrogen production [J]. Applied Catalysis B:Environmental, 2017, 218: 555-569. doi: 10.1016/j.apcatb.2017.06.082 [15] JIANG H L, LIU B, AKITA T, et al. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal–organic framework [J]. Journal of the American Chemical Society, 2009, 131(32): 11302-11303. doi: 10.1021/ja9047653 [16] XIONG W P, ZENG Z T, LI X, et al. Multi-walled carbon nanotube/amino-functionalized MIL-53(Fe) composites: Remarkable adsorptive removal of antibiotics from aqueous solutions [J]. Chemosphere, 2018, 210: 1061-1069. doi: 10.1016/j.chemosphere.2018.07.084 [17] XU H Q, YANG S Z, MA X, et al. Unveiling charge-separation dynamics in CdS/metal–organic framework composites for enhanced photocatalysis [J]. ACS Catalysis, 2018, 8(12): 11615-11621. doi: 10.1021/acscatal.8b03233 [18] LIU Y, DENG L, SHENG J P, et al. Photostable core-shell CdS/ZIF-8 composite for enhanced photocatalytic reduction of CO2 [J]. Applied Surface Science, 2019, 498: 143899. doi: 10.1016/j.apsusc.2019.143899 [19] SAHA S, DAS G, THOTE J, et al. Photocatalytic metal–organic framework from CdS quantum dot incubated luminescent metallohydrogel [J]. Journal of the American Chemical Society, 2014, 136(42): 14845-14851. doi: 10.1021/ja509019k [20] DHAKSHINAMOORTHY A, GARCIA H. Catalysis by metal nanoparticles embedded on metal-organic frameworks [J]. Chemical Society Reviews, 2012, 41(15): 5262-5284. doi: 10.1039/c2cs35047e [21] ZENG M, CHAI Z G, DENG X, et al. Core-shell CdS@ZIF-8 structures for improved selectivity in photocatalytic H2 generation from formic acid [J]. Nano Research, 2016, 9(9): 2729-2734. doi: 10.1007/s12274-016-1161-3 [22] TIAN F Y, ZHANG H L, LIU S, et al. Visible-light-driven CO2 reduction to ethylene on CdS: Enabled by structural relaxation-induced intermediate dimerization and enhanced by ZIF-8 coating [J]. Applied Catalysis B:Environmental, 2021, 285: 119834. doi: 10.1016/j.apcatb.2020.119834 [23] QIU J H, ZHANG X F, ZHANG X G, et al. Constructing Cd0.5Zn0.5S@ZIF-8 nanocomposites through self-assembly strategy to enhance Cr(VI) photocatalytic reduction [J]. Journal of Hazardous Materials, 2018, 349: 234-241. doi: 10.1016/j.jhazmat.2018.02.009 [24] ZHANG H F, ZHAO M, YANG Y, et al. Hydrolysis and condensation of ZIF-8 in water [J]. Microporous and Mesoporous Materials, 2019, 288: 109568. doi: 10.1016/j.micromeso.2019.109568 [25] MORAIS P C, QU F Y. The quantum mechanical description of the dot-dot interaction in ionic colloids [J]. Journal of Alloys and Compounds, 2007, 434/435: 565-568. doi: 10.1016/j.jallcom.2006.08.184 -



 下载:
下载: