-
由于磷肥的施用以及含磷废水的无组织排放,湖泊等缓流水体中磷素的浓度超过水体自净能力,造成藻类和浮游生物大量繁殖,水体溶解氧量下降,水质恶化,最终导致鱼类及其他生物大量死亡. 水体中磷素主要以正磷酸盐(H2PO4–、HPO42–和PO43–)的形态存在,水体中磷酸盐去除方法包括:化学沉淀法、生物修复法和吸附法,其中吸附法由于修复周期短、操作简单、成本低廉等优势广泛应用于磷酸盐的去除中[1].
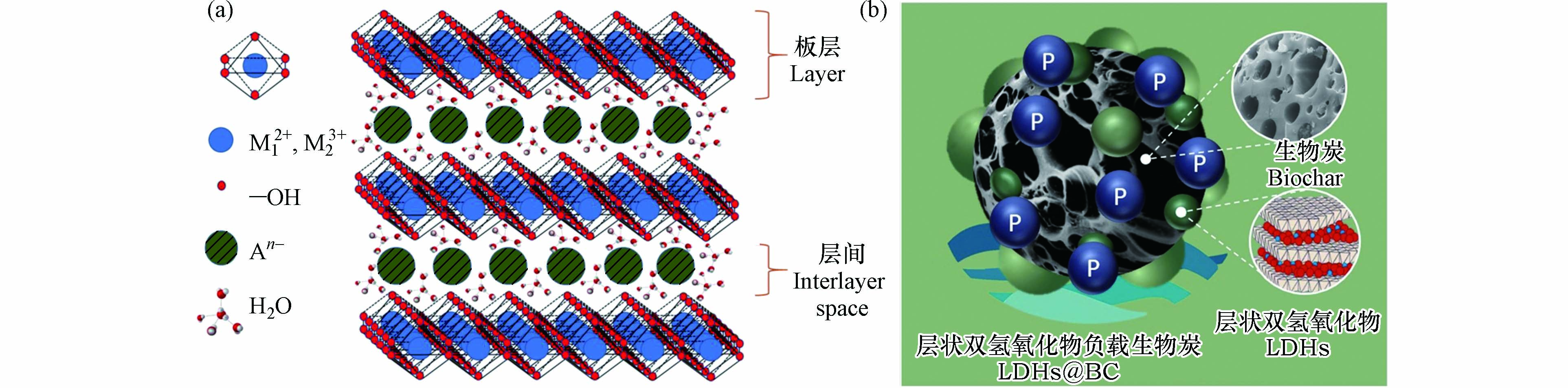
层状双氢氧化物(layered double hydroxides, LDHs)是一类由带正电荷的金属类水镁石层和层间填充带负电荷的阴离子所构成的层状化合物[2]. LDHs典型通式为[M12+(1-x)M23+x(OH)2]x+[An–]x/n·mH2O,其中M12+和M23+分别代表具有正二价和正三价的阳离子,An–是层间可交换的阴离子,x通常在0.13—0.33之间[3](图1). LDHs不仅具有生物相容性好、低毒等特点,还具有制备简单、成本低和便于保存的优势.通过调控二价、三价金属离子的组合以及物质的量比,可以制备出各种各样具有独特理化性质的LDHs[4-5]. LDHs是一种优良的磷酸盐吸附剂,其层间的阴离子可交换性较强,板层上存在着大量羟基,并且在大部分pH范围内,LDHs表面带呈正电性,能通过静电作用吸附磷酸盐. 但是,LDHs通常呈紧密层堆积,易形成致密颗粒或块,导致其在实际应用中水力传导率较低,暴露的活性位点有限,吸附性能显著降低[6]. 通过分散剂,如生物炭、黏土矿物等能够有效分散LDHs,强化其吸附性能[7-8].
生物炭是生物质原料在无氧或限氧的气氛条件下,经热化学转化产生的一种含碳量丰富、物化性质稳定的高度芳香化有机物[11]. 根据热化学转化方式的不同,生物炭可分为水热炭和、热解炭和气化炭,其热转化温度分别为350—550 K、650—1100 K和900—1500 K[12]. 如图1(b)所示,生物炭比表面积大,孔隙结构和表面官能团丰富,是一种理想的LDHs载体,能够显著增强其分散性和稳定性,提高其比表面积和表面活性,从而增强其对磷酸盐的吸附能力[13]. 生物炭来源广泛且容易制备,能有效实现农牧业废弃物的资源化利用. 同时生物炭作为一种被广泛应用的环境修复剂,兼具养分保留和固碳能力[14-16],能有效推动我国早日实现碳达峰和碳中和目标.
近年来,层状双氢氧化物负载生物炭(layered double hydroxides functionalized biochar, LDHs@BC)在水体磷酸盐去除的研究和应用逐渐增多[17-19]. 基于文献调研,本文系统地介绍了LDHs@BC的制备方法和理化性质,详细讨论了LDHs@BC对磷酸盐的吸附性能和机制,分析了LDHs@BC的应用优势,展望了LDHs@BC的应用前景,以期推动LDHs@BC进一步的推广和应用.
-
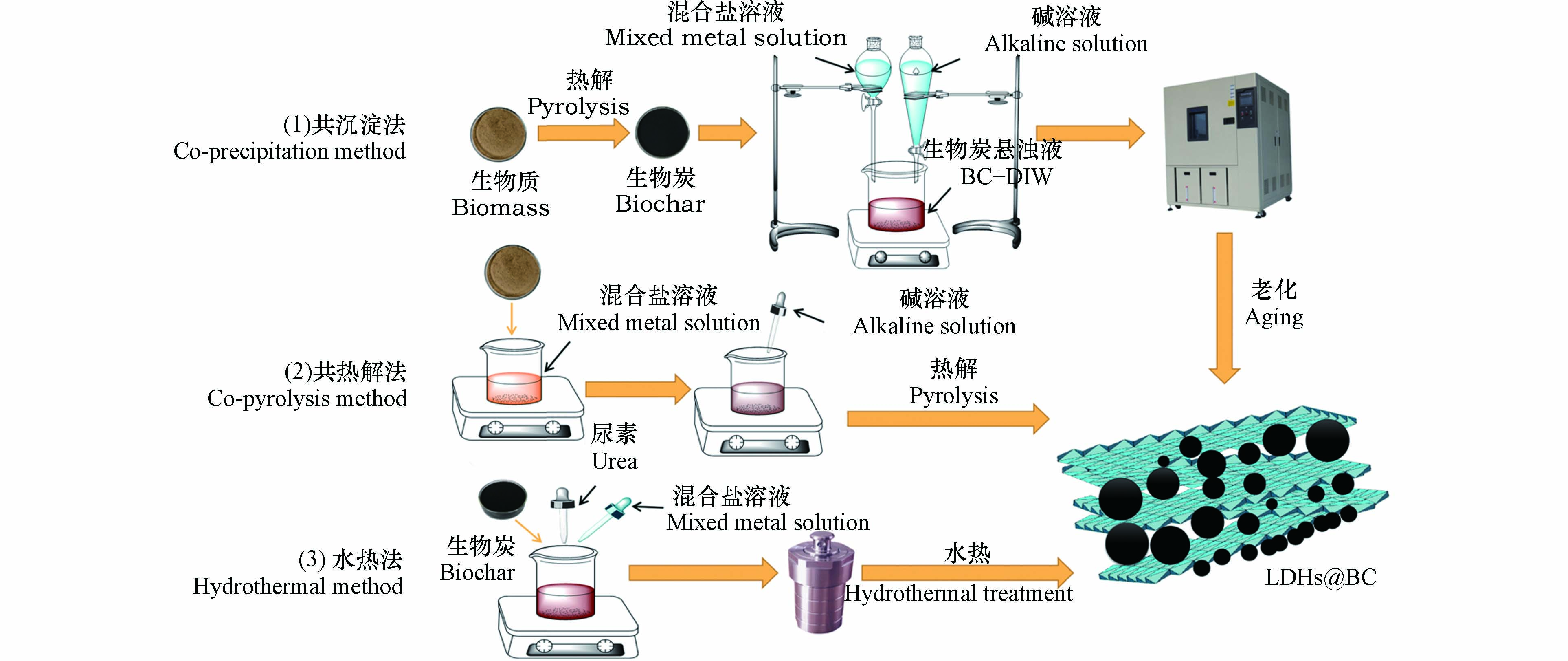
制备方法显著影响LDHs@BC的理化性质,从而影响其对磷酸盐的吸附性能. 根据生物质的热解方式以及生物质热解与LDHs负载的先后顺序,LDHs@BC的制备方法可分为共沉淀法、共热解法和水热法3种. 如图2所示,共沉淀法是指先热解生物质制备为生物炭后,再将LDHs负载于生物炭上. 较常用的方法是向生物炭、混合盐溶液中缓慢滴加氢氧化钠溶液,使混合溶液维持在碱性,然后在一定温度下老化数天后,用去离子水多次洗涤,最终制得LDHs@BC[20]. 共热解法是指将LDHs直接负载于生物质上,再将负载了LDHs的生物质放入马弗炉中热解. 热解过程中,超过一定温度后,LDHs的原始层状结构被破坏,但能够通过捕获水中的氢氧根和磷酸根等阴离子来重建原始层状结构[21]. 水热法是将碱源、混合盐溶液与生物炭或生物质混合于水热反应器中,在一定的温度下反应制得LDHs@BC,如图2(3)所示水热法通常使用尿素作为碱源维持体系pH[22-24]. pH是所有制备LDHs@BC方法中最重要的控制因素,研究表明[25],在制备过程中,pH控制在10左右对LDHs的形成最有利.以Mg/Al-LDHs为例,pH < 6时,LDHs无法生成,产物以氢氧化铝沉淀为主;pH 6—10时,LDHs开始生成,并且产率随pH上升而逐渐增加;pH > 10时,溶液中镁离子逐渐生成氢氧化镁沉淀而非LDHs,LDHs产率随pH上升而逐渐下降[26]. 共沉淀法由于制备程序简单、制备条件灵活可控并且可直接用于大规模生产,因此是最为常用的制备方法. 但该方法在制备过程中由于LDHs颗粒形成速度快,无法对其粒径进行精确控制[27],造成LDHs粒径分布范围较大、样品不均质等问题[28]. 水热法制备的LDHs@BC具有比表面积大、结晶度高和官能团丰富的特点,但制备过程所需反应温度较高(110—160 ℃),耗能较大,同时用于水热反应的高压反应釜昂贵,并且在该过程中无法随时对反应进行调控,因此水热法难以应用于批量生产[29]. 共热解法制备的LDHs@BC表面性能和LDHs结晶度均低于共沉淀法和水热法,由于LDHs的存在,热解过程中生物炭多孔表面结构的形成受到抑制,并且,热解也会破坏LDHs的层状结构,导致层间阴离子的流失[30-31].
-
生物炭和LDHs的比表面积均显著影响LDHs@BC的比表面积. 一般来讲,制备LDHs@BC时会选用孔隙结构丰富和比表面积较大的生物炭,而使用共沉淀法制备LDHs@BC会使生物炭的比表面积大幅降低,这主要是由于LDHs颗粒沉积到生物炭的表面及孔隙结构中,导致其比表面积显著降低.例如,小麦秸秆生物炭比表面积为182 m2∙g−1,而以共沉淀法制备LDHs@BC,其比表面积相较小麦秸秆生物炭降低了97.8%[32]. 但是,当负载的LDHs比表面积大于生物炭时,LDHs@BC的比表面积相比生物炭会有所增加.例如,西雅棕内果皮生物炭比表面积为72 m2∙g–1,而负载LDHs后,LDHs@BC比表面积增加了133%,这主要是由于负载LDHs的比表面积(213 m2∙g−1)相较生物炭更大[33].
制备方法也会对LDHs@BC的比表面积产生影响.相比于共沉淀法,共热解法和水热解法则相对能够得到比表面积更大的LDHs@BC. 研究表明[34],在相同的制备条件下,共热解法制备的LDHs@BC比表面积(387 m2∙g–1)远大于共沉淀法制备的LDHs@BC(174 m2∙g–1). 另外,共热解温度显著影响LDHs@BC比表面积. 研究表明[30],共热解温度从300 ℃增加到到500 ℃,LDHs@BC比表面积从52 m2∙g–1增加到246 m2∙g–1,当热解温度进一步增加至在700 ℃时则下降到104 m2∙g–1。这是由于温度的进一步升高会导致纤维素进一步分解,生物炭孔隙结构被破坏,比表面积降低. 使用水热法制备LDHs@BC则能够大幅提高生物炭比表面积,例如使用水热合成制备新型磁性Mg/Fe-LDHs@BC,其比表面积(87 m2∙g–1)比原始磁性生物炭(38 m2∙g–1)高2倍以上[35]. 因此,通过调控LDHs@BC的制备参数和热解温度能够获得优良比表面积的LDHs@BC,从而有利于其对磷酸盐的吸附.
-
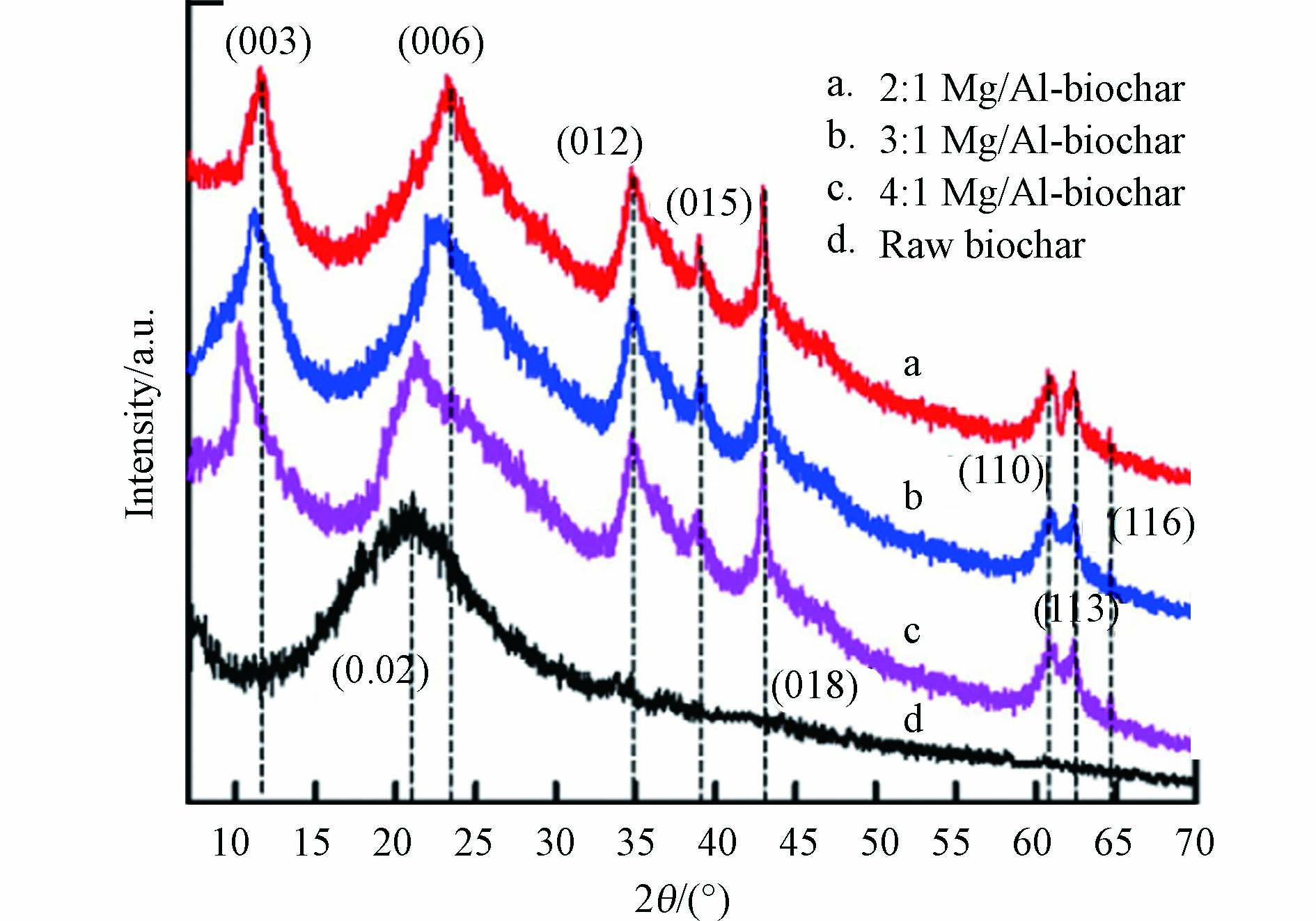
LDHs@BC的晶体结构特征一般通过X射线衍射(X-ray Diffraction, XRD)来表征. 如图3所示,对比原始生物炭和LDHs@BC的XRD图谱能够直接证实生物炭上是否成功负载LDHs晶体. 制备LDHs@BC时,不同金属物质的量比会导致LDHs的层间距发生变化进而使各个晶面的峰位发生偏移,因此通过XRD图谱数据能够获取LDHs的层间距大小,评估LDHs@BC的层间阴离子交换能力,从而判断磷酸盐吸附性能[24, 36].
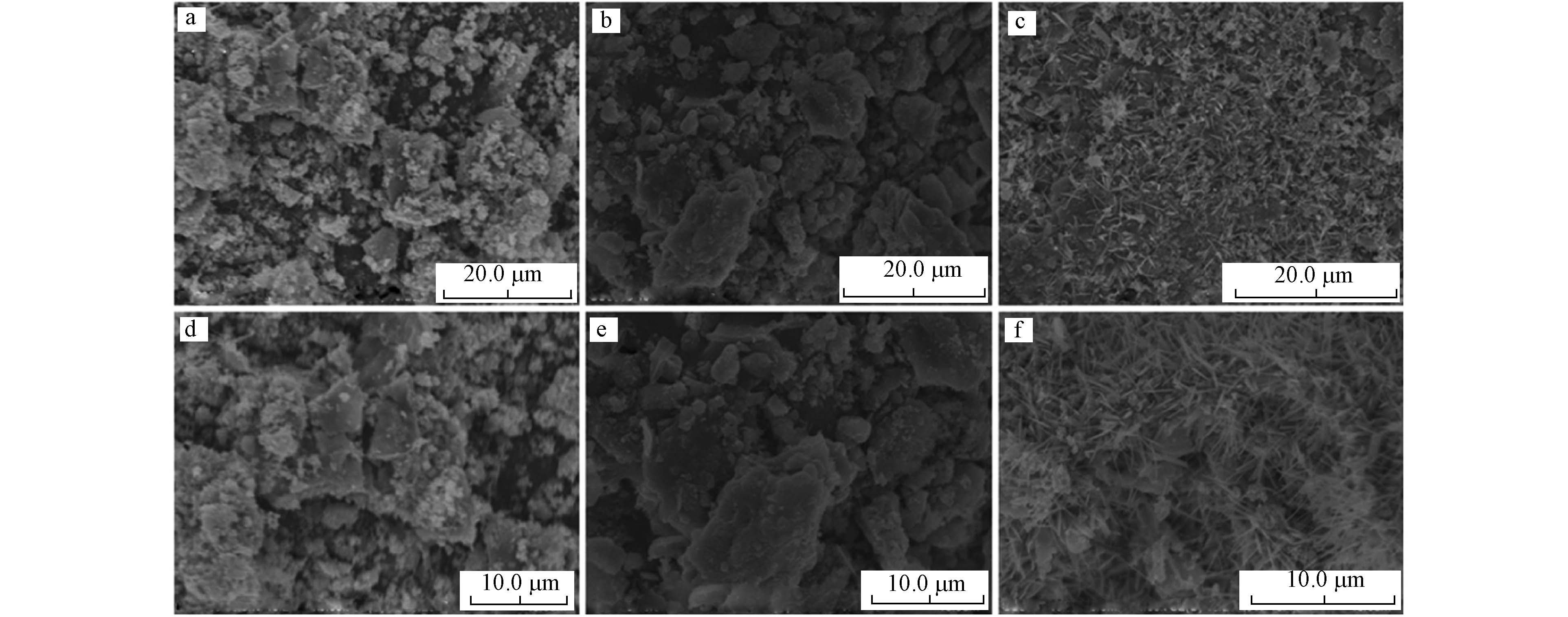
生物炭具有丰富的孔隙结构,表面较为光滑.当LDHs负载到生物炭上后,通过扫描电子显微镜(Scanning Electron Microscope, SEM)图像能够观察到LDHs薄片密集沉积在生物炭表面(图4),生物炭孔隙由于LDHs的大量沉积而堵塞,比表面积下降,表面也由光滑变的粗糙.[36]. 金属组成影响着LDHs的表面形貌,Mg/Al-LDHs@BC和Zn/Al-LDHs@BC呈薄片状,而Ni/Fe-LDHs@BC呈针状[37]. SEM分析还能够获取各元素在LDHs@BC中的分布和占比等信息[20, 34]. 共热解法制备的LDHs@BC层状结构遭到破坏,煅烧导致镁氧化物和铝氧化物的生成[38],从而影响对正磷酸盐的吸附性能.
-
LDHs@BC的热稳定性可以通过热重分析进行评估,即在氮气气氛下以一定的加热速率,分析LDHs@BC的质量和温度变化关系[39]. LDHs@BC第一个明显的质量损失发生在200 ℃,为表面吸附的水分子挥发所致,第二个质量损失发生在200—500 ℃,此温度范围内LDHs@BC开始分解,主要为生物质(纤维素、半纤维素和木质素)的分解以及金属形态由氢氧化物向氧化物的转化导致[40]. 因此,在一般使用温度下,LDHs@BC具有良好的相对热稳定性,能够保证其稳定的磷酸盐吸附性能.
-
LDHs@BC对磷酸盐的吸附能力较强,在22 ℃下Langmiur模型拟合得到的饱和吸附容量可达410 mg∙g–1[20](如表1,文中所指浓度和吸附容量均以磷酸盐计). 研究结果表明[31, 40],LDHs@BC对磷酸盐的吸附与准二级动力学模型拟合程度较高,这是由于吸附涉及化学吸附,并包含液膜扩散及生物炭和LDHs内部的孔扩散等复杂吸附过程.膜扩散为LDHs@BC吸附磷酸盐的控速步骤,有效扩散系数值在10–6到10–8范围之间,并且LDHs@BC对磷酸盐的吸附达到平衡较快[30]. 例如使用水热法制备的LDHs@BC对50 mg∙L–1磷酸盐溶液的吸附平衡时间为40 min[41]. 而共热解法制备的LDHs@BC吸附速率相对更快,同样在50 mg∙L–1磷酸盐溶液条件下,共热解法制备的LDHs@BC在5 min内可去除95.2%的磷酸盐[31]. 其对磷酸盐的快速去除来源于LDHs的记忆效应,LDHs在热解时被破坏的层状结构,在重新进入水溶液中时会重新恢复,而磷酸根离子会快速进入LDHs层间充当平衡性阴离子.
-
LDHs中二价(M12+ = Mg2+、Ni2+、Cu2+、Co2+或Mn2+)和三价(M23+ = Al3+、Fe3+)金属阳离子的种类显著影响LDHs@BC的理化特性,进而影响其磷酸盐的吸附能力[29]. 在相同制备条件下,Mg/Al-LDHs@BC对磷酸盐的去除性能优于它种类的LDHs@BC.例如,相同的吸附条件下,Mg/Al-LDHs@BC对磷酸盐的吸附容量相比于Mg/Fe-LDHs@BC高70%以上[8, 37],归因于Mg/Al-LDHs@BC具有更高的亲水性和溶解性,从而暴露更多的的吸附位点. 研究表明[39],Mg/Al-LDHs@BC的最大饱和吸附容量为152 mg∙g−1,显著高于Ni/Fe-LDHs@BC和Zn/Al-LDHs@BC(分别为78.3 mg∙g–1和64.9 mg∙g–1)。这是由于Mg/Al-LDHs@BC更低的分子量和电负性,使其具有更强的阴离子交换能力,因而对磷酸根阴离子具有更好的吸附性能.
LDHs@BC中二价和三价金属阳离子的物质的量比显著影响LDHs层间距和电荷特性,从而影响其层间阴离子交换能力和静电吸引作用,进而对LDHs@BC磷酸盐的吸附性能产生不同影响. 研究表明[37],随着Mg/Al-LDHs@BC中Mg/Al物质的量比由2:1增加至4:1,Mg/Al-LDHs层间电荷密度逐渐减少,导致其层间距由0.280 nm增加到0.310 nm,而层间距的增加会增强层间阴离子交换作用,显著增强了其对磷酸盐的吸附性能. 并且pH 3—7,较高Mg/Al比(4:1)的Mg/Al-LDHs@BC对pH缓冲作用更强,因而吸附稳定性更强.然而,另一项研究显示[30],LDHs@BC中Mg/Al比从2:1增加到5:1,Mg/Al-LDHs@BC对磷酸盐的吸附率从81.4%逐渐下降到51.8%. 作者归因于Mg:Al比的增加会减小LDHs的电荷密度,使带正电的LDHs板层和磷酸根静电吸引力下降,造成吸附量下降. 造成此差异的原因可能源于两项研究中制备Mg/Al-LDHs@BC的具体参数不同,造成各自Mg/Al-LDHs@BC的理化性质不同,因此静电作用和层间阴离子交换作用在其吸附磷酸盐时的贡献占比不同. 因此,采用不同的制备方法和参数时要进一步确定最优M12+/M23+比值,使LDHs@BC达到最佳的磷酸盐吸附性能.
-
LDHs@BC中对磷酸盐起主要吸附作用的是LDHs,因而LDHs的含量显著影响其对磷酸盐的吸附性能(表1). 研究表明[8],随着LDHs@BC中Mg/Al和Mg/Fe-LDHs的质量含量从25%增加到40%,LDHs@BC对磷酸盐的吸附率增加近1倍. 另外,将LDHs负载于BC后显著提升LDHs的吸附性能,研究表明[19],LDHs@BC中单位质量的LDHs对磷酸盐的吸附率较未负载于BC的LDHs高16.2%—68.4%,这是因为生物炭丰富的孔隙结构能促进LDHs和磷酸盐阴离子之间的相互作用,从而增强对磷酸盐的吸附性能. 但LDHs占比过高时,LDHs会大量堆积在生物炭孔隙和表面,导致LDHs的结晶度和分散性变差,LDHs@BC的比表面积降低,从而导致单位质量的LDHs的吸附能力下降[42]. 因此,优选BC和LDHs比例有助于提升LDHs@BC吸附性能.
-
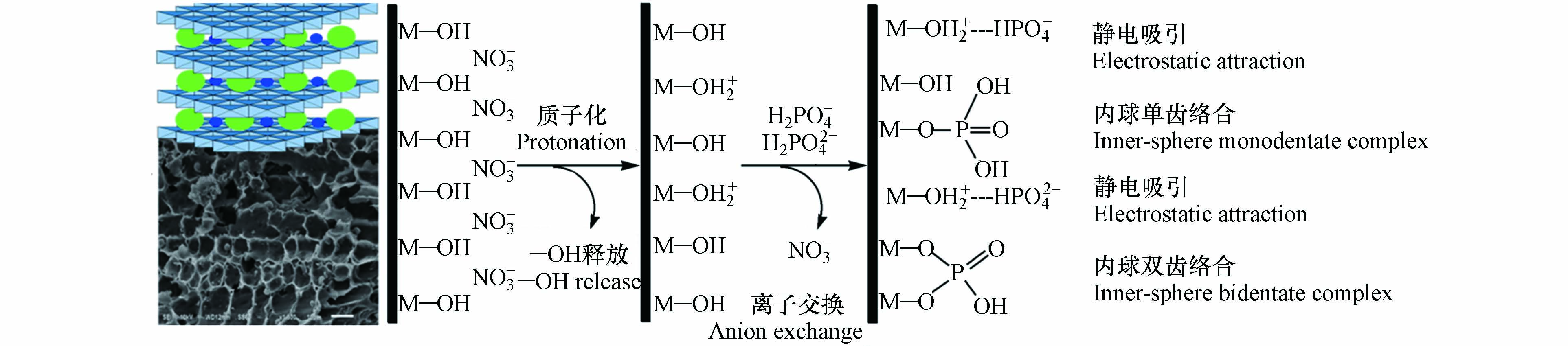
LDHs@BC对磷酸盐的吸附机制包括静电吸引、层间阴离子交换和配体交换[46]. 如图5所示,静电吸附主要是利用异性电荷相互吸引的静电感应现象,将磷酸盐吸附于LDHs@BC上,通常受LDHs@BC表面电荷和磷酸盐形态的影响,pH显著影响LDHs@BC表面电荷和溶液中的磷酸盐形态,从而影响LDHs@BC对磷酸盐的静电吸附.
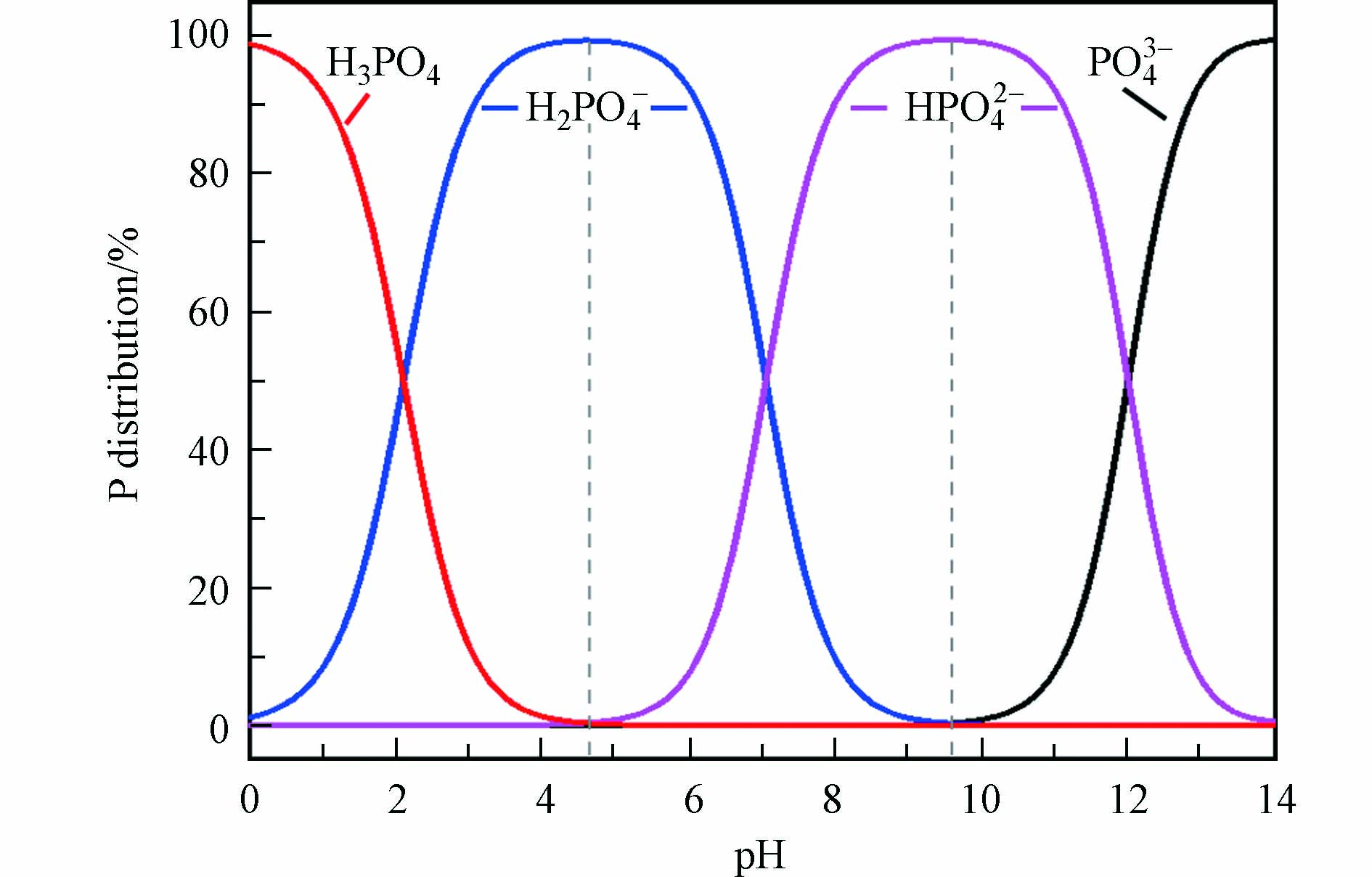
研究表明[37],pH 3时,LDHs@BC对磷酸盐的吸附容量达到最大,这是因为LDHs@BC表面由于质子化而呈较强的正电性,而溶液中的磷酸盐主要以H2PO4–和HPO42–阴离子形态存在,因而能通过强烈的静电作用吸附磷酸盐[47].但pH < 2时,虽然LDHs@BC表面带正电,但溶液中的磷酸盐主要以H3PO4中性分子形态存在(图6,导致带正电的LDHs@BC难以通过静电作用吸附磷酸盐[9]. 当体系pH 3增至LDHs@BC的零点电荷(pHzpc,—8)时,LDHs@BC对磷酸盐的静电吸附作用力逐渐减弱,这是因为尽管LDHs@BC带正电,但磷酸盐主要以HPO42–形态存在,而HPO42–的吸附自由能高于H2PO4–,不利于磷酸盐在LDHs@BC上的吸附,并且pH的升高导致LDHs@BC质子化程度降低,从而削弱了LDHs@BC对磷酸盐的静电吸附[48-49]. 当pH > pHzpc时,LDHs@BC的表面电荷由正转负,与磷酸根阴离子之间的静电斥力增加,导致对磷酸盐的静电吸附作用力显著降低.同时,在较高的pH值下,溶液中氢氧根离子与磷酸根离子之间的竞争也会降低LDHs@BC对磷酸盐的吸附[50-51].
LDHs@BC能通过层间阴离子交换能够吸附磷酸盐. 层间阴离子交换主要与LDHs的层状结构有关,制备过程中正三价金属阳离子能够以一定比例同晶取代正二价金属阳离子,导致层板间电荷不平衡,产生净正电荷,因此阴离子进入层间以平衡电荷,分布在层间的阴离子能与磷酸盐发生离子交换. 傅里叶变换红外吸收光谱分析显示吸附了磷酸盐后的Cu/Al-LDHs@BC在1370 cm–1处CO32–特征峰的强度显著降低,证实了LDHs@BC层间的CO32–与磷酸根发生了离子交换[41].
LDHs@BC表面羟基也能与磷酸根发生配位体交换. 配位体交换主要是由于LDHs@BC表面存在丰富的—OH官能团,能够与磷酸根离子中未质子化的氧原子之间发生配体交换形成表面络合物从而实现溶液中磷酸根的去除. 大量研究通过X射线光电子能谱中Mg(1s)、Cu(1s)、Al(2p)和Fe(2p)等金属结合能的变化、傅里叶变换红外吸收光谱图中新出现的P—O振动峰(1090—1118 cm–1)以及反应后溶液pH的升高,证实了LDHs@BC表面羟基与磷酸盐进行了配体交换,并且与吸附上的磷酸盐形成了表面络合物[17, 30, 45].
-
LDHs@BC对磷酸盐不仅具有优良的吸附性能,同时还对环境中共存的阴离子有显著的抗干扰能力. 研究表明[41],当水体中同时存在相同浓度的常见阴离子,例如Cl–、NO3–、SO42–或CO32–时,LDHs@BC对磷酸盐的吸附性能仅下降0.8%,这主要是由于LDHs@BC主要通过层间阴离子交换以及与磷酸盐形成内球络合物等专性吸附来实现磷酸盐的去除. 但是当水体中还存在BrO3–时. 则会小幅抑制LDHs@BC对磷酸盐的吸附(5.6%),归因于BrO3–相比磷酸盐能够与LDHs形成更稳定的内球络合物[16].
LDHs@BC还具有良好的再生性和可回收性. LDHs@BC可通过多种试剂,如氢氧化钠、乙酸、甲醇和氯化钠等解吸LDHs@BC吸附的磷酸盐,实现LDHs@BC的多次重复使用. 研究表明[52],使用10% NaOH和5% NaCl混合溶液作为La/Fe-LDHs@BC的解吸溶液,经历5次吸附和解吸后,其仍然表现出对磷酸盐优异的去除效率(> 90%).
吸附磷酸盐后的Mg/Al(Fe)-LDHs@BC还可作为生物炭基缓释肥,提高肥料养分的缓释性,降低肥料的淋溶风险,从而促进植物生长. 研究表明[8],将吸附磷酸盐后的Mg/Al-LDHs@BC施用于生菜幼苗,12 d后生菜叶片颜色更绿,幼苗的长度和鲜生物量相比对照组高出60.6%,说明吸附磷酸盐后的LDHs@BC可用于磷酸盐的控释以促进植物生长.
-
本文从LDHs@BC的制备方法、理化性质和对磷酸盐吸附性能及机制等方面总结了LDHs@BC的研究进展. LDHs@BC不仅能够方便低成本的快速获得,还具备生物炭绿色、生物相容性好、透水系数高、比表面积大等特点. 此外,生物炭巨大的表面积和丰富的孔隙结构能够为LDHs的负载提供大量位点,增强了LDHs的分散性,从而显著增强了LDHs@BC对磷酸盐的吸附性能. 另外,通过优化制备方法靶向调控LDHs@BC理化性质,例如比表面积、热稳定性、功能组分的组成和配比,能够显著提升LDHs@BC对磷酸盐的选择性吸附以及环境抗干扰能力. 总之,LDHs@BC对磷酸盐的选择性吸附和优异的再生性均展示了其在水体修复中的应用潜力.吸附磷酸盐后的LDHs@BC在生物炭基缓释肥的应用方面也具有一定的潜力.
同时,LDHs@BC仍具有一些研究空白需要填补和探索. 首先,实际应用中吸附材料不仅需要具有优异的理化性质,还应考量材料的制备效率和成本,因此需要探索优化出同时能兼顾LDHs@BC理化性质、吸附性能和制备效率的制备方法,以获得比表面积大、晶型好、更均质的LDHs@BC.其次,虽然LDHs@BC对磷酸盐的吸附机制已经明确,但不同机制在不同条件下对LDHs@BC吸附磷酸盐的贡献占比还需要进一步研究. 另外,在筛选LDHs@BC制备原料过程中,不仅要考量其吸附性能,还要结合其使用量、使用场景、受体等因素,分析其可能带来的环境风险,尤其是当LDHs中二价阳离子若为Ni、Cu、Mn、Zn等重金属时。并且,目前针对LDHs@BC的研究大多是在实验室规模下的研究,尚需现场或中试规模的研究论证其吸附性能、稳定性及可再生性. 最后,对于原始LDHs@BC以及吸附磷酸盐后的LDHs@BC用作炭基缓释肥和土壤改良剂的潜力仍需要探究.
层状双氢氧化物负载生物炭对磷酸盐的吸附性能研究进展
The adsorption performance of layered double hydroxides functionalized biochar on phosphate: Research advances
-
摘要: 层状双氢氧化物负载生物炭(layered double hydroxides functionalized biochar, LDHs@BC)对水体中磷酸盐具有优异的吸附性能,近年来受到了广泛关注.本文详细介绍了LDHs@BC的制备方法以及制备条件对其理化性质的影响,探讨了LDHs@BC对水体中磷酸盐的吸附性能及机制,并且阐述了生物炭和层状双氢氧化物对磷酸盐吸附的协同作用及其机制,以期通过优化制备工艺参数定向调控LDHs@BC性能,显著提升LDHs@BC对磷酸盐的吸附效率.本文展望了LDHs@BC的应用前景,以期进一步推动LDHs@BC在水体修复中的应用和推广.Abstract: Layered double hydroxides functionalized biochar (LDHs@BC) has drawn wide attention for its good adsorption performance on phosphate in the aqueous system. This paper introduces the synthesis methods of LDHs@BC and summarizes the impacts of different methods on the physicochemical properties and phosphate adsorption performance. The mechanism of using LDHs@BC adsorb phosphate in the aqueous system was discussed. The synergistic mechanism of biochar and layered double hydroxides for phosphate adsorption were elucidated. The synthesis parameters could be optimized to regulate the performance of LDHs@BC, which the adsorption efficiency could be significantly enhanced. Lastly, perspectives were provided to further promote the potential application of LDHs@BC on water remediation.
-

-
表 1 LDHs@BC组成、制备方法及磷酸盐吸附性能的比较
Table 1. Comparison of different composition, synthesis methods, and adsorption performance of LDHs@BC on phosphate removal
生物质原料
BiomassLDHs种类
LDHs typeLDHs金属
物质的量比
LDHs molar ratio制备方法
Synthesis method制备条件
Synthesis condition动力学
Kinetics饱和吸附容量a/
(mg∙g–1)
Maximum adsorption
capacitya参考文献
Reference棉花 Mg/Al 3:1 共沉淀法 老化温度:80 ℃ 老化时间:3 d Ps2 410 [20] 甘蔗叶 Mg/Al 2:1/3:1/4:1 共沉淀法 老化温度:80 ℃ 老化时间:3 d Ps2 165.5/223.5
/253.7[37] 竹 Mg/Al 3:1 共沉淀法 老化温度:80 ℃ 老化时间:3 d Ps2 172 [8] 松果 Mg/Fe 3:1 共沉淀法 老化温度:70 ℃ 老化时间:24 h Ps2 54.3 [36] 椰枣废叶 Mg/Al 3:1 共沉淀法 老化温度:90 ℃ 老化时间:24 h Ps1 178 [18] 水稻秸秆 Mg/Al 3:1 共沉淀法 老化温度:80 ℃ 老化时间:3 d Ps2 192 [10] 小麦秸秆 Mg/Al 1:1 共沉淀法 老化温度:85 ℃ 老化时间:2 d Ps2 153 [43] 玉米秸秆、杏仁壳、牛粪 Fe/Al 1:1 共沉淀法 老化温度:60 ℃ 老化时间:12 h Ps2 215/180/208 [19] 白菜、油菜 Mg/Al 2:1 共热解法 老化温度:60 ℃ 老化时间:6 h
热解温度:500 ℃;2 hPs2 127/133 [31] 玉米秸秆 Zn/Al Mg/Al Ni/Fe 2:1 共热解法 老化温度:室温 老化时间:18 h
热解温度:600 ℃E 64.9/152/78.3 [39] 稻壳 Mg/Al 2:1 共热解法 老化温度:80 ℃ 老化时间:3 d Ps2 64.0 [30] 碳纤维(剑麻) Cu/Al 2:1 水热法 水热温度:110 ℃ 水热时间:12 h Ps2 98.0 [41] 烟梗 Mg/Al 3:1 先负载再水热 水热温度:180 ℃ Ps2 127.6 [44] 香蕉秸秆 Zn/Al 15:1 水热负载后共热解 水热温度:110 ℃ 4 h;60℃ 8 h热解温度:500 ℃ Ps2 185 [23] 柠条 Mg/Al
—电化学法 老化温度:80 ℃ 老化时间:3 d Ps2 253 [45] 注: Ps1: Pseudo-first order, Ps2: Pseudo-second order, E: Elovich., 饱和吸附容量(Maximum adsorption capacity)a:Langmuir最大饱和吸附容量(Maximum adsorption capacity estimated by Langmuir equation). -
[1] WANG Q P, LIAO Z Y, YAO D X, et al. Phosphorus immobilization in water and sediment using iron-based materials: A review [J]. Science of the Total Environment, 2021, 767: 144246. doi: 10.1016/j.scitotenv.2020.144246 [2] VACCARI A. Layered double hydroxides: Present and future [J]. Applied Clay Science, 2002, 22(1-2): 75-76. doi: 10.1016/S0169-1317(02)00112-6 [3] HU H, XIU K M, XU S L, et al. Functionalized layered double hydroxide nanoparticles conjugated with disulfide-linked polycation brushes for advanced gene delivery [J]. Bioconjugate Chemistry, 2013, 24(6): 968-978. doi: 10.1021/bc300683y [4] JELLICOE T C, FOGG A M. Synthesis and characterization of layered double hydroxides intercalated with sugar phosphates [J]. Journal of Physics and Chemistry of Solids, 2012, 73(12): 1496-1499. doi: 10.1016/j.jpcs.2011.10.002 [5] CUNHA V R R, PETERSEN P A D, SOUZA R B, et al. Phytochemical species intercalated into layered double hydroxides: Structural investigation and biocompatibility assays [J]. New Journal of Chemistry, 2020, 44(24): 10011-10021. doi: 10.1039/D0NJ00238K [6] NALAWADE P, AWARE B, KADAM V, et al. Layered double hydroxides: A review [J]. Journal of Scientific and Industrial Research, 2009, 68(4): 267-272. [7] WANG S S, GAO B, LI Y C, et al. Sorption of arsenate onto magnetic iron–manganese (Fe–Mn) biochar composites [J]. RSC Advances, 2015, 5(83): 67971-67978. doi: 10.1039/C5RA12137J [8] WAN S, WANG S S, LI Y C, et al. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions [J]. Journal of Industrial and Engineering Chemistry, 2017, 47: 246-253. doi: 10.1016/j.jiec.2016.11.039 [9] de SOUZA dos SANTOS G E, LINS P V D S, de MAGALHÃES OLIVEIRA L M T, et al. Layered double hydroxides/biochar composites as adsorbents for water remediation applications: recent trends and perspectives [J]. Journal of Cleaner Production, 2021, 284: 124755. doi: 10.1016/j.jclepro.2020.124755 [10] BUATES J, IMAI T. Biochar functionalization with layered double hydroxides composites: Preparation, characterization, and application for effective phosphate removal [J]. Journal of Water Process Engineering, 2020, 37: 101508. doi: 10.1016/j.jwpe.2020.101508 [11] AHMAD M, RAJAPAKSHA A U, LIM J E, et al. Biochar as a sorbent for contaminant management in soil and water: A review [J]. Chemosphere, 2014, 99: 19-33. doi: 10.1016/j.chemosphere.2013.10.071 [12] 李怡冰, 李涵, 黄文轩, 等. 生物炭的制备及其在强化电子传递和催化性能等方面的研究进展 [J]. 环境科学研究, 2021, 34(5): 1157-1167. doi: 10.13198/j.issn.1001-6929.2020.10.01 LI Y B, LI H, HUANG W X, et al. Research progress on the biochar production and its applications in enhancing electron transport and catalysis performance [J]. Research of Environmental Sciences, 2021, 34(5): 1157-1167(in Chinese). doi: 10.13198/j.issn.1001-6929.2020.10.01
[13] WANG S S, GAO B, LI Y C, et al. Manganese oxide-modified biochars: Preparation, characterization, and sorption of arsenate and lead [J]. Bioresource Technology, 2015, 181: 13-17. doi: 10.1016/j.biortech.2015.01.044 [14] EDUAH J O, NARTEY E K, ABEKOE M K, et al. Mechanism of orthophosphate (PO4-P) adsorption onto different biochars [J]. Environmental Technology & Innovation, 2020, 17: 100572. [15] 罗元, 谢坤, 张克强, 等. 生物炭及其金属改性材料脱除水体磷酸盐研究进展 [J]. 环境化学, 2020, 39(8): 2175-2186. doi: 10.7524/j.issn.0254-6108.2019052701 LUO Y, XIE K, ZHANG K Q, et al. Research progress on removal of phosphate from aqueous solution by biochar and its metal modified materials [J]. Environmental Chemistry, 2020, 39(8): 2175-2186(in Chinese). doi: 10.7524/j.issn.0254-6108.2019052701
[16] LI R H, WANG J J, ZHOU B Y, et al. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment [J]. Journal of Cleaner Production, 2017, 147: 96-107. doi: 10.1016/j.jclepro.2017.01.069 [17] JUNG K W, LEE S, LEE Y J. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions [J]. Bioresource Technology, 2017, 245: 751-759. doi: 10.1016/j.biortech.2017.09.035 [18] ALAGHA O, MANZAR M S, ZUBAIR M, et al. Comparative adsorptive removal of phosphate and nitrate from wastewater using biochar-MgAl LDH nanocomposites: Coexisting anions effect and mechanistic studies [J]. Nanomaterials, 2020, 10(2): 336. doi: 10.3390/nano10020336 [19] PENG Y T, SUN Y Q, SUN R Z, et al. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model [J]. Journal of Cleaner Production, 2019, 237: 117770. doi: 10.1016/j.jclepro.2019.117770 [20] ZHANG M, GAO B, YAO Y, et al. Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition [J]. Chemosphere, 2013, 92(8): 1042-1047. doi: 10.1016/j.chemosphere.2013.02.050 [21] TAN X F, LIU Y G, GU Y L, et al. Biochar-based nano-composites for the decontamination of wastewater: A review [J]. Bioresource Technology, 2016, 212: 318-333. doi: 10.1016/j.biortech.2016.04.093 [22] WANG T, LI C, WANG C Q, et al. Biochar/MnAl-LDH composites for Cu (Ⅱ) removal from aqueous solution [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2018, 538: 443-450. doi: 10.1016/j.colsurfa.2017.11.034 [23] JIANG Y H, LI A Y, DENG H, et al. Phosphate adsorption from wastewater using ZnAl-LDO-loaded modified banana straw biochar [J]. Environmental Science and Pollution Research, 2019, 26(18): 18343-18353. doi: 10.1007/s11356-019-05183-1 [24] WANG H B, WANG S Q, CHEN Z L, et al. Engineered biochar with anisotropic layered double hydroxide nanosheets to simultaneously and efficiently capture Pb2+ and CrO42− from electroplating wastewater [J]. Bioresource Technology, 2020, 306: 123118. doi: 10.1016/j.biortech.2020.123118 [25] FANG Q Z, YE S J, YANG H L, et al. Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook [J]. Journal of Hazardous Materials, 2021, 420: 126569. doi: 10.1016/j.jhazmat.2021.126569 [26] GAO W C, LI Z B. Solubility and KSP of Mg4Al2(OH)14·3H2O at the various ionic strengths [J]. Hydrometallurgy, 2012, 117-118: 36-46. doi: 10.1016/j.hydromet.2012.02.003 [27] CHEN S X, HUANG Y F, HAN X X, et al. Simultaneous and efficient removal of Cr(Ⅵ) and methyl orange on LDHs decorated porous carbons [J]. Chemical Engineering Journal, 2018, 352: 306-315. doi: 10.1016/j.cej.2018.07.012 [28] RANE A V, KANNY K, ABITHA V K, et al. Synthesis of Inorganic Nanomaterial[M]. Amsterdam Woodhead Publishing, 2018: 121-139. [29] BUKHTIYAROVA M V. A review on effect of synthesis conditions on the formation of layered double hydroxides [J]. Journal of Solid State Chemistry, 2019, 269: 494-506. doi: 10.1016/j.jssc.2018.10.018 [30] LEE S Y, CHOI J W, SONG K G, et al. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis [J]. Composites Part B:Engineering, 2019, 176: 107209. doi: 10.1016/j.compositesb.2019.107209 [31] ZHANG Z R, YAN L G, YU H Q, et al. Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: Fast removal and mechanistic studies [J]. Bioresource Technology, 2019, 284: 65-71. doi: 10.1016/j.biortech.2019.03.113 [32] XUE, L H, GAO B, WAN Y S, et al. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 63: 312-317. doi: 10.1016/j.jtice.2016.03.021 [33] de SOUZA dos SANTOS G E, IDE A H, DUARTE J L S, et al. Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar [J]. Powder Technology, 2020, 364: 229-240. doi: 10.1016/j.powtec.2020.01.083 [34] WANG S S, GAO B, LI Y C, et al. Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites [J]. RSC Advances, 2016, 6(22): 17792-17799. doi: 10.1039/C5RA17490B [35] JIA Y, ZHANG Y S, FU J G, et al. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 567: 278-287. [36] BOLBOL H, FEKRI M, HEJAZI-MEHRIZI M. Layered double hydroxide–loaded biochar as a sorbent for the removal of aquatic phosphorus: Behavior and mechanism insights [J]. Arabian Journal of Geosciences, 2019, 12(16): 503. doi: 10.1007/s12517-019-4694-4 [37] LI R H, WANG J J, ZHOU B Y, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios [J]. Science of the Total Environment, 2016, 559: 121-129. doi: 10.1016/j.scitotenv.2016.03.151 [38] TAN X F, LIU S B, LIU Y G, et al. One-pot synthesis of carbon supported calcined-Mg/Al layered double hydroxides for antibiotic removal by slow pyrolysis of biomass waste [J]. Scientific Reports, 2016, 6: 3969. [39] YANG F, ZHANG S S, SUN Y Q, et al. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery [J]. Journal of Hazardous Materials, 2019, 365: 665-673. doi: 10.1016/j.jhazmat.2018.11.047 [40] MEILI L, LINS P V, ZANTA C L P S, et al. MgAl-LDH/Biochar composites for methylene blue removal by adsorption [J]. Applied Clay Science, 2019, 168: 11-20. doi: 10.1016/j.clay.2018.10.012 [41] HU F P, WANG M, PENG X M, et al. High-efficient adsorption of phosphates from water by hierarchical CuAl/biomass carbon fiber layered double hydroxide [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2018, 555: 314-323. [42] ZUBAIR M, MANZAR M S, MU'AZU N D, et al. Functionalized MgAl-layered hydroxide intercalated date-palm biochar for Enhanced Uptake of Cationic dye: Kinetics, isotherm and thermodynamic studies [J]. Applied Clay Science, 2020, 190: 105587. doi: 10.1016/j.clay.2020.105587 [43] ZHENG Q, YANG L F, SONG D L, et al. High adsorption capacity of Mg-Al-modified biochar for phosphate and its potential for phosphate interception in soil [J]. Chemosphere, 2020, 259: 127469. doi: 10.1016/j.chemosphere.2020.127469 [44] HE H, ZHANG N, CHEN N, et al. Efficient phosphate removal from wastewater by MgAl-LDHs modified hydrochar derived from tobacco stalk [J]. Bioresource Technology Reports, 2019, 8: 100348. doi: 10.1016/j.biteb.2019.100348 [45] CUI Q L, JIAO G J, ZHENG J Y, et al. Synthesis of a novel magnetic Caragana korshinskii biochar/Mg–Al layered double hydroxide composite and its strong adsorption of phosphate in aqueous solutions [J]. RSC Advances, 2019, 9(32): 18641-18651. doi: 10.1039/C9RA02052G [46] ZUBAIR M, IHSANULLAH I, ABDUL AZIZ H, et al. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook [J]. Bioresource Technology, 2021, 319: 124128. doi: 10.1016/j.biortech.2020.124128 [47] LI W, PIERRE-LOUIS A M, KWON K D, et al. Molecular level investigations of phosphate sorption on corundum (α-Al2O3) by 31P solid state NMR, ATR-FTIR and quantum chemical calculation [J]. Geochimica et Cosmochimica Acta, 2013, 107: 252-266. doi: 10.1016/j.gca.2013.01.007 [48] CHOWDHURY S R, YANFUL E K. Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal [J]. Journal of Environmental Management, 2010, 91(11): 2238-2247. doi: 10.1016/j.jenvman.2010.06.003 [49] CHUBAR N I, KANIBOLOTSKYY V A, STRELKO V V, et al. Adsorption of phosphate ions on novel inorganic ion exchangers [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2005, 255(1-3): 55-63. [50] JUNG K W, JEONG T U, HWANG M J, et al. Phosphate adsorption ability of biochar/Mg-Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl2 as electrolyte [J]. Bioresource Technology, 2015, 198: 603-610. doi: 10.1016/j.biortech.2015.09.068 [51] LALLEY J, HAN C, LI X, et al. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests [J]. Chemical Engineering Journal, 2016, 284: 1386-1396. doi: 10.1016/j.cej.2015.08.114 [52] YANG B, FENG Y F, YU Y L, et al. Lanthanum ferrite nanoparticles modification onto biochar: Derivation from four different methods and high performance for phosphate adsorption [J]. Environmental Science and Pollution Research, 2019, 26(21): 22010-22020. doi: 10.1007/s11356-019-04553-z -



 下载:
下载: