-
中国心理健康调查报告显示国内精神障碍疾病负担较重,抑郁和焦虑症患病率较高[1]. 心理健康受到社会和环境等多种因素影响,引发的炎症、氧化应激和神经递质紊乱都可能是神经系统疾病的致病因素. 长期压力应激状态会诱发焦虑、抑郁等精神类疾病,还具有导致器官功能性病变的风险[2]. 环境污染也可影响疾病的发生发展过程,污染物暴露可通过影响下丘脑-垂体-肾上腺轴(HPA)的功能和应激反应而引发心理疾病[3]. 研究表明心理应激和交通或工业空气污染对健康的不良影响存在明显协同效应[4]. 慢性压力应激状态下,全身易感性增强,环境污染物的毒性作用更为明显. 据报道,慢性压力应激和150 mg·kg−1醋酸铅同时暴露2个月可显著影响大鼠基底皮质酮水平与HPA轴功能[5]. Zhou等[6]发现妊娠期孕妇社会心理压力较大时,铅金属污染物暴露与婴幼儿神经缺陷、认知发育不全等不良健康的关联性增加. Clougherty等[7]研究发现,处于慢性压力下的大鼠对浓缩细颗粒空气污染的呼吸反应更强,并可能存在不同易感性的途径. 现有流行病学数据无法确定差异易感性的生理机制[8],因此,本文基于毒理代谢组学阐明慢性压力应激和污染暴露之间的潜在相互作用.
B[a]P是环境和食品中常见的污染物,主要通过呼吸和饮食途径进入体内,在肝脏蓄积和代谢活化,导致肝功能异常或肝损伤[9]. B[a]P及其代谢物可与体内氨基酸、有机酸、脂质等代谢物相互作用而具有肝毒性效应[10]. 长期慢性心理应激状态下机体生理功能和免疫力显著降低[11],因此可能对B[a]P暴露的应答会产生更复杂影响,但目前未有明确的影响机制研究. 本研究选用慢性不可预知温和刺激(chronic unpredictable mild stimulation,CUMS)作为慢性压力应激模型,采用靶向代谢组学分析方法研究了不同剂量B[a]P和CUMS单独或两者同时暴露对雄性C57BL/6J小鼠肝脏中氨基酸、TCA循环和胆汁酸代谢的影响,并通过统计学分析筛选出了差异代谢物,拟从代谢物差异变化初步探讨肝脏对B[a]P和慢性压力应激的应答和代谢机制.
-
B[a]P(≥96%,HPLC级)购自Sigma化学公司(St Louis,MO,USA);甲酸(LC-MS级)、乙腈(LC-MS级)、甲醇(LC-MS级)和甲酸铵(LC-MS级)购自J&K化学公司(Beijing,China);超纯水由美国Milli-Q 超纯水系统(Millipore,Billerica,USA)净化.
-
雄性C57BL/6J小鼠(7周龄)购自北京维通利华实验动物技术有限公司(Beijing,China),并在中国科学院深圳先进技术研究院喂养,保持12 h光/暗循环,恒温20—26 ℃,相对湿度40% —70%. 动物实验经中国科学院深圳先进技术研究院动物实验和实验动物福利委员会批准,所有涉及动物的实验程序都严格按照国家动物实验法律法规和参考美国国立卫生研究院出版的《实验动物护理和使用指南》执行.
-
小鼠适应环境一周后被随机分成6组(n = 7):对照组(给予等体积橄榄油)、低剂量B[a]P暴露组(2.0 mg·kg−1·d−1)、高剂量B[a]P暴露组(20.0 mg·kg−1·d−1)、CUMS组(给予等体积的橄榄油和CUMS刺激)、低/高剂量B[a]P和CUMS同时暴露组(不同剂量B[a]P暴露基础上进行CUMS刺激). B[a]P溶于橄榄油中采用口服灌胃方式暴露;低/高剂量B[a]P和CUMS同时暴露组在灌胃2 h后以随机排序给予以下CUMS刺激操作:①小鼠置于相对湿度40%—70%的潮湿环境中12 h,②每只小鼠置于50 mL管中禁食禁水2 h进行约束处理,③将4只小鼠同时置于3 cm × 5 cm × 7 cm的盒子中2 h,并禁食禁水. 持续暴露21 d,暴露实验开始和结束时记录小鼠体重. 暴露结束后颈部脱臼法处死小鼠,立即切除肝组织,称取肝脏湿重用于计算肝脏脏器系数(肝脏脏器系数=肝脏湿重/体重 × 100%),随后在液氮中速冻、转移至-80 ℃冰箱储存.
-
取适量肝脏组织样本置于2.0 mL离心管中,加入超纯水(肝组织重量:水重量 = 4:1),在高通量组织研磨仪(SCIENTZ-48L,Scientz,China)中进行研磨(4 ℃, 2.0 min). 称取约15 mg肝组织匀浆转移至1.5 mL新离心管中,加入0.2 mL甲醇,涡旋振荡20 min,在4 ℃和12000 r·min−1条件下离心15 min,取上清液进行仪器分析.
-
使用超高效液相色谱-三重四极杆串联质谱仪(UPLC-MS/MS 8060,Shimadzu,Japan)对肝脏组织中75种氨基酸、有机酸和胆汁酸类相关代谢物(表1、表2和表3)进行测定,采用外标法进行定量. 具体分析条件如下.
色谱参数:色谱柱柱温为40 ℃,流动相流速为0.2 mL·min−1,进样量为1.0 μL.
氨基酸类:Waters ACQUITY UPLC®HSS T3色谱柱(2.1 mm × 100 mm,1.8 μm),流动相A为0.1%甲酸-水,流动相B为0.1%甲酸-乙腈,梯度洗脱程序为:0 — 0.5 min,0% B;6.0 min,75% B;6.1 min,0% B,维持2.0 min.
有机酸类:Waters ACQUITY UPLC®HSS C18 色谱柱(2.1 mm × 100 mm,1.7 μm),流动相A为0.01mol· L−1甲酸铵-水,流动相B为0.01mol· L−1甲酸铵-甲醇,梯度洗脱顺序为:0 min,5% B;2.0 min,60% B;2.1 min,5% B,维持2 min.
胆汁酸类:Waters ACQUITY UPLC®BEH C18 色谱柱(2.1 mm × 100 mm,1.7 μm),流动相A为0.1%甲酸-水,流动相B为0.1%甲酸-乙腈,梯度洗脱程序:0.0 min,40%B;0.5 min,45%B;1.0 min,50%B;2.0 min,52%B;2.5 min,53%B;4.5 min,54%B;5.0 min,55%B,维持1.0 min;7.0 min,70%B;8.0 min,95%B;8.5 min,100%B,维持1.0 min;10.5 min,40% B,平衡2.0 min.
质谱参数:正离子和负离子模式下电喷雾电压分别为4.0 kV和-3.0 kV,雾化气体流量3.0 L·min−1,干燥气流量10 L·min−1,加热气体流量10 L·min−1,接口温度300 ℃,DL温度250 ℃,加热模块温度400 ℃. 各代谢物具体质谱参数如表1、表2和表3所示,定量限为0.02—220.44 μg·L−1,检出限为0.01—77.44 μg·L−1.
-
代谢扰乱度(Metabolic effect level index, MELI)是一种用于评估暴露后生物体代谢产物、特定代谢途径或整体代谢反应的定量终点指标[12]. 首先根据公式(1)计算不同组别样品中每个代谢物的变化(MCi). 其中,Ai为暴露组与对照组中单个代谢物i平均浓度的比值,ln(1)用于抵消对照组代谢水平.
将各代谢物的代谢变化汇总为累积代谢变化,根据公式(2)计算MELI值:
当MELIComb值接近MELIB[a]P和MELICUMS平方和的算术平方根时,B[a]P和CUMS的联合效应类型为加和效应;当MELIComb值大于MELIB[a]P和MELICUMS之和时,联合效应类型为协同效应;当MELIComb值小于MELIB[a]P或MELICUMS时,联合效应类型为拮抗效应.
-
使用MetaboAnalyst 5.0在线分析软件进行多变量统计分析,基于KEGG数据库进行代谢通路分析(https://www.metaboanalyst.ca/),GraphPad Prism 9.0软件进行统计学分析和绘图. 使用单因素方差分析P < 0.05,偏最小二乘判别分析模型变量影响重要性因子(Variable importance in the projection,VIP)值 > 1.0和ROC曲线下面积(Area under curve,AUC)值 > 0.75,作为显著差异代谢物的筛选准则. 所有数据均以平均数 ± 标准差(Mean ± SD)表示,采用单因素方差分析(One-way ANOVA)分析组间差异,P < 0.05为组间差异有统计学意义.
-
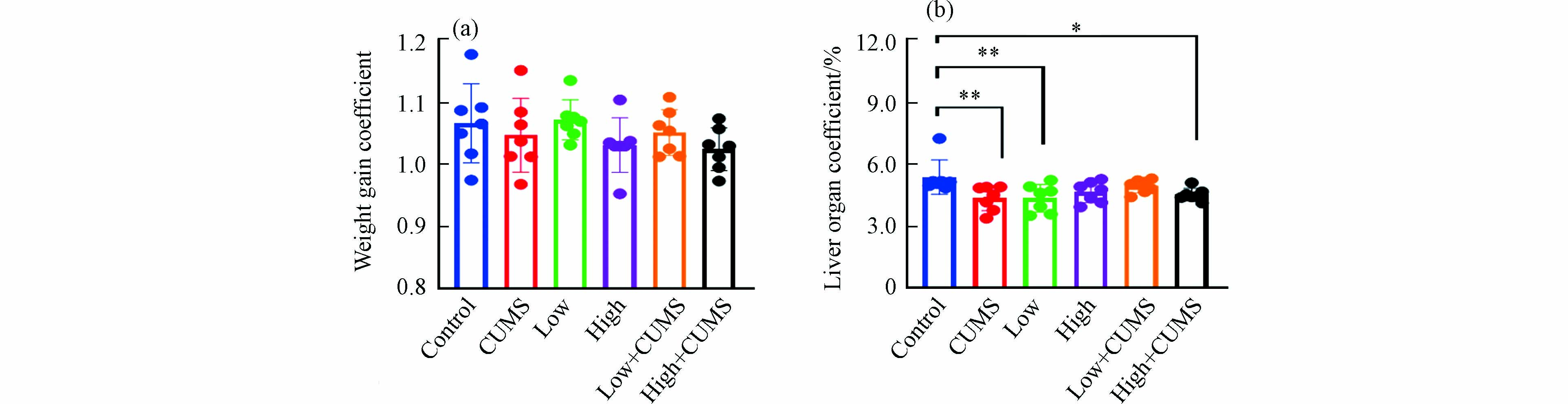
暴露实验结束时小鼠体重增加系数见图1a. 连续暴露21 d,除2.0 mg·kg−1·d−1的B[a]P暴露组外,20.0 mg·kg−1·d−1 B[a]P与CUMS单独暴露及不同剂量B[a]P与CUMS共同暴露的小鼠体重平均增长系数均低于对照组,表明高剂量B[a]P与CUMS单独暴露及B[a]P与CUMS同时暴露均可在一定程度上抑制小鼠的体重增长,减缓小鼠的生长速率. 如图1b所示,不同剂量B[a]P与CUMS单独或二者同时暴露的小鼠肝脏脏器系数均低于对照组,表明B[a]P与CUMS均可引发肝脏出现一定程度萎缩,说明肝脏是B[a]P与CUMS暴露的重要靶器官.
-
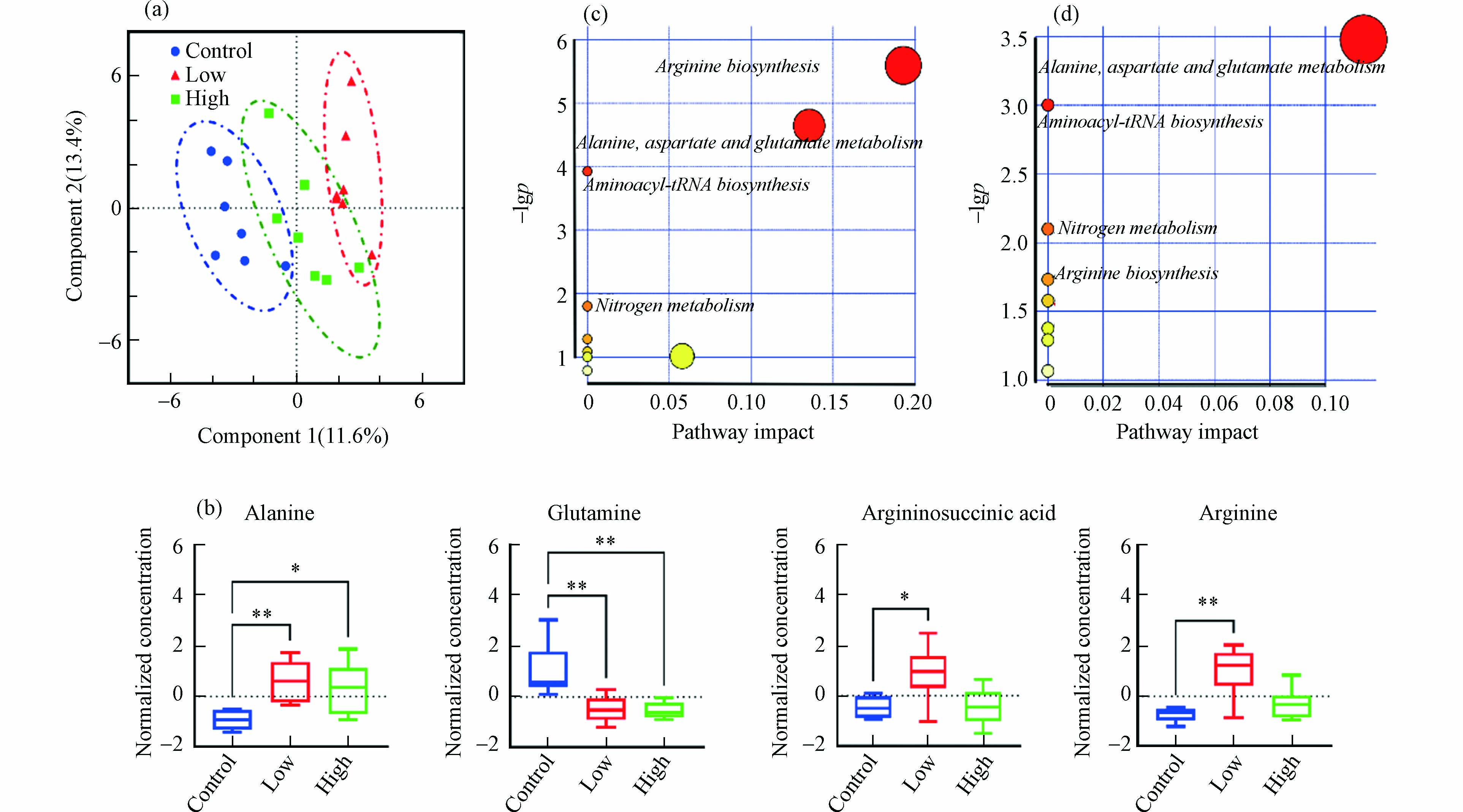
采用PLS-DA评估不同剂量B[a]P暴露后小鼠肝脏氨基酸、TCA循环和胆汁酸代谢的变化. 模型累计贡献率(R2)和模型预测能力(Q2)分别为0.947和0.676,置换检验P值为0.021,表明建立的PLS-DA模型具有良好的解释和预测能力,且不存在过拟合情况. 如图2a所示,两个暴露组和对照组在第一主成分上得到良好区分,说明两个剂量B[a]P暴露均可引起小鼠肝脏代谢紊乱.
筛选出两种B[a]P暴露剂量下的显著差异代谢物,结果如图2b所示(筛选准则见1.6节所述). 与对照组相比,2.0 mg·kg−1·d−1 B[a]P可使小鼠肝脏中精氨琥珀酸、精氨酸、丙氨酸水平显著上升(P < 0.05)而谷氨酰胺水平显著下降(P < 0.01);20.0 mg·kg−1·d−1 B[a]P导致小鼠肝脏中谷氨酰胺水平显著降低(P < 0.01)和丙氨酸显著升高(P < 0.05). 以往研究也发现,腹腔注射1 mg·kg−1·d−1的B[a]P可使SD大鼠肝脏组织中氨基酸代谢发生紊乱,导致谷氨酰胺显著下降,精氨酸、丙氨酸等显著升高,谷草转氨酶显著升高[13]. 作为细胞增殖分化的必需营养物质,肝脏中谷氨酰胺含量下降可能与癌细胞增殖摄取有关,并有可能促发肝细胞凋亡[14]. Wang等[15]发现,10 mg·kg−1和20 mg·kg−1 B[a]P暴露3个月大鼠肝癌发生率分别为26.3%和35.3%,Michurina等[16]也发现,200 mg·kg−1 B[a]P暴露3 d后可致大鼠肝细胞凋亡. 同时,谷氨酰胺与丙氨酸还是肝糖原异生的重要底物[17],推测B[a]P暴露也会影响小鼠肝脏中糖异生能量转化途径. 此外,精氨酸与精氨琥珀酸是尿素循环的中间代谢产物,2.0 mg·kg−1 B[a]P暴露后两者显著上升表明尿素循环代谢被扰乱,可能会引发肝功能衰竭、化学性肝损伤等肝脏疾病[18].
如图2c和2d所示,通过对显著差异代谢物进行KEGG代谢通路分析发现,两个不同剂量B[a]P暴露主要影响小鼠肝脏丙氨酸、天冬氨酸和谷氨酸代谢、氨基酰基-tRNA生物合成、氮代谢、精氨酸代谢合成,而2.0 mg·kg−1·d−1B[a]P影响的9个代谢通路,20.0 mg·kg−1·d−1B[a]P影响的8个代谢通路。 这些结果表明,B[a]P经口暴露后主要影响肝脏氨基酸代谢,低剂量B[a]P暴露的影响更为明显.
-
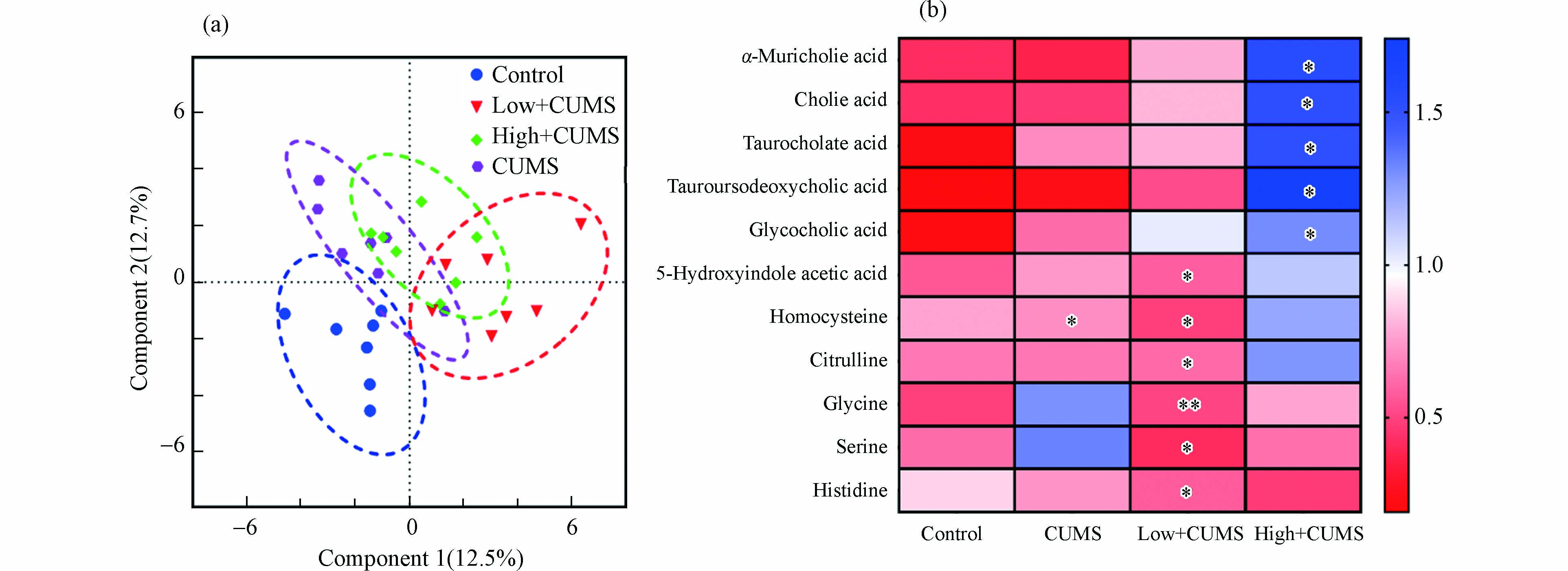
如图3a所示,从PLS-DA因子得分图(R2 = 0.729,Q2 = 0.357,P < 0.001)可以看出,CUMS单独暴露和与B[a]P同时暴露对小鼠肝脏氨基酸和胆汁酸代谢都有明显影响. 根据差异代谢物筛选准则,发现CUMS刺激后,小鼠肝组织中同型半胱氨酸水平显著下降(P < 0.05),这与以往有关CUMS刺激后小鼠肝脏组织代谢变化的结果一致[19]. 同时,发现两个剂量B[a]P和CUMS同时暴露小鼠肝脏的氨基酸和胆汁酸代谢物变化,如图3b中所示,2.0 mg·kg−1·d−1 B[a]P与CUMS同时暴露使得小鼠肝组织中5-羟基吲哚乙酸、同型半胱氨酸、组氨酸、瓜氨酸、甘氨酸、丝氨酸水平显著降低(P < 0.05);而20.0 mg·kg−1·d−1 B[a]P与CUMS同时暴露则主要干扰肝脏胆汁酸代谢途径,表现为甘胆酸、牛磺熊脱氧胆酸、牛磺胆酸、胆酸和α-鼠胆酸水平显著升高(P < 0.05). 即2.0 mg·kg−1·d−1B[a]P与CUMS共同暴露后肝脏氨基酸代谢紊乱而20.0 mg·kg−1·d−1 B[a]P与CUMS共同暴露后肝脏胆汁酸代谢异常. 作为参与机体胆固醇和脂质代谢的内源性小分子代谢物,胆汁酸在肝组织中的累积会诱导肝损伤的发生[20]. 此外,次级胆汁酸如甘胆酸、牛磺熊脱氧胆酸的水平变化与肝性脑病和2-型糖尿病有关[21]. 同时氨基酸代谢与肝脏正常生理功能密切相关,其紊乱还与肝炎、肝硬化等疾病的发生有关[22]. 已有文献报道,CUMS和B[a]P暴露都可使小鼠肝功能血清生化指标天冬氨酸氨基转移酶和丙氨酸氨基转移酶活性升高[23-24],且肝组织HE染色后可观察到明显的组织病理学变化[25-26],表明CUMS和B[a]P可导致肝功能异常和肝损伤. 因此,推测B[a]P与CUMS暴露引发的小鼠肝脏氨基酸和胆汁酸代谢物的变化是其诱导肝功能异常和肝损伤可能的机制之一.
-
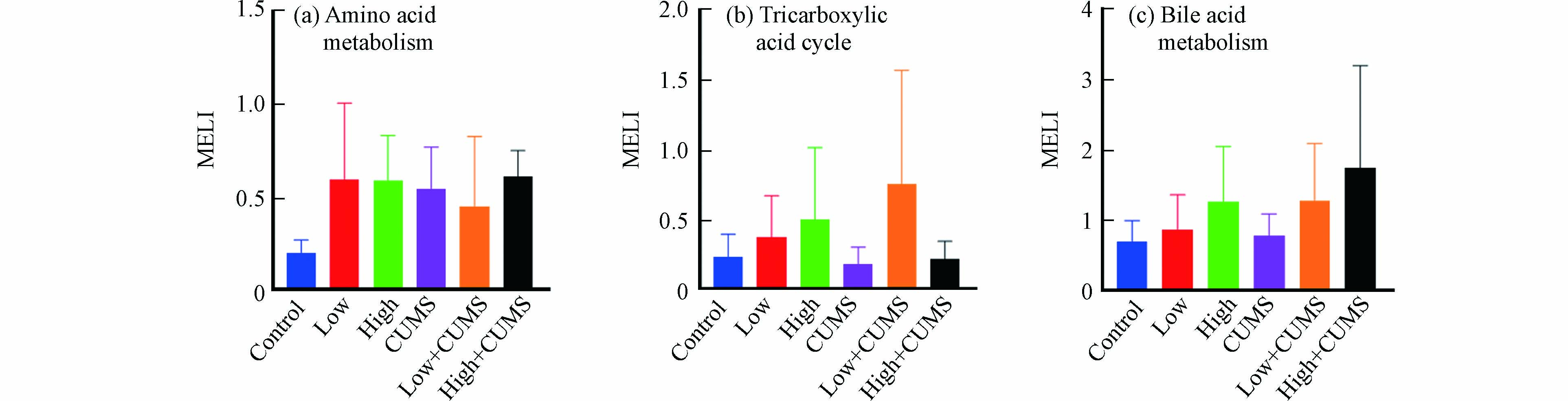
环境污染和心理健康是疾病发生与发展的两大因素,流行病学调查发现空气污染暴露对处于慢性压力状态机体的不良影响显著增加[27],但具体的生理机制及两者间相互作用尚不完全清楚. 毒理代谢组学可从代谢物角度揭示压力应激和环境污染暴露之间的效应,代谢扰乱度(MELI)可将信息量丰富的代谢组学数据转化成一个综合的定量终点,目前已被用于评估反映PM2.5、氯化石蜡等污染物暴露后细胞代谢的的总体变化情况[28-29]. 小鼠肝组织中氨基酸代谢、TCA循环和胆汁酸代谢在B[a]P与CUMS单独或同时暴露后MELI值见图4.
从图4中看出,20.0 mg·kg−1·d−1的B[a]P对氨基酸代谢、TCA循环和胆汁酸代谢的影响更大. 如图4a所示,氨基酸代谢MELILow-CUMS值小于MELILow和MELICUMS表明低剂量B[a]P与CUMS同时暴露对小鼠肝脏氨基酸代谢的联合效应为拮抗效应;MELIHigh-CUMS值接近于MELILow2+MELICUMS2算术平方根的值,说明高剂量B[a]P与CUMS同时暴露对小鼠肝脏氨基酸的联合效应为加和效应. TCA循环的MELILow-CUMS值大于MELILow+MELICUMS,而MELIHigh-CUMS值小于MELIHigh且大于MELICUMS(图4b),说明低剂量B[a]P与CUMS同时暴露对小鼠肝脏TCA循环具有协同效应,而高剂量B[a]P与CUMS同时暴露对小鼠肝脏TCA循环具有拮抗效应. 不同剂量B[a]P与CUMS同时暴露对小鼠肝脏氨基酸代谢和TCA循环的联合效应均不同,其效应的类型与共同暴露时B[a]P的剂量有关. 此外,胆汁酸代谢的MELILow-CUMS与MELIHigh-CUMS值分别接近MELILow2+MELICUMS2和MELIHigh2+MELICUMS2(图4c),表明不同剂量B[a]P与CUMS同时暴露对小鼠肝脏胆汁酸代谢的联合效应类型均为加和效应.
-
本研究通过靶向代谢组学分析方法,发现了暴露在B[a]P下主要影响氨基酸代谢且不同剂量条件下小鼠肝脏代谢应答存在明显差异. 不同剂量B[a]P均可导致谷氨酰胺水平显著下降,丙氨酸显著上升;同时2.0 mg·kg−1·d−1 B[a]P还可使精氨琥珀酸、精氨酸显著上升. 不同剂量B[a]P对肝脏代谢影响的机制是不同的. 慢性压力应激影响小鼠肝脏氨基酸代谢,同型半胱氨酸含量显著下降. B[a]P与CUMS同时暴露,使得小鼠肝脏氨基酸和胆汁酸代谢发生显著变化,差异的代谢物与两者单独暴露明显不同. 利用MELI评估污染物和精神因素对小鼠肝脏代谢的联合作用,不同剂量B[a]P与CUMS对氨基酸代谢、TCA循环和胆汁酸代谢具有不同的作用,差异的机制还需要进一步研究. 本研究初步阐明了两大因子对肝脏氨基酸、有机酸和胆汁酸代谢的影响,但对脂质、激素等代谢的影响及具体作用机制还需进一步研究. 综上,本研究发现的代谢异常与肝损伤、肝功能异常、肝细胞炎症和肝癌等病变的发生有关,这有助于进一步理解环境污染物与精神因素对机体健康的作用.
苯并[a]芘和慢性压力应激暴露对小鼠肝脏代谢的影响
Effects of exposure to benzo[a]pyrene and chronic stress on hepatic metabolism of mice
-
摘要: 环境污染和慢性压力是影响人类健康的两大类常见风险因子,本研究通过表征苯[a]并芘(benzo[a]pyrene,B[a]P)与慢性压力应激(chronic unpredictable mild stimulation,CUMS刺激)暴露对小鼠肝脏代谢的影响,并探讨了同时暴露时两大风险因子之间潜在的相互作用. 采用靶向代谢组学结合多元统计学方法分析了两个剂量B[a]P(2.0 mg·kg−1 ·d−1和20.0 mg·kg−1·d−1)与CUMS刺激单独或联合暴露21 d后,雄性C57BL/6J小鼠肝脏中氨基酸代谢、TCA循环和胆汁酸代谢的变化. 研究发现,2.0 mg·kg−1·d−1 B[a]P可使小鼠肝脏中精氨琥珀酸、精氨酸和丙氨酸显著升高,谷氨酰胺显著降低;20.0 mg·kg−1·d−1 B[a]P则主要导致丙氨酸显著上升,谷氨酰胺下降. 慢性压力应激使小鼠肝脏同型半胱氨酸的含量显著下降. B[a]P与慢性压力应激同时暴露时,小鼠肝脏氨基酸和胆汁酸代谢会发生显著变化. 进一步采用代谢扰乱度(metabolic effect level index,MELI)评估了两个风险因子的联合作用效果,发现2.0 mg·kg−1·d−1 B[a]P和慢性压力应激同时暴露对小鼠氨基酸代谢、TCA循环和胆汁酸代谢的影响分别为拮抗、协同和加和作用;而20 mg·kg−1·d−1 B[a]P和慢性压力应激同时暴露的小鼠氨基酸代谢和胆汁酸代谢的影响均为加和作用,TCA循环为拮抗作用. 结果表明,不同剂量的B[a]P和CUMS暴露造成小鼠肝脏氨基酸代谢和胆汁酸代谢紊乱,差异的代谢物与两者单独暴露时明显不同,且代谢的变化与B[a]P暴露剂量密切相关.Abstract: Environmental pollutants and chronic stress are two common risk factors that have great impact on human health. In this study, potential interactions between these two factors were explored by characterizing the effects of benzo[a]pyrene (B[a]P), or chronic stress, or their combination, on liver metabolism of male C57BL/6J mice using targeted metabolomics followed by multivariate statistical analysis. It was found that the hepatic levels of alanine and glutamine were significantly up- and down-regulated respectively after 21 days of exposure to 2.0 mg·kg−1·d−1 or 20.0 mg·kg−1·d−1 of B[a]P. But only in the 2.0 mg·kg−1·d−1 case did we observe the up-regulation of argininosuccinic acid and arginine, suggesting that the B[a]P-induced alterations in amino acid metabolism were dose dependent. Moreover, chronic stress stimulus was found associated with a significant decrease in the hepatic level of homocysteine. Also, we evaluated the effects of combined exposure on the hepatic metabolism based on the metabolic effect level index (MELI). Specifically, the combined exposure to 2.0 mg·kg−1·d−1 B[a]P and chronic stress exhibited antagonistic, synergistic and additive effects on amino acid metabolism, TCA cycle and bile acid metabolism, respectively. In contrast, the combined exposure to 20.0 mg·kg−1·d−1 B[a]P and chronic stress showed additive effects on both amino acid metabolism and bile acid metabolism, but antagonistic effect on TCA cycle. We conclude that the exposure to both B[a]P and chronic stress can trigger significant disorders in amino acid and bile acid metabolism, which, notably, differ from those caused by exposure to either alone and were closely related to the exposure dose of B[a]P.
-
Key words:
- benzo[a]pyrene /
- chronic stress /
- exposomics /
- mice liver /
- metabolomics
-

-
图 2 不同剂量B[a]P暴露对小鼠肝脏氨基酸、TCA循环和胆汁酸代谢的影响
Figure 2. Effects of exposure to B[a]P on amino acids, TCA cycle and bile acid metabolism in the liver of mice (a) PLS-DA score plot;(b) Box plots of amino acids with the significant difference in different groups;(c) the relevant metabolic pathways after exposure to B[a]P with low doses ;(d) the relevant metabolic pathways after exposure to B[a]P with high doses
表 1 氨基酸代谢物的质谱参数
Table 1. Mass spectrometry parameters for amino acid metabolites
化合物
Compounds检测离子对
Transitions化合物
Compounds检测离子对
Transitions化合物
Compounds检测离子对
Transitions肾上腺素 183.9→166.1a
183.9→107.2b去甲肾上腺素 170.2→107.1
170.2→152.23-羟基苯甲酸 154.1→136.1
154.1→80.15-羟色胺 177.2→160.2
177.2→115.1褪黑素 233.2→174.2
233.2→159.13-羟基犬尿氨酸 224.9→208.1
224.9→162.1胆碱 104.2→60.2
104.2→58.2肌酸 132.1→90.2
132.1→44.25-氨基戊酸 118.4→55.2
118.4→101.1谷氨酰胺 147.2→84.1
147.2→130.1多巴胺 153.9→91.2
153.9→136.95-羟基吲哚乙酸 192.3→146.1
192.3→91.2甘氨酸 76.1→30.2 肌酐 114.3→44.2 二羟基苯乙酸 166.4→122.9 组胺 112.3→95.2
112.3→41.2犬尿酸 190.1→144.1
190.1→89.1γ-氨基丁酸 104.1→87.1
104.1→69.1多巴 198.1→152.2
198.1→107.2二羟基苯乙醇 152.8→123.0
152.8→95.1犬尿氨酸 209.2→192.2
209.2→94.1丙氨酸 90.1→56.2
90.1→44.2苯丙氨酸 166.1→120.2
166.1→103.1牛磺酸 126.0→107.8
126.0→43.95色氨酸 205.2→188.0
205.2→146.1酪胺 138.2→121.1
138.2→77.1酪氨酸 182.1→91.1
182.1→136.2黄尿酸 206.1→160.1
206.1→132.14-羟基脯氨酸 132.1→86.2
132.1→68.1乙酰胆碱 146.2→97.1
146.2→43.1精氨酸 175.2→70.2
175.2→60.2精氨琥珀酸 291.2→70.1
291.2→116.2天冬精氨 133.1→87.2
133.1→28.2天冬氨酸 134.1→74.2
134.1→88.1肉碱 162.2→60.3
162.2→85.2瓜氨酸 176.2→70.2
176.2→159.2半胱氨酸 122.2→59.2
122.2→76.1胱氨酸 241.3→74.1
241.3→152.1谷氨酸 148.1→84.1
148.1→56.1组氨酸 156.2→110.2
156.2→56.2同型半胱氨酸 136.2→90.1
136.2→56.2异亮氨酸 132.2→86.2
132.2→69.2亮氨酸 132.4→86.2
132.4→30.2赖氨酸 147.4→84.2 甲硫氨酸 150.2→56.1
150.2→104.1烟酰胺 123.1→80.1
123.1→78.1鸟氨酸 133.2→70.2
133.2→116.2丝氨酸 106.1→60.1 脯氨酸 116.1→70.1 苏氨酸 120.1→104.1
120.1→74.3缬氨酸 118.1→72.2
118.1→55.2表 2 有机酸代谢物的质谱参数
Table 2. Mass spectrometry parameters for organic acid metabolites
化合物
Compounds检测离子对
Transitions化合物
Compounds检测离子对
Transitions化合物
Compounds检测离子对
Transitionsα-酮戊二酸 145.0→100.9
145.0→56.8乌头酸 173.0→84.9
173.0→129.0柠檬酸 191.0→111
191.0→86.9富马酸 115.1→71.1
115.1→26.9衣康酸 129.0→60.1
129.0→40.8乳酸 89.1→42.9 苹果酸 133.0→71.1
133.0→73.0丙酮酸 87.1→43.0 琥珀酸 117→73.1 表 3 胆汁酸代谢物的质谱参数
Table 3. Mass spectrometry parameters for bile metabolites
化合物
Compounds检测离子对
Transitions化合物
Compounds检测离子对
Transitions化合物
Compounds检测离子对
Transitions甘氨胆酸 464.0→74.0a
464.0→402.2b甘氨鹅脱氧胆酸 448.1→74.0
448.1→386.0牛磺胆酸 514.2→124.0
514.2→107.0α-鼠胆酸 373.1→355.3
373.1→373.2牛磺鹅脱氧胆酸 498.0→124.0
498.0→80.0β-鼠胆酸 391.1→355.3
391.1→373.2ω-鼠胆酸 373.1→159.2
373.1→337.5鹅去氧胆酸 357.1→105.1
357.1→135.4猪去氧胆酸 357.1→161.2
357.1→135.2石胆酸 359.1→135.2
359.1→95.1去氧胆酸 391.0→345.3
391.0→327.1胆酸 407.3→343.3
407.3→288.9熊去氧胆酸 357.1→161.2
357.1→135.2牛磺熊脱氧胆酸 498.0→124.0
498.0→80.0甘氨熊脱氧胆酸 448.05→74.0
448.05→386.2注:a定量离子对:quantitative ion; b定性离子对:qualitative ion. -
[1] HUANG Y Q, WANG Y, WANG H, et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study [J]. The Lancet Psychiatry, 2019, 6(3): 211-224. doi: 10.1016/S2215-0366(18)30511-X [2] NAGARAJA A S, SADAOUI N C, DORNIAK P L, et al. SnapShot: stress and disease [J]. Cell Metabolism, 2016, 23(2): 388-388.e1. doi: 10.1016/j.cmet.2016.01.015 [3] SIRIVELU M P, MOHANKUMAR S M J, WAGNER J G, et al. Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease [J]. Environmental Health Perspectives, 2006, 114(6): 870-874. doi: 10.1289/ehp.8619 [4] CLOUGHERTY J E, KUBZANSKY L D. A framework for examining social stress and susceptibility to air pollution in respiratory health [J]. Environmental Health Perspectives, 2009, 117(9): 1351-1358. doi: 10.1289/ehp.0900612 [5] VIRGOLINI M B, BAUTER M R, WESTON D D, et al. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress [J]. NeuroToxicology, 2006, 27(1): 11-21. doi: 10.1016/j.neuro.2005.05.012 [6] ZHOU L L, XU J, ZHANG J S, et al. Prenatal maternal stress in relation to the effects of prenatal lead exposure on toddler cognitive development [J]. NeuroToxicology, 2017, 59: 71-78. doi: 10.1016/j.neuro.2017.01.008 [7] CLOUGHERTY J E, ROSSI C A, LAWRENCE J, et al. Chronic social stress and susceptibility to concentrated ambient fine particles in rats [J]. Environmental Health Perspectives, 2010, 118(6): 769-775. doi: 10.1289/ehp.0901631 [8] KUBZANSKY L D, KAWACHI I, SPARROW D. Socioeconomic status, hostility, and risk factor clustering in the normative aging study: Any help from the concept of allostatic load? [J]. Annals of Behavioral Medicine, 1999, 21(4): 330-338. doi: 10.1007/BF02895966 [9] ZHANG L B, LIU X L, YOU L P, et al. Benzo(a)Pyrene-induced metabolic responses in Manila clam Ruditapes philippinarum by proton nuclear magnetic resonance (1H NMR) based metabolomics [J]. Environmental Toxicology and Pharmacology, 2011, 32(2): 218-225. [10] KALKHOF S, DAUTEL F, LOGUERCIO S, et al. Pathway and time-resolved benzo[a]Pyrene toxicity on Hepa1c1c7 cells at toxic and subtoxic exposure [J]. Journal of Proteome Research, 2015, 14(1): 164-182. doi: 10.1021/pr500957t [11] OH T W, KIM K Y, DO H J, et al. Comparative analysis of acute and chronic stress-induced neurobehavioral alteration and liver injury in mice [J]. Molecular & Cellular Toxicology, 2020, 16(4): 367-375. [12] RIEDL J, SCHREIBER R, OTTO M, et al. Metabolic effect level index links multivariate metabolic fingerprints to ecotoxicological effect assessment [J]. Environmental Science & Technology, 2015, 49(13): 8096-8104. [13] 石磊. 苯并(a)芘及大气PM2.5染毒大鼠肝和脑代谢组学研究[D]. 太原: 山西医科大学, 2017. SHI L. Metabolism studies on liver and brain in rats exposed to benzo(a) Pyrene and atmospheric fine particulate matter[D]. Taiyuan: Shanxi Medical University, 2017(in Chinese).
[14] MEYNIAL-DENIS D. Glutamine metabolism in advanced age [J]. Nutrition Reviews, 2016, 74(4): 225-236. doi: 10.1093/nutrit/nuv052 [15] WANG Q L, XUE Y J. Characterization of solid tumors induced by polycyclic aromatic hydrocarbons in mice [J]. Medical Science Monitor Basic Research, 2015, 21: 81-85. doi: 10.12659/MSMBR.893945 [16] MICHURINA S V, BORODIN I I, KOLESNIKOV S I, et al. [Liver and Its Lymph Region at Benzo[a]pyrene Effects in an Experiment] [J]. Vestnik Rossiiskoi akademii meditsinskikh nauk, 2015, 2: 242-248. [17] BRÖER S. Amino acid transporters as modulators of glucose homeostasis [J]. Trends in Endocrinology & Metabolism, 2022, 33(2): 120-135. [18] BIGOT A, TCHAN M C, THOREAU B, et al. Liver involvement in urea cycle disorders: A review of the literature [J]. Journal of Inherited Metabolic Disease, 2017, 40(6): 757-769. doi: 10.1007/s10545-017-0088-5 [19] LIU X J, LIU H L, ZHAO D, et al. Hepatic metabolomics of the compatibility effect of Xiaoyaosan on CUMS-induced depression based on the TCM theory of “Treating Diseases via Regulating the Liver's Function” [J]. Journal of Pharmaceutical and Biomedical Analysis, 2021, 201: 114123. doi: 10.1016/j.jpba.2021.114123 [20] 林珠灿, 易开, 许文, 等. 超高效液相色谱-四极杆飞行时间质谱法研究菊三七总生物碱致肝毒性的血清代谢组学 [J]. 分析科学学报, 2018, 34(3): 297-302. doi: 10.13526/j.issn.1006-6144.2018.03.001 LIN Z C, YI K, XU W, et al. A serum metabonomic evaluation of the total alkaloids of Gynura segetum merr.-induced liver toxicity in rats by ultra-high performance liquid chromatography-quadrupole-time of flight mass spectrometry [J]. Journal of Analytical Science, 2018, 34(3): 297-302(in Chinese). doi: 10.13526/j.issn.1006-6144.2018.03.001
[21] WEISS N, BARBIER SAINT HILAIRE P, COLSCH B, et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy [J]. Journal of Hepatology, 2016, 65(6): 1120-1130. doi: 10.1016/j.jhep.2016.07.046 [22] LIU X, WANG H, LIANG X, et al. Hepatic metabolism in liver health and disease[M]//Liver Pathophysiology. Amsterdam: Elsevier, 2017: 391-400. [23] LI H, YUAN L, WANG Z L, et al. Effects of dietary whole grain buckwheat and oat on benzo[a]Pyrene-induced genotoxicity, oxidative and pyroptotic injury in liver of mice [J]. Journal of Functional Foods, 2022, 93: 105082. doi: 10.1016/j.jff.2022.105082 [24] JIA K K, PAN S M, DING H, et al. Chaihu-Shugan San inhibits inflammatory response to improve insulin signaling in liver and prefrontal cortex of CUMS rats with glucose intolerance [J]. Biomedicine & Pharmacotherapy, 2018, 103: 1415-1428. [25] LIU S Z, LUO Y H, MORAIS C L M, et al. Spectrochemical determination of effects on rat liver of binary exposure to benzo[a]Pyrene and 2, 2', 4, 4'-tetrabromodiphenyl ether [J]. Journal of Applied Toxicology:JAT, 2021, 41(11): 1816-1825. doi: 10.1002/jat.4165 [26] MEHRANFARD N, YAZDI A, SARDOOI A R, et al. Honey protects against chronic unpredictable mild stress induced- intestinal barrier disintegration and hepatic inflammation [J]. Molecular Biology Reports, 2020, 47(11): 8475-8484. doi: 10.1007/s11033-020-05888-4 [27] CLOUGHERTY J E, LEVY J I, KUBZANSKY L D, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology [J]. Environmental Health Perspectives, 2007, 115(8): 1140-1146. doi: 10.1289/ehp.9863 [28] SONG X Y, LIU J H, GENG N B, et al. Multi-omics analysis to reveal disorders of cell metabolism and integrin signaling pathways induced by PM2.5 [J]. Journal of Hazardous Materials, 2022, 424: 127573. doi: 10.1016/j.jhazmat.2021.127573 [29] WANG F D, ZHANG H J, GENG N B, et al. A metabolomics strategy to assess the combined toxicity of polycyclic aromatic hydrocarbons (PAHs) and short-chain chlorinated paraffins (SCCPs) [J]. Environmental Pollution, 2018, 234: 572-580. doi: 10.1016/j.envpol.2017.11.073 -



 下载:
下载: