-
近年来我国有机废水排放量仍居高位,据统计2021年我国有机废水排放量突破589. 64亿m3,对水体环境和水生态平衡均造成巨大威胁. 不同行业的有机废水中主要成分为各自工艺流程带入的特征有机物,如农药、医药中间体、抗生素、高分子染料、芳香族化合物以及含硫、氮等有机化合物,具有COD高、生物毒害效应、处理难度大、生物难降解等特点,未经有效处理后排放会造成水体富营养化等环境问题[1-5]. 因此,有机废水的高效治理是从源头上遏制水体富营养化、改善水质和饮水安全的必要途径. 此外,在碳中和碳达峰的目标要求下,对有机废水的高效降解和深度治理符合我国双碳目标的发展路径[6-8].
在有机废水处理方法方面,典型水处理工艺(如吸附、膜过滤等)仅能实现水中有机污染物转移,易造成产生固废等二次污染,不能实现有机成分的深度降解. 高级氧化技术(advanced oxidation processes, AOPs)自1987年被Glaze[9]提出以来各种高级氧化方式不断得到开发,而以过硫酸盐(persulfate, PS)为氧化剂的高级氧化技术近年来引起了更多关注,其特点在于可通过多种活化方式产生活性氧物种(reactive oxygen species, ROS),进而无选择性地将有机污染物降解并矿化为CO2、无机盐和水等产物,具有高效、易储运等优点[10-15]. 通过活化过硫酸盐产生的ROS主要包括硫酸根自由基(
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ )(2.5—3.1 eV, 30—40 μs)、羟基自由基(·OH)(1.8—2.7 eV, 20 ns)、超氧自由基$ {\text{O}}_{\text{2}}^{\text{·–}} $ (2.4 eV)和单态氧1O2(1.52 eV)等,在较宽的pH值范围(2—8)均可有效降解和矿化各类有机污染物[16-18].利用外加能量如紫外光照[19-20]、热活化[21-22]、电化学[23-25]、超声波[26-28]、微波[29]等方式均能有效活化过硫酸盐. 而通过引入外加催化剂的非均相活化方法无需外加能量即可活化过硫酸盐,具有操作方便、成本低、反应条件温和、
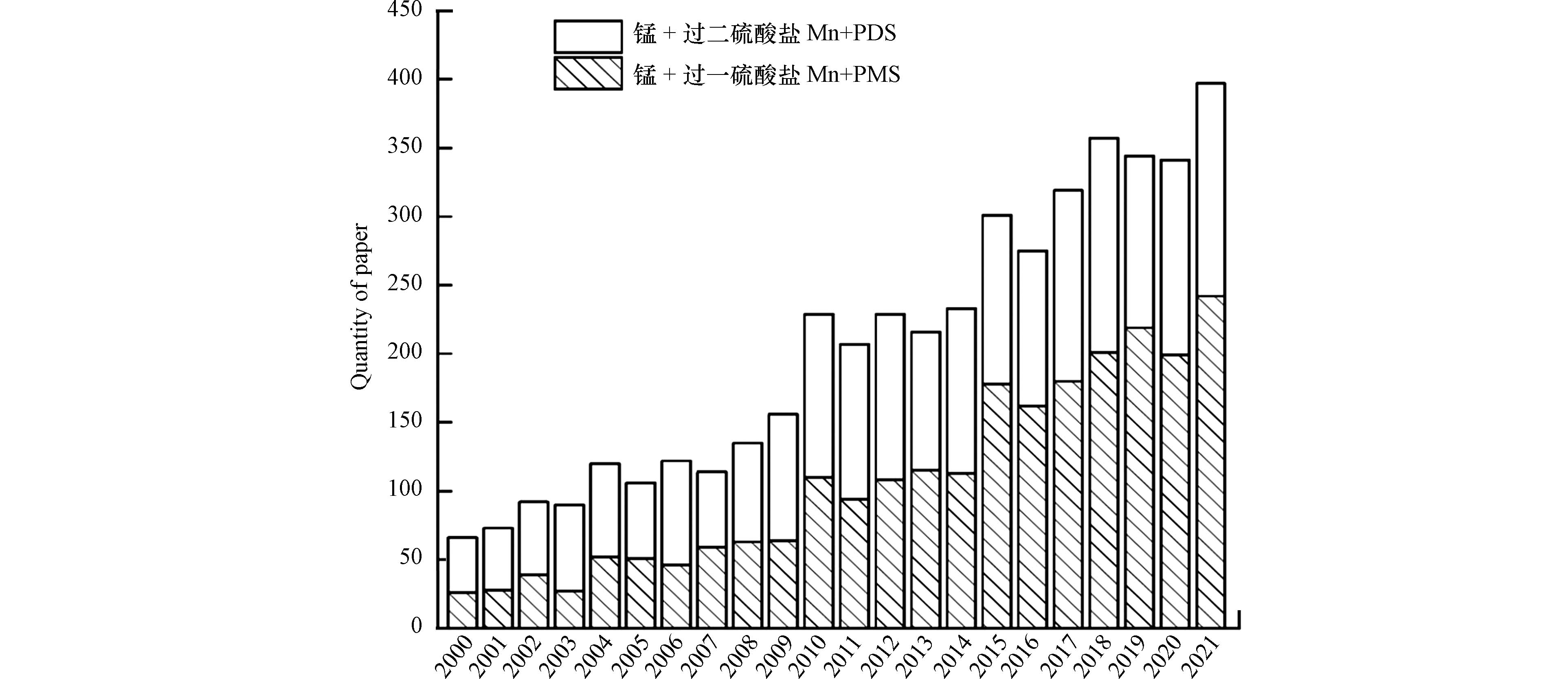
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 生成速率高等优点,其中过渡金属催化剂(如Co,Fe,Mn等)由于来源广泛和可变价态是优异的过硫酸盐催化活化材料[30-33]. 在众多过渡金属中,锰基催化剂因为其高催化活性被广泛应用于环境催化方面[34-35],在催化臭氧分解[36]、电化学氧化[37-38]、光催化[39]、VOCs热催化氧化[40-42]以及过硫酸盐活化方面均有大量应用和报道. 近年来,国内外众多学者对锰基催化剂用于过硫酸盐活化进行了广泛而深入的研究,已成为高级氧化领域的热点研究方向. 以“锰”、“过硫酸盐”为关键词,对Scopus数据库近二十年相关论文发表进行了检索(见图1),可以看出锰基催化剂活化过硫酸盐的研究热度持续上升,其中2021年共计发表文章近400篇. 过氧一硫酸盐(peroxymonosulfate,PMS)和过氧二硫酸盐(peroxydisulfate,PDS)由于结构的不同,在活化途径和非自由基反应方面存在一定的差异. PMS比PDS更活跃[43],具有更高的溶解度,且PMS具有较短的键长(0.146 nm)和更高的键解离能(377 kJ mol−1)[44]. 因此,近几年锰基催化剂活化过氧PMS方面的文章发表数量高于PDS,约占论文发表总数的70%.本文综述了近年来锰基催化材料在催化活化过硫酸盐降解有机废水领域的研究进展,系统总结了零价锰、单一锰氧化物、复合锰氧化物、特殊晶型锰氧化物以及负载型锰氧化物的相关报道,为针对性地设计高活性锰基过硫酸盐活化材料提供思路和借鉴,并对其存在问题和发展趋势进行了初步展望.
-
通过梳理已报道用于活化过硫酸盐的锰基催化剂类别,可以分为零价锰、单一锰氧化物、复合锰氧化物、特殊晶型锰氧化物以及负载型锰氧化物,围绕催化剂类别、催化剂活性组分、制备方法、目标污染物、主要活性物种(ROS)及污染物去除效率等方面,对部分代表性研究进行了总结,具体见表1.
-
零价锰(Mn0)是高度单晶的零价纳米材料,结构呈球形,其具有较高的还原电位,还具有粒径小、活性位多和吸附还原能力强的优点,已被广泛地应用在多种环境介质的修复方面. 当Mn0与水溶液接触时,被氧化生成的电子和Mn2+可与PMS发生连锁反应生成自由基,进而降解矿化有机污染物. Xiao等[45]通过一步液相还原法合成了不同Fe/Mn摩尔比的纳米零价铁锰(NZVIM)双金属材料,分别在pH=3.0、6.0和8.0条件下活化PMS去除SMT. 在纳米零价铁(NZVI)中加入Mn(0)进一步增强了NZVIM内部电子转移的能力,并提高了电子传递速率. 在O2的作用下Mn2+/Mn3+的氧化还原循环为自由基的生成提供了电子,Fe、Mn之间的耦合效应增强了Fe2+/Fe3+循环和自由基的生成速率,从而提高了PMS的活化效率,在Fe/Mn分别为2:1和1:1的条件下,NZVIM在pH=3.0时对SMT的降解效率最高. 零价锰还能有效活化PDS生成
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 和·OH,Shah等[46]采用化学还原法制备了纳米零价锰(nZVMn,Mn0)并用于活化PDS降解CIP,研究了单独Mn0和添加S2O82-后CIP的降解效果,当[CIP]0=10 mg·L−1,[Mn0]0=1.0 g·L−1,反应时间为80 min时,单独Mn0对CIP的降解率为63%,S2O82-引入后CIP的去除率提高到95%. 此外,零价锰还被用于催化活化ClO-去除铊(Tl)[64],以及吸附去除水中As3+、As5+、Cd2+和Cu2+等重金属离子方面[65,66]. -
自然环境中元素锰的存在形式以MnO、MnO2和Mn3O4等单一锰氧化物和天然矿石为主. 锰氧化物的催化活性与Mn元素的混合价态密切相关,相比零价锰通过氧化为高价态活化过硫酸盐产生自由基,单一锰氧化物中Mn的可变价态使其具备较好的氧化还原能力,可提供更多电子活化过硫酸盐生成自由基,是优良的催化剂活性组分[67],且不同形态结构的单一锰氧化物活化过硫酸盐时展现出差异的催化反应活性.
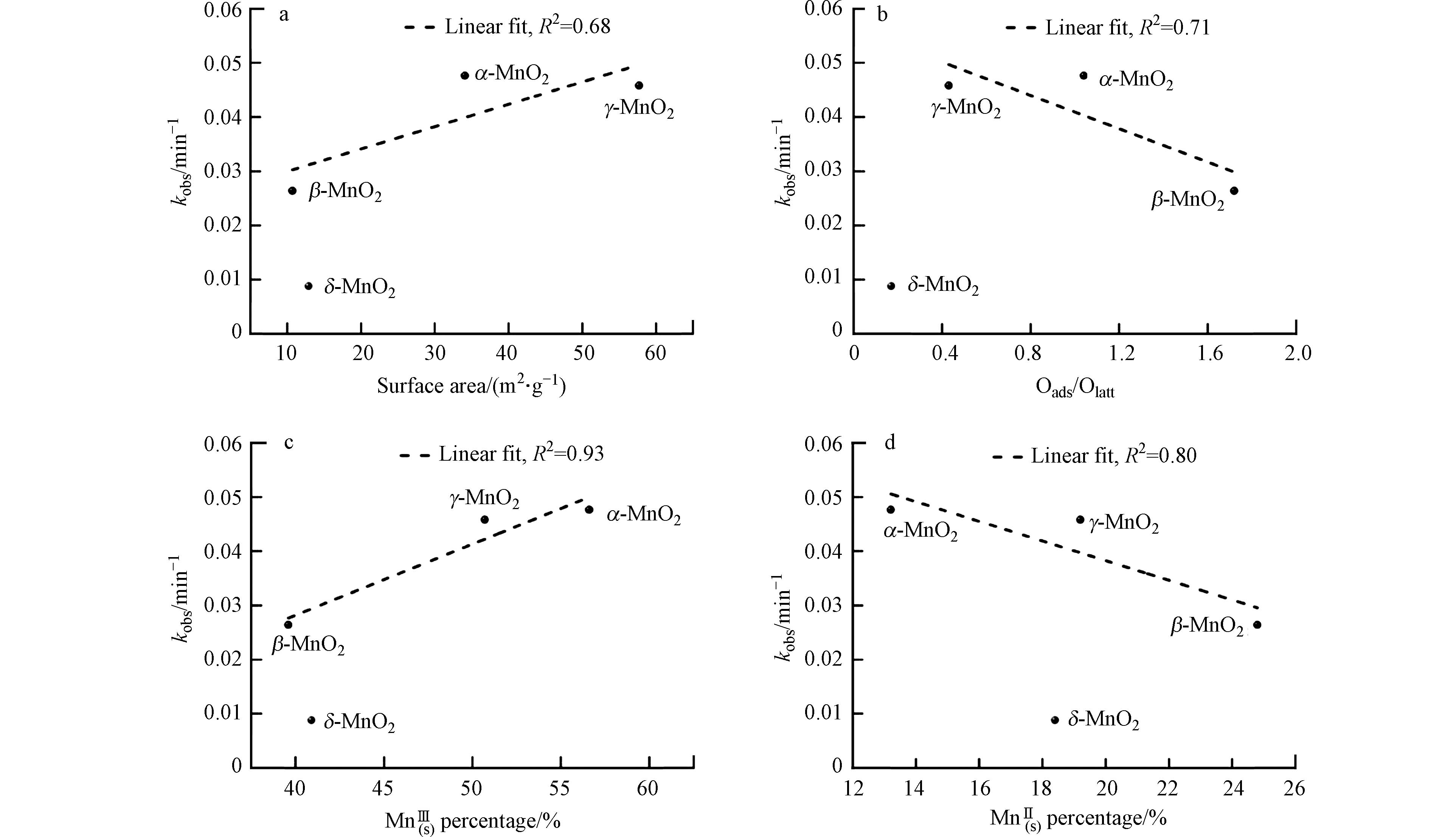
在众多单一锰氧化物中,MnO2存在α-MnO2、β-MnO2、γ-MnO2和δ-MnO2等4种晶型,表现出不同的催化过硫酸盐活性. Shen等[68]成功合成了α-、β-、γ-和δ-MnO2,研究了上述4种不同晶型MnO2活化PMS降解乙酰氨基酚(acetaminophen, ACE)的效果差异,对ACE的去除率为α-MnO2>γ-MnO2>β-MnO2>δ-MnO2. 如图2所示,将不同MnO2的反应速率分别与催化剂比表面积、表面吸附氧与晶格氧、
$ {\text{Mn}}_{\left(\mathrm{s}\right)}^{\text{Ⅲ}} $ 及$ {\text{Mn}}_{\left(\mathrm{s}\right)}^{\text{Ⅱ}} $ 含量进行了线性拟合,发现比表面积、吸附氧和晶格氧不是影响催化效率的关键因素(图2a-b),催化剂表面$ {\text{Mn}}_{\left(\mathrm{s}\right)}^{\text{Ⅲ}} $ 是主要的活性位点,α-、β-和γ-MnO2的活性与$ {\text{Mn}}_{\left(\mathrm{s}\right)}^{\text{Ⅲ}} $ 含量呈正相关(R2=0.93,图2c). 此外,α-、β-和γ-MnO2的催化性能与反应后$ {\text{Mn}}_{\left(\mathrm{s}\right)}^{\text{Ⅱ}} $ 含量呈负相关(R2=0.80,图2d). 因此,表面结合的PMS配合物和$ {\text{Mn}}_{\left(s\right)}^{\text{(Ⅳ}\text{,}\text{Ⅲ)}} $ 的直接氧化是催化活化PMS降解ACE的主要原因. 单一锰氧化物的形貌会对降解反应活性有不同影响. 氧空位可以有效地提高MnO2的催化活性,不仅增强了Mn3+/Mn4+的氧化还原循环,还促进了氧的迁移和交换,从而加速了PMS的活化. Ndayiragije等[69]通过将商用MnO2进行球磨制备得到富氧空位的MnO2并用于活化PMS降解TBBPA,极大提高了PMS活化产生1O2降解TBBPA的效率. 单一锰氧化物制备过程中外界条件的辅助也能提高其催化降解有机物的效率,Yi等[70]将在超声辅助下制备得到ultrasonic(u)-α-MnO2用于PMS活化降解邻苯二甲酸二甲酯(Dimethyl phthalate, DMP),DMP初始浓度为10 mg·L−1时90 min几乎实现完全降解. 不同形貌的Mn2O3暴露出不同晶面,对应晶格氧迁移率不同,对污染物的降解行为和效率也存在较大差异. Li等[71]分别制备了棒状、球状、片状和颗粒状(Mn2O3-R, Mn2O3-S, Mn2O3-F和Mn2O3-P)Mn2O3催化剂活化PMS降解BPA,发现颗粒状Mn2O3-P的催化效率优于其他3种形貌的Mn2O3,在10 min内可使BPA降解率达93%,Mn2O3-P催化剂的暴露晶面更容易产生氧空位和活性氧,PMS分子也更容易吸附在Mn2O3-P晶面上. Mn2O3-P中Mn4+/Mn3+/2+的转化产生的电子,不但向PMS转移生成$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 、·OH和Mn4+,而且与晶格氧结合形成氧空位和活性氧,在氧空位的作用下,活性氧和吸附氧可以进一步转化为1O2,同时$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 与·OH也能生成1O2,从而提高了有机污染物的降解效率.反应条件如阴离子、pH值等对单一锰氧化物的催化活性均有影响,Zhao等[49]以α-MnO2活化PMS降解DIN,实验表明DIN在该体系中降解速度较快,降解过程符合准一级动力学模型,阴离子共存和酸性条件更有利于DIN的降解. Hao等[72]在不同pH值条件下制备了结构相似、Mn3+含量不同的δ-MnO2样品,未加入PMS时Mn3+含量较低的δ-MnO2样品已表现出较高的BPA去除能力,主要由于直接电子转移的影响. 吸附的BPA在羟基化后降解,引入PMS后,Mn3+含量越高的样品
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 和·OH的数量越高,表现出较高的BPA降解率,δ-MnO2-PMS体系对BPA的催化氧化和直接氧化对BPA降解呈现出协同效应.上述研究表明催化剂的理化性质会对单一锰氧化物的催化活性有显著影响,而制备方法和合成条件能够显著改变催化剂的表面和体相结构,进而影响其催化活性. 现有合成单一锰基氧化物的方法主要包括水热法、溶胶凝胶法、共沉淀法和微乳液法等,其中水热合成法的研究最多. Xu等[48]以水热法制备了MnOOH并用于活化PMS,分别考察该反应体系对RhB、TC、杀虫剂(IMI)和PCA等多种难降解有机污染物的降解效果,随着pH从3提高到9,上述污染物的降解率均略有下降,然而MnOOH仍表现出良好的稳定性和重复性,反应过程存在少量金属离子的浸出. Zhou等[73]通过水热法合成了纳米纤维形貌的α-MnO2(OMS-2)和δ-MnO2(OL-1),分别活化PMS降解4-硝基苯酚(4-nitrophenol,4-NP). 由于OMS-2具有更好的理化性能和更快的电子转移速率,4-NP在OMS-2/PMS体系中可以快速完全降解,而OL-1/PMS体系中反应80 min后4-NP降解率仅为31.11%. 王淑敏等[74]采用水热法以制备了α-MnO2、γ-MnO2及β-MnO2,研究了水热时间对MnO2晶型的影响及MnO2的生长过程. 随着水热时间延长,由于阳离子的不足,α-MnO2与γ-MnO2的晶格结构转变形成结晶度更高、晶体生长更加完整的β-MnO2. 哈工大马军院士团队[47]通过控制水热条件制备了以110和100为主要暴露晶面的α-MnO2,进一步活化PMS降解OI,纳米线形貌的α-MnO2-100具有更丰富的表面羟基和更强的还原能力.
单一锰氧化物虽然具有较为优异的催化活性,但其重复稳定性较差,活性点位相对不足,在实际废水中受污水中pH和阴离子的影响较大,该反应体系的研究仍然针对污水中单一有机污染物的降解,限制了在实际有机废水方面的应用.
-
对单一锰氧化物进行金属/非金属复合可以有效增加催化活性位点,同时利用形貌调控增大比表面积以强化吸附和传质效果,催化剂的稳定性有所增强,并且不同金属的协同作用会强化自由基的产率和生成速率[75],因此复合锰氧化物通常具有更好的氧化还原性能和优异的催化性能. 已报道的与单一锰氧化物进行复合的金属包括Fe、Ce、Cu、Mg、Co和Cr等[76-78].
在众多复合锰氧化物的研究中,Fe和Mn的复合氧化物研究较多,其原因主要是Fe的可变价态以及具备磁性可回收的优点. Xu等[55]通过水热法制备了α-MnO2/MnFe2O4复合材料并用于活化PMS降解NOF,实验结果表明α-MnO2/MnFe2O4在多个条件下均对NOF有较好的去除效果,在[α-MnO2/MnFe2O4]0=0.2 g·L−1、[PMS]0=1 mmol·L−1、pH=7和[NOF]0=20 mg·L−1的条件下可获得最佳催化降解效果. Thao等[79]用水热法制备了质量比分别为1:5、1:7和1:9的磁性MnFe2O4/α-MnO2复合催化材料用于活化PMS降解偶氮染料橙G(Orange G, OG). 在[OG]0= 50 mg·L−1、[PMS]0=1000 mg·L−1、[MnFe2O4/α-MnO2]0=100 mg·L−1、[pH]0=3.0和[T]0=30 °C的条件下,30 min内OG的降解率达96.8%. Shi等[80]以Fe3O4颗粒为载体负载MnO2,制备了Fe3O4-MnO2双金属复合锰氧化物(BFMN)活化PMS降解RhB. 当[BFMN]0=0.3 g·L−1、[T]0=25 °C、[RhB]0=20 mg·L−1、[PMS]0=0.3 g·L−1和[pH]0=5. 6时,15 min内RhB降解率达到98%. Chen等[53]采用水热法制备了不同摩尔比的磁性MnO2/MnFe2O4复合锰氧化物活化PMS降解RhB,得出摩尔比为7:1的MnO2/MnFe2O4纳米复合材料降解效果最好,在5 min内对RhB的降解效率达90%.
采用多种金属复合的锰氧化物也有报道,Anushree等[81]通过氧化-沉淀-水热的步骤制备了掺杂Ag+、Zn2+的Fe2O3-MnOx核壳型纳米粒子活化PMS降解溴酚蓝(Bromophenol blue, BPB),金属离子的掺杂引入强化了PMS的活化效率,显著改变了样品中Mn3+与Mn4+的比例、晶格氧的反应活性和迁移速率,而氧化铁磁芯提高了催化剂的回收利用率. 不同晶型MnO2的复合锰氧化物在PMS活化方面也表现出差异的催化性能,Wang等[82]采用水热法合成了不同晶型MnO2并分别负载Co3O4制备得到α-MnO2@Co3O4、β-MnO2@Co3O4、γ-MnO2@Co3O4和δ-MnO2@Co3O4复合锰氧化物,并综合比较了其PMS活化降解磺胺恶唑(sulfisoxazole, SIZ)的效果. 单独MnO2的催化降解性能依次为α-MnO2>γ-MnO2>β-MnO2>δ-MnO2,这主要与其晶体结构和比表面积有关,如图3a-d所示,α-MnO2呈分枝状交错的纳米针结构,而β-MnO2呈现纳米棒状,γ-MnO2为纳米针组成的海胆状结构,δ-MnO2呈纳米片组成的花瓣形貌. 负载Co3O4后催化剂表面更为粗糙(图3e-h),Co3O4纳米颗粒有效负载于MnO2表面,丰富的Co2+和Mn2+可以有效催化PMS生成
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ ,一部分$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 与H2O或OH-反应生成·OH,$ \mathrm{H}{\text{SO}}_{\text{5}}^{\text{–}} $ 可以还原高价Co3+和Mn3+/4+生成$ {\text{SO}}_{\text{5}}^{\text{·–}} $ 并进一步转化生成1O2. SIZ在$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 、·OH和1O2共同作用下实现了高效降解,降解效率依次为γ-MnO2@Co3O4>α-MnO2@Co3O4>β-MnO2@Co3O4>δ-MnO2@Co3O4. γ-MnO2@Co3O4由于其较大的表面积,暴露出更多的活性位点,对SIZ的去除表现出最优的催化性能. Jiang等[83]通过在海绵内部原位生长CoMnOx制备出具有弹性的三维块状CoMnOx@sponge催化剂用于活化PMS降解SIZ,在[CoMnOx@sponge]0=0.1 g·L−1、[PMS]0=1 mmol·L−1条件下,反应5 min可将初始浓度为30 mg·L−1的SIZ完全降解. Chen等[84]采用固相烧结法制备了复合锰基陶瓷膜(Mn-CCM)活化PMS降解11种药物和护理用品(pharmaceutical and personal care products, PPCPs),在[PPCPs]0=500 µg·L−1、[PMS]0=0.01—0.3 mmol·L−1和[pH]0=4.05—11.0反应条件下,各类PPCPs混合物在Mn-CCMs/PMS体系的降解率均在10 min内达到90%以上. Zhao等[85]在氮气氛围下煅烧制备得到Co纳米颗粒均匀分布在碳层包裹的MnO纳米棒(Co/MnO-X@C),用于活化PMS降解BPA,在[Co/MnO-0.32@C]0=0.1 g·L−1、[BPA]0=20 mg·L−1和[PMS]0=1 g·L−1条件下,Co/MnO-0.32@C在1 min内可去除98%以上的BPA. 反应后Co/MnO-0.32@C中浸出的Co离子仅为Co@C的1/5,Co的高活性和低浸出率也表明在PMS活化过程中Co与MnO存在一定的协同作用.此外,在复合锰氧化物的体系中,利用光、热等物理条件与PMS活化进行耦合可实现协同催化效果. Chen等[86]采用共沉淀法-浸渍法制备了Mn2O3/Bi2O3复合材料并活化PMS降解盐酸四环素(tetracycline hydrochloride, TC-HCl),Mn2O3/Bi2O3具有较好的可见光响应,在可见光照条件下与PMS具有协同效应,[Mn2O3/Bi2O3]0=0.2 g·L−1、[PMS]0=0.3 g·L−1和[TC-HCl]0=45 mg·L−1时,100 min内Mn2O3/Bi2O3/Vis/PMS体系的去除率为73.34% ± 1.6%,而未加光照条件下Mn2O3/Bi2O3/PMS体系的去除率仅为51.78% ± 1.5%.
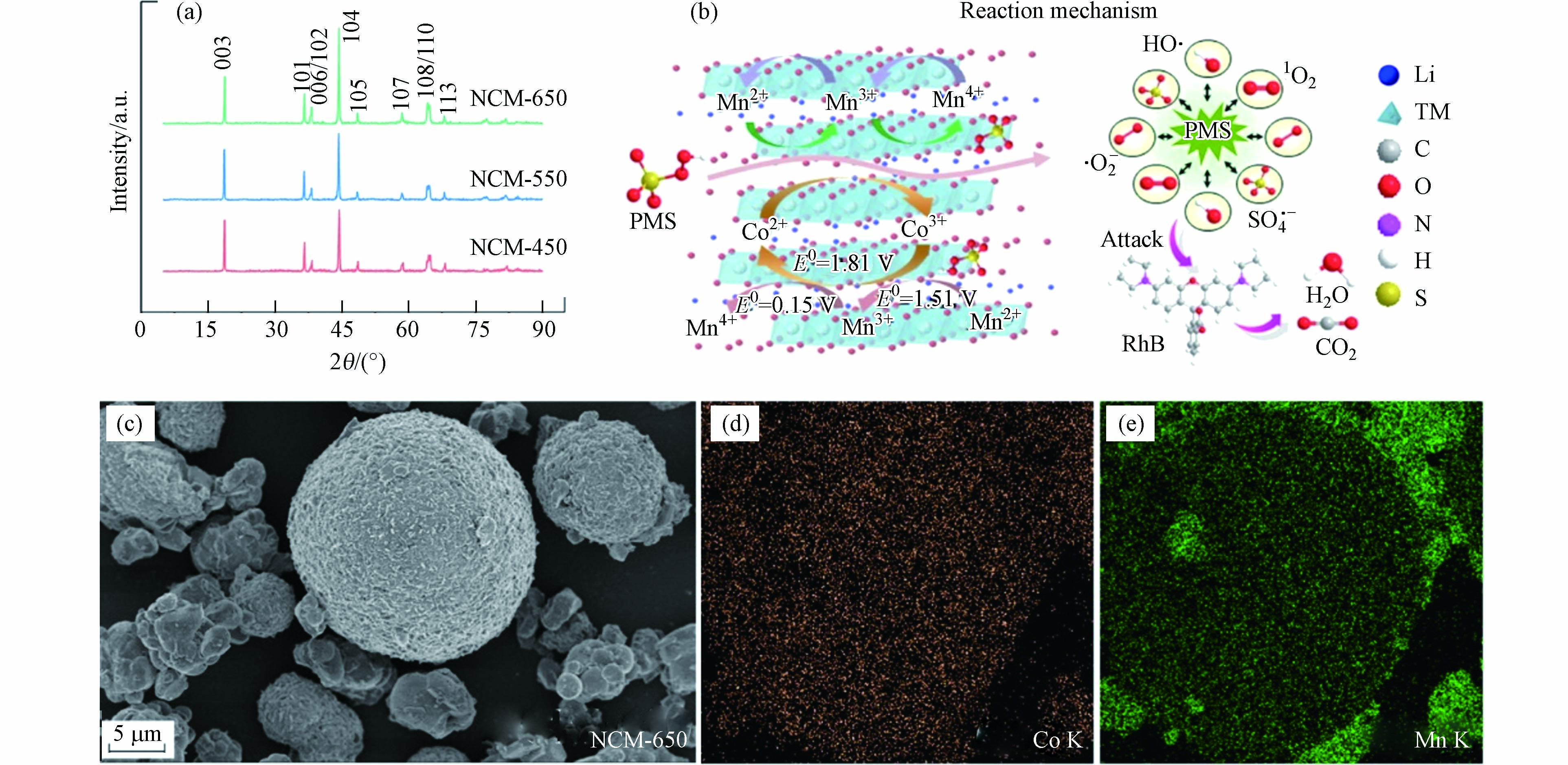
自然界中存在大量天然锰矿复合氧化物,Wang等[87]报道了天然含锰矿物(NMM)可以有效活化过硫酸盐降解双酚AF(bisphenol AF, BPAF),发现PMS与NMM的耦合体系可通过直接电子转移降解BPAF. He等[88]研究了天然锰砂(NMS)活化PMS对盐酸四环素(chlortetracycline hydrochloride, CTC)的降解效果,通过响应曲面法对初始pH值、催化剂用量、PMS用量等实验条件进行了优化,在[PMS]0=2.02 g·L−1、[NMS]0=0.29 g·L−1和[pH]0=3.87的条件下,CTC的降解率为81.65%. 复合锰氧化物还可以由废旧锂离子电池(LIBs)提取制备,实现变废为宝. Zhao等[54]以废LIBs的阴极材料为原料,通过热合成方法制备了LiNi0.5Co0.2Mn0.3O2(NCM)催化剂用于活化PMS去除RhB,NCM/PMS可以适应较宽的pH范围,反应25 min可实现对RhB的完全去除. NCM均为结晶度较高的球形颗粒(图4a, c),不同价态的Co和Mn元素均匀分布于催化剂表面(图4d-e). 其催化活化PMS机理如图4b所示,不同价态锰(Mn2+、Mn3+和Mn4+)之间的快速氧化还原和Co2+/Co3+的电子转移活化PMS产生
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 和·OH,富余电子也与溶解氧反应生成·O2-,该体系中1O2可能由·O2-与HO-/H2O的进一步反应或者$ \mathrm{H}{\text{SO}}_{\text{5}}^{\text{–}} $ 自分解产生,上述ROS的综合作用使RhB被逐步降解和矿化.综上所述,复合锰氧化物可以有效活化过硫酸盐降解多种有机污染物,其活性位点较单一锰氧化物更多,从而产生更多的自由基种类和数量,催化剂的稳定性也有所提高. 然而,复合锰氧化物活化过硫酸盐的反应体系大多在酸性条件下降解效果更为显著,而且复合金属催化剂在降解过程中容易团聚造成比表面积和活性位点减少,一定程度上会降低催化剂与污染物的传质面积和反应速率.
-
特殊晶型锰氧化物也被用于活化过硫酸盐降解有机污染物,代表性物质主要包括锰基钙钛矿型化合物[89]、尖晶石型化合物[90-92]以及类水滑石化合物等. 以锰基钙钛矿为例,较单一和复合锰氧化物通常具有更好的稳定性和持久性,能够通过掺杂、离子取代等方式进一步改善配位环境和晶体结构,以进一步提高钙钛矿的催化活性. 特殊晶型锰氧化物的活化途径主要依靠吸附氧、表面氧空位和晶格氧之间的协同作用,其降解机理也依赖于不同价态锰之间的相互转化.
近年来钙钛矿型氧化物在催化活化过硫酸盐方面有较多研究. 煅烧温度会影响钙钛矿的晶体生长过程和晶相,Utama等[93]采用固相合成方法经600 ℃、700 ℃、800 ℃煅烧制备了LaMnO3钙钛矿并用于活化PMS降解棕榈油(palm oil, POMSE),800 ℃煅烧获得的LaMnO3结晶度和比表面积最大,表现出更高的催化活性. Wang等[94]采用溶胶-凝胶结合高温煅烧法合成了CaMnO3,在pH为4—12的范围内可以高效活化PDS降解PhOH,CaMnO3催化剂中Mn的可变价态和丰富的氧空位有利于Mn3+/Mn4+的氧化还原. 引入金属掺杂钙钛矿也能够提高其催化活性,Gao等[95]成功合成了Cu掺杂LaMnO3并活化PMS降解磺胺甲恶唑(sulfamethoxazole, SMX),随着Cu含量增加,LaMnO3由无序结构逐渐转化为有序La2CuO4,生成了更多的氧空位和氧缺陷,除了1O2外,在Cu-LaMnO3与PMS反应体系产生了较多的·OH和
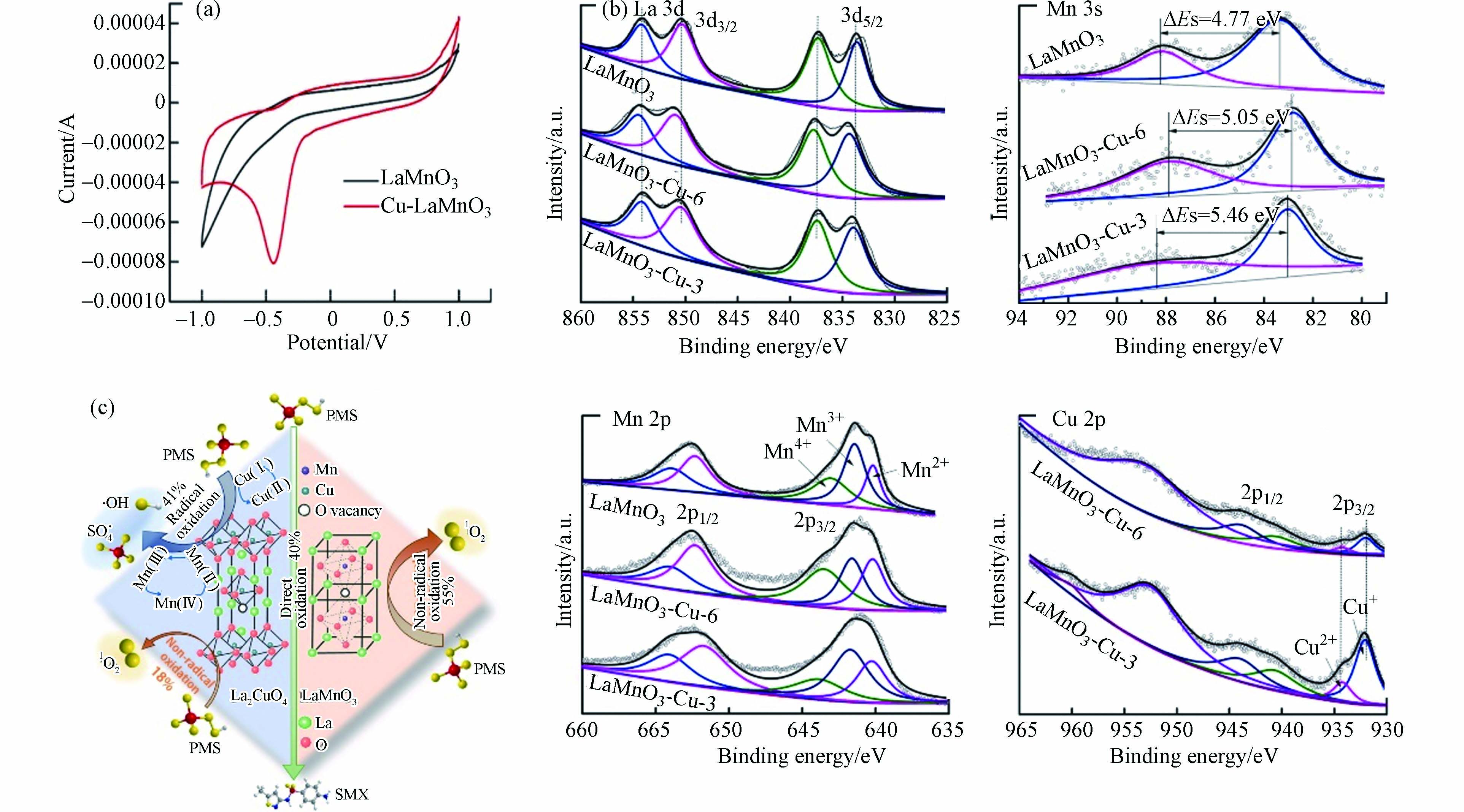
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ ,而LaMnO3中仅检测到1O2. 如图5a所示,Cu-LaMnO3比LaMnO3具有更强的电流密度和还原能力,说明Cu的引入增强了钙钛矿与PMS之间的电子转移过程,对SMX实现了100%的降解和高效矿化,而LaMnO3对SMX的降解率仅为60%. 图5b XPS的测试结果表明,Cu-LaMnO3钙钛矿中存在Mn2+/Mn3+、Mn3+/Mn4+和Cu/Cu2+的氧化还原过程,增强了催化剂与PMS之间的电子转移速率. 其活化PMS机理如图5c所示,具体地,$ {\text{H}\text{SO}}_{\text{5}}^{\text{–}} $ 得到电子生成·OH和$ {\text{SO}}_{\text{4}}^{\text{·–}} $ ,一部分$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 与OH-生成·OH,同时,Cu-LaMnO3上丰富的氧空位可以促进PMS自分解生成1O2,SMX降解产物可被·OH和$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 进一步矿化. Guo等[96]采用溶胶-凝胶法制备了Sr掺杂La1-xSrxMnO3用于降解RhB染料废水,研究发现溶液初始pH值对RhB的降解有较大影响,在强酸条件下,Sr掺杂增强了LaMnO3对RhB的降解能力,降解过程中钙钛矿结构保持稳定,通过调节Sr掺杂含量可以调控催化剂表面Mn4+/Mn3+的含量,从而改善了La1-xSrxMnO3的氧化还原性能. Luo等[97]采用柠檬酸络合溶胶-凝胶法合成了LaMnO3纳米颗粒,并用于降解RhB. 在[LaMnO3]0=2.0 g·L−1、[RhB]0=50 mg·L−1、[pH]0=1—2条件下,RhB的降解效率为99.7%,TOC去除率为65.8%. 锰基钙钛矿活化PMS体系中初始pH值有较大影响,强酸条件下通常具有最佳降解效果.无独有偶,也有利用废LIBs为原料制备特殊晶型锰基催化剂的报道. Liang等[58]利用废LIBs以溶胶-凝胶法制备了磁性锰基尖晶石铁氧体(MnFe2O4-LIBs)用于活化PMS降解BPA,其降解活性是纯MnFe2O4的2.8倍,三元LIBs中多种元素的掺杂(如N、Co等)提高了催化剂的比表面积和ROS数量,在外加磁场下MnFe2O4-LIBs易于分离,具有优异的重复使用性和再生能力,高盐度条件下也能够保持有效的催化活性. Nie等[57]研究了典型有机配体甘氨酸(glycine, GLY)、草酸(oxalic acid, OA)、柠檬酸(citric acid, CA)、硝化三乙酸(nitrilotriacetic acid, NTA)和乙二胺四乙酸(ethylenediaminetetraacetic acid, EDTA)在LaMnO3活化PMS降解OFX过程中的不同作用. 与对照实验相比,EDTA和NTA显著促进OFX降解,而GLY、CA和OA对OFX降解有不利影响,主要原因在于不同配体的引入对LaMnO3表面的Mn2+和氧空位产生了差异影响,LaMnO3的催化活性与配体结构、初始浓度和溶液pH等因素均有关系.
-
将催化剂负载于载体上不但解决了粉体催化剂回收利用和团聚的问题,载体自身也能够吸附去除污染物,通过调控活性组分的负载量和负载方式可适用不同的反应条件和污染物,负载锰氧化物的分散性和稳定性均得到提升. 载体材料需具备无毒、热稳定性好、孔隙率高、多孔、比表面积大等特点,常用的载体有氧化石墨烯(Graphene oxide, GO)、活性炭(Activated carbon, AC)、生物炭(biochar, BC)、碳纳米管(Carbon nanotubes, CNTs)和坡缕石(palychite, Pal)等.
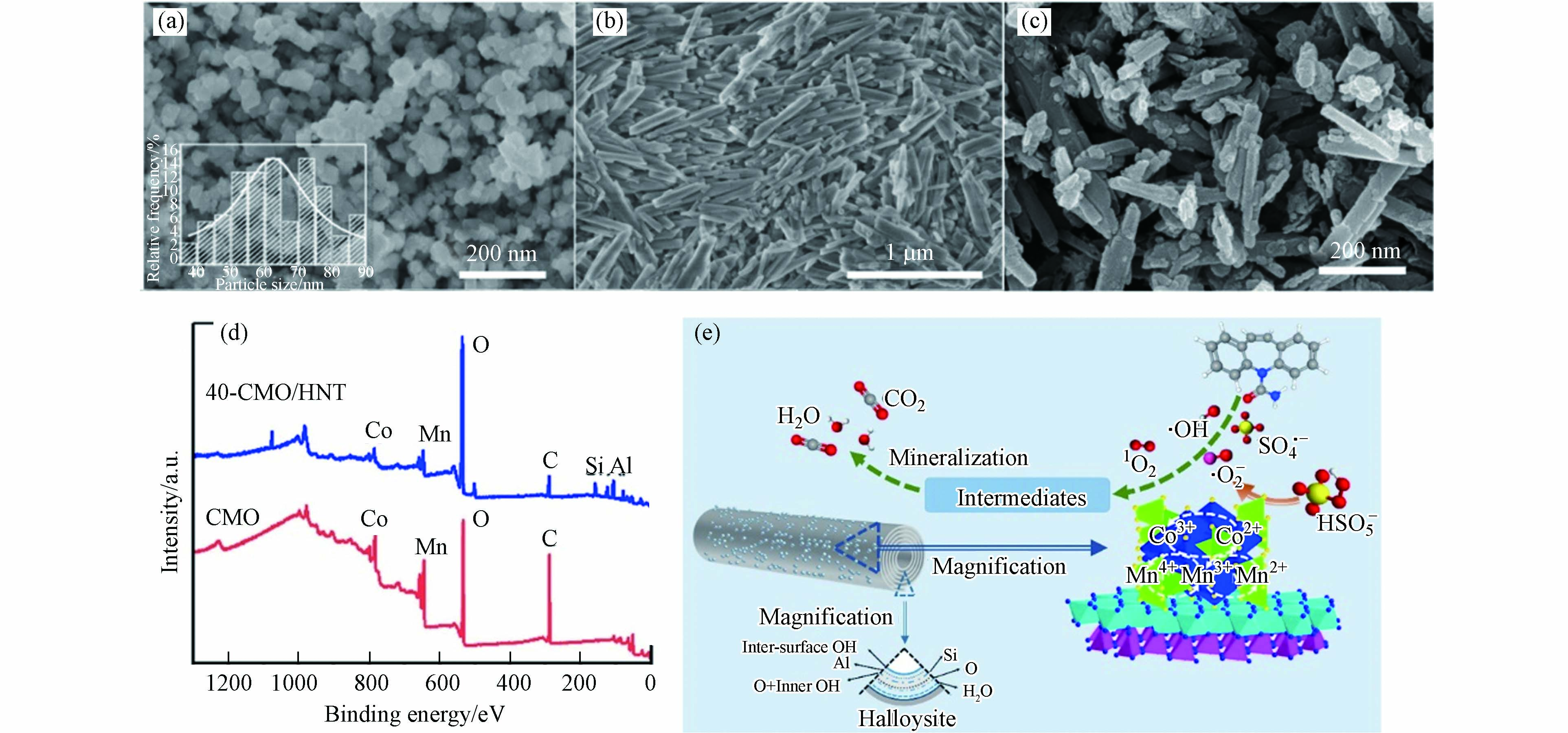
生物炭材料来源广泛,是优良的过硫酸盐载体. Du等[59]将纳米MnFe2O4和壳聚糖经高温烧结制备了CM-600复合催化剂并活化PMS降解酸AO7,与MnFe2O4/PMS体系相比,PMS/CM-600的催化降解活性明显提高,反应30 min AO7降解率达99.0%. Yang等[61]以玉米秸秆为载体制备得到天然锰矿-玉米秸秆生物炭复合材料(MCC)用于活化PMS降解BPA,研究发现在MCC中引入天然锰矿有效提高了生物炭的理化性能和电子传递能力,从而促进了PMS的活化和污染物降解效率. Jiang等[98]将纳米Co3O4-MnO2负载于稻草生物炭上制备了Co3O4-MnO2/BC催化剂活化PMS降解磺胺嘧啶(sulfadiazine, SDZ),在[PMS]0=1 mmol·L−1和[Co3O4-MnO2/BC]0=0.1 g·L−1条件下,10 min内可将初始浓度为25 mg·L−1的SDZ降解完全. Yang等[99]采用共沉淀法在天然矿物纳米管高岭土(HNT)上负载不同含量的CoMn2O4尖晶石(CMO)制备得到x-CMO/HNT催化剂,用于活化PMS降解卡马西平(carbamazepine, CBZ)、OFX、TC和磺胺甲恶唑(sulfamethoxazole, SMZ)等医药中间体. 结果表明,在HNT上负载40%的CMO(即40-CMO/HNT)具有最好的催化活性,CBZ在20 min内能够得到完全降解,这和催化剂负载之后的形貌结构变化有一定联系,CMO的形貌为规则的立方体颗粒并呈现一定的团聚现象(图6a),原始HNT为表面光滑管状(图6b),当CMO颗粒负载于HNT上时,CMO颗粒分布均匀分布在HNT表面(图6c),形成更大的比表面积和孔体积,能够扩大与有机物的接触面积并提供更多的反应位点. XPS分析表明负载后催化剂表面Mn2+与Co2+的含量增加(图6d),Mn与Co的持续氧化还原和再生保证ROS的稳定生成,并保持催化剂表面电荷平衡. 如图6e所示,由于Co2+/Co3+(1.81 V)的标准还原电位高于Mn3+/Mn2+(1.51 V)和Mn3+/Mn4+(0.15 V),因此在热力学上有利于Co3+→Co2+的还原过程,Co3+/Co2+与Mn4+/Mn3+/Mn2+之间的氧化还原反应传递电子活化PMS生成
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ ,额外电子可以结合溶解氧生成·O2-,并与·OH和H2O反应生成1O2.GO具有较大比表面积,也是理想的锰氧化物载体. Nguyen等[100]将NiCo2O4和GO负载在MnOOH上合成了NiCo2O4/MnOOH/GO催化剂,并用于活化PMS降解CIP. 在[PMS]0=0.2 mmol·L−1、[NiCo/Mn/GO]0=0.15 g·L−1、[CIP]0=0.02 mmol·L−1和[pH]0=7条件下,CIP在30 min内的去除率达99%. Wu等[101]采用回流冷凝法在低温下合成了MnO2/GO活化PMS降解NOR,比较了石墨烯负载前后的活性差异. 与MnO2/PMS体系相比,MnO2/GO活化PMS降解NOR的性能更为优异,这是由于MnO2/GO比MnO2纳米棒具有更大的表面积和更多的表面含氧官能团,GO和MnO2的协同作用有利于PMS活化生成更多
$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 和·OH. 在[PMS]0=0.01 mmol·L−1、[MnO2/GO]0=0.8 g·L−1、[NOR]0=0.05 mmol·L−1和[pH]0=7条件下,NOR在20 min内的去除率达85%.催化活性组分的负载方式对过硫酸盐氧化体系的降解效果也有影响,Huang等[62]采用水热法制备了负载纳米棒状α-MnO2的坡缕石(α-MnO2/Pal)活化PMS降解RhB,在[α-MnO2/Pal]0=0.10 g·L−1和[PMS]0=0.10 g·L−1条件下,α-MnO2/Pal+PMS体系180 min可以完全降解RhB. Wang等[63]采用一锅法合成了MnO2/C@CNT壳核材料对TC进行降解,在MnO2/C@CNT(1.75:1)-PMS体系下,活化PMS反应10 min TC降解效果最好为85.6%. Pan等[102]采用水热法制备了聚磷石/MnO2/Fe3O4(PMM)纳米复合材料活化PMS降解酸性红(Acid Scarlet, GR),MnO2、Fe3O4和PG三种材料之间存在显著的协同作用,在[PMM]0=1 g·L−1、[PMS]0=0.7 g·L−1、[pH]0=5和[GR]0=200 mg·L−1的最佳条件下,GR在300 min内完全降解,且具有良好的磁回收能力和可重用性. Hu等[52]利用溶剂热法制备得到锰氧化物负载的Mn3O4/ZIF-8材料活化PMS降解RhB,当Mn3O4与ZIF-8的质量比为0.5:1时,复合材料具有最高的催化活性,Mn的浸出几乎为零,在[Mn3O4/ZIF-8]0=0.4 g·L−1、[PMS]0=0.3 g·L−1的条件下,40 min RhB降解率达到98%. Hu等[103]采用1,10-邻菲罗啉原位锚定经高温热解制备了Mn掺杂C3N4复合材料(PMCN),用于PMS活化降解SMX,PMCN催化剂在PMS活化和SMX降解去除均表现出良好的效果. Gong等[104]在室温条件下用不同量的高锰酸钾直接氧化聚丙烯酸酯-苯乙烯(PAS)聚合物,经氧化还原反应将锰氧化物固定在聚合物衬底上,制备出一系列的Mn@PAS复合微球催化剂并用于活化PMS降解酚类物质,其主要作用机理为非自由基氧化途径.
-
基于硫酸盐自由基的过硫酸盐高级氧化技术因其氧化电位高、便于储存、pH适应范围广等优点近年来在有机废水降解方面研究众多,其中以催化剂为主要活化方式的非均相催化活化具有无需外加能量、氧化剂投入量少等优点. 本文综述了锰基催化材料用于该方向的研究进展,分别就零价锰、单一锰氧化物、复合锰氧化物以及特殊晶型锰氧化物的研究应用进行了总结,并对锰基催化剂的载体和负载方式进行了讨论. 其中零价锰具有较强的吸附还原能力,单一锰氧化物催化氧化活性较高,但重复使用性和活性点位较差,构建复合锰基催化剂和特殊晶型锰氧化物可以有效增加比表面积和催化活性位点,也可提高催化剂的稳定性;通过载体负载可有效解决粉体催化剂的分离回收和团聚问题.
尽管理化性能各异的锰基催化材料在不断发展,然而在催化材料日新月异的今天,利用锰基催化材料活化过硫酸盐降解有机废水仍要持续提高催化活化效率,同时也要考虑和拓展其实际应用场景. 在催化活化效率提升方面,可开发单原子锰和纳米限域锰基催化剂,利用分子筛、介孔碳、碳纳米管、MOF等载体有效分散活化组分,并借助更大的比表面积提高对有机物的吸附富集和降解效率.
在实际应用方面,需要考虑和改善的问题主要有以下方面。1)金属离子的溶出问题,目前研究大多以催化降解效率和探究活化反应机理为主要目标,较少考虑锰离子及复合锰氧化物中其他金属离子的溶出问题;2)目前多以单一有机污染物为研究对象,不能够满足实际工业废水的应用场景,应将不同行业实际废水作为研究对象,综合评价多种阴阳离子共存及高盐度条件下的降解反应效果,探索该技术的适用场景和污染处理对象;3)提高批次废水处理量,或开发连续流式/序批次反应体系,逐步对实验进行放大,以提高过硫酸盐高级氧化技术在实际废水处理过程中的适用性;4)拓展非均相催化活过硫酸盐技术的用途和应用情景,如重金属污染修复、黑臭水体、富营养化水体的修复等;5)目前已开发的催化剂仍存在制备流程复杂的问题,增加了投入实际使用的难度,应研究更简易的催化剂制备方法,并开发催化剂的绿色合成方法以及环境友好型载体;6)进一步梳理不同类型催化剂活化过硫酸盐降解有机物的反应路径,结合降解中间产物的表征、自由基原位表征和同位素示踪等技术,发现并提出新的活性氧物种及降解机理;7)研究并评价过硫酸盐反应后溶液中
$ {\text{SO}}_{\text{4}}^{\text{2–}} $ 的含量变化,以减少过硫酸盐的投加量,进一步分析不同有机降解产物的生物毒性,将过硫酸盐高级氧化技术逐步推向实际引用.
锰基催化剂用于活化过硫酸盐降解有机废水的研究进展
Research progress on manganese based catalysts for activating persulfate degradation of organic wastewater
-
摘要: 基于过硫酸盐的高级氧化技术具有便于储运、活化方式多、pH适应范围广等优点,近年来成为有机废水处理领域的热点研究方向. 该技术可通过物理、化学及多种耦合途径活化过硫酸盐产生多种自由基和单线态氧进而实现对水中有机污染物的高效降解和矿化. 在众多过硫酸盐的活化方式中,非均相催化活化途径具有高效的活化效果,且无需引入额外能量、氧化剂投加量少. 过渡金属催化剂是优异的过硫酸盐催化活化材料,其中锰基催化剂因其自然存量高、价态和晶型丰富等优势,已被广泛用于活化过硫酸盐降解各类有机物. 本文综述了不同锰基催化剂在催化活化过硫酸盐处理有机废水方面的研究进展,分别介绍零价锰、单一锰氧化合物、复合锰氧化合物、特殊晶型锰氧化物及负载型锰氧化物的国内外研究现状,并对锰基催化剂在活化过硫酸盐方面存在的问题和应用前景进行了展望.Abstract: Persulfate (PS) activation based advanced oxidation process is featuring of easy storage and transportation, various activation approach and wide pH value range, which has been a hot issue on organic wastewater treatment in recent years. PS can be activated by physical, chemical and hybrid technologies, thus generating various reactive oxidation species (ROS) and singlet oxygen to efficiently degrade and mineralize organic compounds. Among various PS activation methods, heterogeneous catalytic activation has the advantages of high catalytic activity, none energy consumption and less oxidant dosage. Transition metal catalysts are excellent PS activation candidates. Among them, manganese (Mn)- based catalysts are characterized by abundant natural reserves, variable valence states and multiple crystal forms, which have been widely applied in PS activation for degradation of various organic pollutants. Herein, recent progress on different Mn-based catalysts for PS activation on degradation of organic wastewater were reviewed. Research on zero valence Mn, single manganese oxides, composite manganese oxides, special crystalline manganese oxides and supporting material were discussed respectively. Finally, the remaining challenges and future direction on Mn based catalysts for PS activation were prospected.
-
Key words:
- advanced oxidation /
- manganese based catalyst /
- persulfate /
- organic wastewater /
- activation /
- degradation.
-

-
图 5 LaMnO3和Cu-LaMnO3钙钛矿的循环伏安曲线(a),La 3d、Mn 3s、Mn 2p和Cu 2p的XPS谱图(b),SMX在Cu-LaMnO3/PMS体系中的降解机理(c)[95]
Figure 5. Cyclic voltammetry curve of LaMnO3 and Cu-LaMnO3 (a), XPS spectra of La 3d, Mn 3s, Mn 2p and Cu 2p in LaMnO3 and Cu-LaMnO3 (b) and degradation mechanism of SMX in Cu-LaMnO3/PMS system (c)[95]
图 6 CMO(a),HNT(b),40-CMO/HNT(c)的TEM图像,CMO和40-CMO/HNT的XPS光谱(d),40-CMO/HNT活化PMS产生自由基的机理示意图(e)[99]
Figure 6. TEM images of bare CMO (a), HNT (b) and 40-CMO/HNT (c),XPS spectra of survey for bare CMO and 40-CMO/HNT (d), Schematic illustration of radical generation mechanism through PMS activated by 40-CMO/HNT (e)[99]
表 1 锰基催化剂分类及特性
Table 1. Classification and characteristics of manganese based catalysts
类别
Classification催化活性组分
Active catalytic components制备方法
Preparation method反应条件
Reaction condition目标污染物
Target contaminants主要ROS
Major
reactive
oxygen
species污染物
去除率
Pollutants
removal
efficiency特点
Features参考文献
Reference零价锰 NZVIM 一步液相还原法 Ccata=75.0 mg·L−1、
CPMS=1.0 mmol·L−1
CSMT=5.0 mg·L−1、
MFe/MMn=2:1、1:1、
pH0=3.0、
T=30±0.5 °C、
t=60 min磺胺甲基嘧啶
(sulfamethazine,
SMT)·OH >95% 粒径小、活性位点多、吸附和还原能力强 [45] nZVMn 化学还原法 CMn0=1.0 g·L−1、
CPDS=50 mg·L−1、
pH0=2.0
CCIP=10 mg·L−1、
t=80 min环丙沙星(ciprofloxacin,CIP) $ {\text{SO}}_{\text{4}}^{\text{·–}} $
·OH95% [46] 单一锰
氧化物α-MnO2 水热法 Ccata=0.05 g·L−1、
COI=5 mg·L−1
CPMS=25 μmol·L−1、
pH0=7.0、T=25±2 °C橙I
(OrangeI,OI)$ {\text{SO}}_{\text{4}}^{\text{·–}} $
·OH、
${\text{O}}_{2}^{\cdot –} $
1O286.2% 较好的活性和持久性 [47] MnOOH 水热法 Ccata=0.4 g·L−1、
CPMS=2.5 mmol·L−1、
pH0=3—9、
CPCA=0.5 mmol·L−1、
T=25 °C、t=180 min对氯苯胺
(p-chloroaniline,
PCA)1O2 100% [48] α-MnO2 水热法 Ccata=100 mg·L−1、
CPMS=100 mg·L−1、
t=25 h、
CDIN=2.09 μmol、
pH0=3.9、T=25 °C二唑(diniconazole,
DIN)$ {\text{SO}}_{\text{4}}^{\text{·–}} $
·OH
89.3% [49] 复合锰
氧化物CuMnFe LDHs 水热法 Ccata=0.6 g·L−1、
CPMS=0.4 mmol·L−1
CBPA=20 mg·L−1、
t=15 min、光照双酚A
(bisphenol
A,BPA)h+
$ {\text{O}}_{\text{2}}^{\text{·–}} $ 93.5% 高活性、耐水性和稳定性 [50] MnFeO 水热法 Ccata=0.1 g·L−1、
CPMS=0.4 mmol·L−1、
t=60 min
CBPA=40 μmol·L−1、
pH0=6.5±0.1、
T=(25±0.5) °C、双酚A
(bisphenol
A,BPA)$ {\text{SO}}_{\text{4}}^{\text{·–}} $
·OH
1O2>96% [51] Fe-Mn 溶胶-凝胶法 Ccata=0.5 g·L−1、
CPMS=2.0 g·L−1
CPhenol=30 mg·L−1、
pH0=5、T=25 ℃、
t=90 min苯酚
(Phenol,
PhOH)·OH 90.59% [12] Mn3O4 /ZIF-8 溶剂热法 Ccata=0.4 g·L−1、
CPMS=0.3 g·L−1
CRhB=10 mg·L−1、
T=23 °C罗丹明B
(rhodamine
B,RhB)·OH 96% [52] MnO2/
MnFe2O4水热法 Ccata=0.2 g·L−1、
CPMS=0.4 g·L−1、
pH0=5.4、
CRhB=0.01 g·L−1、
T=30 °C罗丹明B
(rhodamine
B,RhB)$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 99% [53] (LiNi0.5Co0.2Mn0.3O2, NCM) 热合成 Ccata=0.5 g·L−1、
CPMS=1 mmol·L−1、
CRhB=20 mg·L−1、
pH0=3、T=25 °C、
t=20 min罗丹明B
(rhodamine
B,RhB)1O2 100% [54] α-MnO2/
MnFe2O4水热法 Ccata=0.2 g·L−1、
CPMS=1 mmol·L−1
CNOF=20 mg·L−1、
pH0=7、T=25 °C、
t=20 min氟沙星(Norfloxacin,
NOF)$ {\text{SO}}_{\text{4}}^{\text{·–}} $
·OH、$ {\text{O}}_{\text{2}}^{\text{·–}} $ 100% [55] 特殊晶型
锰氧化物La0.5Sr0.5Co0.8Mn0.2O3-δ 煅烧法 Ccata=0.1 g·L−1、
CPMS=0.12 g·L−1
CTBBPA0=20 mg·L−1、
pH0=6.7、T=25 °C、
t=40 min四溴双酚A
(tetrrabromobisphenol
A ,TBBPA)$ {\text{SO}}_{\text{4}}^{\text{·–}} $
>95% 低成本、高热稳定性、高孔隙度、优良的导电性 [56] LaMnO3 溶胶-凝胶法 Ccata=40 mg·L−1、
CPMS=0.2 g·L−1
COFX=10 mg·L−1、
pH0=5.0、t=15 min氧氟沙星(ofloxacin,
OFX)1O2 96.3% [57] MFO-LIBs 溶胶-凝胶法 Ccata=0.3 g·L−1、
CPMS=0.1 g·L−1
CBPA=20 mg·L−1、
pH0=6.2、T=25 °C、
t=15 min双酚A
(bisphenol
A,BPA)$ {\text{SO}}_{\text{4}}^{\text{·–}} $
·OH、$ {\text{O}}_{\text{2}}^{\text{·–}} $
1O2100% [58] 负载型
锰氧化物MnFe2O4/
(CM-600)煅烧法 Ccata=0.4 g·L−1、
nAO7/nPMS=1:15
CAO7=50 g·L−1、
pH0=6.5±0.05、
T=25±0.5 °C酸性橙7 (Orange7,
AO7)$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 99.0% 热稳定性、价格低廉、比表面积大 [59] MMBC 共热解 Ccata=0.2 g·L−1、
CPDS=8 mmol·L−1
CTC=20 mg·L−1、
pH0=3、t=180 min四环素(tetracycline,
TC)·OH 93% [60] 负载型
锰氧化物MCC 浸渍法 Ccata=1.0 g·L−1、
CPMS=1.0 mmol·L−1、
T=25 °C
CBPA=20 mg·L−1、
pH0=6.5±0.2、
t=120 min双酚A
(bisphenol
A,BPA)1O2 90% 热稳定性、价格低廉、比表面积大 [61] α-MnO2/Pal 水热法 Ccata=0.1 g·L−1、
CPMS=0.1 g·L−1
CRhB=20 mg·L−1、
pH0=5.5±0.1、
T=300 min罗丹明B
(rhodamine
B,RhB)$ {\text{O}}_{\text{2}}^{\text{·–}} $
1O2100% [62] MnO2/C@CNT 一锅法 Ccata=0.10 g·L−1、
CPMS=3.0 mmol·L−1
CTC=10 mg·L−1、
pH0=5.2、T=20 °C、
t=10 min四环素(tetracycline,
TC)$ {\text{SO}}_{\text{4}}^{\text{·–}} $ 85.6% [63] (注:Ccata:催化剂添加量;CPMS:过硫酸盐浓度;pH0:初始pH值;T:温度;t:反应时间)
(Note: Ccata: catalyst concentration; CPMS: persulfate concentration; pH0: initial pH value; T: temperature; t: reaction time) -
[1] 杜明辉, 王勇, 高群丽, 等. 臭氧微气泡处理有机废水的效果与机制 [J]. 化工进展, 2021, 40(12): 6907-6915. DU M H, WANG Y, GAO Q L, et al. Mechanism and efficiency of ozone microbubble treatment of organic wastewater [J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6907-6915(in Chinese).
[2] 李珏秀, 刘纪林, 杨草原, 等. 微生物燃料电池辅助转盘液膜光催化降解染料废水 [J]. 中原工学院学报, 2022, 33(2): 51-57. LI J X, LIU J L, YANG C Y, et al. Degradation of dye wastewater by microbial fuel cell assisting rotating disk thin-film photocatalysis integrated system [J]. Journal of Zhongyuan University of Technology, 2022, 33(2): 51-57(in Chinese).
[3] 申大伟. 锰基催化剂对印染废水的降解研究 [J]. 印染助剂, 2022, 39(5): 21-26. SHEN D W. Degradation of printing and dyeing wastewater by Manganese based catalyst [J]. Textile Auxiliaries, 2022, 39(5): 21-26(in Chinese).
[4] 王渊源, 阎鑫, 艾涛, 等. 碳化泡沫负载Co3O4活化过硫酸盐降解罗丹明B [J]. 环境科学, 2022, 43(4): 2039-2046. WANG Y Y, YAN X, AI T, et al. Carbonized foam supported Co3O4 activated peroxymonosulfate towards rhodamine B degradation [J]. Environmental Science, 2022, 43(4): 2039-2046(in Chinese).
[5] 吴健森, 孟耀庭, 潘月燕, 等. 亚临界水氧化技术处理高盐难降解有机废水研究进展 [J]. 能源环境保护, 2022, 36(3): 30-36. doi: 10.3969/j.issn.1006-8759.2022.03.005 WU J S, MENG Y T, PAN Y Y, et al. Research progress in the treatment of refractory organic wastewater with high salinity by subcritical water oxidation technology [J]. Energy Environmental Protection, 2022, 36(3): 30-36(in Chinese). doi: 10.3969/j.issn.1006-8759.2022.03.005
[6] 马博雅, 孙立坤, 杨春维. 碳中和背景下我国污水处理技术思考 [J]. 应用化工, 2022, 51(10): 2997-3000. MA B Y, SUN L K, YANG C W. The feasible wastewater treatment technology in China under the background of carbon neutrality [J]. Applied Chemical Industry, 2022, 51(10): 2997-3000(in Chinese).
[7] 孙小淇, 郝泽伟, 陈家斌, 等. 碳中和背景下高盐废水中盐分的高效分离和资源化 [J]. 工业水处理, 2023, 43(2): 14-22. SUN X Q, HAO Z W, CHEN J B, et al. Efficient separation and resource recovery technology of salts in highly saline wastewater in the context of carbon neutrality [J]. Industrial Water Treatment, 2023, 43(2): 14-22(in Chinese).
[8] 吴百苗, 张一梅, 栗帅, 等. 基于LCA的污水处理方案碳中和综合影响评价 [J]. 环境工程, 2022, 40(6): 130-137. WU B M, ZHANG Y M, LI S, et al. Comprehensive impact assessment on carbon neutralization of wastewater treatment plants based on hybrid LCA [J]. Environmental Engineering, 2022, 40(6): 130-137(in Chinese).
[9] GLAZE W H, KANG J W, CHAPIN D H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation [J]. Ozone:Science & Engineering, 1987, 9(4): 335-352. [10] WEI Y, MIAO J, GE J X, et al. Ultrahigh peroxymonosulfate utilization efficiency over CuO nanosheets via heterogeneous Cu(III) formation and preferential electron transfer during degradation of phenols [J]. Environmental Science & Technology, 2022, 56(12): 8984-8992. [11] 齐亚兵. 活化过硫酸盐高级氧化法降解抗生素的研究进展 [J]. 化工进展, 2022, 41(12): 6627-6643. QI Y B. Research progress on degradation of antibiotics by activated persulfate advanced oxidation [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6627-6643(in Chinese).
[12] 邵强, 郭轶琼. 铁锰催化剂活化过硫酸盐去除水中苯酚的研究 [J]. 工业水处理, 2020, 40(7): 94-97. SHAO Q, GUO Y Q. Removal of phenol from water by activation of persulfate with Fe-Mn catalyst [J]. Industrial Water Treatment, 2020, 40(7): 94-97(in Chinese).
[13] 王庆宏, 李思雨, 牛皓, 等. 活化过硫酸盐氧化处理难降解废水的技术研究进展 [J]. 工业水处理, 2022, 42(8): 8-16,26. WANG Q H, LI S Y, NIU H, et al. An overview of activated persulfate oxidation processes in treatment of refractory wastewaters [J]. Industrial Water Treatment, 2022, 42(8): 8-16,26(in Chinese).
[14] 杨鹤云, 郑兴. 高级氧化法降解有机污染物的应用及研究进展 [J]. 水处理技术, 2021, 47(12): 13-18. YANG H Y, ZHENG X. Application and research progress of advanced oxidation process for degradation of organic pollutants [J]. Technology of Water Treatment, 2021, 47(12): 13-18(in Chinese).
[15] 杨淼淼. 高级氧化技术处理印染废水的研究进展 [J]. 生物化工, 2022, 8(1): 149-152. YANG M M. Research progress of advanced oxidation process for dyeing wastewater treatment based on free radical [J]. Biological Chemical Engineering, 2022, 8(1): 149-152(in Chinese).
[16] JIANG S F, LING L L, CHEN W J, et al. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors [J]. Chemical Engineering Journal, 2019, 359: 572-583. doi: 10.1016/j.cej.2018.11.124 [17] OLMEZ-HANCI T, ARSLAN-ALATON I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol [J]. Chemical Engineering Journal, 2013, 224: 10-16. doi: 10.1016/j.cej.2012.11.007 [18] ZHANG X W, LAN M Y, WANG F, et al. ZIF-67-based catalysts in persulfate advanced oxidation processes (PS-AOPs) for water remediation [J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107997. doi: 10.1016/j.jece.2022.107997 [19] LIU Y H, KUO Y S, LIU W C. et al. Photoelectrocatalytic activity of perovskite YFeO3/carbon fiber composite electrode under visible light irradiation for organic wastewater treatment [J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 128: 227-236. doi: 10.1016/j.jtice.2021.08.029 [20] 马祯, 宋小三, 张轩. 紫外/过硫酸盐高级氧化技术在饮用水处理中的研究进展 [J]. 应用化工, 2022, 51(5): 1466-1471. MA Z, SONG X S, ZHANG X. Research progress of ultraviolet/persulfate advanced oxidation technology in drinking water treatment [J]. Applied Chemical Industry, 2022, 51(5): 1466-1471(in Chinese).
[21] 王静, 邓黎玲, 薛罡, 等. 物化-生化-高级氧化处理涂料废渣热催化废液 [J]. 水处理技术, 2019, 45(10): 100-105. WANG J, DENG L L, XUE G, et al. Physicochemical-biochemical advanced treatment for paint sludge thermocatalytic waste liquor [J]. Technology of Water Treatment, 2019, 45(10): 100-105(in Chinese).
[22] 吴秀, 方迪, 危亚云, 等. 热活化过一硫酸盐调理强化厌氧消化污泥脱水的研究 [J]. 环境科学学报, 2021, 41(11): 4547-4553. WU X, FANG D, WEI Y Y, et al. Improved dewaterability of anaerobically digested sewage sludge by thermally activated peroxymonosulfate [J]. Acta Scientiae Circumstantiae, 2021, 41(11): 4547-4553(in Chinese).
[23] LONG L L, BAI C W, ZHOU X Y, et al. A novel strategy for promoting PMS activation: Enhanced utilization of side reactions [J]. Separation and Purification Technology, 2022, 297: 121432. doi: 10.1016/j.seppur.2022.121432 [24] 程佳鑫, 李荣兴, 杨海涛, 等. 三维电催化氧化处理难生化降解有机废水研究进展 [J]. 环境化学, 2022, 41(1): 288-304. doi: 10.7524/j.issn.0254-6108.2020082804 CHENG J X, LI R X, YANG H T, et al. Review of three-dimensional electrodes for bio-refractory organic wastewater treatment [J]. Environmental Chemistry, 2022, 41(1): 288-304(in Chinese). doi: 10.7524/j.issn.0254-6108.2020082804
[25] 左静, 秦丰林, 方国东. 电化学活化过硫酸盐修复土壤有机污染研究进展 [J]. 现代农业科技, 2021(13): 179-185. ZUO J, QIN F L, FANG G D. Research progress on remediation of soil organic pollution by electrochemical activation of persulfate [J]. Modern Agricultural Science and Technology, 2021(13): 179-185(in Chinese).
[26] 杨晴, 孙昕, 李鹏飞, 等. 超声活化过硫酸盐降解甲基橙的影响因素研究 [J]. 环境科学学报, 2020, 40(8): 2715-2721. YANG Q, SUN X, LI P F, et al. Influencing factors of methyl orange degradation by ultrasound activated persulfate [J]. Acta Scientiae Circumstantiae, 2020, 40(8): 2715-2721(in Chinese).
[27] 张楠, 陈蕾. 超声活化过硫酸盐降解废水中有机污染物的研究进展 [J]. 应用化工, 2021, 50(10): 2805-2808,2813. ZHANG N, CHEN L. Research progress of ultrasonic activated persulfate for degradation of organic pollutants in wastewater [J]. Applied Chemical Industry, 2021, 50(10): 2805-2808,2813(in Chinese).
[28] 朱佳, 宋慧, 高敏, 等. 超声协同MoS2/g-C3N4活化过硫酸盐降解二甲基亚砜的研究 [J]. 化工新型材料, 2022, 50(9): 179-184,190. ZHU J, SONG H, GAO M, et al. Degradation of dimethyl sulfoxide by ultrasonic synergistic [J]. New Chemical Materials, 2022, 50(9): 179-184,190(in Chinese).
[29] 张磊, 祝思频, 张青青, 等. 微波活化过硫酸盐降解典型选矿药剂水杨羟肟酸 [J]. 环境化学, 2022, 41(8): 3414-3424. doi: 10.7524/j.issn.0254-6108.2021030801 ZHANG L, ZHU S P, ZHANG Q Q, et al. Degradation of salicylhydroxamic acid by microwave activated persulfate [J]. Environmental Chemistry, 2022, 41(8): 3414-3424(in Chinese). doi: 10.7524/j.issn.0254-6108.2021030801
[30] 程爱华, 马万超, 徐哲. 等离子体改性海绵铁活化过硫酸盐处理含酚废水 [J]. 化工进展, 2020, 39(2): 798-804. CHENG A H, MA W C, XU Z. Treatment of phenol wastewater with persulfate activated by plasmamodified sponge iron [J]. Chemical Industry and Engineering Progress, 2020, 39(2): 798-804(in Chinese).
[31] 李春琴, 邹亚辰, 贾小宁. 过硫酸盐高级氧化技术活化方法及降解机理的研究进展 [J]. 化学与生物工程, 2022, 39(6): 1-6,27. LI C Q, ZOU Y C, JIA X N. Research progress in activation methods of persulfate and degradation mechanism of organic pollutants by persulfate advanced oxidation process [J]. Chemistry & Bioengineering, 2022, 39(6): 1-6,27(in Chinese).
[32] 凌良雄, 陆建, 周易, 等. 铁基材料活化过硫酸盐降解水中抗生素的研究进展 [J]. 环境科学研究, 2022, 35(1): 290-298. LING L X, LU J, ZHOU Y, et al. Persulfate activated by iron-based materials for degradation of antibiotics in water: A review [J]. Research of Environmental Sciences, 2022, 35(1): 290-298(in Chinese).
[33] 田婷婷, 李朝阳, 王召东, 等. 过渡金属活化过硫酸盐降解有机废水技术研究进展 [J]. 化工进展, 2021, 40(6): 3480-3488. TIAN T T, LI C Y, WANG S D, et al. Research progress of transition metal activated persulfate to degrade organic wastewater [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3480-3488(in Chinese).
[34] 李书典, 郑德山, 郭峰. 二氧化锰催化氧化性能的研究进展 [J]. 现代化工, 2020, 40(3): 52-56. LI S D, ZHENG D S, GUO F. Research progress on catalytic oxidation performance of Manganese dioxide [J]. Modern Chemical Industry, 2020, 40(3): 52-56(in Chinese).
[35] 周自成, 刘悦, 李英, 等. 纳米Mn3O4的快速制备及其对亚甲基蓝的类芬顿催化氧化性能 [J]. 矿冶工程, 2020, 40(4): 153-155,160. ZHOU Z C, LIU Y, LI Y, et al. Simple and rapid preparation of nano-Mn3O4 and its Fenton-like catalytic oxidation of methylene blue [J]. Mining and Metallurgical Engineering, 2020, 40(4): 153-155,160(in Chinese).
[36] 张磊, 王胜, 汪明哲, 等. CoMnOx/Al2O3/monolith整体催化剂的制备及其催化臭氧分解性能 [J]. 工业催化, 2020, 28(1): 17-23. ZHANG L, WANG S, WANG M Z, et al. Preparation of CoMnOx/Al2O3/monolith catalyst for ozone elimination [J]. Industrial Catalysis, 2020, 28(1): 17-23(in Chinese).
[37] FU R, ZHANG P S, JIANG Y X, et al. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods [J]. Chemosphere, 2023, 311: 136993. doi: 10.1016/j.chemosphere.2022.136993 [38] LIN C, LI J L, LI X P, et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation [J]. Nature Catalysis, 2021, 4(12): 1012-1023. doi: 10.1038/s41929-021-00703-0 [39] ZHOU Y, FENG S, DUAN X M, et al. MnO2/UIO-66 improves the catalysed degradation of oxytetracycline under UV/H2O2/PMS system [J]. Journal of Solid State Chemistry, 2021, 300: 122231. doi: 10.1016/j.jssc.2021.122231 [40] GUO M N, FANG R M, LIU X W, et al. Experimental study of volatile organic compounds catalytic combustion on Cu-Mn catalysts with different carriers [J]. International Journal of Energy Research, 2021, 45(6): 8749-8762. doi: 10.1002/er.6411 [41] QIN C H, GUO M K, JIANG C C, et al. Simultaneous oxidation of toluene and ethyl acetate by dielectric barrier discharge combined with Fe, Mn and Mo catalysts [J]. Science of the Total Environment, 2021, 782: 146931. doi: 10.1016/j.scitotenv.2021.146931 [42] 向宁, 韩小金, 郑剑锋, 等. 锰改性对ZIF-67衍生Co3O4低温催化氧化甲醛性能的影响 [J]. 燃料化学学报, 2022, 50(7): 859-867. XIANG N, HAN X J, ZHENG J F, et al. Effect of Manganese modification on catalytic oxidation of formaldehyde at low temperature by Co3O4 derived from ZIF-67 [J]. Journal of Fuel Chemistry and Technology, 2022, 50(7): 859-867(in Chinese).
[43] DING Y B, WANG X R, FU L B, et al. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective [J]. Science of the Total Environment, 2021, 765: 142794. doi: 10.1016/j.scitotenv.2020.142794 [44] WACLAWEK S, LUTZE H V, GRüBEL K, et al. Chemistry of persulfates in water and wastewater treatment: A review [J]. Chemical Engineering Journal, 2017, 330: 44-62. doi: 10.1016/j.cej.2017.07.132 [45] XIAO J Y, LI R, DONG H R, et al. Activation of sulfite via zero-valent iron-Manganese bimetallic nanomaterials for enhanced sulfamethazine removal in aqueous solution: Key roles of Fe/Mn molar ratio and solution pH [J]. Separation and Purification Technology, 2022, 297: 121479. doi: 10.1016/j.seppur.2022.121479 [46] SHAH N S, ALI KHAN J, SAYED M, et al. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent Manganese catalyzed S2O82− [J]. Chemical Engineering Journal, 2019, 356: 199-209. doi: 10.1016/j.cej.2018.09.009 [47] YANG Y S, ZHAO Y, ZONG Y, et al. Activation of peroxymonosulfate by α-MnO2 for Orange Ⅰ removal in water [J]. Environmental Research, 2022, 210: 112919. doi: 10.1016/j.envres.2022.112919 [48] XU X M, ZHANG Y Q, ZHOU S Q, et al. Activation of persulfate by MnOOH: Degradation of organic compounds by nonradical mechanism [J]. Chemosphere, 2021, 272: 129629. doi: 10.1016/j.chemosphere.2021.129629 [49] ZHAO M J, XU R S, CHEN Z Q, et al. Kinetics and mechanisms of diniconazole degradation by α-MnO2 activated peroxymonosulfate [J]. Separation and Purification Technology, 2022, 281: 119850. doi: 10.1016/j.seppur.2021.119850 [50] 刘畅, 王宇寒, 胡清, 等. 太阳光/CuMnFe LDHs催化剂/过一硫酸盐体系降解双酚A [J]. 环境工程学报, 2021, 15(11): 3545-3560. LIU C, WANG Y H, HU Q, et al. Degradation of bisphenol A using CuMnFe LDHs catalyst and peroxymonosulfate under solar light [J]. Chinese Journal of Environmental Engineering, 2021, 15(11): 3545-3560(in Chinese).
[51] 李广英, 杜敏洁, 谈成英, 等. 锰铁氧体活化PMS降解双酚A的过程机制 [J]. 环境工程学报, 2021, 15(9): 2952-2962. LI G Y, DU M J, TAN C Y, et al. Mechanism of BPA degradation in a system of peroxymonosulfate activated by a Mn/Fe bimetallic oxide catalysts [J]. Chinese Journal of Environmental Engineering, 2021, 15(9): 2952-2962(in Chinese).
[52] HU L X, DENG G H, LU W C, et al. Peroxymonosulfate activation by Mn3O4/metal-organic framework for degradation of refractory aqueous organic pollutant rhodamine B [J]. Chinese Journal of Catalysis, 2017, 38(8): 1360-1372. doi: 10.1016/S1872-2067(17)62875-4 [53] CHEN G, ZHANG X Y, GAO Y J, et al. Novel magnetic MnO2/MnFe2O4 nanocomposite as a heterogeneous catalyst for activation of peroxymonosulfate (PMS) toward oxidation of organic pollutants [J]. Separation and Purification Technology, 2019, 213: 456-464. doi: 10.1016/j.seppur.2018.12.049 [54] ZHAO Y L, WANG H, JI J Q, et al. Recycling of waste power lithium-ion batteries to prepare nickel/cobalt/Manganese-containing catalysts with inter-valence cobalt/Manganese synergistic effect for peroxymonosulfate activation [J]. Journal of Colloid and Interface Science, 2022, 626: 564-580. doi: 10.1016/j.jcis.2022.06.112 [55] XU L S, SUN X B, HONG J M, et al. Peroxymonosulfate activation by α-MnO2/MnFe2O4 for norfloxacin degradation: Efficiency and mechanism [J]. Journal of Physics and Chemistry of Solids, 2021, 153: 110029. doi: 10.1016/j.jpcs.2021.110029 [56] 毛韦达, 胡翔. La0.5Sr0.5Co0.8Mn0.2O3-δ钙钛矿对过一硫酸盐降解水中四溴双酚A影响实验研究 [J]. 地学前缘, 2019, 26(3): 255-262. MAO W D, HU X. Exoerinentai studies of the effect of perovskite La0.5Sr0.5Co0.8Mn0.2O3-δ on tetrabromobisphenol A degradation in water by peroxy-monosulfate [J]. Earth Science Frontiers, 2019, 26(3): 255-262(in Chinese).
[57] NIE Y L, ZHOU H, TIAN S, et al. Anionic ligands driven efficient ofloxacin degradation over LaMnO3 suspended particles in water due to the enhanced peroxymonosulfate activation [J]. Chemical Engineering Journal, 2022, 427: 130998. doi: 10.1016/j.cej.2021.130998 [58] LIANG J X, GUO M M, XUE Y X, et al. Constructing magnetically separable Manganese-based spinel ferrite from spent ternary lithium-ion batteries for efficient degradation of bisphenol A via peroxymonosulfate activation [J]. Chemical Engineering Journal, 2022, 435: 135000. doi: 10.1016/j.cej.2022.135000 [59] DU J Y, XU W S, LIU J, et al. Efficient degradation of Acid Orange 7 by persulfate activated with a novel developed carbon-based MnFe2O4 composite catalyst [J]. Journal of Chemical Technology & Biotechnology, 2020, 95(4): 1135-1145. [60] HUANG D L, ZHANG Q, ZHANG C, et al. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline [J]. Chemical Engineering Journal, 2020, 391: 123532. doi: 10.1016/j.cej.2019.123532 [61] YANG Z, WANG Z W, LIANG G W, et al. Catalyst bridging-mediated electron transfer for nonradical degradation of bisphenol A via natural Manganese ore-cornstalk biochar composite activated peroxymonosulfate [J]. Chemical Engineering Journal, 2021, 426: 131777. doi: 10.1016/j.cej.2021.131777 [62] HUANG C, WANG Y L, GONG M, et al. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B [J]. Separation and Purification Technology, 2020, 230: 115877. doi: 10.1016/j.seppur.2019.115877 [63] WANG Z X, HAN Y F, FAN W L, et al. Shell-core MnO2/Carbon@Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline [J]. Separation and Purification Technology, 2021, 278: 119558. doi: 10.1016/j.seppur.2021.119558 [64] LI Y T, LI H S, LIU F L, et al. Zero-valent iron-Manganese bimetallic nanocomposites catalyze hypochlorite for enhanced thallium(I) oxidation and removal from wastewater: Materials characterization, process optimization and removal mechanisms [J]. Journal of Hazardous Materials, 2020, 386: 121900. doi: 10.1016/j.jhazmat.2019.121900 [65] DADA A O, ADEKOLA F A, ODEBUNMI E O. Liquid phase scavenging of Cd (II) and Cu (II) ions onto novel nanoscale zerovalent Manganese (nZVMn): Equilibrium, kinetic and thermodynamic studies [J]. Environmental Nanotechnology, Monitoring & Management, 2017, 8: 63-72. [66] PANDA A P, ROUT P, JENA K K, et al. Core–shell structured zero-valent Manganese (ZVM): A novel nanoadsorbent for efficient removal of As(iii) and As(v) from drinking water [J]. Journal of Materials Chemistry A, 2019, 7(16): 9933-9947. doi: 10.1039/C9TA00428A [67] 贺君, 陈思琦, 唐首锋, 等. MnO2/EGM电极制备及电化学降解罗丹明B的研究 [J]. 环境科学学报, 2020, 40(11): 3922-3930. HE J, CHEN S Q, TANG S F, et al. Preparation of MnO2/EGM electrode and electrochemical degradation of Rhodamine B [J]. Acta Scientiae Circumstantiae, 2020, 40(11): 3922-3930(in Chinese).
[68] SHEN S T, ZHOU X Q, ZHAO Q D, et al. Understanding the nonradical activation of peroxymonosulfate by different crystallographic MnO2: The pivotal role of MnIII content on the surface [J]. Journal of Hazardous Materials, 2022, 439: 129613. doi: 10.1016/j.jhazmat.2022.129613 [69] NDAYIRAGIJE S, ZHANG Y F, ZHOU Y Q, et al. Mechanochemically tailoring oxygen vacancies of MnO2 for efficient degradation of tetrabromobisphenol A with peroxymonosulfate [J]. Applied Catalysis B:Environmental, 2022, 307: 121168. doi: 10.1016/j.apcatb.2022.121168 [70] YI H L, WANG Y H, DIAO L L, et al. Ultrasonic treatment enhances the formation of oxygen vacancies and trivalent Manganese on α-MnO2 surfaces: Mechanism and application [J]. Journal of Colloid and Interface Science, 2022, 626: 629-638. doi: 10.1016/j.jcis.2022.06.144 [71] LI M, ZHANG H, LIU Z L, et al. Surface lattice oxygen mobility inspired peroxymonosulfate activation over Mn2O3 exposing different crystal faces toward bisphenol A degradation [J]. Chemical Engineering Journal, 2022, 450: 138147. doi: 10.1016/j.cej.2022.138147 [72] OUYANG H, WU C, QIU X H, et al. New insight of Mn(III) in δ-MnO2 for peroxymonosulfate activation reaction: Via direct electron transfer or via free radical reactions [J]. Environmental Research, 2023, 217: 114874. doi: 10.1016/j.envres.2022.114874 [73] ZHOU Z G, DU H M, DAI Z H, et al. Degradation of organic pollutants by peroxymonosulfate activated by MnO2 with different crystalline structures: Catalytic performances and mechanisms [J]. Chemical Engineering Journal, 2019, 374: 170-180. doi: 10.1016/j.cej.2019.05.170 [74] 王淑敏, 林海龙, 侯俊斌, 等. MnO2的生长机理及其对罗丹明B的快速降解 [J]. 材料科学与工程学报, 2022, 40(1): 34-39. WANG S M, LIN H L, HOU J B, et al. Growth mechanism of MnO2 and its rapid degradation of rhodamine B [J]. Journal of Materials Science and Engineering, 2022, 40(1): 34-39(in Chinese).
[75] 闵弘扬, 王赟, 冉献强, 等. Cu-Mn/ZSM-5催化剂的制备及其催化降解酸性红的研究 [J]. 工业用水与废水, 2015, 46(1): 52-56,64. MIN H Y, WANG Y, RAN X Q, et al. Study on Cu-Mn/ZSM-5 catalyst preparation and its catalytic degradation performance on acid red [J]. Industrial Water & Wastewater, 2015, 46(1): 52-56,64(in Chinese).
[76] GHASEMI H, MOZAFFARI S, MOUSAVI S H, et al. Decolorization of wastewater by heterogeneous Fenton reaction using MnO2-Fe3O4/CuO hybrid catalysts [J]. Journal of Environmental Chemical Engineering, 2021, 9(2): 105091. doi: 10.1016/j.jece.2021.105091 [77] LIU M, YIN W, ZHAO T L, et al. High-efficient removal of organic dyes from model wastewater using Mg(OH)2-MnO2 nanocomposite: Synergistic effects of adsorption, precipitation, and photodegradation [J]. Separation and Purification Technology, 2021, 272: 118901. doi: 10.1016/j.seppur.2021.118901 [78] PANIMALAR S, SUBASH M, CHANDRASEKAR M, et al. Reproducibility and long-term stability of Sn doped MnO2 nanostructures: Practical photocatalytic systems and wastewater treatment applications [J]. Chemosphere, 2022, 293: 133646. doi: 10.1016/j.chemosphere.2022.133646 [79] THAO L T, van NGUYEN T, NGUYEN V Q, et al. Orange G degradation by heterogeneous peroxymonosulfate activation based on magnetic MnFe2O4/α-MnO2 hybrid [J]. Journal of Environmental Sciences, 2023, 124: 379-396. doi: 10.1016/j.jes.2021.10.008 [80] SHI Q Q, PU S Y, YANG X, et al. Enhanced heterogeneous activation of peroxymonosulfate by boosting internal electron transfer in a bimetallic Fe3O4-MnO2 nanocomposite [J]. Chinese Chemical Letters, 2022, 33(4): 2129-2133. doi: 10.1016/j.cclet.2021.07.063 [81] ANUSHREE C, NANDA GOPALA KRISHNA D, PHILIP J. Efficient dye degradation via catalytic persulfate activation using iron oxide-Manganese oxide core-shell particle doped with transition metal ions [J]. Journal of Molecular Liquids, 2021, 337: 116429. doi: 10.1016/j.molliq.2021.116429 [82] WANG Z M, WANG Z H, LI W, et al. Performance comparison and mechanism investigation of Co3O4-modified different crystallographic MnO2 (α, β, γ, and δ) as an activator of peroxymonosulfate (PMS) for sulfisoxazole degradation [J]. Chemical Engineering Journal, 2022, 427: 130888. doi: 10.1016/j.cej.2021.130888 [83] JIANG Z R, WANG P F, ZHOU Y X, et al. Fabrication of a 3D-blocky catalyst (CoMnOx@sponge) via mooring Co-Mn bimetallic oxide on sponge to activate peroxymonosulfate for convenient and efficient degradation of sulfonamide antibiotics [J]. Chemical Engineering Journal, 2022, 446: 137306. doi: 10.1016/j.cej.2022.137306 [84] CHEN L, MAQBOOL T, NAZIR G, et al. Peroxymonosulfate activated by composite ceramic membrane for the removal of pharmaceuticals and personal care products (PPCPs) mixture: Insights of catalytic and noncatalytic oxidation [J]. Water Research, 2023, 229: 119444. doi: 10.1016/j.watres.2022.119444 [85] ZHAO Y, LI B, LI Y, et al. Synergistic activation of peroxymonosulfate between Co and MnO for bisphenol A degradation with enhanced activity and stability [J]. Journal of Colloid and Interface Science, 2022, 623: 775-786. doi: 10.1016/j.jcis.2022.05.105 [86] CHEN L J, LI Y H, ZHANG J W, et al. Oxidative degradation of tetracycline hydrochloride by Mn2O3/Bi2O3 photocatalysis activated peroxymonosulfate [J]. Inorganic Chemistry Communications, 2022, 140: 109414. doi: 10.1016/j.inoche.2022.109414 [87] WANG L H, XU H D, JIANG N, et al. Effective activation of peroxymonosulfate with natural Manganese-containing minerals through a nonradical pathway and the application for the removal of bisphenols [J]. Journal of Hazardous Materials, 2021, 417: 126152. doi: 10.1016/j.jhazmat.2021.126152 [88] HE B, YANG Y, LIU B R, et al. Degradation of chlortetracycline hydrochloride by peroxymonosulfate activation on natural Manganese sand through response surface methodology [J]. Environmental Science and Pollution Research, 2022, 29(54): 82584-82599. doi: 10.1007/s11356-022-21556-5 [89] CHEN T, ZHU Z L, WANG Z Y, et al. 3D hollow sphere-like Cu-incorporated LaAlO3 perovskites for peroxymonosulfate activation: Coaction of electron transfer and oxygen defect [J]. Chemical Engineering Journal, 2020, 385: 123935. doi: 10.1016/j.cej.2019.123935 [90] FAYYAZ A, SARAVANAKUMAR K, TALUKDAR K, et al. Catalytic oxidation of naproxen in cobalt spinel ferrite decorated Ti3C2Tx MXene activated persulfate system: Mechanisms and pathways [J]. Chemical Engineering Journal, 2021, 407: 127842. doi: 10.1016/j.cej.2020.127842 [91] HUANG M J, PENG S S, XIANG W, et al. Strong metal-support interaction between carbon nanotubes and Mn-Fe spinel oxide in boosting peroxymonosulfate activation: Underneath mechanisms and application [J]. Chemical Engineering Journal, 2022, 429: 132372. doi: 10.1016/j.cej.2021.132372 [92] MANOS D, MISERLI K, KONSTANTINOU I. Perovskite and spinel catalysts for sulfate radical-based advanced oxidation of organic pollutants in water and wastewater systems [J]. Catalysts, 2020, 10(11): 1299. doi: 10.3390/catal10111299 [93] UTAMA P S, WIDAYATNO W B, AZHAR M R, et al. LaMnO3 perovskite activation of peroxymonosulfate for catalytic palm oil mill secondary effluent degradation [J]. Journal of Applied Materials and Technology, 2020, 2(1): 27-35. doi: 10.31258/Jamt.2.1.27-35 [94] WANG T, QIAN X F, YUE D T, et al. CaMnO3 perovskite nanocrystals for efficient peroxydisulfate activation [J]. Chemical Engineering Journal, 2020, 398: 125638. doi: 10.1016/j.cej.2020.125638 [95] GAO P P, TIAN X K, FU W, et al. Copper in LaMnO3 to promote peroxymonosulfate activation by regulating the reactive oxygen species in sulfamethoxazole degradation [J]. Journal of Hazardous Materials, 2021, 411: 125163. doi: 10.1016/j.jhazmat.2021.125163 [96] GUO J X, JING Y, SHEN T, et al. Effect of doped strontium on catalytic properties of La1‒xSrxMnO3 for rhodamine B degradation [J]. Journal of Rare Earths, 2021, 39(11): 1362-1369. doi: 10.1016/j.jre.2020.12.017 [97] LUO H D, GUO J X, SHEN T, et al. Study on the catalytic performance of LaMnO3 for the RhB degradation [J]. Journal of the Taiwan Institute of Chemical Engineers, 2020, 109: 15-25. doi: 10.1016/j.jtice.2020.01.011 [98] JIANG Z R, LI Y X, ZHOU Y X, et al. Co3O4-MnO2 nanoparticles moored on biochar as a catalyst for activation of peroxymonosulfate to efficiently degrade sulfonamide antibiotics [J]. Separation and Purification Technology, 2022, 281: 119935. doi: 10.1016/j.seppur.2021.119935 [99] YANG X, WEI G L, WU P Q, et al. Novel halloysite nanotube-based ultrafine CoMn2O4 catalyst for efficient degradation of pharmaceuticals through peroxymonosulfate activation [J]. Applied Surface Science, 2022, 588: 152899. doi: 10.1016/j.apsusc.2022.152899 [100] NGUYEN T B, LE V R, HUANG C P, et al. Construction of ternary NiCo2O4/MnOOH/GO composite for peroxymonosulfate activation with enhanced catalytic activity toward ciprofloxacin degradation [J]. Chemical Engineering Journal, 2022, 446: 137326. doi: 10.1016/j.cej.2022.137326 [101] WU Y H, LI Y L, HE J Y, et al. Nano-hybrids of needle-like MnO2 on graphene oxide coupled with peroxymonosulfate for enhanced degradation of norfloxacin: A comparative study and probable degradation pathway [J]. Journal of Colloid and Interface Science, 2020, 562: 1-11. doi: 10.1016/j.jcis.2019.11.121 [102] PAN M, WANG N, WENG Z T, et al. The synergistic activation of peroxymonosulfate for the degradation of Acid Scarlet GR by palygorskite/MnO2/Fe3O4 nanocomposites [J]. Dalton Transactions (Cambridge, England:2003), 2023, 52(4): 1009-1020. doi: 10.1039/D2DT02998G [103] HU Y Y, SUN S Y, GUO J L, et al. In situ anchoring strategy to enhance dual nonradical degradation of sulfamethoxazole with high loading manganese doped carbon nitride [J]. Chemosphere, 2022, 303: 135035. doi: 10.1016/j.chemosphere.2022.135035 [104] GONG Y X, WU Y N, SHEN J M, et al. Generation of interfacial high-spin Manganese intermediates as reactive oxidant during peroxymonosulfate activation mediated by amorphous MnOx supported on polymeric substrate [J]. Applied Catalysis B:Environmental, 2022, 316: 121671. doi: 10.1016/j.apcatb.2022.121671 -



 下载:
下载: