-
据统计,我国纺织工业废水年排放量超过20.0亿t,印染作为纺织品加工的关键环节,废水排放量占全行业的70%—80%[1]. 印染废水中含有多种致癌染料化合物,具有较高生物毒性,严重影响地表水水环境和人类健康[2]. 染料是印染废水中的主要污染物,按其结构可分为多种类型,如偶氮类、三苯基甲烷类和噻嗪类. 其中偶氮类染料约占世界上染料总量的70%[3],而甲基红(methyl red,MR)是最常用的偶氮染料之一,因其结构稳定,可生化性差,常规技术较难处理[4];孔雀石绿(malachite green,MG)是一种典型的三苯基甲烷类染料,广泛用于印染、皮革、酿酒和水产养殖等行业的杀菌和防腐[5 − 6],现有研究表明孔雀石绿可能会诱发人体和动物细胞肿瘤的形成[7];同样,亚甲基蓝(methylene Blue,MB)作为典型的噻嗪类染料,在印染、化工等行业应用广泛,体现出较高的生物毒性[8].
目前,印染废水常用的处理方法有高级氧化法、生化法、吸附法、离子交换法等多种方法[9]. 吸附法具有操作简便、成本低廉等优势,适合处理色度高、成分复杂的印染废水,但存在效率不高等缺点. 生物炭具有疏松多孔结构、比表面积较大、表面含有羧基、羟基、酯和酸酐等官能团的特点[10],因此被开发成各种吸附剂. 然而,原始的生物炭材料对污染物去除效果欠佳,需改性后才能获得更高价值. 常见的改性方法可对生物炭进行金属负载改性或二次热解改性. 金属负载的方式又分为浸渍热解法、液相还原法、生物沥滤法等. 浸渍热解法[11]改性效率低,液相还原法[12]药剂浪费严重,能耗高,容易产生二次污染. 生物沥滤法[13]又称微生物湿法冶金法,能将固相中某些不溶性成分(如重金属、硫及其它金属)有效分离和浸提出来,工艺简单,快捷有效,在新型催化材料合成领域具有较好的应用前景. 对生物炭进行二次热解可进一步增加其孔隙度和活性点位,改善其吸附能力. 因此,将某些金属离子经生物沥滤负载后再进行二次热解,理论上可大幅提高材料的吸附降解性能. 本论文通过生物沥滤法和二次热解制备了铁改性生物炭材料,探讨了对几种染料的吸附能力和去除机理.
-
原始生物炭材料来自于湖北省通山县某生物质能厂的各种混合农林废弃物在1000—1100 ℃下解制气的副产物. 本实验所用氧化亚铁硫杆菌由本实验室从活性污泥中筛选,命名为Acidithiobacillus ferrooxidans FD319B. 负载材料为市售的硫酸亚铁(FeSO4·7H2O)试剂,分析纯.
-
将活性污泥置于9 K培养基中,37 ℃驯化培养一周,待pH降至2.0以下对菌液进行平板划线分离,得到氧化亚铁硫杆菌纯化菌株,菌株活化后接种在1 L的9 K液体培养基中,加入10 g初始生物炭和10 g FeSO4·7H2O,摇床震荡培养一周,将混合固体抽滤、干燥,经水蒸气气氛、1000 ℃下管式炉二次热解,得到铁改性生物炭材料. 原始生物炭命名为BC,单一的生物沥滤后固体命名为MBC,生物沥滤+热解改性生物炭命名为FeBC.
-
X射线衍射分析仪(D8,德国Bruker)和傅里叶红外光谱仪(640-IR,美国瓦里安)测定FeBC的晶体结构和表面官能团种类. 表面积及孔径分析仪(麦克2460,美国康塔)测定FeBC的比表面积和孔径. X射线光电子能谱(ThermoFisher Nexsa,美国赛默飞)测定FeBC反应前后主要元素含量和价态的变化.
-
染料浓度设置为100 mg·L−1,考察BC、MBC及FeBC不同材料(添加量固定为1.25 g·L−1)对孔雀石绿、亚甲基蓝和甲基红染料吸附试验,使用紫外-可见分光光度计测吸光度;染料浓度设置为100 mg·L−1,考察不同FeBC投加量(0.50、0.75、1.00、1.25、1.50 g·L−1)对3种染料的吸附试验;考察FeBC对3种染料在不同初始浓度(50、100、150、200、300 mg·L−1)下的去除影响;考察了不同温度条件(25、35、45、55、65 ℃)对3种染料去除影响.
-
把吸附平衡的FeBC浸泡在一定初始浓度的盐酸溶液中,使其对染料进行解吸,脱附平衡后计算其脱附率. 改变盐酸浓度,探究最适解析液浓度.
-
将FeBC投加到最佳吸附条件下的染料中,进行吸附试验,吸附平衡后进行脱附试验,经多次吸附脱附,观察每次的脱附率及循环次数.
-
图1是在衍射角为10°—80°的区域内MBC和FeBC的XRD图,在2θ=25°和43°的特征衍射峰,对应石墨的(002)和(100)晶面[14],表明C—C结构的存在. 且两种材料的XRD图有明显差异,其中,FeBC与Fe3O4的标准卡片(PDF#88-0866)对比可知,FeBC在2θ 在18.4°、30°、35.5°、44°、54°、57.0°、62.5°、74°处的特征峰与Fe3O4有相同的衍射峰,表明材料经过二次热解后表面成功负载Fe3O4.
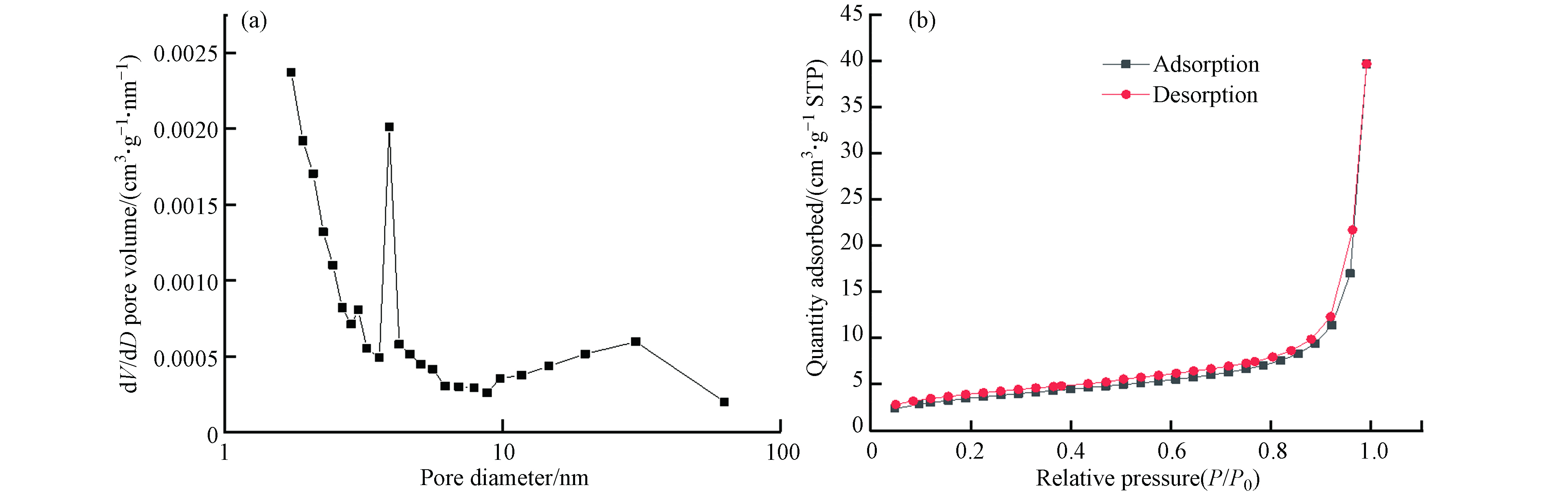
图2(a)是FeBC的N2吸附-脱附曲线,结果符合BDDT(Brunauer-Deming-Demin-Teller)Ⅱ型等温吸附曲线,且属于IUPAC的H4型,同时,由图2(b)也共同说明吸附剂的孔结构是微孔和介孔.
比表面积和官能团结构是决定活性炭吸附能力的重要因素,也分别影响吸附过程中的物理吸附和化学吸附. 由BET结果可知(表1),BC的比表面积最大,有利于物理吸附,同时也有利于Fe元素的负载和改性. 因此,FeBC可以在较强物理吸附的基础上,增加官能团,从而提高材料的吸附性能.
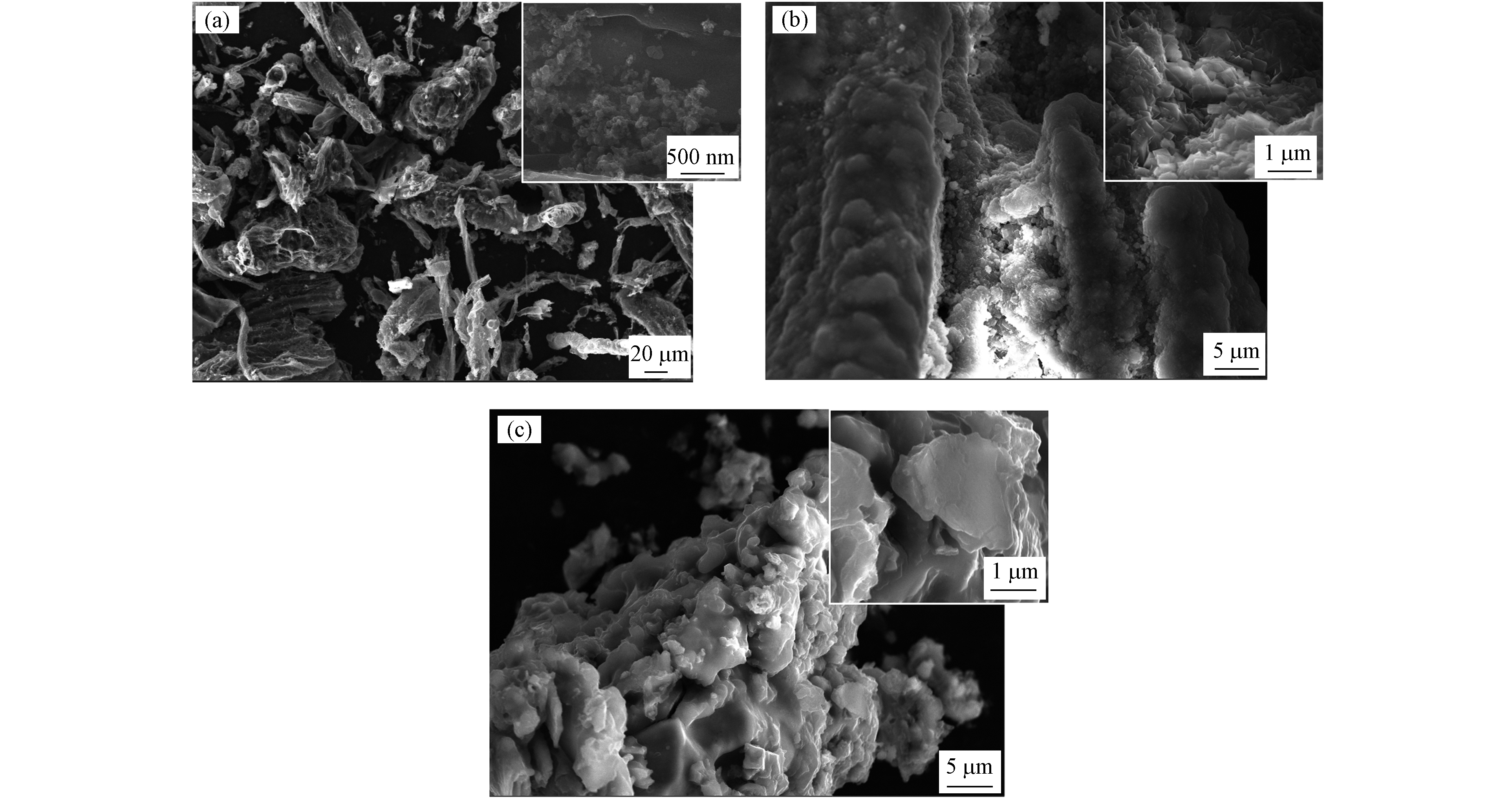
图3为BC、MBC、FeBC的电镜图. 由废弃木材气化得到的BC表面有许多大小不一的孔及不规则的碎片,增大了比表面积的同时也为Fe元素提供了更多的负载位点(图3a). MBC的表面为一层较光泽透明且呈版状材料,符合中间产物黄钾铁矾的特征(图3b). FeBC的表面负载碎片状固体为Fe3O4,证明Fe元素被成功负载,改性成功(图3c).
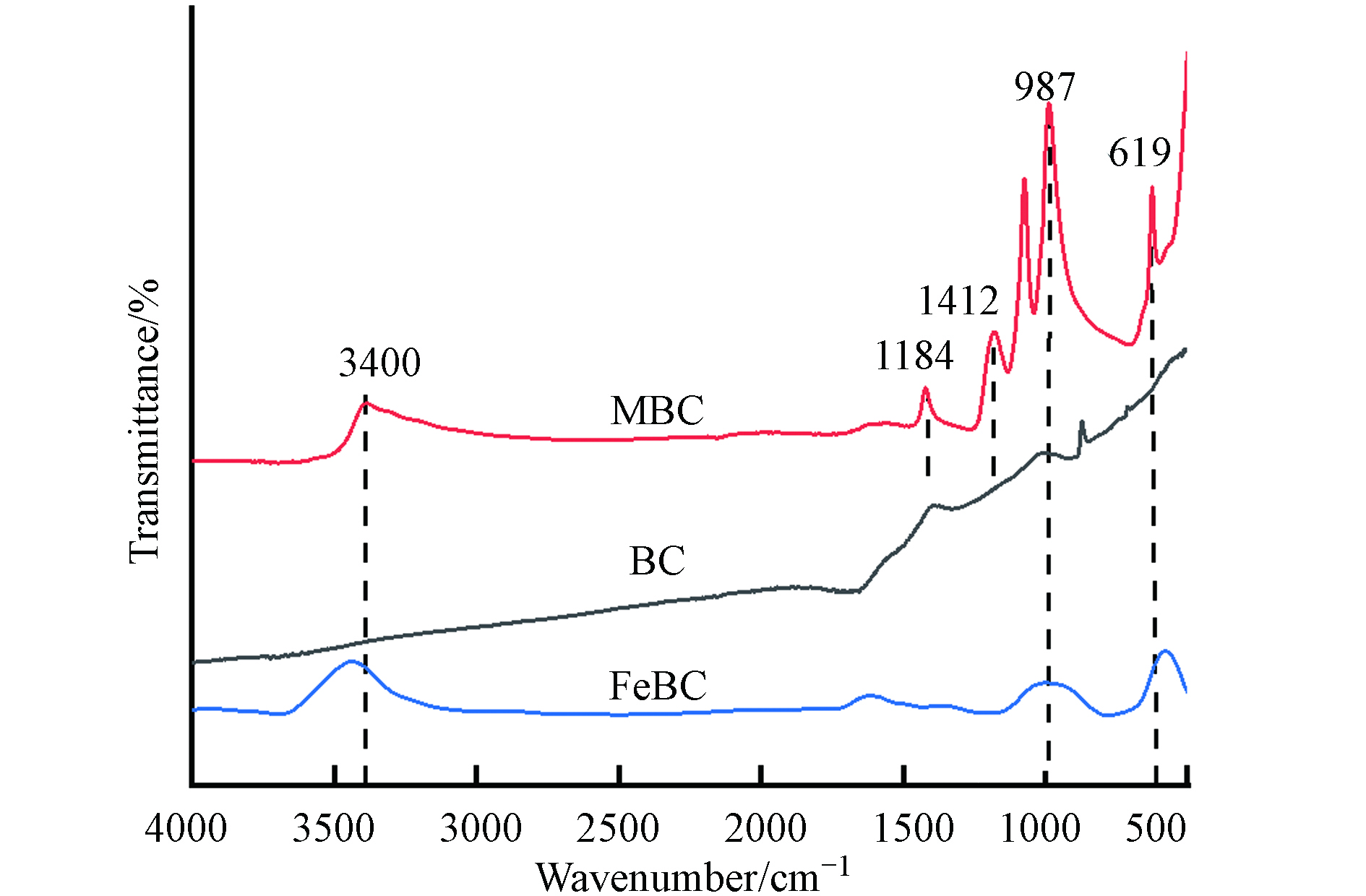
图4是BC、MBC、FeBC的红外光谱图. BC上的官能团较少,1184 cm−1处是—C—O的特征峰. 经生物沥滤后,MBC表面的化学基团大量增加.
图4中,3400 cm−1的特征峰是由于—OH伸缩振动产生,987 cm−1和619 cm−1的峰值对应于Fe—O的延伸,说明生物沥滤过程可以有效的将铁基团引入BC中. 再经二次热解后,部分含氧官能团被高温破坏,FeBC中只有3400、987、619 cm−1处的峰被保留下来,Fe—O的存在也证实了上述表征.
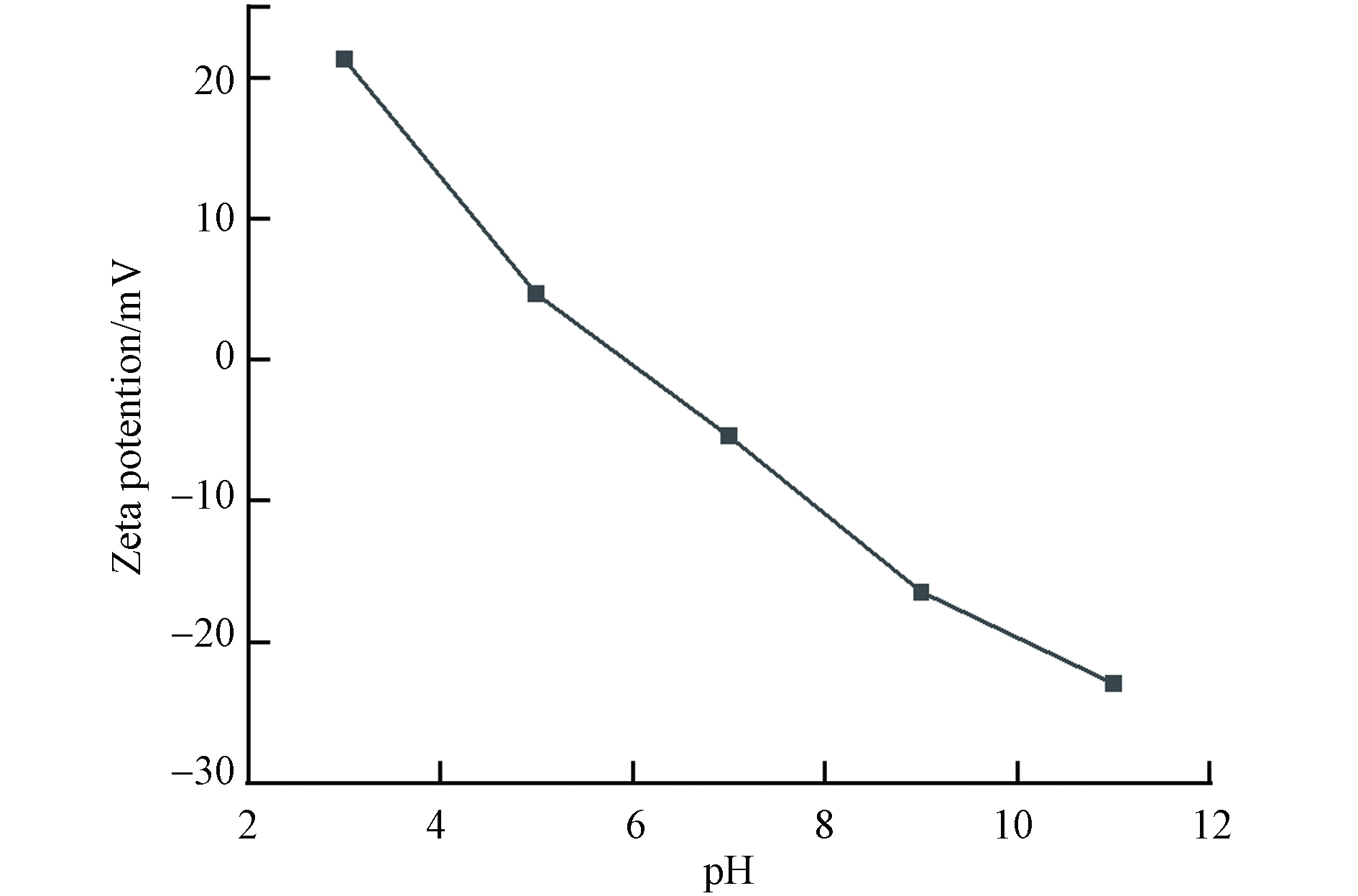
图5为不同pH下FeBC的Zeta电位. 从图5可以看出,FeBC的电离会受溶液酸碱的影响,导致其表面电荷出现不同. 孔雀石绿为阴离子染料,亚甲基蓝和甲基红都是阳离子染料. 在酸性条件下,FeBC表面电荷为正,对阴离子染料吸附较强;在碱性条件下,FeBC表面电荷为正,对阳离子染料吸附较强.
-
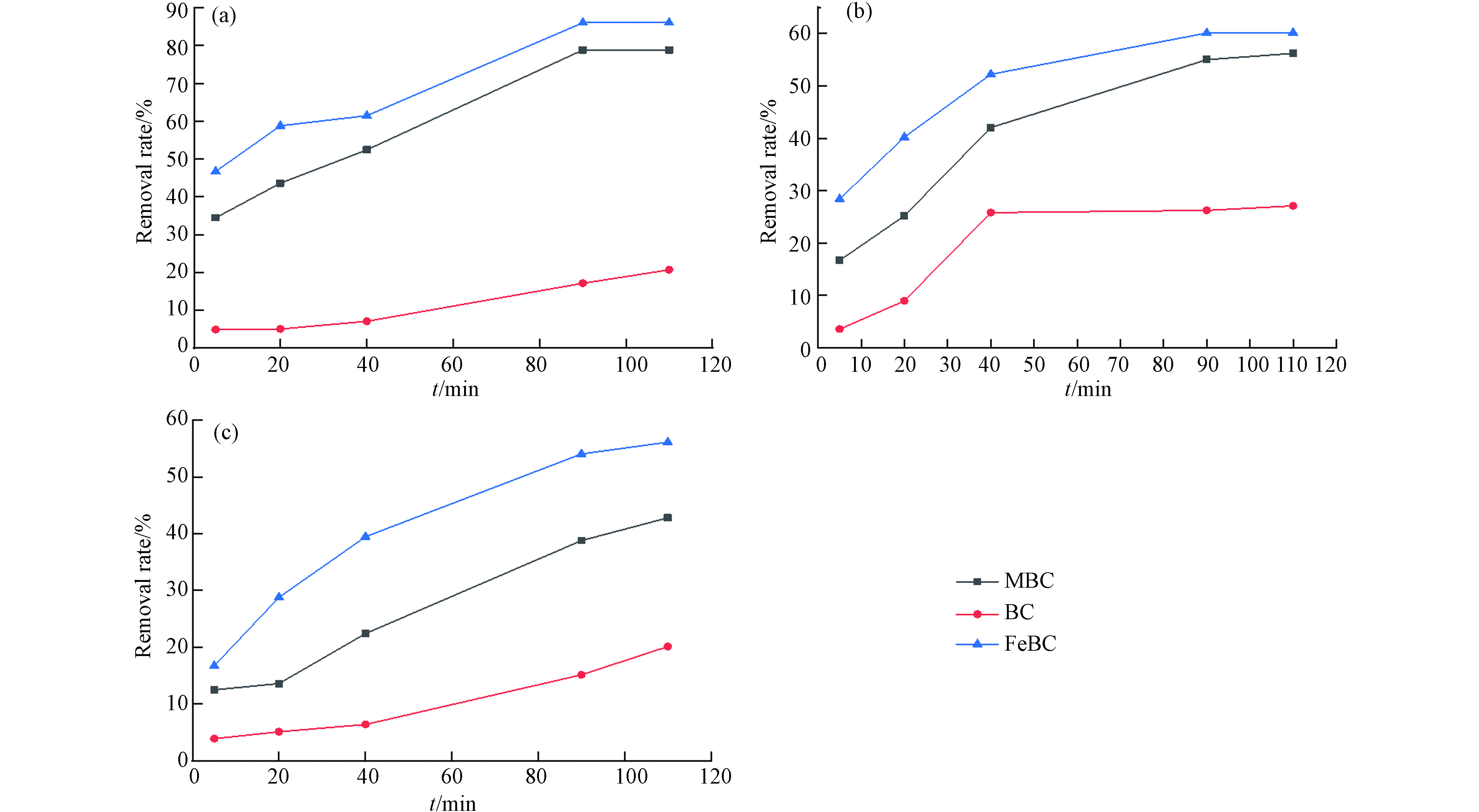
与BC、MBC相比,FeBC对孔雀石绿、甲基红和亚甲基蓝染料的去除率均有极显著提高(P<0.01). 对于3种染料来说,改性后材料吸附量是未改性材料的302.2%、314.7%、409.5%,吸附平衡时的去除效果也更好(见图6). 推测FeBC改性后其孔隙结构、官能团种类更丰富,铁离子热解后的成功负载使FeBC上的金属活性位点与染料苯环上的π电子形成π—π共轭作用,增强了对染料的化学吸附能力[15].
-
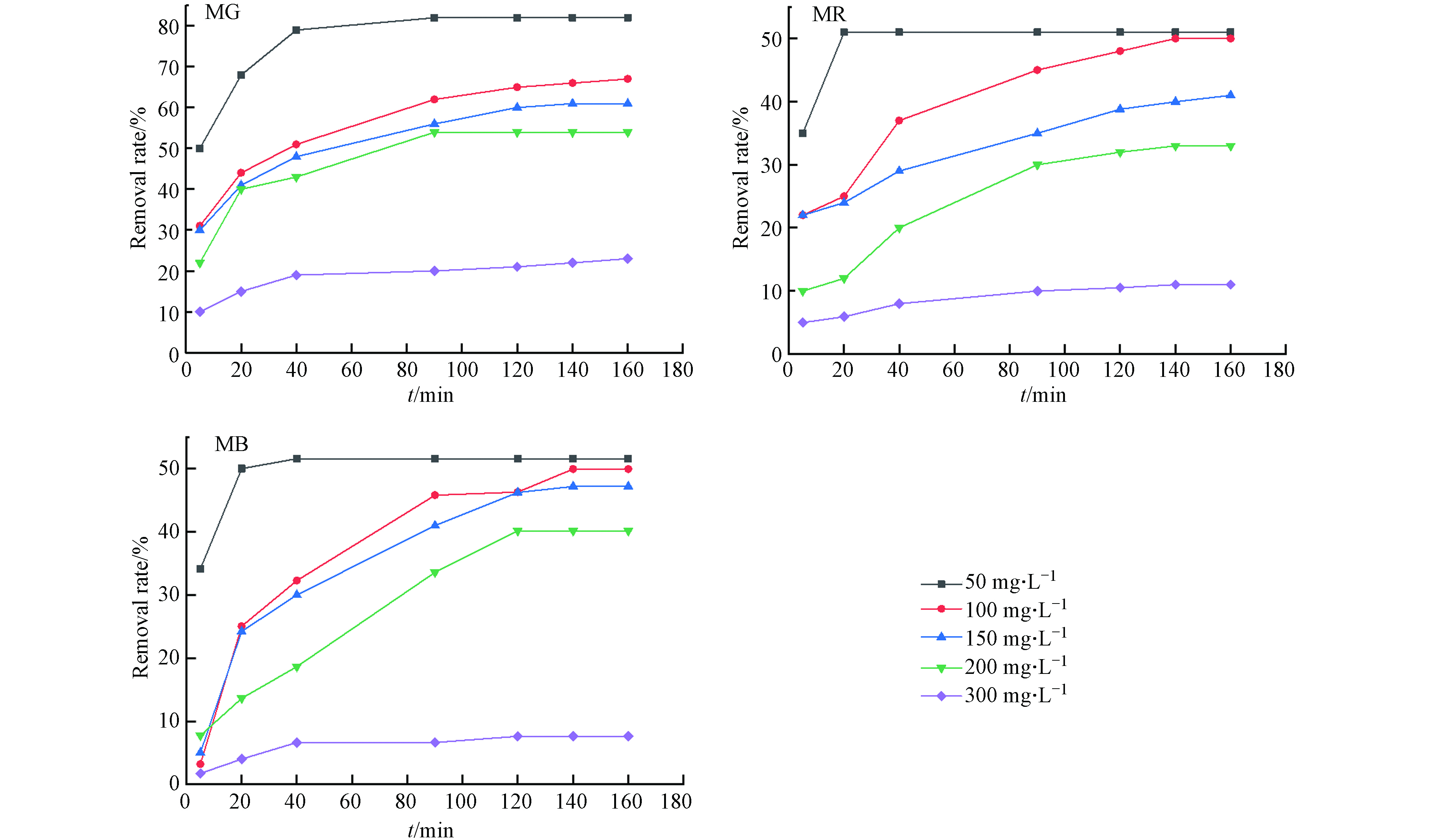
试验表明,孔雀石绿、甲基红和亚甲基蓝染料吸附率随FeBC投加量增多而增加(图7). 推测是因材料的改性后为染料提供了更多的可吸附位点,提高了染料的吸附总量. 值得注意的是,材料投加量过多并不能无限提高对染料的去除率(图7). 本试验体系中FeBC对3种染料最大吸附容量分别为160.0、54.3、49.3 mg·g−1,较原始生物炭(31.4、13.5、14.1 mg·g−1)有大幅提高,因3种染料分子量相差不大,推测其最大吸附容量差异主要与染料的空间构象有关(表2).
-
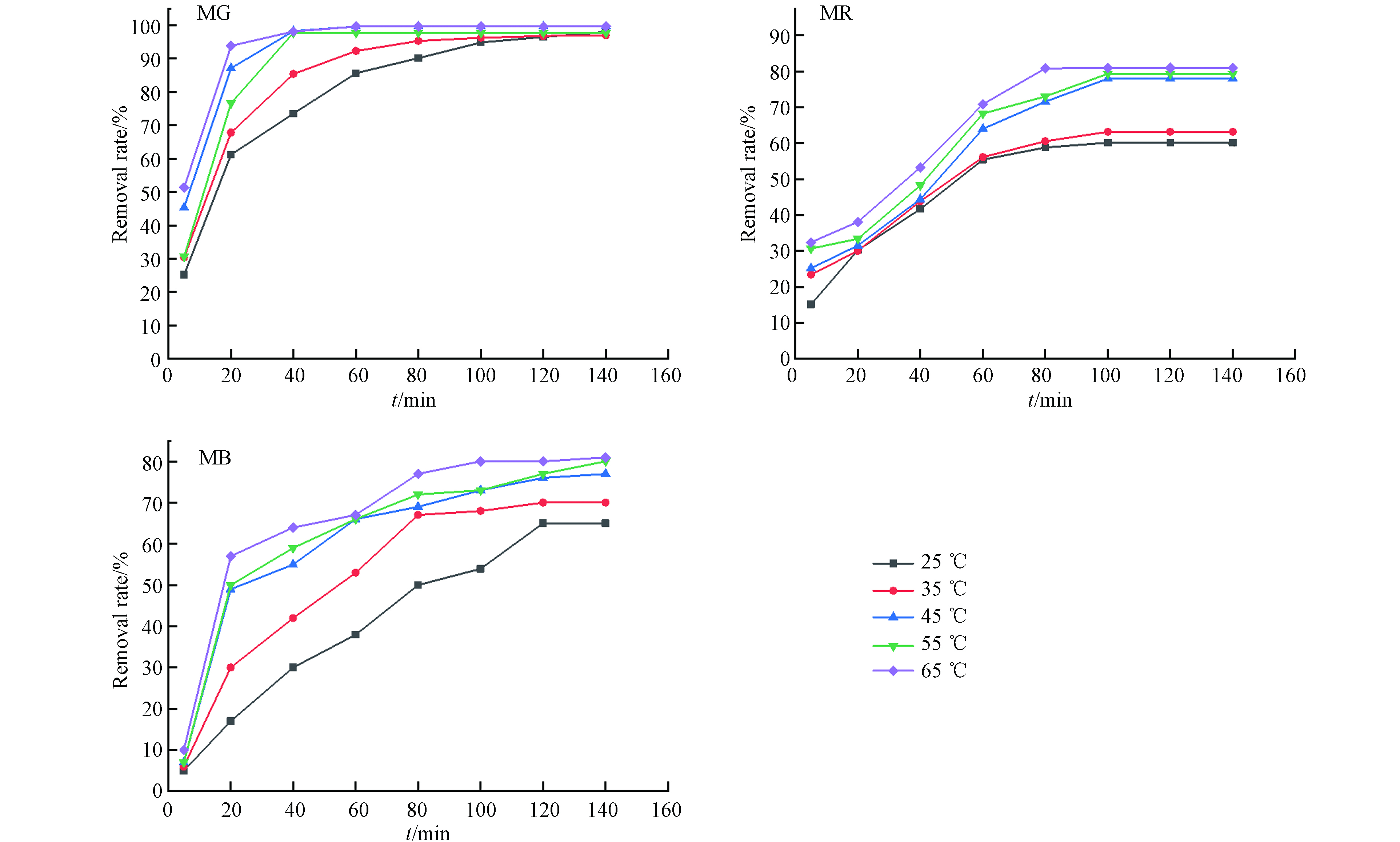
染料的初始浓度影响了其去除效果,如图8所示. 本试验中,染料初始浓度与去除率呈极显著负相关性(P<0.01). FeBC在不同染料浓度的吸附趋势均为先迅速上升,后趋于平缓,推测是因反应初期染料分子迅速占据材料表面活性位点后,溶液中剩余的染料将无法被吸附去除.
-
由图9知,染料的去除率与温度成显著正相关关系(P<0.01). 吸附热力学计算结果显示,孔雀石绿、甲基红和亚甲基蓝的ΔH分别为55.176、47.946、35.791 kJ·mol−1,证实了染料的吸附过程均为吸热反应[16].
-
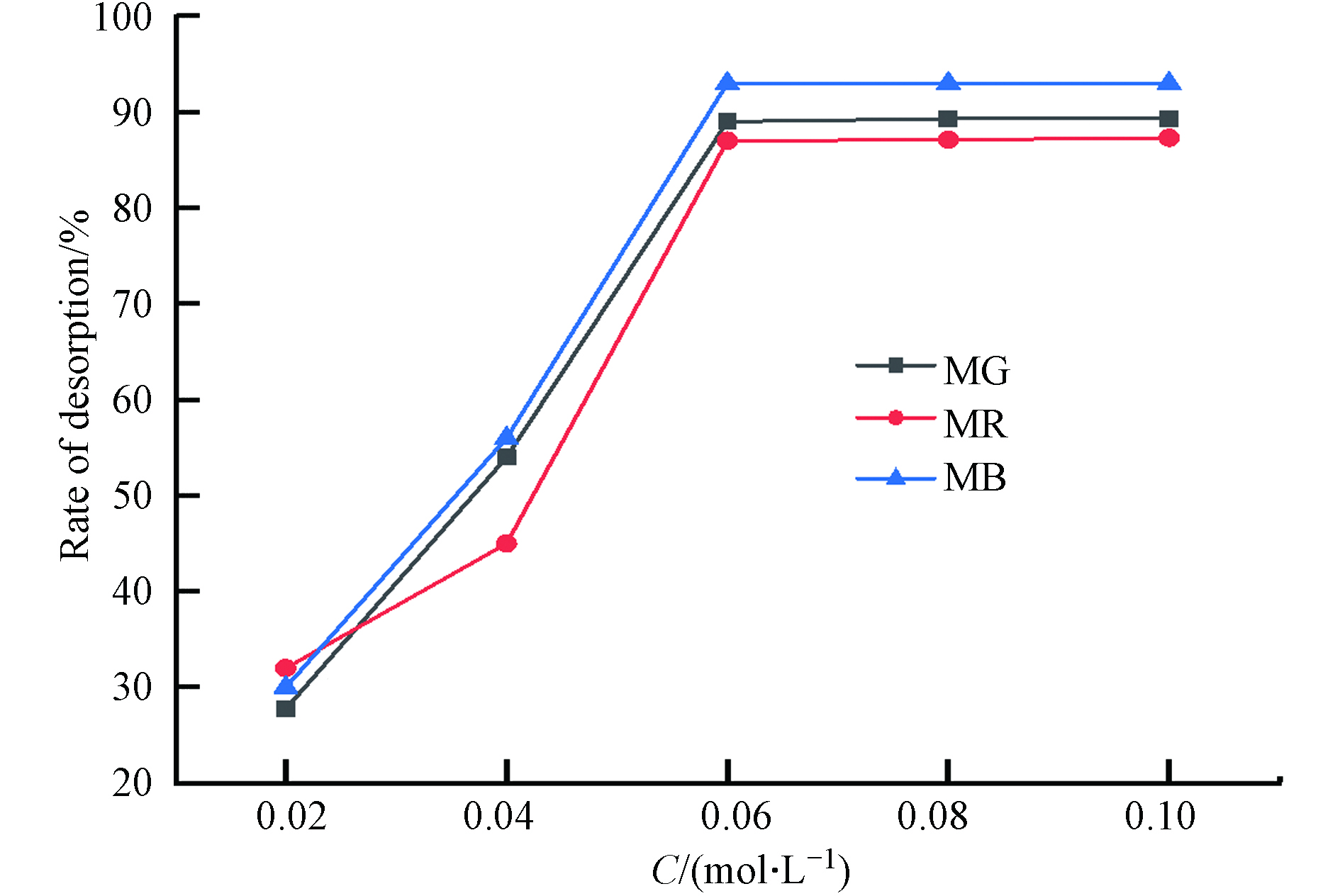
在图10中,3种染料的脱附率都呈现出随着盐酸浓度增大,先增大后不变的趋势. 染料中的阴离子和H+结合,从而实现从材料到溶液的解吸,这也是选择盐酸溶液做脱附剂的原因. 盐酸浓度的增加使H+浓度增加,脱附率提升,在盐酸浓度为0.06 mol·L−1时,孔雀石绿、甲基红和亚甲基蓝染料脱附率都达到了最高,分别为89.77 %,94.32%,87.38 %,此时大量的染料已被脱附,因此盐酸浓度增大脱附率也不变.
-
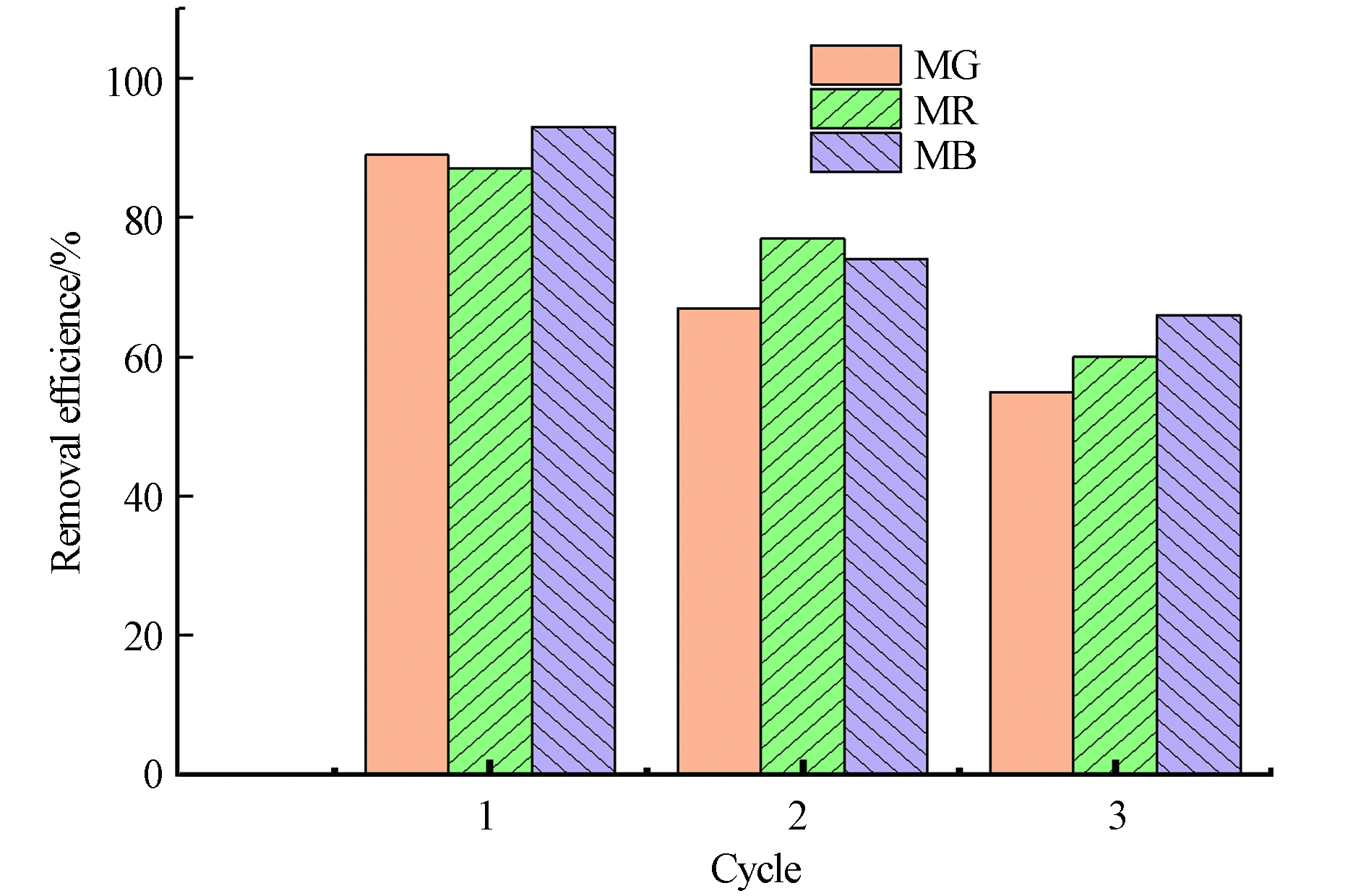
经3次循环后,孔雀石绿、甲基红和亚甲基蓝染料的脱附率分别为54.21%、59.74%和64.72%(图11). FeBC对染料的吸附是物理吸附和化学吸附共存的过程,其中,化学吸附的染料较难洗脱,因此,3次循环后只有较一般的脱附率.
-
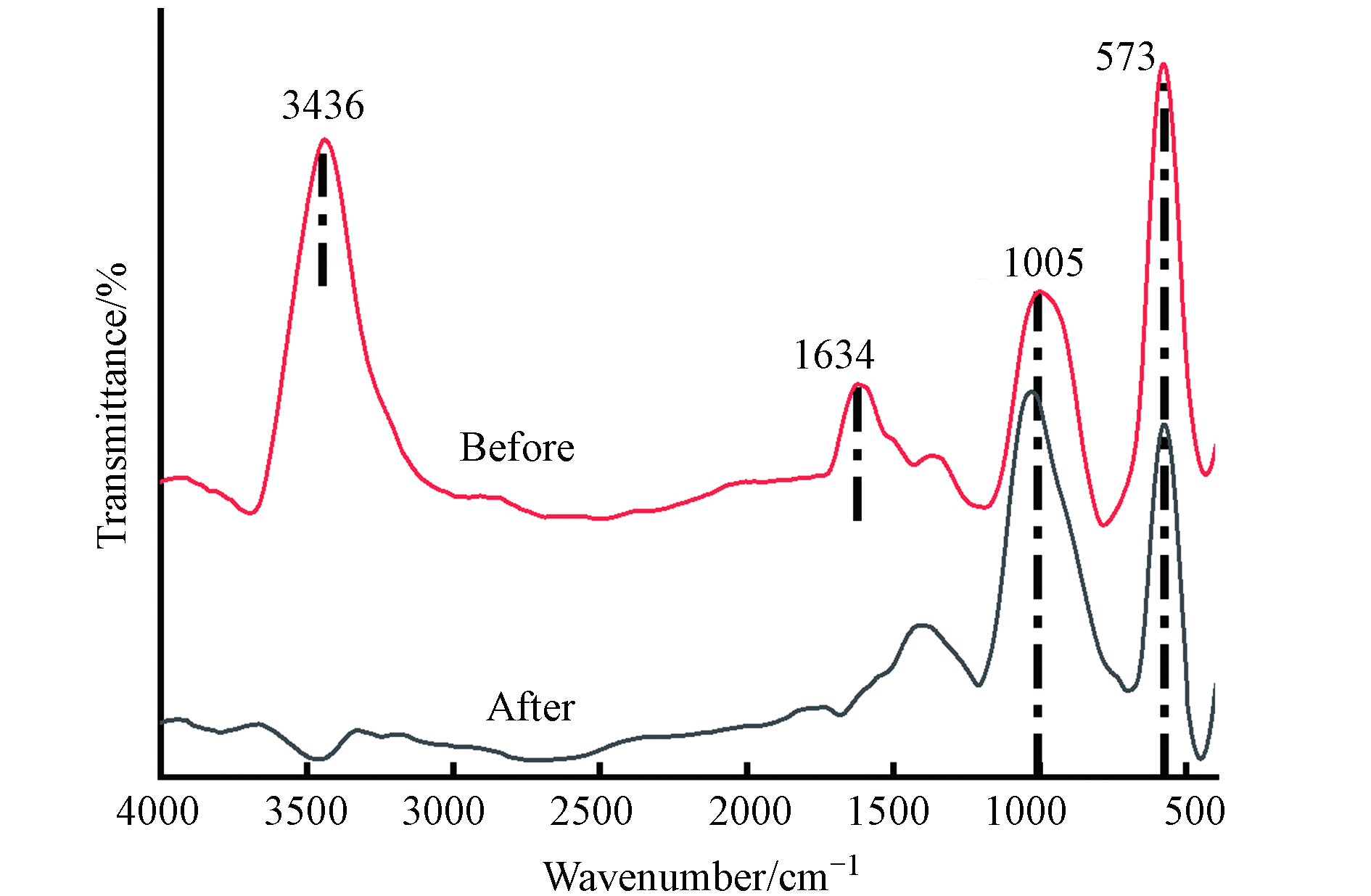
图12是FeBC反应前后的红外光谱图.
图12中,3436 cm−1处是—OH的吸收峰[17],是由于吸附水引起的. 1634 cm−1处为C=C的特征峰[18],其在反应后消失,说明材料中的C=C参与了染料的去除. 1005 cm−1处的C—O特征峰在反应后出现了蓝移,与氢键的形成有关,证明氢键参与了反应. 在573 cm−1处为Fe—O特征峰,反应后仍存在,说明吸附时Fe—O只是部分参与了反应.
-
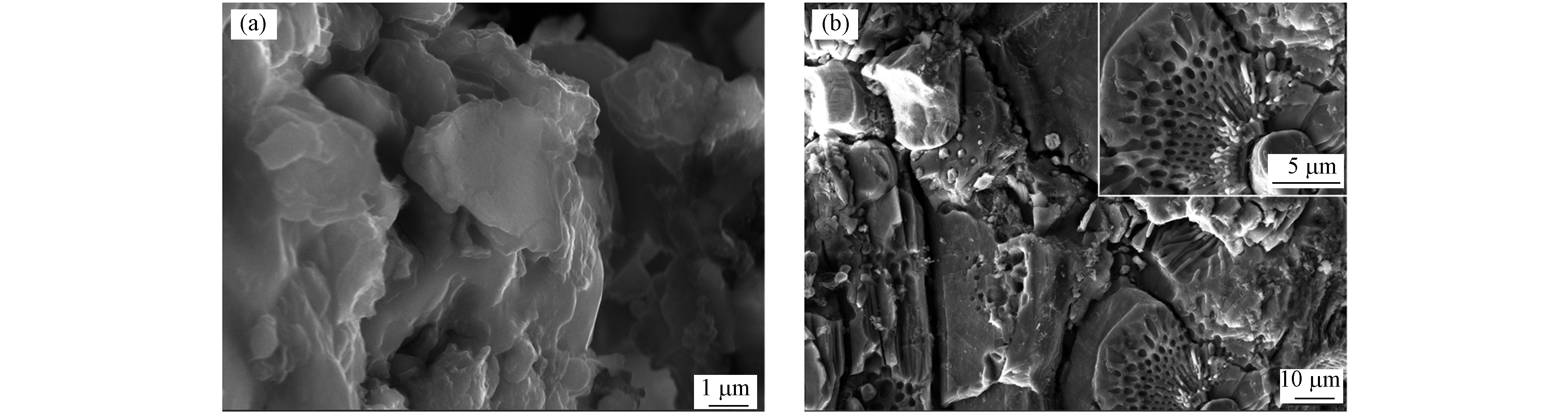
由图13可知,与反应前材料相比(图13a),反应后材料表面大部分是明显孔状结构(图13b),且孔中有小分子物质存在,这是染料分子被吸附到FeBC孔隙中. 但仍存在小部分片状结构,与反应前的Fe3O4结构相同,证明只有部分Fe元素参与染料吸附反应中,与上述的FITR结果相同.
-
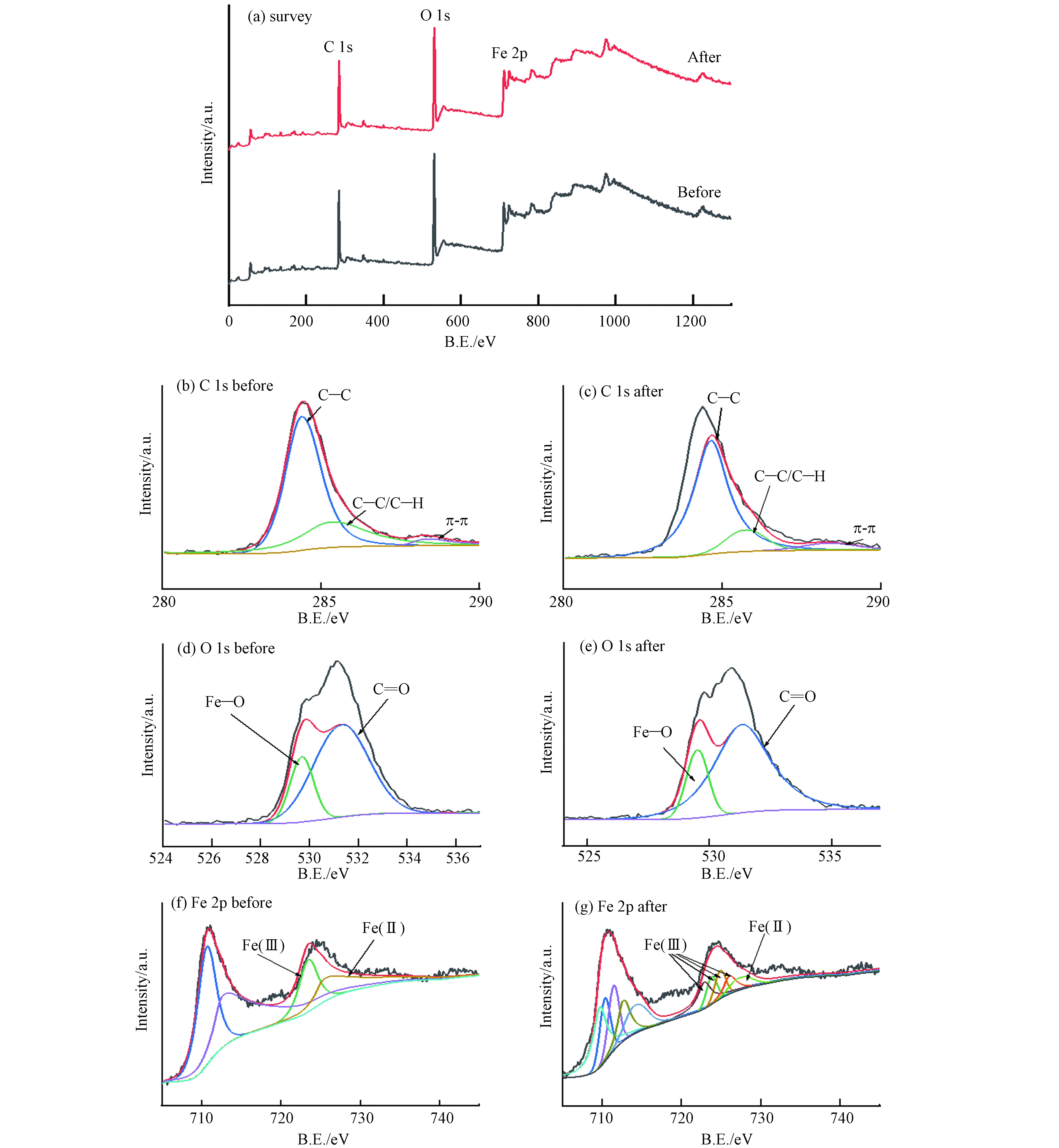
通过FeBC反应前后的XPS图,进一步证明其反应机理. 反应前后的全波扫描图谱(图14)中,无新元素的出现,证明染料的去除是一个吸附过程. 由精细谱知,C元素(图14b、c)主要以C—C、C—H和π—π键存在,对应位置为284.39 eV,285.28 eV和288.31 eV,反应前含量分别为68.55%、27.09%和4.36 %,反应后含量为74.71%、13.29%和12.01 %. 其中,π—π键明显增多,是部分染料苯环上π电子与Fe3O4金属位点形成π—π键的共轭作用. O元素(图14d、e)主要以C=O、Fe—O键存在,对应位置513.3 eV和529.69 eV,反应前含量分别为75.96%和24.04 %,反应后含量分别为89.42%和20.86 %,Fe—O含量的下降证明有部分Fe参与反应,但不是全部,与前面的FITR结果符合. Fe元素(图14f、g)主要以Fe2+、Fe3+存在,分别对应位置710.66 eV和712.63 eV,且反应后Fe3+变少,证明有部分Fe2+参与了π—π键的形成.
-
分别选取几组染料吸附数据进行动一阶动力学(Pseudo-first-order kinetic model)和二阶动力学(Pseudo-second-order kinetic model)拟合,方程的数学表达式分别为:
$ {q}_{\mathrm{e}} $ 为吸附平衡时单位质量的吸附量,(mg·g−1);$ {q}_{t} $ 为t时刻,单位质量的吸附量,(mg·g−1);$ {k}_{1} $ 为一阶动力学方程的吸附速率常数,(min−1);$ {k}_{2} $ 是二阶动力学模型的吸附速率常数(g·mg−1·min−1).经计算,孔雀石绿染料的一阶和二阶动力学模型相关系数R2分别为0.9793和0.9770;甲基红染料一阶和二阶动力学模型相关系数R2分别为0.9721和0.9457. 其他参数见表3. 一阶和二阶模型都可描述吸附的初始阶段,但两种染料与一阶动力学模型的拟合度更高,表明FeBC对两种染料的去除以物理吸附为主,同时存在部分化学吸附. 本文进一步推测出该材料对几种染料的吸附机制可能以分子间范德华力吸附主要,部分染料苯环π电子与Fe3O4金属位点形成π—π共轭作用产生化学吸附,该吸附特征增加了对染料的吸附稳定性.
-
本试验选取了孔雀石绿和甲基红进行等温吸附模型拟合,Langmuir吸附模型和Freundlich吸附模型方程的数学表达分别为:
式中,KL为Langmuir常数,(L·mg−1);Ce为吸附平衡时吸附质的浓度,(mg·L−1);qe为吸附平衡时吸附剂的吸附量,(mg·g−1);qm为吸附剂的最大吸附量,(mg·g−1);1/n为不均匀性因数;KF为Freundlich常数,(mg·g−1)·(L·mg−1)1/n.
在Langmuir模型中,孔雀石绿染料的拟合方程为
$ \dfrac{{C}_{\mathrm{e}}}{{q}_{\mathrm{e}}} $ =0.00452$ C\mathrm{e} $ +1.04387,$ {q}_{\mathrm{m}} $ 为221.239 mg·g−1,R2=0.9962; 在Freundlich模型中,拟合方程为$ \mathrm{ln}{q}_{\mathrm{e}} $ =0.76675$ \mathrm{ln}{C}_{\mathrm{e}} $ +0.63688,n=1.304,R2=0.9881. 其他参数见表4. 本试验中Langmuir模型能更好的拟合FeBC对染料的吸附过程. 同时,Langmuir模型中KL值的正负可判断FeBC是否有利于吸附反应的进行,若KL>0,有利于吸附反应进行,若KL<0,则不利于吸附. 从拟合结果(KL=0.004)可进一步明确几种染料的吸附特征:FeBC对染料的吸附主要发生在单分子层特定位点吸附,一旦吸附剂表面吸附位点被占据后,那么该吸附位点就不会再发生将无法吸附其他染料分子. -
本文通过生物沥滤和二次热解法制备了一种新型铁改性生物炭材料,开展了对3种典型染料的去除效果试验. 与改性前相比,该材料极显著提高了对3种染料的吸附性能(P<0.01),反应机理是以孔径吸附为主,同时因Fe元素的负载改性,也存在氢键、π—π键的作用,且少量的化学吸附显著提升了染料的吸附稳定性.
铁改性生物炭对几种染料的吸附机理研究
Study on the adsorption mechanism of several dyes by iron-modified biochar
-
摘要: 本文以生物炭为原料,经氧化亚铁硫杆菌生物沥滤,将离子态的铁负载到生物炭上再进行二次热解,制备出铁改性生物炭材料(FeBC). 开展该材料对三苯基甲烷、偶氮和噻嗪类染料等典型染料进行吸附试验,考察材料投加量、染料浓度以及温度因素对染料去除的影响,利用XRD、FT-IR、XPS和BET等分析方法对FeBC进行表征并做吸附动力学模型和等温吸附模型拟合. 结果表明,FeBC负载了Fe3O4后表面官能团和孔隙结构更加丰富. FeBC对几种染料的吸附结果符合一阶动力学方程和Langmuir等温吸附模型,证明FeBC对染料的去除是以物理吸附为主的单分子层吸附过程;染料去除率与初始浓度呈极显著负相关(P<0.01),与温度呈极显著正相关(P<0.01). 本文初步揭示了该材料对几种染料的去除机理,是以孔隙吸附、氢键、π—π电子共轭吸附以及静电吸附等方式去除,改性后对不同染料吸附量提高达302.2%—409.5%. 该研究为新型炭改性材料在染料废水中的处理提供了新思路.Abstract: In this paper, iron-modified biochar material (FeBC) was prepared by biolestrating by thiobacter ferrooxidans and loading ionic iron onto biochar for secondary pyrolysis. The adsorption tests on several typical dyes, such as triphenylmethane, azo and thiazine dyes, were carried out to investigate the effects of material dosage, dye concentration and temperature on dye removal. FeBC was characterized by XRD, FT-IR, XPS and BET, and the adsorption kinetics model and isothermal adsorption model were fitted. The results show that FeBC loaded with Fe3O4 has more functional groups and pore structures. The adsorption results of FeBC on several dyes conform to the first-order kinetic equation and Langmuir isothermal adsorption model, which proves that the removal of dyes by FeBC is a single-molecule layer adsorption process based on physical adsorption. The dye removal rate was negatively correlated with initial concentration (P<0.01), and positively correlated with temperature (P<0.01). In this paper, the removal mechanism of several dyes by the material was preliminically revealed, including pore adsorption, hydrogen bond, π—π electron conjugated adsorption and electrostatic adsorption. After modification, the adsorption capacity of different dyes was increased to 302.2% — 409.5%. This study provides a new idea for the treatment of new carbon modified materials in dye wastewater.
-
Key words:
- iron-modified biochar /
- dye wastewater /
- adsorption.
-

-
表 1 BC、MBC和FeBC的BET结果
Table 1. BET results of BC, MBC and FeBC
比表面积 /(m2·g−1)
Surface area平均孔径/nm
Average pore diameter总孔容 /(cm3·g−1)
Total pore volumeBC 52.7430 4.8348 0.063751 MBC 45.6641 5.4489 0.053971 FeBC 12.7462 6.2280 0.019846 表 2 染料的性质
Table 2. Properties of dyes
名称
Name分子式
Molecular formula分子结构
Molecular structures相对分子质量
Molecular weight孔雀石绿 Cu2(OH)2CO3 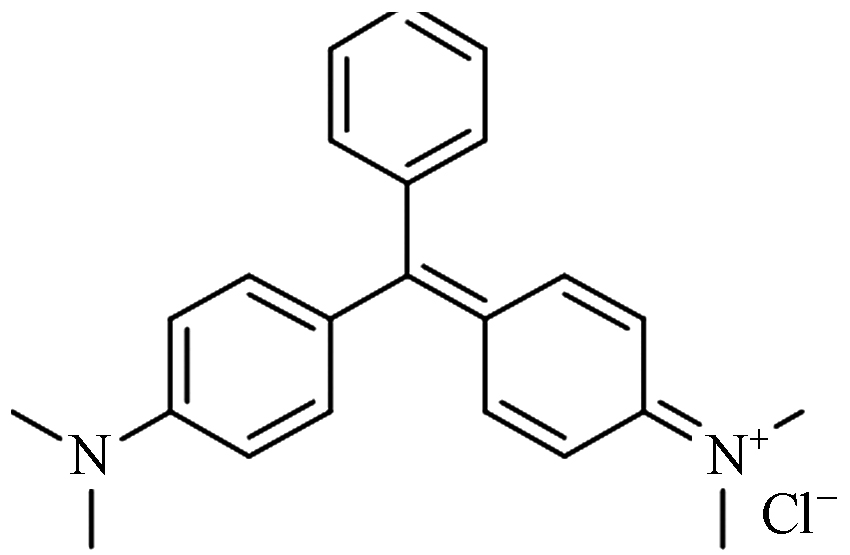
239 甲基红 C15H15N3O2 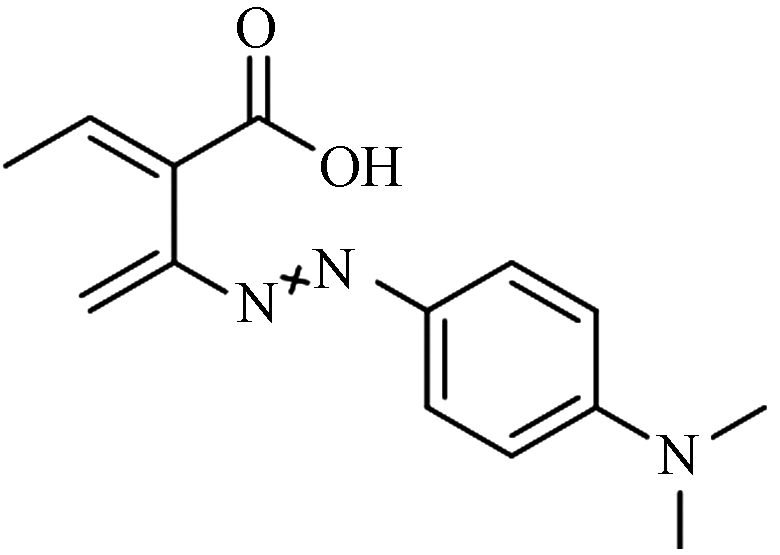
269 亚甲基蓝 C16H18N3ClS 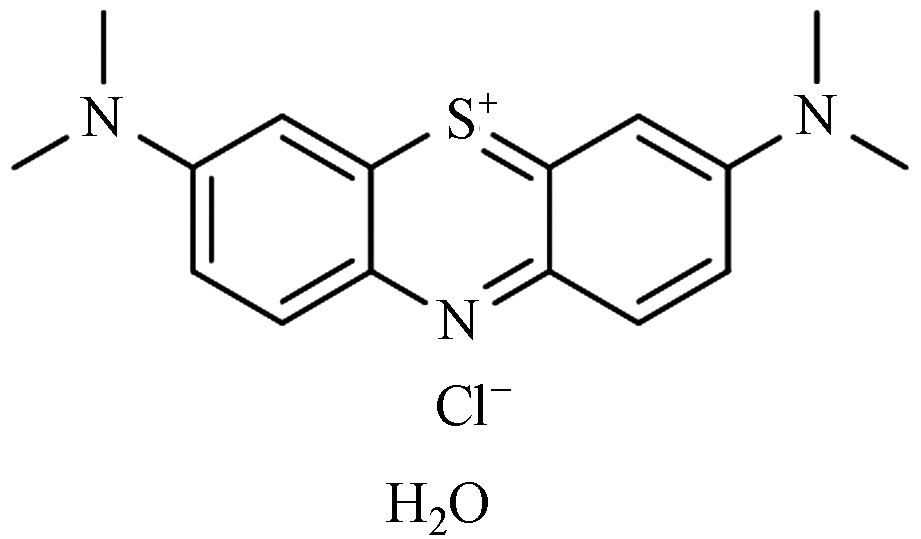
319.5 表 3 三种染料的准一阶动力学模型、准二阶动力学模型拟合参数
Table 3. The fitting parameters of quasi-first-order kinetic model and quasi-second-order kinetic model of three dyes were obtained
染料名称
Dye of name准一阶动力学模型
Pseudo-first-order kinetic model准二阶动力学模型
Pseudo-second-order kinetic modelk1/h−1 qe/(mg·g−1) R2 K2/(g·mg−1·min−1) qe/(mg·g−1) R2 MG 7.68 298.91 0.9793 9.6293 302.11 0.9770 MR 16.40 80.77 0.9721 74.0740 87.71 0.9457 MB 12.97 298.71 0.9955 2.6761 313.48 0.9686 表 4 三种染料吸附等温线Freundlich模型和Langmuir模型拟合参数
Table 4. Fitting parameters of the Freundlich model and Langmuir model for three kinds of dye adsorption isotherms
染料
Dye of nameFreundlich模型 Langmuir模型 KF/(mg·g−1)·(L·mg−1)1/n 1/n R2 qm/(mg·g−1) kL/(L·mg−1) R2 MG 1.8776 0.7668 0.9881 221.239 0.004 0.9962 MR 78.7195 0.1332 0.9772 76.394 0.014 0.9988 MB 6.8895 0.0026 0.9555 6.3291 0.102 0.9861 -
[1] SHAMSIZADEH A, GHAEDI M, ANSARI A, et al. Tin oxide nanoparticle loaded on activated carbon as new adsorbent for efficient removal of malachite green-oxalate: Non-linear kinetics and isotherm study[J]. Journal of Molecular Liquids, 2014, 195: 212-218. doi: 10.1016/j.molliq.2014.02.035 [2] 邓耀棠. 工业废水处理现状及方法探讨[J]. 城市建筑, 2017(9): 406. DENG Y T. Discussion on the present situation and methods of industrial wastewater treatment[J]. Urbanism and Architecture, 2017(9): 406in Chinese).
[3] IMRAN M, SHAHAROONA B, CROWLEY D E, et al. The stability of textile azo dyes in soil and their impact on microbial phospholipid fatty acid profiles[J]. Ecotoxicology and Environmental Safety, 2015, 120: 163-168. doi: 10.1016/j.ecoenv.2015.06.004 [4] 赵玉英, 王琳琳, 宋娟娟, 等. 负载型广枣多糖镧降解甲基红[J]. 西北民族大学学报(自然科学版), 2021, 42(3): 14-17. ZHAO Y Y, WANG L L, SONG J J, et al. Degradation of methyl red by supported fructus choerospondiatis polysaccharide lanthanum[J]. Journal of Northwest Minzu University (Natural Science), 2021, 42(3): 14-17 (in Chinese).
[5] CHENG W, WANG S G, LU L, et al. Removal of malachite green (MG) from aqueous solutions by native and heat-treated anaerobic granular sludge[J]. Biochemical Engineering Journal, 2008, 39(3): 538-546. doi: 10.1016/j.bej.2007.10.016 [6] ZHANG J, LI Y, ZHANG C L, et al. Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root[J]. Journal of Hazardous Materials, 2008, 150(3): 774-782. doi: 10.1016/j.jhazmat.2007.05.036 [7] BHAVANI R, SIVASAMY A. Sonocatalytic degradation of malachite green oxalate by a semiconductor metal oxide nanocatalyst[J]. Ecotoxicology and Environmental Safety, 2016, 134: 403-411. doi: 10.1016/j.ecoenv.2015.10.029 [8] 李文兵, 黄犇, 王光华. 核桃壳炭载铈材料对亚甲基蓝的吸附性能研究[J]. 水处理技术, 2022, 48(12): 48-52, 58. LI W B, HUANG B, WANG G H. Study on adsorption properties of cerium-loaded walnut shell carbon for methylene blue[J]. Technology of Water Treatment, 2022, 48(12): 48-52, 58 (in Chinese).
[9] 周书葵, 曾光明, 刘迎九, 等. 改性羧甲基纤维素对铀吸附机理的试验研究[J]. 中国环境科学, 2011, 31(9): 1466-1471. ZHOU S K, ZENG G M, LIU Y J, et al. Equilibrium, kinetic and thermodynamic study of the adsorption of uranium(Ⅵ) onto modified CMC[J]. China Environmental Science, 2011, 31(9): 1466-1471 (in Chinese).
[10] 肖琴, 刘有才, 曹占芳, 等. 生物炭吸附废水中重金属离子的研究进展[J]. 环境科技, 2019, 32(1): 68-73. doi: 10.3969/j.issn.1674-4829.2019.01.014 XIAO Q, LIU Y C, CAO Z F, et al. Research progress on the absorption of heavy metals from wastewater by biochar[J]. Environmental Science and Technology, 2019, 32(1): 68-73 (in Chinese). doi: 10.3969/j.issn.1674-4829.2019.01.014
[11] NGARMKAM W, SIRISATHITKUL C, PHALAKORNKULE C. Magnetic composite prepared from palm shell-based carbon and application for recovery of residual oil from POME[J]. Journal of Environmental Management, 2011, 92(3): 472-479. doi: 10.1016/j.jenvman.2010.08.031 [12] DEVI P, SAROHA A K. Synthesis of the magnetic biochar composites for use as an adsorbent for the removal of pentachlorophenol from the effluent[J]. Bioresource Technology, 2014, 169: 525-531. doi: 10.1016/j.biortech.2014.07.062 [13] 周顺桂, 周立祥, 黄焕忠. 生物淋滤技术在去除污泥中重金属的应用[J]. 生态学报, 2002, 22(1): 125-133. ZHOU S G, ZHOU L X, HUANG H Z . Removal of heavy metals from sewage sludge by bioleaching[J]. Acta Ecologica Sinica, 2002, 22(1): 125-133 (in Chinese).
[14] 吴思雅, 江敏, 吴昊, 等. 磁性双壁碳纳米管(m-DWCNTs)对水中全氟辛烷磺酸(PFOS)的吸附[J]. 环境化学, 2023, 42(1): 277-287. doi: 10.7524/j.issn.0254-6108.2021090308 WU S Y, JIANG M, WU H, et al. Adsorption of perfluorooctane sulfonic acid (PFOS) in water by magnetic double-walled carbon nanotubes (m-DWCNTs)[J]. Environmental Chemistry, 2023, 42(1): 277-287 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021090308
[15] LE V T, DAO M U, LE H S, et al. Adsorption of Ni(II) ions by magnetic activated carbon/chitosan beads prepared from spent coffee grounds, shrimp shells and green tea extract[J]. Environmental Technology, 2020, 41(21): 2817-2832. doi: 10.1080/09593330.2019.1584250 [16] HOSLETT J, GHAZAL H, KATSOU E, et al. The removal of tetracycline from water using biochar produced from agricultural discarded material[J]. Science of the Total Environment, 2021, 751: 141755. doi: 10.1016/j.scitotenv.2020.141755 [17] AYED L, MAHDHI A, CHEREF A, et al. Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimobilis: Biotoxicity and metabolites characterization[J]. Desalination, 2011, 274(1/2/3): 272-277. [18] 李艳春, 张鹏会, 张强, 等. 4种生物炭对阳离子染料吸附性能[J]. 环境科学与技术, 2020, 43(7): 101-110. LI Y C, ZHANG P H, ZHANG Q, et al. Adsorption of cationic dye in aqueous solution by four biochars[J]. Environmental Science & Technology, 2020, 43(7): 101-110 (in Chinese).
-




 下载:
下载: