全文HTML
1 材料方法
1.1 实验材料
1.2 实验仪器
1.3 CO2吸附材料的制备
1.3.1 类水滑石(LDH)的制备
1.3.2 碱金属硝酸盐负载LDH基材料的制备
1.4 材料CO2吸附性能测试
1.4.1 吸附CO2性能(TGA)测试
1.4.2 CO2吸附-脱附性能测试
1.5 材料的表征
1.5.1 X射线衍射(XRD)分析
1.5.2 傅里叶红外光谱(FT-IR)分析
2 结果与分析
2.1 材料CO2吸附性能测试
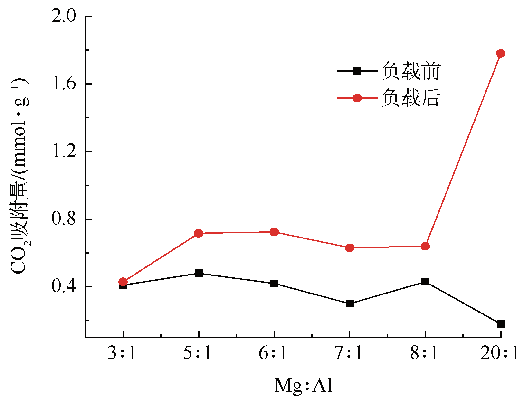
2.1.1 镁铝比对CO2吸附性能的影响
Fig. 1 Effect of different Mg/Al molar ratio on the CO2 capture capacity (calcination temperature 500 ℃ and sorption temperature 240 ℃)
Fig. 1 Effect of different Mg/Al molar ratio on the CO2 capture capacity (calcination temperature 500 ℃ and sorption temperature 240 ℃)
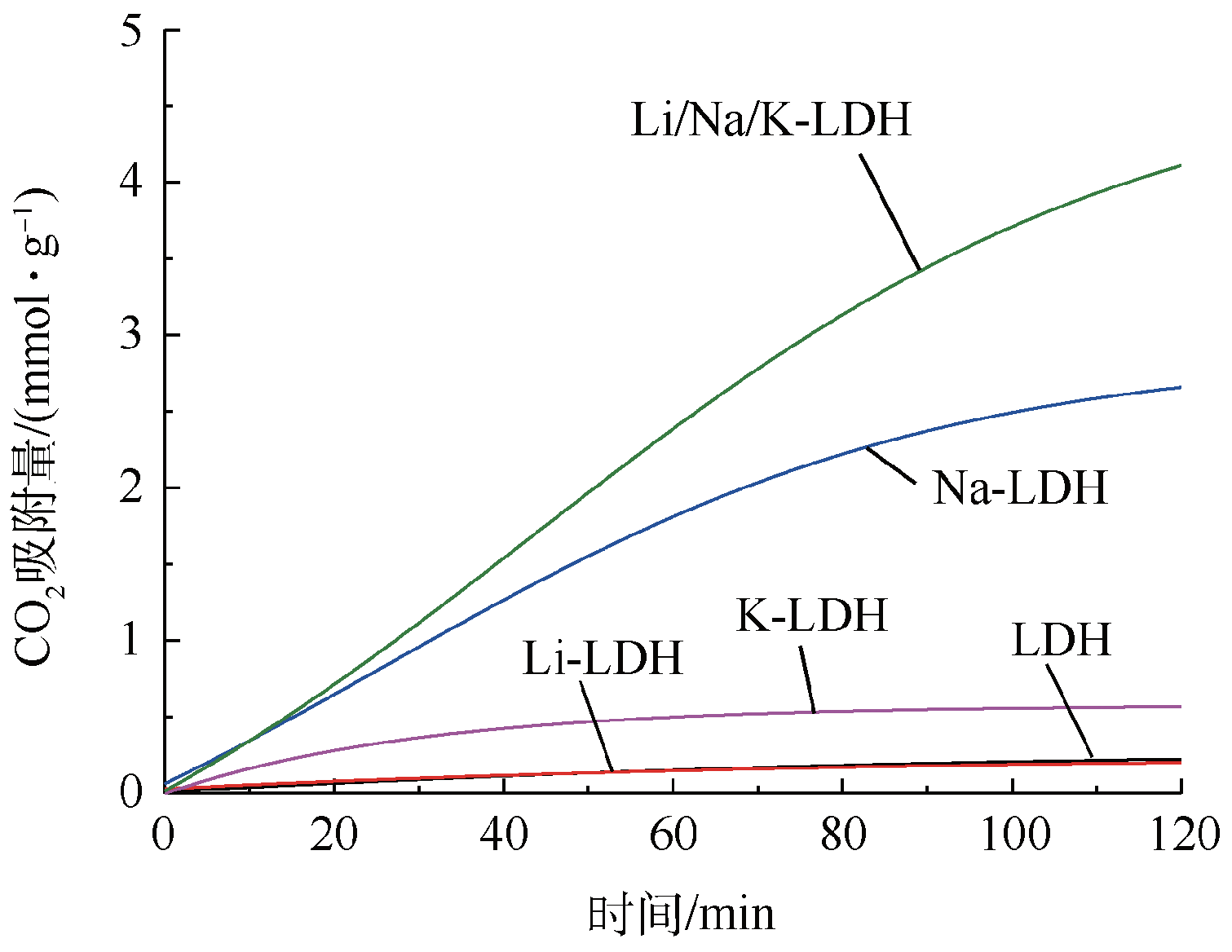
2.1.2 碱金属硝酸盐负载种类对CO2吸附性能的影响
Fig. 2 CO2 capture capacity of Mg20Al-CO3 LDH with different alkali nitrates species loading(calcination temperature 500 ℃ and sorption temperature 240 ℃)
Fig. 2 CO2 capture capacity of Mg20Al-CO3 LDH with different alkali nitrates species loading(calcination temperature 500 ℃ and sorption temperature 240 ℃)
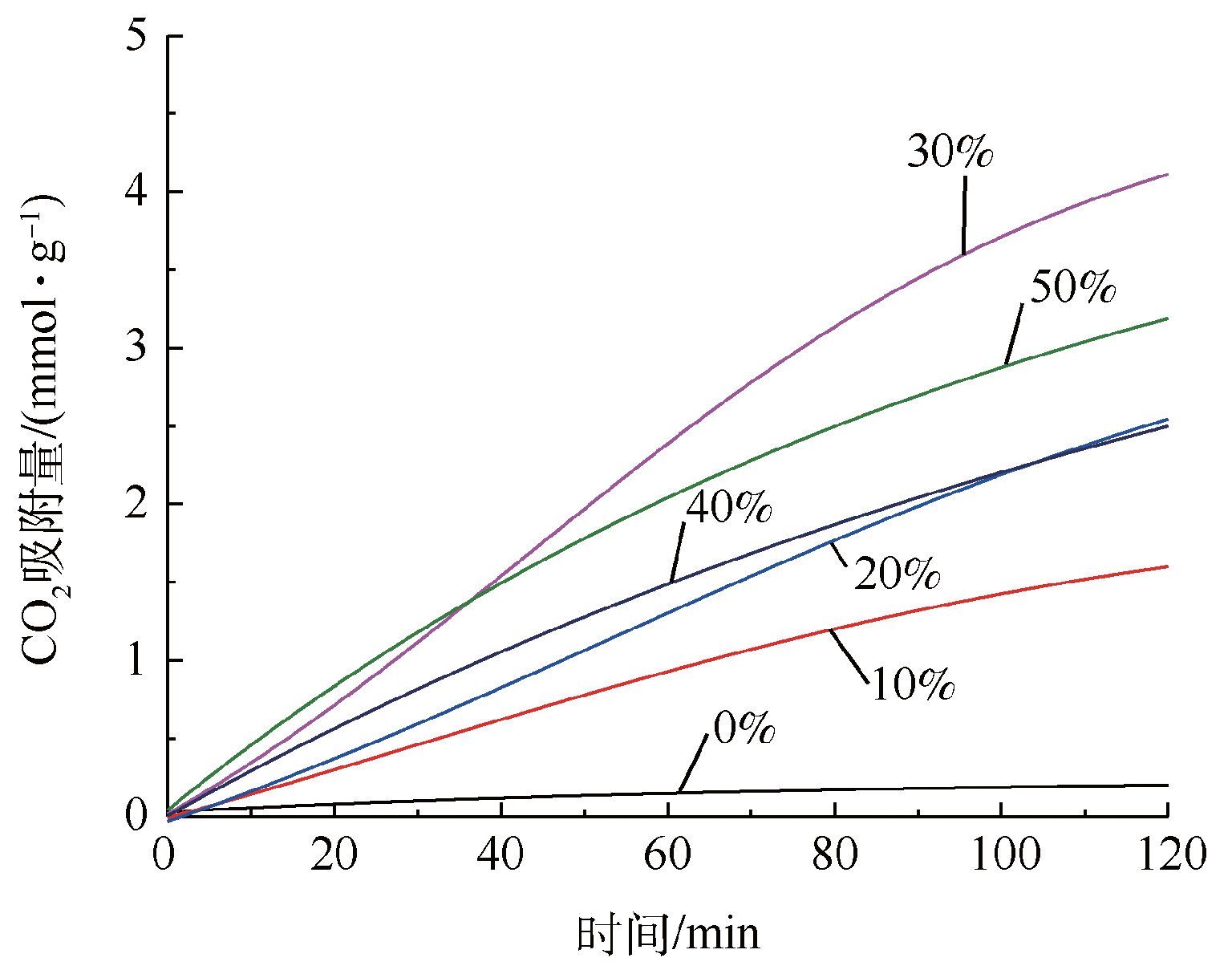
2.1.3 碱金属硝酸盐负载量对CO2吸附性能的影响
Fig. 3 CO2 capture capacity of Mg20Al-CO3 LDH with different alkali metal nitrates loading amount(calcination temperature 500 ℃ and sorption temperature 240 ℃)
Fig. 3 CO2 capture capacity of Mg20Al-CO3 LDH with different alkali metal nitrates loading amount(calcination temperature 500 ℃ and sorption temperature 240 ℃)
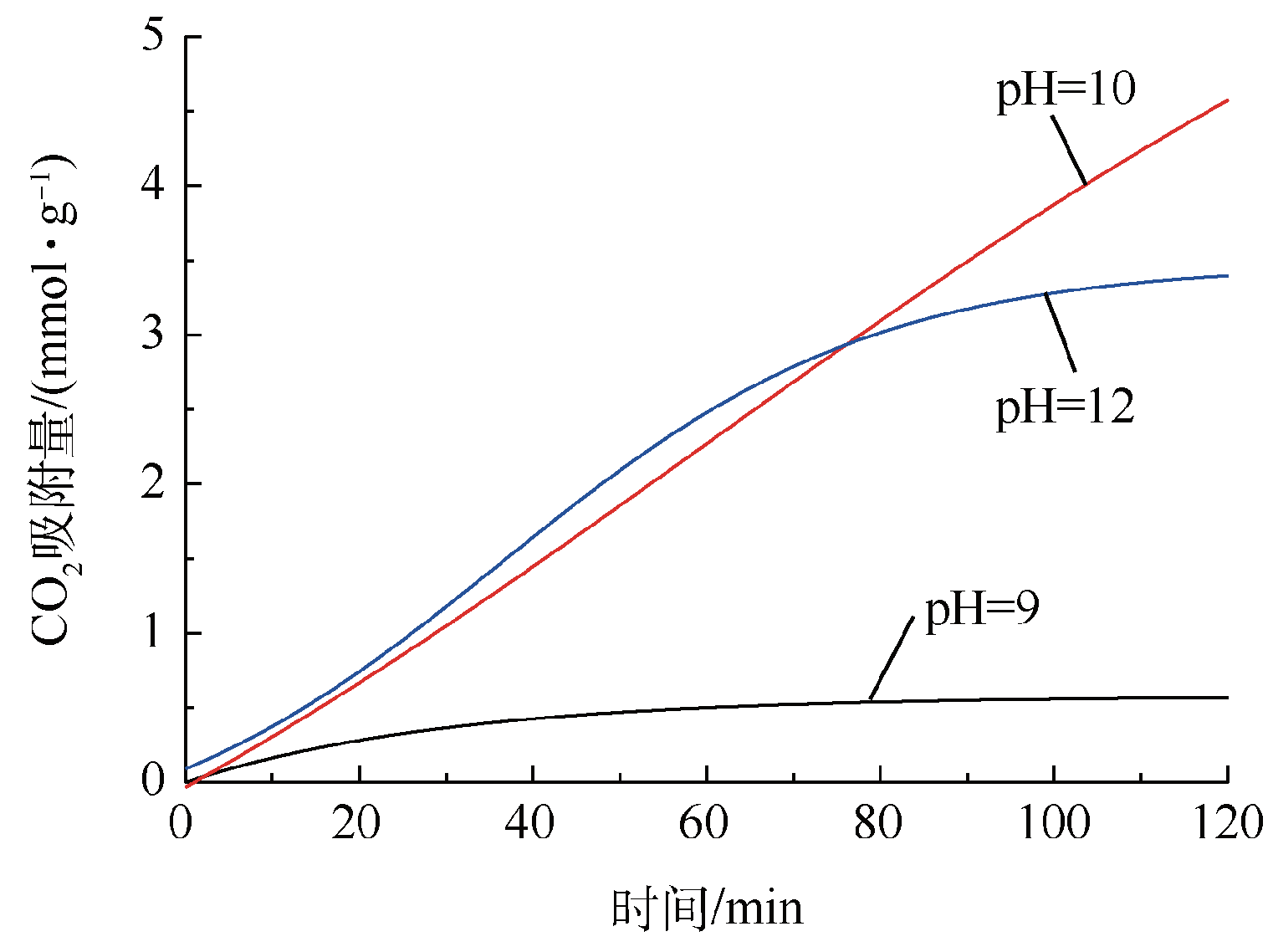
2.1.4 合成pH对CO2吸附性能的影响
Fig. 4 CO2 capture capacity of Mg20Al-CO3 LDH with different synthesis pH (calcination temperature 500 ℃ and sorption temperature 260 ℃)
Fig. 4 CO2 capture capacity of Mg20Al-CO3 LDH with different synthesis pH (calcination temperature 500 ℃ and sorption temperature 260 ℃)
2.1.5 煅烧温度对CO2吸附性能的影响
Fig. 5 Influence of calcination temperature on the CO2 capture capacity of Li/Na/K-LDH(different calcination temperature and sorption temperature 240 ℃)
Fig. 5 Influence of calcination temperature on the CO2 capture capacity of Li/Na/K-LDH(different calcination temperature and sorption temperature 240 ℃)
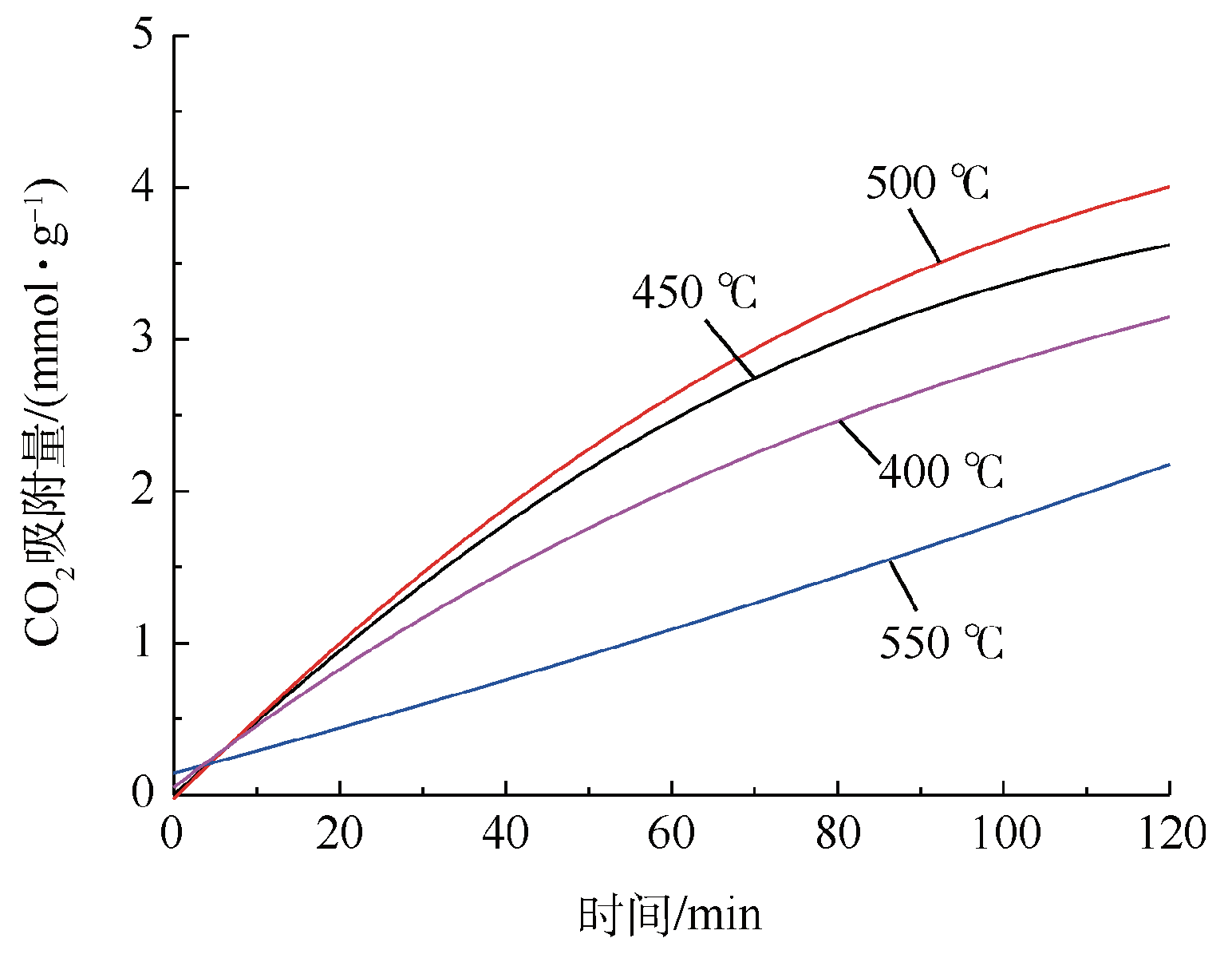
2.1.6 吸附温度对CO2吸附性能的影响
Fig. 6 Influence of adsorption temperature on the CO2 capture capacity of Li/Na/K-LDH (calcination temperature 500 ℃ and different sorption temperature)
Fig. 6 Influence of adsorption temperature on the CO2 capture capacity of Li/Na/K-LDH (calcination temperature 500 ℃ and different sorption temperature)
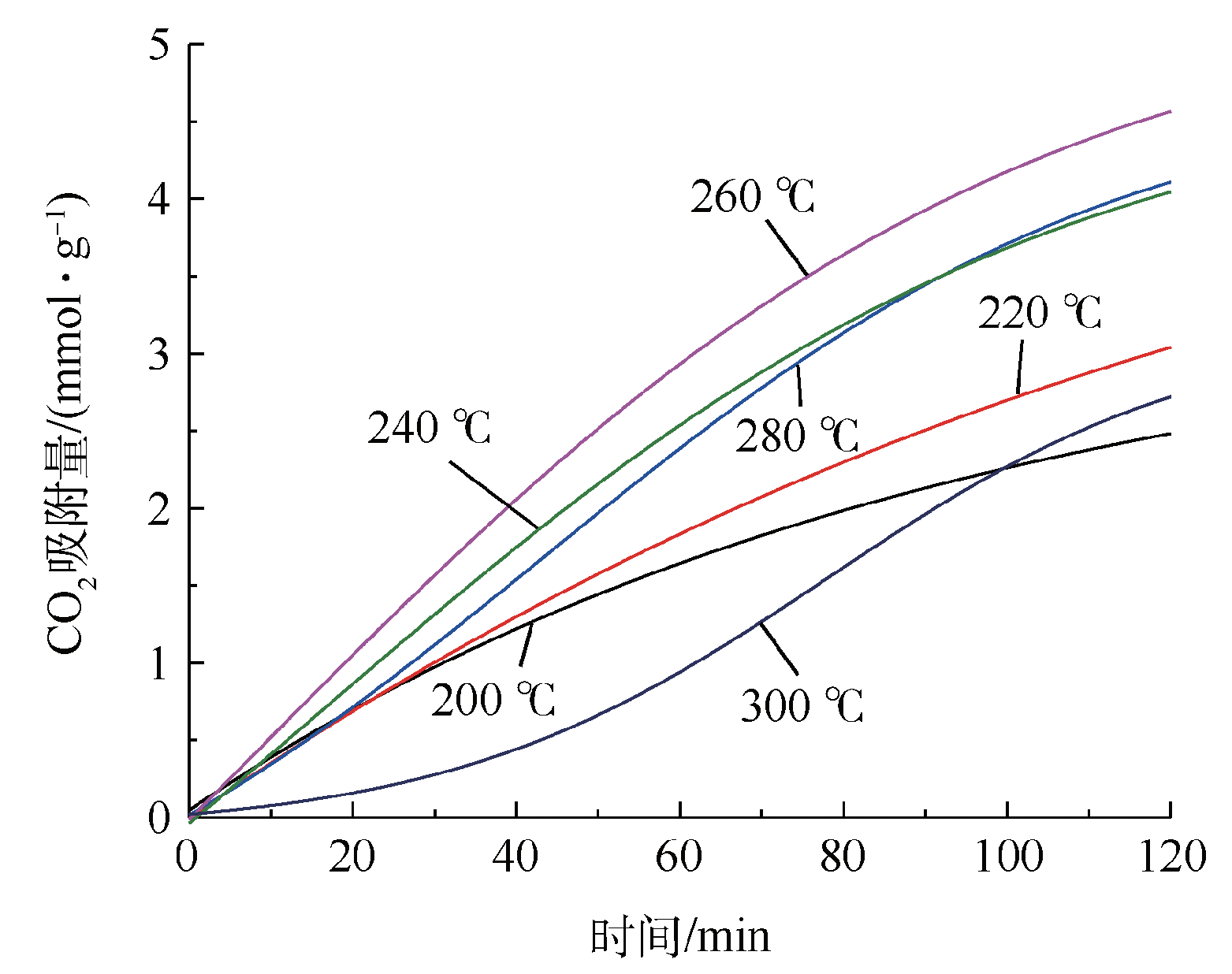
2.1.7 循环稳定性能测试
Fig. 7 CO2 capture capacity of the Li/Na/K-LDH during the 10 adsorption/desorption cycling tests (calcination temperature 500 ℃ and sorption temperature 240 ℃)
Fig. 7 CO2 capture capacity of the Li/Na/K-LDH during the 10 adsorption/desorption cycling tests (calcination temperature 500 ℃ and sorption temperature 240 ℃)

Table 1 Different types of alkali metal loading LDH
LDH | 碱金属盐种类 | 负载方法 | 负载量/% | 吸附温度/℃ | 吸附量/(mmol·g−1) | 来源 |
Mg3Al-CO3 | K2CO3 | 浸渍法 | 12.5 | 300 | 1.11 | |
Mg3Al-CO3 | K2CO3 | 旋转蒸发法 | 20 | 400 | 1.33 | |
Mg3Al-CO3 | Li2CO3 | 浸渍法 | 20 | 400 | 1.05 | |
Mg3Al-CO3 | Na2CO3 | 浸渍法 | 20 | 400 | 0.83 | |
Mg3Al-CO3 | Rb2CO3 | 浸渍法 | 20 | 400 | 0.79 | |
Mg3Al-CO3 | Cs2CO3 | 浸渍法 | 20 | 400 | 0.75 | |
Mg3Al-CO3 | K2CO3 | 浸渍法 | 35 | 400 | 2.1 | |
Mg20Al-CO3 | LiNO3 | 浸渍法 | 30 | 240 | 0.15 | 本实验 |
Mg20Al-CO3 | NaNO3 | 浸渍法 | 30 | 240 | 2.55 | 本实验 |
Mg20Al-CO3 | KNO3 | 浸渍法 | 30 | 240 | 0.62 | 本实验 |
Mg20Al-CO3 | Li/Na/K | 浸渍法 | 30 | 260 | 4.64 | 本实验 |
2.2 材料的表征
2.2.1 材料的 X-射线衍射分析
Fig. 8 XRD patterns of Mg20Al-CO3 LDH with different alkali metal nitrates species loading
Fig. 8 XRD patterns of Mg20Al-CO3 LDH with different alkali metal nitrates species loading
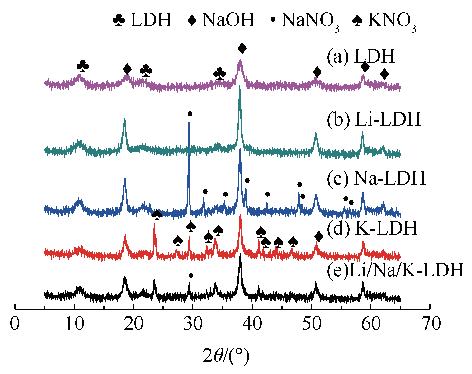
Fig. 9 XRD patterns of Li/Na/K-LDH after calcination
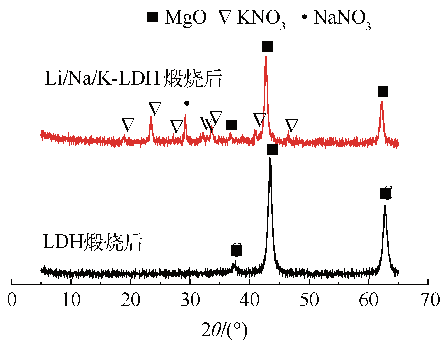
2.2.2 材料的傅里叶红外光谱(FT-IR)分析
Fig. 10 FT-IR spectra of fresh Mg20Al-CO3 LDH with different alkali metal nitrates species loading
Fig. 10 FT-IR spectra of fresh Mg20Al-CO3 LDH with different alkali metal nitrates species loading
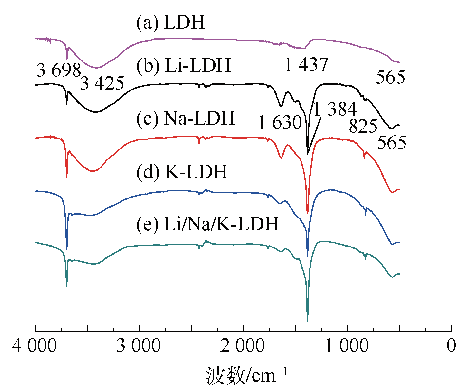
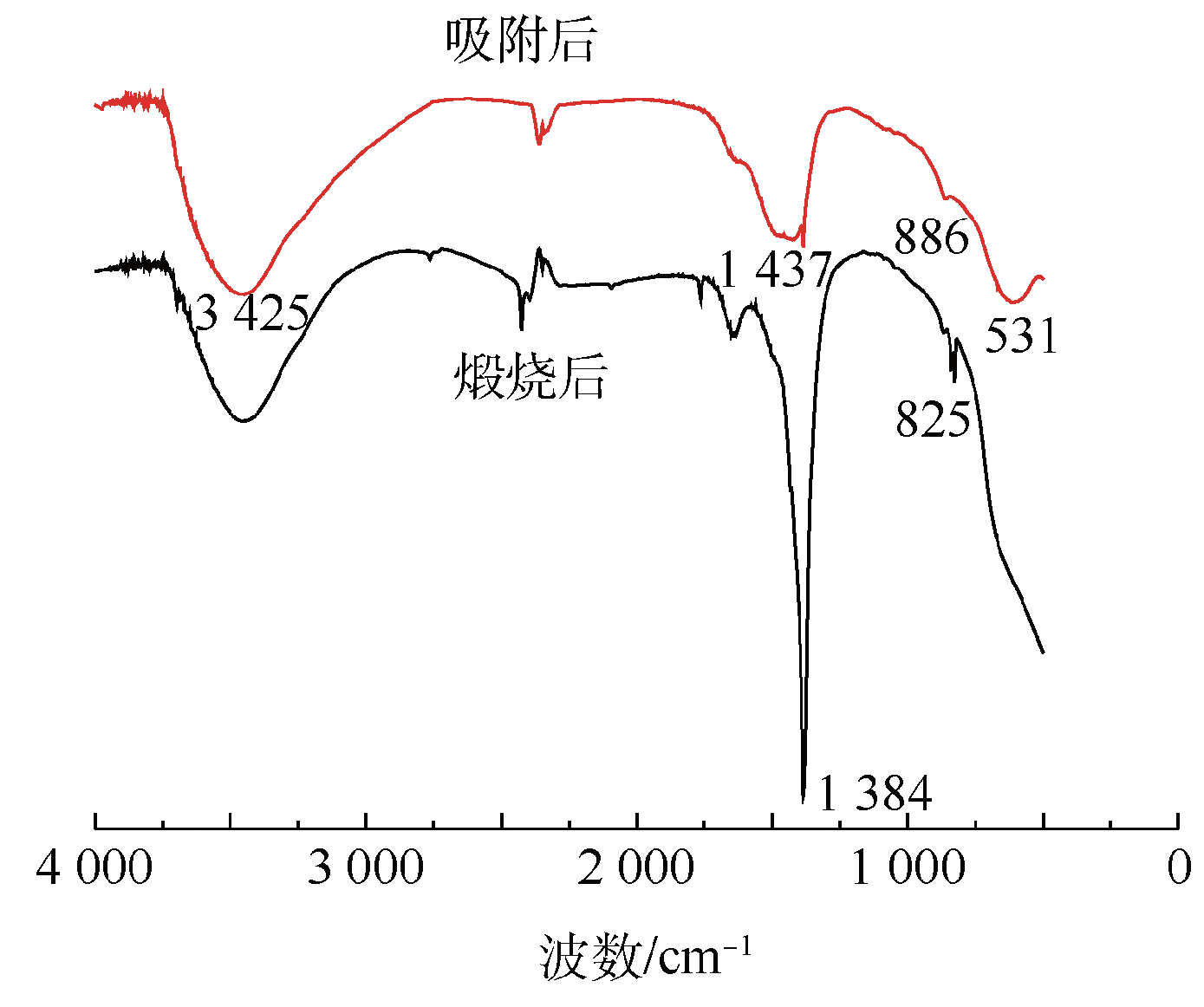
2.2.3 碱金属硝酸盐负载LDH煅烧前后的FT-IR分析
Fig. 11 FT-IR spectra of Li/Na/K-LDH after adsorption and calcined





 百度学术
百度学术


 下载:
下载:
