-
近年来,天然和人工合成激素的不断排放严重影响了生态和环境健康[1-3],这引起了研究学者的关注[4-5]. 其中天然雌激素17β-雌二醇(E2)是一种广泛用于人类内分泌疾病治疗的天然雌激素[6],是天然水体中雌激素活性的主要贡献者[7]. E2已被检出于多种天然水体,如夏季在来自日本109条河流的256个样品中检出率为86.72%,平均浓度2.1 ng·L−1[8];德国的一些饮用水水样中E2的平均检出浓度为0.7 ng·L−1[9];此外E2在阿肯色州西北部的地下水体中也被检测到,浓度范围为6—66 ng·L−1[10]. 有文献报道E2在低浓度甚至环境浓度下足以干扰内分泌系统的功能,损害水生生物和人类健康,如对鱼性别产生诱导的最低可见效应浓度为10 ng·L−1[11],雌性虹鳟鱼中E2诱导卵黄蛋白原的阈值浓度为4.7 ng·L−1和7.9 ng·L−1[12]. E2等污染物在天然水体中的自然衰减途径主要有物理、化学和生物过程,如蒸发、稀释、水解、吸附、光降解和生物降解等,其中光降解和生物降解是污染物自然衰减的两个重要过程[13],对污染物的转化和归趋有着极其重要的影响.
光解分为直接光解和间接光解,前者是污染物可以有效地吸收光子从而发生光解,且污染物直接光解的量子产率越高,光化学活性越高;后者是水体中存在的光敏剂会产生活性氧物种(ROS)或将能量转移至污染物,从而导致污染物发生光解[14]. 据报道,E2可直接吸收290—320 nm范围内的太阳光而发生直接光降解,且水质成分,如腐殖酸,Mn(Ⅲ)和溶解性黑碳等,可明显促进E2的光降解[4, 15-16]. 生物转化主要是通过酶来降解这些污染物[17],其中过氧化物酶(POD)广泛存在于天然环境[18],且活性相当高,如在芬兰Mekkojärvi湖表层水中活性范围为73.6—272.9 nmol·L−1·h−1[19];在德国湖泊中浓度可达到0.06—4.71 mmol·L−1·h–1[20]. 有文献报道,辣根过氧化物酶(HRP)、木质素过氧化物酶以及大豆过氧化物酶等POD可通过氧化偶联反应有效去除三氯生、E2和乙酰氨基酚等多种污染物,且主要底物为酚类污染物[21-23]. 在自然水体中,光降解和酶促反应通常同时发生,且已有研究学者关注光降解和酶转化的复杂过程. 如HRP可利用腐殖质光解产生的H2O2进行氧化偶联反应去除E2,同时,腐殖质生成ROS促进E2的光降解[24],但是HRP在无腐殖质存在的条件下是否可直接影响E2的光转化尚不清楚,因此有必要进行这方面的研究.
本文通过降解动力学、活性氧物种鉴定、光酶结合产物鉴定以及常见水质成分比较等实验系统地研究了HRP对E2光解的影响机制,对光酶协同转化过程有更深的理解,为E2的环境归趋和酶处理技术在水处理方面的应用提供参考数据.
-
17β-雌二醇(E2,纯度98%)、2,2’-连氮基-双-(3-乙基苯并二氢噻唑啉-6-磺酸)二铵盐(ABTS,纯度98%)和辣根过氧化物酶(HRP,EC 1.11.1.7, 52 U·mg−1)购自美国Sigma-Aldrich有限公司;色谱级甲醇来自美国Tedia 公司;Suwannnee河的腐殖酸(SRHA)购自于国际腐殖质协会(IHSS). 实验所用水均来自Milli-Q纯化系统的去离子水18.25 MΩ·cm.
-
用ABTS方法测定辣根过氧化物酶HRP的酶活,酶活的定义为:1单位酶活是每分钟催化氧化1 μmol ABTS的酶量.具体步骤为:在1 cm比色皿中,加入2.3 mL 0.01 mol·L−1磷酸盐缓冲液(PBS)、0.3 mL 2 mmol·L−1H2O2和0.3 mL 2 mmol·L−1ABTS,最后加入0.1 mL的HRP溶液,迅速混合启动反应. 并立即将比色皿放置到紫外分光光度计中,每15 s记录1次在420 nm处的吸光度,共记录3 min,计算出k值,然后根据公式1计算酶活.
-
光降解实验均在50 mL带有石英塞的石英比色管中进行,反应体积为25 mL,含有1.6 μmol·L−1的E2溶液,酶活范围为0.001—0.1 U·mL−1或失活的HRP,反应介质为0.01 mol·L−1磷酸缓冲溶液(PBS, pH=7.0). 将比色管置于XPA-Ⅶ型光化学反应仪(南京胥江机电厂,中国)中,并使其围绕光源旋转保证反应溶液受光均匀,并以铝箔纸完全包裹的相同反应溶液为黑暗对照. 使用带有通过型滤光片(λ> 290 nm)的1000 W氙灯作为光源,并用紫外线辐照器(北京师范大学光电仪器厂,中国)测得365 nm处的辐照强度为46.5 mW·cm−2左右,接近南京(32°20′N)夏季中午的光照强度. 通过冷却的循环水浴将反应溶液的温度保持在(25.0±1.0 )℃. 在预设的反应间隔取样,用于HRP和E2的分析.
HRP失活方法:将HRP溶液在90 ℃水浴锅中保持60 min,结束后用1.2节HRP酶活测定方法测定不到HRP酶活.
鉴定E2反应过程中的活性氧物种(ROS)实验:分别用10 μmol·L−1山梨酸(sorbic acid)、100 mmol·L−1异丙醇(2-propanol)、1.0 mmol·L−1呋喃甲醇(FFA)和0.5 mmol·L−1硝基蓝四唑(NBT)捕获三重态、羟基自由基(·OH)、超氧负离子(O2·−)和单线态氧(1O2)[4, 25-26].
常见水质成分对E2光降解的影响实验:(1)1.6 μmol·L−1 E2溶液;(2)1.6 μmol·L−1的E2和0.01 U·mL−1 HRP;(3)1.6 μmol·L−1的E2和5 mgC·L−1 SRHA;(4)1.6 μmol·L−1的E2和1 mmol·L−1
${\rm{NO}}_3^{-} $ 和(5)1.6 μmol·L−1的E2和30 μmol·L−1 Fe3+.所有实验均设3个平行,标准偏差在95%置信区间.
-
为了更好地阐明雌激素与HRP之间的相互作用机制,进一步研究了E2在HRP溶液中的光降解产物. 反应体系为1.6 μmol·L−1 E2和1.6 μmol·L−1 E2 + 0.01 U·mL−1 HRP,按照1.3节光照条件对这两个反应体系进行光照实验,光照结束后,立刻加入0.5 mL 2.4 mol·L−1 盐酸将反应溶液pH值调至2.0以下,使HRP完全失活.最后用CNW LC-C18柱进行进行固相萃取(SPE),具体步骤如下:1)将C18小柱先用10 mL甲醇活化,再用10 mL去离子水平衡;2)以约0.5 mL·min−1的流速加载光降解溶液,然后用10 mL去离子水淋洗;3)真空抽干C18小柱后,用3×1 mL甲醇洗脱;4)收集洗脱液后用N2吹干,并用1 mL甲醇重溶后进液相色谱飞行时间质谱联用仪(LC-TOF-MS)测定.
-
E2的浓度用配备有荧光检测器的安捷伦高效液相色谱(HPLC1200,美国)测定,激发和发射波长分别为220 nm和310 nm. 色谱分离柱为安捷伦Eclipse XDB-C18柱(250 mm × 4 mm, 5 μm)(安捷伦,美国),流速为0.7 mL·min−1,流动相为80%的乙腈和20%的水,进样量30 μL.
LC-TOF-MS:用配有电喷雾离子源的Triple TOF 5600高效液相/飞行时间质谱联用仪(AB SCIEX,美国)分析E2的光降解产物. 离子源参数如下:离子喷雾电压-5500 V,气帘气电压35 psi,载气电压55 psi,辅助气电压55 psi和温度550 ℃. 液相条件是:Eclipse XDB-C18反相色谱柱(150 mm × 2.1 mm, 2.4 μm),200 μL·min−1流速,5.00 μL进样量,90%甲醇和10%水的流动相和30 ℃柱温.
-
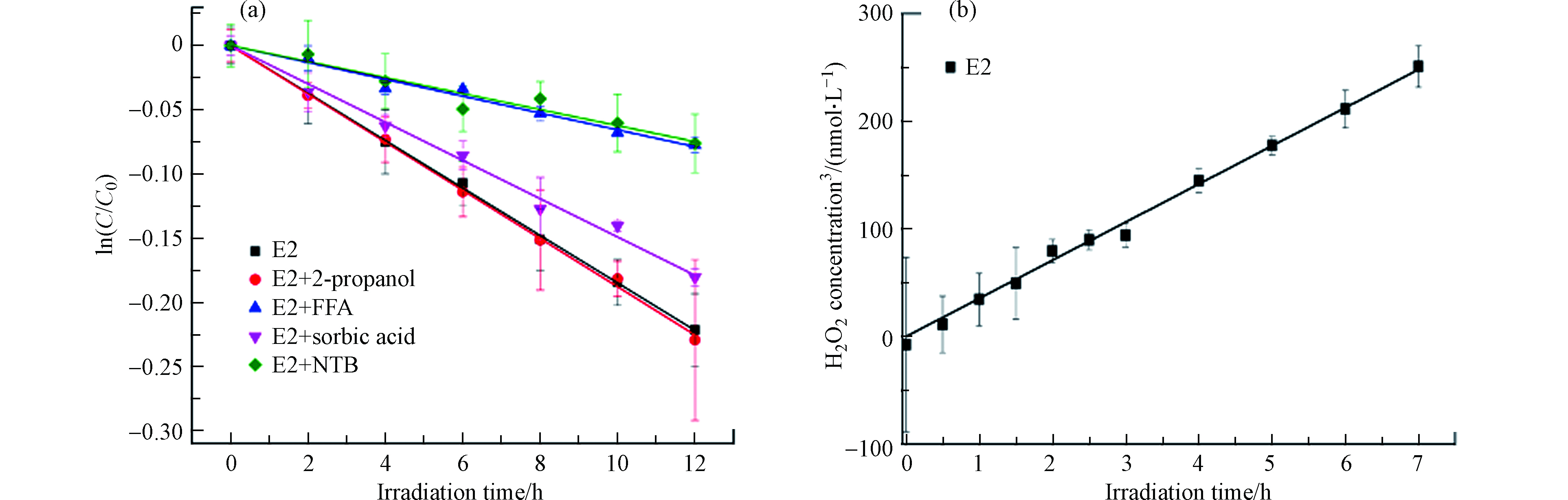
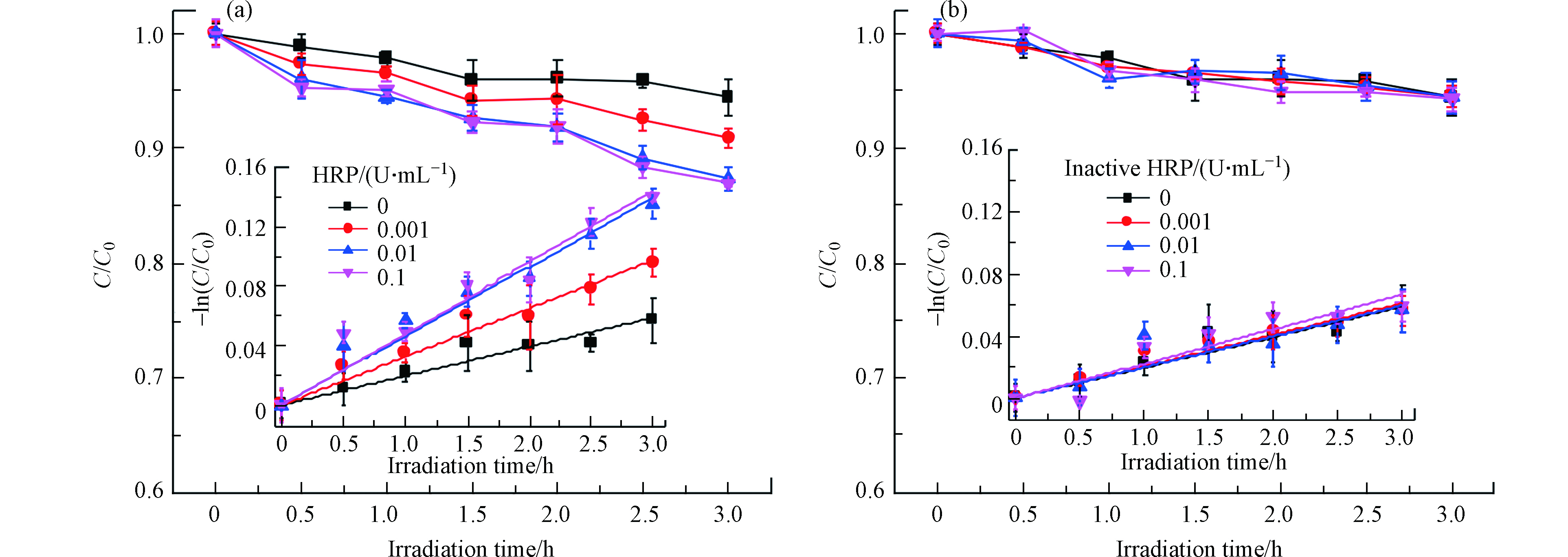
在黑暗条件,E2的浓度在24 h内未观察到显著变化,这表明水解、挥发和石英管壁上的吸附对E2的去除没有影响. E2在模拟太阳辐射下在PBS中的直接光解符合假一级动力学(图1),速率常数为0.0197 h−1(R2=0.972),半衰期为35.19 h,说明E2直接光解很慢,这与E2具有低的量子产率(0.07)是一致的[27],这是因为E2在290—320 nm波长范围内的吸收比较微弱,与文献一致[4].
加入HRP,E2的光降解被显著的促进,但是促进程度随着酶活的增加先增加后趋于饱和,且当酶活高于0.01 U·mL−1时促进效果不再显著增加,说明HRP促进E2光解的最佳酶活为0.01 U·mL−1. E2在HRP存在下的光降解仍然符合假一级动力学,且R2均大于0.98,说明线性很好. E2在0.001、0.01、0.1 U·mL−1HRP溶液中的光解速率常数分别为0.0325、0.0465、0.0480 h−1,其中,在最佳酶活0.01 U·mL−1 HRP下的光解速率常数约为直接光解速率常数的3倍,表明环境水体中的HRP可显著影响雌激素的光化学行为. 将相对应不同初始酶活的HRP失活,研究失活HRP对E2光降解的影响,结果表明,失活的HRP对E2的光解几乎没有影响,表明只有有活性的HRP才能和光协同促进E2的光解.
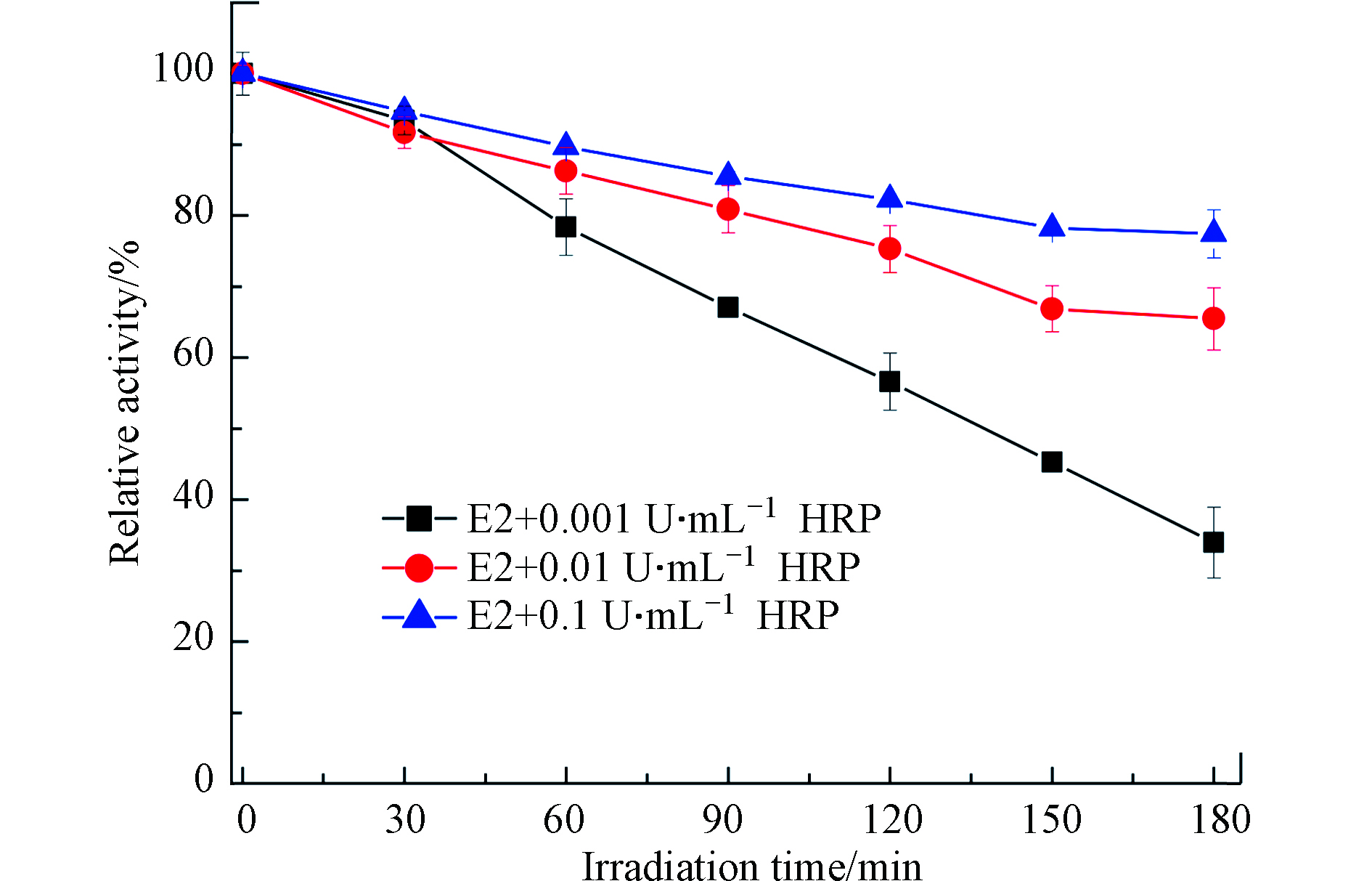
由于酶活影响E2的光转化过程,接下来,测定了E2+HRP溶液中酶活随光照时间的变化. 结果发现,酶活随着光照时间的增加逐渐下降,这是因为HRPFe(Ⅲ)在光照条件下会被还原为HRPFe(Ⅱ),而HRPFe(Ⅱ)无法提供空轨道与H2O2配位,从而导致酶活性降低[28]. 但对于不同酶活来讲,活性越高,失活越慢(图2). 初始酶活为0.001、0.01、0.1 U·mL−1的HRP在光照3 h后分别降低了70%、35%和20%,表明E2在环境水体中较长时间内都能被光酶协同降解.
-
HRP中含有色氨酸、组氨酸和酪氨酸等氨基酸,这些氨基酸能够吸收光从而可能会与E2竞争光而抑制其直接光解[29],类似于腐殖质的光屏蔽作用,因此,通过公式2—3计算不同活性下HRP的光屏蔽因子Sλ,扣除其对E2光降解的屏蔽作用.
其中,Sλ是屏蔽因子,A (cm−1)是特定波长下的衰减系数,b(2 cm)是光化学反应中比色管的路径长度. 由计算可得,在考察的酶活范围内HRP的Sλ值均接近于1,即没有发现HRP的光屏蔽作用,这可能是因为HRP对光的吸收较弱或浓度较低. 这些结果表明HRP只促进了E2的光降解,没有光屏蔽作用.
有学者认为光照条件下量子点CdS QDs产生的活性氧物种(ROS)如·OH和O2·-可激活P450单加氧酶[30],且Fruk等人证明了该量子点可激活活性中心为亚铁血红素的多种过氧化物酶,包括细胞色素C、HRP和肌红蛋白过氧化物酶[31]. 因此,通过活性氧猝灭实验测定E2体系中产生的ROS,结果如图3所示. 分别加入O2·-捕获剂NBT、1O2捕获剂和三重激发态捕获剂山梨酸后,与对照相比,E2的光解分别被抑制了约64.5%、66.3%和19.4%(根据假一级动力学速率常数计算),表明O2·-和1O2对E2的光解起着不可或缺的作用,且三重激发态也参与了E2的光解,而在·OH猝灭剂100 mmol·L−1异丙醇存在下,E2的光降解动力学曲线与直接光解接近,表明·OH对E2光降解的贡献微不足道. 除此之外,本实验通过过氧化物酶-莨菪亭荧光衰减法测到了E2体系中的H2O2,且H2O2的生成符合零级动力学,生成速率为35 nmol·L−1·h−1,上述结果说明E2在光照条件下可生成O2·-、1O2和H2O2. 根据相关文献[32-34]推测E2自敏化生成ROS的过程为:E2在光照激发下形成单重态,然后通过系间窜越(intersystem crossing, ISC)形成三重态,接下来,三重态将能量传递给O2形成1O2或通过自电离形成自由基(E2+·、E2−·)和
$ {\text{e}}_{\text{aq}}^{-} $ ,而$ {\text{e}}_{\text{aq}}^{-} $ 可与O2发生电子转移形成O2−·,然后O2−·会和溶液中的H+结合生成H2O2. 因此可以推测E2自敏化产生的ROS激活了HRP,从而促进了E2的酶降解.另外,有学者认为·OH也能激活酶[30-31],因而导致光酶协同降解雌激素. ·OH、1O2、O2·-和H2O2普遍存在于天然水体中,浓度范围分别为10−14—10−19 mol·L−1、10−12—10−14 mol·L−1、10−9—10−11 mol·L−1和10−9—10−7 mol·L−1[35],且与过氧化物酶和污染物并存,因此,过氧化物酶会对污染物的光化学归趋产生重大影响.除此之外,本研究对于酶处理技术在水处理方面的应用同样有参考价值. 在酶处理技术中,H2O2的存在需要严格的条件,又会影响酶的稳定性[36],同时,过量的H2O2会排入天然水环境,从而影响水生生态系统.若能将过氧化物酶与光结合,既不用像之前一样通过添加辅助因子来激活酶,又能促进污染物的降解,还可精准控制酶活,形成多赢的局面.
-
为了进一步研究HRP和光协同促进雌激素光酶降解的机制,通过SPE/LC/MS方法分析了E2在PBS和HRP共存溶液中的光转化产物以及黑暗对照组(E2和HRP及外加H2O2反应体系)中的酶降解产物.结果如图4所示,在酶降解体系中,共鉴定出5种二聚物(Dimer1-5),其中,二聚物3(Dimer3)峰面积最大.在E2的直接光解体系中,鉴定出3种聚合物(Dimer1-3),说明二聚物4、5(Dimer4、5)是酶降解的专一产物. 在E2和HRP共存的光降解溶液中鉴定出来的产物种类与酶降解相同(Dimer1-5),这说明了光解过程伴随着酶降解的发生;而产物峰面积有所差异,二聚物2(Dimer2)和二聚物3(Dimer3)峰面积接近,这也进一步暗示了酶降解和光降解共存. 另外,Mao 等研究E2在过氧化物酶催化转化下的聚合产物与本文光酶体系中检测到的产物类似,且通过雌激素活性测定发现耦合产物是没有雌激素效应的[23],说明E2通过光酶协同降解后,生态风险被降低.
-
硝酸根(
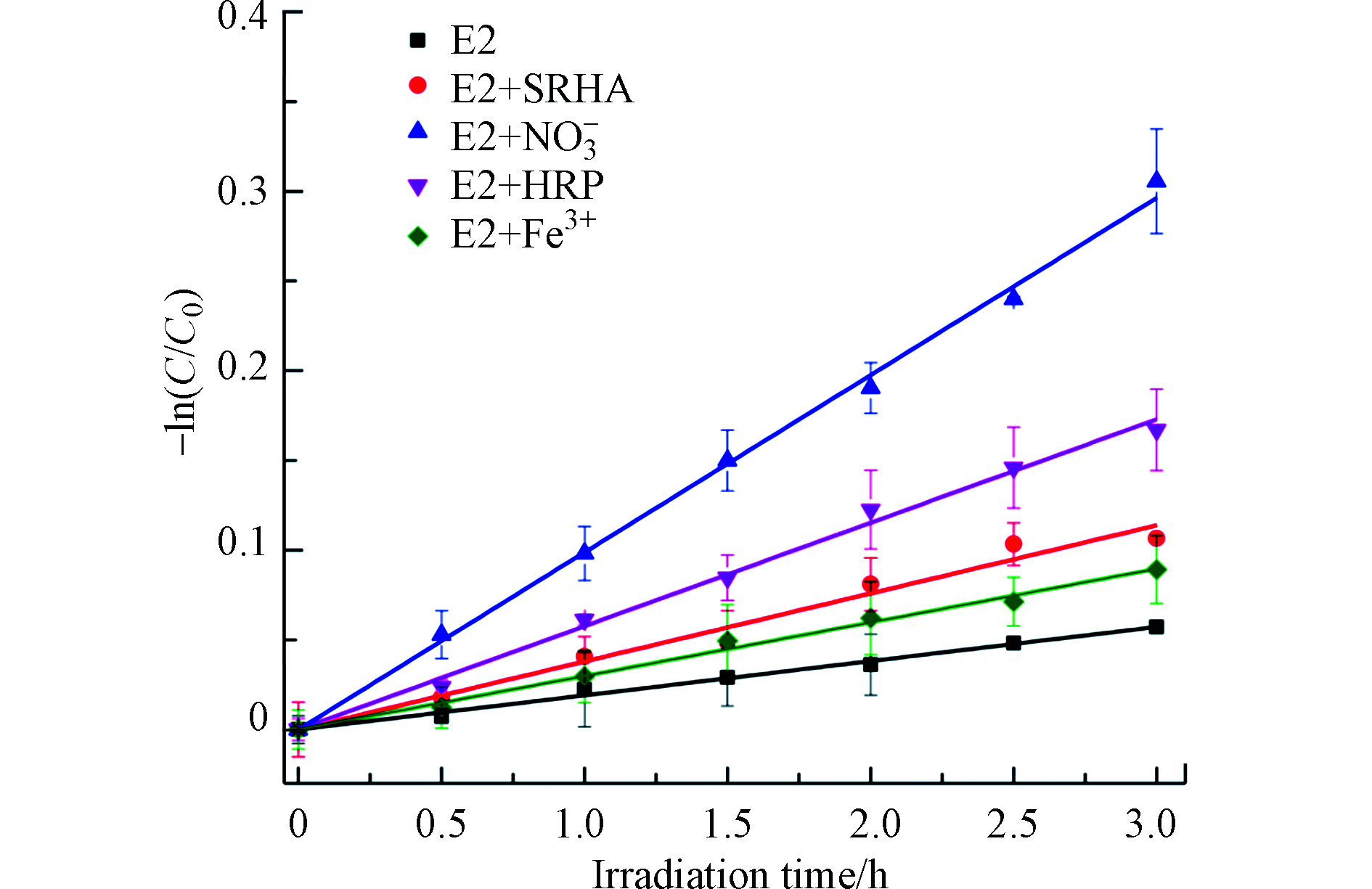
${\rm{NO}}_3^{-} $ )、腐殖质和铁离子(Fe3+)普遍存在于天然水体,并有光化学活性,一般会影响污染物在天然水体的光化学行为,因此,本实验比较了环境浓度下的HRP(0.01 U·mL−1)、${\rm{NO}}_3^{-} $ (1 mmol·L−1)、Fe3+(30 μmol·L−1)和SRHA(5 mg·L−1 C)对E2光解的影响. 结果如图5所示,E2在这4个水质成分作用下的光降解仍呈假一级动力学,且这4个水质成分均促进了E2的光解,假一级速率常数kobs从大到小依次为${\rm{NO}}_3^{-} $ >HRP>SRHA>Fe3+(表1).但由于这几个环境因子都能与E2竞争吸收光子,kobs不能代表其对E2光解的真正贡献,因此扣除这几个因子的光屏蔽作用,校正E2的直接光解速率(式4—5),并计算这4个环境因子引起的其他光解速率常数(表1):其中,kPBS是E2在PBS缓冲液中的直接光解速率常数,kdp和kother分别是E2在不同溶液中的校正直接光解速率常数和环境因子导致的其他光解速率常数.
SRHA和Fe3+的Sλ分别为0.784和0.926(表1),均小于1.0,表明SRHA和Fe3+均具有光屏蔽效应,对E2的光降解有抑制作用,而HRP和
${\rm{NO}}_3^{-} $ 基本上没有. 扣除SRHA和Fe3+的光屏蔽因子之后,E2在这俩溶液中的kother值仍为正值,表明SRHA和Fe3+对E2的光降解还存在促进作用,且促进作用占主导低位. 对于E2在SRHA和${\rm{NO}}_3^{-} $ 溶液中的光降解,由它们导致的间接光解是E2转化的主要途径,而对于E2在HRP溶液中的光降解,HRP引起的酶降解是E2转化的主要途径,但对于E2在Fe3+溶液中的光降解,直接光解是其转化的主要途径. 比较这些水质成分对E2转化的真正贡献率发现HRP的贡献率小于${\rm{NO}}_3^{-} $ 、大于SRHA和Fe3+导致的贡献,表明研究污染物在天然水环境中的光化学行为及其环境归趋时,应重视HRP对这个过程的影响. -
本文系统地研究了HRP对E2光降解过程的影响. 研究发现有活性的HRP可显著促进E2的光化学转化,且促进作用随着活性的增大而增强. 这是由于E2自敏化产生的活性物种可激活HRP,从而促进了E2在光照条件下的酶降解,且E2在HRP溶液中的光转化中间体中有酶降解产生的聚合产物进一步证实了这个推测. HRP对E2酶降解的效率高于常见水质成分SRHA和Fe3+的效率,弱于
${\rm{NO}}_3^{-} $ 引起的贡献率,表明应重视HRP对污染物在天然水体中的环境归趋的影响. 然而,敏化剂在实际天然水体中往往是共存的,情况非常复杂,因此,本文的研究结果具有一定的局限性,在后续研究中,应逐渐将反应体系复杂化. 另外,若能将光和过氧化物酶结合,既不用像之前一样通过添加辅助因子来激活酶,又可促进污染物的降解,还可精准控制酶活,这将为酶处理技术在水处理方面的应用带来巨大前景.
过氧化物酶对水中17β-雌二醇光降解的影响机制
The effect mechanism of peroxidase on the photodegradation of 17β-estradiol in water
-
摘要: 过氧化物酶广泛存在于天然水体,在外加过氧化氢(H2O2)的条件下可有效地降解水体中的有机污染物.17β-雌二醇(E2)是水体中常见的雌激素,低浓度下仍具有极强的内分泌干扰效应,过氧化物酶可通过利用腐殖质产生的H2O2从而影响腐殖质介导的E2的光化学过程,但其是否可直接影响E2的光转化尚不清楚.本文系统地研究了辣根过氧化物酶(HRP)对E2光降解过程的影响.研究发现有活性的HRP可显著促进E2的光化学转化,且促进作用随着活性的增大而增强.活性氧猝灭实验结果表明E2自敏化产生的活性物种可激活HRP,从而促进了E2在光照条件下的酶降解,且E2在HRP溶液中的光解产物实验进一步证实了这一过程.HRP对E2酶降解的效率高于常见水质成分Suwannnee River Humic Acid(SRHA)和Fe3+的促进效率,弱于
${\rm{NO}}_3^{-} $ 引起的促进效率,表明HRP对污染物在天然水体中的环境归趋的影响是值得关注的.本文为深入认识雌激素的环境归趋提供科学数据,并为酶处理技术在水处理方面的应用带来巨大前景.Abstract: Peroxidase is widely widespread in aqueous environment, it can effectively degrade organic pollutants in the presence of hydrogen peroxide (H2O2). 17β-estradiol (E2), as a common estrogen, exhibits strong endocrine disrupting effect even at relatively low concentration. It is well known that peroxidase can affect humus induced photochemical process of E2 via the interaction with H2O2, while whether peroxidase can directly interact with E2 still remains unclear. In this study, the impact of horseradish peroxidase (HRP) on photochemical transformation of E2 was systematically evaluated. Our results revealed that active HRP could significantly promote the photodegradation of E2, and the promotion effect increased with the augment of enzyme activity. Self-sensitization of E2 was proposed to activate HRP, leading to the formation of a series of reactive oxygen species (ROS), which in turn oxidized E2. Such mechanisms were further verified by ROS quenching experiments and photoproducts identification. In addition, the promotion effect of HRP was found to be more pronounced than water components Suwannnee River Humic Acid (SRHA) and Fe3+, while less effective than nitrate, indicating that the involvement of HRP in the environmental fate of pollutants in natural water is noteworthy. The present study would provide scientific data for better understanding of the environmental fate of estrogen and bring great prospects for the application of enzyme treatment technology in water treatment.-
Key words:
- horseradish peroxidase /
- 17β-estradiol /
- enzyme degradation /
- photodegradation /
- water components
-

-
表 1 E2在不同反应溶液中的假一级动力学速率常数(kobs)和直接光解、其他降解预测贡献率
Table 1. Observed pseudo-first-order rate constants (kobs) and predicted contributions from direct photolysis and other degradation of E2 in different water solutions.
溶液Solution kobs/d−1 Sλ kdp/d−1 kother/d−1 DP/% Other/% E2 0.456 1.00 0.456 — 100 — E2+SRHA 0.911 0.784 0.358 0.553 40 60 E2+ ${\rm{NO}}_3^{-} $ 2.37 0.995 0.454 1.916 19 81 E2+ HRP 1.383 1 0.456 0.927 33 67 E2 + Fe3+ 0.717 0.926 0.422 0.295 59 41 -
[1] MILLS L J, CHICHESTER C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? [J]. Science of the Total Environment, 2005, 343(1/2/3): 1-34. [2] VOS J G, DYBING E, GREIM H A, et al. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation [J]. Critical Reviews in Toxicology, 2000, 30(1): 71-133. doi: 10.1080/10408440091159176 [3] ZUO Y G, ZHANG K, ZHOU S. Determination of estrogenic steroids and microbial and photochemical degradation of 17α-ethinylestradiol (EE2) in lake surface water, a case study [J]. Environmental Science. Processes & Impacts, 2013, 15(8): 1529-1535. [4] ZHOU Z C, CHEN B N, QU X L, et al. Dissolved black carbon as an efficient sensitizer in the photochemical transformation of 17β-estradiol in aqueous solution [J]. Environmental Science & Technology, 2018, 52(18): 10391-10399. [5] PAPAEVANGELOU V A, GIKAS G D, TSIHRINTZIS V A, et al. Removal of Endocrine Disrupting Chemicals in HSF and VF pilot-scale constructed wetlands [J]. Chemical Engineering Journal, 2016, 294: 146-156. doi: 10.1016/j.cej.2016.02.103 [6] 李晓曼, 黄斌, 孙雯雯, 等. 类固醇雌激素环境行为研究进展 [J]. 环境化学, 2014, 33(8): 1276-1286. doi: 10.7524/j.issn.0254-6108.2014.08.022 LI X M, HUANG B, SUN W W, et al. Research progress on the environmental behavior of steroid estrogens [J]. Environmental Chemistry, 2014, 33(8): 1276-1286(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.08.022
[7] ONDA K, NAKAMURA Y, TAKATOH C, et al. The behavior of estrogenic substances in the biological treatment process of sewage [J]. Water Science and Technology, 2003, 47(9): 109-116. doi: 10.2166/wst.2003.0504 [8] TABATA A, KASHIWADA S, OHNISHI Y, et al. Estrogenic influences of estradiol-17 beta, p-nonylphenol and bis-phenol-A on Japanese medaka (Oryzias latipes) at detected environmental concentrations [J]. Water Science and Technology, 2001, 43(2): 109-116. doi: 10.2166/wst.2001.0079 [9] KUCH H M, BALLSCHMITER K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC–(NCI)–MS in the picogram per liter range [J]. Environmental Science & Technology, 2001, 35(15): 3201-3206. [10] YING G G, KOOKANA R S, RU Y J. Occurrence and fate of hormone steroids in the environment [J]. Environment International, 2002, 28(6): 545-551. doi: 10.1016/S0160-4120(02)00075-2 [11] METCALFE C D, METCALFE T L, KIPARISSIS Y, et al. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes) [J]. Environmental Toxicology and Chemistry, 2001, 20(2): 297-308. doi: 10.1002/etc.5620200210 [12] MA L, YATES S R. Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: A review [J]. Science of the Total Environment, 2018, 640/641: 529-542. doi: 10.1016/j.scitotenv.2018.05.301 [13] WRITER J H, RYAN J N, KEEFE S H, et al. Fate of 4-nonylphenol and 17β-estradiol in the redwood river of Minnesota [J]. Environmental Science & Technology, 2012, 46(2): 860-868. [14] 马哲, 王杰琼, 陈景文, 等. pH对不同来源溶解性有机质光致生成活性物种量子产率的影响 [J]. 环境化学, 2017, 36(9): 1889-1895. doi: 10.7524/j.issn.0254-6108.2017012301 MA Z, WANG J Q, CHEN J W, et al. Effect of pH on the quantum yield of reactive photo-induced species generated in different sources of DOM [J]. Environmental Chemistry, 2017, 36(9): 1889-1895(in Chinese). doi: 10.7524/j.issn.0254-6108.2017012301
[15] WANG X H, YAO J Y, WANG S Y, et al. Phototransformation of estrogens mediated by Mn(Ⅲ), not by reactive oxygen species, in the presence of humic acids [J]. Chemosphere, 2018, 201: 224-233. doi: 10.1016/j.chemosphere.2018.03.003 [16] SILVA C P, LIMA D L D, OTERO M, et al. Photosensitized degradation of 17β-estradiol and 17α-ethinylestradiol: Role of humic substances fractions [J]. Journal of Environmental Quality, 2016, 45(2): 693-700. doi: 10.2134/jeq2015.07.0396 [17] GURR C J, REINHARD M. Harnessing natural attenuation of pharmaceuticals and hormones in rivers [J]. Environmental Science & Technology, 2006, 40(9): 2872-2876. [18] SENESI N, XING B, HUANG P M. Biophysico-chemical processes involving natural nonliving organic matter in environmental systems//formation mechanisms of humic substances in the environment[M]. John Wiley & Sons, 2009: 41-109. [19] MÜNSTER U, HEIKKINEN E, SALONEN K, et al. Tracing of peroxidase activity in humic lake water [J]. Acta Hydrochimica et Hydrobiologica, 1998, 26(3): 158-166. doi: 10.1002/(SICI)1521-401X(199805)26:3<158::AID-AHEH158>3.0.CO;2-Q [20] BUCK U, BABENZIEN H D, ZWIRNMANN E. Extracellular peroxidase activity in an experimentally divided lake (Große Fuchskuhle, northern Germany) [J]. Aquatic Microbial Ecology, 2008, 51: 97-103. doi: 10.3354/ame01188 [21] LU J H, HUANG Q G, MAO L. Removal of acetaminophen using enzyme-mediated oxidative coupling processes: I. reaction rates and pathways [J]. Environmental Science & Technology, 2009, 43(18): 7062-7067. [22] LI J H, ZHANG Y, PENG J B, et al. The effect of dissolved organic matter on soybean peroxidase-mediated removal of triclosan in water [J]. Chemosphere, 2017, 172: 399-407. doi: 10.1016/j.chemosphere.2017.01.013 [23] MAO L, HUANG Q G, LU J H, et al. Ligninase-mediated removal of natural and synthetic estrogens from water: I. reaction behaviors [J]. Environmental Science & Technology, 2009, 43(2): 374-379. [24] LI J H, ZHANG Y, HUANG Q G, et al. Degradation of organic pollutants mediated by extracellular peroxidase in simulated sunlit humic waters: A case study with 17β-estradiol [J]. Journal of Hazardous Materials, 2017, 331: 123-131. doi: 10.1016/j.jhazmat.2017.02.033 [25] XU L P, LI H, MITCH W A, et al. Enhanced phototransformation of tetracycline at smectite clay surfaces under simulated sunlight via a lewis-base catalyzed alkalization mechanism [J]. Environmental Science & Technology, 2019, 53(2): 710-718. [26] SHI H H, WANG G W, HUANG Q G, et al. The mutual promotion of photolysis and laccase-catalysis on removal of dichlorophen from water under simulated sunlight irradiation [J]. Chemical Engineering Journal, 2018, 338: 392-400. doi: 10.1016/j.cej.2018.01.026 [27] YAN S W, SONG W H. Photo-transformation of pharmaceutically active compounds in the aqueous environment: A review [J]. Environmental Science. Processes & Impacts, 2014, 16(4): 697-720. [28] 唐乾, 宫婷婷, 曹洪玉, 等. 光诱导辣根过氧化物酶催化活性变化及机理研究 [J]. 光谱学与光谱分析, 2018, 38(12): 3692-3698. TANG Q, GONG T T, CAO H Y, et al. Mechanism of the variation of horseradish peroxidase catalytic activity induced by light [J]. Spectroscopy and Spectral Analysis, 2018, 38(12): 3692-3698(in Chinese).
[29] BOREEN A L, EDHLUND B L, COTNER J B, et al. Indirect photodegradation of dissolved free amino acids: The contribution of singlet oxygen and the differential reactivity of DOM from various sources [J]. Environmental Science & Technology, 2008, 42(15): 5492-5498. [30] IPE B I, NIEMEYER C M. Nanohybride aus Quantenpunkten und Cytochrom P450 als Photokatalysatoren [J]. Angewandte Chemie, 2006, 118(3): 519-522. doi: 10.1002/ange.200503084 [31] FRUK L, RAJENDRAN V, SPENGLER M, et al. Light-induced triggering of peroxidase activity using quantum dots [J]. Chembiochem, 2007, 8(18): 2195-2198. doi: 10.1002/cbic.200700594 [32] ZHANG S Y, CHEN J W, QIAO X L, et al. Quantum chemical investigation and experimental verification on the aquatic photochemistry of the sunscreen 2-phenylbenzimidazole-5-sulfonic acid [J]. Environmental Science & Technology, 2010, 44(19): 7484-7490. [33] JI Y F, ZHOU L, ZHANG Y, et al. Photochemical degradation of sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid in different water matrices [J]. Water Research, 2013, 47(15): 5865-5875. doi: 10.1016/j.watres.2013.07.009 [34] ZHANG Y, del VECCHIO R, BLOUGH N V. Investigating the mechanism of hydrogen peroxide photoproduction by humic substances [J]. Environmental Science & Technology, 2012, 46(21): 11836-11843. [35] 周磊. 天然水体中防晒剂对氨基苯甲酸和碳纳米管的光化学行为研究[D]. 南京: 南京大学, 2014. ZHOU L. Photochemical behavior of sunscreen agent p-aminobenzoic acid and carbon nanotubes in natural waters[D]. Nanjing: Nanjing University, 2014(in Chinese).
[36] ARNAO M B, ACOSTA M, del RÍO J A, et al. A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide [J]. Biochimica et Biophysica Acta, 1990, 1041(1): 43-47. doi: 10.1016/0167-4838(90)90120-5 -
 王梦杰.pdf
王梦杰.pdf
-




 下载:
下载: