-
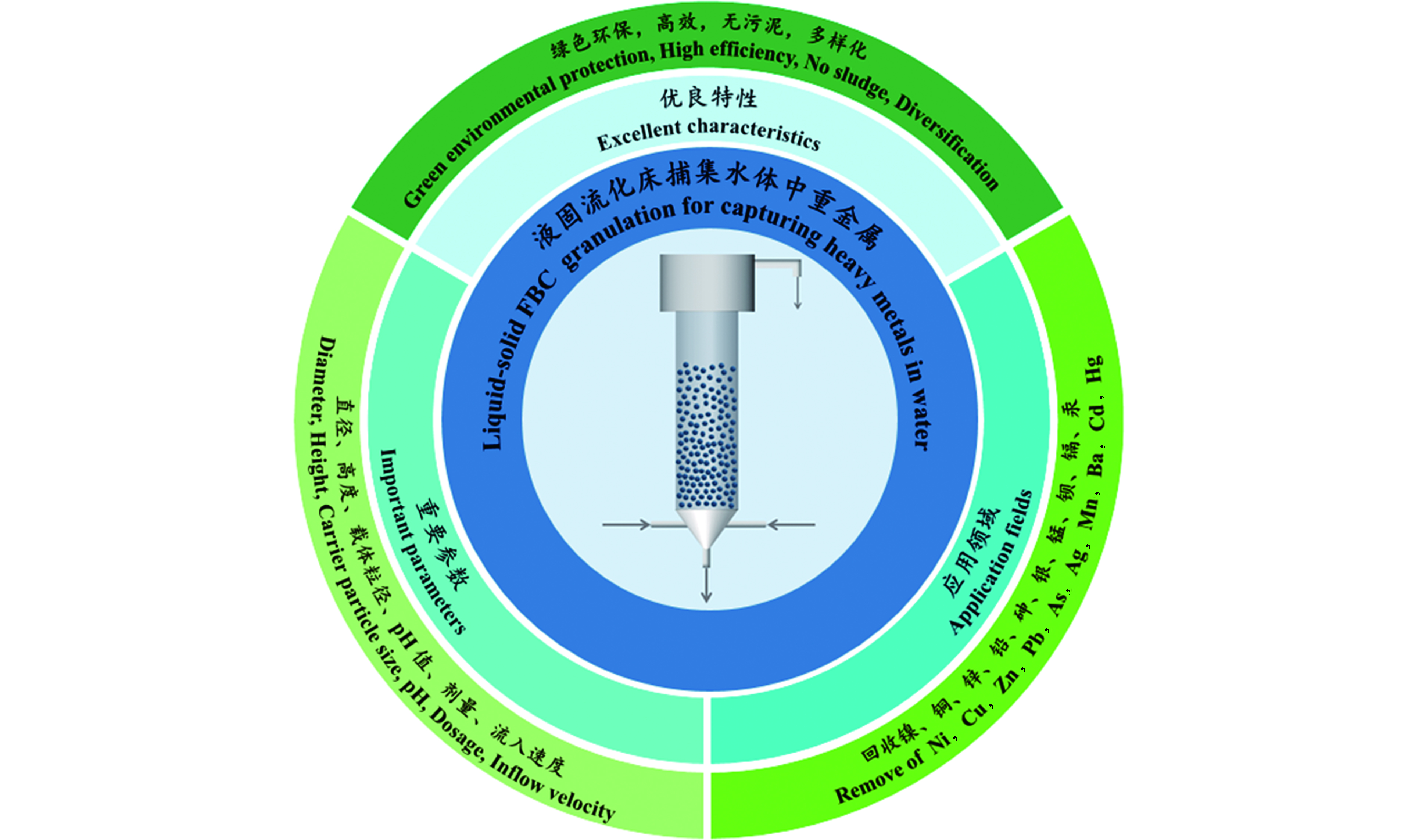
随着现代工业的迅猛发展,更多的工业废水和人工合成物质不断地流入水体,造成水体污染严重[1]。重金属是水源水和处理水中最重要的污染物之一[2],主要来自电镀、采矿、冶金、化工等工业,具有潜在的危害性,特别是汞、镉、铅等重金属具有显著的生物毒性[3]。如果未经处理或处理不彻底的废水渗入到自然环境中,对环境的污染是持久性的,它无法被降解和破坏,当水中的重金属达到一定含量时,将会破坏生态,毒害水体生物[4-5]。随着绿色环保的可持续发展战略的提出,重金属废水处理迫在眉睫[6]。目前,重金属废水的处理方法有沉淀法、电化学法、吸附法、膜分离法、离子交换法等[7]。在实际处理过程中,采用组合工艺对重金属废水进行处理,尽管最终可以使出水重金属浓度达标排放,但是处理成本较高,且不能回收产生的沉淀污泥。本文介绍的流化床结晶造粒技术具有传质速率高、占地面积小、不产生污泥、颗粒含水率低等优点,其相关概述见图1,涵盖了该技术的优良特性、影响因素和参数、应用领域及其优点,流化床结晶造粒技术在国内外已取得了成功探究和实际应用,它在重金属废水处理领域有着较好的应用前景。
-
流态化是一种固体颗粒与流体接触的方法。流体向上流经固体颗粒床,使固体颗粒具有流体性能,这种现象称为颗粒流态化[8]。当流体为液体时,称为液固流化床。
流态化过程的实现需要达到最小流化速度。最小流化速度指当流体通过颗粒床层时,随着流速的增加,颗粒由静止状态转为运动状态,当流体向上所产生的曳力等于颗粒床层的重力时,或当流体通过床层时的压力降刚好等于单位床截面上颗粒重量时,颗粒开始流态化,此时的流体表观线速度称为最小流化速度,或临界流态化速度[9]。
-
液固流化床结晶造粒是采用流态化原理强化结晶过程的一种技术。通过预先添加结晶载体,诱导异相形核,具有反应面积大,结晶效率高[10],结晶体含水率低,易于液固分离的特点。通过流化床结晶造粒回收利用水体中的重金属,是一个重要的应用领域。
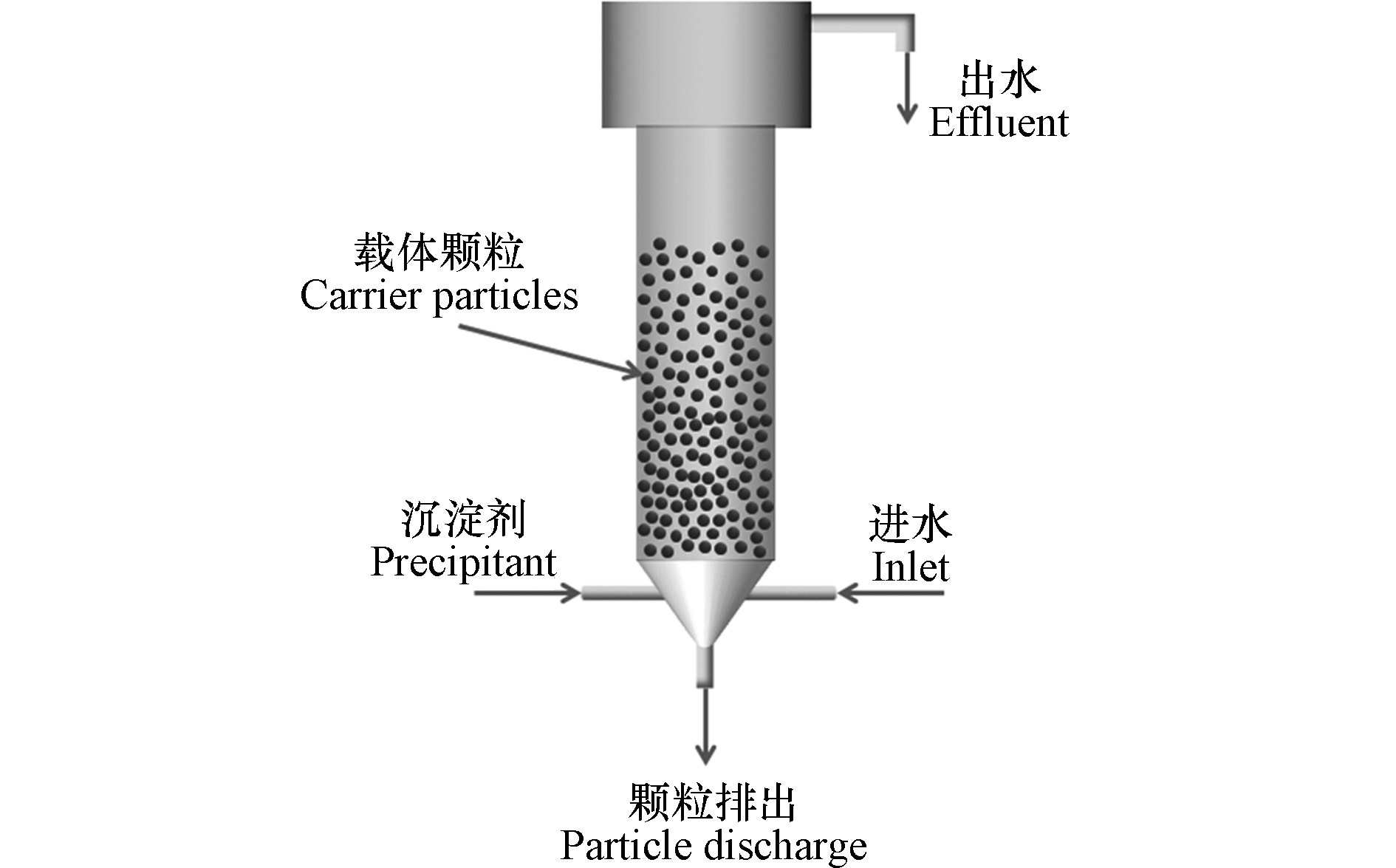
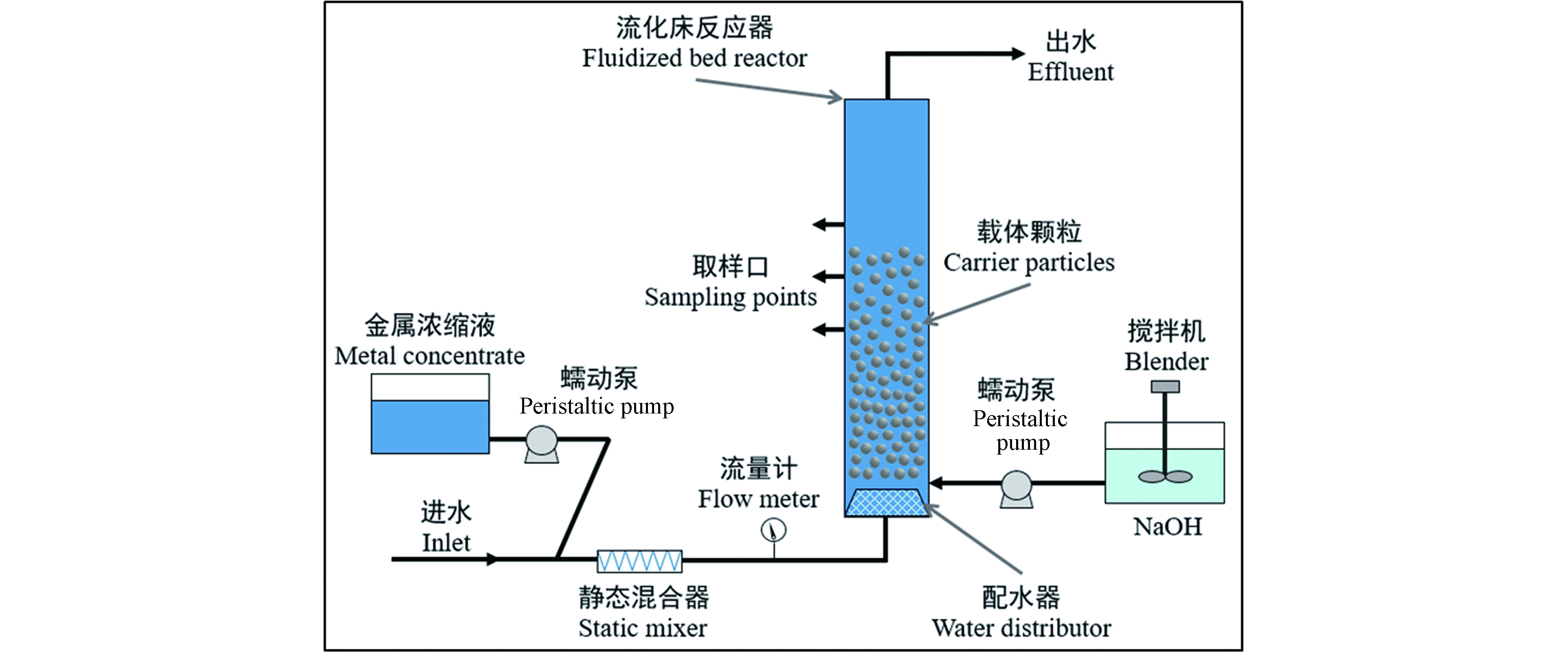
流化床结晶造粒技术从废水中去除和回收重金属是在反应器底部填充载体颗粒,以一定的流速从流化床底部引入废水,使载体处于流化状态,同时向反应器内添加合适的沉淀药剂(Na2CO3、NaS等),其酸碱度调节剂一般采用NaOH、H2SO4等。沉淀药剂能使溶液达到特定过饱和状态,生成的不溶性物质(碳酸盐沉淀、硫化物沉淀、磷酸盐沉淀或氢氧化物沉淀)在载体上非均相结晶,如图2所示。随着结晶过程的进行,载体颗粒直径和重量不断增加,并逐渐沉降到反应器的底部。沉降到反应器底部的颗粒被定期回收,同时向反应器中添加新的载体种子,流化床反应器继续运行。
流化床结晶造粒技术是根据物质的溶度积原理来实现的,促使废水中的金属离子向难溶沉淀物方向进行结晶。溶度积的大小反映了物质的溶解能力,溶度积越小,就越难溶解。表1列出了常见重金属难溶盐的溶度积常数。
-
(1)进水浓度及沉淀药剂
废水中金属离子的浓度和性质决定了所选择的沉淀药剂的种类和投加量。一般来说,废水中金属离子浓度较低时,相应的沉淀药剂的投加量较少,废水中金属粒子浓度较高时,沉淀药剂的投加量就相应成比例增加。在现有的研究中,主要根据离子的不同来选择沉淀药剂的种类。例如,若要捕集废水中的镍离子,会选择Na2CO3为沉淀药剂,得到碳酸镍的晶体颗粒[21-22]。若捕集废水中的砷,则采用NaS作为沉淀剂,得到AsS或者As2S3的结晶[23]。但采用流化床结晶造粒捕集回收重金属离子大部分情况下采用的沉淀剂为Na2CO3。因为流化床结晶造粒捕集到的金属碳酸盐是纯净的,可以通过在强酸中溶解颗粒来重复利用,碳酸盐以二氧化碳的形式逸出,产生一种纯净的浓缩金属溶液,而载体颗粒砂可以再次用于反应器中,纯浓缩的金属溶液可在金属表面处理、化学和金属加工行业中重复使用[21]。从经济和环境的角度来看,碳酸盐金属结晶时最具有吸引力的,因为苏打是一种相对便宜的化学品,并且将捕集颗粒溶解在强酸中进行金属回收再利用是容易且经济的。
(2)载体选择
选择载体的基本条件是,流化性能强、诱导沉淀反应能力强、与沉淀物结合性好、沉降性能好、性质稳定、比重大、无磁性、无腐蚀性、具有经济性。流化床结晶造粒捕集重金属离子中常常采用石英砂作为载体,少部分应用研究采用沉淀目标物晶种为载体。对于载体尺寸的选择为毫米级,常用粒径一般在0.1—0.5 mm之间[24-28]。为保证加入的载体满足工艺要求,加入前需要对载体进行洗涤和水力筛选,达到去除载体中的污染物和过于细小的颗粒。
(3)水力条件
流化床中的水力条件需要兼顾载体流态化、沉淀结晶效率以及载体和沉淀物不会破裂等因素。适当的水力条件可削减结晶颗粒物晶界膜的厚度,从而有利于结晶反应的进行;过大的水力作用会导致载体颗粒物磨损或破碎而导致细小晶体随出水流出,从而降低流化床结晶造粒的废水处理效果。
(4)进药比
进药比指沉淀药剂与目标金属离子的物质的量比。其值决定了反应体系的溶液过饱和度,进药比的适当增加,结晶反应过程的推动力变大,从而可以促使处理废水的效率提高,但过大的进药比会造成药剂利用率的下降、工艺成本的升高。针对不同离子有最佳的摩尔比范围,例如,镍离子捕集中,进药比的[CO32−]/[Ni2+]比值为2时能得到较好的去除效果[22]。铜离子捕集中,进药比[CO32−]/[Cu2+]的比值为1—2时效果较好[29-31]。
-
Ni、Cu、Zn、Pb、As、Ag、Mn、Ba、Cd和Hg是重金属废水处理中受关注度较高的主要微量元素。流化床结晶造粒技术处理重金属废水相比较于传统的化学沉淀技术具有显著的优势。传统的沉淀法通过控制特定的工艺条件和系统设置,得到含水率为60%—80%的污泥。流化床结晶造粒技术捕集回收的颗粒是纯净的,含水量仅为1%—5%的结晶颗粒,含水率低,容易固液分离,颗粒可回收利用,且处理后的废水达到排放标准,节省了大量的废物处理成本。这些发现完全符合国家倡导的可持续发展和循环经济政策。表2显示了流化床结晶造粒处理重金属废水的研究报告。
-
1980年,荷兰的DHV就研发了一种球团反应器中结晶系统,用于从化工、金属加工和电镀行业的几乎所有类型的废水中回收重金属。1987年,在重金属回收领域的第一个全规模的镍回收工厂已投入运行,反应器直径为0.6 m,反应器系统结构紧凑,投资成本相对较低,结晶过程稳定,操作容易[32]。Wilms等[21]利用流化床反应器结晶碳酸镍回收废镀液中的镍,载体采用石英砂,填充高度为1 m。沉淀药剂采用浓度为1.06 g·L−1的碳酸钠溶液,合成镍废水浓度为1350 mg·L−1。结果表明,出水镍浓度低于0.5 mg·L−1,得到直径约为1 mm的碳酸氢镍颗粒,通过将颗粒溶解在HCl或H2SO4中,获得氯化镍的纯溶液,说明了流化床结晶造粒技术处理镍废水是有效的。
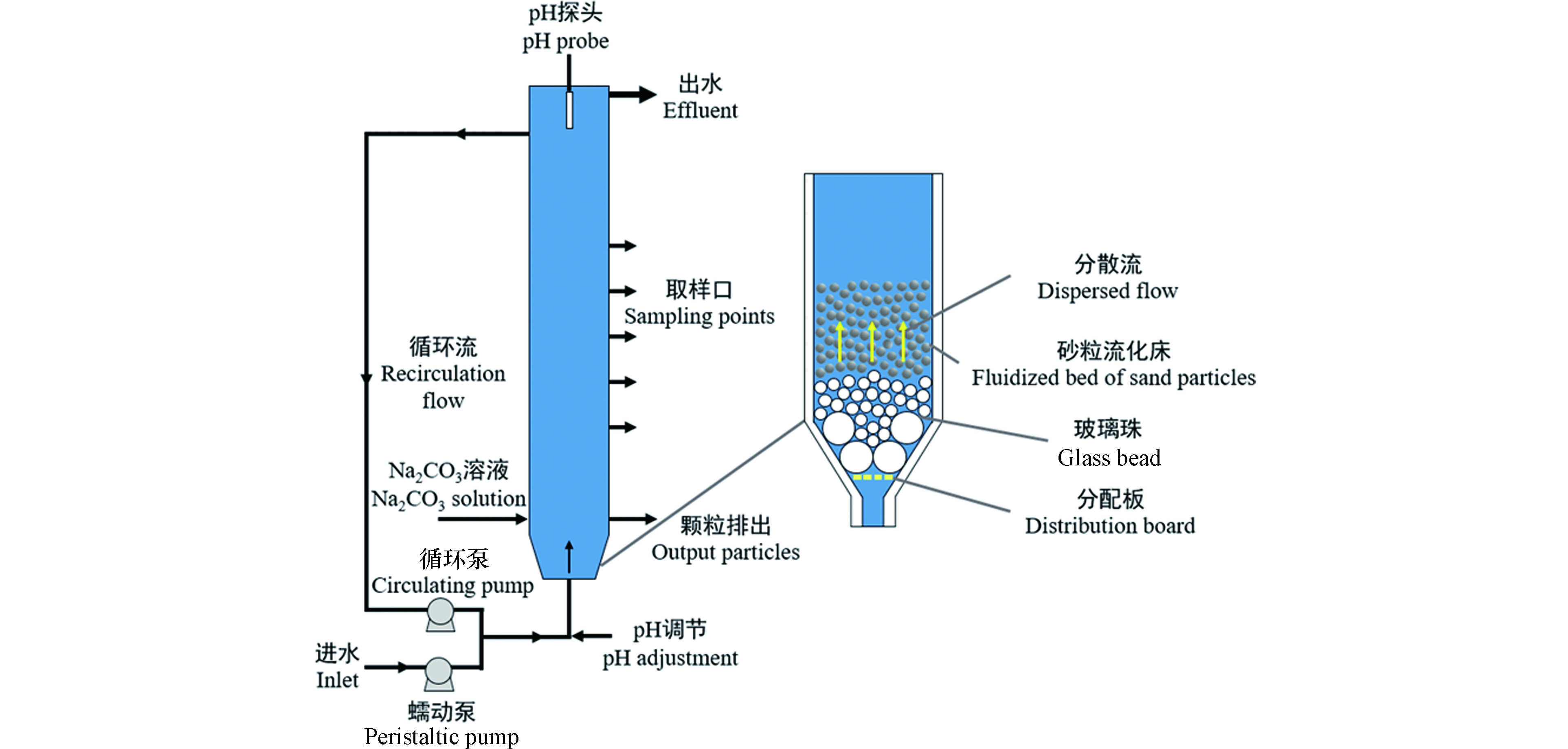
Guillard等[22]研究了流化床中碳酸镍的沉淀。实验装置如图3所示,反应器内载体采用粒径为0.21—0.30 mm的白色石英砂,填充高度0.6 m。沉淀剂为3.6 g·L−1的无水碳酸钠溶液。合成废水的镍浓度为50—150 mg·L−1,进水流量为3.6 L·h−1,沉淀剂流量为1.08 L·h−1,温度为24 ℃,当反应pH为9.8,[CO32−]/[Ni2+]物质的量比为2∶1和4∶1时,合成废水镍的去除率分别为99.6%和97.2%。在前述研究的基础上,Guillard等[33]研究了去除镍的最佳工艺并讨论了系统参数对去除效率的影响程度,当反应pH 9.97,[CO32−]/[Ni2+]物质的量比为3.5,废水镍浓度为69.5 mg·L−1,进水泵转速为43.9 r·min−1时,出水中降至1.37 mg·L−1,镍的去除率达到98%。该研究中[CO32−]/[Ni2+]物质的量比、酸碱度、进水镍浓度具有最显著的影响,再循环比和进料点数目对工艺效率的影响最小。
Costodes等[34]研究了进料点数量变化对结晶造粒过程的影响,对比了2个进药口与6个进药口两系统的情况,研究结果表明,2个进药口系统在床层中存在着底部高、顶部低的过饱和再分配不均的现象,导致了均匀形核导致细粒的过量产生。用6个进药口系统得到了更均匀的过饱和再分配,可有效控制和降低局部过饱和水平,提高除镍效率。并且前者结晶是通过均相成核进行,后者由于过饱和的均匀分布,镍的去除通过异相成核到硅砂上生长聚集。Salcedo等[35]研究了在流化床中均相结晶回收合成废水中的镍,通过改变进水镍浓度、进药比和沉淀剂的酸碱度,当进水镍浓度为300 mg·L−1,[CO32−]/[Ni2+]物质的量比为1.5时,pH 10.8时,镍离子去除率为84.93%。在合成瓦槽废水中添加钙离子提高了造粒效率,但降低了合成废水中镍的去除率。钙离子的存在可能已经在碳酸盐的反应中与镍竞争,发生CaCO3和Ca(OH)2的形成,形成的颗粒很脆,当它们相互碰撞时,就会发生磨损。因此,随着钙浓度的增加,去除效率降低。
Ballesteros等[11]采用流化床均相造粒去除镍,通过测定不同的进水镍浓度、进药比和pH,确定了最佳工艺条件:镍浓度为200 mg·L−1,[CO32−]/[Ni2+]物质的量比为2.0,pH 10.7,镍去除率为98.80%,制粒效率为97.80%,但由于反应时间的延长、流化引起的粉末碰撞等因素,形成的颗粒团聚程度较高,通过能谱分析结果碳、氧、镍的原子百分比约为12%、35%、50%,与碳酸镍一致。在卢明俊等[36]的专利中,以流化床均相结晶技术合成碱式氧化镍(NiOOH)结晶物,将含镍溶液与氧化药剂引入反应器混合以产生碱式氧化镍颗粒晶体,调整水质条件包括pH、截面负荷、水体停留时间,所获得的处理效率与结晶颗粒纯度高。
流化床结晶造粒工艺处理镍废水可同时实现废水中镍的去除和回收,已有良好的应用实例,因此可认为该方法在镍废水处理领域极具研究和应用价值。对于镍废水的处理,下一步可着重对实际废水中其他成分对结晶过程的影响以及如何减少不利因素等方面进行系统地研究。
-
Lee等[29]研究了含铜废水在砂粒表面的沉淀,考察了pH值、进药比、水力负荷和化学试剂类型等参数的影响。随着水力负荷的增大,流化床中颗粒受到相互碰撞会在溶液中形成微粒,从而导致铜的去除量减小。最佳工艺条件下:进水铜浓度为10 mg·L−1,pH 8.4—8.6,[CO32−]/[Cu2+]的物质的量比为2,水力负荷不超过25 m·h−1,铜去除率达到96%,结晶产物结构致密,具有一定的强度,且主要构成元素为铜和氧,其中铜约占总量的50%,适于回收。
阎中等[30]研究了诱导结晶处理含铜废水,在最佳工艺条件下,进药比为1—2,水力负荷为13 m·h−1,水力停留时间为30 min,连续运行145 d,处理浓度为20 mg·L−1、50 mg·L−1、100 mg·L−1的含铜废水,去除率均可以达到90%以上,去除和回收铜总计212 g,结晶颗粒为碱式碳酸铜。
卢明俊等[31]的专利中提出了一种以流化床结晶技术合成均质碱式碳酸铜及氧化铜结晶物,废水初始铜浓度为1300 mg·L−1,[CO32−]/[Cu2+]物质的量比为2,水力停留时间为16.7 min,pH 6—8,得到碱式碳酸铜颗粒;当反应pH>8时,可获得氧化铜颗粒。
Lertratwattana等[37]通过流化床均相结晶从冶金工业废水中去除和回收铜,废水铜浓度为400 mg·L−1时,在双酸碱度操作模式下,pH 6—8,[CO32−]/[Cu2+]物质的量比为1.5,进水流速为0.6 L·h−1,铜去除率为92%,颗粒回收率为96.3%。对球状晶体产物的表征确定了孔雀石是唯一的晶相,氢氧化铜沉淀是孔雀石的主要晶相组成。有专利[38]提到一种结晶铜的硫化物的方法和设备,使用流化床反应器将铜结晶成硫化物,达到去除污水中的铜的目的,同时使材料能够再循环。药剂采用硫化钙(CaS)或者九水合硫化钠(Na2S·6H2O),最终得到硫化铜结晶。
在流化床结晶造粒工艺下,含铜废水可成功从废水中结晶为碱式碳酸盐、氧化物及硫化物,并且废水中铜的去除率较高,具有易控制、效率高、出水水质好、无二次污染等优点,实现了铜废水的高效处理。
-
Jansen[39]提出了一种从废水中去除锌的方法,在反应器载体上结晶为相应的重金属碳酸盐沉淀。圆柱形反应器中采用砂粒作为结晶载体,沉淀药剂为碱金属碳酸盐或重碳酸盐(例如:Na2CO3、NaHCO3、K2CO3、KHCO3)。对于锌浓度为45 mg·L−1的废水,载体填料高度为2 m,进水流速为40 m·h−1,pH 7.5—8时,锌去除率可达到95%。工艺取出的碳酸盐颗粒(粒径约为1—3 mm)含水率低于0.5%,非常适用于电镀工艺的废水处理。
杨艳等[40]研究了诱导结晶处理含锌废水,对进药比、进水pH、等工艺参数进行了讨论,对不同浓度的进水进行了对比试验。得到了最佳工艺条件:沉淀药剂为硫化钠,废水锌浓度为80—100 mg·L−1,进药比[S2−]/[Zn2+]为1—2,pH 9,水力负荷15 m·h−1,水力停留时间30 min,出水含锌量为1.0 mg·L−1左右,去除率达到95%。
Udomkitthaweewat等[19]研究流化床造粒工艺去除螺杆生产废水中的锌。不添加载体,沉淀药剂为Na2CO3,进水锌浓度为500 mg·L−1,进水流量和药剂流量为1.5 L·h−1,[CO32−]/[Zn2+]物质的量比为1.2,pH 7.2,出水锌浓度为19 mg·L−1,锌去除效率为97.56%,结晶率为93.39%。
专利[41]提出了一种采用流化床结晶技术合成均质含锌结晶物的方法,当锌废水的浓度为100 mg·L−1,[CO32−]/[Zn2+]物质的量比为1.2,反应pH 8,停留时间为10—50 min时,工艺除锌效率为99.9%,出水的锌浓度为0.15 mg·L−1。
-
Chen等[42]在流化床反应器中结晶去除合成废水中的铅。最佳工艺条件:载体为白色石英砂,填充高度为反应器总高度的0.25—0.3,沉淀剂为Na2CO3,进水铅浓度为40 mg·L−1,pH 8—9,进水[CO32−]/[Pb2+]物质的量比为3:1,进水流速小于22 m·h−1,反应器运行380 min时,出水铅浓度为1 mg·L−1,铅去除率达到99%。
Luna等[43]用结晶法在流化床反应器中去除和回收铅。证明了流化床反应器结晶过程去除和回收合成废水中铅的潜力,并且确定了流化床中种子结晶可以产生较高的铅转化效率。载体采用粒径为0.053—0.062 mm的PbCO3颗粒,沉淀剂为Na2CO3,废水铅浓度为200 mg·L−1,pH 8—9,[CO32−]/[Pb2+]物质的量比为3,进水和沉淀药剂的流量为0.36 L·h−1,再循环流量为11.1 L·h−1,采用2.5 g种子晶粒,铅去除率达到98%。
Chen等[20]研究了以硅砂为种子的流化床结晶工艺和无载体的流化床均相结晶工艺对含铅废水的处理效果。在不同的pH条件下,两种晶相(PbCO3和Pb3(CO3)2(OH)2)的碳酸铅被回收,其中均相结晶颗粒尺寸为100 μm,表明通过流化床均相结晶去除铅是可行的。
-
Lee等[23]的专利中通过使用填充载体流化床的反应器从水中去除砷。沉淀剂采用硫化钠溶液。进水砷浓度为611 mg·L−1,进水流量为0.3 L·h−1,沉淀药剂流量为1.6 L·h−1,进药比为2,pH 1,结晶率为93%,反应器内排出橙色晶体(雄黄AsS和雌黄As2S3的混合物)直径为1—3 mm,处理出水砷浓度为7.2 mg·L−1,经过超滤系统后砷浓度为0.5 mg·L−1。
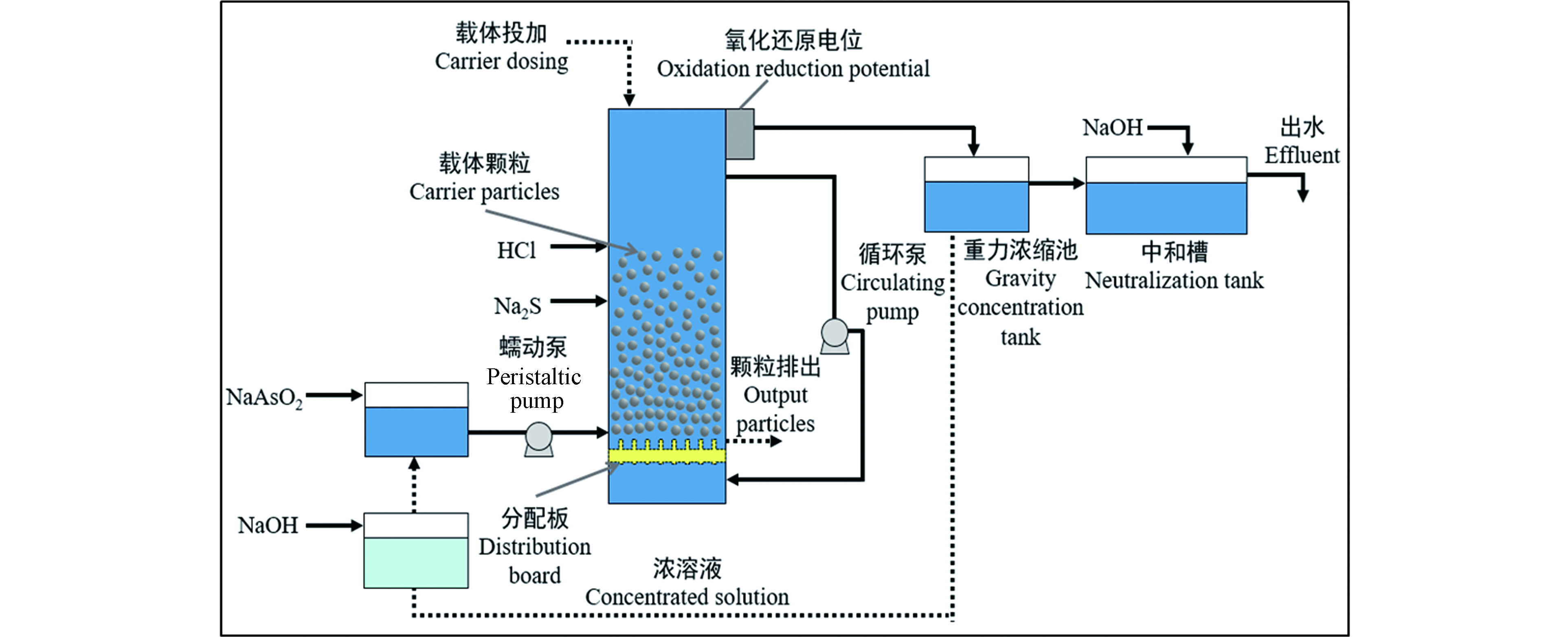
Huang等[44]研究了流化床结晶法处理高浓度含砷废水。实验装置如图4所示,载体为硅砂。沉淀药剂为硫化钠(Na2S)和亚砷酸钠(NaAsO2),对进药比、pH和进水砷浓度进行了变量实验,硫化物投加量和操作pH值是确定出水残余As浓度的两个最重要的参数,实验结果表明满足砷排放条件的最佳pH 2,进药比为2.2,反应器出水中的砷浓度小于0.5 mg·L−1。
-
Wilms等[45]以石英砂为载体在流化床反应器中结晶碳酸银,在最佳工艺条件下:石英砂粒径为0.2—0.3 mm,填充高度为0.6 m,沉淀药剂为Na2CO3,[CO32−]/[Ag+]物质的量比为3,水力负荷为45 m·h−1,出水pH 10.2,出水中的银浓度低于8 mg·L−1,排出颗粒为Ag2CO3,粒径为0.6 mm,质量分数大于99%,研究表明,流化床结晶造粒捕集回收银是从废水中回收银的经典方法的一种有价值的替代方法。
有一篇专利[46]提出了一种流化床结晶回收锰的方法。可用于工业废水,特别是含有高浓度溶解性锰的水的处理。载体材料采用锰砂(也可采用粒径为0.1—5.0 mm的粒状物质:砂、蒽石、活性炭、碳化物、树脂等),填充高度优选1—3 m,药剂采用氢氧化钠、碳酸钠等,反应器设置多个进药口。原水溶解锰浓度为8.5 mg·L−1,pH 9.5,进水速度为25 m·h−1,处理水锰浓度为0.5 mg·L−1。
Su等[47]采用流化床结晶法回收钡,研究了流体上升速度、载体加入量、载体粒度等工艺条件对钡盐晶体生长的影响。当上升流速为2.8 L·h−1,进药比为1.0,pH 8.4—8.8时,钡的去除率为98%,回收得到平均粒径为0.36 mm的颗粒。XRD结果表明,在pH小于10时,结晶产物为BaHPO4以及微量BaO,在pH为11时,结晶产物为Ba3(PO4)2。
Dotremont等[48]通过在流化床反应器内研究了碳酸镉结晶的最佳条件,载体石英砂采用粒径为0.2—0.3 mm,填充高度为1 m。当[CO32−]/[Cd2+]物质的量比为1.6,pH 7.9时,处理镉浓度为1120 mg·L−1的合成废水,出水镉浓度为1 mg·L−1,捕集到的颗粒为CdCO3和少量Cd(OH)2的混合物,粒径为1 mm,溶解于强酸可得到镉离子浓溶液,进行回收利用。
Janssen[24]的专利中研究了从废水中去除汞金属的方法。载体采用粒径为0.1—0.3 mm的沙子,沉淀药剂可用碱金属硫化物(例如Na2S、K2S)或者碱金属硫氢化物(例如HS、KHS)或者硫化铵或硫化亚铁。pH 4—5,处理Hg浓度为5—20 mg·L−1的废水,出水Hg浓度为0.020—0.055 mg·L−1,经过滤后Hg浓度为0.002 mg·L−1,工艺结晶产物为粒径1—3 mm的硫化物颗粒。
从采用流化床结晶造粒处理镍、铜废水及其它各类单一金属废水综合可以得知,该技术处理镍、铜废水方面已被证实了长期稳定性,但在锌、铅、砷等金属方面的研究只是进行了一些可行性的探究,需要进行深入的探讨和优化工艺参数。
-
流化床结晶造粒工艺处理单一重金属废水取得了良好的效果,为了考察处理多种重金属废水的可行性,学者们做了大量的研究,Nielsen等[25]利用流化床反应器去除烟气脱硫废水中的重金属,研究表明流化床结晶造粒技术已被证明能够处理工业废水和地下水中的几种溶解重金属,载体颗粒为石英砂,沉淀药剂为KMnO4,进水镍、镉、锌浓度分别为510 、640、1900 mg·L−1,当废水流量为32 L·h−1,pH 7.2时,出水镍、镉、锌浓度分别为7 、53 、50 mg·L−1,镍、镉、锌的回收率分别为99%、92%、97%。产生的致密颗粒密度为2.5—3.0 kg·L−1,人工脱水后含水量低于20%。
Zhou等[18]开发了一种去除工业废水中重金属的新工艺。串联两个相同规模的流化床反应器进行实验,载体采用粒径为0.15—0.30 mm的沙子,填充高度为0.4 m,沉淀剂Na2CO3,重金属废水为铜、镍、锌的合成废水,当pH 9—9.1,废水中每种重金属离子浓度为10 mg·L−1和20 mg·L−1时,去除率达到92%和95%;当反应pH大于8.7时,92.4%以上的沉淀为更加不溶于水的氢氧化物。
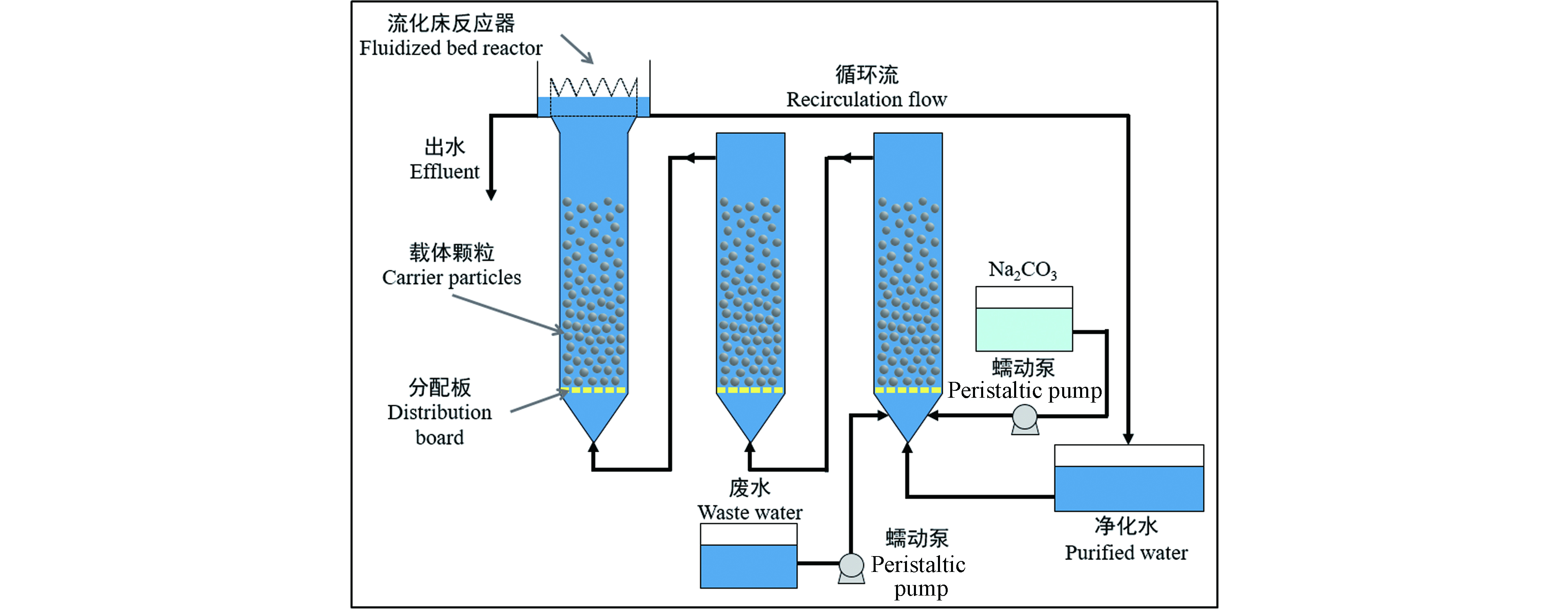
Lee等[26]采用顺序流化床反应器去除合成废水中的重金属,对比于单个流化床反应器,顺序式流化床反应器的重金属去除效率和进水金属浓度限值均占优势。如图5所示,载体采用粒径为0.25—0.42 mm的石英砂,填充高度为0.3 m。
合成废水中Cu浓度为250 mg·L−1、Pb浓度为130 mg·L−1、Ni浓度为130 mg·L−1。当[CO32−]/[Cu2+]=1.5、[CO32−]/[Pb2+]=3、[CO32−]/[Ni2+]=3时,三者的去除率分别为97%、96%、93%。经滤纸过滤后Cu浓度几乎为0 mg·L−1、Pb浓度为0.3 mg·L−1、Ni浓度为0.8 mg·L−1。结晶产物每颗砂中金属体量约为1.16 g。
孙杰等[27]研究了诱导结晶新工艺处理重金属废水,考察了不同浓度的重金属废水的处理情况,当进水流量为25 L·h−1,进药流量为4 L·h−1,水力停留时间为1.8 min,进药比为1.2:1时,沉积在硅砂表面的重金属的去除率最高可达95%,对反应饱和后的硅砂可采取加酸溶解回收重金属会采用水泥固化硅砂的方法,从而达到对重金属废水的最终无害化处理。
唐章程等[28]通过中试实验考察了结晶造粒流化床技术对水中铁、锰的同步去除效果及影响因素,确定了相关工艺条件。实验装置如图6,载体为方解石,填充高度为0.5 m,进水铁浓度为0.87—0.94 mg·L−1,锰浓度为1.86—1.95 mg·L−1,pH 9.6时,出水中锰浓度降低至0.061 mg·L−1,铁浓度为0.246 mg·L−1,结晶颗粒物中铁和锰分别以FeOOH和Mn3O4、MnO2形态存在,所占比例分别为50.14%、49.86%。
-
虽然目前研究中的数据大部分处于实验研究阶段,但在荷兰、中国台湾等发展基础较深厚的地区已经得到了应用。这已经充分说明流化床结晶造粒工业应用的可行性和具有良好的发展前景。这些综合的信息可以帮助学者们进行进一步的探索研究。重金属废水经流化床结晶造粒技术处理后,重金属离子去除率较高,处理后的废水可进行标准化排放,且系统不产生污泥。金属包覆颗粒含水率低,且结构密实,易溶解于强酸,可重新投入工业使用。由所综述的综合文献可知,研究中的流化床反应器内径由2—60 cm,高度由0.6—5.5 m,可处理废水浓度由5—1900 mg·L−1,既能够处理单金属废水,也可以处理多金属混合废水,采用流化床结晶造粒工艺去除重金属是一种回收金属、实现资源化利用的好方法。
尽管该技术已得到了令人鼓舞的结果,但仍需要进行更多的研究来解决文献中的一些空白和相关问题:
(1)液固流态化的实现很大程度上依赖于以往的经验方法。众所周知,流化床反应器的操作很复杂,而且针对于不同的水质,需要灵活调节操作参数,因此,使用其处理重金属废水是具有挑战性的。已有的研究大多报道了处理效果以及一些参数对处理效果的影响。但是基于流态化模型的理论研究不多,在此基础上,还需要对流化床反应器在废水处理中的工艺优化进行进一步的理论研究。
(2)从实验室小规模的研究转型至实际应用的大装置,该过程药剂的使用量和能耗不容易被接受。而且还应考虑流化床反应器的扩大设计以便于使其得到实际应用。虽然已有部分大型设备得到了应用,但是其工艺能耗等方面还需要改进。例如:在颗粒排出方面,装置的自动化程度还需要进行优化。
(3)处理每种金属离子的反应条件不相同,对于不同的工业废水需要进行不同处理研究,从实验室的探索研究,到中试试验,再进行工业化处理,最后投入到工业生产中。在这个长期的研究过程中需要科研工作者克服各种困难,不断前进。
从开始应用到现在,该技术研究的仍然火热。近年来,人们对这一课题的兴趣与日俱增,很明显,流化床结晶造粒技术在重金属废水处理中能够得到充分的发挥使用。总体而言,该技术具有改善工艺性能和降低各种重金属废水处理的成本。若处理得当,在解决实际排放问题的同时还能够实现废水资源化应用。因此,采用流化床结晶造粒技术处理重金属废水的规模化研究具有巨大的发展空间和应用前景。
液固流化床结晶造粒技术捕集水体中的重金属
Liquid solid fluidized bed crystallization granulation technology for capturing heavy metals in water
-
摘要: 流化床结晶(FBC)造粒技术是一种强化诱导结晶的过程,可以同时实现水体净化和回收捕集水体中有价资源的目标。该技术具有传质速率高、不产生污泥、结晶颗粒含水率低、易于固液分离、绿色环保的优点。本文综述了技术的发展背景、工艺基础、工作原理,分析了进水浓度、沉淀药剂、载体、水力条件等因素对FBC处理重金属废水效果的影响。综述了国内外FBC造粒技术处理镍、铜、锌、铅、砷等单一废水和多金属混合废水的研究进展,应用研究表明该技术处理重金属废水是无害化、资源化的优良工艺。不仅解决重金属废水的排放问题,还将水体中的重金属转化为金属盐,可再利用于金属行业,对环境十分友好。最后指出了目前研究中存在的问题以及今后的研究方向和重点,对该技术的发展进行了展望。Abstract: Fluidized bed crystallization (FBC) granulation technology refers to the process of enhanced induced crystallization, which can simultaneously achieve the goal of water purification and recovery of valuable resources in water. This technology have multiple advantages, namely, high mass transfer rate, no sludge, low moisture content of crystalline particles, easy solid-liquid separation and environmental protection. This work summarized the development background, process basis and working principle of the technology by analyzing the influence of influent concentration, precipitant, carrier, hydraulic conditions and other factors on the treatment of heavy metal wastewater by FBC. Moreover, the research progress and optimal process conditions of single wastewater and multi metal mixed wastewater treatment by FBC granulation technology at home and abroad have been summarized. Used in the metal industry and being very friendly to the environment, the application research reveals that this technology is harmless and resource-base, solving heavy metal wastewater discharge problems as well as converting the heavy metals in water into metal salts. Finally, the paper sheds light on the existing problems and future research directions and made it prospective argument on the development of the technology.
-
Key words:
- fluidized-bed /
- fluidization /
- heavy metal /
- waste water /
- crystallization
-

-
图 3 流化床反应器示意图[22]
Figure 3. Schematic representation of the fluidized bed reactor
图 4 FBC处理高浓度砷废水工艺流程图[44]
Figure 4. Diagram of FBC process for the treatment of wastewater containing high-strength As
图 5 顺序流化床实验装置示意图[26]
Figure 5. Schematic diagram of sequential fluidized bed experimental device
图 6 实验装置示意图[28]
Figure 6. Schematic diagram of experimental device
表 1 常见重金属难溶盐的溶度积
Table 1. Solubility product of common heavy metal insoluble salt
金属
Metals沉淀物
Precipitate溶度积常数
Ksp来源
SourceNi NiCO3 1.40×10−7 [11] Ni(OH)2 5.50×10−16 [11] Cu CuCO3 1.40×10−10 [12] Cu(OH)2 4.80×10−20 [13] Cu2(OH)2CO3 5.99×10−35 [14-16] CuS 6.31×10−36 [17] Zn ZnCO3 1.46×10−10 [18] Zn5(CO3)2(OH)6 2.00×10−9 [19] Zn Zn(OH)2 3.00×10−17 [18] Pb PbCO3 7.41×10−14 [20] Pb(CO3)2(OH)2 1.58×10−19 [20] As As2S3
Ca5(AsO4)3(OH)4.00×10−29 [20] 9.10×10−39 [20] Ag Ag2CO3 7.94×10−12 [17] Mn MnCO3 2.50×10−11 [17] Mn(OH)2 2.00×10−13 [17] Ba Ba3(PO4)2 5.01×10−30 [17] Cd CdCO3 1.0×10−12 [17] Cd(OH)2 2.51×10−14 [17] Hg HgS 3.98×10−53 [17] 表 2 流化床结晶造粒技术回收重金属的应用
Table 2. Application of fluidized bed crystallization granulation technology for heavy metal recovery.
金属类型
Type of metal反应器和载体特性
Reactor and carrier properties操作条件
Operational conditions性能
Performance来源
SourceNi D: 2.1 cm,H: 240 cm
Carrier: SiO2
DC: 0.4—0.5 mmPre: Na2CO3,pH: 10
FH: 0.1 mCout Ni2+: 0.5 mg·L−1
ECS: 1.0 mm[21] Ni D: 2.5 cm,H: 100 cm
Carrier: SiO2 and CaCO3
DC: 0.21—0.30 mmCin Ni2+: 150 mg·L−1
Pre: Na2CO3,pH: 9.8
CO3:Ni= 2:1
FH: 0.6 m
Qin: 3.6 L·h−1Removal rate: 99.6% [22] Ni D: 2.5 cm,H: 160 cm
Carrier: SiO2
DC: 0.25 mmCin Ni2+: 100 mg·L−1
Pre: Na2CO3,pH: 9.68
CO3:Ni= 2:1
FH: 0.2 m
Qin: 1 mL·min−1Removal rate: 99% [34] Ni DL: 2 cm,HL: 80 cm
DU: 4 cm
HU: 20 cmCin Ni2+: 300 mg·L−1
Pre: Na2CO3,pH: 10.8
CO3:Ni= 3:1Removal rate: 97.08%
ECS: 0.25—2 mm[11] Ni HL: 80 cm
Carrier: NiOOHCin Ni2+: 1470 mg·L−1
Pre: Na2CO3,pH: 9.68
CO3:Ni= 1:1
FH: 0.5 m
Vin: 42.9 m·h−1Removal rate: 99.6%
Cout Ni2+: 2.31 mg·L−1[36] Cu D: 3 cm,H: 120 cm
Carrier: SiO2
DC: 0.25—0.42 mmCin Cu2+: 10 mg·L−1
Pre: Na2CO3,CO3:Cu= 2:1
FH: 0.45 m
Vin: 25 m·h−1Removal rate: 96% [29] Cu DL: 3 cm,HL: 50 cm
DU: 9 cm,HU: 10 cm
Carrier: SiO2
DC: 0.2—0.3 mmCin Cu2+: 100 mg·L−1
Precipitant: Na2CO3
CO3:Cu= 2:1
Vin: 13 m·h−1
HRT: 30 minRemoval rate: 90% [30] Cu HL: 80 cm
Carrier: CuCO3Cin Cu2+: 1600 mg·L−1
Pre: Na2CO3,pH: 6.0—8.0
CO3:Cu= 3:1
FH : 0.2m
HRT: 16.7 minRemoval rate: 95% [31] Cu DL: 2 cm,HL: 80 cm
DU: 4 cm,HU: 15 cm
V: 0.55 LCin Cu2+: 400 mg·L−1
Pre: Na2CO3,pH: 6.0—8.0
CO3:Cu= 3:1
Qin: 10 mL·min−1Removal rate: 92% [37] Zn Carrier: SiO2 Cin Zn2+: 45 mg·L−1
Pre: Na2CO3,pH: 7.5—8.0
FH : 2 m
Vin: 40 m·h−1Removal rate: 95%
ECS: 1—3 mm
Mc: 5%[39] Zn DL: 3 cm,HL: 50 cm
DU: 9 cm,HU: 10 cm
V: 0.55 L
Carrier: SiO2
DC: 0.2—0.3 mmCin Zn2+: 20 mg·L−1
Pre: Na2S,pH: 9.0
FH: 0.1 m
Vin: 15 m·h−1
HRT: 30 minRemoval rate: 95%
Cout Zn2+: 1.0 mg·L−1[40] Zn DL: 2 cm,HL: 80 cm
DU: 4 cm,HU: 20 cmCin Zn2+: 500 mg·L−1
Pre: Na2CO3,pH: 7.2
CO3:Zn= 1.2:1
Qin: 1.5 L·h−1Removal rate: 97.56%
Cout Zn2+: 19 mg·L−1
ECS: 0.5—1.0 mm[19] Pb D: 2.5 cm,H: 66 cm
Carrier: SiO2
DC: 0.2—0.3 mmCin Pb2+: 40 mg·L−1
Pre: Na2CO3,pH: 8—9
CO3:Pb= 3:1
Vin: 22 m·h−1
HRT: 380 minRemoval rate: 99%
Cout Pb2+: 1 mg·L−1[42] Pb D: 5.2 cm,H: 133 cm
V: 1.35 L
Carrier: PbCO3
DC: 0.053—0.062 mmCin Pb2+: 200 mg·L−1
Pre: Na2CO3,pH: 8—9
CO3:Pb= 3:1
Qin: 6 mL·min−1Removal rate: 98% [43] Pb DL: 2 cm,HL: 80 cm
DU: 4 cm,HU: 20 cm
V: 0.55 LCin Pb2+: 200 mg·L−1
Pre: Na2CO3,pH: 7
CO3:Pb= 1.2:1Cout Pb2+: 1 mg·L−1 [20] As D: 2 cm,H: 160 cm
Carrier: SiO2
DC: 0.2—0.5 mmCin As: 611 mg·L−1
Pre: Na2S,pH: 1.0
S:As= 2:1
Qin: 1.62 L·h−1Cout As: 7.2 mg·L−1
ECS: 1—3 mm[23] As D: 2 cm,H: 185 cm
V: 0.66 L
Carrier: SiO2Cin As: 200 mg·L−1
Pre: Na2S,pH: 2
Dosage C: 400 g
S:As= 2.2:1Cout As: 0.5 mg·L−1 [44] Ag D: 1.8 cm,H: 150 cm
Carrier: SiO2
DC: 0.2—0.3 mmCin Ag: 1080 mg·L−1
Pre: Na2CO3,pH: 10.2
CO3:Ag= 3:1Cout As: 10 mg·L−1
ECS: 0.6 mm[45] Mn D: 10 cm,Carrier: Manganese sand
DC: 0.4 mmCin Mn: 8.5 mg·L−1
Pre: Na2CO3,pH: 9.5
Vin: 25 m·h−1Cout Mn: 0.5 mg·L−1 [46] Ba D: 4 cm,H: 100 cm pH: 8.8
Ba:P= 1:1
Qin: 2.88 L·h−1Removal rate: 98%
ECS: 0.36 mm[47] Cd D: 2 cm,H: 240 cm
Carrier: SiO2
DC: 0.2—0.3 mmCin Cd: 2080 mg·L−1
Pre: Na2CO3。pH: 7.9
CO3:Cd= 1.6:1
FH: 1 mCout Cd: 1 mg·L−1
ECS: 1 mm[48] Hg Carrier: SiO2
DC: 0.1—0.3 mmCin Hg: 20 mg·L−1
Pre: Na2S,pH: 4—5Cout Hg: 0.002 mg·L−1
ECS: 1—3 mm[24] Polymetallic D: 14.5 cm,H: 550 cm
Carrier: SiO2Cin Ni: 510 mg·L−1
Cin Cd: 640 mg·L−1
Cin Zn: 1900 mg·L−1
pH: 7.2
Qin: 32 L·h−1Removal rate: 99%、92%、97%
Cout Ni: 7 mg·L−1
Cout Cd: 53 mg·L−1
Cout Zn: 50 mg·L−1[25] Polymetallic D: 10 cm,H: 220 cm
Carrier: SiO2
DC: 0.15—0.30 mmCin: 20 mg·L−1
Pre: Na2CO3,pH: 9.0
FH: 0.4 mRemoval rate: 95% [18] Polymetallic D: 10 cm,H: 90 cm
Carrier: SiO2
DC: 0.25—0.42 mmCin Cu: 250 mg·L−1
Cin Pb: 130 mg·L−1
Cin Ni: 130 mg·L−1
Pre: Na2CO3,pH: 8.7—9.1
FH: 0.3 mRemoval rate: 97%、96%、93%
Cout Cu: 0.5 mg·L−1
Cout Pb: 0.5 mg·L−1
Cout Ni: 0.9 mg·L−1[26] Polymetallic Carrier: SiO2
DC: 0.25 mmPre: Na2CO3
CO3: Metal= 1.2:1
Qin: 25 L·h−1
HRT: 1.8 minRemoval rate: 95% [27] Polymetallic D: 5 cm,H: 200 cm
Carrier: CaCO3
DC: 0.12—0.18 mmCin Fe: 0.94 mg·L−1
Cin Mn: 1.95 mg·L−1
Pre: NaOH,pH: 9.6
FH: 0.5 mCout Fe: 0.246 mg·L−1
Cout Mn: 0.061 mg·L−1[28] D: Reactor diameter; DL: Lower diameter; DU: Upper diameter; H: Total height of reactor; HL: Lower height; HU: Upper height; V: Reactor volume; DC: Carrier particle size; Dosage C: Carrier dosage; Cin: Influent concentration; Cout: Effluent concentration; FH: Fill Height; Pre: Precipitant; HRT: Hydraulic Retention Time; A:B: The molar ratio of A and B; Vin: Inlet flow rate; Qin: Into the liquid flow; ECS: Carrier excluded particle size; Mc: Moisture content. -
[1] 王小攀, 林璟, 张发明, 等. 重金属工业废水处理技术的研究进展 [J]. 山东化工, 2020, 49(9): 69-71,76. doi: 10.3969/j.issn.1008-021X.2020.09.025 WANG X P, LIN J, ZHANG F M, et al. Research progress of the industrial wastewater treatment technology for heavy metal [J]. Shandong Chemical Industry, 2020, 49(9): 69-71,76(in Chinese). doi: 10.3969/j.issn.1008-021X.2020.09.025
[2] SUN J, TIAN Q F, DENG N S. Notice of retraction: Harmless treatment method for the removal of heavy metals from simulative wastewater by inducing crystallization[C]//2011 5th International Conference on Bioinformatics and Biomedical Engineering. May 10-12, 2011, Wuhan, China. IEEE, 2011: 1-5. [3] 于萍, 任月明, 张密林. 处理重金属废水技术的研究进展 [J]. 环境科学与管理, 2006, 31(7): 103-105,108. doi: 10.3969/j.issn.1673-1212.2006.07.032 YU P, REN Y M, ZHANG M L. The reseachful progress on treatment techniques of heavy metal water [J]. Environmental Science and Management, 2006, 31(7): 103-105,108(in Chinese). doi: 10.3969/j.issn.1673-1212.2006.07.032
[4] HALYSH V, TRUS I, GOMELYA M, et al. Utilization of modified biosorbents based on walnut shells in the processes of wastewater treatment from heavy metal ion [J]. Journal of Ecological Engineering, 2020, 21(4): 128-133. doi: 10.12911/22998993/119809 [5] CELIK A, DEMIRBAŞ A. Removal of heavy metal ions from aqueous solutions via adsorption onto modified lignin from pulping wastes [J]. Energy Sources, 2005, 27(12): 1167-1177. doi: 10.1080/00908310490479583 [6] 孙国庆. 新型吸附剂处理重金属废水的研究进展 [J]. 智能城市, 2020, 6(11): 136-137. SUN G Q. Research progress of new adsorbents for heavy metal wastewater treatment [J]. Intelligent City, 2020, 6(11): 136-137(in Chinese).
[7] 王卓然, 王广智, 耿钰萱, 等. 电镀废水中重金属处理的研究进展 [J]. 电镀与环保, 2017, 37(1): 1-3. doi: 10.3969/j.issn.1000-4742.2017.01.001 WANG Z R, WANG G Z, GENG Y X, et al. Research progress on treatment of electroplating wastewater containing heavy metal [J]. Electroplating & Pollution Control, 2017, 37(1): 1-3(in Chinese). doi: 10.3969/j.issn.1000-4742.2017.01.001
[8] GRACE J R. Hydrodynamics of gas fluidized beds[M]//Fluidized Bed Boilers. Amsterdam: Elsevier, 1984: 13-30. [9] CHENG J X, YANG H T, FAN C L, et al. Review on the applications and development of fluidized bed electrodes [J]. Journal of Solid State Electrochemistry, 2020, 24(10): 2199-2217. doi: 10.1007/s10008-020-04786-w [10] ZHENG D, ZOU W, YAN J, et al. Coupling of contact nucleation kinetics with breakage model for crystallization of sodium chloride crystal in fluidized bed crystallizer [J]. Journal of Chemistry, 2019, 2019: 1-11. [11] BALLESTEROS F C, SALCEDO A F S, VILANDO A C, et al. Removal of nickel by homogeneous granulation in a fluidized-bed reactor [J]. Chemosphere, 2016, 164: 59-67. doi: 10.1016/j.chemosphere.2016.08.081 [12] REITERER F, JOHANNES W, GAMSJÄGER H. Semimicro determination of solubility constants: Copper(Ⅱ) carbonate and iron(Ⅱ) carbonate [J]. Microchimica Acta, 1981, 75(1/2): 63-72. [13] PATNAIK P. Handbook of inorganic chemicals[M]. New York: McGraw-Hill, 2003. [14] SCAIFE J F. The solubility of malachite [J]. Canadian Journal of Chemistry, 1957, 35(11): 1332-1340. doi: 10.1139/v57-177 [15] STELLA R, GANZERLI-VALENTINI M T. Copper ion-selective electrode for determination of inorganic copper species in fresh waters [J]. Analytical Chemistry, 1979, 51(13): 2148-2151. doi: 10.1021/ac50049a021 [16] KISELEVA I A, OGORODOVA L P, MELCHAKOVA L V, et al. Thermodynamic properties of copper carbonates—malachite Cu2(OH)2CO3 and azurite Cu3(OH)2(CO3)2 [J]. Physics and Chemistry of Minerals, 1992, 19(5): 322-333. [17] BLAIS J F, DJEDIDI Z, CHEIKH R B, et al. Metals precipitation from effluents: Review [J]. Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management, 2008, 12(3): 135-149. doi: 10.1061/(ASCE)1090-025X(2008)12:3(135) [18] ZHOU P, HUANG J C, LI A W F, et al. Heavy metal removal from wastewater in fluidized bed reactor [J]. Water Research, 1999, 33(8): 1918-1924. doi: 10.1016/S0043-1354(98)00376-5 [19] UDOMKITTHAWEEWAT N, ANOTAI J, CHOI A E S, et al. Removal of zinc based on a screw manufacturing plant wastewater by fluidized-bed homogeneous granulation process [J]. Journal of Cleaner Production, 2019, 230: 1276-1286. doi: 10.1016/j.jclepro.2019.05.192 [20] CHEN C S, SHIH Y J, HUANG Y H. Remediation of lead (Pb(Ⅱ)) wastewater through recovery of lead carbonate in a fluidized-bed homogeneous crystallization (FBHC) system [J]. Chemical Engineering Journal, 2015, 279: 120-128. doi: 10.1016/j.cej.2015.05.013 [21] WILMS D, VAN HAUTE A, VAN DIJK J, et al. Recovery of nickel by crystallization of nickel carbonate in a fluidized-bed reactor[M]//Water Pollution Control in Asia. Amsterdam: Elsevier, 1988: 449-456. [22] GUILLARD D, LEWIS A E. Nickel carbonate precipitation in a fluidized-bed reactor [J]. Industrial & Engineering Chemistry Research, 2001, 40(23): 5564-5569. [23] LEE M, CHANG W, HUANG C, et al. Method of arsenic immobilization in crystalline form water: US7045066 B2[P]. 05/16/2006. [24] JANSSEN C W. Process for the removal of metals, in particular heavy metals, from waste water: EP0279964 A2[P]. 12/18/1987. [25] NIELSEN P B, CHRISTENSEN T C, VENDRUP M. Continuous removal of heavy metals from FGD wastewater in a fluidised bed without sludge generation [J]. Water Science and Technology, 1997, 36(2/3): 391-397. [26] LEE C I, YANG W F. Heavy metal removal from aqueous solution in sequential fluidized-bed reactors [J]. Environmental Technology, 2005, 26(12): 1345-1353. doi: 10.1080/09593332608618613 [27] 孙杰, 赵晖, 邓南圣. 无害化诱导结晶新工艺处理重金属废水 [J]. 水处理技术, 2006, 32(9): 63-65. doi: 10.3969/j.issn.1000-3770.2006.09.017 SUN J, ZHAO H, DENG N S. A new technology for heavy metal ion removal from wastewater with a harmless treatment method [J]. Technology of Water Treatment, 2006, 32(9): 63-65(in Chinese). doi: 10.3969/j.issn.1000-3770.2006.09.017
[28] 唐章程, 黄廷林, 胡瑞柱, 等. 结晶造粒流化床同步去除水中铁、锰及硬度的中试实验 [J]. 环境工程学报, 2018, 12(11): 3090-3098. doi: 10.12030/j.cjee.201806146 TANG Z C, HUANG T L, HU R Z, et al. Simultaneous removal of iron, manganese and hardness by pellet fluidized bed reactor in pilot-scale experiment [J]. Chinese Journal of Environmental Engineering, 2018, 12(11): 3090-3098(in Chinese). doi: 10.12030/j.cjee.201806146
[29] LEE C I, YANG W F, HSIEH C I. Removal of Cu(II) from aqueous solution in a fluidized-bed reactor [J]. Chemosphere, 2004, 57(9): 1173-1180. doi: 10.1016/j.chemosphere.2004.08.028 [30] 阎中, 熊娅, 王凯军, 等. 诱导结晶工艺处理含铜废水 [J]. 化工学报, 2009, 60(10): 2603-2608. YAN Z, XIONG Y, WANG K J, et al. Copper removal by induced crystallization from copper-containing wastewater [J]. Journal of the Chemical Industry and Engineering Society of China, 2009, 60(10): 2603-2608(in Chinese).
[31] 卢明俊, 黄耀辉, 施育仁. 以流体化床结晶技术合成均质碱式碳酸铜及氧化铜结晶物之方法: TWI640477 B[P]. 11/11/2018. LU M J, HUANG Y H, SHI Y R. Method of synthesizing homogeneous granular basic cupric carbonate and copper oxide by using fluidized-bed crystallization technology: TWI640477 B[P]. 11/11/2018(in Chinese).
[32] SCHÖLLER M, V DIJK J C, V HAUTE A, et al. Recovery of heavy metals by crystallization in the pellet reactor, A promising development [J]. Studies in Environmental Science, 1988, 34: 77-90. doi: 10.1016/S0166-1116(08)71280-9 [33] UILLARD D, LEWIS A E. Optimization of nickel hydroxycarbonate precipitation using a laboratory pellet reactor [J]. Industrial & Engineering Chemistry Research, 2002, 41(13): 3110-3114. [34] OSTODES V C T, LEWIS A E. Reactive crystallization of nickel hydroxy-carbonate in fluidized-bed reactor: Fines production and column design [J]. Chemical Engineering Science, 2006, 61(5): 1377-1385. doi: 10.1016/j.ces.2005.08.038 [35] SALCEDO A F M, BALLESTEROS F C, VILANDO A C, et al. Nickel recovery from synthetic Watts bath electroplating wastewater by homogeneous fluidized bed granulation process [J]. Separation and Purification Technology, 2016, 169: 128-136. doi: 10.1016/j.seppur.2016.06.010 [36] 卢明俊, 黄耀辉. 以流体化床结晶技术从含镍废水中合成碱式氧化镍结晶物之方法: TW201938495 A[P]. 10/01/2019. LU M J, HUANG Y H. Method of synthesizing granular basic nickel oxide from nickel-contained wastewater by using fluidized-bed crystallization technology. TW201938495 A[P]. 10/01/2019(in Chinese).
[37] LERTRATWATTANA K, KEMACHEEVAKUL P, GARCIA-SEGURA S, et al. Recovery of copper salts by fluidized-bed homogeneous granulation process: High selectivity on malachite crystallization [J]. Hydrometallurgy, 2019, 186: 66-72. doi: 10.1016/j.hydromet.2019.03.015 [38] HONG X Y, ZHENG E H, SHEN X Z. Apparatus and method for sulfide crystallization of Cu and Ni using fluidized bed reactor: KR101681701 B1[P]. 12/01/2016. [39] JANSEN C. Process for removing of heavy metal from water in particular from waste water: US4764284 A[P]. 08/16/1988. [40] 杨艳, 陈坚, 马力强. 诱导结晶工艺处理含锌废水[EB/OL]. 北京: 中国科技论文在线[2012-06-12]. [2012-06-12]. YANG Y, CHEN J, MA L Q. Zinc wastewater treatment by induced crystallization[EB/OL]. Beijing: Sciencepaper Online [2012-06-12].
[41] 卢明俊, 黄耀辉. 以流体化床结晶技术合成均质含锌结晶物之方法: TW201902821 A[P]. 01/16/2019. LU M J, HUANG Y H. Method of synthesizing homogeneous zinc-containing crystals by using fluidized-bed crystallization technology: TW201902821 A[P]. 01/16/2019(in Chinese).
[42] CHEN J P, YU H. Lead removal from synthetic wastewater by crystallization in a fluidized-bed reactor [J]. Journal of Environmental Science and Health, Part A, 2000, 35(6): 817-835. doi: 10.1080/10934520009377005 [43] DE LUNA M D G, BELLOTINDOS L M, ASIAO R N, et al. Removal and recovery of lead in a fluidized-bed reactor by crystallization process [J]. Hydrometallurgy, 2015, 155: 6-12. doi: 10.1016/j.hydromet.2015.03.009 [44] HUANG C, PAN J R, LEE M, et al. Treatment of high-level arsenic-containing wastewater by fluidized bed crystallization process [J]. Journal of Chemical Technology & Biotechnology, 2007, 82(3): 289-294. [45] WILMS D, VERCAEMST K, VAN DIJK J C. Recovery of silver by crystallization of silver carbonate in a fluidized-bed reactor [J]. Water Research, 1992, 26(2): 235-239. doi: 10.1016/0043-1354(92)90223-Q [46] NOMURA J, SUGITA Y. The manganese-containing water treatment method and apparatus: JP3729365 B2[P]. 12/21/2005. [47] SU C C, REANO R L, DALIDA M L P, et al. Barium recovery by crystallization in a fluidized-bed reactor: Effects of pH, Ba/P molar ratio and seed [J]. Chemosphere, 2014, 105: 100-105. doi: 10.1016/j.chemosphere.2014.01.005 [48] DOTREMONT C, WILMS D, DEVOGELAERE D, et al. Recovery of cadmium by crystallization of cadmium carbonate in a fluidized-bed reactor[M]//Chemistry for the Protection of the Environment. Springer, Boston, MA, 1991: 741-751. -



 下载:
下载: