-
锑(Sb)是一种天然微量元素,位于元素周期表第VA族,有以下4种价态:Sb(-Ⅲ), Sb(0), Sb(Ⅲ)和 Sb(Ⅴ),在自然环境中主要以Sb(Ⅲ)和Sb(Ⅴ)存在[1]。Sb及其化合物常在聚酯纤维、阻燃剂、电池合成过程中作为催化剂,此外Sb在医疗行业还被用于治疗利什曼病、血吸虫病、蛔虫病等[2-4]。Sb的环境背景值低,未受污染水体中的Sb含量小于1 µg·L−1[5-6],然而锑矿开采和含锑产品的生产使用使得环境中的Sb含量远远高于其背景值,在人类活动区域的地表水中Sb含量甚至高达7 mg·L−1 [7]。Sb作为一种有毒类金属元素,已被美国环境保护协会(USEPA)和欧盟(EU)列为优先污染物,我国规定饮用水中的Sb浓度不得超过5 μg·L−1,工业废水排放限值为1 mg·L−1[8]。因此,探索水中Sb的净化处理技术具有重大意义。
常见的含Sb废水处理技术包括絮凝沉淀、吸附、电化学、膜过滤等方法,其中吸附法由于操作简单、污泥产生量少、价格低廉等优点而受到广泛关注[9]。生物吸附剂(细菌、真菌、藻类、植物等)来源广、成本低,逐渐受到研究者们的青睐[10-11]。酵母是世界上最常见的微生物之一,易大规模培养且生物产量高,作为发酵或制药工业过程中的副产物,供应稳定[12]。目前已有许多研究利用酵母作为吸附剂去除水中的重金属离子(如Cu2+、Pb2+、Cd2+、Hg2+等)[12-14],然而多数研究集中于阳离子重金属的吸附,对含氧阴离子重金属的关注较少,且酵母对Sb(Ⅴ)的吸附去除鲜有报道,故本文选择酵母粉作为吸附材料来探究其对废水中Sb(Ⅴ)净化处理的应用潜力。
一般来说,生物吸附剂含有大量带负电荷的官能团,其阳离子吸附能力较强而阴离子吸附能力较弱[15],因此,人们通过一系列的表面改性操作来提高它们对阴离子的吸附能力。研究表明,铁改性能提高吸附剂对水中含氧阴离子(如磷酸根、砷酸根、锑酸根等)的去除能力[16-18],例如刘爱平[19-20]通过FeCl3改性使天然凹凸棒石和斜发沸石对Sb(Ⅴ)的吸附能力提升了8倍以上。因此本文采用FeCl3对酵母粉进行改性,旨在提高酵母粉对Sb(Ⅴ)的吸附性能,研究FeCl3浓度、吸附剂投加量以及pH对Sb(Ⅴ)去除率的影响,在最佳吸附条件下进行铁改性酵母粉对Sb(Ⅴ)的吸附动力学、吸附等温线实验,采用扫描电子显微镜-能谱仪(SEM-EDS)、X射线衍射(XRD)、X射线光电子能谱(XPS)、衰减全反射傅里叶变换红外光谱分析(ATR-FTIR)等手段对样品进行表征,探究改性酵母粉对Sb(Ⅴ)的吸附机理,为Sb(Ⅴ)的吸附去除提供新的思路与方法。
-
实验所使用酵母粉(yeast)为灭活型干酵母。三氯化铁(FeCl3· 6 H2O)、六羟基锑酸钾(KSbH6O6)、氢氧化钠(NaOH)、盐酸(HCl)等试剂纯度均为分析纯,购于Sigma-Aldrich公司;1000 mg·L−1锑标准溶液(GB04-1748-2004)购自国家有色金属及电子材料分析测试中心。
-
称取10 g干酵母粉,分别加入100 mL 0.01、0.02、0.05、0.1、0.5、1.0、1.5 mol·L−1 FeCl3溶液中,在30 ℃、200 r·min−1条件下振荡24 h,离心洗涤数次至上清液澄清,冷冻干燥后得到不同浓度FeCl3改性酵母粉(分别记为0.01 Fe-Y、0.02 Fe-Y、0.05 Fe-Y、0.1 Fe-Y、0.5 Fe-Y、1.0 Fe-Y、1.5 Fe-Y)。
-
准确称取0.2 g改性/未改性酵母粉(未改性酵母粉记为CK)于50 mL浓度为 10 mg·L−1 Sb(Ⅴ)溶液中,用HCl调节溶液pH=3,在30 ℃、200 r·min−1条件下振荡24 h后离心收集上清液,用0.22 μm水系滤头过滤,待测。
-
准确称取一定质量0.5 Fe-Y于50 mL 浓度为10 mg·L−1 Sb(Ⅴ)溶液中,使固液比(S/L)分别为0.05、0.1、0.2、0.5、1、2、4、8、10 g·L−1,在pH=3、30 ℃、200 r·min−1条件下振荡24 h后离心收集上清液,用0.22 μm水系滤头过滤,待测。
-
准确称取0.1 g 0.5 Fe-Y于50 mL浓度为 10 mg·L−1 Sb(Ⅴ)溶液中,用HCl和NaOH调节溶液pH值至2.0—10.0,在30 ℃、200 r·min−1条件下振荡吸附24 h后离心收集上清液,用0.22 μm水系滤头过滤,待测。
-
准确称取0.3 g 0.5 Fe-Y于150 mL浓度为 10 mg·L−1 Sb(Ⅴ)溶液中,在pH=3、30 ℃、200 r·min−1条件下振荡吸附,分别于0、1、2、4、8、12、24、48、72、96 h取2 mL上清液,用0.22 μm水系滤头过滤,待测。
-
分别称取0.1g 0.5 Fe-Y于50 mL 浓度为 0.01、0.2、1、10、20、50、100、500 mg·L−1 Sb(Ⅴ)溶液中,在pH=3、30 ℃、200 r·min−1条件下振荡吸附24 h后离心收集上清液,用0.22 μm水系滤头过滤,待测。
-
溶液pH值用pH计(Sartorius PB-21,Germany)测定,溶液Sb含量采用电感耦合等离子体质谱仪(NexION 2000, PerkinElmer, USA)测定,测定前用1000 mg·L−1锑标准溶液梯度稀释配制标准曲线(R2>0.999)。采用扫描电子显微镜(Carl Zeiss Supra 55 system, Germany)能谱分析和Zeta电位分析仪(Zetasizer Nano ZS90, England Malvern)分别确定酵母粉表面形貌/元素分布和表面电位;衰减全反射傅里叶变换红外光谱仪(Thermo Fisher Scientific Inc., Madison, USA)记录母粉的红外光谱图,以确定样品表面官能团类型;X射线衍射仪(XRD, SmartLab(3KW), Japan Rigaku)测定酵母粉的X射线衍射谱图,以确定样品的晶体结构;X射线光电子能谱仪(Thermo ESCALAB 250XI, USA)分析Sb(Ⅴ)吸附前后样品的元素组成、化学键等信息。
-
酵母粉吸附量按公式(1)计算:
其中,qt表示t时刻酵母粉对锑吸附量(mg·g−1); c0和 ct表示初始溶液和t时刻溶液中的锑浓度(mg·L−1); V为溶液体积(L); m为酵母粉添加量(g)。
实验均进行3组重复,数据采用Origin 2021、XPSPeak41软件进行拟合分析。
-
由图1可知,FeCl3改性能够提高酵母粉的Sb(Ⅴ)去除能力,且提高效果与FeCl3浓度有关,与CK相比,经FeCl3改性的酵母粉对Sb(Ⅴ)的吸附量最高可提高8倍。随着FeCl3浓度的提高,改性酵母粉对Sb(Ⅴ)的去除率越高,最高达95.9%。原因可能是可溶性锑酸盐与沉积在酵母粉表面的铁形成难溶性络合物,从而将Sb(Ⅴ)从水溶液中去除[21-22],因此FeCl3改性能够显著提高酵母粉对Sb(Ⅴ)的去除能力。当Fe3+浓度大于0.5 mol·L−1时,改性酵母粉对的Sb(Ⅴ)吸附能力趋于稳定,基于FeCl3投加量和Sb(Ⅴ)去除能力综合考量,采用经0.5 mol·L−1 FeCl3改性的酵母粉(0.5 Fe-Y)进行后续实验。
-
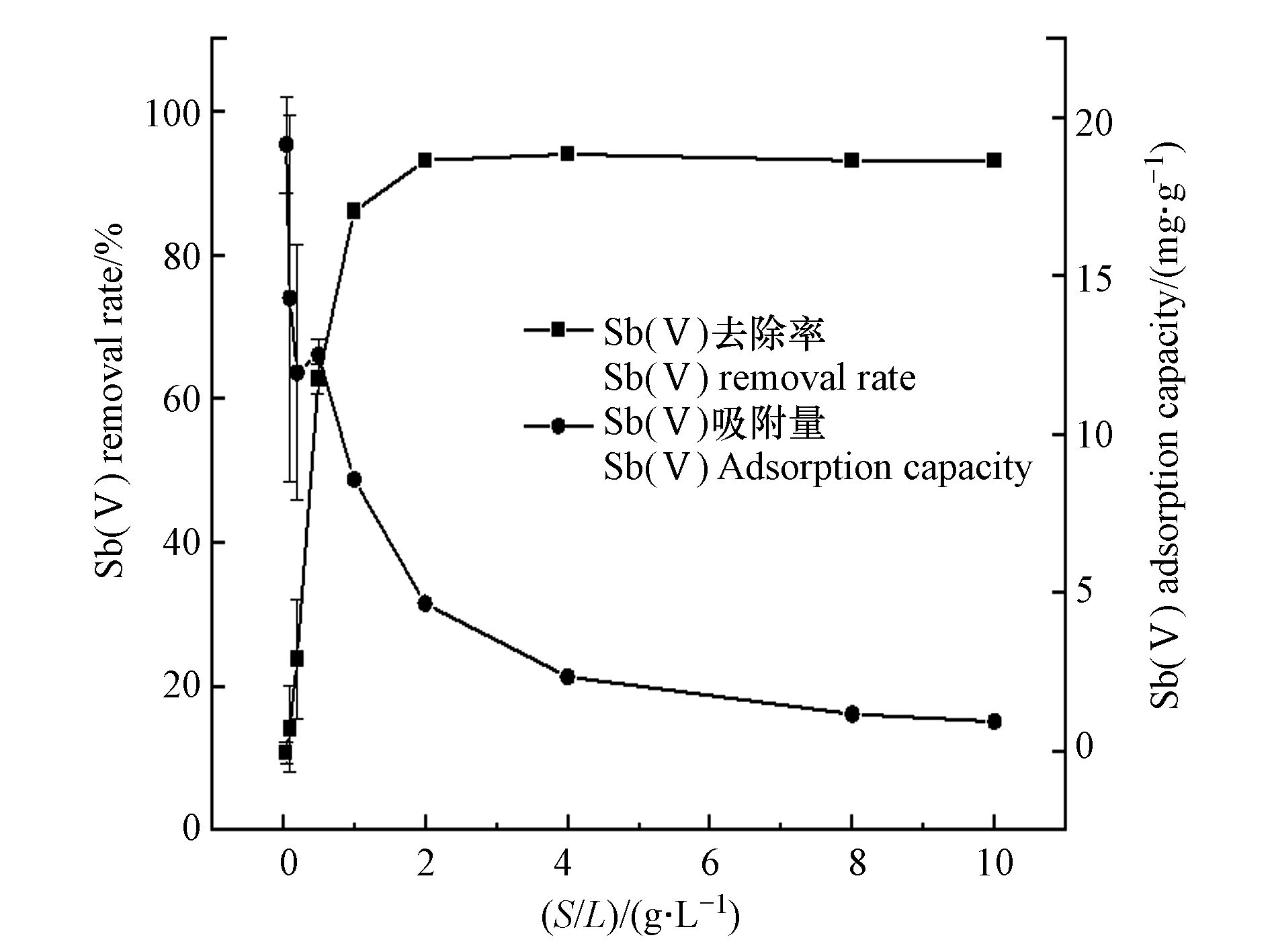
由图2可知,加大吸附剂投加量有利于增加Sb(Ⅴ)去除率,当S/L从0.05 g·L−1增加至2 g·L−1时,Sb(Ⅴ)去除率显著提高,在S/L为2 g·L−1时达93%,继续增加投加量,则变化不明显,而随着0.5 Fe-Y投加量的增加,平衡时的Sb(Ⅴ)吸附量逐渐降低。这是由于当溶液中Sb(Ⅴ)一定时,0.5 Fe-Y越多,Sb(Ⅴ)的吸附位点越多,吸附剂总表面积越大,因此提高了Sb(Ⅴ)去除率[17]。但随着0.5 Fe-Y剂量的增加,Sb(Ⅴ)浓度与0.5 Fe-Y质量的比例下降,所以单位质量0.5 Fe-Y对Sb(Ⅴ)的吸附量降低[23]。当0.5 Fe-Y投加量大于2 g·L−1时,Sb(Ⅴ)去除率趋于稳定,因此,选择2 g·L−1 作为后续实验的0.5 Fe-Y投加量。
-
0.5 Fe-Y对Sb(Ⅴ)的吸附去除率随溶液pH的变化如图3所示。当pH=3时,0.5 Fe-Y对Sb(Ⅴ)的去除率达到最大值(91.2%),此后随着pH值升高,去除率逐渐降低,这与前人的研究一致[24-25]。pH会影响Sb(Ⅴ)在溶液中的存在形式,当溶液pH=2—2.7时,Sb(Ⅴ)以中性的H3SbO4存在,pH>2.7时,Sb(Ⅴ)主要以带负电荷的Sb(OH)6- 存在,而在pH为3左右时,0.5 Fe-Y质子化程度较高,带负电荷的Sb(OH)6- 由于静电引力更容易被吸附,此时的Sb(Ⅴ)去除率大于pH=2的Sb(Ⅴ)去除率。当pH>3时,Sb(Ⅴ)去除率随pH升高而逐渐降低则可归因于溶液中的OH−与同样带负电荷的Sb(OH)6- 竞争吸附位点[24]。由于在pH=3时,0.5 Fe-Y有着最好的Sb(Ⅴ)去除能力,因此选择pH=3作为后续实验条件。
-
在吸附动力学实验中,常采用数学模型来描述重金属离子与吸附剂之间的相互作用,本研究选择伪一级动力学方程和伪二级动力学方程来拟合Sb(Ⅴ)在0.5 Fe-Y表面的吸附动力学。伪一级动力学方程常被用于描述物理吸附,伪二级动力学方程则与化学吸附有关,它们的方程表示如式(2)和(3):
其中,qt表示t时Sb(Ⅴ)吸附量, mg·g−1;qe表示吸附平衡时Sb(Ⅴ)吸附量, mg·g−1;k1表示伪一级速率常数, h−1;k2表示伪二级速率常数, g·mg·h−1
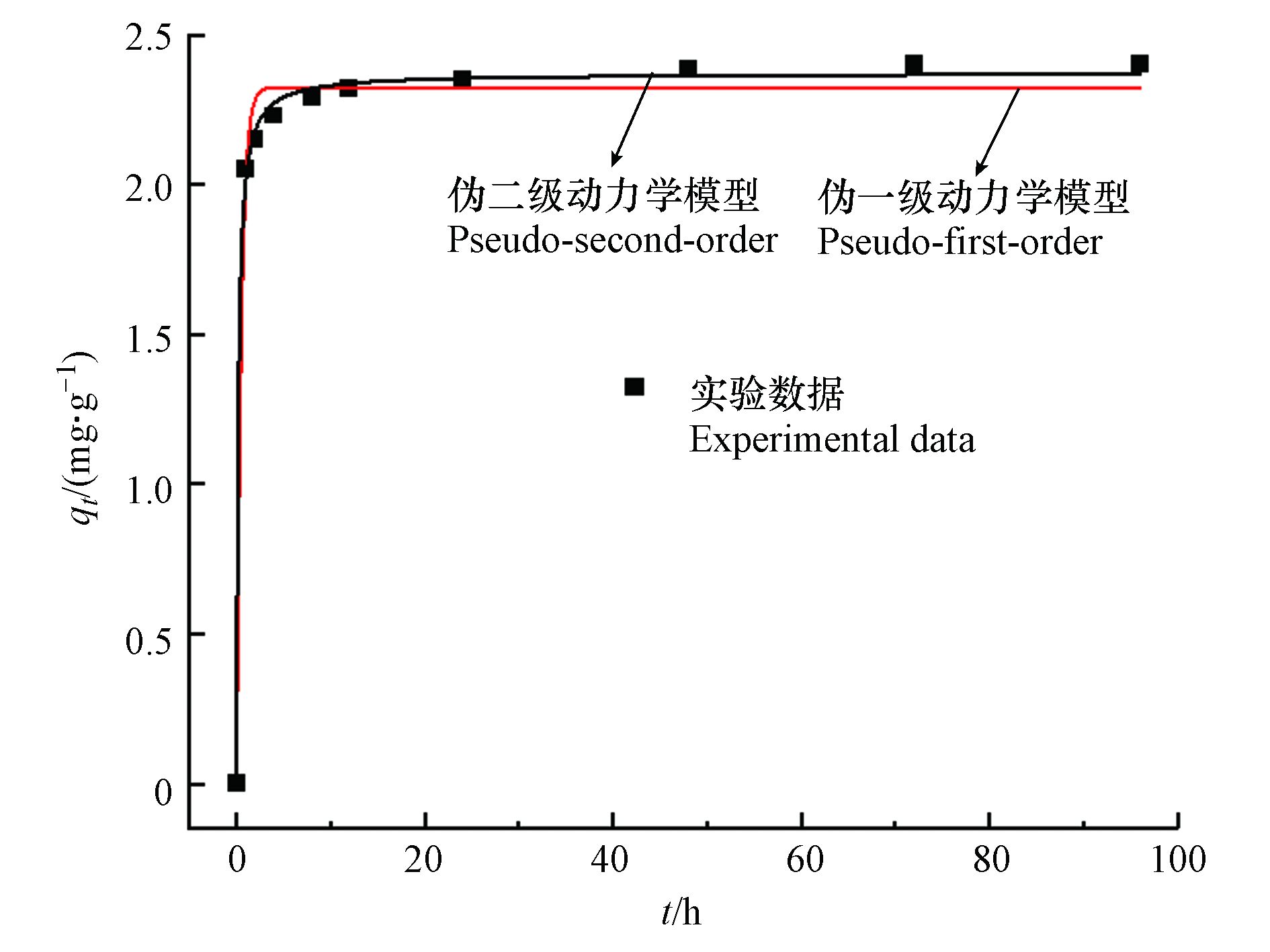
图4展示了两种动力学模型对不同时间吸附数据的拟合情况,拟合参数列于表1。0.5 Fe-Y对Sb(Ⅴ)的吸附可分为两个过程,包括0—4 h的快速吸附过程,以及4—24 h的慢速吸附过程,24 h后吸附达到平衡,最终的平衡吸附量为2.4 mg·g−1。模型拟合结果(表1)表明,伪二级动力学模型能更好地拟合0.5 Fe-Y对Sb(Ⅴ)的吸附过程,其相关系数R2=0.998,模型预测的吸附平衡量qe =2.37 mg·g−1,与实验数据2.4 mg·g−1相符合。动力学分析表明Sb(Ⅴ)在0.5 Fe-Y上的吸附过程受化学吸附过程控制,符合伪二级动力学反应机理,限速过程可能涉及Sb(Ⅴ)与0.5 Fe-Y之间的电子交换与共享[26]。
-
吸附等温线模型常用于描述吸附平衡,并预测吸附剂对污染物的吸附能力,本研究采用Langmuir和Freundlich两种模型来拟合实验数据。Langmuir模型是基于这样的假设:吸附剂上的吸附能是均匀的,吸附质之间没有相互作用,属于单分子层吸附,而Freundlich则假设吸附剂表面的活性位点具有不同的能量[26]。两种模型的表达式如式(4)(5):
其中,ce表示平衡时Sb(Ⅴ)浓度,mg·L−1;qe表示吸附平衡时Sb(Ⅴ)吸附量,mg·g−1;qm表示Sb(Ⅴ)最大吸附量(常数),mg·g−1;kl表示Langmuir常数,L·mg−1;kf表示与吸附容量有关的Freundlich常数,mg1−(1/n)·L1/n g−1;1/n 表示吸附强度的常数。
在30 ℃、pH=3条件下,0.5 Fe-Y对Sb(Ⅴ)的吸附等温线如图5所示,拟合参数列于表2。结果显示,0.5 Fe-Y的Sb(Ⅴ)吸附量在低浓度范围内迅速增加,之后随着浓度的增大而逐渐增加。1/n可作为表面异质性和活性位点的指标,用于表示交换强度或表面不均匀性,有研究表明当1/n<0.5时很容易发生吸附反应[26]。本研究拟合得到较低的1/n值(0.28,表2),说明Sb(Ⅴ)很容易被0.5 Fe-Y吸附。与Freundlich模型相比,Langmuir模型能更好的拟合0.5 Fe-Y对Sb(Ⅴ)的吸附过程,这表明该吸附过程是均匀介质表面的单层吸附,说明Sb(Ⅴ)在0.5 Fe-Y表面的吸附是可能是一个化学过程而非物理过程。计算得到0.5 Fe-Y对Sb(Ⅴ)的最大吸附量为68.15 mg·g−1,吸附能力高于文献报道的多数吸附材料(表3),证明0.5 Fe-Y是一种极具潜力的Sb(Ⅴ)吸附剂。
-
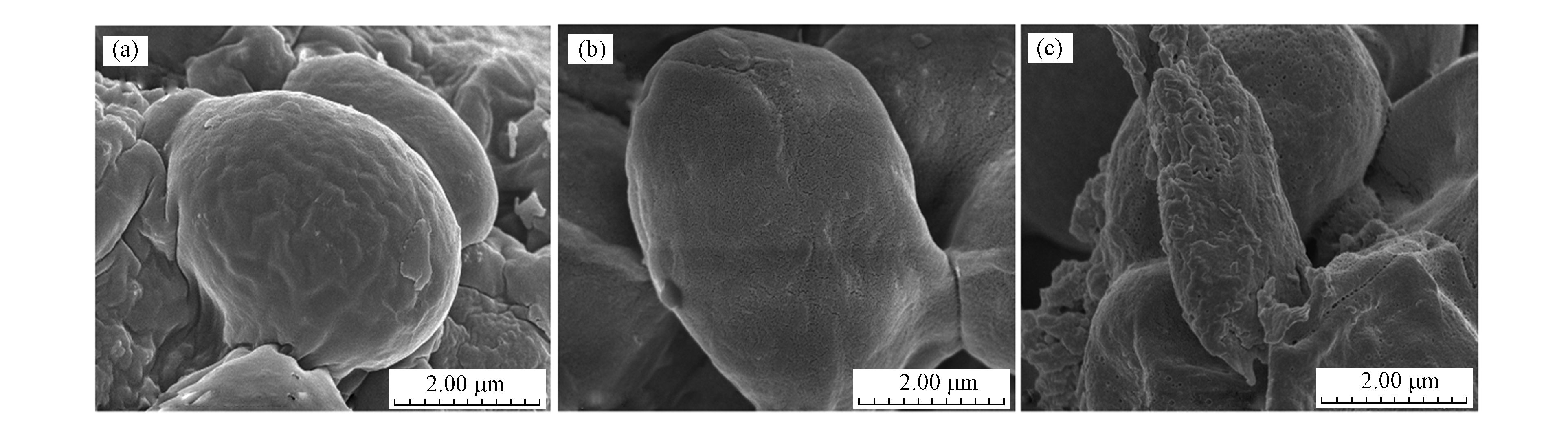
通过SEM-EDS观察Sb(Ⅴ)吸附前后酵母粉的微观形貌(图6)和Sb(Ⅴ)吸附后酵母粉表面元素分布(图7)。由图6可知,改性前酵母粉表面光滑,经FeCl3改性后酵母粉表面细孔明显增加,这可以为Sb(Ⅴ)的吸附提供更大的接触面积和更多的吸附点位。Sb(Ⅴ)的吸附(0.5 Fe-Y-Sb)导致改性酵母粉表面粗糙度进一步扩大,且出现明显的孔状结构,元素映射图(图7)也表明Fe和Sb均匀覆盖在改性酵母粉表面,证实改性操作效果良好,并且0.5 Fe-Y能够吸附溶液中的Sb(Ⅴ)。
-
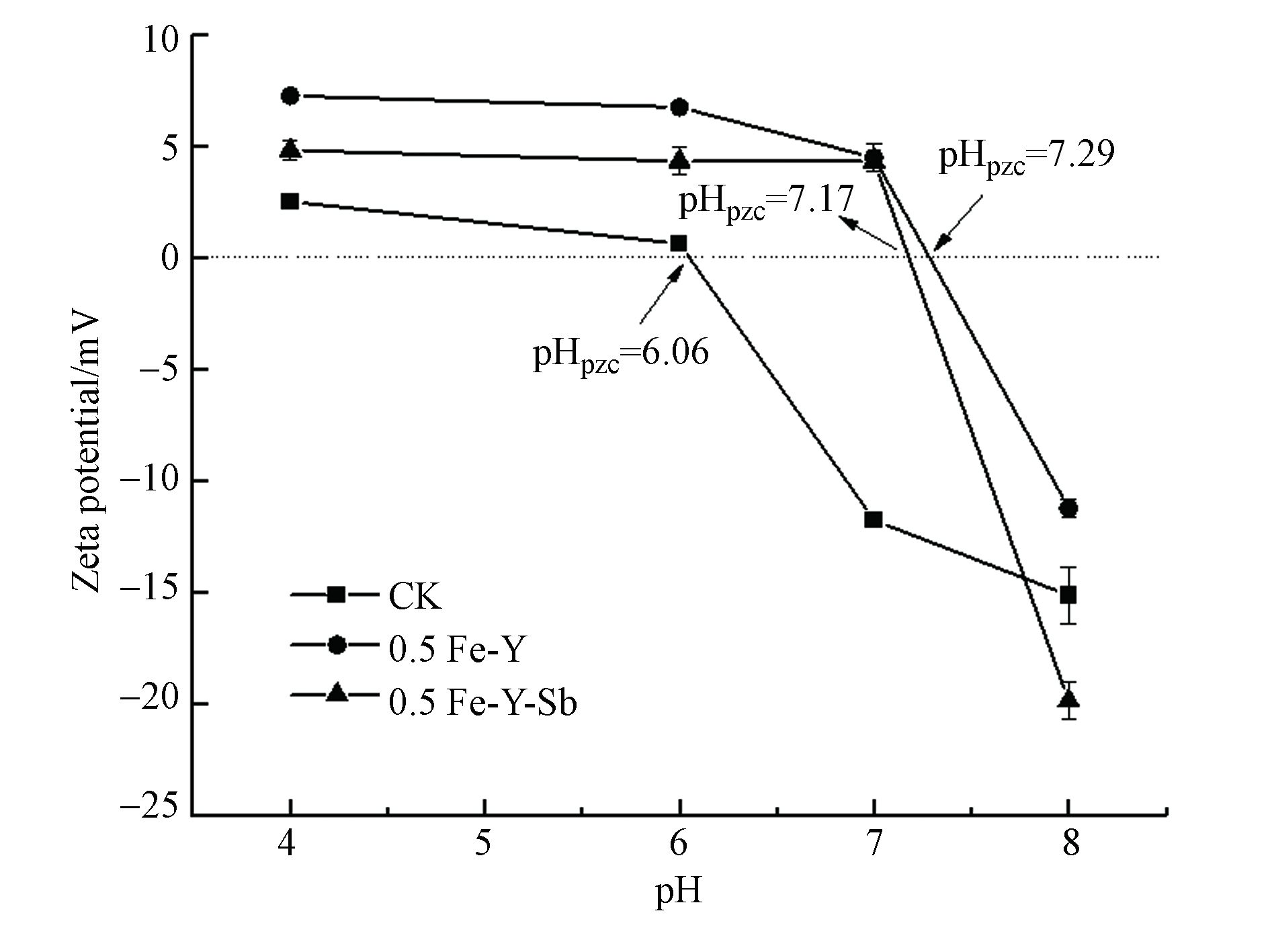
图8为酵母粉在不同pH条件下的Zeta电位图,CK、0.5 Fe-Y和0.5 Fe-Y-Sb的Zeta电位随pH的增大而不断减小。FeCl3改性增加了0.5 Fe-Y表面电位,零电位点从改性前的6.06变为7.29,这主要是由于在酵母粉表面沉积的Fe3+带有较多的正电荷[38],较高的电位利于Sb(Ⅴ)(主要是Sb(OH)6- )的吸附,这导致0.5 Fe-Y比CK有着更好的Sb(Ⅴ)吸附能力。随着pH增加,0.5 Fe-Y的Zeta电位减小,表面带更多的负电荷,与Sb(OH)6- 之间产生静电斥力,这可以导致0.5 Fe-Y对Sb(Ⅴ)的吸附随pH增大而减小(图3),而带负电荷的Sb(OH)6- 的吸附使得0.5 Fe-Y-Sb表面电位降低(图8)。
-
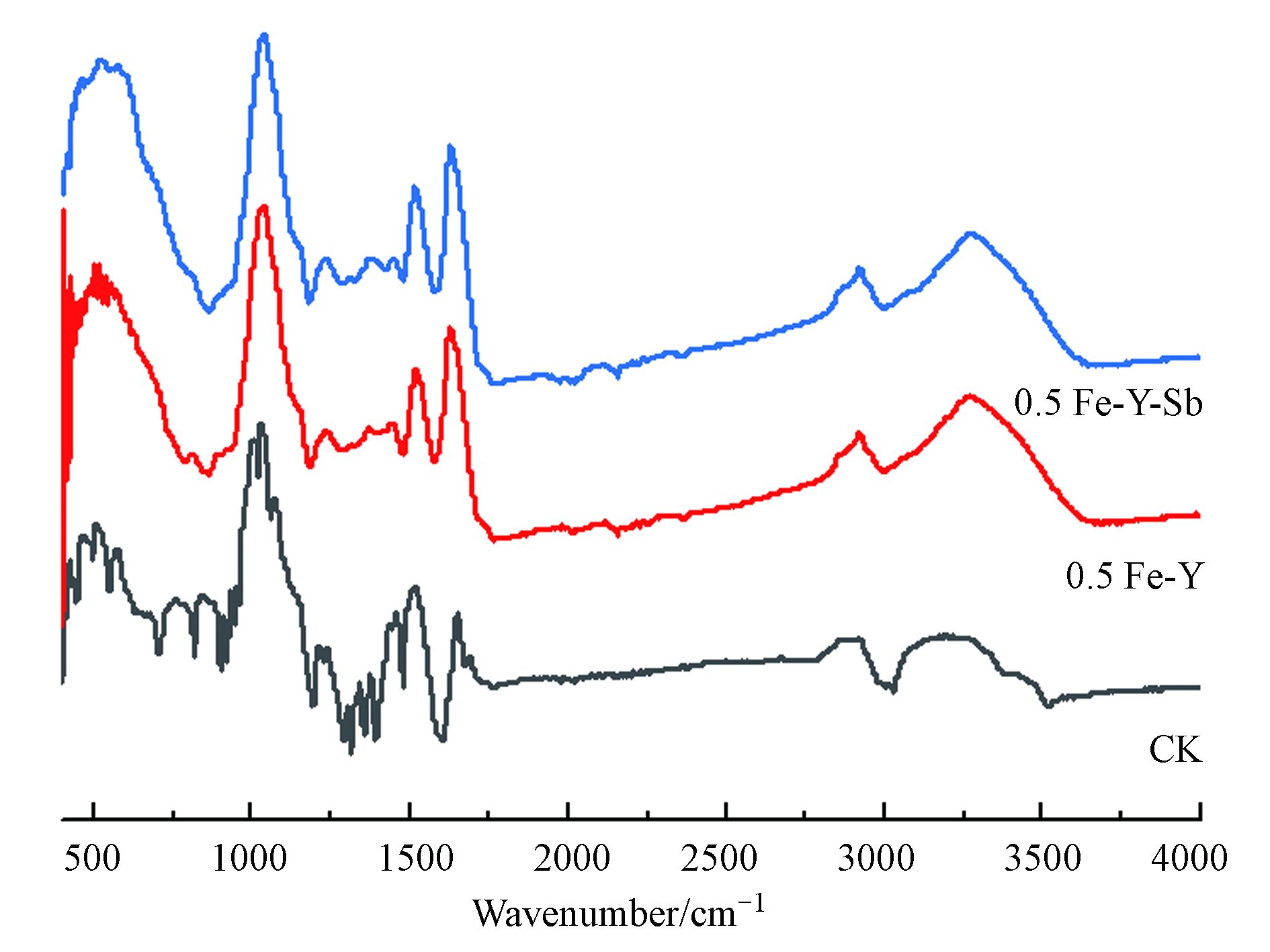
图9为CK、0.5 Fe-Y和0.5 Fe-Y-Sb的红外光谱图。CK的ATR-FTIR谱图中,3000—3500 cm−1的宽谱带归属于—OH/N—H的拉伸振动,2855—2960 cm−1归属于C—H伸缩振动,1600—1800 cm−1归属于C=O拉伸振动,1514 cm−1附近的谱峰归属于N—H弯曲振动,1000—1300 cm−1谱带归属于C—O拉伸振动[39-40]。与CK相比,0.5 Fe-Y的ATR-FTIR谱图在700—1000 cm−1(细胞壁中葡聚糖和甘露聚糖吸收谱带)和1300—1480 cm−1(脂质、蛋白质中CH2拉伸振动和形变)波数范围内的谱峰明显减弱甚至消失,说明FeCl3改性破坏了酵母细胞的细胞壁结构[39]。3000—3500 cm−1的峰振动增强、变得尖锐,可能是由于FeCl3处理能够增加菌粉表面的羟基,参与Sb(Ⅴ)吸附过程[41]。Sb(Ⅴ)吸附前后的ATR-FTIR谱图变化不大,说明Sb(Ⅴ)吸附过程中改性酵母粉表面官能团种类没有发生改变。Sb(Ⅴ)吸附后,3277 cm−1处的—OH/N—H的拉伸振动峰偏移至3274 cm−1,说明—OH/N—H 参与了Sb(Ⅴ)的吸附,0.5 Fe-Y表面的羟基官能团可能与Sb(Ⅴ)发生络合作用[41]。
-
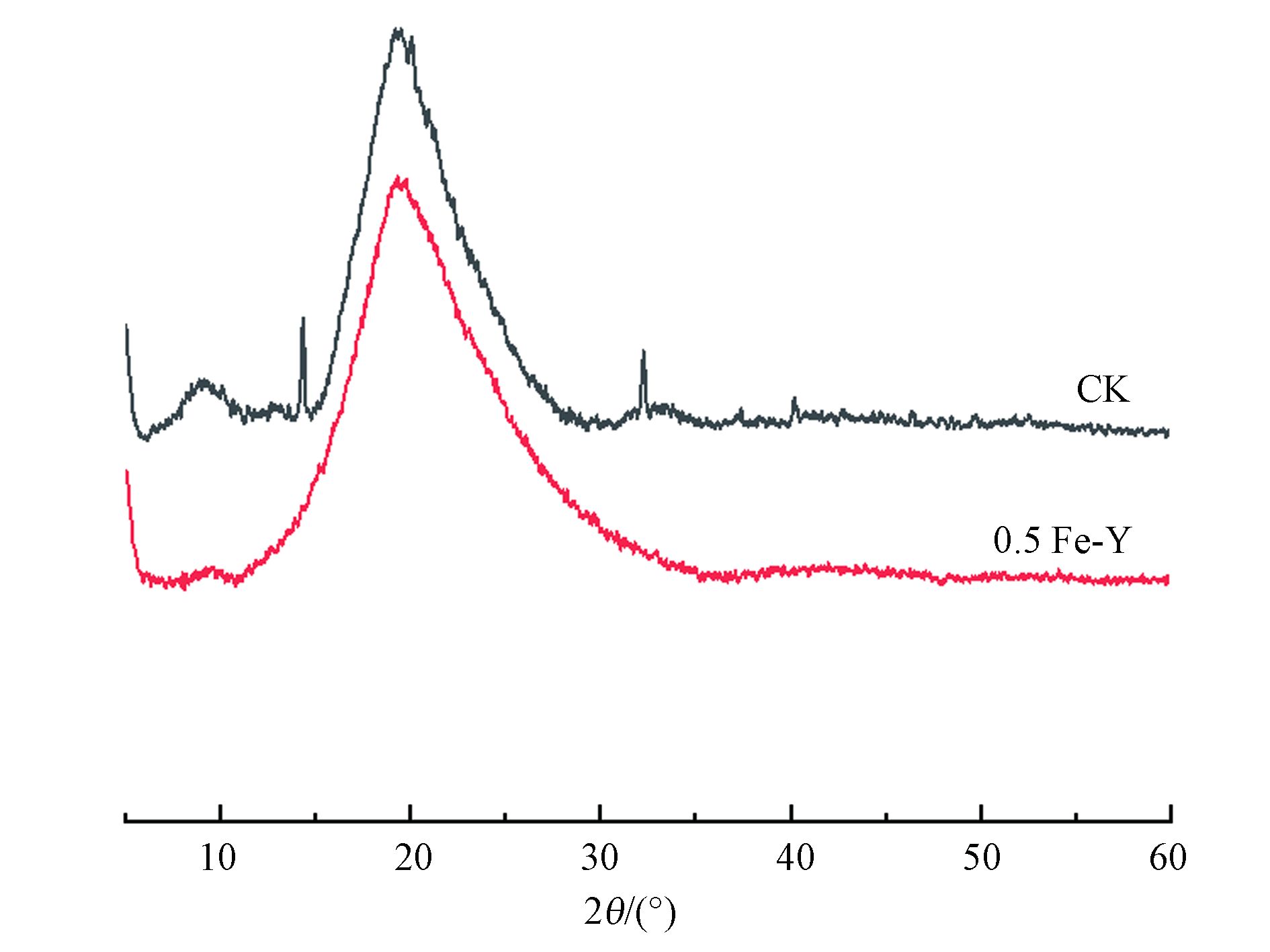
为了解吸附剂表面晶型结构,对改性前后的酵母粉XRD谱图(图10)进行分析,改性前后的酵母粉均在19.3 °处出现强而宽的峰,这归属于酵母的无定形相[42]。CK在19.3 °、32.2 °、37.4 °、40.2 °能观察到明显的谱峰,这与De Rossi等[43]观察到的结果类似。然而0.5 Fe-Y只存在19.3 °处的一个峰,且与CK相比,0.5 Fe-Y在19.3 °处的谱峰更宽,其无定形性质更加突出[44],这表明FeCl3改性会破坏酵母粉表面的结晶度,使酵母粉的晶型更加无序[45]。在0.5 Fe-Y表面没有观察到明显的铁氧化物衍射峰,这与Wang等[46]的研究结果相似,推测Fe以无定形形式沉积到菌粉表面。
-
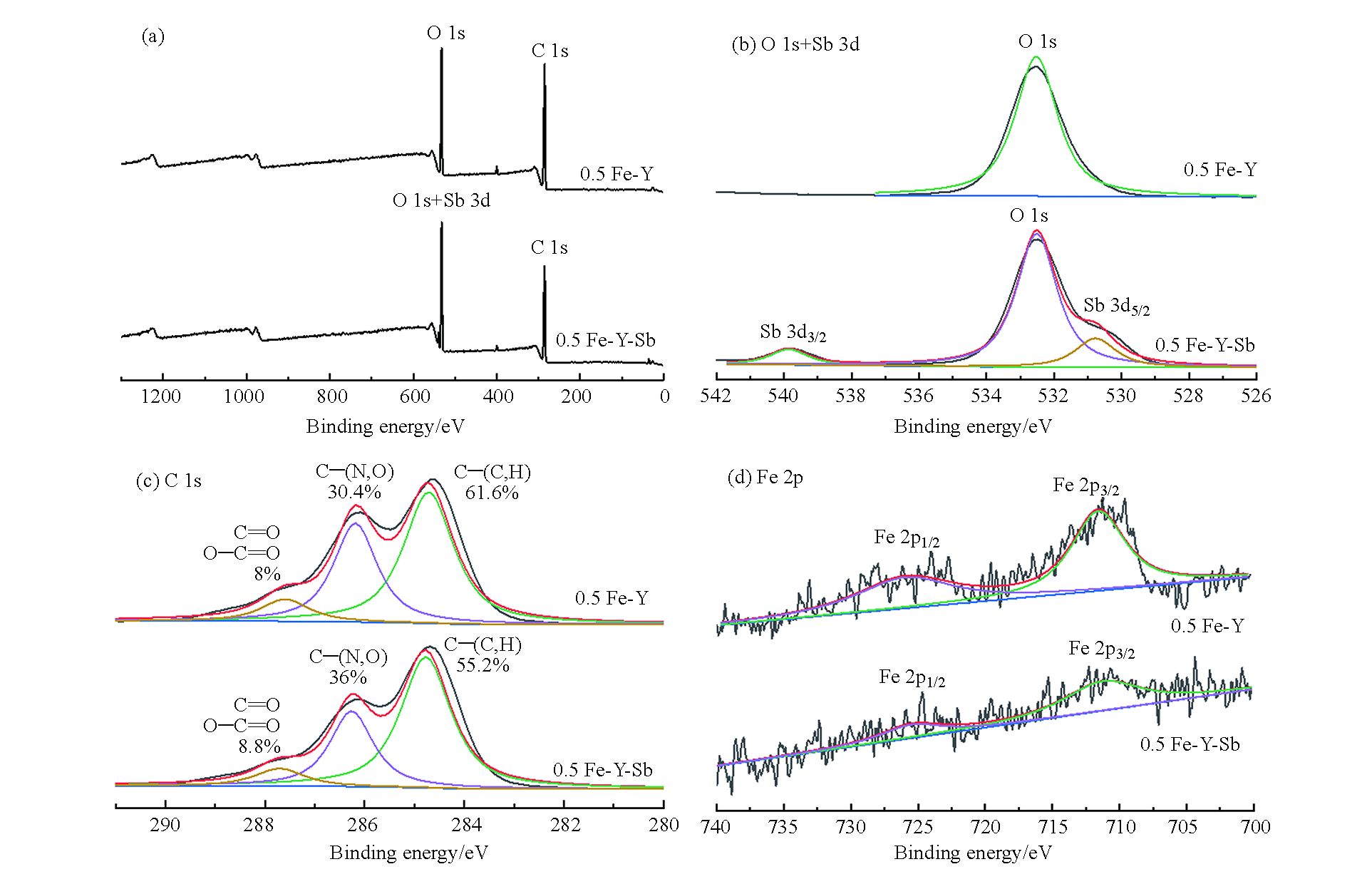
由0.5 Fe-Y吸附Sb(Ⅴ)前后的XPS谱图(图11)可知,0.5 Fe-Y-Sb在540.15 eV处出现了明显的Sb 3d3/2峰(图11(a)),图11(b)中0.5 Fe-Y- Sb也有明显的Sb 3d3/2和Sb 3d5/2峰,Sb 3d3/2和Sb 3d5/2是Sb物种的代表峰,两峰之间的距离大约9.3 eV,未出现与KSb(OH)6沉淀相关的谱峰[47-49]。研究表明,Sb(Ⅲ)负载物的Sb 3d3/2结合能为539.7 eV,低于Sb(Ⅴ)的Sb 3d3/2结合能,说明Sb(Ⅴ)在0.5 Fe-Y上没有发生还原反应[50]。由图11(c)可见,C 1s峰可分成三部分,结合能在284.8 eV处与C、H结合的C,在286.2 eV处与N、O结合的C以及在287.7 eV与O结合形成C=O或O—C=O的C,287.7 eV处的C=O或O—C=O主要来自羰基、羧基和醛基[51]。吸附前后C 1s和O 1s峰强和位置变化不大,说明吸附前后酵母粉结构没有明显变化[52]。0.5 Fe-Y-Sb的Fe 2p峰位向低结合能方向偏移(图11(b)),且峰强明显减弱,说明Fe与Sb之间形成络合物,且与酵母粉的结合属于化学结合[25, 53-54]。通过测定发现,吸附平衡后上清液中Fe含量极低(<1 mg·L−1),因此吸附过程中改性酵母粉性能较为稳定,不易向溶液中释放Fe离子,因此Fe 2p峰强的减弱可能是部分Fe离子被形成的络合物覆盖,不易被仪器检测[55]。
-
前人用FeCl3改性好氧污泥颗粒时, Fe3+以羟基铁(≡Fe-OH)的形式存在于颗粒表面[56]。有研究认为≡Fe-OH与Sb(Ⅴ)之间可以通过以下模型(式(6))形成内层表面络合物[7]:
本研究通过SEM-EDS和XRD分析证实Fe以无定形性质沉积于改性酵母粉表面,此外在pH=3,25 ℃实验条件下,0.5 Fe-Y 与10 mg·L−1 Sb(Ⅴ) 溶液混合24 h后,体系pH略有下降,降低至2.8左右。结合实验结果和前人研究[56-59]可以推断,利用FeCl3对酵母粉进行改性时,Fe3+在溶液中水解形成Fe(OH)3,然后稳定地附着在酵母粉表面,使得改性后的酵母粉带有大量≡Fe-OH。在pH=3的酸性实验条件下,0.5 Fe-Y表面的≡Fe-OH质子化为≡Fe-OH2+ (式(7)),此时带负电荷的由于静电作用被吸引至颗粒表面,两者通过电子交换形成外层络合物≡Fe-OH2+·Sb(OH)6-(式(8)),然后释放H+形成稳定的化学键(Fe-O-Sb),最终形成内层络合物(式(9))。
-
(1)FeCl3改性能显著提高酵母粉对Sb(Ⅴ)的吸附性能,在初始Sb(Ⅴ)浓度为10 mg·L−1、pH=3、S/L = 2 g·L−1时,0.5 Fe-Y 对Sb(Ⅴ)的吸附效果最佳,吸附容量最大值达68.15 mg·g−1。
(2)0.5 Fe-Y对Sb(Ⅴ)的吸附符合伪二级动力学模型和Langmuir模型,该吸附属于化学吸附,主要表现为络合反应和静电吸附。
(3)FeCl3改性会改变酵母粉表面性质,酵母粉表面羟基等官能团增多、电位增加、晶形结构更加无序以及表面羟基铁与Sb(Ⅴ)形成内层络合物是酵母粉Sb(Ⅴ)吸附能力增强的原因。
铁改性酵母粉对锑酸盐的吸附机理
Adsorption mechanism of antimonate by iron modified yeast powder
-
摘要: 采用氯化铁(FeCl3)制备了铁改性酵母粉,测试其对溶液中锑酸盐(Sb(V))的吸附性能,考察了FeCl3浓度、溶液pH以及酵母粉投加量对Sb(V)去除的影响,研究了Sb(V)在铁改性酵母粉表面的吸附动力学、吸附等温线特征,并用SEM-EDS、ATR-FTIR、XRD、XPS等方法对改性酵母粉进行表征以探究其吸附机理。结果表明,改性酵母粉吸附Sb(V)的最佳条件为:FeCl3浓度0.5 mol·L−1、酵母粉投加量2 g·L−1、pH 3。0.5 mol·L−1 FeCl3改性酵母粉(0.5 Fe-Y)对溶液中Sb(V) 的最大去除率为91.2%,吸附容量达68.15 mg·g−1。0.5 Fe-Y对Sb(V)的吸附符合伪二级动力学和Langmuir等温线模型,该吸附过程属于化学吸附,涉及络合反应和静电吸附。FeCl3改性能显著增强酵母粉对Sb(V)的吸附能力,酵母粉表面羟基等官能团增多、电位增加、晶形结构更加无序以及表面羟基铁与Sb(V)形成内层络合物是其Sb(V)吸附能力增强的原因。Abstract: Iron modified yeast powder was synthesized and tested for its ability to adsorb antimonate (Sb(V)) from aqueous solution. Batch experiments were performed to investigate the effects of various parameters, including FeCl3 concentration, pH, and dosage of yeast powder on the Sb(V) removal. Antimonate adsorption kinetics and isotherm characteristics were investigated and the Sb(V) adsorption mechanism was explored by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS), attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The optimal conditions for Sb(V) adsorption by the modified yeast powder were as follows: FeCl3 concentration 0.5 mol·L−1, dosage 2 g·L−1, and pH 3. The maximum Sb(V) removal rate and adsorption capacity of 0.5 mol·L−1 FeCl3 modified yeast powder (0.5 Fe-Y) were 91.2% and 68.15 mg·g−1, respectively. The adsorption data well fitted the pseudo-second-order kinetic model and Langmuir isotherm model. The Sb(V) adsorption process showed chemical nature, involving complexation and electrostatic adsorption. The FeCl3 modification significantly enhanced the Sb(V) adsorption capacity of yeast powder. This could be attributed to increase of hydroxyl and other functional groups, zeta potential, more disordered crystal structure on the surface of iron modified yeast powder and formation of inner-sphere complexes between surface hydroxyl iron and Sb(V).
-
Key words:
- iron modified /
- yeast /
- Sb(V) /
- adsorption
-

-
表 1 0.5 Fe-Y吸附Sb(Ⅴ)的动力学模型拟合参数
Table 1. Kinetic model parameters for the Sb(Ⅴ) adsorption by 0.5 Fe-Y
伪一级动力学模型
pseudo-first-order kinetics model伪二级动力学模型
pseudo-second-order kinetics modelqe/(mg·g−1) k1/ h−1 R2 qe/(mg·g−1) k2/(g·mg·h−1) R2 2.32 2.02 0.91 2.37 2.39 0.998 表 2 0.5 Fe-Y对Sb(Ⅴ)的吸附等温线模型拟合参数
Table 2. Isotherm model parameters of Sb(Ⅴ) adsorption by 0.5 Fe-Y
Langmuir Freundlich qm /(mg·g−1) kl /(L·mg−1) R2 kf /(mg1−(1/n)·L1/n·g−1) 1/n R2 68.15 0.14 0.995 13.52 0.28 0.911 表 3 0.5 Fe-Y与其他吸附剂的Sb(Ⅴ)吸附容量对比
Table 3. Comparisons of Sb(Ⅴ) adsorption capacity between 0.5 Fe-Y and other adsorbents
吸附剂
AdsorbentSb(Ⅴ)浓度/(mg·L−1)
Sb(Ⅴ) concentrationpH S/L/ (g·L−1) 吸附容量/(mg·g−1)
Adsorption capacity文献
Reference膨润土 0.05 — 4 6.0 25 0.556 [27] 纳米零价铁 0 — 20 4 — 10 2 1.65 [28] 大型绿藻 25 2.0 0.25 3.1 [29] 微囊藻 0.2 — 10 2.8 50 7.34 [11] 高岭石 1 6.0 25 12 [30] 铈掺杂磁性生物炭 10 — 100 7.5 1 25 [31] 松树皮提取物 20 2 0.5 27 [32] 太湖蓝藻 10 — 600 2.5 50 38.2 [33] TiO2 0.5 — 150 7.0 1 43 [34] 铁锆双氧化物 0 — 25 7.0 0.2 51 [35] ZrO2-碳纳米纤维 10 — 500 7.0 1 57.17 [36] 合成锰矿 0.5 — 98 3.0 0.6 95 [37] 0.5 Fe-Y 10 3.0 2 68.15 本研究 -
[1] SANTOS S, UNGUREANU G, BOAVENTURA R, et al. Biosorption of antimony by brown algae S. muticum and A. nodosum [J]. Environmental Engineering and Management Journal, 2015, 14(2): 455-463. doi: 10.30638/eemj.2015.047 [2] WANG L L, LI H, YU D Y, et al. Hyperbranched polyamide-functionalized sodium alginate microsphere as a novel adsorbent for the removal of antimony(Ⅲ) in wastewater [J]. Environmental Science and Pollution Research, 2019, 26(26): 27372-27384. doi: 10.1007/s11356-019-05914-4 [3] WANG L L, LUO Y P, LI H, et al. Preparation and selective adsorption of surface-imprinted microspheres based on hyperbranched polyamide-functionalized sodium alginate for the removal of Sb(Ⅲ) [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2020, 585: 124106. doi: 10.1016/j.colsurfa.2019.124106 [4] FILELLA M, BELZILE N, CHEN Y W. Antimony in the environment: A review focused on natural waters: Ⅰ. Occurrence [J]. Earth-Science Reviews, 2002, 57(1/2): 125-176. [5] FILELLA M, BELZILE N, LETT M C. Antimony in the environment: A review focused on natural waters. Ⅲ. Microbiota relevant interactions [J]. Earth-Science Reviews, 2007, 80(3/4): 195-217. [6] 任杰, 刘晓文, 李杰, 等. 我国锑的暴露现状及其环境化学行为分析 [J]. 环境化学, 2020, 39(12): 3436-3449. REN J, LIU X W, LI J, et al. Analysis of exposure status quo and environmental chemical behaviors of antimony in China [J]. Environmental Chemistry, 2020, 39(12): 3436-3449(in Chinese).
[7] GUO X J, WU Z J, HE M C. Removal of antimony(Ⅴ) and antimony(Ⅲ) from drinking water by coagulation-flocculation-sedimentation (CFS) [J]. Water Research, 2009, 43(17): 4327-4335. doi: 10.1016/j.watres.2009.06.033 [8] 蒋丹丹, 王丹, 金鑫, 等. 锑在印染废水高比例循环利用过程中的富集 [J]. 环境化学, 2018, 37(3): 591-599. doi: 10.7524/j.issn.0254-6108.2017072001 JIANG D D, WANG D, JIN X, et al. Accumulation of antimony in a high rate printing and dyeing wastewater recycling process [J]. Environmental Chemistry, 2018, 37(3): 591-599(in Chinese). doi: 10.7524/j.issn.0254-6108.2017072001
[9] UNGUREANU G, SANTOS S, BOAVENTURA R, et al. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption [J]. Journal of Environmental Management, 2015, 151: 326-342. [10] 孙福红, 扈学文, 郭飞, 等. 微囊藻吸附剂对Sb(Ⅲ)的生物吸附特征 [J]. 环境科学研究, 2016, 29(12): 1857-1864. doi: 10.13198/j.issn.1001-6929.2016.12.14 SUN F H, HU X W, GUO F, et al. Biosorption characteristics of Sb(Ⅲ) by Microcystis biosorbent [J]. Research of Environmental Sciences, 2016, 29(12): 1857-1864(in Chinese). doi: 10.13198/j.issn.1001-6929.2016.12.14
[11] SUN F H, YAN Y B, LIAO H Q, et al. Biosorption of antimony(Ⅴ) by freshwater cyanobacteria Microcystis from Lake Taihu, China: Effects of pH and competitive ions [J]. Environmental Science and Pollution Research, 2014, 21(9): 5836-5848. doi: 10.1007/s11356-014-2522-7 [12] ZHANG Y S, LIU W G, ZHANG L, et al. Application of bifunctional Saccharomyces cerevisiae to remove lead(Ⅱ) and cadmium(Ⅱ) in aqueous solution [J]. Applied Surface Science, 2011, 257(23): 9809-9816. doi: 10.1016/j.apsusc.2011.06.026 [13] BANIAMERIAN M J, MORADI S E, NOORI A, et al. The effect of surface modification on heavy metal ion removal from water by carbon nanoporous adsorbent [J]. Applied Surface Science, 2009, 256(5): 1347-1354. doi: 10.1016/j.apsusc.2009.08.106 [14] YAVUZ H, DENIZLI A, GÜNGÜNEŞ H, et al. Biosorption of mercury on magnetically modified yeast cells [J]. Separation and Purification Technology, 2006, 52(2): 253-260. doi: 10.1016/j.seppur.2006.05.001 [15] WANG B, LI F Y, WANG L. Enhanced hexavalent chromium (Cr(Ⅵ)) removal from aqueous solution by Fe-Mn oxide-modified cattail biochar: Adsorption characteristics and mechanism [J]. Chemistry and Ecology, 2020, 36(2): 138-154. doi: 10.1080/02757540.2019.1699537 [16] YANG Q, WANG X L, LUO W, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge [J]. Bioresource Technology, 2018, 247: 537-544. doi: 10.1016/j.biortech.2017.09.136 [17] WANG Y L, WANG S F, WANG X, et al. Adsorption behavior and removal mechanism of arsenic from water by Fe(Ⅲ)-modified 13X molecular sieves [J]. Water, Air, & Soil Pollution, 2016, 227(8): 1-10. [18] 许光眉, 施周, 邓军. 石英砂负载氧化铁吸附除锑的研究 [J]. 环境化学, 2006, 25(4): 481-484. doi: 10.3321/j.issn:0254-6108.2006.04.020 XU G M, SHI Z, DENG J. Removal of antimony from water by iron-oxide coated sand [J]. Environmental Chemistry, 2006, 25(4): 481-484(in Chinese). doi: 10.3321/j.issn:0254-6108.2006.04.020
[19] 刘爱平, 黄阳, 王维清, 等. 铁改性凹凸棒土对Sb(Ⅴ)的吸附研究 [J]. 非金属矿, 2018, 41(6): 26-29. doi: 10.3969/j.issn.1000-8098.2018.06.008 LIU A P, HUANG Y, WANG W Q, et al. Study on the adsorption of Sb(Ⅴ) by iron modified attapulgite [J]. Non-Metallic Mines, 2018, 41(6): 26-29(in Chinese). doi: 10.3969/j.issn.1000-8098.2018.06.008
[20] 刘爱平, 黄阳, 王维清, 等. 铁改性斜发沸石的制备及吸附Sb(Ⅴ)效果研究 [J]. 矿物岩石, 2018, 38(2): 7-12. LIU A P, HUANG Y, WANG W Q, et al. Preparation of iron mdified clinoptilolite and its adsorption results on Sb(ⅴ) [J]. Journal of Mineralogy and Petrology, 2018, 38(2): 7-12(in Chinese).
[21] 秦培瑞, 郗敏, 李纪华, 等. 经三氯化铁改性的沸石除磷效果 [J]. 湿地科学, 2017, 15(3): 464-469. doi: 10.13248/j.cnki.wetlandsci.2017.03.021 QIN P R, XI M, LI J H, et al. Effect of phosphorus removal by zeolite modified by ferric trichloride [J]. Wetland Science, 2017, 15(3): 464-469(in Chinese). doi: 10.13248/j.cnki.wetlandsci.2017.03.021
[22] 黄永炳, 李晓娟, 王丽丽, 等. 铁改性锰矿对砷的吸附性能研究 [J]. 武汉理工大学学报, 2011, 33(9): 115-119. doi: 10.3963/j.issn.1671-4431.2011.09.024 HUANG Y B, LI X J, WANG L L, et al. Study of the arsenic adsorption properties on modified manganese ore of iron ore [J]. Journal of Wuhan University of Technology, 2011, 33(9): 115-119(in Chinese). doi: 10.3963/j.issn.1671-4431.2011.09.024
[23] 刘喜, 敖鸿毅, 刘剑彤. 铁改性竹炭去除水中的As(Ⅲ)和As(Ⅴ) [J]. 环境工程学报, 2012, 6(9): 2958-2962. LIU X, AO H Y, LIU J T. Removal of As(Ⅲ) and As(Ⅴ) from water by iron-modified bamboo charcoal [J]. Chinese Journal of Environmental Engineering, 2012, 6(9): 2958-2962(in Chinese).
[24] GUO W J, FU Z Y, ZHANG Z Y, et al. Synthesis of Fe3O4 magnetic nanoparticles coated with cationic surfactants and their applications in Sb(V) removal from water [J]. Science of the Total Environment, 2020, 710: 136302. doi: 10.1016/j.scitotenv.2019.136302 [25] ZHANG C, JIANG H Y, DENG Y M, et al. Adsorption performance of antimony by modified iron powder [J]. RSC Advances, 2019, 9(54): 31645-31653. doi: 10.1039/C9RA05646G [26] TU Y H, REN L F, LIN Y X, et al. Adsorption of antimonite and antimonate from aqueous solution using modified polyacrylonitrile with an ultrahigh percentage of amidoxime groups [J]. Journal of Hazardous Materials, 2020, 388: 121997. doi: 10.1016/j.jhazmat.2019.121997 [27] XI J H, HE M C, LIN C Y. Adsorption of antimony(Ⅲ) and antimony(Ⅴ) on bentonite: Kinetics, thermodynamics and anion competition [J]. Microchemical Journal, 2011, 97(1): 85-91. doi: 10.1016/j.microc.2010.05.017 [28] ZHAO X Q, DOU X M, MOHAN D, et al. Antimonate and antimonite adsorption by a polyvinyl alcohol-stabilized granular adsorbent containing nanoscale zero-valent iron [J]. Chemical Engineering Journal, 2014, 247: 250-257. doi: 10.1016/j.cej.2014.02.096 [29] UNGUREANU G, FILOTE C, SANTOS S C R, et al. Antimony oxyanions uptake by green marine macroalgae [J]. Journal of Environmental Chemical Engineering, 2016, 4(3): 3441-3450. doi: 10.1016/j.jece.2016.07.023 [30] XI J H, HE M C, LIN C Y. Adsorption of antimony(Ⅴ) on kaolinite as a function of pH, ionic strength and humic acid [J]. Environmental Earth Sciences, 2010, 60(4): 715-722. doi: 10.1007/s12665-009-0209-z [31] WANG L, WANG J Y, WANG Z X, et al. Synthesis of Ce-doped magnetic biochar for effective Sb(Ⅴ) removal: Performance and mechanism [J]. Powder Technology, 2019, 345: 501-508. doi: 10.1016/j.powtec.2019.01.022 [32] BACELO H, VIEIRA B R C, SANTOS S C R, et al. Recovery and valorization of tannins from a forest waste as an adsorbent for antimony uptake [J]. Journal of Cleaner Production, 2018, 198: 1324-1335. doi: 10.1016/j.jclepro.2018.07.086 [33] 吴珊, 孙福红, 鄢元波, 等. 太湖蓝藻对Sb(Ⅴ)的生物吸附作用 [J]. 环境科学研究, 2012, 25(7): 764-769. doi: 10.13198/j.res.2012.07.37.wush.001 WU S, SUN F H, YAN Y B, et al. Biosorption of Sb(Ⅴ) by cyanobacteria from Taihu lake [J]. Research of Environmental Sciences, 2012, 25(7): 764-769(in Chinese). doi: 10.13198/j.res.2012.07.37.wush.001
[34] JIANG Y X, YAN L, NIE X, et al. Remediation of antimony-contaminated tap water using granular TiO2 column [J]. Environmental Chemistry, 2020, 17(4): 323. doi: 10.1071/EN19170 [35] LI X H, DOU X M, LI J Q. Antimony(Ⅴ) removal from water by iron-zirconium bimetal oxide: Performance and mechanism [J]. Journal of Environmental Sciences, 2012, 24(7): 1197-1203. doi: 10.1016/S1001-0742(11)60932-7 [36] LUO J M, LUO X B, CRITTENDEN J, et al. Removal of antimonite (Sb(Ⅲ)) and antimonate (Sb(Ⅴ)) from aqueous solution using carbon nanofibers that are decorated with zirconium oxide (ZrO2) [J]. Environmental Science & Technology, 2015, 49(18): 11115-11124. [37] WANG X Q, HE M C, LIN C Y, et al. Antimony(Ⅲ) oxidation and antimony(Ⅴ) adsorption reactions on synthetic manganite [J]. Geochemistry, 2012, 72: 41-47. doi: 10.1016/j.chemer.2012.02.002 [38] 李敏, 朱润良, 葛飞, 等. 磷酸根和镉离子在羟基铁改性膨润土表面的协同吸附机制研究 [J]. 环境科学学报, 2013, 33(12): 3205-3210. doi: 10.13671/j.hjkxxb.2013.12.011 LI M, ZHU R L, GE F, et al. Synergistic adsorption of cadmium and phosphate on hydroxyl-Fe- bentonite complex [J]. Acta Scientiae Circumstantiae, 2013, 33(12): 3205-3210(in Chinese). doi: 10.13671/j.hjkxxb.2013.12.011
[39] BURATTINI E, CAVAGNA M, DELL’ANNA R, et al. A FTIR microspectroscopy study of autolysis in cells of the wine yeast Saccharomyces cerevisiae [J]. Vibrational Spectroscopy, 2008, 47(2): 139-147. doi: 10.1016/j.vibspec.2008.04.007 [40] ROSSI A, RIGON M R, ZAPAROLI M, et al. Chromium (Ⅵ) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments [J]. Environmental Science and Pollution Research, 2018, 25(19): 19179-19186. doi: 10.1007/s11356-018-2377-4 [41] 莫贞林, 曾鸿鹄, 林华, 等. 高锰酸钾改性桉木生物炭对Pb(Ⅱ)的吸附特性[J]. 环境科学, 2021, 42(11): 5440-5449 MO Z L, ZENG H H, LIN H, et al. Adsorption Characteristics of Pb(Ⅱ) on Eucalyptus Biochar Modified by Potassium Permanganate[J]. Environmental Science, 2021, 42(11): 5440-5449(in Chinese).
[42] ZHANG W, WANG F H, WANG P L, et al. Facile synthesis of hydroxyapatite/yeast biomass composites and their adsorption behaviors for lead (Ⅱ) [J]. Journal of Colloid and Interface Science, 2016, 477: 181-190. doi: 10.1016/j.jcis.2016.05.050 [43] de ROSSI A, RIGUETO C V T, DETTMER A, et al. Synthesis, characterization, and application of Saccharomyces cerevisiae/alginate composites beads for adsorption of heavy metals [J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 104009. doi: 10.1016/j.jece.2020.104009 [44] B A, TALASILA S, RAJESH V, et al. Removal of Europium from aqueous solution using Saccharomyces cerevisiae immobilized in glutaraldehyde cross-linked chitosan [J]. Separation Science and Technology, 2019, 54(10): 1620-1631. doi: 10.1080/01496395.2018.1556303 [45] LI Y C, XU Z, WU J X, et al. Efficiency and mechanisms of antimony removal from wastewater using mixed cultures of iron-oxidizing bacteria and sulfate-reducing bacteria based on scrap iron [J]. Separation and Purification Technology, 2020, 246: 116756. doi: 10.1016/j.seppur.2020.116756 [46] WANG L, WAN C L, LEE D J, et al. Biosorption of antimony(Ⅴ) onto Fe(Ⅲ)-treated aerobic granules [J]. Bioresource Technology, 2014, 158: 351-354. doi: 10.1016/j.biortech.2014.02.046 [47] BIRCHALL T, CONNOR J A, HILLIER L H. High-energy photoelectron spectroscopy of some antimony compounds [J]. Journal of the Chemical Society, Dalton Transactions, 1975(20): 2003. doi: 10.1039/dt9750002003 [48] AVILEZ GARCIA R G, MEZA AVENDAÑO C A, PAL M, et al. Antimony sulfide (Sb2S3) thin films by pulse electrodeposition: Effect of thermal treatment on structural, optical and electrical properties [J]. Materials Science in Semiconductor Processing, 2016, 44: 91-100. doi: 10.1016/j.mssp.2015.12.018 [49] DOU X M, MOHAN D, ZHAO X Q, et al. Antimonate removal from water using hierarchical macro-/mesoporous amorphous alumina [J]. Chemical Engineering Journal, 2015, 264: 617-624. doi: 10.1016/j.cej.2014.11.123 [50] HE X Y, MIN X B, PENG T, et al. Enhanced adsorption of antimonate by ball-milled microscale zero valent iron/pyrite composite: Adsorption properties and mechanism insight [J]. Environmental Science and Pollution Research International, 2020, 27(14): 16484-16495. doi: 10.1007/s11356-020-08163-y [51] LEI M, TAO J, YANG R J, et al. Binding of Sb(Ⅲ) by Sb-tolerant Bacillus cereus cell and cell-goethite composite: Implications for Sb mobility and fate in soils and sediments [J]. Journal of Soils and Sediments, 2019, 19(6): 2850-2858. doi: 10.1007/s11368-019-02272-z [52] LIU C, LI Y, WANG X L, et al. Efficient extraction of antimony(Ⅲ) by titanate nanosheets: Study on adsorption behavior and mechanism [J]. Ecotoxicology and Environmental Safety, 2021, 207: 111271. doi: 10.1016/j.ecoenv.2020.111271 [53] HUDCOVÁ B, ERBEN M L, VÍTKOVÁ M, et al. Antimonate adsorption onto Mg-Fe layered double hydroxides in aqueous solutions at different pH values: Coupling surface complexation modeling with solid-state analyses [J]. Chemosphere, 2019, 229: 236-246. doi: 10.1016/j.chemosphere.2019.05.008 [54] ZHAO T H, TANG Z, ZHAO X L, et al. Efficient removal of both antimonite (Sb(Ⅲ)) and antimonate (Sb(Ⅴ)) from environmental water using titanate nanotubes and nanoparticles [J]. Environmental Science:Nano, 2019, 6(3): 834-850. doi: 10.1039/C8EN00869H [55] HE X Y, MIN X B, PENG T, et al. Highly efficient antimonate removal from water by pyrite/hematite Bi-mineral: Performance and mechanism studies [J]. Journal of Chemical & Engineering Data, 2019, 64(12): 5910-5919. [56] WANG L, WAN C L, ZHANG Y, et al. Mechanism of enhanced Sb(Ⅴ) removal from aqueous solution using chemically modified aerobic granules [J]. Journal of Hazardous Materials, 2015, 284: 43-49. doi: 10.1016/j.jhazmat.2014.10.041 [57] LEUZ A K, MÖNCH H, JOHNSON C A. Sorption of Sb(Ⅲ) and Sb(Ⅴ) to goethite: Influence on Sb(Ⅲ) oxidation and mobilization [J]. Environmental Science & Technology, 2006, 40(23): 7277-7282. [58] ARYAL M, ZIAGOVA M, LIAKOPOULOU-KYRIAKIDES M. Comparison of Cr(Ⅵ) and As(Ⅴ) removal in single and binary mixtures with Fe(Ⅲ)-treated Staphylococcus xylosus biomass: Thermodynamic studies [J]. Chemical Engineering Journal, 2011, 169(1/2/3): 100-106. [59] ARYAL M, ZIAGOVA M, LIAKOPOULOU-KYRIAKIDES M. Study on arsenic biosorption using Fe(Ⅲ)-treated biomass of Staphylococcus xylosus [J]. Chemical Engineering Journal, 2010, 162(1): 178-185. doi: 10.1016/j.cej.2010.05.026 -




 下载:
下载: