-
氰化提金过程中会产生大量的氰化废水,并且为了保证后续浸出效果,在碱浸过程中需要脱药。经脱药后,原精矿粉中的黄药、乙硫氮、浮选油等药剂被脱出,使水中COD升高。这类废水中氰化物主要以金属氰络合物以及SCN−的形式存在,溶液中的有机物的含量大,且难处理。现有的酸化法[1]、化学沉淀法[2]、生物氧化法[3-4]、溶剂萃取法[5-6]等由于普遍存在环境污染大、成本高、过程难控制、处理不达标等问题,难以大规模推广应用。因此,探索一条对环境污染较小、成本低、过程控制简单、高效的处理工艺对我国黄金产业发展有着至关重要的意义。
电解氧化法具有处理效果好、环境污染小等特点,常被用于处理电镀废水[7]、焦化废水[8-9]以及印染废水[10]等。电解过程中阳极反应产生的过氧化氢[11]、羟基自由基[12]、活性氯(Cl2、ClO−、HClO)[13-14]等的氧化作用是污染物去除的主要原因。NIDHEESH等[14]采用石墨作阳极对工业废水进行电化学氧化法处理,额外添加氯化钠可显著提高COD和色度的去除率,强调了Cl−在间接氧化过程中的重要性。GAO等[15]以不锈钢圆柱为阴极,多孔石墨为阳极电解处理氰化废水,发现NaCl的加入可以促进氰化物在阳极表面的氧化及Ag+的阴极回收,相比未加入NaCl之前,当添加2.9 g·L−1 NaCl,电解3.0 h下氰化物的去除率从57.5%提升至大于99%,Ag+去除率达到≥ 95.0%的时间从2.5 h降至1.0 h,实现了氰化物的去除的同时回收废水中的有价元素。LI[16]等采用电解氧化法对垃圾渗滤液中难降解污染物进行去除,以Ti/RuO2-IrO2为阳极,Al为阴极,在电流密度为0.1 A·cm−2,pH为6.37,Cl−质量浓度为6.5 g·L−1的条件下电解150 min,COD和NH3-N去除率分别为83.7%和100%,通过GC-MS分析表明,在电解过程中有机物的种类和质量百分比明显下降,有机物的去除主要依靠溶液中的Cl−在阳极生成氯化物衍生物种(HClO、ClO−等)对其进行氧化去除。FAJARDO [17]等采用Ti/RuO2阳极电化学氧化工艺处理含酚废水,总酚和COD的初始质量浓度为323 m g·L−1和1 118 mg·L−1,当NaCl质量浓度为10 g·L−1,电流密度为119 mA·cm2,初始pH为3.4,电解3.0 h后总酚和COD的去除率可以达到100%,但处理后的废水中仍剩有大量Cl−。电解氧化法对氰化物与有机物的处理效果显著,其中Cl−的引入对污染物的降解起着至关重要的作用,但额外的电解质如氯盐的加入会提升废水的处理成本,还会引入Cl−等新的成分,同时现有研究基本都停留在对低浓度、成分单一以及模拟溶液的研究阶段,而实际工业混合废水中成分复杂、污染物浓度高,需要综合考量其废水特性,以选择最优的处理方案。
本文以某黄金冶炼厂选冶混合废水为研究对象,废水中含大量Cl−,可为直接电解氧化处理奠定基础,无需加入其他电解质,大大节约处理成本。因此,本研究利用电解氧化法对该金矿废水中高浓度氰化物及有机物处理效果的影响以及过程机理进行了研究,重点考察了电压、电解时间等对氰化物与有机物的去除影响,分析了可能的降解机理,以期为此类金矿废水中氰化物与有机物的综合治理提供新的途径。
-
实验水样为某黄金冶炼厂选冶混合废水,水质特征如表1所示。该废水在工厂生产运行期间需要不断循环利用,氰化物与有机物不断富集,导致氰化物(CNT)、Cu、COD、SCN−等浓度较高,分别为1 700.00、2 600.00、33 275.00、22 571.45 mg·L−1,同时还富集了高达3 130.00 mg·L−1的氯离子,这对于电化学处理的有效运行至关重要。该废水在工厂内已经过酸化法预处理,原水pH为6.0,Zn和Fe含量很低,仅为0.30 mg·L−1和1.23 mg·L−1,且未检测到游离氰(CN−)的存在,因此,实验过程不用考虑。
-
以2块石墨板作阳极一块钛合金板作阴极组成两阳一阴三电极体系,尺寸均为50 mm×30 mm×2 mm。采用LP305DE型稳压电源控制电压,采用CJJ78-1型磁力搅拌器进行搅拌,实验装置图见文献[18]。
-
室温下准确量取100 mL废水置于电解槽中,用NaOH、H2SO4调节pH,电解过程中使用磁力搅拌器进行搅拌,一定时间后进行取样过滤,将滤液进行分析测定CNT、COD、Cu、Fe、SCN−、Zn和Cl−等含量,沉淀经去离子水洗至中性,在60 ℃下烘干后分析。
-
废水中Cu、Zn等金属离子采用火焰原子吸收法(GB 7475-87、GB11911-89)测定;CNT采用《水质 氰化物的测定容量法和分光光度法》(HJ484-2009)中的硝酸银滴定法测定;Cl−采用《水质 氯化物的测定 硝酸银滴定法》(GB 11896-89)测定;COD采用《水质 化学需氧量的测定 重铬酸盐法》(HJ828-2017)测定,其中加入硫酸汞溶液来消除氯离子的干扰;SCN−采用7200型分光光度计进行测定;ClO−和Cl2含量采用硫代硫酸钠滴定法进行滴定。采用X'Pert PRO MPD型X射线衍射仪对电解后生成沉淀物的组成进行分析,阴极板的元素组成及形貌采用Zeiss Sigma 300型扫描电子显微镜进行分析,溶液中有机物组成采用PY-3030D型气相色谱质谱联用进行分析。去除率η按式(1)进行计算。
式中:C0为废水中氰化物、铜、铁、锌初始质量浓度与初始COD,mg·L−1;Ct为氧化处理后废水中氰化物、铜、铁、锌、SCN−的质量浓度与COD,mg·L−1。
-
在温度为室温、极板间距为1.5 cm,外加电压分别为3.0、3.5、4、4.5、5 V的条件下电解3 h,污染物去除率及废水温度的变化结果如图1所示。随着外加电压的增大,Cu、CNT、SCN−与COD的去除率逐渐增大,在3.5 V时Cu、CNT去除率达到最大值96.2%、98.5%。由于溶液中COD、SCN−的浓度较高,去除时所需的氧化剂量更高,因此,COD和SCN−在外加电压为4.5 V时去除率才达到90%以上,随后电压的增加对去除率影响不明显。废水中存在Cl−,析氯与析氧标准电位分别为1.36 V和0.40 V[15],因此,当电压大于3.0 V时,阳极以析氯反应为主。Cl−在阳极生成Cl2、HClO、ClO−等活性氯物种(式(2)~式(4)),电压越大越有利于活性氯的生成[19],形成的HClO、ClO−等强氧化性物质将氰化物、有机物等污染物氧化,实际上这与含氰废水常用的处理方法碱性氯化法类似。同时部分金属氰络合物及释放出来的金属阳离子会被还原为锌、铜等金属单质沉积在阴极上从而得以回收[20]。但电压过高时,剧烈的电极反应可能会导致温度升高(图1(b)),从而导致能耗增大,而温度的升高也会影响反应速率等,综合考虑选取最优外加电压为4.5 V。
-
在外加电压为4.5 V,电解时间分别为1、2、3、4、5 h条件下,污染物去除率及废水中Cl−与活性氯质量浓度的变化结果如图2所示。随着电解反应的进行,Cu、CNT、SCN−、COD的去除率逐渐升高,在3 h时达到最高,分别为96.2%、98.5%、99.9%、90.6%,之后不再发生明显的变化。在反应初期,在电场作用下离子快速移动,在阳极不断生成的HClO、ClO−等可对有机物和氰化物进行氧化降解;随着反应的进行,溶液中各离子浓度降低,反应强度降低,各离子去除率的增幅开始减小。由于反应过程包含一系列的氧化还原反应,电解时间过短会造成反应过程不充分、离子接触时间过短,因此,足够的反应时间是污染物降解的重要保障。但电解时间过长会导致能耗的增加,因此,确定最佳电解时间为3 h。
在电解过程中Cl−与活性氯浓度的变化结果如图2(b)所示。可以发现,随着反应的进行,Cl−逐渐减少,活性氯浓度则逐渐升高。这是因为Cl−在阳极被氧化为活性氯,导致Cl−大量消耗。而活性氯会参与氰化物和有机物的氧化降解,随着电解的进行,废水中污染物含量逐渐降低,参与污染物氧化反应的活性氯也随之减少,导致溶液中残留的活性氯含量开始逐渐增加[21]。
-
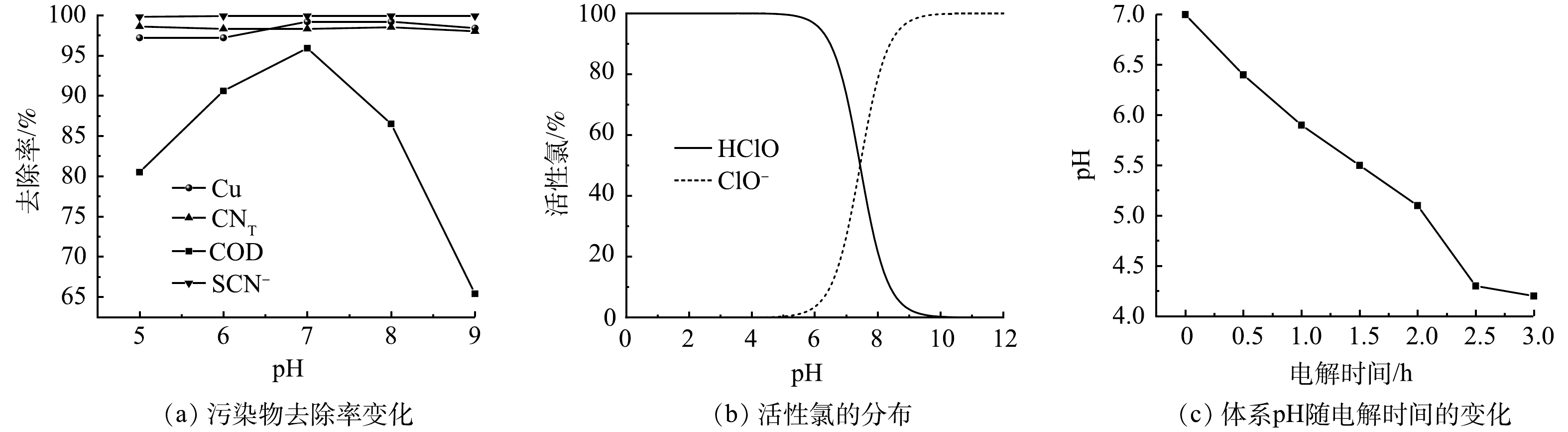
在初始pH为5、6、7、8、9条件下电解3 h,污染物去除率、活性氯分布及废水pH的变化情况如图3所示。体系初始pH的变化对Cu、CNT、SCN−的去除率影响并不明显,但COD去除率随pH的增大先升高后降低,pH为7时达到最大值96%。在pH为5~9的条件下,电解产生的Cl2发生水解(式(4)),产生的HClO是一种弱酸,在水溶液中分解(式(5))[22],。但酸性条件不利于Cl2的水解反应[23],导致Cl2转化为HClO和ClO−的比率降低,同时pH还会影响HClO和ClO−在废水中的平衡,从而影响对污染物的降解。采用Visual MINTEQ软件对25 ℃、Cl−质量浓度为3 130.00 mg·L−1时,溶液中活性氯在不同pH条件下的分布平衡曲线进行模拟如图3(b)所示。可知在pH< 7.5时,主要以HClO的形式存在,当pH<5.0时,ClO−相较HClO含量几乎可以忽略不计。而当pH大于7.5时,HClO还会与ClO−发生反应转换为ClO3−(式(6)),ClO3−的氧化性较弱[17],导致COD去除率下降。此外,生成的ClO3−会与溶液中的钠离子反应产生氯酸钠(NaClO3),氯酸钠可作为COD的掩蔽剂[24],这对COD的测定会产生不利的影响,导致测定结果偏低。因此,综合考虑选择pH为7。
图3(c)反映了在初始pH为7条件下电解过程的pH变化。可知随着电解时间的延长,废水的pH逐渐降低,这是因为Cl−在阳极与OH−反应生成ClO−,如式(3),溶液中生成Cl2还会与H2O反应生成HClO[25](式(4)),此外,SCN−的降解过程也会消耗OH−(式(7)~式(8)),最终导致溶液的pH降低。从图3(c)可知在电解2 h后pH降至5.1,说明在电解时间为0~2 h内,HClO与ClO−均存在于废水中并参与污染物的氧化降解,如式(7)~式(12),但以HClO为主;电解2 h后,废水中几乎只有HClO存在,此时HClO将剩余污染物降解。
-
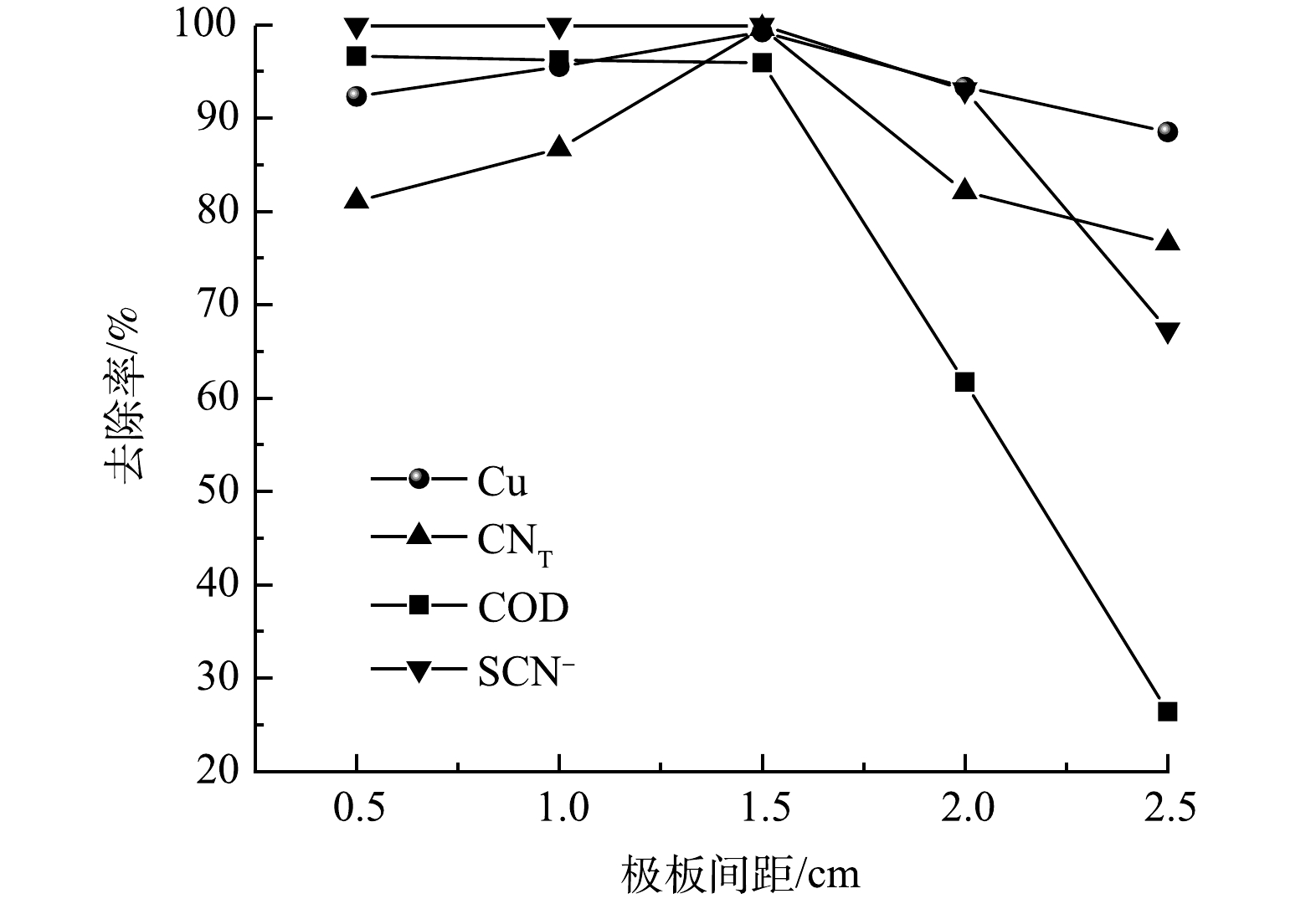
取pH为7,在极板间距为0.5、1.0、1.5、2.0 cm的条件下电解3 h,结果如图4所示。随着极板间距的增大,Cu、CNT、SCN−、COD的去除率先增加后减少,在极板间距达到1.5 cm时各离子的去除率达到最大,分别为99.2%、99.6%、99.9%、96.0%。当极板间距过小时,极板间的电压差越大,易形成瞬时的强电流,从而造成短路现象[18]。极板间距越大,溶液体系中电子传递距离和阻力越大,溶液中各离子扩散距离长、速率慢,并且极板间距越大,电阻越大,电流效率越低,活性氯产量降低,从而影响到氰化物和有机物的去除率。因此,选择最佳极板间距为1.5 cm。
-
在室温,电压4.5 V,pH=7,极板间距1.5 cm,电解时间3 h条件下进行3组平行实验,CNT、Cu、COD及SCN−的平均去除率分别为99.58%、99.22%、95.93%、99.99%。实验结果稳定,说明采用电解氧化法处理黄金冶炼厂选冶混合废水中的氰化物与有机物是有效可行的。
-
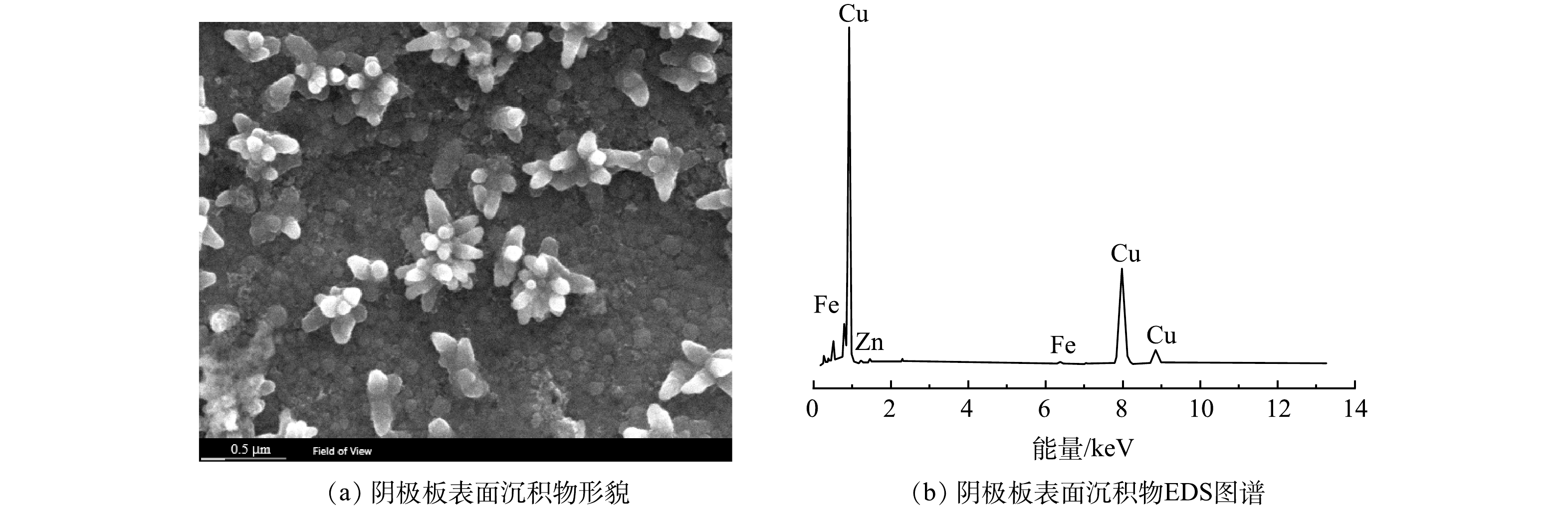
1)SEM-EDS分析。在最佳条件下电解后阴极板的SEM-EDS分析结果如图5所示。可以看出,阴极板表面的析出物主要是铜,少量锌在阴极板上分布。废水中部分金属氰络合离子在阳极被氧化,释放出的Cu2+、Zn2+在电场作用下定向迁移至阴极表面析出铜和锌单质,反应如式(13)~式(14)。
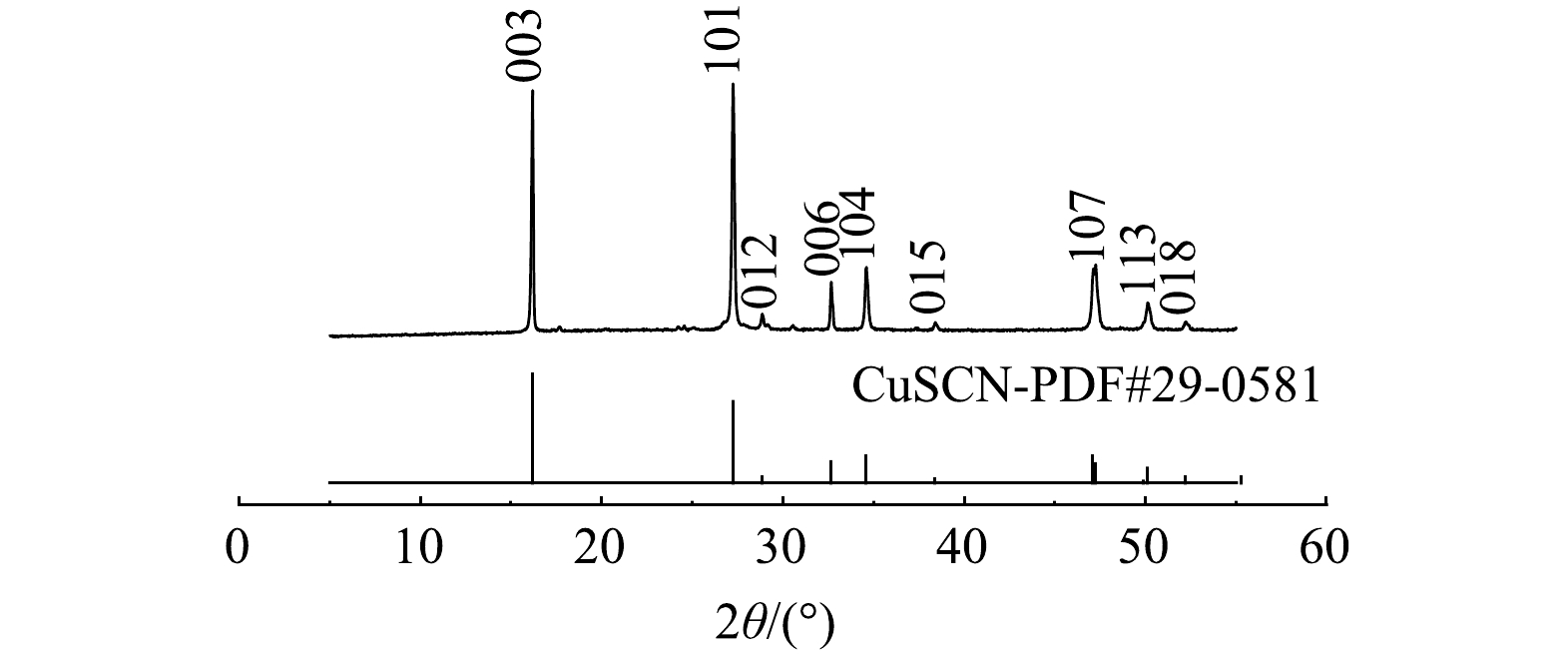
2)沉淀物分析。最佳条件下电解后溶液中沉淀物的XRD图谱分析如图6所示。样品的XRD图谱中显示出典型的CuSCN衍射峰(PDF#29-0581)。在电解过程中,阳极附近OH−参与反应而被消耗,导致H+浓度局部增大,使得一部分迁移富集在阳极板周围的Cu(CN)32-、SCN−之间发生沉淀反应,生成CuSCN沉淀[26],反应如式(15)。将电解在最佳条件下生成的沉淀物进行过滤,水洗至中性,在60 ℃下烘干,之后取出进行称量得到0.18 g的CuSCN,说明废水中有36.17% Cu、3.81% SCN−是通过生成CuSCN沉淀形式而除去,剩余的Cu沉积于阴极极板上,SCN−则被HClO、ClO−氧化为N2、CO2和SO42−,反应如式(7)~(8)。
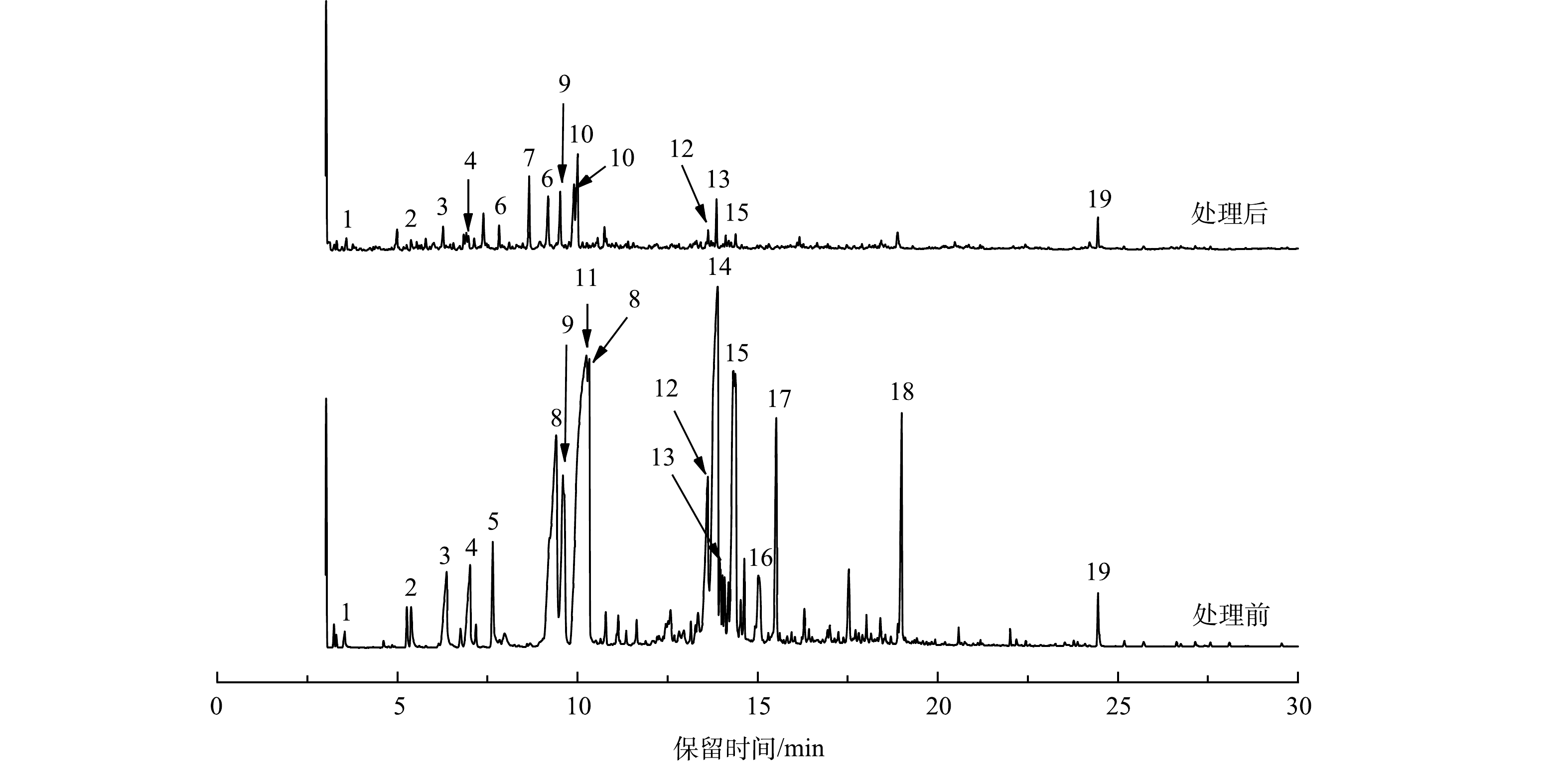
3)气相色谱质谱联用分析。电解处理前后的水样的GC-MS谱图如图7所示。原水中含近26种有机污染物,经电解氧化处理后,其残留有机物种类虽仍有26种,但其强度得到了很大程度的降低。这表明电解氧化处理法对选冶废水中的有机污染物有着较好的处理效果。
利用质谱分析,对一些主要且吻合度超过80%的有机组分的峰进行积分,将其与数据库中的标准物质进行匹配,得到特征污染物及其相关参数见表1。可见,未经处理的选冶废水所含有机物大部分为结构极其复杂的有机大分子化合物,而处理后其有机物污染物大部分为结构较为简单的短链类有机小分子化合物。此外处理后废水中出现了新的组分,这可能是电解过程中大部分有机物被HClO、ClO−等氧化为H2O、CO2、N2及其他一些小分子物质[27-29],但随着电解时间的延长,在3 h之后溶液中的活性氯的生成量趋于平缓,而生成的HClO、ClO−等强氧化性物质不足以将剩余的有机物完全氧化降解,而是将其转化成小分子有机物。此外,表2中有机物基本按照其出峰时间进行排列,可大致看出其规律性,结构较简单且分子质量偏小的物质出峰时间靠前,反之,结构复杂且分子质量大的则出峰时间靠后。出峰时间靠后的有机物中大部分存在于选冶废水中,而处理后水样未检出该有机物,也可定性反映出反应过程中的一些有机大分子被降解。
依照物质结构和所含官能团等可将表1中的有机物大致分为6类,结果见表2。可见,选冶废水中主要含有醇类、酯类、酰胺类、酮类和长链碳氢化合物等,但氯代有机物较少。一方面,可能是该类有机物分子量较大,沸点较高;另一方面,该类有机物浓度低,富集未达到检出下限[16]。选冶废水中酯类、烷烃类和酮类等物质占比明显减少而醇类等物质有所上升,说明C=C键、酯基、羰基等化学键被氧化断链,酯类、酮类等大分子被分解成简单的短链类有机小分子化合物,且烷烃类和酮类物质在处理后水样中未检出,说明反应中该类有机物被分解为小分子物质后继续被HClO、ClO−氧化为H2O、CO2、N2,从而被彻底去除。此外,处理前后酚类物质的峰面积未发生变化,这可能因为选冶废水中还存在一些难降解有机物如2,4-二叔丁基苯酚,其具有较好的稳定性,很难断裂[30-31],从而导致电解后该部分有机污染物仍未被完全氧化降解,还需进一步深入处理。
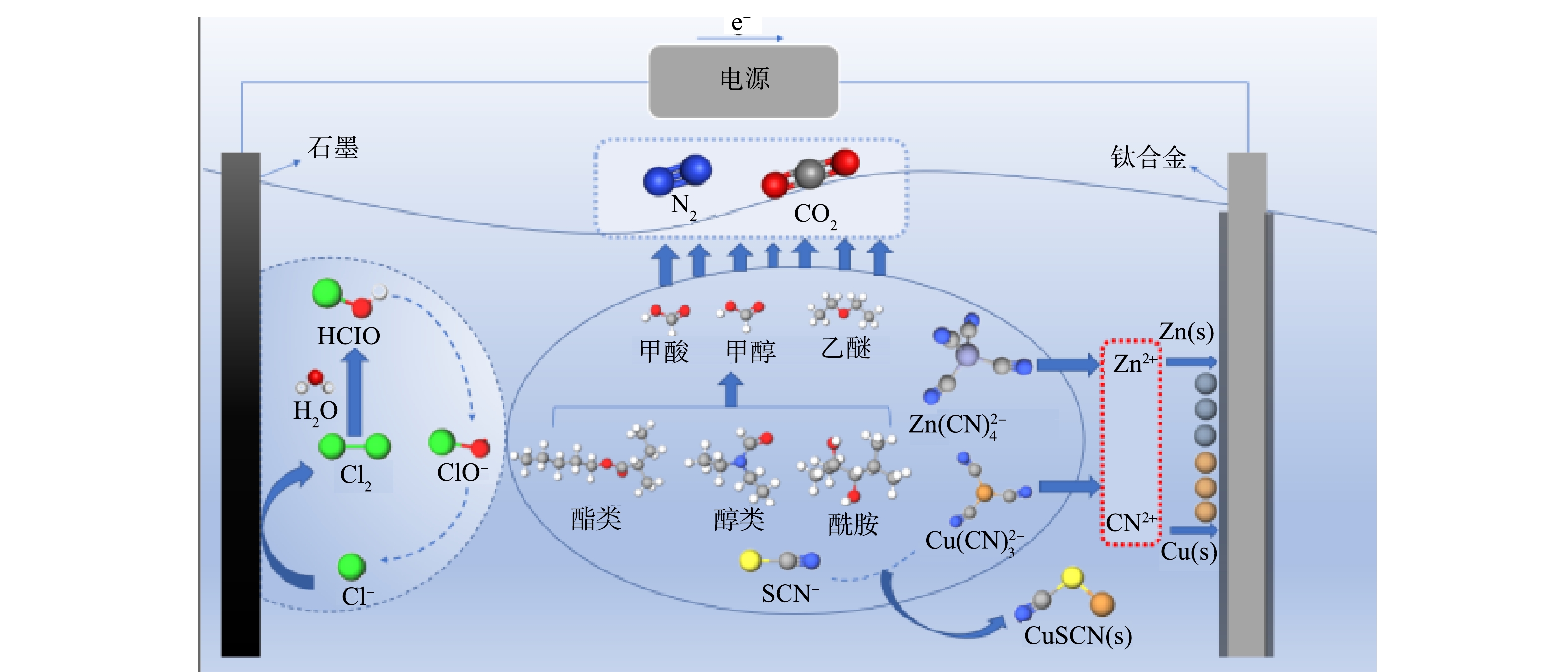
图8简要描述了电解过程中氰化物及有机物的降解路径。在电解过程中,废水中的Cl−会在阳极生成大量活性氯,其中强氧化性的HClO、ClO−等将废水中的氰化物氧化降解,氧化破络释放出的Cu2+、Zn2+在电场作用下定向迁移至阴极表面进而析出铜和锌单质;同时一部分迁移富集在阳极板周围的Cu(CN)32-、SCN−之间发生沉淀反应,形成CuSCN沉淀而被除去。废水中的有机物如酯类、酮类、烷烃类等大分子的C=C键、酯基、羰基等化学键被HClO、ClO−等氧化断链成小分子,之后又将这些小分子物质氧化为H2O、CO2和N2。
-
1)采用电解氧化法处理黄金冶炼厂选冶混合废水中的氰化物和有机物的思路是可行的。以石墨板为阳极,钛合金板为阴极,当电压为4.5 V、pH为7、电解时间为3 h、极板间距为1.5 cm的条件下,CNT、COD、SCN−及Cu的去除率分别可达到99.6%、96.0%、99.9%、99.2%。
2)选冶废水中的Cl−对污染物的降解起着至关重要的作用。在电解过程中体系pH逐渐降低,电场作用下定向迁移至阳极附近的污染物的去除主要归因于HClO及ClO−的间接氧化作用,在电解时间为0-2 h内,体系pH大于5.0,此阶段污染物以HClO的氧化为主,ClO−的氧化为辅,而电解大于2 h后,此时体系pH小于5.0污染物的降解则归因于HClO的氧化。
3)在电解过程中生成的HClO、ClO−等将迁移至阳极附近的氰化物氧化为N2和CO2,将醇类、酯类、烷烃类等有机物先氧化为小分子物质,之后又将这些小分子物质氧化为H2O、CO2和N2。部分Cu(CN)32-与SCN−迁移富集在阳极板周围,形成CuSCN沉淀。废水中的部分金属氰络合离子在阳极被氧化释放出的金属阳离子会被还原为锌、铜等金属单质沉积在阴极上。
电解氧化法处理黄金冶炼厂选冶废水
Treatment of selection and smelting wastewater from gold smelter by electrolytic oxidation
-
摘要: 采用电解氧化法处理同时含有高浓度氰化物与COD的黄金冶炼厂选冶废水,主要考查了外加电压、溶液pH、电解时间及极板间距等因素对氰化物和COD去除率的影响,充分利用原水中Cl-的阳极氧化效应,深入探讨了电化学氧化过程及污染物氧化去除机理。结果表明,随着外加电压和电解时间的增大,氰化物和COD去除率逐渐增大。以石墨为阳极,钛合金为阴极,采用一阴两阳电解体系对废水进行氧化,当电压为4.5 V、初始pH为7、电解时间为3 h、极板间距为1.5 cm的条件下,总氰化物 (CNT) 、COD、SCN-及Cu的去除率最高可达99.6%、96%、99.9%与99.2%。电解过程中体系pH逐渐降低,电场作用下定向迁移至阳极附近的污染物去除主要归因于HClO及ClO-的间接氧化作用,当pH大于5.0时以HClO的氧化为主,ClO-的氧化为辅,而pH小于5.0时则主要是HClO的氧化。电解氧化过程中约有36.17%的Cu与SCN-形成CuSCN沉淀而被除去,剩余的铜则扩散至阴极沉积析出。GC-MS分析表明,烷烃类、酯类、酮类等大分子的C=C键、酯基、羰基等化学键和官能团被氧化断链成小分子物质,随后继续被氧化为H2O、CO2和N2。Abstract: The electrolytic oxidation method was used to treat the selection and smelting wastewater from gold smelter containing both high concentrations of cyanide and COD, the effects of applied voltage, solution pH, electrolysis time and plate spacing on the removal rate of cyanide and COD were studied. The anodic oxidation effect of Cl- in raw water were fully used to deeply explore the electrochemical oxidation process and pollutant oxidation removal mechanism. The results showed that with the increase of applied voltage and electrolysis time, the removal rates of cyanide and COD gradually increased. With graphite as the anode and titanium alloy as the cathode, the one-cathode and two-anode electrolysis system was taken to oxidize the wastewater, and the maximum removal rates of the total cyanide (CNT), COD, SCN- and Cu could reach 99.6%, 96%, 99.9% and 99.2%, respectively, when the voltage was 4.5 V, the initial pH was 7, the electrolysis time was 3 h, and the plate spacing was 1.5 cm. During the electrolysis process, the pH value of the system gradually decreased, and the removal of pollutants that directionally migrated to the anode under the action of the electric field was mainly attributed to the indirect oxidation of HClO and ClO-, at pHs higher than 5.0, HClO oxidation was the main pathway, while ClO- oxidation was an auxiliary one, at pHs lower than 5.0, only HClO dominated the oxidation. During the electrolysis and oxidation process, the CuSCN precipitate was formed through the reaction of 36.17% Cu with SCN-, which could be removed accordingly. The surplus of Cu diffused to anode and the electrodeposition occurred. GC-MS test indicated that the chemical bonds and function groups such as C=C bond, ester group, carbonyl group in large molecular organics such as paraffins, ester and ketones were oxidized and degraded into small molecular organics, then were continuously oxidized to H2O、CO2 and N2.
-

-
表 1 处理前后选冶废水中有机物列表
Table 1. List of organic compounds in selection and smelting wastewater before and after treatment
编号 有机物名称 原水 处理后水样 峰面积 百分比/% 峰面积 百分比/% 1 氨基甲酸乙酯 1.23×108 0.21 8.53×107 1.63 2 N,N-二乙基甲酰胺 3.69×108 0.62 8.58×107 1.64 3 氨基甲酸丙酯 1.28×109 2.14 1.46×108 2.78 4 氨基甲酸丁酯 1.42×109 2.37 1.48×108 2.83 5 1-辛醇 9.38×108 1.57 — — 6 2,2,4-三甲基-1,3-戊二醇 — — 5.50×108 10.48 7 异丁酸己酯 — — 5.04×108 9.62 8 2,4-二甲基-2,3-戊二醇 1.04×1010 17.30 — — 9 5-乙基-1,3-二恶烷-5-甲醇 3.36×109 5.61 3.94×108 7.51 10 2-乙基-1,3-己二醇 — — 1.63×109 31.05 11 2-甲基丁酸戊酯 1.55×1010 25.80 — — 12 3-异硫代氰酰丙酸乙酯 2.63×109 4.39 1.02×108 1.94 13 2,4-二叔丁基苯酚 2.59×108 0.43 2.59×108 5.02 14 4,4-二甲基-2-环己烯-1-醇 7.99×109 13.35 — — 15 2,7-二甲基-1-辛醇 6.73×109 11.24 7.21×107 1.37 16 3-十五烷酮 1.43×109 2.38 — — 17 2-甲基-2-丙烯-1-基(2E)-2-丁烯二酸异丁酯 2.47×109 4.13 — — 18 2,3-二丁基环氧乙烷 1.81×109 3.01 — — 19 双 (2-丙基戊基) 邻苯二甲酸酯 3.94×108 0.66 1.79×108 3.41 注:“—”表示未检出。 表 2 处理前后选冶废水中有机组分变化
Table 2. Changes of organic components in selection and smelting wastewater before and after treatment
有机物类别 处理前溶液产物分布 处理后溶液产物分布 峰面积 峰面积占比/% 峰面积 峰面积占比/% 醇类 2.94×109 51.54 2.64×108 63.58 酯类 2.38×109 41.70 1.16×108 28.01 酚类 2.59×107 0.45 2.59×107 6.33 酰胺类 3.70×107 0.65 8.58×106 2.07 烷烃类 1.80×108 3.16 0 0 酮类 1.43×108 2.50 0 0 -
[1] AKCIL A, ERUST C, GAHAN C S, et al. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants: A review[J]. Waste Management, 2015, 45(3): 258-271. [2] 宋永辉, 屈学化, 吴春晨, 等. 硫酸锌沉淀法处理高铜氰化废水的研究[J]. 稀有金属, 2015, 39(4): 357-364. doi: 10.13373/j.cnki.cjrm.2015.04.010 [3] MEKUTO L, NTWAMPE SKO, AKCIL A, et al. An integrated biological approach for treatment of cyanidation wastewater[J]. Science of the Total Environment, 2016, 47(8): 435-443. [4] ABIDIN C Z A, RIDWAN F, ONG S A, et al. Decolourization and cod reduction of textile wastewater by ozonation in combination with biological treatment[J]. International Journal of Automotive and Mechanical Engineering, 2016, 13(1): 2229-8649. [5] SONG Y H, LI Y F, HE X H, et al. Treatment of cyanide-bearing wastewater by the N263-TBP synergistic extraction system[J]. Chemosphere, 2021: 133052. [6] SONG Y H, Li Y F, He X H, et al. Enrichment of metal and recycling of cyanide in cyanide wastewater by N263-TBP synergistic extraction system[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106774. doi: 10.1016/j.jece.2021.106774 [7] YANG W L, LIU G S, CHEN Y H, et al. Persulfate enhanced electrochemical oxidation of highly toxic cyanide-containing organic wastewater using boron-doped diamond anode[J]. Chemosphere, 2020, 252(11): 326-334. [8] WANG W D, WANG K, HAO W T, et al. Preparation of Ti-based Yb-doped SnO2-RuO2 electrode and electrochemical oxidation treatment of coking wastewater[J]. Journal of Rare Earths, 2021, 257(6): 246-253. [9] ZHU X P, NI J R, LAI P. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using boron-doped diamond electrodes.[J]. Water Research, 2009, 43(17): 4347-4355. doi: 10.1016/j.watres.2009.06.030 [10] WANG J D, YAO J C, WANG L, et al. Multivariate optimization of the pulse electrochemical oxidation for treating recalcitrant dye wastewater[J]. Separation and Purification Technology, 2019, 230(4): 247-254. [11] WANG Z, LI J B, TAN W H, et al. Removal of COD from landfill leachate by advanced Fenton process combined with electrolysis[J]. Separation and Purification Technology, 2018, 208(10): 3-10. [12] HU Y N, ZHANG T, GONG X Z, et al. Roles of Ultrasound on hydroxyl radical generation and bauxite desulfurization from water electrolysis[J]. Journal of the Electrochemical Society, 2018, 165(5): 177-183. [13] HUANG X Y, ZHANG Y, BAI J, et al. Efficient degradation of N-containing organic wastewater via chlorine oxide radical generated by a photoelectrochemical system[J]. Chemical Engineering Journal, 2019, 392(13): 453-462. [14] NIDHEESH P V, KUMAR A, BABU D S, et al. Treatment of mixed industrial wastewater by electrocoagulation and indirect electrochemical oxidation[J]. Chemosphere, 2020, 251(5): 579-587. [15] GAO Y, ZHOU Y, WANG H T, et al. Simultaneous silver recovery and cyanide removal from electroplating wastewater by pulse current electrolysis using static cylinder electrodes[J]. Industrial & Engineering Chemistry Research, 2013, 52(17): 5871-5879. [16] LI J, YANG Z H, XU H Y, et al. Electrochemical treatment of mature landfill leachate using Ti/RuO2IrO2 and Al electrode: Optimization and mechanism[J]. RSC Advances, 2016, 6(53): 367-375. [17] FAJARDO A S, SECA H F, MARTINS R C, et al. Electrochemical oxidation of phenolic wastewaters using a batch-stirred reactor with NaCl electrolyte and Ti/RuO2 anodes[J]. Journal of Electroanalytical Chemistry, 2017, 785(9): 378-385. [18] 董萍, 宋永辉, 张红菊, 等. 利用矿浆电解技术处理氰化尾渣[J]. 环境工程学报, 2021, 15(5): 1662-1669. doi: 10.12030/j.cjee.202012002 [19] ISKURT C, KEYIKOGLU R, KOBYA M, et al. Treatment of coking wastewater by aeration assisted electrochemical oxidation process at controlled and uncontrolled initial pH conditions[J]. Separation and Purification Technology, 2020, 248(8): 358-365. [20] DUTRA A, ROCHA G P, POMBO F R. Copper recovery and cyanide oxidation by electrowinning from a spent copper-cyanide electroplating electrolyte[J]. Journal of Hazardous Materials, 2008, 152(2): 648-655. doi: 10.1016/j.jhazmat.2007.07.030 [21] SONG Y, XIAO M Y, LI Z Y, et al. Degradation of antibiotics, organic matters and ammonia during secondary wastewater treatment using boron-doped diamond electro-oxidation combined with ceramic ultrafiltration[J]. Chemosphere, 2021, 286(2): 3257-3264. [22] DEBORDE M, GUNTEN U V. Reactions of chlorine with inorganic and organic compounds during water treatment: Kinetics and mechanisms: A critical review[J]. Water Research, 2008, 42(2): 13-51. [23] WANG T X, MARGERUM D W. Kinetics of reversible chlorine hydrolysis: Temperature dependence and general-acid/base-assisted mechanisms[J]. Inorganic Chemistry, 1994, 33(6): 1050-1055. doi: 10.1021/ic00084a014 [24] 缪佳, 陈开榜, 朱佳, 等. 氯酸盐对电镀废水COD检测的掩蔽机理初步分析[J]. 中国给水排水, 2018, 34(23): 80-84. doi: 10.19853/j.zgjsps.1000-4602.2018.23.016 [25] AJAB H, ISA M H, YAQUB A. Electrochemical oxidation using Ti/RuO2 anode for COD and PAHs removal from aqueous solution[J]. Sustainable Materials and Technologies, 2020, 26(3): 2573-2581. [26] LEI S M, SONG Y H. Comparative study on electrochemical treatment of cyanide wastewater[J]. Frontiers in Chemistry, 2021, 9(6): 235-243. [27] 毕强, 薛娟琴, 郭莹娟, 等. 电芬顿法去除兰炭废水COD[J]. 环境工程学报, 2012, 6(12): 4310-4314. [28] FU P F, WANG L H, MA Y H, et al. A comparative study on the degradation of ethyl xanthate collector by O3, UV254nm, UV185+254nm, O3/UV254nm and O3/UV185+254nm processes[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 108-119. [29] 聂蕊, 李天国, 徐晓军, 等. 浮选废水中烷基黄药的电催化内电解降解特征及机制[J]. 中国有色金属学报, 2018, 28(3): 594-603. doi: 10.19476/j.ysxb.1004.0609.2018.03.19 [30] WANG Q F, SHAO Y S, GAO N Y, et al. Degradation kinetics and mechanism of 2, 4-Di-tert-butylphenol with UV/persulfate[J]. Chemical Engineering Journal, 2016, 304(4): 201-208. [31] LI J N, WANG S Z, LI Y H, et al. Supercritical water oxidation of semi-coke wastewater: Effects of operating parameters, reaction mechanism and process enhancement[J]. Science of the Total Environment, 2019, 710(11): 4796-4804. -




 下载:
下载: