-
含卤阻燃剂是一类能阻止聚合物材料引燃或抑制火焰传播的含卤有机化合物,包括“氯系阻燃剂”和“溴系阻燃剂”,由于其阻燃效果好,添加量少,性能优异,广泛应用于各类电子产品的生产过程中[1]. 在工业生产及日常使用过程中,一些含卤阻燃剂被废弃后会在环境中残留[2]. 相关研究表明,四氯双酚A(TCBPA)在河流沉积样中被检测出浓度达到542.6 ng·g−1;四溴双酚A(TBBPA)在废水中浓度为102—103 ng·L−1,十溴二苯乙烷(DBDPE)在制造工厂土壤中浓度最高达34000 ng·g−1[3-5]. 部分含卤阻燃剂由于具有持久性、生物富集毒性和长距离迁移等特性,经过在环境中的长期累积[6-9],成为威胁环境安全的污染物,对生物和人类的健康造成不良影响[10-13]. 因此,对含卤阻燃剂进行降解减毒研究具有重要意义.
目前,对含卤阻燃剂的降解方法主要有微生物降解、高温热处理、高级氧化等技术. 微生物降解技术虽然成本较低,但降解缓慢,降解周期长;高温热处理技术通过使污染物在高温下氧化热解作用而达降解,该方法过程简单、降解效率高,但在高温环境降解过程中可能产生有毒气体和其它有毒副产物,造成二次污染;高级氧化技术具有反应速度快,降解效率高,氧化能力强等优点,是近年来较被广泛关注的阻燃剂降解工艺 [14-17]. 相较于上述工艺,紫外/次氯酸 (UV/Cl)体系能够为饮用水处理系统提供多种消毒屏障[18],体系中的余氯具有持续的消毒作用,是新兴的高级氧化工艺,它常用作自来水厂、污水处理厂中水体的消毒. 该体系通过产生高活性的含氯自由基 (RCS ) [19-20],对污染物的富电子基团进行攻击,从而实现对污染物的降解,但降解后的产物种类和产物的毒性鉴定需要高端昂贵仪器或复杂实验检测分析[21-22],一般实验室研究人员无法企及. 该研究采用Gaussian模型和Multiwfn程序通过非实验测试的方法计算出有机物分子中各化学键的键能和Fukui指数,判断各活性位点的化学属性,从而实现降解产物的推测. ECOSAR能够通过定量构效关系(QSAR )来对有机物的毒性进行预测[23],但采用Gaussian计算、ECOSAR模型对阻燃剂的降解路径和毒性预测仍鲜见研究报道.
该研究选择模拟UV/Cl的高级氧化工艺,对TCBPA、TBBPA、DBDPE这3种代表性的含卤阻燃剂的降解产物进行预测与毒性评估. 采用Gaussian软件与Multiwfn程序对TCBPA、TBBPA、DBDPE上位点的键能及Fukui指数进行计算,从而预测出降解产物路径,并用ECOSAR软件对预测出的降解产物进行毒性评估,旨在为难降解毒害性有机物光催化降解路径、机理与产物毒性特征的预测分析提供新思路.
-
通过GaussView5.0构建含卤阻燃剂各组分结构后,选择几何优化与振动频率分析(Opt+Freq),以密度泛函理论 (DFT) 为基础,使用B3LYP方法,基组水平设置为6-31G (2df,2p),设置100 MB的计算储存空间用于保存input文件,并将input文件在Gaussian 09W软件中打开,将循环次数(maxcycle)设置为200,以确保计算结果的准确性. Gaussian 09W计算完成后,将含卤阻燃剂各组分结构的out文件中的结果参照公式(1)进行计算,将化学键断裂后两个离解部分的分子能量之和扣除初始化合物的分子能量,即为化学键离解能[24].
式中D1、D2分别表示两个离解部分的分子能量,D3表示键离解能,D表示初始化合物的分子能量.
-
基于密度函数理论 (DFT) 通过Multiwfn程序计算上述含卤阻燃剂的Fukui指数. Fukui指数是指由于分子中的电子数的变化从而引起的分子中的电子密度函数变化[25-27],它能够提供分子得到或失去电子的电势信息,从而通过数值的高低预测分子中更易受到亲电(f-)或者亲核攻击(f+)的位点[28].
-
QSAR模型能够将化学活性与分子结构和组成相联系,从而预测有机物毒性[29]. 研究表明,ECOSAR软件被认为是估计水生毒性的最佳效果预测程序之一,并且通过将ECOSAR软件的预测值与实测值进行比较,证实了ECOSAR软件的可靠性及有效性[30-35]. 因此采用ECOSAR Version 2.0 (2000-2016 U.S. Environmental Protection Agency)软件能够预测含卤阻燃剂降解产物对鱼类的毒性,通过毒性数据判断所预测的阻燃剂及其降解产物的生态危害水平.
-
Gaussian 09对阻燃剂各位点键能的计算结果如表1所示,可以发现在TCBPA结构中,键C5—Cl13、C8—Cl15的键能最小,均为256.0496 kJ·mol−1,键能较接近的还有键C1—Cl14、C10—Cl16,它们的键能均为261.7385 kJ·mol−1,这4个键的键能较其他位点低,更容易受到自由基的攻击而产生取代、加成等反应. 在TBBPA结构中,C9—C10、C9—C11是结构中键能最小的两个键,且两个键的键能接近,分别为272.2355 kJ·mol−1、272.6038 kJ·mol−1,因此这两个键断裂所需的能量相较于其他键最小,较容易断裂. 在DBDPE结构中, 最小键能的C1—C2仅为258.8407 kJ·mol−1,相比之下更容易断裂,在UV/Cl体系中受到RCS (活性氯自由基)、·OH等自由基的攻击的可能性最大.
-
Fukui指数中f-的数值能够表征活性位点受到自由基攻击的难易程度,f-的数值越大表明越容易受到氧化性自由基的攻击[36]. 因此,该研究通过引入Fukui指数,对键能预测断键点位的准确性加以佐证. 通过分析表2可以发现,在TBBPA结构中,位点Br18(f - = 0.1164 )、Br20(f - = 0.0889 )、Br21(f - = 0.0814 )的 f -指数最高,与结构中键能低的位点基本吻合.在TCBPA结构(表3)中,位点Cl13 (f- = 0.0685)、Cl14 (f- = 0.0765)、Cl15 (f- = 0.0685)、Cl16 (f- = 0.0765)的f-指数相对较高,因此,在UV/Cl体系中,上述四位点最容易受到RCS与·OH的攻击,与表1键能计算所预测的位点基本一致. 在DBDPE结构 (表4 )中,具有最高的f-指数的位点为Br9 (f- = 0.0909 )、Br10 (f- = 0.0841)、Br12 (f- = 0.1009)、Br21 (f- = 0.1009)、Br23 (f- = 0.0841)、Br24 (f- = 0.0908),与Gaussian计算出的DBDPE结构中的键能较低的位点相吻合,推测在降解过程中更容易产生断裂.
-
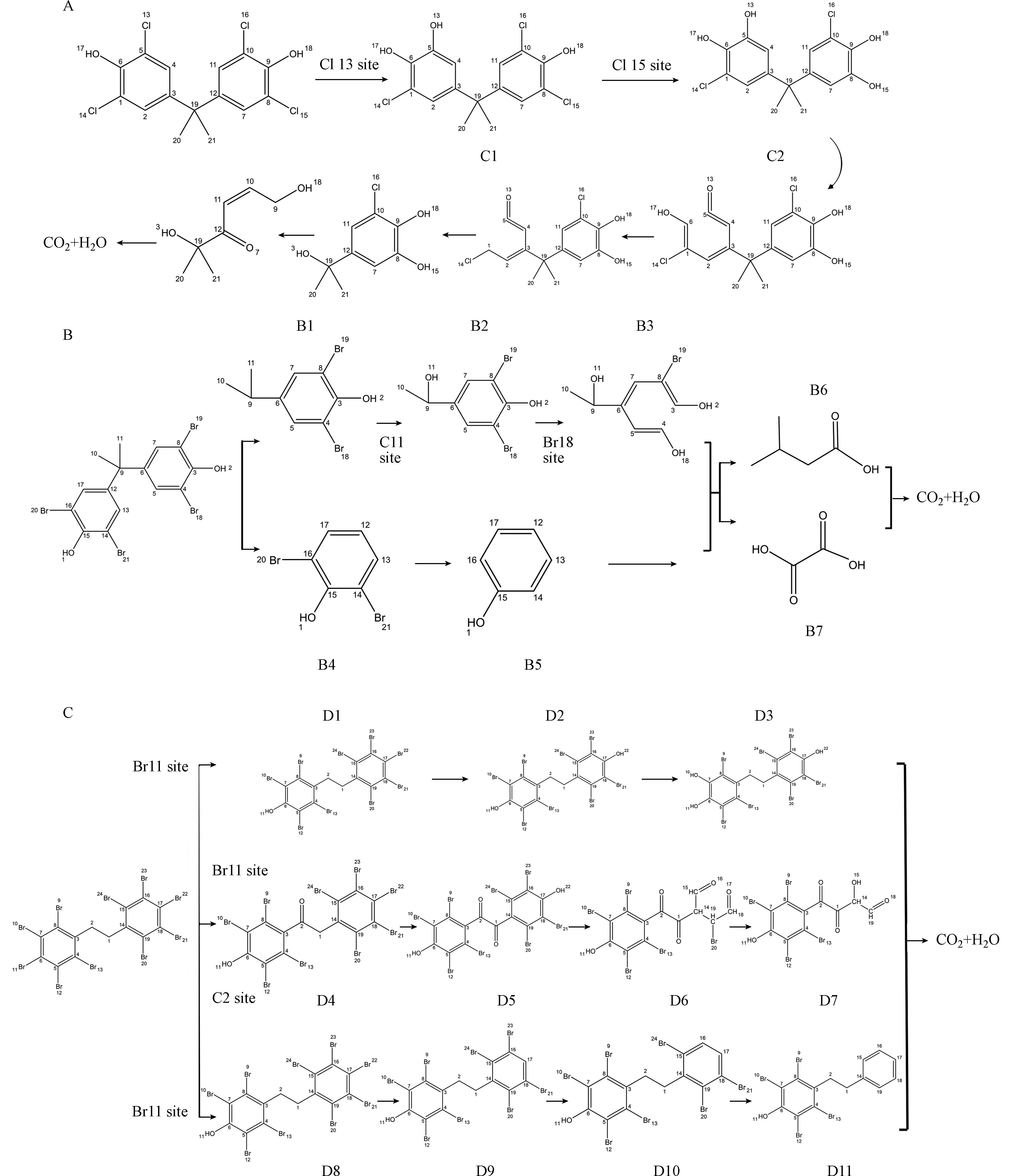
在UV/Cl体系中,RCS与·OH对TCBPA的降解起主导作用,由于键C5—Cl13、C8—Cl15的键能最低,RCS与·OH首先攻击这两个位点,位点随之被·OH取代,随着反应的进一步进行,TCBPA上的键进一步断裂,降解路径如图1(A)所示,预测的最终产物与Wan等[37]的实验研究所得一致. TBBPA结构中,键C9—C11的键能最小,因此在UV/Cl体系中的RCS与·OH使其断裂为B1、B4两部分,B4通过·OH的氧化作用产生脱溴从而进一步转化为苯酚,B1的降解路径如图1(B)所示. 经研究,预测产物与Guo等[38]实验研究结论基本一致,再次验证了反应体系中的RCS和·OH会对键能较低,即活性较高的位点进行攻击. 反应体系中RCS与·OH对活性较高的C1—C2进行攻击,由于二者的强氧化性,使DBDPE在降解过程中产生一系列电子转移、脱氢与脱溴反应,如图1(C)所示. 预测降解产物与Chen等[39-40]实验研究的最终降解产物相一致. 因此,具有较低键能与较高f-指数的位点活性更高,自由基对这一类位点的反应性更高,更容易对其进行攻击.
-
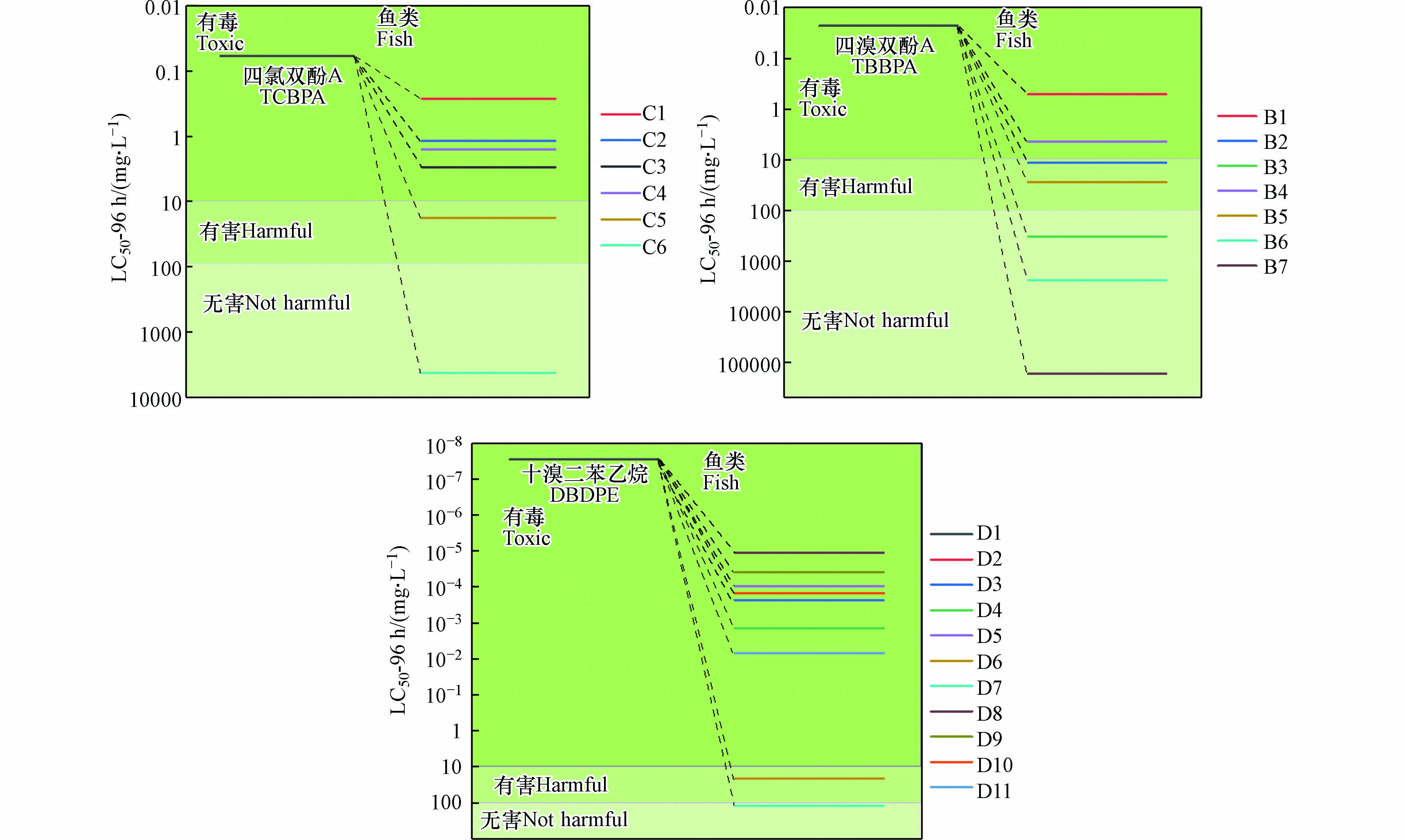
该研究采用ECOSAR软件对模拟出的各阻燃剂的降解产物进行了毒性推测与分析(图2),可以看出鱼类在TCBPA的溶液中96 h后的LC50为0.06 mg·L−1,具有非常强的毒性,然而通过UV/Cl体系中自由基氧化的作用下,TCBPA降解产物的毒性相较于TCBPA本身明显下降,尤其是产物C6的LC50为4230 mg·L−1. 如图2(B)所示,鱼类在TBBPA溶液中96 h后的LC50也达0.023 mg·L−1, 显示TBBPA的降解产物毒性随着降解的逐步进行,也出现了显著的降低,产物B3、B6、B7的LC50分别为3.28×102、2.43 × 103、1.68 × 105 mg·L−1,都达到了无害水平.
在图2(C)中,对于DBDPE来说,鱼类在其溶液中96 h的LC50为2.8 × 10−8 mg·L−1,毒性明显大于TCBPA与TBPA,但最终通过UV/Cl体系高效的降解,DBDPE降解产物的毒性也逐步降低,D11的LC50也达到了124 mg·L−1. 通过对各含卤阻燃剂的降解产物毒性推测,说明UV/Cl体系中产生的RCS与·OH攻击了键能较低的位点,被快速有效降解. 因此,UV/Cl体系对含卤阻燃剂具有高效的降解效果,同时降解产物的毒性较产物本身普遍降低.
-
(1)该研究通过Gaussian 09软件和Mutiwfn程序对3种典型的含卤阻燃剂(TCBPA、TBBPA、DBDPE)的键能与Fukui指数进行计算,进而通过比较分析各化学键位的断裂势,推测出各降解路径和产物.
(2)ECOSAR软件是评估降解产物水生毒性效应的有效可靠预测工具. 该研究通过 ECOSAR Version 2.0 (2000-2016 U.S. Environmental Protection Agency)软件对含卤阻燃剂的降解产物进行毒性预测与分析,得出阻燃剂降解产物的毒性均低于阻燃剂母体的评估结果,说明通过UV/Cl体系降解含卤阻燃剂可有效降低潜在的环境危害.
(3)尽管采用模型与软件对阻燃剂的降解路径和毒性预测不能完全代替实验分析,但通过非实验测试的方法不仅能够为研究提供简便有效的路径方法,实现对含卤阻燃剂及其他持久毒害性有机污染物降解机理和产物毒性快速预测.
基于Gaussian、ECOSAR模型的紫外/次氯酸体系降解含卤阻燃剂的产物预测与毒性评估
Product prediction and toxicity assessment of halogen-containing flame retardants degraded by UV/hypochlorous acid systems based on Gaussian and ECOSAR models
-
摘要: 含卤阻燃剂广泛应用于各类电子产品的生产,难降解且具有生物毒性. 在生产和使用过程中,部分含卤阻燃剂会残留在水体并排放到水环境造成累积污染,威胁水环境安全,亟需探寻有效的降解去毒方法. 本研究通过Gaussian与ECOSAR模型预测了四氯双酚A (TCBPA)、四溴双酚A (TBBPA)、十溴二苯乙烷 (DBDPE) 等3种典型含卤阻燃剂在紫外/次氯酸 (UV/Cl)体系中的光催氧化降解路径与产物毒性. 结果表明, UV/Cl体系中的含氯自由基 (RCS) 与羟基自由基 (·OH) 易攻击阻燃剂分子结构上键能较低、Fukui指数较高的位点,促使C—Cl键、C—Br键、C—C键等因为受到攻击而断裂,进而降解阻燃剂. 同时,利用ECOSAR模型评估发现降解产物的急性毒性LC50-96 h均低于100 mg·L−1,佐证了UV/Cl体系对含卤阻燃剂降解的有效性,并降低其环境危害. 因此,采用Gaussian计算、ECOSAR模型相结合的分析方法,能够更加便捷地预测阻燃剂降解路径与产物毒性特征,为深入揭示UV/Cl体系光催氧化降解含卤阻燃剂机理提供新思路.Abstract: Halogen-containing flame retardants are extensively used in the production of various electronic products, which will inevitably be discharged into surface water, causing cumulative pollution, and threatening the safety of the aquatic environment for their persistent stability and biological toxicity. Thus, it is urgent to explore effective degradation and detoxification methods. In this study, the Gaussian and ECOSAR models were employed to predict the performance of three typical halogen-containing flame retardants including TCBPA (tetrachlorobisphenol A), TBBPA (tetrabromobisphenol A), and DBDPE (decabromodiphenylethane) in ultraviolet excited hypochlorous acid (UV/Cl) systems for evaluating their photocatalytic oxidation degradation pathways and products toxicity. The results showed that the chlorine-containing radicals (RCS) and hydroxyl radicals (·OH) in the UV/Cl system were prone to attack the sites with lower bond energy and higher Fukui index on the molecular structure of the flame retardant, which promoted the degradation of flame retardants through the cleavage of C—Cl Bonds, C—Br bonds, C—C bonds, etc. Meanwhile, the ECOSAR model was used to evaluate the acute toxicity LC50-96 h of the degradation products, which were all lower than 100 mg·L−1, and proved the effectiveness of halogen-containing flame retardants in the UV/Cl process and reduced their environmental hazards. Therefore, the combined analysis method of Gaussian calculation and ECOSAR model conveniently predicted the degradation path and product toxicity characteristics of flame retardants, and provided new insights into further revealing the mechanism of photocatalytic oxidation degradation of halogen-containing flame retardants in UV/Cl system.
-

-
表 1 各阻燃剂不同点位键能分布
Table 1. Different point bond energy distribution of each flame retardant
位点
Sites键能/(kJ·mol−1)
Bond energy四氯双酚A 5C-13Cl 256.0496 
8C-15Cl 256.0496 1C-14Cl 261.7385 10C-16Cl 261.7385 19C-21C 273.4115 19C-20C 273.4115 3C-19C 329.8949 12C-19C 329.8952 17O-26H 364.9065 18O-27H 364.9082 20C-30H 450.0166 20C-28H 450.0167 21C-33H 450.0167 21C-31H 450.0168 20C-29H 450.0169 21C-32H 450.0169 6C-17O 454.1277 2C-22H 496.3993 11C-25H 496.3993 4C-23H 500.8255 7C-24H 500.9393 9C-18O 527.4504 四溴双酚A 9C-11C 272.2355 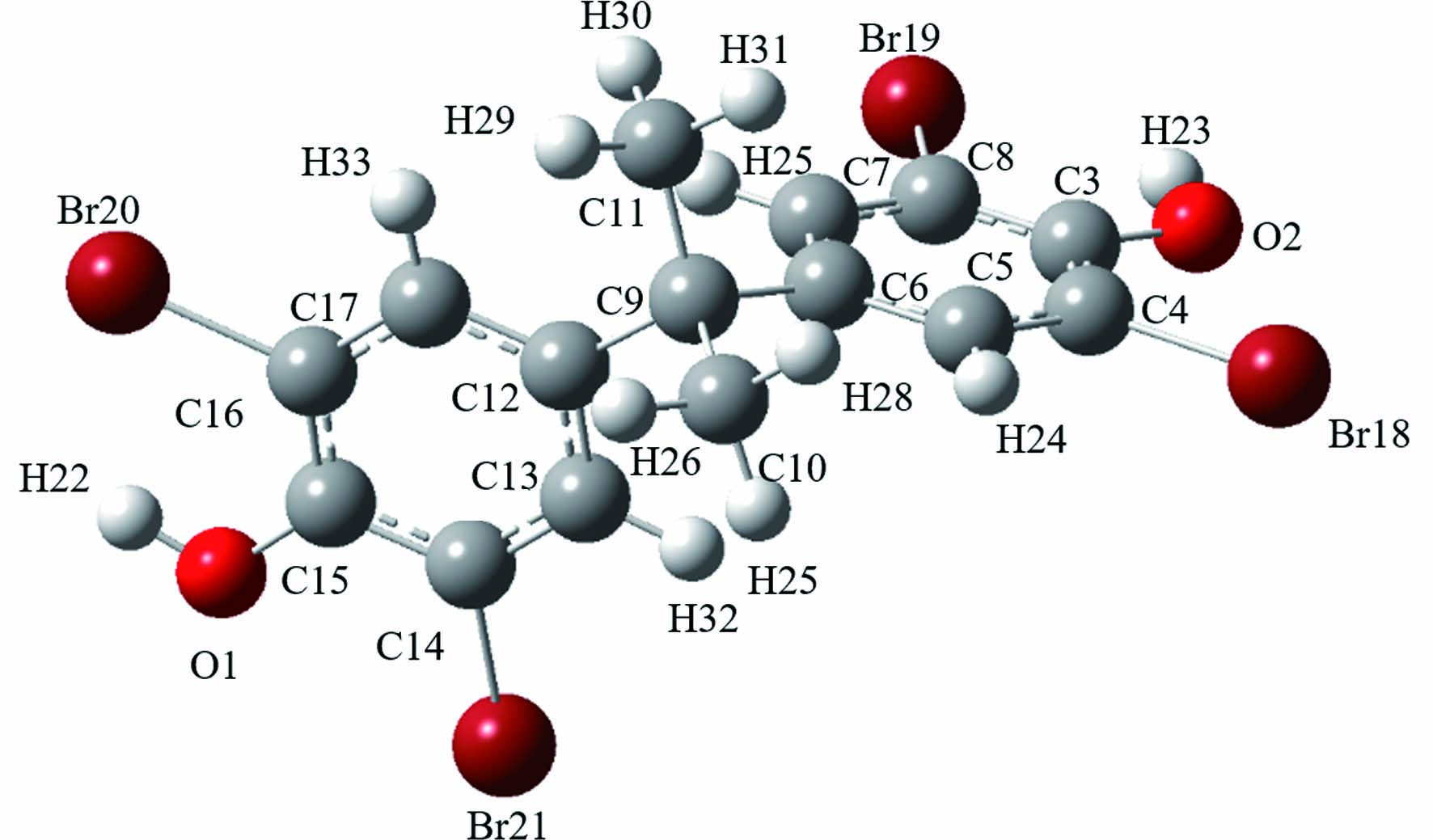
9C-10C 272.6038 9C-12C 331.2436 6C-9C 331.2438 14C-21Br 343.9736 4C-18Br 345.0387 8C-19Br 351.9484 16C-20Br 352.7075 1O-22H 366.4833 2O-23H 366.8199 11C-29H 449.7797 11C-30H 449.7797 11C-31H 449.7797 10C-26H 449.9446 10C-27H 449.9446 10C-28H 449.9446 15C-1O 461.6979 3C-2O 461.9418 17C-33H 494.5618 5C-24H 495.2022 7C-25H 498.5851 13C-32H 499.4345 十溴二苯乙烷 1C-2C 258.8407 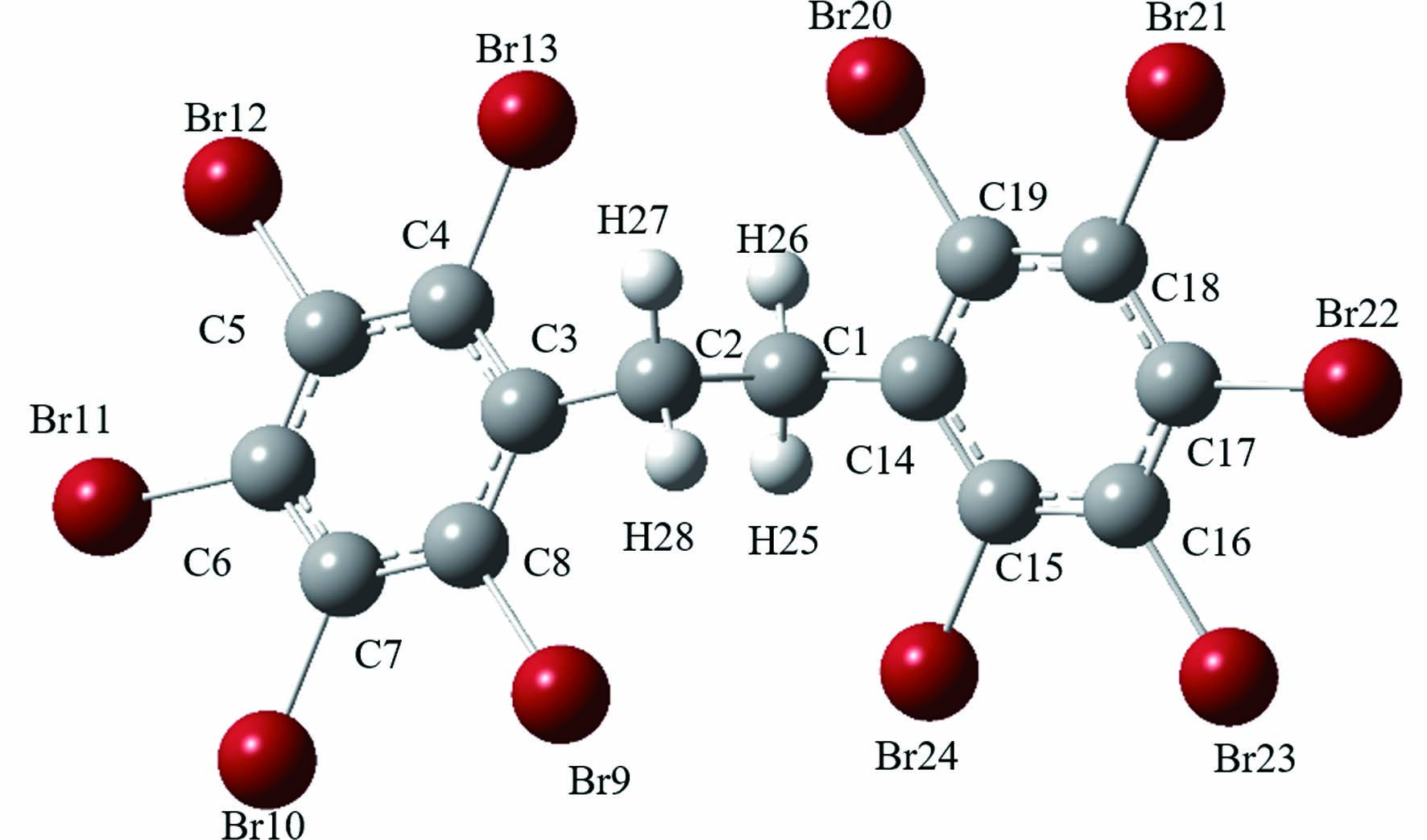
6C-11Br 318.5690 17C-22Br 318.5690 7C-10Br 320.1635 16C-23Br 320.1635 5C-12Br 321.4881 18C-21Br 321.4881 4C-13Br 321.5290 19C-20Br 321.5290 8C-9Br 322.8489 15C-24Br 322.8489 1C-25H 407.1854 2C-27H 407.1854 2C-28H 407.1854 1C-26H 407.1857 1C-14C 433.1090 2C-3C 433.1090 表 2 四溴双酚A不同点位Fukui指数
Table 2. Fukui index at different points of tetrabromobisphenol A
原子
Atom序号
Serial numberq(N) q (N+1) q (N-1) f− f+ Br 18 −0.0369 −0.1557 0.0795 0.1164 0.1188 Br 20 −0.0259 −0.1863 0.063 0.0889 0.1604 Br 21 −0.0338 −0.1413 0.0476 0.0814 0.1075 Br 19 −0.0224 −0.1674 0.0479 0.0703 0.145 O 1 −0.1869 −0.2114 −0.1251 0.0618 0.0246 O 2 −0.1872 −0.2116 −0.1254 0.0618 0.0244 C 3 0.0682 0.0499 0.1125 0.0442 0.0183 C 15 0.0682 0.0493 0.1119 0.0437 0.019 C 6 0.0004 −0.0094 0.0379 0.0375 0.0098 C 12 0.0006 −0.0096 0.0374 0.0367 0.0102 C 4 −0.0079 −0.0364 0.0246 0.0325 0.0285 C 16 −0.0147 −0.054 0.0127 0.0274 0.0392 C 7 −0.0447 −0.065 −0.0179 0.0268 0.0203 C 17 −0.0489 −0.0692 −0.026 0.023 0.0202 C 14 −0.0055 −0.0297 0.0124 0.0179 0.0242 C 5 −0.0457 −0.067 −0.0287 0.017 0.0213 C 13 −0.0417 −0.065 −0.026 0.0157 0.0233 C 8 −0.0115 −0.0451 0.0039 0.0154 0.0336 C 10 −0.0826 −0.0885 −0.0728 0.0098 0.0059 C 11 −0.0833 −0.0887 −0.0735 0.0097 0.0054 C 9 0.0304 0.0271 0.0333 0.0029 0.0033 表 3 四氯双酚A不同点位Fukui指数
Table 3. Fukui index at different points of tetrachlorobisphenol A
原子
Atom序号
Serial numberq (N) q (N+1) q (N-1) f− f+ Cl 14 −0.0648 −0.2085 0.0118 0.0765 0.1437 Cl 16 −0.0648 −0.2084 0.0118 0.0765 0.1436 Cl 13 −0.0652 −0.1624 0.0033 0.0685 0.0972 Cl 15 −0.0652 −0.1623 0.0033 0.0685 0.0971 O 18 −0.1842 −0.2135 −0.1166 0.0676 0.0294 O 17 −0.1842 −0.2135 −0.1166 0.0676 0.0293 C 9 0.0695 0.0423 0.1167 0.0472 0.0273 C 6 0.0695 0.0423 0.1167 0.0472 0.0272 C 3 0.0015 −0.0144 0.0426 0.0411 0.0159 C 12 0.0015 −0.0145 0.0426 0.0411 0.0159 C 1 0.0086 −0.0291 0.0407 0.0321 0.0377 C 10 0.0086 −0.0291 0.0407 0.0321 0.0377 C 2 −0.0481 −0.072 −0.0222 0.026 0.0239 C 11 −0.0481 −0.0719 −0.0222 0.026 0.0238 C 5 0.0195 −0.0063 0.0413 0.0219 0.0258 C 8 0.0195 −0.0063 0.0413 0.0219 0.0258 C 7 −0.0421 −0.0598 −0.0225 0.0195 0.0177 C 4 −0.0421 −0.0597 −0.0226 0.0195 0.0176 C 20 −0.0828 −0.0884 −0.0723 0.0106 0.0055 C 21 −0.0828 −0.0884 −0.0723 0.0106 0.0055 C 19 0.0306 0.026 0.0339 0.0033 0.0046 表 4 十溴二苯乙烷不同点位Fukui指数
Table 4. Fukui index of decabromodiphenylethane at different points
原子
Atom序号
Serial numberq (N) q (N+1) q (N-1) f− f+ Br 12 −0.0037 −0.0893 0.0972 0.1009 0.0856 Br 21 −0.0037 −0.0893 0.0972 0.1009 0.0856 Br 9 0.0079 −0.0547 0.0987 0.0909 0.0626 Br 24 0.0079 −0.0547 0.0987 0.0908 0.0626 Br 10 −0.0042 −0.0874 0.0798 0.0841 0.0831 Br 23 −0.0042 −0.0874 0.0799 0.0841 0.0831 Br 13 0.0122 −0.0545 0.0741 0.0619 0.0667 Br 20 0.0122 −0.0545 0.0741 0.0619 0.0666 Br 11 −0.0016 −0.1035 0.0537 0.0554 0.1018 Br 22 −0.0016 −0.1035 0.0537 0.0554 0.1018 C 5 −0.0054 −0.0219 0.0204 0.0258 0.0165 C 18 −0.0054 −0.0219 0.0204 0.0258 0.0165 C 8 0.004 −0.0086 0.0265 0.0225 0.0127 C 15 0.004 −0.0086 0.0265 0.0225 0.0127 C 7 −0.0052 −0.0212 0.0131 0.0184 0.016 C 16 −0.0052 −0.0212 0.0131 0.0184 0.016 C 4 0.0037 −0.0099 0.0219 0.0182 0.0136 C 19 0.0037 −0.0099 0.0219 0.0182 0.0136 C 6 −0.0037 −0.022 0.0036 0.0072 0.0183 C 17 −0.0037 −0.022 0.0036 0.0072 0.0183 C 3 0.0059 −0.0017 0.0109 0.0049 0.0076 C 14 0.0059 −0.0017 0.0108 0.0049 0.0076 C 1 −0.056 −0.0611 −0.0541 0.0019 0.0051 C 2 −0.056 −0.0611 −0.0542 0.0019 0.0051 -
[1] 朱冰清, 史薇, 胡冠九,等. 中国海洋环境中卤代阻燃剂的污染现状与研究进展 [J]. 环境化学, 2017, 36(11): 2408-2423. doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2017032702 ZHU B Q, SHI W, HU G J et al. The pollution status and research progress on halogenated flame retardants in China marine environment [J]. Environmental Chemistry, 2017, 36(11): 2408-2423(in Chinese). doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2017032702
[2] 童艺, 程伊雪, 吴冠履, 等. 新型溴系阻燃剂环境污染现状研究进展 [J]. 环境化学, 2021, 40(1): 83-101. doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2020042501 TONG Y, CHENG Y X, WU G Y, et al. Current research progress on environmental pollution of novel brominated flame retardant [J]. Environmental Chemistry, 2021, 40(1): 83-101(in Chinese). doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2020042501
[3] LIU W T, PAN Y F, YANG L, et al. Developmental toxicity of TCBPA on the nervous and cardiovascular systems of zebrafish (Danio rerio): A combination of transcriptomic and metabolomics [J]. Journal of Environmental Sciences, 2023, 127: 197-209. doi: 10.1016/j.jes.2022.04.022 [4] WANG S T, LI W L, CHEN Y Y, et al. Toxicity evaluation of decabromodiphenyl ethane (DBDPE) to Pleurotus ostreatus: Oxidative stress, morphology and transcriptomics [J]. Journal of Hazardous Materials, 2022, 431: 128625. doi: 10.1016/j.jhazmat.2022.128625 [5] YAO Y R, YIN L, HE C, et al. Removal kinetics and mechanisms of tetrabromobisphenol A (TBBPA) by HA-n-FeS colloids in the absence and presence of oxygen [J]. Journal of Environmental Management, 2022, 311: 114885. doi: 10.1016/j.jenvman.2022.114885 [6] 朱婧文, 耿存珍, 张丽珠, 等. 溴系阻燃剂的环境毒理学研究进展 [J]. 环境科技, 2012, 25(5): 62-67. ZHU J W, GENG C Z, ZHANG L Z, et al. Progress on environmental toxicology of brominated flame retardants [J]. Environmental Science and Technology, 2012, 25(5): 62-67(in Chinese).
[7] 高玉娟, 谢承劼, 余红, 等. 溴代阻燃剂在土壤中的迁移转化研究进展 [J]. 环境科学研究, 2021, 34(2): 479-490. GAO Y J, XIE C J, YU H, et al. Research progress on migration and transformation of brominated flame retardants in soil [J]. Research of Environmental Sciences, 2021, 34(2): 479-490(in Chinese).
[8] 徐建林, 王涛, 康成虎, 等. 阻燃剂研究与应用进展及问题思考 [J]. 材料导报, 2022, 36(10): 235-243. XU J L, WANG T, KANG C H, et al. Research and applications of flame retardants: A review and thoughts [J]. Materials Reports, 2022, 36(10): 235-243(in Chinese).
[9] 王爽, 路珍, 李斐, 等. 典型溴系阻燃剂四溴双酚A和十溴二苯乙烷的污染现状及毒理学研究进展 [J]. 生态毒理学报, 2020, 15(6): 24-42. WANG S, LU Z, LI F, et al. A review of pollution status and toxicological researches of typical brominated flame retardants tetrabromobisphenol A (TBBPA) and decabromodiphenyl ethane (DBDPE) [J]. Asian Journal of Ecotoxicology, 2020, 15(6): 24-42(in Chinese).
[10] 高佳楠, 王树涛, 齐虹, 等. 4种类型土壤中十溴二苯乙烷的光降解行为 [J]. 环境科学与技术, 2021, 44(10): 66-74. GAO J N, WANG S T, QI H, et al. Photodegradation of decabromodiphenyl ethane in four different soils [J]. Environmental Science & Technology, 2021, 44(10): 66-74(in Chinese).
[11] 李海燕. 广州城市污水厂中卤系阻燃剂和污泥重金属研究[D]. 广州: 广州大学, 2015. LI H Y. Study of HFRs and heavy metals in municipal sewage treatment plant in Guangzhou[D]. Guangzhou: Guangzhou University, 2015(in Chinese).
[12] WANG J, CHEN S J, NIE X, et al. Photolytic degradation of decabromodiphenyl ethane (DBDPE) [J]. Chemosphere, 2012, 89(7): 844-849. doi: 10.1016/j.chemosphere.2012.05.006 [13] 范荣桂, 魏来, 张泽伟, 等. 水中典型溴系阻燃剂的降解与测定方法 [J]. 应用化工, 2020, 49(8): 2116-2121. FAN R G, WEI L, ZHANG Z W, et al. Degradation and determination method of typical bromine flame retardant in water [J]. Applied Chemical Industry, 2020, 49(8): 2116-2121(in Chinese).
[14] 刘舒巍, 杨兴桐, 张从轩, 等. 氯系阻燃剂四氯双酚A的毒性及降解技术研究进展 [J]. 化工环保, 2017, 37(2): 145-151. LIU S W, YANG X T, ZHANG C X, et al. Research progresses on ecotoxicology and degradation technology of tetrachlorobisphenol-a as chlorine flame retardant [J]. Environmental Protection of Chemical Industry, 2017, 37(2): 145-151(in Chinese).
[15] 孙家宁, 孙韶华, 宋娜, 等. 高级氧化技术去除水中溴系阻燃剂的研究进展 [J]. 工业水处理, 2022, 42(2): 19-26. SUN J N, SUN S H, SONG N, et al. Research progress of advanced oxidation technology to remove brominated flame retardants from water [J]. Industrial Water Treatment, 2022, 42(2): 19-26(in Chinese).
[16] 郭耀广. 基于光化学高级氧化技术降解水中典型卤代酚类污染物的研究[D]. 上海: 东华大学, 2014. GUO Y G. Photochemical advanced oxidation processes-based degradation of typical halogenated organic phenolic pollutants in water[D]. Shanghai: Donghua University, 2014(in Chinese).
[17] 张静, 严静娜, 郭悦宁, 等. 阻燃剂四溴双酚A的厌氧-好氧生物降解 [J]. 环境化学, 2016, 35(9): 1776-1784. doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2016.09.2016013001 ZHANG J, YAN J N, GUO Y N, et al. Anaerobic and aerobic biodegradation of flame retardant tetrabromobisphenol A [J]. Environmental Chemistry, 2016, 35(9): 1776-1784(in Chinese). doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2016.09.2016013001
[18] DONG H Y, QIANG Z M, HU J, et al. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes [J]. Water Research, 2017, 121: 178-185. doi: 10.1016/j.watres.2017.05.030 [19] PAN M W, WU Z H, TANG C Y, et al. Emerging investigators series: Comparative study of naproxen degradation by the UV/chlorine and the UV/H2O2 advanced oxidation processes[J]. Environmental Science: Water Research & Technology, 2018, 4(9): 1219-1230. [20] YEOM Y, HAN J R, ZHANG X R, et al. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants [J]. Chemical Engineering Journal, 2021, 424: 130053. doi: 10.1016/j.cej.2021.130053 [21] HUA Z C, LI D, WU Z H, et al. DBP formation and toxicity alteration during UV/chlorine treatment of wastewater and the effects of ammonia and bromide [J]. Water Research, 2021, 188: 116549. doi: 10.1016/j.watres.2020.116549 [22] ZHOU Y J, CHENG F Y, HE D Y, et al. Effect of UV/chlorine treatment on photophysical and photochemical properties of dissolved organic matter [J]. Water Research, 2021, 192: 116857. doi: 10.1016/j.watres.2021.116857 [23] 程艳, 陈会明, 于文莲, 等. QSAR技术对高关注化学物质生态环境毒理风险预测 [J]. 环境科学研究, 2009, 22(7): 817-822. doi: 10.13198/j.res.2009.07.67.chengy.010 CHENG Y, CHEN H M, YU W L, et al. Eco-environmental toxicity risk prediction for substances of very high concern with QSAR approach [J]. Research of Environmental Sciences, 2009, 22(7): 817-822(in Chinese). doi: 10.13198/j.res.2009.07.67.chengy.010
[24] YIN R, BLATCHLEY E R 3rd, SHANG C. UV photolysis of mono- and dichloramine using UV-LEDs as radiation sources: Photodecay rates and radical concentrations[J]. Environmental Science & Technology, 2020, 54(13): 8420-8429. [25] DEMIRCIOĞLU Z, ALBAYRAK KAŞTAŞ Ç, BÜYÜKGÜNGÖR O, et al. The spectroscopic (FT-IR, UV-vis), Fukui function, NLO, NBO, NPA and tautomerism effect analysis of (E)-2-[(2-hydroxy-6-methoxybenzylidene)amino]benzonitrile [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2015, 139: 539-548. doi: 10.1016/j.saa.2014.11.078 [26] LU T, CHEN F W, et al. Multiwfn: A multifunctional wavefunction analyzer [J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. doi: 10.1002/jcc.22885 [27] GEERLINGS P, de PROFT F, LANGENAEKER W, et al. Conceptual density functional theory [J]. Chemical Reviews, 2003, 103(5): 1793-1874. doi: 10.1021/cr990029p [28] ZACHARIAS A O, VARGHESE A, AKSHAYA K B, et al. DFT, spectroscopic studies, NBO, NLO and Fukui functional analysis of 1-(1-(2, 4-difluorophenyl)-2-(1H-1, 2, 4-triazol-1-yl)ethylidene) thiosemicarbazide [J]. Journal of Molecular Structure, 2018, 1158: 1-13. doi: 10.1016/j.molstruc.2018.01.002 [29] BELFIELD S J, ENOCH S J, FIRMAN J W, et al. Determination of “fitness-for-purpose” of quantitative structure-activity relationship (QSAR) models to predict (eco-) toxicological endpoints for regulatory use [J]. Regulatory Toxicology and Pharmacology, 2021, 123: 104956. doi: 10.1016/j.yrtph.2021.104956 [30] WANG J J, QIN J Z, LIU B J, et al. Reaction mechanisms and toxicity evolution of Sulfamethoxazole degradation by CoFe-N doped C as Electro-Fenton cathode [J]. Separation and Purification Technology, 2022, 298: 121655. doi: 10.1016/j.seppur.2022.121655 [31] LI Z Y, WANG F, ZHANG Y M, et al. Activation of peroxymonosulfate by CuFe2O4-CoFe2O4 composite catalyst for efficient bisphenol a degradation: Synthesis, catalytic mechanism and products toxicity assessment [J]. Chemical Engineering Journal, 2021, 423: 130093. doi: 10.1016/j.cej.2021.130093 [32] FAN Y J, YANG J, CAI K C, et al. Mechanism of 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide degradation in UV light-emitting diodes lamp driven sodium sulfite activation process [J]. Process Safety and Environmental Protection, 2022, 165: 704-715. doi: 10.1016/j.psep.2022.07.064 [33] HEGER S, BRENDT J, HOLLERT H, et al. Green toxicological investigation for biofuel candidates [J]. Science of the Total Environment, 2021, 764: 142902. doi: 10.1016/j.scitotenv.2020.142902 [34] MA C, LIU X G, WU X H, et al. Kinetics, mechanisms and toxicity of the degradation of imidaclothiz in soil and water [J]. Journal of Hazardous Materials, 2021, 403: 124033. doi: 10.1016/j.jhazmat.2020.124033 [35] ZHU Y X, ZHENG Y Q, JIAO B, et al. Photodegradation of enestroburin in water by simulated sunlight irradiation: Kinetics, isomerization, transformation products identification and toxicity assessment [J]. Science of the Total Environment, 2022, 849: 157725. doi: 10.1016/j.scitotenv.2022.157725 [36] LIU W, LI Y Y, LIU F Y, et al. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation [J]. Water Research, 2019, 151: 8-19. doi: 10.1016/j.watres.2018.11.084 [37] WAN D, WANG H Y, POZDNYAKOV I P, et al. Formation and enhanced photodegradation of chlorinated derivatives of bisphenol A in wastewater treatment plant effluent [J]. Water Research, 2020, 184: 116002. doi: 10.1016/j.watres.2020.116002 [38] GUO Y G, ZHOU J, LOU X Y, et al. Enhanced degradation of Tetrabromobisphenol A in water by a UV/base/persulfate system: Kinetics and intermediates [J]. Chemical Engineering Journal, 2014, 254: 538-544. doi: 10.1016/j.cej.2014.05.143 [39] CHEN J, XU X X, PAN X X, et al. Mechanism insights into the oxidative degradation of decabromodiphenyl ethane by potassium permanganate in acidic conditions [J]. Chemical Engineering Journal, 2018, 332: 267-276. doi: 10.1016/j.cej.2017.09.071 [40] LI C G, ZUO J L, LIANG S J, et al. Photodegradation of decabromodiphenyl ethane (DBDPE) adsorbed on silica gel in aqueous solution: Kinetics, products, and theoretical calculations [J]. Chemical Engineering Journal, 2019, 375: 121918. doi: 10.1016/j.cej.2019.121918 -



 下载:
下载: