-
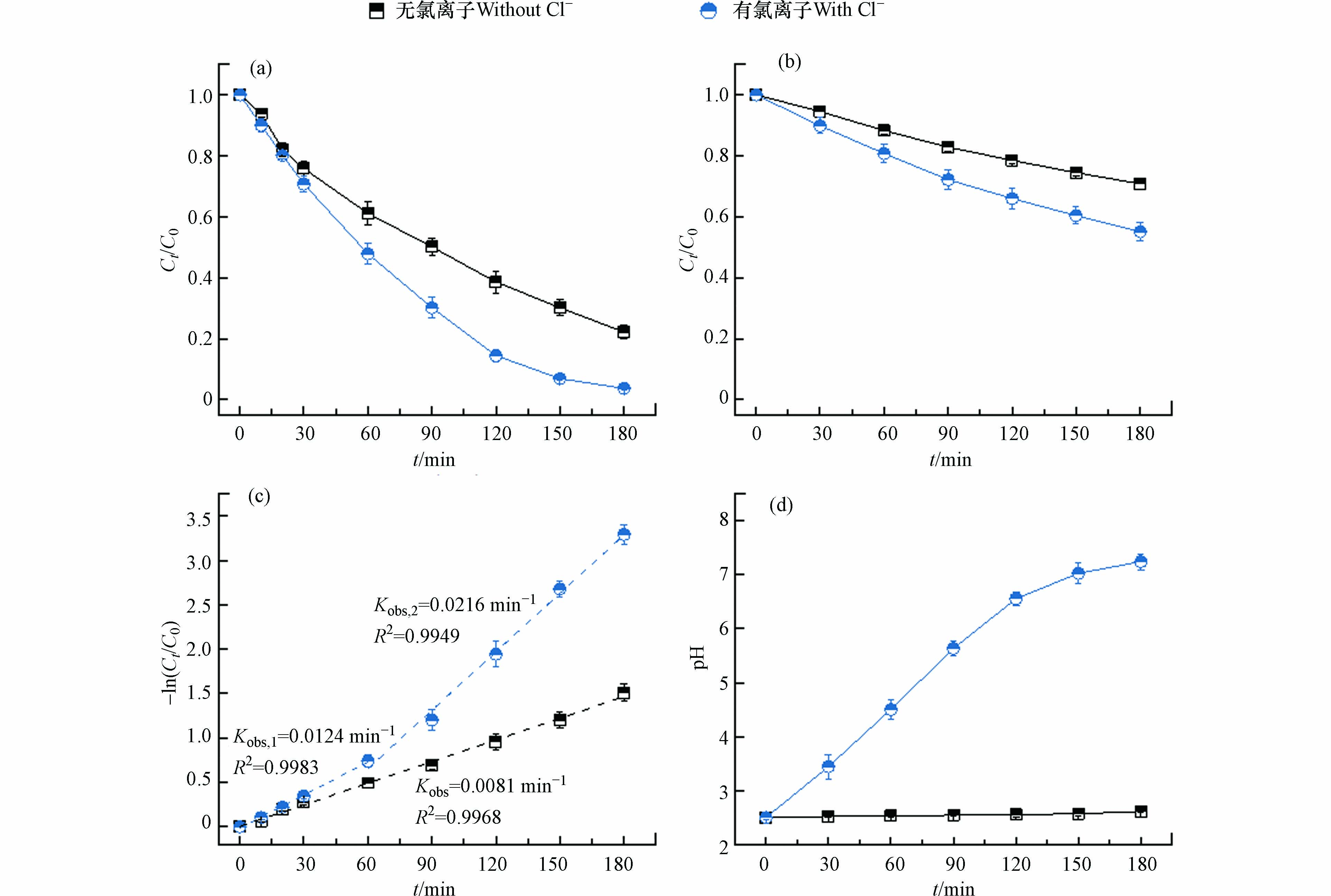
图 1 有无氯情形下电化学体系中(a)Ni-EDTA降解效果;(b)TOC去除效果;(c)Ni-EDTA降解动力学拟合;(d)pH变化.
Figure 1. Comparisons of time-course (a) Ni-EDTA concentration, (b) TOC concentration, (c) kinetic-fitting results, and (d) pH variations in the electrochemical systems in the presence and absence of chloride.
-
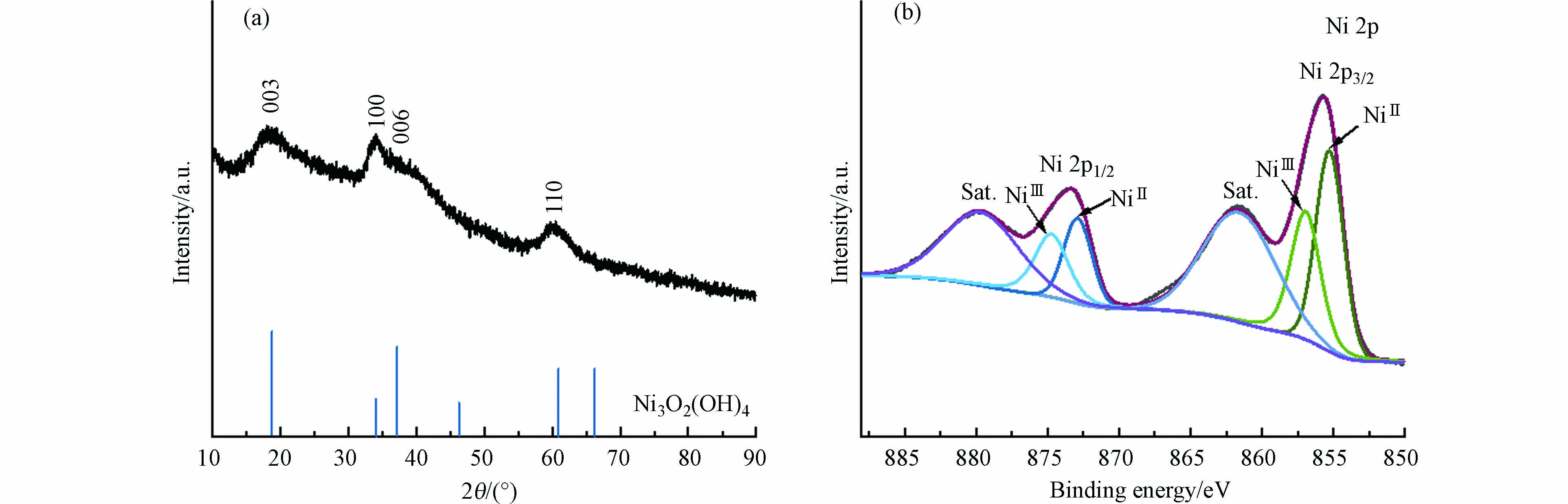
图 2 含氯离子的电化学体系反应后分离出灰黑色固体的表征分析
Figure 2. (a) The XRD pattern and (b) XPS curves of gray black solid obtained from the chloride-containing electrochemical system.
-
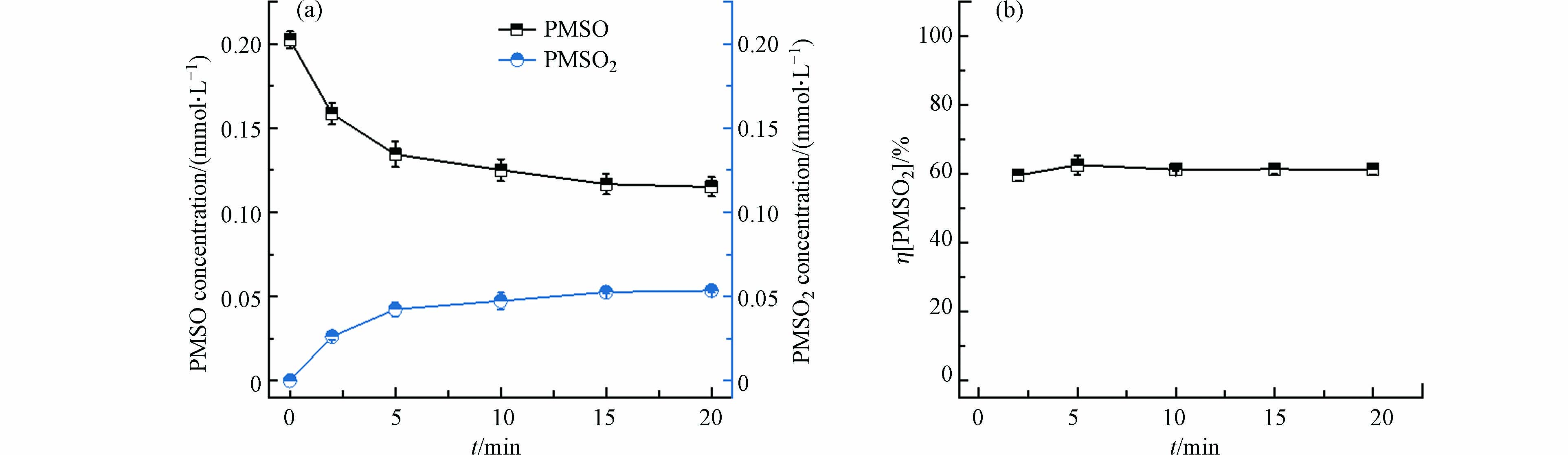
图 3 电化学体系中灰黑色固体氧化PMSO效果
Figure 3. Oxidation of PMSO by the gray black solid obtained from the chloride-containing electrochemical system
-
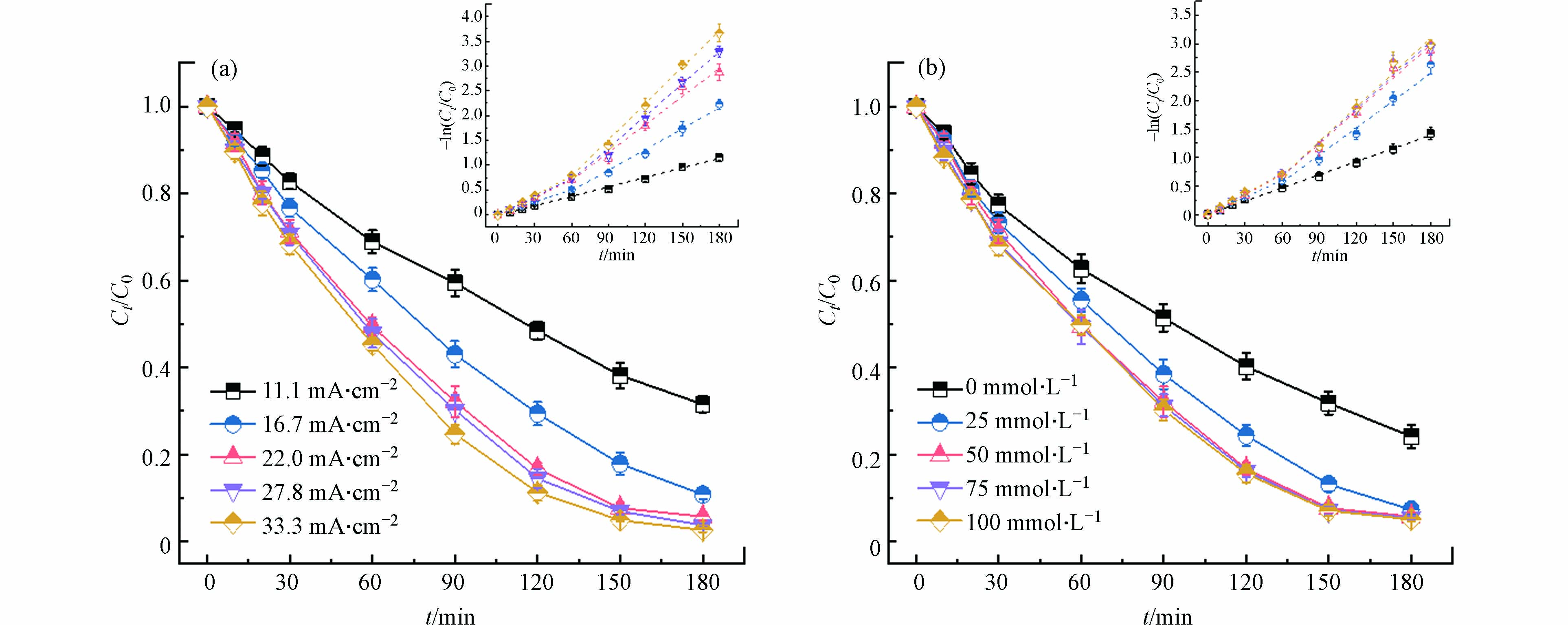
图 4 (a)电流密度和(b)氯离子浓度对Ni-EDTA降解的影响(内插图为Ni-EDTA降解动力学拟合)
Figure 4. Effects of (a) current density and (b) chloride concentration on time-course Ni-EDTA concentration (insert: kinetic-fitting results)
-

图 5 电化学体系中Fe(Ⅲ)-EDTA(a)降解效果(b)降解动力学拟合;不同Ni2+浓度下Fe(Ⅲ)-EDTA(c)降解效果(d)降解动力学拟合.
Figure 5. Comparisons of time-course (a) Fe-EDTA concentration and (b) kinetic-fitting results in the electrochemical systems. The effects of concentration of added Ni2+ on (c) degradation of Fe(Ⅲ)-EDTA and (d) kinetic-fitting results
-
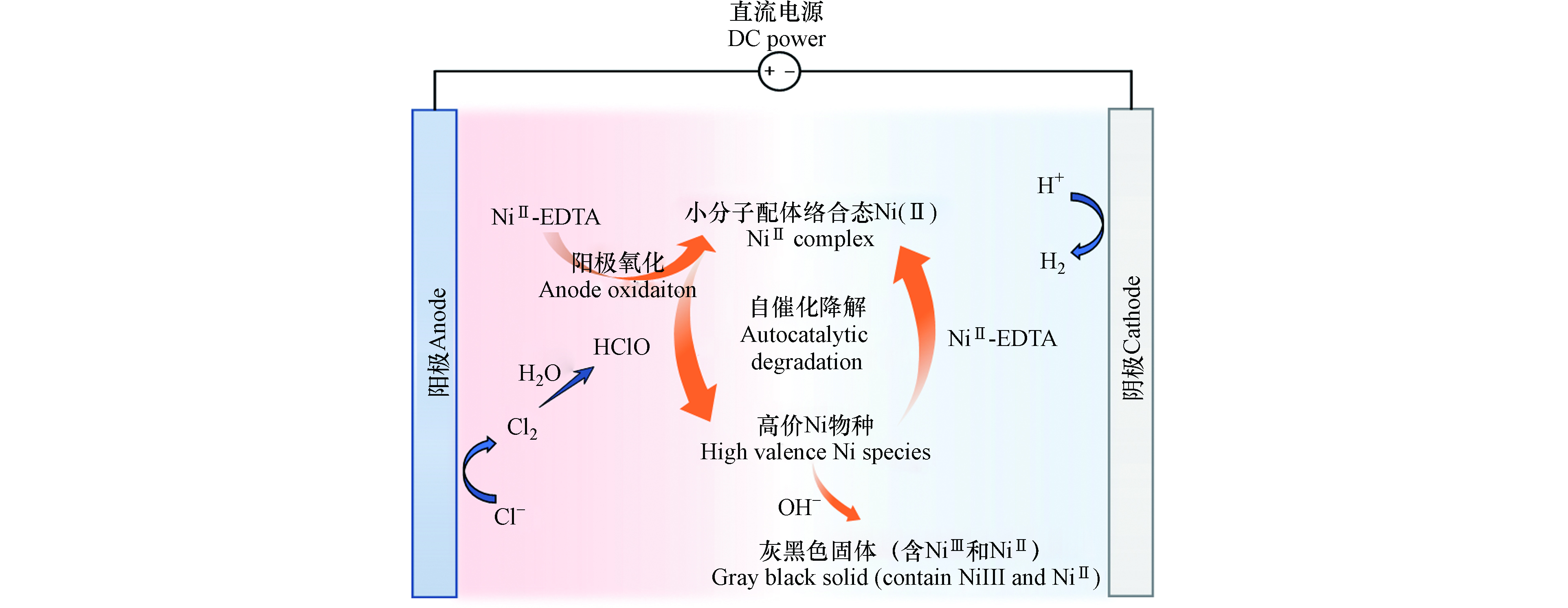
图 6 Ni-EDTA在含氯电化学氧化体系中自催化降解途径示意图.
Figure 6. Schematic representation of the proposed pathways for Ni-EDTA autocatalytic degradation in the chloride-containing electrochemical oxidation system.
Figure
6 ,Table
3 个