镉(cadmium,Cd)是一种毒性大、难降解且污染面积广的主要环境污染物[1]。近年来,随着科技的发展和进步,镉被广泛应用于冶炼、电池、农药、化肥等工业和农业领域,致使环境中的镉含量超出正常范围,生态系统平衡遭到破坏[2]。镉可以通过工业制造活动或食品、水、空气污染以及许多其他潜在来源进入人体[3],食物一般是镉暴露的最主要途径[4]。与其他重金属相比,残留于环境中的镉及其代谢产物由于较强的流动性、持久性和生物累积性[5],易被作物根系吸收并积累,从而沿食物链富集于人类体内并长期蓄积[6-7],造成多种器官、组织以及细胞和DNA方面的损伤[8]。已有研究证实,镉可引发动物机体内大脑皮质损伤[9]、心肌细胞凋亡[10]、肾脏淋巴细胞浸润和肝脏血管炎[11]。此外,镉能通过参与Fenton反应引起细胞内活性氧(reactive oxygen species,ROS)水平增加,造成动植物细胞氧化损伤[12]。镉还可诱导细胞内超氧化物歧化酶(superoxide dismutase,SOD)和过氧化氢酶(catalase,CAT)活性下降,肝脏丙二醛(malonaldehyde,MDA)水平增加,细胞自由基代谢失调,导致氧化伤害[13]。
壳寡糖(chitooligosaccharide,COS)又名壳聚寡糖、几丁寡糖、低聚壳聚糖,其化学名为β-1,4-寡聚-葡萄糖胺,是天然、无毒副作用的低聚糖。COS具有良好的水溶性,有助于提高动物机体免疫力[14],减少炎症[15]、各种应激[16-17]带来的损伤等。相关动物试验表明,COS可以通过使动物机体内的抗氧化酶活性提升,增强机体对自由基的清除作用,从而降低氧化破坏[18-19]。COS还能对炎症因子的分泌进行抑制,或促进抗氧化物质的产生,以此减少ROS的产生[20]。COS还是一部分金属离子的良好配体,且对重金属离子有较好的吸附效果,可以作为一种吸附剂、配位体或螯合剂[21]。同时,COS处理可以介导核因子红细胞2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)和核因子-κB(nuclear factor-kappa B,NF-κB)抗氧化信号通路,上调其下游抗氧化靶基因的表达,在减轻外源性有害物质诱导的细胞氧化应激中发挥重要作用[22-23]。
NF-κB是细胞内氧化信号通路相关的关键转录因子[24]。p38 MAPK信号分子则是丝裂原激活蛋白激酶(mitogen activated protein kinase,MAPK)中的一个亚族,主要参与调节细胞的应激、炎症和凋亡等生物学进程[25]。研究表明,磷酸化的p38 MAPK作用于NF-κB,激活机体的炎症通路,促进炎症因子的释放;炎症因子反过来促进炎症信号通路的活化,加剧炎症反应的发生[26]。有研究显示,镉可通过MAPK途径诱导氧化应激[27]。Nrf2也是氧化应激反应的重要转录因子之一[28]。如图1所示,在重金属诱导的氧化应激条件下,Nrf2可以结合抗氧化反应元件(antioxidant response element,ARE)诱导多种抗氧化酶的表达[29],在抗炎和减少氧化应激引起的损伤中起着至关重要的作用[30]。既往的研究表明,镉诱导的肝脏氧化应激常伴随着Nrf2信号通路的激活[31],提示该通路在镉的主要防御机制中有重要意义。
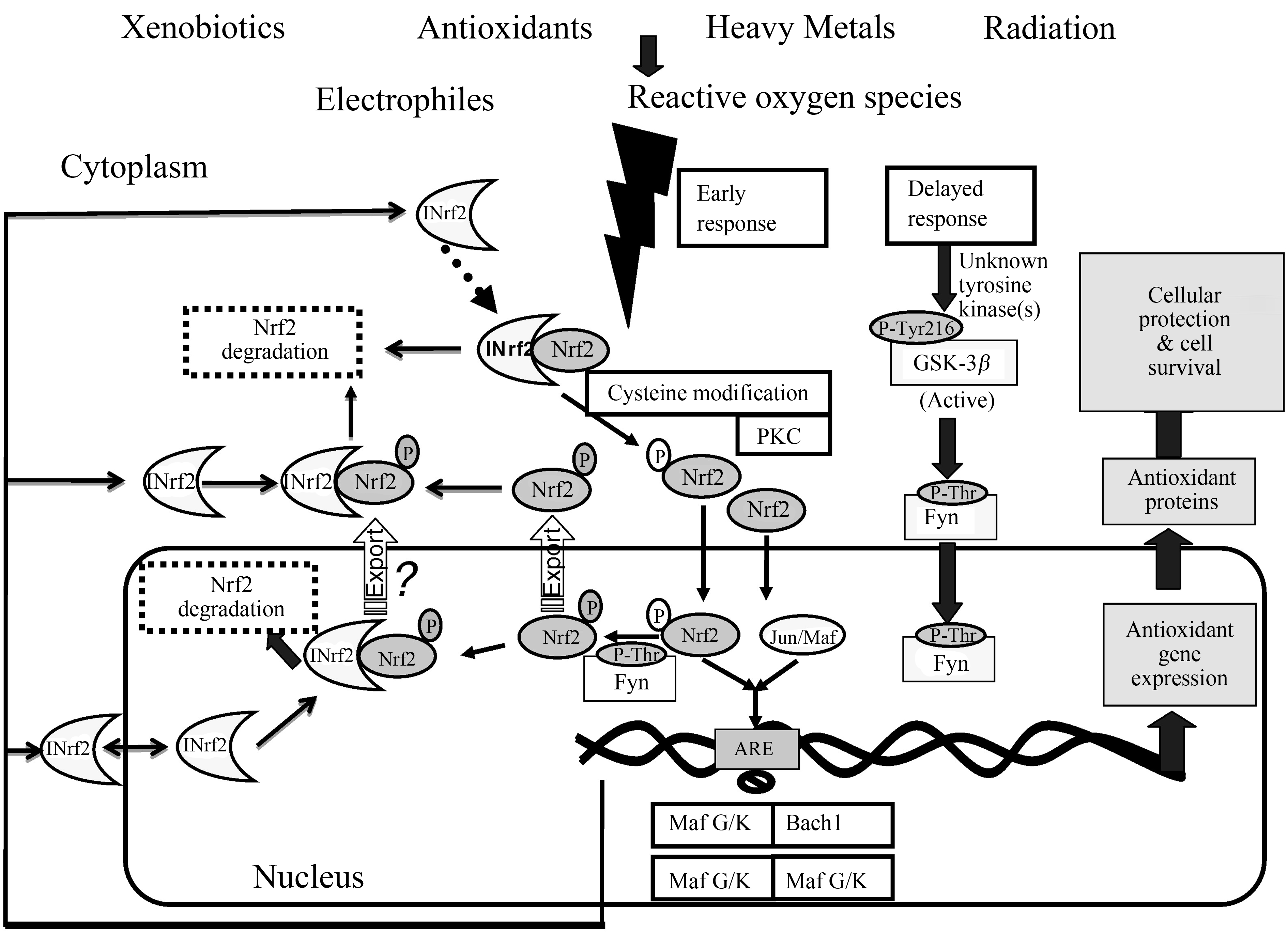
图1 重金属诱导的Nrf2信号通路[32]
Fig. 1 Heavy metals mediated Nrf2 signaling pathway[32]
综合以上研究结果可知,镉可通过p38 MAPK/NF-кB p65/Nrf2介导的信号通路启动炎症反应,但具有缓解氧化应激损伤的COS对镉致肝脏损伤的影响方面的研究还鲜有报道,确切的机制还需要进一步研究探讨。本研究采用腹腔注射CdCl2溶液的方式来建立急性镉暴露小鼠模型,通过在小鼠饮水中添加COS,并对小鼠进行肝脏内ROS水平变化的检测和病理学观察,结合血液中抗氧化相关指标的检测和肝脏氧化应激通路关键蛋白p38 MAPK/NF-кB p65/Nrf2表达水平的检测,研究COS在缓解重金属致肝脏氧化应激方面的作用和可能机制,对通过添加COS预防或改善镉累积致动物机体的毒性损伤具有一定的参考价值,有望为COS作为天然生物制剂在重金属解毒方面的应用提供理论依据和数据支撑,降低部分镉污染地区动物体和人体的健康风险,达到缓解生态危害的目的。
1 材料与方法(Materials and methods)
1.1 材料与试剂
COS为水溶性寡糖,聚合度在2~10之间,以3~4糖为主,分子质量≤2 000,纯度为10%,购于中泰和(北京)科技发展有限公司。谷胱甘肽(glutathione,GSH)试剂盒、谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px,GPx)试剂盒、氧化型谷胱甘肽(oxidized glutathione,GSSG)试剂盒购于南京建成生物工程研究所;MDA试剂盒购于碧云天生物公司,其他试剂均为分析纯,购自国药集团化学试剂有限公司。
1.2 试验动物
选用健康雌性C57BL/6小鼠32只,体质量16~19 g,购自湖南斯莱克景达实验动物有限公司。小鼠饲养环境室内平均气温22 ℃,室内照明控制在12 h/12 h光暗周期节律。小鼠的食物为小鼠标准颗粒饲料,自由饮用纯净水及经过COS处理后的纯净水,实验期为28 d。
1.3 方法
1.3.1 动物分组与处理
32只雌性小鼠适应饲养4 d后,随机分为4个处理组,每个处理组8个重复,每个重复1只小鼠,单栏饲养。4个组分别为:空白对照组(Control)、氯化镉造模组(Cd)、壳寡糖组(COS)、壳寡糖+氯化镉组(COS+Cd)。空白对照组和氯化镉造模组小鼠每日饲喂基础日粮,给予纯净水;壳寡糖组和壳寡糖+氯化镉组小鼠每日饲喂基础日粮,在饮水中添加0.3 g·L-1 COS。在第28天向氯化镉造模组和壳寡糖+氯化镉组小鼠腹腔注射5.45 mg·kg-1氯化镉(以体质量计),12 h后眼框取血,置于1.5 mL EP管中,4 ℃下静置2 h。于3 000 r·min-1、4 ℃离心30 min后,取上清液分装,在-20 ℃下保存备用。采血后脱颈椎处死,取小鼠肝脏组织在福尔马林中固定,4 ℃静置避光保存,每12 h更换固定液。另取一份放入包埋盒,加入适量OCT包埋剂,使组织完全被包埋剂覆盖,置于液氮中速冻,-80 ℃保存。
1.3.2 肝脏组织病理学检测
福尔马林固定好的肝脏组织按常规方法制作石蜡组织切片,苏木精-伊红(hematoxylin-eosin,HE)染色,光镜下观察肝脏组织结构及肝脏组织病理学变化等。
1.3.3 血清抗氧化相关指标检测
检测小鼠血清中GPx活性以及GSH、MDA和GSSG含量,并计算出GSSG/GSH的比值。试验操作严格按照试剂盒说明书进行。
1.3.4 肝脏中ROS水平检测
使用活性氧荧光探针测定,按照说明书建议,以二甲基亚砜(dimethyl sulfoxide,DMSO)溶解二氢乙锭(dihydroethidium,DHE)配制成溶液,避光放置备用,临用前将溶液按照1∶1 000的比例稀释后备用。
将OCT包被好的冷冻组织用冰冻切片机切片。制片后,将稀释好的探针溶液滴加到组织中。于37 ℃孵育30 min。用PBS洗去多余探针溶液,用抗荧光淬灭剂封片。用荧光显微镜观察并拍照,计算各组的相对荧光强度。
1.3.5 肝脏蛋白水平检测
Western blot方法检测肝脏组织中Nrf2、p-NF-кB、NF-кB、p-p38 MAPK、p38 MAPK的蛋白表达水平。剪取适量肝脏组织研磨成干粉状,倒入EP管中,提取组织总蛋白,然后检测并将蛋白浓度调至相同,按照说明书进行配制溶液、封闭及孵育等操作,最后使用ECL方法显色。
1.4 数据处理
应用SPSS 22.0统计软件对数据进行单因素方差分析(one-way analysis of variance,one-way ANOVA),比较组间的统计学差异。数据以“平均值±标准差”表示,P<0.05、P<0.01表示差异显著。
2 结果(Results)
2.1 饲粮中添加COS对小鼠体质量的影响
由图2可知,本试验中氯化镉急性处理和COS添加均未对小鼠体质量产生显著影响(P>0.05)。

图2 各组小鼠体质量
注:Control表示空白对照组;Cd表示氯化镉造模组;COS表示壳寡糖组;COS+Cd表示壳寡糖+氯化镉组。
Fig.2 Body mass of mice in different groups
Note:Control stands for Control group;Cd stands for cadmium chloride model group;COS stands for COS group;COS+Cd stands for COS+cadmium chloride group.
2.2 小鼠肝脏组织病理学观察
由图3可知,空白对照组小鼠肝小叶染色均匀,形态结构正常,细胞间有明显的界限,未见显著的变性与坏死;壳寡糖组肝脏细胞未产生明显变化;氯化镉造模组肝脏组织产生了明显病理变化,细胞间界限模糊、炎症和凋亡增多,且细胞核出现萎缩现象(黑色圆圈及箭头部分),表明镉染毒处理对肝脏细胞造成了一定的损伤;壳寡糖+氯化镉组相对氯化镉造模组,细胞坏死程度和炎症减轻,肝脏结构较为清晰。

图3 光镜下各组小鼠肝脏细胞形态结构(100倍)
注:(a) Control;(b) Cd;(c) COS+Cd;(d) COS;黑色圆圈及箭头部分表示肝脏细胞核萎缩。
Fig.3 Morphology and structure of liver tissue of mice (×100)
Note:(a) Control;(b) Cd;(c) COS+Cd;(d) COS;the black circles and arrow indicated liver nuclear atrophy.
2.3 饲粮中添加COS对其血液抗氧化指标的影响
由图4可知,与氯化镉造模组相比,通过饮水添加COS显著降低了小鼠血清中GSH含量和GPx活性(P<0.05),提高了GSSG含量(P<0.05),缓解了由于重金属镉富集导致的氧化应激,激活了小鼠体内的GSSG/GSH系统(P<0.05)。在正常饲养条件下,饮水中添加COS使小鼠血清中GSH含量呈显著提升趋势(P<0.05),明显降低了GPx活性和GSSG含量(P<0.05),使得GSSG/GSH比值大幅升高(P<0.05)。与空白对照组相比,氯化镉造模组小鼠血清中GSH含量和GSSG/GSH的比值有一定程度的上升(P<0.05),MDA的含量呈显著上升趋势(P<0.01),而壳寡糖+氯化镉组的数据显示,COS显著抑制了镉中毒小鼠血清内MDA的产生P<0.05)。
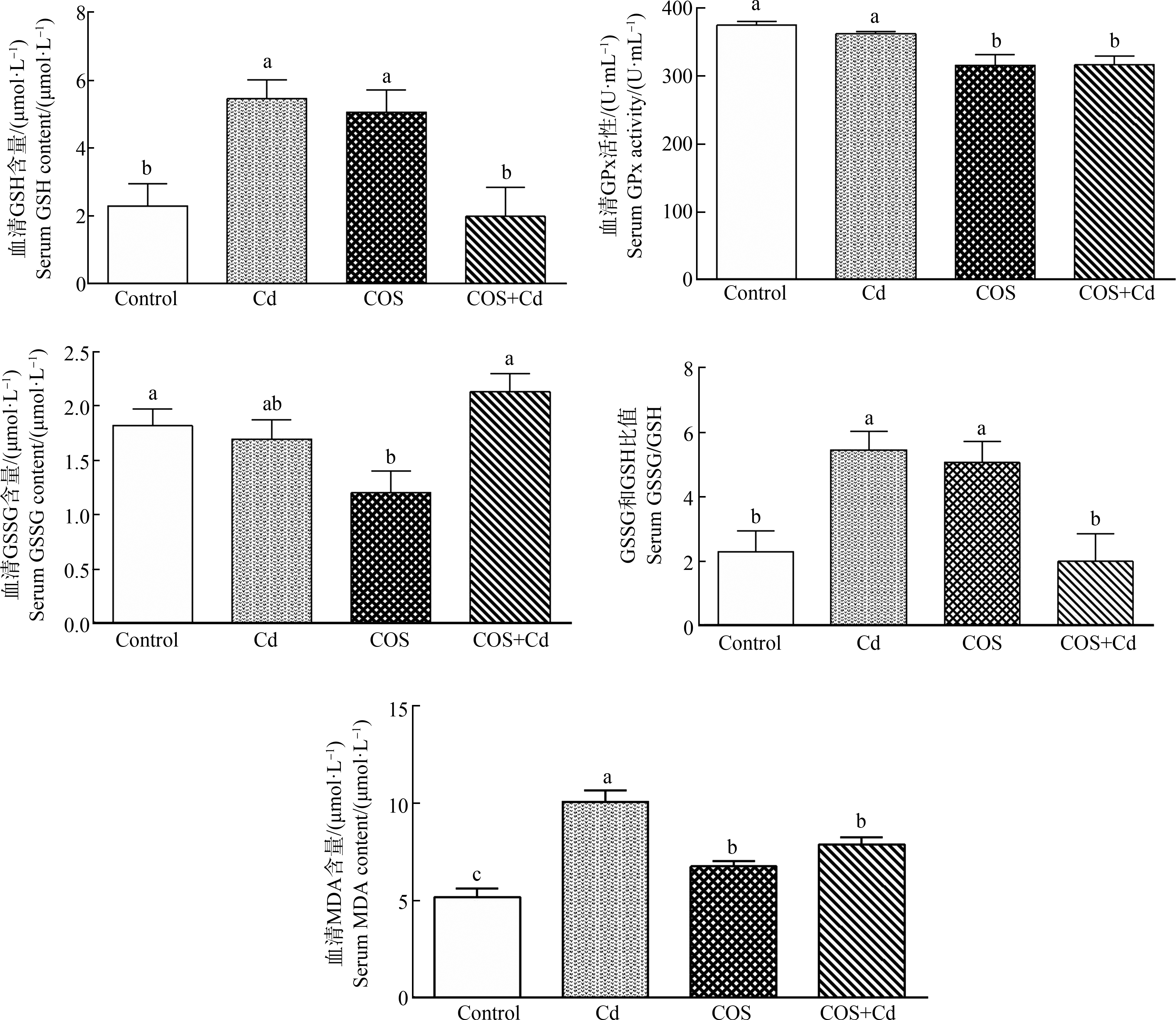
图4 小鼠血清抗氧化相关指标
注:GSH表示谷胱甘肽,GSSG表示氧化型谷胱甘肽,GPx表示谷胱甘肽过氧化物酶,MDA表示丙二醛;不同字母表示差异显著(P<0.05),相同字母表示差异不显著(P>0.05)。
Fig.4 Serum antioxidant indexes of mice
Note:GSH stands for glutathione;GSSG stands for oxidized glutathione;GPx stands for glutathione peroxidase;MDA stands for malonaldehyde;different letters indicated significant difference (P<0.05),and the same letters indicated no significant difference (P>0.05).
2.4 饲粮中添加COS对肝脏中ROS含量的影响
由图5可知,与空白对照组相比,氯化镉造模组小鼠肝脏内代表ROS的红色荧光强度明显升高(P<0.01),添加COS之后,壳寡糖+氯化镉组红色荧光强度降低,整体状态趋近于空白对照组,说明COS可通过减少肝脏中ROS水平,缓解重金属镉蓄积而导致的肝脏损伤。

图5 小鼠肝脏细胞中活性氧(ROS)相对含量
注:DAPI表示4’,6-二脒基-2-苯基吲哚,DHE表示二氢乙啶;不同字母表示差异显著(P<0.05),相同字母表示差异不显著(P>0.05)。
Fig.5 Relative hepatic reactive oxygen species (ROS) content of mice
Note:DAPI stands for 4’,6-diamidino-2-phenylindole,and DHE stands for dihydroethidium;different letters indicated significant difference (P<0.05),and the same letters indicated no significant difference (P>0.05).
2.5 肝脏氧化应激通路关键蛋白的相对表达
由图6可知,重金属镉在机体的富集可以有效激活小鼠肝脏内Nrf2(P<0.05),同时抑制炎症相关的信号分子磷酸化NF-кB p65的表达(P<0.05)。和空白对照组相比,在小鼠饮水中添加COS后,可显著抑制Nrf2的表达(P<0.05),对磷酸化p38 MAPK的表达也有抑制的趋势,但无显著性差异(P>0.05),对其总蛋白的表达亦无明显影响(P>0.05)。氯化镉造模组和壳寡糖+氯化镉组对比结果显示,添加COS后小鼠肝脏内Nrf2水平显著降低(P<0.05),磷酸化p38 MAPK表达量也有一定程度的降低(P>0.05)。

图6 小鼠肝脏氧化应激通路相关蛋白表达水平
注:NF-κB表示核因子-κB,MAPK表示丝裂原激活蛋白激酶,Nrf2表示核因子红细胞2相关因子2;不同字母表示差异显著(P<0.05),相同字母表示差异不显著(P>0.05)。
Fig.6 Relative expression levels of proteins related to oxidative stress in the liver of mice
Note:NF-κB stands for nuclear factor-kappa B,MAPK stands for mitogen activated protein kinase,and Nrf2 stands for nuclear factor erythroid 2-related factor 2;different letters indicated significant difference (P<0.05),and the same letters indicated no significant difference (P>0.05).
3 讨论(Discussion)
作为一种重金属环境污染物,镉可对细胞产生毒性,导致细胞功能异常,对多种组织和系统造成不可逆的损伤[33-35]。食源性镉是畜禽镉暴露的主要来源。动物饲料中的镉多来自于原料和矿补剂添加,会导致动物体生长缓慢,免疫力下降等,进而影响后期的生长性能及胴体性状[36]。肝脏是镉损伤的重要靶器官之一,也是最主要的镉积累器官[37]。Kuester等[38]研究发现,肝脏在急性或慢性镉暴露后几小时内便可以积累大量的镉。镉还能导致肝脏出现多种病理性变化,以50 mg·L-1镉的饮水饲喂大鼠12周,大鼠肝脏MDA含量增加,发生了显著的脂质过氧化反应[39]。陈梦妍等[40]还发现,急性镉暴露可导致小鼠肝脏细胞局部出现气球样变、炎症细胞浸润和坏死等较为明显的损伤。本试验采用氯化镉对小鼠建立急性镉中毒模型,发现氯化镉造模组小鼠肝脏中出现肝脏结构不清楚、肝细胞颗粒变性且细胞核萎缩等病理变化,和陈梦妍等[40]的发现基本一致。GSH、GSSG和GPx参与动物体内氧化还原过程,能和过氧化物结合,以对抗氧化剂对脏器细胞的损害。本实验发现,急性镉中毒处理可以导致小鼠血清中GSH、MDA的含量和GSSG/GSH比值显著升高,表明氯化镉导致小鼠产生了剧烈脂质过氧化反应,并导致机体启动了相应的抗氧化体系,说明本研究成功建立了小鼠的急性镉应激模型。
COS具有抗炎[41-42]、抗菌[43]、提高免疫力[44]和改善肠道微生物[45]等作用。许青松等[46]的研究表明,COS还有缓解氧化应激的作用,能抑制肝组织中MDA含量升高,提高肝组织中SOD活性,对四氯化碳造成的小鼠急性肝损伤进行保护。研究表明,COS可增加细胞内CAT活性,提高GSH含量[47],且对重金属离子也有一定的吸附效果,可以促进动物机体对镉的脱除[48]。在本试验中,与氯化镉造模组相比,COS添加有助于小鼠肝脏组织损伤的的恢复,减缓炎症和细胞凋亡,说明COS在保护急性镉中毒引起的肝脏损伤中具有一定效果。与氯化镉造模组相比,壳寡糖+氯化镉组小鼠血清中GSH、MDA的含量和GPx活性显著降低,GSSG含量显著提升,动物机体内的GSSG/GSH系统被激活。这显示COS能够通过激活动物机体内抗氧化防御系统,来缓解脂质过氧化物的产生而导致肝脏遭受的氧化损害。
镉能通过参与Fenton反应诱导细胞产生过量ROS,引起细胞内ROS水平增加,造成动物细胞的损伤,其毒性与氧化损伤密切相关[49]。研究表明,COS有清除活性氧自由基或离子的功能[50]。在葡聚糖硫酸钠诱导的小鼠结肠炎模型中,COS能抑制活化的中性粒细胞产生过量ROS,从而起到抗炎作用[51]。翟星辰[52]认为,COS可以调控细胞内ROS的积累,达到抑制肾癌对机体损伤的效果。GSH是ROS的天然清除剂,是细胞氧化还原的主要调节因子,可在酶类的催化下氧化生成GSSG[53]。GSH和GSSG之间处于动态平衡,共同缓解机体的氧化应激,二者的氧化还原循环是机体抵抗氧化损伤的重要机制之一,GSSG/GSH比值则是反映机体氧化还原状态的重要指标[54]。本研究中氯化镉造模组小鼠肝脏细胞ROS含量急剧上升,而添加COS后小鼠肝脏细胞ROS含量降低,GSSG/GSH比值下降,细胞组织结构有一定的恢复,提示COS可通过调控GSSG/GSH比例减少肝脏ROS产生,具有修复肝脏氧化损伤的功能。
研究表明,镉可通过增加细胞ROS诱发细胞内氧化还原敏感性转录因子的表达,如Nrf2、NF-кB等[55]。氧化应激条件下Nrf2通路被诱导激活,抵抗ROS导致的细胞损伤,是肝脏迄今为止发现的最为重要的内源性抗氧化应激通路[56],且在降低镉致毒性方面发挥了重要作用[57]。NF-кB是重要的炎症相关信号因子,激活后能够以NF-кB p65的形式存在,在机体的炎性反应和细胞凋亡等过程中发挥着重要作用[58]。本试验发现,镉导致小鼠肝脏组织内抗氧化蛋白Nrf2及磷酸化p38 MAPK的相对表达量升高,磷酸化NF-кB p65的表达降低,进一步证明镉诱发了自由基的产生,使机体产生氧化损伤。Luo等[59]对乙醇诱导的小鼠细胞内氧化应激进行COS处理后发现,COS可通过抑制p38 MAPK的磷酸化和调控Nrf2的活化,影响下游抗氧化酶的表达,进而清除细胞内积累的ROS。在本试验中,相较氯化镉造模组,COS添加在一定程度上抑制了小鼠肝脏组织p38 MAPK的磷酸化水平,和Luo等[59]的发现一致。研究表明,机体处于氧化应激状态时,Nrf2进入细胞核参与抗氧化反应[60],机体恢复稳态后Nrf2转移回细胞质中,并通过泛素化降解或负反馈调节回归正常水平[61]。本试验数据显示,对急性镉暴露小鼠进行COS处理后,小鼠肝脏细胞中Nrf2的表达显著降低,这可能因为COS在激活机体抗氧化系统的同时,降低了肝脏组织ROS含量,使机体内恢复氧化还原稳态。根据本研究结果,我们初步推断:镉暴露引起小鼠细胞内ROS的产生,诱导MAPK信号通路激活,进而导致其中细胞外信号调节激酶p38 MAPK的激活,激活后的磷酸化的p38 MAPK信号分子主要调动细胞氧化应激和炎症反应,说明大量镉蓄积导致肝脏处于急性的炎症活跃期。同时,肝脏在镉诱导的过量ROS的刺激下负反馈抑制磷酸化NF-κB的激活,提高氧化应激关键信号分子Nrf2的表达,减慢炎症发展的速度。机体添加COS后p38的磷酸化水平降低,缓解了肝脏的炎症程度,同时通过抑制磷酸化NF-κB的表达,使Nrf2的表达下降至正常水平,从而维持肝脏的氧化还原稳态(图7)。
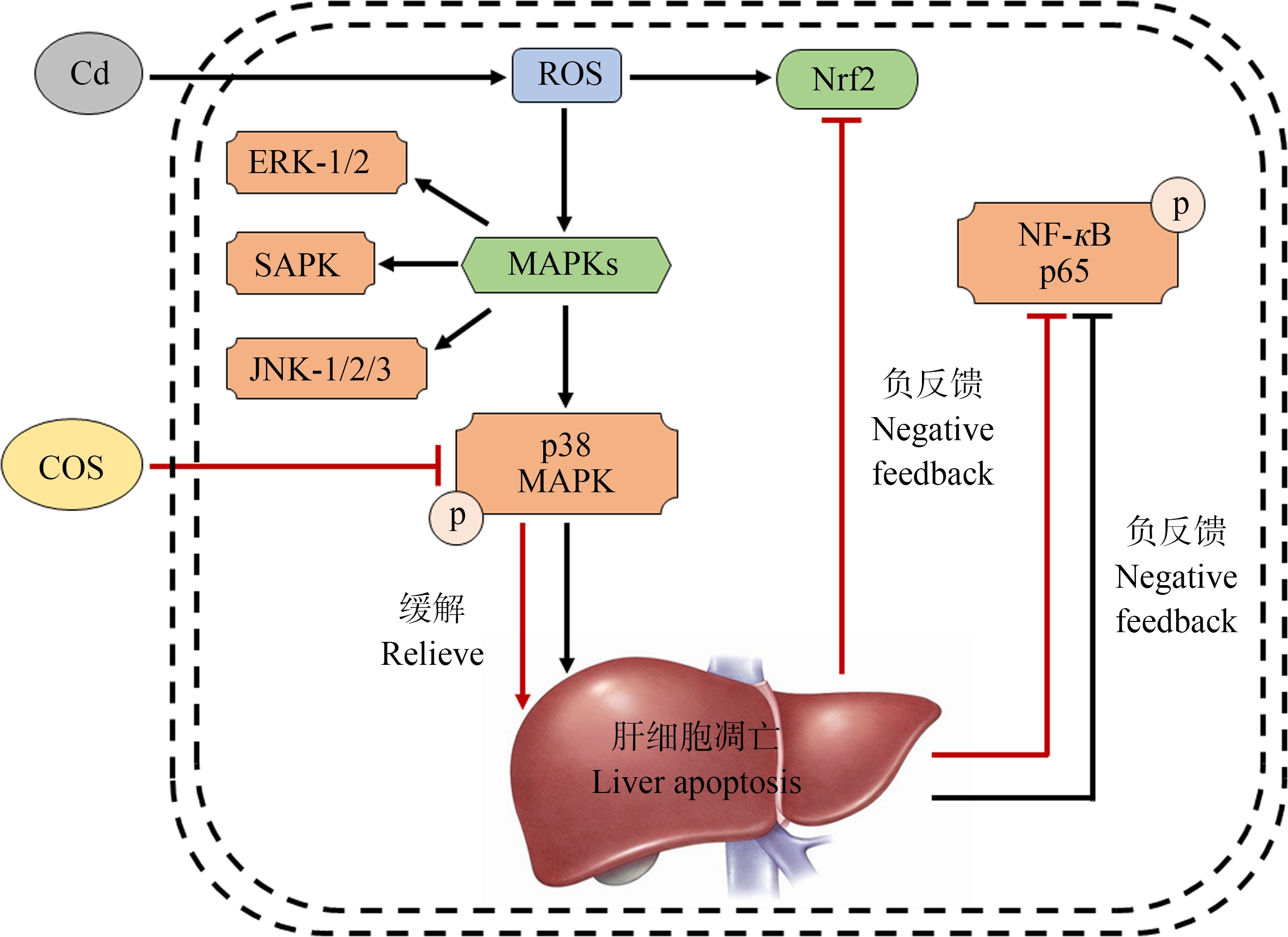
图7 Cd和COS联合作用下小鼠肝脏p38 MAPK/NF-кB p65/Nrf2氧化应激相关信号通路
注:ERK-1/2表示细胞外信号调节激酶;SAPK表示应激活化蛋白激酶;JNK表示c-Jun氨基末端激酶;黑色实线表示Cd产生的影响,红色实线表示COS产生的影响。
Fig.7 Oxidative stress related signaling pathways of p38 MAPK/NF-кB p65/Nrf2 in mouse liver under the combined action of Cd and COS
Note:ERK stands for extracellular regulated kinase,SAPK stands for stress-activated protein kinase,and JNK stands for c-Jun N-terminal kinase;the black solid line shows the effect of Cd,and the red solid line shows the effect of COS.
综上所述,COS可通过调控肝脏主要抗氧化信号通路,来缓解镉对小鼠的应激,减少肝脏组织氧化损伤,达到保护肝脏组织的目的。为COS调控重金属富集的机制研究提供了实验数据支持,为天然多糖类复合物作为生物解毒剂应用于重金属暴露的动物和人体提供依据。
[1] Yang R Y,He Y H,Luo L F,et al.The interaction between selenium and cadmium in the soil-rice-human continuum in an area with high geological background of selenium and cadmium [J].Ecotoxicology and Environmental Safety,2021,222:112516
[2] DalCorso G,Fasani E,Manara A,et al.Heavy metal pollutions:State of the art and innovation in phytoremediation [J].International Journal of Molecular Sciences,2019,20(14):3412
[3] Vesna M,Aleksandra B,Danijela D,et al.Insight into the oxidative stress induced by lead and/or cadmium in blood,liver and kidneys [J].Food and Chemical Toxicology:An International Journal Published for the British Industrial Biological Research Association,2015,78:130-140
[4] Wang K,Ma J Y,Li M Y,et al.Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells:Oxidative stress,cell cycle arrest and apoptosis [J].Science of the Total Environment,2021,756:143951
[5] Chang C,Yin R S,Zhang H,et al.Bioaccumulation and health risk assessment of heavy metals in the soil-rice system in a typical seleniferous area in central China [J].Environmental Toxicology and Chemistry,2019,38(7):1577-1584
[6] Gupta N,Yadav K K,Kumar V,et al.Trace elements in soil-vegetables interface:Translocation,bioaccumulation,toxicity and amelioration:A review [J].Science of the Total Environment,2019,651:2927-2942
[7] Pecina V,![]() M,Baltazár T,et al.Human health and ecological risk assessment of trace elements in urban soils of 101 cities in China:A meta-analysis [J].Chemosphere,2021,267:129215
M,Baltazár T,et al.Human health and ecological risk assessment of trace elements in urban soils of 101 cities in China:A meta-analysis [J].Chemosphere,2021,267:129215
[8] Knoell D L,Wyatt T A.The adverse impact of cadmium on immune function and lung host defense [J].Seminars in Cell &Developmental Biology,2021,115:70-76
[9] 王莉,闻双全,贺双江,等.慢性镉暴露对小鼠大脑皮质的毒性损伤作用[J].畜牧与兽医,2021,53(2):50-55
Wang L,Wen S Q,He S J,et al.Toxic damage effect of chronic cadmium exposure on the cerebral cortex of mouse [J].Animal Husbandry &Veterinary Medicine,2021,53(2):50-55 (in Chinese)
[10] 龚频,高浩天,杨文娟,等.蓝莓花青素对镉致小鼠心脏损伤的改善作用[J].陕西科技大学学报,2020,38(6):48-53
Gong P,Gao H T,Yang W J,et al.The amelioration effect of blueberry anthocyanins on cadmium-induced mice heart damage [J].Journal of Shaanxi University of Science &Technology,2020,38(6):48-53 (in Chinese)
[11] 陶灿.日粮镉对蛋鸡生产性能、蛋品质、肝脏和肾脏的影响[D].武汉:华中农业大学,2019:42
Tao C.Effects of dietary cadmium on performance,egg quality,liver and kidney damage of laying hens [D].Wuhan:Huazhong Agricultural University,2019:42 (in Chinese)
[12] Marín-García J,Akhmedov A T.Mitochondrial dynamics and cell death in heart failure [J].Heart Failure Reviews,2016,21(2):123-136
[13] 张文华,闻双全,王莉,等.葛根素对镉致大鼠肝毒性损伤的保护作用[J].中国兽医科学,2020,50(10):1333-1339
Zhang W H,Wen S Q,Wang L,et al.Protective effect of puerarin on toxic damage caused by cadmium in livers of rats [J].Chinese Veterinary Science,2020,50(10):1333-1339 (in Chinese)
[14] Wan J,Xu Q S,He J.Maternal chitosan oligosaccharide supplementation during late gestation and lactation affects offspring growth [J].Italian Journal of Animal Science,2018,17(4):994-1000
[15] Gu M,Pan S H,Li Q,et al.Chitosan and chitooligosaccharides attenuate soyabean meal-induced intestinal inflammation of turbot (Scophthalmus maximus):Possible involvement of NF-кB,activator protein-1 and mitogen-activated protein kinases pathways [J].British Journal of Nutrition,2021,126(11):1651-1662
[16] Ahmed S M U,Luo L,Namani A,et al.Nrf2 signaling pathway:Pivotal roles in inflammation [J].Biochimica et Biophysica Acta Molecular Basis of Disease,2017,1863(2):585-597
[17] Wang Y M,Xiong Y L,Zhang A P,et al.Oligosaccharide attenuates aging-related liver dysfunction by activating Nrf2 antioxidant signaling [J].Food Science &Nutrition,2020,8(7):3872-3881
[18] 彭媛媛,欧阳富龙,贺建华.壳寡糖在动物体内抗氧化功能研究进展[J].饲料博览,2015(8):16-18
Peng Y Y,Ouyang F L,He J H.Research advances of chitosan oligosaccharide on the antioxidant function in animal [J].Feed Review,2015(8):16-18 (in Chinese)
[19] 郑雯静,杨靖亚,刘克海.壳寡糖对三氧化二砷致大鼠肝细胞毒性的保护作用[J].安徽农业大学学报,2021,48(3):412-417
Zheng W J,Yang J Y,Liu K H.Protective effect of chitosan oligosaccharide against the toxicity of arsenic trioxide toward Buffalo rat liver cells [J].Journal of Anhui Agricultural University,2021,48(3):412-417 (in Chinese)
[20] Zhao Q N,Yin L Q,Zhang L R,et al.Chitoheptaose promotes heart rehabilitation in a rat myocarditis model by improving antioxidant,anti-inflammatory,and antiapoptotic properties [J].Oxidative Medicine and Cellular Longevity,2020,2020:2394704
[21] 孙继鹏.壳寡糖金属配合物对扇贝体内重金属镉的影响[D].青岛:中国海洋大学,2009:14
Sun J P.The effect of chitosan oligosaccharide complexes with metal elements on cadmium in viscera of Chlamys ferrari [D].Qingdao:Ocean University of China,2009:14 (in Chinese)
[22] Montes S,Juárez-Rebollar D,Nava-Ruíz C,et al.Immunohistochemical study of Nrf2-antioxidant response element as indicator of oxidative stress induced by cadmium in developing rats [J].Oxidative Medicine and Cellular Longevity,2015,2015:570650
[23] Zhang Y T,Ahmad K A,Khan F U,et al.Chitosan oligosaccharides prevent doxorubicin-induced oxidative stress and cardiac apoptosis through activating p38 and JNK MAPK mediated Nrf2/ARE pathway [J].Chemico-Biological Interactions,2019,305:54-65
[24] Rius-Pérez S,Pérez S,Martí-Andrés P,et al.Nuclear factor kappa B signaling complexes in acute inflammation [J].Antioxidants &Redox Signaling,2020,33(3):145-165
[25] Kim E K,Choi E J.Pathological roles of MAPK signaling pathways in human diseases [J].Biochimica et Biophysica Acta,2010,1802(4):396-405
[26] Gorska M M,Liang Q L,Stafford S J,et al.MK2 controls the level of negative feedback in the NF-κB pathway and is essential for vascular permeability and airway inflammation [J].Journal of Experimental Medicine,2007,204(7):1637-1652
[27] Cheng C Y,Mruk D D.The blood-testis barrier and its implications for male contraception [J].Pharmacological Reviews,2012,64(1):16-64
[28] Huang G Q,Sun J P,Wang D F,et al.Chitosan oligosaccharide-Ca complex accelerates the depuration of cadmium from Chlamys ferrari [J].Journal of Ocean University of China,2012,11(2):219-226
[29] Nguyen T,Nioi P,Pickett C B.The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress [J].Journal of Biological Chemistry,2009,284(20):13291-13295
[30] Radan M,Dianat M,Badavi M,et al.In vivo and in vitro evidence for the involvement of Nrf2-antioxidant response element signaling pathway in the inflammation and oxidative stress induced by particulate matter (PM10):The effective role of gallic acid [J].Free Radical Research,2019,53(2):210-225
[31] Ren L F,Qi K,Zhang L,et al.Glutathione might attenuate cadmium-induced liver oxidative stress and hepatic stellate cell activation [J].Biological Trace Element Research,2019,191(2):443-452
[32] Kaspar J W,Niture S K,Jaiswal A K.Nrf2:INrf2 (Keap1) signaling in oxidative stress [J].Free Radical Biology and Medicine,2009,47(9):1304-1309
[33] Ben P L,Zhang Z P,Zhu Y Y,et al.L-theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation [J].NeuroToxicology,2016,57:95-103
[34] Mohajeri M,Rezaee M,Sahebkar A.Cadmium-induced toxicity is rescued by curcumin:A review [J].BioFactors,2017,43(5):645-661
[35] Chen X M,Bi M Y,Yang J,et al.Cadmium exposure triggers oxidative stress,necroptosis,Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine [J].Journal of Hazardous Materials,2022,421:126704
[36] Adamse P,van der Fels-Klerx H J I,Jong J D.Cadmium,lead,mercury and arsenic in animal feed and feed materials - trend analysis of monitoring results [J].Food Additives &Contaminants Part A,Chemistry,Analysis,Control,Exposure &Risk Assessment,2017,34(8):1298-1311
[37] Ma X Q,Hou M,Liu C B,et al.Cadmium accelerates bacterial oleic acid production to promote fat accumulation in Caenorhabditis elegans [J].Journal of Hazardous Materials,2022,421:126723
[38] Kuester R K,Waalkes M P,Goering P L,et al.Differential hepatotoxicity induced by cadmium in Fischer 344 and Sprague-Dawley rats [J].Toxicological Sciences,2002,65(1):151-159
[39] Yeh C M,Hsiao L J,Huang H J.Cadmium activates a mitogen-activated protein kinase gene and MBP kinases in rice [J].Plant and Cell Physiology,2004,45(9):1306-1312
[40] 陈梦妍,谢佳,田丽,等.ZKSCAN3介导的自噬在急性镉暴露肝毒性中的作用[J].局解手术学杂志,2020,29(12):944-949
Chen M Y,Xie J,Tian L,et al.Effect of ZKSCAN3 mediated autophagy in acute cadmium exposure-induced liver injury [J].Journal of Regional Anatomy and Operative Surgery,2020,29(12):944-949 (in Chinese)
[41] Sun X R,Su F M,Chen X L,et al.Doppler ultrasound and photoplethysmographic assessment for identifying pregnancy-induced hypertension [J].Experimental and Therapeutic Medicine,2020,19(3):1955-1960
[42] Affinati A H,Auchus R J.Endocrine causes of hypertension in pregnancy [J].Gland Surgery,2020,9(1):69-79
[43] Silva N S D,Araújo N K,Daniele-Silva A,et al.Antimicrobial activity of chitosan oligosaccharides with special attention to antiparasitic potential [J].Marine Drugs,2021,19(2):110
[44] Xie W M,Xu P X,Liu Q.Antioxidant activity of water-soluble chitosan derivatives [J].Bioorganic &Medicinal Chemistry Letters,2001,11(13):1699-1701
[45] Zhang X Y,Yang H B,Zheng J P,et al.Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice [J].Carbohydrate Polymers,2021,253:117218
[46] 许青松,宫德正,邹原,等.两种壳寡糖对急性肝损伤模型小鼠的保护作用[J].医药导报,2008,27(2):153-155
Xu Q S,Gong D Z,Zou Y,et al.Protective effect of two types of oligochitosans on CCl4-induced acute liver injury in mice [J].Herald of Medicine,2008,27(2):153-155 (in Chinese)
[47] Azuma K,Osaki T,Minami S,et al.Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides [J].Journal of Functional Biomaterials,2015,6(1):33-49
[48] 朱常龙,汪东风,孙继鹏,等.壳寡糖配合物对扇贝产品中镉的脱除作用[J].农产品加工:创新版,2010(7):10-13,20
Zhu C L,Wang D F,Sun J P,et al.The removal of cadmium from Chlamys ferrari by chitosan oligosaccharide complexes with Ca and Mg [J].Innovational Edition of Farm Products Processing,2010(7):10-13,20 (in Chinese)
[49] Liu J,Qu W,Kadiiska M B.Role of oxidative stress in cadmium toxicity and carcinogenesis [J].Toxicology and Applied Pharmacology,2009,238(3):209-214
[50] Fernandes J C,Eaton P,Nascimento H,et al.Antioxidant activity of chitooligosaccharides upon two biological systems:Erythrocytes and bacteriophages [J].Carbohydrate Polymers,2010,79(4):1101-1106
[51] Qiao Y,Bai X F,Du Y G.Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress [J].International Immunopharmacology,2011,11(1):121-127
[52] 翟星辰.壳寡糖免疫增强及对肾癌抑制作用的研究[D].哈尔滨:哈尔滨工业大学,2019:71-72
Zhai X C.Research on immune enhancement of chitosan oligosaccharides and its inhibitory effects against renal carcinoma [D].Harbin:Harbin Institute of Technology,2019:71-72 (in Chinese)
[53] 张梦龙,赵璧忱,邢菲菲,等.热休克蛋白27通过调节氧化应激影响奶牛胎衣不下发生机制研究[J].黑龙江八一农垦大学学报,2021,33(1):21-26,75
Zhang M L,Zhao B C,Xing F F,et al.Mechanism of retained fetal membranes in cow affected by HSP27 through regulating oxidative stress [J].Journal of Heilongjiang Bayi Agricultural University,2021,33(1):21-26,75 (in Chinese)
[54] Beiraghi-Toosi A,Askarian R,Sadrabadi Haghighi F,et al.Burn-induced oxidative stress and serum glutathione depletion;a cross sectional study [J].Emergency,2018,6(1):e54
[55] Copple I M,Goldring C E,Kitteringham N R,et al.The Keap1-Nrf2 Cellular Defense Pathway:Mechanisms of Regulation and Role in Protection against Drug-Induced Toxicity [M]//Uetrecht J.Handbook of Experimental Pharmacology.Springer,2010:233-266
[56] Wu K C,Liu J J,Klaassen C D.Nrf2 activation prevents cadmium-induced acute liver injury [J].Toxicology and Applied Pharmacology,2012,263(1):14-20
[57] Casalino E,Calzaretti G,Landriscina M,et al.The Nrf2 transcription factor contributes to the induction of alpha-class GST isoenzymes in liver of acute cadmium or manganese intoxicated rats:Comparison with the toxic effect on NAD(P)H:Quinone reductase [J].Toxicology,2007,237(1-3):24-34
[58] 李凯群.高胆固酵通过激活ROS介导的NF-кB通路抑制肌腱干细胞的腱系分化[D].广州:南方医科大学,2019:40-41
Li K Q.High cholesterol inhibits tenogenic differentiation in tendon-derived stem cells through ROS-activated NF-κB signaling [D].Guangzhou:Southern Medical University,2019:40-41 (in Chinese)
[59] Luo Z G,Dong X X,Ke Q,et al.Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation [J].Oncology Reports,2014,32(5):2215-2222
[60] Shaw P,Chattopadhyay A.Nrf2-ARE signaling in cellular protection:Mechanism of action and the regulatory mechanisms [J].Journal of Cellular Physiology,2020,235(4):3119-3130
[61] 林谦,邱磊,云龙,等.核因子E2相关因子2调控机体抗氧化途径特性及其与畜禽的健康和肉品质的关系[J].动物营养学报,2014,26(6):1421-1429
Lin Q,Qiu L,Yun L,et al.Nuclear factor erythroid-2-related factor 2 mediated antioxidant pathway character and its relation on health and meat quality of livestock and poultry [J].Chinese Journal of Animal Nutrition,2014,26(6):1421-1429 (in Chinese)