6-乙酰基-1,1,2,4,4,7-六甲基四氢萘(CAS:1506-02-1),又称吐纳麝香(tonalide,AHTN),是一种人工合成的半挥发性环状有机化合物,具有与天然麝香相似的气味,被广泛应用于个人护理品和空气清新剂等产品中[1-2]。AHTN具有与持久性有机污染物(persistent organic pollutants,POPs)类似的化学结构(图1),自然状态下很难进行化学和生物降解。目前,在大气[1-2]、室内灰尘[3]、水环境[4-7]、污泥和沉积物[5-6,8-9]等环境介质中均检测到AHTN的存在。AHTN具有亲脂性,很容易发生生物富集,目前已在鱼类[6,10-11]、贝类[12-14]、人体的母乳[9,15-16]、脂肪组织[15,17-19]和血液[20-21]中发现AHTN的蓄积。根据Hu等[21]的研究,AHTN在中国11个城市人群血液的中位浓度为0.53 ng·g-1,换算后约为10-8 mol·L-1浓度范围。
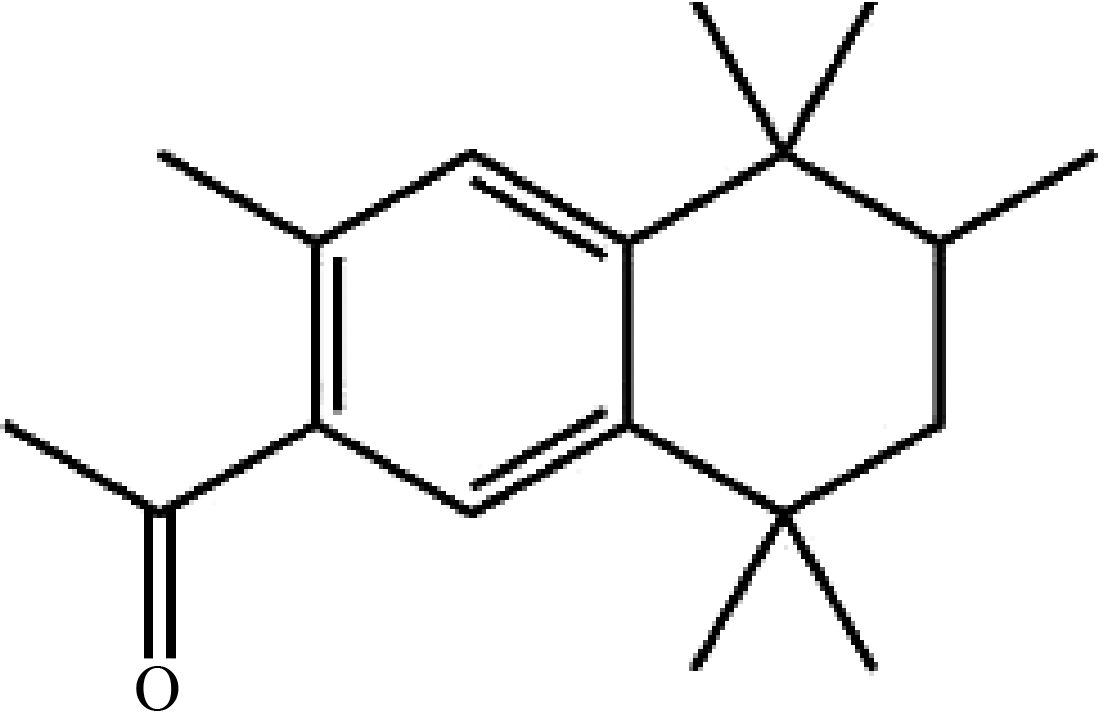
图1 吐纳麝香(AHTN)化学结构式
Fig. 1 The chemical structure of tonalide (AHTN)
对AHTN的毒性研究已有近30年的历史,主要集中在基础毒性[22]、内脏毒性[23]、酶活性毒性[24]、氧化损伤毒性[25]、神经毒性[26]以及内分泌干扰效应[27]等方面。研究显示,AHTN具有弱雌激素活性,且由雌激素受体(estrogen receptor,ER)介导[27-28]。Schreurs等[29]利用体外报告基因检测发现AHTN是弱的ERα激动剂和ERβ拮抗剂。Li等[30]对人体肾上腺皮质癌细胞H295R在AHTN暴露后类固醇激素及类固醇生成途径相关基因的表达进行了定量分析,发现AHTN主要通过抑制3βHSD2和CYP21来抑制孕酮和皮质醇的产生。此外,Schreurs等[31]发现0.01、0.1和1 μmol·L-1 AHTN暴露对斑马鱼的ER产生剂量依赖的拮抗作用,0.01 μmol·L-1 AHTN暴露会产生抗孕激素效应[32]。
AHTN的暴露途径为皮肤接触、食物摄入和吸入吸收[33]。其中,皮肤接触是AHTN进入人体最主要的途径[33-34]。研究表明,AHTN的皮肤暴露量约为5.96 μg·kg-1·d-1[34]。目前AHTN对皮肤的毒性研究非常有限,据香料材料研究所(Research Institute for Fragrance Materials,RIFM)的报告显示,AHTN对大鼠和家兔急性皮肤毒性的LD50分别为7.94 g·kg-1和>5 g·kg-1 [35]。鉴于富含人工麝香的日化品引起皮肤过敏的案例时有报道,有必要开展其对人体皮肤效应的深入研究。
细胞间隙连接通讯(gap junction intercellular communication,GJIC)是调节细胞增殖、迁移、凋亡、致癌作用、信号转导、炎症及免疫反应的重要生物学机制,是近年来皮肤毒性的重要研究内容。GJIC通道由连接蛋白(Connexin,Cx)构成,Cx的表达或磷酸化状态的改变,会引起多种皮肤疾病[36]。Cx26和Cx43是人角质形成细胞间隙连接的主要成分,Cx26表达的改变会增加皮肤癌的易感性,Cx43是一种预防皮肤癌发病的保护因子[36]。雌激素可以通过ER介导的基因组[37]和非基因组机制[38]调节Cx在细胞中的表达、分布和功能状态,进而影响细胞的GJIC功能。其中,基因组机制主要调节Cx的基因转录和蛋白表达[37],非基因组机制(如MAPK/ERK和PI3K/AKT信号通路等)主要调节Cx的磷酸化状态、空间结构及降解等[38]。
本研究采用人永生化角质形成细胞HaCaT,探究环境相关浓度AHTN对皮肤细胞GJIC功能的影响及可能的分子机制,为客观评价AHTN的生物毒性和致癌风险提供依据。
1 材料与方法(Materials and methods)
1.1 化学药品和试剂
分析纯AHTN购自上海源叶,雌二醇(E2)、二甲基亚砜(DMSO)和荧光黄染料购自Sigma,ERα抑制剂ICI182780购自MCE。细胞培养用DMEM培养基购自Gibco;特级胎牛血清购自万泽;青链霉素混合液和胰蛋白酶-EDTA消化液购自Solarbio。分子生物学试剂均购自博瑞德,IP裂解液、PMSF和BCA蛋白浓度试剂盒购自碧云天;氯仿、异丙醇等化学试剂均购自上海生工,所用抗体购于江苏亲科和武汉三鹰公司。
1.2 细胞培养
人永生化角质形成细胞HaCaT由大连海事大学环境系统生物学研究所提供,采用10% FBS、1%链霉素和青霉素及88% DMEM高糖培养基,置于37 ℃、5% CO2的Thermo 3110细胞培养箱(赛默飞,美国)进行培养。
1.3 暴露处理
采用0.1% DMSO为助溶剂,分别配制10-3 mol·L-1 AHTN和10-2 mol·L-1 E2母液,超净台中使用0.22 μm的滤膜过滤器抽滤,再用DMSO逐级稀释为10-4~10-7 mol·L-1 AHTN和10-6 mol·L-1 E2工作液。工作液与细胞培养基按1∶1 000的比例稀释后用于暴露处理,即AHTN的暴露浓度为10-7~10-10 mol·L-1,10-9 mol·L-1 E2为阳性对照,0.1% DMSO为空白对照。每个处理组均6个平行重复。
1.4 细胞活性检测
MTT法测定细胞存活率。吸出培养基,PBS溶液清洗3遍,加入150 μL配好的培养基和50 μL 1×MTT溶液,37 ℃培养箱中孵育4 h后,弃上清。加入150 μL DMSO溶液溶解甲瓒,用Molecular Devices SpectraMax M5酶标仪(Molecular Devices,美国)测定490 nm波长下各孔的光吸收,计算细胞存活率。细胞存活率=(暴露孔OD/对照孔OD)×100%。
1.5 GJIC功能检测
细胞划痕标记染料示踪技术(scrape-loading and dye transfer,SLDT)检测GJIC功能。吸出培养基,PBS溶液清洗3遍,加入质量浓度为0.05%荧光黄染料1 mL。用锐利手术刀片轻划3次,放入37 ℃培养箱避光标记10 min。吸出荧光黄染料,PBS冲洗细胞3次,去除游离荧光染料及脱落的细胞。加入4%多聚甲醛溶液1 mL,室温固定10 min,弃去固定液。每孔加入1 mL PBS溶液,倒置荧光显微镜(Nikon TE2000E)下观察并拍照(蓝色激发波长为470 nm,绿色发射波长为505 nm)。荧光图片采用Photoshop软件进行反相处理,Image J软件进行荧光强度数字化。
1.6 实时荧光定量PCR检测
Trizol法提取总RNA,反转为cDNA,采用SYBR Green为DNA结合染料,实时荧光定量PCR(RT-qPCR)法检测相关基因的表达。引物设计使用Primer Express(ABI)进行(表1),Gapdh为内参基因。预实验确定引物特异性及扩增效率。RT-qPCR使用ABI7300进行,反应条件为95 ℃预变性10 min,95 ℃变性15 s,60 ℃退火延伸1 min,40个循环。获得的Ct值采用2-△△Ct法进行分析。
表1 实时荧光定量PCR引物序列
Table 1 Sequences of qRT-PCR primers
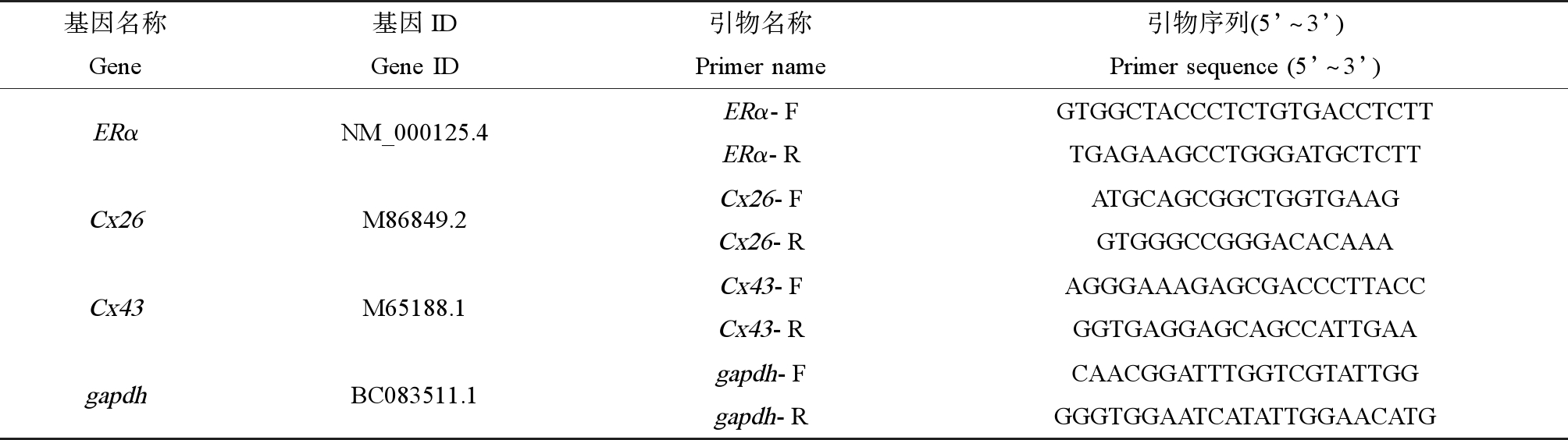
基因名称Gene基因IDGene ID引物名称Primer name引物序列(5’~3’)Primer sequence (5’~3’)ERαNM_000125.4ERα-FERα-RGTGGCTACCCTCTGTGACCTCTTTGAGAAGCCTGGGATGCTCTTCx26M86849.2Cx26-FCx26-RATGCAGCGGCTGGTGAAGGTGGGCCGGGACACAAACx43M65188.1Cx43-FCx43-RAGGGAAAGAGCGACCCTTACCGGTGAGGAGCAGCCATTGAAgapdhBC083511.1gapdh-Fgapdh-RCAACGGATTTGGTCGTATTGGGGGTGGAATCATATTGGAACATG
1.7 蛋白质印迹法
蛋白定量分析采用蛋白质印迹(Western blot)法。提取总蛋白,Bradford法进行蛋白定量。Bio-Rad 164-5056电泳仪进行SDS-PAGE,分离胶浓度为10%。转膜、封闭后,与一抗杂交过夜。一抗浓度分别:GAPDH(1∶000),PI3K和AKT(1∶2000),Cx43、p-AKT和p-Cx43(1∶1000),ERα、p-PI3K、MEK和ERK(1∶500),Cx26、p-MEK和p-ERK(1∶250)。TBST清洗后,分别与二抗(1∶2000)杂交1 h。清洗及发光显色后,采用UVP GelDoc-It 310凝胶成像系统进行图像采集,Image J软件进行条带的灰度分析。
1.8 统计学分析
所有实验至少进行3次生物学重复,数据以mean±SD表示。统计分析采用T-TEST进行,*P≤0.05、**P≤0.01,具有“显著性”差异。GraphPad Prism 8软件绘制图片。
2 结果(Results)
2.1 AHTN暴露对HaCaT细胞活性的影响
与DMSO对照组相比,10-8 mol·L-1 AHTN暴露24 h和10-9 mol·L-1 AHTN暴露48 h均可显著促进HaCaT细胞的增殖(P≤0.01),细胞存活率分别增加12%和13%(图2),增殖效率略逊于10-9 mol·L-1 E2阳性对照组。而10-6 mol·L-1 AHTN暴露48 h显著抑制HaCaT细胞的增殖(P≤0.01),细胞存活率较对照组降低10%(图2(b))。
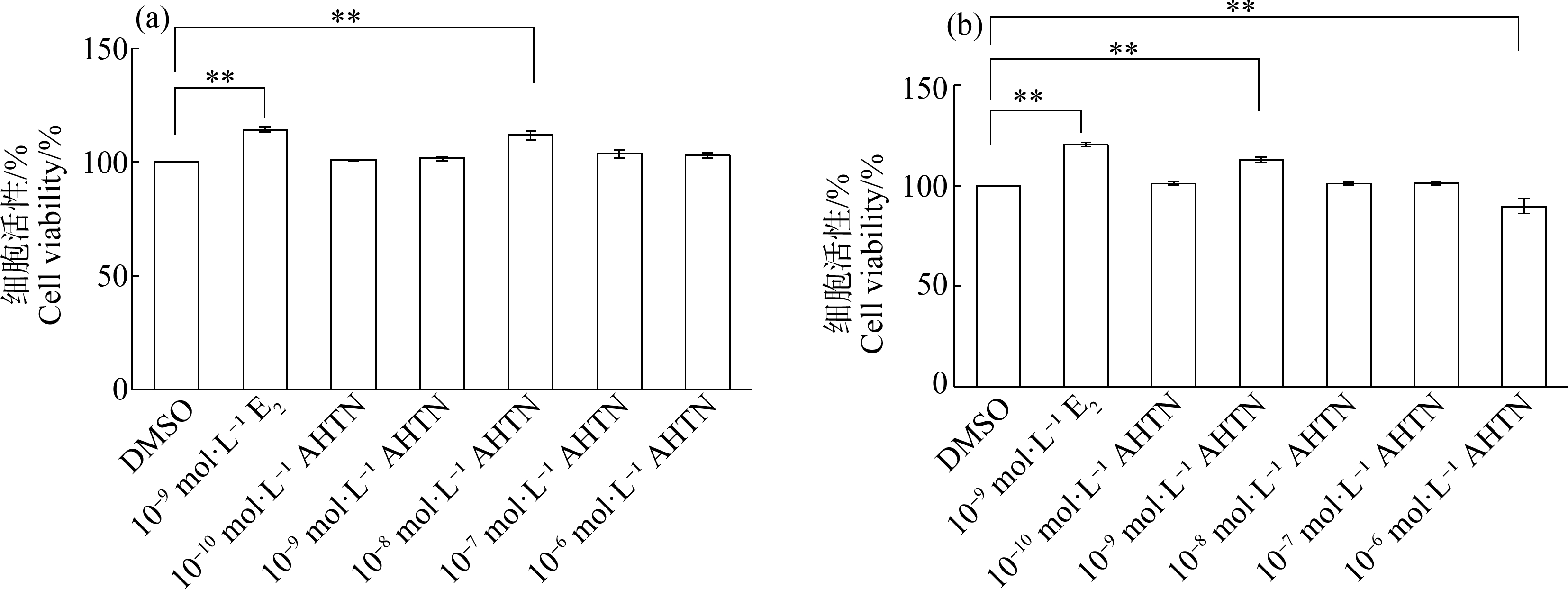
图2 AHTN暴露对HaCaT细胞活性的影响
注:DMSO表示二甲基亚砜,E2表示雌二醇,AHTN表示吐纳麝香;(a)暴露24 h,(b)暴露48 h;**表示P≤0.01。
Fig.2 Effects of AHTN exposure on cell viability in HaCaT
Note:DMSO stands for dimethyl sulfoxide,E2 stands for estradiol,and AHTN stands for tonalide;(a) 24 h exposure,(b) 48 h exposure;**represents P≤0.01.
2.2 AHTN暴露对HaCaT细胞GJIC功能的影响
SLDT检测结果显示,10-8 mol·L-1 AHTN暴露24 h,荧光强度较DMSO对照组上调8%(图3(a)和(b)),10-9 mol·L-1和10-8 mol·L-1 AHTN暴露48 h,荧光强度较对照组分别减弱13%和9%(图3(c)和(d));而10-9 mol·L-1 E2暴露均会导致荧光强度增强,24 h增强14%(图3(a)和(b)),48 h增强6%(图3(c)和(d))。由此可见,10-8 mol·L-1 AHTN对HaCaT细胞GJIC功能的影响与10-9 mol·L-1 E2类似,均随着暴露时间的延长,荧光强度逐渐降低。
对荧光染料的传输范围进行分析发现,与对照组相比,10-8 mol·L-1 AHTN暴露24 h荧光染料传输了一层细胞(图3(a)),其余组均没有明显的传输现象;而10-9 mol·L-1 E2阳性对照组在24 h和48 h均发现荧光染料传输了一层细胞(图3(a)和(c))。
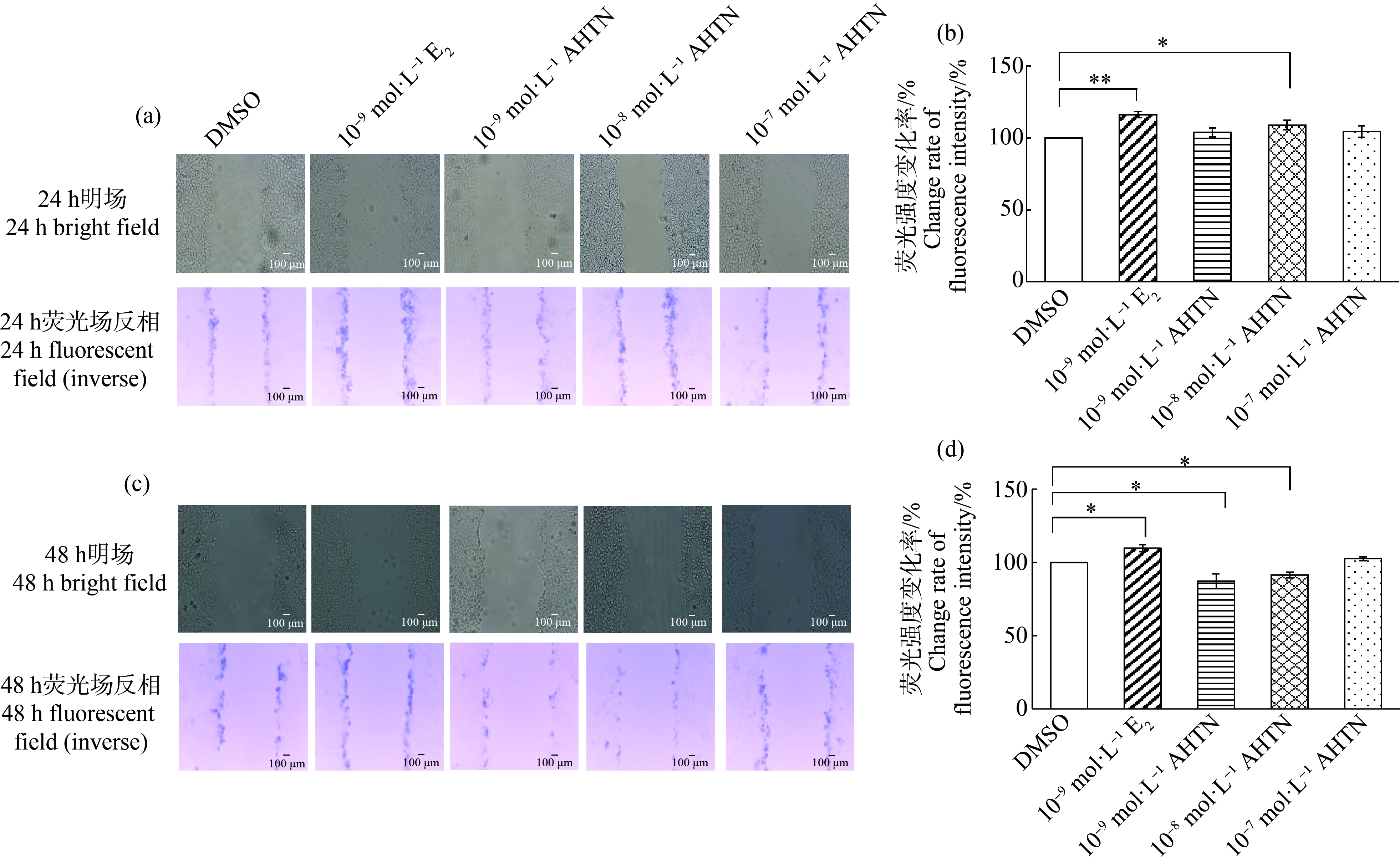
图3 AHTN暴露对HaCaT细胞的细胞间隙连接通讯(GJIC)功能的影响
注:(a) 暴露24 h后显微成像图(bar=100 μm),(b) 暴露24 h后荧光强度变化率,(c) 暴露48 h后显微成像图(bar=100 μm),(d) 暴露48 h后荧光强度变化率;*表示P≤0.05,**表示P≤0.01。
Fig.3 Effects of AHTN exposure on gap junction intercellular communication (GJIC) function in HaCaT
Note:(a) Microscopic images of 24 h exposure (bar=100 μm),(b) Change rate of fluorescence intensity after 24 h exposure,(c) Microscopic images of 48 h exposure (bar=100 μm),(d) Change rate of fluorescence intensity after 48 h exposure;*represents P≤0.05,and **represents P≤0.01.
2.3 AHTN通过ERα介导的基因组途径影响HaCaT细胞的GJIC功能
分别采用RT-qPCR和Western blot,检测10-8 mol·L-1和10-9 mol·L-1 AHTN暴露对ERα及其下游连接蛋白(Cx26和Cx43)表达的影响。结果表明,10-8 mol·L-1 AHTN暴露24 h,ERα、Cx26和Cx43的基因和蛋白水平均发生显著性上调(图4(a)和(c))。但暴露时间延长至48 h后,10-9 mol·L-1和10-8 mol·L-1 AHTN暴露仅显著上调ERα的基因和蛋白水平(图4(b)和(d)),而Cx26和Cx43的基因和蛋白水平均发生显著性下调(图4(b)和(d))。阳性对照10-9 mol·L-1 E2暴露则始终会导致ERα、Cx26和Cx43基因和蛋白水平的显著上调(图4)。

图4 AHTN暴露对HaCaT细胞ERα及相关Cx表达的影响
注:(a)暴露24 h基因表达情况,(b)暴露48 h基因表达情况,(c)暴露24 h蛋白表达情况,(d)暴露48 h蛋白表达情况;*表示P≤0.05,**表示P≤0.01。
Fig.4 Expression of ERα and related connexins in HaCaT cells after exposure to AHTN
Note:(a) Gene expression after 24 h exposure,(b) Gene expression after 48 h exposure,(c) Protein expression after 24 h exposure,(d) Protein expression after 48 h exposure;*represents P≤0.05,and **represents P≤0.01.
为验证ERα在AHTN对HaCaT细胞GJIC功能影响中的作用,加入ERα抑制剂ICIl82780重新进行SLDT实验。结果发现,10-8 mol·L-1 AHTN与抑制剂联合暴露24 h,荧光强度较未加抑制剂组下降10%(图5(a));10-9 mol·L-1和10-8 mol·L-1 AHTN与抑制剂联合暴露48 h,荧光强度较未加抑制剂组分别降低10%和16%(图5(b))。而抑制剂的加入会导致10-9 mol·L-1 E2的荧光强度大幅降低,24 h下降10%(图5(a)),48 h下降18%(图5(b))。
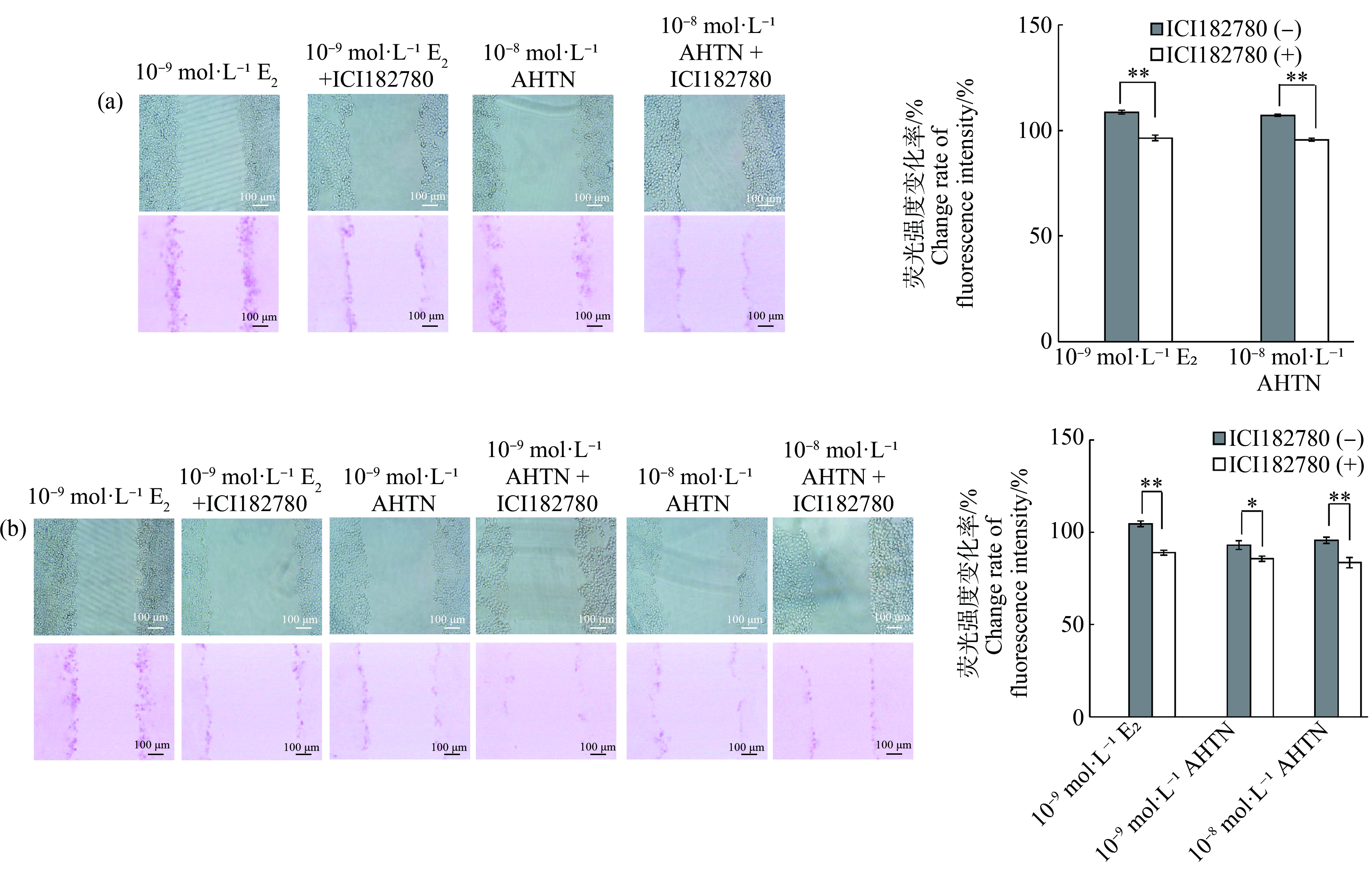
图5 ERα抑制剂对HaCaT细胞GJIC功能的影响
注:(a)暴露24 h,(b)暴露48 h;bar=100 μm;*表示P≤0.05,**表示P≤0.01。
Fig.5 Effects of ERα inhibitor on GJIC function in HaCaT
Note:(a) 24 h exposure,(b) 48 h exposure;bar=100 μm;*represents P≤0.05,and **represents P≤0.01.
2.4 AHTN通过激活MAPK/ERK和PI3K/AKT信号通路影响HaCaT细胞的GJIC功能
为探究AHTN对HaCaT细胞GJIC功能影响的其他分子机制,选取与GJIC调控相关的2条重要信号传导通路,对其级联分子进行定量分析。结果表明,10-8 mol·L-1 AHTN暴露24 h和48 h,以及10-9 mol·L-1 AHTN暴露48 h,对MAPK/ERK信号通路中级联分子MEK和ERK的含量无显著性影响,但均可导致其磷酸化水平(p-MEK和p-ERK)的显著上调(图6(a)和(b))。对PI3K/AKT信号通路的分析则表明,上述AHTN暴露既能导致级联分子PI3K和AKT含量的增加,也会导致二者磷酸化水平(p-PI3K和p-AKT)的显著上调(图6(c)和(d))。Cx43作为2条通路下游的靶分子,其磷酸化水平(p-Cx43)在上述AHTN暴露后均发生显著性上调(图6)。
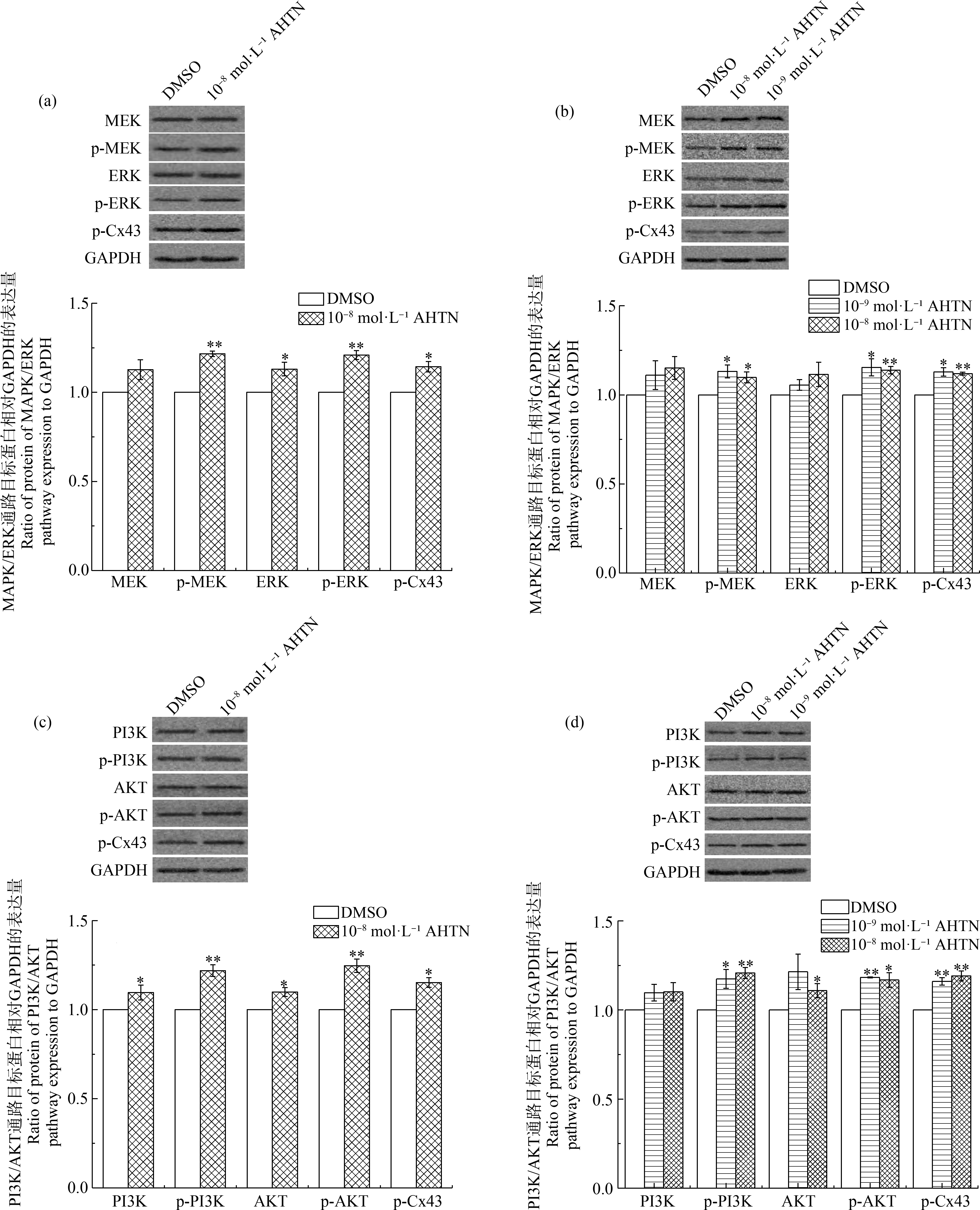
图6 AHTN暴露对HaCaT细胞MAPK/ERK和PI3K/AKT信号通路的影响
注:(a)暴露24 h对MAPK/ERK通路的检测,(b)暴露48 h对MAPK/ERK通路的检测,(c)暴露24 h对PI3K/AKT通路的检测,(d)暴露48 h对PI3K/AKT通路的检测;*表示P≤0.05,**表示P≤0.01。
Fig.6 Effects of AHTN exposure on MAPK/ERK and PI3K/AKT pathways in HaCaT
Note:(a) Analysis of MAPK/ERK pathway after 24 h exposure,(b) Analysis of MAPK/ERK pathway after 48 h exposure,(c) Analysis of PI3K/AKT pathway after 24 h exposure,(d) Analysis of PI3K/AKT pathway after 48 h exposure;*represents P≤0.05,and **represents P≤0.01.
3 讨论(Discussion)
AHTN在中国城市人群血液的中位浓度为0.53 ng·g-1[21],中国台湾地区AHTN的皮肤暴露量约为5.96 μg·kg-1·d-1[34],据此,我们推算AHTN在表皮中的暴露剂量在10-8 mol·L-1数量级。本文探究了10-10~10-7 mol·L-1 AHTN对人永生化角质形成细胞HaCaT的影响。结果表明,与许多天然激素和环境内分泌干扰物类似[39],AHTN表现出低剂量刺激、高剂量抑制细胞活性的特点(图2)。现有研究表明,AHTN对不同细胞内分泌干扰的浓度范围与效应不同。10-5 mol·L-1 AHTN暴露可导致人乳腺癌MCF-7细胞增殖[27],0.25、2.5和25 μmol·L-1 AHTN暴露会引起人肾上腺皮质癌细胞系H295R的增殖[30]。本研究使用的HaCaT细胞,与正常皮肤角质形成细胞特性类似,可无限传代,不具有肿瘤特性[40]。本研究结果表明环境相关浓度的AHTN暴露可导致HaCaT细胞活性的改变,且具有复杂的剂量-效应关系。
GJIC功能对细胞具有重要的生理作用,其功能异常会诱发癌症,皮肤瘢痕癌的发生发展与GJIC功能的变化相关[41]。SLDT是检测GJIC功能最常用的方法之一,具有实验周期短、设备需求少等特点,被广泛应用于体外毒性研究中。在本研究中,环境相关浓度(10-8 mol·L-1)AHTN暴露24 h和48 h出现相反的变化趋势。为避免因误差所致,我们进行了3次重复实验,并分别采用荧光成像、荧光强度分析和荧光染料传输范围3种不同的表征方法,均证明10-8 mol·L-1 AHTN暴露24 h,导致GJIC功能短暂性增强,暴露48 h后,GJIC功能逐渐受到抑制(图3)。上述结果与AHTN对HaCaT细胞活性的影响趋势(图2)相同。与本研究结果类似,Zhang等[42]在双酚A的研究中发现,暴露48 h可抑制HaCaT细胞的GJIC功能,而24 h未检测到显著性改变。
GJIC功能受多种因素调控。研究表明,雌激素可通过ER介导的基因组[37]和非基因组机制[38]调节Cx在细胞中的表达、分布和功能状态,进而影响细胞的GJIC功能。
为探究AHTN对HaCaT细胞GJIC功能影响的分子机制,首先对10-8 mol·L-1和10-9 mol·L-1 AHTN暴露后的ERα进行了定量分析,结果发现ERα基因和蛋白的表达均发生显著上调(图4),而加入ERα抑制剂后,GJIC功能受到不同程度的抑制(图5),说明AHTN对HaCaT细胞GJIC功能的影响是由ERα介导的。对ERα介导基因组途径中的下游分子(连接蛋白Cx26和Cx43)进行定量分析发现,10-8 mol·L-1 AHTN暴露48 h,二者的基因和蛋白水平均发生显著性下调(图4(b)和(d)),且与该浓度下GJIC功能受到抑制(图3(b))的效应吻合。后续可以对加入ERα抑制剂后Cx26和Cx43的表达情况进行定量分析,为AHTN通过ERα介导的基因组途径影响HaCaT细胞GJIC功能提供更可靠的科学证据。
另外,Cx26和Cx43作为人角质形成细胞间隙连接的主要成分,与GJIC功能直接相关,常被用作各类皮肤癌症发生和发展的生物标志物。在皮肤瘢痕癌中,Cx26的mRNA表达下调,GJIC功能减弱[43];在黑色素瘤中,Cx26、Cx43表达下调导致GJIC功能减弱,诱发癌症的发生[44]。本研究发现10-8 mol·L-1 AHTN暴露48 h,Cx26和Cx43表达下调(图4(b)和(d)),提示AHTN暴露可能具有潜在诱发皮肤癌的风险。虽然目前尚缺乏AHTN暴露与皮肤癌发生相关性的流行病学调查数据,但有学者采用实验与理论计算相结合的研究发现,AHTN可以作为光敏化剂,诱导人体氨基酸发生光敏化氧化,并且猜测其可能像其他光敏化剂一样,导致生物分子损伤,引起细胞凋亡或坏死,影响信号通路,甚至诱发皮肤癌的发生[45]。
在GJIC功能调控的非基因组机制中,MAPK/ERK和PI3K/AKT信号通路可通过调节下游Cx的磷酸化状态、空间结构及降解等发挥作用。Rivedal和Opsahl[46]发现12-O-十四烷酰佛波醇-13-醋酸酯(12-O-tetradecanoylphorbol-13-acetate,TPA)暴露处理大鼠肝上皮细胞WB-F344,可激活ERK,提高Cx43的磷酸化水平,降低GJIC功能。Klotz等[47]发现维生素K3(甲萘醌)可通过激活MAPK激酶,导致Cx43磷酸化增加,从而降低WB-F344细胞的GJIC。Lee等[48]发现褪黑素可显著增加Cx26和Cx43的表达,下调ERK的磷酸化水平,促进H2O2处理后HaCaT细胞的GJIC功能。本研究发现10-8 mol·L-1和10-9 mol·L-1 AHTN暴露可上调级联分子MEK、ERK、PI3K和AKT的磷酸化水平(图6),激活MAPK/ERK和PI3K/AKT信号通路,进而上调Cx43磷酸化水平,抑制GJIC功能。
综上所述,本研究通过体外实验,证明了环境相关浓度AHTN对人皮肤细胞活性及GJIC的影响,并对其可能的分子机制进行了分析。本研究发现AHTN一方面可以上调ERα的表达,通过ERα介导的基因组途径,改变下游GJIC标志物Cx26和Cx43的表达,发挥弱的类雌激素效应;另一方面,AHTN可通过上调级联分子MEK、ERK、PI3K和AKT的磷酸化水平,激活MAPK/ERK和PI3K/AKT信号通路,提高下游Cx43的磷酸化水平,进而导致HaCaT细胞的GJIC功能受到抑制(图7)。本研究的结果将为客观评价AHTN的生物毒性和致癌风险提供了科学依据。

图7 AHTN对HaCaT细胞GJIC功能可能分子机制推测
Fig. 7 Putative mechanism of AHTN on GJIC function in HaCaT
[1] Kallenborn R,Gatermann R,Rimkus G G.Synthetic musks in environmental samples:Indicator compounds with relevant properties for environmental monitoring [J].Journal of Environmental Monitoring,1999,1(4):70N-74N
[2] Peck A M,Hornbuckle K C.Synthetic musk fragrances in urban and rural air of Iowa and the Great Lakes [J].Atmospheric Environment,2006,40(32):6101-6111
[3] Sofuoglu A,Kiymet N,Kavcar P,et al.Polycyclic and nitro musks in indoor air:A primary school classroom and a women’s sport center [J].Indoor Air,2010,20(6):515-522
[4] Zhou H D,Huang X,Gao M J,et al.Distribution and elimination of polycyclic musks in three sewage treatment plants of Beijing,China [J].Journal of Environmental Sciences (China),2009,21(5):561-567
[5] Zhang X L,Yao Y,Zeng X Y,et al.Synthetic musks in the aquatic environment and personal care products in Shanghai,China [J].Chemosphere,2008,72(10):1553-1558
[6] Hu Z J,Shi Y L,Cai Y Q.Concentrations,distribution,and bioaccumulation of synthetic musks in the Haihe River of China [J].Chemosphere,2011,84(11):1630-1635
[7] 高艳蓬,李桂英,马盛韬,等.合成麝香的研究新进展与当前挑战:从人体护理、环境污染到人体健康[J].化学进展,2017,29(9):1082-1092
Gao Y P,Li G Y,Ma S T,et al.Research progress and challenge of synthetic musks:From personal care,environment pollution to human health [J].Progress in Chemistry,2017,29(9):1082-1092 (in Chinese)
[8] Zeng X Y,Sheng G Y,Xiong Y,et al.Determination of polycyclic musks in sewage sludge from Guangdong,China using GC-EI-MS [J].Chemosphere,2005,60(6):817-823
[9] Zeng X Y,Mai B X,Sheng G Y,et al.Distribution of polycyclic musks in surface sediments from the Pearl River Delta and Macao Coastal Region,South China [J].Environmental Toxicology and Chemistry,2008,27(1):18-23
[10] Osemwengie L I,Gerstenberger S L.Levels of synthetic musk compounds in municipal wastewater for potential estimation of biota exposure in receiving waters [J].Journal of Environmental Monitoring,2004,6(6):533-539
[11] Saraiva M,Cavalheiro J,Lanceleur L,et al.Synthetic musk in seafood products from south Europe using a quick,easy,cheap,effective,rugged and safe extraction method [J].Food Chemistry,2016,200:330-335
[12] Shek W M,Murphy M B,Lam J C,et al.Polycyclic musks in green-lipped mussels (Perna viridis) from Hong Kong [J].Marine Pollution Bulletin,2008,57(6-12):373-380
[13] Vallecillos L,Pocurull E,Borrull F.Influence of pre-treatment process on matrix effect for the determination of musk fragrances in fish and mussel [J].Talanta,2015,134:690-698
[14] Ziarrusta H,Olivares M,Delgado A,et al.Multiscreening determination of organic pollutants in molluscs using matrix solid phase dispersion [J].Journal of Chromatography A,2015,1391:18-30
[15] Rimkus G G,Wolf M.Polycyclic musk fragrances in human adipose tissue and human milk [J].Chemosphere,1996,33(10):2033-2043
[16] Yin J,Wang H,Zhang J,et al.The occurrence of synthetic musks in human breast milk in Sichuan,China [J].Chemosphere,2012,87(9):1018-1023
[17] Müller S,Schmid P,Schlatter C.Occurrence of nitro and non-nitro benzenoid musk compounds in human adipose tissue [J].Chemosphere,1996,33(1):17-28
[18] Kannan K,Reiner J L,Yun S H,et al.Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States [J].Chemosphere,2005,61(5):693-700
[19] Moon H B,Lee D H,Lee Y S,et al.Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females [J].Chemosphere,2012,86(5):485-490
[20] Hutter H P,Wallner P,Moshammer H,et al.Blood concentrations of polycyclic musks in healthy young adults [J].Chemosphere,2005,59(4):487-492
[21] Hu Z J,Shi Y L,Niu H Y,et al.Occurrence of synthetic musk fragrances in human blood from 11 cities in China [J].Environmental Toxicology and Chemistry,2010,29(9):1877-1882
[22] Wollenberger L,Breitholtz M,Ole Kusk K,et al.Inhibition of larval development of the marine copepod Acartia tonsa by four synthetic musk substances [J].The Science of the Total Environment,2003,305(1-3):53-64
[23] Api A M,Ford R A.Evaluation of the oral subchronic toxicity of HHCB (1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran) in the rat [J].Toxicology Letters,1999,111(1-2):143-149
[24] Randelli E,Rossini V,Corsi I,et al.Effects of the polycyclic ketone tonalide (AHTN) on some cell viability parameters and transcription of P450 and immunoregulatory genes in rainbow trout RTG-2 cells [J].Toxicology in Vitro:An International Journal Published in Association with BIBRA,2011,25(8):1596-1602
[25] Parolini M,Magni S,Traversi I,et al.Environmentally relevant concentrations of galaxolide (HHCB) and tonalide (AHTN) induced oxidative and genetic damage in Dreissena polymorpha [J].Journal of Hazardous Materials,2015,285:1-10
[26] Ayuk-Takem L,Amissah F,Aguilar B J,et al.Inhibition of polyisoprenylated methylated protein methyl esterase by synthetic musks induces cell degeneration [J].Environmental Toxicology,2014,29(4):466-477
[27] Bitsch N,Dudas C,Körner W,et al.Estrogenic activity of musk fragrances detected by the E-screen assay using human MCF-7 cells [J].Archives of Environmental Contamination and Toxicology,2002,43(3):257-264
[28] van der Burg B,Schreurs R,van der Linden S,et al.Endocrine effects of polycyclic musks:Do we smell a rat?[J].International Journal of Andrology,2008,31(2):188-193
[29] Schreurs R H,Quaedackers M E,Seinen W,et al.Transcriptional activation of estrogen receptor ERalpha and ERbeta by polycyclic musks is cell type dependent [J].Toxicology and Applied Pharmacology,2002,183(1):1-9
[30] Li Z N,Yin N Y,Liu Q,et al.Effects of polycyclic musks HHCB and AHTN on steroidogenesis in H295R cells [J].Chemosphere,2013,90(3):1227-1235
[31] Schreurs R H,Legler J,Artola-Garicano E,et al.In vitro and in vivo antiestrogenic effects of polycyclic musks in zebrafish [J].Environmental Science &Technology,2004,38(4):997-1002
[32] Schreurs R H,Sonneveld E,Jansen J H,et al.Interaction of polycyclic musks and UV filters with the estrogen receptor (ER),androgen receptor (AR),and progesterone receptor (PR) in reporter gene bioassays [J].Toxicological Sciences:An Official Journal of the Society of Toxicology,2005,83(2):264-272
[33] Cadby P A,Troy W R,Vey M G H.Consumer exposure to fragrance ingredients:Providing estimates for safety evaluation [J].Regulatory Toxicology and Pharmacology,2002,36(3):246-252
[34] Tseng W J,Tsai S W.Assessment of dermal exposures for synthetic musks from personal care products in Taiwan [J].Science of the Total Environment,2019,669:160-167
[35] Research Institute for Fragrance Materials,Inc.(RIFM).1975.Acute toxicity studies on rats and rabbits [R].Woodcliff:RIFM,2020
[36] Richard G.Connexins:A connection with the skin [J].Experimental Dermatology,2000,9(2):77-96
[37] Ren J,Wang X H,Wang G C,et al.17β estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells [J].Bone,2013,53(2):587-596
[38] Chung T H,Wang S M,Liang J Y,et al.The interaction of estrogen receptor alpha and caveolin-3 regulates connexin43 phosphorylation in metabolic inhibition-treated rat cardiomyocytes [J].The International Journal of Biochemistry &Cell Biology,2009,41(11):2323-2333
[39] Vandenberg L N,Colborn T,Hayes T B,et al.Hormones and endocrine-disrupting chemicals:Low-dose effects and nonmonotonic dose responses [J].Endocrine Reviews,2012,33(3):378-455
[40] Boukamp P,Petrussevska R T,Breitkreutz D,et al.Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line [J].The Journal of Cell Biology,1988,106(3):761-771
[41] 王娟,郭瑞珍.皮肤瘢痕癌与细胞间隙连接通讯的研究现状[J].医学综述,2008,14(11):1617-1619
Wang J,Guo R Z.Current study of skin scar carcinoma and GJIC [J].Medical Recapitulate,2008,14(11):1617-1619 (in Chinese)
[42] Zhang Q,Wu S,Liu L,et al.Effects of bisphenol A on gap junctions in HaCaT cells as mediated by the estrogen receptor pathway [J].Journal of Applied Toxicology,2019,39(2):271-281
[43] 陈世玖,郭瑞珍,李纳.皮肤瘢痕上皮及瘢痕癌癌巢中Cx26 Cx26 mRNA的表达及意义[J].中国现代医学杂志,2012,22(5):6-9
Chen S J,Guo R Z,Li N.Expressions and significance of Cx26 and Cx26 mRNA in skin scar epithelium and cancer nestes of skin scar carcinoma [J].China Journal of Modern Medicine,2012,22(5):6-9 (in Chinese)
[44] Orellana V P,Tittarelli A,Retamal M A.Connexins in melanoma:Potential role of Cx46 in its aggressiveness [J].Pigment Cell &Melanoma Research,2021,34(5):853-868
[45] Fang H S,Gao Y P,Wang H H,et al.Photo-induced oxidative damage to dissolved free amino acids by the photosensitizer polycyclic musk tonalide:Transformation kinetics and mechanisms [J].Water Research,2017,115:339-346
[46] Rivedal E,Opsahl H.Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells [J].Carcinogenesis,2001,22(9):1543-1550
[47] Klotz L O,Patak P,Ale-Agha N,et al.2-methyl-1,4-naphthoquinone,vitamin K(3),decreases gap-junctional intercellular communication via activation of the epidermal growth factor receptor/extracellular signal-regulated kinase cascade [J].Cancer Research,2002,62(17):4922-4928
[48] Lee H J,Lee H J,Sohn E J,et al.Inhibition of connexin 26/43 and extracellular-regulated kinase protein plays a critical role in melatonin facilitated gap junctional intercellular communication in hydrogen peroxide-treated HaCaT keratinocyte cells [J].Evidence-Based Complementary and Alternative Medicine,2012,2012:589365