杀生剂在广义上指能有效控制或杀死细菌、真菌、病毒及藻类等微生物的一类具有广谱性质的化学制剂[1]。按化学功能基团划分[2],杀生剂可分为以下7类:(1)无机化合物,如银、汞和铜等重金属;(2)烃类、卤代烃和硝基化合物,如低毒的联苯用于水果的包装和储存;(3)醇类、酚类及其衍生物,如六氯苯用于化妆品;(4)醛类、酮类、有机酸及其衍生物,如甲醛用于仓库等的消毒;(5)胺类、胺盐和季铵盐类化合物,如苯扎氯铵等;(6)有机元素化合物,如有机汞、有机锡和有机砷等化合物;(7)杂环化合物,如添加于润滑剂的呋喃西林等。按用途划分,可分为抗菌剂、消毒剂和防腐剂[3]。其中,抗菌剂和消毒剂具有几乎相同的作用,即杀死或控制微生物的生命周期[4]。抗菌剂通常存在于化妆品、洗手液、洗发水、面霜、牙膏、漱口水及消毒湿巾等个人护理品中[3]。消毒剂广泛用于环境或物体表面清洁,如饮用水和游泳池水的氯化,医疗设备和空气加湿器及空调设备的有效消毒等[3]。此外,消毒剂还可抑制孢子生长繁殖[1]。防腐剂则用于制药和食品中,以防止微生物在这些产品中繁殖,也用于保存木材、皮革、塑料、涂层膜和纺织品。杀生剂类化合物固有的杀菌、消毒和防腐等属性使其对环境中土著微生物群落具有潜在风险[3-5]。
抗生素耐药性是全球关注的焦点[6-7]。这是一种微生物对用于治疗或预防的抗生素药物产生耐药性的现象,该现象妨碍临床疾病的治疗,导致广泛的公共卫生和经济挑战[8]。世界卫生组织将“抗生素耐药性”列为“2019年全球健康十大威胁”之一,2020年又提出将“对抗耐药性”列入“2021年需要追踪的10个全球健康问题之一”[9]。抗生素耐药快速传播的主要原因是人类医疗健康和畜牧业中抗生素的不合理使用和滥用。然而,越来越多的研究表明,除抗生素作为细菌耐药性的直接选择压力外,杀生剂、重金属等非抗生素物质对细菌耐药性的发展也发挥着共选择作用[10-12]。
近十几年来,杀生剂作为一类新型有机污染物得到人们广泛关注[13-15]。我国是人口和消费大国,杀生剂的使用量不可小觑,加之2019年新型冠状病毒肺炎的暴发,杀生剂的消费量更急剧上升[16]。虽然杀生剂的使用确实极大地降低了人类与许多传染病相关的发病率和死亡率,然而使用后的杀生剂会残留于空气、土壤和水环境等受纳环境中,其土著微生物群落受杀生剂胁迫,通过交叉选择和共同选择来发展抗性特征,从而引起环境中耐药性的发展和传播,危害生态系统的稳态[17]。尽管已有研究报道了杀生剂的作用机理,但关于杀生剂对细菌的耐受机制及对抗生素耐药性传播的影响机制仍然不够明了,本文结合杀生剂作用机制及其环境归趋,对杀生剂的抗生素耐药性影响机制研究展开进一步概述。
1 杀生剂的作用机制及其环境归趋(Mechanisms of biocide action and its environmental fate)
1.1 杀生剂使用现状及环境检出率
各类杀生剂充斥于人类的生活中,它们不仅限于医学领域,也作为日常消费品广泛使用,消费者几乎每天都接触含有杀生剂成分的产品[17-18]。在新冠疫情持续暴发的背景下,2020年全球表面消毒剂的销售额总计45亿美元,比2019年增长超过30%[19]。据估算,我国三氯生(triclosan,TCS)和氯咪巴唑(climbazole,CBZ)的年使用量分别高达100 t和345 t[20-21]。Data Bridge Market Research公司在一份全面的杀生剂行业市场研究报告中指出,2020—2027年间杀生剂市场预计将以5.0%年增长率增长,预计2027年将达到97.9亿美元[22]。
近年来,杀生剂的环境归趋得到广泛关注。杀生剂在水、土壤及空气等受纳环境中大量检出,甚至有研究在人体乳腺组织中检出尼泊金酯类防腐剂[23]。表1汇总了近年来不同国家从不同受纳环境中检出的典型杀生剂的浓度分布。已有大量调查研究发现杀生剂普遍存在于污泥农用土壤、地表水环境和沉积物、污水处理厂进出水口及其受纳河流等受纳环境中,且部分浓度可高达μg·L-1或μg·g-1级别[5,24-28]。
表1 全球各种受纳环境中检出的典型杀生剂的浓度分布
Table 1 Distribution of typical biocide concentrations in various receiving environment around the world
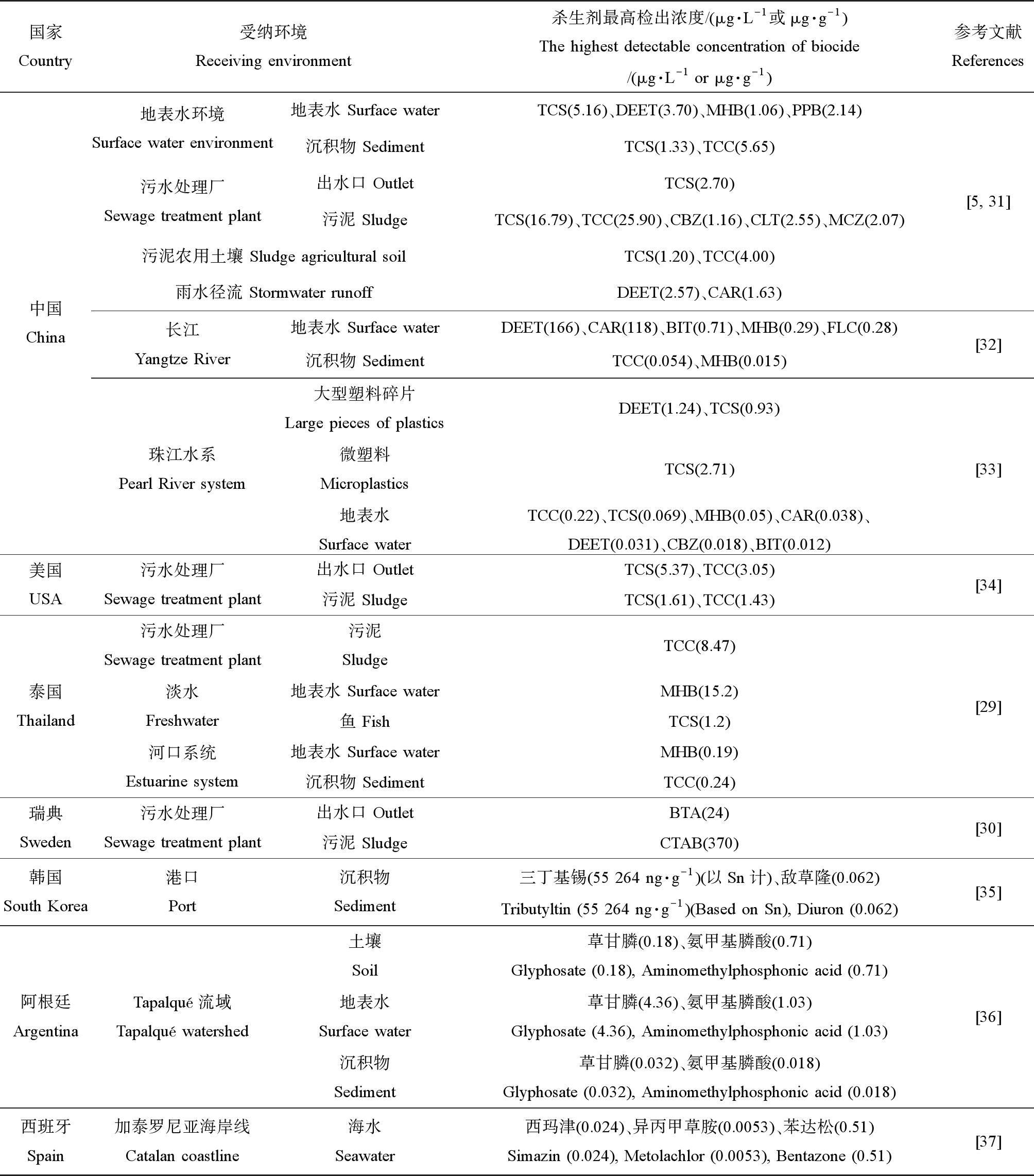
国家Country受纳环境Receiving environment杀生剂最高检出浓度/(μg·L-1或μg·g-1)The highest detectable concentration of biocide/(μg·L-1 or μg·g-1)参考文献References中国China地表水环境Surface water environment地表水 Surface waterTCS(5.16)、DEET(3.70)、MHB(1.06)、PPB(2.14)沉积物 SedimentTCS(1.33)、TCC(5.65)污水处理厂Sewage treatment plant出水口 OutletTCS(2.70)污泥 SludgeTCS(16.79)、TCC(25.90)、CBZ(1.16)、CLT(2.55)、MCZ(2.07)污泥农用土壤 Sludge agricultural soilTCS(1.20)、TCC(4.00)雨水径流 Stormwater runoffDEET(2.57)、CAR(1.63)长江Yangtze River地表水 Surface waterDEET(166)、CAR(118)、BIT(0.71)、MHB(0.29)、FLC(0.28)沉积物 SedimentTCC(0.054)、MHB(0.015)珠江水系Pearl River system大型塑料碎片Large pieces of plasticsDEET(1.24)、TCS(0.93)微塑料MicroplasticsTCS(2.71)地表水Surface waterTCC(0.22)、TCS(0.069)、MHB(0.05)、CAR(0.038)、DEET(0.031)、CBZ(0.018)、BIT(0.012)[5, 31][32][33]美国USA污水处理厂Sewage treatment plant出水口 OutletTCS(5.37)、TCC(3.05)污泥 SludgeTCS(1.61)、TCC(1.43)[34]泰国Thailand污水处理厂Sewage treatment plant淡水Freshwater河口系统Estuarine system污泥SludgeTCC(8.47)地表水 Surface waterMHB(15.2)鱼 FishTCS(1.2)地表水 Surface waterMHB(0.19)沉积物 SedimentTCC(0.24)[29]瑞典Sweden污水处理厂Sewage treatment plant出水口 OutletBTA(24)污泥 SludgeCTAB(370)[30]韩国South Korea港口Port沉积物Sediment三丁基锡(55 264 ng·g-1)(以Sn计)、敌草隆(0.062)Tributyltin (55 264 ng·g-1)(Based on Sn), Diuron (0.062)[35]阿根廷ArgentinaTapalqué流域Tapalqué watershed土壤Soil草甘膦(0.18)、氨甲基膦酸(0.71)Glyphosate (0.18), Aminomethylphosphonic acid (0.71)地表水Surface water草甘膦(4.36)、氨甲基膦酸(1.03)Glyphosate (4.36), Aminomethylphosphonic acid (1.03)沉积物Sediment草甘膦(0.032)、氨甲基膦酸(0.018)Glyphosate (0.032), Aminomethylphosphonic acid (0.018)[36]西班牙Spain加泰罗尼亚海岸线Catalan coastline海水Seawater西玛津(0.024)、异丙甲草胺(0.0053)、苯达松(0.51)Simazin (0.024), Metolachlor (0.0053), Bentazone (0.51)[37]
注:TCS为三氯生;DEET为避蚊胺;MHB为尼泊金甲酯;PPB为尼泊金丙酯;TCC为三氯卡班;CBZ为氯咪巴唑;CLT为克霉唑;MCZ为咪康唑;CAR为多菌灵;BIT为苯并异噻唑酮;FLC为氟康唑;BTA为苯并三唑;CTAB为十六烷基三甲基溴化铵;当受纳环境为沉积物、污泥、大型塑料碎片、微塑料、鱼和土壤时,相对应的杀生剂最高检出浓度单位为μg·g-1,其余为μg·L-1。
Note:TCS is triclosan;DEET is diethyltoluamide;MHB is methylparaben;PPB is propylparaben;TCC is triclocarban;CBZ is clomiprazole;CLT is clotrimazole;MCZ is miconazole;CAR is carbendazim;BIT is benzisothiazolone;FLC is fluconazole;BTA is benzotriazole;CTAB is cetyl trimethyl ammonium bromide;when the receiving environment was sediment,sludge,large pieces of plastics,microplastics,fish and soil,the corresponding highest detectable concentration of biocides was μg·g-1,and the rest were μg·L-1.
2018年有研究者调查了华南高度城市化地区水生环境中19种目标杀生剂的检出情况[5],河流系统中检测到目标杀生剂中的17种,在地表水中羟苯甲酯(methylparaben,MHB)、氯咪巴唑和避蚊胺(diethyltoluamide,DEET)的平均检出浓度相对较高,其中氯咪巴唑浓度高达276 ng·L-1。在惠州和东莞采集到的降雨径流样品中广泛检测到18种目标杀生剂,两地检出浓度较高的杀生剂都为避蚊胺和多菌灵(carbendazim,CAR)。对于惠州的降雨径流,避蚊胺和多菌灵的最高浓度均>300 ng·L-1;而对于东莞的降雨径流,二者最高浓度甚至高达1 629 ng·L-1和2 572 ng·L-1。2019年,在对泰国8个污水处理厂和受纳水环境(淡水和河口系统)中19种杀生剂的发生和归宿的研究结果表明,在污水和地表水中检测到羟苯甲酯的最大浓度为15.2 μg·L-1,污泥和底泥中三氯卡班(triclocarban,TCC)的最大浓度为8.47 μg·g-1,且在鱼样品中检测到三氯生的最大浓度为1.2 μg·g-1[29]。2017年,Östman等[30]在11个瑞典污水处理厂的进水口、出水口及消化污泥中检测到此前尚未报道过或报告数据非常有限的季铵化合物(quaternary ammonium compounds,QACs),例如氯己定(chlorhexidine,CHX)、氯化苄氧乙铵(benzoxyethylamine chloride,BEC)、氯化十六烷基吡啶(cetylpyridine chloride,CPC)和地喹氯铵(dequalinium chloride,DQC)。季铵化合物是颗粒相中含量最高的物质,其中十六烷基三甲基溴化铵(cetyl trimethyl ammonium bromide,CTAB)含量高达370 μg·g-1,而苯并三唑(benzotriazole,BTA)是水相中高检出率物质,含量高达24 μg·L-1。
1.2 杀生剂对细菌的作用机制
不同于具有特异性细胞靶标的抗生素,杀生剂的活性范围很广,在细菌细胞上通常具有多个非特异性靶位点[38-40]。许多学者对杀生剂的作用方式进行了广泛的综述和总结[41-42]。杀生剂的作用机制可根据其作用于微生物细胞的不同关键组成部分来确定,主要分为3个层次的作用机制:与细胞外部组分的相互作用、与细胞质膜的相互作用和与细胞质组分的相互作用(表2)[1]。某些杀生剂兼具2个或2个以上的作用机制。
表2 常见杀生剂细胞作用位点
Table 2 Targeted site of typical biocides in cell

作用位点Site of action常见杀生剂Common biocides参考文献References细胞外部组分Extracellular components阳离子膜活性剂(BAC、CHX)Cationic membrane active agent (BAC, CHX)[44-46]阴离子膜活性剂(SLS、SLES)Anionic membrane active agent (SLS, SLES)[1, 42, 48]氧化剂(次氯酸盐)Oxidizer (Hypochlorite)[1, 42]其他(苯酚、福尔马林、氯化汞)Others (Phenol, Formalin, Mercuric chloride)[1, 42, 47]细胞质膜: 膜裂解Cytoplasmic membrane: Membrane lysisQACs(溴代十六烷基三甲胺、SLS、十二烷基胍醋酸盐)QACs (Cetyltrimethyl bromide, SLS, Dodecylguanidine acetate)[42, 53-54]双胍类(CHX、PHMB)Biguanides (CHX, PHMB)[42, 45, 52, 55-56]强氧化剂(过氧化氢)Strong oxidants (Hydrogen peroxide)[57]醇类(乙醇、异丙醇、苯乙醇、苯氧乙醇)Alcohols (Ethanol, Isopropanol, Phenethyl alcohol, Phenoxyethanol)[42, 58]其他(有机酸、酯类、肉桂醛、PCMX)Others (Organic acids, Esters, Cinnamaldehyde, PCMX)[1, 59-60]细胞质膜: 质子动力耗散JCytoplasmic membrane: Proton dynamic dissipationPCP、CHX、二硝基苯酚、苯氧乙醇、BITPCP, CHX, Dinitrophenol, Phenoxyethanol, BIT[64-68]细胞质膜: 与其他酶系统Cytoplasmic membrane: Other enzymatic systemsFLC、六氯苯酚、乙醇、BIT、苯氧乙醇FLC, Hexachlorophenol, Ethanol, BIT, Phenoxyethanol[1, 42, 69]细胞质组分Cytoplasmic components核酸(结晶紫、吖啶、奎纳克林、环氧乙烷、甲醛、戊二醛、OPA、苯氧乙醇)Nucleic acids (Crystal violet, Acridine, Quinacrine, Ethylene oxide, Formaldehyde, Glutaraldehyde, OPA, Phenoxyethanol)[1, 74-76, 79, 87]核糖体(过氧化氢、BAC、乙醇)Ribosomes (Hydrogen peroxide, BAC, Ethanol)[66, 79]其他(TCS、CHX、环氧乙烷、过氧化氢、甲醛、戊二醛、OPA)Others (TCS, CHX, Ethylene oxide, Hydrogen peroxide, Formaldehyde, Glutaraldehyde, OPA)[1, 52, 79, 82-85]
注:BAC为苯扎氯铵;CHX为氯己定;SLS为十二烷基硫酸钠;SLES为十二烷基醚硫酸钠;QACs为季铵化合物;PHMB为聚六亚甲基双胍;PCMX为氯二甲苯酚;PCP为五氯苯酚;BIT为苯并异噻唑酮;FLC为氟康唑;OPA为邻苯二醛;TCS为三氯生。
Note:BAC is benzalkonium chloride;CHX is chlorhexidine;SLS is sodium lauryl sulfates;SLES is sodium lauryl ether sulfate;QACs is quaternary ammonium compounds;PHMB is polyhexamethylene biguanide;PCMX is p-chloro-xylenol;PCP is pentachlorophenol;BIT is benzisothiazolone;FLC is fluconazole;OPA is o-phthalaldehyde;TCS is triclosan.
1.2.1 与细胞外部组分相互作用
不同于革兰氏阳性菌细胞,革兰氏阴性菌因具有磷脂双分子层构成的内膜和细胞外部不对称的外膜组成的多层包膜结构,形成低渗透性保护[43]。一些阳离子表面活性杀生剂专门作用于这道渗透屏障。它们与细菌细胞的细胞外部组分相互作用,导致细胞疏水性的变化,但自身活力可能不受影响[1]。常用的苯扎氯铵(benzalkonium chloride,BAC)和CHX通过与革兰氏阴性菌细胞壁和外膜的负电荷相互作用,破坏并穿透其细胞壁和外膜,达到细胞质膜和细胞质内的靶位点,导致细胞失去渗透调节能力[44-46]。还有些杀生剂能诱导细菌的裂解或溶解。如次氯酸盐,除与细胞壁相互作用外,还可诱导革兰氏阴性菌裂解[1,42]。低浓度的苯酚、福尔马林和氯化汞也能导致正在生长的大肠杆菌(Escherichia coli)、链球菌(Streptococcus)和葡萄球菌(Staphylococcus)被迅速溶解[1,42,47]。高浓度的阴离子表面活性剂,如十二烷基硫酸钠(sodium lauryl sulfates,SLS)和十二烷基醚硫酸钠(sodium lauryl ether sulfate,SLES),也能裂解革兰氏阴性菌[1,42,48]。
1.2.2 与细胞质膜相互作用
细胞质膜常被认为是杀生剂的主要攻击靶点[45,49-51]。由于不同杀生剂的化学结构不同,其对细胞质膜产生的作用效果也各不相同。“膜活性剂”一词多用于抗菌药物,泛指在细菌细胞的细胞质膜水平上具有活性的化合物,如季铵化合物、双胍类、多尼泊金酯类、酚类和醇类等[1]。对膜的损伤大致分为以下3种形式:物理破坏、质子动力(PMF)的耗散和相关酶活性的抑制。
1.2.2.1 细胞质膜的裂解
细胞质膜的裂解通常表现为细胞内成分的渗漏,先是钾离子,后是无机磷酸盐、在260 nm处有吸收的氨基酸和物质、核酸及蛋白质[52]。这一类作用机制的杀生剂主要包括:季铵化合物、双胍类、强氧化剂和醇类等。
季铵化合物不仅作用于细胞外部成分,还会导致膜损伤,进而引起细胞内成分渗漏。阳离子表面活性剂CTAB在杀菌浓度下作用于细胞膜的脂质成分进而裂解细胞[42]。阴离子活性剂,例如低浓度SLS可诱导大肠杆菌原生质球的裂解[42,53];十二烷基胍醋酸盐也被证明在低浓度下能迅速渗透丁香假单胞菌(Pseudomonas syringae)的外膜和胞质膜,并与细胞磷脂和蛋白质结合,引起细胞内容物的渗漏,最终导致细胞裂解甚至细胞死亡[54]。
双胍类杀生剂中最常见的包括CHX和聚六亚甲基双胍(polyhexamethylene biguanide,PHMB)。CHX在低浓度时导致细胞内容物的高泄漏率[52]。CHX与细胞膜上相邻的2个酸性磷脂基团相结合,导致膜的渗透性降低,并引起膜及其相关酶的渗透调节和代谢能力以及转运系统功能的伴随改变[45,52]。PHMB在低浓度下具抑菌作用,而在高浓度时具杀菌作用[55]。其生物杀灭机制为作用于膜磷脂[42],随后非特异性破坏细胞膜内成分[55-56]。
强氧化剂中过氧化氢也能诱导膜损伤,其产生的自由基可作用于细胞内外的一系列靶点[3]。其靶细胞膜磷脂内的多不饱和酸被羟基自由基氧化,导致细胞裂解,随后释放的细胞成分被氧化[57]。醇类中的乙醇和异丙醇[58]、苯乙醇和苯氧乙醇[42]都是膜裂解剂,诱导细胞质膜功能的丧失。其他化合物如有机酸及其酯也可能导致细胞内成分的渗漏[1]。肉桂醛被证实显著增加大肠杆菌和金黄色葡萄球菌(S. aureus)的细胞膜通透性,导致细胞质膜与细胞壁分离、细胞壁和细胞膜裂解及细胞质内容物泄漏[59]。还有研究指出酚类杀生剂氯二甲苯酚(p-chloro-xylenol,PCMX)可能干扰细胞膜并导致细胞内容物渗漏[60]。
1.2.2.2 质子动力的耗散
质子动力指细菌建立并维持的一种跨越细胞质膜的质子电化学梯度[61]。质子动力参与细菌的ATP合成、氧化磷酸化、主动运输、细胞分裂及鞭毛运动等关键过程[62-63]。质子动力的崩溃直接造成这些功能途径被抑制,从而导致细菌活力的丧失。例如五氯苯酚(pentachlorophenol,PCP)通过强化细胞膜对质子的渗透性来解偶联氧化磷酸化,从而导致跨膜质子梯度的电势耗散[64]。有研究证实氯己定能抑制粪肠球菌(Enterococcus faecalis)与膜的结合并抑制可溶性ATP酶的活性[65-66]。二硝基苯酚已被证明在弱酸性环境下作为解偶联剂破坏线粒体中的电化学质子梯度,导致ATP合成的驱动力丧失[67]。低浓度苯氧乙醇诱导大肠杆菌的质子易位且抑制氧化磷酸化途径[68]。苯并异噻唑酮(benzoisothiazolone,BIT)通过破坏质子动力影响金黄色葡萄球菌中葡萄糖的主动转运和氧化、含硫醇酶、ATP酶及3-磷酸甘油醛脱氢酶的活性[65]。
1.2.2.3 与其他酶系统的相互作用
杀生剂还可与嵌于细胞质膜的蛋白质酶类相互作用而达到抑菌或杀菌效果。例如,三唑类药物氟康唑(fluconazole,FLC)通过作用于麦角甾醇途径的羊毛甾醇去甲基化酶,进而抑制真菌质膜上麦角甾醇的生物合成[69]。低浓度六氯苯酚抑制电子传递链参与酶类的膜结合部分[1]。乙醇对大肠杆菌参与糖酵解、脂肪酸和磷脂合成及溶质摄取过程中的酶有抑制作用[42]。苯氧乙醇有抑制细菌TCA循环酶的作用[70]。此外,一些杀生剂能与蛋白酶类活性的关键基团——硫醇基团反应或使其氧化而影响其活性,导致细胞被抑制或失活。如氯和释氧剂,其杀菌效果可能由一系列膜结合酶和胞内酶的硫醇(或其他基团)的氧化作用引起[71];又如广泛用作防腐剂的苯并异噻唑酮和异噻唑啉酮(methylisothiazolinone,MIT)[42],MIT作为亲电子试剂特异性作用于呼吸酶,抑制细菌新陈代谢并导致其死亡[72-73]。
1.2.3 与细胞质组分相互作用
细胞质中含有各种具有不同功能的酶、核酸、核糖体、蛋白质和脂质等组分,它们虽不是杀生剂的主要靶点,但仍可造成可逆或不可逆的细胞损伤。
1.2.3.1 与核酸的相互作用
作为抗菌染料的结晶紫和吖啶均被证明能与核酸相互作用。吖啶除与质子竞争细胞表面的阴离子位点外还能与胞内DNA分子结合[1,74];结晶紫能与大肠杆菌中的核酸分子发生络合反应[75]。有研究证实8×10-4 mol·L-1的抗疟药奎纳克林,通过阻断DNA合成并强烈抑制RNA和蛋白质的合成实现对大肠杆菌的杀菌作用[76]。邻苯二醛(o-phthalaldehyde,OPA)被指出会引起荧光假单胞菌的DNA损伤[77]。基于乙醇的杀生剂影响细胞壁形成的同时还抑制DNA和RNA的合成[78]。烷基化剂如环氧乙烷,与细菌蛋白质和核酸中的氨基、巯基和羟基相互作用[79]。苯氧乙醇通过抑制胸腺嘧啶、尿嘧啶和葡萄糖的同化作用进而抑制大肠杆菌DNA和RNA生物合成[70]。
1.2.3.2 与核糖体的相互作用
核糖体通常作为被杀生剂破坏的次要目标位点。过氧化氢形成的羟基自由基会攻击细胞成分(包括脂类和蛋白质),作用于核糖体并抑制细菌代谢[66,79]。在使用杀生剂根除石油和天然气作业中脱硫弧菌(Desulfovibrio vulgaris)的机理研究中,BAC被指出具有特异性靶向核糖体结构[80]。还有研究指出乙醇可能通过作用于核糖体和RNA聚合酶进而使mRNA和蛋白质合成解偶联[81]。
1.2.3.3 与其他细胞成分的相互作用
除核酸和核糖体外,杀生剂还会与细胞质其他成分发生反应。例如,TCS作用于细菌脂肪酸合成中的烯酰基-酰基载体蛋白还原酶[82-84]。CHX已被证明能引起细胞质凝固[52]。烷基化剂和氧化剂因具有高度活性而与细菌发生强烈反应。其中环氧乙烷作用于核酸的同时攻击其他细胞成分,包括蛋白质[79];过氧化氢作用于核糖体的同时氧化脂质、蛋白质和酶中的硫醇基团[1]。OPA通过与氨基酸的亲核中心反应进而促进蛋白质交联[77]。致死剂量下的MIT会影响蛋白质上的硫醇基团[73]。醛类杀生剂甲醛和戊二醛可与蛋白质或核酸的游离氨基发生交联,戊二醛主要作用于外膜的脂蛋白,阻止膜结合酶的释放[85-86]。
2 杀生剂耐药菌的形成和耐受机制(Emergence of biocide-resistant bacteria and its tolerant mechanisms)
2.1 不同环境中杀生剂耐受细菌的流行情况
细菌对杀生剂的耐受性早在20世纪50年代就被提及,随着杀生剂在各行各业的广泛使用,细菌杀生剂耐受性现象在食品加工、医疗和养殖等多种环境中普遍发生,耐药菌株检出率呈现明显增加(表3)。
表3 全球分离得到的杀生剂耐药菌株
Table 3 Isolated biocide-resistant strain around the world
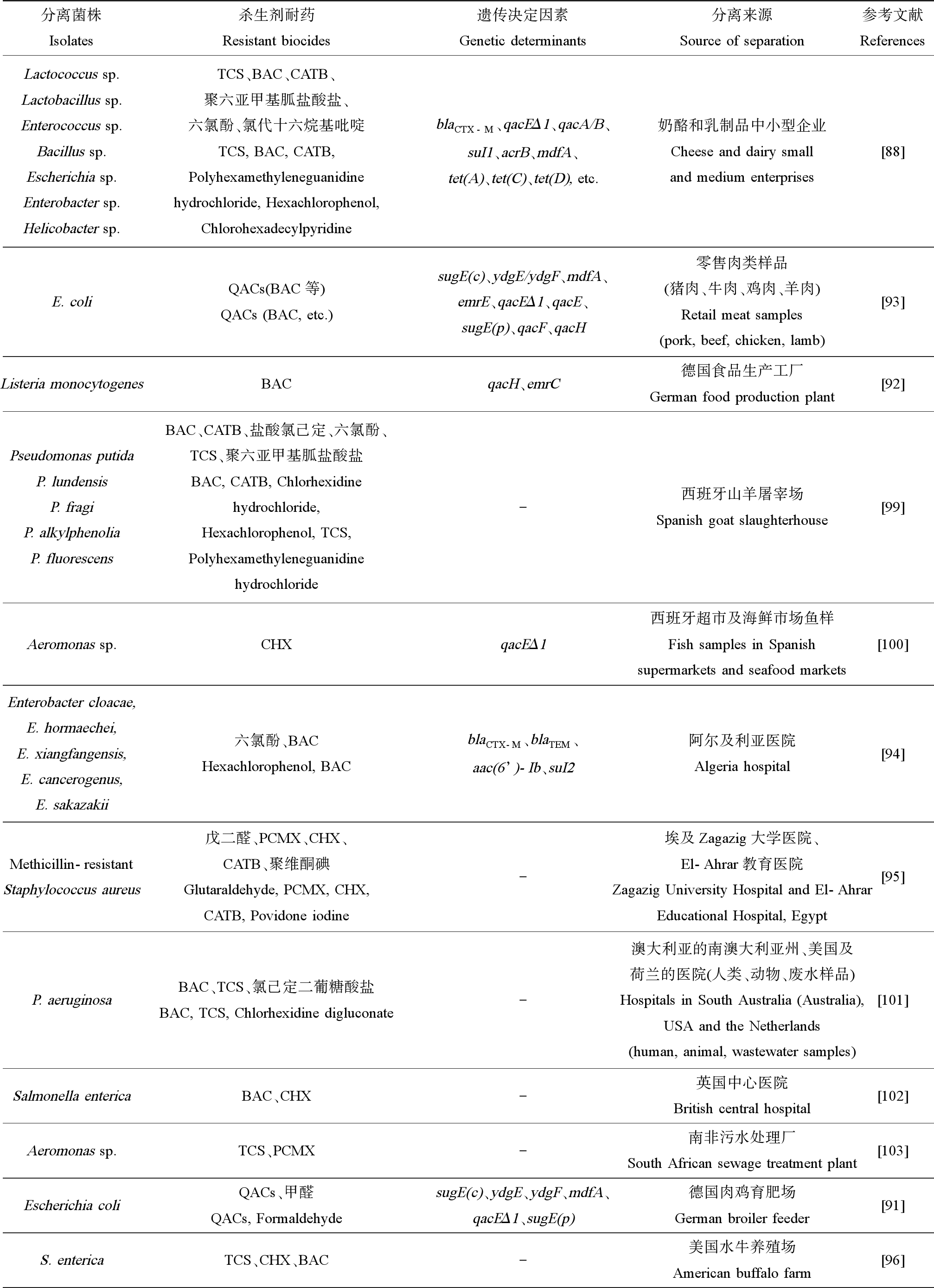
分离菌株Isolates杀生剂耐药Resistant biocides遗传决定因素Genetic determinants分离来源Source of separation参考文献ReferencesLactococcus sp. Lactobacillus sp. Enterococcus sp.Bacillus sp. Escherichia sp.Enterobacter sp.Helicobacter sp.TCS、BAC、CATB、聚六亚甲基胍盐酸盐、六氯酚、氯代十六烷基吡啶TCS, BAC, CATB, Polyhexamethyleneguanidine hydrochloride, Hexachlorophenol, ChlorohexadecylpyridineblaCTX-M、qacEΔ1、qacA/B、suI1、acrB、mdfA、tet(A)、tet(C)、tet(D), etc.奶酪和乳制品中小型企业Cheese and dairy small and medium enterprises[88]E. coliQACs(BAC等)QACs (BAC, etc.)sugE(c)、ydgE/ydgF、mdfA、emrE、qacEΔ1、qacE、sugE(p)、qacF、qacH零售肉类样品(猪肉、牛肉、鸡肉、羊肉)Retail meat samples (pork, beef, chicken, lamb)[93]Listeria monocytogenesBACqacH、emrC德国食品生产工厂German food production plant[92]Pseudomonas putidaP. lundensisP. fragiP. alkylphenoliaP. fluorescensBAC、CATB、盐酸氯己定、六氯酚、TCS、聚六亚甲基胍盐酸盐BAC, CATB, Chlorhexidine hydrochloride, Hexachlorophenol, TCS, Polyhexamethyleneguanidine hydrochloride-西班牙山羊屠宰场Spanish goat slaughterhouse[99]Aeromonas sp.CHXqacEΔ1西班牙超市及海鲜市场鱼样Fish samples in Spanish supermarkets and seafood markets[100]Enterobacter cloacae, E. hormaechei, E. xiangfangensis, E. cancerogenus, E. sakazakii六氯酚、BACHexachlorophenol, BACblaCTX-M、blaTEM、aac(6’)-Ib、suI2阿尔及利亚医院Algeria hospital[94]Methicillin-resistantStaphylococcus aureus戊二醛、PCMX、CHX、CATB、聚维酮碘Glutaraldehyde, PCMX, CHX, CATB, Povidone iodine-埃及Zagazig大学医院、El-Ahrar教育医院Zagazig University Hospital and El-Ahrar Educational Hospital, Egypt[95]P. aeruginosaBAC、TCS、氯己定二葡糖酸盐BAC, TCS, Chlorhexidine digluconate-澳大利亚的南澳大利亚州、美国及荷兰的医院(人类、动物、废水样品)Hospitals in South Australia (Australia), USA and the Netherlands (human, animal, wastewater samples)[101]Salmonella entericaBAC、CHX-英国中心医院British central hospital[102]Aeromonas sp.TCS、PCMX-南非污水处理厂South African sewage treatment plant[103]Escherichia coliQACs、甲醛QACs, FormaldehydesugE(c)、ydgE、ydgF、mdfA、qacEΔ1、sugE(p)德国肉鸡育肥场German broiler feeder[91]S. entericaTCS、CHX、BAC-美国水牛养殖场American buffalo farm[96]

续表3分离菌株Isolates杀生剂耐药Resistant biocides遗传决定因素Genetic determinants分离来源Source of separation参考文献ReferencesMRSA CC30BAC、过氧化氢、甲醛、次氯酸钠、氢氧化钠BAC, Hydrogen peroxide, Formaldehyde, Sodium hypochlorite, Sodium hydroxideqacG、qacC丹麦猪Denmark pig[97]Campylobacter jejuniTCS、CHX-美国肉鸡鸡舍垫料The litter of American broiler chicken houses[98]
注:TCS为三氯生;BAC为苯扎氯铵;CATB为十六烷基三甲基溴化铵;QACs为季铵化合物;CHX为氯己定;PCMX为氯二甲苯酚。
Note:TCS is triclosan;BAC is benzalkonium chloride;CATB is cetyl trimethyl ammonium bromide;QACs is quaternary ammonium compounds;CHX is chlorhexidine;PCMX is p-chloro-xylenol.
食品中存在的抗杀生剂及抗生素的人畜共患病原体对公众健康构成直接威胁。有研究在乳制品制造环境中分离纯化出了杀生剂耐药细菌[88]。该研究对来自山羊奶酪或牛奶生产的中小型企业的120株细菌分离株进行筛选,获得19株杀生剂耐药菌株,分别属于乳球菌属(Lactococcus)、乳杆菌属(Enterococcus)、肠球菌属(Lactobacillus)、芽孢杆菌属(Bacillus)、埃希氏菌属(Escherichia)、肠杆菌属(Enterobacter)和螺杆菌属(Helicobacter)。部分菌株对杀生剂和抗生素具有多重耐药特征。除检出sul1、acrB、blaCTX-M、blaPSE和mdfA等抗生素耐药基因外,还检出了qacEΔ1和qacA/B等杀生剂耐药基因。还有一些研究报道了食源性大肠杆菌降低了对QACs的敏感性[89-92]。其中Jiang等[93]从645份零售肉类样品中分离出179株大肠杆菌菌株,并从中检出了sugE(c)、ydgE/ydgF、mdfA、emrE、qacEΔ1、qacE、sugE(p)、qacF和qacH等QAC抗性基因。同时有研究者从德国食品生产工厂分离到的93株李斯特菌(Listeria monocytogenes)菌株中检测到15株苯扎氯铵耐药株,在其中13株菌株中发现了qacH和emrC等赋予苯扎氯铵耐受性的耐药基因[92]。
医院及医疗机构等卫生保健环境中,具杀生剂和抗生素耐药性的高致病性病原体同样令人担忧。有研究发现源自阿尔及利亚医院的大部分大肠杆菌分离菌株对杀生剂有耐受性,其中六氯酚和苯扎氯铵的最小抑制浓度(minimum inhibitory concentrations,MICs)均可高达128 mg·L-1。同时该研究指出反复暴露于杀生剂不仅会增加对杀生剂耐药菌的选择,且可能有助于抗生素耐药机制的表达和传播[94]。最近,Youssef等[95]调查了埃及医院内耐多药的耐甲氧西林金黄色葡萄球菌(methicillin-resistant Staphylococcus aureus,MRSA)临床和环境分离株的杀生剂敏感性情况。该研究收集到114株临床和8株环境MRSA,其中75株临床菌株和6株环境菌株对戊二醛、氯甲酚、氯己定、溴棕三甲铵和聚维酮碘等杀生剂的敏感性降低。
养殖相关环境是杀生剂使用频率较高的场所之一,包括畜牧业(家禽养殖场、养猪场和养牛场)和渔业(水产养殖场)等都会利用杀生剂进行消杀,因此相关杀生剂耐药菌株的分离情况也层出不穷。在德国3个肉鸡育肥场收集到93株大肠杆菌菌株,其中有9株分离株显示出对至少一种杀生剂(甲醛、氯甲酚、过氧乙酸和苯扎氯铵)的敏感性降低[91]。从美国水牛养殖场的水牛牛皮和粪便样本中分离得到145株肠道沙氏门菌(Salmonella enterica)菌株,研究结果显示所有菌株均对三氯生敏感且均对氯己定产生耐药,近1/3的菌株对苯扎氯铵具有低水平抗性[96]。有研究从使用猪场杀生剂(苯扎氯铵、过氧化氢、甲醛、次氯酸钠和氢氧化钠)的丹麦猪中分离出MRSA CC30,并首次从中检测到qacG和qacC耐药基因[97]。从美国肉鸡鸡舍垫料分离到96株空肠弯曲杆菌菌株,其中99%的菌株对三氯生具有抗性,32%的菌株对氯己定具有抗性[98]。
2.2 细菌对杀生剂的耐受机制
杀生剂对细菌细胞造成的选择压力诱导其引起应激反应,导致细菌表达相应的抵抗机制来防止杀生剂的有害影响[104]。细菌对杀生剂的耐药机制分为先天固有,即由染色体控制的内在自然特性;后天获得,即由于遗传物质突变或通过水平基因转移(以转座子或质粒等可移动遗传元件的形式)获得耐药基因[1,85,105]。细菌对杀生剂的耐受机制如下。
2.2.1 细胞膜渗透性降低
细菌细胞中渗透屏障的存在会限制杀生剂的渗透,降低杀生剂的吸收浓度或使其无法进入靶细胞,最终导致失效[85]。相较于革兰氏阳性菌,革兰氏阴性菌对杀生剂具有更高的耐受性。因为革兰氏阴性菌具富含脂多糖(磷脂双分子层对杀生剂不渗透为主要原因)的不对称外膜,加之外膜蛋白的存在,使得细胞渗透性降低,阻碍杀生剂的吸收和扩散,所以导致革兰氏阴性菌对杀生剂不敏感[106]。同时,细菌细胞膜表面较小孔径的孔蛋白基因ompC表达上调,能降低细菌细胞膜对杀生剂的透过性[107]。其中铜绿假单胞菌(P. aeruginosa)、洋葱假单胞菌(P. cepacia)、变形杆菌(Proteus sp.)和斯氏普罗威登斯菌(Providencia stuartii)等革兰氏阴性菌对某些杀生剂具有较强抗性[108]。
2.2.2 生物膜形成
生物膜指细胞通过胞外聚合物附着在基质表面所形成的微生物群落,这是一种细菌应对外界刺激的机制,能阻遏杀生剂的渗透[81]。生物膜的形成赋予细菌对杀生剂的耐受性,其降低药物敏感性的机理一直是实验研究的主题[81,109]。这些机制除减少杀生剂进入细菌细胞外,还包括生物膜和杀生剂之间的化学相互作用、微环境的调节(产生营养和氧气受限且饥饿的细胞)、生物膜内杀生剂降解酶的产生及群体感应等[110]。现已累积了大量生物膜形成对杀生剂耐药性的影响研究。例如,有研究首次报道了亚致死浓度苯扎氯铵胁迫下的肠道沙门氏菌的生物膜细胞群体能对其产生适应性反应[111]。Henly等[112]对长期暴露于杀生剂的8株菌株的生物膜形成情况进行比较,发现杀生剂暴露在很大程度上导致生物膜形成的增加,特别是暴露于苯扎氯铵和三氯生后均有7株分离株的生物膜形成增加。Buzón-Durán等[113]研究了亚最低抑菌浓度下(sub-MICs)苯扎氯铵、磷酸三钠和次氯酸钠对MRSA形成的生物膜结构和活力影响,发现次氯酸钠实验组的菌株生物膜形成能力增长。还有研究报道当铜绿假单胞菌和表皮葡萄球菌(S. epidermidis)以生物膜的形式存在时,对妥布霉素和苯扎氯铵的耐受性增加了100倍[73,105,114]。
2.2.3 外排泵
外排泵是最常见的耐药机制之一,它广泛存在于细菌中,是一种依赖能量驱动主动向外泵出杀生剂等化合物且不发生目标物改变或降解的药物外排系统[106]。外排泵通过降低细菌胞内杀生剂有效浓度而实现耐受。与细菌多重耐药有关的外排泵主要有以下5类:主要易化家族(major facilitator superfamily,MFS)、小多药耐药家族(small multi-drug resistance,SMR)、多药与毒物外排家族(multidrug and toxic efflux,MATE)、耐药结节分化家族(resistance-nodulation-division,RND)和ATP结合盒家族(ATP binding cassette,ABC)[115-116]。外排作为降低杀生剂敏感性的机制已得到充分证实。多项研究为质粒介导的苯扎氯铵耐药性提供证据。有研究报道来自肉类加工设施中分离得到的李斯特菌分离株存在编码MdrL和Lde外排泵的染色体定位基因,且证实了分离株对苯扎氯铵的耐受性与质粒携带的bcr ABC盒有关[117]。有学者观察到金黄色葡萄球菌临床分离株对苯扎氯铵和氯己定敏感性的降低与季铵化合物诱导编码的外排泵QacA、QacB、QacC和QacG有关[118-119]。Maseda等[120-121]将粘质沙雷氏菌(Serratia marcescens)反复暴露于浓度递增的氯化十六烷基吡啶,发现细菌通过表达SdeAB外排泵获得对杀生剂和抗生素的耐药性,并证实了耐药菌株中SdeAB外排泵的增强表达是受sdeS基因突变的影响。
2.2.4 杀生剂失活
杀生剂失活或降解是微生物对杀生剂的另一个固有抗性,即通过酶降解、活性基团替换或化学转化直接使化合物失活[105,122]。目前已有相关报道描述了细菌中将杀生剂酶促转化或灭活成无毒形式的现象[123]。Kümmerle等[124]对大肠杆菌耐甲醛菌株VU3695的甲醛耐药机制的研究发现,其抗性机制是基于甲醛脱氢酶对甲醛的酶降解。有研究发现源自土壤的恶臭假单胞菌(P. putida)TriRY菌株和木糖氧化产碱反硝化菌(Alcaligenes xylosoxidans subsp.denitrificans)TR1菌株的高水平三氯生抗性,有赖于细菌对三氯生的降解[125-126]。Hay等[127]分离自污水处理厂活性污泥的营养缺陷型鞘氨醇单胞菌株(Sphingomonas),在复杂培养基上生长时能够部分矿化三氯生,将约35%的[14C]三氯生转化为[14C]CO2。Nishihara等[128]发现一株能降解双十烷基二甲基氯化铵(didecyldimethylammonium chloride,DDAC)的荧光假单胞菌(P. fluorescens)TN4,该菌株能通过N-脱烷基化过程将季铵化合物降解,产生对季铵化合物的高度耐受性。
2.2.5 靶位修饰
此外,细菌可通过在结合位点处或附近产生突变或酶促修饰对靶位进行改变,使杀生剂无法与细菌结合,从而减少杀害作用[129]。抗生素由于作用靶点的特异性,靶位变更介导的耐药性被广泛研究。而杀生剂耐药性和抗生素耐药性的靶位改变机制不同,通常细菌不太可能通过靶位改变对杀生剂产生耐受性,因为杀生剂往往具有多个作用位点[129]。目前关于导致杀生剂耐药的靶位改变的研究主要集中于三氯生耐药性。已有大量研究证实大肠杆菌对三氯生的耐药性是通过细菌脂肪酸合成中的烯酰基-酰基载体蛋白还原酶编码基因fabI基因的错义突变获得[82-84]。
3 杀生剂对细菌抗生素耐药性的影响(Influence of biocide on the bacterial antibiotic-resistance)
随着对细菌抗生素耐药性控制问题的全球共识的达成和疫情背景下杀生剂消耗量的增加,越来越多的研究证实环境中常检出的杀生剂是细菌耐药性发展和扩散的驱动因素。环境中多种低浓度水平杀生剂的积累可对细菌造成长期胁迫,通过细菌抗生素耐药性共同选择作用,导致细菌耐药性的演变和传播[130-131]。杀生剂对细菌抗生素耐药性的影响主要表现在促进抗生素耐药基因(antibiotic resistance genes,ARGs)的水平转移和共选择机制。
3.1 水平基因转移
抗生素耐药细菌携带的ARGs可以在细菌种内和种间进行水平基因转移(horizontal gene transfer,HGT),加剧了抗生素耐药在全球的出现和传播[132]。因此HGT被认为是抗生素耐药性传播和扩散的重要途径。HGT通常由转化、转导和接合3种机制介导[133]。细菌通过HGT获得具有杀生剂耐药基因的可移动遗传元件(mobile genetic elements,MGEs)来获得抗性。其中,插入序列、转座子、整合子和基因盒可以在细菌内的DNA分子间移动,共轭转座子和质粒可以在细菌之间移动,它们都是传播抗生素耐药性遗传决定因子的重要载体。抗生素是传播抗生素耐药性的关键驱动力,但非抗生素物质对ARGs转化的贡献通常被忽视。最近,有研究首次提供证据[134],证明非甾体抗炎药、布洛芬和降脂药等6种常见的非抗生素药物显著促进了外源性ARGs的细菌转化,且可能是通过促进细菌活性、增强应激水平、过度产生活性氧和增加细胞膜通透性来助力非抗生素药物的ARGs转化。该研究强调了非抗生素药物通过促进转化途径促进ARGs水平基因转移的重要性,这无疑引发对杀生剂对细菌抗生素耐药性传播的思考。
现已有研究报道了亚抑制浓度杀生剂通过提高接合转移频率,进而促进抗生素耐药性的传播。例如Jutkina等[135]首次证明TCS和CHX能在亚抑制浓度下显著诱导耐药性的接合转移频率。Han等[132]选择了5种QACs揭示影响抗生素耐药性传播的机制,结果显示QACs耐药基因在浙江省三大流域中普遍存在,并且与整合子基因intI1及7个ARGs之间存在显著相关性;QACs通过增强细菌细胞的膜通透性并刺激细菌产生活性氧,进而可能促进细菌之间质粒RP4的接合转移。有研究证实了常用杀生剂乙醇对枯草芽孢杆菌(B. subtilis)菌株之间接合转座子Tn 916的影响,结果显示亚抑制浓度乙醇将Tn 916的转移频率显著增加5倍,表明暴露于亚抑制浓度的乙醇可能会诱导Tn 916及其抗性基因的转移[136]。Jin等[137]发现了饮用水中的氯消毒对抗生素耐药性传播构成威胁,研究表明,氯化作用使处于生理感受态细胞的耐氯损伤细菌的质粒转化频率比未处理的细菌高550倍,且极易从周围环境中吸收游离的ARGs,从而促进ARGs的水平转移。需要注意的是,目前收集的关于由于杀生剂持续暴露而导致抗生素耐药基因水平转移的信息仍不够充分。
另外,有研究指出细菌SOS反应会促进HGT的发生[138]。细菌SOS反应是对环境不断变化的响应系统之一,该系统通过诱导一系列参与DNA修复和重组的基因表达来应对DNA损伤,直接干扰DNA复制、抑制细胞壁合成或产生活性氧都是SOS反应的潜在激活因素。抗生素能有效激活细菌的SOS反应已在一些研究中得到证实[139-141]。同时,人类会向环境中释放大量包括杀生剂在内的非抗生素污染物,造成的环境状态波动会诱发细菌SOS反应。确有研究指出杀生剂造成的选择压力会触发细菌SOS反应[17,142]。
3.2 共选择机制
杀生剂与抗生素不同,前者通常以杀灭浓度被有意地置于各种外界环境中。尽管对杀菌剂本身的耐药性发展存在一些担忧,但关于耐药性最大的担忧是它们主要通过共同和交叉选择机制共同选择抗生素耐药性的潜力。与抗生素相比,对选择和共同选择所需的杀生物剂浓度的研究较少。
接触杀生剂也可能增加抗生素耐药性并导致多重耐药的事实已经被人们慢慢认可。大量文献描述了杀生剂的使用与细菌抗生素耐药性增长之间的联系[1,85,143]。AKimitsu等[144]发现,QACs暴露会增加MRSA菌株对苯唑西林和β-内酰胺的耐药性。Langsrud等[145]将大肠杆菌暴露于亚抑制浓度的BAC,观察到细菌对BAC和氯霉素的交叉耐药性。Mc Cay等[146]在添加BAC的情况下对铜绿假单胞菌进行富集连续培养,结果显示该适应性细菌对BAC的耐受性提高了12倍,同时对环丙沙星的耐药性显著增加了265倍。Kim等[147]阐明了BAC暴露共同选择抗生素耐药性的潜在遗传机制,包括BAC耐受基因和ARGs位于同一MGEs中、pmrB基因的突变以及外排泵基因的上调。Tandukar等[148]还探讨了BAC暴露和微生物群落抗生素耐药性的联系,研究发现,暴露后的微生物群落对BAC及3种抗生素(青霉素G、四环素和环丙沙星)的敏感性显著降低,其中BAC和青霉素耐药性增加的耐药机制为降解或转化,而对四环素和环丙沙星的耐药性增加主要归因于外排泵的活性提高。
杀生剂对抗生素耐药性的共同选择的潜力已被广泛报道,杀生剂驱动共同选择主要通过协同抗性(cross resistance)和交叉抗性(co-resistance)2种机制实现。协同抗性指编码杀生剂抗性和抗生素抗性的基因位于同一可移动遗传元件上,能在新的微生物-宿主系统中转移和表达[17,85,149]。Roedel等[91]发现从德国肉鸡育肥场分离到的杀生剂耐药株,其杀生剂耐药基因qacEΔ1和sugE(p)同时位于含有抗生素耐药基因sul1和blaCMY-2的移动遗传元件上。杀生剂和抗生素的细菌耐药机制相似,因此杀生剂暴露会引起抗生素的交叉耐药。交叉抗性指赋予杀生剂和抗生素产生耐药性的基因编码于同一耐药机制[17,85,149]。有研究首次证明临床上分离得到的铜绿假单胞菌中三氯生和抗生素的交叉耐药性是由三氯生暴露后过度表达MexCD-OprJ多药外排泵介导的[82]。MexAB-OprM多药外排泵的过度表达是导致不同生态位铜绿假单胞菌中苯扎氯铵和抗生素的交叉耐药性的主要促成因素[101]。有研究者针对空肠弯曲杆菌(Campylobacter jejuni)和结肠弯曲杆菌(Campylobacter coli)开展进化实验,确定了5种杀生剂(三氯生、苯扎氯铵、氯化十六烷基吡啶、醋酸氯己定和磷酸三钠)对抗生素(红霉素和环丙沙星)的交叉耐药性[150]。食品工业中的空肠弯曲杆菌对杀生剂的适应通过增强生物膜的形成(生物量、表面覆盖率、粗糙度和生物膜的表面粘附力显著增加)来介导与抗生素之间的交叉耐药性[151]。有研究对亚抑制浓度杀生剂(氯酚、苯扎氯铵、戊二醛和氯己定)条件下的肠道细菌大肠杆菌进行了实验室适应性驯化,发现与多药外排蛋白、孔蛋白和RNA聚合酶上相关基因(mdfA、acrR、envZ、ompR、rpoA和rpoBC)的突变以及分别调控的双组分系统和生物膜形成等途径,是与抗生素产生交叉抗性背后的机制[152]。Wand等[153]利用氯己定适应性诱导肺炎克雷伯菌(Klebsiella pneumoniae)对粘菌素的交叉抗性,发现其抗性与双组分调节剂phoPQ以及与MFS外排泵基因smvA毗邻的TET抑制基因smvR的突变相关。最近的一篇研究[142]对消毒剂次氯酸钠的耐药机制进行深入探讨,结果显示MuxABC-OpmB外排泵上muxA和muxB多药外排基因的表达及细胞膜渗透性的降低介导了假单胞菌对杀生剂和抗生素的交叉耐药。
由此可见,随着杀生剂的广泛使用而导致其在受纳环境的残留,胁迫环境微生物抗生素耐药性的产生和传播成为一大环境挑战。
4 展望(Research prospect)
结合杀生剂的受纳环境检出率、环境耐药菌株分离率及其对细菌耐药性传播与发展的影响,现有研究表明杀生剂广泛存在于环境介质中并对人类健康及生态环境构成潜在威胁。目前,关于杀生剂对细菌耐药性影响的研究仍存在一些不足,主要表现在:(1)评价杀生剂抗性的临界点仍不够清晰,缺少相应的标准评价体系;(2)关于杀生剂的耐药机制研究仍不够明确,尤其是杀生剂失活及靶位变更引起的杀生剂抗性;(3)关于针对具体某一杀生剂的深入研究仍较为缺乏,目前仅对三氯生的作用机制研究得较为透彻;(4)关于杀生剂驱动抗生素耐药性传播的机制研究仍不够成熟,对杀生剂抗性基因水平转移的研究较为有限,尤其对于杀生剂抗性基因是否能通过转导或转化机制转移,值得进一步研究。
鉴于目前全球杀生剂使用量的日益增加、环境中杀生剂耐药菌株的频繁检出以及对抗生素耐药性传播的促进,显然需要加强谨慎使用现有杀生剂的意识。为了正确评估和防控杀生剂对抗生素耐药性污染的生态风险,最大限度地减少杀生剂对细菌抗生素耐药性的发展和传播,需要抓紧进一步明确杀生剂耐药性的标准评价体系,利用多学科研究手段加强对杀生剂耐药机制的研究,进而为新药的开发和揭示杀生剂与抗生素耐药性的共选择机制提供理论依据。
[1] Ortega Morente E,Fernández-Fuentes M A,Grande Burgos M J,et al.Biocide tolerance in bacteria [J].International Journal of Food Microbiology,2013,162(1):13-25
[2] Pekhtasheva E,Neverov A,Kubica S,et al.Classification of biodamages,evaluation and protection methods [J].Chemistry &Chemical Technology,2012,6(4):459-472
[3] Jones I A,Joshi L T.Biocide use in the antimicrobial era:A review [J].Molecules,2021,26(8):2276
[4] Alrashdi A S M.The impact of household biocides and antibiotics on aquatic microbial community composition [D].Houghton,Michigan:Michigan Technological University,2019:2-3
[5] Liu W R,Yang Y Y,Liu Y S,et al.Biocides in the river system of a highly urbanized region:A systematic investigation involving runoff input [J].The Science of the Total Environment,2018,624:1023-1030
[6] Suhail Hamdani S,Ahmad Bhat B,Tariq L,et al.Antibiotic resistance:The future disaster [J].International Journal for Research in Applied Sciences and Biotechnology,2020,7(4):133-145
[7] Shao Y T,Wang Y P,Yuan Y W,et al.A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China [J].The Science of the Total Environment,2021,798:149205
[8] Jansen K U,Gruber W C,Simon R,et al.The impact of human vaccines on bacterial antimicrobial resistance.A review [J].Environmental Chemistry Letters,2021,19(6):4031-4062
[9] Gothwal R,Shashidhar T.Antibiotic pollution in the environment:A review [J].CLEAN-Soil,Air,Water,2015,43(4):479-489
[10] Chapman J S.Disinfectant resistance mechanisms,cross-resistance,and co-resistance [J].International Biodeterioration &Biodegradation,2003,51(4):271-276
[11] Stepanauskas R,Glenn T C,Jagoe C H,et al.Elevated microbial tolerance to metals and antibiotics in metal-contaminated industrial environments [J].Environmental Science &Technology,2005,39(10):3671-3678
[12] Sidhu M S,Heir E,Sørum H,et al.Genetic linkage between resistance to quaternary ammonium compounds and beta-lactam antibiotics in food-related Staphylococcus spp.[J].Microbial Drug Resistance,2001,7(4):363-371
[13] Arvanitoyannis I,Tserkezou P,Choreftaki S.Presentation and comments on EU legislation related to food industries-environment interactions:Organic contaminants (chemicals,pesticides,dioxins,furans,biocides and their waste management) [J].International Journal of Food Science and Technology,2006,41:833-853
[14] Hernando M D,Rodríguez A,Vaquero J J,et al.Environmental risk assessment of emerging pollutants in water:Approaches under horizontal and vertical EU legislation [J].Critical Reviews in Environmental Science and Technology,2011,41(7):699-731
[15] Marcotte S,Estel L,Leboucher S,et al.Occurrence of organic biocides in the air and dust at the Natural History Museum of Rouen,France [J].Journal of Cultural Heritage,2014,15(1):68-72
[16] Paul D,Mondal S K,Mandal S M.Biologia futura:Use of biocides during COVID-19-global reshuffling of the microbiota [J].Biologia Futura,2021,72(3):273-280
[17] Paul D,Chakraborty R,Mandal S M.Biocides and health-care agents are more than just antibiotics:Inducing cross to co-resistance in microbes [J].Ecotoxicology and Environmental Safety,2019,174:601-610
[18] Kähkönen E,Nordström K.Toward a nontoxic poison:Current trends in (European Union) biocides regulation [J].Integrated Environmental Assessment and Management,2008,4(4):471-477
[19] Lewis D.COVID-19 rarely spreads through surfaces.So why are we still deep cleaning?[J].Nature,2021,590(7844):26-28
[20] Zhang Q Q,Ying G G,Chen Z F,et al.Multimedia fate modeling and risk assessment of a commonly used azole fungicide climbazole at the river basin scale in China [J].Science of the Total Environment,2015,520:39-48
[21] Zhao J L,Liu Y S,Liu W R,et al.Tissue-specific bioaccumulation of human and veterinary antibiotics in bile,plasma,liver and muscle tissues of wild fish from a highly urbanized region [J].Environmental Pollution,2015,198:15-24
[22] Data Bridge Market Research.Biocides 2021 Global Industry Extensive Competitive Landscape on Size,Volume,Trends,Share and Revenue Regional Forecast By 2027 The Courier [R].Maharashtra,India:Data Bridge Market Research,2020
[23] Darbre P D,Aljarrah A,Miller W R,et al.Concentrations of parabens in human breast tumours [J].Journal of Applied Toxicology,2004,24(1):5-13
[24] Kahle M,Buerge I J,Hauser A,et al.Azole fungicides:Occurrence and fate in wastewater and surface waters [J].Environmental Science &Technology,2008,42(19):7193-7200
[25] Chen Z F,Ying G G.Occurrence,fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment:A review [J].Environment International,2015,84:142-153
[26] Liu W R,Yang Y Y,Liu Y S,et al.Biocides in wastewater treatment plants:Mass balance analysis and pollution load estimation [J].Journal of Hazardous Materials,2017,329:310-320
[27] Mulder I,Siemens J,Sentek V,et al.Quaternary ammonium compounds in soil:Implications for antibiotic resistance development [J].Reviews in Environmental Science and Bio/Technology,2018,17(1):159-185
[28] Merel S,Benzing S,Gleiser C,et al.Occurrence and overlooked sources of the biocide carbendazim in wastewater and surface water [J].Environmental Pollution,2018,239:512-521
[29] Juksu K,Zhao J L,Liu Y S,et al.Occurrence,fate and risk assessment of biocides in wastewater treatment plants and aquatic environments in Thailand [J].The Science of the Total Environment,2019,690:1110-1119
[30] Östman M,Lindberg R H,Fick J,et al.Screening of biocides,metals and antibiotics in Swedish sewage sludge and wastewater [J].Water Research,2017,115:318-328
[31] 柳王荣.典型杀生剂在污水处理厂与受纳水环境中的分布、归趋及生态风险研究[D].北京:中国科学院大学,2016:49-56
Liu W R.Occurrence,fate and ecological risk of biocides in the wastewater treatment plants and receiving aquatic environment [D].Beijing:University of Chinese Academy of Sciences,2016:49-56 (in Chinese)
[32] Liu W R,Zhao J L,Liu Y S,et al.Biocides in the Yangtze River of China:Spatiotemporal distribution,mass load and risk assessment [J].Environmental Pollution,2015,200:53-63
[33] Jia Y W,Huang Z,Hu L X,et al.Occurrence and mass loads of biocides in plastic debris from the Pearl River system,South China [J].Chemosphere,2020,246:125771
[34] Kumar K S,Priya S M,Peck A M,et al.Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah,Georgia,USA [J].Archives of Environmental Contamination and Toxicology,2010,58(2):275-285
[35] Kim N S,Hong S H,An J G,et al.Distribution of butyltins and alternative antifouling biocides in sediments from shipping and shipbuilding areas in South Korea [J].Marine Pollution Bulletin,2015,95(1):484-490
[36] Pérez D J,Iturburu F G,Calderon G,et al.Ecological risk assessment of current-use pesticides and biocides in soils,sediments and surface water of a mixed land-use basin of the Pampas region,Argentina [J].Chemosphere,2021,263:128061
[37] Köck-Schulmeyer M,Postigo C,Farré M,et al.Medium to highly polar pesticides in seawater:Analysis and fate in coastal areas of Catalonia (NE Spain) [J].Chemosphere,2019,215:515-523
[38] Curiao T,Marchi E,Grandgirard D,et al.Multiple adaptive routes of Salmonella enterica Typhimurium to biocide and antibiotic exposure [J].BMC Genomics,2016,17:491
[39] Almeida A C,Gomes T,Langford K,et al.Oxidative stress in the algae Chlamydomonas reinhardtii exposed to biocides [J].Aquatic Toxicology,2017,189:50-59
[40] Bock L J.Bacterial biocide resistance:A new scourge of the infectious disease world?[J].Archives of Disease in Childhood,2019,104(11):1029-1033
[41] Jones R D.Bacterial resistance and topical antimicrobial wash products [J].American Journal of Infection Control,1999,27(4):351-363
[42] Maillard J Y.Bacterial target sites for biocide action [J].Journal of Applied Microbiology,2002,92:16S-27S
[43] Gravel J,Paradis-Bleau C,Schmitzer A R.Adaptation of a bacterial membrane permeabilization assay for quantitative evaluation of benzalkonium chloride as a membrane-disrupting agent [J].MedChemComm,2017,8(7):1408-1413
[44] Fazlara A,Ekhtelat M.The disinfectant effects of benzalkonium chloride on some important foodborne pathogens [J].American-Eurasian Journal of Agricultural and Environmental Sciences,2012,12(1):23-29
[45] Gilbert P,Moore L E.Cationic antiseptics:Diversity of action under a common epithet [J].Journal of Applied Microbiology,2005,99(4):703-715
[46] Gerba C P.Quaternary ammonium biocides:Efficacy in application [J].Applied and Environmental Microbiology,2015,81(2):464-469
[47] Pulvertaft R J V,Lumb G D.Bacterial lysis and antiseptics [J].The Journal of Hygiene,1948,46(1):62-64
[48] El-Falaha B M A,Furr J R,Russell A D.Effect of anionic detergents on wild-type and envelope mutants of Escherichia coli and Pseudomonas aeruginosa [J].Letters in Applied Microbiology,1989,8(1):15-19
[49] McBain A J,Gilbert P.Biocide tolerance and the harbingers of doom [J].International Biodeterioration &Biodegradation,2001,47(2):55-61
[50] Wessels S,Ingmer H.Modes of action of three disinfectant active substances:A review [J].Regulatory Toxicology and Pharmacology,2013,67(3):456-467
[51] Wales A D,Davies R H.Co-selection of resistance to antibiotics,biocides and heavy metals,and its relevance to foodborne pathogens [J].Antibiotics,2015,4(4):567-604
[52] Al-Adham I I,Dinning A J,Eastwood I M,et al.Cell membrane effects of some common biocides [J].Journal of Industrial Microbiology and Biotechnology,1998,21(1-2):6-10
[53] Razin S,Argaman M.Lysis of mycoplasma,bacterial protoplasts,spheroplasts and L-forms by various agents [J].Journal of General Microbiology,1963,30:155-172
[54] Cabral J P.Damage to the cytoplasmic membrane and cell death caused by dodine (dodecylguanidine monoacetate) in Pseudomonas syringae ATCC 12271 [J].Antimicrobial Agents and Chemotherapy,1991,35(2):341-344
[55] Chindera K,Mahato M,Sharma A K,et al.The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes [J].Scientific Reports,2016,6:23121
[56] Machuca J,Lopez-Rojas R,Fernandez-Cuenca F,et al.Comparative activity of a polyhexanide-betaine solution against biofilms produced by multidrug-resistant bacteria belonging to high-risk clones [J].The Journal of Hospital Infection,2019,103(1):e92-e96
[57] Linley E,Denyer S P,McDonnell G,et al.Use of hydrogen peroxide as a biocide:New consideration of its mechanisms of biocidal action [J].The Journal of Antimicrobial Chemotherapy,2012,67(7):1589-1596
[58] Fitzgerald K A,Davies A,Russell A D.Sensitivity and resistance of Escherichia coli and Staphylococcus aureus to chlorhexidine [J].Letters in Applied Microbiology,1992,14(2):33-36
[59] Shen S X,Zhang T H,Yuan Y,et al.Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane [J].Food Control,2015,47:196-202
[60] Poger D,Mark A E.Effect of triclosan and chloroxylenol on bacterial membranes [J].The Journal of Physical Chemistry B,2019,123(25):5291-5301
[61] Le D,Krasnopeeva E,Sinjab F,et al.Active efflux leads to heterogeneous dissipation of proton motive force by protonophores in bacteria [J].mBio,2021,12(4):e0067621
[62] Farha M A,Verschoor C P,Bowdish D,et al.Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus [J].Chemistry &Biology,2013,20(9):1168-1178
[63] Mitchell P.Chemiosmotic coupling in oxidative and photosynthetic phosphorylation [J].Biochimica et Biophysica Acta (BBA) - Bioenergetics,2011,1807(12):1507-1538
[64] Abdel-Ghani N T,El-Chaghaby G A,Zahran E M.Pentachlorophenol (PCP) adsorption from aqueous solution by activated carbons prepared from corn wastes [J].International Journal of Environmental Science and Technology,2015,12(1):211-222
[65] Ferrario D,Rabbit R R.Analysis of the proposed EU regulation concerning biocide products and its opportunities for alternative approaches and a toxicology for the 21st Century [J].ALTEX,2012,29(2):157-172
[66] McDonnell G,Russell A D.Antiseptics and disinfectants:Activity,action,and resistance [J].Clinical Microbiology Reviews,1999,12(1):147-179
[67] Hua X,Du G L,Han J,et al.Bioprocess intensification for whole-cell catalysis of catabolized chemicals with 2,4-dinitrophenol uncoupling [J].ACS Sustainable Chemistry &Engineering,2020,8(41):15782-15790
[68] Montforts M H M M,de Jonge R,Franz E,et al.Development of a protocol to evaluate bacterial resistance in response to household disinfectants:A feasibility study [R].Bilthoven:National Institute for Public Health and the Environment,2015
[69] White T C,Marr K A,Bowden R A.Clinical,cellular,and molecular factors that contribute to antifungal drug resistance [J].Clinical Microbiology Reviews,1998,11(2):382-402
[70] Gilbert P,Beveridge E G,Crone P B.Effect of 2-phenoxyethanol upon RNA,DNA and protein biosynthesis in Escherichia coli NCTC 5933 [J].Microbios,1980,28(111):7-17
[71] Beumer R,Bloomfield S,Exner M,et al.Microbial resistance and biocides:A review by the International Forum on Home Hygiene (IFH) [R].The IFH Scientific Advisory Board,2000
[72] Ali A,Xiao Y,Song L N,et al.Biodegradable polyurethane based clay composite and their anti-biofouling properties [J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2021,625:126946
[73] Gilbert P,McBain A J.Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance [J].Clinical Microbiology Reviews,2003,16(2):189-208
[74] Gittelson B L,Walker I O.The interaction of proflavine with deoxyribonucleic acid and deoxyribonucleohistone [J].Biochimica et Biophysica Acta,1967,138(3):619-621
[75] Adams E.Binding of crystal violet by nucleic acids of Escherichia coli [J].The Journal of Pharmacy and Pharmacology,1968,20(Suppl):18S
[76] Ciak J,Hahn F E.Quinacrine (atebrin):Mode of action [J].Science,1967,156(3775):655-656
[77] Simões M,Simões L C,Cleto S,et al.Antimicrobial mechanisms of ortho-phthalaldehyde action [J].Journal of Basic Microbiology,2007,47(3):230-242
[78] Hendry E,Conway B,Worthington T.Antimicrobial efficacy of a novel Eucalyptus oil,chlorhexidine digluconate and isopropyl alcohol biocide formulation [J].International Journal of Molecular Sciences,2012,13(11):14016-14025
[79] Lin W,Yi J,Zhang Y,et al.Effects of glutaraldehyde-didecyldimethylammonium bromide combined disinfectant on the cell surface of Staphylococcus aureus [J].Journal of Applied Microbiology,2018,124(5):1060-1070
[80] Lee M H,Caffrey S M,Voordouw J K,et al.Effects of biocides on gene expression in the sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough [J].Applied Microbiology and Biotechnology,2010,87(3):1109-1118
[81] Milani E S,Hasani A,Varschochi M,et al.Biocide resistance in Acinetobacter baumannii:Appraising the mechanisms [J].Journal of Hospital Infection,2021,117:135-146
[82] Chuanchuen R,Beinlich K,Hoang T T,et al.Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps:Exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ [J].Antimicrobial Agents and Chemotherapy,2001,45(2):428-432
[83] Li M Z,He Y N,Sun J,et al.Chronic exposure to an environmentally relevant triclosan concentration induces persistent triclosan resistance but reversible antibiotic tolerance in Escherichia coli [J].Environmental Science &Technology,2019,53(6):3277-3286
[84] Lu J,Jin M,Nguyen S H,et al.Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation [J].Environment International,2018,118:257-265
[85] Elekhnawy E,Sonbol F,Abdelaziz A,et al.Potential impact of biocide adaptation on selection of antibiotic resistance in bacterial isolates [J].Future Journal of Pharmaceutical Sciences,2020,6:97
[86] Cloete T E,Jacobs L,Brözel V S.The chemical control of biofouling in industrial water systems [J].Biodegradation,1998,9(1):23-37
[87] Graham D M,Nelson F E.Inhibition of lactic Streptococcus bacteriophage by crystal violet and other agents [J].The Journal of General Physiology,1953,37(1):121-138
[88] Fernández Márquez M L,Grande Burgos M J,López Aguayo M C,et al.Characterization of biocide-tolerant bacteria isolated from cheese and dairy small-medium enterprises [J].Food Microbiology,2017,62:77-81
[89] Zhang A Y,He X M,Meng Y,et al.Antibiotic and disinfectant resistance of Escherichia coli isolated from retail meats in Sichuan,China [J].Microbial Drug Resistance,2016,22(1):80-87
[90] Zou L K,Meng J H,McDermott P F,et al.Presence of disinfectant resistance genes in Escherichia coli isolated from retail meats in the USA [J].Journal of Antimicrobial Chemotherapy,2014,69(10):2644-2649
[91] Roedel A,Vincze S,Projahn M,et al.Genetic but no phenotypic associations between biocide tolerance and antibiotic resistance in Escherichia coli from German broiler fattening farms [J].Microorganisms,2021,9(3):651
[92] Roedel A,Dieckmann R,Brendebach H,et al.Biocide-tolerant Listeria monocytogenes isolates from German food production plants do not show cross-resistance to clinically relevant antibiotics [J].Applied and Environmental Microbiology,2019,85(20):e01253-e01219
[93] Jiang X B,Xu Y M,Li Y,et al.Characterization and horizontal transfer of qacH-associated class 1 integrons in Escherichia coli isolated from retail meats [J].International Journal of Food Microbiology,2017,258:12-17
[94] Boutarfi Z,Rebiahi S A,Morghad T,et al.Biocide tolerance and antibiotic resistance of Enterobacter spp.isolated from an Algerian hospital environment [J].Journal of Global Antimicrobial Resistance,2019,18:291-297
[95] Youssef C R B,Kadry A A,Shaker G H,et al.The alarming association between antibiotic resistance and reduced susceptibility to biocides in nosocomial MRSA isolates from two regional hospitals in Egypt [J].Archives of Microbiology,2021,203(6):3295-3303
[96] Beier R C,Callaway T R,Andrews K,et al.Disinfectant and antimicrobial susceptibility profiles of Salmonella strains from feedlot water-sprinkled cattle:Hides and feces [J].Journal of Food Chemistry and Nanotechnology,2017,3(2):50-59
[97] Seier-Petersen M A,Nielsen L N,Ingmer H,et al.Biocide susceptibility of Staphylococcus aureus CC398 and CC30 isolates from pigs and identification of the biocide resistance genes,qacG and qacC [J].Microbial Drug Resistance,2015,21(5):527-536
[98] Beier R C,Byrd J A,Andrews K,et al.Disinfectant and antimicrobial susceptibility studies of the foodborne pathogen Campylobacter jejuni isolated from the litter of broiler chicken houses [J].Poultry Science,2021,100(2):1024-1033
[99] Lavilla Lerma L,Benomar N,Casado Mu oz M D C,et al.Correlation between antibiotic and biocide resistance in mesophilic and psychrotrophic Pseudomonas spp.isolated from slaughterhouse surfaces throughout meat chain production [J].Food Microbiology,2015,51:33-44
oz M D C,et al.Correlation between antibiotic and biocide resistance in mesophilic and psychrotrophic Pseudomonas spp.isolated from slaughterhouse surfaces throughout meat chain production [J].Food Microbiology,2015,51:33-44
[100] Romero J L,Grande Burgos M J,Pérez-Pulido R,et al.Resistance to antibiotics,biocides,preservatives and metals in bacteria isolated from seafoods:Co-selection of strains resistant or tolerant to different classes of compounds [J].Frontiers in Microbiology,2017,8:1650
[101] Amsalu A,Sapula S A,de Barros Lopes M,et al.Efflux pump-driven antibiotic and biocide cross-resistance in Pseudomonas aeruginosa isolated from different ecological niches:A case study in the development of multidrug resistance in environmental hotspots [J].Microorganisms,2020,8(11):1647
[102] Braoudaki M,Hilton A C.Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents [J].Journal of Clinical Microbiology,2004,42(1):73-78
[103] Mann B C,Bezuidenhout J J,Bezuidenhout C C.Biocide resistant and antibiotic cross-resistant potential pathogens from sewage and river water from a wastewater treatment facility in the North-West,Potchefstroom,South Africa [J].Water Science and Technology:A Journal of the International Association on Water Pollution Research,2019,80(3):551-562
[104] Maillard J Y.Resistance of bacteria to biocides [J].Microbiology Spectrum,2018,6(2):ARBA-0006-2017
[105] Gnanadhas D P,Marathe S A,Chakravortty D.Biocides:Resistance,cross-resistance mechanisms and assessment [J].Expert Opinion on Investigational Drugs,2013,22(2):191-206
[106] Kumar A,Schweizer H P.Bacterial resistance to antibiotics:Active efflux and reduced uptake [J].Advanced Drug Delivery Reviews,2005,57(10):1486-1513
[107] Lin X M,Yang M J,Li H,et al.Decreased expression of LamB and Odp1 complex is crucial for antibiotic resistance in Escherichia coli [J].Journal of Proteomics,2014,98:244-253
[108] Russell A D,Gould G W.Resistance of Enterobacteriaceae to preservatives and disinfectants [J].Journal of Applied Bacteriology,2008,65:167S-195S
[109] Mah T F C,O’Toole G A.Mechanisms of biofilm resistance to antimicrobial agents [J].Trends in Microbiology,2001,9(1):34-39
[110] Russell A D.Similarities and differences in the responses of microorganisms to biocides [J].Journal of Antimicrobial Chemotherapy,2003,52(5):750-763
[111] Mangalappalli-Illathu A K,![]() S,Korber D R.Differential adaptive response and survival of Salmonella enterica serovar Enteritidis planktonic and biofilm cells exposed to benzalkonium chloride [J].Antimicrobial Agents and Chemotherapy,2008,52(10):3669-3680
S,Korber D R.Differential adaptive response and survival of Salmonella enterica serovar Enteritidis planktonic and biofilm cells exposed to benzalkonium chloride [J].Antimicrobial Agents and Chemotherapy,2008,52(10):3669-3680
[112] Henly E L,Dowling J A R,Maingay J B,et al.Biocide exposure induces changes in susceptibility,pathogenicity,and biofilm formation in uropathogenic Escherichia coli [J].Antimicrobial Agents and Chemotherapy,2019,63(3):e01892-e01818
[113] Buzón-Durán L,Alonso-Calleja C,Riesco-Peláez F,et al.Effect of sub-inhibitory concentrations of biocides on the architecture and viability of MRSA biofilms [J].Food Microbiology,2017,65:294-301
[114] Gilbert P,Collier P J,Brown M R.Influence of growth rate on susceptibility to antimicrobial agents:Biofilms,cell cycle,dormancy,and stringent response [J].Antimicrobial Agents and Chemotherapy,1990,34(10):1865-1868
[115] Li X Z,Nikaido H.Efflux-Mediated Antimicrobial Resistance in Bacteria [M].New York:Springer,2016:219-259
[116] Poole K.Efflux pumps as antimicrobial resistance mechanisms [J].Annals of Medicine,2007,39(3):162-176
![]() á J,Véghová A,Mikulášová M,et al.Benzalkonium chloride tolerance of Listeria monocytogenes strains isolated from a meat processing facility is related to presence of plasmid-borne bcrABC cassette [J].Antonie Van Leeuwenhoek,2018,111(10):1913-1923
á J,Véghová A,Mikulášová M,et al.Benzalkonium chloride tolerance of Listeria monocytogenes strains isolated from a meat processing facility is related to presence of plasmid-borne bcrABC cassette [J].Antonie Van Leeuwenhoek,2018,111(10):1913-1923
[118] Buffet-Bataillon S,Tattevin P,Maillard J Y,et al.Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria [J].Future Microbiology,2016,11(1):81-92
[119] Furi L,Ciusa M L,Knight D,et al.Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus [J].Antimicrobial Agents and Chemotherapy,2013,57(8):3488-3497
[120] Maseda H,Hashida Y,Konaka R,et al.Mutational upregulation of a resistance-nodulation-cell division-type multidrug efflux pump,SdeAB,upon exposure to a biocide,cetylpyridinium chloride,and antibiotic resistance in Serratia marcescens [J].Antimicrobial Agents and Chemotherapy,2009,53(12):5230-5235
[121] Maseda H,Hashida Y,Shirai A,et al.Mutation in the sdeS gene promotes expression of the sdeAB efflux pump genes and multidrug resistance in Serratia marcescens [J].Antimicrobial Agents and Chemotherapy,2011,55(6):2922-2926
[122] Zhang R,Yang S,An Y W,et al.Antibiotics and antibiotic resistance genes in landfills:A review [J].Science of the Total Environment,2022,806:150647
[123] Cloete T E.Resistance mechanisms of bacteria to antimicrobial compounds [J].International Biodeterioration &Biodegradation,2003,51(4):277-282
[124] Kümmerle N,Feucht H H,Kaulfers P M.Plasmid-mediated formaldehyde resistance in Escherichia coli:Characterization of resistance gene [J].Antimicrobial Agents and Chemotherapy,1996,40(10):2276-2279
[125] Meade M J,Waddell R L,Callahan T M.Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp.denitrificans inactivate triclosan in liquid and solid substrates [J].FEMS Microbiology Letters,2001,204(1):45-48
[126] Schweizer H P.Triclosan:A widely used biocide and its link to antibiotics [J].FEMS Microbiology Letters,2001,202(1):1-7
[127] Hay A G,Dees P M,Sayler G S.Growth of a bacterial consortium on triclosan [J].FEMS Microbiology Ecology,2001,36(2-3):105-112
[128] Nishihara T,Okamoto T,Nishiyama N.Biodegradation of didecyldimethylammonium chloride by Pseudomonas fluorescens TN4 isolated from activated sludge [J].Journal of Applied Microbiology,2000,88(4):641-647
[129] Tong C Y,Hu H,Chen G,et al.Disinfectant resistance in bacteria:Mechanisms,spread,and resolution strategies [J].Environmental Research,2021,195:110897
[130] McBain A J,Rickard A H,Gilbert P.Possible implications of biocide accumulation in the environment on the prevalence of bacterial antibiotic resistance [J].Journal of Industrial Microbiology &Biotechnology,2002,29(6):326-330
[131] Rezasoltani S,Yadegar A,Hatami B,et al.Antimicrobial resistance as a hidden menace lurking behind the COVID-19 outbreak:The global impacts of too much hygiene on AMR [J].Frontiers in Microbiology,2020,11:590683
[132] Han Y,Zhou Z C,Zhu L,et al.The impact and mechanism of quaternary ammonium compounds on the transmission of antibiotic resistance genes [J].Environmental Science and Pollution Research International,2019,26(27):28352-28360
[133] Sidhu M S,Sørum H,Holck A.Resistance to quaternary ammonium compounds in food-related bacteria [J].Microbial Drug Resistance,2002,8(4):393-399
[134] Wang Y,Lu J,Engelstädter J,et al.Non-antibiotic pharmaceuticals enhance the transmission of exogenous antibiotic resistance genes through bacterial transformation [J].The ISME Journal,2020,14(8):2179-2196
[135] Jutkina J,Marathe N P,Flach C F,et al.Antibiotics and common antibacterial biocides stimulate horizontal transfer of resistance at low concentrations [J].The Science of the Total Environment,2018,616-617:172-178
[136] Seier-Petersen M A,Jasni A,Aarestrup F M,et al.Effect of subinhibitory concentrations of four commonly used biocides on the conjugative transfer of Tn916 in Bacillus subtilis [J].The Journal of Antimicrobial Chemotherapy,2014,69(2):343-348
[137] Jin M,Liu L,Wang D N,et al.Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation [J].The ISME Journal,2020,14(7):1847-1856
[138] Gillings M R.Lateral gene transfer,bacterial genome evolution,and the anthropocene [J].Annals of the New York Academy of Sciences,2017,1389(1):20-36
[139] Kohanski M A,Dwyer D J,Hayete B,et al.A common mechanism of cellular death induced by bactericidal antibiotics [J].Cell,2007,130(5):797-810
[140] Laureti L,Matic I,Gutierrez A.Bacterial responses and genome instability induced by subinhibitory concentrations of antibiotics [J].Antibiotics,2013,2(1):100-114
[141] Shapiro R S.Antimicrobial-induced DNA damage and genomic instability in microbial pathogens [J].PLoS Pathogens,2015,11(3):e1004678
[142] Tong C Y,Hu H,Chen G,et al.Chlorine disinfectants promote microbial resistance in Pseudomonas sp.[J].Environmental Research,2021,199:111296
[143] Wand M E.Bacterial Resistance to Hospital Disinfection [M]//Modeling the Transmission and Prevention of Infectious Disease.Cham:Springer,2017:19-54
[144] AKimitsu N,Hamamoto H,Inoue R,et al.Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride,a disinfectant widely used in hospitals [J].Antimicrobial Agents and Chemotherapy,1999,43(12):3042-3043
[145] Langsrud S,Sundheim G,Holck A L.Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers [J].Journal of Applied Microbiology,2004,96(1):201-208
[146] Mc Cay P H,Ocampo-Sosa A A,Fleming G T A.Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture [J].Microbiology,2010,156(Pt 1):30-38
[147] Kim M,Weigand M R,Oh S,et al.Widely used benzalkonium chloride disinfectants can promote antibiotic resistance [J].Applied and Environmental Microbiology,2018,84(17):e01201-e01218
[148] Tandukar M,Oh S,Tezel U,et al.Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance [J].Environmental Science &Technology,2013,47(17):9730-9738
[149] Baker-Austin C,Wright M S,Stepanauskas R,et al.Co-selection of antibiotic and metal resistance [J].Trends in Microbiology,2006,14(4):176-182
[150] Mavri A,Smole Možina S.Development of antimicrobial resistance in Campylobacter jejuni and Campylobacter coli adapted to biocides [J].International Journal of Food Microbiology,2013,160(3):304-312
[151] Techaruvichit P,Takahashi H,Kuda T,et al.Adaptation of Campylobacter jejuni to biocides used in the food industry affects biofilm structure,adhesion strength,and cross-resistance to clinical antimicrobial compounds [J].Biofouling,2016,32(7):827-839
[152] Merchel Piovesan Pereira B,Wang X K,Tagkopoulos I.Biocide-induced emergence of antibiotic resistance in Escherichia coli [J].Frontiers in Microbiology,2021,12:640923
[153] Wand M E,Bock L J,Bonney L C,et al.Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine [J].Antimicrobial Agents and Chemotherapy,2017,61(1):e01162-e01116