内分泌干扰物(endocrine disrupting chemicals, EDCs)对于生物体的健康危害已经受到广泛关注,目前已被证实具有内分泌干扰活性的环境污染物包括农药和除草剂(利谷隆、除草醚和莠去津等)、工业化合物(双酚A、多氯联苯和壬基酚等)、类固醇雌激素(17α-乙炔雌二醇、17β-雌二醇和己烷雌酚等)和重金属(砷、镉和汞等)等[1]。这些EDCs广泛分布在水、土壤甚至是大气等环境介质中,已有诸多综述总结了这类物质对动物内分泌系统、发育过程和生殖过程等产生的不良影响,以及对肿瘤和癌症的诱导作用[2-5]。然而,以往的研究大多只关注了EDCs对亲本的毒性效应;最近的研究却发现,EDCs引起的亲代生理功能异常还会跨世代传递给子代,即使子代并没有受到EDCs的直接暴露[6-8]。这种跨世代毒性效应会影响子代的存活能力、生长发育水平和生理功能,改变种群的性别比例,损害子代捕食、求偶和躲避敌害等行为能力,从而对种群发展和生态系统健康产生深远影响[9]。因此,了解EDCs的跨世代毒性效应及机制对于全面认识EDCs的生态风险具有重要意义。
鱼类作为一种被广泛使用的模式生物,对于评估EDCs的生态风险,特别是对水生生态系统的风险具有重要价值。本文按照EDCs单独暴露雌鱼、单独暴露雄鱼以及同时暴露雌雄鱼的方式,分别从母本、父本和父母双亲的角度综述了EDCs对鱼类的跨世代毒性效应;并从EDCs的跨世代传递、内分泌激素的跨世代传递以及表观遗传学3个角度探讨了其可能的作用机制,以期为全面认识EDCs对水生生态系统的风险提供参考。
1 EDCs对鱼类的跨世代毒性效应(Transgenerational toxicity of EDCs on fish)
有性生殖的亲本或无性、孤雌生殖的F0代受到外界环境因素(如营养状况、环境温度、电离辐射和化学品暴露等)的影响,导致子代在内分泌系统、生理和发育等方面产生异常的现象,可以称之为环境因素的跨世代毒性效应[10]。即使子代在正常环境条件下培养,这种异常环境因素暴露亲本引起的毒性效应可能会一直持续好几代,这表明跨世代毒性效应是依赖于生殖细胞系来完成亲本到子代的传递的[11]。
1.1 EDCs单独暴露鱼类母本对后代的影响
由于鱼类繁殖方式的差异,EDCs单独暴露母本的方式也存在差别。对于卵生鱼来说,EDCs单独暴露母本是指仅暴露雌鱼或卵细胞,然后与未暴露过的雄鱼或精子交配产卵(图1(a));对于卵胎生或者胎生的鱼类来说,还可能是指未暴露过的雌雄鱼交配后,再用EDCs暴露怀卵雌鱼[10](图1(b))。目前关于EDCs单独暴露鱼类母本对后代的影响研究如表1所示,这种暴露方式产生的跨世代毒性效应主要包括以下几个方面。
降低子代受精率、孵化率,或延长孵化时间,从而降低子代存活率、影响种群数量。单独暴露鱼类母本可产生这类跨世代毒性的EDCs包括重金属、类固醇雌激素、工业化合物和杀虫剂等物质。其中,重金属Cd是一种制造合金的原料,我国地表水中Cd2+的检出浓度可以达到10.7 μmol·L-1[12];Wu等[6]采用4.45 μmol·L-1的Cd2+暴露雌性斑马鱼3 d,然后与未暴露的雄鱼配对产卵,发现F1代24 hpf (hour past fertilization, hpf)的死亡率显著升高。17β-雌二醇(17β-estradiol, E2)是体内合成的天然雌激素,环境中存在的E2也是一种EDCs,其在地表水中的浓度高达13.66 ng·L-1[13];E2短期暴露雌性斑马鱼,无论是采取水体暴露方式(0.73 μmol·L-1和1.1 μmol·L-1,即199 μg·L-1和299 μg·L-1)还是体内注射(1 nmol·kg-1和1 000 nmol·kg-1)方式,均会显著降低F1代存活率[7-8]。环境雌激素辛基酚(octyphenol, OP)是非离子表面活性剂烷基酚聚乙氧基化物的分解产物之一,在地表水中检出浓度为1.7×103~15.7×103 ng·L-1[14],25 μg·L-1和100 μg·L-1的OP单独暴露欧洲绵鳚母本17 d或35 d,显著升高了F1代的死亡率,同时显著降低了F1代体长和体质量[15]。塑料添加剂双酚A (bisphenol A, BPA)在地表水中检出浓度达到21 μg·L-1[16];15 μg·L-1和225 μg·L-1的BPA仅暴露雌性稀有鮈鲫21 d后,能显著降低F1代受精率和受精卵的直径,同时降低孵化率。工业用导热介质多氯联苯(polychlorinated biphenyls, PCBs)在地表水中的检出浓度为2.99~32.7 ng·L-1[17];Westerlund等[8]向斑马鱼雌鱼体内注射1 μmol·kg-1的10种PCBs同系物(PCB60、PCB104、PCB112、PCB126、PCB143、PCB173、PCB184、PCB190、羟基化的OH-PCB30和OH-PCB61),10 d后与正常雄鱼配对产卵,发现与对照组相比F1代仔鱼在8 dpf的死亡率显著升高,不同同系物之间跨世代毒性由高到低分别是OH-PCB30>PCB104>PCB60>PCB143>PCB173>OH-PCB61>PCB112>PCB126>PCB184>PCB190。有机氯类杀虫剂滴滴涕(DDT)在地表水中的检出浓度为5.03~28.3 ng·L-1[18];Metcalfe等[19]发现2.5 μg·L-1的o,p’-DDT暴露雌性青鳉14 d后与未暴露的雄鱼产卵,F1代胚胎的孵化时间显著延长,可能导致胚胎更容易受到捕食者和其他环境因子的侵害。

图1 内分泌干扰物(EDCs)单独暴露鱼类母本或父本对子代的影响
Fig. 1 Effects on fish offspring produced by only maternal or only paternal exposed to endocrine disrupting chemicals (EDCs)
诱发骨骼发育畸形,升高子代畸形率。研究发现,BPA(15 μg·L-1和225 μg·L-1)仅暴露雌鱼21 d能够诱发F1代骨骼发育异常,延迟F1代颅面软骨中的筛骨、鳃盖骨、角鳃骨和腭方骨的成骨化过程,下调成骨化相关基因的表达水平和赖氨酰氧化酶的活性,显著升高子代胚胎畸形率[20]。4.45 μmol·L-1的Cd2+仅暴露雌鱼3 d也能导致F1代仔鱼(72 hpf)颅面畸形,出现角舌软骨角度增大的现象[6]。
产生神经行为毒性。脊椎动物的行为受神经系统控制,不同神经元之间通过突触传递兴奋或者抑制,从而支配肌肉的收缩活动[21];神经行为毒性的产生会影响子代觅食和躲避敌害等行为。有研究发现重金属Pb的母本暴露能够影响鱼类子代神经系统,继而改变子代的运动活性、诱发行为异常[7]。重金属Pb被广泛用于化妆品制造、食品行业和石油化工等产业中,水环境中其浓度可高达400 μg·L-1[22]。Wang等[7]采用10 μg·L-1和100 μg·L-1的Pb暴露雌性斑马鱼14 d后与正常的雄鱼配对产卵,同时也采用Pb继续暴露F1代受精卵,结果发现母本和子代继续暴露于Pb导致了F1代在15 dpf (day past fertilization, dpf)运动的回转角度和角速度显著性降低,在30 dpf时运动距离和平均速度显著性降低;此外,在鱼缸上半部分游泳的幼鱼所占比例以及幼鱼个体之间的距离显著增加,可能和受损的脑部神经元相关。
产生内分泌干扰效应。由于目前的研究大多数采用具有雌激素效应的物质暴露母本,因此在子代中主要观察到跨世代传递的雌激素效应,表现为雌性特异性蛋白(卵黄原蛋白)表达量异常、性腺发育异常甚至性别比例失调等。例如,向雌性斑马鱼体内注射1 nmol·kg-1和1 000 nmol·kg-1的E2后,观察到F1代仔鱼体内雌激素受体表达水平显著升高[8];25 μg·L-1和100 μg·L-1的OP暴露雌性欧洲绵鳚17 d或35 d,F1代胚胎体内雌激素受体和卵黄原蛋白表达水平显著升高,同时性腺分化过程中出现雌雄兼性现象[15];2.5 μg·L-1的o,p’-DDT暴露雌性青鳉14 d,会导致F1代雌鱼卵巢发育加速,同时在E2诱导条件下F1代雄鱼肝脏能够产生更多的卵黄原蛋白[19]。
此外,研究还发现某些EDCs单独暴露母本会损伤F1代心脏功能,如Fan等[20]报道15 μg·L-1和225 μg·L-1的BPA暴露雌性稀有鮈鲫21 d后,子代的心脏功能出现异常,表现为F1代胚胎心率显著升高。
1.2 EDCs单独暴露鱼类父本对后代的影响
大多数研究中EDCs单独暴露鱼类父本包括2种情况。一方面,通过只暴露雄鱼或精子、然后与未暴露过的雌鱼或卵细胞结合受精,可以研究EDCs单独暴露鱼类父本对后代的影响(图1(d));另一方面,通过对比暴露双亲与EDCs只暴露雌鱼(未暴露雄鱼)的实验结果,也可以从侧面了解父本暴露与否对后代的影响(图1(c))。与单独暴露母本的研究相比,EDCs单独暴露父本对后代的影响研究较少(表1),这种暴露方式产生的跨世代毒性效应主要包括以下几个方面。
降低子代受精率和存活率。17α-乙炔雌二醇(17α-ethynyl estradiol, EE2)是一种人工合成的雌激素类药物,其在地表水中的浓度约9 ng·L-1[23]。多项研究发现EE2单独暴露鱼类父本会影响F1代存活,如10 ng·L-1和100 ng·L-1的EE2暴露雄性虹鳟鱼62 d[23]、0.8~65 ng·L-1的EE2暴露雄性虹鳟鱼50 d或56 d均会显著降低F1代胚胎存活率[24-25];通过比较分析,Nash等[26]的研究也证实5 ng·L-1的EE2暴露斑马鱼雄鱼整个生活史,会降低F1代受精率。
诱发鱼鳔、骨骼和心脏发育缺陷,产生多种畸形表型。2.5 ng·L-1和5 ng·L-1的EE2仅暴露雄性斑马鱼14 d后,F1代仔鱼会出现鱼鳔膨胀缺陷、体轴骨骼弯曲、软骨异常、耳石紧缩和淋巴水肿等畸形表型,其中鱼鳔膨胀缺陷和骨骼发育异常等畸形表型,会通过损伤仔鱼的平衡能力和游泳能力,影响其觅食和逃避天敌等行为[27]。而较高浓度(2 000 μg·L-1)的BPA仅暴露雄鱼,也会诱发F1代仔鱼心脏发育缺陷,表现为心脏发育相关基因表达异常,心脏出现异位心跳、心律失常、心脏骤停和心包囊肿等功能障碍和畸形表型,石蜡切片的结果也发现心脏内腔组织结构混乱[28]。
此外,父本暴露EDCs后还可影响F2代的发育过程。如Lombó等[28]发现,较高浓度(2 000 μg·L-1)的BPA除能够通过父本干扰F1代的心脏发育外,还能导致F2代的仔鱼出现心包囊肿等畸形表型。
1.3 EDCs同时暴露鱼类母本和父本对后代的影响
这种情况指的是雌鱼或卵细胞以及雄鱼或精子都受到EDCs暴露,之后配对繁殖,后代所表现出的毒性效应。但是这并不意味着父本和母本对后代产生的影响是相等的,因为双亲都受到了EDCs暴露,所以实际上并不能区分母本和父本对后代的单独影响[10]。与以上2小节中母本或父本单独暴露相比,EDCs同时暴露双亲这种方式是最常见的,很多化合物都能通过暴露双亲产生跨世代毒性(表1),这种暴露方式产生的跨世代毒性效应主要包括以下几个方面。
降低子代受精卵、存活率,升高畸形率。BPA(0.1~20 μg·L-1暴露成年雌雄斑马鱼120 d)、EE2(0.5~2 ng·L-1全世代暴露斑马鱼、3.2 ng·L-1和5.3 ng·L-1暴露黑头软口鲦104 d、1.73~723 ng·L-1暴露杂色鳉59 d)和重金属Cd (1 μg·L-1暴露三刺鱼90 d)暴露鱼类双亲后,均能显著降低F1代胚胎孵化率和存活率[29-34],BPA还能升高F1代胚胎畸形率[29-30]。此外,研究发现能产生类似跨世代毒性的EDCs还有壬基酚(nonylphenol, NP)、全氟辛烷磺酸(perfluorooctane sulfonic acid, PFOS)和多溴联苯醚(polybrominated diphenyl ethers, PBDEs)。与OP类似,NP也是非离子表面活性剂烷基酚聚乙氧基化物的分解产物之一,水环境中NP的检出浓度为0.1~336 μg·L-1[35];NP暴露斑马鱼(50 μg·L-1暴露21 d、10~100 μg·L-1暴露2~60 dph)和东方彩虹鱼(50~5 000 μg·L-1暴露24 h)双亲后,显著降低了F1代孵化率、存活率,升高了畸形率[36-38]。PFOS是一种用途广泛的全氟化合物,在阻燃剂、润滑剂、粘合剂和纸张涂料等的生产中均有使用[39],其在地表水中的检出浓度为2.4~46.88 ng·L-1[40];PFOS暴露斑马鱼(0.5 μmol·L-1暴露1~120 dpf、5~250 μg·L-1暴露8 hpf~150 dpf)和青鳉(0.01~1 mg·L-1暴露100 d)双亲后,也能显著升高F1代胚胎死亡率、畸形率,显著降低孵化率并延迟胚胎孵化时间。PBDEs是一种溴化阻燃剂,我国地表水和沉积物中PBDEs浓度分别达到24.4 ng·L-1和4 250 ng·g-1[41];依据溴原子的数目和位置不同,PBDEs同系物多达209种,其中最常用的商业混合物DE-71含有BDE-47和BDE-209等多种同系物。研究发现DE-71 (1~10 μg·L-1暴露全世代)、BDE-47 (1~10 μg·L-1暴露全世代)和BDE-209 (3~300 μg·L-1暴露28 d)暴露斑马鱼双亲,均可显著降低F1代胚胎的孵化率和仔鱼体质量[42-44],其中BDE-209暴露后还会造成F1代仔鱼存活率和体长显著降低、畸形率显著升高[44]。
诱发神经行为毒性。暴露鱼类双亲后能产生这类跨世代毒性的EDCs主要包括BPA、PBDEs和PFOS等工业化学品以及类固醇激素EE2。2 μg·L-1和20 μg·L-1的BPA暴露成年雌雄斑马鱼120 d后,F1代仔鱼中枢神经系统基因表达和神经递质的水平以及运动活性均显著降低[45];而采用200 μg·L-1的BPA在不同发育时期暴露青鳉,会导致F1代促性腺激素释放激素3(GnRH3)神经元数目异常(过多或过少),4 dpf时F1代仔鱼脑的面积均显著性降低,同时GnRH3神经系统的紊乱显著影响了仔鱼的游泳行为,F1代仔鱼游泳距离和游泳速度也显著降低[46]。0.16~4.0 μg·L-1的DE-71暴露性成熟雌雄斑马鱼150 d后,F1代仔鱼脑中的乙酰胆碱酯酶活性降低,中枢神经系统基因表达水平异常,仔鱼运动活性降低[47];不同浓度的BDE-209长期暴露斑马鱼,也可损伤运动神经元的发育和功能,影响肌纤维排列、运动行为和视觉功能[22, 48]。0.5 μmol·L-1的PFOS在不同发育阶段暴露斑马鱼双亲后,能够改变F1代仔鱼在光暗转换实验中的游泳速度,产生神经行为毒性[49]。此外,重金属Hg暴露双亲也可诱发子代的神经行为毒性。环境中的Hg污染主要来源于煤炭燃烧和金矿开采等人类活动,进入水环境中的Hg被细菌代谢为甲基汞(methylmercury, MeHg)继而产生生物毒性[50],地表水中MeHg的检出浓度为0.053~0.33 ng·L-1[51];采用食物暴露方式,研究发现MeHg暴露斑马鱼全世代会降低F1代仔鱼在视觉-运动响应中的反应速度[52],暴露绒须石首鱼双亲30 d会导致F1代游泳速度和对震动刺激的响应能力降低[53]。最近的研究还发现EE2暴露亲本也会引起子代神经行为异常:新缸实验和趋光实验的结果发现,EE2暴露孔雀鱼(20 ng·L-1暴露怀卵雌鱼至-F1出生)和斑马鱼(1.2 ng·L-1和1.6 ng·L-1暴露1~80 dpf)亲本后,会显著延迟F1和/或F2代成鱼首次进入新缸上部的时间和首次进入光照空间的时间,且在上部空间和光照空间停留的总时间均显著缩短,表明EE2暴露亲本导致子代产生了明显的焦虑状行为[54-55]。
产生内分泌干扰效应。目前的研究主要关注了EDCs暴露鱼类双亲后,对子代产生的雌激素效应和生殖毒性,以及对子代甲状腺内分泌系统的干扰。其中,类固醇激素E2和EE2、工业化学品NP以及重金属Hg和Pb均能对F1代产生雌激素效应和生殖毒性[56-60, 22],主要的毒性终点包括改变F1代受精卵中性激素水平、改变F1代成鱼肝脏卵黄原蛋白含量、降低雌鱼性腺指数、延迟产卵时间、减少产卵量和诱导性腺出现雌雄兼性现象等。而多种PBDEs暴露鱼类双亲后则能够引发F1代甲状腺内分泌系统紊乱,如1~10 μg·L-1的DE-71暴露斑马鱼2 hpf~150 dpf、1~10 μg·L-1的BDE-47暴露斑马鱼2 hpf~180 dpf以及3~300 μg·L-1的BDE-209暴露斑马鱼28 d,均会显著改变F1代受精卵和仔鱼的甲状腺素(T4)和三碘甲状腺原氨酸(T3)水平、扰乱下丘脑-垂体-甲状腺(hypothalamic-pituitary-thyroid, HPT)轴基因表达水平[22, 42-44]。此外,研究也发现2 μg·L-1和20 μg·L-1的BPA暴露成年斑马鱼120 d后,也能显著降低F1代受精卵和仔鱼体内T4水平[45]。
损伤免疫系统功能。BPA的研究中还发现,0.1~10 μg·L-1的BPA暴露亲本斑马鱼全生命周期后,F1代仔鱼免疫系统相关基因的表达紊乱;同时在受细菌或病毒感染时,仔鱼体内溶菌酶的活性显著升高、而“呼吸爆发”反应和氧化防御相关基因的表达水平显著降低,表明BPA暴露损伤了F1代的先天性免疫功能[29]。
影响F2代的发育过程。EE2和MeHg的研究中发现,双亲暴露不仅会影响F1代发育,甚至还会将这种不良影响传递至F2代。如20 ng·L-1的EE2暴露孔雀鱼亲代,对F1和F2代均产生显著的神经行为毒性,表现为成年雄鱼在新缸环境中首次进入上部的时间延长、且在上部停留的时间减少[54]。MeHg的研究中发现,0.5~11 μg·g-1的MeHg经食物暴露底鳉双亲42 d,除显著降低F1代成鱼的产卵量外,也显著降低了F2代受精率[60]。
表1 内分泌干扰物(EDCs)单独暴露鱼类母本、父本或同时暴露双亲对后代的影响
Table 1 Effects on fish offspring produced by only maternal, only paternal, or parental fish exposed to endocrine disrupting chemicals (EDCs)
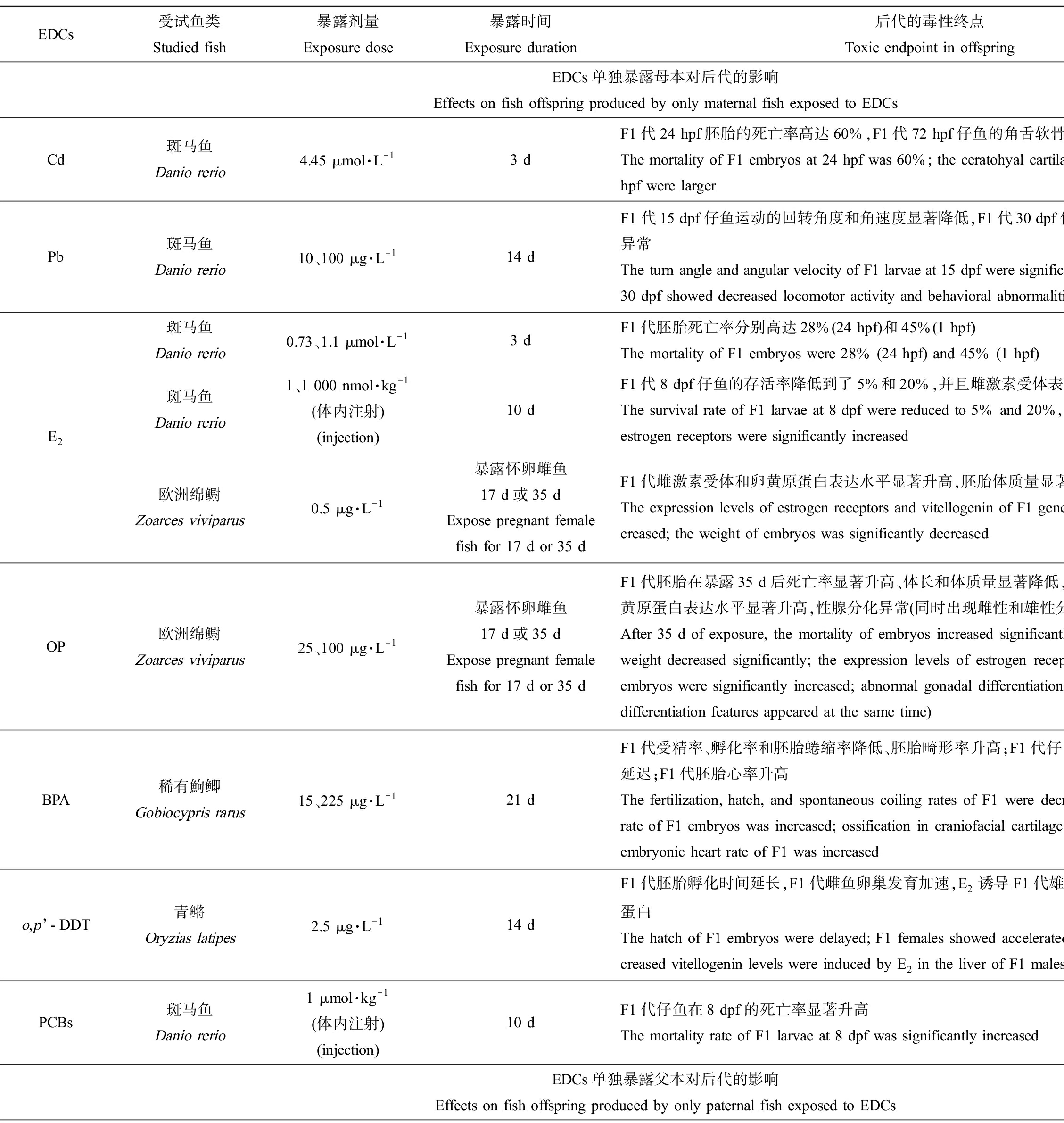
EDCs受试鱼类Studied fish暴露剂量Exposure dose暴露时间Exposure duration后代的毒性终点Toxic endpoint in offspring参考文献ReferenceEDCs单独暴露母本对后代的影响 Effects on fish offspring produced by only maternal fish exposed to EDCsCd斑马鱼Danio rerio4.45 μmol·L-13 dF1代24 hpf胚胎的死亡率高达60%,F1代72 hpf仔鱼的角舌软骨角度变大The mortality of F1 embryos at 24 hpf was 60%; the ceratohyal cartilage angle of F1 larvae at 72 hpf were larger[6]Pb斑马鱼Danio rerio10、100 μg·L-114 dF1代15 dpf仔鱼运动的回转角度和角速度显著降低,F1代30 dpf仔鱼的运动活性降低、行为异常The turn angle and angular velocity of F1 larvae at 15 dpf were significantly decreased; F1 larvae at 30 dpf showed decreased locomotor activity and behavioral abnormalities[7]E2斑马鱼Danio rerio0.73、1.1 μmol·L-13 dF1代胚胎死亡率分别高达28%(24 hpf)和45%(1 hpf)The mortality of F1 embryos were 28% (24 hpf) and 45% (1 hpf)[6]斑马鱼Danio rerio1、1 000 nmol·kg-1(体内注射)(injection)10 dF1代8 dpf仔鱼的存活率降低到了5%和20%,并且雌激素受体表达水平显著升高The survival rate of F1 larvae at 8 dpf were reduced to 5% and 20%, and the expression levels of estrogen receptors were significantly increased[8]欧洲绵鳚Zoarces viviparus0.5 μg·L-1暴露怀卵雌鱼17 d或35 dExpose pregnant female fish for 17 d or 35 dF1代雌激素受体和卵黄原蛋白表达水平显著升高,胚胎体质量显著降低The expression levels of estrogen receptors and vitellogenin of F1 generation were significantly in-creased; the weight of embryos was significantly decreased[15]OP欧洲绵鳚Zoarces viviparus25、100 μg·L-1暴露怀卵雌鱼17 d或35 dExpose pregnant female fish for 17 d or 35 dF1代胚胎在暴露35 d后死亡率显著升高、体长和体质量显著降低,胚胎体内雌激素受体和卵黄原蛋白表达水平显著升高,性腺分化异常(同时出现雌性和雄性分化特征) After 35 d of exposure, the mortality of embryos increased significantly, and the body length and weight decreased significantly; the expression levels of estrogen receptors and vitellogenin of F1 embryos were significantly increased; abnormal gonadal differentiation occurred (female and male differentiation features appeared at the same time)[15]BPA稀有鮈鲫Gobiocypris rarus15、225 μg·L-121 dF1代受精率、孵化率和胚胎蜷缩率降低、胚胎畸形率升高;F1代仔鱼颅面软骨的成骨化过程延迟;F1代胚胎心率升高The fertilization, hatch, and spontaneous coiling rates of F1 were decreased but the malformation rate of F1 embryos was increased; ossification in craniofacial cartilage of F1 larvae were delayed; embryonic heart rate of F1 was increased[20]o,p’-DDT青鳉Oryzias latipes2.5 μg·L-114 dF1代胚胎孵化时间延长,F1代雌鱼卵巢发育加速,E2诱导F1代雄鱼肝脏产生更多的卵黄原蛋白The hatch of F1 embryos were delayed; F1 females showed accelerated development of ovary; in-creased vitellogenin levels were induced by E2 in the liver of F1 males[19]PCBs斑马鱼Danio rerio1 μmol·kg-1(体内注射)(injection)10 dF1代仔鱼在8 dpf的死亡率显著升高The mortality rate of F1 larvae at 8 dpf was significantly increased [8]EDCs单独暴露父本对后代的影响 Effects on fish offspring produced by only paternal fish exposed to EDCs
续表1
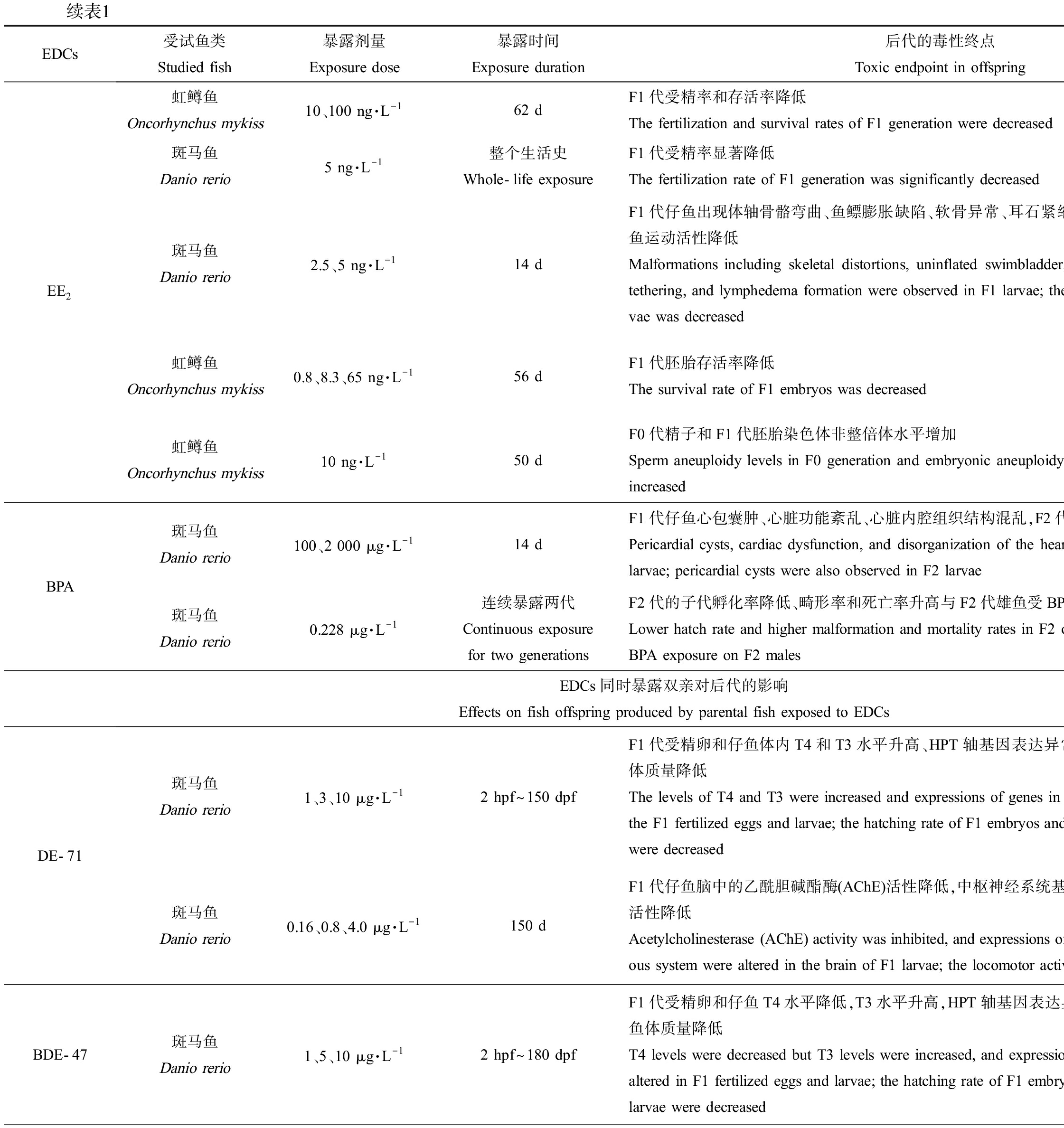
EDCs受试鱼类Studied fish暴露剂量Exposure dose暴露时间Exposure duration后代的毒性终点Toxic endpoint in offspring参考文献ReferenceEE2虹鳟鱼Oncorhynchus mykiss10、100 ng·L-162 dF1代受精率和存活率降低The fertilization and survival rates of F1 generation were decreased[23]斑马鱼Danio rerio5 ng·L-1整个生活史Whole-life exposureF1代受精率显著降低The fertilization rate of F1 generation was significantly decreased[26]斑马鱼Danio rerio2.5、5 ng·L-114 dF1代仔鱼出现体轴骨骼弯曲、鱼鳔膨胀缺陷、软骨异常、耳石紧缩、淋巴水肿等畸形表型,仔鱼运动活性降低Malformations including skeletal distortions, uninflated swimbladder, cartilage deformities, otolith tethering, and lymphedema formation were observed in F1 larvae; the locomotor activity of F1 lar-vae was decreased[27]虹鳟鱼Oncorhynchus mykiss0.8、8.3、65 ng·L-156 dF1代胚胎存活率降低The survival rate of F1 embryos was decreased[24]虹鳟鱼Oncorhynchus mykiss10 ng·L-150 dF0代精子和F1代胚胎染色体非整倍体水平增加Sperm aneuploidy levels in F0 generation and embryonic aneuploidy levels in F1 generation were increased[25]BPA斑马鱼Danio rerio100、2 000 μg·L-114 dF1代仔鱼心包囊肿、心脏功能紊乱、心脏内腔组织结构混乱,F2代仔鱼也出现心包囊肿Pericardial cysts, cardiac dysfunction, and disorganization of the heart lumen were observed in F1 larvae; pericardial cysts were also observed in F2 larvae[28]斑马鱼Danio rerio0.228 μg·L-1连续暴露两代Continuous exposure for two generationsF2代的子代孵化率降低、畸形率和死亡率升高与F2代雄鱼受BPA暴露相关Lower hatch rate and higher malformation and mortality rates in F2 offspring were associated with BPA exposure on F2 males[61]EDCs同时暴露双亲对后代的影响 Effects on fish offspring produced by parental fish exposed to EDCsDE-71斑马鱼Danio rerio1、3、10 μg·L-12 hpf~150 dpfF1代受精卵和仔鱼体内T4和T3水平升高、HPT轴基因表达异常,F1代胚胎孵化率和仔鱼体质量降低The levels of T4 and T3 were increased and expressions of genes in the HPT axis were altered for the F1 fertilized eggs and larvae; the hatching rate of F1 embryos and the body weight of F1 larvae were decreased[42]斑马鱼Danio rerio0.16、0.8、4.0 μg·L-1150 dF1代仔鱼脑中的乙酰胆碱酯酶(AChE)活性降低,中枢神经系统基因表达水平异常,仔鱼运动活性降低Acetylcholinesterase (AChE) activity was inhibited, and expressions of genes related to central nerv-ous system were altered in the brain of F1 larvae; the locomotor activity of larvae were decreased[47]BDE-47斑马鱼Danio rerio1、5、10 μg·L-12 hpf~180 dpfF1代受精卵和仔鱼T4水平降低,T3水平升高,HPT轴基因表达异常,F1代胚胎孵化率和仔鱼体质量降低T4 levels were decreased but T3 levels were increased, and expressions of genes in HPT axis were altered in F1 fertilized eggs and larvae; the hatching rate of F1 embryos and the body weight of F1 larvae were decreased[43]
续表1
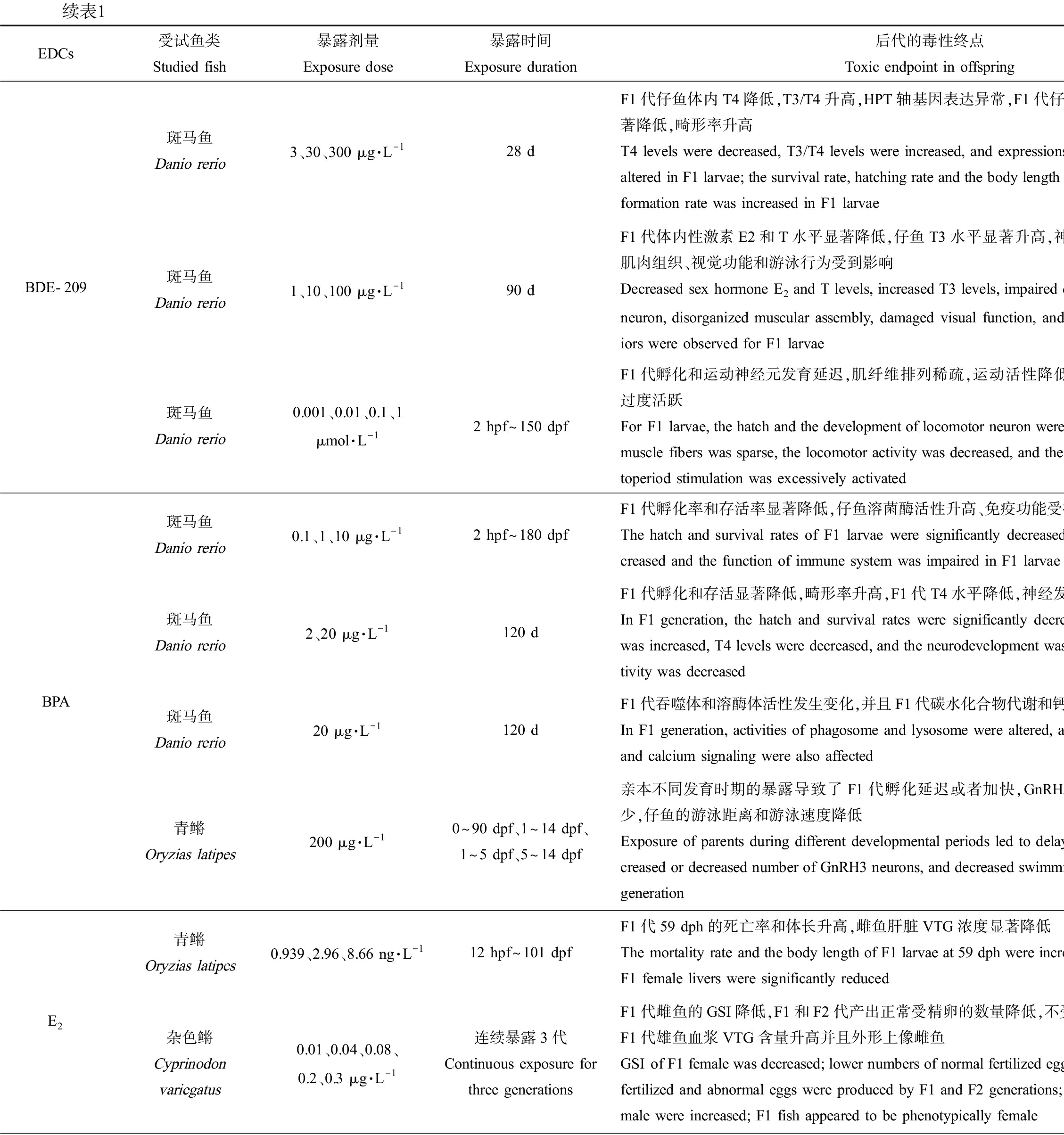
EDCs受试鱼类Studied fish暴露剂量Exposure dose暴露时间Exposure duration后代的毒性终点Toxic endpoint in offspring参考文献ReferenceBDE-209斑马鱼Danio rerio3、30、300 μg·L-128 dF1代仔鱼体内T4降低,T3/T4升高,HPT轴基因表达异常,F1代仔鱼存活率、孵化率、体长显著降低,畸形率升高T4 levels were decreased, T3/T4 levels were increased, and expressions of genes in HPT axis were altered in F1 larvae; the survival rate, hatching rate and the body length were decreased and the mal-formation rate was increased in F1 larvae[44]斑马鱼Danio rerio1、10、100 μg·L-190 dF1代体内性激素E2和T水平显著降低,仔鱼T3水平显著升高,神经元的发育和功能受损,肌肉组织、视觉功能和游泳行为受到影响Decreased sex hormone E2 and T levels, increased T3 levels, impaired development and function of neuron, disorganized muscular assembly, damaged visual function, and defected swimming behav-iors were observed for F1 larvae[22]斑马鱼Danio rerio0.001、0.01、0.1、1 μmol·L-12 hpf~150 dpfF1代孵化和运动神经元发育延迟,肌纤维排列稀疏,运动活性降低,仔鱼对明暗刺激的响应过度活跃For F1 larvae, the hatch and the development of locomotor neuron were delayed, the arrangement of muscle fibers was sparse, the locomotor activity was decreased, and the response to light-dark pho-toperiod stimulation was excessively activated[48]BPA斑马鱼Danio rerio0.1、1、10 μg·L-12 hpf~180 dpfF1代孵化率和存活率显著降低,仔鱼溶菌酶活性升高、免疫功能受损The hatch and survival rates of F1 larvae were significantly decreased; lysozyme activity was in-creased and the function of immune system was impaired in F1 larvae[29]斑马鱼Danio rerio2、20 μg·L-1120 dF1代孵化和存活显著降低,畸形率升高,F1代T4水平降低,神经发育缺陷和运动活性降低In F1 generation, the hatch and survival rates were significantly decreased, the malformation rate was increased, T4 levels were decreased, and the neurodevelopment was defected and locomotor ac-tivity was decreased[45]斑马鱼Danio rerio20 μg·L-1120 dF1代吞噬体和溶酶体活性发生变化,并且F1代碳水化合物代谢和钙信号通路传导也受到影响In F1 generation, activities of phagosome and lysosome were altered, and carbohydrate metabolism and calcium signaling were also affected[30]青鳉Oryzias latipes200 μg·L-10~90 dpf、1~14 dpf、1~5 dpf、5~14 dpf亲本不同发育时期的暴露导致了F1代孵化延迟或者加快,GnRH3神经元数目增多或者减少,仔鱼的游泳距离和游泳速度降低Exposure of parents during different developmental periods led to delayed or accelerated hatch, in-creased or decreased number of GnRH3 neurons, and decreased swimming distance and speed of F1 generation [46]E2青鳉Oryzias latipes0.939、2.96、8.66 ng·L-112 hpf~101 dpfF1代59 dph的死亡率和体长升高,雌鱼肝脏VTG浓度显著降低The mortality rate and the body length of F1 larvae at 59 dph were increased, and the VTG levels in F1 female livers were significantly reduced [56]杂色鳉Cyprinodon variegatus0.01、0.04、0.08、0.2、0.3 μg·L-1连续暴露3代Continuous exposure for three generationsF1代雌鱼的GSI降低,F1和F2代产出正常受精卵的数量降低,不受精的和异常的数目升高,F1代雄鱼血浆VTG含量升高并且外形上像雌鱼GSI of F1 female was decreased; lower numbers of normal fertilized eggs but higher numbers of un-fertilized and abnormal eggs were produced by F1 and F2 generations; plasma VTG contents of F1 male were increased; F1 fish appeared to be phenotypically female[57]
续表1

EDCs受试鱼类Studied fish暴露剂量Exposure dose暴露时间Exposure duration后代的毒性终点Toxic endpoint in offspring参考文献ReferenceEE2斑马鱼Danio rerio0.5、1、2 ng·L-12 hpf~8 mpf子代在8 hpf发育异常,导致8~24 hpf之间死亡率升高The offspring developed abnormally at 8 hpf, resulting in increased mortality at 8~24 hpf[31]黑头软口鲦Pimephales promelas3.2、5.3 ng·L-1成鱼暴露至104 d、部分F1代继续全世代暴露Adults were exposed for 104 d; partial larvae were continuously exposed for the whole life F1代和F2代的个体数量均降低The count of individuals in both F1 and F2 (a part of individuals was early-life exposed and the other part was whole-life exposed) generations were decreased [32]EE2杂色鳉Cyprinodon variegatus1.73、18.1、117、328、723 ng·L-159 d (从未性成熟期到性成熟期)59 d (from subadult stages to sexual maturity)F1代孵化率降低The hatch rate of F1 generation was decreased[33]斑马鱼Danio rerio2 ng·L-1连续暴露2代Continuous exposure for two generationsF2代35 dpf和75 dpf幼鱼的平均体长降低,成鱼首次产卵时间延迟且产卵量、受精率降低The average body length of F2 larvae at 35 dpf and 75 dpf were decreased; the first spawning time of adult fish was delayed, and the spawning quantity and fertilization rate were decreased[58]孔雀鱼Poecilia reticulata20 ng·L-1从雌鱼怀卵开始暴露至F0代仔鱼出生Expose pregnant female fish until F0 larvae were born F1和F2成年雄鱼在新缸环境中首次进入上部的时间延长、且在上部停留的时间减少In the novel tank test, latency to enter the top area for the first time was delayed and the time spent in the top area in the novel tank test was less for F1 and F2 adult male fish [54]斑马鱼Danio rerio1.2、1.6 ng·L-11~80 dpfF1代雌雄成鱼在新缸环境中进入上部的时间、以及在趋光实验中首次进入光照空间的时间均显著延迟,且停留时间均显著缩短For F1 adult male and female fish, both latency to enter the top area for the first time in the novel tank test, and latency to enter the light area for the first time in the scototaxis test were delayed; in addition, the residence time in both tests were shortened [55]NP斑马鱼Danio rerio50 μg·L-121 dF1代仔鱼畸形率升高The malformation rate of F1 larvae was increased[36]斑马鱼Danio rerio10、30、100 μg·L-12~60 dphF1代受精卵的存活率、孵化率和正常游泳仔鱼数目降低The survival and hatch rates of F1 fertilized eggs and the count of normal swimming larvae were de-creased [37]东方彩虹鱼Melanotaenia fluviatilis50、100、500、1 000、2 250、5 000 μg·L-124 hF1代孵化率显著降低The hatch rate of F1 embryos was significantly decreased[38]青鳉Oryzias latipes17.7 μg·L-10~104 dpfF1代(60 dph)性腺组织同时出现卵巢和精巢的结构The testis-ova structure was observed in the gonad of F1 generation (60 dph) [59]PFOS斑马鱼Danio rerio0.5 μmol·L-11~20 dpf、21~120 dpf、1~120 dpfF1代胚胎畸形率和死亡率升高,改变F1代仔鱼在光暗转换实验中的游泳速度The malformation and mortality rates of F1 embryos were increased, and the swimming speed in a light-to-dark behavior test was altered in F1 larvae[49]斑马鱼Danio rerio5、50、250 μg·L-18 hpf~150 dpf高浓度组F1代7 dpf死亡率达到100%,低浓度组F1代仔鱼的运动活性异常增强The mortality rate of F1 larvae at 7 dpf was 100% in the high-dose group; the locomotor activity of F1 larvae were abnormally enhanced in lower-dose group[62]
续表1
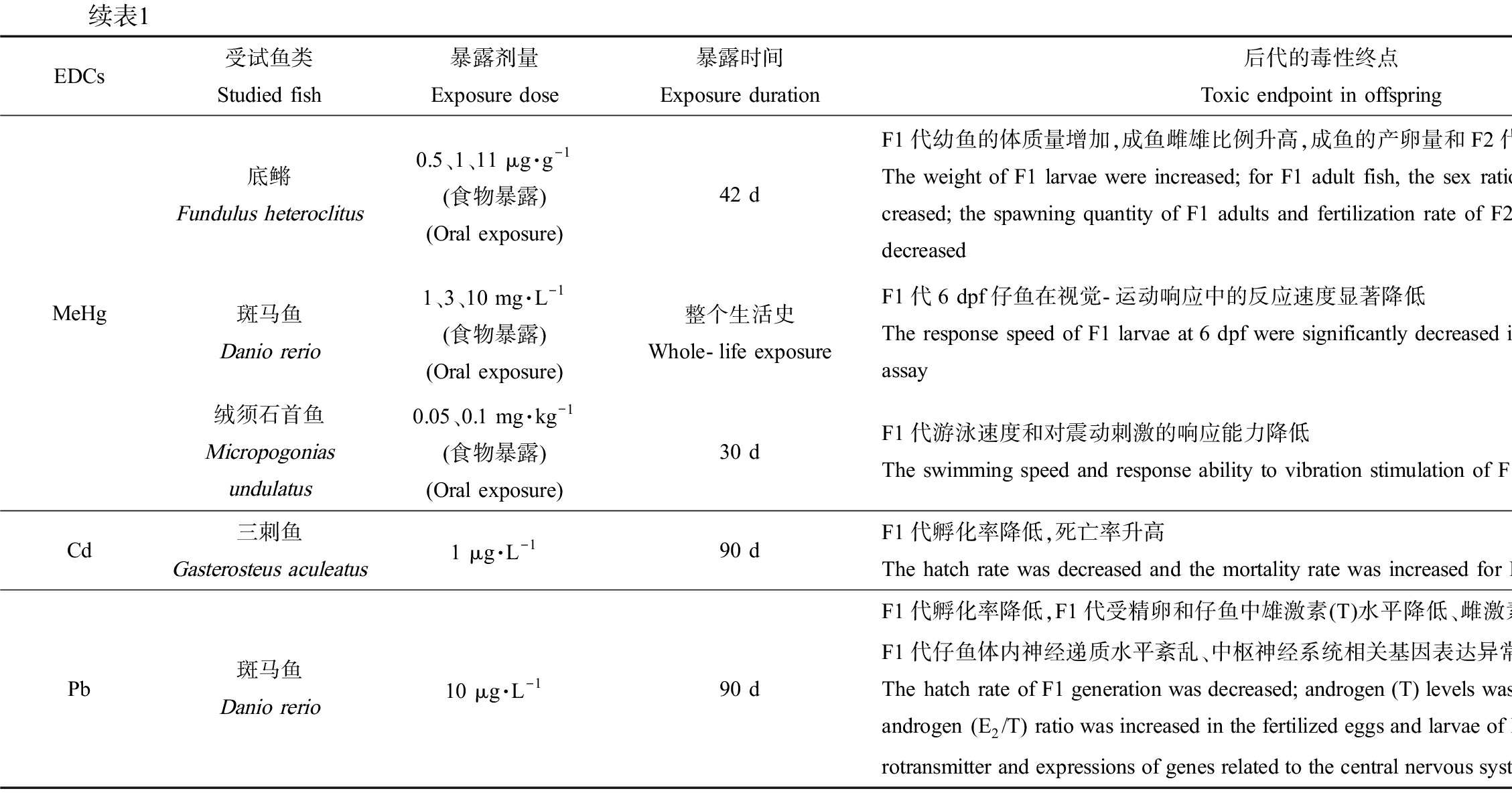
EDCs受试鱼类Studied fish暴露剂量Exposure dose暴露时间Exposure duration后代的毒性终点Toxic endpoint in offspring参考文献ReferenceMeHg底鳉Fundulus heteroclitus0.5、1、11 μg·g-1(食物暴露)(Oral exposure)42 dF1代幼鱼的体质量增加,成鱼雌雄比例升高,成鱼的产卵量和F2代受精率显著降低The weight of F1 larvae were increased; for F1 adult fish, the sex ratio of female to male was in-creased; the spawning quantity of F1 adults and fertilization rate of F2 embryos were significantly decreased[60]斑马鱼Danio rerio1、3、10 mg·L-1(食物暴露)(Oral exposure)整个生活史Whole-life exposureF1代6 dpf仔鱼在视觉-运动响应中的反应速度显著降低The response speed of F1 larvae at 6 dpf were significantly decreased in the visual-motor response assay[52]绒须石首鱼Micropogonias undulatus0.05、0.1 mg·kg-1(食物暴露)(Oral exposure)30 dF1代游泳速度和对震动刺激的响应能力降低The swimming speed and response ability to vibration stimulation of F1 generation were decreased[53]Cd三刺鱼Gasterosteus aculeatus1 μg·L-190 dF1代孵化率降低,死亡率升高The hatch rate was decreased and the mortality rate was increased for F1 generation[34]Pb斑马鱼Danio rerio10 μg·L-190 dF1代孵化率降低,F1代受精卵和仔鱼中雄激素(T)水平降低、雌激素/雄激素(E2/T)显著升高,F1代仔鱼体内神经递质水平紊乱、中枢神经系统相关基因表达异常The hatch rate of F1 generation was decreased; androgen (T) levels was decreased and the estrogen/androgen (E2/T) ratio was increased in the fertilized eggs and larvae of F1 generation; levels of neu-rotransmitter and expressions of genes related to the central nervous system were altered in F1 larvae[22]
注:E2表示17β-雌二醇;OP表示辛基酚;BPA表示双酚A;o,p’-DDT表示滴滴涕;PCBs表示多氯联苯;EE2表示17α-乙炔雌二醇;DE-71表示多溴联苯醚混合物;BDE-47表示2,2’,4,4’-四溴联苯醚;BDE-209表示十溴联苯醚;NP表示壬基酚;PFOS表示全氟辛烷磺酸;hpf表示受精后小时数;dpf表示受精后天数;mpf表示受精后月数;HPT表示下丘脑-垂体-甲状腺;GnRH3表示促性腺激素释放激素3;VTG表示卵黄蛋白原;GSI表示性腺指数;T3表示三碘甲状腺原氨酸;T4表示甲状腺素;dph表示孵化后的天数;T表示睾酮。
Note:E2 represents 17β-estradiol; OP represents octyphenol; BPA represents bisphenol A; o,p’-DDT represents o, p’-dichlorodiphenyltrichloroethane; PCBs represents polychlorinated biphenyls; EE2 represents 17α-ethynyl estradiol; DE-71 represents pentabromodiphenyl ether mixture; BDE-47 represents 2,2’,4,4’-tetrabromodiphenyl ether; BDE-209 represents decabromodiphenyl ether; NP represents nonylphenol; PFOS represents perfluorooctane sulfonate; hpf represents hour past fertilization; dpf represents day past fertilization; mpf represents month past fertilization; HPT represents hypothalamic-pituitary-thyroid; GnRH3 represents gonadotropin-releasing hormone 3; VTG represents vitellogenin; GSI represents gonadosomatic index; T3 represents triiodothyronine; T4 represents tetraiodothyronine; dph represents day post hatch; T represents testosterone.
2 EDCs对鱼类跨世代毒性效应的作用机制(Mechanisms underlying the transgenerational toxicity of EDCs on fish)
2.1 EDCs的跨世代传递
大部分EDCs特别是持久性有机污染物因具有很强的亲脂和疏水性,容易在鱼类体内富集,能够通过生殖细胞传递给子代。如研究发现PBDEs、BPA、PFOS和重金属等EDCs不仅能在F0代雌雄体内富集,还能在F1代受精卵、仔鱼体内残留,并且亲代暴露剂量与子代体内负荷量之间存在显著的正相关关系[22,44-45,62]。而且,EDCs的跨世代传递还可能持续2代或者更长时间[63]。此外,除了母体化合物,EDCs的代谢产物也可能随着生殖细胞传递至下一代[22]。由于鱼类受精卵中的主要营养物质来源于卵细胞,精子主要携带父本的遗传信息,因此目前的研究认为亲代体内的EDCs及其代谢产物主要由卵细胞传递至子代受精卵,即母源性传递[62-64],这些传递到子代的EDCs可能最终诱发各种毒性效应。
2.2 内分泌激素和其他生理因子的跨世代传递
尽管脂溶性的EDCs能够富集在卵细胞中并传递至子代,但研究发现EDCs的母源性传递并不能完全解释子代中出现的毒性效应[65-66]。事实上,EDCs暴露会导致亲代内分泌系统的生理学变化,如改变体内激素和其他生理因子的含量,而激素和其他生理因子的变化也会随着卵细胞传递至子代受精卵,这是跨世代毒性产生的另一个重要机制。
鱼类受精卵的卵黄中储存着较高含量的甲状腺激素、性激素和皮质醇等内分泌激素,这些激素均为母源性传递而来[67-69];研究发现EDCs暴露不仅会改变母本血浆中的甲状腺激素、性激素和皮质醇等内分泌激素含量,还会导致子代体内的激素出现相似变化[22, 30, 42]。如Wei等[9]发现BPA替代物双酚S(BPS)暴露斑马鱼全世代后,不仅升高了母本血浆中的T3水平,也导致F1代胚胎中T3水平显著升高,导致子代出现发育延迟、鱼鳔膨胀缺陷、运动能力减退和神经发育毒性等表型。这些结果表明,内分泌激素的跨世代传递是EDCs对子代产生毒性的重要原因。
除了改变内分泌激素水平外,EDCs还能改变亲代生殖细胞特别是卵细胞中的各种生理因子的水平,继而对子代产生毒性效应。Zhang等[66]采用有机磷酸酯磷酸三(1, 3-二氯异丙基)酯(TDCIPP)长期暴露斑马鱼F0和F1代,发现TDCIPP的母源性传递不能解释F2代中出现的跨世代毒性效应,但转录组和蛋白组分析发现,F1代卵细胞中包括核糖体和内质网蛋白加工通路在内的诸多生理途径受到暴露影响,这些基因和蛋白的异常表达可能通过卵细胞传递至子代受精卵,最终对子代产生毒性效应[66]。Valcarce等[27]也发现EE2仅暴露雄性斑马鱼后,F1代仔鱼发育畸形和运动活性的降低可能和受精卵中父本来源的雌激素受体ER mRNA水平升高相关[27]。
2.3 表观遗传修饰的跨世代继承
表观遗传的研究内容主要包括DNA甲基化、组蛋白修饰、染色质重塑和非编码RNA等[70]。研究发现EDCs的暴露能直接改变生殖系细胞的表观遗传修饰,导致表观遗传信息和相关表型的变化,而且即使后代并未受到EDCs的暴露,改变的表观遗传修饰和表型仍能世代遗传,这种现象称作表观遗传修饰的跨世代继承[71]。关于哺乳动物表观遗传的跨世代继承研究较多,而在鱼类中则非常有限,且主要集中在DNA甲基化修饰调节方面[72]。
鱼类胚胎发生期和原始生殖细胞分化期会发生DNA甲基化的重编程,且这2个时期DNA甲基化模式对于EDCs的暴露极其敏感,甲基化水平一旦改变难以恢复,甚至会持续至鱼类性成熟且会传递至子代中[73]。Kamstra等[74]分别采用塑化剂邻苯二甲酸酯代谢物邻苯二甲酸单乙基己基酯(MEHP)和DNA甲基转移酶抑制剂5AC暴露F0代斑马鱼0~6 dpf,发现2种化合物特别是5AC显著降低了F1和F2代仔鱼的体长、阻碍了鱼鳔膨胀过程,甚至显著升高了F1代中雄鱼的性别比例。通过分析全基因组和个别位点的DNA甲基化水平,Kamstra等[74]发现MEHP和5AC均显著降低了F0代基因组DNA甲基化水平,而且未暴露的F1和F2代基因组中出现了类似F0代的差异性甲基化区域;这些差异性甲基化区域主要富集在DNA的远端非编码区域,可能参与调控能量代谢和胚胎发育相关基因的表达,从而对F1和F2代产生发育毒性[74]。另一项关于MeHg的研究中也发现,DNA甲基化修饰的跨世代继承可能是MeHg产生跨世代毒性的重要原因。Carvan等[75]采用含nmol·L-1级别的MeHg水体暴露斑马鱼0~24 hpf (F0代),然后转移到清水中培养至成鱼产卵,发现F0和F2代都表现出过度活跃、视觉缺陷和视觉电生理异常的表型。进一步分析发现,F2代精子DNA中差异性甲基化区域与F0代高度重合,这些差异性甲基化区域可能通过影响神经配体-受体相互作用通路基因、以及肌动蛋白-细胞骨架通路基因的表达,导致子代出现神经行为的异常表型[75]。除了DNA甲基化外,其他表观遗传修饰的跨世代继承在鱼类跨世代毒性中发挥的作用还需要进一步研究。
3 结论和展望(Conclusion and prospects)
(1)以往的研究主要关注了EDCs对亲代的影响,最近的研究发现很多EDCs在环境浓度水平即可对鱼类产生多种跨世代毒性,包括降低子代受精率、孵化率和存活率,诱发骨骼、鱼鳔和心脏等组织器官发育畸形,产生多种神经行为毒性和内分泌干扰效应等,甚至能将这种跨世代毒性传递多代。这些跨世代毒性的产生既可以直接降低子代存活率,又可以通过影响子代生长发育水平、觅食和躲避敌害的行为能力、以及求偶生殖过程,间接威胁个体生存,从而影响种群的长远发展和生态系统健康。因此,认识EDCs的跨世代毒性及其作用机制,有助于全面认识EDCs的生态风险。然而,一方面与EDCs暴露亲代的研究相比,跨世代毒性效应的研究仍然较少;另一方面与单一EDCs暴露的情形相比,自然环境条件下多种EDCs或EDCs与其他污染物复合暴露的情形更多,涉及的作用机制更加复杂。因此,今后的研究应进一步关注EDCs特别是新污染物的跨世代毒性效应,同时应从多个角度分析复合暴露情形下的跨世代毒性及其作用机制。
(2)目前鱼类中的研究主要关注了EDCs暴露F0代对F1代的跨世代毒性,对F2代乃至F3代影响的研究较少,未来的研究应继续关注EDCs的多世代毒性效应,以进一步探究污染物暴露对种群发展的动态影响。此外,在探讨跨世代毒性时,EDCs、内分泌激素和其他生理因子的母源传递往往只持续一代,这些机制只能解释F1代中的变化;尽管有少量研究从上述机制的角度阐释了F2代中出现的毒性效应,但值得注意的是,这些研究中的F1代仍然是直接暴露在EDCs中的[58, 61, 66]。因此,在探讨EDCs多世代毒性效应机制时,今后的研究应重点关注能够多世代遗传的表观遗传修饰机制。
[1] Bergman Å, Heindel J, Jobling S, et al. State-of-the-science of endocrine disrupting chemicals, 2012 [J]. Toxicology Letters, 2012, 211: S3
[2] Koch C A, Diamanti-Kandarakis E. Introduction to endocrine disrupting chemicals - Is it time to act? [J]. Reviews in Endocrine and Metabolic Disorders, 2015, 16(4): 269-270
[3] Annamalai J, Namasivayam V. Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife [J]. Environment International, 2015, 76: 78-97
[4] Sun Y, Huang H, Sun Y, et al. Occurrence of estrogenic endocrine disrupting chemicals concern in sewage plant effluent [J]. Frontiers of Environmental Science & Engineering, 2014, 8(1): 18-26
[5] Futran Fuhrman V, Tal A, Arnon S. Why endocrine disrupting chemicals (EDCs) challenge traditional risk assessment and how to respond [J]. Journal of Hazardous Materials, 2015, 286: 589-611
[6] Wu S M, Su C K, Shu L H. Effects of calcium and estrogen on the development of the ceratohyal cartilage in zebrafish (Danio rerio) larvae upon embryo and maternal cadmium exposure [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2018, 213: 47-54
[7] Wang Y C, Shen C, Wang C G, et al. Maternal and embryonic exposure to the water soluble fraction of crude oil or lead induces behavioral abnormalities in zebrafish (Danio rerio), and the mechanisms involved [J]. Chemosphere, 2018, 191: 7-16
[8] Westerlund L, Billsson K, Andersson P. Early life-stage mortality in zebrafish (Danio rerio) following maternal exposure to polychlorinated biphenyls and estrogen [J]. Environmental Toxicology and Chemistry, 2000, 19(6): 1582-1588
[9] Wei P H, Zhao F, Zhang X N, et al. Transgenerational thyroid endocrine disruption induced by bisphenol S affects the early development of zebrafish offspring [J]. Environmental Pollution, 2018, 243: 800-808
[10] Schwindt A R. Parental effects of endocrine disrupting compounds in aquatic wildlife: Is there evidence of transgenerational inheritance? [J]. General and Comparative Endocrinology, 2015, 219: 152-164
[11] Skinner M K, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors [J]. Reproductive Toxicology, 2011, 31(3): 337-343
[12] Ke X, Gui S F, Huang H, et al. Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China [J]. Chemosphere, 2017, 175: 473-481
[13] Hassani G, Babaei A A, Takdastan A, et al. Occurrence and fate of 17β-estradiol in water resources and wastewater in Ahvaz, Iran [J]. Global Nest Journal, 2016, 18(4): 855-866
[14] 陈茹. 珠江河口水体和沉积物中壬基酚和辛基酚的分布特征及风险评价[D]. 广州: 暨南大学, 2014: 28
Chen R. Distribution characteristics and risk assessment of nonylphenol and octylphenol in water and sediments from riverine runoff of the Pearl River Delta [D]. Guangzhou: Jinan University, 2014: 28 (in Chinese)
[15] Rasmussen T H, Andreassen T K, Pedersen S N, et al. Effects of waterborne exposure of octylphenol and oestrogen on pregnant viviparous eelpout (Zoarces viviparus) and her embryos in ovario [J]. The Journal of Experimental Biology, 2002, 205(Pt 24): 3857-3876
[16] Kang J H, Asai D, Katayama Y. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms [J]. Critical Reviews in Toxicology, 2007, 37(7): 607-625
[17] 邵阳, 杨国胜, 刘韦华, 等. 北京地区地表水中OCPs和PCBs的污染分析[J]. 中国环境科学, 2016, 36(9): 2606-2613
Shao Y, Yang G S, Liu W H, et al. The study of organochlorine pesticides and polychlorinated biphenyls in surface water around Beijing [J]. China Environmental Science, 2016, 36(9): 2606-2613 (in Chinese)
[18] 罗冬莲. 福建漳江口水环境中滴滴涕(DDTs)的分布与溯源[J]. 应用生态学报, 2014, 25(12): 3664-3672
Luo D L. Distribution characteristics and source apportionment of dichloro-diphenyl-tricgloroethanes in Zhangjiang River Estuary of Fujian, China [J]. Chinese Journal of Applied Ecology, 2014, 25(12): 3664-3672 (in Chinese)
[19] Metcalfe T L, Metcalfe C D, Kiparissis Y, et al. Gonadal development and endocrine responses in Japanese medaka (Oryzias latipes) exposed to o,p’-DDT in water or through maternal transfer [J]. Environmental Toxicology and Chemistry, 2000, 19(7): 1893
[20] Fan X T, Wu L, Hou T T, et al. Maternal bisphenol A exposure impaired endochondral ossification in craniofacial cartilage of rare minnow (Gobiocypris rarus) offspring [J]. Ecotoxicology and Environmental Safety, 2018, 163: 514-520
[21] Brustein E, Saint-Amant L, Buss R R, et al. Steps during the development of the zebrafish locomotor network [J]. Journal of Physiology-Paris, 2003, 97(1): 77-86
[22] Chen L G, Wang X F, Zhang X H, et al. Transgenerational endocrine disruption and neurotoxicity in zebrafish larvae after parental exposure to binary mixtures of decabromodiphenyl ether (BDE-209) and lead [J]. Environmental Pollution, 2017, 230: 96-106
[23] Schultz I R, Skillman A, Nicolas J M, et al. Short-term exposure to 17 alpha-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout (Oncorhynchus mykiss) [J]. Environmental Toxicology and Chemistry, 2003, 22(6): 1272-1280
[24] Brown K H, Schultz I R, Nagler J J. Reduced embryonic survival in rainbow trout resulting from paternal exposure to the environmental estrogen 17alpha-ethynylestradiol during late sexual maturation [J]. Reproduction, 2007, 134(5): 659-666
[25] Brown K H, Schultz I R, Cloud J G, et al. Aneuploid sperm formation in rainbow trout exposed to the environmental estrogen 17{alpha}-ethynylestradiol [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(50): 19786-19791
[26] Nash J P, Kime D E, Van der Ven L T M, et al. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish [J]. Environmental Health Perspectives, 2004, 112(17): 1725-1733
[27] Valcarce D G, Vuelta E, Robles V, et al. Paternal exposure to environmental 17-alpha-ethinylestradiol concentrations modifies testicular transcription, affecting the sperm transcript content and the offspring performance in zebrafish [J]. Aquatic Toxicology, 2017, 193: 18-29
[28] Lombó M, Fernández-Díez C, González-Rojo S, et al. Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure [J]. Environmental Pollution, 2015, 206: 667-678
[29] Dong X, Zhang Z, Meng S L, et al. Parental exposure to bisphenol A and its analogs influences zebrafish offspring immunity [J]. Science of the Total Environment, 2018, 610-611: 291-297
[30] Chen L G, Hu C Y, Guo Y Y, et al. TiO2 nanoparticles and BPA are combined to impair the development of offspring zebrafish after parental coexposure [J]. Chemosphere, 2019, 217: 732-741
[31] Soares J, Coimbra A M, Reis-Henriques M A, et al. Disruption of zebrafish (Danio rerio) embryonic development after full life-cycle parental exposure to low levels of ethinylestradiol [J]. Aquatic Toxicology, 2009, 95(4): 330-338
[32] Schwindt A R, Winkelman D L, Keteles K, et al. An environmental oestrogen disrupts fish population dynamics through direct and transgenerational effects on survival and fecundity [J]. Journal of Applied Ecology, 2014, 51(3): 582-591
[33] Zillioux E J, Johnson I C, Kiparissis Y, et al. The sheepshead minnow as an in vivo model for endocrine disruption in marine teleosts: A partial life-cycle test with 17alpha-ethynylestradiol [J]. Environmental Toxicology and Chemistry, 2001, 20(9): 1968-1978
[34] Hani Y M I, Turies C, Palluel O, et al. Effects of chronic exposure to cadmium and temperature, alone or combined, on the threespine stickleback (Gasterosteus aculeatus): Interest of digestive enzymes as biomarkers [J]. Aquatic Toxicology, 2018, 199: 252-262
[35] Kang I, Yokota H, Oshima Y, et al. Effects of 4-nonylphenol on reproduction of Japanese medaka, Oryzias latipes [J]. Environmental Toxicology and Chemistry: An International Journal, 2003, 22(10): 2438-2445
[36] Yang F X, Xu Y, Hui Y. Reproductive effects of prenatal exposure to nonylphenol on zebrafish (Danio rerio) [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2006, 142(1-2): 77-84
[37] Hill R L Jr, Janz D M. Developmental estrogenic exposure in zebrafish (Danio rerio): Ⅰ. Effects on sex ratio and breeding success [J]. Aquatic Toxicology, 2003, 63(4): 417-429
[38] Holdway D A, Hefferman J, Smith A. Multigeneration assessment of nonylphenol and endosulfan using a model Australian freshwater fish, Melanotaenia fluviatilis [J]. Environmental Toxicology, 2008, 23(2): 253-262
[39] Wang Y, Wang L, Chang W G, et al. Neurotoxic effects of perfluoroalkyl acids: Neurobehavioral deficit and its molecular mechanism [J]. Toxicology Letters, 2019, 305: 65-72
[40] Jin Y H, Liu W, Sato I, et al. PFOS and PFOA in environmental and tap water in China [J]. Chemosphere, 2009, 77(5): 605-611
[41] Wu J P, Luo X J, Zhang Y, et al. Bioaccumulation of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in wild aquatic species from an electronic waste (e-waste) recycling site in South China [J]. Environment International, 2008, 34(8): 1109-1113
[42] Yu L Q, Lam J C W, Guo Y Y, et al. Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish [J]. Environmental Science & Technology, 2011, 45(24): 10652-10659
[43] Zhao X S, Ren X, Ren B X, et al. Life-cycle exposure to BDE-47 results in thyroid endocrine disruption to adults and offsprings of zebrafish (Danio rerio) [J]. Environmental Toxicology and Pharmacology, 2016, 48: 157-167
[44] Han Z H, Li Y F, Zhang S H, et al. Prenatal transfer of decabromodiphenyl ether (BDE-209) results in disruption of the thyroid system and developmental toxicity in zebrafish offspring [J]. Aquatic Toxicology, 2017, 190: 46-52
[45] Guo Y Y, Chen L G, Wu J, et al. Parental co-exposure to bisphenol A and nano-TiO2 causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring [J]. Science of the Total Environment, 2019, 650: 557-565
[46] Inagaki T, Smith N L, Sherva K M, et al. Cross-generational effects of parental low dose BPA exposure on the Gonadotropin-Releasing Hormone3 system and larval behavior in medaka (Oryzias latipes) [J]. Neurotoxicology, 2016, 57: 163-173
[47] Chen L G, Yu K, Huang C J, et al. Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae [J]. Environmental Science & Technology, 2012, 46(17): 9727-9734
[48] He J H, Yang D R, Wang C Y, et al. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring [J]. Ecotoxicology, 2011, 20(8): 1813-1822
[49] Chen J F, Das S R, Du J L, et al. Chronic PFOS exposures induce life stage-specific behavioral deficits in adult zebrafish and produce malformation and behavioral deficits in F1 offspring [J]. Environmental Toxicology and Chemistry, 2013, 32(1): 201-206
[50] Risch M R, Gay D A, Fowler K K, et al. Spatial patterns and temporal trends in mercury concentrations, precipitation depths, and mercury wet deposition in the North American Great Lakes region, 2002-2008 [J]. Environmental Pollution, 2012, 161: 261-271
[51] 何天容, 吴玉勇, 冯新斌. 富营养化对贵州红枫湖水库汞形态和分布特征的影响[J]. 湖泊科学, 2010, 22(2): 208-214
He T R, Wu Y Y, Feng X B. The impact of eutrophication on distribution and speciation of mercury in Hongfeng Reservoir, Guizhou Province [J]. Journal of Lake Sciences, 2010, 22(2): 208-214 (in Chinese)
[52] Mora-Zamorano F X, Klingler R, Murphy C A, et al. Parental whole life cycle exposure to dietary methylmercury in zebrafish (Danio rerio) affects the behavior of offspring [J]. Environmental Science & Technology, 2016, 50(9): 4808-4816
[53] Alvarez M D C, Murphy C A, Rose K A, et al. Maternal body burdens of methylmercury impair survival skills of offspring in Atlantic croaker (Micropogonias undulatus) [J]. Aquatic Toxicology, 2006, 80(4): 329-337
[54] Volkova K, Reyhanian Caspillo N, Porseryd T, et al. Developmental exposure of zebrafish (Danio rerio) to 17α-ethinylestradiol affects non-reproductive behavior and fertility as adults, and increases anxiety in unexposed progeny [J]. Hormones and Behavior, 2015, 73: 30-38
[55] Volkova K, Reyhanian Caspillo N, Porseryd T, et al. Transgenerational effects of 17α-ethinyl estradiol on anxiety behavior in the guppy, Poecilia reticulata [J]. General and Comparative Endocrinology, 2015, 223: 66-72
[56] Seki M, Yokota H, Maeda M, et al. Fish full life-cycle testing for 17β-estradiol on medaka (Oryzias latipes) [J]. Environmental Toxicology and Chemistry, 2005, 24(5): 1259-1266
[57] Raimondo S, Hemmer B L, Goodman L R, et al. Multigenerational exposure of the estuarine sheepshead minnow (Cyprinodon variegatus) to 17β-estradiol. Ⅱ. Population-level effects through two life cycles [J]. Environmental Toxicology and Chemistry, 2009, 28(11): 2409-2415
[58] Schäfers C, Teigeler M, Wenzel A, et al. Concentration- and time-dependent effects of the synthetic estrogen, 17alpha-ethinylestradiol, on reproductive capabilities of the zebrafish, Danio rerio [J]. Journal of Toxicology and Environmental Health Part A, 2007, 70(9): 768-779
[59] Yokota H, Seki M, Maeda M, et al. Life-cycle toxicity of 4-nonylphenol to medaka (Oryzias latipes) [J]. Environmental Toxicology and Chemistry, 2001, 20(11): 2552
[60] Matta M B, Linse J, Cairncross C, et al. Reproductive and transgenerational effects of methylmercury or aroclor 1268 on Fundulus heteroclitus [J]. Environmental Toxicology and Chemistry, 2001, 20(2): 327-335
[61] Chen J F, Xiao Y Y, Gai Z X, et al. Reproductive toxicity of low level bisphenol A exposures in a two-generation zebrafish assay: Evidence of male-specific effects [J]. Aquatic Toxicology, 2015, 169: 204-214
[62] Wang M Y, Chen J F, Lin K F, et al. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring [J]. Environmental Toxicology and Chemistry, 2011, 30(9): 2073-2080
[63] Shi G H, Wang J X, Guo H, et al. Parental exposure to 6: 2 chlorinated polyfluorinated ether sulfonate (F-53B) induced transgenerational thyroid hormone disruption in zebrafish [J]. Science of the Total Environment, 2019, 665: 855-863
[64] Xu C, Niu L L, Liu J S, et al. Maternal exposure to fipronil results in sulfone metabolite enrichment and transgenerational toxicity in zebrafish offspring: Indication for an overlooked risk in maternal transfer? [J]. Environmental Pollution, 2019, 246: 876-884
[65] Cheng H C, Yan W, Wu Q, et al. Parental exposure to microcystin-LR induced thyroid endocrine disruption in zebrafish offspring, a transgenerational toxicity [J]. Environmental Pollution, 2017, 230: 981-988
[66] Zhang Y K, Su G Y, Li M, et al. Chemical and biological transfer: Which one is responsible for the maternal transfer toxicity of tris(1,3-dichloro-2-propyl) phosphate in zebrafish? [J]. Environmental Pollution, 2018, 243: 1376-1382
[67] Power D M, Llewellyn L, Faustino M, et al. Thyroid hormones in growth and development of fish [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2001, 130(4): 447-459
[68] Miccoli A, Dalla Valle L, Carnevali O. The maternal control in the embryonic development of zebrafish [J]. General and Comparative Endocrinology, 2017, 245: 55-68
[69] Sopinka N M, Capelle P M, Semeniuk C A D, et al. Glucocorticoids in fish eggs: Variation, interactions with the environment, and the potential to shape offspring fitness [J]. Physiological and Biochemical Zoology: PBZ, 2017, 90(1): 15-33
[70] Bird A. DNA methylation patterns and epigenetic memory [J]. Genes & Development, 2002, 16(1): 6-21
[71] Youngson N A, Whitelaw E. Transgenerational epigenetic effects [J]. Annual Review of Genomics and Human Genetics, 2008, 9: 233-257
[72] Head J A. Patterns of DNA methylation in animals: An ecotoxicological perspective [J]. Integrative and Comparative Biology, 2014, 54(1): 77-86
[73] Cavalieri V, Spinelli G. Environmental epigenetics in zebrafish [J]. Epigenetics & Chromatin, 2017, 10(1): 46
[74] Kamstra J H, Sales L B, Aleström P, et al. Differential DNA methylation at conserved non-genic elements and evidence for transgenerational inheritance following developmental exposure to mono(2-ethylhexyl) phthalate and 5-azacytidine in zebrafish [J]. Epigenetics & Chromatin, 2017, 10: 20
[75] Carvan M J Ⅲ, Kalluvila T A, Klingler R H, et al. Mercury-induced epigenetic transgenerational inheritance of abnormal neurobehavior is correlated with sperm epimutations in zebrafish [J]. PLoS One, 2017, 12(5): e0176155