根据2014年我国发布的《全国土壤污染状况调查公报》可知,我国受污染土壤中由重金属和类金属污染所致的占82.4%,其他为有机污染物所致[1]。重金属通常指的是密度>5 g·cm-3的金属和类金属[2],砷(arsenic, As)由于具有与重金属相似的化学性质和环境行为,通常也被归类为重金属[3];砷是土壤重金属污染的5种主要元素之一[4],重金属污染具有隐蔽、持续且不可逆转的特性[5]。砷是一种常见的广泛分布在环境中的有毒污染物[6];流行病学研究和临床观察表明,砷与许多人类癌症和非癌性疾病有关[7],对人体健康构成严重的威胁。相对于重金属砷的总量而言,砷的生物可给性与生物有效性更能精确地评估其对人体产生的健康风险;因此本文对重金属砷的来源与危害、砷的生物可给性与生物有效性的定义与关系、相关研究方法以及影响两者的因素进行了综述,以期对重金属人体健康风险评估工作提供参考。
1 砷的来源与危害(Sources and hazards of arsenic)
1.1 人体中砷的来源
砷是已知的致癌物质,对人体健康构成严重的威胁,人类在土壤环境中接触砷是人们广泛关注的问题。人体暴露于土壤砷环境之中通常包括吸入、经口摄入和皮肤接触3种方式[8-9];其中经口摄入是人体吸收土壤砷的重要途径之一,尤其是儿童在户外通过手口活动摄食土壤砷[10]。另外,人类接触砷的主要途径就是通过食用受污染的食物和水,例如食用受砷污染的水稻产品。大米是世界上占一半人口的主食,广泛被人类所食用,其积累砷的能力也比其他谷物作物更高效[11]。水稻中砷的浓度通常可达到小麦和大麦中砷浓度的10倍之多[12]。这种现象在一定程度上是由于世界上一些地区通常使用地下水灌溉水稻,使其在浸水(厌氧条件)状态下生长繁殖,而处于厌氧状态下的土壤则有利于砷释放到土壤溶液中,进而水稻可通过土壤溶液吸收砷并在稻米籽粒、秸秆等部位积累[13]。同时,水稻在厌氧培育条件下还可使五价的无机砷酸盐(iAsⅤ)转化为生物可利用性更好的三价的无机亚砷酸盐(iAsⅢ)[14],更有利于水稻对土壤砷的吸收利用。含砷农药和化肥的使用也是使水稻等农副产品中砷含量增加的重要原因。
人类不仅可以通过食用受砷污染的稻米吸收砷,食用由稻谷加工产生的副产品制成的富含营养元素的食品也是接触砷的重要途径之一。米糠作为一些国家(如日本和萨尔瓦多)受欢迎的超级食品或健康食品的补充,富含维生素、蛋白质及其他营养元素,其主要针对的是营养食品的消费者。然而,研究发现米糠中含有大量的无机砷(iAs),商业米糠中砷的含量为0.4~1.1 mg·kg-1,其中无机砷含量占比高达93.4%~97.7%[15]。相关研究还表明,米糠中总砷和无机砷的含量要高于其相应精米中砷的含量[16-17]。因此,人类在食用米糠类食品时也有可能会摄入砷,来自米糠中的砷暴露也成为一种潜在的健康风险问题。
1.2 砷的危害
人类所产生的诸多健康问题,如心血管疾病、糖尿病、肝肾损伤、膀胱癌及肺和皮肤出现癌症等皆可由接触砷所致[18-19]。砷是一种存在于土壤、水和食物等介质中的致癌物质[20-21],其中慢性砷的存在促进了癌症和皮肤病等健康问题发生频率的升高[22]。
砷的毒性在很大程度上取决于其存在的形态和种类[23]。通常,无机砷的毒性大于有机砷单甲基砷酸(MMA)和二甲基砷酸(DMA),三价砷(AsⅢ)的毒性大于五价砷(AsⅤ)[24];绝大部分的砷氧化物(如三氧化二砷)和盐类都具有高毒性的特点。三价无机砷会干扰细胞的正常代谢,影响细胞呼吸、谷胱甘肽和脂质氧化及相关酶和蛋白的基因表达过程,促进细胞坏死,而无机五价砷类对肠道细胞几乎没有毒性作用[25]。有机砷类物质在人体内的代谢产物,如二甲基砷酸(DMAV)和硫代二甲基砷酸(thio-DMAV)等,不仅可以对人体细胞膜的屏障产生破坏作用,还可以增加无机砷类物种进入人体时的生物有效性[26-27]。五价无机砷(iAsV)的毒性比单甲基砷酸(MMAV)和二甲基砷酸(DMAV)的毒性高10倍[28]。另外,砷的毒性也会受到一些化合物和元素的影响。砷毒性主要的作用机制就是氧化应激,维生素可以通过增加砷的甲基化程度和抗氧化酶来拮抗氧化应激作用以降低砷的毒害作用[29-30]。
研究表明,在结肠提取液中已经检测到了包括无机砷(iAs)、低毒性的单甲基砷酸(MMAⅤ)和二甲基砷酸(DMAⅤ)、高毒性的单甲基砷酸(MMAⅢ)以及毒性未知的单甲基单硫砷酸(MMMTAⅤ)等在内的砷物种[31],对人类健康产生严重的威胁;因此,亟需进一步加强对不同形态砷的毒性、代谢途径及生物有效性等方面的研究。
2 砷生物可给性与生物有效性的定义与关系(Definition and relationship between arsenic bioaccessibility and bioavailability)
在评价重金属砷对人体健康风险的过程中,生物可给性和生物有效性是2个重要的指标。砷生物可给性是指可溶于人体胃肠道环境且可被吸收的砷含量,而砷生物有效性是指经吸收进入人体血液循环系统的部分[32-33]。
砷生物可给性与生物有效性并不是2个相互独立的概念,已有许多研究表明生物可给性与生物有效性之间具有一定的相关性。Li等[34]分别用欧洲标准法(Unified BARGE Method, UBM)、体外胃肠道法(In Vitro Gastrointestinal, IVG)、生理原理提取法(Physiologically Based Extraction Test, PBET)、溶解度/生物利用度研究联合会方法(the Solubility/Bioavailability Research Consortium, SBRC)和德国标准研究院法(Deutsches Institut für Normung e.v. Method, DIN)共5种方法对受污染土壤中砷的生物有效性进行预测,结果表明与小鼠体内试验数据拟合度最强的是IVG方法(R2=0.83),并且UBM和SBRC方法也具有预估土壤中砷生物有效性的潜力,相关系数(r2)在0.57~0.80之间。郑小曼[35]将利用5种体外模拟方法(RIVM、IVG、DIN、PBET和SBRC)得到的砷生物可给性数据与对12种叶菜中砷生物有效性数据进行相关性拟合分析,结果表明,5种体外方法下叶菜的砷生物可给性与其生物有效性呈正相关,且预测强度范围内r2在0.3407~0.8539之间,其中PBET模型肠阶段的相关系数值(r2=0.8539)最大。Wang等[36]在砷生物可给性(As-BA)和相对生物有效性(As-RBA)的对比试验中发现,体内-体外试验的相关系数(IVIVCs)r2在胃(r2=0.392)和结肠(r2=0.362)阶段是偏低的,而在小肠阶段体内-体外试验的r2=0.544要高于前二者,因此,基于As-BA与As-RBA之间的相关性,利用肾脏和肝脏中砷的浓度、小肠阶段中砷生物可给性或许可以更好地预测米糠中砷的相对生物有效性。然而,Li等[37]通过改良的PBET胃阶段(MPBETGP)方法测定的砷生物可给性值与砷相对生物有效性的相关性不强,然而拟合砷生物有效性和生物可给性之间差异和比率的Bland-Altman曲线却吻合良好。
砷的生物可给性与生物有效性之间虽具有一定的相关性,但根据研究结果可知,不同消化阶段以及不同方法之间二者的相关性存在一定的差异[36-37];因此,并不能把砷生物可给性与生物有效性二者等同起来。这可能是由于不同消化部位砷的存在形态具有一定的差异以及不同形态的砷在胃肠环境中的吸收特性也存在很大的差异[38]。因而,在探究土壤及食物基质中砷生物可给性的同时,也应注重土壤及食物基质中砷生物有效性的相关研究,进一步明确二者间的关系式及相关修正参数;另外,目前涉及二者相关性的研究较少,没有统一的研究方法和标准,需进一步细致探究二者存在的内在联系及传递机制。
3 砷生物有效性与生物可给性的研究方法(Research methods for arsenic bioavailability and bioaccessibility)
3.1 体内方法(in vivo)
通常采用体内试验又称活体实验(in vivo)评估土壤和食物基质中砷生物有效性[34, 39]。研究砷生物有效性常用的方法一般采用动物模型活体实验,常用的模型动物有老鼠、小猪等[36, 40]。目前已经开发出来的测量土壤及食物基质中重金属的相对生物有效性的体内动物实验测定法[41],由于其试验成本高、周期长、动物个体间的差异以及涉及伦理等问题,限制了其在人类健康风险评估中的广泛使用[42-43]。近年来,源于人体结肠癌细胞的Caco-2细胞模型的发展[44],为评估土壤及食物基质中重金属的生物有效性提供了可能的方向。Caco-2细胞与小肠吸收细胞具有许多相似的特征[45],实验具有易操作、高通量、成本低等特点[46];但是,Caco-2细胞与人体小肠上皮细胞也存在一定的差异,如缺少黏液层、细胞培养条件存在差异,使得不同实验室出来的结果缺乏可比性。
由于成本及伦理等方面的考虑,体外方法(in vitro)提供了一种简单、快速和经济的方法来测量重金属的生物可给性[47];且体外方法也被认为是预测生物体内相对生物有效性(RBA)的合适的替代方法[48-49];因此,通过体外模拟(in vitro)方法评估重金属砷的生物可给性来预测其生物有效性的发展为研究土壤及食物基质中砷生物有效性提供了新的发展方向(表1)[36, 44, 48-55]。
表1 体内方法与Caco-2细胞模型的特点
Table 1 The characteristics of in-vivo methods and Caco-2 cell model
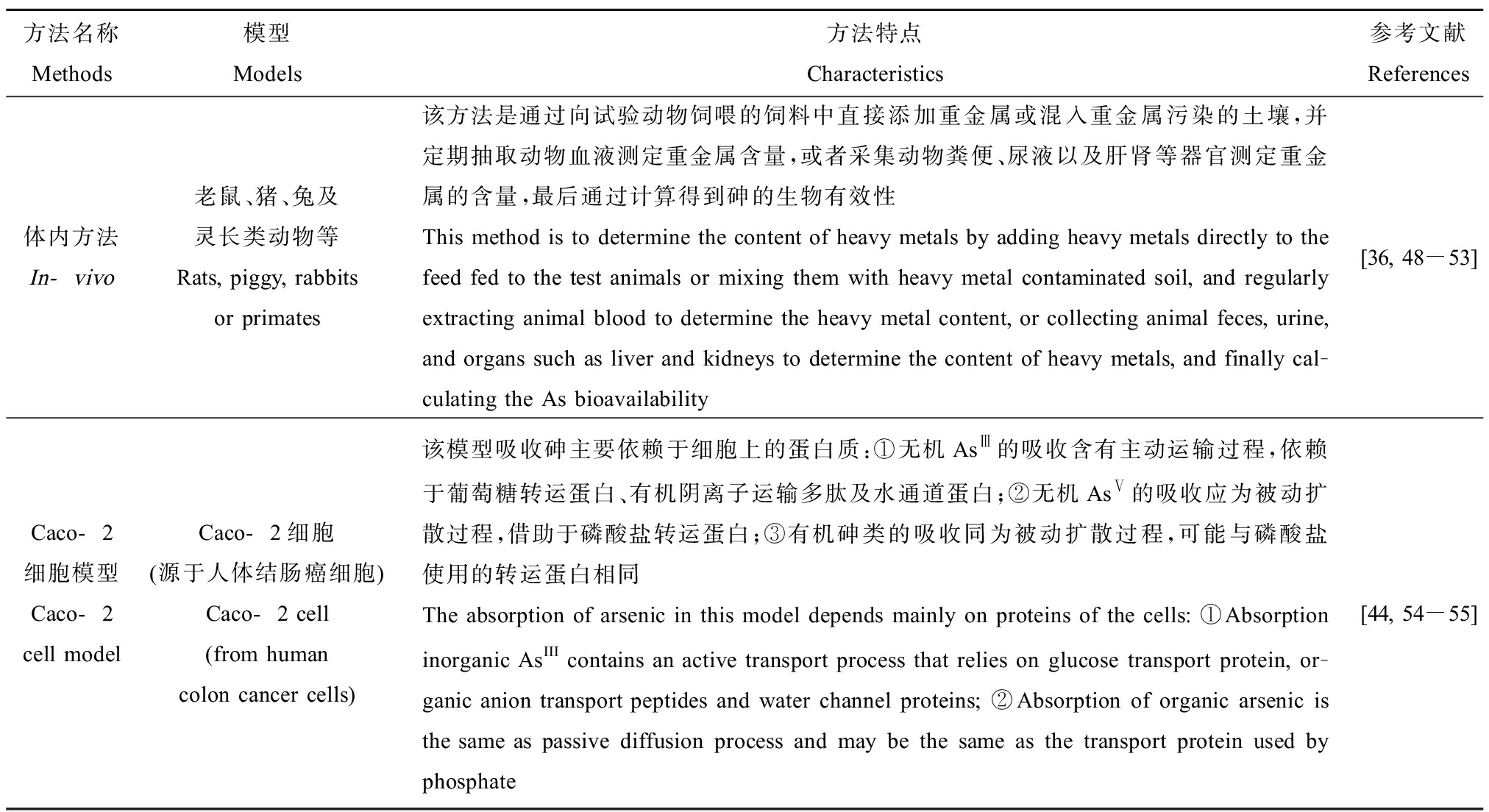
方法名称Methods模型Models方法特点Characteristics参考文献References体内方法In-vivo老鼠、猪、兔及灵长类动物等Rats, piggy, rabbits or primates该方法是通过向试验动物饲喂的饲料中直接添加重金属或混入重金属污染的土壤,并定期抽取动物血液测定重金属含量,或者采集动物粪便、尿液以及肝肾等器官测定重金属的含量,最后通过计算得到砷的生物有效性This method is to determine the content of heavy metals by adding heavy metals directly to the feed fed to the test animals or mixing them with heavy metal contaminated soil, and regularly extracting animal blood to determine the heavy metal content, or collecting animal feces, urine, and organs such as liver and kidneys to determine the content of heavy metals, and finally cal-culating the As bioavailability[36, 48-53]Caco-2细胞模型Caco-2 cell modelCaco-2细胞(源于人体结肠癌细胞)Caco-2 cell (from human colon cancer cells)该模型吸收砷主要依赖于细胞上的蛋白质:①无机AsⅢ的吸收含有主动运输过程,依赖于葡萄糖转运蛋白、有机阴离子运输多肽及水通道蛋白;②无机AsⅤ的吸收应为被动扩散过程,借助于磷酸盐转运蛋白;③有机砷类的吸收同为被动扩散过程,可能与磷酸盐使用的转运蛋白相同The absorption of arsenic in this model depends mainly on proteins of the cells: ①Absorption inorganic AsIII contains an active transport process that relies on glucose transport protein, or-ganic anion transport peptides and water channel proteins; ②Absorption of organic arsenic is the same as passive diffusion process and may be the same as the transport protein used by phosphate[44, 54-55]
3.2 体外方法(in vitro)
体外方法(in vitro)是研究重金属生物可给性常用的方法[56],在近几十年的研究中,体外模拟方法得到了较好的发展和应用。目前,国际上通常采用体外胃肠模拟方法来评估重金属砷的生物可给性[32-33, 57-59]。常用的方法各有特点,没有统一的模式,被广泛采用的体外方法主要包括生理原理提取法(PBET)、荷兰公共卫生与环境国家研究院法(RIVM)、荷兰应用科学研究院胃肠法(TIM)、德国标准研究院法(DIN)、生物可给性简化提取法(SBET)和体外胃肠道法(IVG)。这些方法各有特色和侧重,如模拟的消化器官、模拟液的pH值、酶的种类和含量、是否进食及停留时间变化、蠕动方式的影响等(表2)。在近些年的应用发展过程中,这些体外研究方法都不同程度地应用于土壤和食物2种样品中砷生物有效性与生物可给性的评估工作中[35]。然而,这些模型大多未考虑消化吸收过程中肠道微生物对食物中砷生物有效性的影响,而结肠中存在着丰富的微生物菌群,这些肠道微生物会影响食物中重金属砷的代谢与存在形态[60-61];因而在土壤及食物基质消化过程中,经过结肠的基质残渣中的部分砷也不能被忽视。由此可见,想要更加全面地评价人体健康风险,在研究胃和小肠阶段中砷的生物可给性时,也应考虑肠道微生物的影响。
表2 6种体外方法的模拟器官及特点简介
Table 2 The simulated organs and characteristics of 6 in-vitro methods
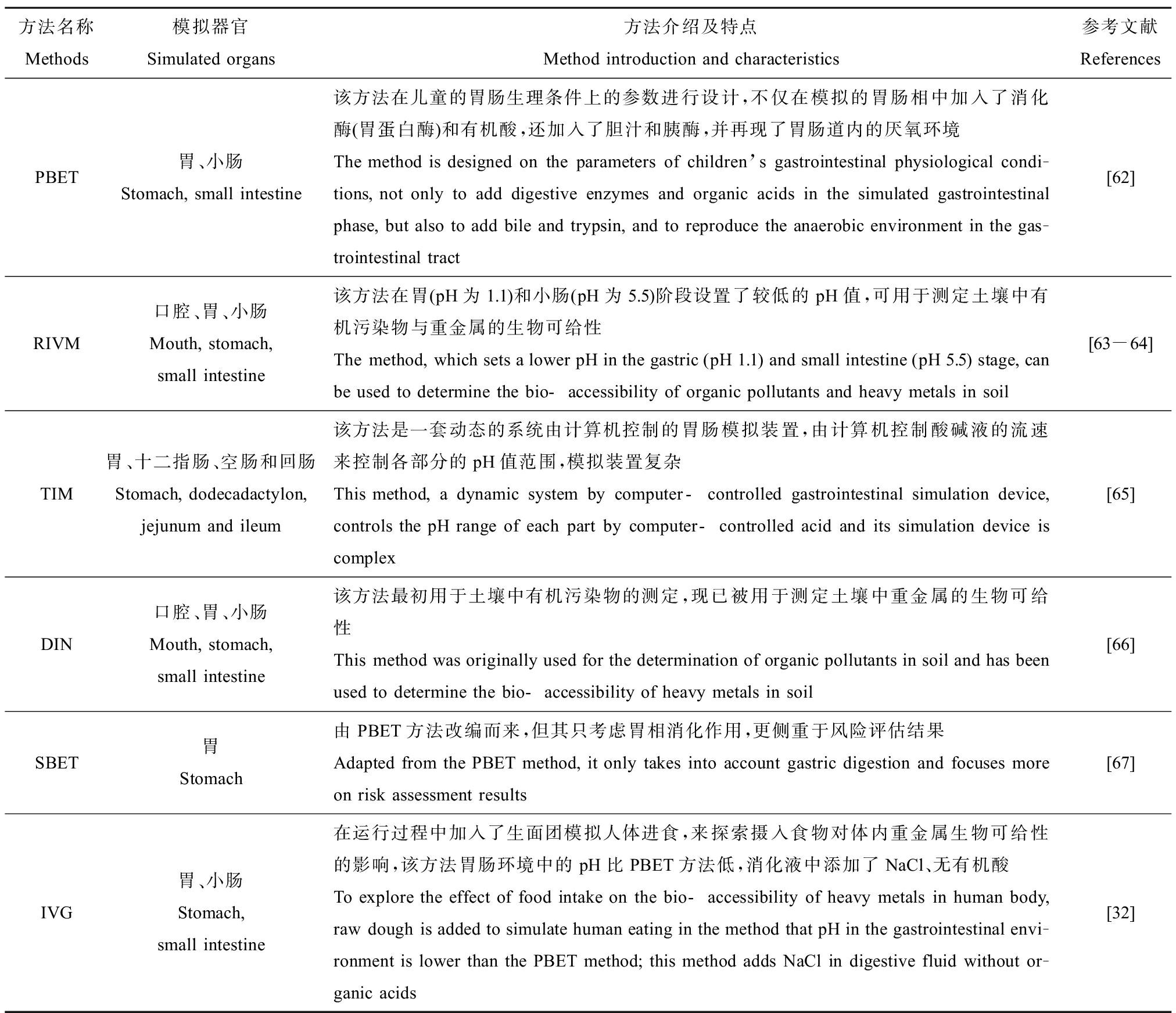
方法名称Methods模拟器官Simulated organs方法介绍及特点Method introduction and characteristics参考文献ReferencesPBET胃、小肠Stomach, small intestine该方法在儿童的胃肠生理条件上的参数进行设计,不仅在模拟的胃肠相中加入了消化酶(胃蛋白酶)和有机酸,还加入了胆汁和胰酶,并再现了胃肠道内的厌氧环境The method is designed on the parameters of children’s gastrointestinal physiological condi-tions, not only to add digestive enzymes and organic acids in the simulated gastrointestinal phase, but also to add bile and trypsin, and to reproduce the anaerobic environment in the gas-trointestinal tract[62]RIVM口腔、胃、小肠Mouth, stomach, small intestine该方法在胃(pH为1.1)和小肠(pH为5.5)阶段设置了较低的pH值,可用于测定土壤中有机污染物与重金属的生物可给性The method, which sets a lower pH in the gastric (pH 1.1) and small intestine (pH 5.5) stage, can be used to determine the bio-accessibility of organic pollutants and heavy metals in soil[63-64]TIM胃、十二指肠、空肠和回肠Stomach, dodecadactylon, jejunum and ileum该方法是一套动态的系统由计算机控制的胃肠模拟装置,由计算机控制酸碱液的流速来控制各部分的pH值范围,模拟装置复杂This method, a dynamic system by computer-controlled gastrointestinal simulation device, controls the pH range of each part by computer-controlled acid and its simulation device is complex[65]DIN口腔、胃、小肠Mouth, stomach, small intestine该方法最初用于土壤中有机污染物的测定,现已被用于测定土壤中重金属的生物可给性This method was originally used for the determination of organic pollutants in soil and has been used to determine the bio-accessibility of heavy metals in soil[66]SBET胃Stomach由PBET方法改编而来,但其只考虑胃相消化作用,更侧重于风险评估结果Adapted from the PBET method, it only takes into account gastric digestion and focuses more on risk assessment results[67]IVG胃、小肠Stomach, small intestine在运行过程中加入了生面团模拟人体进食,来探索摄入食物对体内重金属生物可给性的影响,该方法胃肠环境中的pH比PBET方法低,消化液中添加了NaCl、无有机酸To explore the effect of food intake on the bio-accessibility of heavy metals in human body, raw dough is added to simulate human eating in the method that pH in the gastrointestinal envi-ronment is lower than the PBET method; this method adds NaCl in digestive fluid without or-ganic acids[32]
近年来,人体肠道微生物生态系统模型(simulator of human intestinal ecosystem, SHIME)的发展与应用为土壤及食物基质中重金属砷生物可给性的研究提供了更为可靠的方法。SHIME是一种在体外条件下模拟人体胃肠环境及肠道微生物群落的系统,动态SHIME模型由5个隔室组成,用于模拟胃、小肠、升结肠、横结肠和降结肠,且温度维持在37 ℃;SHIME反应器内升结肠、横结肠和降结肠室中pH值范围依次为5.6~5.9、6.2~6.5和6.7~6.9;在研究前6个月内没有抗生素治疗史的成年志愿者的新鲜粪便中提取肠道菌群,在厌氧条件下培养微生物群至稳定状态后用于实验[57, 68]。结肠阶段是食物消化和代谢的一个基本阶段,作为一个动态的人体胃肠道模拟器,SHIME模型被应用于培养结肠微生物群落[69]。SHIME模型因考虑到肠道微生物在食物基质消化吸收的重要作用,能够更好地反映不同人群对食物中污染物的消化、吸收过程,而被研究者广泛认可和应用[61, 70-72]。不同的体外消化模型都有其独有的特点和侧重,若考虑将这些方法相互结合应用在土壤及食物基质中重金属砷在胃肠阶段的消化吸收特征的研究中,将有助于人体健康风险评估工作的优化和完善。如PBET方法模拟胃和小肠阶段,而SHIME模型则因引入了含有结肠微生物的结肠阶段,研究者将2种方法相结合用于研究土壤砷的代谢、形态与分布及米糠中砷的生物可给性等[36, 61]工作中,充分发挥2种方法的优越性,为准确评估由土壤和食物基质中砷暴露对人体健康潜在的风险提供了可靠的根据。
4 影响砷生物有效性与可给性的因素(Factors affecting arsenic bioavailability and bioaccessibility)
4.1 砷总浓度及其形态
人体内砷的生物有效性与生物可给性通常与其总浓度及存在形态有着密切联系。在稻米砷的健康风险评估工作中经常会考虑砷的总浓度及其存在形态的影响[73-74]。一方面,土壤及食品基质中砷的总浓度可直接对生物有效性砷的含量产生影响。研究发现,随着土壤总砷浓度的升高和草酸盐可萃取锰含量的降低,生物可利用性砷的浓度增加[61];在连续提取实验(PBET)和体外实验SHIME模型相结合的条件下,研究发现稻米中总砷的浓度在胃、小肠和结肠阶段中与生物可给性砷的总浓度呈正相关[38]。
而Wang等[75]在对北方典型水稻产区(南四湖)水稻中砷的分布和来源进行Spearman等级相关性分析和主成分分析的结果表明,水稻中砷的浓度也会受土壤和灌溉水中砷浓度的影响。另一方面,砷的形态通常在人类健康风险评估工作中发挥着关键作用。Juhasz等[40]利用小猪模型对稻米中砷生物有效性进行了研究,发现砷的生物有效性主要与稻米中砷的形态有关;如亚砷酸盐(iAsⅢ)比砷酸盐(iAsⅤ)的生物可利用性更好[14]。
4.2 矿物质元素
矿物质是构成人体组织的重要原料,人们会从食物基质中摄取钙、铁、锌和磷等矿物质元素来满足机体生长代谢的需求。在测定食物中砷生物有效性时应考虑钙、铁等矿质元素的影响,因为这些元素不仅有益于人体健康而且还可以降低砷的生物有效性[76]。水稻和海藻样品中的钙可降低砷的生物可给性,并且还可以使二甲基砷酸(DMAⅤ)在胃肠道消化环境中的溶解度有效地降低[77]。在小鼠体内试验和体外PBET与SHIME模型结合试验的对比研究结果表明,胃肠阶段中生物可给性钙、铁的含量与砷生物可给性呈显著负相关,同时,食物中Ca、Fe和Zn的含量与砷生物有效性也呈显著负相关[78]。
另外,Fe(3 mg·L-1)可以在模拟的胃肠消化道上进行絮凝,并在消化期间与溶解性的砷结合,导致可溶性砷的含量减少[79],相似的研究也表明体外试验中在小肠阶段Fe通过共絮凝作用使砷的生物可给性降低[80];可溶性的二价铁盐和三价铁盐降低砷生物可给性的效率比金属铁更高,添加可溶性三价铁盐是通过增加Fe(Ⅲ)氢氧化物和降低土壤pH值以减少土壤中砷生物可给性的[81]。磷也可能会增加土壤砷的流动性,使其更易溶于胃肠溶液中,从而增加砷的生物可给性[82];然而,在小肠阶段中砷酸盐可能与磷酸盐、磷等共享转运蛋白,溶解的磷或许也可以抑制砷在胃肠道内的吸收[83]。
4.3 营养状态(营养物质)
营养物质对砷生物可给性有较大的影响。Oomen等[84]使用体外胃肠模拟法研究发现,奶粉(包括蛋白质和碳水化合物)可使受污染土壤中的砷生物可给性增加约7%~14%;葡萄糖作为一种碳水化合物,是溶解性的有机碳,可以增加土壤中砷的释放[85];由于砷不与碳水化合物的疏水胶束结合,液相中游离砷的生物有效性更好[86];Laird等[87]的研究表明,碳水化合物的混合物可以增加模拟胃和小肠阶段中砷的生物可给性。Wang等[71]利用人体肠道微生物生态模拟系统对4种不同营养状态(维生素C、蛋白粉、葡萄糖和禁食)对砷生物可给性的影响进行了研究,结果表明,维生素C可以增强胃和小肠阶段的砷生物可给性,蛋白粉可以显著增强胃和结肠阶段中砷生物可给性,葡萄糖可以增强小肠阶段中砷生物可给性。维生素C可以有效地从非晶体氧化铁形式的土壤砷中提取砷[88],砷还可以与蛋白质的硫醇基结合[89],这都可以对砷生物可给性产生影响。然而,Clemente等[90]研究发现,也存在某些营养物质(如半胱氨酸)可以降低水稻和海藻中砷的生物可给性。在评估土壤砷的健康风险时,应注意营养物质对砷生物可给性的影响。
4.4 肠道微生物
肠道微生物涵盖了许多具有重要功能细菌物种的大量可变基因,在人类健康中发挥着许多重要作用[69]。肠道微生物菌群可以通过影响土壤及基质中砷的代谢作用,从而对砷生物可给性与生物有效性及形态产生影响。研究表明,肠道微生物菌群可以通过还原、甲基化及硫醇化等作用对砷的代谢产生显著影响[60]。肠道微生物对砷代谢影响的研究主要以动物试验为基础进行。Sun等[91]在研究肠道微生物对稻米中砷生物可给性的影响时,发现肠道微生物可以降低稻米中砷的生物可给性,这可能是由于结肠悬浊液中含有大量的有机质吸附了砷;类似地,Wang等[78]研究米糠在不同消化阶段的砷生物有效性时发现,与小肠阶段比,结肠段的砷生物可给性较低,由此推断可能是由于结肠悬浮液中的微生物将砷吸附到了有机物质上,从而降低了砷的生物可给性。人体肠道微生物还可直接释放土壤中的砷,尤其是非晶态铁铝氧化物结合的砷,显著提高了土壤砷的生物有效性[61, 92];Yin等[93]研究了5种米糠产品在不同消化阶段砷生物有效性的变化,结果表明砷的生物有效性在胃消化阶段为52.8%~78.8%,在小肠阶段有所提高,为胃阶段的1.2倍(66.0%~95.8%),而结肠阶段砷的生物有效性明显降低(11.3%~63.6%)。肠道微生物不仅对砷的生物可利用性产生影响,对其他重金属也存在着或正或负的影响;如Wang等[94]利用PBET方法与SHIME方法相结合对小麦籽粒中Cu、Cd、Pb和Zn等重金属的生物有效性进行研究时发现,小麦籽粒经小肠和结肠消化4 h后,Cu的生物利用率呈上升趋势,而Cd、Pb和Zn的生物利用率则降低。因此,肠道微生物对砷的代谢、消化吸收及生物有效性的影响不容小觑,需进一步探究其作用机制及在砷的人体健康风险评估中考虑该因素所占的权重。
4.5 其他因素
稻米中砷生物可给性与生物有效性还受基质组分、消化场所的酸性条件和营养成分变化等因素的影响。体外消化方法实验结果表明,稻米中的蛋白对砷生物可给性有较大的影响,胃肠消化液中的生物可给性砷的含量与白蛋白、球蛋白和胶原蛋白中砷的含量呈显著正相关[38]。pH也可通过影响小肠中Fe的溶解性间接地影响砷生物有效性;研究表明,Fe对总可溶性砷浓度的影响主要发生在pH值剧烈变化的小肠阶段中[80],通过减少可溶性砷以降低生物可给性砷的吸收并导致毒性降低。在小肠液中添加碳酸盐也可影响金属的生物可给性[95-96],这也可能是小肠中可溶性砷和Fe减少的原因之一。此外,有机硒可以改变砷在萝卜中的分布,促进砷从无机形态向有机形态的转化,降低砷的生物可给性,从而减轻砷的毒理学作用,降低砷的健康风险[97];而由于Se和砷之间存在复杂的相互作用,Se对砷的作用机制尚不清楚。
5 总结与展望(Summary and outlook)
相对于土壤及食物基质中重金属砷的总量而言,其生物可给性与生物有效性的值更能准确地评估土壤及食物基质中砷的健康风险。
(1)在近几十年的发展过程中,研究者们开发出许多种评估重金属生物可给性与生物有效性的方法,从Caco-2细胞模型到体内(in vivo)方法再到体外(in vitro)方法;尽管如此,但都具有各自的优点与不足之处,没有统一的标准,评估的结果也多有出入;未来亟待建立针对不同状态下砷生物有效性与生物可给性的标准评估方法。通过标准化砷生物有效性和生物可给性评估方法将有助于这一问题的解决。
体外胃肠消化模型中的SHIME动态模型引入了结肠微生物群,更加真实地模拟了人体胃肠道的消化环境,研究者们也利用此模型对土壤及食物基质中砷的代谢、生物可给性等进行了一定的研究;而肠道微生物的种类受到饮食、性别、年龄、地区及环境化学品等外源性因素的影响[98-101],加上pH梯度及药物非特异性影响,可能会导致微生物组分发生变化。因此,需要综合考虑多种因素条件下人类肠道微生物对砷代谢及生物可给性的影响,尝试将肠道微生物培养体系标准化。
此外,由于砷的毒性与其物种形态有关,对人体肠道微生物产生的影响也应存在差异[102-103];在今后的研究中也应注重不同形态下的砷对人体肠道菌群结构和组成的影响及两者相互作用诱导疾病发生的机制,这对进一步加强砷消化吸收过程中形态及生物可给性的研究具有重要的科学意义。
(2)与单一的砷污染相比,砷与其他污染物的相互作用,对环境产生机制更为复杂的联合污染,不能孤立的看待某一种污染物对环境造成的影响。土壤和食物基质中单一砷污染在胃肠吸收消化的机制相对来说已非常明确,未来可借助体外胃肠消化模型进行砷与其他有机或无机污染物的联合污染机制研究。通过探明砷与其他污染物的联合污染机制不仅可以为砷的毒理学作用提供更加全面的信息,也对健康风险评估具有十分重要的意义。
土壤和水体中的砷污染经由食物链的富集作用最终进入人体并对人体产生毒害作用,传统的砷生物有效性与生物可给性评价仅将土壤或食物作为单一样品进行研究,对砷在土壤-食物-人体系统中的传递机制缺少探索,而利用人体肠道微生物生态系统模型进行相应的研究,能更全面地评估砷的健康风险。
(3)在肠道微生物群的影响下,不同食物来源中砷的生物可给性存在差异,以及在人体消化过程中砷物种形态是发生变化的[104],胃肠道消化期间污染土壤中砷的释放和生物转化受所处营养状态的影响[71];因此,进一步研究以确定不同食物基质中砷生物可给性的水平及影响砷生物可给性的因素具有重要意义。
[1] 中华人民共和国环境保护部,中华人民共和国国土资源部. 全国土壤污染状况调查公报[R]. 北京: 中华人民共和国环境保护部, 中华人民共和国国土资源部, 2014
[2] Oves M, Khan M S, Zaidi A, et al. Soil contamination, nutritive value, and human health risk assessment of heavy metals: An overview [M]//Toxicity of Heavy Metals to Legumes and Bioremediation. Vienna: Springer Vienna, 2012: 1-27
[3] 陈怀满, 郑春荣, 涂从, 等. 中国土壤重金属污染现状与防治对策[J]. AMBIO-人类环境杂志, 1999, 28(2): 130-134, 207
Chen H M, Zheng C R, Tu C, et al. Heavy metal pollution in soils in Chian: Status and countermeasures[J]. AMBIO-A Journal of the Hunman Environment, 1999, 28(2): 130-134, 207(in Chinese)
[4] Vodyanitskii Y N. Contamination of soils with heavy metals and metalloids and its ecological hazard (analytic review) [J]. Eurasian Soil Science, 2013, 46(7): 793-801
[5] Dong W Q Y, Cui Y, Liu X. Instances of soil and crop heavy metal contamination in China [J]. Soil and Sediment Contamination: An International Journal, 2001, 10(5): 497-510
[6] Bhattacharya P, Welch A H, Stollenwerk K G, et al. Arsenic in the environment: Biology and chemistry [J]. Science of the Total Environment, 2007, 379(2-3): 109-120
[7] Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: Toxicity, oxidative stress and human disease [J]. Journal of Applied Toxicology: JAT, 2011, 31(2): 95-107
[8] US EPA National Center for Environmental Assessment, Exposure Analysis Group. Child-Specific Exposure Factors Handbook [R]. Washington DC: US EPA National Center for Environmental Assessment, Exposure Analysis and Risk Characterization Group, 2002
[9] Zhu Y G, Sun G X, Lei M, et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice [J]. Environmental Science & Technology, 2008, 42(13): 5008-5013
[10] Ljung K, Selinus O, Otabbong E, et al. Metal and arsenic distribution in soil particle sizes relevant to soil ingestion by children [J]. Applied Geochemistry, 2006, 21(9): 1613-1624
[11] Su Y H, McGrath S, Zhao F. Rice is more efficient in arsenite uptake and translocation than wheat and barley [J]. Plant and Soil, 2010, 328: 27-34
[12] Williams P N, Villada A, Deacon C, et al. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley [J]. Environmental Science & Technology, 2007, 41(19): 6854-6859
[13] Chen H P, Tang Z, Wang P, et al. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice [J]. Environmental Pollution, 2018, 238: 482-490
[14] Arao T, Kawasaki A, Baba K, et al. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice [J]. Environmental Science & Technology, 2009, 43(24): 9361-9367
[15] Signes-Pastor A J, Carey M, Meharg A A. Inorganic arsenic removal in rice bran by percolating cooking water [J]. Food Chemistry, 2017, 234: 76-80
[16] Ruangwises S, Saipan P, Tengjaroenkul B, et al. Total and inorganic arsenic in rice and rice bran purchased in Thailand [J]. Journal of Food Protection, 2012, 75(4): 771-774
[17] Sun G X, Williams P N, Carey A M, et al. Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain [J]. Environmental Science & Technology, 2008, 42(19): 7542-7546
[18] Twaddle N C, Vanlandingham M, Beland F A, et al. Metabolism and disposition of arsenic species after repeated oral dosing with sodium arsenite in drinking water. Ⅱ. Measurements in pregnant and fetal CD-1 mice [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2018, 115: 178-184
[19] Brahman K D, Kazi T G, Afridi H I, et al. Exposure of children to arsenic in drinking water in the Tharparkar region of Sindh, Pakistan [J]. The Science of the Total Environment, 2016, 544: 653-660
[20] Cheyns K, Waegeneers N, van de Wiele T, et al. Arsenic release from foodstuffs upon food preparation [J]. Journal of Agricultural and Food Chemistry, 2017, 65(11): 2443-2453
[21] Wang X, Geng A J, Dong Y, et al. Comparison of translocation and transformation from soil to rice and metabolism in rats for four arsenic species [J]. Journal of Agricultural and Food Chemistry, 2017, 65(41): 8992-8998
[22] Molin M, Ulven S M, Meltzer H M, et al. Arsenic in the human food chain, biotransformation and toxicology: Review focusing on seafood arsenic [J]. Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (GMS), 2015, 31: 249-259
[23] Alava P, Tack F, Laing G D, et al. Arsenic undergoes significant speciation changes upon incubation of contaminated rice with human colon micro biota [J]. Journal of Hazardous Materials, 2013, 262: 1237-1244
[24] Sun H J, Rathinasabapathi B, Wu B, et al. Arsenic and selenium toxicity and their interactive effects in humans [J]. Environment International, 2014, 69: 148-158
[25] Calatayud M, Devesa V, Vélez D. Differential toxicity and gene expression in Caco-2 cells exposed to arsenic species [J]. Toxicology Letters, 2013, 218(1): 70-80
[26] Leffers L, Wehe C A, Hüwel S, et al. In vitro intestinal bioavailability of arsenosugar metabolites and presystemic metabolism of thio-dimethylarsinic acid in Caco-2 cells [J]. Metallomics: Integrated Biometal Science, 2013, 5(8): 1031-1042
[27] Leffers L, Ebert F, Taleshi M S, et al. In vitro toxicological characterization of two arsenosugars and their metabolites [J]. Molecular Nutrition & Food Research, 2013, 57(7): 1270-1282
[28] Hirner A V, Hartmann L M, Hippler J, et al. Organometal(loid) Compounds Associated with Human Metabolism [M]//Organic Metal and Metalloid Species in the Environment. Berlin, Heidelberg: Springer Berlin Heidelberg, 2004: 181-203
[29] Majumdar S, Maiti A, Karmakar S, et al. Antiapoptotic efficacy of folic acid and vitamin B12 against arsenic-induced toxicity [J]. Environmental Toxicology, 2012, 27(6): 351-363
[30] Herrera A, Pineda J, Antonio M T. Toxic effects of perinatal arsenic exposure on the brain of developing rats and the beneficial role of natural antioxidants [J]. Environmental Toxicology and Pharmacology, 2013, 36(1): 73-79
[31] Van de Wiele T, Gallawa C M, Kubachka K M, et al. Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils [J]. Environmental Health Perspectives, 2010, 118(7): 1004-1009
[32] Rodriguez R R, Basta N T, Casteel S W, et al. An In vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media [J]. Environmental Science & Technology, 1999, 33(4): 642-649
[33] Juhasz A L, Weber J, Smith E, et al. Assessment of four commonly employed in vitro arsenic bioaccessibility assays for predicting in vivo relative arsenic bioavailability in contaminated soils [J]. Environmental Science & Technology, 2009, 43(24): 9487-9494
[34] Li J, Li K, Cui X Y, et al. In vitro bioaccessibility and in vivo relative bioavailability in 12 contaminated soils: Method comparison and method development [J]. The Science of the Total Environment, 2015, 532: 812-820
[35] 郑小曼. 叶菜类蔬菜中砷的生物有效性研究[D]. 南宁: 广西大学, 2017: 33-39
Zheng X M. Bioavailability of arsenic in leafy vegetables [D]. Nanning: Guangxi University, 2017: 33-39 (in Chinese)
[36] Wang P F, Yin N Y, Cai X L, et al. Comparison of bioaccessibility and relative bioavailability of arsenic in rice bran: The in vitro with PBET/SHIME and in vivo with mice model [J]. Chemosphere, 2020, 259: 127443
[37] Li J, Chen S, Li H B, et al. Arsenic bioaccessibility in rice grains via modified physiologically-based extraction test (MPBET): Correlation with mineral elements and comparison with As relative bioavailability [J]. Environmental Research, 2021, 198: 111198
[38] Wang P F, Yin N Y, Cai X L, et al. Assessment of arsenic distribution, bioaccessibility and speciation in rice utilizing continuous extraction and in vitro digestion [J]. Food Chemistry, 2021, 346: 128969
[39] van de Wiele T R, Oomen A G, Wragg J, et al. Comparison of five in vitro digestion models to in vivo experimental results: Lead bioaccessibility in the human gastrointestinal tract [J]. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances & Environmental Engineering, 2007, 42(9): 1203-1211
[40] Juhasz A L, Smith E, Weber J, et al. In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment [J]. Environmental Health Perspectives, 2006, 114(12): 1826-1831
[41] Li H B, Li M Y, Zhao D, et al. Oral bioavailability of As, Pb, and Cd in contaminated soils, dust, and foods based on animal bioassays: A review [J]. Environmental Science & Technology, 2019, 53(18): 10545-10559
[42] 蔡美芳, 吴仁人, 李开明, 等. 植物性食物中重金属生物可利用性研究进展[J]. 环境科学与技术, 2014, 37(11): 99-104
Cai M F, Wu R R, Li K M, et al. Bioavailability of heavy metals in vegetable food grown in contaminated soils [J]. Environmental Science & Technology, 2014, 37(11): 99-104 (in Chinese)
[43] Yin N Y, Zhao Y L, Wang P F, et al. Effect of gut microbiota on in vitro bioaccessibility of heavy metals and human health risk assessment from ingestion of contaminated soils [J]. Environmental Pollution, 2021, 279: 116943
[44] 王振洲, 崔岩山, 张震南, 等. Caco-2细胞模型评估金属人体生物有效性的研究进展[J]. 生态毒理学报, 2014, 9(6): 1027-1034
Wang Z Z, Cui Y S, Zhang Z N, et al. Evaluation on the human bioavailability of metals using Caco-2 cell model: A review [J]. Asian Journal of Ecotoxicology, 2014, 9(6): 1027-1034 (in Chinese)
[45] 张东平, 余应新, 张帆, 等. 环境污染物对人体生物有效性测定的胃肠模拟研究现状[J]. 科学通报, 2008, 53(21): 2537-2545
Zhang D P, Yu Y X, Zhang F, et al. Current status of gastrointestinal simulation research on determination of bioavailability of environmental pollutants to human body [J]. Chinese Science Bulletin, 2008, 53(21): 2537-2545 (in Chinese)
[46] Vázquez M, Devesa V, Vélez D. Characterization of the intestinal absorption of inorganic mercury in Caco-2 cells [J]. Toxicology in Vitro, 2015, 29(1): 93-102
[47] Li M Y, Wang P, Wang J Y, et al. Arsenic concentrations, speciation, and localization in 141 cultivated market mushrooms: Implications for arsenic exposure to humans [J]. Environmental Science & Technology, 2019, 53(1): 503-511
[48] Juhasz A L, Smith E, Nelson C, et al. Variability associated with as in vivo-in vitro correlations when using different bioaccessibility methodologies [J]. Environmental Science & Technology, 2014, 48(19): 11646-11653
[49] Bradham K D, Scheckel K G, Nelson C M, et al. Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils [J]. Environmental Health Perspectives, 2011, 119(11): 1629-1634
[50] Bradham K D, Diamond G L, Scheckel K G, et al. Mouse assay for determination of arsenic bioavailability in contaminated soils [J]. Journal of Toxicology and Environmental Health Part A, 2013, 76(13): 815-826
[51] Li S W, Sun H J, Wang G, et al. Lead relative bioavailability in soils based on different endpoints of a mouse model [J]. Journal of Hazardous Materials, 2017, 326: 94-100
[52] Mandal B K, Ogra Y, Suzuki K T. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in west Bengal, India [J]. Chemical Research in Toxicology, 2001, 14(4): 371-378
[53] Li S W, Sun H J, Li H B, et al. Assessment of cadmium bioaccessibility to predict its bioavailability in contaminated soils [J]. Environment International, 2016, 94: 600-606
[54] Calatayud M, Barrios J A, Vélez D, et al. In vitro study of transporters involved in intestinal absorption of inorganic arsenic [J]. Chemical Research in Toxicology, 2012, 25(2): 446-453
[55] Calatayud M, Gimeno J, Vélez D, et al. Characterization of the intestinal absorption of arsenate, monomethylarsonic acid, and dimethylarsinic acid using the Caco-2 cell line [J]. Chemical Research in Toxicology, 2010, 23(3): 547-556
[56] 崔岩山, 陈晓晨, 付瑾. 污染土壤中铅、砷的生物可给性研究进展[J]. 生态环境学报, 2010, 19(2): 480-486
Cui Y S, Chen X C, Fu J. Progress in study of bioaccessibility of lead and arsenic in contaminated soils [J]. Ecology and Environmental Sciences, 2010, 19(2): 480-486 (in Chinese)
[57] Van de Wiele T R, Verstraete W, Siciliano S D. Polycyclic aromatic hydrocarbon release from a soil matrix in the in vitro gastrointestinal tract [J]. Journal of Environmental Quality, 2004, 33(4): 1343-1353
[58] Tang X Y, Zhu Y G, Cui Y S, et al. The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China [J]. Environment International, 2006, 32(5): 682-689
[59] Wragg J, Cave M, Basta N, et al. An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil [J]. The Science of the Total Environment, 2011, 409(19): 4016-4030
[60] Lu K, Cable P H, Abo R P, et al. Gut microbiome perturbations induced by bacterial infection affect arsenic biotransformation [J]. Chemical Research in Toxicology, 2013, 26(12): 1893-1903
[61] Yin N Y, Zhang Z N, Cai X L, et al. In vitro method to assess soil arsenic metabolism by human gut Microbiota: Arsenic speciation and distribution [J]. Environmental Science & Technology, 2015, 49(17): 10675-10681
[62] Ruby M V, Davis A, Link T E, et al. Development of an in vitro screening test to evaluate the in vivo bioaccessibility of ingested mine-waste lead [J]. Environmental Science & Technology, 1993, 27(13): 2870-2877
[63] Oomen A G, Hack A, Minekus M, et al. Comparison of five In vitro digestion models to study the bioaccessibility of soil contaminants [J]. Environmental Science & Technology, 2002, 36(15): 3326-3334
[64] Ljung K, Oomen A, Duits M, et al. Bioaccessibility of metals in urban playground soils [J]. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances & Environmental Engineering, 2007, 42(9): 1241-1250
[65] Minekus M, Smeets-Peeters M, Bernalier A, et al. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products [J]. Applied Microbiology and Biotechnology, 1999, 53(1): 108-114
[66] Hack A, Selenka F. Mobilization of PAH and PCB from contaminated soil using a digestive tract model [J]. Toxicology Letters, 1996, 88(1-3): 199-210
[67] Medlin E A. An in vitro method for estimating the relative bioavailability of lead in humans [D]. Colorado: University of Colorado, 1997, 23(3): 243-249
[68] Van de Wiele T, Vanhaecke L, Boeckaert C, et al. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites [J]. Environmental Health Perspectives, 2005, 113(1): 6-10
[69] Yin N Y, Du H L, Wang P F, et al. Interindividual variability of soil arsenic metabolism by human gut microbiota using SHIME model [J]. Chemosphere, 2017, 184: 460-466
[70] 尹乃毅, 都慧丽, 张震南, 等. 应用SHIME模型研究肠道微生物对土壤中镉、铬、镍生物可给性的影响[J]. 环境科学, 2016, 37(6): 2353-2358
Yin N Y, Du H L, Zhang Z N, et al. Effects of human gut Microbiota on bioaccessibility of soil Cd, Cr and Ni using SHIME model [J]. Environmental Science, 2016, 37(6): 2353-2358 (in Chinese)
[71] Wang P F, Yin N Y, Cai X L, et al. Nutritional status affects the bioaccessibility and speciation of arsenic from soils in a simulator of the human intestinal microbial ecosystem [J]. The Science of the Total Environment, 2018, 644: 815-821
[72] Du H L, Yin N Y, Cai X L, et al. Lead bioaccessibility in farming and mining soils: The influence of soil properties, types and human gut microbiota [J]. The Science of the Total Environment, 2020, 708: 135227
[73] Ruttens A, Blanpain A C, De Temmerman L, et al. Arsenic speciation in food in Belgium [J]. Journal of Geochemical Exploration, 2012, 121: 55-61
[74] Sharafi K, Yunesian M, Nodehi R N, et al. The reduction of toxic metals of various rice types by different preparation and cooking processes: Human health risk assessment in Tehran households, Iran [J]. Food Chemistry, 2019, 280: 294-302
[75] Wang L H, Gao S L, Yin X X, et al. Arsenic accumulation, distribution and source analysis of rice in a typical growing area in North China [J]. Ecotoxicology and Environmental Safety, 2019, 167: 429-434
[76] Clemente M J, Cimbalo A, Chiocchetti G, et al. Dietary compounds to reduce in vivo inorganic arsenic bioavailability [J]. Journal of Agricultural and Food Chemistry, 2019, 67(32): 9032-9038
[77] Clemente M J, Devesa V, Vélez D. Dietary strategies to reduce the bioaccessibility of arsenic from food matrices [J]. Journal of Agricultural and Food Chemistry, 2016, 64(4): 923-931
[78] Wang P F, Yin N Y, Cai X L, et al. Comparison of bioaccessibility and relative bioavailability of arsenic in rice bran: The in vitro with PBET/SHIME and in vivo with mice model [J]. Chemosphere, 2020, 259: 127443
[79] Mohan D, Pittman C U Jr. Arsenic removal from water/wastewater using adsorbents: A critical review [J]. Journal of Hazardous Materials, 2007, 142(1-2): 1-53
[80] Yu H Y, Wu B, Zhang X X, et al. Arsenic metabolism and toxicity influenced by ferric iron in simulated gastrointestinal tract and the roles of gut Microbiota [J]. Environmental Science & Technology, 2016, 50(13): 7189-7197
[81] Subacz J L, Barnett M O, Jardine P M, et al. Decreasing arsenic bioaccessibility/bioavailability in soils with iron amendments [J]. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances & Environmental Engineering, 2007, 42(9): 1317-1329
[82] Impellitteri C A. Effects of pH and phosphate on metal distribution with emphasis on As speciation and mobilization in soils from a lead smelting site [J]. The Science of the Total Environment, 2005, 345(1-3): 175-190
[83] Villa-Bellosta R, Sorribas V. Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate [J]. Toxicology and Applied Pharmacology, 2008, 232(1): 125-134
[84] Oomen A G, Hack A, Minekus M, et al. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants [J]. Environmental Science & Technology, 2002, 36(15): 3326-3334
[85] Mohapatra D, Mishra D, Rout M, et al. Adsorption kinetics of natural dissolved organic matter and its impact on arsenic(V) leachability from arsenic-loaded ferrihydrite and Al-ferrihydrite [J]. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances & Environmental Engineering, 2007, 42(1): 81-88
[86] Moreda-Pi eiro J, Alonso-Rodríguez E, Romarís-Hortas V, et al. Assessment of the bioavailability of toxic and non-toxic arsenic species in seafood samples [J]. Food Chemistry, 2012, 130(3): 552-560
eiro J, Alonso-Rodríguez E, Romarís-Hortas V, et al. Assessment of the bioavailability of toxic and non-toxic arsenic species in seafood samples [J]. Food Chemistry, 2012, 130(3): 552-560
[87] Laird B D, Yeung J, Peak D, et al. Nutritional status and gastrointestinal microbes affect arsenic bioaccessibility from soils and mine tailings in the simulator of the human intestinal microbial ecosystem [J]. Environmental Science & Technology, 2009, 43(22): 8652-8657
[88] Kim E J, Lee J C, Baek K. Abiotic reductive extraction of arsenic from contaminated soils enhanced by complexation: Arsenic extraction by reducing agents and combination of reducing and chelating agents [J]. Journal of Hazardous Materials, 2015, 283: 454-461
[89] Narukawa T, Chiba K. Heat-assisted aqueous extraction of rice flour for arsenic speciation analysis [J]. Journal of Agricultural and Food Chemistry, 2010, 58(14): 8183-8188
[90] Clemente M J, Devesa V, Vélez D. In vitro reduction of arsenic bioavailability using dietary strategies [J]. Journal of Agricultural and Food Chemistry, 2017, 65(19): 3956-3964
[91] Sun G X, van de Wiele T, Alava P, et al. Arsenic in cooked rice: Effect of chemical, enzymatic and microbial processes on bioaccessibility and speciation in the human gastrointestinal tract [J]. Environmental Pollution, 2012, 162: 241-246
[92] Yin N Y, Cai X L, Du H L, et al. In vitro study of soil arsenic release by human gut microbiota and its intestinal absorption by Caco-2 cells [J]. Chemosphere, 2017, 168: 358-364
[93] Yin N Y, Wang P F, Li Y, et al. Arsenic in rice bran products: In vitro oral bioaccessibility, arsenic transformation by human gut Microbiota, and human health risk assessment [J]. Journal of Agricultural and Food Chemistry, 2019, 67(17): 4987-4994
[94] Wang L H, Yin X X, Gao S L, et al. In vitro oral bioaccessibility investigation and human health risk assessment of heavy metals in wheat grains grown near the mines in North China [J]. Chemosphere, 2020, 252: 126522
[95] Laird B D, Peak D, Siciliano S D. Bioaccessibility of metal cations in soil is linearly related to its water exchange rate constant [J]. Environmental Science & Technology, 2011, 45(9): 4139-4144
[96] Pokrovsky O S, Schott J. Surface chemistry and dissolution kinetics of divalent metal carbonates [J]. Environmental Science & Technology, 2002, 36(3): 426-432
[97] Hu L, Wang X L, Wu D S, et al. Effects of organic selenium on absorption and bioaccessibility of arsenic in radish under arsenic stress [J]. Food Chemistry, 2021, 344: 128614
[98] Tramontano M, Andrejev S, Pruteanu M, et al. Nutritional preferences of human gut bacteria reveal their metabolic idiosyncrasies [J]. Nature Microbiology, 2018, 3(4): 514-522
[99] Chiu K, Warner G, Nowak R A, et al. The impact of environmental chemicals on the gut microbiome [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2020, 176(2): 253-284
[100] David L A, Maurice C F, Carmody R N, et al. Diet rapidly and reproducibly alters the human gut microbiome [J]. Nature, 2014, 505(7484): 559-563
[101] Greenblum S, Carr R, Borenstein E. Extensive strain-level copy-number variation across human gut microbiome species [J]. Cell, 2015, 160(4): 583-594
[102] Yang Y F, Chi L, Lai Y J, et al. The gut microbiome and arsenic-induced disease-iAs metabolism in mice [J]. Current Environmental Health Reports, 2021, 8(2): 89-97
[103] Alava P, Tack F, Laing G D, et al. Arsenic undergoes significant speciation changes upon incubation of contaminated rice with human colon micro biota [J]. Journal of Hazardous Materials, 2013, 262: 1237-1244
[104] Fu Y Q, Yin N Y, Cai X L, et al. Arsenic speciation and bioaccessibility in raw and cooked seafood: Influence of seafood species and gut microbiota [J]. Environmental Pollution, 2021, 280: 116958