药物和个人护理品(pharmaceuticals and personal care products, PPCPs)包括用于预防或治疗人类和动物疾病的药物,以及提高生活质量的个人护理品[1]。药物按照作用机理和作用部位,可分为抗生素类、非类固醇抗炎药、激素及内分泌调节剂、血压和血脂调节药和β-阻断剂等;个人护理品主要包括杀菌消毒剂、防腐剂、驱虫剂、芳香剂和紫外线防护剂等[2-3]。作为与人类活动密切相关的一类化合物,PPCPs在地表水、地下水、饮用水和城市污水等多种水环境中频繁检出,浓度范围为ng·L-1~μg·L-1[2-3]。PPCPs可通过制药生产过程、医疗废水、生活垃圾、生活污水、畜牧业、水产养殖业、污水处理厂出水、垃圾填埋场渗滤液和农田径流等多种途径进入受纳水环境[4-6]。此外,经人类和动物服用的PPCPs,只有小部分能在生物体内代谢转化,40%~90%会以母体化合物或活性代谢物的形式经尿液和粪便排泄到水环境中[7-8]。随着人类的不断使用,大量PPCPs持续不断输入环境中,造成“假持久性”现象。水环境中残留的PPCPs,会引起水生生物生理过程异常、造成生殖损伤、干扰内分泌功能等。此外,部分PPCPs会在生物体内蓄积,进而通过食物链(网)传递,影响人类健康[4]。水环境中的PPCPs污染会对生态系统和人类健康造成潜在威胁,因此亟待加强PPCPs的污染管控,探寻有效去除PPCPs的新方法。
现有的常规污水处理工艺如沉淀、曝气和絮凝等,主要针对化学需氧量(COD)、生化需氧量(BOD5)等常规污染物指标进行设计,工艺出水仍有较多PPCPs检出[3]。相对而言,膜过滤、活性碳吸附和臭氧氧化等工艺对PPCPs的去除效果较好,但同时伴随着一定的潜在风险,且较高的运行和维护成本限制了其进一步大规模应用[1, 3, 5]。近年来,以植物为基础、通过太阳能驱动的生物过程去除污染物的植物修复技术,因其具有能耗和成本较低、环境友好等独特优势,日益引起广泛关注[5]。水生漂浮植物浮萍是植物修复中常用模式生物,常见的物种有青萍(Lemna minor)、紫背浮萍(Spirodela polyrhiza)、少根紫萍(Landoltia punctata)、稀脉浮萍(Lemna aequinoctialis)和芜萍(Wolffia globosa)。浮萍体型微小,结构简单,无性繁殖速度快,对环境具有较强的适应能力,广泛分布于世界各种水生环境中[9]。研究发现,浮萍不仅可以高效去除氮磷营养盐、重金属等常规污染物,还对农药、放射性污染物、纳米材料、石油和PPCPs等有机污染物具有一定的去除能力[10]。浮萍具有较好的污水修复能力和较强的应用潜力,易于回收利用,且可应用于食品、饲料和生物能源开发[9]。本文针对浮萍在PPCPs修复中的研究进展和应用现状进行综述,重点总结了浮萍对PPCPs的去除机制,并对其未来的研究方向提出展望,以期为浮萍在PPCPs修复中的应用提供科学依据。
1 浮萍在PPCPs修复中的应用研究(Application of duckweed in PPCPs bioremediation)
本文对浮萍在PPCPs修复中的应用进行了概况总结,如表1所示。浮萍对抗生素、非类固醇抗炎药和β-阻断剂等多种药物具有较好的去除效果,且相比于其他类型的植物,浮萍有一定的优势。Zhou等[11]对比分析了漂浮植物(青萍Lemna minor)、沉水植物狐尾藻(Myriophyllum verticillatum)和挺水植物香蒲(Typha orientalis Presl)等3种不同类型植物对13种药物的去除效果,发现青萍(L. minor)对药物的去除效果相对较好,其对醋氨酚的去除效率高达98.9%。此外,还有研究发现浮萍能在生活污水、农业污水和养猪废水等多种水生环境中正常生长[12-14],且对布洛芬、咖啡因和磺胺甲噁唑(SMZ)等典型药物具有较强去除能力。Di Baccio等[15]通过考察圆瘤浮萍(Lemna gibba)对布洛芬的去除效果,发现布洛芬在空白对照组中的去除率仅为8.2%,而其在L. gibba实验组中的去除率高达89%~92.5%。Matamoros等[16]进行了为期38 d的暴露实验,发现暴露14 d时,青萍(L.minor)实验组中的咖啡因去除率高达100%;而经38 d暴露,空白对照组中咖啡因的去除率仅为34%。Iatrou等[12]探讨了不同培养条件对青萍(L. minor)生长的影响,发现当温度为24 ℃、以储存1 d的人体尿液(稀释倍数为1∶200)为培养基时,L. minor的生长速率最高,对COD、总磷、总氮和SMZ的去除率分别为80%、90%、50%和80%。而Grenni等[17]研究发现,当暴露浓度为500 μg·L-1,暴露时间为28 d时,在河水中青萍(L. minor)对SMZ的去除率仅为17%,分析其原因除了暴露浓度不同外,河水不能像培养基一样给浮萍提供丰富的营养物质,致使浮萍对SMZ的去除率较低。浮萍具有去除多种药物的潜力且不同培养条件对药物去除效果的影响明显,因此有必要进一步开展提高浮萍对药物去除能力的相关研究。
除了药物外,浮萍对个人护理品也具有的一定的去除能力。研究表明,不同种类的浮萍(少根浮萍(Landoltia punctata)、青萍(L. minor)和紫背浮萍(Spirodela polyrhiza))均能有效去除典型个人护理品三氯生,且三氯生去除率超过95%[16, 18-19]。而三氯生在水-沉积物-浮萍系统中的总去除率仅为29%,分析其原因三氯生疏水性较强,大部分吸附于沉积物[11]。此外,还有研究发现在青萍(L. minor)湿地系统中,疏水性较强的驱蚊胺的去除率几乎为零[18]。Li等[19]基于正交实验分析,发现光强为240 μmmol m-2
m-2 s-1、曝气和大肠杆菌丰度为1.0×106 CFU·(100 mL)-1、浮萍生物量为1.00 kg·m-2的最优组合条件下,紫背浮萍(S. polyrhiza)湿地系统对三氯生的去除率高达95.4%,驱蚊胺去除率仅为17.1%。Gatidou等[20]研究发现青萍(L. minor)对5种苯并三唑类化合物(苯并三氮唑(BTR)、4-甲基苯基-1,2,3-三噻唑(4TTR)、5-甲基-苯并三氮唑(5TTR)、5,6-二甲基-1H-苯并三唑(XTR)、5-氯代苯并三唑(CBTR))均具有一定的去除能力;经36 d处理,反应体系中苯并三唑的去除率均可达100%(4HTR,(48.2±4.1)%),半衰期为(1.6±0.2) d(CBTR)到(25±3.6) d(4TTR),且浮萍对苯并三唑类化合物的去除以浮萍介导的生物吸收为主导。
s-1、曝气和大肠杆菌丰度为1.0×106 CFU·(100 mL)-1、浮萍生物量为1.00 kg·m-2的最优组合条件下,紫背浮萍(S. polyrhiza)湿地系统对三氯生的去除率高达95.4%,驱蚊胺去除率仅为17.1%。Gatidou等[20]研究发现青萍(L. minor)对5种苯并三唑类化合物(苯并三氮唑(BTR)、4-甲基苯基-1,2,3-三噻唑(4TTR)、5-甲基-苯并三氮唑(5TTR)、5,6-二甲基-1H-苯并三唑(XTR)、5-氯代苯并三唑(CBTR))均具有一定的去除能力;经36 d处理,反应体系中苯并三唑的去除率均可达100%(4HTR,(48.2±4.1)%),半衰期为(1.6±0.2) d(CBTR)到(25±3.6) d(4TTR),且浮萍对苯并三唑类化合物的去除以浮萍介导的生物吸收为主导。
表1 浮萍在PPCPs修复中的研究
Table 1 Studies on the bioremediation of PPCPs by duckweed

药物和个人护理品PPCPs浮萍种类Duckweed species暴露条件Exposure conditions研究结果Research results参考文献Reference阿替洛尔、醋氨酚、磺胺噻唑、甲氧苄啶、咖啡因、阿奇霉素、普萘洛尔、卡马西平、克拉霉素、氯贝酸、双氯芬酸、布洛芬、三氯生Atenolol, paracetamol, sulfathiazole, trimethoprim, caffeine, azithromycin, propranolol, carbamazepine, clarithromycin, clofibric acid, diclofenac, ibuprofen, triclosan青萍Lemna minor暴露浓度/(μg·L-1):10暴露时间/d:15Exposure concentration/(μg·L-1): 10Exposure time/d: 15去除率分别为:35.9%、98.9%、53.7%、22.0%、53.1%、31.3%、59.8%、22.7%、68.4%、25.6%、49.0%、27.1%、29.0%Removal rates: 35.9%, 98.9%, 53.7%, 22.0%, 53.1%, 31.3%, 59.8%, 22.7%, 68.4%, 25.6%, 49.0%, 27.1%, 29.0%, respectively[11]环丙沙星、磺胺甲噁唑Ciprofloxacin, sulfamethoxazole青萍Lemna minor暴露浓度/(μg·L-1):50暴露时间/d:14Exposure concentration/(μg·L-1): 50Exposure time/d: 14去除率:> 80%Removal rates: > 80%[12]醋氨酚、阿莫西林、氨苄青霉素、双氯酚酸Acetaminophen, amoxicillin, ampicillin, diclofenac圆瘤浮萍Lemna gibba进水浓度/(μg·L-1):190.5±42.5、303.0±74.7、277.5±59.1、111.1±32.8水力停留时间/d:12The influent concentration/(μg·L-1): 190.5±42.5, 303.0±74.7, 277.5±59.1, 111.1±32.8Hydraulic retention time/d: 12出水浓度分别为/(μg·L-1):21.9±7.2, 54.1±15.5, 20.2±5.2, 23.4±5.3 The effluent concentration/(μg·L-1): 21.9±7.2, 54.1±15.5, 20.2±5.2, 23.4±5.3, respectively[13]氧四环素Oxytetracycline稀脉浮萍Lemna aequinoctialis暴露浓度/(mg·L-1):0.05~1.00暴露时间/d:10Exposure concentration/(mg·L-1): 0.05~1.00Exposure time/d: 10去除率:70.80%~96.79%Removal rate: 70.80%~96.79%[14]三氯生、双氯芬酸、萘普生、咖啡因、布洛芬、氯贝酸Triclosan, diclofenac, naproxen, caffeine, ibuprofen, clofibric acid青萍Lemna minor暴露浓度/(μg·L-1):10暴露时间/d:38Exposure concentration/(μg·L-1): 10 Exposure time/d: 38去除率分别为:97%、99%、40%、99%、44%、16%Removal rates: 97%, 99%, 40%, 99%, 44%, 16%, respectively[16]布洛芬、氟西汀、三氯生Ibuprofen, fluoxetine, triclosan青萍、少根紫萍Lemna minor, Landoltia punctata暴露浓度/(μmol·L-1):10暴露时间/d:5~26Exposure concentration/(μmol·L-1): 10 Exposure time/d: 5~26去除率分别为:100%、约80%、约96%Removal rates: 100%, about 80%,about 96%, re-spectively[18]醋氨酚、咖啡因、三氯生、避蚊胺Paracetamol, caffeine, triclosan, N,N-diethyl-meta-toluamide紫背浮萍Spirodela polyrhiza暴露浓度/(μg·L-1):25暴露时间/d:7Exposure concentration/(μg·L-1): 25Exposure time/d: 7去除率分别为:98.8%、96.4%、95.4%、17.1%Removal rates: 98.8%, 96.4%, 95.4%, 17.1%, re-spectively[19]三氯蔗糖Sucralose青萍Lemna minor暴露浓度/(nmol·L-1):0~15 000暴露时间/d:21Exposure concentration/(nmol·L-1): 0~15 000 Exposure time/d: 21去除率:56%Removal rate: 56%[21]

续表1药物和个人护理品PPCPs浮萍种类Duckweed species暴露条件Exposure conditions研究结果Research results参考文献Reference顺铂Cisplatin青萍Lemna minor暴露浓度/(μmol·L-1):0~160暴露时间/d:4Exposure concentration/(μmol·L-1): 0~160 Exposure time/d: 4顺铂在浮萍中的最高累积量/(ng∙g-1)(以鲜质量计):320The highest content of cisplatin in duckweed/(ng∙g-1)(Based on fresh weight): 320[22]毒死蜱Chlorpyrifos青萍Lemna minor暴露浓度/(mg·L-1):0、0.1、0.5暴露时间/d:7Exposure concentration/(mg·L-1): 0, 0.1, 0.5 Exposure time/d: 7最高去除率:87%Maximum removal rate: 87%[23]醋氨酚、双氯芬酸、黄体酮Paracetamol, diclofenac, progesterone 圆瘤浮萍Lemna gibba暴露浓度/(μg·L-1):1 000暴露时间/d:10Exposure concentration/(μg·L-1): 1 000 Exposure time/d: 10去除率分别为:66.12%、47.50%、66.50%Removal rates: 66.12%, 47.50%, 66.50%, respec-tively[24]头孢羟氨苄、甲硝唑、磺胺甲噁唑、甲氧苄啶Cefadroxil, metronidazole, sulfamethoxazole, trimethoprim青萍Lemna minor暴露浓度/(μg·L-1):250暴露时间/d:24Exposure concentration/(μg·L-1): 250Exposure time/d: 24去除率分别为:100%、96%、73%、59%Removal rates: 100%, 96%, 73%, 59%,respec-tively[25]文拉法辛Venlafaxine青萍Lemna minor暴露浓度/(mg·L-1):0.5暴露时间/d:90Exposure concentration/(mg·L-1): 0.5Exposure time/d: 90去除率:58%~96%Removal rate: 58%~96%[26]阿莫西林Amoxicillin紫背浮萍Spirodela polyrhiza暴露浓度/(mg·L-1):0.0001~1暴露时间/d:7Exposure concentration/(mg·L-1): 0.0001~1Exposure time/d: 7去除率:84.6%~100%Removal rate: 84.6%~100%[27]吡虫啉、噻虫嗪Imidacloprid, thiacloprid鳞根萍Lemna turionifera暴露浓度/(μg·L-1):18.6、2.4暴露时间/d:6Exposure concentration/(μg·L-1): 18.6, 2.4, respectivelyExposure time/d: 6去除速率分别为/d-1:0.63±0.07、0.62±0.05Removal rates/d-1: 0.63±0.07, 0.62±0.05, re-spectively[28]四环素Tetracycline圆瘤浮萍Lemna gibba暴露浓度/(μg·L-1):50、100、300暴露时间/d:10Exposure concentration/(μg·L-1): 50, 100, 300Exposure time/d: 10去除率:> 99%Removal rate: 99%[29]亚甲基蓝Methylene blue青萍Lemna minor暴露浓度/(mg·L-1):15暴露时间/d:1Exposure concentration/(mg·L-1): 15Exposure time/d: 1去除率:98%Removal rate: 98%[30]
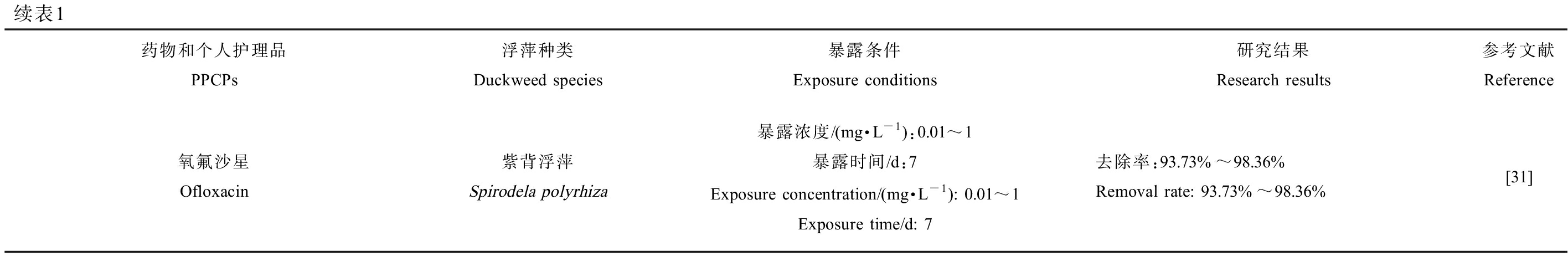
续表1药物和个人护理品PPCPs浮萍种类Duckweed species暴露条件Exposure conditions研究结果Research results参考文献Reference氧氟沙星Ofloxacin紫背浮萍Spirodela polyrhiza暴露浓度/(mg·L-1):0.01~1暴露时间/d:7Exposure concentration/(mg·L-1): 0.01~1Exposure time/d: 7去除率:93.73%~98.36%Removal rate: 93.73%~98.36%[31]
注:PPCPs是药物和个人护理品的英文缩写。
Note: PPCPs is the abbreviation of pharmaceuticals and personal care products.
浮萍对多种典型药物和个人护理品具有较好的去除效果,展现出了其较强的PPCPs污染修复能力和应用潜力。同一种浮萍对不同PPCPs的去除效率存在一定差异,这可能与化合物本身的理化性质(如疏水性)相关。另外,不同的培养条件(如培养基类型、系统类型、暴露浓度和暴露时间)也会影响PPCPs的去除效率。然而,当前针对浮萍在PPCPs修复中的应用研究普遍局限于污染物去除效果方面,浮萍对污染物的去除机理仍尚不清楚。
2 浮萍在PPCPs修复中的机理研究(Mechanisms of PPCPs bioremediation mediated by duckweed)
目前,关于浮萍对PPCPs去除机制的研究并不多,有限的研究表明浮萍可通过生物吸附、生物吸收和生物降解等途径去除PPCPs[18, 24-25]。一般而言,PPCPs通过各种物理化学作用吸附到浮萍表面;随后被吸附的部分PPCPs通过生物吸收进入浮萍胞内,并在细胞内累积;累积在浮萍胞内的PPCPs在各种酶的作用下进一步被降解。
2.1 生物吸附
生物吸附是利用生物本身的化学结构和成分特性来结合水溶液中特定离子或分子,从而去除水中污染物的方法[32-33]。生物吸附不需要消耗能量,且目标化合物不发生代谢转化作用,既可以发生在活细胞表面,也可以发生在灭活的细胞表面[34-35]。Reinhold等[18]将10 μmol·L-1的氟西汀分别暴露于鲜活青萍(L. minor)和化学灭活青萍(L. minor),发现2个实验组中氟西汀的去除率分别为(77.7±0.4)%和(55.6±3.9)%,推测浮萍对氟西汀的去除以生物吸附为主。PPCPs的生物吸附与化合物辛醇-水分配系数(logKow)所决定的疏水性密切相关,通常疏水性较强的化合物对应生物吸附作用越强,青萍(L. minor)对氟西汀(logKow=4.05)的吸附作用较强,而对氯贝酸(logKow=2.57)的吸附作用不明显[18, 36]。然而,化合物的疏水性不足以评估PPCPs在环境中的吸附特性和分配规律。PPCPs的生物吸附受多种因素影响,包括化合物理化性质、化合物暴露浓度、浮萍初始生物量、pH和温度等[36]。Can-Terzi等[30]采用响应面分析法探讨多种因素对青萍(L. minor)去除亚甲基蓝的影响,研究发现,当亚甲基蓝的初始浓度为15 mg·L-1、L. minor的初始生物量为4.9 g、pH为6.8时,L. minor对亚甲基蓝的去除率可高达98%。Imron等[37]基于傅里叶红外变换光谱分析,发现生物吸附是亚甲基蓝去除的主要机制;L. minor主要通过氢键作用和静电作用吸附亚甲基蓝,其自身含有的O—H、C—H和C—O官能团直接参与了亚甲基蓝的吸附过程,在亚甲基蓝的去除过程中发挥重要作用。此外,研究发现有机污染物的吸附机理还可能包括阳离子交换、阳离子桥接、络合和离子相互作用等[36]。
2.2 生物吸收
生物吸收是指污染物从植物环境介质转移进入植物组织内的过程[6]。研究表明,浮萍可以从水环境中吸收PPCPs并将其累积于体内。Supalkova等[22]以抗癌药顺铂对青萍(L. minor)进行暴露实验,发现顺铂可在L. minor胞内累积且最高累积量可达320 ng g-1(鲜质量)。Zhou等[11]通过13种药物在水-沉积物-浮萍系统中的定量分析和质量平衡核算发现,青萍(L. minor)对咖啡因、卡马西平和氯贝酸的去除以L. minor介导的生物吸收为主导。此外,还有研究发现浮萍对PPCPs的降解产物也具有一定的吸收作用,四环素及其降解产物4-差向四环素、4-差向脱水四环素和无水四环素均能在圆瘤浮萍(L. gibba)胞内累积,且其最高累积量分别可达(123±2.0)、(129±3.2)、(42.7±0.5)和(31.9±0.3) ng
g-1(鲜质量)。Zhou等[11]通过13种药物在水-沉积物-浮萍系统中的定量分析和质量平衡核算发现,青萍(L. minor)对咖啡因、卡马西平和氯贝酸的去除以L. minor介导的生物吸收为主导。此外,还有研究发现浮萍对PPCPs的降解产物也具有一定的吸收作用,四环素及其降解产物4-差向四环素、4-差向脱水四环素和无水四环素均能在圆瘤浮萍(L. gibba)胞内累积,且其最高累积量分别可达(123±2.0)、(129±3.2)、(42.7±0.5)和(31.9±0.3) ng g-1(干质量) [38]。
g-1(干质量) [38]。
目前,浮萍吸收PPCPs的机理尚未明晰,但有限的前期研究表明水环境中的PPCPs主要通过根部组织进入植物体内[39-40]。首先,PPCPs通过根尖表皮和根毛进入根内;然后,依次穿过皮层、内皮层和凯氏带,到达维管组织;最后,在木质部或韧皮部的作用下运输到叶等其他植物组织[41]。除了一些激素类化合物的吸收为主动吸收外,植物根系对PPCPs等人造有机化学品的吸收是被动扩散过程[42]。经生物吸收累积在浮萍胞内的PPCPs,会对浮萍产生一定的毒性作用,诸如抑制生长、降低光色素含量等[43-44];还会诱导浮萍发生氧化应激反应,促进过氧化氢酶(CAT)、超氧化物歧化酶(SOD)、过氧化物酶(POD)和抗坏血酸过氧化物酶(APX)等多种抗氧化酶和活性氧(ROS)产生[27]。研究发现,稀脉浮萍(L. aequinoctialis)能够抵御低浓度的PPCPs胁迫。当氧四环素(OTC)浓度低于0.50 mg·L-1时,暴露前8 d,稀脉浮萍(L. aequinoctialis)胞内H2O2和过氧化物酶(POD)增加;暴露18 d,H2O2和POD恢复正常水平,OTC诱导L.aequinoctialis发生的氧化应激反应消失[14]。另一方面,浮萍胞内累积的PPCPs过量时,产生的ROS超出其自身氧化胁迫清除能力时,导致细胞膜、蛋白质等发生氧化损伤,严重时引起细胞死亡。Gomes等[45]发现青萍(L. minor)暴露于环丙沙星时,其在L. minor胞内的生物富集系数(BCF)随着暴露浓度的升高而降低;分析其原因过量的环丙沙星暴露引起浮萍膜系统损伤,进而导致其在浮萍胞内的吸收累积量降低。
2.3 生物降解
生物降解是指在生物作用下,污染物结构和理化性质发生改变,转化为生物体的组成部分或是最终转化为没有生物毒性的有机或无机小分子的过程[46-47]。PPCPs的植物降解,与植物复杂的酶系统相关,主要包Ⅰ相、Ⅱ相和Ⅲ相反应。Ⅰ相反应是指PPCPs在细胞色素P450酶(Cyt P450)、细胞色素b5(Cyt b5)、NADPH-细胞色素P450还原酶(P450R)等Ⅰ相酶的作用发生羟基化、脱甲基化、脱烷基化、环氧化、异构化或脱羧基等氧化、还原或水解反应;Ⅱ相反应指在糖基转移酶(UGTs)、谷胱甘肽巯基转移酶(GSTs)Ⅱ相酶的作用下,PPCPs及其Ⅰ相代谢产物与糖、有机酸或谷胱甘肽等亲水官能团发生加合反应;Ⅲ相反应是指Ⅱ相产物在液泡中隔离或结合在细胞壁上[5, 48-49]。Qu等[26]通过考察微塑料对文拉法辛(venlafaxine, VFX)在5种不同水生系统中的去除效果影响,研究发现当微塑料为1 mg L-1时VFX在水-青萍(L. minor)体系中的水相去除率高达65%,且在L. minor胞内检测到VFX及其代谢产物去甲基文法拉辛(O-desmethylvenlafaxine, O-DVFX)累积。而Kingbäck等[50]研究发现,VFX主要通过Cyt P450酶催化作用进行生物转化,推测青萍(L. minor)通过胞内Ⅰ相代谢酶Cyt P450将VFX降解为O-DVFX。Pietrini等[51]在圆瘤浮萍(L. gibba)体内检测出了布洛芬及其11种代谢产物,包括羟基布洛芬、二羟基布洛芬、羟基布洛芬葡糖苷酸和己糖苷化合物等;其中,羟基布洛芬葡糖苷酸可能与浮萍的解毒机制相关,而己糖苷化合物是Ⅱ相反应中常见的产物。
此外,PPCPs的生物降解与植物根际环境和根际微生物群落等密切相关。植物生长的过程中,根系不仅从环境中摄取养分和水分,同时也不断向生长介质中分泌大量的有机物质,包含低分子量的有机酸、氨基酸和酚类等,高分子凝胶物质如多糖、脂肪酸和酶等。植物根系分泌物为根际微生物提供丰富的营养和能量物质,促进微生物生长,进而产生明显的根际效应[52]。不同环境条件下,植物根系分泌物可改变根际微环境的pH、氧化还原电位等理化性质和根际微生物的活性,从而改变根际微环境中污染物的形态及其生物有效性,最终影响植物对营养物质和污染物的吸收和累积。植物根系分泌物促进有机污染物的降解主要表现为3个方面:植物根系分泌物中的部分酶直接参与有机污染物的降解;根系微生物作用间接促进有机污染物降解;根系分泌物改变有机污染物的生物有效性[47, 53-54]。水生植物对环境的作用是利用植物-微生物-环境三者之间的相互作用,吸附、吸收和降解环境污染物[55-58]。Reinhold等[18]通过设置不同的处理组探讨青萍(L. minor)对布洛芬的去除机理,研究发现在鲜活浮萍组、搅碎浮萍组、化学灭活浮萍组和空白对照组(无浮萍)对布洛芬的去除率(暴露9 d)分别为(47.5±3.9)%、(24.8±2.0)%、0%和0%,推测布洛芬的去除以微生物降解为主导。此外,Muerdter和LeFevre[28]研究发现当鳞根萍(L. turionifera)和微生物共存时,吡虫啉和噻虫啉的去除速率可达(0.63±0.07) d-1和(0.62±0.05) d-1,而其在浮萍处理组、微生物处理组和非生物对照组的去除效果均不明显,说明浮萍和微生物通过协同作用去除吡虫啉和噻虫啉。
3 展望(Prospect)
浮萍因其独特的优势而广泛应用于水体污染物修复方面的研究,不仅可以有效去除氮磷营养盐、重金属等常规污染物[59-60],对多种典型药物和个人护理品也具有较强的去除能力。近年来,针对浮萍在水体污染修复方面开展了大量的基础研究和应用研究,取得了较大的进展。利用浮萍处理水环境中PPCPs污染问题,已然成为未来的重要发展方向。然而,现阶段相关研究仍存在许多挑战,有待进一步研究。
(1)现阶段浮萍在PPCPs修复中的研究大多局限于实验室内研究,暴露条件与真实环境条件存在较大差异,不足以准确评估浮萍对真实水生环境中PPCPs的去除效果。因此,未来可采集实际污水开展微宇宙、中宇宙实验模拟真实水生环境,这将有助于研究者们更好地了解浮萍对实际生活污水和工业废水中PPCPs处理的潜力。
(2)目前针对浮萍去除PPCPs的研究相对较少,且大多侧重于PPCPs去除效率方面,而浮萍对其吸收转化迁移机制仍尚未明晰。因此,有必要深入探究PPCPs在浮萍中的吸收、迁移和转化机制,结合高分辨质谱技术和基因组学、转录组学、代谢组学等多组学技术,从转化产物、差异基因、代谢物、代谢通路等多水平阐明浮萍对PPCPs的去除机理,相关研究结果有望为浮萍在PPCPs修复中的应用提供科学依据。
(3)鉴于部分PPCPs为难生物降解化合物,浮萍对其去除效果很有限。因此,未来可利用浮萍与其他水生植物、或微生物混合培养,充分发挥不同生物对PPCPs的吸收能力,提高浮萍对难生物降解PPCPs的去除能力,进而扩大浮萍在PPCPs污染水体修复中的应用。
(4)目前近10个浮萍物种已建立遗传转化体系,常见的浮萍也已完成全基因组测序[9],为浮萍的品质改良奠定了基础。未来可以利用分子生物学和生物信息学技术手段,开发具有较强特异性的浮萍,有针对性地高效处理不同类型的PPCPs污染水体。
[1] Boxall A B, Rudd M A, Brooks B W, et al. Pharmaceuticals and personal care products in the environment: What are the big questions? [J]. Environmental Health Perspectives, 2012, 120(9): 1221-1229
[2] Liu J L, Wong M H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China [J]. Environment International, 2013, 59: 208-224
[3] Yang Y, Ok Y S, Kim K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review [J]. The Science of the Total Environment, 2017, 596-597: 303-320
[4] Wang H, Xi H, Xu L L, et al. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review [J]. The Science of the Total Environment, 2021, 788: 147819
[5] Kurade M B, Ha Y H, Xiong J Q, et al. Phytoremediation as a green biotechnology tool for emerging environmental pollution: A step forward towards sustainable rehabilitation of the environment [J]. Chemical Engineering Journal, 2021, 415: 129040
[6] Keerthanan S, Jayasinghe C, Biswas J K, et al. Pharmaceutical and personal care products (PPCPs) in the environment: Plant uptake, translocation, bioaccumulation, and human health risks [J]. Critical Reviews in Environmental Science and Technology, 2021, 51(12): 1221-1258
[7] Tasho R P, Cho J Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review [J]. Science of the Total Environment, 2016, 563-564: 366-376
[8] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782
[9] 杨晶晶, 赵旭耀, 李高洁, 等. 浮萍的研究及应用[J]. 科学通报, 2021, 66(9): 1026-1045
Yang J J, Zhao X Y, Li G J, et al. Research and application in duckweeds: A review [J]. Chinese Science Bulletin, 2021, 66(9): 1026-1045 (in Chinese)
[10] Ekperusi A O, Sikoki F D, Nwachukwu E O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective [J]. Chemosphere, 2019, 223: 285-309
[11] Zhou H D, Liu X J, Chen X M, et al. Characteristics of removal of waste-water marking pharmaceuticals with typical hydrophytes in the urban rivers [J]. The Science of the Total Environment, 2018, 636: 1291-1302
[12] Iatrou E I, Stasinakis A S, Aloupi M. Cultivating duckweed Lemna minor in urine and treated domestic wastewater for simultaneous biomass production and removal of nutrients and antimicrobials [J]. Ecological Engineering, 2015, 84: 632-639
[13] Bassuney D, Tawfik A. Baffled duckweed pond system for treatment of agricultural drainage water containing pharmaceuticals [J]. International Journal of Phytoremediation, 2017, 19(8): 774-780
[14] Hu H, Li X, Wu S H, et al. Effects of long-term exposure to oxytetracycline on phytoremediation of swine wastewater via duckweed systems [J]. Journal of Hazardous Materials, 2021, 414: 125508
[15] Di Baccio D, Pietrini F, Bertolotto P, et al. Response of Lemna gibba L. to high and environmentally relevant concentrations of ibuprofen: Removal, metabolism and morpho-physiological traits for biomonitoring of emerging contaminants [J]. Science of the Total Environment, 2017, 584-585: 363-373
[16] Matamoros V, Nguyen L X, Arias C A, et al. Evaluation of aquatic plants for removing polar microcontaminants: A microcosm experiment [J]. Chemosphere, 2012, 88(10): 1257-1264
[17] Grenni P, Patrolecco L, Rauseo J, et al. Sulfamethoxazole persistence in a river water ecosystem and its effects on the natural microbial community and Lemna minor plant [J]. Microchemical Journal, 2019, 149: 103999
[18] Reinhold D, Vishwanathan S, Park J J, et al. Assessment of plant-driven removal of emerging organic pollutants by duckweed [J]. Chemosphere, 2010, 80(7): 687-692
[19] Li J N, Zhou Q Z, Campos L C. Removal of selected emerging PPCP compounds using greater duckweed (Spirodela polyrhiza) based lab-scale free water constructed wetland [J]. Water Research, 2017, 126: 252-261
[20] Gatidou G, Oursouzidou M, Stefanatou A, et al. Removal mechanisms of benzotriazoles in duckweed Lemna minor wastewater treatment systems [J]. Science of the Total Environment, 2017, 596-597: 12-17
[21] Amy-Sagers C, Reinhardt K, Larson D M. Ecotoxicological assessments show sucralose and fluoxetine affect the aquatic plant, Lemna minor [J]. Aquatic Toxicology, 2017, 185: 76-85
[22] Supalkova V, Beklova M, Baloun J, et al. Affecting of aquatic vascular plant Lemna minor by cisplatin revealed by voltammetry [J]. Bioelectrochemistry, 2008, 72(1): 59-65
[23] Prasertsup P, Ariyakanon N. Removal of chlorpyrifos by water lettuce (Pistia stratiotes L.) and duckweed (Lemna minor L.) [J]. International Journal of Phytoremediation, 2011, 13(4): 383-395
[24] Allam A, Tawfik A, Negm A, et al. Treatment of drainage water containing pharmaceuticals using duckweed (Lemna gibba) [J]. Energy Procedia, 2015, 74: 973-980
[25] Iatrou E I, Gatidou G, Damalas D, et al. Fate of antimicrobials in duckweed Lemna minor wastewater treatment systems [J]. Journal of Hazardous Materials, 2017, 330: 116-126
[26] Qu H, Ma R X, Wang B, et al. Effects of microplastics on the uptake, distribution and biotransformation of chiral antidepressant venlafaxine in aquatic ecosystem [J]. Journal of Hazardous Materials, 2018, 359: 104-112
[27] Singh V, Pandey B, Suthar S. Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: Growth, oxidative stress, biochemical traits and antibiotic degradation [J]. Chemosphere, 2018, 201: 492-502
[28] Muerdter C P, LeFevre G H. Synergistic Lemna duckweed and microbial transformation of imidacloprid and thiacloprid neonicotinoids [J]. Environmental Science & Technology Letters, 2019, 6(12): 761-767
[29] Topal M, Öbek E, Uslu  enel G, et al. Removal of tetracycline antibiotic by Lemna gibba L. from aqueous solutions [J]. Water and Environment Journal, 2020, 34(1): 37-44
enel G, et al. Removal of tetracycline antibiotic by Lemna gibba L. from aqueous solutions [J]. Water and Environment Journal, 2020, 34(1): 37-44
[30] Can-Terzi B, Goren A Y, Okten H E, et al. Biosorption of methylene blue from water by live Lemna minor [J]. Environmental Technology & Innovation, 2021, 22: 101432
[31] Singh V, Pandey B, Suthar S. Phytotoxicity and degradation of antibiotic ofloxacin in duckweed (Spirodela polyrhiza) system [J]. Ecotoxicology and Environmental Safety, 2019, 179: 88-95
[32] Volesky B. Biosorption and me [J]. Water Research, 2007, 41(18): 4017-4029
[33] 王建龙, 陈灿. 生物吸附法去除重金属离子的研究进展[J]. 环境科学学报, 2010, 30(4): 673-701
Wang J L, Chen C. Research advances in heavy metal removal by biosorption [J]. Acta Scientiae Circumstantiae, 2010, 30(4): 673-701 (in Chinese)
[34] Tsezos M. Biosorption: A Mechanistic Approach [M]//Advances in Biochemical Engineering/Biotechnology. Berlin, Heidelberg: Springer Berlin Heidelberg, 2013: 173-209
[35] Xiong Q, Hu L X, Liu Y S, et al. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems [J]. Environment International, 2021, 155: 106594
[36] Zhang D Q, Gersberg R M, Ng W J, et al. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review [J]. Environmental Pollution, 2014, 184: 620-639
[37] Imron M F, Ananta A R, Ramadhani I S, et al. Potential of Lemna minor for removal of methylene blue in aqueous solution: Kinetics, adsorption mechanism, and degradation pathway [J]. Environmental Technology & Innovation, 2021, 24: 101921
[38] Topal M, Uslu  enel G, Öbek E, et al. Bioaccumulation of tetracycline and degradation products in Lemna gibba L. exposed to secondary effluents [J]. Desalination and Water Treatment, 2016, 57(18): 8270-8277
enel G, Öbek E, et al. Bioaccumulation of tetracycline and degradation products in Lemna gibba L. exposed to secondary effluents [J]. Desalination and Water Treatment, 2016, 57(18): 8270-8277
[39] Madikizela L M, Ncube S, Chimuka L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review [J]. Science of the Total Environment, 2018, 636: 477-486
[40] Zhang C, Feng Y, Liu Y W, et al. Uptake and translocation of organic pollutants in plants: A review [J]. Journal of Integrative Agriculture, 2017, 16(8): 1659-1668
[41] Miller E L, Nason S L, Karthikeyan K G, et al. Root uptake of pharmaceuticals and personal care product ingredients [J]. Environmental Science & Technology, 2016, 50(2): 525-541
[42] Collins C, Fryer M, Grosso A. Plant uptake of non ionic organic chemicals [J]. Environmental Science & Technology, 2006, 40(1): 45-52
[43] Brain R A, Johnson D J, Richards S M, et al. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewal test [J]. Environmental Toxicology and Chemistry, 2004, 23(2): 371-382
[44] Pomati F, Netting A G, Calamari D, et al. Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor [J]. Aquatic Toxicology, 2004, 67(4): 387-396
[45] Gomes M P, Gonçalves C A, de Brito J C M, et al. Ciprofloxacin induces oxidative stress in duckweed (Lemna minor L.): Implications for energy metabolism and antibiotic-uptake ability [J]. Journal of Hazardous Materials, 2017, 328: 140-149
[46] 李伟明, 鲍艳宇, 周启星. 四环素类抗生素降解途径及其主要降解产物研究进展[J]. 应用生态学报, 2012, 23(8): 2300-2308
Li W M, Bao Y Y, Zhou Q X. Degradation pathways and main degradation products of tetracycline antibiotics: Research progress [J]. Chinese Journal of Applied Ecology, 2012, 23(8): 2300-2308 (in Chinese)
[47] 刘伟, 王慧, 陈小军, 等. 抗生素在环境中降解的研究进展[J]. 动物医学进展, 2009, 30(3): 89-94
Liu W, Wang H, Chen X J, et al. Progress on degradation of antibiotics in environment [J]. Progress in Veterinary Medicine, 2009, 30(3): 89-94 (in Chinese)
[48] Ohta D, Mizutani M. Redundancy or flexibility: Molecular diversity of the electron transfer components for P450 monooxygenases in higher plants [J]. Frontiers in Bioscience: A Journal and Virtual Library, 2004, 9: 1587-1597
[49] He Y J, Langenhoff A A M, Sutton N B, et al. Metabolism of ibuprofen by Phragmites australis: Uptake and phytodegradation [J]. Environmental Science & Technology, 2017, 51(8): 4576-4584
[50] Kingbäck M, Karlsson L, Zackrisson A L, et al. Influence of CYP2D6 genotype on the disposition of the enantiomers of venlafaxine and its major metabolites in postmortem femoral blood [J]. Forensic Science International, 2012, 214(1-3): 124-134
[51] Pietrini F, Di Baccio D, Ace a J, et al. Ibuprofen exposure in Lemna gibba L.: Evaluation of growth and phytotoxic indicators, detection of ibuprofen and identification of its metabolites in plant and in the medium [J]. Journal of Hazardous Materials, 2015, 300: 189-193
a J, et al. Ibuprofen exposure in Lemna gibba L.: Evaluation of growth and phytotoxic indicators, detection of ibuprofen and identification of its metabolites in plant and in the medium [J]. Journal of Hazardous Materials, 2015, 300: 189-193
[52] Shaw L J, Burns R G. Biodegradation of organic pollutants in the rhizosphere [J]. Advances in Applied Microbiology, 2003, 53: 1-60
[53] 黄俊伟, 闯绍闯, 陈凯, 等. 有机污染物的植物-微生物联合修复技术研究进展[J]. 浙江大学学报: 农业与生命科学版, 2017, 43(6): 757-765
Huang J W, Chuang S C, Chen K, et al. Progress on plant-microorganism combined remediation of organic pollutants [J]. Journal of Zhejiang University: Agriculture and Life Sciences, 2017, 43(6): 757-765 (in Chinese)
[54] 张昕怡, 田卓炎, 张成, 等. 植物修复多环芳烃污染土壤的根际效应机制研究进展[J]. 土壤通报, 2021, 52(5): 1251-1260
Zhang X Y, Tian Z Y, Zhang C, et al. The mechanism of rhizosphere effect on phytoremediation of polycyclic aromatic hydrocarbons in soil: A review [J]. Chinese Journal of Soil Science, 2021, 52(5): 1251-1260 (in Chinese)
[55] Toyama T, Yu N, Kumada H, et al. Accelerated aromatic compounds degradation in aquatic environment by use of interaction between Spirodela polyrrhiza and bacteria in its rhizosphere [J]. Journal of Bioscience and Bioengineering, 2006, 101(4): 346-353
[56] Kristanti R A, Kanbe M, Hadibarata T, et al. Isolation and characterization of 3-nitrophenol-degrading bacteria associated with rhizosphere of Spirodela polyrrhiza [J]. Environmental Science and Pollution Research International, 2012, 19(5): 1852-1858
[57] Ogata Y, Toyama T, Yu N, et al. Occurrence of 4-tert-butylphenol (4-t-BP) biodegradation in an aquatic sample caused by the presence of Spirodela polyrrhiza and isolation of a 4-t-BP-utilizing bacterium [J]. Biodegradation, 2013, 24(2): 191-202
![]() S, Uzelac B, et al. Phenol removal capacity of the common duckweed (Lemna minor L.) and six phenol-resistant bacterial strains from its rhizosphere: In vitro evaluation at high phenol concentrations [J]. Plants, 2020, 9(5): E599
S, Uzelac B, et al. Phenol removal capacity of the common duckweed (Lemna minor L.) and six phenol-resistant bacterial strains from its rhizosphere: In vitro evaluation at high phenol concentrations [J]. Plants, 2020, 9(5): E599
[59] Cheng J J, Stomp A. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed [J]. Clean-Soil Air Water, 2009, 37: 17-26
[60] Ali S, Abbas Z, Rizwan M, et al. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review [J]. Sustainability, 2020, 12(5): 1927