1 研究背景(Research background)
土壤是农业的基石,土壤健康是农业可持续发展与人类健康的前提。近年来,由于金属冶炼厂、矿山、油漆工业和涂装工艺等废弃物不合理排放,导致农业土壤中铅(Pb)、锌(Zn)、铜(Cu)、镉(Cd)、铬(Cr)、砷(As)和汞(Hg)等重金属的积累不断加剧[1-2]。如Huang等[3]分析了中国农业土壤重金属污染的现状。结果表明,与背景值相比,Cd和Hg的水平有所增加,而Pb、Zn、Cu、Cr、As和镍(Ni)在正常农田中没有明显的积累,重金属含量在西南和南部沿海地区普遍较高,但在西北地区较低。
由于重金属在土壤-农作物系统中的毒性、不可生物降解性和隐蔽性,寻求在受污染的土壤中去除或钝化重金属的有效方法具有重要意义,生物炭作为一种生态安全、无污染的材料引起了广泛关注。生物炭是指生物质热解或气化的固体产物,可以制备具有高阳离子交换量和碱性的多孔碳材料,其外表面和内表面可以通过不同功能的金属离子络合吸附土壤中的重金属,也可以通过改变土壤的氧化还原状态来降低重金属的流动性,以防止重金属从土壤转移到作物中。此外,重金属还可以与生物炭中Ca2+、Mg2+等离子交换,或与生物炭中含氧官能团发生反应,进而钝化重金属。生物炭材料的土壤修复和钝化效果取决于生物炭的表面积、金属含量和碱度等指标,这些指标可能因生物质原材料类型和热解条件的变化而发生显著变化。不同性质的生物炭对土壤中重金属的修复效果会有所不同。农林废弃物是一种可持续、经济的生物质生产资源,由于成本低、储量丰富和广泛可用性,是生产生物炭最常用原材料。其中最常用的农业废弃物是棉花秸秆、稻麦秸秆、玉米秸秆、甘蔗渣、花生秸秆和柳枝稷等。研究者们对农林废弃物生物炭钝化土壤重金属机制进行了大量的研究[4]。基于此,本文综述了农林废弃物生物炭修复土壤重金属的机制,并对今后的研究方向提出了建议与展望。
2 生物炭的制备及其性能影响因素(Preparation of biochar and factors affecting its properties)
利用生物炭作为吸附剂可以去除土壤环境中的污染物,特别是重金属。然而,不同条件下制得的生物炭材料钝化土壤重金属的效果也不一样,其性能与原材料类型、热解温度和热解停留时间等因素有关。
2.1 原材料类型
根据原料来源的不同,农林废弃物生物炭主要包括植物源生物炭(木质生物炭、竹生物炭、秸秆生物炭和稻壳生物炭)和动物源生物炭(动物粪便生物炭)。相比之下,动物源生物炭中磷(P)、钾(K)、钙(Ca)和镁(Mg)等微量元素含量高于植物源生物炭。植物生物炭比表面积、吸附性能和重金属固定性能均高于牛粪生物炭,其中芳香结构和烷基是生物炭的主要成分[5]。Xu等[6]对麦秸和猪粪生物炭的元素组成进行了研究,发现猪粪生物炭中含有Ca和Mg,而麦秸生物炭中不含有这些元素。Liu和Zhang[7]的研究表明松木生物炭吸附Pb的能力约为稻壳生物炭的2倍。此外,不同生物质原料制备的生物炭的pH值、表面电荷和比表面积也存在显著差异。如Nzediegwu等[8]挑选出3种木质纤维素原料(锯末、油菜秸秆和小麦秸秆)和一种非木质纤维素原料(粪肥颗粒)在不同温度下进行热解,结果表明,与比表面积相比,原料类型对pH和表面电荷的影响较小,对生物炭的比表面积和孔径有显著影响。可见,生物质原料对生物炭的性能影响较大,而生物炭的性能又直接影响到生物炭对各种重金属的吸附能力。生物炭的灰分成分在不同的原料之间有很大的差异。来自生物固体和动物粪便的生物炭灰分比例较高,而来自草本饲料(如玉米、甜菜和甘薯)的生物炭灰分含量较低[9]。研究表明,灰分含量越高,阳离子交换容量(CEC)越大,对特定金属(如Pb(Ⅱ))的吸附能力越强[10]。也有研究发现,过量的灰分含量不利于重金属的吸附,这可能是因为无机矿物成分堵塞生物炭的孔隙,从而限制对重金属的吸附[9]。
2.2 热解温度
不同热解温度对生物炭的性能也有显著影响。Zhang等[11]研究了杨木在不同温度下热解得到的生物炭的功能,拉曼表征表明,羧基的稳定性不如羰基,醛和酮的官能团在200 ℃形成,并在300~500 ℃达到峰值。Cao和Harris[12]研究了不同热解温度下制备的牛粪和植物生物炭比表面积的变化,发现生物炭的比表面积随着热解温度的升高而增大。在300 ℃高温热解制得的生物炭含有更多的含氧官能团,而在500~700 ℃高温热解制得的生物炭含有更多的微孔和更大的表面积。生物炭中灰分和矿物质含量也随着热解温度的升高而增加。Cantrell等[13]比较了几种动物粪便(如牛、家禽、猪和火鸡粪便)在350~700 ℃温度范围内制备的生物炭。他们发现高温条件下生产的生物炭的pH值和灰分含量高于低温条件下生产的生物炭,可能是农业废弃物生物炭灰分含量较低,热解温度升高导致酸性官能团分解,易于有机酸和有机物质挥发,使生物炭pH值升高和残留更多的灰分。然而,过高的热解温度(>900 ℃)会导致孔隙膨胀和相邻孔隙的合并,导致孔隙堵塞和孔数减少,以及灰的软化和熔化。焦炭与挥发物的二次反应引起的微孔结构的坍塌,也会在较高温度下引起比表面积的减小。Zhang等[14]测试了热解温度对生物炭除Pb能力的影响。他们发现生物炭对的Pb去除率从400 ℃时的81.7 mg·g-1增加到700 ℃时的742 mg·g-1,然后在800 ℃时下降到368 mg·g-1。Park等[15]的研究表明生物炭除Cd的最佳热解温度为500 ℃。
随着热解温度的升高,生物炭中氢(H)、氮(N)和硫(S)等元素的含量、阳离子交换量和表面含氧官能团数量减少,但芳构化程度增加,这些条件不利于重金属的吸附。然而,也有研究发现,随着热解温度的升高,生物炭的比表面积、孔隙结构和碱度都增加,有利于对重金属的吸附[5]。由此可见,热解温度过高或过低均不利于生物炭的吸附性能。因此,热解温度的选择对生物炭吸附重金属能力至关重要。
2.3 热解停留时间
停留时间对热解过程的影响与热解温度有关。Williams和Besler[16]研究发现,当热解温度低于400 ℃时,生物炭的C含量随着停留时间的延长而增加,当热解温度>500 ℃时,生物炭的生成过程在短时间内发展到最大,因此生物炭的性质不随停留时间的变化而变化,与Braadbaart和Poole[17]的研究结果一致。停留时间对生物炭产量的影响随热解温度的变化而变化,在低热解温度下,产量随停留时间的增加而增加,而在350 ℃时,停留时间的增加主要改变了生物炭的表面和内部结构,但对产量没有影响。此外,更长的停留时间,有利于总磷和钾的积累,Ca、Mg和硅(Si)的释放,以及铁(Fe)、锰(Mn)和硫(S)的保留。Zhao等[18]研究了热解停留时间(15~120 min)对榛子壳生物炭特性的影响。随着热解持续时间的增加(15~120 min),比表面积表现出火山类型的变化,并在90 min时达到598.7 m2·g-1的最大值。比表面积的增加与相对较长时间内的充分燃烧相关,从而形成更发达的孔隙网络,而热解时间的进一步延长可能会通过蚀刻效应将微孔扩大到中孔,甚至大孔,从而导致比表面积的降低,不利于对重金属的吸附。
3 农林废弃物生物炭对重金属离子的作用(Effect of biochar from agricultural and forestry waste on heavy metal ions)
生物炭固定重金属的方法通常是通过阳离子交换、吸附、沉淀和表面络合来降低重金属的生物有效性(指穿过细胞膜被植物体吸收进入器官的部分),生物炭对重金属的物理吸附主要受生物炭的比表面积、表面能和孔容的影响。一般情况下,生物炭具有的比表面积和孔隙结构越大,物理吸附作用则越强。生物炭的高pH值和生物炭中金属的存在有助于重金属离子与![]() 反应生成氢氧化物、磷酸盐或碳酸盐沉淀,有效地固化了重金属污染物。氧化还原通过改变元素的存在形式来影响元素的化学行为、迁移能力和生物有效性,主要存在变价态的重金属中。一些生物炭材料表面有大量的K+、Ca2+、Na+和Mg2+等碱金属离子的存在,为重金属在污染土壤中与之交换创造了可能性。生物炭表面富含—COOH、—COH、—NH2和—OH等含氧官能团,与重金属离子配合形成了含有特定金属-配体相互作用的多原子结构[1]。生物炭阳离子π函数与生物炭的芳构化有关,π共轭芳香族结构越多,π轨道上的负电荷变化越大,从而官能团失去电子的能力增加,吸附效果更显著。生物炭对重金属的作用机理如图1所示。
反应生成氢氧化物、磷酸盐或碳酸盐沉淀,有效地固化了重金属污染物。氧化还原通过改变元素的存在形式来影响元素的化学行为、迁移能力和生物有效性,主要存在变价态的重金属中。一些生物炭材料表面有大量的K+、Ca2+、Na+和Mg2+等碱金属离子的存在,为重金属在污染土壤中与之交换创造了可能性。生物炭表面富含—COOH、—COH、—NH2和—OH等含氧官能团,与重金属离子配合形成了含有特定金属-配体相互作用的多原子结构[1]。生物炭阳离子π函数与生物炭的芳构化有关,π共轭芳香族结构越多,π轨道上的负电荷变化越大,从而官能团失去电子的能力增加,吸附效果更显著。生物炭对重金属的作用机理如图1所示。
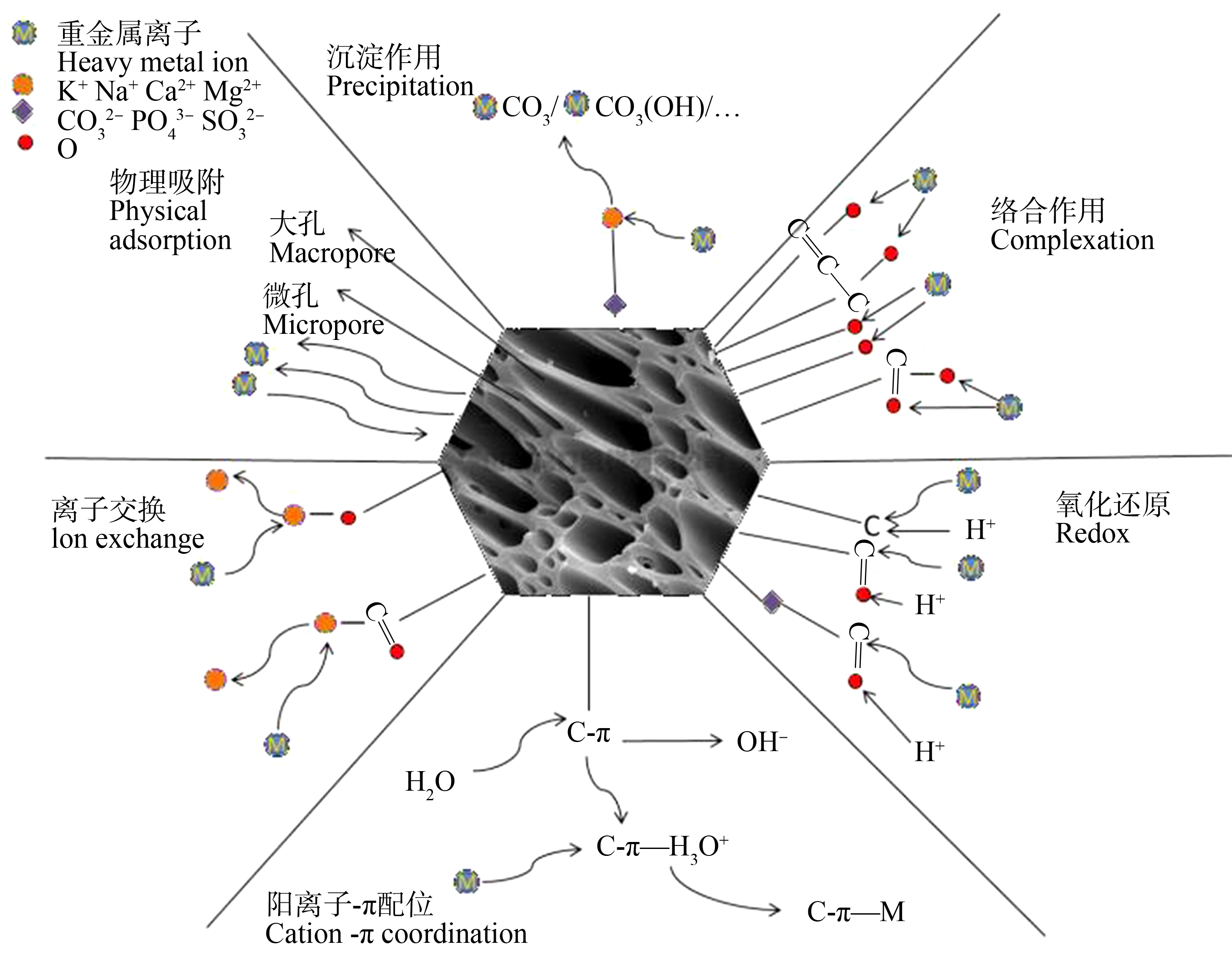
图1 农林废弃物生物炭钝化土壤重金属的作用机制(改自Li等[19])
Fig. 1 Mechanism of inactivation of heavy metals in soil by agricultural and forestry waste biochar(modified from Li et al.[19])
4 生物炭对不同重金属的吸附机理(Adsorption mechanism of biochar for different heavy metals)
生物炭被认为是一种很有前途的去除重金属的吸附剂,对不同重金属的吸附行为不同,且与重金属的性质有很好的相关性。不同的重金属离子在不同环境条件下形成不同的金属离子基团,导致生物炭的吸附机理不同[19]。此外,微生物利用生物炭作为电子传递介质,促进细胞间的电子转移,加速重金属迁移转化,微生物细胞还可以通过呼吸作用将重金属离子转运到细胞内部,使其转化为固定形态。所以生物炭可以通过改变土壤细菌群落结构(增加细菌多样性和微生物生物量)来吸附重金属。生物炭的性质(如矿物组成和官能团)和吸附条件(如pH值、反应温度和共存离子)也会影响其吸附行为和吸附机理,对不同金属离子的吸附机理的研究有助于环境污染的针对性修复。
4.1 Hg
Hg通常由燃烧过程释放,包括城市、医疗垃圾焚烧和燃煤发电,作为一种特殊的金属,Hg会对人中枢神经系统产生毒性作用,如震颤、妄想、记忆丧失和神经认知障碍。生物炭是一种有效的土壤Hg修复工具,如Wang等[20]研究发现,硬木生物炭表面的—COOH与土壤中的Hg(Ⅱ)离子发生配位反应,生成—COOHg+配合物沉淀,从而降低了Hg的迁移率,硫醇官能团和亚砜基也能与Hg(Ⅱ)反应形成-S(Hg)-和![]() 沉淀。此外,Xing等[21]发现稻壳生物炭比麦秸生物炭具有更高的硫酸盐浓度,更有效地促进汞-硫配位反应,产生硫化物沉淀。Hg的甲基化是一种特殊的环境生物地球化学行为,一旦土壤中的无机汞转化为甲基汞(MeHg),其毒性和生物积累将大大增强,而生物炭可以抑止Hg的甲基化。研究表明,水稻根系分泌物的释放降低了土壤pH值,从而促进了Hg的甲基化,而施用碱性生物炭提高了土壤pH值,有效抑制了甲基化。此外,稻壳生物炭和麦稻秸秆生物炭混合后携带hgcA细菌相对丰度的变化使土壤中的甲基汞浓度降低[22]。松木生物炭和假单胞菌属的共同作用下使残渣态Hg含量从(98.9±5.7)mg·kg-1下降至(76.0±5.7)mg·kg-1,对Hg去除率达到23.2%[23]。
沉淀。此外,Xing等[21]发现稻壳生物炭比麦秸生物炭具有更高的硫酸盐浓度,更有效地促进汞-硫配位反应,产生硫化物沉淀。Hg的甲基化是一种特殊的环境生物地球化学行为,一旦土壤中的无机汞转化为甲基汞(MeHg),其毒性和生物积累将大大增强,而生物炭可以抑止Hg的甲基化。研究表明,水稻根系分泌物的释放降低了土壤pH值,从而促进了Hg的甲基化,而施用碱性生物炭提高了土壤pH值,有效抑制了甲基化。此外,稻壳生物炭和麦稻秸秆生物炭混合后携带hgcA细菌相对丰度的变化使土壤中的甲基汞浓度降低[22]。松木生物炭和假单胞菌属的共同作用下使残渣态Hg含量从(98.9±5.7)mg·kg-1下降至(76.0±5.7)mg·kg-1,对Hg去除率达到23.2%[23]。
综上,生物炭对Hg的钝化机制主要是络合沉淀,且在络合沉淀的过程中还能抑制Hg的甲基化,所以生物炭是一种有效的修复Hg污染土壤的工具,尤其是碱性生物炭。生物炭钝化Hg的机制如表1所示。
表1 生物炭钝化Hg的机制
Table 1 Mechanism of Hg passivation by biochar

重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesHg负载Fe(NO3)3的木材Wood loaded with Fe(NO3)3700氧化还原Redox在铁的催化下,比表面积和CO官能团均有所增加,增加了Hg的去除活性位点Under the catalysis of iron, the specific surface area and CO functional groups were in-creased, which increased the active sites for Hg removal[24]Hg竹子Bamboo600络合作用Complexation生物炭中有机硫官能团与甲基汞形成络合物Organic sulfur functional groups in biochar form complexes with methylmercury[25]Hg水稻壳Rice husk480~660沉淀作用Precipitation生物炭更有效地促进汞-硫配位反应,产生硫化物沉淀Biochar can more effectively promote mercury sulfur coordination reaction and produce sulfide precipitation[21]Hg核桃壳Walnut shell800吸附和氧化作用Adsorption and oxidation孔结构和化学官能团分别是Hg的主要物理和化学吸附活性中心,晶格氧、化学吸附氧是主要的氧化位点,它们共同将Hg氧化为HgO,Pore structure and chemical functional groups are the main physical and chemical adsorption active centers of Hg respectively; lattice oxygen and chemisorption oxygen are the main oxidation sites; they jointly oxidize Hg to HgO[26]Hg(Ⅱ)玉米秸秆Corn straw500络合反应Complexation羧基和羟基与Hg2+的络合反应是主要的作用The complexation reaction of carboxyl and hy-droxyl groups with Hg2+ is the main role[27]
4.2 Zn
研究表明,造纸、制药、油漆和颜料工业产生的废水中含有丰富的Zn,Zn是一种重要的微量营养素,在植物的代谢和生理过程中起着积极的作用,缺Zn会影响土壤的pH、有机质含量和碳酸氢盐含量等特征,阻碍Mg和Fe在土壤中的作用。然而,过量的Zn会对植物体产生毒害。Rahmani-Sani等[28]以鸡毛和蛋壳为原料合成生物炭对重金属的吸附进行了实验及动力学和热力学计算。他们认为,Zn2+的吸附是一个静电相互作用的过程,而不是物理吸附。阳离子交换机制也被认为是生物炭共热解吸附Zn(Ⅱ)的重要机制。如Dai等[29]的研究表明阳离子交换机制在300 ℃下猪粪和稻草生物炭共热解吸附Zn至关重要。此外,在700 ℃的条件下,氧化官能团(—OH和—COOH)在生物炭对Zn的吸附中也起着重要的作用。Song等[30]发现,蒙脱石和玉米秸秆共热解生物炭的主要吸附机理是羟基和硅醇基团与Zn(Ⅱ)脱氢和络合。同时,蒙脱石提供的Ca(Ⅱ)或Na(Ⅰ)也有利于通过提高溶液pH值来去除重金属。此外,鸡粪和稻壳生物炭使Zn的生物有效性降低了73.4%,其中细菌和有机成分的相互作用是最关键的驱动因素。细菌与有机组分的相互作用也是Zn失活过程中的关键驱动因素。由此可见,Zn的过量和缺少都会对植物体和土壤造成一定的影响,生物炭对Zn的钝化机制是静电吸附、络合作用和阳离子交换作用。生物炭钝化Zn的机制如表2所示。
表2 生物炭钝化Zn的机制
Table 2 Mechanism of Zn passivation by biochar
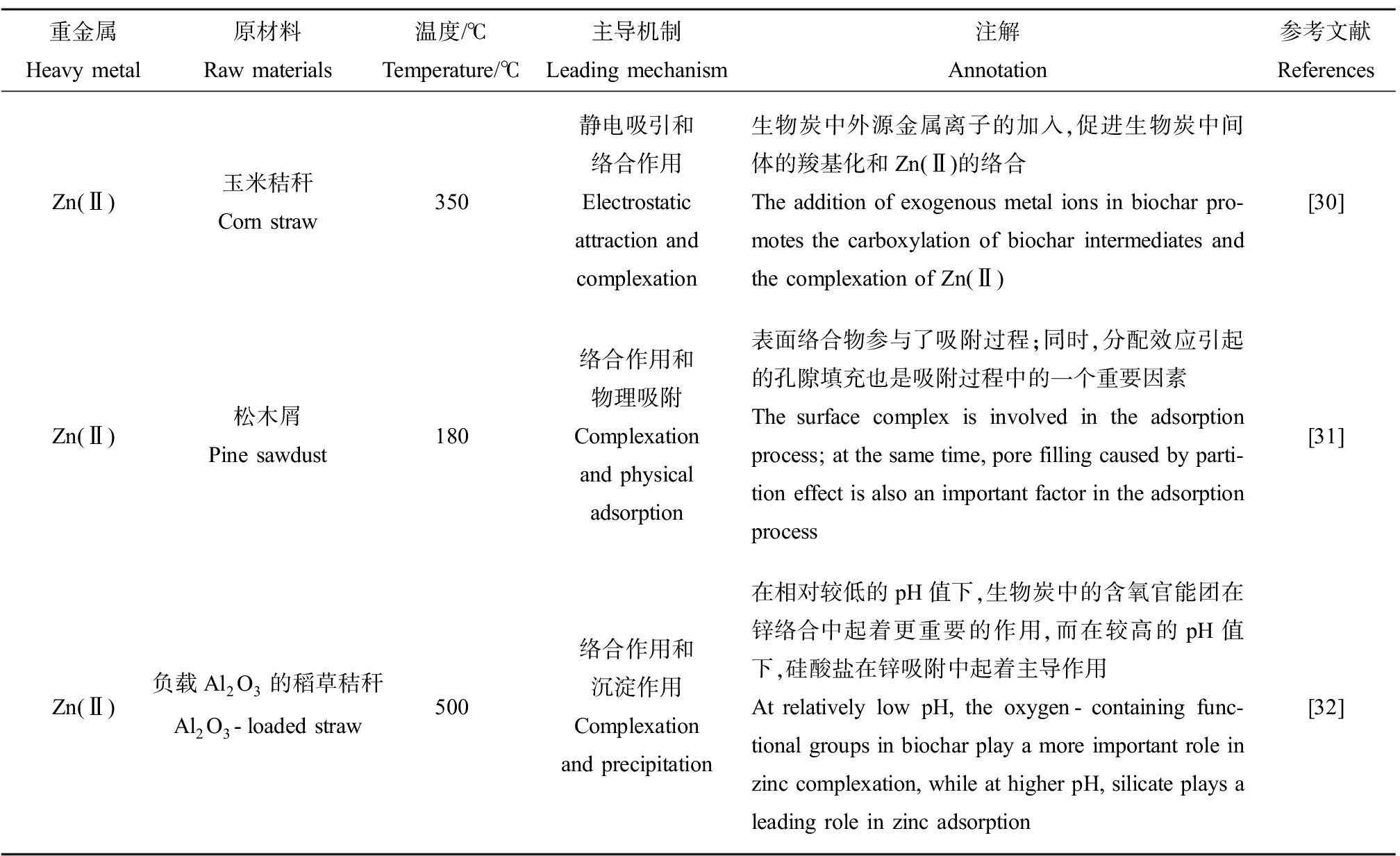
重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesZn(Ⅱ)玉米秸秆Corn straw350静电吸引和络合作用Electrostatic attraction and complexation生物炭中外源金属离子的加入,促进生物炭中间体的羧基化和Zn(Ⅱ)的络合The addition of exogenous metal ions in biochar pro-motes the carboxylation of biochar intermediates and the complexation of Zn(Ⅱ)[30]Zn(Ⅱ)松木屑Pine sawdust180络合作用和物理吸附Complexation and physical adsorption表面络合物参与了吸附过程;同时,分配效应引起的孔隙填充也是吸附过程中的一个重要因素The surface complex is involved in the adsorption process; at the same time, pore filling caused by parti-tion effect is also an important factor in the adsorption process[31]Zn(Ⅱ)负载Al2O3的稻草秸秆Al2O3-loaded straw500络合作用和沉淀作用Complexation and precipitation在相对较低的pH值下,生物炭中的含氧官能团在锌络合中起着更重要的作用,而在较高的pH值下,硅酸盐在锌吸附中起着主导作用At relatively low pH, the oxygen-containing func-tional groups in biochar play a more important role in zinc complexation, while at higher pH, silicate plays a leading role in zinc adsorption[32]
4.3 Pb
一般来说,Pb大部分是在废旧铅酸电池的回收过程中产生的,可能对人和动物造成致畸和致病性影响。生物炭固定土壤中铅的过程相对复杂,Ahmad等[33]认为,大豆秸秆生物炭激发Pb的固定化过程是由π-阳离子电子供体-受体相互作用引起的。Kazak和Tor[34]阐明了废酒渣和赤泥通过共水热处理对Pb(Ⅱ)的吸附机理,包括阳离子交换、静电吸引、“阳离子-π”相互作用、沉淀和络合。羧基(—COOH)和羟基(—OH)官能团之间可以通过络合作用加速Pb(Ⅱ)离子的吸附[19],Pb(Ⅱ)的吸附是与K(Ⅰ)和Na(Ⅰ)进行金属交换,从而以静电作用与表面—COOH和—OH官能团络合,内部与自由—OH络合。此外,Pb(Ⅱ)与生物炭中碱性物质![]() 及其他碱土金属Ca2+、Mg2+之间的阳离子交换和沉淀反应能够实现显著的Pb固定效应,如形成Pb3(CO3)2(OH)2沉淀。畜禽粪生物炭和污泥生物炭中含有丰富的磷酸盐,这些磷酸盐能与Pb(Ⅱ)形成Pb5(PO4)3Cl、Pb5(PO4)3OH和β-Pb9(PO4)6等不溶性化合物,降低Pb的迁移率。Gao等[35]指出在热解过程中加入正磷酸盐,Pb(Ⅱ)析出形成Pb5(PO4)3Cl、Pb2P2O7和Pbn/2(PO3)n的沉淀。含氮官能团对Pb的固定也有一定的影响,如Igalavithana等[36]报道,蔬菜废弃物生物炭不仅增加土壤pH,同时与生物炭表面—NH2的共价键作用促进了Pb的固定,从而有效地减少了Pb的生物有效态浓度。此外,微生物可以分泌更多的细胞外聚合物通过络合/螯合反应钝化土壤Pb[37]。生物炭钝化Pb的机制如表3所示。
及其他碱土金属Ca2+、Mg2+之间的阳离子交换和沉淀反应能够实现显著的Pb固定效应,如形成Pb3(CO3)2(OH)2沉淀。畜禽粪生物炭和污泥生物炭中含有丰富的磷酸盐,这些磷酸盐能与Pb(Ⅱ)形成Pb5(PO4)3Cl、Pb5(PO4)3OH和β-Pb9(PO4)6等不溶性化合物,降低Pb的迁移率。Gao等[35]指出在热解过程中加入正磷酸盐,Pb(Ⅱ)析出形成Pb5(PO4)3Cl、Pb2P2O7和Pbn/2(PO3)n的沉淀。含氮官能团对Pb的固定也有一定的影响,如Igalavithana等[36]报道,蔬菜废弃物生物炭不仅增加土壤pH,同时与生物炭表面—NH2的共价键作用促进了Pb的固定,从而有效地减少了Pb的生物有效态浓度。此外,微生物可以分泌更多的细胞外聚合物通过络合/螯合反应钝化土壤Pb[37]。生物炭钝化Pb的机制如表3所示。
表3 生物炭钝化Pb的机制
Table 3 Mechanism of Pb passivation by biochar

重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesPb(Ⅱ)废酒糟Waste distiller’s grains180阳离子交换Cation exchange废酒糟和赤泥中的Ca(Ⅱ)在与Pb(Ⅱ)的阳离子交换中起着重要作用Ca(Ⅱ) in waste distiller’s grains and red mud plays an important role in cation exchange with Pb(Ⅱ)[34]Pb(Ⅱ)油菜秸秆Rape straw500沉淀作用Precipitation热解过程之前,加入正磷酸盐,Pb(Ⅱ)沉淀形成Pb5(PO4)3Cl、Pb2P2O7、Pbn/2(PO3)nBefore pyrolysis, orthophosphate was added and Pb(Ⅱ) precipitated to form Pb5(PO4)3Cl, Pb2P2O7, Pbn/2(PO3)n[38]Pb(Ⅱ)稻草秸秆Straw550络合吸附Complex adsorption生物炭的碳和氢含量最高,官能团数量最多,pH值最高,表面负电荷最多,物理稳定性最高,羟基和羧基在与Pb(Ⅱ)的络合中起主导作用,是由于Pb2+的结合能较低、电负性较高Biochar has the highest carbon and hydrogen content, the largest number of functional groups, the highest pH value, the largest surface negative charge and the highest physical stability; hydroxyl and carboxyl groups play a leading role in the complexation with Pb(Ⅱ), which is due to the low binding energy and high electronegativity of Pb2+[39]
综上,生物炭钝化Pb的机制是复杂的,既可以与含氧官能团—COOH和—OH之间产生络合作用,还可以与表面重金属离子K(Ⅰ)和Na(Ⅰ)进行离子交换,还有阳离子-π作用等最后形成沉淀,降低Pb的迁移率。
4.4 Cu
Cu是一种持久性重金属,通常从电镀池、炼油厂、造纸和纸浆厂的工业废水中排放出来。Cu是植物必需的重要微量元素,也是土壤中的重要元素。然而,当土壤中过量的Cu可能会对农作物产生毒害作用。Deng等[31]研究表明,加入硅酸盐和镁盐的松木屑水热处理合成的生物炭对Cu(Ⅱ)的吸附过程中均存在空隙吸附、共沉淀和表面络合作用。硅酸盐和镁盐的添加有利于生物炭比表面积和孔隙结构的增大,促进了Cu(Ⅱ)孔隙的填充。同时,Cu吸附后的沉淀物中存在CuSiO3以及Cu(Ⅱ)与官能团的配合物,证实了硅酸盐与Cu(Ⅱ)之间存在共沉淀和络合现象。Yu等[40]发现N-Cu键存在较强的共轭效应,有利于Cu(Ⅱ)在N掺杂生物炭上的吸附。正如Meier等[41]所描述的,鸡粪生物炭中存在的官能团(尤其是—OH)已被证明对Cu有很高的亲和力。Cu的固定化过程是通过提高土壤pH值和诱导石灰效应,刺激Cu(Ⅱ)与生物炭表面官能团(如C![]() O和酚-OH)络合而实现的。此外,Rechberger等[42]发现木屑生物炭灰中的碳酸盐和氢氧化物是Cu(Ⅱ)的重要吸附剂,能够促进CuCO3和Cu(OH)2的形成,Zhang等[43]制备的毛竹生物炭也证明了这一点。因此,固定重金属Cu的关键是利用生物炭的碱度来提高土壤的pH值,通过在高的pH下,Cu的溶解度降低,更容易络合沉淀,从而实现有效固定土壤中的Cu。生物炭钝化Cu的机制如表4所示。
O和酚-OH)络合而实现的。此外,Rechberger等[42]发现木屑生物炭灰中的碳酸盐和氢氧化物是Cu(Ⅱ)的重要吸附剂,能够促进CuCO3和Cu(OH)2的形成,Zhang等[43]制备的毛竹生物炭也证明了这一点。因此,固定重金属Cu的关键是利用生物炭的碱度来提高土壤的pH值,通过在高的pH下,Cu的溶解度降低,更容易络合沉淀,从而实现有效固定土壤中的Cu。生物炭钝化Cu的机制如表4所示。
表4 生物炭钝化Cu的机制
Table 4 Mechanism of Cu passivation by biochar
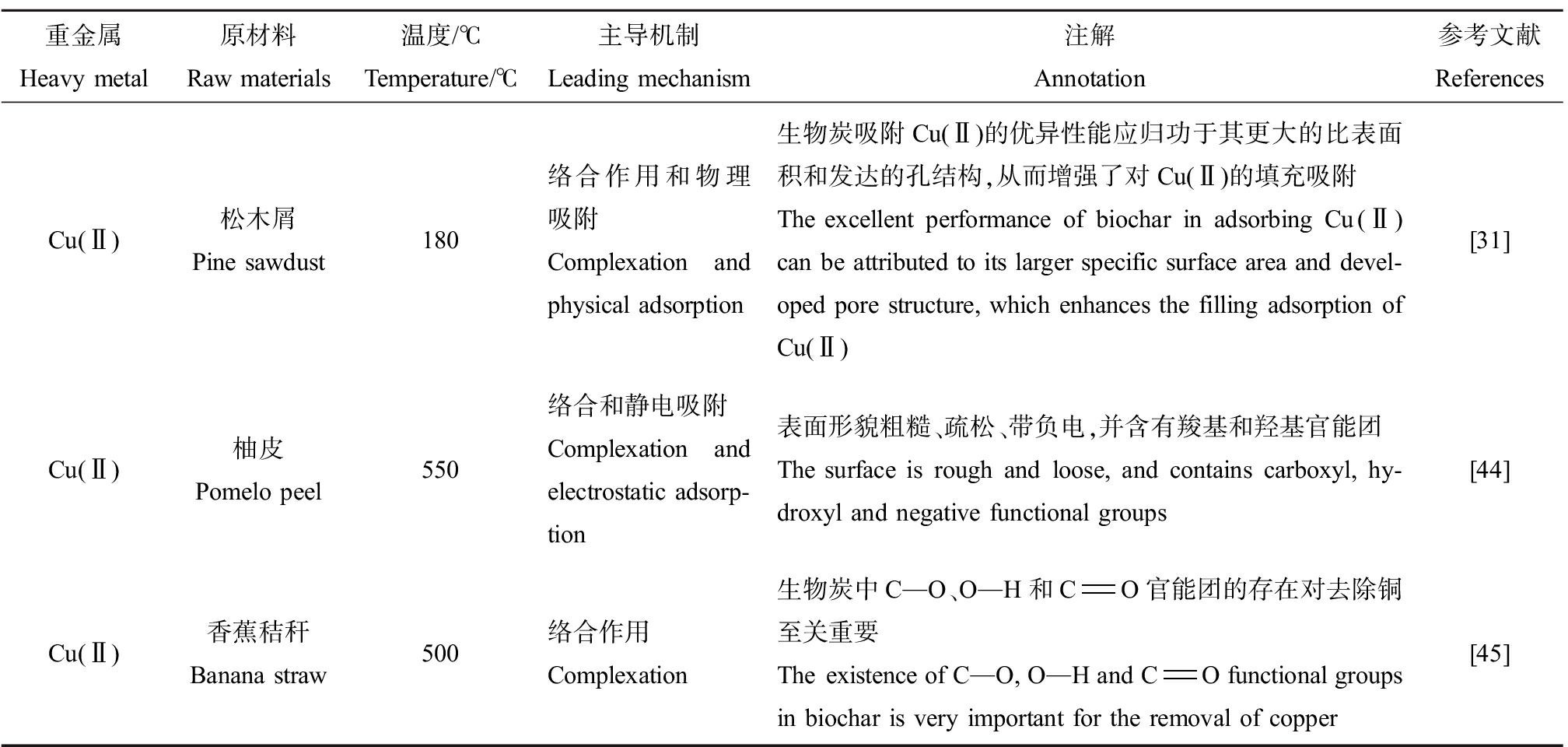
重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesCu(Ⅱ)松木屑Pine sawdust180络合作用和物理吸附Complexation and physical adsorption生物炭吸附Cu(Ⅱ)的优异性能应归功于其更大的比表面积和发达的孔结构,从而增强了对Cu(Ⅱ)的填充吸附The excellent performance of biochar in adsorbing Cu(Ⅱ) can be attributed to its larger specific surface area and devel-oped pore structure, which enhances the filling adsorption of Cu(Ⅱ)[31]Cu(Ⅱ)柚皮Pomelo peel550络合和静电吸附Complexation and electrostatic adsorp-tion表面形貌粗糙、疏松、带负电,并含有羧基和羟基官能团The surface is rough and loose, and contains carboxyl, hy-droxyl and negative functional groups[44]Cu(Ⅱ)香蕉秸秆Banana straw500络合作用Complexation生物炭中C—O、O—H和CO官能团的存在对去除铜至关重要The existence of C—O, O—H and CO functional groups in biochar is very important for the removal of copper[45]
4.5 Cd
Cd被认为是使用最广泛的有毒金属,土壤Cd是我国土壤重金属点位污染最为严重的重金属,且容易在食物链中积累。土壤中Cd的活性强烈依赖于土壤pH值。之前的研究表明生物炭中羟基是通过形成Cd-O键参与Cd(Ⅱ)吸附的主要活性物质[40]。Liang等[46]证实了Cd(Ⅱ)去除的主要机制是Cd(Ⅱ)与羟基络合生成Cd-O或Cd-OH物质。且生物炭中所含的![]() 和OH-等碱性物质通常对土壤中Cd具有较强的吸附和结合能力,使游离Cd(Ⅱ)转化为Cd(OH)2、Cd3(PO4)2和CdCO3沉淀[42]。土壤方解石(CaCO3)与Cd(Ⅱ)的阳离子交换效应产生的高吸附亲和力是降低Cd生物有效性的主要因素;当然,生物炭中丰富的官能团和较大的比表面积对Cd固定化也至关重要。如Yin等[47]使用1%~2%的水稻秸秆生物炭处理矿区农田土壤,发现土壤根际孔隙水Cd浓度显著降低,Gao等[48]制备的玉米秸秆生物炭使农田土壤中可提取态Cd含量降低了91%。此外,麦秆生物炭还可以通过增加土壤溶解有机质的丰度和种类,同时提高土壤微生物群落的多样性和种类,如短食单胞菌和窄食单胞菌数量增加,使Cd(Ⅱ)的去除量达到137.89 mg·g-1[49]。值得一提的是,生物炭中的碱性物质还可以刺激生物炭的酸性官能团去质子化,从而进一步增强对Cd的吸附能力。生物炭钝化Cd的机制如表5所示。
和OH-等碱性物质通常对土壤中Cd具有较强的吸附和结合能力,使游离Cd(Ⅱ)转化为Cd(OH)2、Cd3(PO4)2和CdCO3沉淀[42]。土壤方解石(CaCO3)与Cd(Ⅱ)的阳离子交换效应产生的高吸附亲和力是降低Cd生物有效性的主要因素;当然,生物炭中丰富的官能团和较大的比表面积对Cd固定化也至关重要。如Yin等[47]使用1%~2%的水稻秸秆生物炭处理矿区农田土壤,发现土壤根际孔隙水Cd浓度显著降低,Gao等[48]制备的玉米秸秆生物炭使农田土壤中可提取态Cd含量降低了91%。此外,麦秆生物炭还可以通过增加土壤溶解有机质的丰度和种类,同时提高土壤微生物群落的多样性和种类,如短食单胞菌和窄食单胞菌数量增加,使Cd(Ⅱ)的去除量达到137.89 mg·g-1[49]。值得一提的是,生物炭中的碱性物质还可以刺激生物炭的酸性官能团去质子化,从而进一步增强对Cd的吸附能力。生物炭钝化Cd的机制如表5所示。
表5 生物炭钝化Cd的机制
Table 5 Mechanism of Cd passivation by biochar
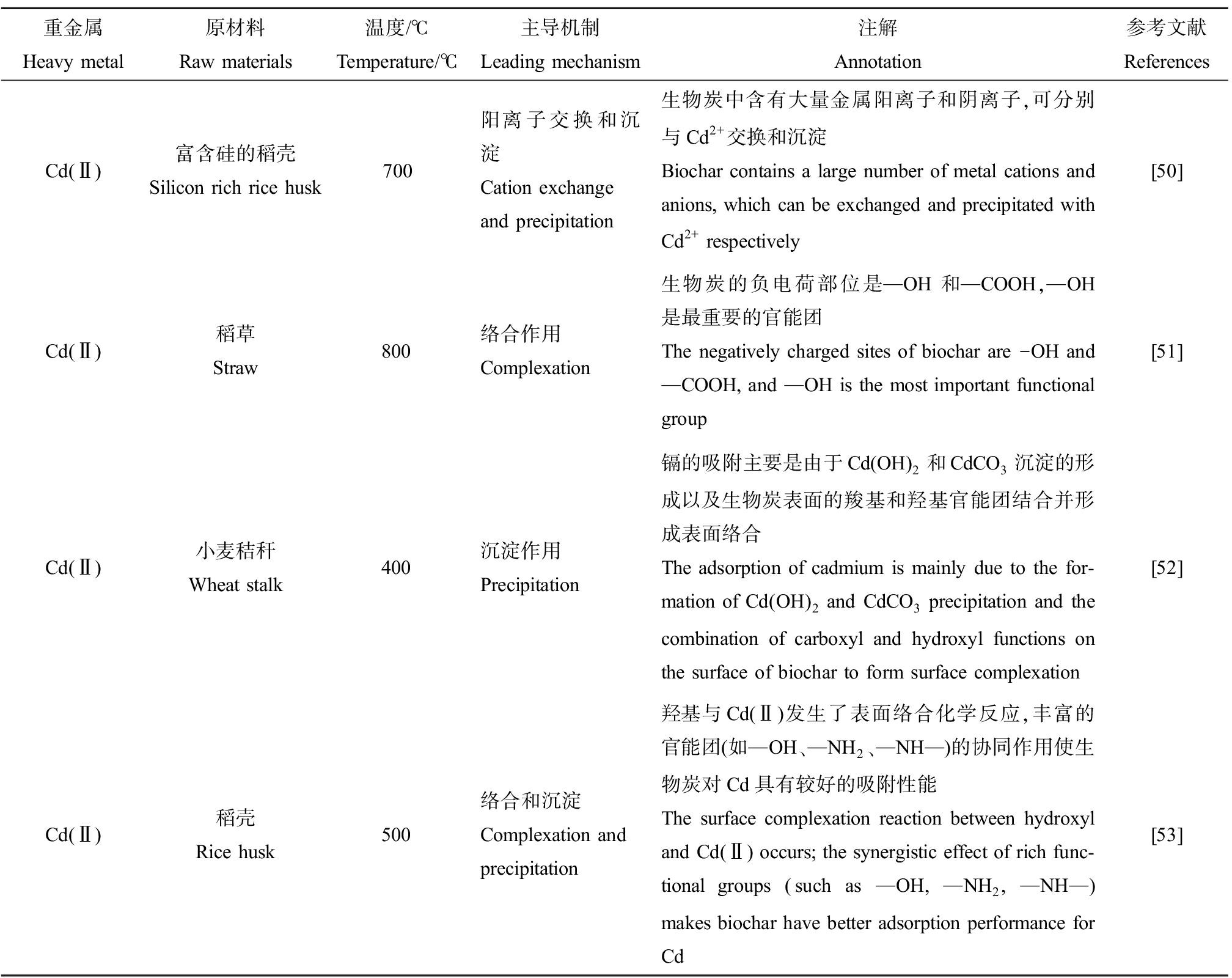
重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesCd(Ⅱ)富含硅的稻壳Silicon rich rice husk700阳离子交换和沉淀Cation exchange and precipitation生物炭中含有大量金属阳离子和阴离子,可分别与Cd2+交换和沉淀Biochar contains a large number of metal cations and anions, which can be exchanged and precipitated with Cd2+ respectively[50]Cd(Ⅱ)稻草Straw800络合作用Complexation生物炭的负电荷部位是—OH和—COOH,—OH是最重要的官能团The negatively charged sites of biochar are -OH and —COOH, and —OH is the most important functional group[51]Cd(Ⅱ)小麦秸秆Wheat stalk400沉淀作用Precipitation镉的吸附主要是由于Cd(OH)2和CdCO3沉淀的形成以及生物炭表面的羧基和羟基官能团结合并形成表面络合The adsorption of cadmium is mainly due to the for-mation of Cd(OH)2 and CdCO3 precipitation and the combination of carboxyl and hydroxyl functions on the surface of biochar to form surface complexation[52]Cd(Ⅱ)稻壳Rice husk500络合和沉淀Complexation and precipitation羟基与Cd(Ⅱ)发生了表面络合化学反应,丰富的官能团(如—OH、—NH2、—NH—)的协同作用使生物炭对Cd具有较好的吸附性能The surface complexation reaction between hydroxyl and Cd(Ⅱ) occurs; the synergistic effect of rich func-tional groups (such as —OH, —NH2, —NH—) makes biochar have better adsorption performance for Cd[53]
4.6 Cr
土壤中Cr一般以2种价态存在,即Cr(Ⅵ)和Cr(Ⅲ)。Cr也被认为是Ⅰ类人类致癌物,通过吸入或食用受Cr污染的食品进入人体。Cr的毒性在植物中作用也很明显,表现为种子萌发延迟、根系受损和生物量减少等。土壤中Cr的固定化是一个吸附-还原-沉淀的复杂组合过程。Cr(Ⅵ)的吸附机理主要是静电作用、还原和络合作用,而Cr(Ⅲ)更倾向于络合吸附。生物炭可以作为电子供体和Cr(Ⅵ)还原的穿梭体,有助于更高的Cr(Ⅵ)去除效率。Mandal等[54]发现动物粪源生物炭在酸性土壤中的Cr(Ⅵ)还原效果显著高于碱性土壤。主要表现在生物炭中C—O、C![]() O、—COOH和—OH等含氧官能团对Cr(Ⅵ)的表面吸附作用以及生物炭提供的电子的还原反应。具体来说,生物炭通过吸附-还原作用将Cr(Ⅵ)转化为毒性和溶解度较低的Cr(Ⅲ),并参与Cr2O3的形成或Cr(OH)3的沉淀,从而达到固定Cr的目的。Choppala等[55]将鸡粪生物炭应用于添加Cr的低有机质含量土壤,降低了Cr的生物毒性。生物炭还可以直接吸附Cr(Ⅲ),其中含氧官能团通过络合作用起主导作用。Sun等[56]在蚕茧与KOH共热解生物炭中发现Cr(Ⅲ)与含氧官能团之间存在很强的相互作用。此外Lian等[57]提出生物炭去除Cr(Ⅵ)的3种机制,即与Cr(Ⅵ)的静电相互作用、Cr(Ⅵ)还原为Cr(Ⅲ)和Cr(Ⅲ)的络合。首先,在酸性条件下,带负电荷的Cr(Ⅵ)在静电驱动下向带正电荷的生物炭表面移动;然后,在氢离子和生物炭提供的电子供给体的参与下,将Cr(Ⅵ)还原为Cr(Ⅲ);最后,将一部分Cr(Ⅲ)与生物炭上的官能团络合。且在纳米零价铁活性污泥生物炭上也可以发现类似的机理[58]。部分Cr(Ⅵ)通过静电相互作用直接吸附在生物炭上,而大部分Cr(Ⅵ)被还原成Cr(Ⅲ),然后通过络合去除[58]。此外,花生壳生物炭提高了土壤有机质含量,提高了土壤脲酶、磷酸酶、过氧化氢酶和蔗糖酶活性,还增加了土壤中细菌、放线菌和真菌的数量,降低了土壤铬的有效性[59]。综上对不同价态的Cr的钝化机制是不同的,具体钝化机制如表6所示,Cr(Ⅵ)的吸附机理主要是静电作用和络合作用,还可以将Cr(Ⅵ)还原为Cr(Ⅲ)然后再络合吸附。
O、—COOH和—OH等含氧官能团对Cr(Ⅵ)的表面吸附作用以及生物炭提供的电子的还原反应。具体来说,生物炭通过吸附-还原作用将Cr(Ⅵ)转化为毒性和溶解度较低的Cr(Ⅲ),并参与Cr2O3的形成或Cr(OH)3的沉淀,从而达到固定Cr的目的。Choppala等[55]将鸡粪生物炭应用于添加Cr的低有机质含量土壤,降低了Cr的生物毒性。生物炭还可以直接吸附Cr(Ⅲ),其中含氧官能团通过络合作用起主导作用。Sun等[56]在蚕茧与KOH共热解生物炭中发现Cr(Ⅲ)与含氧官能团之间存在很强的相互作用。此外Lian等[57]提出生物炭去除Cr(Ⅵ)的3种机制,即与Cr(Ⅵ)的静电相互作用、Cr(Ⅵ)还原为Cr(Ⅲ)和Cr(Ⅲ)的络合。首先,在酸性条件下,带负电荷的Cr(Ⅵ)在静电驱动下向带正电荷的生物炭表面移动;然后,在氢离子和生物炭提供的电子供给体的参与下,将Cr(Ⅵ)还原为Cr(Ⅲ);最后,将一部分Cr(Ⅲ)与生物炭上的官能团络合。且在纳米零价铁活性污泥生物炭上也可以发现类似的机理[58]。部分Cr(Ⅵ)通过静电相互作用直接吸附在生物炭上,而大部分Cr(Ⅵ)被还原成Cr(Ⅲ),然后通过络合去除[58]。此外,花生壳生物炭提高了土壤有机质含量,提高了土壤脲酶、磷酸酶、过氧化氢酶和蔗糖酶活性,还增加了土壤中细菌、放线菌和真菌的数量,降低了土壤铬的有效性[59]。综上对不同价态的Cr的钝化机制是不同的,具体钝化机制如表6所示,Cr(Ⅵ)的吸附机理主要是静电作用和络合作用,还可以将Cr(Ⅵ)还原为Cr(Ⅲ)然后再络合吸附。
表6 生物炭钝化Cr的机制
Table 6 Mechanism of Cr passivation by biochar

重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesCr(Ⅲ)稻壳Rice husk390离子交换Ion exchange生物炭中矿物质通过与溶液中Cr(Ⅲ)交换提高了吸附能力Minerals in biochar improve the adsorption capacity by exchanging with Cr(Ⅲ) in solution[60]Cr(Ⅲ)负载Fe3O4的高梁秸秆Sorghum straw loaded with Fe3O4600络合作用Complexation重金属吸附过程中发生了表面络合,其中羧基氧向金属离子提供电子,导致相邻碳原子的电子密度降低,且Cr(Ⅲ)离子半径相对较小Surface complexation occurs in the process of heavy metal adsorption, in which carboxyl oxygen provides electrons to metal ions, resulting in the reduction of e-lectron density of adjacent carbon atoms, and the radi-us of Cr(Ⅲ) ion is relatively small[61]Cr(Ⅵ)柚皮Pomelo peel300还原、静电作用和络合作用Reduction, electrostatic interaction and complexationK2FeO4的加入促进了含氧官能团的形成,从而与Cr(Ⅵ)络合The addition of K2FeO4 promoted the formation of oxygen-containing functional groups, which com-plexed with Cr(Ⅵ)[62]
4.7 As
环境中As含量的增加是人为(如采矿、化工生产等)和自然(如火山爆发、地热等)活动的结果。As暴露会导致植物生长抑制和果实减少,也会导致人类和动物的肠道、肺部和心脏疾病。
磷(P)和As具有相似的化学性质,土壤P含量是控制As迁移的关键因素。As污染土壤中磷含量与砷含量呈显著正相关。含磷生物炭处理后,土壤颗粒吸附部位发生的可溶性磷酸盐与砷酸盐的竞争促进了固相土壤中As的解吸,提高了孔隙水中As的浓度,如豆粕、松针和稻秆生物炭。土壤可溶性有机碳的作用与P相似,但可溶性有机碳还有另一种作用。如Wang等[63]的研究表明,厌氧条件下施用稻秆生物炭增加了水稻土中铁还原菌(如梭状芽胞杆菌、芽孢杆菌和发热菌)的丰度,促进了Fe/Al非晶态氧化物上As(Ⅴ)的还原。总之,土壤中可溶性有机碳含量的增加增强了As(Ⅴ)的微生物还原效应,最终促进了As(Ⅲ)从水稻土中释放出来。在缺氧条件下,Qiao等[64]制备的油棕榈纤维生物炭的应用也得到了类似的结果。生物炭还具有高芳香度和高碱度,像腐殖质一样能同时促进微生物对Fe(Ⅲ)和As(Ⅴ)的还原,表现为电子穿梭的作用[64]。Choppala等[55]还报道了鸡粪生物炭表面官能团提供的π电子可以促进As(Ⅴ)的还原,这是提高As迁移率的另一个重要因素。因此,As更倾向于通过静电吸引和氧化还原作用吸附在生物炭上。生物炭钝化As的机制如表7所示。此外,添加松果炭后,微生物群落丰富度和代谢活动强度增强,溶解性有机物种类和含量显著增加。尤其增加了土壤中的芽孢杆菌和不动杆菌,从而增强了无机离子的运输和代谢功能,促进重金属的钝化和稳定,使As的形态由交换态向残留态转变[37]。
表7 生物炭钝化As的机制
Table 7 Mechanism of biochar passivation of As
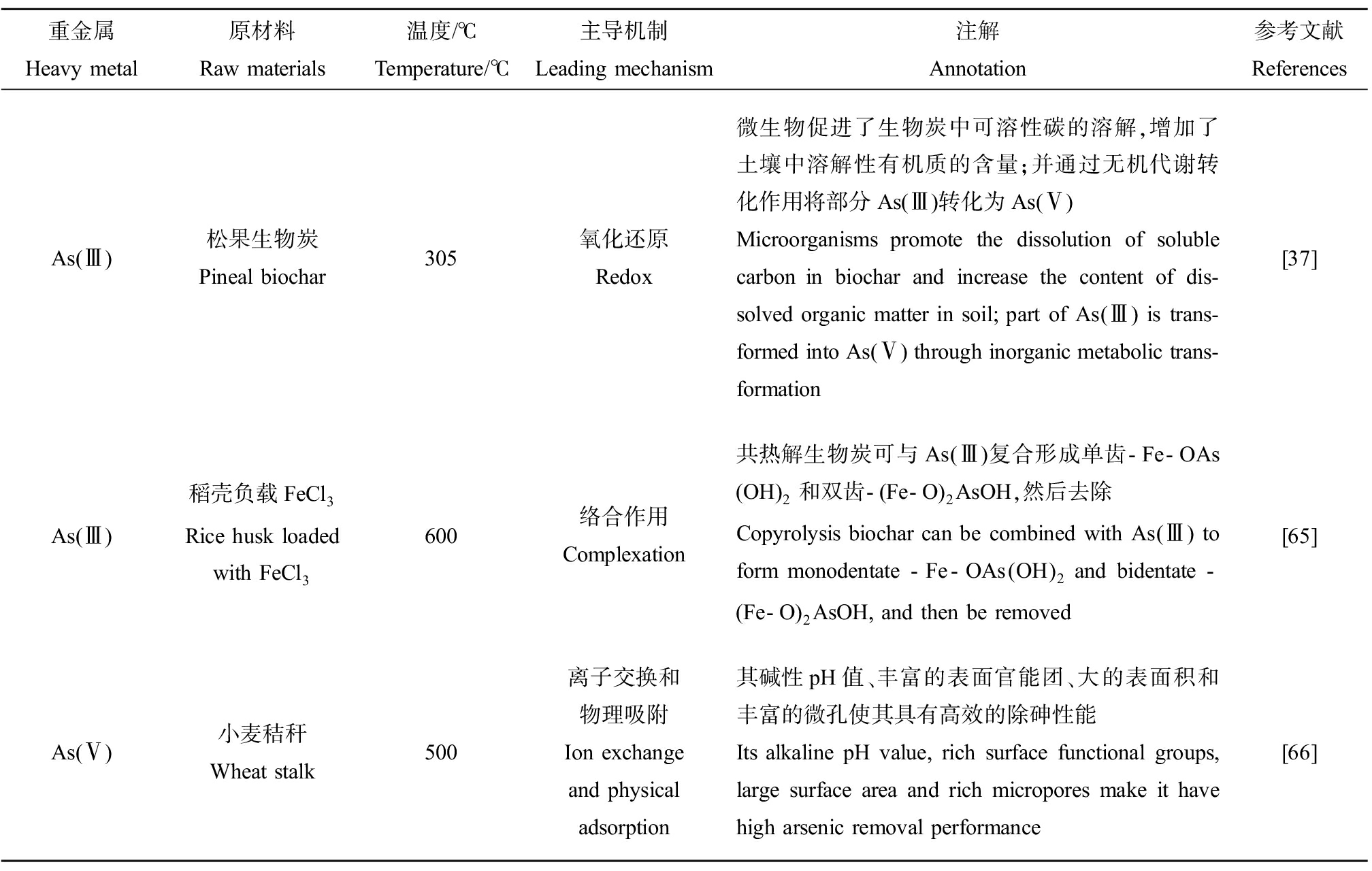
重金属Heavy metal原材料Raw materials温度/℃Temperature/℃主导机制Leading mechanism注解Annotation参考文献ReferencesAs(Ⅲ)松果生物炭Pineal biochar305氧化还原Redox微生物促进了生物炭中可溶性碳的溶解,增加了土壤中溶解性有机质的含量;并通过无机代谢转化作用将部分As(Ⅲ)转化为As(Ⅴ)Microorganisms promote the dissolution of soluble carbon in biochar and increase the content of dis-solved organic matter in soil; part of As(Ⅲ) is trans-formed into As(Ⅴ) through inorganic metabolic trans-formation[37]As(Ⅲ)稻壳负载FeCl3Rice husk loaded with FeCl3600络合作用Complexation共热解生物炭可与As(Ⅲ)复合形成单齿-Fe-OAs(OH)2和双齿-(Fe-O)2AsOH,然后去除Copyrolysis biochar can be combined with As(Ⅲ) to form monodentate -Fe-OAs(OH)2 and bidentate -(Fe-O)2AsOH, and then be removed[65]As(Ⅴ)小麦秸秆Wheat stalk500离子交换和物理吸附Ion exchange and physical adsorption其碱性pH值、丰富的表面官能团、大的表面积和丰富的微孔使其具有高效的除砷性能Its alkaline pH value, rich surface functional groups, large surface area and rich micropores make it have high arsenic removal performance[66]
综上所述,农林废弃物生物炭对不同金属离子的钝化机理并不完全相同,多种钝化作用共存。生物炭表面富含大量的含氧官能团(—COOH、—COH和—OH)、碱金属离子(K+、Ca2+、Na+和Mg2+)、微生物群落为重金属离子的钝化提供了重要的条件。生物炭对重金属的固定是一个吸附-还原-络合-沉淀的复杂过程。
5 局限性与展望(Limitations and prospects)
应用生物炭修复土壤重金属是一种可行的绿色、经济和环保方法。然而,随着时间的推移,土壤成分会发生不同程度的理化变化,其中包括生物炭的性质。虽然生物炭固定土壤重金属的研究已经发展到室内盆栽试验和短期田间试验,但最终目标是将其扩展到大规模的工程应用。因此,在不同类型重金属污染的土壤进行长期定位测试是非常有必要性的,以进一步验证生物炭的长期稳定性及其对土壤环境的长期影响,确保农业土地的质量和安全。还应注意的是,土壤中生物炭的老化过程会增加重金属的不稳定组分和施用生物炭的环境风险。虽然生物炭具有较高的吸附能力和较低的潜在生态风险,在重金属吸附和固定化方面具有重要的应用前景。然而,进一步改进目前研究的一些局限性仍是其开发和利用的必要条件。针对目前研究的局限性,提出未来可能的研究方向如下。(1)生物炭的吸附性能进一步提高和优化。可能的方法包括增加比表面积和孔体积,用活性表面官能团改性,通过优化合成条件(如热解温度、热解时间、加热速率和气体),改变原料及其配比,提高矿物含量,引入活性添加剂,甚至将生物质转化为功能性碳纳米材料。(2)生物炭与微生物相互作用下对重金属的吸附机理研究还存在许多不足,需要详细和及时的研究。(3)吸附和固定化过程中产生的残留副产物的处理和处置在其广泛应用之前仍是一个未解决的问题,这往往会加剧重金属污染。因此,为了实现生物炭的商业化,需要对共热解生物炭应用进行生命周期分析,评估其经济效益、资源损失、能源和环境影响。生物炭的经济成本与效益价值之间的优劣需要仔细衡量。为了提高经济可用性,需要发现更容易的生产工艺和更便宜的生物质原料来源,可以为提高生产效率和减少经济负担提供一个平台,即达到商业实用性的目的。(4)土壤是一个复杂的生态系统,生物炭钝化土壤重金属的目的很大程度上是为了让农作物少吸收或不吸收重金属,因此,在未来的研究中生物炭修复对农作物本身吸附重金属特性的影响也值得关注。
[1] Mandal S, Pu S Y, Adhikari S, et al.Progress and future prospects in biochar composites: Application and reflection in the soil environment[J].Critical Reviews in Environmental Science and Technology, 2021, 51(3): 219-271
[2] Li C F, Zhou K H, Qin W Q, et al.A review on heavy metals contamination in soil: Effects, sources, and remediation techniques[J].Soil and Sediment Contamination: An International Journal, 2019, 28(4): 380-394
[3] Huang Y, Wang L Y, Wang W J, et al.Current status of agricultural soil pollution by heavy metals in China: A meta-analysis[J].The Science of the Total Environment, 2019, 651(Pt 2): 3034-3042
[4] Wang Y, Wang H S, Tang C S, et al.Remediation of heavy-metal-contaminated soils by biochar: A review[J].Environmental Geotechnics, 2020, 9(3): 135-148
[5] Qiu B, Tao X, Wang H, et al.Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review[J].Journal of Analytical and Applied Pyrolysis, 2021, 155: 105081
[6] Xu D Y, Zhao Y, Sun K, et al.Cadmium adsorption on plant-and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties[J].Chemosphere, 2014, 111: 320-326
[7] Liu Z G, Zhang F S.Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass[J].Journal of Hazardous Materials, 2009, 167(1-3): 933-939
[8] Nzediegwu C, Arshad M, Ulah A, et al.Fuel, thermal and surface properties of microwave-pyrolyzed biochars depend on feedstock type and pyrolysis temperature[J].Bioresource Technology, 2021, 320(Pt A): 124282
[9] Li S M, Harris S, Anandhi A, et al.Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses[J].Journal of Cleaner Production, 2019, 215: 890-902
[10] Hopkins D, Hawboldt K.Biochar for the removal of metals from solution: A review of lignocellulosic and novel marine feedstocks[J].Journal of Environmental Chemical Engineering, 2020, 8(4): 103975
[11] Zhang C T, Zhang Z M, Zhang L J, et al.Evolution of the functionalities and structures of biochar in pyrolysis of poplar in a wide temperature range[J].Bioresource Technology, 2020, 304: 123002
[12] Cao X D, Harris W.Properties of dairy-manure-derived biochar pertinent to its potential use in remediation[J].Bioresource Technology, 2010, 101(14): 5222-5228
[13] Cantrell K B, Hunt P G, Uchimiya M, et al.Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar[J].Bioresource Technology, 2012, 107: 419-428
[14] Zhang J Z, Hou D Y, Shen Z T, et al.Effects of excessive impregnation, magnesium content, and pyrolysis temperature on MgO-coated watermelon rind biochar and its lead removal capacity[J].Environmental Research, 2020, 183: 109152
[15] Park J H, Wang J J, Kim S H, et al.Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures[J].Journal of Colloid and Interface Science, 2019, 553: 298-307
[16] Williams P T, Besler S.The influence of temperature and heating rate on the slow pyrolysis of biomass[J].Renewable Energy, 1996, 7(3): 233-250
[17] Braadbaart F, Poole I.Morphological, chemical and physical changes during charcoalification of wood and its relevance to archaeological contexts[J].Journal of Archaeological Science, 2008, 35(9): 2434-2445
[18] Zhao B, Xu X Y, Zeng F Q, et al.The hierarchical porous structure bio-char assessments produced by co-pyrolysis of municipal sewage sludge and hazelnut shell and Cu(Ⅱ)adsorption kinetics[J].Environmental Science and Pollution Research, 2018, 25(20): 19423-19435
[19] Li Y L, Yu H, Liu L N, et al.Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates[J].Journal of Hazardous Materials, 2021, 420: 126655
[20] Wang A O, Ptacek C J, Blowes D W, et al.Application of hardwood biochar as a reactive capping mat to stabilize mercury derived from contaminated floodplain soil and riverbank sediments[J].The Science of the Total Environment, 2019, 652: 549-561
[21] Xing Y, Wang J X, Xia J C, et al.A pilot study on using biochars as sustainable amendments to inhibit rice uptake of Hg from a historically polluted soil in a Karst Region of China[J].Ecotoxicology and Environmental Safety, 2019, 170: 18-24
[22] Man Y, Wang B, Wang J X, et al.Use of biochar to reduce mercury accumulation in Oryza sativaL.: A trial for sustainable management of historically polluted farmlands[J].Environment International, 2021, 153: 106527
[23] Chen J Q, Dong J, Chang J J, et al.Characterization of an Hg(Ⅱ)-volatilizing Pseudomonassp.strain, DC-B1, and its potential for soil remediation when combined with biochar amendment[J].Ecotoxicology and Environmental Safety, 2018, 163: 172-179
[24] Xu Y, Luo G Q, He S W, et al.Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe(NO3)3-laden wood and polyvinyl chloride waste[J].Fuel, 2019, 239: 982-990
[25] Wang Y J, Dang F, Zheng X M, et al.Biochar amendment to further reduce methylmercury accumulation in rice grown in selenium-amended paddy soil[J].Journal of Hazardous Materials, 2019, 365: 590-596
[26] Jia L, Yu Y, Li Z P, et al.Study on the Hg(0)removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals[J].Bioresource Technology, 2021, 332: 125086
[27] Guo X L, Li M H, Liu A C, et al.Adsorption mechanisms and characteristics of Hg2+removal by different fractions of biochar[J].Water, 2020, 12(8): 2105
[28] Rahmani-Sani A, Singh P, Raizada P, et al.Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions[J].Bioresource Technology, 2020, 297: 122452
[29] Dai Z M, Brookes P C, He Y, et al.Increased agronomic and environmental value provided by biochars with varied physiochemical properties derived from swine manure blended with rice straw[J].Journal of Agricultural and Food Chemistry, 2014, 62(44): 10623-10631
[30] Song J P, Zhang S S, Li G X, et al.Preparation of montmorillonite modified biochar with various temperatures and their mechanism for Zn ion removal[J].Journal of Hazardous Materials, 2020, 391: 121692
[31] Deng J, Li X, Wei X, et al.Hybrid silicate-hydrochar composite for highly efficient removal of heavy metal and antibiotics: Coadsorption and mechanism[J].Chemical Engineering Journal, 2020, 387: 124097
[32] Wu P, Cui P X, Alves M E, et al.Interactive effects of rice straw biochar and γ-Al2O3on immobilization of Zn[J].Journal of Hazardous Materials, 2019, 373: 250-257
[33] Ahmad M, Ok Y S, Kim B Y, et al.Impact of soybean stover-and pine needle-derived biochars on Pb and As mobility, microbial community, and carbon stability in a contaminated agricultural soil[J].Journal of Environmental Management, 2016, 166: 131-139
[34] Kazak O, Tor A.In situpreparation of magnetic hydrochar by co-hydrothermal treatment of waste vinasse with red mud and its adsorption property for Pb(Ⅱ)in aqueous solution[J].Journal of Hazardous Materials, 2020, 393: 122391
[35] Gao Y, Jiang Z, Li J J, et al.A comparison of the characteristics and atrazine adsorption capacity of co-pyrolysed and mixed biochars generated from corn straw and sawdust[J].Environmental Research, 2019, 172: 561-568
[36] Igalavithana A D, Kwon E E, Vithanage M, et al.Soil lead immobilization by biochars in short-term laboratory incubation studies[J].Environment International, 2019, 127: 190-198
[37] Lan J R, Zhang S S, Dong Y Q, et al.Stabilization and passivation of multiple heavy metals in soil facilitating by pinecone-based biochar: Mechanisms and microbial community evolution[J].Journal of Hazardous Materials, 2021, 420: 126588
[38] Gao R L, Fu Q L, Hu H Q, et al.Highly-effective removal of Pb by co-pyrolysis biochar derived from rape straw and orthophosphate[J].Journal of Hazardous Materials, 2019, 371: 191-197
[39] Zhao M, Dai Y, Zhang M Y, et al.Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies[J].The Science of the Total Environment, 2020, 717: 136894
[40] Yu W C, Lian F, Cui G N, et al.N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution[J].Chemosphere, 2018, 193: 8-16
[41] Meier S, Curaqueo G, Khan N, et al.Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil[J].Journal of Soils and Sediments, 2017, 17(3): 741-750
[42] Rechberger M V, Kloss S, Wang S L, et al.Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors?[J].Chemosphere, 2019, 216: 463-471
[43] Zhang C, Shan B Q, Zhu Y Y, et al.Remediation effectiveness of Phyllostachys pubescensbiochar in reducing the bioavailability and bioaccumulation of metals in sediments[J].Environmental Pollution, 2018, 242(Pt B): 1768-1776
[44] Chen Y N, Liu Y H, Li Y P, et al.Novel magnetic pomelo peel biochar for enhancing Pb(Ⅱ)and Cu(Ⅱ)adsorption: Performance and mechanism[J].Water, Air, &Soil Pollution, 2020, 231(8): 404
[45] Deng H, Li Q Y, Huang M J, et al.Removal of Zn(Ⅱ), Mn(Ⅱ)and Cu(Ⅱ)by adsorption onto banana stalk biochar: Adsorption process and mechanisms[J].Water Science and Technology, 2020, 82(12): 2962-2974
[46] Liang J, Li X, Yu Z, et al.Amorphous MnO2modified biochar derived from aerobically composted swine manure for adsorption of Pb(Ⅱ)and Cd(Ⅱ)[J].ACS Sustainable Chemistry &Engineering, 2017, 5(6): 5049-5058
[47] Yin D X, Wang X, Peng B, et al.Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system[J].Chemosphere, 2017, 186: 928-937
[48] Gao X, Peng Y T, Zhou Y Y, et al.Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi(Brassica chinensisL.)[J].Journal of Environmental Management, 2019, 251: 109610
[49] Li J H, Xia C G, Cheng R, et al.Passivation of multiple heavy metals in lead-zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution[J].The Science of the Total Environment, 2022, 803: 149866
[50] Huang F, Gao L Y, Wu R R, et al.Qualitative and quantitative characterization of adsorption mechanisms for Cd2+by silicon-rich biochar[J].The Science of the Total Environment, 2020, 731: 139163
[51] Tan Z X, Yuan S N, Hong M F, et al.Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd[J].Journal of Hazardous Materials, 2020, 384: 121370
[52] Chen D, Wang X B, Wang X L, et al.The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil[J].The Science of the Total Environment, 2020, 714: 136550
[53] Xiang J X, Lin Q T, Yao X S, et al.Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in situremediation of Cd-contaminated soil[J].Environmental Research, 2021, 195: 110650
[54] Mandal S, Sarkar B, Bolan N, et al.Enhancement of chromate reduction in soils by surface modified biochar[J].Journal of Environmental Management, 2017, 186(Pt 2): 277-284
[55] Choppala G, Bolan N, Kunhikrishnan A, et al.Differential effect of biochar upon reduction-induced mobility and bioavailability of arsenate and chromate[J].Chemosphere, 2016, 144: 374-381
[56] Sun J T, Li M F, Zhang Z H, et al.Unravelling the adsorption disparity mechanism of heavy-metal ions on the biomass-derived hierarchically porous carbon[J].Applied Surface Science, 2019, 471: 615-620
[57] Lian G Q, Wang B, Lee X Q, et al.Enhanced removal of hexavalent chromium by engineered biochar composite fabricated from phosphogypsum and distillers grains[J].The Science of the Total Environment, 2019, 697: 134119
[58] Liu L H, Liu X, Wang D Q, et al.Removal and reduction of Cr(Ⅵ)in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge[J].Journal of Cleaner Production, 2020, 257: 120562
[59] Guo X F, Ji Q, Rizwan M, et al.Effects of biochar and foliar application of selenium on the uptake and subcellular distribution of chromium in Ipomoea aquaticain chromium-polluted soils[J].Ecotoxicology and Environmental Safety, 2020, 206: 111184
[60] Dias D, Bernardo M, Matos I, et al.Activation of co-pyrolysis chars from rice wastes to improve the removal of Cr3+from simulated and real industrial wastewaters[J].Journal of Cleaner Production, 2020, 267: 121993
[61] Xie J X, Lin R Y, Liang Z J, et al.Effect of cations on the enhanced adsorption of cationic dye in Fe3O4-loaded biochar and mechanism[J].Journal of Environmental Chemical Engineering, 2021, 9(4): 105744
[62] Yin Z B, Xu S, Liu S, et al.A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium[J].Bioresource Technology, 2020, 300: 122680
[63] Wang N, Xue X M, Juhasz A L, et al.Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic[J].Environmental Pollution, 2017, 220(Pt A): 514-522
[64] Qiao J T, Li X M, Li F B.Roles of different active metal-reducing bacteria in arsenic release from arsenic-contaminated paddy soil amended with biochar[J].Journal of Hazardous Materials, 2018, 344: 958-967
[65] Singh P, Sarswat A, Pittman C U Jr, et al.Sustainable low-concentration arsenite[As(Ⅲ)]removal in single and multicomponent systems using hybrid iron oxide-biochar nanocomposite adsorbents—A mechanistic study[J].ACS Omega, 2020, 5(6): 2575-2593
[66] Kumar A, Bhattacharya T.Removal of arsenic by wheat straw biochar from soil[J].Bulletin of Environmental Contamination and Toxicology, 2022, 108(3): 415-422