手性是化合物的一种立体结构属性,手性分子具有一对互为镜像又不能够完全重合的对映异构体(enantiomer),这对对映异构体具有不同的旋光性,分别称为左旋体和右旋体,等量的对映异构体混合物不能够使偏振光旋转,称为外消旋体(racemate)。手性分子的2个对映异构体在结构上的差别很小,一般情况下具有相同的物理化学性质(如,溶解度、空气-水交换、吸附和非生物转化),但在与酶、受体或其他手性分子(如,蛋白质、核酸和糖类)相互作用时会产生立体选择性,发生不同的生物响应,从而导致手性药物对映异构体在药效学、毒理学和代谢动力学等方面的表现不一致[1-2]。因此,手性分子的研究在生命科学、药物学和现代医学等领域具有重要的意义。美国食品与药物监督管理局(Food and Drug Administration, FDA)规定,外消旋体类药物在进行申请的过程中,必须提供每个对应异构体在药效和毒理方面的详细数据,而不能将它们视为一种物质。
据统计,在1 850种常用人类药物中,有1 045种为手性药物,其中45.5%以外消旋体的形式使用,抗癫痫药、β肾上腺素受体激动药、β受体阻断药和口服抗凝剂药中外消旋体占90%以上,目前世界上手性药物销售以每年15%以上的速度增长[3]。药物在人体或动物体内代谢后,以尿液或粪便的形式排入市政污水管道,部分未被代谢的药物以原形排出,由于现有的污水处理技术很难将其有效清除,这部分药物通过污水处理厂出水的方式进入水环境。手性药物在环境中的迁移、降解、生物富集以及对生物的毒性效应存在对映体选择性,但是一直以来,在研究手性分子的环境行为及生态效应时,都把它们视为单一化合物,而且,几乎所有的环境法规也把其当成单一化合物管理,这有可能会高估或者低估该类污染物的生态风险和健康安全效应。目前,手性分子在化学领域,尤其是在环境化学领域中越来越受到人们的青睐,药物的环境手性特征及环境行为已引起人们的广泛关注[4-5]。欧美、日本等发达国家已开展了一系列手性药物环境行为和效应的对映体选择性研究,相比之下,我国针对环境中手性药物的研究较少,亟待开展广泛深入的研究[6]。
1 手性药物在地表水环境中的暴露与归趋(Exposure and fate trends of chiral pharmaceuticals in surface water environments)
1.1 地表水环境暴露特征
随着新型污染物药物和个人护理品(pharmaceuticals and personal care products, PPCPs)引起广泛的关注,关于手性药物在我国地表水的污染特征调查也越来越多,报道较多的10种手性药物包括咖啡因、布洛芬、卡马西平、诺氟沙星、吉非贝齐、普萘洛尔、氧氟沙星、恩诺沙星、萘普生和环丙沙星[7]。在我国地表水中,有5种手性药物的最高浓度为μg·L-1水平,暴露浓度最高的是氧氟沙星(1 6953 ng·L-1),在89个点位中检出77个,检出率为86.5%;其次是咖啡因(9 785 ng·L-1),在52个点位中全部检出;卡马西平的最高检出浓度是1 090 ng·L-1,在46个点位中检出43个,检出率为93%[8]。对比国外地表水中手性药物的暴露浓度,欧洲地表水中达到μg·L-1水平的手性药物有7种,检出浓度最高的是咖啡因,高达39 813 ng·L-1,其次是布洛芬(31 323 ng·L-1)[9-13],美国地表水中的手性药物浓度较低,只有3种达到μg·L-1水平,分别是布洛芬(1 620 ng·L-1)、吉非贝齐(1 360 ng·L-1)和咖啡因(1 276 ng·L-1)[14]。
以上调查结果均基于非手性检测方法,研究表明,生物降解过程对手性药物具有立体选择性[15-17],可能导致水环境中2个对映体的含量有所差异。在手性环境化学中,一般使用对映体含量比值(enantiomer ratio, ER)或对映体分数(enantiomer fraction, EF)来描述环境中的对映体选择性行为,其中ER为S对映体与R对映体含量的比值,取值范围为0到无穷;EF为S对映体与外消旋体的比值,取值范围是0~1[6, 18]。目前关于地表水中手性药物对映体构成的报道较少(表1),Ma等[19]调查了北京河流中5种手性药物(阿替洛尔、美托洛尔、普萘洛尔、文拉法辛和氟西汀)及其降解产物的浓度和对映体构成,EF值为0.40~0.57。Kasprzyk-Hordern和Baker[20]检测了英国地表水中5种手性药物(苯丙胺、安非他明、文拉法辛和阿替洛尔)的浓度和对映体组成,其中阿替洛尔最高浓度可达3 048.5 ng·L-1,EF值为0.39~0.56,麻黄碱的EF值较高,在某些点位为1。萘普生在澳大利亚[21]地表水中的浓度可达604.5 ng·L-1,EF值>0.98,而日本[22]多摩河中萘普生的EF值均<0.98。手性药物对映体的分离是分析环境中手性药物的前提,而复杂环境中ng·L-1级浓度的手性药物给环境分析技术带来巨大挑战。
表1 地表水中手性药物浓度和对映体构成
Table 1 Chiral pharmaceuticals concentrations and enantiomeric composition in surface water
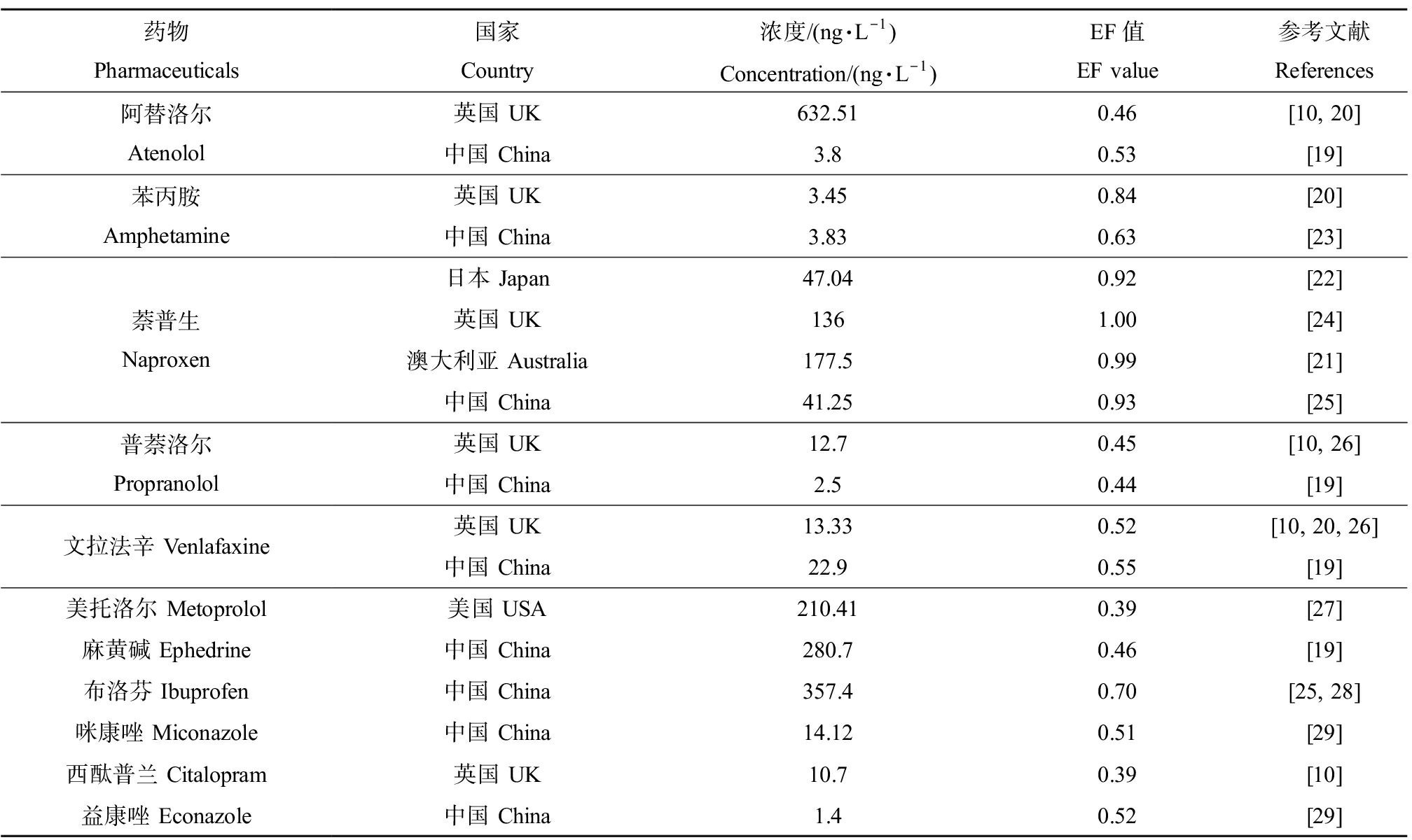
药物Pharmaceuticals国家Country浓度/(ng·L-1)Concentration/(ng·L-1)EF值EF value参考文献References阿替洛尔Atenolol英国 UK632.510.46[10, 20]中国 China3.80.53[19]苯丙胺Amphetamine英国 UK3.450.84[20]中国 China3.830.63[23]萘普生Naproxen日本 Japan47.040.92[22]英国 UK1361.00[24]澳大利亚 Australia177.50.99[21]中国 China41.250.93[25]普萘洛尔Propranolol英国 UK12.70.45[10, 26]中国 China2.50.44[19]文拉法辛 Venlafaxine英国 UK13.330.52[10, 20, 26]中国 China22.90.55[19]美托洛尔 Metoprolol美国 USA210.410.39[27]麻黄碱 Ephedrine中国 China280.70.46[19]布洛芬 Ibuprofen中国 China357.40.70[25, 28]咪康唑 Miconazole中国 China14.120.51[29]西酞普兰 Citalopram英国 UK10.70.39[10]益康唑 Econazole中国 China1.40.52[29]
注:EF为对映体分数。
Note: EF means enantiomer fraction.
1.2 生物降解转化规律及应用
1.2.1 不同手性药物的立体选择性
Fono等[27]分析了美国特里尼蒂河中美托洛尔在污水排放口及其下游的浓度和EF值,发现美托洛尔的浓度从污水处理厂排放口处的390 ng·L-1降到40 ng·L-1时,EF值从0.45降低到0.31,说明地表水环境对美托洛尔的迁移转化过程具有立体选择性。手性药物在水环境中的迁移转化过程主要为生物降解和物理吸附,另外有研究表明,在水环境中,布洛芬分子可以自动实现从S型向R型转变[30]。
研究表明,生物降解对手性药物具有立体选择性,导致污水处理厂出水中手性药物对映体的浓度分布和EF值不同[31-35](图1)。Evans等[36]对英国某污水处理厂进出水中的药物进行分析发现,9种化合物在进水和出水中的EF值明显不同。例如,3,4-亚甲基二氧基-甲基苯丙胺在进水中以外消旋体的形式存在(EF值为0.5),而在出水中的EF值则为0.7,S对映体的比例提高,说明微生物优先降解R对映体;伪麻黄碱进水EF值为1,出水中则为0.2,说明S-伪麻黄碱先转化为R-伪麻黄碱,然后再被降解。

图1 手性药物在生物降解过程中的立体选择性[9, 11, 20-22, 24, 26, 32, 41-49]
Fig. 1 Stereoselectivity of chiral pharmaceuticals in the biodegradation process[9, 11, 20-22, 24, 26, 32, 41-49]
Rudolf等[9]调查了瑞士污水处理厂中布洛芬的浓度及对映体构成,发现污水处理厂进水中布洛芬的浓度高达3.3 μg·L-1,EF值为0.84~0.95,而出水中的最高浓度为81 ng·L-1,EF值为0.47~0.67。类似的,西班牙5个污水厂中布洛芬的进水EF值为0.73~0.90,出水为0.60~0.76[37];另有研究表明,经膜生物反应器处理后,布洛芬的降解率可以达到99%,EF值从0.52降至0.39[38]。这证明在适宜的条件下,微生物可以优先降解S-布洛芬[39]。
对于部分手性药物,微生物降解过程中的对映体选择性则不太明显。例如,唑类抗真菌药益康唑、咪康唑和酮康唑在污水处理厂进水和出水中的EF值范围为0.47~0.50,没有明显变化[40]。Evans等[36]得到了污水处理厂进出水中18种手性药物的分析结果,其中8种手性药物的EF值没有变化。
水温、水深和营养物质的可利用率和酶作用的接触时间等因素都会对手性药物的生物转化过程产生影响,EF值可能会随着污水处理厂处理工艺的不同有所变化。Matamoros等[50-53]分析了布洛芬和萘普生在5种不同污水处理系统中生物降解的立体选择性,当溶解氧的浓度>1 mg·L-1时,布洛芬的EF值由0.9降至0.7左右,而在溶解氧为0.5 mg·L-1时,进出水中布洛芬EF值无明显变化。萘普生的降解过程则不受溶解氧的影响,无论是在厌氧还是好氧环境中,EF值均由0.9降至0.7。
Evans等[36]发现9种手性药物的EF值在厌氧消化污泥与污水处理厂出水中有显著差异。例如,阿普雷诺尔的出水EF值为0.5,消化污泥中为0.7;安非他明的出水EF值为0.6,消化污泥中则为0.3。由此推断,这些手性药物在厌氧环境中的对映体选择性降解与好氧环境下相反,主要受微生物种群和环境条件影响。
在地表水环境中,布洛芬的降解机理与污水处理工艺中的好氧生物法基本一致[37]。另外有研究表明,在水环境中,布洛芬分子可以自动实现从S型向R型转变[30]。如果S-布洛芬比例较高,说明采样点附近有未经处理的污水排放口,反之则说明进入水体的污水已经经过处理,或采样点距离排污口较远[54]。因此,有学者指出,地表水中布洛芬的对映体EF值可以作为污染源示踪的特征污染物[21]。
如图2所示,地表水中的手性药物,如普萘洛尔、文拉法辛、布洛芬和阿替洛尔EF值和浓度随时间不断将变化,Rudolf等[9]调查了瑞士水域中布洛芬在不同季节的浓度及对映体构成,发现布洛芬在地表水中的浓度普遍较低(≤7.8 ng·L-1),ER值为0.7~4.2,在温度较高的季节ER值较低,而冬季ER值较高,推测原因为温度改变使冬季和夏季微生物群落不同,而导致微生物转化途径不同。
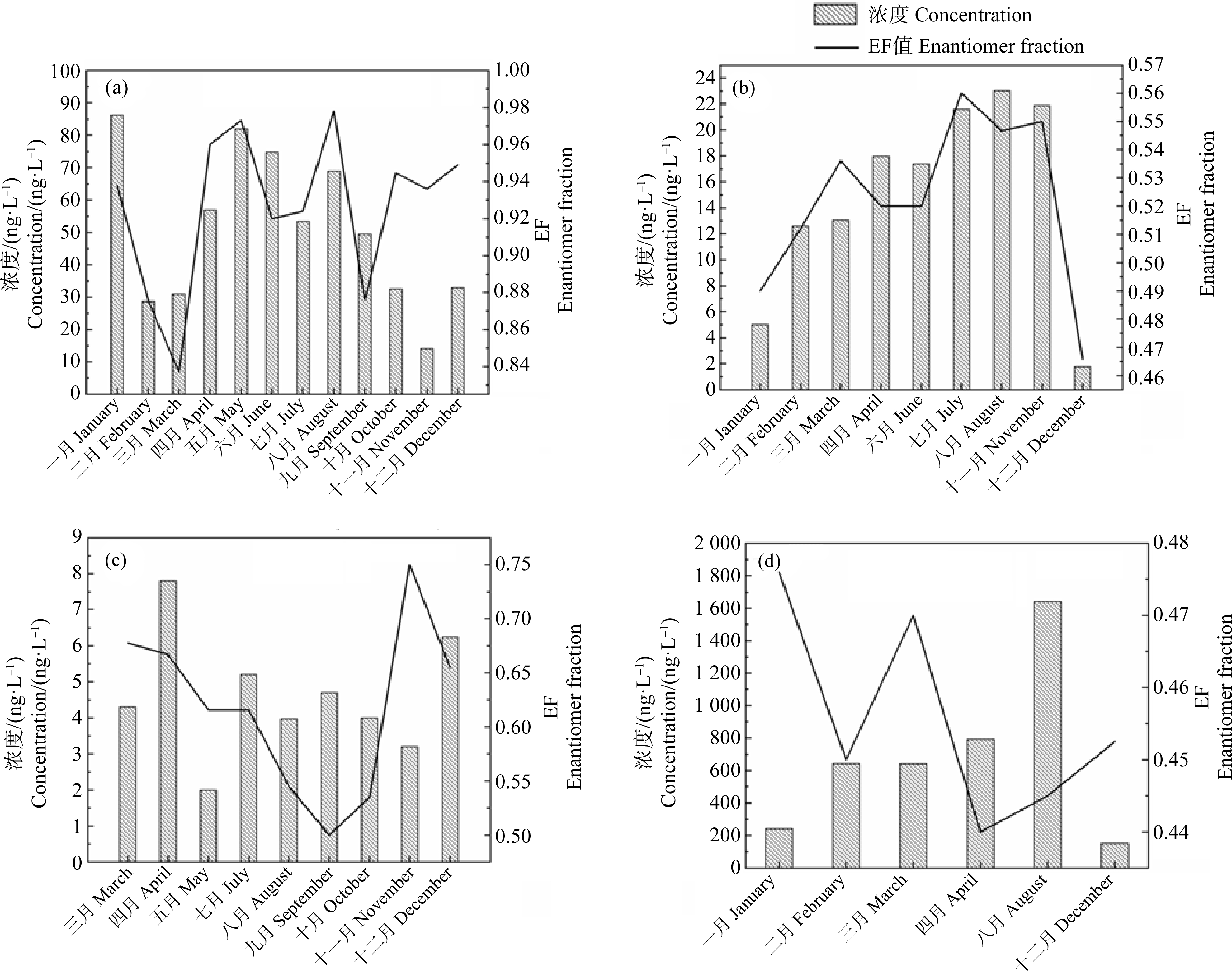
图2 地表水中手性药物普萘洛尔(a)、文拉法辛(b)、布洛芬(c)、阿替洛尔(d)浓度和EF值随月份变化[9-10, 19-20, 26]
Fig. 2 Variation of chiral pharmaceuticals propranolol(a), venlafaxine(b), ibuprofen(c)and atenolol(d)concentrations and EF values in surface water with month[9-10, 19-20, 26]
Selke和Hühnerfuss[55]检测易北河和阿尔斯特湖中的普萘洛尔,结果表明,S-普萘洛尔在夏季的降解速率较高;Fono和Sedlak[32]发现经污水厂处理后,普萘洛尔的EF值由0.49~0.54降至0.31~0.42,而在地表水微宇宙实验和湿地中,普萘洛尔的EF值未发生变化,结论与Suzuki等[22]的研究一致。因此可以认为,当在地表水中检测到普萘洛尔时,其EF值为0.50时,则表示其来源为未经处理的污水;EF值为0.42或更低时,表示其来源为经处理的污水;EF在0.42和0.5之间时,则表示来自多个来污染源。
1.2.2 物理吸附的立体选择性
污水厂活性污泥和地表水沉积物中的腐殖质含有手性中心[56],对手性药物的吸附存在一定的立体选择性。此外,污水中经常含有手性表面活性剂,从而改变手性药物在污泥和水之间的相分布[57]。
Sanganyado等[11]研究了污水处理厂活性污泥对5种常用β受体阻滞剂对映体的吸附效果,发现吸附的立体选择性随药物疏水性的降低而升高,污泥中醋丁洛尔和美托洛尔的EF值分别为0.27和0.32,S对映异构体的吸附系数(Kd)约为R对映异构体的2倍;用非离子表面活性剂聚乙二醇辛基苯基醚(Triton X 100)对活性污泥改性后,阿替洛尔在污泥中的EF值从0.55降至0.44,而吲哚洛尔和普萘洛尔异构体的Kd值在统计学上没有显著差异。这些结果表明,表面活性剂改变了污泥对β阻滞剂的吸附,可能是通过形成离子对配合物,促进与固体表面的疏水作用。Wedyan和Preston[58]证明,一些不含有机物的沉积物可以对手性化合物进行选择性吸附,实验表明石英、黏土矿物蒙脱石和高岭土等矿物质也存在对手性药物的选择性吸附[59]。
目前,多种新的检测方法被用于手性药物的分析中,包括高效液相色谱法、毛细管电泳法和气相色谱法等,然而环境样品中存在大量的非目标药物,检测仪器的分析精度、灵敏度和方法检出水平可能会受到干扰,并且检测不同环境介质中的多种手性药物,增加了分析的难度。与地表水和污水处理厂进出水中手性药物的检测方法相比,污泥、土壤和沉积物等更复杂的环境介质中手性药物的检测方法较少,因此,复杂环境介质中手性药物的检测方法仍需不断探索。
2 手性药物的毒性特征(Toxicity characteristics of chiral pharmaceuticals)
2.1 毒性效应
目前国内外对手性药物的研究主要集中在药物代谢与药物动力学,对非靶标生物的毒性效应研究较少。给药后,手性药物会经历吸收、分布、代谢和排泄等多种生理过程,其中许多过程涉及手性药物和手性生物大分子之间的相互作用,表现出立体选择性[60-61]。因此外消旋体与对映体、对映体之间的毒性可能有较大差异(表2),主要表现在3个方面[62]:对同一靶点的毒性效应相同但强度不同、对不同作用靶点表现不同的特性、体内生物转化改变对映体的毒性。
表2 手性药物对映体对水生生物的毒性效应差异
Table 2 Differences in toxic effects of chiral pharmaceuticals enantiomers on aquatic organisms
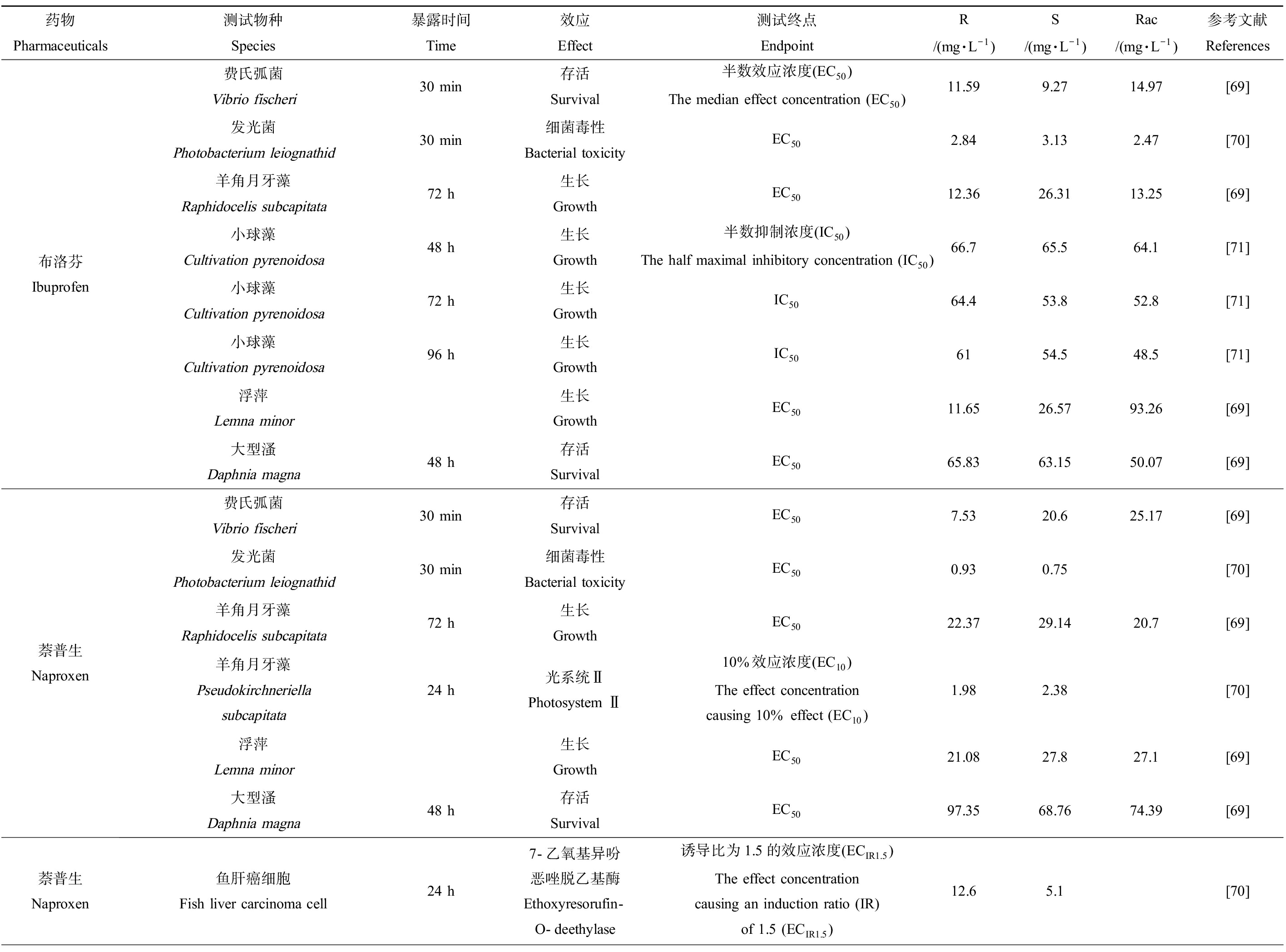
药物Pharmaceuticals测试物种Species暴露时间Time效应Effect测试终点EndpointR/(mg·L-1)S/(mg·L-1)Rac/(mg·L-1)参考文献References布洛芬Ibuprofen费氏弧菌Vibrio fischeri30 min存活Survival半数效应浓度(EC50)The median effect concentration (EC50)11.599.2714.97[69]发光菌Photobacterium leiognathid30 min细菌毒性Bacterial toxicityEC502.843.132.47[70]羊角月牙藻Raphidocelis subcapitata72 h生长GrowthEC5012.3626.3113.25[69]小球藻Cultivation pyrenoidosa48 h生长Growth半数抑制浓度(IC50)The half maximal inhibitory concentration (IC50)66.765.564.1[71]小球藻Cultivation pyrenoidosa72 h生长GrowthIC5064.453.852.8[71]小球藻Cultivation pyrenoidosa96 h生长GrowthIC506154.548.5[71]浮萍Lemna minor生长GrowthEC5011.6526.5793.26[69]大型溞Daphnia magna48 h存活SurvivalEC5065.8363.1550.07[69]萘普生Naproxen费氏弧菌Vibrio fischeri30 min存活SurvivalEC507.5320.625.17[69]发光菌Photobacterium leiognathid30 min细菌毒性Bacterial toxicityEC500.930.75[70]羊角月牙藻Raphidocelis subcapitata72 h生长GrowthEC5022.3729.1420.7[69]羊角月牙藻Pseudokirchneriella subcapitata24 h光系统ⅡPhotosystem Ⅱ10%效应浓度(EC10)The effect concentration causing 10% effect (EC10)1.982.38[70]浮萍Lemna minor生长GrowthEC5021.0827.827.1[69]大型溞Daphnia magna48 h存活SurvivalEC5097.3568.7674.39[69]萘普生Naproxen鱼肝癌细胞Fish liver carcinoma cell24 h7-乙氧基异吩恶唑脱乙基酶Ethoxyresorufin-O-deethylase诱导比为1.5的效应浓度(ECIR1.5)The effect concentration causing an induction ratio (IR) of 1.5 (ECIR1.5)12.65.1[70]
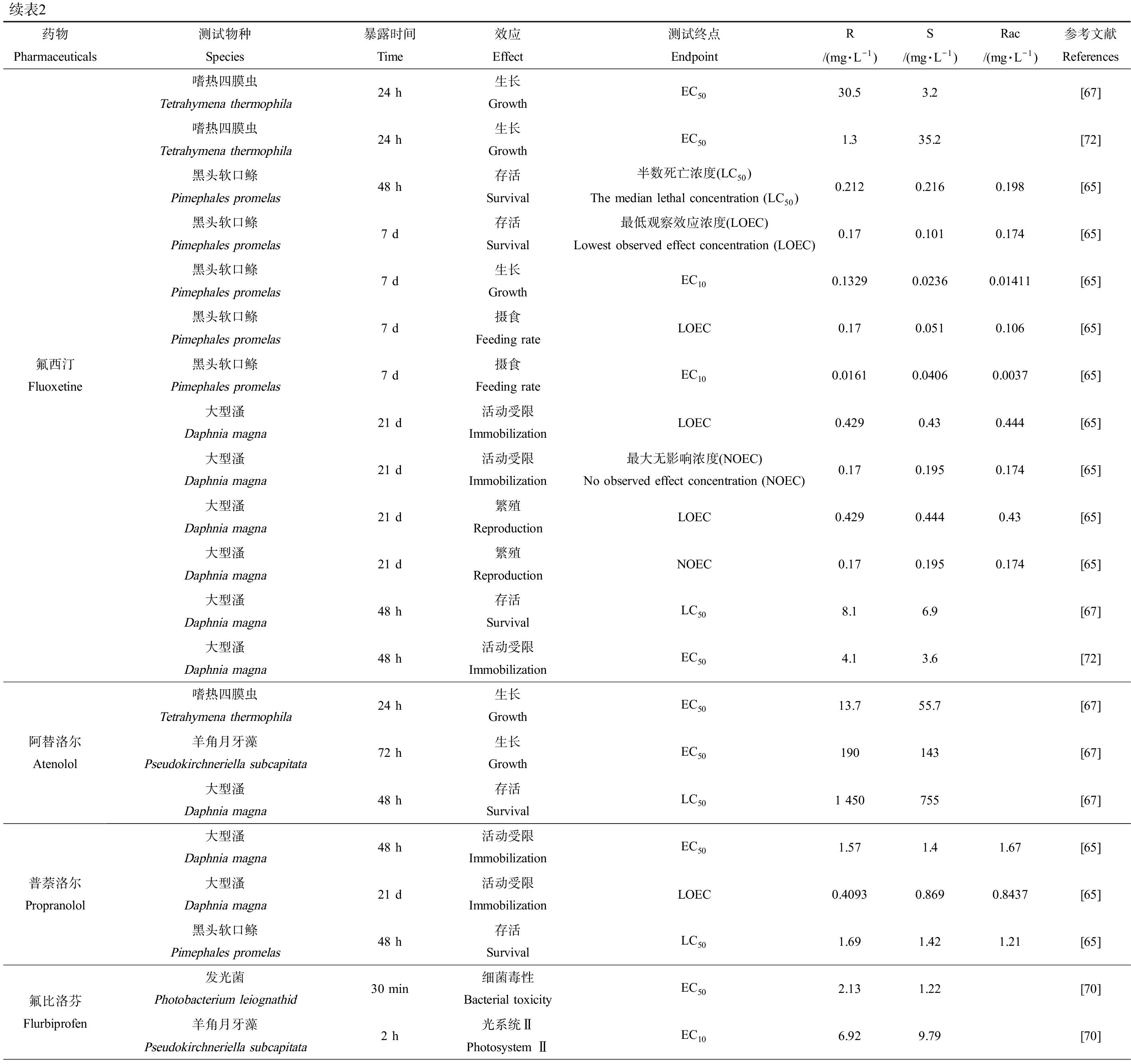
续表2药物Pharmaceuticals测试物种Species暴露时间Time效应Effect测试终点EndpointR/(mg·L-1)S/(mg·L-1)Rac/(mg·L-1)参考文献References氟西汀Fluoxetine嗜热四膜虫Tetrahymena thermophila24 h生长GrowthEC5030.53.2[67]嗜热四膜虫Tetrahymena thermophila24 h生长GrowthEC501.335.2[72]黑头软口鲦Pimephales promelas48 h存活Survival半数死亡浓度(LC50)The median lethal concentration (LC50)0.2120.2160.198[65]黑头软口鲦Pimephales promelas7 d存活Survival最低观察效应浓度(LOEC)Lowest observed effect concentration (LOEC)0.170.1010.174[65]黑头软口鲦Pimephales promelas7 d生长GrowthEC100.13290.02360.01411[65]黑头软口鲦Pimephales promelas7 d摄食Feeding rateLOEC0.170.0510.106[65]黑头软口鲦Pimephales promelas7 d摄食Feeding rateEC100.01610.04060.0037[65]大型溞Daphnia magna21 d活动受限ImmobilizationLOEC0.4290.430.444[65]大型溞Daphnia magna21 d活动受限Immobilization最大无影响浓度(NOEC)No observed effect concentration (NOEC)0.170.1950.174[65]大型溞Daphnia magna21 d繁殖ReproductionLOEC0.4290.4440.43[65]大型溞Daphnia magna21 d繁殖ReproductionNOEC0.170.1950.174[65]大型溞Daphnia magna48 h存活SurvivalLC508.16.9[67]大型溞Daphnia magna48 h活动受限ImmobilizationEC504.13.6[72]阿替洛尔Atenolol嗜热四膜虫Tetrahymena thermophila24 h生长GrowthEC5013.755.7[67]羊角月牙藻Pseudokirchneriella subcapitata72 h生长GrowthEC50190143[67]大型溞Daphnia magna48 h存活SurvivalLC501 450755[67]普萘洛尔Propranolol大型溞Daphnia magna48 h活动受限ImmobilizationEC501.571.41.67[65]大型溞Daphnia magna21 d活动受限ImmobilizationLOEC0.40930.8690.8437[65]黑头软口鲦Pimephales promelas48 h存活SurvivalLC501.691.421.21[65]氟比洛芬Flurbiprofen发光菌Photobacterium leiognathid30 min细菌毒性Bacterial toxicityEC502.131.22[70]羊角月牙藻Pseudokirchneriella subcapitata2 h光系统ⅡPhotosystem ⅡEC106.929.79[70]

续表2药物Pharmaceuticals测试物种Species暴露时间Time效应Effect测试终点EndpointR/(mg·L-1)S/(mg·L-1)Rac/(mg·L-1)参考文献References去甲氟西汀Norfluoxetine羊角月牙藻 Pseudokirchneriella subcapitata24 h光系统ⅡPhotosystem ⅡEC105.479.07[70]大型溞Daphnia magna48 h活动受限ImmobilizationEC502.92.8[72]嗜热四膜虫Tetrahymena thermophila26 h生长GrowthEC505.83.8[72]酮洛芬Ketoprofen发光菌Photobacterium leiognathid30 min细菌毒性Bacterial toxicityEC504.234.563.42[70]
注:rac为药物的外消旋体。
Note: rac is the racemate of pharmaceuticals.
对同一种生物,手性药物对映异构体可能具有不同甚至相反的作用,例如,S-巴比妥酸盐是镇静剂,而R-巴比妥酸盐对映异构体具有惊厥作用[63]。最常见的现象是只有一个异构体有药理活性,而另一个没有或活性很低,在毒理学方面,表现为不同的毒性效应。例如,当布洛芬浓度为5 μg·L-1时,斑马鱼体内的抗氧化酶超氧化物歧化酶(superoxide dismutase, SOD)和过氧化氢酶(catalase, cat)的活性受到显著影响,且S-布洛芬的毒性强于R-布洛芬和布洛芬外消旋体[64]。药物对不同组织的作用机制不同,因此同一生物的不同测试终点可能存在毒性效应差异。Stanley等[65]研究了氟西汀对黑头软口鲦(Pimephales promelas)不同测试终点的对映体选择性行为,发现S-氟西汀和外消旋体对黑头软口鲦7 d生长抑制的最低观察效应浓度(lowest observed effect concentration, LOEC)分别为51 μg·L-1和53 μg·L-1,明显比R-氟西汀的毒性强(170 μg·L-1);以摄食率为测试终点,S-氟西汀的毒性最强,LOEC为51 μg·L-1,其次是外消旋体,LOEC为106 μg·L-1,R-氟西汀的毒性最弱,LOEC为170 μg·L-1;以生存为测试终点,S-氟西汀、R-氟西汀和外消旋体的LOEC分别为101、170和174 μg·L-1,S-氟西汀的毒性最强,外消旋体与R-氟西汀的毒性差别不大。类似的,池剑[66]研究发现普萘洛尔和美托洛尔的手性对映体对斑马鱼胚胎发育和孵化率基本无差异,然而,在基因转录水平上则表现出显著的手性特征。在0.01mg·L-1的暴露条件下,R-普萘洛尔能够显著诱导4种解毒基因和3种凋亡基因的表达,而S-普萘洛尔没有影响,表现出对映体差异;在0.1 mg·L-1的处理组中,S-普萘洛尔显著诱导氧化应激基因cat的表达,R-普萘洛尔则没有影响。S-美托洛尔和R-美托洛尔在0.01 mg·L-1暴露组均能诱导cat基因表达,S型诱导强度是R型的2倍。
手性药物在不同水生生物类群体内的受体或生物利用率有所差异,从而表现出不同的立体选择性。De Andrés等[67]研究了氟西汀和阿替洛尔对映异构体对不同营养级水生生物的毒性效应,发现四膜虫(Tetrahymena thermophila)的立体选择性最强,S-氟西汀的毒性是R-氟西汀的10倍,R-阿替洛尔的毒性是S-阿替洛尔的4倍;其次是大型溞,虽然对氟西汀的立体选择性不明显,但S-阿替洛尔的毒性是R-阿替洛尔的2倍;月牙藻(Pseudokirchneriella subcapitata)对2种手性药物的立体选择性均不明显。Stanley等[68]研究了黑头软口鲦(Pimephales promelas)和大型溞(Daphnia magna)对普萘洛尔的对映体选择性行为,在黑头软口鲦的7 d生长试验中,S-普萘洛尔和外消旋体具有相似的生长抑制效应,R-普萘洛尔未表现出显著的生长抑制,对于大型溞,外消旋体和对映异构体的毒性效应差别不大。
2.2 体内转化与毒性作用机理
手性药物对映异构体在体内代谢转化过程中具有立体选择性,导致2个对映体表现出不同的药效或毒性效应。例如,局麻药丙胺卡因(prilocaine, PRI)的2种对映体的局麻作用相近,其S-PRI水解缓慢,但R-PRI可迅速水解生成导致高铁血红蛋白症的甲苯胺,具有血液毒性[73]。在20世纪60年代,曾发生过震惊世界的沙立度胺(thalidomide, TD)事件。R-TD和S-TD都有镇静作用,可用于缓解妊娠妇女的晨吐反应,但S-TD的二酰亚胺能够在体内进行酶促水解,生成邻苯二甲酰亚胺基戊二酸,然后通过干扰胎儿的谷氨酸类物质转变为叶酸的生化反应来干扰胎儿发育,最终导致胎儿畸形。此外,有研究表明,在某些情况下,外消旋体对有机体的有害影响(包括致癌性)大于2种对映体的单独影响,这可能是由于一种对映体对另一种对映异构体起催化作用[74]。
体外抑制前列腺素合成试验显示,S-布洛芬抑制前列腺素合成的能力是R-布洛芬的160倍,但在体内仅为1.4倍[75],原因是在动物体内,R-布洛芬可以转化为S-布洛芬,从而达到与S-布洛芬近似的抑制效果。Janjikhel等[76]对给药后大鼠血浆中的布洛芬浓度进行测定,发现大鼠血浆中S-布洛芬的浓度明显高于R-布洛芬;Lee等[77]发现,4位健康男性受试者分别口服布洛芬消旋体以及2种对映体后,血液中平均63%的R-布洛芬转化为S-布洛芬,没有观察到S-布洛芬转化为R-布洛芬;刘扬[78]研究发现,志愿者口服布洛芬缓释胶囊和片剂后,血清中的S-布洛芬消除速度均显著慢于R-布洛芬,服用缓释胶囊的志愿者血清中S/R比值显著高于服用片剂的志愿者血清,说明在体内发生了R-布洛芬向S-布洛芬的生物转化,且转化程度受药物的释放速率影响,释放越慢转化程度越高。
对映体选择性转化意味着参与此类化合物转化的酶能够区分对映体,酶的活性部位的细微差异决定了它们的对映体选择性。林文辉等[79]研究发现,布洛芬在体内的手性转化机制是,先与辅酶A(CoA)形成硫酯,然后在异构化酶的作用下实现对映体之间的转化,最后水解酶将硫酯水解为相应的对映体。由于涉及硫酯生成的乙酰辅酶A(actyl-CoA)合成酶只能以R-布洛芬为底物,因此在部分动物体内布洛芬只能从R型转化为S型[80]。另外有研究表明,在豚鼠体内,布洛芬的手性转化可能是双向进行的[81],说明手性转化具有较大的种属差异。
3 手性药物在水环境中的生态风险(Ecological risks of chiral pharmaceuticals in the aquatic environment)
研究表明,在目前环境浓度水平下,药物不会对水生生物造成急性致死,但长期低浓度暴露会对水生生物的生长发育和繁殖,特别是对鱼类的繁殖具有一定的影响[82]。Sui等[83]根据我国PPCPs的使用量、污水处理厂去除率及其生态效应,筛选出17种需要优先控制的药物,其中包括手性药物布洛芬、吉非贝齐、酮康唑、萘普生和卡马西平;Zhao等[84]、Wang等[85]和Liu等[7]运用风险商法(risk quotients, RQ)对我国珠江、黄河、海河和辽河等河流中PPCPs进行了风险评价,结果表明布洛芬、卡马西平和咖啡因在我国部分水体中存在较高的环境风险,萘普生、氧氟沙星和吉非贝齐等手性药物也具有一定的潜在风险。
Rivera-Jaimes等[86]对墨西哥莫雷洛斯州库埃纳瓦卡地表和废水中的PPCPs进行风险评估,发现卡马西平在地表水中表现出中等生态风险,其危险商(hazard quotients, HQ)值为0.19;布洛芬的HQ值为111;萘普生的HQ值为14.8。根据计算出的HQ值,在大多数情况下,阿帕特拉科河河水中出现的药物浓度比污水处理厂排放的药物浓度具有更高的生态风险。Guruge等[87]基于最敏感的水生物种,对斯里兰卡地表水中布洛芬进行风险评估,其RQ值为8.4,具有较高的生态风险,普萘洛尔和卡马西平的RQ值均低于0.1,二者则处于较低的生态风险水平。对丹麦水域中存在的药物进行风险评估,布洛芬处于高风险水平(RQ>1)[88];普萘洛尔对西班牙地表水生态系统中淡水桡足类的毒性增加了对水体的环境风险[89];结合化学和生态毒理学数据,布洛芬在巴西圣保罗州桑托斯湾附近的RQ高于1,表明布洛芬对海洋生物具有高风险[90]。总体而言,手性药物对全球构成威胁,并有可能对水生生物群造成重大损害。
基于外消旋体的生态风险评估研究表明,布洛芬、卡马西平和萘普生等手性药物对水生生物具有潜在的风险,而不同对映体对水生生物的毒性存在一定差异。因此,有必要将手性药物的2种对映体当作2种独立的药品,进行更为精确的风险评估[91-93]。目前尚缺乏基于对映体毒性差异和暴露差异的风险评估,但大多数研究人员已经意识到手性药物在风险评估方面的特殊性。Stanley和Brooks[94]指出,对手性化合物进行生态风险评估时,往往将2种对映体当成一种物质,忽略了手性化合物对映体的毒性差异,增加了生态风险评估的不确定性。例如,如果毒性更大的对映体优先降解,就会高估该药物的生态风险[2],进而制定较严格的环境法规,增加不必要的环境修复成本;反之,如果优先降解的是毒性较小的对映异构体,生态风险将被低估,可能导致环境保护不足。同时认为,在风险评估过程中,应将由2个对映异构体构成的手性化合物看作混合物,如果手性药物的2个对映体仅存在毒性大小差异且两者毒性效应机制一致,可以将二者的风险商直接相加;如果对映体毒性效应机制不相同,则应该将其看作2种不同的物质,按照独立作用规则评估手性药物的生态风险,2种对映异构体的总和是它们各自影响的总和减去对2种对映异构体的敏感性重叠的群体部分(2种对映体影响的乘积)。
4 结论与展望(Conclusion and outlook)
(1)手性药物在地表水中不断被检测到,在自然环境条件下会发生一定的转化,表现出立体选择性,并具有季节性差异,其中布洛芬的表现更为明显。
(2)手性药物在多种环境介质中的分析检测技术仍需完善,对映体拆分、目标分析物的识别和量化,是多种手性药物同时监测的关键。
(3)微生物对手性药物的降解转化的对映体选择性受温度、溶解氧条件和水深等因素影响。
(4)布洛芬的生态风险高于其他手性药物,对布洛芬在环境中的行为进行研究发现,其对映体之间在环境介质、微生物以及生物体内可以发生转变,而布洛芬的毒性因对映体、靶器官不同而不同。
(5)目前针对手性药物的环境行为与生态毒理学研究较少,用基于外消旋体的数据进行生态风险评估存在很大的不确定性。为精确评估其生态风险,应加强对手性药物,特别是对映体药理活性不同和生物转化后毒性增强的手性药物的研究。
[1] Wong C S.Environmental fate processes and biochemical transformations of chiral emerging organic pollutants[J].Analytical and Bioanalytical Chemistry, 2006, 386(3): 544-558
[2] Zhu W T, Dang Z H, Qiu J, et al.Stereoselective toxicokinetics and tissue distribution of ethofumesate in rabbits[J].Chirality, 2007, 19(8): 632-637
[3] Sharma B.Nature of chiral drugs and their occurrence in environment[J].Journal of Xenobiotics, 2014, 4(1): 14-19
[4] Sanganyado E, Lu Z J, Fu Q G, et al.Chiral pharmaceuticals: A review on their environmental occurrence and fate processes[J].Water Research, 2017, 124: 527-542
[5] 刘瑞霞, 李佳熹, 刘晓玲.环境介质中手性药物对映体分离分析及环境行为研究进展[J].环境工程学报, 2017, 11(6): 3341-3351
Liu R X, Li J X, Liu X L.Research progress on separation, analysis and environmental behavior of chiral pharmaceutical enantiomers in environmental matrix[J].Chinese Journal of Environmental Engineering, 2017, 11(6): 3341-3351(in Chinese)
[6] 尹立娜, 王斌, 马瑞雪, 等.手性PPCPs环境行为与效应的对映体选择性[J].化学进展, 2016, 28(5): 744-753
Yin L N, Wang B, Ma R X, et al.Enantioselective environmental behavior and effect of chiral PPCPs[J].Progress in Chemistry, 2016, 28(5): 744-753(in Chinese)
[7] Liu N, Jin X W, Feng C L, et al.Ecological risk assessment of fifty pharmaceuticals and personal care products(PPCPs)in Chinese surface waters: A proposed multiple-level system[J].Environment International, 2020, 136: 105454
[8] 褚莹倩, 陈溪, 张晓林, 等.中国地表水环境中药物与个人护理品生态风险评价的研究进展[J].生态毒理学报, 2021, 16(4): 80-92
Chu Y Q, Chen X, Zhang X L, et al.Ecological risk assessment of pharmaceutical and personal care products in the surface water of China: A review[J].Asian Journal of Ecotoxicology, 2021, 16(4): 80-92(in Chinese)
[9] Rudolf B H, Thomas P, Müller Markus D.Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater[J].Environmental Science &Technology, 1999, 33(15): 2529-2535
[10] Petrie B, Moffat C F.Occurrence and fate of chiral and achiral drugs in estuarine water—A case study of the Clyde Estuary, Scotland[J].Environmental Science Processes &Impacts, 2022, 24(4): 547-556
[11] Sanganyado E, Fu Q G, Gan J.Enantiomeric selectivity in adsorption of chiral β-blockers on sludge[J].Environmental Pollution, 2016, 214: 787-794
[12] Roberts P H, Thomas K V.The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne Catchment[J].The Science of the Total Environment, 2006, 356(1-3): 143-153
[13] Stafiej A, Pyrzynska K, Regan F.Determination of anti-inflammatory drugs and estrogens in water by HPLC with UV detection[J].Journal of Separation Science, 2007, 30(7): 985-991
[14] Bradley P M, Journey C A, Romanok K M, et al.Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S.streams[J].Environmental Science &Technology, 2017, 51(9): 4792-4802
[15] Can Guven E, Bolat D, Gedik K, et al.Chiral pollutants and their significance in the environment[J].Pamukkale University Journal of Engineering Sciences, 2016, 22(6): 486-496
[16] Xu C Y, Lin X M, Yin S S, et al.Enantioselectivity in biotransformation and bioaccumulation processes of typical chiral contaminants[J].Environmental Pollution, 2018, 243(Pt B): 1274-1286
[17] Maia A, Ribeiro A R, Castro P, et al.Chiral analysis of pesticides and drugs of environmental concern: Biodegradation and enantiomeric fraction[J].Symmetry, 2017, 9: 196
[18] Tom H, Karin W, Ross N.Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis[J].Environmental Science &Technology, 2000, 34(1): 218-220
[19] Ma R X, Qu H, Wang B, et al.Widespread monitoring of chiral pharmaceuticals in urban rivers reveals stereospecific occurrence and transformation[J].Environment International, 2020, 138: 105657
[20] Kasprzyk-Hordern B, Baker D R.Enantiomeric profiling of chiral drugs in wastewater and receiving waters[J].Environmental Science &Technology, 2012, 46(3): 1681-1691
[21] Khan S J, Wang L L, Hashim N H, et al.Distinct enantiomeric signals of ibuprofen and naproxen in treated wastewater and sewer overflow[J].Chirality, 2014, 26(11): 739-746
[22] Suzuki T, Kosugi Y, Hosaka M, et al.Occurrence and behavior of the chiral anti-inflammatory drug naproxen in an aquatic environment[J].Environmental Toxicology and Chemistry, 2014, 33(12): 2671-2678
[23] Xu Z Q, Du P, Li K Y, et al.Tracing methamphetamine and amphetamine sources in wastewater and receiving waters via concentration and enantiomeric profiling[J].The Science of the Total Environment, 2017, 601-602: 159-166
[24] Camacho-Mu oz D, Kasprzyk-Hordern B.Multi-residue enantiomeric analysis of human and veterinary pharmaceuticals and their metabolites in environmental samples by chiral liquid chromatography coupled with tandem mass spectrometry detection[J].Analytical and Bioanalytical Chemistry, 2015, 407(30): 9085-9104
oz D, Kasprzyk-Hordern B.Multi-residue enantiomeric analysis of human and veterinary pharmaceuticals and their metabolites in environmental samples by chiral liquid chromatography coupled with tandem mass spectrometry detection[J].Analytical and Bioanalytical Chemistry, 2015, 407(30): 9085-9104
[25] Ma R X, Qu H, Wang B, et al.Simultaneous enantiomeric analysis of non-steroidal anti-inflammatory drugs in environment by chiral LC-MS/MS: A pilot study in Beijing, China[J].Ecotoxicology and Environmental Safety, 2019, 174: 83-91
[26] Bagnall J P, Evans S E, Wort M T, et al.Using chiral liquid chromatography quadrupole time-of-flight mass spectrometry for the analysis of pharmaceuticals and illicit drugs in surface and wastewater at the enantiomeric level[J].Journal of Chromatography A, 2012, 1249: 115-129
[27] Fono L J, Kolodziej E P, Sedlak D L.Attenuation of wastewater-derived contaminants in an effluent-dominated river[J].Environmental Science &Technology, 2006, 40(23): 7257-7262
[28] Yuan X C, Li X H, Guo P, et al.Simultaneous enantiomeric analysis of chiral non-steroidal anti-inflammatory drugs in water, river sediment, and sludge using chiral liquid chromatography-tandem mass spectrometry[J].Analytical Methods, 2018, 10(36): 4404-4413
[29] Huang Q X, Wang Z F, Wang C W, et al.Chiral profiling of azole antifungals in municipal wastewater and recipient rivers of the Pearl River Delta, China[J].Environmental Science and Pollution Research International, 2013, 20(12): 8890-8899
[30] 潘宇, 李冰, 姜春旭, 等.水液相环境下布洛芬分子手性对映体转变的机理[J].复旦学报(自然科学版), 2019, 58(6): 784-792
Pan Y, Li B, Jiang C X, et al.Mechanism of chiral enantiomer transition of ibuprofen molecules in water/liquid phase environment[J].Journal of Fudan University(Natural Science), 2019, 58(6): 784-792(in Chinese)
[31] Barclay V K, Tyrefors N L, Johansson I M, et al.Chiral analysis of metoprolol and two of its metabolites, α-hydroxymetoprolol and deaminated metoprolol, in wastewater using liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography A, 2012, 1269: 208-217
[32] Fono L J, Sedlak D L.Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters[J].Environmental Science &Technology, 2005, 39(23): 9244-9252
[33] Matamoros V, Uggetti E, García J, et al.Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study[J].Journal of Hazardous Materials, 2016, 301: 197-205
[34] Moreira I S, Ribeiro A R, Afonso C M, et al.Enantioselective biodegradation of fluoxetine by the bacterial strain Labrys portucalensisF11[J].Chemosphere, 2014, 111: 103-111
[35] Ribeiro A R, Afonso C, Castro P, et al.Enantioselective HPLC analysis and biodegradation of atenolol, metoprolol and fluoxetine[J].Environmental Chemistry Letters, 2013, 11(1): 83-90
[36] Evans S E, Davies P, Lubben A, et al.Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry[J].Analytica Chimica Acta, 2015, 882: 112-126
[37] Matamoros V, Rodríguez Y, Albaigés J.A comparative assessment of intensive and extensive wastewater treatment technologies for removing emerging contaminants in small communities[J].Water Research, 2016, 88: 777-785
[38] Hashim N H, Khan S J.Enantioselective analysis of ibuprofen, ketoprofen and naproxen in wastewater and environmental water samples[J].Journal of Chromatography A, 2011, 1218(29): 4746-4754
[39] Thomason M J, Rhys-Williams W, Hung Y F, et al.A mechanistic investigation of the microbial chiral inversion of 2-phenylpropionic acid by Verticillium lecanii[J].Chirality, 1997, 9(3): 254-260
[40] Huang Q X, Zhang K, Wang Z F, et al.Enantiomeric determination of azole antifungals in wastewater and sludge by liquid chromatography-tandem mass spectrometry[J].Analytical and Bioanalytical Chemistry, 2012, 403(6): 1751-1760
[41] Castrignanò E, Lubben A, Kasprzyk-Hordern B.Enantiomeric profiling of chiral drug biomarkers in wastewater with the usage of chiral liquid chromatography coupled with tandem mass spectrometry[J].Journal of Chromatography A, 2016, 1438: 84-99
[42] Castrignanò E, Yang Z G, Bade R, et al.Enantiomeric profiling of chiral illicit drugs in a pan-European study[J].Water Research, 2018, 130: 151-160
[43] Estévez-Danta A, Montes R, Bijlsma L, et al.Source identification of amphetamine-like stimulants in Spanish wastewater through enantiomeric profiling[J].Water Research, 2021, 206: 117719
[44] Kasprzyk-Hordern B, Kondakal V V R, Baker D R.Enantiomeric analysis of drugs of abuse in wastewater by chiral liquid chromatography coupled with tandem mass spectrometry[J].Journal of Chromatography A, 2010, 1217(27): 4575-4586
[45] MacLeod S L, Sudhir P, Wong C S.Stereoisomer analysis of wastewater-derived beta-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography A, 2007, 1170(1-2): 23-33
[46] Caballo C, Sicilia M D, Rubio S.Enantioselective determination of representative profens in wastewater by a single-step sample treatment and chiral liquid chromatography-tandem mass spectrometry[J].Talanta, 2015, 134: 325-332
[47] Vazquez-Roig P, Kasprzyk-Hordern B, Blasco C, et al.Stereoisomeric profiling of drugs of abuse and pharmaceuticals in wastewaters of Valencia(Spain)[J].The Science of the Total Environment, 2014, 494-495: 49-57
[48] Duan L, Zhang Y Z, Wang B, et al.Occurrence, elimination, enantiomeric distribution and intra-day variations of chiral pharmaceuticals in major wastewater treatment plants in Beijing, China[J].Environmental Pollution, 2018, 239: 473-482
[49] Archer E, Castrignanò E, Kasprzyk-Hordern B, et al.Wastewater-based epidemiology and enantiomeric profiling for drugs of abuse in South African wastewaters[J].The Science of the Total Environment, 2018, 625: 792-800
[50] Matamoros V, Arias C, Brix H, et al.Removal of pharmaceuticals and personal care products(PPCPs)from urban wastewater in a pilot vertical flow constructed wetland and a sand filter[J].Environmental Science &Technology, 2007, 41(23): 8171-8177
[51] Matamoros V, Bayona J M.Elimination of pharmaceuticals and personal care products in subsurface flow constructed wetlands[J].Environmental Science &Technology, 2006, 40(18): 5811-5816
[52] Matamoros V, Caselles-Osorio A, García J, et al.Behaviour of pharmaceutical products and biodegradation intermediates in horizontal subsurface flow constructed wetland.A microcosm experiment[J].The Science of the Total Environment, 2008, 394(1): 171-176
[53] Matamoros V, Hijosa M, Bayona J M.Assessment of the pharmaceutical active compounds removal in wastewater treatment systems at enantiomeric level.Ibuprofen and naproxen[J].Chemosphere, 2009, 75(2): 200-205
[54] 梅泽民, 王佐成, 赵衍辉, 等.水环境下布洛芬分子的手性转变机理[J].吉林大学学报(理学版), 2015, 53(2): 331-339
Mei Z M, Wang Z C, Zhao Y H, et al.Chiral shift mechanism of ibuprofen molecule in water[J].Journal of Jilin University(Science Edition), 2015, 53(2): 331-339(in Chinese)
[55] Selke S, Hühnerfuss H.Enantioselective transformation of the betablocker propranolol in the aquatic environment[J].Organohalogen Compounds, 2011, 73: 190-193
[56] Oravec M, Simek Z, Holoubek I.The effect of humic acid and ash on enantiomeric fraction change of chiral pollutants[J].Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 359(1-3): 60-65
[57] Hari A C, Paruchuri R A, Sabatini D A, et al.Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material[J].Environmental Science &Technology, 2005, 39(8): 2592-2598
[58] Wedyan M, Preston M R.Isomer-selective adsorption of amino acids by components of natural sediments[J].Environmental Science &Technology, 2005, 39(7): 2115-2119
[59] Belden J B, Maul J D, Lydy M J.Partitioning and photodegradation of ciprofloxacin in aqueous systems in the presence of organic matter[J].Chemosphere, 2007, 66(8): 1390-1395
[60] 孙璐, 杨亚楠, 严静.手性药物的立体选择性药物动力学[J].中国药物化学杂志, 2009, 19(6): 498-501
Sun L, Yang Y N, Yan J.Stereoselectivity pharmacokinetics of chiral drugs[J].Chinese Journal of Medicinal Chemistry, 2009, 19(6): 498-501(in Chinese)
[61] Lu H.Stereoselectivity in drug metabolism[J].Expert Opinion on Drug Metabolism &Toxicology, 2007, 3(2): 149-158
[62] Kasprzyk-Hordern B.Pharmacologically active compounds in the environment and their chirality[J].Chemical Society Reviews, 2010, 39(11): 4466-4503
[63] Ari⊇ns E J.QSAR methods and stereoselectivity in action[J].Progress in Clinical and Biological Research, 1989, 291: 3-6
[64] Song Y, Chai T T, Yin Z Q, et al.Stereoselective effects of ibuprofen in adult zebrafish(Danio rerio)using UPLC-TOF/MS-based metabolomics[J].Environmental Pollution, 2018, 241: 730-739
[65] Stanley J K, Ramirez A J, Chambliss C K, et al.Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate[J].Chemosphere, 2007, 69(1): 9-16
[66] 池剑.β肾上腺受体阻滞剂对模式鱼的毒性效应研究[D].杭州: 浙江工业大学, 2012: 76-77
Chi J.Toxic effects of beta-adrenergic receptor antagonist on the mode fish[D].Hangzhou: Zhejiang University of Technology, 2012: 76-77(in Chinese)
[67] De Andrés F, Casta eda G, Ríos A.Use of toxicity assays for enantiomeric discrimination of pharmaceutical substances[J].Chirality, 2009, 21(8): 751-759
eda G, Ríos A.Use of toxicity assays for enantiomeric discrimination of pharmaceutical substances[J].Chirality, 2009, 21(8): 751-759
[68] Stanley J K, Ramirez A J, Mottaleb M, et al.Enantiospecific toxicity of the beta-blocker propranolol to Daphnia magnaand Pimephales promelas[J].Environmental Toxicology and Chemistry, 2006, 25(7): 1780-1786
[69] Grabarczyk  , Mulkiewicz E, Stolte S, et al.Ecotoxicity screening evaluation of selected pharmaceuticals and their transformation products towards various organisms[J].Environmental Science and Pollution Research, 2020, 27(21): 26103-26114
, Mulkiewicz E, Stolte S, et al.Ecotoxicity screening evaluation of selected pharmaceuticals and their transformation products towards various organisms[J].Environmental Science and Pollution Research, 2020, 27(21): 26103-26114
[70] Neale P A, Branch A, Khan S J, et al.Evaluating the enantiospecific differences of non-steroidal anti-inflammatory drugs(NSAIDs)using an ecotoxicity bioassay test battery[J].The Science of the Total Environment, 2019, 694: 133659
[71] Wang F, Wang B, Qu H, et al.The influence of nanoplastics on the toxic effects, bioaccumulation, biodegradation and enantioselectivity of ibuprofen in freshwater algae Chlorella pyrenoidosa[J].Environmental Pollution, 2020, 263(Pt B): 114593
[72] Andrés-Costa M J, Proctor K, Sabatini M T, et al.Enantioselective transformation of fluoxetine in water and its ecotoxicological relevance[J].Scientific Reports, 2017, 7(1): 15777
[73] Sidebotham D A, Schug S A.Stereochemistry in anaesthesia[J].Clinical and Experimental Pharmacology &Physiology, 1997, 24(2): 126-130
[74] Ali I, Aboul-Enein H Y, Ghanem A.Enantioselective toxicity and carcinogenesis[J].Current Pharmaceutical Analysis, 2004, 1(1): 109-125
[75] 向瑾, 余勤, 梁茂植, 等.布洛芬对映体在健康人体的药动学研究[J].四川大学学报(医学版), 2010, 41(1): 176-178
[76] Janjikhel R K, Douglas Bricker J, Borochovitz D, et al.Stereoselective disposition of sustained release microspheres of ibuprofen enantiomers in rats: Ⅱ.Acute gastrointestinal toxicity[J].Drug Delivery, 1999, 6(3): 163-170
[77] Lee E J, Williams K, Day R, et al.Stereoselective disposition of ibuprofen enantiomers in man[J].British Journal of Clinical Pharmacology, 1985, 19(5): 669-674
[78] 刘扬.手性药物布洛芬药物动力学研究[D].哈尔滨: 黑龙江中医药大学, 2009: 45-46
Liu Y.Studies on pharmacokinetics of chiral drug ibuprofen[D].Harbin: Heilongjiang University of Chinese Medicine, 2009: 45-46(in Chinese)
[79] 林文辉, 崔福德, 吴斌, 等.布洛芬离体肝代谢中对映体间的相互作用[J].沈阳药科大学学报, 2005, 22(1): 1-4
Lin W H, Cui F D, Wu B, et al.Interaction of the ibuprofen enantiomers during the chiral inversion in vitro[J].Journal of Shenyang Pharmaceutical University, 2005, 22(1): 1-4(in Chinese)
[80] Nakamura Y, Yamaguchi T, Takahashi S, et al.Optical isomerization mechanism of r-(-)-hydrotropic acid-derivatives[J].Journal of Pharmacobio-Dynamics, 1981, 4(2): S1-S1
[81] Chen C S, Shieh W R, Lu P H, et al.Metabolic stereoisomeric inversion of ibuprofen in mammals[J].Biochimica et Biophysica Acta, 1991, 1078(3): 411-417
[82] 刘娜, 金小伟, 王业耀, 等.我国地表水中药物与个人护理品污染现状及其繁殖毒性筛查[J].生态毒理学报, 2015, 10(6): 1-12
Liu N, Jin X W, Wang Y Y, et al.Pharmaceuticals and personal care products(PPCPs)caused reproductive toxicity in surface water of China: A review[J].Asian Journal of Ecotoxicology, 2015, 10(6): 1-12(in Chinese)
[83] Sui Q, Wang B, Zhao W T, et al.Identification of priority pharmaceuticals in the water environment of China[J].Chemosphere, 2012, 89(3): 280-286
[84] Zhao J L, Ying G G, Liu Y S, et al.Occurrence and a screening-level risk assessment of human pharmaceuticals in the Pearl River system, South China[J].Environmental Toxicology and Chemistry, 2010, 29(6): 1377-1384
[85] Wang L, Ying G G, Zhao J L, et al.Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of North China[J].The Science of the Total Environment, 2010, 408(16): 3139-3147
[86] Rivera-Jaimes J A, Postigo C, Melgoza-Alemán R M, et al.Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment[J].The Science of the Total Environment, 2018, 613-614: 1263-1274
[87] Guruge K S, Goswami P, Tanoue R, et al.First nationwide investigation and environmental risk assessment of 72 pharmaceuticals and personal care products from Sri Lankan surface waterways[J].The Science of the Total Environment, 2019, 690: 683-695
[88] Stuer-Lauridsen F, Birkved M, Hansen L P, et al.Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use[J].Chemosphere, 2000, 40(7): 783-793
[89] di Lorenzo T, Casta o-Sánchez A, di Marzio W D, et al.The role of freshwater copepods in the environmental risk assessment of caffeine and propranolol mixtures in the surface water bodies of Spain[J].Chemosphere, 2019, 220: 227-236
o-Sánchez A, di Marzio W D, et al.The role of freshwater copepods in the environmental risk assessment of caffeine and propranolol mixtures in the surface water bodies of Spain[J].Chemosphere, 2019, 220: 227-236
[90] Pusceddu F H, Choueri R B, Pereira C D S, et al.Environmental risk assessment of triclosan and ibuprofen in marine sediments using individual and sub-individual endpoints[J].Environmental Pollution, 2018, 232: 274-283
[91] 刘维屏, 徐超, 周珊珊.手性污染物与环境安全[J].环境化学, 2006, 25(3): 247-251
Liu W P, Xu C, Zhou S S.Chiral pollutants and environmental safety[J].Environmental Chemistry, 2006, 25(3): 247-251(in Chinese)
[92] 唐梦龄, 王丹, 傅柳松, 等.手性农药毒性机制的对映体选择性[J].农药学学报, 2011, 13(4): 335-340
Tang M L, Wang D, Fu L S, et al.Enantioselectivity on toxic mechanisms of chiral pesticides[J].Chinese Journal of Pesticide Science, 2011, 13(4): 335-340(in Chinese)
[93] Ye J, Zhao M R, Liu J, et al.Enantioselectivity in environmental risk assessment of modern chiral pesticides[J].Environmental Pollution, 2010, 158(7): 2371-2383
[94] Stanley J K, Brooks B W.Perspectives on ecological risk assessment of chiral compounds[J].Integrated Environmental Assessment and Management, 2009, 5(3): 364-373