外源性雌激素(exoestrogens, EE)是指具有雌激素活性的外源性化学物质,又被称为环境雌激素(environmental estrogens)或异源雌激素(xenoestrogens),这类物质具有雌激素样活性,可以通过模拟或抑制内源性雌激素来发挥广泛的雌激素作用,从而改变内分泌与生殖系统的生理功能[1-2]。EE主要来源于植物或真菌中的天然产物,如大豆和鹰嘴豆中的异黄酮、亚麻籽中的木脂素、黄豆芽和绿豆芽籽中的香豆素等,以及人工合成衍生剂,如某些药物(口服避孕药等)、杀虫剂、化妆品、塑料、烟雾、工业副产品和金属等。因此,EE广泛存在于食品和环境中。
多项研究表明,摄入EE会影响生物体的生理功能。人工合成类的外源性雌激素对生物体具有毒害作用,不仅会损伤女性和男性的生殖系统发育,并且相关代谢疾病以及骨骼健康与EE的摄入也有显著的关联性[3-4]。而植物源性的外源性雌激素一方面也会扰乱生物体的正常内分泌活动,另一方面在正常的剂量范围内对人体有一定的益处[3-4]。
妊娠期孕妇作为一个独特的群体,摄入过量的EE不仅会对自身产生危害,还会对其胎儿产生不利影响。在生殖系统方面,多项研究表明,女性在妊娠期摄入双酚A(bisphenol A, BPA)和己烯雌酚(diethylstilbestrol, DES)等EE不仅会增加自身乳腺癌和子宫内膜异位症等生殖系统疾病的发病率,还会增加下一代未来患乳腺癌和子宫内膜异位症等生殖系统疾病的风险,并且风险随着年龄的增加更加显著[5-8]。胚胎期暴露于对羟基苯甲酸丙酯会加剧雌性大鼠成年后卵巢氧化应激、炎症和纤维化,从而通过激活线粒体凋亡途径促进卵泡闭锁,加速卵巢衰老[9]。男性生殖系统健康方面的问题(如精液质量下降、睾丸癌、尿道下裂和隐睾症)也与EE的过量摄入有关,且在胚胎期和婴幼儿时期过量摄入EE造成的不利影响更为显著[10]。在代谢方面,妊娠期内摄入EE会影响胎儿的代谢调节过程,例如在子宫内暴露于极低剂量BPA的雌性大鼠胎儿的肝脏中参与胆固醇和脂肪酸生物合成和运输的蛋白质受到显著的调节作用[11]。此外,在影响婴幼儿发育方面,孕妇在妊娠期摄入BPA等酚类EE与胎儿生长参数的不利影响相关(与出生身长呈负相关,与体质量指数呈正相关,与胎龄呈负相关),且在女婴中的影响更大[12-13];孕妇在妊娠期摄入EE还会影响其胎儿的神经发育过程,例如有研究表明妊娠期摄入BPA与注意缺陷多动障碍(尤其在男孩中)相关联[14]。
鉴于妊娠期孕妇因为EE摄入引起的代谢过程和生理病理变化与EE摄入程度具有关联性,因此,本研究首先基于超高效液相色谱-三重四极杆质谱建立孕妇尿液样本中8种EE的定量方法并测定EE含量,然后根据样本中的EE含量将样本分为低、中和高EE水平组;再借助基于高分辨质谱的非靶向代谢组学策略分析不同EE水平的孕妇尿液样本的代谢组,通过多维统计分析、差异代谢物筛选和代谢通路分析等手段,探索EE摄入程度与孕妇代谢过程和生理病理变化之间的关联性。
1 材料与方法(Materials and methods)
1.1 孕妇尿液样本的收集
本研究中所用尿液样本由上海市生殖研究所提供,所有采集的尿液样本均与提供者签署了书面知情同意书,并经伦理委员会批准。本次实验共采集了90例尿液样本,尿液样本经采集后置于-80 ℃保存直至使用。
1.2 仪器、试剂与材料
液相色谱-三重四级杆质谱联用仪(6490 QqQ-MS,Agilent,美国);ACQUITY UPLC BEH C18色谱柱(100 mm×2.1 mm,1.7 μm,Waters,美国);Vanquish UHPLC system &Q Exactive plus Mass spectrometer (Thermofisher, 美国);ACQUITY UPLC HSS T3(100 mm×2.1 mm,1.7 μm,Waters,美国);XW-80A涡旋混合器(上海青浦沪西仪器厂,中国);离心浓缩干燥仪T120(江苏太仓华利达仪器公司,中国);冷冻离心机(Centrifuge 5417R,Eppendorf,德国);KQ5200DE超声波清洗器(昆山市超声仪器有限公司,中国),SHB-III循环水多用真空泵(郑州长城科工贸有限公司,中国)。
超纯水由Molcell 1805V摩尔细胞型纯水机(重庆摩尔水处理设备有限公司,中国)制备;甲醇、乙腈(色谱纯,Merck,德国);甲酸、甲酸铵、乙酸铵(色谱纯,CNW,德国);乙酸乙酯(色谱纯,国药,中国);黄豆苷原(daidzein, DAD)、染料木黄酮(genistein, GEN)、黄豆黄素(glycitein, GLY)、肠二醇(enterodiol, END)、肠内酯(enterolactone, ENT)、雌马酚(3-(4-hydroxyphenyl)chroman-7-ol, EQU)、松脂醇(pinoresinol, PRS)、罗汉松脂素(matairesinol, MTR)和磺胺甲噁唑(sulfamethoxazole, SMZ)均购自Sigma公司;β-葡萄糖醛酸酶(Tyep HP-2, 100 000 units·mL-1, Sigma,英国);L-2-氯苯丙氨酸(Sigma Aldrich,美国);LPC 17:0(Avanti公司,美国)。
1.3 基于三重四极杆质谱的外源性雌激素定量方法建立
准确称取外源性雌激素各对照品适量,分别以甲醇溶解,得到103 mg·L-1的贮备液。稀释后得0.2 mg·L-1工作液,经QqQ质谱优化各对照品的质谱参数。
色谱条件:色谱柱为ACQUITY UPLC BEH C18色谱柱(100 mm×2.1 mm, 1.7 μm,Waters,美国);流动相A为0.1%甲酸水溶液,流动相B为乙腈;流速为0.25 mL·min-1;进样量为5 μL;柱温为室温;梯度洗脱如下:0 min,30% B;0.0~1.0 min,30%~50% B;1.0~4.0 min,50%~90% B;4.0~4.5 min,90%~100% B。每2针样品间以初始流动相梯度(30% B)平衡1 min,即4.5~4.6 min,100%~30% B;4.6~5.6 min,30% B。
质谱条件:ESI电离源,负离子模式,毛细管电压为3 kV,干燥气温度(gas temp.)为130 ℃,干燥气流量11 L·min-1,雾化器压力344.738 kPa, 鞘流气温度275 ℃,鞘流气流速12 L·min-1,采用MRM模式,各化合物MRM参数如表1所示。
表1 8种外源性雌激素和内标的质谱参数
Table 1 MS parameters of 8 exoestrogens and internal standard sulfamethoxazole
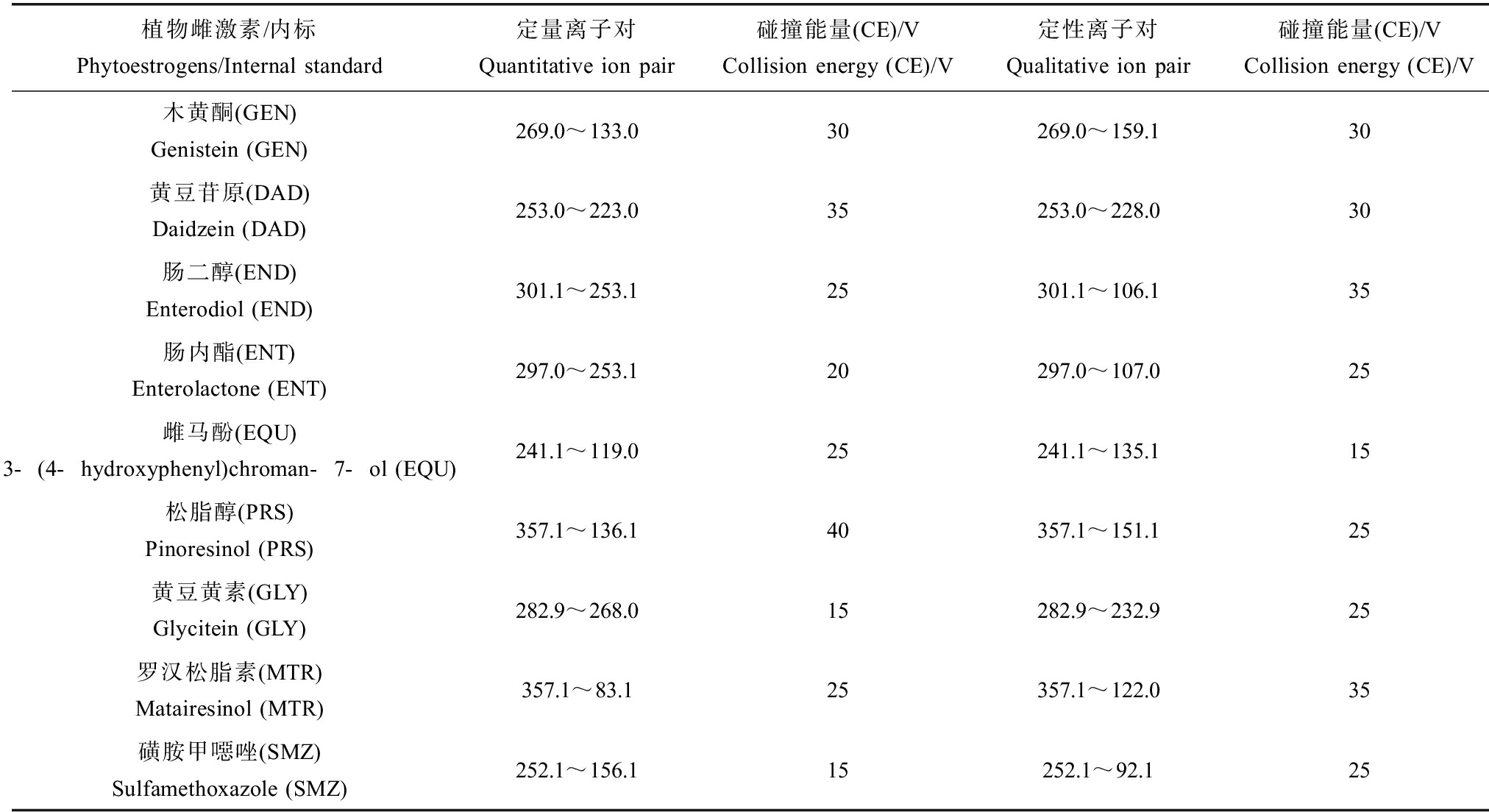
植物雌激素/内标Phytoestrogens/Internal standard定量离子对Quantitative ion pair碰撞能量(CE)/VCollision energy (CE)/V定性离子对Qualitative ion pair碰撞能量(CE)/VCollision energy (CE)/V木黄酮(GEN)Genistein (GEN)269.0~133.030269.0~159.130黄豆苷原(DAD)Daidzein (DAD)253.0~223.035253.0~228.030肠二醇(END)Enterodiol (END)301.1~253.125301.1~106.135肠内酯(ENT)Enterolactone (ENT)297.0~253.120297.0~107.025雌马酚(EQU)3-(4-hydroxyphenyl)chroman-7-ol (EQU)241.1~119.025241.1~135.115松脂醇(PRS)Pinoresinol (PRS)357.1~136.140357.1~151.125黄豆黄素(GLY)Glycitein (GLY)282.9~268.015282.9~232.925罗汉松脂素(MTR)Matairesinol (MTR)357.1~83.125357.1~122.035磺胺甲噁唑(SMZ)Sulfamethoxazole (SMZ)252.1~156.115252.1~92.125
1.4 尿液样本中的外源性雌激素含量测定
标准曲线配制:取适量103 mg·L-1 8种EE对照品贮备液,用甲醇配成浓度为1.25×10-3、2.5×10-3、5×10-3、10×10-3、20×10-3、40×10-3、0.1、0.2、0.5、1和2 mg·L-1的混合对照品溶液;取0.1 mL各浓度的混合标准品溶液,分别加入10 μL内标SMZ(0.2 mg·L-1)后待测。线性以标准品与内标的峰面积比值和标准品浓度梯度进行线性回归,得到线性回归方程。在本研究建立的分析方法中,检测限(LOD)是生物样品按照分析方法的要求进行提取处理并检测,能区分于噪声的最低检出浓度,检出限对应的响应值一般为基线噪声的3倍;定量限(LOQ)是通过对一系列含有已知浓度被测物的试样进行分析,在准确度和精密度都符合要求的情况下,来确定被测物能被定量的最小量,即标准曲线浓度的最小值。
分析方法验证:本研究中分析方法验证包括精密度、回收率和基质效应。精密度通常包括日内(批内)精密度和日间(批间)精密度。在标准曲线范围的高、中、低3个浓度(1、0.2和5×10-3 mg·L-1)分别制备6个样品,连续测定3批,以测定该方法的精密度。变异系数(CV%)常用于表示精密度,当日间和日内精密度的CV%不超过±15%时,该方法精密度良好。其中,CV%=(标准偏差(SD)/平均值(Mean))×100%。本研究使用内标法进行提取回收率和基质效应测定。选取浓度为200 ng·mL-1的混合对照品溶液用作验证,提取回收率为内标加入后提取的峰面积与内标溶液峰面积的百分比,基质效应的测定则是提取后添加内标峰面积与内标标准品的峰面积的百分比,所有的测定均进行3组平行。
样本前处理:取0.1 mL尿液样品,加入0.5 mL 20 mmol·L-1醋酸铵缓冲液和20 μL β-葡萄糖醛酸酶(20 KU·mL-1,20 mmol·L-1醋酸铵缓冲液配制),充分混匀后置于37 ℃恒温摇床中水解过夜。水解后加入10 μL内标SMZ溶液(200 ng·mL-1)和1 mL乙酸乙酯,充分振荡3 min,于4 000 r·min-1,4 ℃离心15 min,准确吸取上清液于另一试管中。重复上述步骤,混匀2次上清液。将上清液在室温条件下于离心浓缩干燥仪中浓缩干燥,干燥后加入110 μL甲醇,充分涡旋,于10 000 r·min-1,4 ℃离心10 min,然后将液体转移至液相进样小瓶中,待测。
1.5 尿液样本分组
尿液样本中测定的8种EE浓度经肌酐进行校正。以GEN为代表,将GEN含量标准化并排序后绘制90例孕妇尿液样本中GEN的含量变化;然后根据GEN含量将90例孕妇尿液样本分为3组:前30例为低EE水平组(Exp-L),中间30例为中EE水平组(Exp-M),后30例为高EE水平组(Exp-H)。然后对3组样本中8种EE的标准化含量变化进行比较。
1.6 基于高分辨质谱的尿液样本非靶向代谢组学分析
非靶向代谢组学尿液样本前处理:取0.1 mL尿液样品,加入0.2 mL甲醇-乙腈溶液(2∶1, V∶V)和10 μL的双内标(L-2-氯苯丙氨酸,甲醇配制,300 mg·L-1;LPC 17:0,甲醇配制,100 mg·L-1),涡旋震荡60 s后,于冰水浴中超声提取10 min,然后放置于-20 ℃中静置30 min。将静置后的混合溶液于13 000 r·min-1,4 ℃下离心15 min,取160 μL上清液装入带内衬管的液相进样小瓶中,待测。此外,所有尿液样本取等量(20 μL)均匀混合,用作质控样本(quality control, QC),并按上述前处理方法相同处理。
色谱条件:色谱柱为ACQUITY UPLC HSS T3柱(100 mm×2.1 mm,1.7 μm);流动相A为0.1%甲酸-水溶液,流动相B为0.1%甲酸-乙腈溶液;流速为0.4 mL·min-1;进样体积为1 μL;柱温为45 ℃;梯度洗脱如下:0 min,1% B;0~12 min,1%~90% B;12~13 min,90% B。每2针样品间以初始流动相梯度(1% B)平衡2 min,即13~13.1 min,90%~1% B;13.1~15.1 min,1% B。
质谱条件:ESI电离源,正负离子模式;碰撞气、雾化气为氮气(99.999%);正离子模式下喷射电压为3.2 kV,负离子模式下喷射电压为2.8 kV;毛细管温度为320 ℃;s-lens RF level为50 V;扫描模式为DDA模式(1 full scan followed by 5 MS/MS scans);一级碰撞能为15 V,二级碰撞能为30 V;质量扫描范围为70~1 000 m/z;全扫扫描分辨率为70 000,自动增益目标值(AGC target)为1e6,注入时间(IT)为100 ms;二级扫描(dd-MS/MS)分辨率为17 500,AGC target为5e5,IT为50 ms。数据通过Xcalibur 3.0软件采集。
1.7 非靶向代谢组学数据分析
非靶向代谢组学分析获得的原始数据经代谢组学处理软件Progenesis QI v2.3软件(Nonlinear Dynamics, Newcastle,英国)进行基线过滤、峰识别、积分、保留时间校正、峰对齐和归一化。其主要参数为precursor tolerance: 0.0005%;product tolerance: 0.001%;product ion threshold: 5%。化合物的鉴定基于精确质量数、二级碎片以及同位素分布,使用The Human Metabolome Database(HMDB)和Lipidmaps(v2.3)以及METLIN数据库进行定性。对提取到的数据,删除缺失值(0值)>50%的离子峰,并将0值以质谱响应峰面积最小值的一半替换,并根据化合物定性结果打分(Score)对定性得到的化合物进行筛选。最后将正负离子数据合并成一个数据矩阵表。
根据QC、低EE水平(Exp-L)组、中EE水平(Exp-M)组和高EE水平(Exp-H)组孕妇尿液样本中鉴定出的代谢物及其相对含量进行主成分分析(principal component analysis, PCA),再将低EE水平组(Exp-L)、中EE水平(Exp-M)和高EE水平组(Exp-H)孕妇尿液样本的代谢物进行两两正交偏最小二乘法判别分析(orthogonal partial least squares discriminant analysis, OPLS-DA),同时根据OPLS-DA得到在样本区分中贡献较大的变量,即变量权重(variable importance for the projection, VIP)。使用GraphPad Prism 8软件通过moderate t-statistic计算P-value,并基于P-value、差异倍数(fold change, FC)和VIP筛选出3组样品之间的差异代谢物,并绘制火山图。基于筛选获得的差异代谢物进行KEGG通路分析,根据-log10 P-value排序的top15通路通过Origin 2019b软件绘制气泡图展示。使用SPSS软件根据筛选获得的差异代谢物进行接受者操作特性曲线(ROC)分析,并计算曲线下面积(AUC)。
2 结果(Results)
2.1 外源性雌激素的定量方法和含量测定
2.1.1 定量方法建立
在本研究中,基于UPLC-QqQ-MS的MRM模式对8种EE进行含量测定,8种EE和内标SMZ定量离子对和定性离子对的总离子流图如图1所示。8种EE的标准曲线如表2所示,回归系数均>0.99,线性关系良好;8种EE检测限和定量限如表2所示;且回收率为55.9%~99.8%,基质效应为74.1%~110.7%,日内精密度为5.0%~8.1%,日间精密度为5.7%~8.8%。上述参数均符合要求,说明建立的LC-QqQ-MS方法能够用于检测孕妇尿液样本中8种EE的含量。

图1 8种外源性雌激素和内标的定量和定性离子对LC-MS/MS分离图
Fig. 1 LC-MS/MS chromatograms of quantitative ion pair and qualitative ion pair of 8 exoestrogens and internal standard
表2 基于LC-QqQ-MS方法的8种外源性雌激素的标准曲线、检测限(LOD)和定量限(LOQ)
Table 2 Standard curves, limit of detection (LOD) and limit of quantification (LOQ) of 8 exoestrogens measured by LC-QqQ-MS method
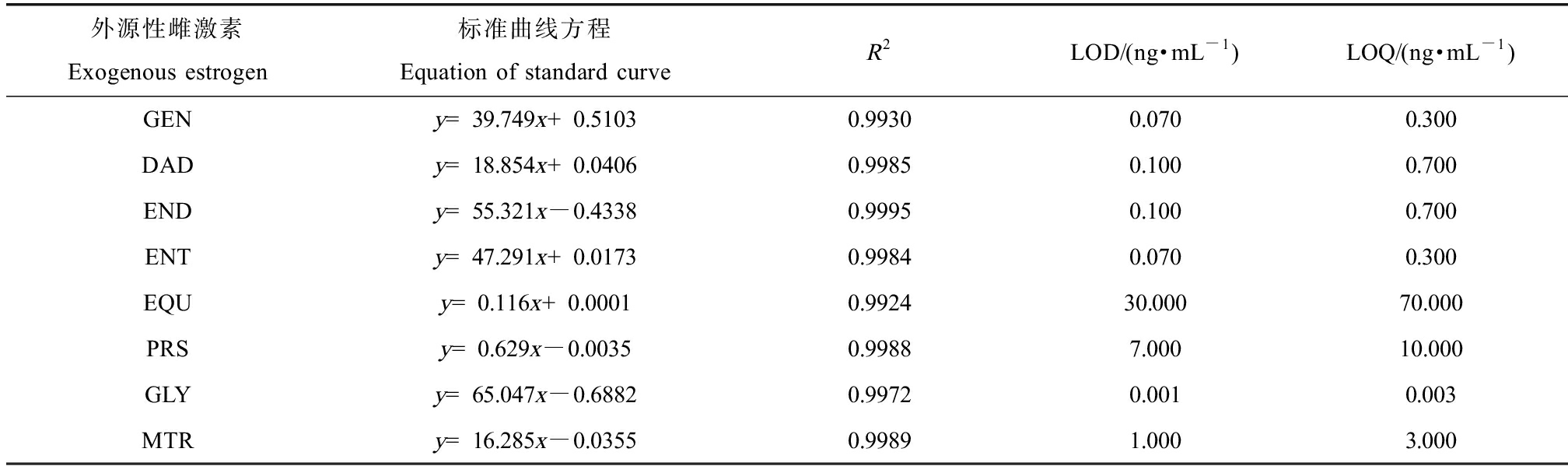
外源性雌激素Exogenous estrogen标准曲线方程Equation of standard curveR2LOD/(ng·mL-1)LOQ/(ng·mL-1)GENy=39.749x+0.51030.99300.0700.300DADy=18.854x+0.04060.99850.1000.700ENDy=55.321x-0.43380.99950.1000.700ENTy=47.291x+0.01730.99840.0700.300EQUy=0.116x+0.00010.992430.00070.000PRSy=0.629x-0.00350.99887.00010.000GLYy=65.047x-0.68820.99720.0010.003MTRy=16.285x-0.03550.99891.0003.000
2.1.2 含量测定分析及样本分组
基于上述LC-QqQ-MS方法测定了90例孕妇尿液样本中的8种EE浓度,并经肌酐进行校正。GEN是一种具有抗氧化特性的异黄酮类植物雌激素,90例孕妇尿液样本中GEN含量的变化幅度较大,如图2(a)所示。根据GEN含量将90例孕妇尿液样本分为Exp-L、Exp-M和Exp-H 3组后,其他7种EE含量趋势与GEN一致,即当孕妇体内GEN水平较低时,其他7种EE水平也是较低的;中水平和高水平亦是如此,如图2(b)所示。这可能是由于饮食等生活习惯或身处环境相似的群体,她们的EE摄入种类和程度也较为相近。并且,90例孕妇尿液样本分为3组后,GEN、DAD和GLY这3种EE含量在Exp-L、Exp-M和Exp-H组间都具有显著性差异;END、ENT和EQU这3种EE含量至少在2组间具有显著性差异;PRS和MTR的含量在3组间尽管有差异,却不具备显著性。但是,大部分EE含量在3组间的显著性差异说明该分组方式的有效性,具有统计学意义。此外,本研究中分析的8种EE中,显著性差异较大的GEN、DAD和ENT已被美国环境保护局(US EPA)列入内分泌干扰素审查程序(Endocrine Disruptor Screening Program, EDSP)清单中。因此,我们可以GEN为代表,将EE水平作为尿液样本的分组依据,并研究不同程度的EE摄入对妊娠期孕妇的生理过程和代谢过程的影响。

图2 孕妇尿液样本中外源性雌激素的含量比较
注:(a) 90例尿液样品中GEN浓度变化趋势;(b) 低EE水平组(Exp-L)、中EE水平组(Exp-M)和高EE水平组(Exp-H)组尿液样本中8种外源性雌激素的含量对比;*P<0.05, **P<0.01, ***P<0.001。
Fig. 2 Content comparison of exoestrogens in the urine samples of pregnant women Note: (a) Trend of GEN concentration in 90 urine samples; (b) Content comparison of 8 exoestrogens in the urine samples of low EE level group (Exp-L), medium EE level group (Exp-M) and high EE level group (Exp-H) groups; *P<0.05, **P<0.01, ***P<0.001.
2.2 不同外源性雌激素水平的孕妇尿液样本代谢组学分析
2.2.1 多维统计分析
根据孕妇尿液样本中的EE含量将90例样本分为低Exp-L、Exp-M和Exp-H组,利用液相色谱-质谱联用技术分析尿液样本中的代谢组。多维统计分析可同时考察所有变量(代谢物)对样本的影响,找出样本与变量之间的关系,并且能够在简化复杂数据的同时,最大程度地保留原始信息。因此,可以通过多维统计分析获得样本的代谢特征,并通过降维可视化不同组之间的分离程度。
首先通过无监督的PCA对Exp-L、Exp-M和Exp-H组孕妇尿液样本以及QC质控样本的代谢组数据做一个总体的趋势分析,确定不同EE水平是否会影响孕妇的代谢过程。由PCA得分图(图3(a))可知,样本基本上均匀分布在95%的可信区间内(圈外个别离奇值需要剔除),其中,QC样本聚集良好,表明检测样本批次之间误差可控,仪器和方法的精密度和稳定性满足实验需求。进一步观察,各组样本分布均较广,且几何位置相近,区分并不明显,不同组别难以通过PCA良好地区分,提示需要进一步的统计分析。
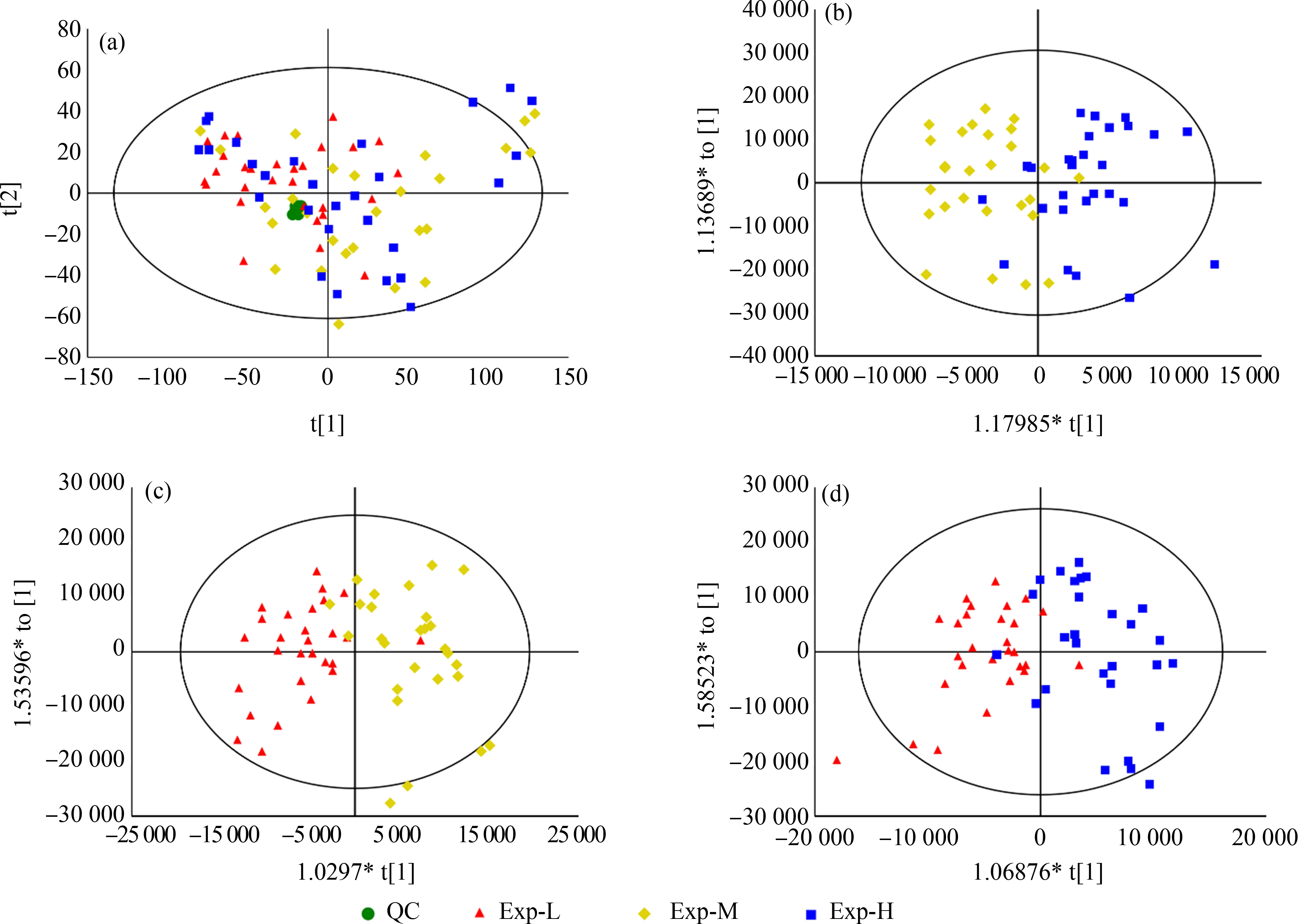
图3 Exp-L、Exp-M和Exp-H组孕妇尿液样本代谢组的多维统计分析得分图
注:(a) QC、Exp-L、Exp-M和Exp-H组的PCA得分图;(b) Exp-H vs Exp-M的OPLS-DA得分图;(c) Exp-M vs Exp-L的OPLS-DA得分图;(d) Exp-H vs Exp-L的OPLS-DA得分图。
Fig. 3 Score plots of multivariate statistical analysis of metabolome in the pregnant women’s urine samples of Exp-L, Exp-M and Exp-H groups
Note: (a) PCA score plot of QC, Exp-L, Exp-M and Exp-H groups; (b) OPLS-DA score plot of Exp-H vs Exp-M; (c) OPLS-DA score plot of Exp-M vs Exp-L; (d) OPLS-DA score plot of Exp-H vs Exp-L.
有监督的OPLS-DA结合了正交信号校正和偏最小二乘法这2种方式,能将主要差距集中在预测主成分中,因此可以更好地模拟和区分结果。Exp-L、Exp-M和Exp-H组孕妇尿液样本两两之间进行OPLS-DA,由OPLS-DA得分图(图3(b):Exp-H vs Exp-M;图3(c):Exp-M vs Exp-L;图3(d):Exp-H vs Exp-L)可知,Exp-L、Exp-M和Exp-H组孕妇尿液样本可以良好地区分开,表明孕妇摄入不同程度的EE与其生理代谢过程变化之间具有关联性。此外,通过OPLS-DA还可以根据VIP值得到在样本区分中贡献较大的变量,用于下一步筛选差异代谢物。
2.2.2 差异代谢物筛选
代谢组学分析的主要目的是寻找不同组间具有重要生物学意义的差异代谢物,以阐明生物体的代谢过程和生理病理变化机制。而差异代谢物的筛选是代谢组学分析中重要的步骤,后续的代谢通路和代谢网络分析等都是基于差异代谢物进行的。在筛选差异代谢物的过程中,一般会选择多标准的评价方法,以提高筛选的准确度和可信度。
FC指的是2组间某一变量的平均值之比,该参数可在宏观角度比较变量在2组间的表现;根据单维统计分析Student’s t-test检验得到的P-value可衡量变量在2组间差异的显著性;考察所有变量的多维统计分析中获得的VIP值也可用于差异代谢物的筛选。因此,在本实验中,结合以上3个参数,即FC>1.11或<0.90,P-value<0.05,VIP>1,对Exp-L、Exp-M和Exp-H组孕妇尿液样本中的差异代谢物进行筛选。
由图4可知,我们能够直观合理地观察并筛选得到3组孕妇尿液样本中的差异代谢物。图4(a)和表3展示了Exp-H组与Exp-M组间筛选到的17个差异代谢物,其中11个差异代谢物上调,6个差异代谢物下调;图4(b)和表4展示了Exp-M组与Exp-L组间筛选到的80个差异代谢物,其中40个差异代谢物上调,40个差异代谢物下调;图4(c)和表5展示了Exp-H组与Exp-L组间筛选到的48个差异代谢物,其中27个差异代谢物上调,21个差异代谢物下调。
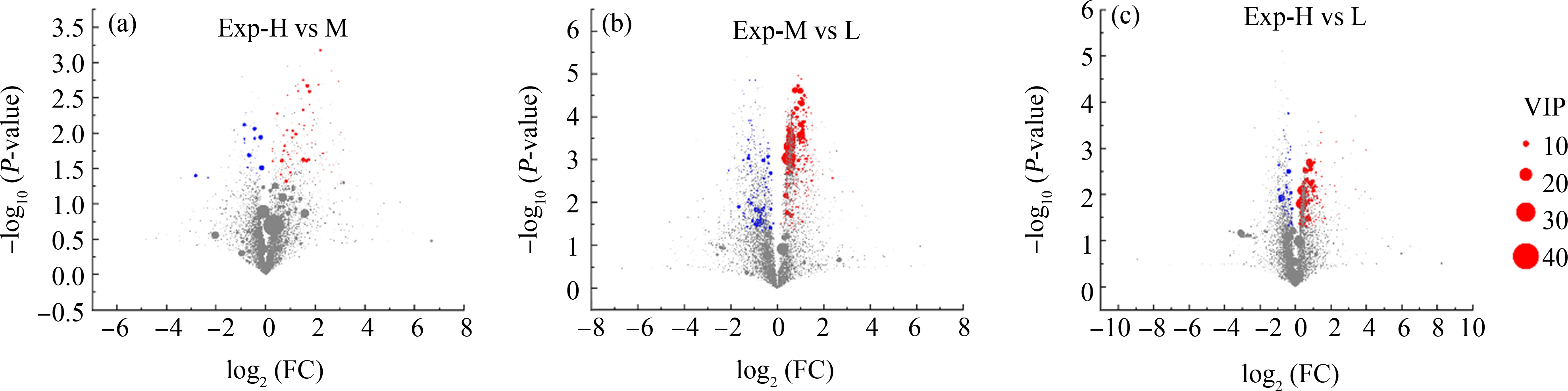
图4 Exp-L、Exp-M和Exp-H组孕妇尿液样本代谢物的火山图
注:(a) Exp-H vs Exp-M;(b) Exp-M vs Exp-L;(c) Exp-H vs Exp-L。
Fig. 4 Volcano plots of metabolites in the pregnant women’s urine samples of Exp-L, Exp-M and Exp-H groups Note: (a) Exp-H vs Exp-M; (b) Exp-M vs Exp-L; (c) Exp-H vs Exp-L.
表3 Exp-H和Exp-M组孕妇尿液样本中的差异代谢物
Table 3 Differential metabolites between urine samples of the Exp-H and Exp-M pregnant women
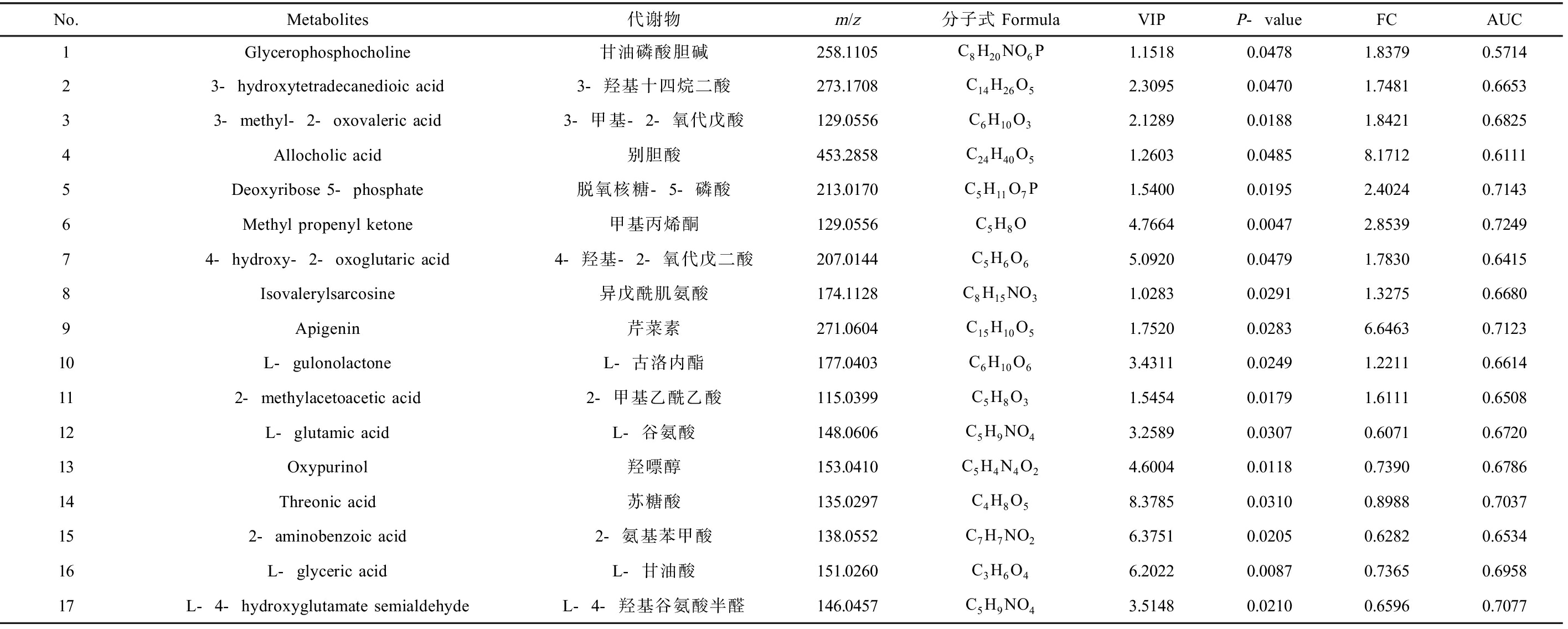
No.Metabolites代谢物m/z分子式FormulaVIPP-valueFCAUC1Glycerophosphocholine甘油磷酸胆碱258.1105C8H20NO6P1.15180.04781.83790.571423-hydroxytetradecanedioic acid3-羟基十四烷二酸273.1708C14H26O52.30950.04701.74810.665333-methyl-2-oxovaleric acid3-甲基-2-氧代戊酸129.0556C6H10O32.12890.01881.84210.68254Allocholic acid别胆酸453.2858C24H40O51.26030.04858.17120.61115Deoxyribose 5-phosphate脱氧核糖-5-磷酸213.0170C5H11O7P1.54000.01952.40240.71436Methyl propenyl ketone甲基丙烯酮129.0556C5H8O4.76640.00472.85390.724974-hydroxy-2-oxoglutaric acid4-羟基-2-氧代戊二酸207.0144C5H6O65.09200.04791.78300.64158Isovalerylsarcosine异戊酰肌氨酸174.1128C8H15NO31.02830.02911.32750.66809Apigenin芹菜素271.0604C15H10O51.75200.02836.64630.712310L-gulonolactoneL-古洛内酯177.0403C6H10O63.43110.02491.22110.6614112-methylacetoacetic acid2-甲基乙酰乙酸115.0399C5H8O31.54540.01791.61110.650812L-glutamic acidL-谷氨酸148.0606C5H9NO43.25890.03070.60710.672013Oxypurinol羟嘌醇153.0410C5H4N4O24.60040.01180.73900.678614Threonic acid苏糖酸135.0297C4H8O58.37850.03100.89880.7037152-aminobenzoic acid2-氨基苯甲酸138.0552C7H7NO26.37510.02050.62820.653416L-glyceric acidL-甘油酸151.0260C3H6O46.20220.00870.73650.695817L-4-hydroxyglutamate semialdehydeL-4-羟基谷氨酸半醛146.0457C5H9NO43.51480.02100.65960.7077
表4 Exp-M和Exp-L组孕妇尿液样本中的差异代谢物
Table 4 Differential metabolites between urine samples of the Exp-M and Exp-L pregnant women

No.Metabolites代谢物m/z分子式FormulaVIPP-valueFCAUC1Adenine腺嘌呤136.0620C5H5N51.51490.00382.18480.71462Betaine甜菜碱140.0685C5H11NO21.06270.00002.07910.81743Citric acid柠檬酸210.0611C6H8O78.16150.01731.36740.66164Fumaric acid富马酸134.0450C4H4O41.17030.00151.74860.75105L-aspartic acidL-天冬氨酸132.0301C4H7NO41.56660.00621.21880.69096Isocitric acid异柠檬酸215.0165C6H8O71.51430.03191.36260.64757Palmitic acid棕榈酸274.2742C16H32O26.27580.00161.37820.75108Thymine胸腺嘧啶125.0355C5H6N2O22.51280.00011.53030.77789delta-hexanolactoneδ-己内酯115.0755C6H10O21.51500.00031.35500.767610Dodecanoic acid十二烷酸218.2118C12H24O21.97390.00031.42390.781611Malonic acid丙二酸103.0035C3H4O41.22620.00111.52280.736912S-sulphocysteineS-磺基半胱氨酸199.9690C3H7NO5S21.83100.00601.90000.710113Myristic acid肉豆蔻酸246.2430C14H28O22.09960.00061.47720.784214Stearic acid硬脂酸302.3056C18H36O23.82320.00221.39450.761215L-valineL-缬氨酸118.0864C5H11NO23.19870.01531.31870.682016Pyridine吡啶80.0496C5H5N1.32740.00891.54080.669217Behenic acid山嵛酸358.3681C22H44O23.83330.00021.37140.7727184-imidazolone-5-propionic acid4-咪唑酮-5-丙酸155.0461C6H8N2O31.86220.00011.52830.7816193-aminopropionaldehyde3-氨基丙醛74.0602C3H7NO1.32520.00041.39630.754820Iminoaspartic acid亚氨基天冬氨酸130.0144C4H5NO41.57590.00091.52500.729221Carbon monoxide一氧化碳72.9930CO1.85630.00101.52230.735622alpha-CEHCα-甲基-4-(异丁基)苯甲基青霉素钠279.1593C16H22O46.53990.00021.38440.772723Palmitaldehyde棕榈醛258.2794C16H32O1.90500.00031.50430.779124Carbon dioxide二氧化碳88.9880CO22.94190.00011.60030.791825Phytol植物醇314.3419C20H40O1.04630.00011.36890.793126Cyanate氰酸盐88.0039CHNO2.87920.00041.53150.765027Aminoacetone氨基丙酮74.0602C3H7NO2.63600.00051.65970.752228Arachidic acid花生酸330.3369C20H40O23.53180.00061.35900.772729Oxalic acid草酸88.9880C2H2O43.40820.00191.50670.721630Pyruvatoxime丙酮肟102.0195C3H5NO31.20540.00171.61830.7599311-methyladenosine1-甲基腺苷282.1199C11H15N5O43.69010.00275.20940.680732Pyrimidine嘧啶81.0449C4H4N22.44610.00061.40600.749733D-ornithineD-鸟氨酸133.0974C5H12N2O21.00300.00011.38560.765034gamma-caprolactoneγ-己内酯115.0755C6H10O21.01500.00031.38450.763735Imidazole-4-acetaldehyde咪唑-4-乙醛109.0406C5H6N2O2.27350.00011.53070.7918365-aminoimidazole5-氨基咪唑84.0558C3H5N31.86990.00071.43420.7522372-O-methylcytosine2-O-甲基胞嘧啶126.0664C5H7N3O1.64050.00111.40310.730538Imidazolone咪唑酮83.0250C3H4N2O3.73490.00031.52090.765039L-glyceric acidL-甘油酸151.0260C3H6O42.85800.01961.30890.671840L-4-hydroxyglutamate semialdehydeL-4-羟基谷氨酸半醛146.0457C5H9NO42.28700.02961.54240.721641Tetrahydrobiopterin四氢生物蝶呤242.1250C9H15N5O31.63800.00150.75840.725442Adenosine腺苷312.0950C10H13N5O41.57470.00400.48300.722943Cyclic AMP循环AMP330.0600C10H12N5O6P1.15100.02170.79510.676944Gentisic acid龙胆酸153.0192C7H6O41.43750.03530.49710.763745Indoleacetic acid吲哚乙酸176.0709C10H9NO21.59170.04100.43860.790546Oxoglutaric acid氧戊二酸191.0196C5H6O56.51870.03910.81940.659047Pantothenic acid泛酸220.1182C9H17NO55.15800.01480.64810.6705

续表4No.Metabolites代谢物m/z分子式FormulaVIPP-valueFCAUC482-methylbutyroylcarnitine2-甲基丁酰肉碱246.1702C12H23NO43.66950.02580.71330.70504918-hydroxycortisol18-羟基皮质醇423.2025C21H30O61.21360.04500.63580.6564505-hydroxy-L-tryptophan5-羟基-L-色氨酸221.0923C11H12N2O32.54580.00490.77130.712651Cinnamic acid肉桂酸166.0866C9H8O26.06550.00080.75790.754852Citraconic acid柠康酸129.0192C5H6O43.12860.00340.70890.722953Homo-L-arginine同型L-精氨酸189.1349C7H16N4O22.23480.00060.78220.756154L-leucineL-亮氨酸132.1021C6H13NO26.72170.00100.66550.796955Kynurenic acid犬尿酸190.0501C10H7NO32.81530.00160.74840.736956Glycylproline甘氨酰脯氨酸173.0924C7H12N2O32.56560.00600.70880.706357Hydroxykynurenine羟基犬尿氨酸225.0872C10H12N2O41.46220.02030.32520.713958N-acetyl-L-aspartic acidN-乙酰-L-天冬氨酸174.0406C6H9NO53.83600.00120.77380.756159Xanthurenic acid黄尿酸204.0300C10H7NO41.57190.03530.72950.655260N-acetylaspartylglutamic acidN-乙酰天冬氨酰谷氨酸305.0981C11H16N2O81.57140.00250.76790.721661Methyl propenyl ketone甲基丙烯酮129.0556C5H8O1.66390.00820.43240.653962Allysine赖氨酸144.0665C6H11NO32.35790.00670.83770.711463Dimethyl-L-arginine二甲基-L-精氨酸203.1505C8H18N4O23.18860.03100.89500.653964Cystathionine ketimine胱硫醚酮亚胺248.0233C7H9NO4S1.09120.02720.69860.6692654-hydroxy-2-oxoglutaric acid4-羟基-2-氧代戊二酸207.0144C5H6O63.75960.00030.42520.768866Carnosol鼠尾草酚375.1812C20H26O41.25380.00810.44240.719067Imidazoleacetic acid riboside咪唑乙酸核苷303.0833C10H14N2O62.16050.00060.75180.762568Aspartyl-L-proline天冬氨酰-L-脯氨酸231.0978C9H14N2O51.47060.04730.82680.6884694-nitrocatechol4-硝基儿茶酚173.0560C6H5NO41.21220.02020.65610.689770N-methylnicotinamideN-甲基烟酰胺137.0712C7H8N2O2.54200.04550.65100.670571Deoxyribose脱氧核糖179.0560C5H10O42.28900.03840.78640.657772Dihydrocortisol二氢皮质醇409.2231C21H32O51.20680.01550.33300.772773Nicotinuric acid烟尿酸179.0461C8H8N2O31.87820.01220.78370.6948744-guanidinobutanoic acid4-胍基丁酸146.0926C5H11N3O24.90930.03240.55240.662875L-gulonolactoneL-古洛内酯177.0403C6H10O62.25320.00040.76030.752276N6-methyladenosineN6-甲基腺苷282.1199C11H15N5O45.38370.01340.67170.6948773-methoxy-4-hydroxyphenylglycolaldehyde3-甲氧基-4-羟基苯乙醇醛200.0920C9H10O41.20960.00390.69020.7305785-methoxyindoleacetate5-甲氧基吲哚乙酸酯250.0720C11H11NO31.47550.02330.44430.645079N4-acetylcytidineN4-乙酰胞苷286.1036C11H15N3O63.15990.00100.64190.749780D.L-methylisocitrateD.L-甲基异柠檬酸盐205.0352C7H10O71.27150.00870.78650.7075
表5 Exp-H和Exp-L组孕妇尿液样本中的差异代谢物
Table 5 Differential metabolites between urine samples of the Exp-H and Exp-L pregnant women

No.Metabolites代谢物m/zFormulaVIPP-valueFCAUC1Adenine腺嘌呤136.0620C5H5N51.31360.04321.77700.60592Betaine甜菜碱140.0685C5H11NO21.12490.00051.86830.70323Citric acid柠檬酸210.0611C6H8O78.10430.01941.28040.65274Thymine胸腺嘧啶125.0355C5H6N2O22.47460.00681.40820.65645delta-hexanolactoneδ-己内酯115.0755C6H10O21.42280.02421.23530.64166Creatinine肌酐112.0515C4H7N3O1.01830.03161.25860.59367Dodecanoic acid十二烷酸218.2118C12H24O21.99130.04741.30560.60968Myristic acid肉豆蔻酸246.2430C14H28O22.17650.03151.29230.63309Behenic acid山嵛酸358.3681C22H44O23.27160.03561.21340.6170104-imidazolone-5-propionic acid4-咪唑酮-5-丙酸155.0461C6H8N2O31.83600.00661.41290.6589113-aminopropionaldehyde3-氨基丙醛74.0602C3H7NO1.19630.01241.27690.640412Iminoaspartic acid亚氨基天冬氨酸130.0144C4H5NO41.24650.02111.32200.637913Carbon monoxide一氧化碳72.9930CO1.45960.02231.32060.645314alpha-CEHCα-甲基-4-(异丁基)苯甲基青霉素钠279.1593C16H22O45.07710.04491.24330.620715Palmitaldehyde棕榈醛258.2794C16H32O1.76250.03101.30820.631816Carbon Dioxide二氧化碳88.9880CO22.51550.00691.39040.653917Cyanate氰酸盐88.0039CHNO2.60900.00991.37860.651518Aminoacetone氨基丙酮74.0602C3H7NO1.94470.02501.36330.629319Oxalic acid草酸88.9880C2H2O42.60610.02951.30710.637920Pyruvatoxime丙酮肟102.0195C3H5NO31.00820.01551.33420.6441211-methyladenosine1-甲基腺苷282.1199C11H15N5O43.49830.03313.21700.570222Pyrimidine嘧啶81.0449C4H4N22.06510.01541.26620.650223gamma-caprolactoneγ-己内酯115.0755C6H10O21.03590.04631.23910.620724Imidazole-4-acetaldehyde咪唑-4-乙醛109.0406C5H6N2O2.39310.00551.43960.6638255-aminoimidazole5-氨基咪唑84.0558C3H5N31.58980.01201.29130.6527262-O-methylcytosine2-O-甲基胞嘧啶126.0664C5H7N3O1.46190.01251.28430.646627Imidazolone咪唑酮83.0250C3H4N2O3.55160.00861.38530.653928α-ketoisovaleric acidα-酮异戊酸115.0399C5H8O31.08980.01220.85540.676129Gentisic acid龙胆酸153.0192C7H6O41.51740.02260.51740.726630Homo-L-arginine同型L-精氨酸189.1349C7H16N4O23.75400.00020.75820.761131Kynurenic acid犬尿酸190.0501C10H7NO32.75820.00950.79890.704432N-acetyl-L-aspartic acidN-乙酰-L-天冬氨酸174.0406C6H9NO53.23210.03980.84710.672433Ribothymidine核苷259.0928C10H14N2O61.14220.03870.81010.665034N-acetylaspartylglutamic acidN-乙酰天冬氨酰谷氨酸305.0981C11H16N2O82.02370.00910.81840.689735N-acetylneuraminosyl(alpha2-6)lactosamineN-乙酰神经氨基(α2-6)乳糖胺673.2311C25H42N2O191.30840.01180.85910.686036Allysine赖氨酸144.0665C6H11NO32.89600.04830.88610.630537L-dopachrome左旋多巴色素211.0716C9H7NO41.04390.01170.41410.752538Dimethyl-L-arginine二甲基-L-精氨酸203.1505C8H18N4O25.34920.02050.86480.668739S-(2-carboxypropyl)-CysteamineS-(2-羧丙基)-半胱胺164.0742C6H13NO2S1.64780.02570.73710.674940Imidazoleacetic acid riboside咪唑乙酸核苷303.0833C10H14N2O62.86880.00170.80520.727841Chenodeoxycholic acid 3-sulfate鹅去氧胆酸-3-硫酸盐471.2421C24H40O7S1.95170.04980.55190.565342Sulfolithocholylglycine硫代胆碱基甘氨酸512.2686C26H43NO7S3.57130.01400.53070.672443Ursodeoxycholic acid 3-sulfate熊去氧胆酸-3-硫酸盐471.2421C24H40O7S1.08230.04930.63120.6170442-methylglutaconic acid2-甲基戊二酸189.0403C6H8O41.12110.04380.86430.6884453-methoxy-4-hydroxyphenylglycolaldehyde3-甲氧基-4-羟基苯乙醇醛200.0920C9H10O41.65600.00060.64790.747546beta-cortolβ-皮质醇543.2813C27H44O111.24060.03020.71450.670047N4-acetylcytidineN4-乙酰胞苷286.1036C11H15N3O62.62980.00860.72850.715548Prolylhydroxyproline脯氨酰羟脯氨酸229.1185C10H16N2O45.03930.04080.83580.7020
2.2.3 差异代谢物的生物信息分析
通过上述筛选过程获得的差异代谢物具有重要的生物学意义,可以阐明生物体的代谢过程和生理病理变化机制。本实验中,我们将获得的差异代谢物通过KEGG数据库寻找与其相关的代谢通路,探究孕妇摄入不同程度EE与孕妇体内的代谢过程和生理病理过程变化之间的关联性。
Exp-H与Exp-M组间差异代谢物相关的通路如图5(a)所示;Exp-M与Exp-L组间差异代谢物相关的通路如图5(b)所示;Exp-H与Exp-L组间差异代谢物相关的通路如图5(c)所示。由差异代谢物相关的代谢通路可知,孕妇摄入不同程度EE造成的孕妇代谢过程和生理病理过程变化主要有氨基酸代谢、脂肪酸生物合成、柠檬酸循环和氮代谢过程等。这与许多研究结果相一致,即生物体摄入EE会对其氨基酸代谢和柠檬酸循环等能量代谢过程造成显著的影响且代谢过程变化与体内EE水平具有关联性。这些代谢过程的变化与孕妇妊娠期EE摄入造成的孕妇生殖系统受损以及婴幼儿发育影响等相关联且与EE摄入水平呈正相关。

图5 Exp-L、Exp-M和Exp-H组孕妇尿液样本间差异代谢物的KEGG通路气泡图
注:(a) Exp-H vs Exp-M;(b) Exp-M vs Exp-L;(c) Exp-H vs Exp-L。
Fig. 5 Bubble diagrams of KEGG pathways of differential metabolites between the pregnant women’s urine samples of Exp-L, Exp-M and Exp-H groups
Note: (a) Exp-H vs Exp-M; (b) Exp-M vs Exp-L; (c) Exp-H vs Exp-L.
为了验证这些差异代谢物用于辅助临床诊断和鉴别的价值,进一步对于上述的差异代谢物进行ROC分析,并根据AUC大小确定哪些关键差异代谢物可以成为用于探究孕妇体内EE水平影响代谢过程的潜在生物标志物。Exp-L、Exp-M和Exp-H组孕妇尿液样本间差异代谢物的AUC值如表3、表4和表5所示。由表可知,单个差异代谢物的AUC值并不是很大,用于预测模型的效果一般。
进一步从3个比较组(Exp-H vs Exp-M,Exp-M vs Exp-L,Exp-H vs Exp-L)中筛选表达差异和诊断价值较大(FC<1/1.85或>1.85,AUC>0.7)的共同差异代谢物,其中至少同时出现在2个比较组中的共同差异代谢物有甜菜碱(betaine)和龙胆酸(gentisic acid)。作Exp-L、Exp-M和Exp-H组孕妇尿液样本中甜菜碱和龙胆酸含量的柱状图。如图6所示,Exp-M组Exp-H组孕妇尿液样本中甜菜碱和龙胆酸含量没有显著差异,而Exp-M,Exp-H组孕妇尿液样本和Exp-L组孕妇尿液样本中甜菜碱和龙胆酸含量具有显著差异。这表明孕妇的EE摄入程度与体内甜菜碱和龙胆酸含量变化具有相关性,但是当EE值到达一定水平区间时(以GEN为代表的EE含量为0.5~12.0 ng·mL-1),将不再会引起甜菜碱和龙胆酸含量的显著变化。
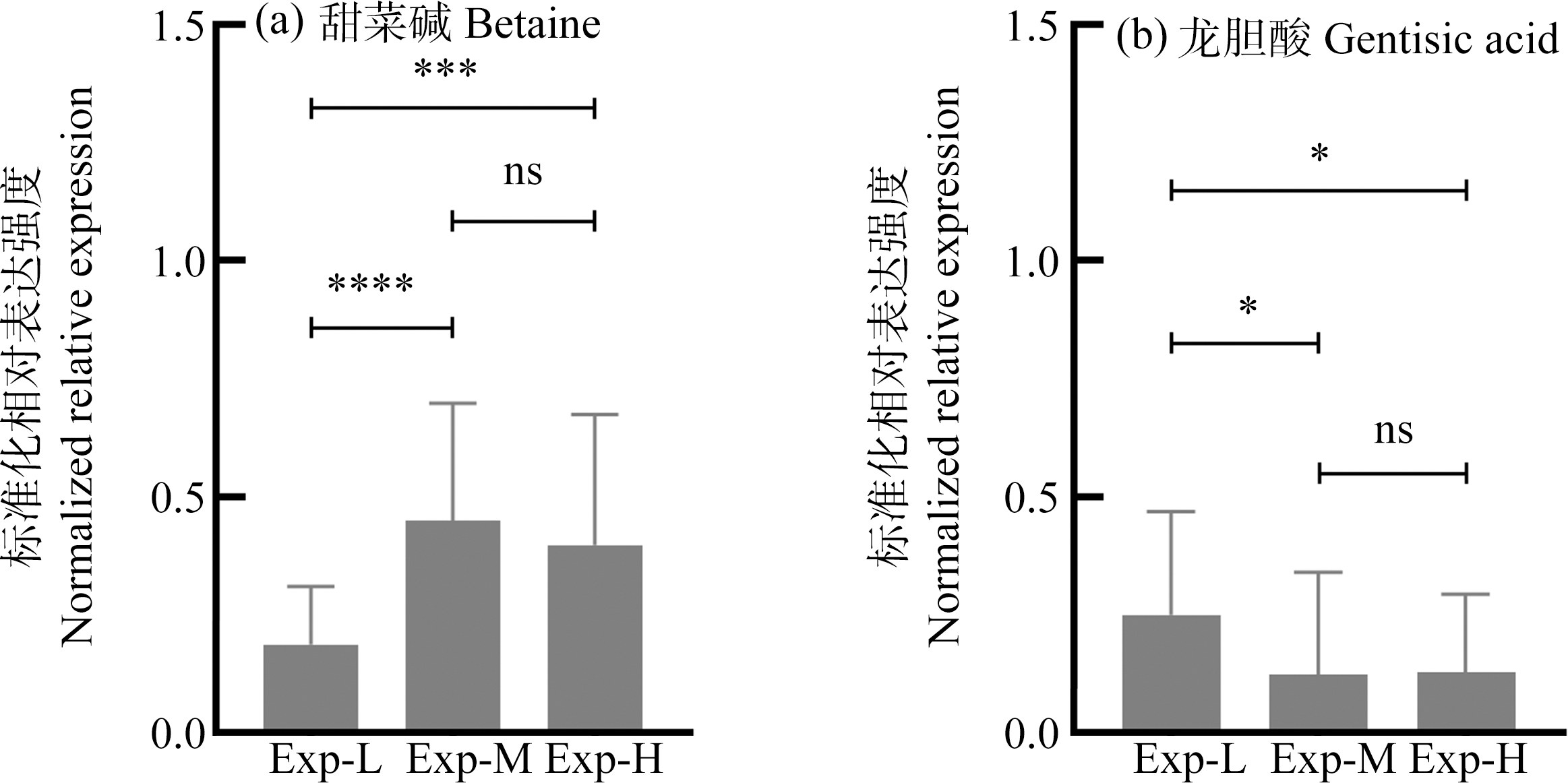
图6 Exp-L、Exp-M和Exp-H组孕妇尿液样本中甜菜碱和龙胆酸相对含量对比
注:(a)甜菜碱;(b)龙胆酸。
Fig. 6 Relative content comparison of betaine and gentisic acid in the pregnant women’s urine samples of Exp-L, Exp-M and Exp-H groups
Note: (a) Betaine; (b) Gentisic acid.
甜菜碱(N,N,N-三甲基甘氨酸)是一种季铵碱类物质,广泛存在于麦麸、小麦胚芽、菠菜、甜菜等植物中。膳食甜菜碱的摄入量对身体的甜菜碱含量起着决定性的作用。除了饮食摄入外,甜菜碱还可以从体内的胆碱合成。甜菜碱具有重要的生物学意义,一方面是一种重要的渗透保护剂,可在细胞中积累而不会破坏细胞功能,在渗透压力下保护细胞、蛋白质和酶,另一方面是体内甲基转移反应的重要甲基供体。甜菜碱可改善含硫氨基酸(SAA)代谢对抗氧化应激,如甜菜碱-同型半胱氨酸甲基转移酶(BHMT)可催化甲基从甜菜碱转移到同型半胱氨酸上以形成蛋氨酸,蛋氨酸进一步转化为产生S-腺苷甲硫氨酸(SAM)[15]。因此,由图6(a)可知,摄入EE可能会降低甜菜碱代谢效率,即甜菜碱体内浓度上升,且与EE摄入程度具有一定的相关性。
龙胆酸(2,5-二羟基苯甲酸)是一种多羟基酸,它是水杨酸经肾代谢之后的次要产物,也是龙胆属植物根部的天然产物,存在于龙胆、葡萄、柑橘、紫檀、芝麻、猕猴桃、苹果、苦瓜、黑莓以及梨等植物和水果中。龙胆酸具有广泛的生物活性,具有抗氧化、抗炎、抗诱变性、保肝、神经保护和抗菌等作用。龙胆酸具有2个彼此处于对位的酚羟基,可以在不同类型的物理和化学刺激下发挥抗氧化剂和自由基清除剂的作用,如诱导性高氧大鼠在口服阿司匹林后血浆中的GSH水平和龙胆酸水平显著升高[16]。由图6(b)可知,孕妇摄入EE会降低体内龙胆酸水平且与EE摄入程度具有一定的相关性。这可能是由于EE会影响龙胆酸代谢,降低其抗氧化剂和自由基清除剂的作用,从而不利于ROS的消除,产生氧化应激作用。
3 讨论(Discussion)
通过对非靶向代谢组学结果的分析,发现了甜菜碱和龙胆酸这2种代谢物在Exp-L与Exp-M和Exp-H组孕妇尿液样本间具有较大的表达差异和诊断价值。甜菜碱作为体内重要的甲基供体可改善SAA代谢以对抗氧化应激;龙胆酸的抗氧化剂和自由基清除功能也能起到消除ROS、对抗氧化应激的作用。在EE摄入程度不同的孕妇体内,甜菜碱和龙胆酸的含量差异揭示了EE摄入能引起孕妇体内的氧化应激,并且氧化应激水平与EE摄入程度具有一定的相关性。如图5所示,在孕妇摄入不同程度EE造成的代谢过程变化中,含有与氧化应激相关的通路也能证实该论点,如谷胱甘肽代谢(glutathione metabolism)和嘧啶代谢(pyrimidine metabolism)等。ROS刺激可通过Nrf2通路诱导下游抗氧化响应元件表达,进而诱导谷胱甘肽S-转移酶等基因表达,从而通过GSH代谢过程调节体内ROS含量[17];同时氧化应激会直接刺激caspase-3活性,导致DNA的损伤,而DNA的损伤和修复过程则与嘧啶代谢密切相关[18-19]。
不仅是本研究中分析的8种EE,动植物等摄入其他种类的EE同样也会引起氧化应激,诱发一系列不良后果。Kanwar等[20]发现经BPA处理的番茄的膜脂过氧化和ROS积累增加且呈剂量依赖性,同时BPA还提高了GSH含量、谷胱甘肽S-转移酶和谷胱甘肽还原酶的活性,用以缓解BPA引起的氧化应激和毒性;Wang等[21]发现BPA能通过增加ROS产生和减弱人类皮质神经元中的抗氧化防御来导致氧化应激,并通过调节Bcl-2家族和caspase依赖性信号通路触发细胞凋亡,产生神经毒性;Le等[22]发现外源性苯甲酸雌二醇能显著降低雄性小鼠睾丸中过氧化氢酶、超氧化物歧化酶和谷胱甘肽过氧化物酶的表达和活性,破坏小鼠睾丸中的氧化还原平衡,引起氧化损伤,进而诱导雄性小鼠无精子症。
已有许多研究表明,氧化应激与孕妇健康和胎儿发育的不利影响具有紧密联系。在怀孕期间,氧化应激的平衡状态能够确保子宫血管适应和胎盘发育正常。而当孕妇摄入外源性雌激素等内分泌干扰物质后,其代谢状态发生改变时,促氧化剂的产生和生物活性超过了抗氧化剂的保护作用,即氧化应激失衡,会导致子宫血管形成失调或异常血管形成等结果,从而进一步引发一系列妊娠疾病,如先兆子痫、妊娠糖尿病和胎儿生长受限等[23-24]。在相关机制研究中,Deng等[25]发现在滋养层细胞模型HTR-8/SVneo中,氧化应激会诱导AKT途径改变从而引发细胞死亡增加;Tang等[26]发现在绒毛外滋养层细胞模型JEG-3中,氧化应激可通过激活ERK1/2、MAPK和JNK蛋白介导细胞凋亡;Curtis等[27]发现在子宫内生长受限女性的足月胎盘中,自噬泡的数量增加并定位于胎盘的滋养层,且BeWo细胞系经氧化应激培养处理后能检测到自噬体的存在,表明氧化应激能增加有害自噬并与子宫内生长受限诱发相关。
因此,通过调节过度氧化应激的健康管理方案可能有益于孕妇及其胎儿。然而,由于潜在的致畸作用,怀孕期间的药物干预很少被研究,被批准使用的药物也相当有限。通过饮食摄入预防妊娠疾病,不仅相当简便且可能具备显著的效果,受到了广泛的关注[24]。选择健康食品的饮食干预可提高孕妇体内的抗氧化能力,如富含脂肪酸、硒等微量元素和维生素D等的食品。二十二碳六烯酸(DHA)等Omega-3脂肪酸具有抗氧化和抗炎特性,能在促进胎盘血管系统中发挥作用[28];硒可以通过抑制多种类型细胞(如HUVEC细胞等)中的NF-κB、toll样受体和MAPK通路来减轻炎症,并能够通过激活D-葡萄糖摄取和代谢,逆转氧化应激,降低妊娠糖尿病患者的高血糖状态[29];维生素D可激活胎盘内皮中的维生素D受体,防止氧化应激和内皮功能失调的代谢,减少线粒体氧化磷酸化[30]。
综上所述,本文首先基于三重四极杆质谱的液相色谱-质谱联用技术测定孕妇尿液样本中的8种EE含量,并根据EE含量将尿液样本分为低EE水平组、中EE水平组和高EE水平组;然后基于液相色谱-高分辨质谱联用技术的非靶向代谢组学策略分析不同EE水平的孕妇尿液样本的代谢组,通过多维统计分析、差异代谢物筛选和差异代谢物生物信息分析,探究EE摄入程度对孕妇体内代谢过程的影响,可以为孕妇的合理饮食和健康管理提供借鉴。
[1] 林涵, 蒋学武. 外源性雌激素与男性生殖系统发育异常[J]. 中国男科学杂志, 2004, 18(4): 65-68
[2] Xu Z X, Liu J, Wu X H, et al. Nonmonotonic responses to low doses of xenoestrogens: A review [J]. Environmental Research, 2017, 155: 199-207
[3] Singleton D W, Khan S A. Xenoestrogen exposure and mechanisms of endocrine disruption [J]. Frontiers in Bioscience: A Journal and Virtual Library, 2003, 8: s110-s118
[4] Wang L H, Chen L R, Chen K H. In vitro and vivo identification, metabolism and action of xenoestrogens: An overview [J]. International Journal of Molecular Sciences, 2021, 22(8): 4013
[5] Hoover R N, Hyer M, Pfeiffer R M, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol [J]. The New England Journal of Medicine, 2011, 365(14): 1304-1314
[6] Signorile P G, Spugnini E P, Mita L, et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring [J]. General and Comparative Endocrinology, 2010, 168(3): 318-325
[7] Missmer S A, Hankinson S E, Spiegelman D, et al. In utero exposures and the incidence of endometriosis [J]. Fertility and Sterility, 2004, 82(6): 1501-1508
[8] Cai L Y, Izumi S, Suzuki T, et al. Dioxins in ascites and serum of women with endometriosis: A pilot study [J]. Human Reproduction, 2011, 26(1): 117-126
[9] Li M L, Zhou S, Wu Y L, et al. Prenatal exposure to propylparaben at human-relevant doses accelerates ovarian aging in adult mice [J]. Environmental Pollution, 2021, 285: 117254
[10] Toppari J, Larsen J C, Christiansen P, et al. Male reproductive health and environmental xenoestrogens [J]. Environmental Health Perspectives, 1996, 104(Suppl 4): 741-803
[11] Tonini C, Segatto M, Bertoli S, et al. Prenatal exposure to BPA: The effects on hepatic lipid metabolism in male and female rat fetuses [J]. Nutrients, 2021, 13(6): 1970
[12] Zhou B, Yang P, Deng Y L, et al. Prenatal exposure to bisphenol A and its analogues (bisphenol F and S) and ultrasound parameters of fetal growth [J]. Chemosphere, 2020, 246: 125805
[13] Yang P, Lin B G, Zhou B, et al. Sex-specific associations of prenatal exposure to bisphenol A and its alternatives with fetal growth parameters and gestational age [J]. Environment International, 2021, 146: 106305
[14] Minatoya M, Kishi R. A review of recent studies on bisphenol A and phthalate exposures and child neurodevelopment [J]. International Journal of Environmental Research and Public Health, 2021, 18(7): 3585
[15] Zhao G F, He F, Wu C L, et al. Betaine in inflammation: Mechanistic aspects and applications [J]. Frontiers in Immunology, 2018, 9: 1070
[16] Abedi F, Razavi B M, Hosseinzadeh H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects [J]. Phytotherapy Research, 2020, 34(4): 729-741
[17] 袁圣武, 黄超, 季晓亚, 等. 环境污染物导致氧化应激的关键信号通路及其检测方法[J]. 生态毒理学报, 2017, 12(1): 25-37
Yuan S W, Huang C, Ji X Y, et al. Main signaling pathways and detection methods of oxidative stress caused by environmental pollutants [J]. Asian Journal of Ecotoxicology, 2017, 12(1): 25-37 (in Chinese)
[18] Zhu Y, Zhang Y K, Li Y B, et al. Integrative proteomics and metabolomics approach to elucidate metabolic dysfunction induced by silica nanoparticles in hepatocytes [J]. Journal of Hazardous Materials, 2022, 434: 128820
[19] Bordin D L, Lirussi L, Nilsen H. Cellular response to endogenous DNA damage: DNA base modifications in gene expression regulation [J]. DNA Repair, 2021, 99: 103051
[20] Kanwar M K, Xie D L, Yang C, et al. Melatonin promotes metabolism of bisphenol A by enhancing glutathione-dependent detoxification in Solanum lycopersicum L. [J]. Journal of Hazardous Materials, 2020, 388: 121727
[21] Wang H O, Zhao P Q, Huang Q S, et al. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons [J]. Chemosphere, 2019, 229: 618-630
[22] Le J H, Lei X C, Ren Y P, et al. Exogenous oestradiol benzoate induces male mice azoospermia through modulation of oxidative stress and testicular metabolic cooperation [J]. Molecular Medicine Reports, 2019, 19(6): 4955-4963
[23] Gómez-Roig M D, Pascal R, Cahuana M J, et al. Environmental exposure during pregnancy: Influence on prenatal development and early life: A comprehensive review [J]. Fetal Diagnosis and Therapy, 2021, 48(4): 245-257
[24] Prins J R, Schoots M H, Wessels J I, et al. The influence of the dietary exposome on oxidative stress in pregnancy complications [J]. Molecular Aspects of Medicine, 2022, 87: 101098
[25] Deng Q Y, Yin N L, Chen Y, et al. Downregulated N-acetylglucosaminyltransferase Ⅲ is involved in attenuating trophoblast migration and invasion under hypoxia-reoxygenation condition [J]. The Journal of Maternal-Fetal &Neonatal Medicine, 2019, 32(14): 2369-2375
[26] Tang C L, Liang J, Qian J F, et al. Opposing role of JNK-p38 kinase and ERK1/2 in hydrogen peroxide-induced oxidative damage of human trophoblast-like JEG-3 cells [J]. International Journal of Clinical and Experimental Pathology, 2014, 7(3): 959-968
[27] Curtis S, Jones C J P, Garrod A, et al. Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction [J]. The Journal of Maternal-Fetal &Neonatal Medicine, 2013, 26(4): 339-346
[28] Jones M L, Mark P J, Waddell B J. Maternal dietary omega-3 fatty acids and placental function [J]. Reproduction, 2014, 147(5): R143-R152
[29] Karamali M, Dastyar F, Badakhsh M H, et al. The effects of selenium supplementation on gene expression related to insulin and lipid metabolism, and pregnancy outcomes in patients with gestational diabetes mellitus: A randomized, double-blind, placebo-controlled trial [J]. Biological Trace Element Research, 2020, 195(1): 1-8
[30] Wu M F, Wu Y, Xu K Z, et al. Protective effects of 1, 25 dihydroxyvitamin D3 against high-glucose-induced damage in human umbilical vein endothelial cells involve activation of Nrf2 antioxidant signaling [J]. Journal of Vascular Research, 2021, 58(4): 267-276