苯并三唑类紫外光稳定剂(benzotriazole ultraviolet stabilizers, BUVSs)因具有良好的紫外吸收能力以及抗氧化性能,被作为添加剂广泛应用于塑料制品和个人护理品中[1-2]。由于需求量大、生产量高,近年来在多种环境介质中被频繁检出[3-4],成为一种新污染物,其在地表水中的浓度高达2 419 ng·L-1,在沉积物和污泥中的浓度高达216.2 μg·g-1[2, 5-8]。BUVSs亲脂性较高,其辛醇水分配系数(log Kow)为4.31~7.67,具有生物富集性[9]。此外,BUVSs具有潜在的环境持久性,其在环境中的半衰期为75~328 d[3, 10-11]。在沿食物链放大和生物转化的过程中,BUVSs可能对鱼类等高等水生生物产生较高的暴露风险和毒性效应[12]。
已有研究报道BUVSs可通过影响水生生物的抗氧化系统,引起氧化损伤[13-15]。同时,体内和体外实验表明BUVSs具有配体活性,能与雌激素受体(estrogen receptor, ER)、雄激素受体(androgen receptor, AR)和芳香烃受体(aryl hydrocarbon receptor, AhR)等多种核激素受体结合,具有内分泌干扰效应[16-17]。Liang等[18]评估了4种BUVSs(UV-234、UV-326、UV-329和UV-P)在1、10和100 μg·L-1暴露96 h后对斑马鱼胚胎的毒性效应,观察到甲状腺激素受体基因的转录水平受到影响,表明BUVSs可能通过影响斑马鱼胚胎甲状腺系统导致内分泌失衡。Fent等[19]将斑马鱼胚胎分别暴露于16 μg·L-1和690 μg·L-1的UV-P和UV-326中6 d后,发现代谢相关基因(ahr1、arnt2、cyp1a1和gstp1)表达具有显著变化,在690 μg·L-1的浓度下具有较高的抗雄激素活性,其结果揭示了BUVSs暴露导致斑马鱼代谢稳态失衡和发育毒性。鱼类的内分泌系统主要通过下丘脑-垂体控制的相关作用轴调控各种生理功能,从而影响鱼类的生长发育和代谢[20]。具有内分泌干扰效应的外源化合物能够激活鱼类体内激素受体信号通路,诱导免疫细胞增殖或功能损伤,从而导致免疫刺激或免疫抑制[21-22]。近年来,已有研究报道了BUVSs对斑马鱼早期发育阶段的免疫毒性。如Liang等[23]发现斑马鱼胚胎分别暴露于0.01、0.1和1 μmol·L-1的UV-234和UV-320中6 d后,免疫细胞因子il8和cxcl-C1c的基因表达显著抑制,且斑马鱼胚胎发育、运动行为和线粒体生物能均受到不同程度的干扰。李雯镜[24]比较了4种BUVSs(UV-234、UV-326、UV-329和UV-P)对斑马鱼幼鱼转录组的影响,结果发现暴露28 d后,4种BUVSs共同影响斑马鱼的免疫系统相关通路。同时,在10 μg·L-1和100 μg·L-1浓度下斑马鱼仔鱼AhR-IL17/IL22介导的免疫通路相关分子受到显著影响[25]。这些研究表明,BUVSs可能通过干扰斑马鱼先天免疫系统引起免疫毒性。然而,目前对BUVSs免疫毒性的研究主要集中于免疫相关因子的基因表达水平,同时,由于斑马鱼早期发育阶段只有先天免疫,并不具备成熟的获得性免疫,因此,BUVSs长期暴露能否干扰斑马鱼成鱼的免疫系统并产生不利影响,需进一步研究。
因此,为了探究不同结构的BUVSs对斑马鱼成鱼的免疫毒性,将斑马鱼成鱼暴露于4种典型BUVSs(UV-234、UV-326、UV-329和UV-P)中28 d,首先通过组织病理学观察初步判别BUVSs长期暴露对斑马鱼成鱼肠道组织的影响,然后测定不同免疫器官(肠道、肾脏和血液)中芳香烃受体(aryl hydrocarbon receptor, AhR)介导的免疫通路相关因子的含量,评估不同结构BUVSs对斑马鱼成鱼的免疫毒性效应。
1 材料与方法(Materials and methods)
1.1 实验动物
野生AB型斑马鱼购自商业公司(一树梨花,南京,中国)并饲养于实验室流水养殖系统内(Z-A-S5,海圣,上海)。养殖系统的光/暗周期设置为14 h∶10 h。控制养殖水条件:水温(28±1) ℃,pH 7.2±0.1,溶解氧(8.0±0.5) mg·L-1,电导率为(500±50) μS·cm-1。每天投喂丰年虾活体(Artemia naupli),同时清除系统内的食物残渣和粪便。
1.2 试剂
UV-234(CAS号70321-86-7;纯度>99%)、UV-326(CAS号3896-11-5;纯度>99%)、UV-329(CAS号3147-75-9;纯度>99%)和UV-P(CAS号2440-224;纯度>99%)均购自美国百灵威(J&K)化工有限公司;内标Allyl-bzt(2-(3-allyl-2-hydroxy-5-methylphenyl)-benzotriazole, 2-(3-烯丙基-2-羟基-5-甲基苯基)-2H-苯并噻唑, CAS号2170-39-0,纯度>98%)购自TCI;斑马鱼白细胞介素IL-17A、IL-22、细胞色素P4501A1(CYP1A1)、芳香烃受体(AhR)、免疫球蛋白Ig-M、Ig-G酶联免疫吸附分析试剂盒均购自江苏酶免实业有限公司(中国)。液相色谱级甲醇、二氯甲烷由默克公司(Merck,德国)提供。
1.3 实验方法
1.3.1 暴露液配制
基于Li等[25]对4种BUVSs暴露后斑马鱼幼鱼免疫毒性的研究和李雯镜等[3]对苯并三唑类紫外稳定剂在环境中的检测和分布等调查,选取10 μg·L-1为暴露浓度。以DMSO为助溶剂制备10 mg·L-1的各BUVSs储备液。暴露实验开始前按照1 mL BUVSs储备液∶1 L养殖水的比例加入相应体积的BUVSs储备液,使暴露溶液的终浓度为10 μg·L-1 BUVSs。实验以0.1% DMSO (V∶V=1∶1 000)作为溶剂对照组。
1.3.2 斑马鱼成鱼暴露
暴露实验前,斑马鱼(~3月鱼龄,野生AB型)已在养殖系统中驯化2周,随机挑选健康成鱼并分别置于15个3 L烧杯中,每个烧杯15条鱼。以含0.1% DMSO的溶液为空白对照,10 μg·L-1的4种BUVSs为暴露组,共5个处理组,每个处理组设置3个平行(n=3)。进行28 d半静态暴露,每天更新90%的暴露液,暴露期间斑马鱼养殖条件与驯化期间相同,暴露结束前1 d不再投喂。将斑马鱼成鱼置于冰上麻醉并解剖,用肝素钠浸泡过的毛细管进行尾部取血,取每10条鱼的血液混合为一个生物样本,每个处理组3个生物学重复(n=3)。每个处理组各取3条鱼的肠道,立即放入组织固定液以便进行后续组织病理学分析(n=3)。每个烧杯中,收集5条鱼的肠道或肾脏组织作为一个生物学样本,每个处理组3个生物学重复(n=3),组织样本用液氮速冻并在-80 ℃保存以用于后续的酶活分析。
1.3.3 暴露液浓度测定
化合物浓度分析测定参照文献报道的方法[2-3, 26]。暴露期间于4个不同时间点,从每个处理组(n=3)中分别采集250 mL水样(暴露液更新后取样,-20 ℃避光保存),将4次采集的暴露液混合为1 L,加入内标Allyl-bzt使其在待处理水样中浓度为10 μg·L-1。水样经0.22 μm的玻璃纤维滤膜(Whatman,英国)过滤后用固相萃取柱(HLB, Waters,美国)进行富集。HLB柱使用前依次加入6 mL二氯甲烷,6 mL甲醇和6 mL纯水进行活化,活化后水样以10 mL·min-1流速经过HLB柱,再用6 mL纯水润洗HLB柱,真空干燥30 min。分别用2 mL甲醇/二氯甲烷(V∶V=1∶1)溶液洗脱3次,共收集6 mL洗脱液至玻璃试管中,然后用高纯度氮气吹干。管中加入1 mL甲醇复溶,转移至检测瓶使用超高效液相质谱联用仪(ACQUITY H-class UPLC;Xevo TQD,美国)分析。色谱柱为Shim-Pack Scepter C18(4.6 mm × 50 mm,3 μm,SHIMADZU)。检测条件见表1,采用内标法进行定量分析。
表1 UPLC-MS检测条件
Table 1 Test conditions of UPLC-MS
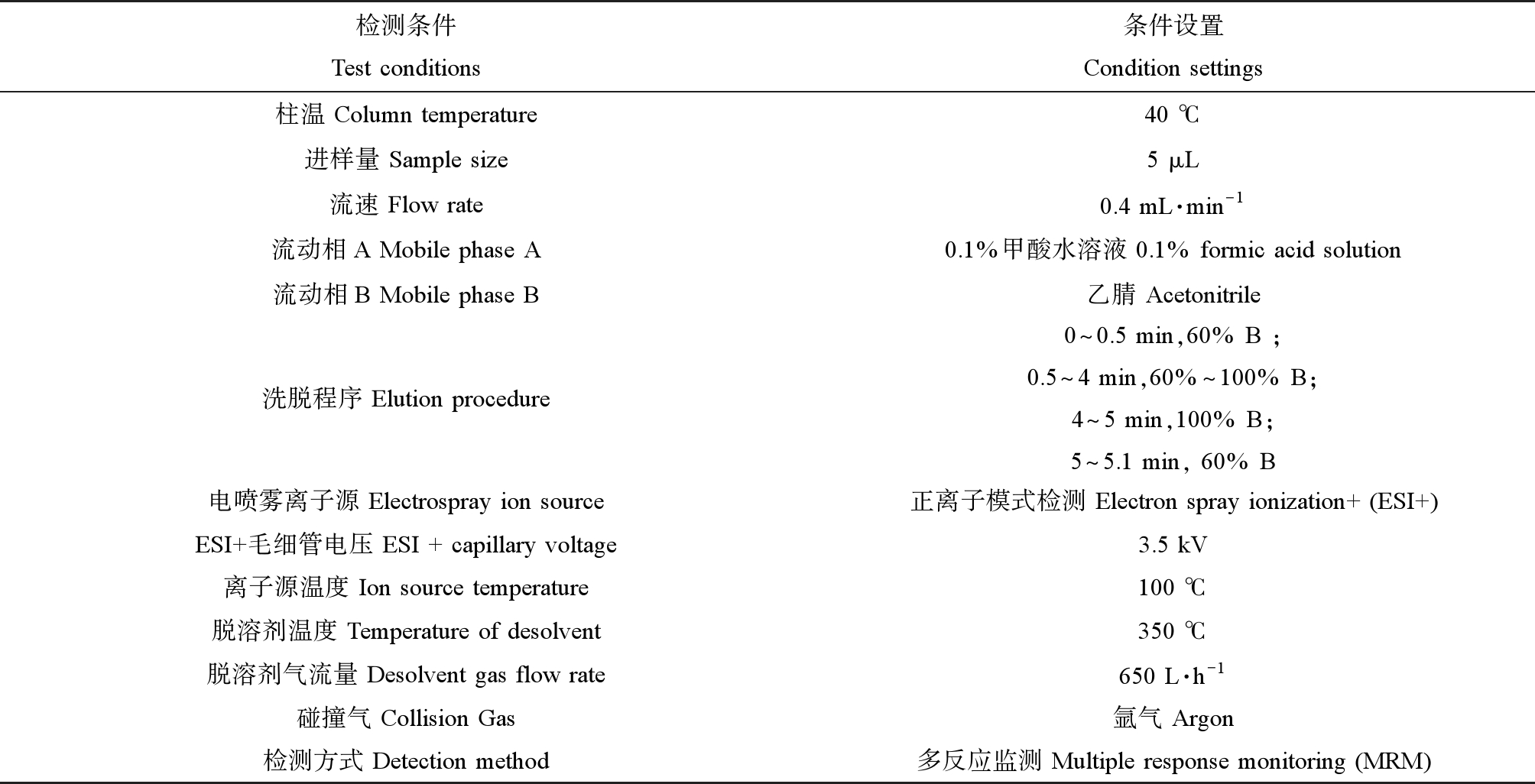
检测条件Test conditions条件设置Condition settings柱温 Column temperature40 ℃进样量 Sample size5 μL流速 Flow rate0.4 mL·min-1流动相A Mobile phase A0.1%甲酸水溶液 0.1% formic acid solution流动相B Mobile phase B乙腈 Acetonitrile洗脱程序 Elution procedure0^0.5 min,60% B ;0.5^4 min,60%^100% B;4^5 min,100% B;5^5.1 min, 60% B电喷雾离子源 Electrospray ion source正离子模式检测 Electron spray ionization+ (ESI+)ESI+毛细管电压 ESI + capillary voltage3.5 kV离子源温度 Ion source temperature100 ℃脱溶剂温度 Temperature of desolvent350 ℃脱溶剂气流量 Desolvent gas flow rate650 L·h-1碰撞气 Collision Gas氩气 Argon检测方式 Detection method多反应监测 Multiple response monitoring (MRM)
1.3.4 组织病理学观察
肠道组织置于4%多聚甲醛固定液中固定,乙醇中脱水后进行石蜡包埋。制成厚度为4 μm的切片,用苏木精和伊红染色(H&E染色)。在显微镜下(Olympus SZX2-ILLB,日本)随机选取3个视野镜检并拍照。组织损伤半定量分析使用ImageJ图像处理软件进行,首先对拍摄的图像进行灰度处理,然后使用ImageJ的计数功能对每张图像中观察到的杯状细胞进行计数,并使用ImageJ的测量功能计算不同处理组肠道组织中黏液量(占肠道组织区域面积的百分比)、肠绒毛高度和肌层厚度(n=9)。以平均数差异显著性T检验,判断肠道损伤情况,P< 0.05表示具有统计学意义。
1.3.5 酶联免疫吸附测定
取适量组织样本放置在1.5 mL离心管中,按照质量(g)∶体积(mL)=1∶9的比例,将PBS(1×)添加到不同组织样本(肠道、肾脏和血液)中,随后将样品放置在冰上进行匀浆,随后于4 ℃、2 500 r·min-1条件下离心10 min,取上清液用于ELISA酶活测试。根据试剂盒说明书测定样本的总蛋白含量、AhR含量、CYP1A1活性、IL17A、IL22、Ig-G和Ig-M含量。各蛋白的检出限分别为AhR(25~800 pg·mL-1)、CYP1A1(3~150 IU·L-1)、IL17A(0.25~9 ng·L-1)、IL22(1~40 ng·L-1)、Ig-G(1~32 μg·mL-1)和Ig-M(0.1~3.5 μg·mL-1)。每个平行组之间的平均变异系数和板内变异系数的平均值分别为<10%和<12%。
1.4 数据分析
使用GraphPad Prism 9进行统计学分析。采用Kolmogorov-Smirnov和Levene检验分别验证数据正态性和方差齐性,数据分析采用ANOVA单因素方差分析和Holm-Sidak多重比较试验。定量数据表示为平均值±标准误差(mean±SEM),P<0.05表示显著差异。
2 结果(Results)
2.1 BUVSs浓度分析
BUVSs化合物定量离子对(m/z)、出峰时间分别为UV-234(448.2>370.2,1.001~1.135 min)、UV-326(316.3>260.1,0.702~0.902 min)、UV-329(324.2>212.1,1.838~1.905 min)和UV-P(225.9>120.0,0.602~0.668 min),如图1所示,方法检出限、定量限分别为UV-234(0.25 μg·L-1, 0.75 μg·L-1)、UV-326(0.57 μg·L-1, 1.70 μg·L-1)、UV-329(0.35 μg·L-1, 0.92 μg·L-1)和UV-P(0.61 μg·L-1, 1.25 μg·L-1)。每个处理组检测到的暴露液浓度如表2所示,由于实际浓度与名义浓度接近(误差<20%),因此下文以BUVSs的名义浓度作为暴露浓度。
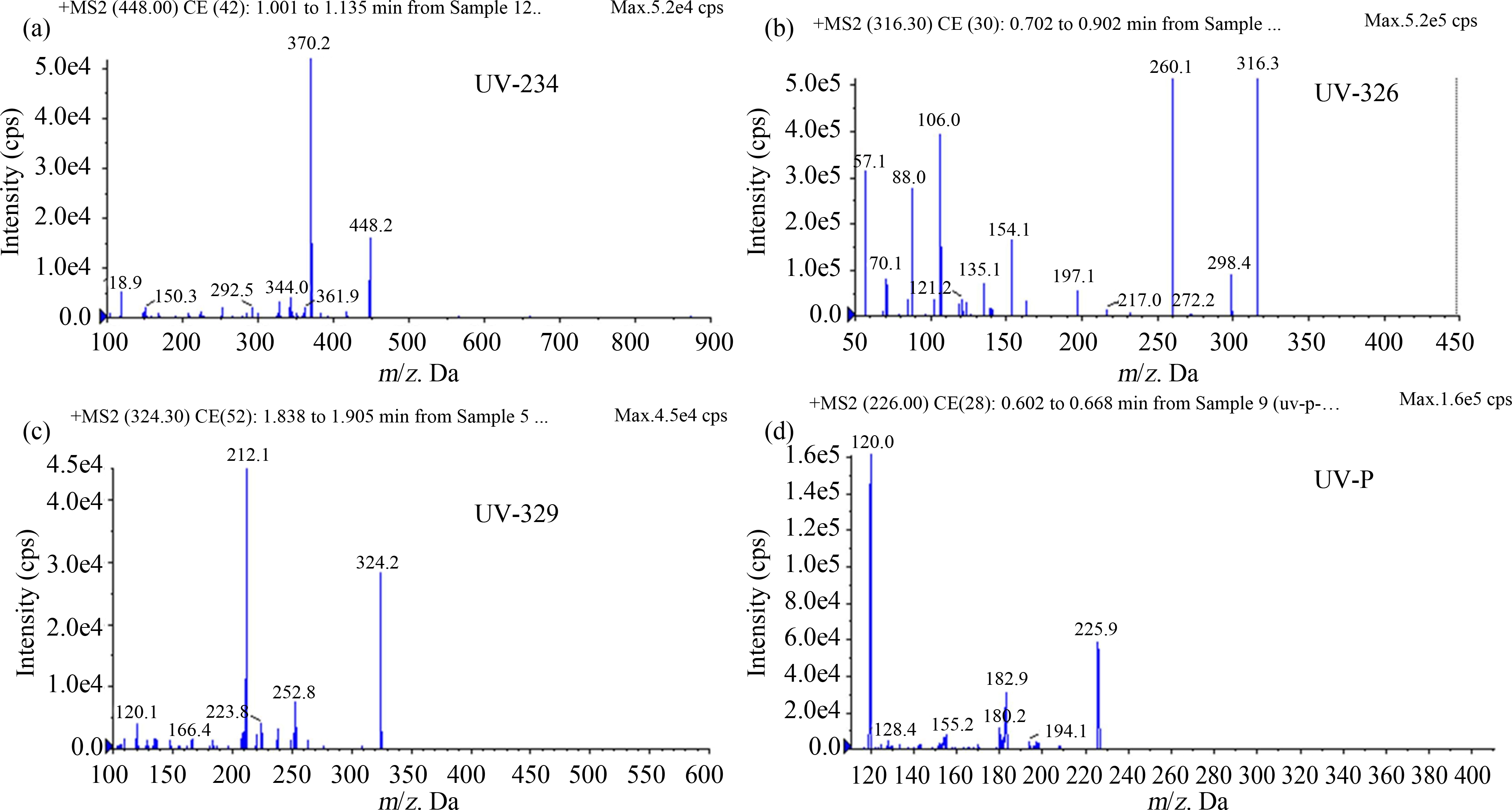
图1 苯并三唑类紫外光稳定剂(BUVSs)离子对和出峰时间
注:(a) UV-234;(b) UV-326;(c) UV-329;(d) UV-P。
Fig. 1 Ion-pair and peak emergence time of benzotriazole ultraviolet stabilizers (BUVSs)
Note: (a) UV-234; (b) UV-326; (c) UV-329; (d) UV-P.
表2 BUVSs暴露液实际浓度
Table 2 BUVSs concentration measured in the exposure solutions

注:ND表示未检测出;数据以平均值±标准偏差表示。
Note: ND stands for not detected; the data are expressed as means±standard deviations.
条件ConditionsControlUV-234UV-326UV-329UV-P名义浓度/(μg·L-1)Nominal concentration/(μg·L-1)010101010实际浓度/(μg·L-1)Measured concentration/(μg·L-1)ND9.73±0.219.18±0.579.67±0.838.47±0.39
2.2 BUVSs破坏斑马鱼成鱼的肠道屏障
在28 d暴露实验期间,斑马鱼成鱼的死亡率和畸形率均无显著变化。然而,10 μg·L-1的4种BUVSs暴露后,斑马鱼肠道均出现不同程度的损伤,包括杯状细胞数量减少、肠道黏液增多、肠绒毛部分脱落以及肠绒毛高度降低等(图2)。在UV-234处理组中,杯状细胞数量减少36%(F (4, 40)=28.23, P<0.0001)(图2(b)和图3(a)),黏液量增加46.8%(F (4, 40)=16.27, P<0.0001)(图2(b)和图3(d));在UV-326暴露组中,部分肠绒毛出现组织溶解现象(图2(c));而在UV-329和UV-P处理组中,肠绒毛平均高度均显著降低,分别由32.9 μm降低到24.69 μm(F (4, 40)=17.18, P<0.0001)和24.86 μm(F (4, 40)=17.18, P<0.0001)(图2(d)和图2(e),图3(b))。这些结果表明4种BUVSs均能破坏斑马鱼肠道屏障。

图2 BUVSs对斑马鱼成鱼肠道的组织病理学分析
注:(a) 0.1% DMSO,(b) 10 μg·L-1 UV-234,(c) 10 μg·L-1 UV-326,(d) 10 μg·L-1 UV-329,(e) 10 μg·L-1 UV-P;黑色箭头表示杯状细胞,红色箭头表示肠道黏液,黄色双向箭头表示肠绒毛高度,蓝色圆圈表示肠绒毛溶解;比例尺为20 μm。
Fig. 2 Histological analysis of the intestine of adult zebrafish following BUVSs exposure
Note: (a) 0.1% DMSO, (b) 10 μg·L-1 UV-234, (c) 10 μg·L-1 UV-326, (d) 10 μg·L-1 UV-329, (e) 10 μg·L-1 UV-P; black arrows indicate the goblet cell; red arrow indicates the mucus; yellow arrows indicate the villus height; blue circles indicate damaged and partially dissolved villi; scale bar is 20 μm.
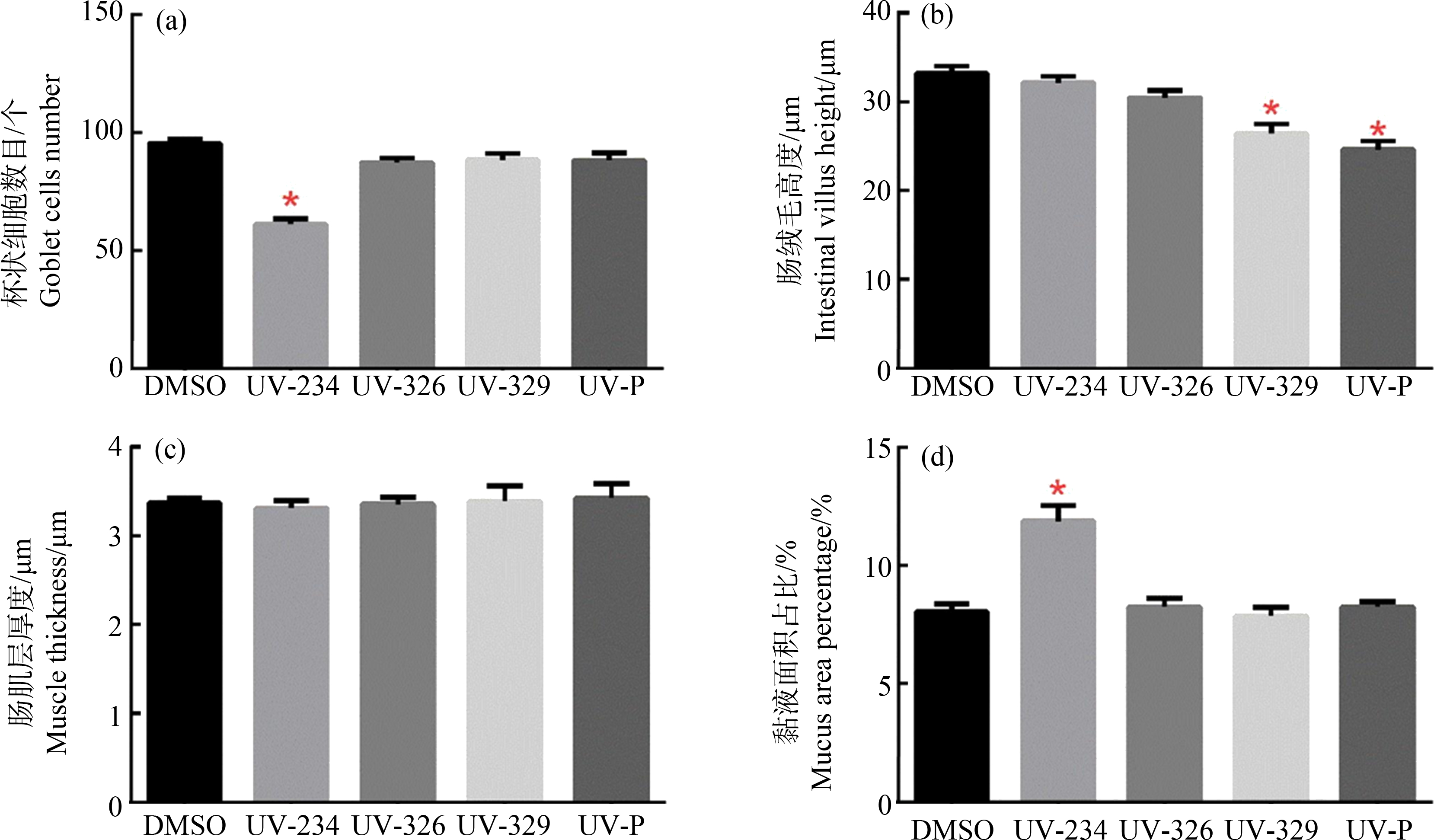
图3 10 μg·L-1 BUVSs暴露对肠道组织表型指标水平影响
注:(a) 杯状细胞数目,(b) 肠绒毛高度,(c) 肠肌层厚度,(d) 黏液占肠切面百分比;所有定量数据均以平均数±SEM表示;*表示暴露组和对照组之间有显著差异(P<0.05)。
Fig. 3 Semi-quantitation of histological changes in intestines of adult zebrafish following 10 μg·L-1 BUVSs exposure
Note: (a) Number of goblet cells, (b) intestinal villus height, (c) muscle thickness, (d) percentage of mucus in intestinal section; all quantitative data are expressed as mean±SEM; the asterisk (*) indicates a significant difference between treatments and controls (*P<0.05).
2.3 BUVSs对斑马鱼免疫相关蛋白的影响
为了进一步研究BUVSs对斑马鱼成鱼的免疫毒性,选取6个免疫相关蛋白(IL-17A、IL-22、CYP1A1、AhR、Ig-G和Ig-M)进行ELISA酶活测试。结果显示,暴露28 d后,不同结构的BUVSs处理组中AhR、CYP1A1、IL-17A和IL-22的表达出现显著差异,同时呈现组织特异性(图4)。在肠道中,UV-234和UV-326处理组中AhR的表达量分别上调1.5倍(F(4, 10)=7.66, P=0.05)和1.53倍(F(4, 10)=7.66, P=0.04),而UV-329和UV-P暴露组无显著差异(图4(a))。另外,IL-22在UV-234和UV-P暴露后分别增加1.75倍(F(4, 10)=3.86, P=0.04)和1.85倍(F(4, 10)=3.86, P=0.02),而其他2个处理组无变化(图4(d))。

图4 10 μg·L-1 BUVSs暴露对斑马鱼肠道、肾脏组织和血液免疫相关蛋白表达的影响
注:测试指标包括AhR、CYP1A1、IL-17A和IL-22分别在肠道组织中(a)~(d)和肾脏组织(e)~(h)中的含量或活性,以及血液中(i)Ig-M和(j)Ig-G的含量;数据以平均值±标准误差表示;*表示实验组与对照组之间有显著性差异(*P<0.05)。
Fig. 4 Effects of 10 μg·L-1 BUVSs exposures on the levels of proteins related to immune response
Note: The biomarkers included the content or activity of AhR, CYP1A1, IL-17A, and IL-22 in intestine (a)~(d) and kidney (e)~(h), as well as the content of (i) Ig-M and (j) Ig-G in serum; the data are expressed as mean±SEM, and asterisk denotes significant differences (*P< 0.05) between treatments and controls.
在肾脏中,除UV-234外,UV-326、UV-329和UV-P暴露组中CYP1A1的活性均受到显著抑制,分别下降了1.26倍(F(4, 10)=23.65, P=0.01)、1.44倍(F(4, 10)=23.65, P=0.0006)和1.58倍(F(4, 10)=23.65, P=0.0002)(图3(f))。同时,UV-326暴露后,IL-17A表达量显著上调1.42倍(F(4, 10)=5.23, P=0.02)(图4(g))。
4种BUVSs暴露后血液中Ig-M含量均无显著变化(图4(i)),仅在UV-P暴露组中检测到Ig-G含量显著上调1.35倍(F(4, 10)=13.59, P=0.0005)(图4(j))。
3 讨论(Discussion)
鱼类肠道是抵抗复杂外部环境压力和病原微生物的最大免疫界面[27],同时也是外源性化合物进入鱼体的重要途径[28]。肠道表面的微绒毛显著增加了肠道表面积,有利于营养物质的吸收和肠道微生物的附着,同时,肠绒毛强烈而规律的运动有助于阻止有害细菌定植。鱼类肠道中的杯状细胞可分泌黏蛋白,是黏液细胞的主要类型[29]。杯状细胞和分泌的黏液在抵抗病原微生物入侵和保护肠黏膜免受损伤等方面起着重要作用[30]。本研究中,在10 μg·L-1 BUVSs暴露后,斑马鱼成鱼的肠道组织出现杯状细胞减少、黏液增加和肠绒毛高度下降等现象。与本研究结果类似,Jin等[30]发现斑马鱼成鱼暴露于1 000 μg·L-1的直径50 μm的球形聚苯乙烯微塑料(microplastics, MP)14 d后,出现肠道中杯状细胞减少和肠腔黏液体积增加的现象,并诱导肠道微生物群失调且诱发肠道炎症反应。Huang等[31]用将斑马鱼成鱼分别暴露于1 μmol·L-1 PFOS(perfluorooctane sulfonate)、F-53B(6:2 chlorinated polyfluorinated ether sulfonate, 6:2 Cl-PFAES)和OBS(sodium p-perfluorous nonenoxybenzene sulfonate)中21 d,发现3种PFAS(per- and polyfluoroalkyl substances)暴露后均降低斑马鱼成鱼肠绒毛高度,F-53B和OBS处理组更具有显著性,同时观察到头肾形态改变和肠道微生物群失调,提示PFAS具有免疫毒性作用。本研究中,肠道组织学分析结果表明斑马鱼成鱼肠道组织可能是BUVSs毒性的靶标,BUVSs暴露能够引起斑马鱼成鱼肠道损伤,可能干扰肠道屏障功能和免疫稳态。
肠道受到感染或损伤时,机体启动免疫应答机制,激活免疫效应细胞并分泌促炎和抗炎细胞因子,通过炎症反应介导细胞凋亡以清除外来有害物质并修复受损细胞和组织[32-33]。细胞因子是免疫系统中细胞间相互作用的信号分子,与细胞膜上受体结合后发挥多种生物效应,在免疫应答、免疫调节和炎症反应中发挥重要作用[34]。很多环境污染物具有免疫毒性,长期暴露后可干扰细胞因子及其相关的生物学通路,导致炎症反应异常或慢性免疫抑制,从而导致免疫失衡[31, 35-37]。已有研究报道,BUVSs对AhR具有显著的配体活性[38],并可激活斑马鱼的AhR通路[19]。笔者先前的研究也表明不同结构的BUVSs均能干扰斑马鱼胚胎和幼鱼免疫相关通路,可能作用于AhR-IL17/22通路并诱导肝脏损伤[25, 39]。上述研究均表明AhR介导的细胞因子途径可能是BUVSs暴露后的免疫毒性机制。AhR是炎症反应中调节组织稳态的关键因子[40-41],AhR信号被激活后,通过促炎因子直接或间接参与转录调控,以维持免疫屏障和控制炎症反应[42]。而在AhR信号通路调节过程中,CYP1类酶,特别是CYP1A1参与生理性AhR配体的降解,提供自动调节反馈机制,终止AhR信号传导并防止外源性物质诱导的免疫抑制[43]。有研究发现,通过腹腔注射将青鳉鱼(Oryzias latipes)暴露于2 μg·g-1 (以体质量计)苯并[a]蒽(benzo[a]pyrene, BaP)后,CYP1A活性受到显著诱导,免疫细胞数量增加,结果表明BaP诱导的免疫毒性依赖于AhR通路的激活或CYP1A介导,而由于苯并[e]芘(benzo[e]pyrene, BeP)不具AhR配体亲和力,因此不能激活AhR通路,未诱导CYP1A活性变化且未影响青鳉鱼的免疫功能[44]。本研究中10 μg·L-1 UV-234和UV-326暴露均增加了斑马鱼成鱼肠道中AhR蛋白的表达,表明UV-234和UV-326可能通过AhR途径影响肠道免疫。同时,除UV-234外,其他3种BUVSs均抑制了斑马鱼肾脏中CYP1A1的表达,这表明UV-326可能通过影响AhR-CYP1A1的相互作用调节斑马鱼免疫系统,而UV-329和UV-P可能通过影响CYP1A1干扰免疫响应。
白细胞介素是一种参与免疫调节和炎症反应的细胞因子,AhR信号的激活可诱导Th17细胞的分化,产生白细胞介素IL-17和IL-22,进而增强体内Th17细胞介导的自身免疫[45]。研究表明,在获得性免疫的发展过程中,IL-17和IL-22的上调可能刺激嗜中性粒细胞的募集,从而诱导炎症[25]。Li等[25]发现BUVSs暴露后,il17a、il22基因和IL-22蛋白表达水平显著下调,可能通过干扰AhR-IL17/IL22通路对斑马鱼仔鱼产生免疫毒性。另有研究表明肠道通透性和促炎细胞因子之间存在正相关性[46]。机体在炎症状态下会产生大量的IL-22,IL-22通过刺激杯状细胞产生黏蛋白形成黏液层,发挥分离肠道内细菌并维持肠道上皮细胞完整性的作用,并能通过调控肠道菌群来降低结肠炎的发生概率[47]。本研究结果显示,UV-234和UV-P暴露导致斑马鱼成鱼肠道IL-22表达上调,而UV-326暴露后斑马鱼肾脏组织中IL-17A蛋白表达显著上调,这些结果表明BUVSs在分子水平上引起免疫应激,可能诱导炎症反应。
在本研究中,免疫相关蛋白在斑马鱼成鱼肠道组织和肾脏组织中的差异表达呈现组织特异性。肠道是鱼类免疫细胞相互作用和抗原呈递的主要场所,肠道相关淋巴组织分布在整个肠黏膜[48]。而肾脏是鱼体内代谢物排泄的主要器官,肾脏中的内皮细胞和巨噬细胞具有高度内吞作用,在清除代谢废物的过程中发挥重要作用[49]。研究报道,BUVSs的生物富集在斑马鱼体内具有组织特异性,BUVSs在肾脏中的生物浓缩系数BCF(bioconcentration factor)高于肠道,更易于在肾脏中蓄积[50]。本研究中,斑马鱼成鱼经BUVSs暴露后,肠道中CYP1A1的活性未发生显著变化,而肾脏中CYP1A1的活性受到显著抑制,这可能是由于BUVSs在肾脏中的大量富集和代谢导致的。
另外,鱼类血浆中的免疫球蛋白是其体液免疫系统的主要成分,由B淋巴细胞产生,专门用于维持内环境稳定[51]。未成熟的B细胞表面只有单体Ig-M,当B细胞识别并激活抗原时,发生类别转换,B细胞开始制造并分泌Ig-G。Ig-M和Ig-G都具有中和活性,能够帮助鱼类抵抗病毒和细菌病原体,Ig-M对鱼类维持黏膜部位微生物群落稳态具有重要作用,Ig-G能帮助免疫细胞增强吞噬能力[52]。本研究中10 μg·L-1 UV-P暴露后,斑马鱼成鱼血液中Ig-G的表达量显著增加,表明UV-P激活了斑马鱼的适应性免疫。
综上所述,BUVSs长期暴露可干扰斑马鱼成鱼免疫相关因子的表达,引起肠道损伤,具有免疫毒性。同时,不同结构的BUVSs其免疫毒性机制可能不同。UV-234可能通过增加肠道AhR的表达并促进分泌IL-22干扰斑马鱼免疫系统,引起肠道损伤;而UV-326通过激活AhR,促进肾脏IL-17A的表达并抑制下游CYP1A1活性引起免疫应激;UV-P可能通过抑制CYP1A1活性,促进细胞因子IL-22表达,增加免疫球蛋白Ig-G的表达影响斑马鱼的免疫系统功能;UV-329可能通过抑制CYP1A1活性引起代谢异常,导致肠道损伤。但不同结构的BUVSs对斑马鱼成鱼的免疫毒性效应需结合其他生物学指标进一步评估。
通信作者简介:梁雪芳(1987—),女,博士,副教授,硕士生导师,主要研究方向为水生态毒理学。
[1] Giokas D L, Salvador A, Chisvert A. UV filters: From sunscreens to human body and the environment [J]. TrAC Trends in Analytical Chemistry, 2007, 26(5): 360-374
[2] Ruan T, Liu R Z, Fu Q, et al. Concentrations and composition profiles of benzotriazole UV stabilizers in municipal sewage sludge in China [J]. Environmental Science &Technology, 2012, 46(4): 2071-2079
[3] 李雯镜, 李志彤, 梁雪芳. 苯并三唑类紫外稳定剂在环境中的检测、分布及其毒性效应[J]. 生态毒理学报, 2018, 13(1): 89-105
Li W J, Li Z T, Liang X F. Detection, distribution and toxicological effects of benzotriazole UV stabilizers [J]. Asian Journal of Ecotoxicology, 2018, 13(1): 89-105 (in Chinese)
[4] 仝天衡, 杨慧婷, 陈辉辉, 等. 紫外吸收剂在湖泊中的分布及其对底栖动物的毒性效应[J]. 生态毒理学报, 2019, 14(3): 1-17
Tong T H, Yang H T, Chen H H, et al. Distribution of UV absorbers in lake environment and their toxicological effects on benthic animals [J]. Asian Journal of Ecotoxicology, 2019, 14(3): 1-17 (in Chinese)
[5] Montesdeoca-Esponda S, Torres-Padrón M E, Sosa-Ferrera Z, et al. Fate and distribution of benzotriazole UV filters and stabilizers in environmental compartments from Gran Canaria Island (Spain): A comparison study [J]. The Science of the Total Environment, 2021, 756: 144086
[6] Tang Z W, Han X, Li G H, et al. Occurrence, distribution and ecological risk of ultraviolet absorbents in water and sediment from Lake Chaohu and its inflowing rivers, China [J]. Ecotoxicology and Environmental Safety, 2018, 164: 540-547
[7] Vimalkumar K, Arun E, Krishna-Kumar S, et al. Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, and fish from Indian rivers [J]. The Science of the Total Environment, 2018, 625: 1351-1360
[8] Zhang Z F, Ren N Q, Li Y F, et al. Determination of benzotriazole and benzophenone UV filters in sediment and sewage sludge [J]. Environmental Science &Technology, 2011, 45(9): 3909-3916
[9] Zhang S Y, Wang Z Y, Chen J W, et al. Tissue-specific accumulation, biotransformation, and physiologically based toxicokinetic modeling of benzotriazole ultraviolet stabilizers in zebrafish (Danio rerio) [J]. Environmental Science &Technology, 2021, 55(17): 11874-11884
[10] Zhao X, Zhang Z F, Xu L, et al. Occurrence and fate of benzotriazoles UV filters in a typical residential wastewater treatment plant in Harbin, China [J]. Environmental Pollution, 2017, 227: 215-222
[11] Lai H J, Ying G G, Ma Y B, et al. Field dissipation and plant uptake of benzotriazole ultraviolet stabilizers in biosolid-amended soils [J]. Environmental Science Processes &Impacts, 2014, 16(3): 558-566
[12] Lu Z, de Silva A O, Peart T E, et al. Distribution, partitioning and bioaccumulation of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in an urban creek in Canada [J]. Environmental Science &Technology, 2016, 50(17): 9089-9097
[13] Giraudo M, Cottin G, Esperanza M, et al. Transcriptional and cellular effects of benzotriazole UV stabilizers UV-234 and UV-328 in the freshwater invertebrates Chlamydomonas reinhardtii and Daphnia magna [J]. Environmental Toxicology and Chemistry, 2017, 36(12): 3333-3342
[14] Hemalatha D, Rangasamy B, Nataraj B, et al. Transcriptional, biochemical and histological alterations in adult zebrafish (Danio rerio) exposed to benzotriazole ultraviolet stabilizer-328 [J]. The Science of the Total Environment, 2020, 739: 139851
[15] He S, Xiao H, Luo S, et al. Benzotriazole ultraviolet stabilizers promote breast cancer cell proliferation via activating estrogen-related receptors α and γ at human-relevant levels [J]. Environmental Science &Technology, 2022, 56(4): 2466-2475
[16] Sakuragi Y, Takada H, Sato H, et al. An analytical survey of benzotriazole UV stabilizers in plastic products and their endocrine-disrupting potential via human estrogen and androgen receptors [J]. The Science of the Total Environment, 2021, 800: 149374
[17] Nagayoshi H, Kakimoto K, Takagi S, et al. Benzotriazole ultraviolet stabilizers show potent activities as human aryl hydrocarbon receptor ligands [J]. Environmental Science &Technology, 2015, 49(1): 578-587
[18] Liang X F, Li J J, Martyniuk C J, et al. Benzotriazole ultraviolet stabilizers alter the expression of the thyroid hormone pathway in zebrafish (Danio rerio) embryos [J]. Chemosphere, 2017, 182: 22-30
[19] Fent K, Chew G, Li J, et al. Benzotriazole UV-stabilizers and benzotriazole: Antiandrogenic activity in vitro and activation of aryl hydrocarbon receptor pathway in zebrafish eleuthero-embryos [J]. The Science of the Total Environment, 2014, 482-483: 125-136
[20] Huang T, Zhao Y H, He J, et al. Endocrine disruption by azole fungicides in fish: A review of the evidence [J]. The Science of the Total Environment, 2022, 822: 153412
[21] Milla S, Depiereux S, Kestemont P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review [J]. Ecotoxicology, 2011, 20(2): 305-319
[22] Yang M, Qiu W H, Chen B, et al. The in vitro immune modulatory effect of bisphenol A on fish macrophages via estrogen receptor α and nuclear factor-κB signaling [J]. Environmental Science &Technology, 2015, 49(3): 1888-1895
[23] Liang X F, Adamovsky O, Souders C L 2nd, et al. Biological effects of the benzotriazole ultraviolet stabilizers UV-234 and UV-320 in early-staged zebrafish (Danio rerio) [J]. Environmental Pollution, 2019, 245: 272-281
[24] 李雯镜. BUVSs对斑马鱼脑组织的毒性效应和作用机制研究[D]. 呼和浩特: 内蒙古大学, 2018: 17-26
Li W J. Toxic effects and mechanisms of BUVSs in zebrafish brain [D]. Hohhot: Inner Mongolia University, 2018: 17-26 (in Chinese)
[25] Li Z T, Liang X F, Liu W, et al. Elucidating mechanisms of immunotoxicity by benzotriazole ultraviolet stabilizers in zebrafish (Danio rerio): Implication of the AHR-IL17/IL22 immune pathway [J]. Environmental Pollution, 2020, 262: 114291
[26] Casado J, Rodríguez I, Carpinteiro I, et al. Gas chromatography quadrupole time-of-flight mass spectrometry determination of benzotriazole ultraviolet stabilizers in sludge samples [J]. Journal of Chromatography A, 2013, 1293: 126-132
[27] MacDonald T T, Monteleone I, Fantini M C, et al. Regulation of homeostasis and inflammation in the intestine [J]. Gastroenterology, 2011, 140(6): 1768-1775
[28] Maharajan A, Kitto M R, Paruruckumani P S, et al. Histopathology biomarker responses in Asian Sea bass, Lates calcarifer (Bloch) exposed to copper [J]. The Journal of Basic &Applied Zoology, 2016, 77: 21-30
[29] Qiao R X, Deng Y F, Zhang S H, et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish [J]. Chemosphere, 2019, 236: 124334
[30] Jin Y X, Xia J Z, Pan Z H, et al. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish [J]. Environmental Pollution, 2018, 235: 322-329
[31] Huang J, Wang Q Y, Liu S, et al. Comparative chronic toxicities of PFOS and its novel alternatives on the immune system associated with intestinal microbiota dysbiosis in adult zebrafish [J]. Journal of Hazardous Materials, 2022, 425: 127950
[32] Na Y R, Stakenborg M, Seok S H, et al. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD [J]. Nature Reviews Gastroenterology &Hepatology, 2019, 16(9): 531-543
[33] Martin S A M, Król E. Nutrigenomics and immune function in fish: New insights from omics technologies [J]. Developmental &Comparative Immunology, 2017, 75: 86-98
[34] Salazar-Mather T P, Hokeness K L. Cytokine and chemokine networks: Pathways to antiviral defense [J]. Current Topics in Microbiology and Immunology, 2006, 303: 29-46
[35] de Farias N O, Oliveira R, Moretti P N S, et al. Fluoxetine chronic exposure affects growth, behavior and tissue structure of zebrafish [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2020, 237: 108836
[36] Zhang L, Hong X S, Zhao X, et al. Exposure to environmentally relevant concentrations of deltamethrin renders the Chinese rare minnow (Gobiocypris rarus) vulnerable to Pseudomonas fluorescens infection [J]. The Science of the Total Environment, 2020, 715: 136943
[37] Liu W, Zhang J Y, Liang X F, et al. Environmental concentrations of 2,4-DTBP cause immunotoxicity in zebrafish (Danio rerio) and may elicit ecological risk to wildlife [J]. Chemosphere, 2022, 308(Pt 3): 136465
[38] Nagayoshi H, Kakimoto K, Takagi S, et al. Benzotriazole ultraviolet stabilizers show potent activities as human aryl hydrocarbon receptor ligands [J]. Environmental Science &Technology, 2015, 49(1): 578-587
[39] 李志彤. 苯并三唑类紫外光稳定剂对斑马鱼的免疫毒性作用研究[D]. 呼和浩特: 内蒙古大学, 2020: 28-35
Li Z T. Immunotoxic effect of BUVSs on Danio rerio [D]. Hohhot: Inner Mongolia University, 2020: 28-35 (in Chinese)
[40] Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology [J]. Pharmacological Reviews, 2015, 67(2): 259-279
[41] Stockinger B, di Meglio P, Gialitakis M, et al. The aryl hydrocarbon receptor: Multitasking in the immune system [J]. Annual Review of Immunology, 2014, 32: 403-432
[42] Rothhammer V, Quintana F J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease [J]. Nature Reviews Immunology, 2019, 19(3): 184-197
[43] Kyoreva M, Li Y, Hoosenally M, et al. CYP1A1 enzymatic activity influences skin inflammation via regulation of the AHR pathway [J]. The Journal of Investigative Dermatology, 2021, 141(6): 1553-1563.e3
[44] Carlson E A, Li Y, Zelikoff J T. The Japanese medaka (Oryzias latipes) model: Applicability for investigating the immunosuppressive effects of the aquatic pollutant benzo[a]pyrene (BaP) [J]. Marine Environmental Research, 2002, 54(3-5): 565-568
[45] Veldhoen M, Hirota K, Westendorf A M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins [J]. Nature, 2008, 453(7191): 106-109
[46] Wang L K, Wang L W, Li X, et al. Ethyl pyruvate prevents inflammatory factors release and decreases intestinal permeability in rats with D-galactosamine-induced acute liver failure [J]. Hepatobiliary &Pancreatic Diseases International, 2013, 12(2): 180-188
[47] Mizuno S, Mikami Y, Kamada N, et al. Cross-talk between RORγt+ innate lymphoid cells and intestinal macrophages induces mucosal IL-22 production in Crohn’s disease [J]. Inflammatory Bowel Diseases, 2014, 20(8): 1426-1434
[48] Wittamer V, Bertrand J Y, Gutschow P W, et al. Characterization of the mononuclear phagocyte system in zebrafish [J]. Blood, 2011, 117(26): 7126-7135
[49] Liu X H, Wu H Z, Liu Q, et al. Profiling immune response in zebrafish intestine, skin, spleen and kidney bath-vaccinated with a live attenuated Vibrio anguillarum vaccine [J]. Fish &Shellfish Immunology, 2015, 45(2): 342-345
[50] 张书莹. 部分有机化学品鱼体生理毒代动力学模型研究[D]. 大连: 大连理工大学, 2021: 88-96
Zhang S Y. Development of physiologically based toxicokinetic models for selective organic chemicals in fish [D]. Dalian: Dalian University of Technology, 2021: 88-96 (in Chinese)
[51] Palaksha K J, Shin G W, Kim Y R, et al. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus) [J]. Fish &Shellfish Immunology, 2008, 24(4): 479-488
[52] Salinas I, Fernández-Montero  , Ding Y, et al. Mucosal immunoglobulins of teleost fish: A decade of advances [J]. Developmental and Comparative Immunology, 2021, 121: 104079
, Ding Y, et al. Mucosal immunoglobulins of teleost fish: A decade of advances [J]. Developmental and Comparative Immunology, 2021, 121: 104079