双酚类化合物(bisphenols)是由2个酚环通过碳桥或其他化学结构连接在一起组成的物质(图1),主要应用于环氧树脂和聚碳酸酯塑料的生产[1]。迄今为止,最广泛使用的双酚类化合物是双酚A(bisphenol A, BPA),目前全球BPA年消费量已超过700万t[2]。大量研究证实,BPA可以与动物内分泌系统的多个受体/蛋白发生相互作用,发挥生殖内分泌干扰作用、甲状腺干扰作用,并具有致肥胖效应,是最经典的环境内分泌干扰物(environmental endocrine disruptors, EEDs)之一[3]。考虑到BPA暴露的广泛性、持续性以及BPA的内分泌干扰作用,一些国家和组织提出了关于BPA使用的限制和法规。例如,加拿大、法国和欧盟分别于2008年、2010年和2011年禁止在婴儿奶瓶中使用BPA;欧盟关于化学品注册、评估、授权和限制的法规(regulation on registration, evaluation, authorization and restriction of chemicals, REACH)规定热敏纸生产过程中BPA使用的最高浓度限值为0.02%(m/m)[4]。
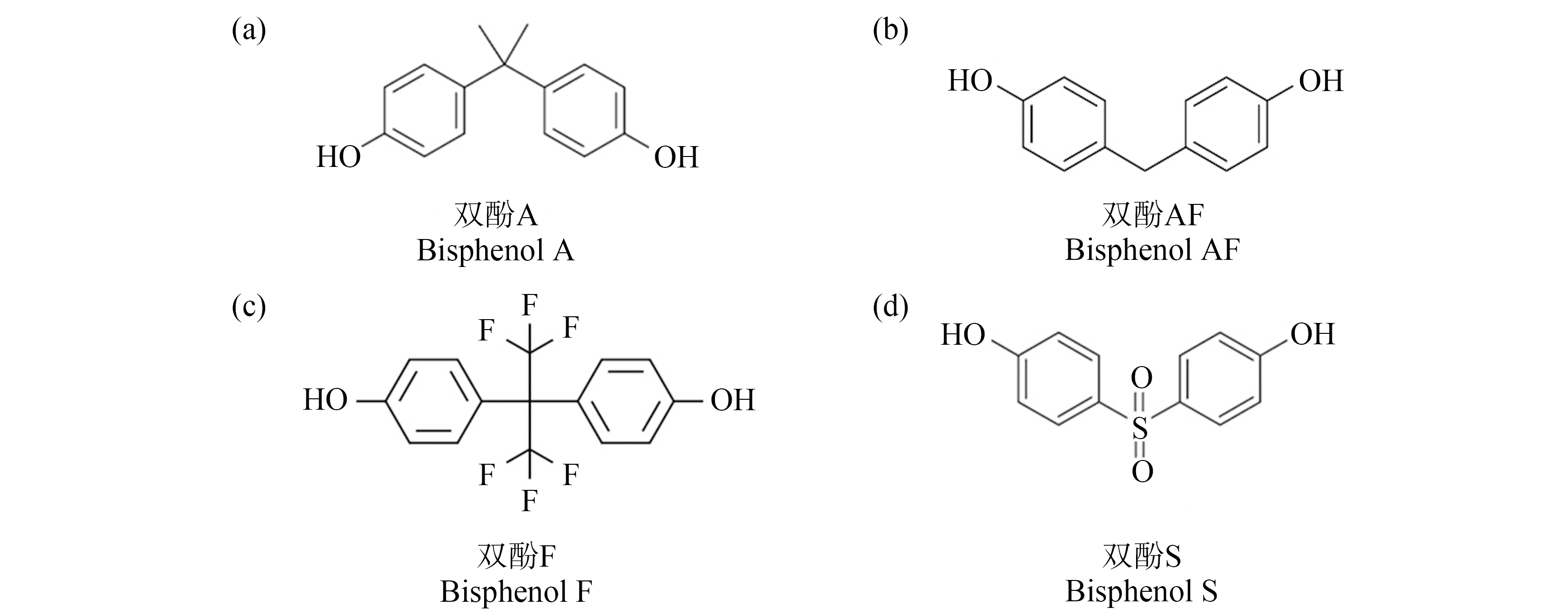
图1 双酚A(BPA)及其替代物的结构图
Fig. 1 Structure diagram of bisphenol A (BPA) and its substitutes
双酚AF(bisphenol AF, BPAF)、双酚F(bisphenol F, BPF)和双酚S(bisphenol S, BPS)等BPA的结构类似物已被用作替代品投入市场使用,以生产BPA-free(不含BPA)的产品。最近的调查数据表明,BPA类似物在环境、食品、消费品甚至人类样本中普遍存在[5-8],且检出频率及检出浓度不断增加。然而,最新的体内和体外实验研究表明,BPAF、BPF和BPS与BPA具有相同/相似的受体激动/拮抗活性,BPF和BPS的效力甚至高于BPA[9]。
本研究总结、回顾了当前BPA及其替代物BPAF、BPF和BPS的内分泌干扰作用的受体信号通路研究,通过分子层面探究双酚类化合物产生内分泌干扰效应的结构基础,揭示其发挥多种内分泌干扰效应的具体机制。可为BPA及其替代物的毒性评估和风险控制提供数据支撑,并有助于BPA及其替代物相关管控法规的制定。
1 BPA及其替代物的雌激素效应受体作用信号通路(Receptor signaling pathways of BPA and its substitutes exerting estrogenic effects)
2种雌激素受体(estrogen receptor, ER):ERα和ERβ被认为是BPA及其替代物的经典作用位点,此外双酚类化合物还可以通过雌激素受体相关受体(estrogen-related receptor, ERR)介导雌激素信号[10-11]。除了基因组效应途径,非基因组效应途径——G蛋白偶联雌激素受体(G-protein-coupled estrogen receptor, GPER)也是BPA及其替代物发挥生殖内分泌干扰作用的靶点之一。
1.1 基因组效应途径
1.1.1 ER途径
ERα和ERβ是核激素受体(nuclear receptor, NR)家族的成员,作为配体诱导的转录因子,是介导雌激素信号的经典基因组途径[2]。ERs包含3个主要的功能域,包括一个具有转录激活功能(activation function-1, AF-1)的N端A/B域,一个DNA结合域(DNA binding domain, DBD)和一个具有配体依赖性转录激活功能(activation function-2, AF-2)的C端配体结合域(ligand binding domain, LBD)[12]。BPA及其结构类似物与ERs的LBD区域具有较高的结合潜力,计算机模拟4种双酚类化合物和3种斑马鱼ER亚型之间的结合,其结合能排序为BPAF>BPA>BPF>BPS,与ERα的结合能分别为-16.86、-14.91、-12.42和-10.05 kcal·mol-1,与ERβ1的结合能分别为-13.77、-13.08、-11.49和-11.23 kcal·mol-1,与ERβ2的结合能分别为-20.47、-18.59、-16.70和-14.26 kcal·mol-1(1 kcal=4.185 kJ)[13]。
BPA及其替代物能够作为配体与经典的核雌激素受体ERα和ERβ结合,直接激动或拮抗雌激素效应基因的转录[11,14-17]。由表1可知,BPA和BPAF都能诱导ERα/雌激素反应元件(ERE)以及ERβ/ERE介导的转录活性,但BPAF的ER激动活性高于BPA[14]。然而,二者也能够拮抗ER/ERE介导的转录活性[11,15-16],其激动或拮抗特性似乎与多种因素有关。首先,暴露时间可能会影响BPA的激动或拮抗特性。例如,BPA暴露7 d抑制了类固醇基因(star和hsd11b2)ERE招募雌激素受体(ER)的能力,而BPA处理14 d则增强了ERE招募ER的能力[15]。其次,由于调节辅助因子具有组织特异性,能够影响与目标基因上的ER和ERE的相互作用,因此组织特异性也是影响双酚类化合物激动或拮抗特性的因素之一。从来自3种不同组织的细胞系中进行的实验结果显示,在E2存在的条件下,在人子宫内膜腺癌细胞(Ishikawa)中观察到BPA对ERα的显著抑制,而在人宫颈上皮癌细胞(HeLa)和人肝细胞癌细胞(HepG2)中没有观察到这种抑制作用[11]。
表1 BPA及其替代物的生殖内分泌干扰作用靶点——基因组途径
Table 1 Targtes of reproductive endocrine-disruption of BPA and its substitutes—Genomic pathways
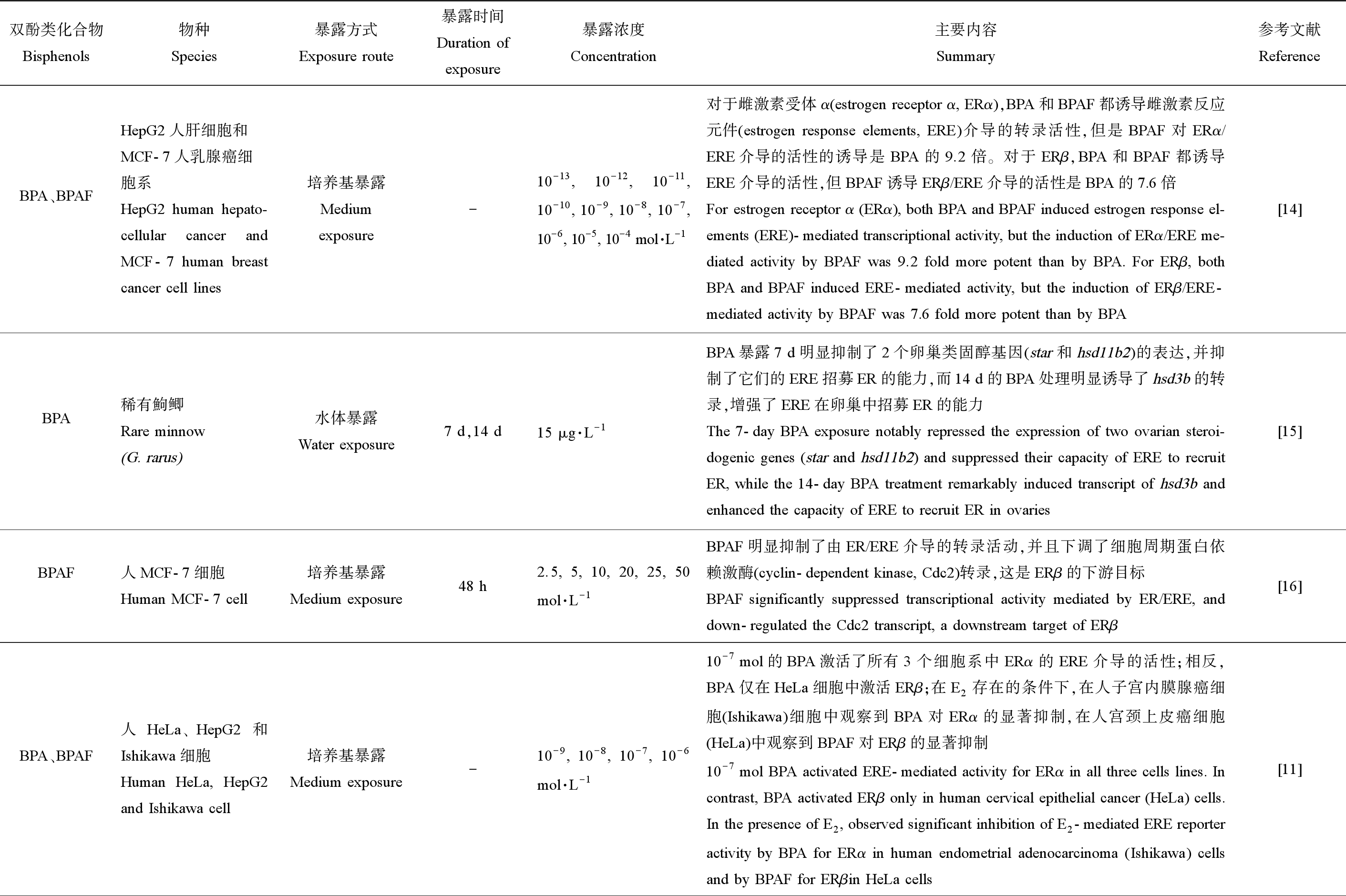
双酚类化合物Bisphenols物种Species暴露方式Exposure route暴露时间Duration ofexposure暴露浓度Concentration主要内容Summary参考文献ReferenceBPA、BPAFHepG2人肝细胞和MCF-7人乳腺癌细胞系HepG2 human hepato-cellular cancer and MCF-7 human breast cancer cell lines培养基暴露Medium exposure-10-13, 10-12, 10-11, 10-10, 10-9, 10-8, 10-7, 10-6, 10-5, 10-4 mol·L-1对于雌激素受体α(estrogen receptor α, ERα),BPA和BPAF都诱导雌激素反应元件(estrogen response elements, ERE)介导的转录活性,但是BPAF对ERα/ERE介导的活性的诱导是BPA的9.2倍。对于ERβ,BPA和BPAF都诱导ERE介导的活性,但BPAF诱导ERβ/ERE介导的活性是BPA的7.6倍For estrogen receptor α (ERα), both BPA and BPAF induced estrogen response el-ements (ERE)-mediated transcriptional activity, but the induction of ERα/ERE me-diated activity by BPAF was 9.2 fold more potent than by BPA. For ERβ, both BPA and BPAF induced ERE-mediated activity, but the induction of ERβ/ERE-mediated activity by BPAF was 7.6 fold more potent than by BPA[14]BPA稀有鮈鲫Rare minnow(G. rarus)水体暴露Water exposure 7 d,14 d15 μg·L-1BPA暴露7 d明显抑制了2个卵巢类固醇基因(star和hsd11b2)的表达,并抑制了它们的ERE招募ER的能力,而14 d的BPA处理明显诱导了hsd3b的转录,增强了ERE在卵巢中招募ER的能力The 7-day BPA exposure notably repressed the expression of two ovarian steroi-dogenic genes (star and hsd11b2) and suppressed their capacity of ERE to recruit ER, while the 14-day BPA treatment remarkably induced transcript of hsd3b and enhanced the capacity of ERE to recruit ER in ovaries[15]BPAF人MCF-7细胞Human MCF-7 cell培养基暴露Medium exposure48 h2.5, 5, 10, 20, 25, 50 mol·L-1BPAF明显抑制了由ER/ERE介导的转录活动,并且下调了细胞周期蛋白依赖激酶(cyclin-dependent kinase, Cdc2)转录,这是ERβ的下游目标BPAF significantly suppressed transcriptional activity mediated by ER/ERE, and down-regulated the Cdc2 transcript, a downstream target of ERβ[16]BPA、BPAF人HeLa、HepG2和Ishikawa细胞Human HeLa, HepG2 and Ishikawa cell培养基暴露Medium exposure-10-9, 10-8, 10-7, 10-6 mol·L-110-7 mol的BPA激活了所有3个细胞系中ERα的ERE介导的活性;相反,BPA仅在HeLa细胞中激活ERβ;在E2存在的条件下,在人子宫内膜腺癌细胞(Ishikawa)细胞中观察到BPA对ERα的显著抑制,在人宫颈上皮癌细胞(HeLa)中观察到BPAF对ERβ的显著抑制10-7 mol BPA activated ERE-mediated activity for ERα in all three cells lines. In contrast, BPA activated ERβ only in human cervical epithelial cancer (HeLa) cells. In the presence of E2, observed significant inhibition of E2-mediated ERE reporter activity by BPA for ERα in human endometrial adenocarcinoma (Ishikawa) cells and by BPAF for ERβin HeLa cells[11]
注:-表示无数据;BPA表示双酚A;BPAF表示BPAF;BPF表示双酚F;BPS表示双酚S;下同。
Note: - means no data available; BPA means bisphenol A; BPA means bisphenol AF; BPF means bisphenol F; BPS means bisphenol S; the same below.
续表1双酚类化合物Bisphenols物种Species暴露方式Exposure route暴露时间Duration ofexposure暴露浓度Concentration主要内容Summary参考文献ReferenceBPF、BPS人Ishikawa细胞Human Ishikawa cell培养基暴露Medium exposure24 h10-6, 10-5mol·L-1在转染的Ishikawa细胞中,BPF和BPS激活了雌激素反应基因,表现为由ERE结合介导的荧光素酶信号增加;并且BPF和BPS对Ishikawa细胞的基因激活主要是通过ERαIn transfected Ishikawa cells, BPF and BPS activated estrogen responsive genes, as demonstrated by increased luciferase signals mediated by ERE binding; and the gene activation by BPF and BPS in Ishikawa cells was mainly through ERα[17]
1.1.2 ERR途径
ERR也被认为是介导雌激素信号的基因组途径之一。ERR家族是一个转录因子核受体超家族,其成员包括:ERRα、ERRβ和ERRγ[18]。ERR与ERs有显著的同源性,特别是在DNA结合结构域以及LBD结构域。而BPA及其替代物也被证实具有与ERR结合的潜力,如BPA与ERRγ-LBD的结合亲和力极高,并且BPA的酚羟基位于ERRγ-LBD的活性口袋中[19];并且一项针对于BPA与ERR结合的结构基础分析认为一个酚羟基就足以使BPA与ERR-γ结合[20]。
BPA及其替代物能够通过ERR介导的基因组途径影响下游生物学反应。例如,BPS可以增加ERRα与纤维连接蛋白(fibronectin, FN1)启动子的结合,从而诱导FN1在嗜铬细胞瘤(PC12)细胞中的表达,触发PC12细胞的迁移和侵袭[21]。
1.2 非基因组效应途径
与ER或ERR介导的“缓慢”的基因组效应不同,膜受体GPER介导的“快速”细胞反应,称为“非基因组”效应[22]。
表2总结了BPA及其替代物通过GPER介导的非基因组效应的相关研究。BPA能够通过GPER介导表皮生长因子受体-细胞外信号相关激酶1/2 (epidermal growth factor receptor-extracellular signal-related kinases 1 and 2, EGFR/ERK1/2)[23-24]和活性氧自由基/Ca2+-细胞凋亡信号调节激酶1-c-Jun氨基末端激酶(reactive oxygen species/Ca2+-apoptosis signal-regulating kinase 1-c-Jun amino terminal kinase, ROS/Ca2+-ASK1-JNK)信号通路[25]的快速激活,发挥非基因组雌激素作用。BPAF、BPF和BPS也能够通过GPER介导不同信号通路以及钙动员等途径发挥雌激素作用[26-30],且BPAF的活性甚至比BPA更高[26]。一项对于GPER与双酚类化合物之间的动态结合过程的模拟研究结果表明,所有测试的双酚类化合物都可以在其经典的正构位点上通过范德华相互作用力和分子间静电能与GPER结合,形成GPER-配体复合物[31]。BPA和BPAF触发不同的GPER构象变化,从而导致不同信号通路的激活[32],这可能是导致BPA及其替代物激素活性差异的原因。
表2 BPA及其替代物的生殖内分泌干扰作用靶点——GPER非基因组途径
Table 2 Targets of reproductive endocrine disrupting effects of BPA and its substitutes—GPER non-genomic pathways
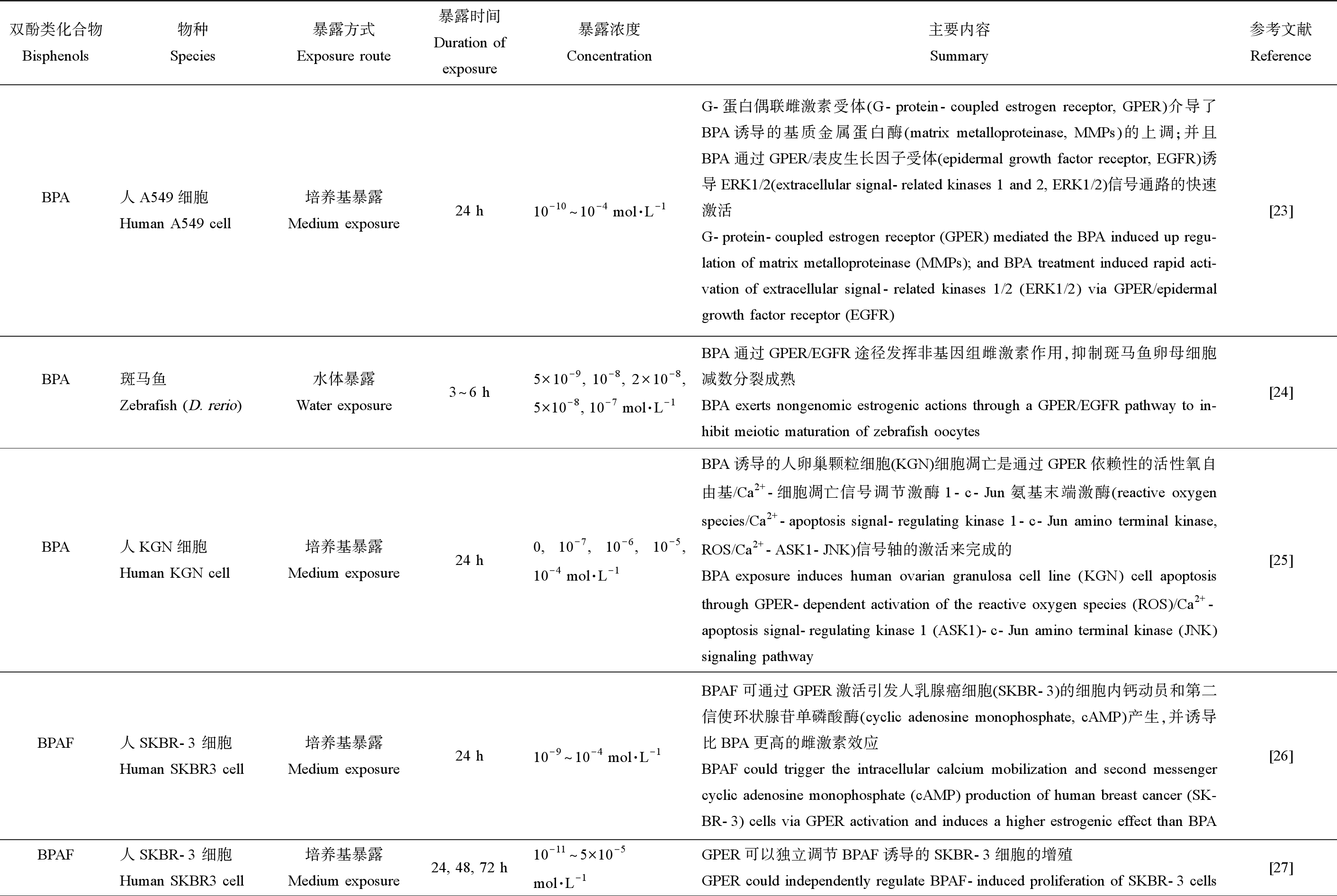
双酚类化合物Bisphenols物种Species暴露方式Exposure route暴露时间Duration ofexposure暴露浓度Concentration主要内容Summary参考文献ReferenceBPA人A549细胞Human A549 cell培养基暴露Medium exposure24 h10-10^10-4 mol·L-1G-蛋白偶联雌激素受体(G-protein-coupled estrogen receptor, GPER)介导了BPA诱导的基质金属蛋白酶(matrix metalloproteinase, MMPs)的上调;并且BPA通过GPER/表皮生长因子受体(epidermal growth factor receptor, EGFR)诱导ERK1/2(extracellular signal-related kinases 1 and 2, ERK1/2)信号通路的快速激活G-protein-coupled estrogen receptor (GPER) mediated the BPA induced up regu-lation of matrix metalloproteinase (MMPs); and BPA treatment induced rapid acti-vation of extracellular signal-related kinases 1/2 (ERK1/2) via GPER/epidermal growth factor receptor (EGFR)[23]BPA斑马鱼Zebrafish (D. rerio)水体暴露Water exposure 3^6 h5×10-9, 10-8, 2×10-8, 5×10-8, 10-7 mol·L-1BPA通过GPER/EGFR途径发挥非基因组雌激素作用,抑制斑马鱼卵母细胞减数分裂成熟BPA exerts nongenomic estrogenic actions through a GPER/EGFR pathway to in-hibit meiotic maturation of zebrafish oocytes[24]BPA人KGN细胞Human KGN cell培养基暴露Medium exposure24 h0, 10-7, 10-6, 10-5, 10-4 mol·L-1BPA诱导的人卵巢颗粒细胞(KGN)细胞凋亡是通过GPER依赖性的活性氧自由基/Ca2+-细胞凋亡信号调节激酶1-c-Jun氨基末端激酶(reactive oxygen species/Ca2+-apoptosis signal-regulating kinase 1-c-Jun amino terminal kinase, ROS/Ca2+-ASK1-JNK)信号轴的激活来完成的BPA exposure induces human ovarian granulosa cell line (KGN) cell apoptosis through GPER-dependent activation of the reactive oxygen species (ROS)/Ca2+-apoptosis signal-regulating kinase 1 (ASK1)-c-Jun amino terminal kinase (JNK) signaling pathway[25]BPAF人SKBR-3 细胞Human SKBR3 cell培养基暴露Medium exposure24 h10-9^10-4 mol·L-1BPAF可通过GPER激活引发人乳腺癌细胞(SKBR-3)的细胞内钙动员和第二信使环状腺苷单磷酸酶(cyclic adenosine monophosphate, cAMP)产生,并诱导比BPA更高的雌激素效应BPAF could trigger the intracellular calcium mobilization and second messenger cyclic adenosine monophosphate (cAMP) production of human breast cancer (SK-BR-3) cells via GPER activation and induces a higher estrogenic effect than BPA[26]BPAF人SKBR-3 细胞Human SKBR3 cell培养基暴露Medium exposure24, 48, 72 h10-11^5×10-5mol·L-1GPER可以独立调节BPAF诱导的SKBR-3细胞的增殖GPER could independently regulate BPAF-induced proliferation of SKBR-3 cells[27]
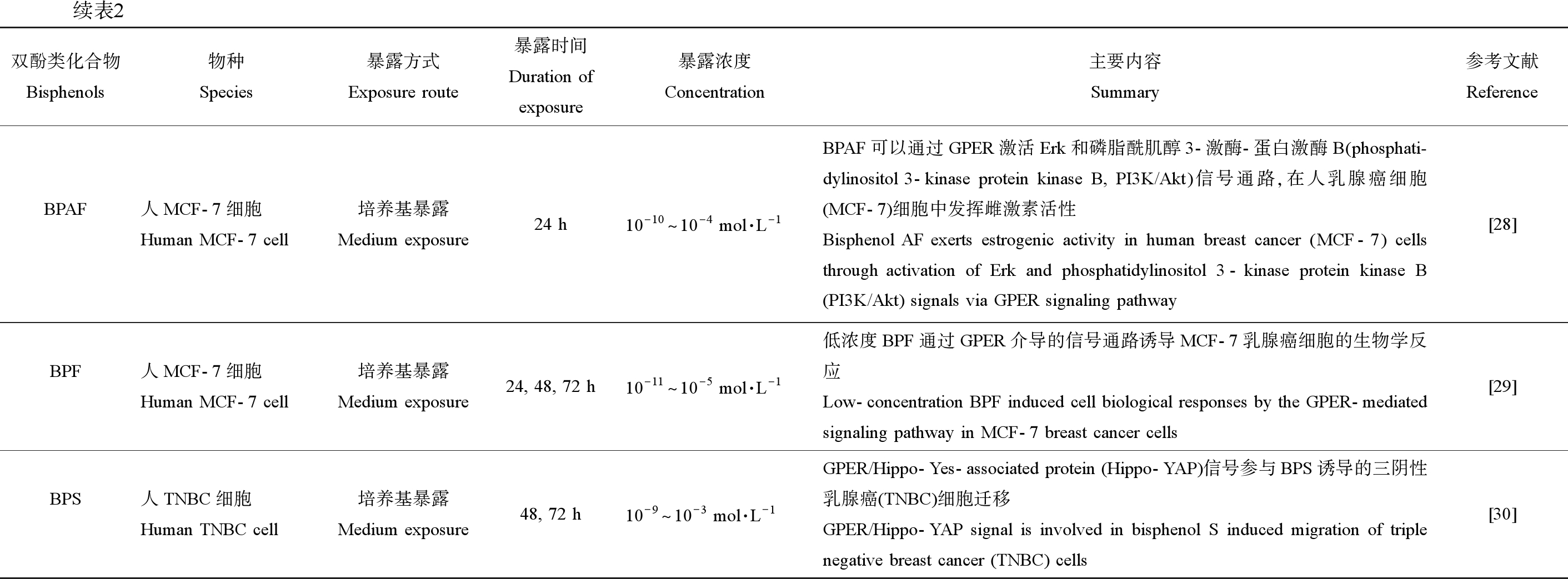
续表2双酚类化合物Bisphenols物种Species暴露方式Exposure route暴露时间Duration ofexposure暴露浓度Concentration主要内容Summary参考文献ReferenceBPAF人MCF-7细胞Human MCF-7 cell培养基暴露Medium exposure24 h10-10^10-4 mol·L-1BPAF可以通过GPER激活Erk和磷脂酰肌醇3-激酶-蛋白激酶B(phosphati-dylinositol 3-kinase protein kinase B, PI3K/Akt)信号通路,在人乳腺癌细胞(MCF-7)细胞中发挥雌激素活性Bisphenol AF exerts estrogenic activity in human breast cancer (MCF-7) cells through activation of Erk and phosphatidylinositol 3-kinase protein kinase B (PI3K/Akt) signals via GPER signaling pathway[28]BPF人MCF-7细胞Human MCF-7 cell培养基暴露Medium exposure24, 48, 72 h10-11^10-5 mol·L-1低浓度BPF通过GPER介导的信号通路诱导MCF-7乳腺癌细胞的生物学反应Low-concentration BPF induced cell biological responses by the GPER-mediated signaling pathway in MCF-7 breast cancer cells[29]BPS人TNBC细胞Human TNBC cell培养基暴露Medium exposure48, 72 h10-9^10-3 mol·L-1GPER/Hippo-Yes-associated protein (Hippo-YAP)信号参与BPS诱导的三阴性乳腺癌(TNBC)细胞迁移GPER/Hippo-YAP signal is involved in bisphenol S induced migration of triple negative breast cancer (TNBC) cells[30]
2 BPA及其替代物甲状腺干扰效应的受体作用信号通路(Receptor signaling pathways of BPA and its substitutes exerting thyroid-disrupting effects)
由于双酚类化合物与甲状腺激素(thyroid hormone, TH, T4和T3)的结构相似[33],甲状腺激素受体(thyroid hormone receptor, TR)介导途径是BPA及其替代物发挥甲状腺干扰效应的重要作用途径[33],其中包括基因组和非基因组途径。此外,与血清转运蛋白的竞争性结合也是双酚类化合物发挥甲状腺激素干扰作用的重要靶点。
2.1 基因组途径
TH的作用通常是由基因组途径介导的,如TH与高亲和力的TRs(如TRβ、TRα)结合,识别TH靶基因启动子中的特定TH反应元件(thyroid hormone response element, TRE),并激活或抑制转录以响应激素调节[34]。竞争性荧光结合试验结果表明BPA、BPF和BPS均能够与TH受体(TRα和TRβ)结合,但是BPF和BPS结合能力比BPA低一个数量级(BPA>BPF>BPS)[35],且这些化学物质对TRβ-LBD(TRβ配体结合结构域)的结合能力大于对TRα-LBD(TRα配体结合结构域),在共激活因子募集试验中,BPF和BPS将共激活因子募集至TRβ,而非TRα[35]。
大部分研究通常认为,BPA的TR-拮抗作用可能是其破坏甲状腺功能的主要机制[36]。由表3所示,BPA暴露(10~100 μmol·L-1)会导致TR活性受到抑制,其中处于较低的浓度(0.001~0.1 μmol·L-1)时TRβ即受到抑制[37]。同时,低浓度的BPA通过向TR募集核受体共抑制因子(nuclear receptor co-repressors, N-CoR)来抑制T3诱导的转录[38];当斑马鱼胚胎暴露于高于12.5 μg·L-1的BPAF时,TR水平显著下降,同样表明BPAF是一种TR拮抗剂[39]。并且BPF和BPS也可以结合TRβ并发挥拮抗活性[40-41]。
表3 BPA及其替代物的甲状腺干扰作用靶点——基因组途径
Table 3 Targets of thyroid-disrupting effects of BPA and its substitutes—Genomic pathways

双酚类化合物Bisphenols物种Species暴露方式Exposure route暴露时间Duration ofexposure暴露浓度Concentration主要内容Summary参考文献ReferenceBPA蝌蚪Tadpoles(X. laevis)水体暴露Water exposure21 d10-7, 10-6 mol·L-1BPA暴露会导致甲状腺激素受体(TR)活性受到抑制,其中处于较低的浓度(10-7 mol)时TRβ即受到抑制Thyroid hormone receptor (TR) was inhibited by BPA treatment, where TRβ inhi-bition was at a lower concentration (10-7 mol)[37]BPA人TSA 201和293细胞Human TSA 201 and 293 cell培养基暴露Medium exposure24 h10-9, 10-8, 10-5mol·L-1BPA通过募集甲状腺受体的核受体共阻遏物(N-CoRs)来抑制三碘甲状腺原氨酸(T3)诱导的转录BPA suppresses triiodothyronine (T3)-induced transcription by recruiting nuclear receptor co-repressors (N-CoRs) to the thyroid receptor[38]BPAF斑马鱼Zebrafish (D. rerio)水体暴露Water exposure7 d12.5, 125 μg·L-1当暴露于≥12.5 μg·L-1的BPAF时,TR水平显著下降,这表明BPAF是一种TR拮抗剂The TR levels decreased significantly upon exposure to ≥12.5 μg·L-1 BPAF, im-plying that BPAF acts as a TR antagonist[39]BPF非洲爪蟾(X. laevis)水体暴露Water exposure48, 96 h10-8, 10-7, 10-6mol·L-1在10-9 mol T3存在的情况下,10-6和/或10-7 mol的BPF拮抗了T3诱导的甲状腺激素(thyroid hormones, TH)反应基因的表达In the presence of 10-9 mol T3, 10-6 and/or 10-7 mol of BPF antagonized T3-in-duced thyroid hormones (TH)-response gene expression[40]BPS人TRβ双杂交酵母菌Human TRβ two-hy-brid yeast培养基暴露Medium exposure-5×10-12^5×10-5mol·L-1BPS以浓度依赖的方式显著抑制β-半乳糖苷酶活性,表明其对TRβ具有拮抗作用BPS significantly suppressed β-galactosidase activity in a concentration-dependent manner, suggesting their antagonistic effect toward TRβ[41]
此外,也有研究观察到在缺乏T3的情况下,双酚类化合物表现为TR激动剂[35,42]。例如,BPA、BPS和BPF单独暴露都能诱导TH依赖性的大鼠垂体瘤细胞(GH3)增殖,促进TR介导的报告基因转录[35]。
2.2 非基因组途径
膜受体β3整合素(integrin beta 3, ITGβ3)主要通过有丝分裂原活化蛋白激酶(mitogen-activated protein kinases, MAPK)信号通路介导快速非基因组效应和转录调节[43-44]。此外,ITGβ3还介导TH对细胞内蛋白质运输和质膜离子泵(包括钠/蛋白质反转运蛋白)的作用[34]。
有研究表明BPA可能通过非基因组机制对TH介导的转录产生干扰作用[43-44]。例如,BPA将N-CoR募集到TR-β1来抑制TR介导的转录,这是通过ITGβ3/酪氨酸激酶(tyrosine kinase, c-Src)/MAPK/TR-β1途径的非基因组机制介导的[44]。
2.3 非受体途径
甲状腺素运载蛋白(transthyretin, TTR)与甲状腺素结合球蛋白(thyroxine-binding globulin, TBG)是血液循环系统中T3、T4的重要载体蛋白。研究证实BPA及其替代物与天然甲状腺激素竞争结合TTR,且BPAF和BPF与TTR的结合能力高于BPA[45]。一项针对于双酚类化合物与TBG的结构基础研究表明BPA、BPAF、BPF、BPS与TBG相互作用的残基数与天然配体T4分别有100%、92%、85%和77%相同,与T3分别有70%、70%、74%和74%相同[46]。
3 BPA及其替代物致肥胖效应的受体作用信号通路(Receptor signaling pathways of BPA and its substitutes exerting obesogenic effects)
过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptor, PPAR)α/γ也是NR家族成员之一,可通过调节成脂基因的表达来控制脂质代谢,如甾醇调节元件结合蛋白(sterol regulatory element binding protein, SREBP)-1c、脂肪酸合酶(fatty acid synthase, FAS)、二酰基甘油酰基转移酶(diacylglycerol acyl transferase, DGAT)和脂蛋白脂肪酶(lipoprotein lipase, LPL)等[47]。多项研究证实BPA及其替代物以PPAR为作用靶点导致脂质生成或代谢紊乱,促进肥胖的发生[48-52]。
PPARγ以其对脂肪生成的主要调节活性而闻名,受刺激后,PPARγ将进一步激活其下游信号分子,导致脂肪细胞过度分化,从而干扰脂质代谢过程[48]。由表4可知,BPA暴露会导致PPARγ过度表达,这与宫旁白色脂肪组织(parametrial white adipose tissue, pWAT)质量增加有关[49],并且BPA对脂肪形成的影响可能会通过表观遗传机制介导,即降低前脂肪细胞中PPARγ启动子甲基化[50]。BPA、BPF和BPS还通过诱导CCAAT/增强子结合蛋白α(CCAAT/enhancer binding proteinα, C/EBPα)的过表达激活核激素受体PPARγ进而刺激与脂肪生成相关的基因表达量增加[51-52],诱导脂质积累。
表4 BPA及其替代物的致肥胖干扰作用靶点——PPARγ途径
Table 4 Mechanism of endocrine disrupting effects of BPA and its substitutes—PPARγ pathways
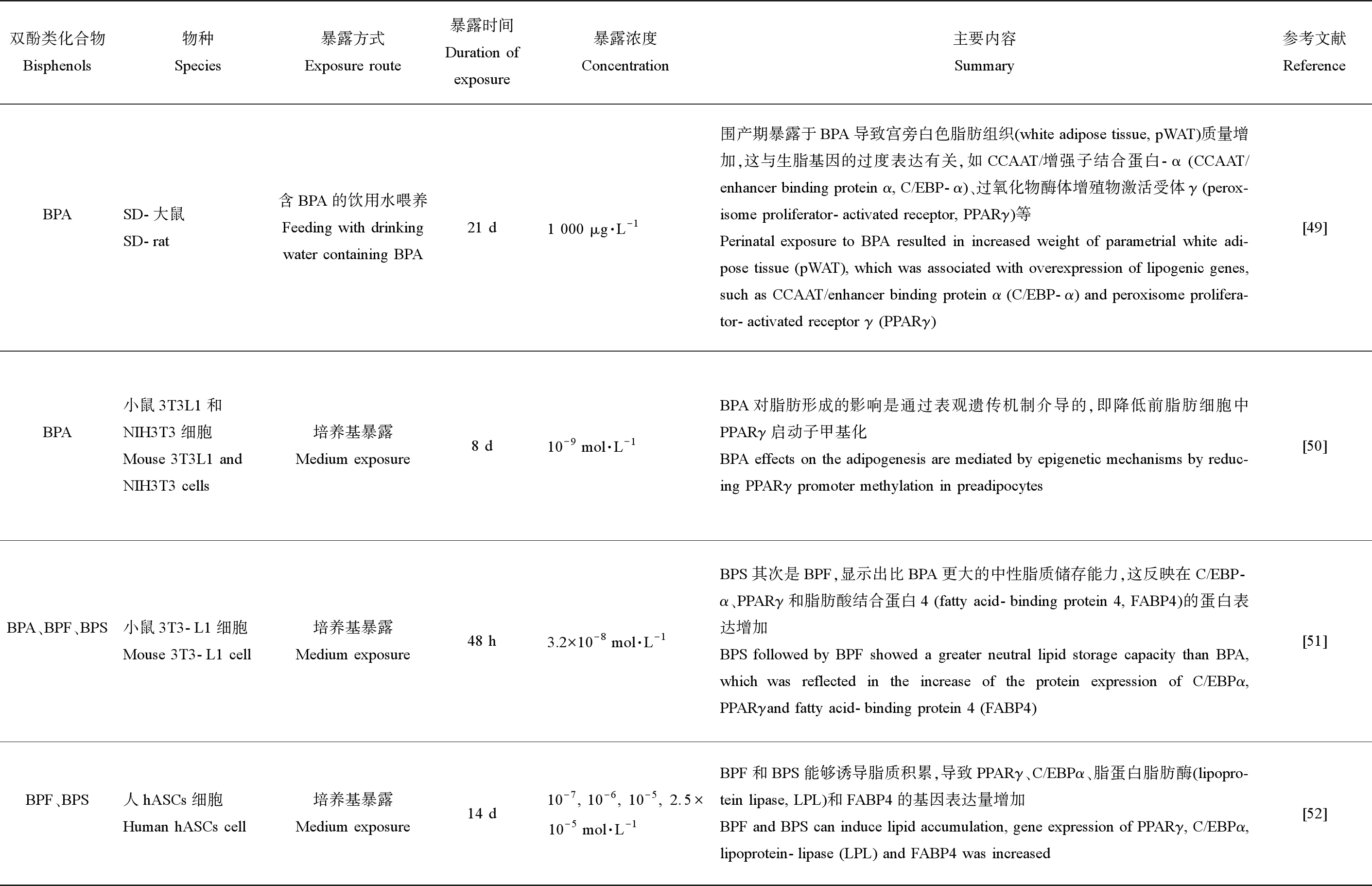
双酚类化合物Bisphenols物种Species暴露方式Exposure route暴露时间Duration ofexposure暴露浓度Concentration主要内容Summary参考文献ReferenceBPASD-大鼠SD-rat含BPA的饮用水喂养Feeding with drinking water containing BPA21 d1 000 μg·L-1围产期暴露于BPA导致宫旁白色脂肪组织(white adipose tissue, pWAT)质量增加,这与生脂基因的过度表达有关,如CCAAT/增强子结合蛋白-α (CCAAT/enhancer binding protein α, C/EBP-α)、过氧化物酶体增殖物激活受体γ (perox-isome proliferator-activated receptor, PPARγ)等Perinatal exposure to BPA resulted in increased weight of parametrial white adi-pose tissue (pWAT), which was associated with overexpression of lipogenic genes, such as CCAAT/enhancer binding protein α (C/EBP-α) and peroxisome prolifera-tor-activated receptor γ (PPARγ)[49]BPA小鼠3T3L1和NIH3T3细胞Mouse 3T3L1 and NIH3T3 cells培养基暴露Medium exposure8 d10-9 mol·L-1BPA对脂肪形成的影响是通过表观遗传机制介导的,即降低前脂肪细胞中PPARγ启动子甲基化BPA effects on the adipogenesis are mediated by epigenetic mechanisms by reduc-ing PPARγ promoter methylation in preadipocytes[50]BPA、BPF、BPS小鼠3T3-L1细胞Mouse 3T3-L1 cell培养基暴露Medium exposure48 h3.2×10-8 mol·L-1BPS其次是BPF,显示出比BPA更大的中性脂质储存能力,这反映在C/EBP-α、PPARγ和脂肪酸结合蛋白4 (fatty acid-binding protein 4, FABP4)的蛋白表达增加BPS followed by BPF showed a greater neutral lipid storage capacity than BPA, which was reflected in the increase of the protein expression of C/EBPα, PPARγand fatty acid-binding protein 4 (FABP4)[51]BPF、BPS人hASCs细胞Human hASCs cell培养基暴露Medium exposure14 d10-7, 10-6, 10-5, 2.5×10-5 mol·L-1BPF和BPS能够诱导脂质积累,导致PPARγ、C/EBPα、脂蛋白脂肪酶(lipopro-tein lipase, LPL)和FABP4的基因表达量增加BPF and BPS can induce lipid accumulation, gene expression of PPARγ, C/EBPα, lipoprotein-lipase (LPL) and FABP4 was increased[52]
PPARα主要在肝脏中表达,它的表达可以减少肝脏脂质积累[47]。而BPA可以通过直接失活PPARα信号通路来刺激与人体脂质代谢相关的基因[48],当PPARα被抑制时,脂质往往会在肝细胞中积聚,进一步加重肥胖。
4 总结与展望(Summary and prospect)
本研究主要归纳总结了BPA及其替代物BPAF、BPF和BPS的核受体(ER、ERR、TR和PPAR)及膜受体(GPER和ITGβ3)的作用通路,如图2所示。我们认为目前的研究忽视了以下3个方面,可作为未来研究的可能性方向。
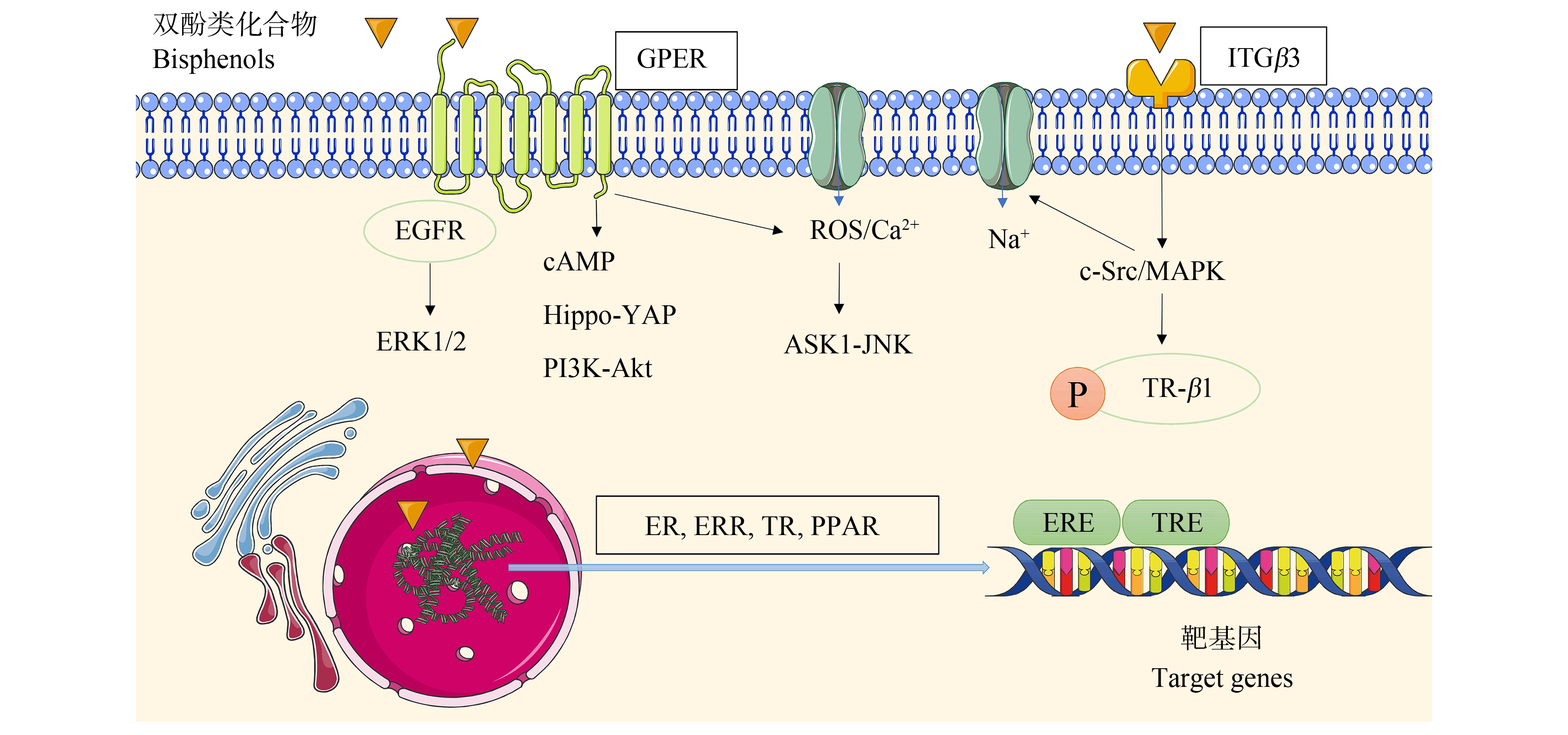
图2 BPA及其替代物的受体作用信号通路
注:GPER为G蛋白偶联雌激素受体,ITGβ3为β3整合素,ER为雌激素受体,ERR为雌激素相关受体,TR为甲状腺激素受体,PPAR为过氧化物酶体增殖物激活受体,EGFR为表皮生长因子受体,ERK1/2为细胞外信号相关激酶1/2,cAMP为环状腺苷单磷酸酶,Hippo-YAP为Hippo-Yes相关蛋白,PI3K-Akt为磷脂酰肌醇3-激酶-蛋白激酶B,ASK1-JNK为细胞凋亡信号调节激酶1-c-Jun氨基末端激酶,c-Src/MAPK为酪氨酸激酶/有丝分裂原活化蛋白激酶,TR-β1为甲状腺激素受体β1,ERE为雌激素反应元件,TRE为甲状腺激素反应元件。
Fig. 2 Receptor signaling pathways of BPA and its substitutes
Note: GPER is G protein-coupled estrogen receptor, ITGβ3 is integrin beta 3, ER is estrogen receptor, ERR is estrogen-related receptor, TR is thyroid hormone receptor, PPAR is peroxisome proliferator-activated receptor, EGFR is epidermal growth factor receptor, ERK1/2 is extracellular signal-related kinases 1/2, cAMP is cyclic adenosine monophosphate, Hippo-YAP is Hippo-Yes-associated protein, PIK3-Akt is phosphatidylinositol 3-kinase protein kinase B, ASK1-JNK is apoptosis signal-regulating kinase 1-c-Jun amino terminal kinase, c-Src/MAPK is tyrosine kinase/mitogen-activated protein kinase, TR-β1 is thyroid hormone receptor β1, ERE is estrogen response element, and TRE is thyroid hormone response element.
(1)有必要关注BPA及其替代物的膜受体作用信号通路
尽管膜受体表达量低,但是其介导的非基因组调控途径具有生物反应快、调控精确的优点,对发挥许多重要的细胞功能至关重要。如前文所述,BPA及其替代物能够通过膜受体GPER介导多个信号通路(EGFR/ERK1/2、ROS/Ca2+-ASK1-JNK、cAMP、PI3K/Akt和Hippo-YAP等)的快速激活发挥雌激素作用,也能通过β3整合素介导的非基因组途径将N-CoR募集到TR-β1来抑制TR介导的转录。然而,相较于BPA及其替代物与核受体家族相互作用的大量研究,关于膜受体途径的研究仍非常有限,这可能成为未来探究BPA及其替代物作用靶点的新切入点。
(2)多种技术手段相结合验证BPA及其替代物的受体作用信号通路
目前研究BPA及其替代物的核受体作用信号通路一般分3个步骤。首先,通过理论计算如分子对接[13]、分子动力学模拟[41]等模拟双酚类化合物与核受体的相互作用;随后,应用荧光偏振检测实验[35]等体外结合实验为双酚类化合物与核受体结合提供直接实验证据;最后,应用细胞增殖实验[29]、酵母双杂交实验[41]和荧光素酶报告基因实验[14]区分拮抗或激动特性。而在研究BPA及其替代物的膜受体作用信号通路时,往往采用化合物与信号通路相关因子激动剂、拮抗剂联合暴露[24-25]。例如,BPA单独暴露抑制斑马鱼卵母细胞成熟,使用EGFR抑制剂和MAPK3/1信号通路抑制剂与GPER激动剂和BPA共同处理斑马鱼卵母细胞,可阻断BPA和GPER激动剂的作用,并促进斑马鱼卵母细胞成熟,表明BPA通过激活GPER/EGFR/MAPK3/1信号通路途径破坏斑马鱼卵母细胞成熟[24]。随着分子生物学的快速发展,siRNA沉默[30]、基因编辑[53]等技术为我们验证BPA及其替代物的核受体、膜受体作用靶点提供了更有力的工具。
(3)多种双酚类化合物的联合作用应当引起重视
关于环境样本、人体样本的双酚类化合物污染状况调查研究表明,多种双酚类化合物之间存在明显的相关性,表明其可能有共同来源。例如,Spearman相关分析表明,室内灰尘中BPA与BPF的等级相关系数为0.51[6]。然而,目前在实验室内开展的BPA及其替代物的受体作用信号通路研究多是采用单一暴露。具有相似或者不同作用机制的化学污染物能够互相影响各自的毒性作用,从而引起一系列的协同、加和或者拮抗效应。对于多种双酚类化合物的联合作用应当引起重视。
通信作者简介:田华(1983—),女,博士,副教授,主要研究方向为生态毒理学。
[1] Jiang D Q, Chen W Q, Zeng X L, et al. Dynamic stocks and flows analysis of bisphenol A (BPA) in China: 2000-2014 [J]. Environmental Science &Technology, 2018, 52(6): 3706-3715
[2] Delfosse V, Grimaldi M, Pons J L, et al. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(37): 14930-14935
[3] Sabry R, Apps C, Reiter-Saunders J A, et al. BPA and BPS affect connexin 37 in bovine cumulus cells [J]. Genes, 2021, 12(2): 321
[4] European Chemicals Agency (ECHA). Seven new substances added to the candidate list entry for bisphenol-A updated [EB/OL]. [2023-01-05]. https://echa.europa.eu/fr/-/seven-newsubstances-added-to-the-candidate-list-entry-for bisphenol-a-updated-toreflect-its-endocrine-disrupting-properties-for-the environment
[5] Siracusa J S, Yin L, Measel E, et al. Effects of bisphenol A and its analogs on reproductive health: A mini review [J]. Reproductive Toxicology, 2018, 79: 96-123
[6] Yang Y, Shi Y M, Chen D, et al. Bisphenol A and its analogues in paired urine and house dust from South China and implications for children’s exposure [J]. Chemosphere, 2022, 294: 133701
[7] González N, Cunha S C, Ferreira R, et al. Concentrations of nine bisphenol analogues in food purchased from Catalonia (Spain): Comparison of canned and non-canned foodstuffs [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2020, 136: 110992
[8] Zhang B, He Y, Zhu H K, et al. Concentrations of bisphenol A and its alternatives in paired maternal-fetal urine, serum and amniotic fluid from an e-waste dismantling area in China [J]. Environment International, 2020, 136: 105407
[9] Rochester J R, Bolden A L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes [J]. Environmental Health Perspectives, 2015, 123(7): 643-650
[10] Kuiper G G, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta [J]. Endocrinology, 1997, 138(3): 863-870
[11] Li Y, Burns K A, Arao Y, et al. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro [J]. Environmental Health Perspectives, 2012, 120(7): 1029-1035
[12] Benecke A, Chambon P, Gronemeyer H. Synergy between estrogen receptor alpha activation functions AF1 and AF2 mediated by transcription intermediary factor TIF2 [J]. EMBO Reports, 2000, 1(2): 151-157
[13] Mu X Y, Huang Y, Li X X, et al. Developmental effects and estrogenicity of bisphenol A alternatives in a zebrafish embryo model [J]. Environmental Science &Technology, 2018, 52(5): 3222-3231
[14] Pelch K E, Li Y, Perera L, et al. Characterization of estrogenic and androgenic activities for bisphenol A-like chemicals (BPs): in vitro estrogen and androgen receptors transcriptional activation, gene regulation, and binding profiles [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2019, 172(1): 23-37
[15] Zhang T, Guan Y J, Wang S, et al. Bisphenol A induced abnormal DNA methylation of ovarian steroidogenic genes in rare minnow Gobiocypris rarus [J]. General and Comparative Endocrinology, 2018, 269: 156-165
[16] Okazaki H, Takeda S, Kakizoe K, et al. Bisphenol AF as an inducer of estrogen receptor β (ERβ): Evidence for anti-estrogenic effects at higher concentrations in human breast cancer cells [J]. Biological &Pharmaceutical Bulletin, 2017, 40(11): 1909-1916
[17] Fan H J, Fernando S R, Jiang L H, et al. Bisphenol A analogues suppress spheroid attachment on human endometrial epithelial cells through modulation of steroid hormone receptors signaling pathway [J]. Cells, 2021, 10(11): 2882
[18] Song H X, Zhang T, Yang P, et al. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRγ signals [J]. Toxicology in Vitro: An International Journal Published in Association with BIBRA, 2015, 30(1 Pt B): 521-528
[19] Liu X H, Matsushima A, Shimohigashi M, et al. A characteristic back support structure in the bisphenol A-binding pocket in the human nuclear receptor ERRγ [J]. PLoS One, 2014, 9(6): e101252
[20] Okada H, Tokunaga T, Liu X, et al. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma [J]. Environmental Health Perspectives, 2008, 116(1): 32-38
[21] Jia Y F, Sun R X, Ding X M, et al. Bisphenol S triggers the migration and invasion of pheochromocytoma PC12 cells via estrogen-related receptor α [J]. Journal of Molecular Neuroscience, 2018, 66(2): 188-196
[22] Qie Y, Qin W P, Zhao K D, et al. Environmental estrogens and their biological effects through GPER mediated signal pathways [J]. Environmental Pollution, 2021, 278: 116826
[23] Zhang K S, Chen H Q, Chen Y S, et al. Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway [J]. Biomedicine &Pharmacotherapy, 2014, 68(8): 1037-1043
[24] Fitzgerald A C, Peyton C, Dong J, et al. Bisphenol A and related alkylphenols exert nongenomic estrogenic actions through a G protein-coupled estrogen receptor 1 (Gper)/Epidermal growth factor receptor (Egfr) pathway to inhibit meiotic maturation of zebrafish oocytes [J]. Biology of Reproduction, 2015, 93(6): 135
[25] Huang M Q, Huang M Z, Li X J, et al. Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca2+-ASK1-JNK pathway in human granulosa cell line KGN [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111429
[26] Cao L Y, Ren X M, Li C H, et al. Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via G protein-coupled estrogen receptor pathway [J]. Environmental Science &Technology, 2017, 51(19): 11423-11430
[27] Lei B L, Xu L B, Tang Q Q, et al. Molecular mechanism study of BPAF-induced proliferation of ERα-negative SKBR-3 human breast cancer cells in vitro/in vivo [J]. The Science of the Total Environment, 2021, 775: 145814
[28] Lei B L, Sun S, Zhang X L, et al. Bisphenol AF exerts estrogenic activity in MCF-7 cells through activation of Erk and PI3K/Akt signals via GPER signaling pathway [J]. Chemosphere, 2019, 220: 362-370
[29] Lei B L, Huang Y Y, Liu Y, et al. Low-concentration BPF induced cell biological responses by the ERα and GPER1-mediated signaling pathways in MCF-7 breast cancer cells [J]. Ecotoxicology and Environmental Safety, 2018, 165: 144-152
[30] Deng Q Q, Jiang G M, Wu Y M, et al. GPER/Hippo-YAP signal is involved in bisphenol S induced migration of triple negative breast cancer (TNBC) cells [J]. Journal of Hazardous Materials, 2018, 355: 1-9
[31] Liu X C, Xue Q, Zhang H Z, et al. Structural basis for molecular recognition of G protein-coupled estrogen receptor by selected bisphenols [J]. The Science of the Total Environment, 2021, 793: 148558
[32] Buoso E, Kenda M, Masi M, et al. Effects of bisphenols on RACK1 expression and their immunological implications in THP-1 cells [J]. Frontiers in Pharmacology, 2021, 12: 743991
[33] Gorini F, Bustaffa E, Coi A, et al. Bisphenols as environmental triggers of thyroid dysfunction: Clues and evidence [J]. International Journal of Environmental Research and Public Health, 2020, 17(8): 2654
[34] Davis P J, Leonard J L, Davis F B. Mechanisms of nongenomic actions of thyroid hormone [J]. Frontiers in Neuroendocrinology, 2008, 29(2): 211-218
[35] Zhang Y F, Ren X M, Li Y Y, et al. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo [J]. Environmental Pollution, 2018, 237: 1072-1079
[36] Kim M J, Park Y J. Bisphenols and thyroid hormone [J]. Endocrinology and Metabolism, 2019, 34(4): 340-348
[37] Heimeier R A, Das B, Buchholz D R, et al. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone [J]. Endocrinology, 2009, 150(6): 2964-2973
[38] Moriyama K, Tagami T, Akamizu T, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist [J]. The Journal of Clinical Endocrinology and Metabolism, 2002, 87(11): 5185-5190
[39] Chen P Y, Wang R H, Chen G, et al. Thyroid endocrine disruption and hepatotoxicity induced by bisphenol AF: Integrated zebrafish embryotoxicity test and deep learning [J]. The Science of the Total Environment, 2022, 822: 153639
[40] Niu Y, Zhu M, Dong M Q, et al. Bisphenols disrupt thyroid hormone (TH) signaling in the brain and affect TH-dependent brain development in Xenopus laevis [J]. Aquatic Toxicology, 2021, 237: 105902
[41] Lu L P, Zhan T J, Ma M, et al. Thyroid disruption by bisphenol S analogues via thyroid hormone receptor β: in vitro, in vivo, and molecular dynamics simulation study [J]. Environmental Science &Technology, 2018, 52(11): 6617-6625
[42] Terasaki M, Kosaka K, Kunikane S, et al. Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two-hybrid assay [J]. Chemosphere, 2011, 84(10): 1527-1530
[43] Sheng Z G, Wang C, Ren F R, et al. Molecular mechanism of endocrine-disruptive effects induced by bisphenol A: The role of transmembrane G-protein estrogen receptor 1 and integrin αvβ3 [J]. Journal of Environmental Sciences (China), 2019, 75: 1-13
[44] Sheng Z G, Tang Y, Liu Y X, et al. Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a nongenomic mechanism [J]. Toxicology and Applied Pharmacology, 2012, 259(1): 133-142
[45] Huang M W, Huang X M, Yong L, et al. Insight on the microscopic binding mechanism of bisphenol compounds (BPs) with transthyretin (TTR) based on multi-spectroscopic methods and computational simulations [J]. Analytical and Bioanalytical Chemistry, 2022, 414(13): 3765-3780
[46] Beg M A, Sheikh I A. Endocrine disruption: Molecular interactions of environmental bisphenol contaminants with thyroid hormone receptor and thyroxine-binding globulin [J]. Toxicology and Industrial Health, 2020, 36(5): 322-335
[47] Yang Z, Roth K, Agarwal M, et al. The transcription factors CREBH, PPARa, and FOXO1 as critical hepatic mediators of diet-induced metabolic dysregulation [J]. The Journal of Nutritional Biochemistry, 2021, 95: 108633
[48] Naomi R, Yazid M D, Bahari H, et al. Bisphenol A (BPA) leading to obesity and cardiovascular complications: A compilation of current in vivo study [J]. International Journal of Molecular Sciences, 2022, 23(6): 2969
[49] Somm E, Schwitzgebel V M, Toulotte A, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat [J]. Environmental Health Perspectives, 2009, 117(10): 1549-1555
[50] Longo M, Zatterale F, Naderi J, et al. Low-dose bisphenol-A promotes epigenetic changes at PPARγ promoter in adipose precursor cells [J]. Nutrients, 2020, 12(11): 3498
[51] Martínez M  , Blanco J, Rovira J, et al. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2020, 140: 111298
, Blanco J, Rovira J, et al. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2020, 140: 111298
[52] Reina-Pérez I, Olivas-Martínez A, Mustieles V, et al. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2021, 152: 112216
[53] Li J Z, Cheng C H K. Evolution of gonadotropin signaling on gonad development: Insights from gene knockout studies in zebrafish [J]. Biology of Reproduction, 2018, 99(4): 686-694