随着塑料制品在生产和生活中的广泛使用,中国已经成为全球最大的塑料生产国。由于使用的需要,这些塑料制品通常耐高温,耐酸碱,耐腐蚀,目前还没有正确的方式处理塑料垃圾,这些塑料垃圾在自然环境下几乎不会被完全降解,而是通过生物以及非生物途径逐渐降解为微米或纳米级的碎片[1-3],更容易发生转移和扩散,导致微塑料和纳米塑料对环境的污染。
根据塑料颗粒的粒径不同,可以将其划分为微塑料(microplastics, MPs)(直径<5 mm)[4]和纳米塑料(nano-plastics, NPs),目前有关NPs的粒径划分标准尚存争议,大部分学者将粒径<1 μm的塑料颗粒定义为NPs[5-6]。随着对人体中MPs和NPs研究的不断深入,研究者们已经在人类血液、胎盘和粪便中发现了MPs和NPs[7-9]。MPs和NPs主要通过食物、水以及空气进入人体[10-11]。小鼠暴露实验发现,MPs和NPs进入体内后在胃、肠、肝脏等器官积累[12-13]。从消化道进入的MPs和NPs由于耐腐蚀性高,消化液会改变MPs和NPs颗粒的表面粗糙度和粒径[12],使它们更稳定地停留在消化道内壁,也更易吸附其他有毒物质从而增加毒性[14]。实际上,组织内的屏障并不能阻止MPs和NPs的入侵,MPs和NPs进入人体后,小粒径的塑料颗粒可以跨越消化系统的上皮屏障[15-17],从而进入淋巴和血液循环,粒径范围在0.1~10 μm的纳米级塑料颗粒甚至可以穿过血脑屏障和胎盘[18-20]。饮食饮水摄入的MPs和NPs颗粒,若其粒径>150 μm,通常很难穿过肠道上皮细胞,导致几乎90%的MPs通过粪便排出体外[5],剩下的只能在肠道上皮细胞膜外产生局部影响,而<150 μm的纳米级塑料颗粒接触到小肠绒毛时,这些小粒径的塑料颗粒则会穿过小肠上皮细胞[21],进入淋巴系统[22]和血液[18, 23],通过毛细血管最终到达门静脉,扩散至全身[24-26]。对于<150 μm的纳米级塑料颗粒物,其中>10 μm的部分塑料颗粒(0.1%)可到达其他器官和细胞膜表面[19],而<5 μm的纳米级颗粒物则会被淋巴细胞吸收[21]。随着有关纳米级塑料颗粒进入哺乳动物细胞机制研究的逐渐深入,可以归纳出以下3种纳米级颗粒进入淋巴和血液循环的机制:(1)小粒径的纳米粒子通过细胞间紧密连接旁路,扩散进入血液[27],肠道上皮杯状细胞分泌的黏液是促进其旁路扩散的因素[21];(2)较大一些的纳米粒子(50~200 nm)更倾向于通过内吞作用穿过肠道上皮细胞[21],并且可能存在黄金摄入尺寸,例如40 nm可能是非吞噬细胞摄取的最佳尺寸[28],而200 nm可能是穿过血脑屏障的最佳尺寸[29]。体内研究发现,肠道细胞可利用不同的内吞机制,甚至多种内吞机制联合使用,内化纳米级颗粒物,例如吞噬细胞可通过吞噬作用将其内化[30],而非吞噬细胞可以借助网格蛋白或细胞膜内陷介导的内吞作用内化较小的纳米颗粒[27],在这一过程中肌动蛋白发挥重要作用[31]。另外,能量依赖性途径也是肠道上皮细胞内吞作用的机制之一[31]。最近的研究发现,<3 μm的纳米级颗粒可通过非特异性内吞机制内化到非吞噬细胞[31],且可利用内吞作用的微粒粒径上限增加到了5 μm[19, 21],肠道Peyer斑中丰富的M细胞的内吞作用是粒径上限增加的主要原因[23],此外肠道黏膜的协助也是可能的影响因素之一[32-33];(3)带正电的粒子与质膜的高度结合会增加表面张力并导致膜穿孔或变形[34],从而使纳米级塑料颗粒进入细胞,进而进入血液循环。除了被消化道吸收的纳米级塑料颗粒,经呼吸系统进入肺部的MPs和NPs通常会滞留在肺部或者通过毛细血管进入体内循环系统,<2.5 μm的塑料颗粒会进入肺部深处或渗透肺泡进入血液循环[35]。这些进入血液循环的纳米级塑料颗粒,其中粒径在100 nm左右的会被血清白蛋白包围[36],形成多层血清白蛋白冠,可能有助于纳米塑料逃避免疫监视,增加其在血液循环系统的时间,并帮助颗粒到达次级器官并在肝脏、肾脏和肠道中积累[36]。血清白蛋白与纳米级塑料颗粒的结合导致蛋白质的二级结构发生改变[37],从而增加了塑料颗粒的细胞毒性[27, 36]。
尽管只有小部分纳米级塑料颗粒能够穿透肺泡和胃肠道的上皮屏障,转移到次级组织器官内,但考虑到人类对塑料颗粒的长期接触以及塑料颗粒可能产生积累,这种低比例的内化仍不容忽视,因为它们能够进一步引起一系列毒性效应(图1),包括氧化应激、局部炎症、细胞凋亡以及肠道菌群的改变[38-41]。流行病学调查发现,吸入MPs和NPs会增加对呼吸道刺激的几率,一部分职业性接触MPs和NPs的工人患有间质性肺病,肺组织病理学检查发现,该病是MPs和NPs作为半抗原刺激呼吸道,引发肺泡炎症产生的[42-43]。体内研究表明,小鼠摄入MPs后,胃中的幽门螺旋杆菌与MPs相互作用,促进了幽门螺旋杆菌在胃黏膜上皮细胞的快速定植[12],这种致病菌的大量繁殖导致小鼠胃部炎症的发生。肠道菌群的失调也是MPs产生的毒性效应之一,在多项体外研究中均发现MPs导致小鼠肠道菌群紊乱,条件致病菌数量增多,同时伴随肠道炎症[44-46]。NPs对肝脏的毒性效应主要表现为糖脂代谢紊乱,NPs暴露后的小鼠,体内葡萄糖含量升高并伴随糖尿病发生[47],脂肪质量减少,肝脏中甘油三酯和总胆固醇水平下降[48]。进入人体的NPs最终进入并积累在组织内的各种细胞中构成潜在威胁。体外研究发现纳米级塑料颗粒可进入细胞,产生毒性效应[10, 49]。人胃黏膜上皮细胞与NPs共培养后,发现细胞增殖速率降低,细胞凋亡增加[50]。在人肠道细胞中也发现NPs会导致细胞产生氧化应激[51]。
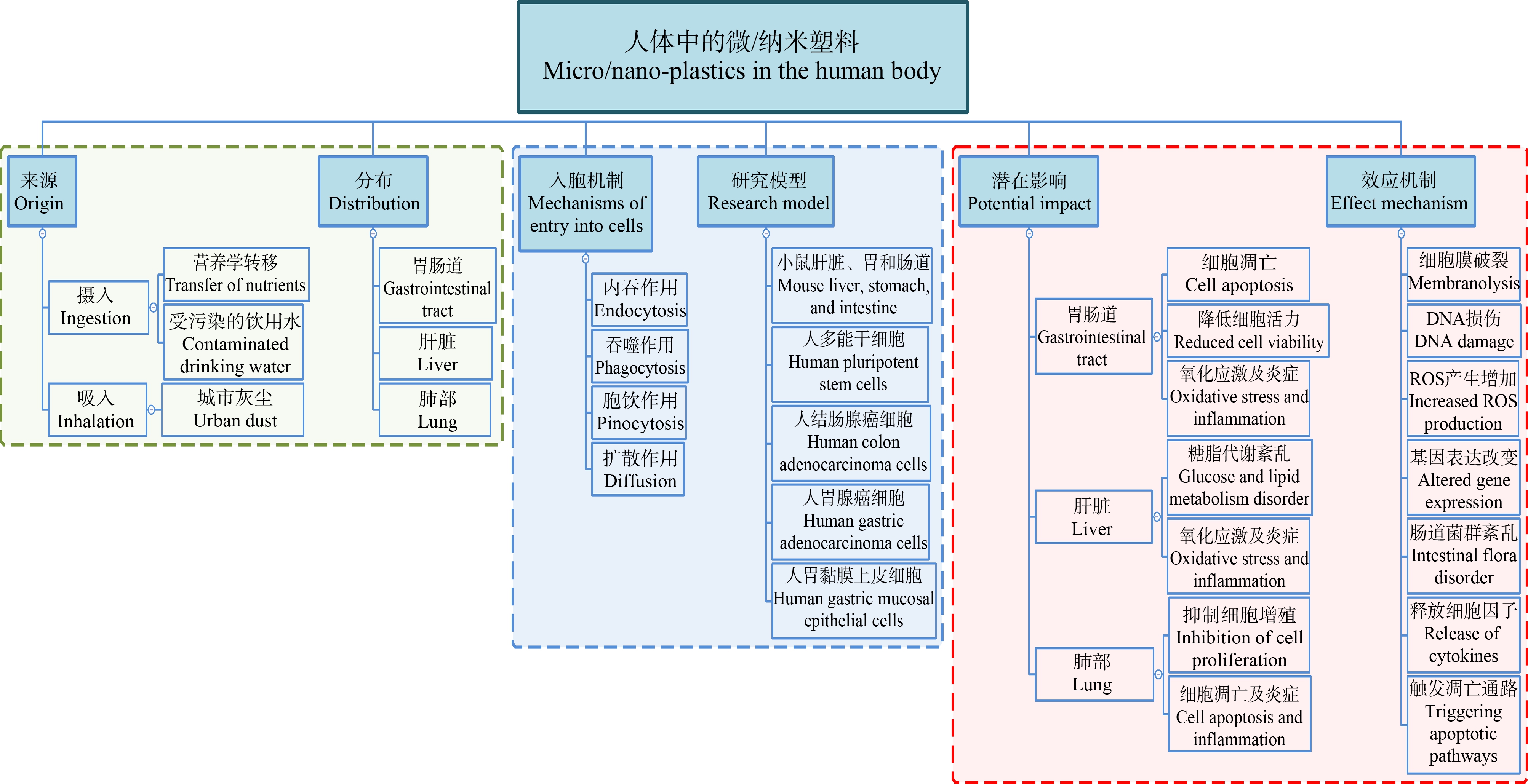
图1 人体中微塑料和纳米塑料的来源、分布及影响
Fig. 1 Source, distribution and impact of microplastics and nano-plastics in human body
尽管目前没有研究证明MPs和NPs会通过食物链传递至人体内,但现有的体内外研究已经展现出MPs和NPs进入体内产生的不良后果。目前MPs和NPs积累对人体的毒性效应研究还不深入,因此聚焦于MPs和NPs对人体产生的毒性效应机制,对了解MPs和NPs对人体健康的影响有重要意义,同时也为今后预防和治疗MPs和NPs导致的人体疾病提供科学依据。本篇综述将归纳总结MPs和NPs对人体胃肠道及肝脏的毒性效应机制,提出目前研究存在的问题和不足以及未来可能的发展方向,为今后研究MPs和NPs对人体的毒性效应及机制提供科学依据。
1 引发胃肠道(gastrointestinal tract, GIT)氧化应激、炎症及细胞凋亡的毒性效应机制(Toxic mechanism of oxidative stress, inflammation and apoptosis in GIT)
人体中的MPs和NPs经不同的内吞机制进入细胞或吸附聚集在胃肠道组织表面,引起氧化应激和炎症,甚至细胞凋亡,该现象已经在多项体外研究及小鼠的体内研究中得到证实(表1)。MPs和NPs对人体胃肠道健康的危害日益显现,因此探究MPs和NPs对胃肠道的毒性效应机制,为防治MPs和NPs引起的胃肠道疾病提供科学依据。
表1 微塑料和纳米塑料对胃肠道及肝脏产生的毒性效应及机制
Table 1 Toxic effects and mechanism of microplastics and nano-plastics on human body

器官/细胞Organs/Cells聚合物Polymer剂量Dose毒性效应Toxicity effect产生机制Generation mechanism体内研究(小鼠)In vivo studies (mice)肝脏、结肠、回肠和盲肠内容物[13]Liver, colon, ileum and cecum contents[13]PS100, 1 000 μg·L-1脂质代谢紊乱Disorder of lipid metabolism肠道屏蔽功能障碍Intestinal shielding dysfunction肠道菌群改变Changes in intestinal flora胃[12]Stomach[12]PE100 μg·mL-1促进幽门螺旋杆菌在胃黏膜上皮细胞快速定植Promote the rapid colonization of Helicobacterpylori in gastric mucosal epithelial cells胃部损伤及炎症Gastric injury and inflammation髓过氧化物酶表达增加Increased expression ofmyeloperoxidaseIL-6和TNF-α表达上调Up-regulation of IL-6 and TNF-α结肠和十二指肠[44]Colon and duodenum[44]PE6, 60, 600 μg·d-1肠道炎症Intestinal inflammationIL-1α表达上调Up-regulation of IL-1α肠道菌群改变Changes in intestinal flora免疫失衡Immune imbalanceTLR4、AP-1和IRF5表达上调Up-regulation of TLR4, AP-1 and IRF5结肠[45]Colon[45]PS500 μg·L-1肠道屏障受损和肠道炎症Impaired intestinal barrier and intestinal inflammation肠道致病菌增加Increased intestinal pathogenic bacteria干扰肠道微生物代谢Interference with intestinal microbial metabolism免疫失衡Immune imbalance肠道菌群改变Changes in intestinal flora炎症因子(TNF-α、IL-1β和IFN-γ)表达上调Up-regulation of inflammatory factors (TNF-α, IL-1β and IFN-γ)小肠和盲肠[46]Small intestines and cecum[46]PE5.25×104 particles·d-1肠道通透性增加Increased intestinal permeability肠道炎症Intestinal inflammation代谢紊乱Metabolic disorders改变肠道菌群组成Changes in the composition of intestinal flora能量代谢和免疫功能相关细菌相对丰度减少Reducing the relative abundance of bacteria related to energy metabolism and immune function氧化应激、免疫应答和脂质代谢相关基因表达下调Down-regulation of genes related tooxidative stress, immune response and lipid metabolism肝、结肠、盲肠内容物、回肠[48]Liver, colon, cecum contents and ileum[48]PS100, 1 000 μg·L-1减少肠道黏液分泌Decreased secretion of intestinal mucus损害肠道屏障功能Damage to intestinal barrier function肠道微生物群失调和代谢紊乱Dysbiosis of the gut microbiome and metabolic disorders/
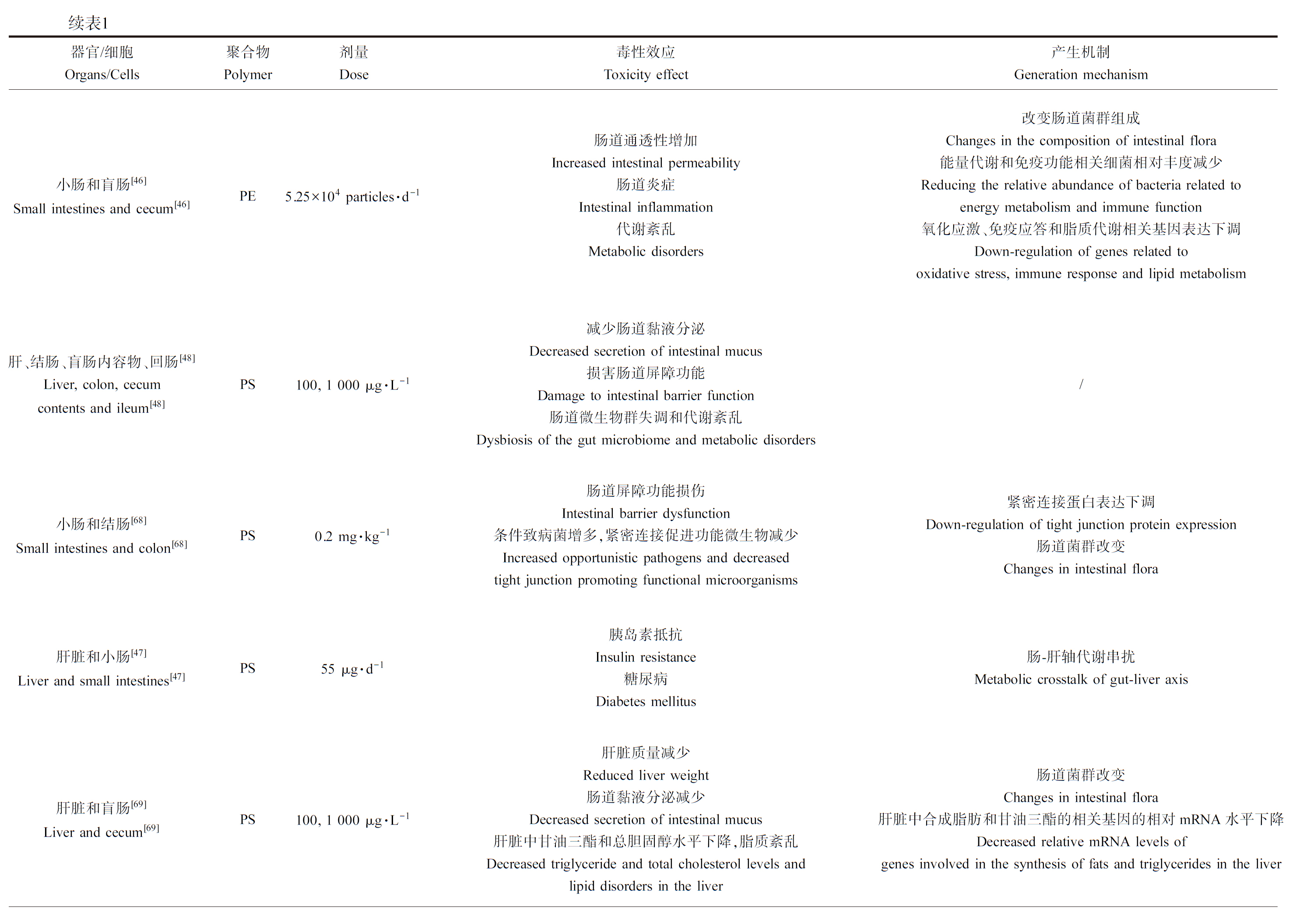
续表1器官/细胞Organs/Cells聚合物Polymer剂量Dose毒性效应Toxicity effect产生机制Generation mechanism小肠和结肠[68]Small intestines and colon[68]PS0.2 mg·kg-1肠道屏障功能损伤Intestinal barrier dysfunction条件致病菌增多,紧密连接促进功能微生物减少Increased opportunistic pathogens and decreasedtight junction promoting functional microorganisms紧密连接蛋白表达下调Down-regulation of tight junction protein expression肠道菌群改变Changes in intestinal flora肝脏和小肠[47]Liver and small intestines[47]PS55 μg·d-1胰岛素抵抗Insulin resistance糖尿病Diabetes mellitus肠-肝轴代谢串扰Metabolic crosstalk of gut-liver axis肝脏和盲肠[69]Liver and cecum[69]PS100, 1 000 μg·L-1肝脏质量减少Reduced liver weight肠道黏液分泌减少Decreased secretion of intestinal mucus肝脏中甘油三酯和总胆固醇水平下降,脂质紊乱Decreased triglyceride and total cholesterol levels andlipid disorders in the liver肠道菌群改变Changes in intestinal flora肝脏中合成脂肪和甘油三酯的相关基因的相对mRNA水平下降Decreased relative mRNA levels ofgenes involved in the synthesis of fats and triglycerides in the liver肝脏[70]Liver[70]PS0.5 mg·d-1影响肝脏免疫微环境Affect the liver immune microenvironment肝脏局部组织炎症Local tissue inflammation in the liver增加NK细胞和巨噬细胞的免疫浸润,减少B细胞的免疫浸润Increased immune infiltration of NK cells and macrophages and decreased immune infiltration of B cells谷丙转氨酶和谷草转氨酶表达增加Increased expression of alanine aminotransferase and aspartate aminotransferase激活NF-κB信号通路Activation of the NF-κB signaling pathway体外研究In vitro studies人胃腺癌细胞[64]Human gastric adenocarcinoma cells[64]PS2~30 μg·mL-1影响细胞活力和形态Decreased cell viability and morphology炎症InflammationIL-6和IL-8表达上调Upregulation of IL-6 and IL-8人胃黏膜上皮细胞[50]Human gastric mucosal epithelial cells[50]PS50 μg·mL-1细胞增殖速率降低Decreased cell proliferation rate细胞凋亡增加Increased cell apoptosis/人结肠腺癌细胞[17, 56]Human colon adenocarcinoma cells[17, 56]PS0~200 μg·mL-1, 0.01~100 μg·mL-1降低细胞活力氧化应激和炎症Decreased cell viability; oxidative stress and inflammation线粒体凋亡 Mitochondrial apoptosisHSP70、HO1表达上调,IL-1β表达上调The expression of HSP70 and HO1 was up-regulated, and the expression of IL-1β was up-regulated 线粒体膜电位增加The mitochondrial membrane potential increased

续表1器官/细胞Organs/Cells聚合物Polymer剂量Dose毒性效应Toxicity effect产生机制Generation mechanism人胃癌细胞株[66]Human gastric carcinoma cell line[66]PS0.1~100 μg·mL-1降低细胞活力Decreased cell viability诱导细胞凋亡或坏死 Apoptosis or necrosis was induced破坏细胞膜完整性Disruption of cell membrane integrity;bax表达上调Up-regulation of bax expression Caspase-3和Caspase-8蛋白酶表达增加 The expression of Caspase-3 and Caspase-8 protease was increased人结肠腺癌细胞[51]Human colon adenocarcinoma cells[51]PS100 μg·mL-1氧化应激Oxidative stress改变氧化应激相关基因表达Altered expression of oxidative stress-related genesHO1和SOD2转录水平显著增加HO1 and SOD2 transcript levels were significantly increased人多能干细胞产生的肝脏类器官[40]Liver organoids[40]PS0.25~25 μg·mL-1破坏代谢酶的功能,增加脂质积累Disrupt the function of metabolic enzymes; increased lipid accumulationROS生成、氧化应激和炎症反应ROS production; oxidative stress and inflammatory response肝细胞毒性HepatotoxicityASL和ALT释放增加The release of ASL and ALT increased破坏肝功能相关基因表达Disruption of gene expression related to liver functionHNF4A和CYP2E1表达上调The expression of HNF4A and CYP2E1 was up-regulatedIL-6和COL1A1表达上调 Up-regulated expression of IL-6 and COL1A1

注: PS表示聚苯乙烯;PE表示聚乙烯;ROS表示活性氧自由基;ASL表示精氨琥珀酸裂解酶;ALT表示谷丙转氨酶;IL-6表示白细胞介素6;IL-8表示白细胞介素8;IL-1β表示白细胞介素1β;IL-1α表示白细胞介素1α;TNF-α表示肿瘤坏死因子α;IFN-γ表示γ干扰素。
Note: PS means polystyrene; PE means polyethylene; ROS means reactive oxide species; ASL means argininosuccinate lyase; ALT means alanine transaminase; IL-6 means interleukin-6; IL-8 means interleukin-8; IL-1β means interleukin-1β; IL-1α means interleukin-1α; TNF-α means tumor necrosis factor α; IFN-γ means interferon-γ.
1.1 细胞中的活性氧(reactive oxygen species, ROS)诱导氧化应激的产生(ROS in cells induce the generation of oxidative stress)
细胞内拥有一套抗氧化防御系统,可以维持细胞内ROS的水平,保护重要的生物分子免受自由基的伤害[52-53]。细胞中活性氧和氧化应激的增加与抗氧化系统失衡和疾病有关[54]。体内外研究表明,MPs和NPs暴露后,细胞内ROS水平升高,一方面是外源颗粒的直接刺激作用诱导细胞内ROS的产生增加[55];另一方面,MPs和NPs抑制抗氧化酶转录因子的产生或降低抗氧化酶的活性,进而抑制ROS代谢,使线粒体膜电位增加,导致线粒体通透性和ROS产生增加,进而加速线粒体中产生的ROS向胞质转移[17, 56]。超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽(GSH)是衡量机体氧化应激程度重要而不可或缺的生物标志物[57]。研究发现,聚苯乙烯纳米塑料可导致小鼠十二指肠内过氧化生物标志物水平升高,SOD、CAT活性和GSH含量明显降低[58-59];人正常结肠黏膜上皮细胞的体外实验也发现,经NPs处理的细胞中ROS水平比未经处理细胞的ROS水平高[58]。因此,MPs和NPs可直接促进ROS产生或通过抑制抗氧化酶活性和谷胱甘肽的产生进而抑制ROS代谢,间接导致ROS增加。随着MPs和NPs与细胞微环境的相互作用,增加的ROS在MPs和NPs颗粒表面沉降,导致细胞产生氧化应激,如果它们不能穿过细胞膜,则会诱导肠道局部炎症[60],如果颗粒足够小,可以穿过肠道上皮细胞,此时位于颗粒表面的ROS毒性就会增强,介导细胞产生应激反应[61](图2)。

图2 引起胃肠道(GIT)氧化应激、炎症及细胞凋亡的潜在机制
注:微塑料和纳米塑料一方面直接刺激细胞产生ROS,另一方面调控细胞抗氧化系统抑制ROS代谢从而导致ROS激增,诱导氧化应激的产生;微塑料和纳米塑料可直接刺激细胞产生炎症细胞因子或通过氧化应激诱导炎症反应,产生的炎症反应和氧化应激最终导致细胞凋亡。
Fig. 2 Potential mechanisms causing oxidative stress, inflammation and apoptosis in the gastrointestinal tract (GIT)
Note: On the one hand, microplastics and nano-plastics directly stimulate cells to produce ROS, on the other hand, they regulate cellular antioxidant system to inhibit ROS metabolism and lead to ROS surge, resulting in a surge of ROS and inducing oxidative stress; microplastics and nano-plastics can directly stimulate cells to produce inflammatory cytokines or induce inflammatory response through oxidative stress; the resulting inflammatory response and oxidative stress eventually lead to cell apoptosis.
1.2 炎症产生的潜在机制(The underlying mechanism of inflammation)
胃肠道是MPs和NPs经摄入途径进入人体后发生积累的组织器官,MPs和NPs带来的机械损伤或刺激很容易造成胃肠道的炎症反应[12, 44],炎症反应的产生机制可以分为2个方面:(1)促炎细胞因子释放产生的炎症反应[12];(2)肠道菌群失调,条件致病菌增多,导致免疫失衡以及脂多糖含量增加刺激炎症产生[46, 62]。
MPs和NPs可通过2种机制诱导促炎细胞因子释放(图2),一种机制是MPs和NPs直接刺激产生促炎细胞因子。在小鼠体内实验中,IL-6和TNF-α升高促进小鼠胃部损伤及炎症[12]。在体外研究中,MPs和NPs处理后的胃上皮细胞和小肠上皮细胞均发现促炎反应相关基因(如IL-1β、IL-6、IL-8)发生不同程度的基因表达上调[63-64],导致促炎细胞因子释放增多。另一种机制是氧化应激促进炎症发生,通过氧化应激激活NF-κB、p53、PPAR-γ和Nrf2等多种转录因子,而这些转录因子可以调控炎症细胞因子的表达[65],从而增加促炎细胞因子的释放。
体内研究表明,MPs和NPs会导致小鼠胃肠道菌群紊乱。MPs和NPs通过与幽门螺旋杆菌相互作用促进其在胃黏膜上皮细胞表面快速定植[12],提高NPs进入组织的效率,促进炎症发生[12]。MPs和NPs引起肠道菌群失调,特别是免疫功能相关的细菌相对丰度显著减少[46],致病菌的菌群数量增加,降低CD4+细胞的Th17/Treg细胞百分比,导致免疫失衡,同时血浆脂多糖含量增加[62],刺激肠道炎症产生[45],还有研究发现,肠道菌群失调后,小鼠体内TLR4、AP-1和IRF5基因表达上调,以此介导肠道炎症反应[44]。
1.3 细胞凋亡产生的潜在机制(Potential mechanisms of apoptosis)
内源性和外源性因子均可导致DNA损伤,已有研究发现粒径足够小的NPs可穿过核膜直接造成DNA损伤。另外MPs和NPs导致细胞内ROS水平激增引起的氧化应激也是导致DNA损伤的原因,若DNA损伤修复不及时则诱导细胞凋亡的发生。在体外实验中经常可以观察到由氧化应激引起的细胞凋亡[63, 66],氧化应激引起细胞凋亡的同时还伴随线粒体膜电位的升高。一项以HaCaT细胞为模型的研究发现,在体外模拟氧化应激的条件下,细胞内INF2表达增加,导致线粒体中的ROS超负荷,打破细胞氧化还原平衡,改变线粒体膜电位,引起线粒体应激,同时抑制HIF-1信号通路介导细胞凋亡[67]。Bcl-2相关X蛋白(Bcl2-associated X, Bax)是Bcl-2蛋白家族的成员,它可以调节凋亡诱导因子的释放,bax的过表达可能是细胞凋亡发生的另一个原因[66]。此外,Bax还可调节线粒体外膜的透化作用,其表达的增加使线粒体膜通透性增加,从而促使凋亡因子从线粒体释放到细胞质中,激活半胱天冬酶,导致细胞凋亡。事实上,Bax的N端乙酰化参与了其线粒体靶向作用,因此bax基因表达的上调导致线粒体膜通透性增加,使线粒体内ROS外溢,导致细胞中ROS激增进而引发凋亡。另外,MPs和NPs导致的炎症反应最终也会引发细胞凋亡。
综上所述,MPs和NPs引起氧化应激、炎症及细胞凋亡的机制为:ROS产生增加或ROS代谢减少造成细胞内ROS激增,引起DNA损伤和氧化应激;胃肠道菌群失调引起的免疫失衡以及炎症相关细胞因子表达上调导致炎症反应发生;氧化应激和炎症反应最终会导致细胞凋亡,此外MPs和NPs还可使促凋亡相关基因过表达直接导致细胞凋亡(图2)。
2 引发肝脏糖脂代谢紊乱的毒性效应机制(Toxic effect mechanism of liver glucose and lipid metabolism disorder)
肝脏是人体重要的排毒解毒器官,MPs和NPs经食物进入人体后聚集在胃肠道上皮细胞表面,纳米级塑料颗粒则被上皮细胞吸收进入淋巴和血液循环,最终经门静脉到达肝脏[11],相关研究还发现NPs富集后,会扰乱肝脏组织的糖脂代谢[47, 69],在人类肝脏类器官的体外研究中也发现了类似的毒性效应(表1)。目前,越来越多的研究聚焦于NPs造成肝脏组织糖脂代谢紊乱的毒性机制[13, 47, 62],主要是从生化和转录组学方面展开分析,发现NPs会从生化和转录水平影响糖脂代谢,其机制大致可以总结为以下2点:(1)在生化水平上影响糖代谢中间代谢物的产生(图3);(2)在转录水平上影响糖脂代谢中关键/限速酶的产生(图4)。

图3 在生化水平影响糖脂代谢的潜在机制
注:在生化水平,纳米塑料通过抑制ChREBP的合成进而抑制丙酮酸激酶、ATP柠檬酸裂解酶、棕榈酸-5-羟基硬脂酸产生,阻碍了乙酰辅酶A的合成,发生胰岛素抵抗,导致葡萄糖含量升高,最终导致Ⅱ型糖尿病风险增加;ChREBP的合成减少还会抑制成纤维细胞因子21的合成,减少脂蛋白分解,从而增加血浆中甘油三酯的含量,最终导致高血脂;纳米塑料还通过抑制脂肪酸转运体和脂肪酸转运蛋白2的合成,激活载脂蛋白和脂肪酸结合蛋白6的合成,使肝细胞中脂肪酸合成减少、转出增多,导致脂肪储存减少,最终增加脂肪营养不良综合征的风险;ChREBP表示碳水化合物调节元件结合蛋白。
Fig. 3 Potential mechanisms affecting glucose and lipid metabolism at the biochemical level
Note: At the biochemical level, nano-plastics inhibit the synthesis of ChREBP and then inhibit the production of pyruvate kinase, ATP citrate lyase, and palmitic acid-5-hydroxystearic acid, which hinder the synthesis of acetyl-CoA and lead to insulin resistance, leading to the increase of glucose content, and ultimately leading to the increased risk of type 2 diabetes; the decreased synthesis of ChREBP can also inhibit the synthesis of fibroblast factor 21 and reduce the decomposition of lipoproteins, thereby increasing the content of triglyceride in plasma and eventually leading to hyperlipidemia; nano-plastics also inhibit the synthesis of fatty acid transporter and fatty acid transporter 2, activate the synthesis of apolipoprotein and fatty acid binding protein 6, reduce the synthesis of fatty acid and increase the export of fatty acid in hepatocytes, resulting in the reduction of fat storage and ultimately increasing the risk of lipodystrophy syndrome; ChREBP means carbohydrate regulatory element-binding proteins.

图4 在转录水平影响糖脂代谢的潜在机制
注:(a) 纳米塑料对糖代谢关键限速酶转录水平的影响:纳米塑料上调HK1的转录水平,下调PKLR和CS的转录水平,促进己糖激酶的合成,抑制丙酮酸激酶和柠檬酸合成酶的合成,导致果糖积累,丙酮酸合成减少,抑制了三羧酸循环和乙酰辅酶A的产生,最终增加高血糖的风险;(b) 纳米塑料对脂质代谢关键限速酶转录水平的影响:纳米塑料上调PPAR-γ、ACC1、SREBP1α和PPAR-α的转录水平,下调DGAT2、Aco1和Cpt1的转录水平,抑制甘油三酯的合成,加速脂肪酸β氧化,最终导致脂肪储存减少,产生脂肪营养不良综合征;DGAT2表示二酰甘油酰基转移酶;PPAR-α表示过氧化物酶体增殖物激活受体α;PPAR-γ表示过氧化物酶体增殖物激活受体γ;ACC1表示乙酰辅酶A羧化酶1;SREBP1α表示固醇调节元件结合蛋白1α;Aco1表示乙酰辅酶A氧化酶1;Cpt1表示肉毒碱棕榈酰基转移酶1。
Fig. 4 Potential mechanisms affecting glucose and lipid metabolism at the transcriptional level
Note: (a) Effects of nano-plastics on transcription levels of key rate-limiting enzymes of glucose metabolism: Nano-plastics up-regulate the transcription level of HK1, down-regulate the transcription level of PKLR and CS, promote the synthesis of hexokinase, inhibit the synthesis of pyruvate kinase and citrate synthase, lead to the accumulation of fructose, reduce the synthesis of pyruvate, inhibit the tricarboxylic acid cycle and the production of acetyl-CoA, and ultimately increase the risk of hyperglycemia; (b) Effects of nano-plastics on transcription levels of key rate-limiting enzymes in lipid metabolism: Nano-plastics can up-regulate the transcriptional levels of PPAR-γ, ACC1, SREBP1α and PPAR-α, down-regulate the transcriptional levels of DGAT2, Aco1 and Cpt1, inhibit the synthesis of triglyceride, accelerate the oxidation of fatty acid β, and eventually lead to the reduction of fat storage and the development of lipodystrophy syndrome; DGAT2 means diacylglycerol acyltransferase; PPAR-α means peroxisome proliferator-activated receptor α; PPAR-γ means peroxisome proliferator-activated receptor γ; ACC1 means acetyl-CoA carboxylase 1; SREBP1α means sterol regulatory element-binding protein 1α; Aco1 means acetyl-CoA oxidase 1; Cpt1 means carnitine palmitoyltransferase 1.
2.1 在生化水平上影响糖脂代谢中间代谢物的产生(Affecting the production of intermediate metabolites for glycolipid metabolism at the biochemical level)
NPs会通过影响中间代谢物的产量继而对糖脂代谢造成影响。丙酮酸是糖酵解途径的重要中间代谢物,也是连接糖脂代谢的重要枢纽,其产量的增加可能是丙酮酸激酶(PK)和磷酸烯醇丙酮酸羧激酶(PEPckc)的水平升高所致[69, 71-72],可促进糖代谢向脂质代谢的转化,导致脂肪酸产生增加;肝脏内葡萄糖和胆固醇水平的升高,则可能增加人体罹患Ⅱ型糖尿病、高血脂和脂肪肝的风险[71]。研究发现,摄入NPs后,肝脏组织内参与糖代谢调控的重要因子和催化酶的生化水平会发生改变,小鼠在摄入NPs后其肝脏细胞中碳水化合物调节元件结合蛋白(ChREBP)的表达量显著降低[69],该蛋白通过抑制PK和ATP-柠檬酸裂解酶(ACL)的产生,阻碍葡萄糖转化为乙酰辅酶A,使肝脏中的糖原不断积累,增加人体罹患Ⅱ型糖尿病的风险[73]。此外ChREBP合成的减少还会导致棕榈酸-5-羟基硬脂酸的合成量减少,研究表明棕榈酸-5-羟基硬脂酸可以增加脂肪组织中胰岛素的敏感性[74],还可以通过激活G蛋白偶联受体40(GPR40)增加胰岛素的分泌[75],因此NPs直接导致ChREBP表达降低后,间接抑制了胰岛素的敏感性和分泌量,进而阻碍糖酵解途径,导致糖代谢紊乱[76],还有研究发现,NPs可增加组织中乳酸脱氢酶(LDH)和柠檬酸合酶(CS)的活性,这2种酶是参与糖酵解和糖异生的关键酶,其活性增加导致糖代谢紊乱,但目前关于NPs影响酶活性的具体机制尚不明确[77]。
在影响脂质代谢方面,NPs造成ChREBP表达量的降低导致肝细胞中成纤维细胞生长因子21(FGF21)的合成量下降,从而抑制了FGF21通过加速脂肪组织中脂蛋白分解降低血浆甘油三酯的功能,因此血浆中甘油三酯堆积,导致人体患高血脂的风险增加[78-79]。血液中的游离脂肪酸进入肝细胞后帮助肝脏组织内脂肪酸的合成,但有研究发现,NPs处理肝细胞后,脂肪酸转运蛋白2(FATP2)和脂肪酸转运体(FAT)合成量降低[69],因此阻碍了血液中的脂肪酸向肝脏运输,间接阻碍了肝脏脂肪酸的合成;同时还有研究发现,NPs处理肝细胞后,载脂蛋白和脂肪酸结合蛋白6(FABP6)的合成量显著升高,这2个蛋白参与脂肪酸的转运出胞过程,因此肝脏脂肪酸的水平降低使得甘油三酯的合成不足,间接影响脂肪储存,脂肪储存严重缺乏的情况下可能导致脂肪营养不良[80]。脂肪营养不良综合征是一种代谢疾病,会导致和肥胖患者类似的代谢并发症,如胰岛素抵抗、糖尿病、肝脂肪变性和血脂异常[81]。
因此,NPs在生化水平影响糖脂代谢的机制为:在糖代谢方面,NPs通过抑制ChREBP的合成,阻碍葡萄糖转化为乙酰辅酶A以及抑制胰岛素的敏感性和分泌量,共同导致葡萄糖的积累,从而造成糖代谢的紊乱;在脂质代谢方面,NPs通过同时抑制脂肪酸产生和促进脂肪酸的转运出胞,间接导致甘油三酯含量降低,使脂肪储存减少。
2.2 在转录水平上影响糖脂代谢中关键/限速酶的产生(Affecting the production of intermediate metabolites for glycolipid metabolism at the biochemical transcription level)
目前的研究已经发现NPs可以对参与糖代谢的关键限速酶包括己糖激酶1(HK1)、丙酮酸激酶(PK)和柠檬酸合酶(CS)产生影响。斑马鱼摄入聚苯乙烯微塑料后,提取实验组和对照组的肝脏组织进行转录组分析,发现实验组中PK的转录水平与对照组相比明显下调,HK1的转录水平明显上调[72];HK1是己糖激酶家族成员,催化葡萄糖转化为果糖,HK1转录水平上调导致HK1合成量增加,加速葡萄糖转化为果糖的过程,又由于PK的转录水平下调,果糖转化为丙酮酸的过程被抑制,导致果糖积累;而积累的果糖经血液循环到达肠道,并积累在肠道中,则被肠道菌群利用产生乙酸盐,在肠道产生的乙酸盐通过门静脉到达肝脏转化为乙酰辅酶A,用作脂肪生成的底物,进而导致脂肪生成增加[82]。CS是三羧酸循环的关键酶,可将草酰乙酸转化为柠檬酸,通过转录组分析发现微塑料导致CS的转录水平降低[69],CS转录水平降低后会导致乌头酸和α-酮戊二酸的合成减少[83],影响三羧酸循环进而导致糖脂代谢紊乱。
在转录水平上影响脂肪酸的合成及β-氧化也是NPs影响脂质代谢的机制之一。脂肪酸合成和β-氧化是糖脂代谢的重要组成部分,在研究NPs对肝脏脂质代谢的影响时通常把脂肪酸合成和β-氧化过程的关键酶作为重要生物标志物[84],检测相关基因的转录水平,经转录组分析,进而发现NPs对糖脂代谢通路产生的影响及涉及的信号通路[85-86]。研究表明,NPs主要通过促进乙酰辅酶A羧化酶1(Acc1)、固醇调节元件结合蛋白1α(SREBP1α)以及脂肪酸合酶(FAS)的mRNA转录,促进脂肪酸的合成,抑制乙酰辅酶A氧化酶(Aco)和肉毒碱棕榈酰基转移酶1(Cpt1)的mRNA转录,抑制脂肪酸的β-氧化[71]。过氧化物酶体增殖物激活受体α(PPAR-α)和过氧化物酶体增殖物激活受体γ(PPAR-γ)是核激素受体家族中的配体激活受体,可作为转录激活蛋白调控过氧化物酶体中氧化酶的表达,过氧化物酶体中包含多种氧化酶,参与多种代谢,包括脂肪酸的β-氧化、胆汁酸和胆固醇代谢[87]。研究发现,NPs会对PPAR信号通路造成影响——上调PPAR-α和PPAR-γ的转录水平[71],因此PPAR-α的转录水平上调后,导致过氧化物酶体中氧化酶的含量增加,进而促进脂肪酸的β-氧化,胆汁酸和胆固醇代谢,如果肝脏中过氧化物酶体的大小和数量显著增加,则会导致肝脏肥大、肝增生和肝癌的发生[84]。PPAR-γ参与脂肪细胞的分化与成熟[88],其转录水平上调会促进脂肪合成进而造成脂质代谢紊乱。二酰甘油酰基转移酶(DGAT)是脂肪细胞中合成甘油三酯和脂滴的关键酶,在调节脂质代谢过程中发挥重要作用[89]。NPs会抑制DGAT的mRNA转录,导致DGAT表达减少,抑制脂滴和脂肪酸形成,进而减少脂肪储存[69, 71]。小鼠实验中发现,DGAT2缺陷的小鼠出生后不久即因能量代谢底物的严重减少和皮肤渗透屏障功能受损最终死亡[90],因此NPs抑制DGAT的mRNA转录,不仅影响脂质代谢,还会对皮肤渗透屏障功能造成一定的损伤,最终危害人体健康。
综上所述,MPs和NPs经食物进入人体后,纳米级塑料颗粒可经循环系统到达肝脏致使肝脏糖脂代谢紊乱,进而导致肝脏肥大、肝增生,诱发Ⅱ型糖尿病、高血脂以及脂肪营养不良综合征,甚至促使肝癌的发生。
3 总结和展望(Summary and prospects)
由于MPs和NPs在地球生物圈广泛存在,其对人类健康产生的影响也日益得到人们关注。近年来,陆续有研究证明人类可通过吸入或摄入的方式不断接触MPs和NPs。MPs和NPs进入人体后,较大的颗粒可通过粪便排除,而较小的颗粒往往经胃液和肠道黏液处理后在胃肠道积累[91],粒径足够小则会被细胞吸收[18]。目前研究表明有一小部分颗粒能够跨越肺部和肠道屏障,在组织和器官中积累,特别是<150 μm的颗粒能够从肠道腔到淋巴和循环系统,引起全身组织的暴露和积累,包括肝脏、肾脏和大脑,并产生一定的毒性效应[10-11]。本文总结了MPs和NPs引起胃肠道产生氧化应激、炎症和细胞凋亡的潜在机制以及造成肝脏糖脂代谢紊乱的潜在机制。首先MPs和NPs从2个方面使细胞产生氧化应激,一方面直接刺激细胞内ROS的产生,另一方面抑制抗氧化酶活性和谷胱甘肽合成进而抑制ROS的代谢;其次,MPs和NPs对胃肠道引发的炎症反应主要通过直接刺激吞噬细胞分泌促炎细胞因子和扰乱肠道菌群,使条件致病菌数量增多,进而导致免疫失衡,间接导致炎症发生,同时氧化应激也是促使炎症反应发生的主要原因之一;最后,炎症、氧化应激以及进入细胞的NPs导致的DNA损伤共同激活细胞凋亡信号通路,最终导致细胞凋亡。纳米级颗粒物对肝脏造成糖脂代谢紊乱的潜在机制可分为生化水平和转录水平2个方面。在生化水平上主要是影响葡萄糖、甘油三酯和脂肪酸的产生与代谢,而在转录水平上主要影响糖脂代谢关键/限速酶的产生,导致糖脂代谢紊乱。MPs和NPs对胃肠道毒性效应以及对肝脏糖脂代谢的影响会增加罹患肠胃炎、高血糖、糖尿病、肝脏肥大、高血脂以及脂肪营养不良综合征的风险,对人体健康造成严重威胁。
目前,关于MPs和NPs对胃肠道及肝脏的毒性效应及机制的研究大多基于人源细胞、啮齿动物和水生物种开展,虽然相关研究取得了一定的进展,提供了MPs和NPs可能对人体胃肠道及肝脏产生毒性效应的证据,但是仍然存在一定的局限性。
(1)目前,有关MPs和NPs在人体内吸收、代谢、排泄的知识缺口仍然存在,关于MPs和NPs跨越人体组织屏障的能力还不明确,因此需要进一步研究MPs和NPs跨越胃肠道屏障机制及造成的毒性效应机制。现有的研究已经发现MPs和NPs通过减少肠道黏液分泌,抑制紧密连接蛋白合成增加肠道通透性以及导致肠道菌群紊乱来损伤肠道屏障功能,但是仍缺乏有关MPs和NPs损伤胃肠道屏障具体机制的研究。
(2)在体内外进行MPs和NPs对胃肠道和肝脏的毒性效应研究时,实验所用的MPs和NPs浓度远大于实际接触浓度,基于实际接触浓度下的研究还很匮乏。因此,我们需要进一步了解MPs和NPs的毒性代谢动力学,以及人类的实际接触浓度。并且不同个体之间存在免疫能力的差异,在进行人体毒理学效应评估时应考虑免疫状态差异。
(3)越来越多的研究发现MPs和NPs诱导肠道菌群失调,进而导致免疫失衡,以及淋巴细胞对NPs的摄取均反映出MPs和NPs可以对免疫系统造成影响。但是有关MPs和NPs对免疫细胞的毒性效应基质研究还不充分,同时也缺乏MPs和NPs对整个免疫系统的毒性效应研究。
(4)肠道微生物群已被发现不仅是免疫和代谢健康的重要组成部分,而且还影响中枢神经系统,通过动物模型,已经确定了“脑肠轴”的几种不同的通信途径[92]。因此,可进一步开展MPs和NPs造成肠道菌群紊乱后对“脑肠轴”的毒性效应及机制研究。
胃肠道和肝脏是人体内重要的吸收、代谢和排毒的器官。MPs和NPs的危害不仅限于“肠肝轴”,引起胃肠道氧化应激、炎症、细胞凋亡和肝脏糖脂代谢紊乱,造成胃肠炎和高血糖、高血脂等疾病,还通过肠道菌群间接影响“脑肠轴”,造成神经系统损伤。因此,关于MPs和NPs对胃肠道及肝脏产生的毒性效应及机制值得进一步研究。
[1] Turner A, Holmes L, Thompson R C, et al. Metals and marine microplastics: Adsorption from the environment versus addition during manufacture, exemplified with lead [J]. Water Research, 2020, 173: 115577
[2] Kazour M, Terki S, Rabhi K, et al. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill [J]. Marine Pollution Bulletin, 2019, 146: 608-618
[3] Lambert S, Wagner M.Characterisation of nanoplastics during the degradation of polystyrene [J]. Chemosphere, 2016, 145: 265-268
[4] 钱亚茹, 石磊磊, 沈茜, 等. 淡水环境中微塑料污染及毒性效应研究进展[J]. 环境工程技术学报, 2022, 12(4): 1096-1104
Qian Y R, Shi L L, Shen Q, et al. Research progress on pollution and toxic effects of microplastics in freshwater environment [J]. Journal of Environmental Engineering Technology, 2022, 12(4): 1096-1104 (in Chinese)
[5] 李娇, 陈大岭, 陈玉立, 等. 微纳米塑料的人体健康风险研究进展[J]. 生态毒理学报, 2023, 18(2): 175-187
Li J, Chen D L, Chen Y L, et al. Effects of micro/nano plastics on human health: A review [J]. Asian Journal of Ecotoxicology, 2023, 18(2): 175-187 (in Chinese)
[6] Alimba C G, Faggio C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile [J]. Environmental Toxicology and Pharmacology, 2019, 68: 61-74
[7] Schwabl P, Köppel S, Königshofer P, et al. Detection of various microplastics in human stool: A prospective case series [J]. Annals of Internal Medicine, 2019, 171(7): 453-457
[8] Amereh F, Amjadi N, Mohseni-Bandpei A, et al. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies [J]. Environmental Pollution, 2022, 314: 120174
[9] Leslie H A, van Velzen M J M, Brandsma S H, et al. Discovery and quantification of plastic particle pollution in human blood [J]. Environment International, 2022, 163: 107199
[10] 刘雅宣, 王兰, 师庆英, 等. 微塑料的人体暴露和健康风险研究进展[J]. 生态毒理学报, 2022, 17(3): 354-365
Liu Y X, Wang L, Shi Q Y, et al. Research progress on human exposure and health risks of microplastics [J]. Asian Journal of Ecotoxicology, 2022, 17(3): 354-365 (in Chinese)
[11] Vethaak A D, Legler J. Microplastics and human health [J]. Science, 2021, 371(6530): 672-674
[12] Tong X H, Li B Q, Li J, et al. Polyethylene microplastics cooperate with Helicobacter pylori to promote gastric injury and inflammation in mice [J]. Chemosphere, 2022, 288(Pt 2): 132579
[13] Luo T, Wang C Y, Pan Z H, et al. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring [J]. Environmental Science &Technology, 2019, 53(18): 10978-10992
[14] Wang L X, Wang Y X, Xu M, et al. Enhanced hepatic cytotoxicity of chemically transformed polystyrene microplastics by simulated gastric fluid [J]. Journal of Hazardous Materials, 2021, 410: 124536
[15] Yee M S L, Hii L W, Looi C K, et al. Impact of microplastics and nanoplastics on human health [J]. Nanomaterials, 2021, 11(2): 496
[16] Hesler M, Aengenheister L, Ellinger B, et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro [J]. Toxicology in Vitro: An International Journal Published in Association with BIBRA, 2019, 61: 104610
[17] Cortés C, Domenech J, Salazar M, et al. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells [J]. Environmental Science: Nano, 2020, 7(1): 272-285
[18] Dong X S, Liu X B, Hou Q L, et al. From natural environment to animal tissues: A review of microplastics(nanoplastics) translocation and hazards studies [J]. The Science of the Total Environment, 2023, 855: 158686
[19] Campanale C, Massarelli C, Savino I, et al. A detailed review study on potential effects of microplastics and additives of concern on human health [J]. International Journal of Environmental Research and Public Health, 2020, 17(4): 1212
[20] Barboza L G A, Dick Vethaak A, Lavorante B R B O, et al. Marine microplastic debris: An emerging issue for food security, food safety and human health [J]. Marine Pollution Bulletin, 2018, 133: 336-348
[21] Bouwmeester H, Hollman P C H, Peters R J B. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: Experiences from nanotoxicology [J]. Environmental Science &Technology, 2015, 49(15): 8932-8947
[22] Domenech J, Hernández A, Rubio L, et al. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier [J]. Archives of Toxicology, 2020, 94(9): 2997-3012
[23] Hussain N, Jaitley V, Florence A T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics [J]. Advanced Drug Delivery Reviews, 2001, 50(1-2): 107-142
[24] Eldridge J H, Meulbroek J A, Staas J K, et al. Vaccine-containing biodegradable microspheres specifically enter the gut-associated lymphoid tissue following oral administration and induce a disseminated mucosal immune response [J]. Advances in Experimental Medicine and Biology, 1989, 251: 191-202
[25] Jani P U, McCarthy D E, Florence A T. Nanosphere and microsphere uptake via Peyer’s patches: Observation of the rate of uptake in the rat after a single oral dose [J]. International Journal of Pharmaceutics, 1992, 86(2-3): 239-246
[26] Volkheimer G. Hematogenous dissemination of ingested polyvinyl chloride particles [J]. Annals of the New York Academy of Sciences, 1975, 246: 164-171
[27] Banerjee A, Shelver W L. Micro- and nanoplastic induced cellular toxicity in mammals: A review [J]. Science of the Total Environment, 2021, 755: 142518
[28] Varela J A, Bexiga M G, Åberg C, et al. Quantifying size-dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells [J]. Journal of Nanobiotechnology, 2012, 10: 39
[29] Nowak M, Brown T D, Graham A, et al. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow [J]. Bioengineering &Translational Medicine, 2020, 5(2): e10153
[30] Firdessa R, Oelschlaeger T A, Moll H. Identification of multiple cellular uptake pathways of polystyrene nanoparticles and factors affecting the uptake: Relevance for drug delivery systems [J]. European Journal of Cell Biology, 2014, 93(8-9): 323-337
[31] Gratton S E, Ropp P A, Pohlhaus P D, et al. The effect of particle design on cellular internalization pathways [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(33): 11613-11618
[32] Carr K E, Smyth S H, McCullough M T, et al. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement [J]. Progress in Histochemistry and Cytochemistry, 2012, 46(4): 185-252
[33] Schmidt C, Lautenschlaeger C, Collnot E M, et al. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa: A first in vivo study in human patients [J]. Journal of Controlled Release: Official Journal of the Controlled Release Society, 2013, 165(2): 139-145
[34] Li S, Malmstadt N. Deformation and poration of lipid bilayer membranes by cationic nanoparticles [J]. Soft Matter, 2013, 9(20): 4969-4976
[35] Xie W, You J, Zhi C X, et al. The toxicity of ambient fine particulate matter (PM2.5) to vascular endothelial cells [J]. Journal of Applied Toxicology, 2021, 41(5): 713-723
[36] Gopinath P M, Saranya V, Vijayakumar S, et al. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics [J]. Scientific Reports, 2019, 9: 8860
[37] Hollóczki O, Gehrke S. Nanoplastics can change the secondary structure of proteins [J]. Scientific Reports, 2019, 9: 16013
[38] Goodman K E, Hare J T, Khamis Z I, et al. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes [J]. Chemical Research in Toxicology, 2021, 34(4): 1069-1081
[39] Xu M K, Halimu G, Zhang Q R, et al. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell [J]. The Science of the Total Environment, 2019, 694: 133794
[40] Cheng W, Li X L, Zhou Y, et al. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids [J]. The Science of the Total Environment, 2022, 806(Pt 1): 150328
[41] Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature [J]. Particle and Fibre Toxicology, 2020, 17(1): 57
[42] Muittari A, Veneskoski T. Natural and synthetic fibers as causes of asthma and rhinitis [J]. Annals of Allergy, 1978, 41(1): 48-50
[43] Pimentel J C, Avila R, Lourenço A G. Respiratory disease caused by synthetic fibres: A new occupational disease [J]. Thorax, 1975, 30(2): 204-219
[44] Li B Q, Ding Y F, Cheng X, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice [J]. Chemosphere, 2020, 244: 125492
[45] Liu S, Li H, Wang J, et al. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance [J]. Science of the Total Environment, 2022, 833: 155198
[46] Deng Y F, Yan Z H, Shen R Q, et al. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut [J]. Environment International, 2020, 143: 105916
[47] Shi C Z, Han X H, Guo W, et al. Disturbedgut-liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance [J]. Environment International, 2022, 164: 107273
[48] Jin Y X, Lu L, Tu W Q, et al. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice [J]. The Science of the Total Environment, 2019, 649: 308-317
[49] Hwang J, Choi D, Han S, et al. An assessment of the toxicity of polypropylene microplastics in human derived cells [J]. Science of the Total Environment, 2019, 684: 657-669
[50] Ding Y F, Zhang R Q, Li B Q, et al. Tissue distribution of polystyrene nanoplastics in mice and their entry, transport, and cytotoxicity to GES-1 cells [J]. Environmental Pollution, 2021, 280: 116974
[51] Domenech J, de Britto M, Velázquez A, et al. Long-term effects of polystyrene nanoplastics in human intestinal caco-2 cells [J]. Biomolecules, 2021, 11(10): 1442
[52] Eleutherio E C A, Silva Magalhães R S, de Araújo Brasil A, et al. SOD1, more than just an antioxidant [J]. Archives of Biochemistry and Biophysics, 2021, 697: 108701
[53] Johnson P. Antioxidant enzyme expression in health and disease: Effects of exercise and hypertension [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2002, 133(4): 493-505
[54] Jakubczyk K, Dec K, Ka duńska J, et al. Reactive oxygen species - sources, functions, oxidative damage [J]. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego, 2020, 48(284): 124-127
duńska J, et al. Reactive oxygen species - sources, functions, oxidative damage [J]. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego, 2020, 48(284): 124-127
[55] Wang X, Zheng H, Zhao J, et al. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis [J]. Environmental Science &Technology, 2020, 54(10): 6202-6212
[56] DeLoid G M, Cao X Q, Bitounis D, et al. Toxicity, uptake, and nuclear translocation of ingested micro-nanoplastics in an in vitro model of the small intestinal epithelium [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2021, 158: 112609
[57] Bhagat J, Ingole B S. Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as a biomarkers of oxidative stress in snails: A review [D]. Modena: University of Modena and Reggio Emilia, 2016: 336-349
[58] He Y J, Li Z, Xu T, et al. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway [J]. Chemosphere, 2022, 307(Pt 1): 135662
[59] Deng Y F, Zhang Y, Lemos B, et al. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure [J]. Scientific Reports, 2017, 7: 46687
[60] Rubio L, Marcos R, Hernández A. Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models [J]. Journal of Toxicology and Environmental Health Part B, Critical Reviews, 2020, 23(2): 51-68
[61] Powell J J, Thoree V, Pele L C. Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract [J]. The British Journal of Nutrition, 2007, 98(Suppl 1): S59-S63
[62] Huang D J, Zhang Y, Long J L, et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation [J]. The Science of the Total Environment, 2022, 838(Pt 1): 155937
[63] Cortés C, Domenech J, Salazar M, et al. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells [J]. Environmental Science: Nano, 2020, 7(1): 272-285
[64] Forte M, Iachetta G, Tussellino M, et al. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells [J]. Toxicology in Vitro, 2016, 31: 126-136
[65] Reuter S, Gupta S C, Chaturvedi M M, et al. Oxidative stress, inflammation, and cancer: How are they linked? [J]. Free Radical Biology and Medicine, 2010, 49(11): 1603-1616
[66] Yan X M, Zhang Y Y, Lu Y Q, et al. The complex toxicity of tetracycline with polystyrene spheres on gastric cancer cells [J]. International Journal of Environmental Research and Public Health, 2020, 17(8): 2808
[67] Chen Z X, Wang C Y, Yu N Z, et al. INF2 regulates oxidative stress-induced apoptosis in epidermal HaCaT cells by modulating the HIF1 signaling pathway [J]. Biomedecine &Pharmacotherapie, 2019, 111: 151-161
[68] Qiao J Y, Chen R, Wang M J, et al. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction [J]. Nanoscale, 2021, 13(19): 8806-8816
[69] Lu L, Wan Z Q, Luo T, et al. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice [J]. The Science of the Total Environment, 2018, 631-632: 449-458
[70] Zhao L T, Shi W Y, Hu F F, et al. Prolonged oral ingestion of microplastics induced inflammation in the liver tissues of C57BL/6J mice through polarization of macrophages and increased infiltration of natural killer cells [J]. Ecotoxicology and Environmental Safety, 2021, 227: 112882
[71] Wan Z Q, Wang C Y, Zhou J J, et al. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish [J]. Chemosphere, 2019, 217: 646-658
[72] Zhao Y, Bao Z W, Wan Z Q, et al. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish [J]. Science of the Total Environment, 2020, 710: 136279
[73] Shi L, Tu B P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences [J]. Current Opinion in Cell Biology, 2015, 33: 125-131
[74] Zhou P, Santoro A, Peroni O D, et al. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms [J]. The Journal of Clinical Investigation, 2019, 129(10): 4138-4150
[75] Syed I, Lee J, Moraes-Vieira P M, et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis [J]. Cell Metabolism, 2018, 27(2): 419-427.e4
[76] Vijayakumar A, Aryal P, Wen J, et al. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport [J]. Cell Reports, 2017, 21(4): 1021-1035
[77] Wen B, Zhang N, Jin S R, et al. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid [J]. Aquatic Toxicology, 2018, 195: 67-76
[78] Schlein C, Talukdar S, Heine M, et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues [J]. Cell Metabolism, 2016, 23(3): 441-453
[79] Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes [J]. FEBS Letters, 2009, 583(17): 2882-2886
[80] Bi Y K, Chang Y, Liu Q, et al. ERp44/CG9911 promotes fat storage in Drosophila adipocytes by regulating ER Ca2+ homeostasis [J]. Aging, 2021, 13(11): 15013-15031
[81] Simha V, Garg A. Lipodystrophy: Lessons in lipid and energy metabolism [J]. Current Opinion in Lipidology, 2006, 17(2): 162-169
[82] Iizuka K, Ken T K, Yabe D. ChREBP-mediated regulation of lipid metabolism: Involvement of the gut microbiota, liver, and adipose tissue [J]. Frontiers in Endocrinology, 2020, 11: 587189
[83] Nunes-Nesi A, Araújo W L, Obata T, et al. Regulation of the mitochondrial tricarboxylic acid cycle [J]. Current Opinion in Plant Biology, 2013, 16(3): 335-343
[84] Bougarne N, Weyers B, Desmet S J, et al. Molecular actions of PPARα in lipid metabolism and inflammation [J]. Endocrine Reviews, 2018, 39(5): 760-802
[85] Wang Q, Wu Y L, Zhang W J, et al. Lipidomics and transcriptomics insight into impacts of microplastics exposure on hepatic lipid metabolism in mice [J]. Chemosphere, 2022, 308(Pt 3): 136591
[86] Fan X P, Wei X J, Hu H L, et al. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice [J]. Chemosphere, 2022, 288(Pt 3): 132607
[87] Islinger M, Cardoso M J R, Schrader M. Be different—The diversity of peroxisomes in the animal kingdom [J]. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 2010, 1803(8): 881-897
[88] Marion-Letellier R, Savoye G, Ghosh S. Fatty acids, eicosanoids and PPAR gamma [J]. European Journal of Pharmacology, 2016, 785: 44-49
[89] Bhatt-Wessel B, Jordan T W, Miller J H, et al. Role of DGAT enzymes in triacylglycerol metabolism [J]. Archives of Biochemistry and Biophysics, 2018, 655: 1-11
[90] Stone S J, Myers H M, Watkins S M, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice [J]. The Journal of Biological Chemistry, 2004, 279(12): 11767-11776
[91] Stock V, Fahrenson C, Thuenemann A, et al. Impact of artificial digestion on the sizes and shapes of microplastic particles [J]. Food and Chemical Toxicology, 2020, 135: 111010
[92] Strandwitz P. Neurotransmitter modulation by the gut microbiota [J]. Brain Research, 2018, 1693(Pt B): 128-133