抗生素在人类疾病防治、畜禽养殖及水产养殖业等领域已经得到广泛应用。中国作为世界上抗生素生产和使用大国,2013年的抗生素生产量就已达到24.8万t,总使用量约16.2万t[1]。用于人类或动物的抗生素一般不能被完全吸收和代谢,大约一半的抗生素被人类和牲畜排泄后,直接或间接途径进入环境[2-3]。近10年来,我国不同环境介质中多种抗生素被广泛检出并报道[4]。抗生素在环境中大多呈现出低剂量、混合污染和假持久等特征[5-6]。在我国主要城市地下水中,已经检测出7类57种抗生素,浓度从<1 ng·L-1到数百μg·L-1不等[7-8]。环境中的抗生素会引起抗性基因的增殖和扩散[8, 10-11],其相关的生态效应和人类健康风险已经引起广泛关注。
相对于传统的生物处理技术,高级氧化法被认为可以更有效的降解抗生素。在常用的氧化剂中,过硫酸盐具有较强的氧化能力和相对较宽的反应条件,可以在含水层中长距离输送,因此得到了广泛应用[12]。过硫酸盐可以通过过渡金属、热、紫外线等方式活化,其中Fe2+活化是污染修复最常用的方法之一[13],硫酸根自由基(SO4·-)和羟基自由基(HO·)是氧化降解污染物的主要活性物种[14]。目前,采用高级氧化法降解抗生素的相关研究中,大多面向废水处理针对一种或一类抗生素在较高浓度下开展[15-16],这些研究结果在用到低浓度复合污染的污染控制和修复时,可能存在较大的偏差。此外,以往的氧化处理对目标抗生素的关注较多,对于氧化降解产物的识别及其可能的生态毒性关注不足。为了更好地认识多种抗生素在低浓度下共存时的氧化降解过程,本研究采用Fe2+活化过硫酸盐法在低浓度水平下降解我国地下水中常见的几种抗生素,包括磺胺类的磺胺甲噁唑(sulfamethoxazole,SMX)、磺胺二甲嘧啶(sulfamethazine,SMT)、喹诺酮类的左氧氟沙星(levofloxacin,LVF)、环丙沙星(ciprofloxacin,CIP)和大环内酯类的罗红霉素(roxithromycin,ROX),研究了抗生素的降解动力学,分析了其降解产物和降解路径,评估了5种抗生素及其降解产物的毒性,为水中抗生素复合低浓度污染场景的控制与修复提供参考。
1 材料与方法(Materials and methods)
1.1 实验药品与仪器
甲醇(色谱纯)及乙腈(色谱纯)购自Sigma-Aldrich;FeSO4·7H2O(纯度≥99%)、甲酸铵(纯度≥98%)及甲酸(分析纯)均购自天津市科密欧化学试剂有限公司;SMX(纯度≥98%)、SMT(纯度≥98%)、CIP(纯度≥98%)、LVF(纯度≥98%)、ROX(纯度≥98%)、Na2S2O8(纯度≥98%)均购自北京百灵威科技有限公司。
实验所用仪器包括蠕动泵(中国兰格恒流泵有限公司,BT100-1L),搅拌器(中国巩义市予华仪器有限责任公司,85-1)等。
1.2 降解实验方法
向2 L烧杯中准确量取999 mL分别含有100 μg·L-1 SMT、SMX、LVF、CIP和ROX的混合溶液,并加入1 mL浓度为3 g·L-1的FeSO4·7H2O水溶液。将烧杯置于搅拌器上持续搅拌,利用蠕动泵将浓度为1.724 g·L-1的过硫酸钠水溶液以0.09667 mL·min-1的速度注入烧杯中,在3 360 min的总反应时间内,于特定时间点取出适量反应溶液用0.22 μm滤膜过滤,取0.5 mL过膜后的反应溶液至液相小瓶内并立即用0.48 mL甲醇淬灭,加入20 μL 1 mg·L-1磺胺二甲氧嘧啶-D6作为内标后避光4 ℃保存待测。
1.3 检测方法
采用超高效液相色谱-四极杆-静电场轨道阱高分辨质谱仪(UPLC-Q-Orbitrap)(美国Thermo Fisher Scientific公司,Exactive)分析测定抗生素及其降解产物。色谱和质谱参数如下。C18色谱柱(1.7 μm, 50 mm×2.1 mm),流动相:流动相A含有体积分数为0.1%甲酸和质量分数为0.1%的甲酸铵溶液,流动相B为乙腈-甲醇(1∶1, V/V)溶液。柱温40 ℃,流速为0.25 mL·min-1,进样量为5 μL;洗脱梯度:0~10 min,95%A~40%A;10~15 min,40%A;15~20 min,40%A~10%A;20~25 min,10%A~95%A。使用ESI正离子模式进行全扫,毛细管温度350 ℃,干燥气(N2)流速15.0 L·min-1,扫描范围:150~900 m/z,分辨率为70 000。抗生素采用内标法进行定量,降解产物采用峰面积进行半定量分析。
1.4 抗生素降解动力学方程拟合
为表征5种抗生素的降解动力学过程,采用方程:ln(Ct/C0)=-kt进行拟合。其中,Ct和C0分别为抗生素t时刻的浓度和初始浓度(μg·L-1);k为准一级动力学反应速率常数(min-1);t为反应时间(min)。
1.5 毒性预测
采用美国环境保护局开发的ECOSAR(v2.2)预测模型软件(https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model),对SMT、SMX、LVF、CIP、ROX及其降解产物的急性毒性进行预测,分别选取鱼类96 h半数致死浓度(LC50, mg·L-1)、水蚤48 h半数致死浓度和绿藻96 h半数效应浓度(EC50, mg·L-1)用于水生生态毒性的表征和评估。
2 结果与讨论(Results and discussion)
2.1 5种抗生素的反应动力学
为了更准确地测定5种抗生素的反应速率常数,本研究使用蠕动泵将过硫酸钠以恒定速率注入烧杯中,以使该氧化体系中氧化剂和自由基处于稳态水平。Fe2+活化过硫酸钠降解5种抗生素的结果如图1(a)所示。在3 360 min反应时间内,除了ROX降解率为91%,SMX、SMT、CIP以及LVF的降解率均达到了100%,表明该体系对磺胺类和喹诺酮类抗生素的降解较为彻底。为获得降解过程的反应速率常数,对其进行了拟合,如图1(b)所示,降解过程符合准一级动力学方程,反应速率常数和拟合方程的相关系数如表1所示。降解速率遵循SMT>LVF>CIP>SMX>ROX的顺序,对应的反应速率常数分别为0.01927、0.00302、0.00222、0.00188和0.00056 min-1。Wang等[17]使用控释材料降解单一种类的抗生素时测得反应速率常数为0.00541 min-1,和本研究相近,表明混合体系对抗生素反应速率没有显著的影响。
表1 5种抗生素3 360 min去除率、反应速率常数和拟合方程的决定系数
Table 1 The removal rate within 3 360 min, reaction rate constant and determination coefficient of fitting equation of five antibiotics

抗生素种类Type of antibiotics3 360 min去除率/%Removal rate within 3 360 min/%反应速率常数/min-1Reaction rate constant/min-1拟合方程决定系数Determination coefficient of fitting equation磺胺甲噁唑(SMX)Sulfamethoxazole (SMX)1000.001880.958磺胺二甲嘧啶(SMT)Sulfamethazine (SMT)1000.019270.965环丙沙星(CIP)Ciprofloxacin (CIP)1000.002220.871左氧氟沙星(LVF)Levofloxacin (LVF)1000.003020.898罗红霉素(ROX)Roxithromycin (ROX)910.000560.974

图1 5种抗生素的降解率(a)和动力学拟合方程(b)
Fig. 1 The degradation rate (a) and kinetic fitting equations of five antibiotics (b)
从表1可知,含有五元杂环的SMX和六元杂环的SMT的反应速率存在显著差异,这是因为六元杂环比五元杂环具有更强的吸电子能力,因此SMT更容易受到亲电试剂SO4·-和HO·的攻击[18-19]。哌嗪环的裂解是氟喹诺酮类抗生素最重要的降解途径,LVF和CIP哌嗪环上的N烷基化程度不同,LVF上叔胺的反应性高于CIP的仲胺,从而使LVF更易被氧化降解[20],这与Fang等[21]发现的趋势相近。本研究发现大环内酯类抗生素ROX降解速率显著低于磺胺类和氟喹诺酮类抗生素,可能是因为ROX结构复杂且饱和度较高,在该氧化体系中较难降解[22]。在Xie和Jin[23]采用脉冲放电技术生成HO·的氧化体系中和Lin等[24]在O3/H2O2氧化体系中均发现ROX降解速率显著低于磺胺等其他抗生素,这些发现与本研究结果一致。
2.2 5种抗生素的降解产物和降解路径推测
采用UPLC-Q-Orbitrap对Fe2+活化过硫酸盐降解5种抗生素的产物进行检测,共检测出33种可能存在的降解产物,其理论质量和实测质量偏差<6×10-6。降解产物的保留时间、SMILES式等信息如表2所示。结合以往的文献报道对SMT、LVF、ROX的降解途径进行了推测,如图2~4所示。
表2 检测到的降解产物信息及ECOSAR软件预测的生态毒性
Table 2 The information of degradation products detected and the ecological toxicity predicted by ECOSAR

产物代号Products name保留时间/minRetention time/minSMILES急性毒性/(mg·L-1)Acute toxicity/(mg·L-1)鱼FishLC50(96 h)水蚤Water fleaLC50(48 h)绿藻Green algaeEC50(96 h)毒性变化Toxicity changeSMX4.48O=S(C1=CC=C(N)C=C1)(NC2=NOC(C)=C2)=O266.86.421.8SMX-10.96O=S(C1=CC=C(N)C=C1)(NC2=NOC(C)(O)C2O)=O8 4506841 210↓SMX-24.48C1(S(=O)(=O)NC2=NC(C)=CO2)CCC(N)CC12676.4321.8↑SMX-310.26O=S(=O)(NC1=NC(O)=C(O1)C)C2CCC(N)CC21895.8318.9↑SMX-43.34O=S(=O)(NC=1C=C(ON=1)C)C2CCC(N)CC2(O)1194.8614.9↓SMX-519.97O=S(=O)(NC=1C=C(ON=1)C)C4CCC(N=NC2CCC(CC2)S(=O)(=O)NC3NOC(C3)C)CC43.292.324.22↑SMT3.64O=S(C1=CC=C(N)C=C1)(NC2=NC(C)=CC(C)=N2)=O1956.0219.5SMT-15.54O=S(=O)(NC1NC(CC(N1)C)C)C2CCC(O)CC21 220641351↓SMT-20.54N=C1N=C(C=C(N1C2CCC(N)CC2)C)C1.210.6971.29↑SMT-39.74O=NC1CCC(CC1)N2C(=N)N=C(C=C2C)C0.6350.4650.990↑SMT-48.17O=NC1CCC(CC1(O))N2C(=N)N=C(C=C2C)C1.831.282.28↑SMT-57.45O=N(=O)C1CCC(CC1)S(=O)(=O)NC2NC(CC(N2)C)C725393241↓CIP4.16O=C(C1=CN(C2CC2)C3=C(C=C(F)C(N4CCNCC4)=C3)C1=O)O13 1001 2401 620CIP-14.67O=C(C1=CN(C2CC2)C3=C(C=C(F)C(N(C=O)CC-NC=O)=C3)C1=O)O401 000183 00055 000↓CIP-25.53O=C(C1=CN(C2CC2)C3=C(C=C(F)C(N(C=O)CCN)=C3)C1=O)O141 00011 10020 800↓CIP-33.68O=C(C1=CN(C2CC2)C3=C(C=C(F)C(NCCN)=C3)C1=O)O47 3004 0206 470↓CIP-45.51O=CNC1CC2C(CC1(F))C(=O)C(=CN2C3CC3)C(=O)O10 40055 3003 140↓CIP-50.53O=C(O)N(C1CC(C(F)CC1(C(=O)CC(=O)O))N2CCNCC2)C3CC3692 00048 600114 000↓CIP-69.15O=C(O)C1=CN(C2CC(C(O)CC2(C1(=O)))N3CCNCC3)C4CC436 5003 1904 880↓CIP-710.02O=C(O)C1=CN(C2CC(NCCN)C(O)CC2(C1(=O)))C3CC3131 00010 30019 400↓LVF4.07O=C(C(C1=O)=CN2C(C)COC3=C(N4CCN(C)CC4)C(F)=CC1=C23)O19 4001 7902 440LVF-15.34O=C(O)C2=CN1C3C(OCC1(C(=O)O))C(C(F)CC3(C2(=O)))N4CCN(C)CC43 200 000201 000589 000↓LVF-28.28O=CC2=CN1C3C(OCC1C)C(C(F)CC3(C2(=O)))N4CCN(C)CC420722.622.2↑LVF-310.08O=C2C=CN1C3C(OCC1C)C(C(F)CC23)N4CCN(C)CC445.85.584.40↑LVF-43.87O=C(O)C2=CN1C3C(OCC1C)C(NCCNC)C(F)CC3(C2(=O))47 7004 0906 490↓

续表2产物代号Products name保留时间/minRetention time/minSMILES急性毒性/(mg·L-1)Acute toxicity/(mg·L-1)鱼FishLC50(96 h)水蚤Water fleaLC50(48 h)绿藻Green algaeEC50(96 h)毒性变化Toxicity changeLVF-53.78O=C(O)C2=CN1C3C(OCC1C)C(NCCN)C(F)CC3(C2(=O))92 5007 50013 300↓LVF-65.47O=C(O)C2=CN1C3C(OCC1C)C(N)C(F)CC3(C2(=O))1 77057.9185↑LVF-75.95O=C(O)C2=CN1C3C(OCC1C)CC(F)CC3(C2(=O))3 7202 0601 390↑LVF-80.53O=CN1C2C(OCC1C)C(C(F)CC2(C(=O)C(=O)C(=O)O))N3CCN(C)CC3728.627.06↑LVF-90.54O=CN1C2C(OCC1C)C(C(F)CC2(C(=O)O))N3CCN(C)CC347858.446.0↑ROX10.12O=C(OC(CC)C(C)(O)C(O)C/1C)C(C)C(OC2CC(OC)(C)C(O)C(C)O2)C(C)C(OC3C(O)C(N(C)C)CC(C)O3)C(C)(O)CC(C)C1=N\OCOCCOC51.66.724.66ROX-17.43OC(C(C)C(OC(CC)C(C)(O)C(O)C/1C)=O)C(C)C(OC2C(O)C(N(C)C)CC(C)O2)C(C)(O)CC(C)C1=N\OCOC-COC7 000614928↓ROX-26.90OC2OC(CC)C(O)(C)C(O)C(C(=NOCOCCO)C(C)CC(O)(C)C(OC1OC(C)CC(N(C)C)C1(O))C(C(=O)C2C)C)C19 7001 6002 840↓ROX-310.12O=C3OC(CC)C(O)(C)C(O)C(C(=NO)C(C)CC(O)(C)C(OC1OC(C)CC(N(C)C)C1(O))C(C)C(OC2OC(C)C(O)C(OC)(C)C2)C3C)C96.311.89.19↓ROX-46.22O=C(OC(CC)C(C)(O)C(O)/C(C)=C\1)C(C)C(OC2CC(OC)(C)C(O)C(C)O2)C(C)C(OC3C(O)C(N(C)C)CC(C)O3)C(C)(O)CC1=C96.911.89.3↓ROX-56.94O=C2OC(CC)C(O)(C)C(O)C(C(=NO)C(C)CC(O)(C)C(OC1OC(C)CC(N(C)C)C1(O))C(C)C(O)C2C)C10612.710.3↓ROX-612.81C=C2C=C(C)C(O)C(O)(C)C(OC(=O)C(C)C(O)C(C)C(OC1OC(C)CC(N(C)C)C1(O))C(O)(C)C2)CC57.07.135.34↓ROX-712.09C=C2C=C(C(O)=C(C)C(OC(=O)C(C)C(O)C(C)C(OC1OC(C)CC(N(C)C)C1(O))C(O)(C)C2)CC)C17.22.351.48↑
注:根据中国新化学物质危害评估导则(HJ/T 154—2004)中生态毒理学危害性分级,毒性数据标红的代表毒性极高,橙色代表毒性高,黄色代表毒性中等,绿色代表低毒性;“↑”代表该降解产物的生态毒性相比于母体有所上升,“↓”代表该降解产物的生态毒性相比于母体有所下降;LC50为半数致死浓度,EC50为半数效应浓度。
Note: According to the classification of ecotoxicological hazards in The Guidelines for the Hazard Evaluation of New Chemical Substance in China (HJ/T 154—2004); the toxicity data of standard: red represents extremely high toxicity; orange represents higher toxicity; yellow represents moderate toxicity; green represents low toxicity; “↑”indicates that the ecotoxicity of the degradation product is higher than that of the parent; “↓”indicate that the ecotoxicity of the degradation product is lower than that of the parent; LC50 is concentration for 50% of lethal effect; EC50 is concentration for 50% of maximal effect.
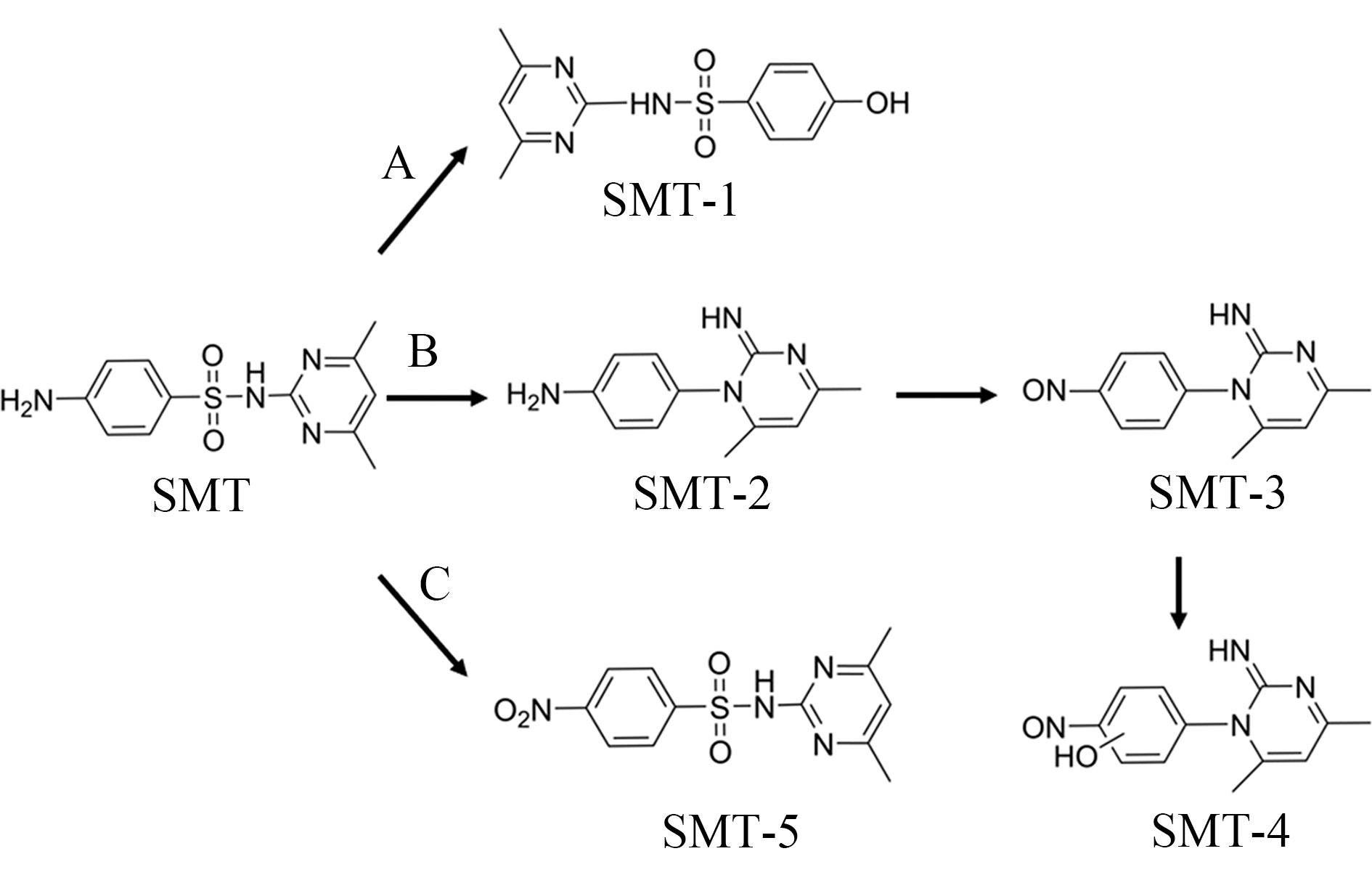
图2 SMT降解途径推测
Fig. 2 Degradation pathway of SMT proposed
SMT可能的降解路径如图2所示。在途径A中,SO4·-通过单电子转移,选择性地与苯胺等富电子有机基团反应形成SMT-1(m/z=280);在途径B中,自由基攻击SMT苯胺部分的反应位点,通过电子转移机制产生苯胺基自由基。自由基靠近磺胺键的芳香族碳具有较强正电荷,易受到分子间的亲核攻击,SMT分子中嘧啶环上的N原子可以作为亲核试剂进攻该位置,最终导致分子结构重排,SO2部分被脱除,从而形成SMT-2(m/z=215),该过程被称为Smiles重排[6]。随后,SO4·-通过亲电反应形成亚硝基和硝基取代的SMT-3(m/z=229)和SMT-5(m/z=309)[25]。之后自由基攻击芳烃使HO·添加到苯环或杂环中形成羟基化中间体SMT-4(m/z=246)。
LVF可能的降解路径如图3所示。LVF主要有4种降解途径,包括羧化、脱羧化、哌嗪环转化和喹诺酮基转化。LVF-1(m/z=392)是LVF的羧化产物;对于途径B,可能是由于SO4·-和HO·攻击LVF通过脱羧生成LVF-2(m/z=346)和LVF-3(m/z=318)。在降解途径C中,LVF通过去哌嗪酰化分解生成LVF-4(m/z=336),LVF-4进一步分解为LVF-5(m/z=322)和LVF-7(m/z=264);降解途径D中,喹诺酮基的转化是自由基攻击LVF的另一个主要途径,并被SO4·-进一步氧化生成产物[26]。

图3 LVF降解途径推测
Fig. 3 Degradation pathway of LVF proposed
ROX可能的降解路径如图4所示。在降解途径A中,ROX丢失支链糖部分后得到ROX-1(m/z=680),ROX-1叔胺部分去甲基化后得到ROX-2(m/z=666),去甲基化首先从叔胺开始,说明叔胺和支链糖部分的氧是自由基的主要攻击位点。在途径B中,ROX-3(m/z=750)可以认为是由肟(C![]() N)侧链上的C—O键裂解形成的,ROX-3丢失支链糖部分后得到ROX-5(m/z=592)。ROX-3和ROX-5随后脱去羟胺(NH2OH)形成ROX-4(m/z=717)和ROX-6(m/z=559)。随后,ROX-6脱去H2O形成ROX-7(m/z=541)[27]。
N)侧链上的C—O键裂解形成的,ROX-3丢失支链糖部分后得到ROX-5(m/z=592)。ROX-3和ROX-5随后脱去羟胺(NH2OH)形成ROX-4(m/z=717)和ROX-6(m/z=559)。随后,ROX-6脱去H2O形成ROX-7(m/z=541)[27]。

图4 ROX降解途径推测
Fig. 4 Degradation pathway of ROX proposed
2.3 5种抗生素及其降解产物生态毒性预测结果评估
前人研究表明,降解产物可能比其母体化合物具有更高的毒性,因此有必要对降解产物的毒性进行评估[28]。美国环境保护局开发的ECOSAR模型通过对分子结构的描述,建立起化学结构与生物活性相关性的模型,该模型可以预测多种化学品及其转化产物的生态毒性[29]。利用ECOSAR模型预测了5种抗生素及其降解产物对鱼类、水蚤和绿藻的急性毒性,结果如表2所示。根据中国新化学物质危害评估导则(HJ/T 154—2004),化学品的生态毒理学危害性依据其对水生生物的急性毒性按半数致死浓度(LC50)或半数效应浓度(EC50)划分为4个类别,即:极高,L(E)C50≤1 mg·L-1;高,1 mg·L-1
本研究中每种抗生素都检测到多种降解产物,其预测的毒性也不尽相同。除了CIP外,其余抗生素均有一些降解产物生态毒性超过母体化合物,其中Smiles重排副产物SMT-2、SMT-3和SMT-4的生态毒性很高,值得特别关注。Lin等[30]用过氧单硫酸盐体系降解SMT时也检测到了SMT-2的存在,T.E.S.T.软件预测结果表明SMT-2的生物蓄积性较高,因此作者认为SMT重排产物毒性更高。我们的研究发现与Lin等关于SMT氧化重排产物毒性增强的结论一致[30]。SMX的五元杂环在降解过程中不会发生Smiles重排,因此总体来看其降解产物生态毒性较低,但SMX-5中含有偶氮键,偶氮键会断裂生成苯胺或氧化为高活性的重氮盐,因此该降解产物可能具有较高的毒性。CIP降解产物对鱼、水蚤和绿藻的生态毒性均较低,而LVF降解过程中产生了对水蚤和绿藻具有高毒性的LVF-3和LVF-8。ROX在急性毒性中被归类为对水蚤和绿藻毒性较高的化合物。除ROX-7外,大多数降解产物的生态毒性均低于母体化合物,表明叔胺部分去甲基化和支链糖部分的丢失可以在一定程度上降低副产物的生态毒性。虽然ECOSAR预测的毒性数据具有一定的参考价值,但预测结果也会存在一定的不确定性,需要进一步的毒性实验验证。
2.4 降解产物的动态变化
发光细菌、斑马鱼等生物常被用于研究抗生素降解前后的毒性变化。前人对于抗生素降解前后生态毒性测试数据存在不一致的情况,如表3所示。Yan等[31]活化过氧单硫酸盐降解SMX时,发现降解后毒性显著降低,但Qi等[32]活化过硫酸盐降解SMX时,发现SMX完全去除后毒性仍显著增加。理论上,反应过程中的毒性变化一方面与降解产物的毒性强弱有关,一方面还与降解产物的动态变化情况有关。根据ECOSAR的预测,SMT降解后产生了毒性较高的SMT-2、SMT-3、SMT-4这3种产物,因此,有必要进一步分析其在降解过程中的动态变化。
表3 抗生素降解前后毒性变化的相关研究
Table 3 Studies on the toxicity changes before and after antibiotic degradation

氧化体系Oxidative system抗生素种类Types of antibiotics指示生物种类Indicator organisms降解后毒性变化Toxicity changes after degradation参考文献References紫外辐射活化K2S2O8K2S2O8 activated by ultraviolet radiation磺胺类Sulfonamides人胚胎肾细胞Human embryonic kidney cells↓[33]H2O2湿式催化氧化H2O2Wet catalytic oxidation of H2O2SMT斑马鱼Zebrafish↓[34]电解芬顿体系Electrolytic Fenton systemSMT费氏弧菌Vibrio fischeri↓[35]硫酸根自由基高级氧化体系Advanced oxidation system of sulfate radicalLVF革兰氏阳性菌/阴性菌Gram positive/negative bacteria↓[36]微波活化过硫酸盐Persulfate activated by microwaveSMX发光细菌Luminescent bacteria↑[32]过渡金属活化过氧单硫酸盐Peroxonosulfate activated by transition metalSMX活性污泥中的微生物Microorganisms in activated sludge↓[31]紫外辐射活化H2O2Hydrogen peroxide activated by ultraviolet radiationROX发光细菌Luminescent bacteria↑[16]
注:“↑”代表该降解产物的生态毒性相比于母体有所上升,“↓”代表该降解产物的生态毒性相比于母体有所下降。
Note: “↑” indicates that the ecotoxicity of the degradation product increases compared with that of the parent, and “↓” indicates that the ecotoxicity of the degradation product decreases compared with that of the parent.
由于这3种转化产物的标准样品不易获得,本研究对3种降解产物所在的色谱峰进行积分,采用峰面积随时间的变化来表征其降解产物的动态变化,结果如图5所示。3种降解产物在整个降解过程中的峰面积总体呈现先上升后下降的趋势。SMT-2和SMT-3的变化规律高度相似,且SMT-3峰面积达到最高值的取样时间晚于SMT-2,这一定程度上说明了SMT-3可能由SMT-2转化而来。SMT-4的峰面积总体也呈现先上升后下降的趋势,但具体变化较为复杂,可能是其三级降解产物,其生成受到前体物生成和降解的影响有关;此外,SMT-4也可能还存在图2中未识别的生成途径。3种高毒性的降解产物在反应1 380min内的峰面积较高,如果在该时刻停止反应,大量的高毒性降解产物仍存在于该体系中,反应后毒性很可能会升高。而经过3 360 min的反应后,峰面积均处于较低水平,表明这3种毒性较高的产物在较长的反应时间内氧化较为彻底,此时体系的毒性效应可能会大大降低。只有母体化合物和有较高生态风险的降解产物均彻底降解,才能够达到降低生态毒性和生态风险的目的。因此,在进行实际的污染修复工作时,为了避免降解过程中毒性升高的风险,不仅要关注母体化合物的动态变化,也要关注高毒性产物的动态变化,从而达到降低生态风险的根本目的。

图5 SMT部分降解产物的峰面积随时间的变化情况
Fig. 5 The change of peak area of SMT degradation products with time
2.5 结论
本研究采用Fe2+活化过硫酸盐法在低浓度水平下降解我国地下水中常见的5种抗生素。该体系对抗生素的降解较为彻底,在3 360 min反应时间内,除了ROX降解率为91%,SMX、SMT、CIP以及LVF的降解率均达到了100%。5种抗生素均符合伪一级动力学规律,降解速率遵循SMT>LVF>CIP>SMX>ROX的趋势。5种抗生素共鉴定出33种可能存在的降解产物,降解产物峰面积随时间一般呈现先上升后下降的趋势。氧化过程中可能会产生生态毒性更强的降解产物。为了避免降解过程中毒性升高的风险,不仅要关注母体化合物的动态变化,也要关注高毒性产物的动态变化,以达到降低生态风险的目的。
[1] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science &Technology, 2015, 49(11): 6772-6782
[2] Pulingam T, Parumasivam T, Gazzali A M, et al. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome [J]. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, 2022, 170: 106103
[3] Li X H, Liu C, Chen Y X, et al. Antibiotic residues in liquid manure from swine feedlot and their effects on nearby groundwater in regions of North China [J]. Environmental Science and Pollution Research International, 2018, 25(12): 11565-11575
[4] 孔慧敏, 赵晓辉, 徐琬, 等. 我国地下水环境抗生素赋存现状及风险评价[J]. 环境工程, 2023, 41(2): 219-226
Kong H M, Zhao X H, Xu W, et al. Occurrence and risk assessment of antibiotics in groundwater environment in China [J]. Environmental Engineering, 2023, 41(2): 219-226 (in Chinese)
[5] 王桂祥, 张琼, 匡少平, 等. 环境浓度下的混合抗生素对普通小球藻的联合毒性[J]. 生态毒理学报, 2019, 14(2): 122-128
Wang G X, Zhang Q, Kuang S P, et al. The joint toxicity of mixed antibiotics on Chlorella vulgaris at normal environmental concentration [J]. Asian Journal of Ecotoxicology, 2019, 14(2): 122-128 (in Chinese)
[6] 梁延鹏, 王婧, 钱丽, 等. 磺胺类抗生素对斜生栅藻的协同和拮抗作用研究[J]. 生态毒理学报, 2022, 17(1): 244-254
Liang Y P, Wang J, Qian L, et al. Synergism and antagonism effects of sulfonamide antibiotics to Scenedesmus obliquus [J]. Asian Journal of Ecotoxicology, 2022, 17(1): 244-254 (in Chinese)
[7] Li S, Shi W Z, Liu W, et al. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005-2016) [J]. The Science of the Total Environment, 2018, 615: 906-917
[8] 卫承芳, 李佳乐, 孙占学, 等. 水-土壤环境中抗生素污染现状及吸附行为研究进展[J]. 生态毒理学报, 2022, 17(3): 385-399
Wei C F, Li J L, Sun Z X, et al. Research progress of antibiotic pollution and adsorption behavior in water-soil environment [J]. Asian Journal of Ecotoxicology, 2022, 17(3): 385-399 (in Chinese)
[9] Zeng H P, Li J X, Zhao W H, et al. The current status and prevention of antibiotic pollution in groundwater in China [J]. International Journal of Environmental Research and Public Health, 2022, 19(18): 11256
[10] Qiao M, Ying G G, Singer A C, et al. Review of antibiotic resistance in China and its environment [J]. Environment International, 2018, 110: 160-172
[11] 王晓洁, 赵蔚, 张志超, 等. 兽用抗生素在土壤中的环境行为、生态毒性及危害调控[J]. 中国科学: 技术科学, 2021, 51(6): 615-636
Wang X J, Zhao W, Zhang Z C, et al. Veterinary antibiotics in soils: Environmental processes, ecotoxicity, and risk mitigation [J]. Scientia Sinica (Technologica), 2021, 51(6): 615-636 (in Chinese)
[12] Zhou Z, Liu X T, Sun K, et al. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review [J]. Chemical Engineering Journal, 2019, 372: 836-851
[13] 刘路明, 高志敏, 邓兆雄, 等. 过硫酸盐的活化及其在氧化降解水中抗生素的机理和应用[J]. 环境化学, 2022, 41(5): 1702-1717
Liu L M, Gao Z M, Deng Z X, et al. Activation of persulfate and its mechanism and application in oxidative degradation of antibiotics in water [J]. Environmental Chemistry, 2022, 41(5): 1702-1717 (in Chinese)
[14] Ji Y F, Ferronato C, Salvador A, et al. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics [J]. Science of the Total Environment, 2014, 472: 800-808
[15] Liu Z T, Hu W P, Zhang H P, et al. Enhanced degradation of sulfonamide antibiotics by UV irradiation combined with persulfate [J]. Processes, 2021, 9(2): 226
[16] Li W, Xu X J, Lv B L, et al. Degradation of typical macrolide antibiotic roxithromycin by hydroxyl radical: Kinetics, products, and toxicity assessment [J]. Environmental Science and Pollution Research International, 2019, 26(14): 14570-14582
[17] Wang T, Xu K M, Yan K X, et al. Comparative study of the performance of controlled release materials containing mesoporous MnOx in catalytic persulfate activation for the remediation of tetracycline contaminated groundwater [J]. Science of the Total Environment, 2022, 846: 157217
[18] Hu J H, Li X Y, Liu F F, et al. Comparison of chemical and biological degradation of sulfonamides: Solving the mystery of sulfonamide transformation [J]. Journal of Hazardous Materials, 2022, 424: 127661
[19] Feng M B, Baum J C, Nesnas N, et al. Oxidation of sulfonamide antibiotics of six-membered heterocyclic moiety by ferrate(Ⅵ): Kinetics and mechanistic insight into SO2 extrusion [J]. Environmental Science &Technology, 2019, 53(5): 2695-2704
[20] Guo H G, Ke T L, Gao N Y, et al. Enhanced degradation of aqueous norfloxacin and enrofloxacin by UV-activated persulfate: Kinetics, pathways and deactivation [J]. Chemical Engineering Journal, 2017, 316: 471-480
[21] Fang Z H, Zhou Z L, Xue G, et al. Application of sludge biochar combined with peroxydisulfate to degrade fluoroquinolones: Efficiency, mechanisms and implication for ISCO [J]. Journal of Hazardous Materials, 2022, 426: 128081
[22] Ashraf A, Liu G J, Yousaf B, et al. Recent trends in advanced oxidation process-based degradation of erythromycin: Pollution status, eco-toxicity and degradation mechanism in aquatic ecosystems [J]. The Science of the Total Environment, 2021, 772: 145389
[23] Xie H L, Jin X H. Pulse discharge plasma coupled with magnetic mesoporous silica for synergistic degradation of pharmaceutical wastewater and mechanism [J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 106815
[24] Lin A Y, Lin C F, Chiou J M, et al. O3 and O3/H2O2 treatment of sulfonamide and macrolide antibiotics in wastewater [J]. Journal of Hazardous Materials, 2009, 171(1-3): 452-458
[25] Su P, Fu W Y, Du X D, et al. Confined Fe0@CNTs for highly efficient and super stable activation of persulfate in wide pH ranges: Radicals and non-radical co-catalytic mechanism [J]. Chemical Engineering Journal, 2021, 420: 129446
[26] Liu L L, Zhan R, Zhang M, et al. Insights into the performance, mechanism, and ecotoxicity of levofloxacin degradation in CoFe2O4 catalytic peroxymonosulfate process [J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107435
[27] 郑琴琴, 张若琦, 闫于飞, 等. 太阳光/Fe(Ⅱ)/柠檬酸/激活过硫酸盐对污水二级出水中罗红霉素的降解效能[J]. 环境化学, 2021, 40(10): 3122-3132
Zheng Q Q, Zhang R Q, Yan Y F, et al. Degradation of roxithromycin in secondary effluent by persulfate activated by the combination of sunlight, Fe(Ⅱ) and citric acid [J]. Environmental Chemistry, 2021, 40(10): 3122-3132 (in Chinese)
[28] 廖洋, 鲁金凤, 曹轶群, 等. 光催化降解对抗生素藻类毒性效应影响研究进展[J]. 环境化学, 2021, 40(1): 111-120
Liao Y, Lu J F, Cao Y Q, et al. Research progress on the effects of photocatalytic degradation on the algae toxicity of antibiotics [J]. Environmental Chemistry, 2021, 40(1): 111-120 (in Chinese)
[29] Buth J M, Arnold W A, McNeill K. Unexpected products and reaction mechanisms of the aqueous chlorination of cimetidine [J]. Environmental Science &Technology, 2007, 41(17): 6228-6233
[30] Lin Y Z, Chen J, Zhou M L, et al. Efficiency and mechanism of zero-valent iron/nitrilotriacetic acid/peroxymonosulfate system for degrading sulfamethazine [J]. Process Safety and Environmental Protection, 2022, 168: 993-1008
[31] Yan J F, Li J, Peng J L, et al. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation [J]. Chemical Engineering Journal, 2019, 359: 1097-1110
[32] Qi C D, Liu X T, Lin C Y, et al. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity [J]. Chemical Engineering Journal, 2014, 249: 6-14
[33] Acosta-Rangel A, Sánchez-Polo M, Polo A M S, et al. Sulfonamides degradation assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, mechanism and byproducts cytotoxicity [J]. Journal of Environmental Management, 2018, 225: 224-231
[34] Liu X Y, Huang F, Yu Y, et al. Determination and toxicity evaluation of the generated byproducts from sulfamethazine degradation during catalytic oxidation process [J]. Chemosphere, 2019, 226: 103-109
[35] Barhoumi N, Oturan N, Olvera-Vargas H, et al. Pyrite as a sustainable catalyst in electro-Fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment [J]. Water Research, 2016, 94: 52-61
[36] Foti, Coviello D, Zuorro A, et al. Comparison of sunlight-AOPs for levofloxacin removal: Kinetics, transformation products, and toxicity assay on Escherichia coli and Micrococcus flavus [J]. Environmental Science and Pollution Research International, 2022, 29(38): 58201-58211