氟化物是全球污染物,F-是一种氧化剂和可逆酶抑制剂,可干扰至少80种蛋白质的酶活性[1],过量的氟积累会对生物体产生严重的毒害作用[2-4]。氟的化学性质活泼,电负性强,易与其他化学元素结合形成稳定的化合物,氟化物通过火山排放、矿物溶解和工业副产品逐渐积聚在环境中[4]。氟可通过空气、饮用水和食物进入机体,并且在活体动物中有累积效应[5-6]。机体摄入的氟易被牙齿和骨骼吸收,长期接触过量的氟化物不仅会导致氟斑牙和氟骨症的产生[7-8],也会对机体其他组织(如脑、肾、肝、生殖器官等)产生不同程度的损伤[9-11]。在脊椎动物中,氟化物对心脏、肾脏、神经系统、呼吸系统、免疫系统、生殖系统等均有危害,甚至可诱发癌症[12]。对于植物,植物中的氟主要是从大气中吸收,通常叶片累积的氟化物最多,据报道,茎叶积累的氟化物比周围空气中的氟化物含量普遍高1 000倍左右,且只要不超过其耐受范围,氟化物能不断地被吸收[13-14],但当氟化物含量过高时也将导致植物体发生病变,叶片萎黄、边缘性坏死、脱落等[15-16]。
昆虫是无脊椎动物中的一大类群,经常被认为植物、动物和人类的媒介,对生态平衡的维持至关重要。已有研究表明氟化物对部分昆虫具有毒害作用,导致其生长发育受阻,数量减少,生活史缩短,大气氟化物对昆虫的毒害引起了越来越多的关注。目前氟化物对昆虫影响主要集中在模式昆虫黑腹果蝇(Drosophila melanogaster)和家蚕(Bombyx mori)[17-18],如:氟中毒的家蚕幼虫表现出明显的生长发育不齐,取食减少,体质量降低,产生黑色环斑,产丝异常,茧小及肠上皮细胞损伤的现象[19-21]。然而,某些害虫却得益于氟污染,例如:松芽麦蛾(Exoteleia dodecella)在氟污染地区的种群密度较未受污染的地区增大[22]。NaF对其他植食性害虫的生长发育和生殖毒性的报道甚少,关于氟化物对植食性害虫潜在的生长发育和生殖能力的影响尚未阐明。美国白蛾(H. cunea)在全球范围内广泛传播,其寄主植物有600余种,在欧洲和亚洲已成为一种主要害虫,对农林业造成了重大的经济损失[23]。本研究以美国白蛾为对象,在考查NaF对美国白蛾急性毒性的基础上,系统研究了亚致死剂量NaF对美国白蛾生长发育和生殖能力的影响,为评价氟化物对植食性昆虫毒性影响及进一步解析环境污染物氟化物对杂食性害虫美国白蛾的毒性机制提供理论依据。
1 材料与方法(Materials and methods)
1.1 供试昆虫及处理
美国白蛾的卵块和人工饲料均来自于中国林业科学研究院森林生态环境与自然保护研究所。在相对湿度75%,温度(25±1) ℃,光周期16 L∶8 D的养虫室孵化后,采用人工饲料饲养。
参考Tang等[20] NaF处理家蚕的方法,略作修改。NaF(分析纯,上海国药集团)溶于去离子水,制成母液,将母液用去离子水稀释成不同浓度梯度(80、160、320和1 000 mg·L-1),置于4 ℃冰箱储存备用。参照《农药室内生物测定试验准则》(NY/T 1154.10—2008)[24]中的人工饲料混药法,将不同浓度的NaF溶液或去离子水加入50~60 ℃的人工饲料中,迅速搅拌使其与饲料充分混合,冷却至室温。选取6龄第1天美国白蛾幼虫,置于含不同浓度NaF的人工饲料中,以加入等量去离子水的人工饲料为对照,每个处理10头幼虫,重复3次。处理后每24 h观察记录美国白蛾的存活情况。
1.2 美国白蛾生长发育指标测定
根据毒力回归线求得LC10(120 mg·L-1),配制相应浓度NaF溶液,以去离子水作空白对照。将同一时期蜕皮至6龄的美国白蛾幼虫,分别置于含120 mg·L-1 NaF和对照(去离子水)的人工饲料养虫盒中饲养,每个处理设置3个生物学重复,每个重复10头幼虫。处理后每24 h更换新鲜不同处理的人工饲料,并观察记录,统计体质量、发育历期、蛹质量、蛹历期、化蛹率和羽化率生长发育指标。
1.3 美国白蛾生殖能力测定
参照曹红妹等[25]的方法,稍加改动。待饲喂含120 mg·L-1 NaF人工饲料的美国白蛾6龄幼虫化蛹、羽化为成虫后,将其与未经NaF处理的对照成虫交配,进行3种交配组合:对照♀×对照![]() ♀×对照
♀×对照![]() 对照♀。每个处理设置3个生物学重复,每个重复10对雌雄成虫。每种交配组合均为一雌一雄的组合形式,待雌蛾死亡后,收集每只雌蛾产下的卵块,计算其产卵量、受精率和孵化率;其中受精率=受精卵数/总产卵量(美国白蛾卵块变黑即为受精);孵化率 = 初孵幼虫数/受精卵数。
对照♀。每个处理设置3个生物学重复,每个重复10对雌雄成虫。每种交配组合均为一雌一雄的组合形式,待雌蛾死亡后,收集每只雌蛾产下的卵块,计算其产卵量、受精率和孵化率;其中受精率=受精卵数/总产卵量(美国白蛾卵块变黑即为受精);孵化率 = 初孵幼虫数/受精卵数。
1.4 数据处理
根据经济合作与发展组织(Organisation for Economic Cooperation and Development, OECD)[26]对最大无观察效应浓度(no observed effect concentration, NOEC)的统计分析指南,将获得的毒性试验数据通过软件SPSS Statistic 25进行计算,采用ANOVA法在5%显著水平下,确定NOEC。利用EPA Probit法[27]通过SPSS Statistic 25进行亚致死剂量浓度LC50、LC30和LC10计算,以及毒力回归方程分析[28]。采用GraphPad Prism 8进行数据分析与作图,并且采用软件中的独立样本T检验分析对照和处理组之间的差异显著性;*、**、***分别表示0.05、0.01和0.001显著性差异水平。
2 结果(Results)
2.1 不同剂量NaF对美国白蛾存活率的影响
美国白蛾6龄幼虫取食不同浓度NaF人工饲料,其生存能力明显降低(图1)。NaF处理组的存活率均显著低于对照组(100%,P<0.05)。随着NaF浓度的增加和暴露时间的延长,美国白蛾幼虫的死亡率显著升高。上述结果显示NaF对美国白蛾死亡率的影响呈现明显的时间-剂量效应(图1)。根据死亡率计算出10 d的毒力回归方程为:y = 7.809x-17.642,其致死中浓度(LC50)为181.70 mg·L-1(95%置信区间为159.60~209.10 mg·L-1),NOEC为80.00 mg·L-1(表1)。
表1 NaF处理10 d对美国白蛾6龄幼虫的毒力
Table 1 Toxicity of NaF to the 6th instar larvae of H. cunea after 10 d exposure
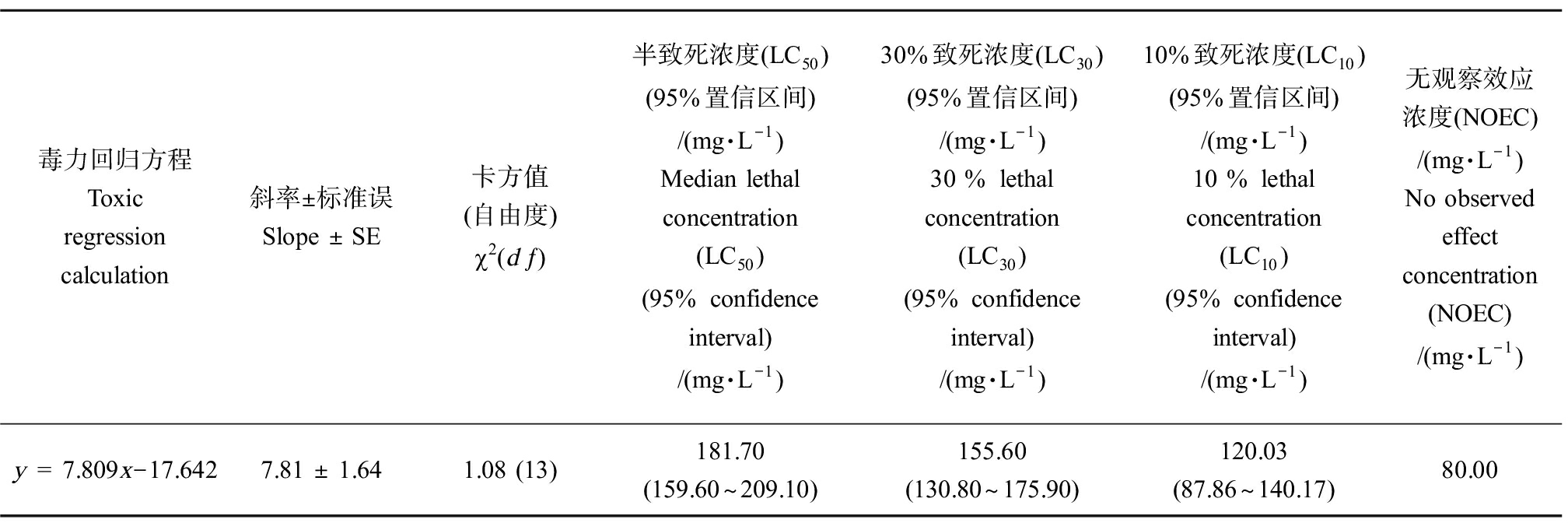
毒力回归方程Toxic regression calculation斜率±标准误Slope ± SE 卡方值(自由度)2(d) 半致死浓度(LC50)(95%置信区间)/(mg·L-1)Median lethal concentration (LC50)(95% confidence interval)/(mg·L-1)30%致死浓度(LC30)(95%置信区间)/(mg·L-1)30 % lethal concentration(LC30)(95% confidence interval)/(mg·L-1)10%致死浓度(LC10)(95%置信区间)/(mg·L-1)10 % lethal concentration(LC10)(95% confidence interval)/(mg·L-1)无观察效应浓度(NOEC)/(mg·L-1)No observed effect concentration (NOEC)/(mg·L-1)y = 7.809x-17.642 7.81 ± 1.641.08 (13)181.70 (159.60~209.10)155.60 (130.80~175.90)120.03 (87.86~140.17)80.00
注:卡方值小于![]() 故毒力回归方程与实际相符;PROBIT模型,PROBIT(p) = 截距 + Bx(协变量x使用底数为10的对数进行转换)。
故毒力回归方程与实际相符;PROBIT模型,PROBIT(p) = 截距 + Bx(协变量x使用底数为10的对数进行转换)。
Note: The Chi-square value was less than ![]() 22.36, so the toxicity regression equation was consistent with the actual; PROBIT model, PROBIT(p) = Intercept + Bx (Covariates x are transformed using the base 10 logarithm).
22.36, so the toxicity regression equation was consistent with the actual; PROBIT model, PROBIT(p) = Intercept + Bx (Covariates x are transformed using the base 10 logarithm).
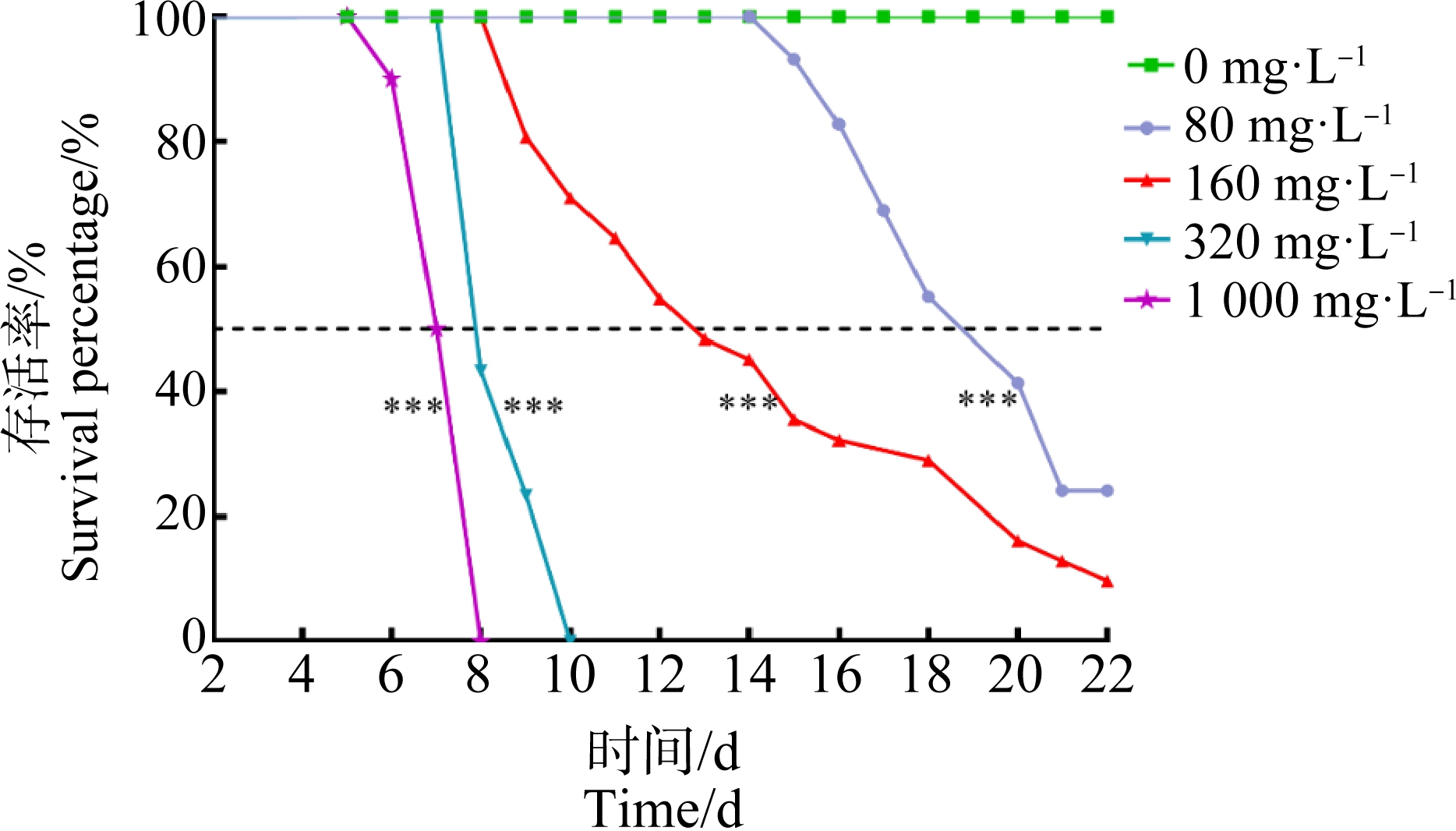
图1 氟化钠(NaF)胁迫下美国白蛾6龄幼虫的存活率
Fig. 1 Survival rate of 6th instar larvae of the H. cuneaunder sodium fluoride (NaF) stress
2.2 NaF处理对美国白蛾的生长发育影响
亚致死浓度(120 mg·L-1) NaF处理对美国白蛾的生长发育具有明显的抑制作用。取食NaF处理人工饲料的美国白蛾与对照组相比,处理24 h,NaF处理组体质量为对照组的75.9%,处理48 h,NaF处理组体质量为对照组体质量的68.5%,体质量均显著下降(图2(a),P<0.001),且NaF处理组幼虫出现缩节、体型缩小等现象(图2(c))。NaF处理美国白蛾的6龄历期显著延长,严重阻碍了美国白蛾的生长发育(图2(b),P<0.001)。
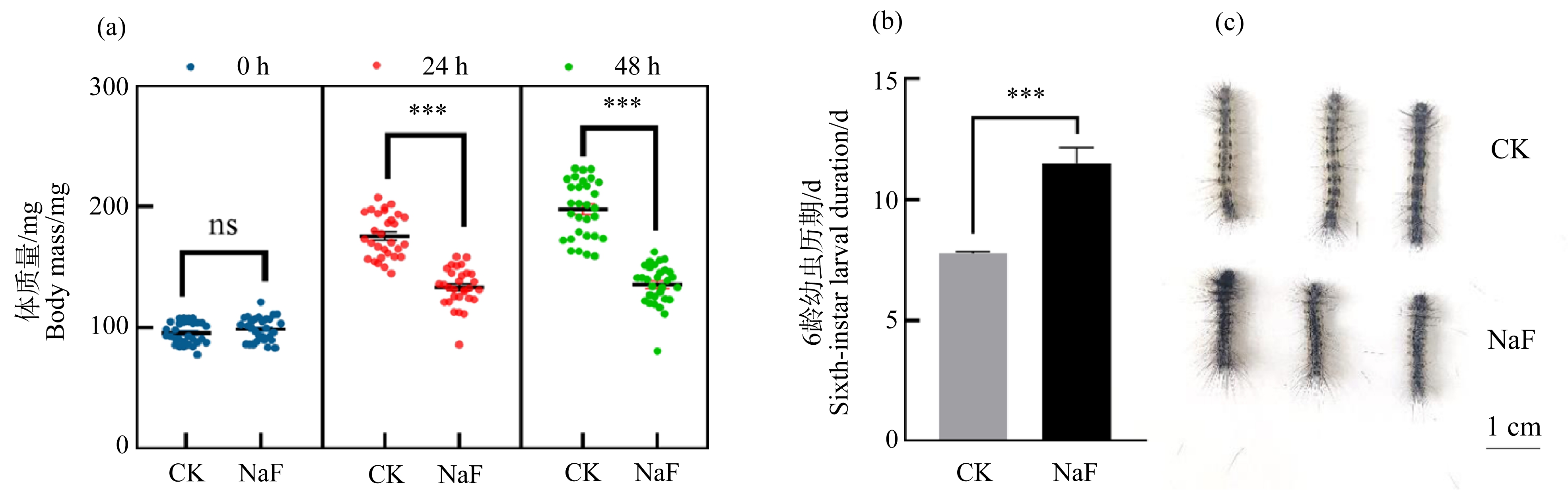
图2 NaF对美国白蛾体质量、体型大小和6龄发育历期的影响
注:(a) 体质量;(b) 6龄幼虫历期;(c) 体型;数据为平均值±标准误(样本重复n=3);ns代表与对照值差异不显著,
*代表与对照值有显著性差异(独立样本T检验,*P<0.05、***P<0.01、***P<0.001)。
Fig. 2 Effects of NaF on body weight, body size and developmental duration of 6th instar H. cunea larvae
Note: (a) Body weight; (b) Developmental duration of 6th instar H. cunea larvae; (c) Body size; results are presented as the mean ± SEM from at least three independent experiments; ns is not considered statistically significant; *indicates significant differences
(*P<0.05, ***P<0.01, ***P<0.001); statistical analyses were carried out employing Student’s t-test.
NaF处理组的美国白蛾化蛹率显著降低,仅为对照组的86.7%(图3(a),P<0.05);NaF处理组的雄蛹质量和雌蛹质量分别为对照组的89.8%和76.7%,且差异显著,NaF处理组雄蛹和雌蛹的体型明显小于对照组(图3(c)和3(d),P<0.001)。NaF延长美国白蛾蛹期,NaF处理组美国白蛾蛹历期比对照组延长了15.4%(图3(b),P<0.001)。
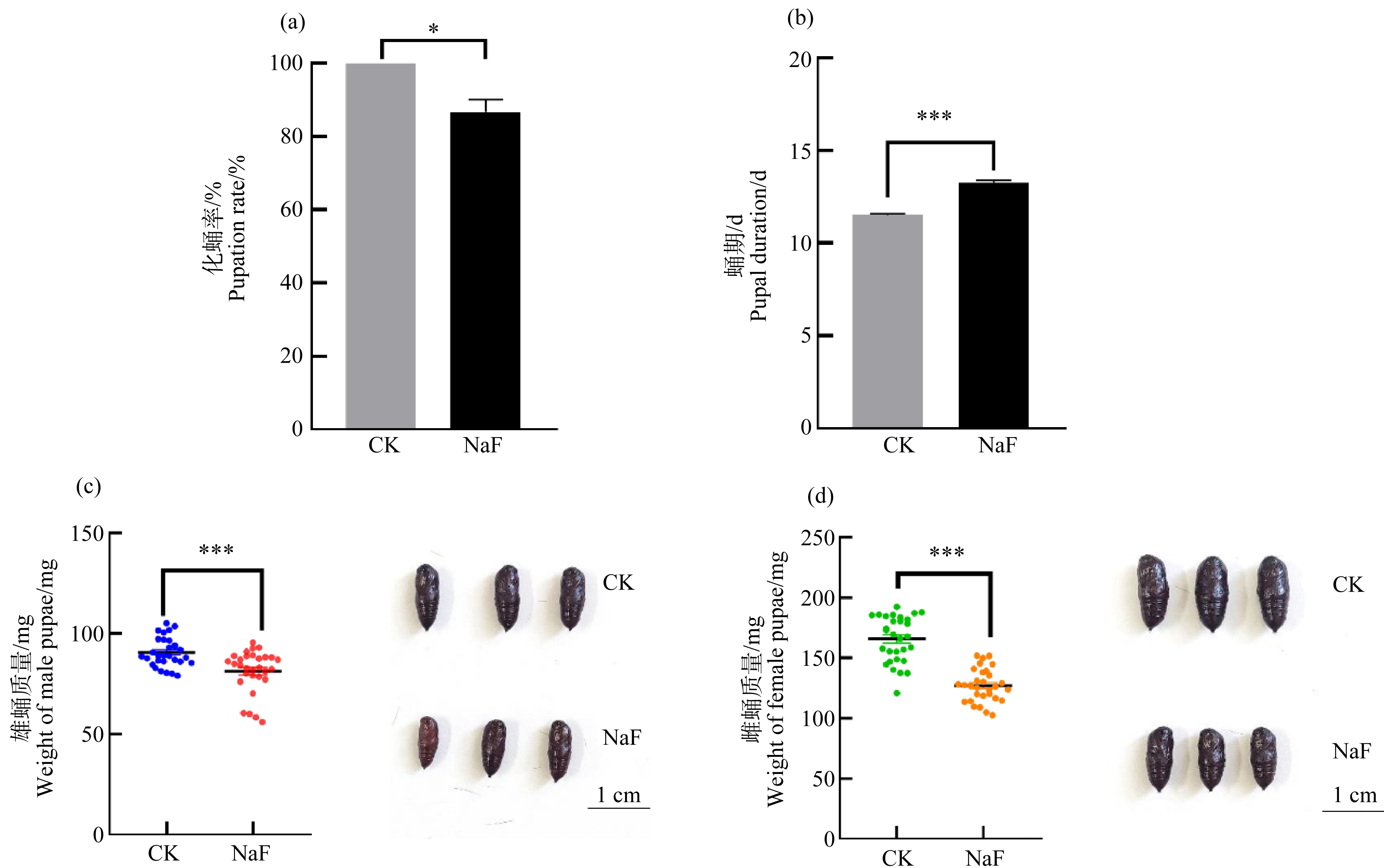
图3 120 mg·L-1 NaF对美国白蛾蛹发育的影响
注:(a) 化蛹率;(b) 蛹期;(c) 雄蛹质量;(d) 雌蛹质量;数据为平均值±标准误(样本重复n=3);
*所指的是与对照值有显著性差异(独立样本T检验,*P<0.05、***P<0.01、***P<0.001)。
Fig. 3 Effects of 120 mg·L-1 NaF on pupal development of H. cunea
Note: (a) Pupation rate; (b) Pupal duration; (c) Male pupal weight; (d) Female pupal weight; results are presented as the mean ± SEM from at least three independent experiments; *indicates significant differences (*P<0.05,
***P<0.01, ***P<0.001); statistical analyses were carried out employing Student’s t-test.
2.3 NaF对美国白蛾生殖能力的影响
亚致死浓度(120 mg·L-1) NaF对美国白蛾羽化率无显著影响(图4(a)),但显著影响了成虫产卵量、受精率和卵的孵化率。NaF处理组的美国白蛾产卵量降低(图4(b)和4(e)),其中![]() 对照♀交配组的成虫产卵量为对照组的48.2%,差异显著(P<0.001);NaF♀×对照
对照♀交配组的成虫产卵量为对照组的48.2%,差异显著(P<0.001);NaF♀×对照![]() 交配组的成虫产卵量为对照组的76.5%,但差异不显著(P>0.05)。
交配组的成虫产卵量为对照组的76.5%,但差异不显著(P>0.05)。

图4 120 mg·L-1 NaF对美国白蛾成虫生殖能力影响
注:(a) 羽化率;(b) (e) 产卵量;(c) 受精率;(d) 孵化率;![]() ♀
♀![]() ♀ (n=15),NaF♀
♀ (n=15),NaF♀![]() 数据为平均值±标准误(样本重复n=3);ns代表与对照值差异不显著(P>0.05),*代表与对照值有显著性差异(独立样本T检验,*P<0.05、***P<0.01、***P<0.001)。
数据为平均值±标准误(样本重复n=3);ns代表与对照值差异不显著(P>0.05),*代表与对照值有显著性差异(独立样本T检验,*P<0.05、***P<0.01、***P<0.001)。
Fig. 4 Effects of 120 mg·L-1 NaF on reproductive capacity of H. cunea
Note: (a) Emergence rate; (b) (e) Fecundity per female; (c) Fertilized eggs rate; (d) Hatchability; ![]() ♀ (n=15),
♀ (n=15), ![]() ♀ (n=15), NaF♀
♀ (n=15), NaF♀![]() (n=14); results are presented as the mean ± SEM from at least three independent experiments; ns is not considered statistically significant; *indicates significant differences (*P<0.05, ***P<0.01, ***P<0.001); statistical analyses were carried out employing Student’s t-test.
(n=14); results are presented as the mean ± SEM from at least three independent experiments; ns is not considered statistically significant; *indicates significant differences (*P<0.05, ***P<0.01, ***P<0.001); statistical analyses were carried out employing Student’s t-test.
对照组成虫交配的受精率为85.78%,而NaF处理组成虫与对照成虫交配后受精率显著降低。![]() 对照♀交配组卵的受精率为22.74%(P<0.001),NaF♀×对照组
对照♀交配组卵的受精率为22.74%(P<0.001),NaF♀×对照组![]() 交配组卵的受精率为56.8%(P<0.01);NaF处理组与对照组之间有显著差异(P<0.01)(图
交配组卵的受精率为56.8%(P<0.01);NaF处理组与对照组之间有显著差异(P<0.01)(图![]() 对照♀交配组的卵孵化率为23.25%(P<0.001),NaF♀×对照
对照♀交配组的卵孵化率为23.25%(P<0.001),NaF♀×对照![]() 交配组的卵孵化率为61.23%(P<0.05),对照组卵孵化率为82.87%,两者间差异显著(P<0.05,图4(d))。而NaF♀×对照
交配组的卵孵化率为61.23%(P<0.05),对照组卵孵化率为82.87%,两者间差异显著(P<0.05,图4(d))。而NaF♀×对照![]() 交配组的受精率为
交配组的受精率为![]() 对照♀交配组的2.5倍(P<0.05),NaF♀×对照
对照♀交配组的2.5倍(P<0.05),NaF♀×对照![]() 交配组卵孵化率为
交配组卵孵化率为![]() 对照♀交配组的2.63倍(P<0.05);NaF处理对雄蛾的生殖影响比雌蛾更为显著。
对照♀交配组的2.63倍(P<0.05);NaF处理对雄蛾的生殖影响比雌蛾更为显著。
3 讨论(Discussion)
大气中过量的氟污染会损害动植物的生长和生殖发育,研究表明过量的氟积累会使植物发生病变,也会对动物的神经系统、免疫系统、消化系统和生殖系统产生损害[29]。庄平等[30]研究发现西伯利亚鲟(Acipenser baeri)初孵仔鱼在氟胁迫下随着暴露时间的延长与暴露浓度的升高,死亡率显著增加;氟离子降低了家蚕幼虫的取食量,导致节间膜病变,生长速度降低,死亡率增加[20,31]。Gong等[32]的研究结果显示,当HF浓度为0.87 μg·dm-2·d-1时,70.4%的黏虫(Mythimna separate)幼虫增加了一个龄期,且幼虫取食量减少。上述研究结果显示过量的氟积累会对动物产生毒害作用,过量的氟积累会引起动物生理功能的改变,例如体色加深、取食减少、体质量减轻、发育历期延长、化蛹率和羽化率降低[25,33],而当高浓度的氟积累到一定程度,动物机体各个系统严重受损而不能负荷的时候便会引起动物死亡。本文研究结果显示中高浓度NaF(80、160、320和1 000 mg·L-1 NaF)持续处理下的美国白蛾死亡率较高,并且死亡率与NaF的浓度呈正相关。亚致死浓度(120 mg·L-1) NaF处理的美国白蛾幼虫取食量减少,体型缩小,体质量显著减轻,发育历期和蛹期延长,化蛹率、蛹质量和羽化率显著降低,我们推断慢性氟中毒可能影响到了美国白蛾幼虫的能量代谢和生长发育,使其代谢紊乱,营养积累差,使其生理功能障碍;故而导致体型缩小、体质量减轻、化蛹率降低、蛹小、生长周期和蛹期发育延长。而NaF诱导机体生理功能损伤的机理较为复杂。据报道,氟化物诱导对生物体的毒性主要是因为它可以催化自由基的过量产生,导致氧化应激,干扰抗氧化酶的活性、导致脂质过氧化,从而对生物体产生不良影响,如免疫系统紊乱、抗氧化系统受损、促炎细胞因子表达减少、细胞凋亡等[34-37],而氟化物对美国白蛾生长发育的影响生化和分子机制有待于进一步研究。然而,未观察幼虫生长发育不齐、体色变深和蛹期节间膜变薄等表型[20],这与NaF暴露下家蚕的表型不同,说明NaF对不同昆虫毒性影响各异。
过量的氟积累会影响动物的生殖能力。慢性氟中毒的雄性小鼠体外受精能力下降,生育力严重降低,这是由于氟胁迫导致内质网应激反应造成细胞凋亡,使其睾丸损伤,精子形态异常且数量减少[38-39];而受到高剂量NaF毒害的雌性小鼠,其甲状腺功能减退,从而影响雌性生殖系统功能,出现卵泡异常及颗粒坏死的现象[40]。研究发现NaF胁迫下家蚕的繁殖力和孵化率显著降低,由于体内抗氧化系统紊乱,使生殖腺形态异常,进而影响了家蚕生殖能力[20,41]。本文的研究结果显示亚致死浓度(120 mg·L-1)NaF处理的美国白蛾产卵量减少、受精率和孵化率均降低,这可能是由于NaF胁迫对美国白蛾的生殖腺造成损伤,进而影响其繁殖能力;NaF处理组的产卵量下降,其中![]() 对照♀交配组的产卵量与对照相比显著降低(P<0.001),有研究表明雌成虫能在交配过程中从雄成虫的精液中获得大量的营养物质或获得了能刺激产卵的物质,从而提高产卵量[42]。故推测可能是NaF对美国白蛾雄成虫生殖腺产生了损伤,导致雄成虫的精液在交配过程中无法提供足够的营养物质到雌成虫的卵巢而造成其产卵量减少,但这需要进一步的研究。而NaF♀×对照
对照♀交配组的产卵量与对照相比显著降低(P<0.001),有研究表明雌成虫能在交配过程中从雄成虫的精液中获得大量的营养物质或获得了能刺激产卵的物质,从而提高产卵量[42]。故推测可能是NaF对美国白蛾雄成虫生殖腺产生了损伤,导致雄成虫的精液在交配过程中无法提供足够的营养物质到雌成虫的卵巢而造成其产卵量减少,但这需要进一步的研究。而NaF♀×对照![]() 交配组成虫的产卵量与对照相比虽有所下降,但差异不显著(P>0.05)。值得注意的是,NaF♀×对照
交配组成虫的产卵量与对照相比虽有所下降,但差异不显著(P>0.05)。值得注意的是,NaF♀×对照![]() 交配组产卵量、受精率和卵孵化率,依次为
交配组产卵量、受精率和卵孵化率,依次为![]() 对照♀交配组的1.59倍、2.50倍和2.63倍,这表明NaF对美国白蛾的影响具有性别差异,NaF对雄成虫生殖腺的损害较雌成虫显著。与本文的研究结果一致,唐文超等[41]发现在NaF胁迫下,家蚕雄成虫比雌成虫对NaF更为敏感。此外,已有研究显示NaF能使家蚕生殖腺脂质过氧化,形成丙二醛(MDA)造成生殖腺细胞损伤,例如各睾丸胞的胞膜消失、睾丸胞数目减少和精巢结构异变;卵巢发生畸形,卵细胞数目减少[20,41]。这些研究表明氟暴露可能引起氧化胁迫,造成机体代谢紊乱,进而对美国白蛾的生殖腺造成了损伤,造成了处理组受精率和孵化率的降低。
对照♀交配组的1.59倍、2.50倍和2.63倍,这表明NaF对美国白蛾的影响具有性别差异,NaF对雄成虫生殖腺的损害较雌成虫显著。与本文的研究结果一致,唐文超等[41]发现在NaF胁迫下,家蚕雄成虫比雌成虫对NaF更为敏感。此外,已有研究显示NaF能使家蚕生殖腺脂质过氧化,形成丙二醛(MDA)造成生殖腺细胞损伤,例如各睾丸胞的胞膜消失、睾丸胞数目减少和精巢结构异变;卵巢发生畸形,卵细胞数目减少[20,41]。这些研究表明氟暴露可能引起氧化胁迫,造成机体代谢紊乱,进而对美国白蛾的生殖腺造成了损伤,造成了处理组受精率和孵化率的降低。
当前氟化物对昆虫的毒性效应已受到大量关注,主要是集中在寡食性昆虫家蚕上,对杂食性害虫的研究较少,无法全面解释氟化物对昆虫毒害作用。本研究考察了NaF对美国白蛾的致死效应及生长发育指标和生殖能力指标的影响,确定亚致死浓度(120 mg·L-1) NaF胁迫导致美国白蛾幼虫体型缩小,体质量减轻,发育历期长,化蛹率低,蛹质量轻和蛹期延长,导致雌蛾的产卵量、受精率和孵化率均显著降低。这些研究结果为评价氟化物对昆虫毒性的影响提供参考依据,也为进一步揭示氟化物毒性机理提供理论基础。
[1] García-Montalvo E A, Reyes-Pérez H, Del Razo L M. Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress [J]. Toxicology, 2009, 263(2-3): 75-83
[2] Abd-Allah E R, El-Rahman H A A. Ameliorative effects of nano Moringa on fluoride-induced testicular damage via down regulation of the StAR gene and altered steroid hormones [J]. Reproductive Biology, 2023, 23(1): 100724
[3] Wu Y J, Zhang X L, Chen J J, et al. Self-recovery study of fluoride-induced ferroptosis in the liver of zebrafish (Danio rerio) [J]. Aquatic Toxicology, 2022, 251: 106275
[4] Johnston N R, Strobel S A. Principles of fluoride toxicity and the cellular response: A review [J]. Archives of Toxicology, 2020, 94(4): 1051-1069
[5] Dehbandi R, Moore F, Keshavarzi B. Geochemical sources, hydrogeochemical behavior, and health risk assessment of fluoride in an endemic fluorosis area, central Iran [J]. Chemosphere, 2018, 193: 763-776
[6] 陈思怀, 刘燕, 唐发书. 氟化物对体外培养猪小肠上皮细胞的影响[J]. 安徽农业科学, 2008, 36(3): 1062-1063, 1098
Chen S H, Liu Y, Tang F S. Effects of fluoride on porcine small intestine epithelial cells cultured in vitro [J]. Journal of Anhui Agricultural Sciences, 2008, 36(3): 1062-1063, 1098 (in Chinese)
[7] Li Y, Wang F, Feng J, et al. Health risk in children to fluoride exposure in a typical endemic fluorosis area on Loess Plateau, North China, in the last decade [J]. Chemosphere, 2020, 243: 125451
[8] Srivastava S, Flora S J S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact [J]. Current Environmental Health Reports, 2020, 7(2): 140-146
[9] Jiang P, Li G Y, Zhou X Y, et al. Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: Role of GSK-3β/β-catenin pathway [J]. Chemosphere, 2019, 214: 430-435
[10] Efe U, Dede S, Yüksek V, et al. Apoptotic and oxidative mechanisms in liver and kidney tissues of sheep with fluorosis [J]. Biological Trace Element Research, 2021, 199(1): 136-141
[11] Zhang J H, Zhu Y C, Shi Y, et al. Fluoride-induced autophagy via the regulation of phosphorylation of mammalian targets of rapamycin in mice Leydig cells [J]. Journal of Agricultural and Food Chemistry, 2017, 65(40): 8966-8976
[12] Takeshita A, Imai K, Kato S, et al. 1alpha, 25-dehydroxyvitamin D3 synergism toward transforming growth factor-beta1-induced AP-1 transcriptional activity in mouse osteoblastic cells via its nuclear receptor [J]. The Journal of Biological Chemistry, 1998, 273(24): 14738-14744
[13] 刘佩琪, 邓志华, 陈奇伯, 等. 昆明市城市森林常见树种对大气氟化物的净化作用动态研究[J]. 中南林业科技大学学报, 2017, 37(8): 108-113
Liu P Q, Deng Z H, Chen Q B, et al. The purification effect research in motion of urban forest on atmosphere in Kunming [J]. Journal of Central South University of Forestry &Technology, 2017, 37(8): 108-113 (in Chinese)
[14] 徐丽珊. 大气氟化物对植物影响的研究进展[J]. 浙江师范大学学报(自然科学版), 2004, 27(1): 66-71
Xu L S. Effects of atmospheric fluoride pollution on plant [J]. Journal of Zhejiang Normal University (Natural Sciences), 2004, 27(1): 66-71 (in Chinese)
[15] 王继臣. 大气氟化物对植物的伤害及其测试分析方法[J]. 安徽农业科学, 2008, 36(11): 4650-4651
Wang J C. Effects of atmospheric fluoride pollution on plant and its testing and analysis methods [J]. Journal of Anhui Agricultural Sciences, 2008, 36(11): 4650-4651 (in Chinese)
[16] Sharma R, Kaur R. Insights into fluoride-induced oxidative stress and antioxidant defences in plants [J]. Acta Physiologiae Plantarum, 2018, 40(10): 181
[17] Perveen S, Kumari S, Raj H, et al. Effects of sodium fluoride and Ocimum sanctum extract on the lifespan and climbing ability of Drosophila melanogaster [J]. The Journal of Basic and Applied Zoology, 2021, 82(1): 32
[18] Liu Y, Liang Y, Yang C F, et al. A deep insight into the transcriptome of midgut and fat body reveals the toxic mechanism of fluoride exposure in silkworm [J]. Chemosphere, 2021, 262: 127891
[19] 戴盛, 李斌, 马晓春, 等. 氟化钠对家蚕幼虫体液免疫功能的影响[J]. 安徽农业科学, 2009, 37(15): 7022-7023, 7036
Dai S, Li B, Ma X C, et al. Effect of sodium fluoride on humoral immune function of silkworm larvae [J]. Journal of Anhui Agricultural Sciences, 2009, 37(15): 7022-7023, 7036 (in Chinese)
[20] Tang W C, Zheng X, Li D, et al. Effects of sodium fluoride on the reproductive development of Bombyx mori [J]. Environmental Toxicology and Pharmacology, 2018, 64: 41-47
[21] Li G N, Zheng X, Zhu Y, et al. In-depth insights into the disruption of the microbiota-gut-blood barrier of model organism (Bombyx mori) by fluoride [J]. Science of the Total Environment, 2022, 838: 156220
[22] 张云, 叶万辉, 李跃林. 大气污染对植食昆虫的影响及作用机制[J]. 农村生态环境, 2002, 18(3): 49-55
Zhang Y, Ye W H, Li Y L. Effect of atmospheric pollution on phytophagous insects and its mechanism [J]. Rural Eco-Environment, 2002, 18(3): 49-55 (in Chinese)
[23] Edosa T T, Jo Y H, Keshavarz M, et al. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development [J]. Journal of Applied Entomology, 2019, 143(1-2): 1-10
[24] 中华人民共和国农业部. 农药室内生物测定试验准则 杀虫剂 第10部分: 人工饲料混药法: NY/T 1154.10—2008 [S]. 北京: 中国农业出版社, 2008
[25] 曹红妹, 郑丽霞, 魏洪义. 重金属Ni2+对亚洲玉米螟生长发育和生殖行为的影响[J]. 昆虫学报, 2015, 58(6): 650-657
Cao H M, Zheng L X, Wei H Y. Effects of heavy metal Ni2+ on the development and reproductive behavior of Ostrinia furnacalis (Lepidoptera: Pyralidae) [J]. Acta Entomologica Sinica, 2015, 58(6): 650-657 (in Chinese)
[26] Organisation for Economic Co-operation and Development (OECD). Test No. 233: Sediment-water chironomid life-cycle toxicity test using spiked water or spiked sediment [S]. Paris: OECD, 2009
[27] 吴鼎勋, 洪万树. 四种重金属对鮸状黄姑鱼胚胎和仔鱼的毒性[J]. 台湾海峡, 1999, 18(2): 186-190, 237
Wu D X, Hong W S. Study on toxicity of four heavy metals to embryo and larval of Nibea miichthioides [J]. Journal of Oceanography in Taiwan Strait, 1999, 18(2): 186-190, 237 (in Chinese)
[28] 武怀恒, 万鹏, 黄民松. 毒力回归计算方法及相应软件使用介绍[J]. 安徽农业科学, 2014, 42(27): 9335-9338, 9340
Wu H H, Wan P, Huang M S. Toxicity regression calculation method and introduction of corresponding software utilization [J]. Journal of Anhui Agricultural Sciences, 2014, 42(27): 9335-9338, 9340 (in Chinese)
[29] Zuo H, Chen L, Kong M, et al. Toxic effects of fluoride on organisms [J]. Life Sciences, 2018, 198: 18-24
[30] 庄平, 王瑞芳, 石小涛, 等. 氟对西伯利亚鲟仔鱼的急性毒性及安全浓度评价[J]. 生态毒理学报, 2009, 4(3): 440-445
Zhuang P, Wang R F, Shi X T, et al. Acute toxicity and safety assessment of fluoride to larval Siberian sturgeon Acipenser baeri [J]. Asian Journal of Ecotoxicology, 2009, 4(3): 440-445 (in Chinese)
[31] 白会钗, 徐安英, 李木旺, 等. 利用SSR标记对家蚕耐氟基因进行连锁定位分析[J]. 蚕业科学, 2008, 34(2): 191-196
Bai H C, Xu A Y, Li M W, et al. SSR based linkage and mapping analysis of dominant endurance to fluoride gene (def) in the silkworm, Bombyx mori [J]. Science of Sericulture, 2008, 34(2): 191-196 (in Chinese)
[32] Gong P Y, Li X Z, Jianmin W K S, et al. Effects of fluoride on growth and reproduction of the army worm, Mythimna separata (Walker) [J]. Journal of Environmental Sciences, 1990(4): 45-50
[33] Barbier O, Arreola-Mendoza L, Del Razo L M. Molecular mechanisms of fluoride toxicity [J]. Chemico-Biological Interactions, 2010, 188(2): 319-333
[34] Cao J L, Feng C P, Xie L T, et al. Sesamin attenuates histological alterations, oxidative stress and expressions of immune-related genes in liver of zebrafish (Danio rerio) exposed to fluoride [J]. Fish &Shellfish Immunology, 2020, 106: 715-723
[35] Dutta M, Rajak P, Khatun S, et al. Toxicity assessment of sodium fluoride in Drosophila melanogaster after chronic sub-lethal exposure [J]. Chemosphere, 2017, 166: 255-266
[36] Chen T, Cui H M, Cui Y, et al. Decreased antioxidase activities and oxidative stress in the spleen of chickens fed on high-fluorine diets [J]. Human &Experimental Toxicology, 2011, 30(9): 1282-1286
[37] Lu Y J, Luo Q, Cui H M, et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse liver [J]. Aging, 2017, 9(6): 1623-1639
[38] 冯婧, 田晓琳, 董妮莎, 等. 氟致内质网应激性凋亡的研究进展[J]. 环境与职业医学, 2018, 35(6): 566-571
Feng J, Tian X L, Dong N S, et al. Research progress on endoplasmic reticulum stress-induced apoptosis caused by fluorine [J]. Journal of Environmental &Occupational Medicine, 2018, 35(6): 566-571 (in Chinese)
[39] 张佳勇, 唐乐, 阮琴, 等. 内质网应激在氟暴露致小鼠睾丸损伤中的作用[J]. 环境科学学报, 2020, 40(5): 1865-1872
Zhang J Y, Tang L, Ruan Q, et al. Role of endoplasmic reticulum stress on testicular injury induced by fluoride exposure in male mice [J]. Acta Scientiae Circumstantiae, 2020, 40(5): 1865-1872 (in Chinese)
[40] Dhurvey V, Patil V, Thakare M. Effect of sodium fluoride on the structure and function of the thyroid and ovary in albino rats (Rattus norvegicus) [J]. Fluoride, 2017, 50(2): 235-246
[41] 唐文超, 肖媛媛, 杨成飞, 等. 氟化物导致的家蚕生殖损伤及氧化应激反应[J]. 昆虫学报, 2016, 59(12): 1308-1316
Tang W C, Xiao Y Y, Yang C F, et al. Reproductive damage and oxidative stress caused by fluoride in the silkworm, Bombyx mori [J]. Acta Entomologica Sinica, 2016, 59(12): 1308-1316 (in Chinese)
[42] Edvardsson M, Canal D. The effects of copulation duration in the bruchid beetle Callosobruchus maculatus [J]. Behavioral Ecology, 2006, 17(3): 430-434