全氟及多氟烷基化合物(per- and polyfluoroalkyl substances, PFASs)是以烷基链为骨架,碳链上的全部或部分氢原子被氟原子取代的一类人工合成的有机化合物[1]。由于碳氟键具有极高的键能,因此PFASs在高温、强酸强碱环境下不易被降解,具有较高的化学稳定性,因此被广泛地应用于工业和消费领域[2]。全氟辛烷磺酸(perfluorooctane sulphonate, PFOS)是应用最为广泛且影响最大的PFASs之一,目前已在世界多个国家和地区被禁用[3]。
随着PFOS的禁用,大量替代物随之产生,其中全氟壬烯基苯磺酸钠(sodium p-perfluorinated noneoxybenzen sulfonate, OBS)是广泛使用的新型替代品之一。近年来,在油田周围区域、自然水体、人体等多种环境介质及生物体检测到OBS的存在,例如大庆油田周边水域OBS含量最高达3.9×103 ng·L-1[4]。与PFOS相比,OBS同样具有持久性、生物累积性和毒性,并且没有太大改善。虽然OBS已有40多年的使用历史,但是其环境行为和许多毒理学效应仍未可知,随着OBS的大范围使用,可以预知OBS将在环境中广泛存在。因此,本文旨在对PFOS及其新型替代物OBS的环境行为、人群暴露的健康危害和毒性效应等方面的研究结果进行总结,为今后相关的研究调查和科学实验提供重要参考。
1 PFOS与OBS的特性和应用(Characteristics and applications of PFOS and OBS)
PFOS(C8HF17O3S,分子量500),是一类人工合成的氟表面活性剂,具有疏水、疏油、高表面活性等优良性能,因此广泛应用于毛毯、皮革、表面活性剂等消费和工业领域[5]。然而自20世纪90年代以来,大量研究表明,PFOS具有环境持久性、生物累积性、长距离迁移能力、生物放大效应以及多种生物毒性[6-7]。鉴于PFOS的多种危害已严重影响了人体健康和生态环境,全球最大的PFOS生产商3M公司于2000年宣布逐步停止PFOS的生产使用;2009年联合国环境规划署通过《斯德哥尔摩公约》,正式将PFOS及其盐类列为新污染物,以期逐步减少并最终禁用该类物质[3]。
OBS(C9F17OC6H4SO3Na,分子量626)是以六氟丙烯为原料通过齐聚反应合成的,在合成过程中产生了4种同分异构体,目前关于OBS的研究主要集中在OBS-c。作为PFOS的替代物,OBS与PFOS结构不同(图1),OBS是支链结构,且多了双键、醚键和苯环,但是二者之间也存在许多相似的特性。OBS是20世纪70年代由中国上海有机氟材料研究所成功合成的[8],主要在我国广泛应用。相比于PFOS,OBS的生产成本更低,性价比相对较高,具有疏水疏油、良好的耐热性、化学惰性高等特性,常用于生产氟蛋白泡沫高效灭火剂和石油开采助剂,还应用于钢板清洗、印刷等工业领域[9-10]。自PFOS禁用后,产量大幅增加,目前年产量大约3 500 t[11]。随着OBS的广泛应用,目前已在环境和人体中检测到,但是关于OBS对环境及人类的潜在风险尚不明确。

图1 全氟辛烷磺酸(PFOS)和全氟壬烯基苯磺酸钠(OBS)异构体结构式
Fig. 1 The structural formulas of perfluorooctane sulphonate (PFOS) and sodium p-perfluorinated noneoxybenzen sulfonate (OBS) isomers
2 PFOS与OBS的环境检出(Environmental detection of PFOS and OBS)
环境中的PFASs的来源可分为直接来源和间接来源,直接来源包括PFASs生产过程中废物处理(污水处理、垃圾填埋等),PFASs产品的使用等;间接来源包括PFASs的降解等[12]。由于PFASs的特性,导致PFASs在各种环境介质广泛存在,并最终通过食物网、食物链进入生物体内(图2)。目前,已在土壤、沉积物、水体、大气等环境介质中检测到PFOS及其替代物OBS的存在,表1中列出了PFOS和OBS在部分区域的环境介质中的检测浓度。
2.1 土壤、沉积物
PFASs可以通过点源(污水处理、垃圾填埋等)或者扩散源(大气沉降等)排放到土壤中,而土壤中的PFASs可以通过挥发、扩散等方式进入到大气、水体、并通过食物链转移到生物体内,因此土壤浓度是环境中PFASs的污染程度的重要参考指标[13]。在我国珠江三角洲区域土壤检测发现,PFOS是主要污染物之一,平均相对百分含量为75%,土壤浓度范围是0.05~2.41 ng·g-1[14]。从我国黄海和东海的沉积物中PFASs检测来看,PFOS平均检测含量为0.002~0.558 ng·g-1[15]。在我国天津渤海湾地区周围区域的沉积物中检测到OBS,检出率为77%[10]。在我国宿迁一家氟化工生产厂附近河流沉积物样品中检测到OBS,浓度范围为0.58~81.50 ng·g-1[16]。在我国大庆油田周边湖泊底泥2次采样结果表明,OBS含量分别为7.25 ng·g-1和59.87 ng·g-1[17]。这说明在OBS生产使用区域,土壤中含量较高,对周边环境、人群存在潜在危害。
2.2 水体
水体中的PFASs来源很多,包括工业废水等的直接排放,土壤和沉积物的渗入、大气沉降、降雨降雪等[18]。PFOS生产使用历史长达60年,由于其结构特性具有环境持久性,虽然PFOS目前已被禁用但是仍可以在世界各地的水体中检测到其存在。在2012—2014年中国上海市黄浦江上层水体检测中发现,PFOS在所检测的14种PFASs中占比最高,平均浓度为139.6 ng·L-1[19]。在我国及国外的各大水系,例如我国黄河[20]、法国塞纳河[21]等水体也都检测到PFOS的存在,且在所检测的PFASs中占比靠前。
在我国渝北河和高碑店湖的地表水中检测到OBS的浓度分别为17.1 ng·L-1和5.93 ng·L-1,高于PFOS的浓度(分别为2.04 ng·L-1和1.61 ng·L-1)[22]。根据李闯修[17]等2015年2次在我国大庆油田周围湖泊及底泥采样的结果来看,油田周围湖泊PFASs含量很高。在9种检测的PFASs中,OBS含量远高于其他PFASs,夏季采样结果为平均浓度409.73 ng·L-1,采样点最高浓度达3 935 ng·L-1;冬季采样结果为平均浓度296.73 ng·L-1。Xu等[4]在我国大庆油田的稀疏油田区域、新开发油田区域、致密油田区域周围水域采样,均测得较高浓度的OBS。Qu等[23]测定了我国江苏省常州市靠近长三角饮用水加工厂的工艺水流、上游水源水(长江和德胜河)和下游公共供水的31种PFASs的浓度,在夏季样品中检测到了OBS的存在。在我国太湖流域周围的8个饮用水加工厂的原水和处理后水的大部分样品中也检测到OBS的存在[24]。
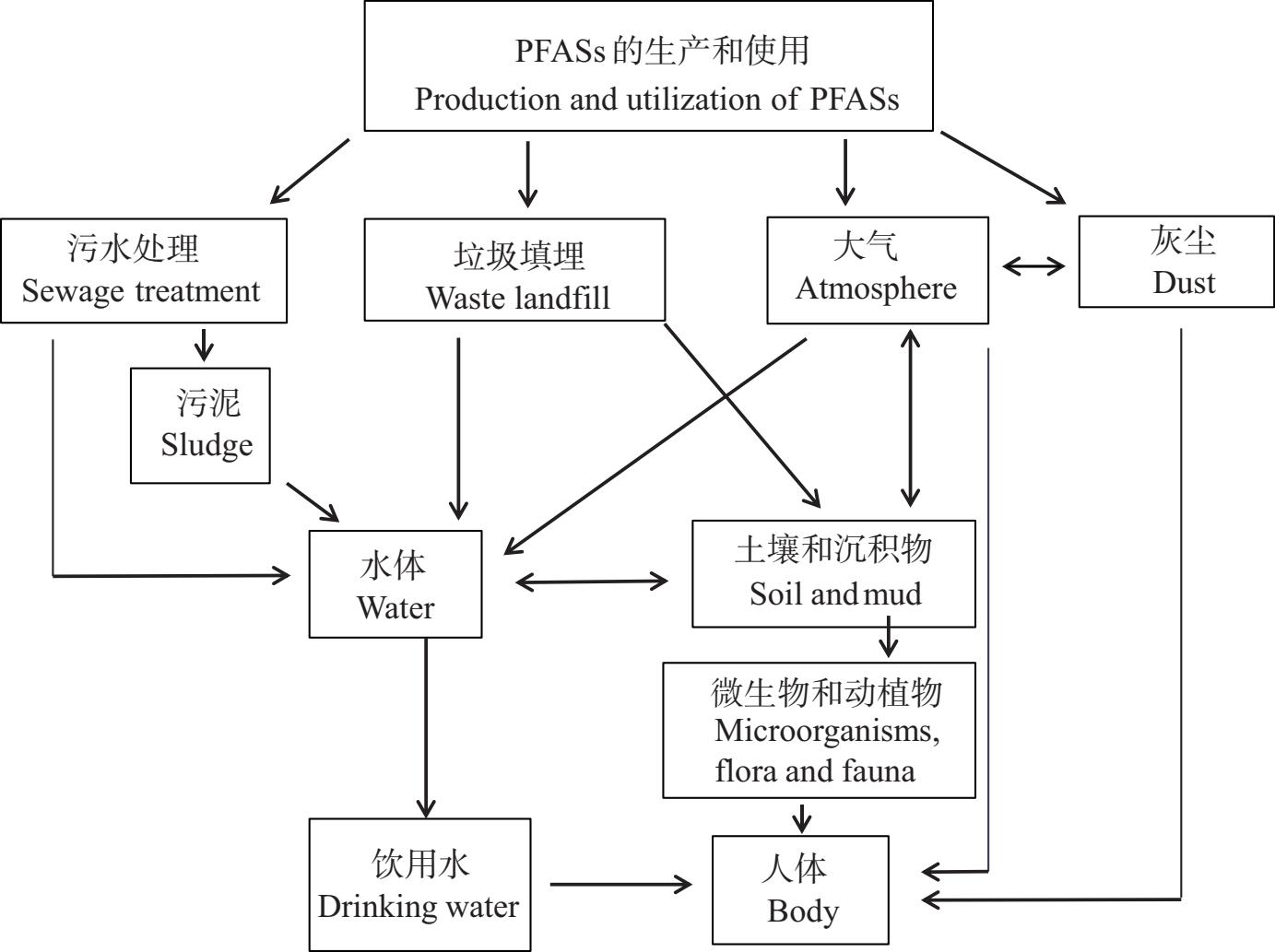
图2 全氟及多氟烷基化合物(PFASs)的环境来源和归趋
Fig. 2 Environmental sources and destinations of per- and polyfluoroalkyl substances (PFASs)
2.3 大气
PFASs可以通过土壤和水体的挥发进入大气,在大气中进行运输和转化[25]。方祥光等[26]调查了2014年中国10个城市空气颗粒物中PFOS的浓度,浓度范围为0.12~14 pg·m-3,平均浓度为2.1 pg·m-3。2017年10月检测我国成都市空气颗粒物中PFASs含量,PFOS浓度为0.966~213 pg·m-3,平均值为(13.9±26.4) pg·m-3[27]。目前,OBS在大气中含量的报道较少,Yu等[28]在中国5个城市(北京市、济南市、南京市、常熟市、贵阳市)的120份空气颗粒物样品中检测到OBS的存在。
3 PFOS与OBS的人群暴露风险(Population exposure to PFOS and OBS)
人体暴露于PFASs的途径主要为经口摄入(饮水饮食)、呼吸道(呼吸、吸入粉尘颗粒等)、皮肤接触(PFASs产品的直接接触),借助的环境媒介包括水体、土壤、空气、室内/室外灰尘等[29](图3)。其中,饮食暴露是PFASs暴露最主要的途径,在蔬菜、谷物种子等食物中检测到PFOS[30]。血液是常用的反映人体内PFASs含量的生物基质,研究发现,PFOS在我国部分地区人体血液中含量较高,在我国多个城市(深圳市、重庆市、宜宾市、沈阳市、大连市)人群血液PFASs分析均检测到较高浓度PFOS[31]。孕妇体内的PFASs可以通过胎盘转移到胎儿体内,因此脐带血是重要的测定指标。在我国广州市、深圳市、贵港市3个城市的孕妇血液样本中检测到PFOS,检出率达99%[32]。研究发现,在我国阜新市氟化工业园区周边孕妇的血清、胎盘、脐带血中均检测到OBS[33]。此外,在我国北京市地区的孕妇血清中检测到OBS浓度为0.711 ng·mL-1,脐带血血清中OBS浓度为0.604 ng·mL-1[34]。
表1 PFOS和OBS的环境检测浓度
Table 1 Environmental detection concentration of PFOS and OBS

环境介质Environmental medium地区AreaPFOS检测浓度Detection concentration of PFOSOBS检测浓度Detection concentration of OBS参考文献Reference土壤、沉积物Soil, sediments珠江三角洲Pearl River Delta0.05~2.41 ng·g-1-[14]黄海和东海Yellow Sea, East Sea0.002~0.558 ng·g-1-[15]江苏省宿迁市Suqian, Jiangsu-0.58~81.50 ng·g-1[16]大庆市油田Daqing Oil Field-7.25 ng·g-1和59.87 ng·g-17.25 ng·g-1 and 59.87 ng·g-1[17]水体Water body上海市黄浦江Huangpu River, Shanghai139.6 ng·L-1-[19]黄河Yellow River157.5 ng·L-1-[20]渝北河Yubei River2.04 ng·L-117.10 ng·L-1[22]高碑店湖Gaobeidian Lake1.61 ng·L-15.93 ng·L-1[22]大庆市油田周围水域Waters around Daqing Oil Field-夏季样品平均浓度(Average concentration of summer samples):409.73 ng·L-1冬季样品平均浓度(Average concentration of winter samples):296.73 ng·L-1[17]大庆油田周围水域Waters around Daqing Oil Field-稀疏油田(Background area):6.90 ng·L-1新油田(New oilfield):50.00 ng·L-1老油田(Old oilfield):560.00 ng·L-1[4]
注:-为无可用数据。
Note: - no data available.
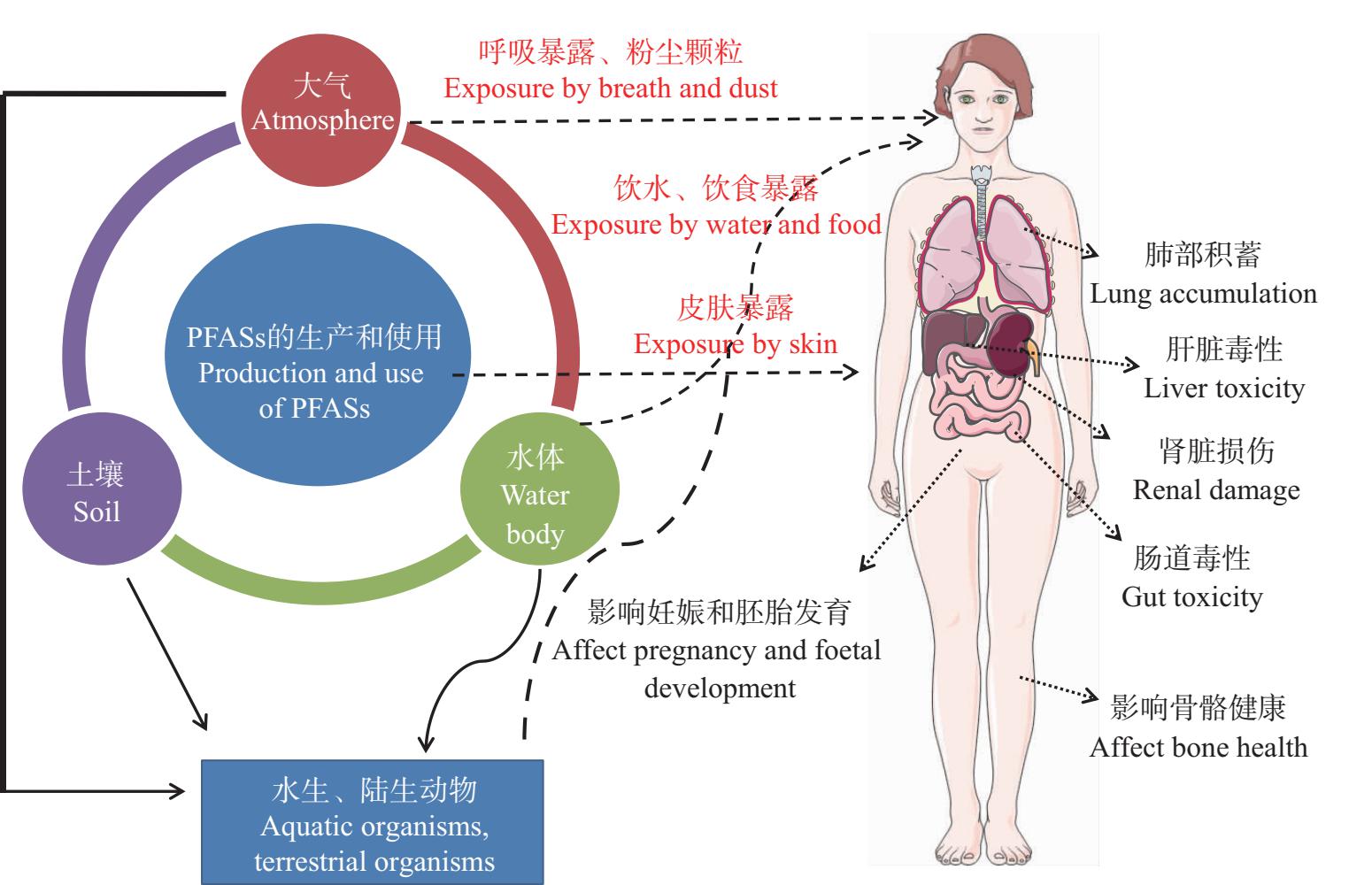
图3 PFASs的暴露途径和对人体的影响
Fig. 3 Exposure pathways and effects of PFASs on human body
4 PFOS与OBS的毒性效应(Toxicity of PFOS and OBS)
环境中存在的PFASs最终能够进入生物体内并在体内蓄积,对生物体造成危害。目前,PFOS已被证实存在着多种毒性,OBS作为PFOS的替代物,其毒性效应与PFOS存在相似与差异(表2)。
4.1 细胞毒性
PFOS对多种细胞具有毒性,能够引起成年小鼠的海马细胞凋亡率显著上升[35];在小脑颗粒细胞凋亡过程中起到促凋亡作用等[36]。PFOS暴露处理人肝癌细胞(HepG2),在300~400 μmol·L-1时,PFOS对细胞活力有轻微抑制,可以破坏细胞膜从而增加细胞通透性[37]。目前,有研究发现,OBS暴露处理大鼠垂体GH3细胞,导致GH3细胞增殖抑制,通过激活p53-p21信号通路从而诱导GH3细胞过早衰老[38]。Wang等[39]发现OBS暴露处理HepG2细胞在80 μmol·L-1时能够抑制HepG2细胞的生长,且存在剂量依赖效应。此外,OBS还能诱导HepG2细胞脂质积累,过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptors γ, PPAR γ)和CD36蛋白水平升高,这说明对于HepG2细胞而言,OBS的毒性可能大于PFOS。
4.2 水生生物毒性
研究发现,大型蚤暴露于PFOS处理24 h,其半数致死浓度(LC50)值为150.34 mg·L-1,暴露48 h的LC50值为120.22 mg·L-1[40]。斑马鱼毒性试验发现PFOS暴露处理48 h和96 h的LC50分别为105.81 mg·L-1和133.53 mg·L-1;且PFOS可以引起斑马鱼发生氧化应激反应,造成氧化损伤,存在剂量依赖效应[41]。Xu等[4]研究发现,OBS对斑马鱼和蝌蚪的96 h-LC50值分别为25.5 mg·L-1和28.4 mg·L-1。就PFOS及OBS对斑马鱼的急性毒性试验结果来看,虽然PFOS在不同文献中实验结果存在差异[42],但是96 h-LC50均>10 mg·L-1,根据全球化学品统一分类和标签制度,PFOS和OBS应被列为急性毒性(对水生生物有害)的Ⅲ类化学品。
此外,在我国渝北河和高碑店湖的野生鲫鱼中检测到了OBS,且组织分布与PFOS相似,血液>肝脏>肾脏>膀胱>性腺>肌肉[22];在斑马鱼中也检测到类似的结果,OBS和PFOS在血液和肝脏中积聚最多,在肌肉中积聚最少[43],这主要是因为PFASs能够和某些蛋白质特异结合而肌肉中蛋白质含量低。分子对接实验发现,OBS与人血清白蛋白、肝脏脂肪酸结合蛋白结合,但是结合能均低于PFOS,因此OBS的生物富集能力小于PFOS[43-44]。斑马鱼暴露实验中测定OBS与PFOS的生物富集因子(bioconcentration factor, BCF)发现,PFOS的BCF大于OBS的BCF,例如在斑马鱼幼体中检测到OBS的BCF值为238.0~242.5,而PFOS的BCF值为644.2[45]。OBS与PFOS在斑马鱼体内的摄取速率常数相当,但是OBS的消除速率常数远大于PFOS,在斑马鱼幼体测得OBS的半衰期为69.7~85 h,PFOS的半衰期为222.2 h[45]。Wen等[46]研究发现,全氟烷烃的BCF随全氟化碳数量增加而呈现指数增加;因此OBS的BCF低于PFOS可能是由于其全氟化碳数低于PFOS。此外。传统PFASs的支链异构体相比直链异构体而言,在体内消除的速率更高[47],因此OBS的支链结构可能是导致其生物富集性低于PFOS的另一个原因。OBS与PFOS暴露除了导致斑马鱼幼体发育异常还能引起严重的氧化应激,并且Nrf2-ARE信号通路可能是引起氧化应激的机制之一[45]。这说明OBS虽然生物蓄积能力、与特定蛋白结合能力均低于PFOS,但是其水生毒性作用依然不能忽视。
表2 PFOS与OBS的毒性作用与机制比较
Table 2 Comparison of toxicity and mechanism between PFOS and OBS

毒性类别Type of toxicity毒性比较Comparison of toxicityPFOS毒性Toxicity of PFOSOBS毒性Toxicity of OBS参考文献Reference细胞毒性Cytotoxicity具有肝细胞毒性,在HepG2细胞上OBS毒性大于PFOS They have hepatotoxicity, and OBS has greater cytotoxicity than PFOS in HepG2 cellsPFOS 300~400 μmol·L-1时对HepG2细胞活力有轻微抑制,破坏细胞膜PFOS (300~400 μmol·L-1) slightly inhibits the cell viability and disrupts cell membrane in HepG2 cellsOBS 80 μmol·L-1时就能够明显抑制HepG2细胞生长,引起脂质积累OBS (from 80 μmol·L-1) obviously inhibits cell growth and induces lipid accumulation in HepG2 cells[35-39]水生生物毒性Aquatic toxicity具有急性毒性而PFOS的生物蓄积能力强于OBSThey have acute toxicity, and PFOS has stronger bioaccumula-tion ability than OBSPFOS的BCF更大,与特定蛋白结合能更大BCF of PFOS is larger with power-fully binding energy to specific pro-teinsOBS的BCF、与特定蛋白结合能比PFOS低BCF of OBS and its binding energy to specific proteins are lower than those of PFOS[4,22,40-47]发育毒性Development toxicity导致胚胎发育异常而PFOS的发育毒性更大They can lead to abnormal em-bryonic development, and PFOS has stronger development toxici-tyPFOS降低斑马鱼胚胎孵化率,升高胚胎心率PFOS reduces the hatch ratio of ze-brafish embryos, and elevates heart rate in zebrafish embryosOBS影响斑马鱼幼体血管形态以及心脏功能OBS affects vascular morphology and cardiac function in zebrafish larva[39,48-51]神经毒性Neurotoxicity诱导多巴胺分泌失调,PFOS的神经毒性更强They cause the dysregulation of dopamine secretion; PFOS has stronger neurotoxicityPFOS破坏钙离子信号通路,多巴胺分泌减少PFOS disrupts calcium signaling pathway and decreases dopamine se-cretionOBS干扰Wnt信号通路,引起多巴胺分泌异常OBS interferes the Wnt signaling pathway and causes abnormal dopa-mine secretion[49,52-53]肝脏毒性Liver toxicityPFOS的肝脏毒性更大,造成肝脏损伤,但是机制与OBS不同PFOS has stronger liver toxicity, and causes liver damage but its mechanism is different from OBSPFOS能够和PPARγ稳定结合,诱导肝脏代谢紊乱破坏肝脏脂质代谢平衡PFOS can bind with PPARγ and in-duce liver metabolic disorders and disrupt lipid metabolism balanceOBS无法和PPARγ结合,但可以影响脂质基因和氨基酸而扰乱肝脏代谢平衡OBS cannot bind with PPARγ, but affects lipid gene and amino acid, so that interferes liver metabolic balance[54-59]肠道毒性Intestinal toxicity破坏肠道屏障,肠道菌群失调,与脂质代谢失调、肝脏毒性密切相关They disrupt intestinal barrier and cause intestinal microbiota imbalance, closely related to lipid metabolism disorders and liver toxicityPFOS引起肠道菌群失衡、肠道屏障损伤和肠道通透性增加,与肝脏损伤和代谢紊乱存在一定关联性PFOS causes intestinal microbiota imbalance, intestinal barrier damage, and increased intestinal permeability, associated with liver damage and metabolic disordersOBS引起肠道黏液分泌减少,离子转运异常,破坏肠道屏障,影响与代谢相关的菌群OBS causes a decrease in intestinal mucus secretion, abnormal ion trans-port, disruption of intestinal barriers, and affects metabolic related microbi-ota[57-58,60-61]肾脏毒性Renal toxicity在肾脏中蓄积,PFOS诱导肾细胞凋亡,但OBS肾毒性未知They can accumulate in the kid-ney; PFOS induces renal cell ap-optosis, but it is not known about OBS nephrotoxicityPFOS引起氧化应激,肾功能障碍PFOS induces oxidative stress, and renal dysfunctionOBS可能具有潜在的肾脏毒性OBS may have potential nephrotoxicity[43,62-65]微生物毒性Microbial toxicity对微生物的活性、结构、基因表达等存在影响They affect microbial activity, structure, and gene expressionPFOS影响土壤中细菌的结构和基因表达PFOS affects the structure, and gene expression of bacteria in soilOBS调节土壤中氨氧化微生物的丰度、结构OBS regulates the abundance and structure of ammonia oxidizing mi-croorganisms in soil[11,66-67]
4.3 发育毒性和神经毒性
研究发现,随着PFOS浓度的升高,斑马鱼胚胎孵化率越低,死亡率越高,存在着明显的剂量效应,并且存活的斑马鱼胚胎往往伴随着明显的心率升高,行动迟缓等现象[48]。PFOS还具有发育神经毒性,有研究发现,哺乳期和胚胎期小鼠PFOS暴露,不仅导致母鼠的妊娠期延长,还导致子代小鼠海马组织受到损伤,学习记忆能力下降[49]。根据Wang等[39]研究发现,孕期小鼠暴露于OBS后,子代小鼠肝脏中出现OBS蓄积,且表现出酪氨酸代谢失衡和干扰脂质代谢。PFOS与OBS暴露引起斑马鱼幼体畸形,包括心包水肿、脊柱弯曲、身长缩短等;还会造成孵化时间延长、运动行为改变以及纤毛功能障碍,且与PFOS相比,OBS虽然毒性效应低于PFOS但是OBS导致血管形态改变并影响心脏功能[50]。OBS与PFOS能够引起斑马鱼幼体组织学改变、氧化应激和免疫畸变,且三者之间互相影响,进一步扰乱斑马鱼幼体发育[51]。此外,有研究证明,斑马鱼胚胎暴露于PFOS与OBS后,对斑马鱼幼体的运动能力具有相似的破坏作用,导致多巴胺能神经元数量显著降低,多巴胺分泌减少,且PFOS影响更强[52]。这在其他研究中也得到证实,成年斑马鱼暴露PFOS和OBS后,二者均引起斑马鱼中脑肿胀,多巴胺分泌减少,但是机制不同。PFOS暴露引起中脑肿胀导致钙离子信号通路失调,导致多巴胺分泌异常,进而影响热反应;OBS暴露引起中脑肿胀和室管膜细胞纤毛稳态破坏,破坏Wnt信号通路,最终导致多巴胺分泌异常,影响昼夜节律[53]。这说明PFOS和OBS暴露均具有发育毒性,导致胚胎发育过程中出现诸多问题,且毒性作用可以通过母体传给后代。由于结构的差异导致PFOS的神经毒性相对OBS更强,且二者在发育毒性上存在差别。
4.4 肝脏毒性
肝脏是重要的代谢器官,许多重要的代谢活动如脂肪酸代谢、葡萄糖代谢等都在肝脏中进行,肝脏也是部分PFASs生物积累的靶器官,目前关于肝脏毒性的研究较多。大量研究发现,PFOS能够在肝脏中积聚,诱导肝脏肿大,引起肝脏生化指标如甘油三酯、丙酮酸、血清总胆固醇增多,破坏肝脏脂质代谢平衡[54]。目前,已有研究阐明了PFOS对肝脏脂质代谢干扰的相关机制,小鼠暴露于一定浓度的PFOS后,肝细胞出现空泡化,外观肿大和变黄;脂质代谢相关酶类、低密度脂蛋白和极低密度脂蛋白等在转录水平分别上调和下调;线粒体β-氧化效率降低,从而导致肝脏肿大以及脂质代谢失衡[55]。Cheng等[56]研究发现,斑马鱼长期暴露于低剂量PFOS也出现类似结果,脂质代谢相关酶、基因在转录水平表达上调,引起脂质代谢失调,且存在性别差异。
OBS的毒性效应研究主要集中在肝脏,Wang等[57]研究发现,小鼠暴露于低剂量OBS,肝脏中OBS残留高于其他组织,且肝细胞空泡化;血清中丙酮酸水平增高,脂质代谢相关酶类、脂肪酸合成基因在转录水平上表达下调。斑马鱼实验发现,血清丙酮酸、甘油三酯含量不变,血清总胆固醇含量显著降低;脂质代谢相关基因在mRNA水平显著变化,肝脏代谢物如琥珀酸以及各种氨基酸如参与尿素循环的瓜氨酸均发生变化,说明OBS暴露通过扰乱肝脏代谢平衡,引起斑马鱼肝脏毒性[58]。此外,有研究证明,PFOS能够和PPARγ稳定结合而OBS无法和PPARγ结合,因此与OBS相比,PFOS暴露能够诱导PPARγ表达显著下调,明显引起肝细胞核固缩,从而诱导肝脏代谢紊乱[59]。以上说明OBS作为PFOS替代物,同样易在肝脏积聚,诱导肝细胞空泡化,但是其毒性作用小于PFOS,影响肝脏代谢紊乱的机制与PFOS存在差别。
4.5 肠道毒性
肠道不仅是重要的营养吸收系统,也是抵御病原体及其他有害物质进入机体的关键屏障,环境中的化学物质可通过肠道进入人体,对人体造成潜在的危害,并且当肠道屏障受损时,有害物质可以通过血液循环至肝脏,造成肝脏损伤。Wang等[60]发现,小鼠暴露于PFOS后,肠道菌群失调,例如厚壁菌门、拟杆菌门相对丰度分别降低和增高,且肠道菌群的改变与代谢物的变化之间存在显著相关性。已有研究证实,PFOS暴露引起肠道菌群失衡、肠道屏障损伤和肠道通透性增加,与肝脏损伤和代谢紊乱存在一定关联性[61]。
OBS暴露也观察到类似结果,小鼠暴露于低剂量的OBS后,结果发现OBS可以与肠道跨膜蛋白稳定结合,引起肠道黏液分泌减少和离子转运异常,从而破坏肠道屏障[58]。通过斑马鱼的暴露实验发现,OBS暴露后引起斑马鱼肠道微生物菌群失调,破坏肠道屏障;300 μg·L-1 OBS暴露21 d后,在门水平上,α-变形菌门、β-变形菌门、拟杆菌门等相对丰度降低;在属水平上,黄杆菌、副球菌、弧菌等菌群的相对丰度发生了显著变化,且这些菌群与代谢存在相关性[57]。已有研究证实,α-变形菌、单胞菌等肠道菌群与PPARγ信号通路中脂质代谢相关基因、脂质合成与分解基因等高度相关[60]。此外,长期暴露与PFOS和OBS导致成年斑马鱼肠绒毛高度缩短,肠道基因表达失调,产生免疫毒性,且二者对肠道菌群丰度有相似的影响[61]。这表明PFOS和OBS暴露均会导致肠道菌群失调,破坏肠道屏障和多条代谢途径,并且能够对肝脏产生毒性效应,与脂质代谢紊乱存在关联。
4.6 肾脏毒性
肾脏是重要的代谢器官,由于其结构和功能的特性,是许多环境污染物攻击的靶器官。已有毒理学研究表明,PFASs暴露导致肾脏发生氧化应激,基因表达失调,出现肾功能障碍[62]。研究发现,PFOS暴露肾上皮细胞是通过NAD-依赖性去乙酰化酶Sirtuin-1和PPAR介导的,导致肾细胞凋亡和相关抗氧化酶的表达下调[63-64]。然而,目前关于OBS对肾脏损伤的具体机制尚未见报道,仅有报道称OBS在斑马鱼暴露过程中,肾脏是仅次于肝脏和肠道OBS蓄积的场所[65]。此外,分子对接发现OBS能够和肾脏的有机阴离子转运蛋白(OAT1)通过非共价结合方式稳定结合,结合能(-11.39 kJ·mol-1)略低于PFOS与OAT1结合能(-15.48 kJ·mol-1)[43],这从侧面说明OBS能够在肾脏积聚,且可能具有潜在的肾脏毒性。
4.7 微生物毒性
PFASs广泛存在于土壤环境中,而土壤微生物是土壤生态系统重要的组成部分。此前,有研究证实PFASs可能影响土壤微生物群落,较高浓度的PFOS可能对土壤中细菌的丰度和多样性产生不利影响[11]。Qiao等[66]研究发现低浓度PFOS能促进土壤中蔗糖酶和脲酶的活性,而高浓度PFOS能够抑制其活性,从而进一步影响土壤中细菌的结构和基因表达;此研究中还发现1 mg·kg-1 OBS能够促进上述2种酶的活性。研究表明,OBS可以通过调节土壤中氨氧化微生物的丰度、结构来影响氨氧化,并且一定浓度的OBS可能对土壤中的细菌和古细菌数量产生影响[67]。
5 PFOS与OBS的去除研究(Study on removal of PFOS and OBS)
PFOS与OBS由于特殊的结构特性,难以通过常规技术手段进行去除。与PFOS相比,OBS在结构上多了醚单元和芳香基团,这使得二者在去除方面可能存在差异。研究发现,曝气-泡沫处理是去除PFOS和OBS的有效方法,在短时间内可以高效率进行去除,但会残余少部分需要进行进一步去除[68]。PFASs光降解研究表明,PFOS溶液在220 nm范围内紫外线(UV)吸收较弱,在220~400 nm范围内无明显吸收[69]。OBS在UV (254 nm)下可以分解,并且在UV/H2O2中分解效果更好,然而在这2种条件下都生成了复杂的副产物[13]。
活性炭(AC)吸附法是近年来去除水中PFOS的一种有效且经济的方法之一,粉状活性炭去除PFOS效率较高,仅为4 h左右;颗粒活性炭(GAC)168 h才达到吸附平衡[70]。然而,其他研究发现,经KOH等活化后的GAC吸附效率大幅提高[71]。OBS的AC吸附研究表明,超细型活性炭的初始吸附速率最快,24 h即可达到吸附平衡,而GAC至少需要72 h,但是活化后的GAC吸附效率及效果均显著提高[72]。以上结果说明,OBS与PFOS的去除方法存在着一定程度的差异。
6 总结与展望(Summary and future)
本文对PFOS及其新型替代物OBS的特性和应用、环境检出、人体暴露、多种毒性及去除等方面进行了全面的归纳总结。随着PFOS的全面禁用,OBS将在更大范围内应用,然而目前关于OBS的风险管控和相关研究都相对较少。因此,将来需要加强OBS在典型环境介质中的分布特征、生物蓄积性及生物毒性等方面的深入研究;全面系统地研究OBS的暴露途径和致病风险,更深层次地研究毒性机制,做好OBS的环境监测和管理,科学评估其对人体的健康风险。
[1] Buck R C, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins [J]. Integrated Environmental Assessment and Management, 2011, 7(4): 513-541
[2] Rumsby P C, McLaughlin C L, Hall T. Perfluorooctane sulphonate and perfluorooctanoic acid in drinking and environmental waters [J]. Philosophical Transactions Series A, Mathematical, Physical, and Engineering Sciences, 2009, 367(1904): 4119-4136
[3] 邓红霞, 郑冬芳, 徐娇, 等. PFOS相关法规及其替代品研究进展[J]. 浙江化工, 2021, 52(7): 1-5
Deng H X, Zheng D F, Xu J, et al. Research progress on relevant laws and regulations of PFOS and its substitutes [J]. Zhejiang Chemical Industry, 2021, 52(7): 1-5 (in Chinese)
[4] Xu L, Shi Y L, Li C X, et al. Discovery of a novel polyfluoroalkyl benzenesulfonic acid around oilfields in northern China [J]. Environmental Science &Technology, 2017, 51(24): 14173-14181
[5] Prevedouros K, Cousins I T, Buck R C, et al. Sources, fate and transport of perfluorocarboxylates [J]. Environmental Science &Technology, 2006, 40(1): 32-44
[6] Conder J M, Hoke R A, de Wolf W, et al. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds [J]. Environmental Science &Technology, 2008, 42(4): 995-1003
[7] Zeng Z T, Song B, Xiao R, et al. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies [J]. Environment International, 2019, 126: 598-610
[8] 程宁. 含氟表面活性剂的生产应用现状及研究进展[J]. 日用化学品科学, 2008, 31(6): 17-20
Cheng N. Status of manufacturing and application and research progress of fluorosurfactant [J]. Detergent &Cosmetics, 2008, 31(6): 17-20 (in Chinese)
[9] Chen H, Munoz G, Duy S V, et al. Occurrence and distribution of per- and polyfluoroalkyl substances in Tianjin, China: The contribution of emerging and unknown analogues [J]. Environmental Science &Technology, 2020, 54(22): 14254-14264
[10] 曹友桂, 李方山, 章于川. 对全氟壬烯氧基苯磺酸钠的合成、表征与应用[J]. 精细化工, 2012, 29(2): 174-177
Cao Y G, Li F S, Zhang Y C. Synthesis, characterization and application of sodium p-perfluorous nonenoxybenzene sulfonate [J]. Fine Chemicals, 2012, 29(2): 174-177 (in Chinese)
[11] Bao Y X, Qu Y X, Huang J, et al. First assessment on degradability of sodium p-perfluorous nonenoxybenzene sulfonate (OBS), a high volume alternative to perfluorooctane sulfonate in fire-fighting foams and oil production agents in China [J]. RSC Advances, 2017, 7(74): 46948-46957
[12] 郭海荣, 刘庆玉, 杨婧, 等. 全氟和多氟烷基物质对生物环境作用的研究进展[J]. 环境工程, 2023, 41(8): 259-269
Guo H R, Liu Q Y, Yang J, et al. Research progress on effects of per- and poly-fluoroalkyl substances on biological environment [J]. Environmental Engineering, 2023, 41(8): 259-269 (in Chinese)
[13] Kim E J, Park Y M, Park J E, et al. Distributions of new Stockholm Convention POPs in soils across South Korea [J]. The Science of the Total Environment, 2014, 476-477: 327-335
[14] 胡国成, 郑海, 张丽娟, 等. 珠江三角洲土壤中全氟化合物污染特征研究[J]. 中国环境科学, 2013, 33(S1): 37-42
Hu G C, Zheng H, Zhang L J, et al. Contamination characteristics of perfluorinated compounds in soil from Pearl River Delta, South China [J]. China Environmental Science, 2013, 33(S1): 37-42 (in Chinese)
[15] 钟慧芳. 我国东部沿海沉积物和土壤中全氟化合物的污染特征研究[D]. 武汉: 江汉大学, 2021: 66-67
Zhong H F. Pollution characteristics of per-and polyfluoroalkyl substances in soils and coastal sediments in eastern China [D]. Wuhan:Jianghan University, 2021: 66-67 (in Chinese)
[16] Hou M M, Jin Q, Na G S, et al. Emissions, isomer-specific environmental behavior, and transformation of OBS from one major fluorochemical manufacturing facility in China [J]. Environmental Science &Technology, 2022, 56(12): 8103-8113
[17] 李闯修. F-53B和OBS等多/全氟化合物在典型区域的污染特征研究[D]. 青岛: 青岛理工大学, 2016: 36-41
Li C X. Contamination characteristics of F-53B, OBS and other poly-and perfluoroalky substances in typical areas [D]. Qingdao: Qingdao Technology University, 2016: 36-41 (in Chinese)
[18] Guelfo J L, Korzeniowski S, Mills M A, et al. Environmental sources, chemistry, fate, and transport of per- and polyfluoroalkyl substances: State of the science, key knowledge gaps, and recommendations presented at the August 2019 SETAC focus topic meeting [J]. Environmental Toxicology and Chemistry, 2021, 40(12): 3234-3260
[19] Sun Z Y, Zhang C J, Yan H, et al. Spatiotemporal distribution and potential sources of perfluoroalkyl acids in Huangpu River, Shanghai, China [J]. Chemosphere, 2017, 174: 127-135
[20] Wang S L, Wang H, Deng W J. Perfluorooctane sulfonate (PFOS) distribution and effect factors in the water and sediment of the Yellow River Estuary, China [J]. Environmental Monitoring and Assessment, 2013, 185(10): 8517-8524
[21] Munoz G, Fechner L C, Geneste E, et al. Spatio-temporal dynamics of per and polyfluoroalkyl substances (PFASs) and transfer to periphytic biofilm in an urban river: Case-study on the River Seine [J]. Environmental Science and Pollution Research International, 2018, 25(24): 23574-23582
[22] Shi Y L, Song X W, Jin Q, et al. Tissue distribution and bioaccumulation of a novel polyfluoroalkyl benzenesulfonate in crucian carp [J]. Environment International, 2020, 135: 105418
[23] Qu Y X, Jiang X S, Cagnetta G, et al. Poly- and perfluoroalkyl substances in a drinking water treatment plant in the Yangtze River Delta of China: Temporal trend, removal and human health risk [J]. Science of the Total Environment, 2019, 696: 133949
[24] Jiao E M, Zhu Z L, Yin D Q, et al. A pilot study on extractable organofluorine and per- and polyfluoroalkyl substances (PFAS) in water from drinking water treatment plants around Taihu Lake, China: What is missed by target PFAS analysis? [J]. Environmental Science Processes &Impacts, 2022, 24(7): 1060-1070
[25] Wang Q, Ruan Y F, Lin H J, et al. Review on perfluoroalkyl and polyfluoroalkyl substances (PFASs) in the Chinese atmospheric environment [J]. The Science of the Total Environment, 2020, 737: 139804
[26] 方祥光, 赵祯, 李军, 等. 中国典型城市和地区大气颗粒物中全氟烷基酸的污染分布特征[J]. 环境化学, 2018, 37(7): 1445-1459
Fang X G, Zhao Z, Li J, et al. Concentrations and distribution of perfluoroalkyl acids in the atmospheric particles in typical cities and regions of China [J]. Environmental Chemistry, 2018, 37(7): 1445-1459 (in Chinese)
[27] Fang S H, Li C, Zhu L Y, et al. Spatiotemporal distribution and isomer profiles of perfluoroalkyl acids in airborne particulate matter in Chengdu City, China [J]. The Science of the Total Environment, 2019, 689: 1235-1243
[28] Yu N Y, Guo H W, Yang J P, et al. Non-target and suspect screening of per- and polyfluoroalkyl substances in airborne particulate matter in China [J]. Environmental Science &Technology, 2018, 52(15): 8205-8214
[29] Sunderland E M, Hu X C, Dassuncao C, et al. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects [J]. Journal of Exposure Science &Environmental Epidemiology, 2019, 29(2): 131-147
[30] Ghisi R, Vamerali T, Manzetti S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review [J]. Environmental Research, 2019, 169: 326-341
[31] 李笑. 我国一般人群血清中PFOS和PFOA分布特征及基准值[D]. 大连: 大连理工大学, 2011: 50-51
Li X. Distribution characteristics and reference values of serum perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in the general population of China [D]. Dalian: Dalian University of Technology, 2011: 50-51 (in Chinese)
[32] 赵之梦. 孕妇血液中全氟化合物和金属元素的暴露水平及相关性研究[D]. 广州: 南方医科大学, 2019: 54-55
Zhao Z M. Study on exposure levels and correlations of perfluorinated compounds and metal elements in pregnant women’s blood [D]. Guangzhou: Southern Medical University, 2019: 54-55 (in Chinese)
[33] Bao J, Shao L X, Liu Y, et al. Target analysis and suspect screening of per- and polyfluoroalkyl substances in paired samples of maternal serum, umbilical cord serum, and placenta near fluorochemical plants in Fuxin, China [J]. Chemosphere, 2022, 307(Pt 1): 135731
[34] Li Y Q, Yu N Y, Du L T, et al. Transplacental transfer of per- and polyfluoroalkyl substances identified in paired maternal and cord sera using suspect and nontarget screening [J]. Environmental Science &Technology, 2020, 54(6): 3407-3416
[35] Lee Y J, Lee H G, Yang J H. Perfluorooctane sulfonate-induced apoptosis of cerebellar granule cells is mediated by ERK 1/2 pathway [J]. Chemosphere, 2013, 90(5): 1597-1602
[36] Long Y, Wang Y B, Ji G X, et al. Neurotoxicity of perfluorooctane sulfonate to hippocampal cells in adult mice [J].PLoS One, 2013, 8(1): e54176
[37] Wang X F, Zhao G P, Liang J T, et al. PFOS-induced apoptosis through mitochondrion-dependent pathway in human-hamster hybrid cells [J]. Mutation Research, 2013, 754(1-2): 51-57
[38] Zhang M, Bi X W, Liu S, et al. The novel polyfluoroalkyl benzenesulfonate OBS exposure induces cell cycle arrest and senescence of rat pituitary cell GH3 via the p53/p21/RB pathway [J]. Toxicology, 2023, 490: 153511
[39] Wang C Y, Fang C L, Wang C H, et al. Maternal sodium p-perfluorous nonenoxybenzene sulfonate exposure disturbed lipid metabolism and induced an imbalance in tyrosine metabolism in the F1 generation of mice [J]. Chemical Research in Toxicology, 2022, 35(4): 651-662
[40] 刘冉, 曹志会, 赵月, 等. PFOA和PFOS对大型蚤急性毒性试验研究[J]. 安全与环境工程, 2015, 22(4): 51-55, 74
Liu R, Cao Z H, Zhao Y, et al. Experiment study on acute toxicity of PFOA and PFOS to Daphnia magna [J]. Safety and Environmental Engineering, 2015, 22(4): 51-55, 74 (in Chinese)
[41] 郭晋姝, 王金有, 武佳琪, 等. PFOS对斑马鱼的急性毒性及安全浓度评价[J]. 安徽农业科学, 2017, 45(7): 92-95
Guo J S, Wang J Y, Wu J Q, et al. Acute toxicity and safe concentration assessment of perfluorooctane sulfonate (PFOS) to Danio rerio [J]. Journal of Anhui Agricultural Sciences, 2017, 45(7): 92-95 (in Chinese)
[42] Wang S W, Huang J, Yang Y, et al. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment [J]. Environmental Science &Technology, 2013, 47(18): 10163-10170
[43] 邹义龙. PFOS替代品OBS在斑马鱼体内的毒代动力学及毒性作用机制研究[D]. 南昌: 南昌大学, 2021: 44-55
Zou Y L. The toxicokinetics and toxic mechanism of PFOS alternative OBS in zebrafish (Danio rerio) [D]. Nanchang: Nanchang University, 2021: 44-55 (in Chinese)
[44] Tu W Q, Martínez R, Navarro-Martin L, et al. Bioconcentration and metabolic effects of emerging PFOS alternatives in developing zebrafish [J]. Environmental Science &Technology, 2019, 53(22): 13427-13439
[45] Zou Y L, Wu Y M, Wang Q Y, et al. Comparison of toxicokinetics and toxic effects of PFOS and its novel alternative OBS in zebrafish larvae [J]. Chemosphere, 2021, 265: 129116
[46] Wen W, Xia X H, Zhou D, et al. Bioconcentration and tissue distribution of shorter and longer chain perfluoroalkyl acids (PFAAs) in zebrafish (Danio rerio): Effects of perfluorinated carbon chain length and zebrafish protein content [J]. Environmental Pollution, 2019, 249: 277-285
[47] Chen M, Qiang L W, Pan X Y, et al. In vivo and in vitro isomer-specific biotransformation of perfluorooctane sulfonamide in common carp (Cyprinus carpio) [J]. Environmental Science &Technology, 2015, 49(23): 13817-13824
[48] Rosen M B, Schmid J E, Das K P, et al. Gene expression profiling in the liver and lung of perfluorooctane sulfonate-exposed mouse fetuses: Comparison to changes induced by exposure to perfluorooctanoic acid [J]. Reproductive Toxicology, 2009, 27(3-4): 278-288
[49] 王玉, 张倩, 刘薇, 等. 胚胎期和哺乳期全氟辛烷磺酸(PFOS)暴露致大鼠学习记忆能力下降[J]. 生态毒理学报, 2013, 8(5): 671-677
Wang Y, Zhang Q, Liu W, et al. Decline of learning and memory abilities in rats induced by prenatal and lactational exposure to perfluorooctane sulfonate (PFOS) [J]. Asian Journal of Ecotoxicology, 2013, 8(5): 671-677 (in Chinese)
[50] Huang J, Sun L W, Mennigen J A, et al. Developmental toxicity of the novel PFOS alternative OBS in developing zebrafish: An emphasis on cilia disruption [J]. Journal of Hazardous Materials, 2021, 409: 124491
[51] Huang J, Wang Q Y, Liu S, et al. Crosstalk between histological alterations, oxidative stress and immune aberrations of the emerging PFOS alternative OBS in developing zebrafish [J]. Science of the Total Environment, 2021, 774: 145443
[52] Kalyn M, Lee H, Curry J, et al. Effects of PFOS, F-53B and OBS on locomotor behaviour, the dopaminergic system and mitochondrial function in developing zebrafish (Danio rerio) [J]. Environmental Pollution, 2023, 326: 121479
[53] Wang Q Y, Gu X Y, Liu Y, et al. Insights into the circadian rhythm alterations of the novel PFOS substitutes F-53B and OBS on adult zebrafish [J]. Journal of Hazardous Materials, 2023, 448: 130959
[54] Guo J S, Wu P H, Cao J L, et al. The PFOS disturbed immunomodulatory functions via nuclear factor-κB signaling in liver of zebrafish (Danio rerio) [J]. Fish &Shellfish Immunology, 2019, 91: 87-98
[55] Wan H T, Zhao Y G, Wei X, et al. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport [J]. Biochimica et Biophysica Acta, 2012, 1820(7): 1092-1101
[56] Cheng J F, Lv S P, Nie S F, et al. Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish [J]. Aquatic Toxicology, 2016, 176: 45-52
[57] Wang C Y, Zhang Y, Deng M, et al. Bioaccumulation in the gut and liver causes gut barrier dysfunction and hepatic metabolism disorder in mice after exposure to low doses of OBS [J]. Environment International, 2019, 129: 279-290
[58] Wang C Y, Zhao Y, Jin Y X. The emerging PFOS alternative OBS exposure induced gut microbiota dysbiosis and hepatic metabolism disorder in adult zebrafish [J]. Comparative Biochemistry and Physiology Part C: Toxicology &Pharmacology, 2020, 230: 108703
[59] Wang Q Y, Huang J, Liu S, et al. Aberrant hepatic lipid metabolism associated with gut microbiota dysbiosis triggers hepatotoxicity of novel PFOS alternatives in adult zebrafish [J]. Environment International, 2022, 166: 107351
[60] Wang G, Sun S S, Wu X B, et al. Intestinal environmental disorders associate with the tissue damages induced by perfluorooctane sulfonate exposure [J]. Ecotoxicology and Environmental Safety, 2020, 197: 110590
[61] Huang J, Wang Q Y, Liu S, et al. Comparative chronic toxicities of PFOS and its novel alternatives on the immune system associated with intestinal microbiota dysbiosis in adult zebrafish [J]. Journal of Hazardous Materials, 2022, 425: 127950
[62] Kim H S, Kwack S J, Han E S, et al. Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate [J]. The Journal of Toxicological Sciences, 2011, 36(2): 201-210
[63] Wen L L, Lin C Y, Chou H C, et al. Perfluorooctanesulfonate mediates renal tubular cell apoptosis through PPARgamma inactivation [J]. PLoS One, 2016, 11(5): e0155190
[64] Chou H C, Wen L L, Chang C C, et al. From the cover: L-carnitine via PPARγ- and Sirt1-dependent mechanisms attenuates epithelial-mesenchymal transition and renal fibrosis caused by perfluorooctanesulfonate [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2017, 160(2): 217-229
[65] McDonough C A, Choyke S, Ferguson P L, et al. Bioaccumulation of novel per- and polyfluoroalkyl substances in mice dosed with an aqueous film-forming foam [J]. Environmental Science &Technology, 2020, 54(9): 5700-5709
[66] Qiao W C, Xie Z Y, Zhang Y H, et al. Perfluoroalkyl substances (PFASs) influence the structure and function of soil bacterial community: Greenhouse experiment [J]. The Science of the Total Environment, 2018, 642: 1118-1126
[67] Ke Y C, Chen J F, Hu X Y, et al. Emerging perfluoroalkyl substance impacts soil microbial community and ammonia oxidation [J]. Environmental Pollution, 2020, 257: 113615
[68] Meng P P, Deng S B, Maimaiti A, et al. Efficient removal of perfluorooctane sulfonate from aqueous film-forming foam solution by aeration-foam collection [J]. Chemosphere, 2018, 203: 263-270
[69] Wang X L, Chen Z Y, Wang Y L, et al. A review on degradation of perfluorinated compounds based on ultraviolet advanced oxidation [J]. Environmental Pollution, 2021, 291: 118014
[70] Yu Q, Zhang R Q, Deng S B, et al. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study [J]. Water Research, 2009, 43(4): 1150-1158
[71] Deng S B, Nie Y, Du Z W, et al. Enhanced adsorption of perfluorooctane sulfonate and perfluorooctanoate by bamboo-derived granular activated carbon [J]. Journal of Hazardous Materials, 2015, 282: 150-157
[72] Wang W, Mi X, Shi H L, et al. Adsorption behaviour and mechanism of the PFOS substitute OBS (sodium p-perfluorous nonenoxybenzene sulfonate) on activated carbon [J]. Royal Society Open Science, 2019, 6(9): 191069