-
土壤重金属污染已成为我国亟需解决的环境问题。而重金属污染场地是我国污染场地数量最多、污染最严重的一类。在我国西南和中南地区,重金属污染场地具有污染浓度高、污染分布不均等特点[1]。2014年,据《全国土壤污染状况调查公报》[2]和《土地整治蓝皮书》[3]统计,全国土壤污染物超标率总数达到了16.1%,其中铬污染点位超标率为1.1%。
铬在土壤中主要以Cr(Ⅲ)和Cr(Ⅵ) 2种价态存在[4],Cr(Ⅵ)是国际公认的3大致癌金属物之一[5-6]。目前,我国每年新增铬渣及副产物量达数十万t[7],受处理成本和技术条件限制,大部分铬渣露天堆放,污染物随风扩散或经雨水淋溶和浸滤,造成严重的土壤铬污染。
土壤淋洗技术工艺简单、修复效率高、处理方量大,已逐渐成为许多规模较大的现场修复示范项目首选技术,可用于高浓度Cr(Ⅵ)污染土壤,具有永久去除Cr(Ⅵ)[8]和经济高效等特点[9],常用草酸、柠檬酸、乙酸、二乙基三胺五乙酸(DTPA)、乙二胺四乙酸(EDTA)和乙二胺二琥珀酸(EDDS)等作为淋洗剂[9-13]。目前,关于土壤淋洗修复Cr(Ⅵ)污染土壤的研究主要集中于淋洗剂的比选和优化淋洗参数等方面,且基本处于实验室层面。由于未考虑修复粒级>2 mm的土壤,因此,在实际修复工程中,不能全面评价Cr(Ⅵ)污染土壤的修复效果[14]。目前评价修复效果大多局限于去除率的研究,有关修复后土壤残留Cr(Ⅵ)含量和Cr(Ⅵ)的淋洗机理研究还鲜有报道。本研究根据土壤淋洗应用的可行性,针对重庆市某工业废弃地Cr(Ⅵ)污染土壤,将土壤粒级分为>2 mm和<2 mm,探究组合淋洗对Cr(Ⅵ)污染土壤的修复能力,同时采用SEM-EDS和XRD分析对淋洗前后土壤进行表征,分析组合淋洗剂对土壤中Cr(Ⅵ)的去除机制,为该类污染土壤的修复提供参考。
全文HTML
-
供试土壤采自重庆市某工业废弃地铬污染土壤,采样深度为0~40 cm。样品取回后自然风干,采用四分法[15]处理,样品充分混匀,除去>50 mm大石块与动植物残体,取充分混匀的土壤置于聚乙烯薄膜封口袋储存,将土壤粒级分为>2 mm和<2 mm,经破碎研磨后,过0.15 mm用于测定土壤基本理化性质、总Cr和Cr(Ⅵ)含量。供试土壤理化性质见表1。
-
实验试剂:二水合草酸、一水合柠檬酸、冰乙酸、DTPA和二苯碳酰二肼,均为分析纯。
实验仪器:DR2800型可见光分光光度计(上海哈希水质分析仪器有限公司);AA-6680型原子吸收分光光度计(日本岛津仪器公司);Regulus8100型冷场发射扫描电镜(日本Hitachi公司);X’ Pert PRO MPD型X射线粉末衍射仪(荷兰Panalytical公司)。
-
在进行不同浓度天然有机酸和DTPA对Cr(Ⅵ)污染土壤的淋洗实验时,准确称取(1 000±1.0) g Cr(Ⅵ)污染土壤置于自制搅拌反应器内(见图1),将草酸、柠檬酸、乙酸的浓度设置为0.01、0.02、0.05、0.1、0.2和0.5 mol·L−1,将DTPA浓度设置为0.5、1、2、5、10和20 g·L−1,分别加入10 L不同浓度淋洗剂,液固比为10∶1,以800 r·min−1转速搅拌淋洗60 min,淋洗后,将土壤筛分为粒级>2 mm和粒级<2 mm,粒级<2 mm土壤在真空过滤机中抽滤,抽滤后烘干称质量,采用碱消解-二苯碳酰二肼分光光度法[16-17]测定土壤中残留Cr(Ⅵ)含量,每个处理设置3个平行。
在进行天然有机酸与DTPA组合淋洗最优比实验时,准确称取(1 000±1.0) g Cr(Ⅵ)污染土壤于自制搅拌反应器内,根据筛选适宜浓度的天然有机酸(草酸、柠檬酸和乙酸)与DTPA进行组合淋洗实验,设置液固比为10∶1,以800 r·min−1转速搅拌淋洗,组合淋洗实验参数条件如表2和表3所示,分为顺序淋洗(S1~S12处理)和混合淋洗(H1~H6处理),S1~S6处理为先进行草酸、柠檬酸和乙酸淋洗30 min,后DTPA淋洗30 min,S7~S12处理为先进行DTPA淋洗30 min,后草酸、柠檬酸和乙酸淋洗30 min,H1~H6处理为草酸、柠檬酸和乙酸分别与DTPA混合淋洗60 min。
-
在进行液固比对Cr(Ⅵ)去除效果实验时,准确称取(1 000±1.0) g Cr(Ⅵ)污染土壤于自制搅拌反应器内,选取确定的最佳组合淋洗剂,体积分别设为1、2、5、8、10、15、20和30 L,以800 r·min−1转速搅拌淋洗60 min,实验方法同1.3。
在进行淋洗时间对Cr(Ⅵ)去除效果实验时,准确称取(1 000±1.0) g Cr(Ⅵ)污染土壤于自制搅拌反应器内,选取最佳液固比的组合淋洗剂,淋洗时间设置为10、20、30、40、60、90、120、150、180和240 min,实验方法同1.3。
-
土壤pH、含水率、质量分数、有机质和阳离子交换量的测定参照《土壤农化分析方法》[15];土壤中Cr(Ⅵ)含量采用USEPA Method 3060A碱消解法[16]进行前处理,使用USEPA 7196A比色法[17]测定;土壤总Cr含量采用《土壤和沉积物 铜、锌、铅、镍、铬的测定 火焰原子吸收分光光度计》(HJ 491-2019)[18]进行测定;土壤SEM-EDS分析采用Regulus8100型冷场发射扫描电镜检测,分辨率:0.8 nm@15 kV,1.1 nm@1 kV,加速电压0.5~30 kV,减速电压0.1~2 kV,倍率20~106倍,电流80 pA~300 nA;土壤XRD分析采用X’ Pert PRO MPD型X射线粉末衍射仪进行土壤矿物学成分表征,使用Cu Kα 辐射在60 kV和55 mA下进行连续扫描,2θ的角度为5°~90°,扫描速度2(°)·min−1,使用软件MDI Jade 6.5进行分析。
1.1. 供试土壤
1.2. 实验试剂与仪器
1.3. 天然有机酸与DTPA组合淋洗实验
1.4. 组合淋洗的单因素实验
1.5. 土壤样品测定与分析
-
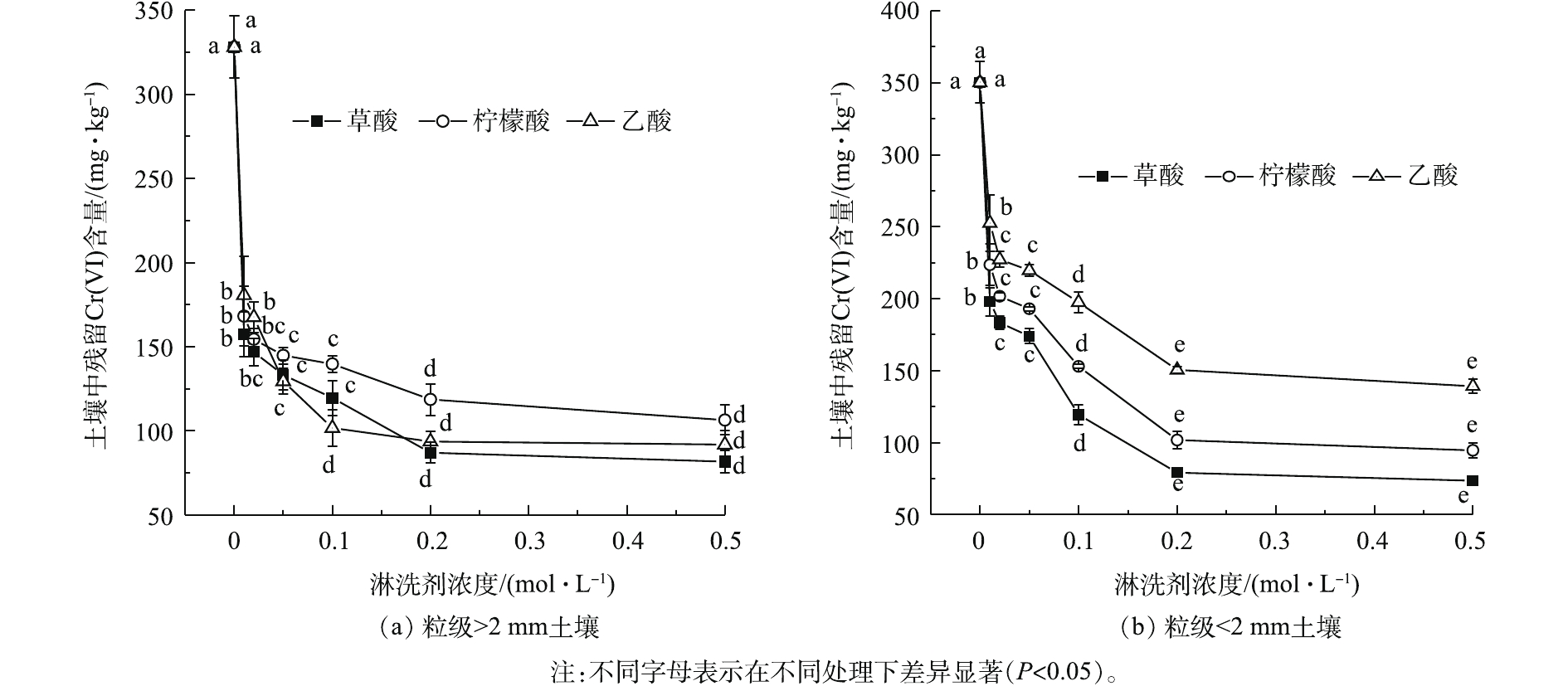
不同浓度天然有机酸对Cr(Ⅵ)污染土壤的淋洗效果见图2。可以看出,Cr(Ⅵ)土壤残留Cr(Ⅵ)含量均随有机酸浓度的升高而减少,逐渐趋于平缓,对不同粒级土壤的淋洗效果高低为草酸>柠檬酸>乙酸(P<0.05)。粒级>2 mm土壤在低浓度条件Cr(Ⅵ)下降幅度较大,当浓度为0.2 mol·L−1时,土壤残留Cr(Ⅵ)含量为86.92~118.54 mg·kg−1;粒级<2 mm土壤残留Cr(Ⅵ)含量在0.2 mol·L−1时为79.22~150.51 mg·kg−1。草酸的淋洗效果最佳,去除率为76.50%,这与王海豹等[19]的研究结果一致。但有机酸浓度过高会导致土壤酸化,破坏土壤微团聚体结构。因此,选取0.1 mol·L−1和0.2 mol·L−1的草酸、柠檬酸和乙酸作为适宜浓度的淋洗剂进行组合淋洗实验。
不同浓度DTPA对Cr(Ⅵ)污染土壤的淋洗效果如图3所示。粒级>2 mm土壤残留Cr(Ⅵ)含量随DTPA浓度的增加而先降低后增高,在浓度5 g·L−1时最低(66.75 mg·kg−1),去除率为79.66%;粒级<2 mm土壤在5 g·L−1时残留Cr(Ⅵ)含量为107.29 mg·kg−1,去除率为69.36%。继续增加浓度,DTPA会被大量Ca2+、Fe3+以及矿物成分消耗,不会增加Cr(Ⅵ)的提取,可能会促进Cr(Ⅵ)的再吸附,抑制重金属的解吸[20]。因此,选取5 g·L−1 DTPA作为最适浓度的淋洗剂进行组合淋洗实验。
-
上述结果表明,单一的天然有机酸和DTPA对Cr(Ⅵ)的淋洗效率明显,但土壤残留Cr(Ⅵ)含量仍较高。同时,由于土壤性质,Cr(Ⅵ)污染途径、程度和时间等差异,土壤中共存的金属阳离子和阴离子增大了淋洗难度。因此,将草酸、柠檬酸、乙酸分别与DTPA进行组合淋洗,探究不同组合淋洗剂对Cr(Ⅵ)土壤的淋洗效果。
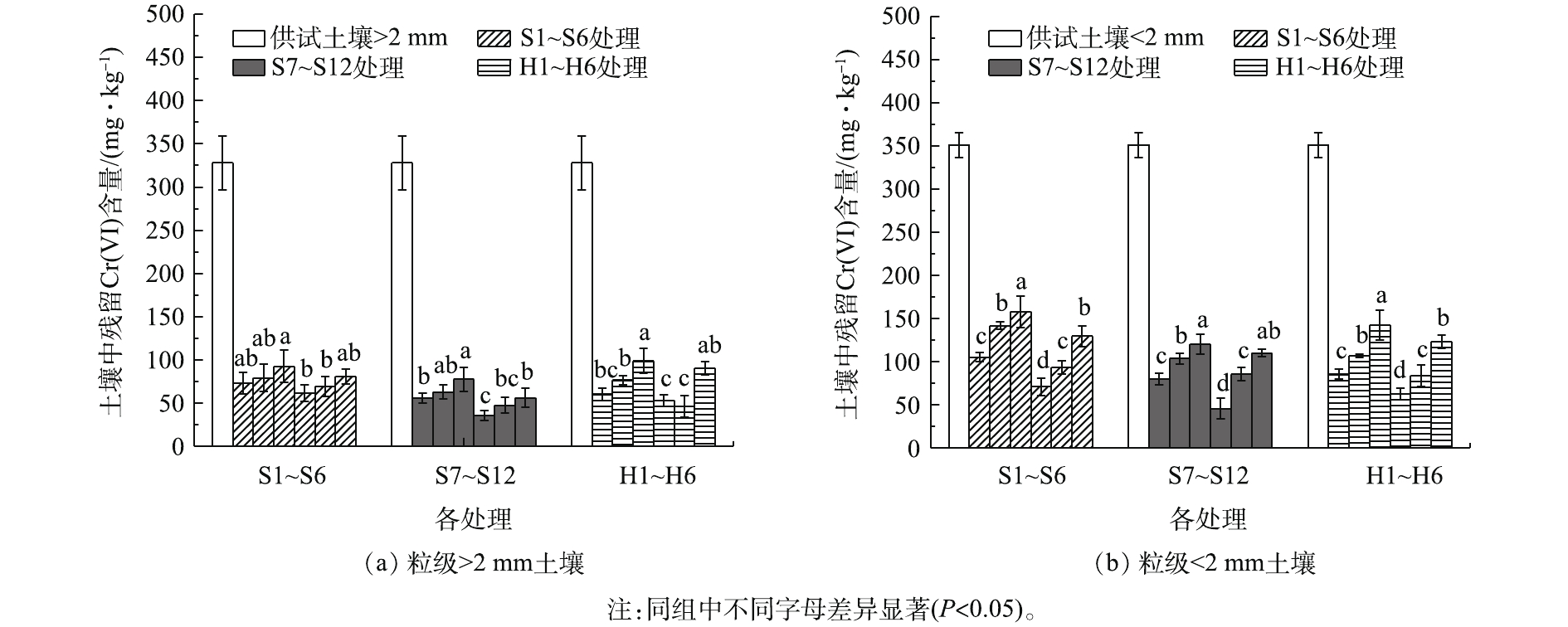
由图4(a)可知,粒级>2 mm土壤经淋洗后残留Cr(Ⅵ)含量为35.42~92.52 mg·kg−1,各处理淋洗能力为S7~S12处理>S1~S6处理>H1~H6处理((80.80%~89.20%)>(75.32%~81.27%)>(69.91%~85.85%))。在S10处理后,土壤残留Cr(Ⅵ)含量仅为35.42 mg·kg−1,去除率达到89.20%。多步淋洗提高了大颗粒土壤相互碰撞,附着于土壤表面的Cr(Ⅵ)极易迁移至液相而被淋洗剂去除,提高了淋洗剂进入大颗粒内部的效率,从而有效地去除稳定态Cr(Ⅵ)。混合淋洗的效果低于顺序淋洗(P<0.05)。这可能是由于天然有机酸使反应环境pH降低,DTPA在酸性条件下容易质子化,两者发生拮抗作用[21],从而对Cr(Ⅵ)的去除率较低。
由图4(b)可知,粒级<2 mm土壤淋洗后残留Cr(Ⅵ)含量为45.57~142.02 mg·kg−1。0.1 mol·L−1和0.2 mol·L−1乙酸与DTPA顺序淋洗的去除率仅为54.95%~63.06%,低于乙酸单独淋洗效果,乙酸很难打破重金属与土壤矿物组分间的化学键结合;同时,Cr(Ⅵ)与DTPA形成的不稳定态金属络合物容易析出,并在土壤中再吸收(固定化),导致土壤残留Cr(Ⅵ)含量较高[22-23]。S4、S10和H4处理后土壤残留Cr(Ⅵ)分别为70.74、45.57和62.30 mg·kg−1,淋洗效果均高于同类各处理(P<0.05),草酸对土壤重金属的增溶作用是DTPA与Cr(Ⅵ)螯合反应的关键[9]。吸附(溶解)是所有与配体有关的表面化学反应的基础,并可能通过配体交换、静电吸引或氢键发生[24]。S10处理先利用DTPA与Cr(Ⅵ)发生络合反应活化重金属,溶解吸附在土壤表面的Cr(Ⅵ)。由于Cr(Ⅵ)极易溶于水,迁移能力强,如
$ {\rm{CrO}}_{\rm{4}}^{{\rm{2 - }}}$ 、$ {\rm{HCrO}}_{\rm{4}}^{\rm{ - }}$ 、$ {\rm{Cr}}_{\rm{2}}^{}{\rm{O}}_{\rm{7}}^{{\rm{2 - }}}$ 等离子与土壤的吸附为非专性吸附,因此土壤表面的Cr(Ⅵ)在浸湿过程中更易解吸暴露出来,这部分易溶于水的阴离子进入液相而被淋洗剂去除。随着以非专性吸附的Cr(Ⅵ)去除,草酸淋洗能够打破土壤团聚体结构,溶解土壤基质,使其与难溶的矿物结晶充分接触,渗入颗粒深层,去除包裹或嵌附在矿物结晶的Cr(Ⅵ)[25]。同时,草酸具有较强的还原性,能将生物毒性较大的Cr(Ⅵ)还原为毒性相对较低的Cr(Ⅲ)。可见,采用适宜浓度的天然有机酸与DTPA组合淋洗,不仅能弥补单一淋洗剂的不足,还能较大程度地提高粒级>2 mm和粒级<2 mm土壤中Cr(Ⅵ)的去除效果。因此,选取S10处理(5 g·L−1 DTPA+0.2 mol·L−1 草酸)作为最优组合淋洗剂进行组合淋洗的单因素实验。
-
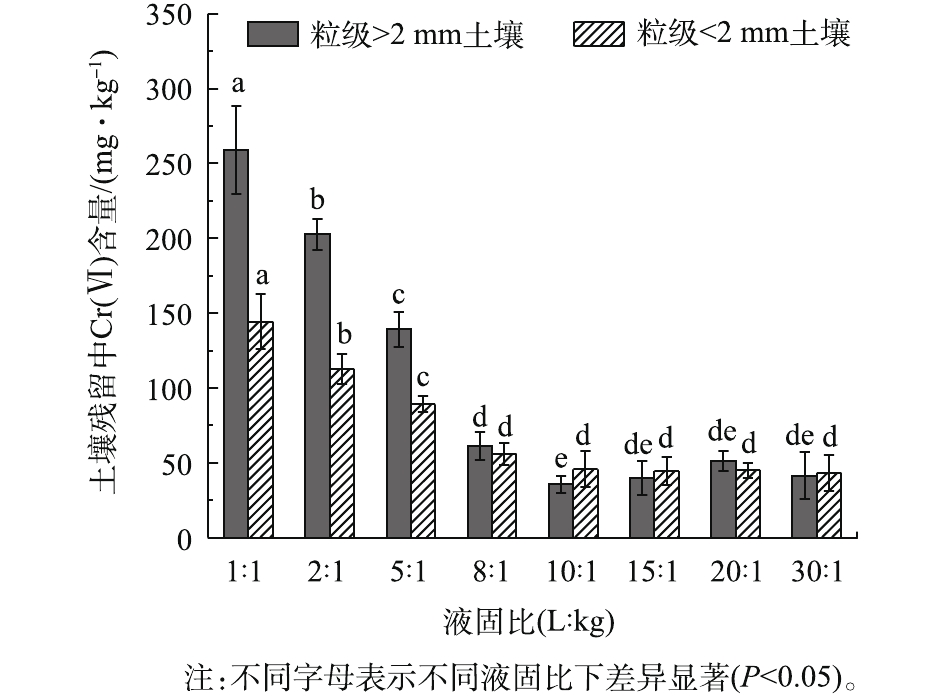
液固比是土壤淋洗技术中重要的技术参数,合适的液固比不仅能提高土壤中Cr(Ⅵ)的整体去除率,而且对淋洗后废液的处理有至关重要的作用。由图5可知:当液固比从1∶1增加至10∶1时,土壤残留Cr(Ⅵ)含量逐渐减少(P<0.05);当液固比为10∶1时,粒级>2 mm和<2 mm土壤残留Cr(Ⅵ)含量分别为35.42 mg·kg−1和45.57 mg·kg−1;随着液固比的继续增加,Cr(Ⅵ)含量几乎保持不变(P>0.05)。液固比的增加使淋洗剂与Cr交换和络合能力增强,附着于土壤表面以非专性吸附存在的Cr(Ⅵ)与淋洗剂充分接触更易进入液相。但当液固比从10∶1增加至30∶1时,土壤中的Cr(Ⅵ)达到解吸平衡(P>0.05),淋洗剂只是不断地被稀释,并没有淋出更多的Cr(Ⅵ)。因此,5 g·L−1 DTPA与0.2 mol·L−1草酸顺序淋洗在液固比为10∶1时淋洗效果最好。
-
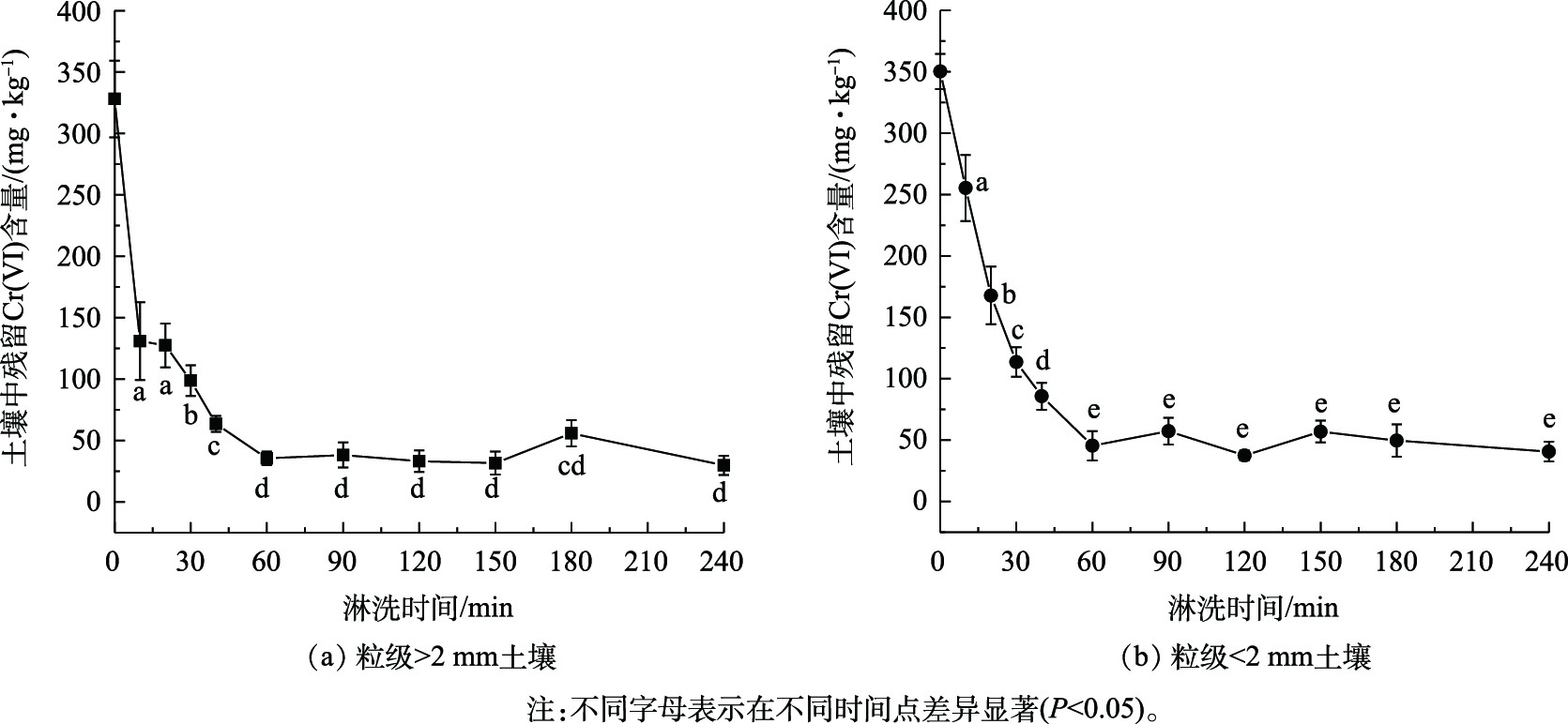
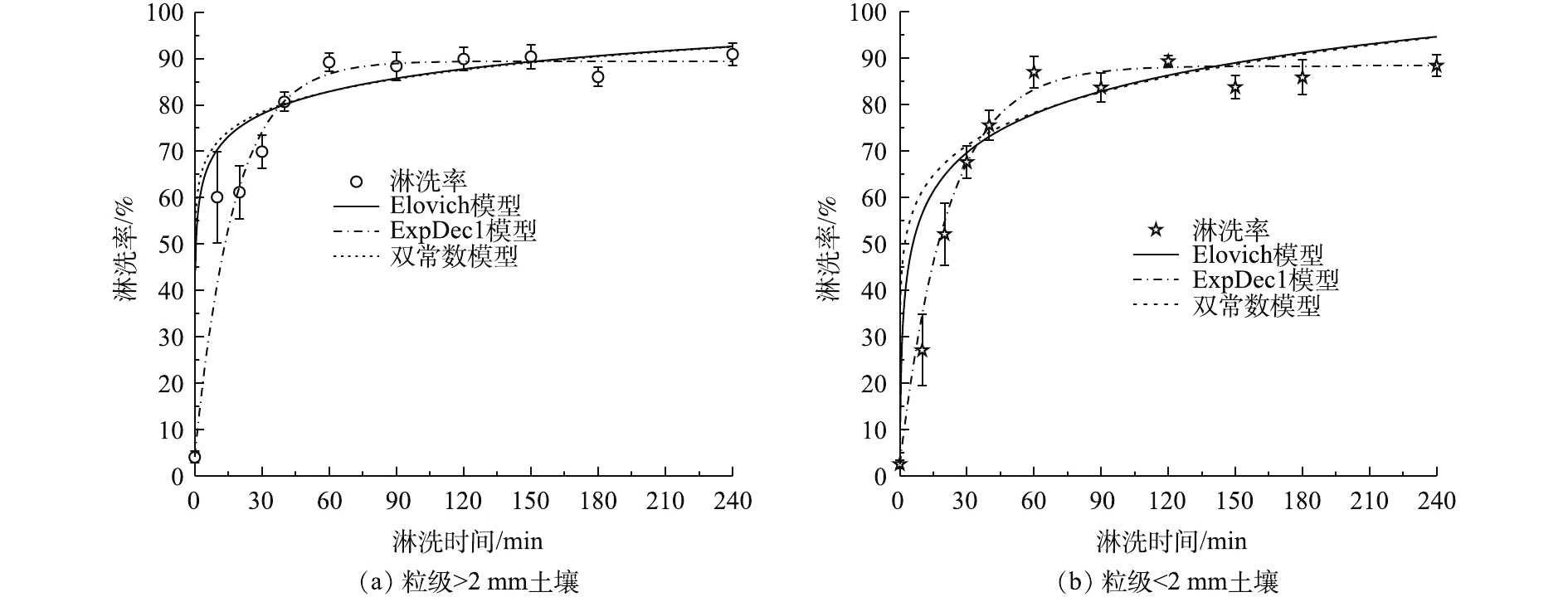
由图6可知,在淋洗初期,组合淋洗剂对Cr(Ⅵ)淋洗速率较快,土壤残留Cr(Ⅵ)含量降幅较大。随着淋洗时间的延长,淋洗速率在60 min逐渐变缓,在60~240 min之间达到吸附解吸平衡,这与黄川等[11]、陈欣园等[12]的研究一致。粒级>2 mm和<2 mm土壤快速反应阶段均在0~40 min(P<0.05)。粒级>2 mm土壤在10 min时土壤残留Cr(Ⅵ)含量为130.92 mg·kg−1,去除率达到60.10%;而粒级<2 mm土壤在10 min时土壤残留Cr(Ⅵ)含量为255.37 mg·kg−1,去除率仅为27.08%。40~60 min为缓慢淋洗阶段,粒级<2 mm土壤残留Cr(Ⅵ)含量在60 min时仅为45.57 mg·kg−1。继续增加淋洗时间,土壤残留Cr(Ⅵ)含量基本保持不变或少量增加(P>0.05),淋洗速率变化不大。因此,60 min是土壤Cr(Ⅵ)吸附解吸平衡的时间点,同时也是最佳淋洗时间。
Cr(Ⅵ)污染土壤的淋洗动力学可通过双常数、Elovich和ExpDec1模型进行拟合,结果如表4和图7所示。整个淋洗过程分为快速反应、慢速反应和淋洗平衡阶段[26],快速反应阶段主要是静电吸附态重金属的解吸,慢速反应阶段一般为专性吸附态重金属的解吸[27]。粒级>2 mm土壤和粒级<2 mm土壤3种模型的R2分别为0.984 7~0.995 9和0.985 4~0.997 8,均适用于Cr(Ⅵ)淋洗动力学过程。土壤中Cr(Ⅵ)淋洗过程主要受扩散因子控制,而不是反应速率控制,按照土壤重金属经典扩散理论,分析Cr(Ⅵ)的解吸为非均相扩散。粒级<2 mm土壤对Cr(Ⅵ)的初始淋洗速率较慢,这是因为细粒土壤具有更大的比表面积和吸附位点[28],Cr(Ⅵ)与土壤表面形成稳定的共价键不易断裂,使其Cr(Ⅵ)初始淋洗速率较慢。
综合考虑,选取5 g·L−1 DTPA与0.2 mol·L−1草酸作为最佳组合淋洗剂,液固比为10∶1,顺序淋洗时间为60 min,此时,粒级>2 mm和<2 mm土壤残留Cr(Ⅵ)含量分别为35.42 mg·kg−1和45.57 mg·kg−1,去除率分别为89.20%和86.99%。
-
由表5可知,以5 g·L−1 DTPA与0.2 mol·L−1草酸顺序淋洗,液固比为10∶1,淋洗60 min后,能明显地降低土壤中总Cr、Cr(Ⅲ)和Cr(Ⅵ)含量(P<0.05),且淋洗液中均未检测出Cr(Ⅵ)。粒级>2 mm土壤总Cr、Cr(Ⅲ)和Cr(Ⅵ)去除率分别为45.99%、41.15%和89.20%;粒级<2 mm土壤为60.62%、60.37%和86.99%。虽土壤残留总Cr、Cr(Ⅲ)含量仍较高,但其对环境的危害和生物毒性明显降低。淋洗后粒级>2 mm和<2 mm土壤pH分别为3.91和4.54,土壤pH对土壤吸持Cr(Ⅲ)和Cr(Ⅵ)能力的影响较大,通过影响液相中离子浓度、吸附解吸和离子交换行为等机制,从而影响淋洗剂提取重金属的能力[29]。DI PALMA等[9]和LIAO等[30]的研究表明,当土壤pH在3~4左右时,Cr(Ⅵ)解吸率较高。土壤在该环境下对Cr(Ⅵ)的吸附能力较弱,H+能够破坏土壤颗粒表面的官能团与Cr离子形成的络合物,从而使吸附于土壤表面的Cr(Ⅵ)解吸,淋洗剂的效果能发挥到最大。草酸和DTPA对土壤Cr(Ⅵ)的淋洗机制主要有3个方面:1) H+的酸化作用溶解了土壤表面部分矿物质,使得Cr(Ⅵ)竞争表面活性位点得到释放[31];2) 阴离子草酸根能促进Cr(Ⅵ)在土壤颗粒表面的离子交换,将迁移性较强的Cr(Ⅵ)还原为稳定的Cr(Ⅲ);3) —COOH能与Cr(Ⅲ)形成高溶解性的配位络合物进入液相,同时阻碍已被释放的Cr(Ⅲ)被土壤再吸附[32-33]。
-
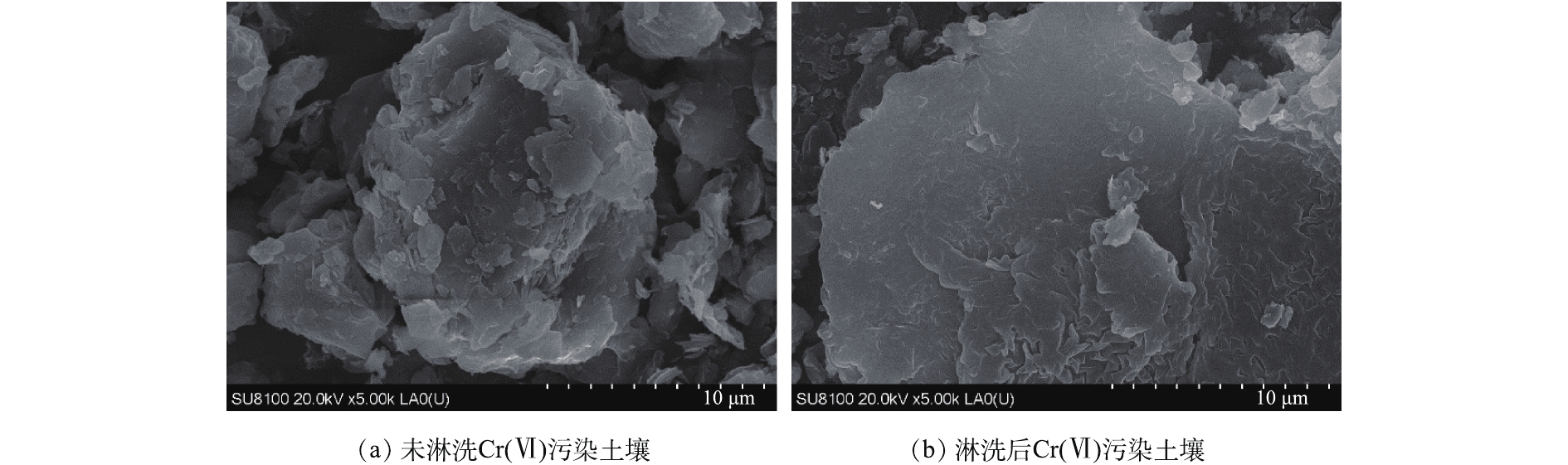
图8(a)和图8(b)为最佳组合淋洗剂淋洗前后土壤SEM电镜扫描图像。未淋洗Cr(Ⅵ)污染土壤表面较粗糙,CERQUEIRA等[34]研究表明,外来污染物与土壤颗粒表面组分会形成不规则的结晶体。Cr作为外来污染物,主要与土壤中钙镁氧化物和钒氧化物结合,判断该原生矿物结合的Cr聚合物在土壤表面呈不规则结晶体分布。淋洗后,Cr(Ⅵ)污染土壤颗粒表面结构形貌发生了较大变化,表面较光滑,并形成一层比较致密的结晶矿物。这是由于组合淋洗剂对Cr具有配位、络合作用,与Cr形成的螯合物从土壤颗粒表面解吸。
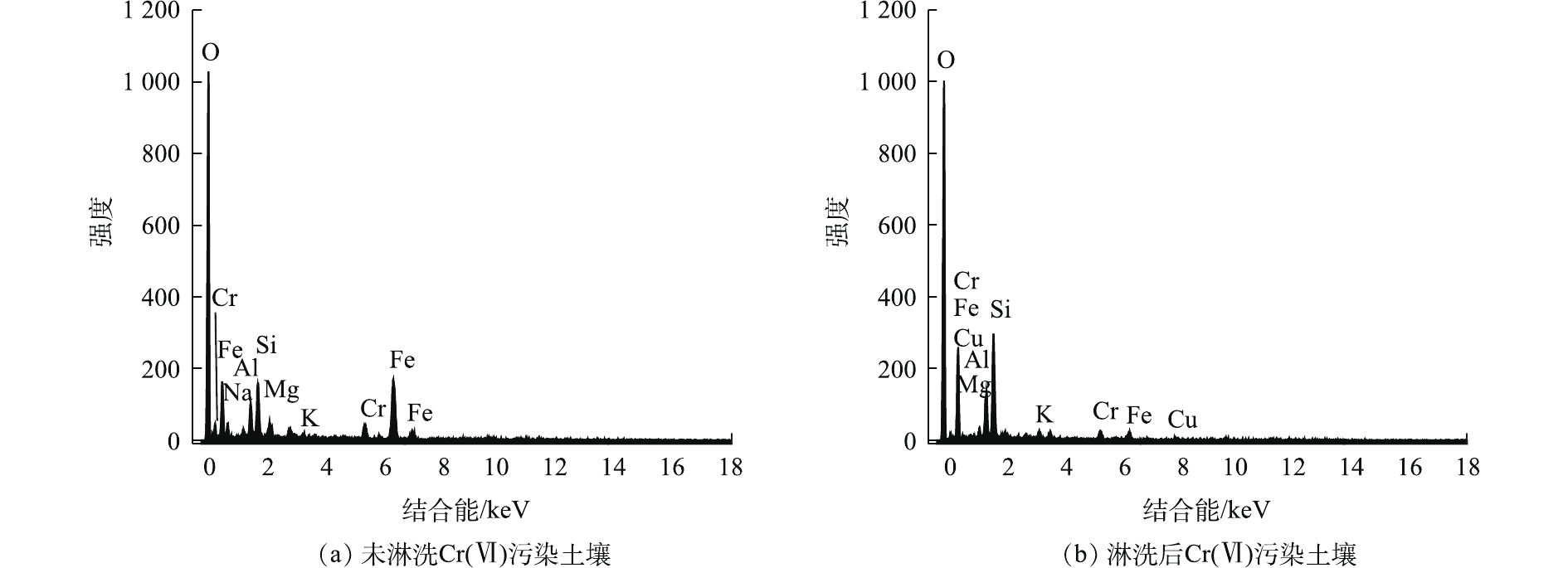
EDS能谱分析如图9所示。图9(a)和图9(b)分别为淋洗前后Cr(Ⅵ)污染土壤。未淋洗Cr(Ⅵ)污染土壤中O、Al、Si、Fe存在较大峰值,淋洗后Cr(Ⅵ)污染土壤中元素分布呈现较大变化,O、Al、Si存在较大峰值。Fe元素经淋洗后从47.78%降至6.36%,大量Fe元素矿物被消耗。淋洗前后土壤中Cr的百分比分别为7.08%、5.94%,组合淋洗后的土壤表面含铬矿物降低,残余的Cr均为稳定的Cr(Ⅲ),大多位于土壤矿物晶格内部。由于Cr峰受其他金属元素的干扰,使得它们在EDS图谱中的衍射峰彼此重叠。
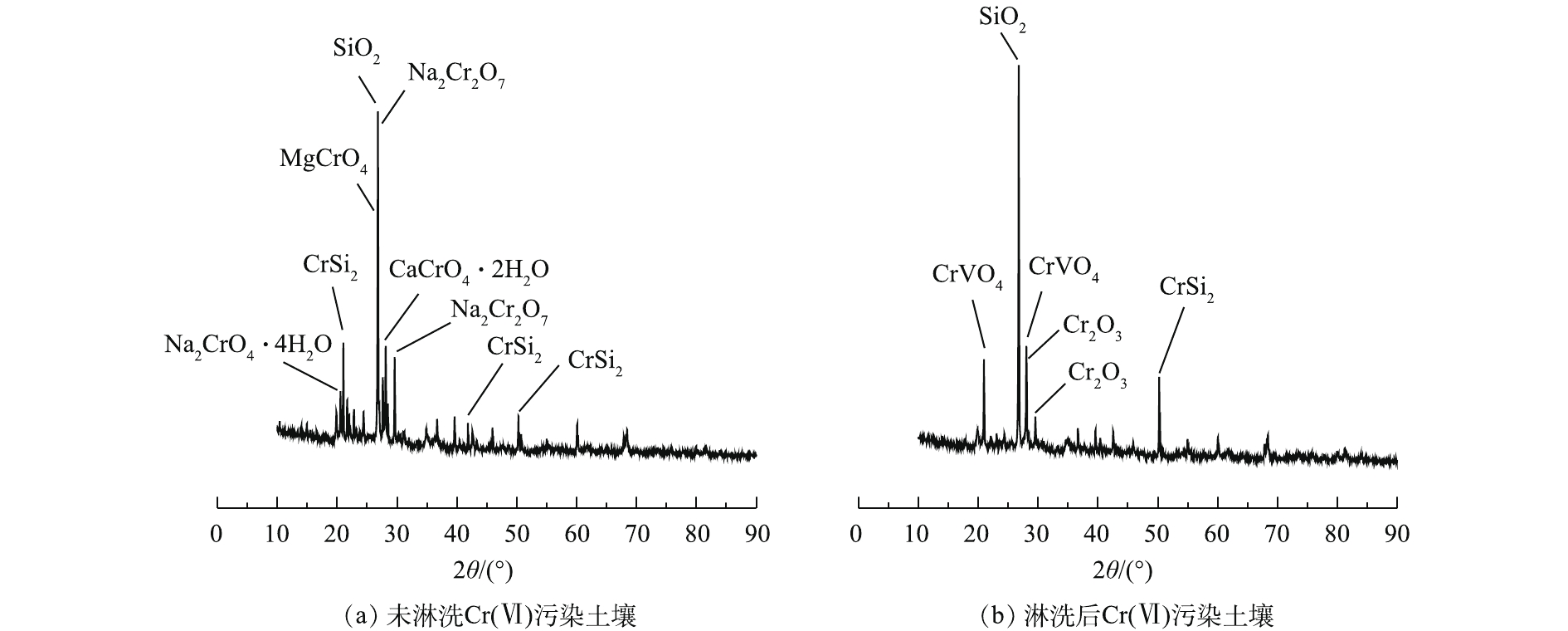
XRD能谱分析如图10所示。图10(a)和图10(b)能谱线上均检出SiO2,未淋洗Cr(Ⅵ)污染土壤以伊利石、石英、钠长石和方解石为主要相,次生黏土矿物则主要由沸石以及由结晶态或非结晶态的硅酸盐组成。GALAN等[35]研究发现,层状硅酸盐和方解石可在极酸环境下大量溶解并消失。DTPA和草酸顺序淋洗使土壤处于低pH环境下,小分子有机酸的酸溶作用对Cr(Ⅵ)阴离子的去除效果较好。图10(a)能谱在2θ为20°~25°、26.7°和27°~29°附近表现出强烈的衍射峰,XRD检索出Cr(Ⅵ)的化合物,分别为四水合铬酸钠(Na2CrO4·4H2O)、二水合铬酸钙(CaCrO4·2H2O)、铬酸镁(MgCrO4)和重铬酸钠(Na2CrO7)[36]。图10(b)能谱未检索出Cr(Ⅵ)化合物,但能检索出Cr(Ⅲ)的钒氧化物(CrVO4)和氧化铬(Cr2O3)[37]。这部分的Cr(Ⅲ)存在于土壤矿物内部晶格中难以析出,其他易溶的矿物在淋洗剂的作用下溶解并进入液相。Cr(Ⅵ)污染土壤经淋洗后,Cr的矿物学形态变化明显,土壤表面的活性Cr(Ⅵ)阴离子较易被淋洗和还原[38],但包裹在土壤中的含铬矿物有待进一步研究。
2.1. 不同浓度天然有机酸和DTPA对Cr(Ⅵ)污染土壤的淋洗效果
2.2. 天然有机酸与DTPA组合淋洗最优比选实验
2.3. 液固比对土壤残留Cr(Ⅵ)含量的影响
2.4. 淋洗时间对土壤残留Cr(Ⅵ)含量的影响
2.5. 最佳组合淋洗修复Cr(Ⅵ)土壤机理
2.6. 土壤微观表征分析
-
1)天然有机酸和DTPA均能有效降低土壤中Cr(Ⅵ)含量,有机酸单一淋洗的优劣顺序为草酸>柠檬酸>乙酸(P<0.05),0.2 mol·L−1草酸和5 g·L−1DTPA去除效果较好。
2)天然有机酸与DTPA组合淋洗对Cr(Ⅵ)污染土壤的去除率高于单一淋洗。淋洗方式、液固比和时间对Cr(Ⅵ)淋洗效果影响较大,5 g·L−1 DTPA和0.2 mol·L−1草酸组合顺序淋洗在液固比为10∶1、淋洗时间为60 min时效果最佳,粒级>2 mm和<2 mm土壤淋洗效果显著(P<0.05),组合淋洗能有效地降低Cr(Ⅵ)污染土壤的生物毒性和环境风险。
3)双常数、Elovich和ExpDec1模型均适用于Cr(Ⅵ)的淋洗动力学过程(R2>0.98),按照经典扩散理论,判断土壤Cr(Ⅵ)的解吸为非均相扩散。结合SEM-EDS和XRD表征分析,土壤表面Cr的矿物学形态变化明显,经组合淋洗后Cr元素含量减少,且土壤表面未检出Cr(Ⅵ)化合物,但残留在土壤矿物晶格的含铬矿物有待进一步研究。



 下载:
下载: