-
3,4-二氯硝基苯广泛应用于工业化学和医药等领域。由于3,4-二氯硝基苯具有一定毒性,对生态环境安全具有潜在风险,因而去除水环境中3,4-二氯硝基苯污染物研究受到了众多学者的关注。3,4-二氯硝基苯可以被光催化降解为CO2、H2O,也可以被催化还原为芳香胺即3,4-二氯苯胺[1]。3,4-二氯苯胺是一种重要的精细化工中间体,在偶氮染料、药品、塑料、农药、纺织印染、阻燃剂等化学工业中被广泛应用[2]。3,4-二氯苯胺的传统制备方法包括贝尚反应、加氢还原法和铁粉还原法[3]。上述反应需要在高温、高压等操作条件下进行,需要较高的生产条件并具有潜在的污染风险和安全隐患[4],因此需要探索常温、常压的清洁生产方法。光催化还原法是一种有效降解有机污染物的绿色方法,具有成本低、安全高效、原子利用率高、几乎不产生副产品等优点,已成为光催化领域备受关注的热点问题。据文献报道,目前光催化技术可处理3 000多种污染水体[5-7]。在室温下,通过二氧化钛(TiO2)光催化氧化可降解绝大多数有机污染物[8];而添加空穴清除剂时,TiO2又可将有机硝基化合物光催化还原为相应的芳香胺[9]。通过掺杂金属[10]或非金属元素[11-12],或以石墨烯、碳纳米管、分子筛作为载体等方法改性TiO2[13-14],可极大地提高TiO2的催化性能。此外,通过调节TiO2的暴露晶面来提高其光催化性能的方式也引起了广泛关注[15-17]。YANG等[16]利用TiF4作为钛源,HF作为形貌控制剂合成了具有(001)面的锐钛型TiO2单晶,其晶面的形成可有效地优化光生电子空穴的迁移路径和分离率,进而显著提高光催化效率。
值得关注的是,反应介质是影响TiO2光催化氧化还原性能的重要因素[18-21]。因此,可通过改变反应溶剂的性质以调节氧化、还原反应之间的竞争关系。质子性有机溶剂作为空穴捕获剂可抑制系统中光生电子-空穴对的复合,从而提高催化剂表面积聚的光生电子的浓度和光催化还原活性。在甲醇、乙醇和异丙醇等质子性有机溶剂中,乙醇和异丙醇作为光催化反应溶剂时,可能会出现醛、酮等中间产物影响反应的进程[22]。甲醇作为光催化反应的溶剂具有最高的还原效率,这表明质子性溶剂的极性是影响光催化还原性能的重要影响因素之一。同时,甲醇具有饱和醇的高活性和极性,能有效吸附在TiO2表面与光生空穴迅速反应[23]。甲醇还具有低粘度和高极化率的特点[24],可以和光生空穴反应产生甲氧基自由基并进一步分解为阴离子自由基,从而为反应系统提供丰富的电子和H+以促进还原反应的进行。本研究采用溶胶-凝胶法制备了TiO2光催化剂,考察了TiO2在甲醇和水混合溶剂中光催化氧化、还原3,4-二氯硝基苯的性能,并通过羟基自由基(·OH)和氧化还原电位(Eh)[25-26]随反应进程的量化变化关系对3,4-二氯硝基苯的光催化反应机制进行进一步探讨。
全文HTML
-
3,4-二氯硝基苯和3,4-二氯苯胺均购自TCI(上海)开发有限公司。无水甲醇购自天津金东天正精密化学试剂厂。盐酸由天津丰川化学试剂技术有限公司提供。香豆素(coumarin, COU)由上海源野生物科技有限公司提供。所有试剂均未进一步纯化。钛酸丁酯购于天津光复精细化工研究所。无水乙醇由天津冀纯化学试剂有限公司提供。
-
本研究中,以钛酸丁酯为前驱体,通过溶胶-凝胶法合成了TiO2。首先,将4.26 mL 36.5%的盐酸加入一定量的乙醇中,得到0.1 mol·L−1的缓冲溶液。然后,10 mL钛酸丁酯和10 mL无水乙醇混合,在搅拌下转入分液漏斗A内;与此同时,2.1 mL去离子水和5 mL缓冲溶液混合,放置在分液漏斗B中。20 mL的缓冲溶液搅拌3 min后转移至烧杯中,然后将分液漏斗A和B中的混合物逐滴加入到烧杯中。将上述悬浮液进行磁性搅拌直到形成浅黄色透明溶胶。溶胶老化12 h后,在110 ℃的干燥箱中放置12 h,最后在500 ℃的马弗炉中煅烧2 h,最终得到纳米级TiO2光催化剂。
-
光催化实验在具有循环冷却水的石英反应器中进行,并以300 W高压汞灯(GGZ300,上海继光特种照明电器厂,中国) 为光源。将0.3 g·L−1的3,4-二氯硝基苯和1.0 g·L−1的TiO2光催化剂分散在50 mL甲醇和水的混合溶剂中,然后悬浮液维持暗反应1 h以达到吸附/解吸平衡。混合溶剂的组成分别为V甲醇∶V水=9∶1、8∶2、5∶5和2∶8。在240 min内,定时提取一定量的悬浮液,离心后收集1 mL滤液,将其分散在9 mL甲醇中,用紫外分光光度计(北京普析通用仪器有限公司,中国,TU-1 900型)分析。
绘制了3,4-二氯硝基苯和3,4-二氯苯胺的浓度与相应吸光度的关系图,以甲醇作溶剂得到的线性拟合曲线如图1所示。拟合方程中C、A、R分别为待测有机物浓度、特征吸收峰(3,4-二氯硝基苯为269 nm,3,4-二氯苯胺为248 nm)的吸光度和可决系数。2个线性回归方程中R2均大于0.999,即吸光度与浓度的线性关系均较好。
-
通过COU与·OH生成的在465 nm具有特征荧光峰的7-羟基香豆素(7-HC),进而测定·OH的浓度。在无3,4-二氯硝基苯情况下,在体系中加入1.0×10−3 mol·L−1的COU,以此作为对照实验。样品经紫外光照射40 min后,取5 mL悬浮液离心,在激发波长为332 nm条件下进行荧光检测。
-
使用X射线衍射仪(BDDX330)表征TiO2粉末的晶型特征;使用JEM-2100透射电镜对TiO2进行形貌分析;使用岛津分光光度计(2501 PC)对光催化剂进行紫外-可见漫反射吸收光谱的测定,使用荧光光度计(F-7000,日立制造有限公司,日本)进行荧光分析;使用激光纳米粒度分析仪(DelsaNano C,贝克曼库尔特)进行TiO2粉末的粒径分析;使用氧化还原电位测量仪测定反应溶液的氧化还原电位。
1.1. 实验试剂和仪器
1.2. TiO2的制备
1.3. 光催化实验
1.4. ·OH的测定方法
1.5. 分析方法
-
通过粉末X射线衍射(PXRD)表征TiO2粉末的晶体结构,结果如图2(a)所示。衍射峰分别对应于(101)、(004)、(200)、(105)、(211)、(204)、(116)、(220)和(215)晶面,这与锐钛矿型TiO2晶面相对应。此外,所得TiO2的XRD图谱中衍射峰是较强且锐利的峰。以上结果表明,结晶性较高的TiO2光催化剂被成功合成。透射电镜对TiO2粉末的微观结构表征结果表明,TiO2粉末是由尺寸约为10 nm的颗粒组成,如图2(b)所示。由图2(c)高分辨透射电镜图(HRTEM)可以看到,晶格条纹的间距为0.351 nm,对应于锐钛矿型TiO2的(101)晶面。为了进一步确定TiO2晶体结构,进行了选区电子衍射(SAED)实验(图2(d)),SAED图中所标注的点相对应于TiO2的(101)、(004)和(200)晶面。此外,光催化剂的紫外-可见漫反射吸收光谱表明,TiO2具有较宽的带隙且在可见光区域没有吸收(图2(e))。图2(f)中的粒径结果表明,TiO2粉末的颗粒粒径集中在150~350 nm,这可能是因为表面能较高的微小TiO2颗粒易团聚成较大的颗粒。
-
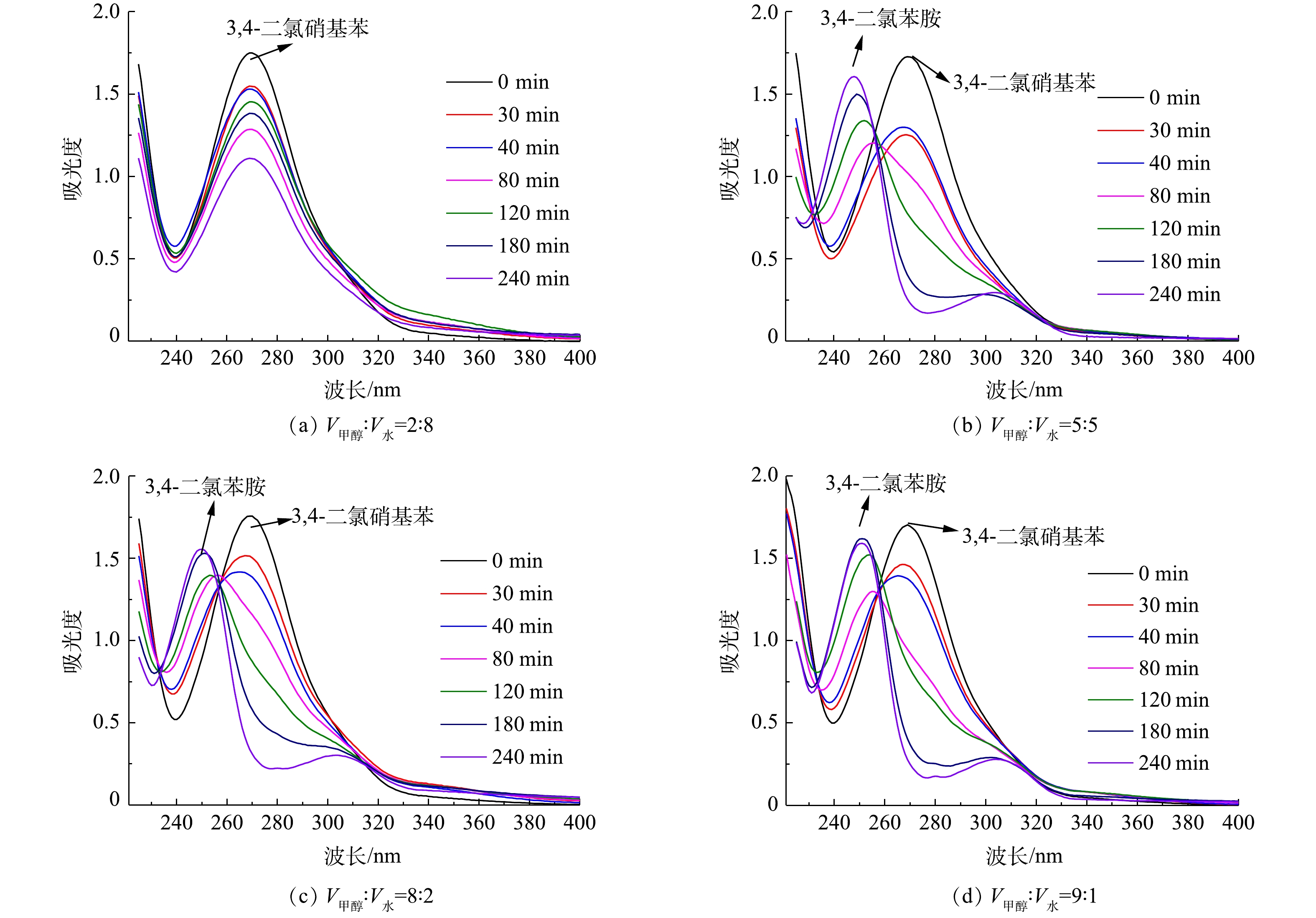
在紫外光照射下,以不同体积比的甲醇和水混合物为溶剂,以TiO2为催化剂进行3,4-二氯硝基苯的光催化对照反应。3,4-二氯硝基苯和3,4-二氯苯胺的紫外特征吸收峰分别在269 nm和248 nm处。在不同的照射时间下,检测了225~400 nm处的紫外吸收光谱的变化(图3)。
由图3可知,溶剂组成比例会导致3,4-二氯硝基苯的光催化氧化和还原反应发生显著变化。当V甲醇∶V水为2∶8时,3,4-二氯硝基苯在269 nm处的峰强度在240 min内随时间的延长而逐渐减弱,但没有出现3,4-二氯苯胺对应的特征吸收峰,这说明3,4-二氯硝基苯在240 min内主要进行了光催化氧化降解反应。当V甲醇∶V水由5∶5升高到9∶1时,3,4-二氯硝基苯在前40 min内的降解过程是显而易见的,随后还原产物则逐渐增加,并且随着混合溶剂中甲醇含量越高,3,4-二氯苯胺的特征峰越为明显。这表明反应的初期主要以氧化反应为主,而反应后期主要以还原反应为主,并且甲醇相对含量越高,越有利于还原反应的发生。
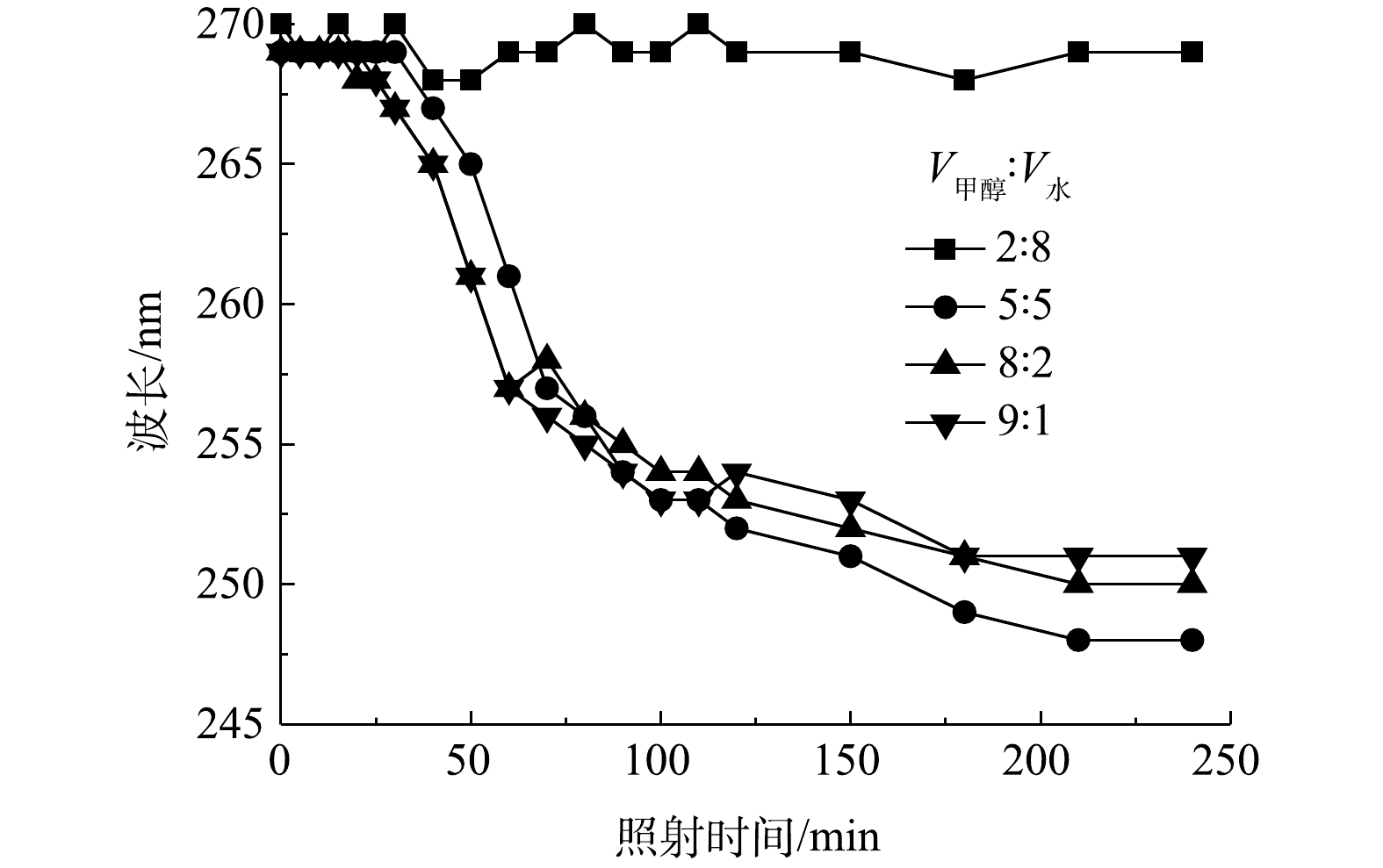
为比较240 min光照内有机物的变化情况,考察了最大紫外吸收峰随混合溶剂比V甲醇∶V水=2∶8、5∶5、8∶2、9∶1时随反应时间的变化情况,结果如图4所示。当V甲醇∶V水为2∶8和5∶5时,最大紫外吸收峰的变化可分为3个阶段:0~40、40~150、150~240 min。在第1阶段,最大紫外吸收峰的波长基本上维持在3,4-二氯硝基苯的特征峰处,与此同时,3,4-二氯硝基苯的峰强度不断减弱;在第2阶段,紫外吸收峰的位置由3,4-二氯硝基苯的特征峰(269 nm)移动到3,4-二氯苯胺的特征峰(248 nm),此时3,4-二氯硝基苯和3,4-二氯苯胺共存;在第3阶段,紫外吸收峰维持在248 nm处,3,4-二氯苯胺的特征峰逐渐升高。以上结果表明,3,4-二氯硝基苯后期主要经过光催化还原而生成3,4-二氯苯胺。由整个光照过程可以看出,当V甲醇∶V水为8∶2时,3,4-二氯硝基苯主要被氧化降解;而当V甲醇∶V水为9∶1时,3,4-二氯硝基苯主要进行还原反应。
因此,溶剂中甲醇和水的含量对光催化氧化和还原过程具有显著的影响。混合溶剂中水的含量越高,越有利于光催化氧化反应;相对地,甲醇的含量越高,越有利于光催化还原反应。
-
光催化降解率以及还原产物的产率是评价光催化过程的重要参数。在本研究中,3,4-二氯硝基苯的降解率、3,4-二氯苯胺的产率可按式(1)和式(2)进行计算。
式中:Ct和C0分别为3,4-二氯硝基苯在一定光照时间内的残留浓度和初始浓度,mg·L−1;Ct'为光照t时间内3,4-二氯苯胺的浓度,mg·L−1;C'为理论上还原所得3,4-二氯苯胺的最高浓度,mg·L−1;X和Y分别为3,4-二氯硝基苯的降解率和3,4-二氯苯胺的产率。
图5(a)为不同甲醇-水溶剂中光催化降解3,4-二氯硝基苯的实验结果。当V甲醇∶V水为5∶5时,3,4-二氯硝基苯的降解率最高,在40 min内达到26.81%。当V甲醇∶V水为9∶1时,3,4-二氯苯胺的产率最高为78%;而当V甲醇∶V水为9∶1~2∶8时,随着溶剂中含水率的增加,还原产物的产率整体为下降趋势(图5(b));但V甲醇∶V水为2∶8时,反应的还原产率略高于在5∶5的溶剂中的还原产率,该结果与图5(a)中所示的结果一致。
-
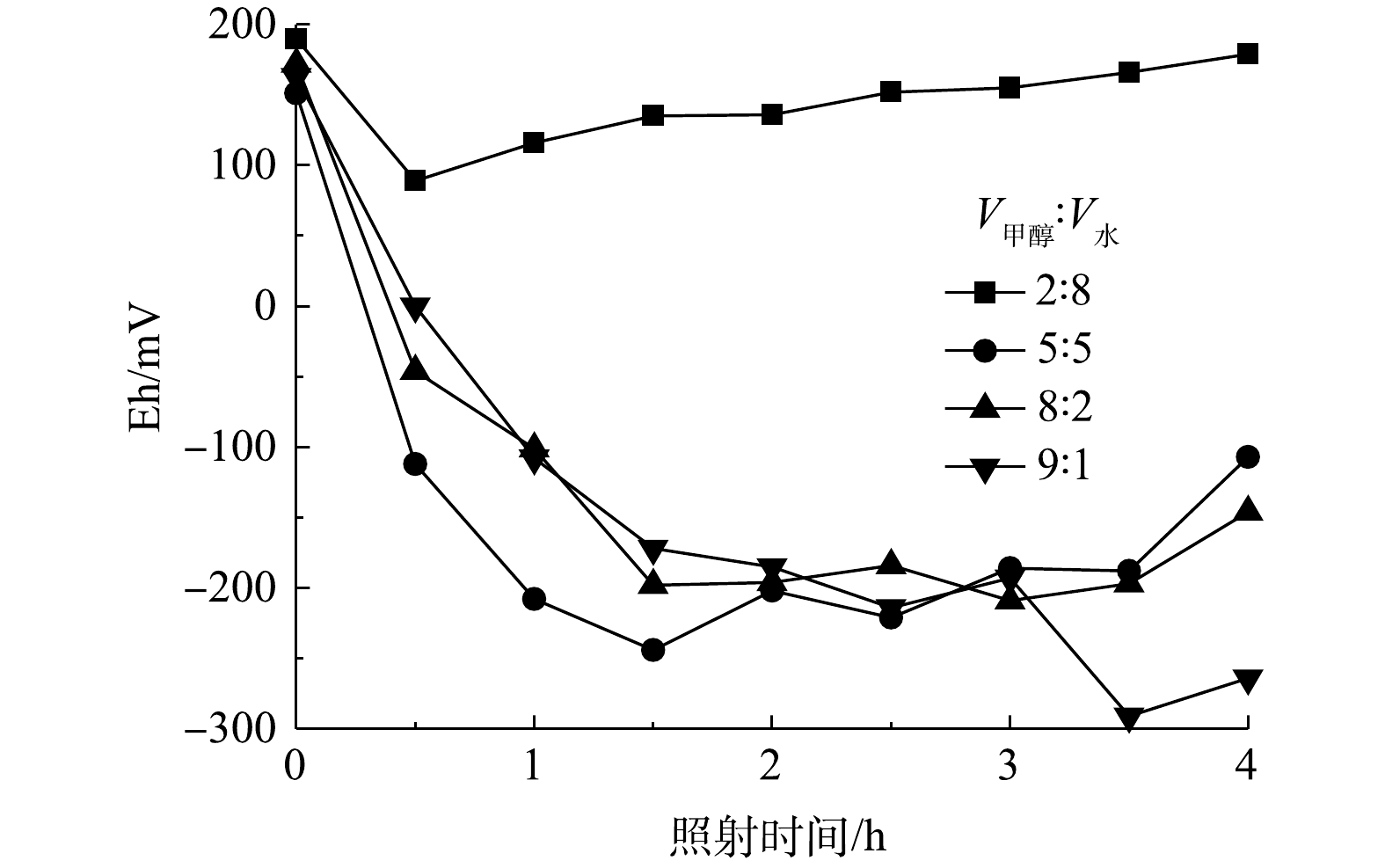
氧化还原电位(Eh)可以直接反映体系的氧化还原能力[27],因此,本研究在不同的V甲醇: V水条件下进行了Eh测定,结果如图6所示。正电势表示系统具有氧化特性,负电势表示系统具有还原特性。在反应阶段初期所有的Eh值均为正值,可以认为在不同甲醇-水比例的溶剂中,3,4-二氯硝基苯以氧化降解反应为主。值得注意的是,当混合溶剂中V甲醇∶V水为2∶8时,Eh值仍为正,这表明整个反应过程以氧化反应为主导。随着反应进行,混合溶剂中V甲醇∶V水由5∶5增大至9∶1时,Eh变为负值,这说明该体系的还原特性促进了3,4-二氯硝基苯向3,4-二氯苯胺转化。
在光催化氧化反应中,水中的溶解氧和羟基与催化剂表面的光生载流子发生反应,形成·OH[27],如式(3)~式(5)所示。
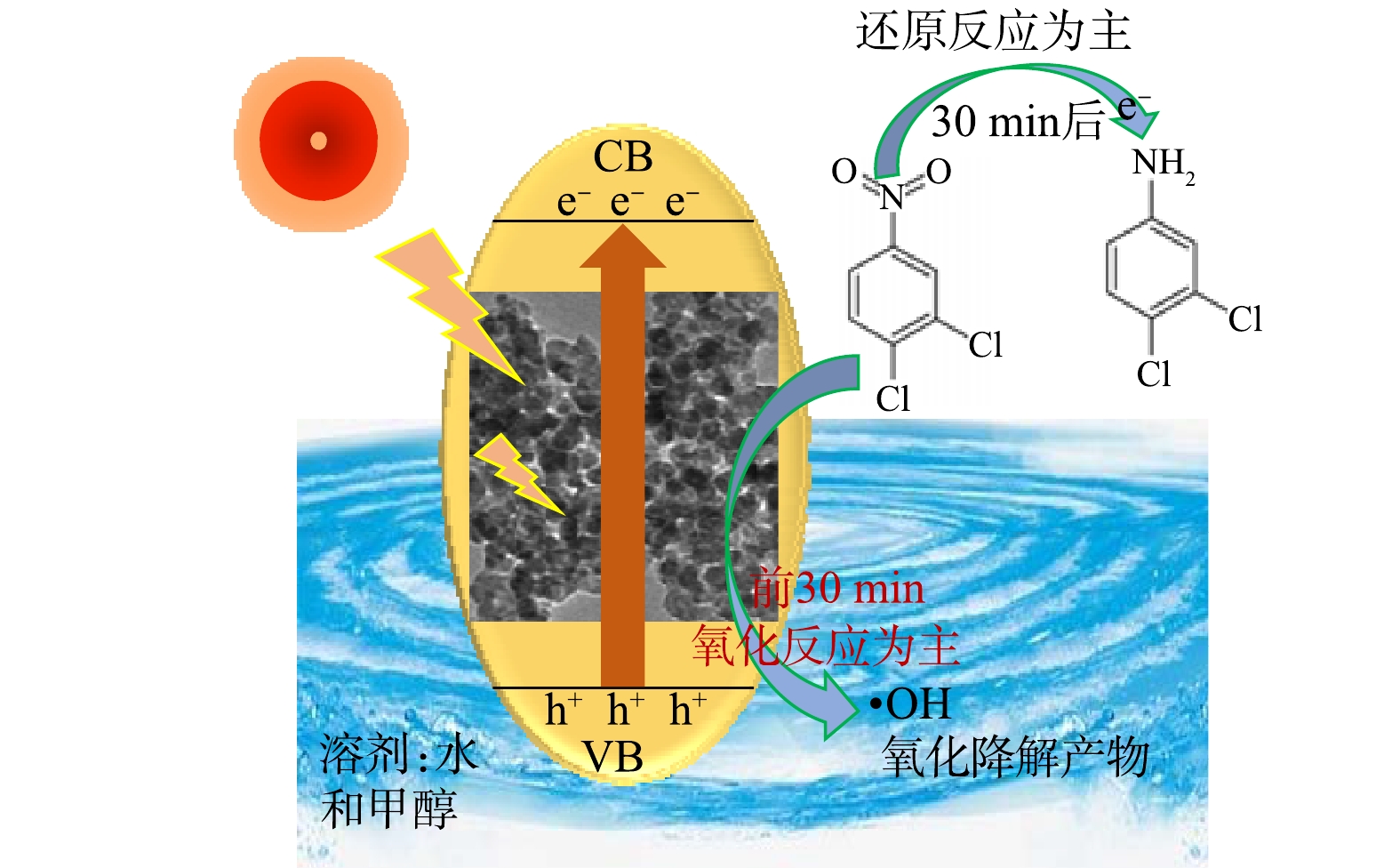
光生空穴与H2O反应生成具有氧化性的·OH,导致3,4-二氯硝基苯进行氧化降解反应;光电子具有很强的还原性,可以将3,4-二氯硝基苯还原为3,4-二氯苯胺(图7)。因此,当反应体系中混合溶剂的水含量较低时,·OH的数量减少,氧化效果较差。反应体系(V甲醇∶V水=2∶8)以水为主时氧化效率同样较差,这可能是由于电子空穴的复合概率增加所致。
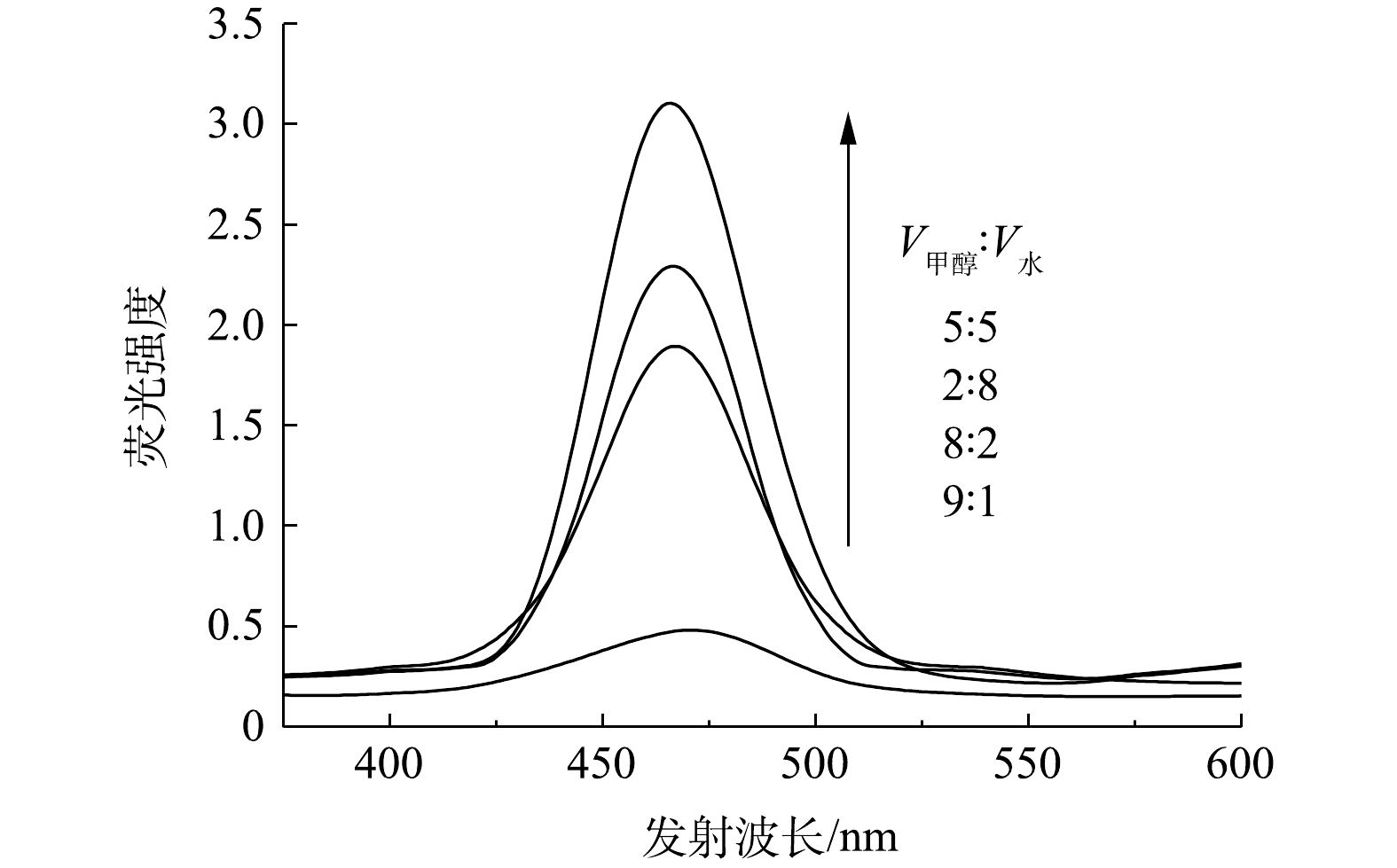
COU与·OH反应生成的7-羟基香豆素在465 nm处具有特征荧光峰。荧光测定实验结果表明,当V甲醇∶V水为5∶5时,7-羟基香豆素的荧光强度最高(图8),这反映出此时该体系中·OH的浓度最高,即3,4-二氯硝基苯的氧化效果最好。·OH的浓度越高越有利于光催化降解过程,由此可推断,光催化过程中·OH对氧化反应具有较高的贡献率。
另一方面,混合溶剂中甲醇与空穴反应形成甲氧基,可导致系统中大量激发态电子[28]被保留,进而促进还原反应进行,如式(6)~式(8)所示。
当混合溶剂以甲醇为主时,体系可表现出良好的还原能力[29]。在V甲醇: V水为5:5的体系中反应物的降解率最高,导致该体系在反应初始阶段消耗反应物最多,故而还原产物3,4-二氯苯胺的产率最低。而当体系中以水为主时,反应以氧化反应为主导[30]。
-
对不同混合溶剂条件下反应40 min内的实验结果进行了光催化氧化过程的动力学研究,根据式(9)对所得结果进行三阶动力学拟合,拟合的结果如图9所示。
式中:C0和Ct分别为3,4-二氯硝基苯的初始浓度和t时间下的浓度,mg·L−1;k为速率常数,L2·(mg2·min)−1;t为光催化反应时间,min。
对比图9(a)~(d)可以看出,拟合方程的可决系数分别为0.917、0.914、0.955和0.946,说明三阶动态模型可以较好地描述3,4-二氯硝基苯的光催化氧化过程。拟合直线的斜率用来估算速率常数k。当V甲醇∶V水为2∶8、5∶5、8∶2和9∶1时,k分别为1.85×10−7、1.66×10−7、8.92×10−8和7.82×10−8 L2·(mg2·min)−1,即当V甲醇∶V水为2∶8和5∶5时,3,4-二氯硝基苯的光催化氧化速率较高。
2.1. TiO2的表征
2.2. 光催化氧化还原过程的紫外吸收光谱
2.3. 溶剂比例对光催化氧化、还原反应的影响
2.4. 3,4-二氯硝基苯光催化氧化还原反应机理
2.5. 光催化氧化反应动力学
-
1) 3,4-二氯硝基苯的氧化反应发生在光照初期的40 min内,当V甲醇∶V水为5∶5时,3,4-二氯硝基苯降解率最好,为26.81%,此时·OH的浓度最高;而3,4-二氯硝基苯的还原反应主要发生在光照60~240 min内,当V甲醇∶V水为9∶1时,3,4-二氯苯胺的产率最高,为78%。
2)在光照60 min后,V甲醇∶V水为5∶5、8∶2和9∶1时,Eh为负值,反应以3,4-二氯硝基苯光催化氧化为主。
3)三级动力学模型与氧化过程吻合较好,当V甲醇∶V水为2∶8和5∶5时,光催化氧化速率较高。




 下载:
下载: