-
活性蓝19(RB19)是一种蒽醌类染料,具有色彩鲜艳、染色度高、固色率好等特点,常用于纺织工业中。随着近年来生产规模的扩大,印染废水不断排入水体中,由于染料废水可生化性差、成分结构复杂且生物毒性高,因此,常规处理手段对染料废水处理效果差,给生态环境与人体健康造成了严重危害[1-4]。
目前,在众多蒽醌染料废水处理方法中,光催化氧化方法是一种有效的处理手段。龚丹[5]利用光催化TiO2降解蒽醌染料茜素绿,在光催化30 min后,茜素绿的脱色率达100%,紫外可见光谱分析结果表明,可见光区的吸收峰基本消失,染料发色基团和共轭结构均被破坏,处理后的废水矿化度高,可生化性较好。为进一步增强光催化的降解效果,压缩处理成本,部分学者在光催化体系中引入过二硫酸盐(PS),以提高体系氧化能力。作为电子受体的PS被活化后,不仅可以产生硫酸根自由基、羟基自由基等活性物质并与污染物发生反应,而且在一定程度上延长了光催化剂表面电子-空穴对的存活寿命,提高了污染物降解效果。GAO等[6]采用vis/MIL-53(Fe)/PS体系降解AO7,90 min内,染料去除率达82%,与单独光催化相比,效率提高58%,极大地提高了体系氧化降解能力。
一些学者[7-9]在探究污染物降解工艺的过程中也发现,污染物本身的分子结构在一定程度下也可以发生活化,以促进体系的降解效果。NIE等[8]发现,在光照条件下,将卡马西平(CBZ)与罗丹明B(RhB)的混合溶液引入NaHSO3后,2种污染物均可以发生降解,RhB在反应过程中充当了电子转移的角色,从而活化NaHSO3,产生过一硫酸根自由基降解CBZ。FANG等[9]发现,在对苯醌与过硫酸盐的体系内,对苯醌在光照条件下活化产生半醌自由基(Q−·),从而活化过硫酸盐,产生硫酸根自由基,使PCB28降解率达80%以上。由此可见,罗丹明B、蒽醌等光敏性染料在光照条件下产生了活性物质,这些活性物质活化过硫酸盐、亚硫酸盐等生成氧化性自由基,促进了污染物的降解。
RB19中含有特定氧化敏感型官能团,在可见光照射条件下可被活化,生成的Q−·进一步参与降解反应,并且在反应过程中可能存在长链式自由基反应,可有效延长体系的降解时间。但迄今为止,对该类体系内污染物中间活性物质的形成、参与降解反应的过程、与氧化体系内其他活性成分的协同机制以及这种长链式自由基反应所带来的“增强效应”受不同环境因素的影响规律的研究甚少,揭示此类反应在降低中间产物生物毒性作用机制的研究则更为鲜见[10]。
本研究以石墨相氮化碳/溴化氧铋(g-C3N4/BiOBr)为光催化材料,引入光照(vis)与PS,对不同体系的光催化效果进行对比,构建最优组合体系,从而高效去除RB19;采用淬灭实验、EPR等手段,探明体系内自由基的种类,揭示光催化降解蒽醌染料RB19中Q−·的生成及其衍生物质参与反应过程的机理;进一步研究不同反应条件(材料投加量、初始pH、活性蓝19初始质量浓度、过硫酸盐投加量、光照强度等)对Q−·增强效应的影响;采用大肠杆菌培养实验、LC-MS等实验手段,分析反应后溶液的毒性和降解路径,以期充分发挥Q−·歧化反应的循环效应优势,为蒽醌染料废水处理的实际应用提供理论基础与参考依据。
-
尿素、溴化钾、乙二醇、乙醇、硫酸、盐酸、氢氧化钠、甲醇、叔丁醇、乙二胺四乙酸二钠盐、过硫酸钠均为分析纯,购自国药集团。活性蓝19(RB19)、活性红24(RR24)、五水合硝酸铋均为分析纯,纯度97%的5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)和纯度98%的甲磺酸去铁胺 (DFO)均购自上海麦克林有限公司。
-
实验中染料污染物的初始质量浓度均为40 mg·L−1,氙灯功率为300 W,PS的质量浓度为400 mg·L−1,g-C3N4/BiOBr投加量为0.1 g·L−1,pH≈6(原始pH),以此条件进行不同体系降解实验。
光催化材料选用质量分数为5%的g-C3N4/BiOBr材料,具体材料制备条件的优化及其物化性质的分析表征见文献中的方法[11]。
配置100 mg·L−1的RB19储备液,备用,根据所需质量浓度配置成250 mL的溶液。实验在250 mL的夹套烧杯中进行。反应伊始,投入光催化材料,暗吸附30 min。达到吸附平衡后,加入PS,进行光催化反应。实验时开启循环冷凝泵,保证反应温度恒定。每隔一段时间,用2 mL的注射器进行取样。水样用0.45 μm聚醚矾膜过滤,以去除水体中影响吸光度测定的光催化材料。在水样稀释5倍后,用分光光度计于592 nm处测量吸光度。 将配制好的初始质量浓度为40 mg·L−1的RB19溶液倒入夹套烧杯中,再分别加入一定量的甲醇(MeOH)、叔丁醇(TBA)、乙二胺四乙酸二钠(EDTA-2Na)和甲磺酸去铁胺(DFO)等淬灭剂。投加光催化材料,在进行30 min暗吸附后,加入PS,开启氙灯进行实验。
在实验过程中,对蒽醌初始质量浓度、pH、光催化剂投量、光照强度及过硫酸盐质量浓度等5个条件进行单因素探究实验。在进行单因素实验时,其他条件不变,蒽醌初始质量浓度设置为10、20、40和80 mg·L−1,反应初始pH分别为3、6、9和11,光催化剂投量分别为0.05、0.1、0.2和0.3 g·L−1,光照强度设置为2、5和7 W·m−2,过硫酸盐的质量浓度分别为50、100、200和400 mg·L−1。
量取一定量胰蛋白胨、酵母浸粉等溶于蒸馏水、反应出水和RB19溶液中,配置LB培养基,pH控制在7.4。高压蒸汽灭菌锅经过121 ℃条件下灭菌后,在超净工作台中接种大肠杆菌菌株,包扎后,放置在37 ℃的恒温振荡器中,培养48 h,每隔6 h测试菌悬液OD600的值。
-
采用紫外可见分光光度法测定RB19的质量浓度;使用Unic UV-2000紫外可见分光光度计于λ= 592 nm处测定吸光度,通过标准曲线换算出RB19的质量浓度。电子自旋共振(electron paramagnetic resonance,EPR)测试条件如下:共振频率为9.77 GHz,微波功率为19.97 mW,调制频率为100 kHz,调制幅度为0.2 mT,扫描中心场强度为344 mT,扫描宽度为10 mT,扫描时间为83.87 s,信号通道分段增益为30 dB,微波衰减系数为25 dB,质谱采用正离子模式。
-
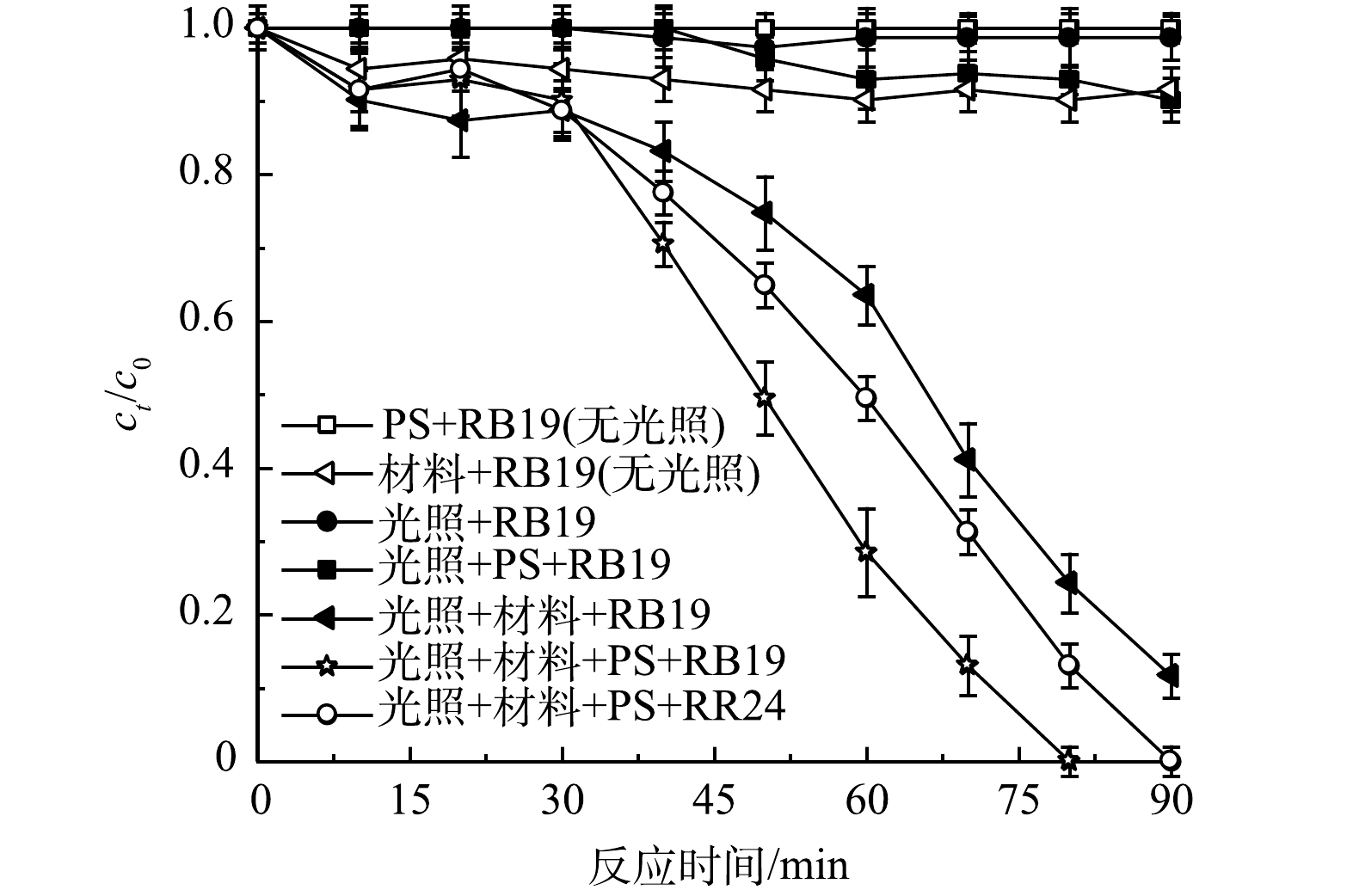
为探究vis/g-C3N4/BiOBr/PS体系降解RB19的效果,对比分析了vis/PS、PS/RB19、vis/PS/RB19、vis/g-C3N4/BiOBr以及vis/g-C3N4/BiOBr/PS等不同体系下的污染物降解情况,实验结果如图1所示。可以看出,RB19在仅有光照条件下以及在黑暗条件下的PS和g-C3N4/BiOBr体系中均没有发生降解。而在光照与投加g-C3N4/BiOBr光催化材料共同作用的体系中,反应90 min,降解率达89.36%。这说明,g-C3N4/BiOBr具有良好的光催化性能,在光照条件下可以迅速降解RB19并使溶液脱色。另外,向vis/g-C3N4/BiOBr体系加入PS时,RB19染料在80 min内就实可完全降解,说明PS的加入促进了该体系内氧化还原反应的正向进行。值得注意的是,在光照条件下,RB19/RR24与PS共存时,观察到约14.03%的RB19在反应进行90 min后被去除。可以推断,在光照条件下蒽醌染料RB19可以活化PS,产生的活性氧物种与RB19发生氧化还原反应。为进一步探明蒽醌染料RB19的特殊性,实验中选用40 mg·L−1的非蒽醌类染料RR24作为对照。实验结果表明,在降解相同质量浓度的RB19和RR24时,RB19比RR24提前10 min全部完成降解。结合已有研究[12-14],可以看出,在vis/PS/g-C3N4/BiOBr处理RB19的体系中, RB19可以活化PS,使体系内·OH、SO4−·的数量增多,可加快自身的降解进程。
-
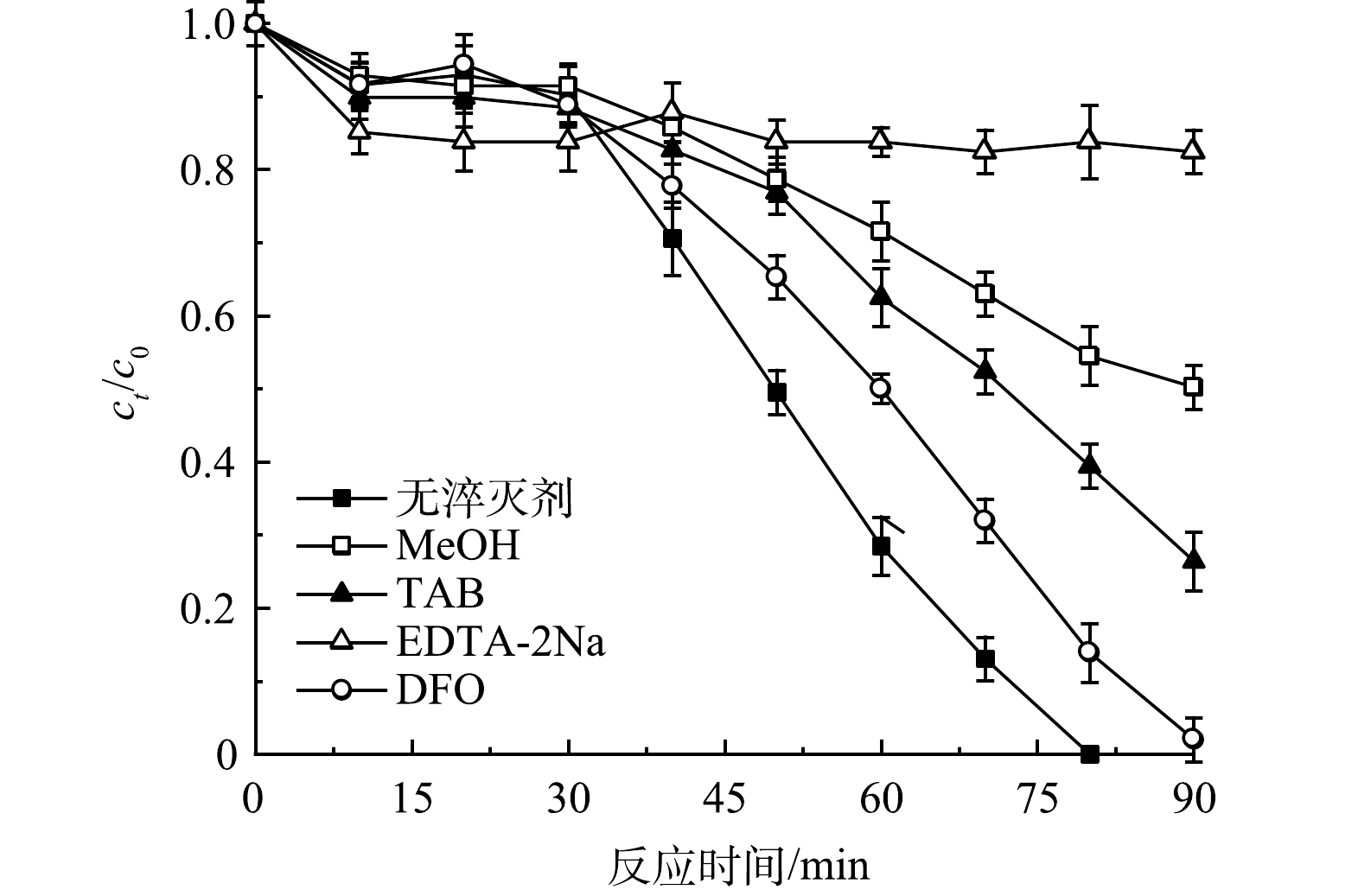
1) 活性物种的淬灭实验。为探究vis/PS/g-C3N4/BiOBr体系内蒽醌染料RB19的降解机理,分别选择甲醇(MeOH)、叔丁醇(TBA)、EDTA-2Na来淬灭·OH、SO4−·以及h+[15-16],结果如图2所示。当加入叔丁醇时,反应90 min后,降解率为73.69%,与空白对照组相比,降解率降低了26.31%。当加入甲醇时,90 min后,降解率为49.77%,与叔丁醇相比,降解率降低了23.92%。由于甲醇可以同时淬灭羟基与硫酸根自由基,结合以上淬灭结果可以看出,在相同条件下,体系内羟基自由基贡献率比硫酸根自由基更高,发挥作用更强。当加入淬灭剂EDTA-2Na时,反应90 min后,降解率仅为17.57%,说明体系内空穴发挥了主要作用。另外,在加入半醌自由基(Q−·)淬灭剂——甲磺酸去铁胺(DFO)后,反应70 min时,降解率仅为68.1%,与空白组相比,此时降解率降低了10.1%。这说明体系内有Q−·产生,且Q−·在光催化体系中发挥了促进降解的作用。这一实验结果也进一步验证了vis/PS/g-C3N4/BiOBr体系内存在蒽醌染料活化PS产生Q−·的反应机理。
由以上分析可以看出,该体系内发挥作用的活性物种为h+、·OH、SO4−·,并且在反应过程中存在RB19和BiOBr对PS的协同活化作用,促进了自由基的产生速率,进而加速对污染物的降解。
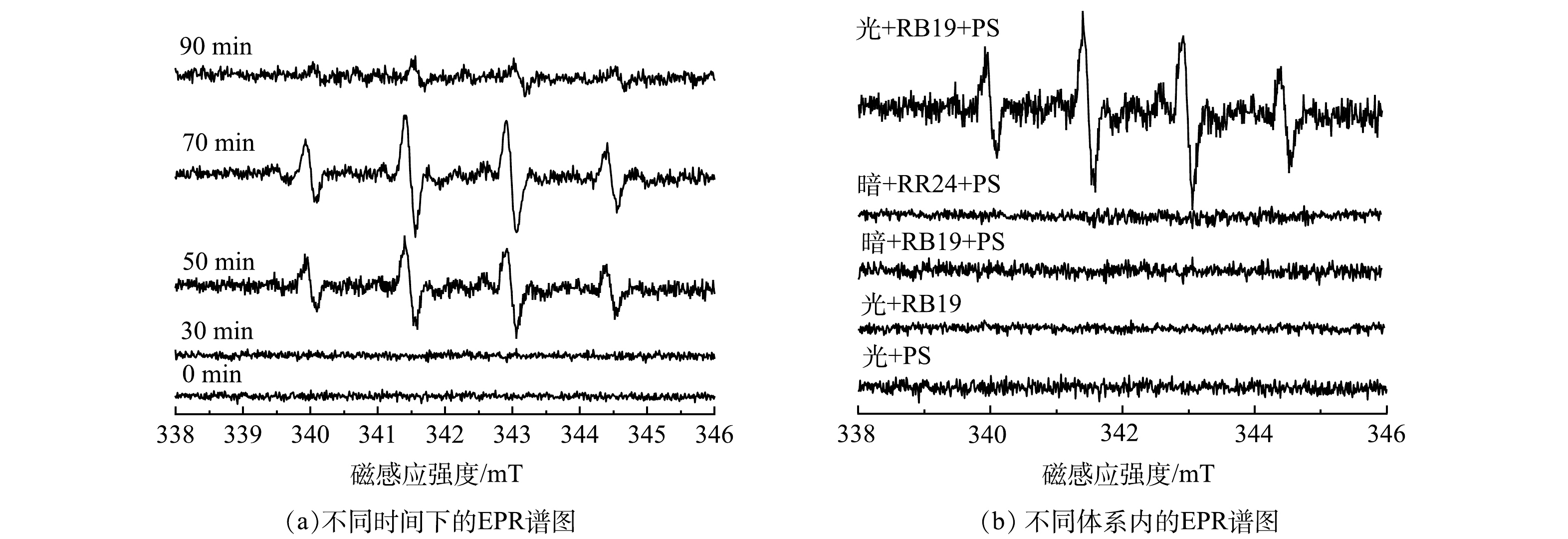
2) 自由基的捕获实验。为了进一步确定体系内的自由基种类,以5,5-二甲基-1-吡咯啉-N-氧化物 (DMPO) 为自由基捕捉剂,使用电子电子顺磁共振仪(EPR)对vis/PS/g-C3N4/BiOBr反应体系中自由基进行鉴定。实验中的溶剂为水溶液,光催化材料投加量为0.1 g·L−1,过硫酸盐投加量为200 mg·L−1,光照功率为300 W,溶液pH为6。在催化反应阶段前,设置30 min 的预吸附实验;随后向体系内加入光照和PS,在催化反应过程中,每隔20 min取样1次,每次取样1 mL,经0.45 μm聚醚矾膜过滤后,加入10 μL DMPO,测试结果如图3(a)所示。可以看出,图谱中存在明显的·OH和SO4−·特征峰,且自由基峰强变化规律呈先增大后减少的趋势,在70 min时达到最大。这说明反应中确实有·OH和SO4−·的生成,同时,70 min时该体系中自由基数量较多,活性较强,持续时间长,反映出该体系内可能存在自由基链式反应。
为验证vis/RB19/PS体系中Q−·的生成与过硫酸盐的活化作用,在光反应5 min时,对vis/PS、vis/RB19、vis/RB19/PS等体系进行EPR测试,结果见图3(b)。可以看出,光照条件下的PS、RB19及黑暗条件下的RB19/PS体系中均没有特征峰的形成。然而当RB19/PS体系添加光照时,EPR图谱中出现了明显的·OH和SO4−·的特征峰,说明光照是氧化性自由基形成的关键。此外,为验证蒽醌在体系内的作用,实验中将体系内蒽醌染料RB19更换成偶氮染料RR24,在相同条件下,EPR测试发现,只有在蒽醌染料RB19存在时,才有·OH等活性物质的形成。这表明,体系内蒽醌染料—RB19被光激发生成的Q−·可以进一步活化PS,进而产生具有强氧化性的活性物种。
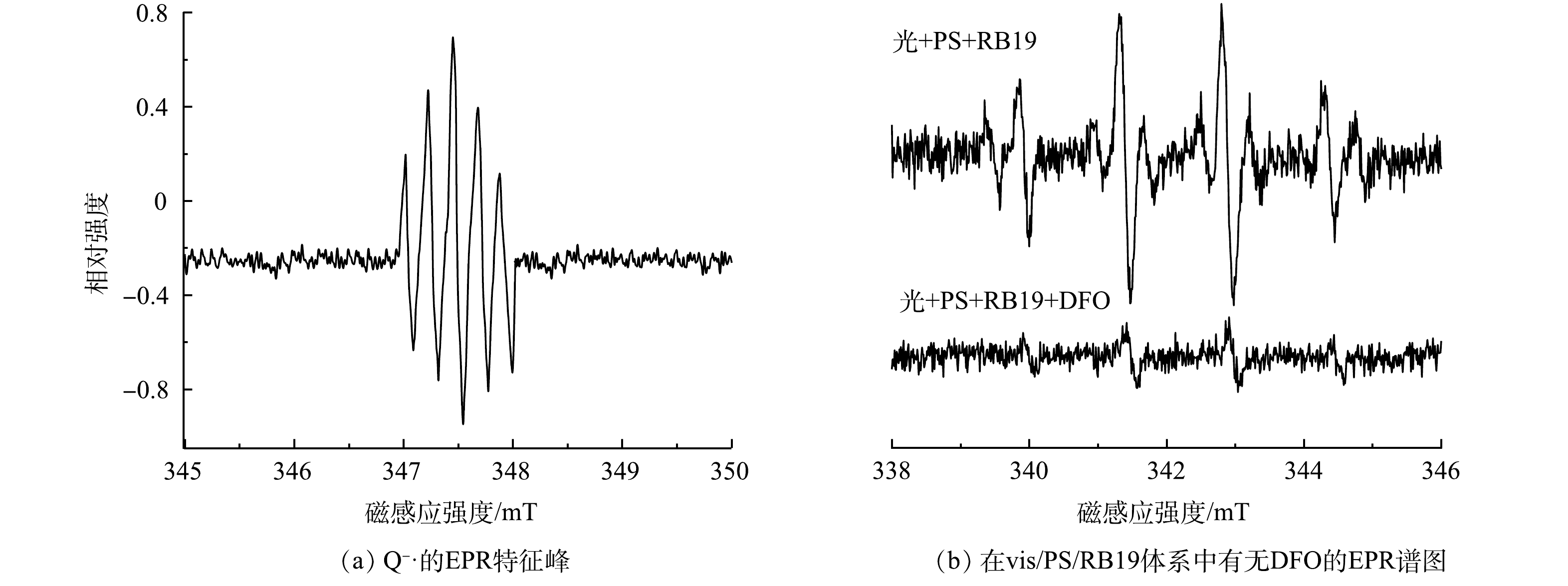
为更加直观证明RB19光照下产生氧化性自由基自由基这一推测,将配置好的RB19在氙灯照射下进行EPR测试,测试结果见图4(a)。从图4(a)中可以明显发现有Q−·特征峰的形成,证明了体系内RB19在光照下产生Q−·这一猜测。此外,为了证明Q−·对PS的活化作用,对添加了Q−·淬灭剂(DFO)[17-18]的vis/PS/RB19体系进行EPR测试,结果如图4(b)所示。可以看出,vis/PS/RB19体系内有明显的羟基与硫酸根自由基特征峰,当加入DFO淬灭剂后,体系内羟基、硫酸根自由基峰度大幅减弱,这是Q−·被淬灭后使得PS活化能力减弱所造成的,也进一步证明Q−·可以对PS进行活化。
-
由上述实验可以发现,在光催化降解过程中,蒽醌可以在光激发作用下产生Q−·[12],可以在体系内发挥PS活化协同降解以及氧化循环的作用,促进污染物的降解。在光催化降解RB19染料的过程中,作用机制主要包括g-C3N4/BiOBr材料的光催化、过硫酸盐协同降解和RB19蒽醌染料自活化降解反应3个方面。
1) 光催化效应及过硫酸盐协同降解机理分析。在可见光的照射下,g-C3N4/BiOBr被激发,价带的电子(e−)被跃迁到导带上,使得价带上形成空穴(h+)[19-21]。由于g-C3N4/BiOBr的异质结属性[22],光生电子从g-C3N4的导带迁移到BiOBr的导带,在BiOBr内部静电场的作用下,BiOBr价带的空穴会移动到g-C3N4的价带,极大地降低了电子-空穴对的复合,提高了光催化氧化降解效果[23-25]。
此外,RB19产生的Q−·在提高体系整体氧化能力方面也发挥了重要作用。Q−·活化PS产生SO4−·,体系内SO4−·的数量增多,从而增强氧化能力;同时,一部分SO4−·可以进一步与H2O反应生成·OH(反应过程见式(1)),进一步提高了体系内自由基种类与数量,加速了RB19的降解[26]。需要指出的是,此效应属于长链自由基反应,一般来说,自由基链式反应分为链引发、链反应与链终止3个阶段,但由于反应的历时有所不同,所以反应效率也有所差异[27]。在本体系中,由Q−·衍生的O2−·等超氧化自由基,可进一步攻击RB19中间产物中的不饱和脂肪酸,形成能够与其他分子发生氧化反应的超氧化脂肪酸自由基,从而形成自由基长链反应,促进污染物降解及矿化。
2) RB19蒽醌染料活化的降解机理分析。在可见光激发下产生的Q−·,不仅可活化PS,而且还可以发生自歧化反应,生成氢醌和醌。氢醌不稳定,会进一步被氧化为醌[9],反应过程见式(2)和式(3),由此形成了蒽醌特有的自循环反应。在转化过程中,Q−·争夺了部分光生电子,从而降低了光催化剂中电子-空穴的复合率,增强了光催化效应。所以,在醌类自循环转化以及Q−·活化PS的共同作用下,活性物种含量的增加不仅提高了污染物降解率,也加速了整体氧化反应的进行。
式中Q−·为醌自由基;Q为醌;H2Q为氢醌。
综上所述,蒽醌染料在光照作用下可以产生能激活PS的Q−·,在污染物的降解过程中能够产生更多的活性物种以实现对RB19的充分去除。为了进一步探究不同条件下Q−·对该体系降解效率增强效应的影响规律,本研究探究了不同实验条件(蒽醌初始质量浓度、pH、光催化剂投量、光照强度及过硫酸盐质量浓度)对RB19降解性能的影响。
-
1) 反应动力学方程的建立。RB19的光催化降解过程符合准一级动力学,降解速率计算方法见式(4)和式(5)。
式中:c0为RB19的初始质量浓度,mg·L−1;kobs为准一级动力学反应速率常数;t为反应时间,min;ct为t时刻RB19的质量浓度,mg·L−1。
本研究以初始pH(A)、初始RB19质量浓度(B)、光照强度(C)、材料投加量(D)、过硫酸盐投加量(E)5个主要因素作为自变量,建立kobs与5种因素的关系式 (式(6))。
式中:kobs为准一级动力学反应速率常数; A、B、C、D、E为主要因素;a、b、c、d、e、ε均为常数,通过自变量数值与体系光催化反应速率计算求得,计算过程见已有研究[28]。
表1反映了在光催化阶段(30~90 min)不同实验因素对RB19降解(一级)动力学的影响。
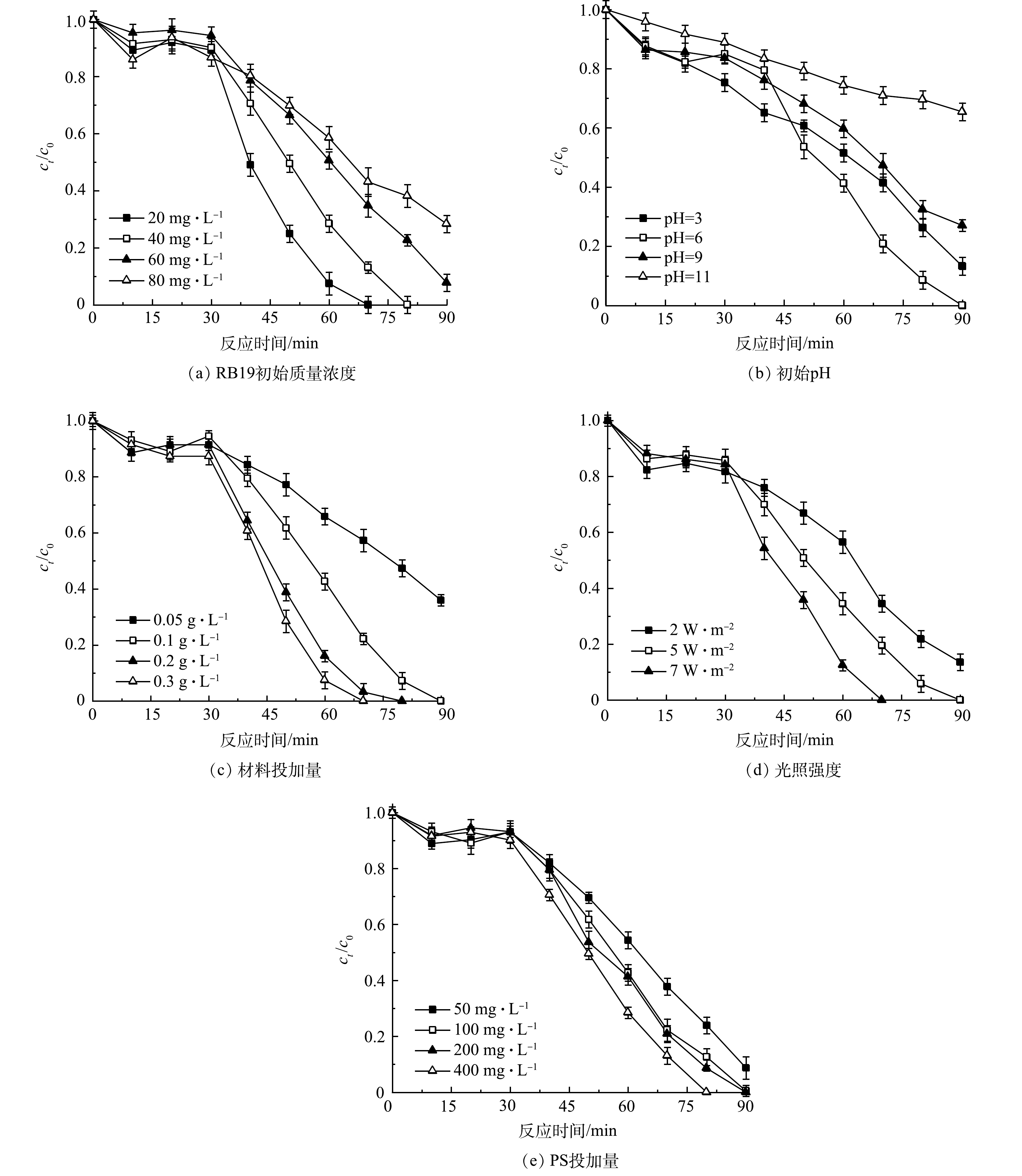
2) 蒽醌初始质量浓度对体系的影响。RB19作为生成Q−·的前体物质,其质量浓度的变化会影响Q−·的生成。为探究不同RB19质量浓度对实验的影响,设置了RB19质量浓度10、20、40和80 mg·L−1,结果见图5(a)。可以看出,质量浓度低于80 mg·L−1的RB19均可完全降解,当质量浓度为80 mg·L−1时,反应结束时降解率降低到71.68%,这是体系内SO4−·和·OH数量限制所造成的。由光反应阶段动力学拟合结果可以看出,随着质量浓度的增加,速率常数逐渐降低。当RB19质量浓度为10 mg·L−1时,反应速率常数最大,因为在低质量浓度下,单位体积溶液中被光活化的RB19分子增多,加快了Q−·的产生,促进了PS的活化,提高了体系内羟基、硫酸根自由基的量,加速了反应的进行。当RB19质量浓度加到80 mg·L−1时,反应速率常数进一步下降,原因是高质量浓度下染料分子间发生了聚集效应,形成了聚集体[29]。这不仅使污染物可降解性变差,同时也减弱了蒽醌染料的光活化效应,导致Q−·数量减少,进而降低了反应速率常数。
3) pH对体系的影响。其他条件不变,本研究还探讨了不同初始pH对Q−·参与降解的影响,结果如图5(b)所示。在60 min的光催化反应过程中,随着pH的升高,污染物降解率与降解速率均呈先增加后减少的趋势。 pH=3时,90 min降解率达87.34%,整个光催化过程反应速率常数为0.010 min−1。pH增加到6时,污染物实现完全降解,反应速率常数达到最大,原因是在偏酸性条件下,染料以质子化形式存在,被光激发后生成Q−·,反应过程[30]见式(7)。同时,溶液中存在的S2O82−也会与光生电子发生反应(式(1)和式(8)),产生大量SO4−·与·OH,加快了污染物的降解。当pH增大到9和11时,材料表面呈现负电性,与带同性电荷的染料分子产生排斥作用,阻碍了RB19的降解;但在碱性条件下,体系内羟基自由基增多,反应(式(2)和式(3))向正方向进行,蒽醌与Q−·的循环加快,促进了体系内电子的消耗,加强了光催化效应,这在一定程度上减缓了降解速率的下降,导致整个氧化体系在较宽的溶液pH范围内均能保持较好的降解效率。
4) 光催化剂投加量对体系的影响。其他实验条件不变,设置光催化剂投量为0.05、0.1、0.2、0.3 g·L−1,实验结果见图5(c)。投加量为0.05 g·L−1时,污染物反应90 min后,降解率仅为64%,降解速率常数为0.009 min−1。随着光催化剂投加量的不断增加,RB19的降解效果也不断提高。当投加量增加到0.3 g·L−1时,反应70 min后,污染物降解率便达到100%,降解速率常数增大至0.027 min−1,增加了2.94倍。RB19的去除率及kobs的显著提升主要有2个原因:一方面,光催化剂投加量的增加提高了PS的活化效率,能够产生更多的活性物种;另一方面,体系内蒽醌、氢醌和Q−·间的自循环效应能够降低光催化剂中电子-空穴的复合率,极大地增强了光催化效应。同时,这一实验结果也说明Q−·的形成能够有效提升光催化材料对PS的催化性能。
5) 光照强度对体系的影响。其他实验条件不变,为研究光照强度对反应的影响,测量了武汉某地一段时间内的正午光照强度。在晴天和阴雨天时,太阳光的辐射强度平均分别为5 W·m−2和 2 W·m−2;因此,在实验中采用2、5、7 W·m−2 3种光照强度,结果如图5(d)所示。反应90 min后,污染物降解率均可达100%。由图5可知,当光照强度增大时,反应速率也提高。这是由于随着光照强度的增加,可利用的光子能量越多,加快了电子空穴对的产生,体系内氧化活性物质的含量也越多,从而加速了RB19的降解。然而,随着光照强度的增加(7 W·m−2),RB19的降解速率并没有得到对应的提升。这是因为,当光照强度达到一定的阈值后,光强的继续增加对体系内Q−·含量变化的影响较小,决定体系内Q−·含量变化的关键因素会转变为其他实验条件(如蒽醌的初始质量浓度等)。因此,选择适宜的光照强度尤为重要。
6) PS质量浓度变化对体系的影响。在所构建的体系内,过硫酸盐投加量直接决定了SO4−·和·OH的数量。本研究探索了PS投加量为50、100、200及400 mg·L−1时RB19的降解效果,结果如图5(e)所示。可以看出,当c(PS)≥100 mg·L−1时,反应90 min,染料均可完全降解,且随着PS投量的增加,染料降解速率也不断加快。这是因为随着投加量的增加,在光生电子活化与Q−·活化的共同作用下,加快了对PS的活化效果,产生较多的SO4−·和·OH,促进污染物的降解。另外, PS投量为50 mg·L−1时,在90 min内也实现了较高的RB19去除率,去除率达到91%,这是由于体系内Q−·的增强效应减缓了PS减少所带来去除效果下降的负面效应。这一现象的出现说明该体系可应用于蒽醌类废水处理工程中,对降低和控制PS的投加量具有重要的实际指导价值。
7) RB19反应动力学模型的建立。根据以上结果,建立RB19光催化反应的速率常数kobs与5种影响因素的关系式(式(9))。
在RB19初始质量浓度为40 mg·L−1、初始pH为6、材料投加量为100 mg·L−1、过硫酸盐投加量为400 mg·L−1、光照强度为5 W·m−2条件下,速率常数kobs=-0.015 min−1,将kobs带入式(8),可得
$ {\varepsilon }=0.052 $ 。速率常数计算方法见式(10)和式(11)。由式(11)可知,5个因素对RB19降解速率的影响从强到弱的顺序为材料投加量>初始pH>RB19初始质量浓度>过硫酸盐投加量>光照强度。
由此可以看出,材料投加量是RB19中影响最大的因素。光催化材料在吸收光子后,不仅可作为体系内光生电子的供体,以活化过硫酸盐等物质加速降解反应,而且所产生的材料空穴也可以直接氧化污染物,这是降解反应能够进行的根本原因。初始pH一方面会影响光催化材料表面的带电性质,进而影响污染物吸附效果;另一方面会影响溶液内羟基、硫酸根自由基等活性氧化物的产生量,进而影响RB19在单位时间的降解率。RB19污染物质量浓度可以直接影响光激发下Q−·的生成,从而间接影响PS的活化速率与蒽醌自循环加强光催化的效果。过硫酸盐投加量可直接影响体系内羟基、硫酸根自由基的数量,但由于降解过程中空穴发挥了主要的降解作用,故导致过硫酸盐产生的影响减小。光照强度可以影响光催化材料光生电子、空穴的产生,间接影响降解的进行,由于实验当地可见光强度变化的限制原因,与前几项因素相比,该光强范围内对体系的降解影响较小,故其影响也最弱。
为了验证模型的准确性,选用RB19初始质量浓度为40 mg·L−1、初始pH为6、过硫酸盐投加量为400 mg·L−1、光照强度为5 W·m−2、材料投加量分别为0.1、0.2、0.3 g·L−1的3组实验数据进行检验,并将实际值与理论值进行对比,结果如图6所示。可以看出,实验值与模型计算值较为接近。这说明通过分析得出的准一级模型对RB19的降解具有较高的拟合度,该模型可以用来描述RB19的降解动力学。
-
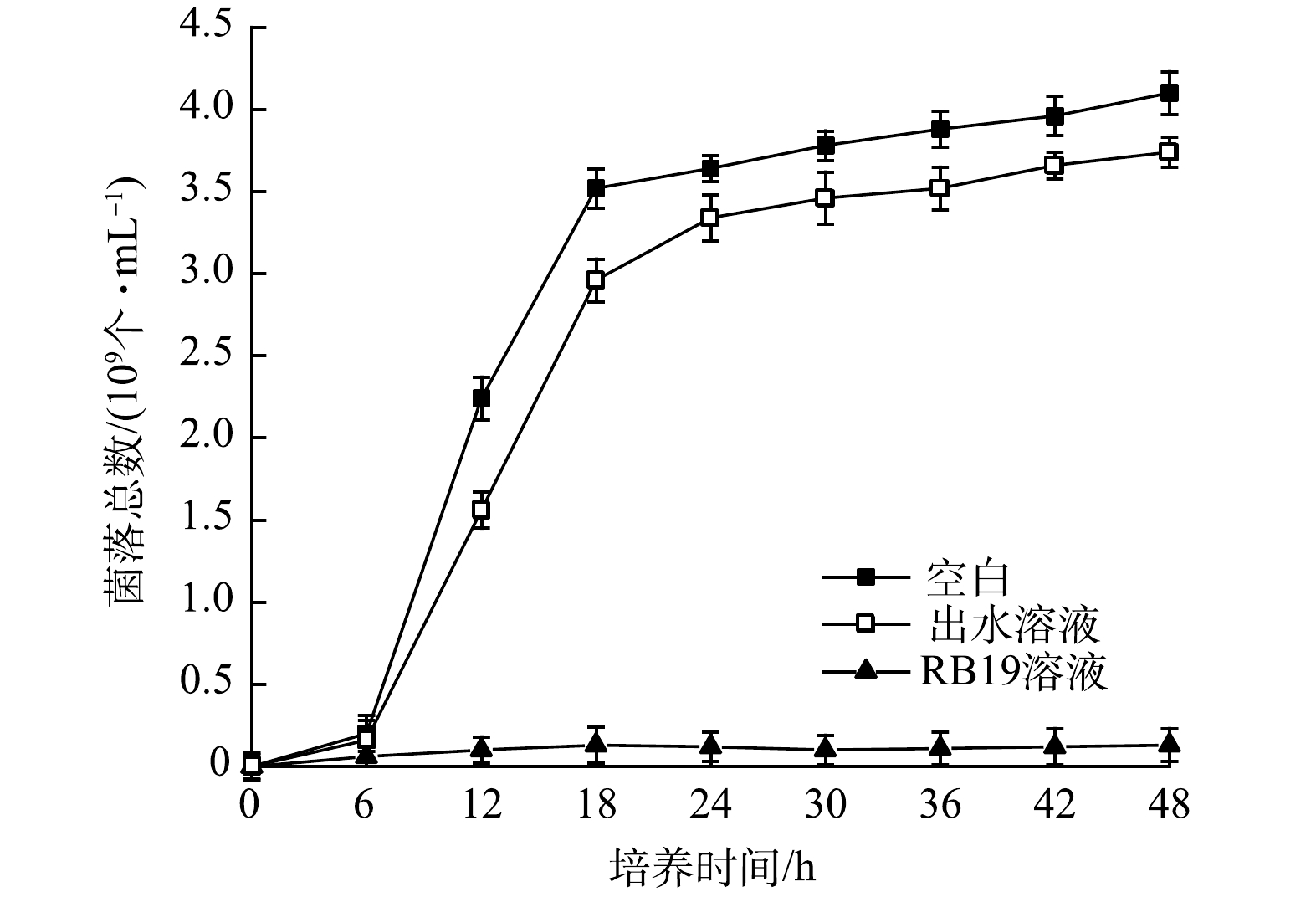
1) 毒理性分析。RB19为蒽醌染料,具有较大毒性,为了评估处理后废水的毒性情况,利用大肠杆菌在空白、出水以及RB19溶液培养基中进行培养实验[31] 。使用紫外分光光度计测定OD600处的光度值,以确定空白、出水培养基的细菌总数[32],RB19溶液培养基采用血球计数法进行测定,结果如图7所示。大肠杆菌在0~6 h均处于缓慢期,6~18 h为对数期,18~48 h为平稳期。在对数期,出水培养的大肠杆菌生长速度比空白缓慢。对数期结束后,出水培养基中的细菌总数为空白的86.6%。培养48 h后,出水培养细菌浓度占空白组的94.5%。而在RB19溶液培养基中,整个培养期间大肠杆菌繁殖生长情况均较差。因此,使用该体系处理后的蒽醌溶液培养大肠杆菌时,对其生长曲线影响较小。这进一步说明降解后的蒽醌染料废水的矿化度高,毒性小。
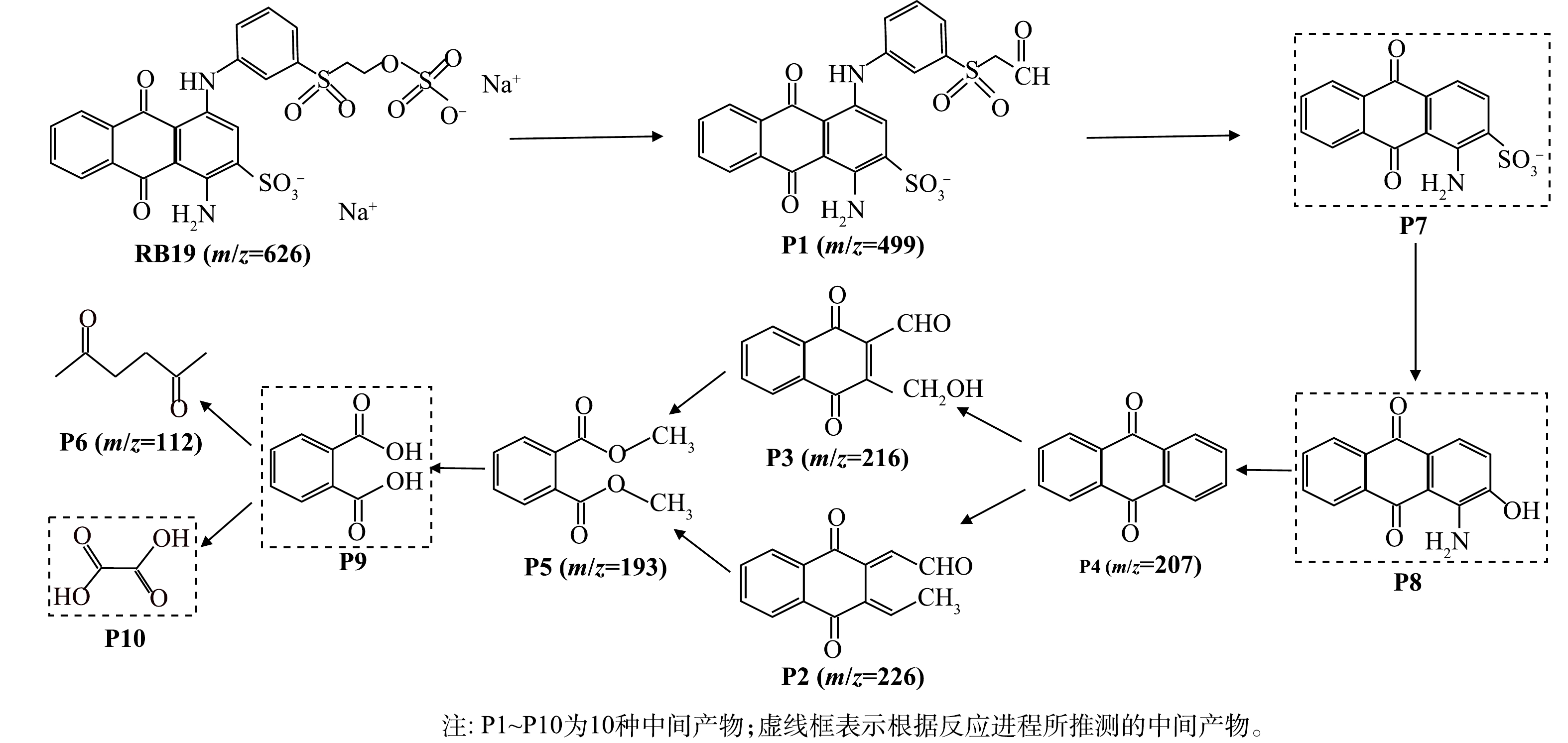
2) 降解路径分析。为进一步从理论上探究RB19的毒性弱化过程,在反应55 min后,在据液面2~3 cm处提取反应后溶液,进行LC/MS中间产物测试。在RB19的降解过程中,发现了6种中间产物。
在分析检测中间产物后,本研究提出了RB19的降解路径(图8)。可以看出,RB19分子中磺酸基上的Na+在水中快速解离,生成—SO3H基团,随后—SO3H基团和—NH—H基团中的H与水溶液中的羟基发生氧化反应,生成醛基,进而生成物质P1。P1中的醇羟基和醛基在自由基的作用下分别被氧化为醛基和C=N键[33];同时,由于C—N的键能较低,在强氧化性物质存在的水溶液中通过电子、质子的先后转移,发生氧化反应,生成物质P7。P7中的磺酸基和—NH2在羟基自由基的作用下被氧化,形成中间产物P4,P4为蓝色系染料的重要中间体,带有一定的生物毒性。P4上苯环在自由基的强氧化作用下逐渐开环,分别生成m/z=216和m/z=226的分子质量更小的中间产物(P2与P3)。P2结构中的甲基带有α—H,极易受到由Q−·活化产生的硫酸根自由基的攻击[34],随着开环反应的发生,逐渐生成中间产物P5。由于P5分子中含有脂基,可发生水解反应,生成P9,进而被开环氧化生成R—COOH和甲醇。这些小分子单链物质进一步被自由基氧化,分解为小分子有机酸等物质。此外,在RB19分子结构中,除了C和H外,有机杂原子N(—NH)和S(—SO3)在氧化降解反应中也会被矿化为无机离子SO42−、NO3−和NH4+[35]。由LOVATO等[36]的氧化体系降解路径结果可以看出,在采用臭氧紫外线技术降解RB19时,反应30 min后,溶液毒性才开始降低,且降解过程中出现了具有较大毒性的苯甲醇、苯胺等中间产物,但在光催化降解条件下,反应25 min时,光催化体系溶液毒性已经开始降低,中间产物更为简单,无苯酚残留,仅检测出P6等小分子物质,且出现时间较早。由此可以推断,随着Q−·蒽醌类染料光降解过程中的产生,通过Q−·促进过硫酸盐活化的作用,提高了溶液中羟基与硫酸根自由基的含量,简化并促进了RB19的降解过程,有效降低了溶液毒性。
-
1)在vis/g-C3N4/BiOBr/PS体系降解RB19的过程中,通过光照蒽醌染料所激发出的Q−·不仅能够激活PS,而且与 H2Q和Q的循环转化有效加强了体系的光催化效应,从而提高了体系的氧化能力。
2)因素对体系影响的强弱顺序为材料投加量>初始pH>RB19初始质量浓度>过硫酸盐投加量>光照强度,其中材料投加量对实验效果影响最为显著。
3) RB19的快速降解主要归因于体系内所产生的Q−·强化了光催化剂对PS的活化效果。采用vis/g-C3N4/BiOBr/PS体系处理后的RB19废水毒性较低,具有较好的环境效益应用前景。
可见光/石墨相氮化碳/溴化氧铋/过二硫酸盐体系高效降解活性蓝19的增强效应及路径
Enhancement effect and path of vis/g-C3N4/BiOBr/PS system for efficient degradation of active blue 19
-
摘要: 为探究光催化降解蒽醌染料活性蓝19(RB19)过程中蒽醌的光敏特性对去除率的影响,以石墨相氮化碳/溴化氧铋(g-C3N4/BiOBr)为光催化材料,引入光照(vis)与过二硫酸盐(PS),构成协同催化氧化体系,考察光激发产物半醌自由基(Q−·)的形成及其参与、增强体系氧化能力的作用机制,采用单因素(材料投加量、初始pH、活性蓝19初始质量浓度、过硫酸盐投加量、光照强度)分析方法,探究Q−·增强效应的影响,使用降解动力学方法及LC/MS评估降解后废水的毒性。结果表明:Q−·的形成不仅加速了过硫酸盐的活化过程,Q−·与氢醌(H2Q)、醌(Q)形成的循环作用也强化了材料的光催化效应,在模拟太阳光照射下(300 W),催化剂用量为0.1 g·L−1和PS投加量为400 mg·L−1时,Q−·引发的长链式自由基反应使该体系在 80 min内对40 mg·L−1的RB19的降解率可达到100%;反应条件对催化效果影响的大小顺序为材料投加量>初始pH>RB19初始质量浓度>过硫酸盐投加量>光照强度;Q−·中间体的形成有效提高了体系内自由基的含量,是反应后废水毒性显著降低的主要原因。由此可知,体系内Q−·所引发的自降解自循环、长链式自由基效应是实现RB19高效降解的主要因素。本研究结果可为开发蒽醌类染料废水处理技术的开发及实际应用提供参考。
-
关键词:
- 可见光/石墨相氮化碳/溴化氧铋/过二硫酸盐 /
- 高级氧化 /
- 半醌自由基 /
- 蒽醌染料活性蓝19 /
- 光催化
Abstract: To investigate the influence of the photosensitive properties of anthraquinone on the removal efficiency of anthraquinone-based Reactive Blue 19 (RB19) by photocatalytic degradation, the graphitic phase carbon nitride/bismuth bromide oxide (g-C3N4/BiOBr) was used as the photocatalytic material, and light (vis) together with peroxydisulfate (PS) were introduced, and a synergistic catalytic oxidation system was built. The formation of semiquinone radicals (Q−·) during the degradation process, and the mechanism of their participation and enhancement of the oxidation capacity were investigated. The effect of Q−·-enhancement effect was investigated by single factor (material dosing, initial pH, initial mass concentration of RB19, PS dosing, and light intensity) analysis method. The toxicity of the degraded wastewater was assessed by the degradation kinetics method and LC/MS. The results showed that the formation of Q−· not only accelerated the activation of PS, but also enhanced the photocatalytic effect of the material due to its cyclic action with hydroquinone (H2Q) and quinone (Q). Under the simulated sunlight (300 W), at the catalyst dosage of 0.1 g·L−1 and the PS dosage of 400 mg·L−1, the long chain radical reaction initiated by Q−· in the process of deriving other free radicals enabled the system to completely degrade RB19 with initial concentration of 40 mg·L−1 within 80 min. The influence of the four factors followed the order: material dosage > initial pH > RB19 initial concentration > light intensity. The significant reduction of wastewater toxicity after the reaction was mainly attributed to the formation of semi-quinone radical intermediates, which could effectively increase the content of free radicals in the system. In conclusion, the self-degradation, self-cycling and long-chain radical effect induced by Q−· within the system was the main reason for achieving an efficient degradation of RB19. The results of this study can provide a reference for the development and practical application of anthraquinone dyestuff wastewater treatment technology. -

-
表 1 不同单因素下的动力学拟合结果
Table 1. Fitting results of different single factor dynamics
影响因素 数值 kobs/min−1 RB19初始质量浓度 20 mg·L−1 0.030 40 mg·L−1 0.026 60 mg·L−1 0.016 80 mg·L−1 0.010 初始pH 3 0.010 6 0.016 9 0.010 11 0.004 光催化剂投加量 0.05 g·L−1 0.009 0.1 g·L−1 0.017 0.2 g·L−1 0.022 0.3 g·L−1 0.027 PS投加量 50 mg·L−1 0.014 100 mg·L−1 0.016 200 mg·L−1 0.018 400 mg·L−1 0.020 光照强度 2 W·m−2 0.015 5 W·m−2 0.016 7 W·m−2 0.016 -
[1] 钱俊, 刘福强, 胡大波, 等. 蒽醌系染料废水的3R处理技术进展与展望[J]. 环境科技, 2009, 22(2): 66-69. doi: 10.3969/j.issn.1674-4829.2009.02.019 [2] 苏营营, 于艳卿, 杨沛珊, 等. 纳米TiO2/硅藻土光催化降解蒽醌染料废水的研究[J]. 中国环境科学, 2009, 29(11): 1171-1176. doi: 10.3321/j.issn:1000-6923.2009.11.009 [3] 丁绍兰, 李郑坤, 王睿, 等. 微电解及微电解芬顿氧化处理染料废水的中试研究[J]. 中国皮革, 2009, 38(11): 5-8. [4] MASI F, RIZZO A, BRESCIANI R, et al. Lessons learnt from a pilot study on residual dye removal by an aerated treatment wetland[J]. Science of the Total Environment, 2019, 648: 144-152. doi: 10.1016/j.scitotenv.2018.08.113 [5] 龚丹. 蒽醌染料的TiO2光催化降解产物分析及其降解过程研究[D]. 武汉: 武汉纺织大学, 2013. [6] GAO Y W, LI S M, LI Y X, et al. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate[J]. Applied Catalysis B:Environmental, 2017, 202: 165-174. doi: 10.1016/j.apcatb.2016.09.005 [7] CAI T, LIU Y T, WANG L L, et al. Activation of persulfate by photoexcited dye for antibiotic degradation: Radical and nonradical reactions[J]. Chemical Engineering Journal, 2019, 375: 122070. doi: 10.1016/j.cej.2019.122070 [8] NIE G, XIAO L. New insight into wastewater treatment by activation of sulfite with photosensitive organic dyes under visible light irradiation[J]. Chemical Engineering Journal, 2020, 389: 123446. doi: 10.1016/j.cej.2019.123446 [9] FANG G, GAO J, DIONYSIOU D D, et al. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs[J]. Environmental Science & Technology, 2013, 47(9): 4605-4611. [10] ROUTOULA E, PATWARDHAN S V. Degradation of anthraquinone dyes from effluents: A review focusing on enzymatic dye degradation with industrial potential[J]. Environmental Science & Technology, 2020, 54(2): 647-664. [11] 翟文琰. g-C3N4/BiOBr/PS光催化氧化体系降解蒽醌类染料废水的机理研究[D]. 武汉: 武汉理工大学, 2021. [12] 朱维晃, 杨瑞, 王宏伟. 蒽醌活化过硫酸盐降解罗丹明B[J]. 环境化学, 2015, 34(10): 1948-1954. doi: 10.7524/j.issn.0254-6108.2015.10.2015030904 [13] 朱维晃, 袁博, 杨瑞. 蒽醌类溶解有机质和模拟太阳光耦合驱动下的Fenton法对罗丹明B脱色特征的影响因素研究[J]. 环境科学学报, 2015, 35(2): 508-513. doi: 10.13671/j.hjkxxb.2014.0762 [14] 朱维晃, 王宏伟, 刘文奇. 对苯醌活化过硫酸盐降解罗丹明B的动力学特征及其影响因素[J]. 中国环境科学, 2016, 36(6): 1732-1737. doi: 10.3969/j.issn.1000-6923.2016.06.019 [15] LI R, CAI M, LIU H, et al. Thermo-activated peroxydisulfate oxidation of indomethacin: Kinetics study and influences of co-existing substances[J]. Chemosphere, 2018, 212: 1067-1075. doi: 10.1016/j.chemosphere.2018.08.126 [16] GAO F R, CHEN J C, ZHAO J Z, et al. Z-scheme heterojunction g-C3N4@pda/BiOBr with biomimetic polydopamine as electron transfer mediators for enhanced visible-light driven degradation of sulfamethoxazole[J]. Chemical Engineering Journal, 2020, 386: 124014. doi: 10.1016/j.cej.2020.124014 [17] ZHU B Z, HAR-EL R, KITROSSKY N, et al. New modes of action of desferrioxamine: Scavenging of semiquinone radical and stimulation of hydrolysis of tetrachlorohydroquinone[J]. Free Radical Biology & Medicine, 1998, 24(2): 360-369. [18] BEN-ZHAN Z, HONG-TAO Z, BALARAMAN K, et al. Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: An ESR spin trapping study[J]. Free Radical Biology and Medicine, 2002, 32(5): 465-473. doi: 10.1016/S0891-5849(01)00824-3 [19] ZHANG B, HU X Y, LIU E Z, et al. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity[J]. Chinese Journal of Catalysis, 2021, 42(9): 1519-1529. doi: 10.1016/S1872-2067(20)63765-2 [20] GENG X, WANG L, ZHANG L, et al. H2O2 production and in situ sterilization over a ZnO/g-C3N4 heterojunction photocatalyst[J]. Chemical Engineering Journal, 2021, 420(1): 129722. [21] QU J N, DU Y, FENG Y B, et al. Visible-light-responsive K-doped g-C3N4/BiOBr hybrid photocatalyst with highly efficient degradation of rhodamine B and tetracycline[J]. Materials Science in Semiconductor Processing, 2020, 112: 105023. doi: 10.1016/j.mssp.2020.105023 [22] ZHANG S, GU P C, MA R, et al. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review[J]. Catalysis Today, 2019, 335: 65-77. doi: 10.1016/j.cattod.2018.09.013 [23] 李扬帆. g-C3N4/BiOBr复合材料的制备及光催化性能研究[D]. 武汉: 武汉理工大学, 2018. [24] SHI Z, ZHANG Y, SHEN X, et al. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light[J]. Chemical Engineering Journal, 2020, 386: 124010. doi: 10.1016/j.cej.2020.124010 [25] HOU Y P, GAN Y Y, YU Z B, et al. Solar promoted azo dye degradation and energy production in the biophotoelectrochemical system with a g-C3N4/BiOBr heterojunction photocathode[J]. Journal of Power Sources, 2017, 371: 26-34. doi: 10.1016/j.jpowsour.2017.10.033 [26] LIU B C, QIAO M, WANG Y B, et al. Persulfate enhanced photocatalytic degradation of bisphenol A by g-C3N4 nanosheets under visible light irradiation[J]. Chemosphere, 2017, 189: 115-122. doi: 10.1016/j.chemosphere.2017.08.169 [27] CHEVES W. Free radical chain reactions[J]. Chemical & Engineering News, 1961, 39(40): 98-110. [28] 唐建伟. 光电耦合催化氧化处理高氯3, 4-二甲基苯胺废水的机理研究[D]. 武汉: 武汉理工大学, 2019. [29] 商李金. 提高C. I. 活性蓝19应用性能的研究[D]. 上海: 东华大学, 2012. [30] 于辉, 金春姬, 王鹏远, 等. Fe2+与Fe0活化过二硫酸盐降解活性艳蓝KN-R[J]. 环境科学研究, 2015, 28(1): 88-95. [31] 蒋梦然. 铁碳微电解耦合微生物深度处理印染废水除碳脱氮研究[D]. 上海: 东华大学, 2015. [32] 傅世磊. 水中纳米材料和重金属对大肠杆菌的毒性研究[D]. 杭州: 浙江农林大学, 2015. [33] WANG J, WANG S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism[J]. Chemical Engineering Journal, 2020, 401: 126158. doi: 10.1016/j.cej.2020.126158 [34] HE W W, JIA H M, WAMER W G, et al. Predicting and identifying reactive oxygen species and electrons for photocatalytic metal sulfide micro-nano structures[J]. Journal of Catalysis, 2014, 320: 97-105. doi: 10.1016/j.jcat.2014.10.004 [35] 李海燕, 龚丹. 蒽醌染料的矿化过程及其相关中间产物分析[J]. 安全与环境学报, 2016, 16(2): 254-257. [36] LOVATO M E, FIASCONARO M L, MARTÍN C A. Degradation and toxicity depletion of RB19 anthraquinone dye in water by ozone-based technologies[J]. Water Science and Technology, 2017, 75(4): 813-822. doi: 10.2166/wst.2016.501 -



 下载:
下载: