-
抗生素在我国被广泛应用于水产、畜牧、养殖、医疗等行业,抗生素滥用造成的环境污染和生态风险越来越受到重视[1]。水产、畜牧、养殖、医疗等行业产生的废水经过处理排入自然水体,由于处理过程中无法去除抗生素污染物,往往会对河流、湖泊造成抗生素污染[2],水源地受到抗生素污染,对人体健康和生态环境会造成严重的危害。
磺胺甲恶唑(C10H11N3O3S,简称SMX)是一种非常典型的磺胺类抗生素,近年来常常在废水、湖泊、河流中被检出[3],因此,去除废水与自然水体中SMX十分重要。不同于传统的化学法、生物法、膜处理法,高级氧化技术(AOPs)处理磺胺类污染物效果好且不会产生二次污染[3]。芬顿法(Fenton)属于高级氧化技术的一种,利用亚铁盐、过氧化氢(H2O2)在催化剂的作用下产生活性羟基自由基(·OH),可以有效去除废水中的SMX[4]。电芬顿反应(electro-Fenton)的原理是溶解氧在阴极接受电子,还原生成H2O2,电芬顿体系能实现H2O2的阴极原位再生,处理过程中不需额外投加H2O2,处理过程易于控制且经济性良好[5-6]。
金属有机骨架(MOFs)是一种由机配体和金属离子组成的新型多孔材料,具有比表面积大、孔隙率高、孔结构可调等优点[7]。近年来,MOFs催化生成羟基自由基从而降解有机物的研究逐渐成为热点[8]。其中铁基MOFs作为芬顿反应催化剂产生·OH已被广泛用于去除水中有机污染物[9]。MIL-88B(Fe)是由对苯二甲酸 (C8H6O4,简称BDC)和铁三聚八面体簇(Fe3-μ3-OXO)构成的一种三维多孔铁基MOFs材料[10],MIL-88B(Fe)作为非均相芬顿催化剂用于催化降解SMX处理效率高、性能优异[11],但单一的MIL-88B(Fe)存在导电性较差的问题。
本文采用有机酸对MIL-88B(Fe)进行刻蚀,在晶体表面构造不饱和金属位点,对MOFs材料的结构和表面性质进行了调控,从而制备出高导电性、高活性且结构稳定的高效自组装的缺陷MOFs催化剂[12],并将制得的缺陷MOFs作为电芬顿催化剂在电芬顿体系下降解水中SMX,并利用扫描电子显微镜(SEM)、X射线衍射技术(XRD)、X射线光电子能谱(XPS)技术对缺陷MOFs催化剂的理化性质进行了表征分析,深入研究了缺陷MOFs催化剂的比表面积、孔结构、电化学性能、降解动力学特征。
-
六水合三氯化铁(FeCl3·6H2O)、对苯二甲酸(C8H6O4)、甲酸(HCOOH)、N,N-二甲基甲酰胺(DMF)、无水乙醇(C2H5OH)、萘酚(Naphthol)、硫酸钠(Na2SO4)、磺胺甲恶唑(SMX)、硫酸(H2SO4)、冰乙酸(CH3COOH)、乙二胺四乙酸二钠(EDTA-2Na)、异丙醇(C3H8O,简称IPA)、对苯醌(C6H4O2,简称BQ)
-
根据文献[13]中提到的合成方法制备MIL-88B(Fe):称量0.756 g (2.770 mmol) FeCl3·6H2O 和0.231 g (1.385 mmol) 对苯二甲酸,溶解于60 mL DMF 中。在室温下磁力搅拌30 min后,将混合溶液转移至高压反应釜中,置于150 ℃恒温加热2 h。待加热结束后取出反应釜,自然冷却至室温,将所得固体离心分离并用乙醇洗涤数次。洗涤结束后将材料置于真空干燥箱中60 ℃条件下干燥12 h,干燥结束后取出材料,使用玛瑙研钵研磨后得到MIL-88B(Fe)粉末材料。
-
在所合成的MIL-88B(Fe)中加入有机酸进行刻蚀,可以对原有MOFs材料进行改性,在其表面引入不饱和金属位点,进而制备出具备高导电性、高活性且结构稳定的高效自组装的缺陷MOFs催化剂[14]。
本研究使用甲酸刻蚀MOFs材料从而引入缺陷,缺陷MOFs催化剂合成方法为:称量0.756 g (2.770 mmol) FeCl3·6H2O、0.231 g (1.385 mmol) 对苯二甲酸和 0.230~0.690 g (5.00~15.0 mmol) 甲酸,溶解于60 mL DMF中。在室温下磁力搅拌30 min后,将混合溶液转移至高压反应釜中,置于150 ℃恒温加热2 h。待加热结束后取出反应釜,自然冷却至室温,将所得固体离心分离并用乙醇洗涤数次。洗涤结束后将材料置于真空干燥箱中60 ℃条件下干燥12 h,干燥结束后取出材料,使用玛瑙研钵研磨后得到缺陷MOFs粉末材料。
使用5.0 mmol甲酸刻蚀制得的缺陷MOFs催化剂称为5A-MIL-88(Fe),使用10.0 mmol甲酸刻蚀制得的缺陷MOFs催化剂称为10A-MIL-88(Fe),使用15.0 mmol甲酸刻蚀制得的缺陷MOFs催化剂称为15A-MIL-88(Fe)。
-
本研究降解以磺胺甲恶唑(SMX)为代表的抗生素污染物,25 mg·L−1 SMX溶液的配制方法如下:配制好0.1 mol·L−1的Na2SO4 溶液,称取12.5 mg SMX于烧杯内,加入0.1 mol·L−1 Na2SO4溶液溶解SMX,缓慢加入0.5 mol浓硫酸,随后用保鲜膜封住烧杯口,并用铝箔完全包裹住烧杯,使用磁力搅拌器搅拌12 h直至溶液完全澄清。待搅拌结束后将澄清液移至500 mL容量瓶中,使用0.1 mol·L−1 Na2SO4溶液定容。
-
将催化剂材料涂附在2 cm×2 cm大小的碳毡上,称量10 mg材料粉末,溶解于1 mL无水乙醇中,超声20 min,结束后加入30 μL 5%(质量分数)萘酚溶液,再次超声20 min。超声结束后使用滴管将液体逐滴滴在碳毡上,使液滴均匀分布在碳毡表面从而将材料完全涂附在碳毡上。碳毡电极作为本研究中MOFs和缺陷MOFs催化剂的载体,在先前的研究中呈现出比表面积大、吸附能力强、导电性能良好的优点[15]。
在降解池中按比例加入25 mg·L−1 SMX溶液和0.1 mol·L−1 Na2SO4溶液,金属铂片和碳毡插入到降解池液面以下,碳毡连接直流稳压电源的阴极,金属铂片连接直流稳压电源的阳极,控制电流为40 mA,电压保持在3.5 V。降解过程中,使用磁力搅拌器低速搅拌降解池内溶液,持续向阴极通氧气。
降解实验进行到0、10、20、30、40、60、90、120 min的时间,从降解池中取样,取出的样品经0.22 μm玻璃纤维滤头过滤后使用高效液相色谱仪(Waters 2695,美国 Waters公司)分析。样品过0.45 μm微孔滤膜后进入C18色谱柱( 4.6 mm×250 mm, 5 μm )进行分离,色谱柱温度为(25±5) ℃,使用标准工作曲线法进行定量,流动相为1% 冰乙酸,流速为1 mL·min−1,进样体积为10 μL,测量波长为289 nm处样品的吸光度,根据标准曲线计算样品浓度。SMX去除率按式(1)进行计算。
式中:η为SMX去除率,%;C0为SMX初始质量浓度,mg·L−1;Ct为t时刻对应的SMX质量浓度, mg·L−1。
-
本实验采用X射线衍射仪(XRD,Bruker D8)对材料结构进行分析;采用扫描电子显微镜(SEM,Merlin德国 卡尔·蔡司公司)观察材料晶形、晶貌;采用X射线光电子能谱(XPS,Thermo Scientific K-Alpha)对材料表面的元素组成及元素化学状态进行分析。
-
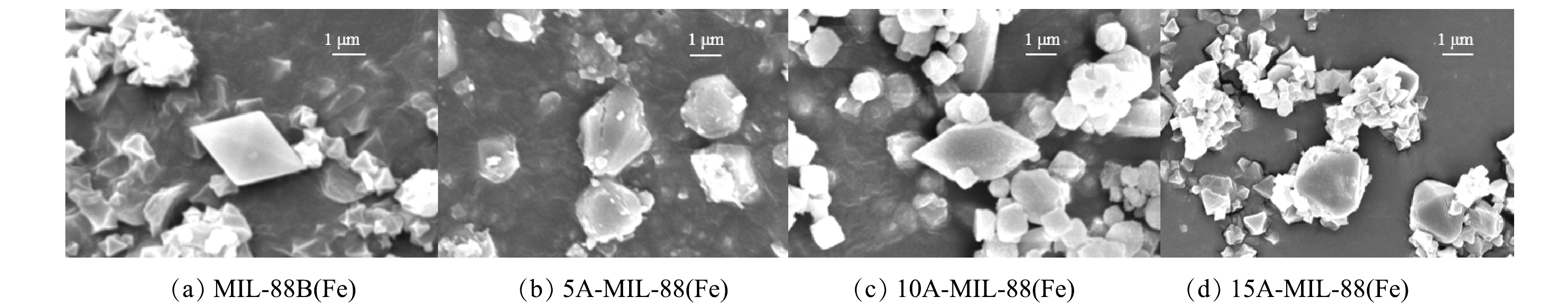
1) SEM分析。采用扫描电镜(SEM)观察催化剂材料的晶体形貌与结构。由图1(a)可见,MIL-88B(Fe) 的晶体结构呈均匀、高度对称的纺锤体形状,这与先前研究中的描述一致[16]。由图1(b)可见,5A-MIL-88(Fe)的晶体表面粗糙,出现明显的缺陷与孔隙结构,纺锤体棱角被有机酸蚀去,比表面积较之甲酸刻蚀前增大。由图1(c)可见,随着刻蚀甲酸量的增大,10A-MIL-88(Fe)晶体基本保留刻蚀前的纺锤体形状,晶粒逐渐成为球形且分布分散。由图1(d)可见,随着刻蚀甲酸量的进一步增大,15A-MIL-88(Fe)晶体难以生长成完整的纺锤体形状,晶粒分布高度分散。5A-MIL-88(Fe)、10A-MIL-88(Fe)、15A-MIL-88(Fe)材料的SEM分析结果表明,不同甲酸用量的刻蚀程度不同,过高的甲酸用量会影响缺陷MOFs材料的结晶度。
2) XPS分析。采用X射线光电子能谱(XPS)对催化剂材料的元素组成和电子层结构进行分析。由图2(a)可知,MIL-88B(Fe)和5A-MIL-88(Fe)主要组成元素均为C、O、Fe元素,此外,5A-MIL-88(Fe)中Fe元素的能谱峰强度高于MIL-88B(Fe),这表明5A-MIL-88(Fe)材料表面Fe元素含量增加,Fe元素暴露更充分。由图2(b)可知,MIL-88B(Fe)结合能为711.08 eV (Fe 2p3/2)、723.88 eV (Fe 2p1/2),Fe 2p3/2、Fe 2p1/2对应的卫星峰峰值分别出现在结合能为715.88 eV、728.68 eV处,这与先前研究中呈现的结果基本一致[17]。依据峰型特征判断,MIL-88B(Fe)中Fe元素为Fe(Ⅲ)、Fe(Ⅱ)混杂形态。5A-MIL-88(Fe)结合能为710.98 eV (Fe 2p3/2)、723.78 eV (Fe 2p1/2),Fe 2p3/2、Fe 2p1/2对应的卫星峰峰值分别出现在结合能为715.88 eV、728.68 eV处。依据峰型特征判断,材料中Fe元素为Fe(Ⅲ)、Fe(Ⅱ)混杂形态。5A-MIL-88(Fe)与MIL-88B(Fe)峰面积与峰值出现的位置基本一致,表明5A-MIL-88(Fe)保留了MIL-88B(Fe)不饱和金属位点富集度高、Fe位点电子密度高的优点[18-19]。
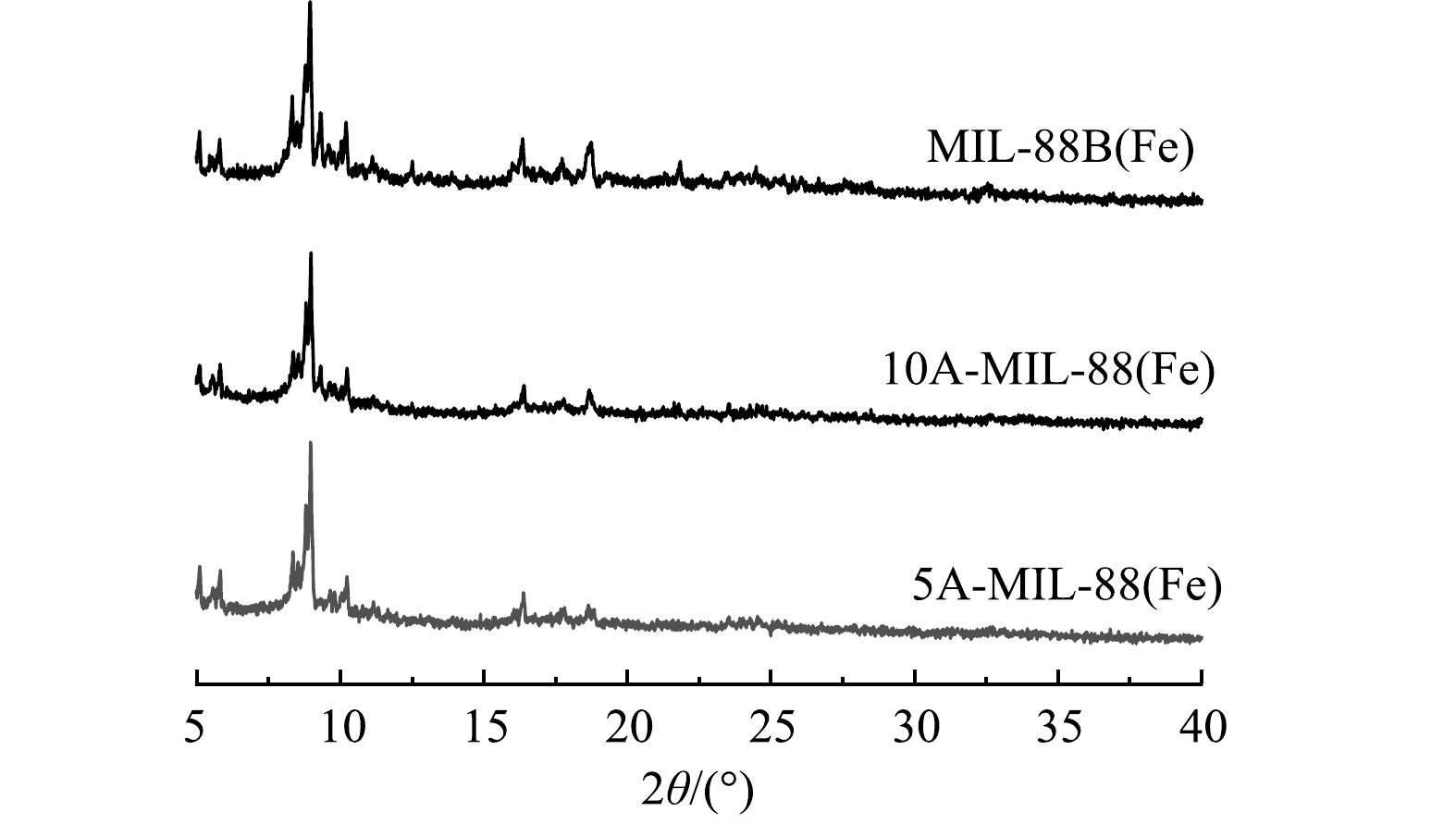
3) XRD分析。采用X射线衍射仪(XRD)对催化剂材料的晶体结构进行分析。由图3可见,MIL-88B(Fe) 在2θ=8.9°处衍射峰峰强最高,材料在2θ=8.9°、9.2°、10.2°、16.4°、18.7°处衍射峰的位置及特征与先前研究中呈现的结果基本一致[20]。5A-MIL-88(Fe)、10A-MIL-88(Fe)衍射峰的位置及特征与MIL-88B(Fe)基本一致,但部分衍射峰 (2θ=16.4°、18.7°)峰强相比MIL-88B(Fe)均略微有所减弱。结合SEM结果可知,缺陷MOFs材料的结构完整性和结晶形态基本保留,甲酸刻蚀没有导致材料晶体结构发生相变,部分衍射峰峰强减弱可能是因为引入缺陷过程中,缺陷MOFs材料结构轻微变形。
-
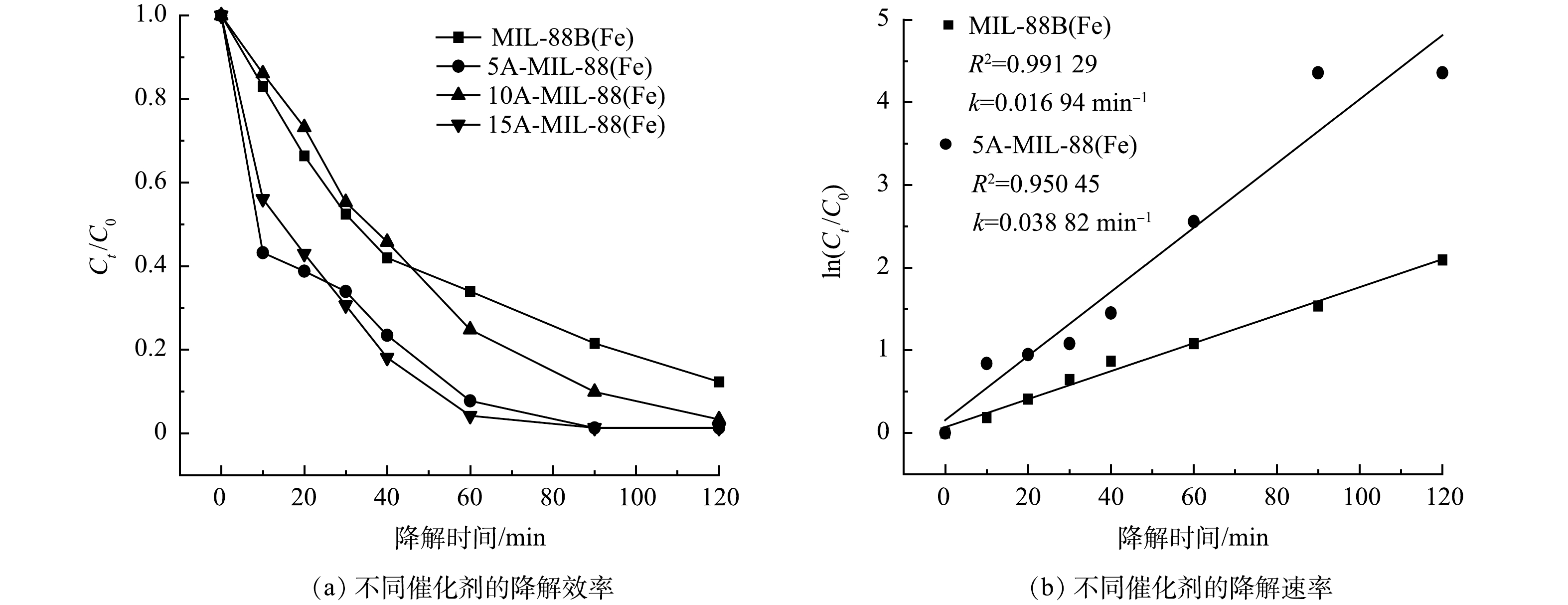
本研究进行了MIL-88B(Fe)催化剂和缺陷MOFs催化剂对10 mg·L−1 SMX的电催化降解实验。由图4(a)可见,反应120 min后,没有经过修饰的MIL-88B(Fe)对SMX去除率为87.67%;相比之下,缺陷MOFs对SMX去除率均高于98%,其中5 mmol甲酸刻蚀制得的5A-MIL-88(Fe)对SMX去除率最高,达98.72%。这是因为甲酸对MOFs材料表面进行修饰后,材料表面存在的不饱和金属位点的位置、数量和暴露程度均发生了改变,从而导致材料孔隙率增加、比表面积增大、电荷转移能力增强、电化学性能和催化性能得到增强[21]。但甲酸的过量添加则会导致MIL-88B(Fe)原有骨架的坍塌,影响其孔道结构,使得催化性能下降,故10A-MIL-88(Fe)、15A-MIL-88(Fe)对SMX去除率低于5A-MIL-88(Fe),这与SEM测试结果相互佐证。由图4(b)可见,MIL-88B(Fe)与缺陷MOFs催化降解SMX反应的动力学拟合曲线符合一级动力学模型,MIL-88B(Fe)对SMX催化降解速率为0.016 94 min−1,相比之下,降解效率最高的5A-MIL-88(Fe)对SMX催化降解速率为0.038 82 min−1,是MIL-88B(Fe)催化降解速率的2.29倍。这同样证明使用甲酸对MOFs刻蚀能够增强材料的电化学性能和催化性能。基于上述实验结果,后期研究均采用5A-MIL-88(Fe)作为最佳催化剂,分析5A-MIL-88(Fe)催化降解SMX的性能。
-
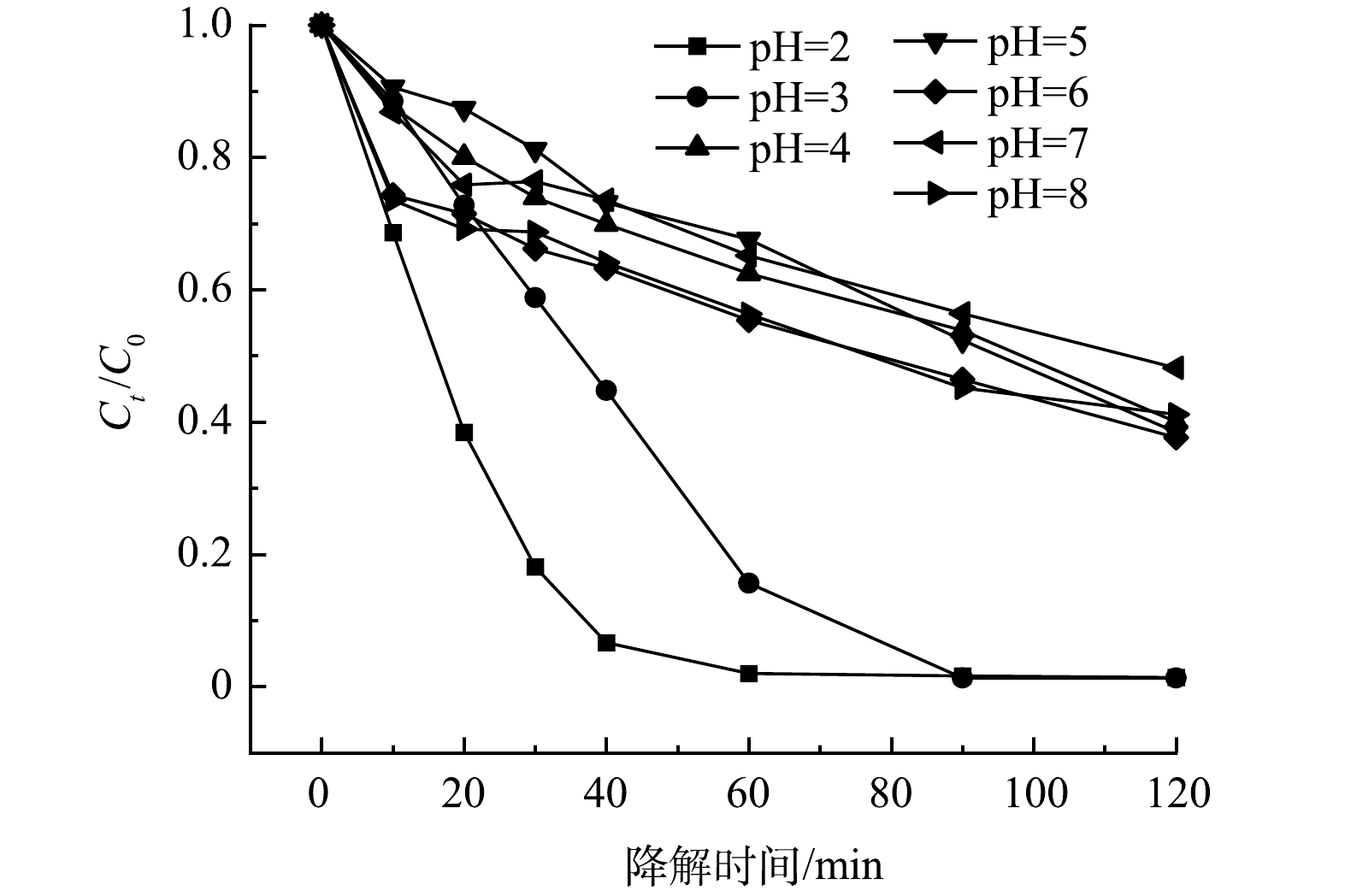
1) pH的影响。pH对5A-MIL-88(Fe)催化降解10 mg·L−1 SMX的影响结果如图5所示。可以看出,当pH为2时,SMX去除率为98.63%;当pH为3时,SMX去除率最高,达到了98.69%;当pH为4时,SMX去除率开始大幅度降低,仅有59.98%;随着环境pH继续升高,当pH为5~8时,SMX去除率均未超过65%。结果表明,5A-MIL-88(Fe)仅能在较强酸性环境中高效降解抗生素污染物,在弱酸性、中性或碱性环境下降解抗生素污染物效率较低。这是因为环境pH会影响溶液中SMX存在形态、材料表面电荷分布、电子转移速率及活性基团的生成速率导致,其中占主要因素的可能是活性基团的生成速率[22-23]。当pH呈弱酸性、中性或碱性时,溶液中H+含量低,不利于阴极产生H2O2,电芬顿体系中阴极产生H2O2的方程如式(2)所示。
当H2O2的产生受到抑制时,活性羟基自由基(·OH)、超氧自由基(·O2−)的产生也会受到影响,电芬顿体系中Fe(Ⅲ)、Fe(Ⅱ)产生·O2H、·OH的反应方程如式(3)和式(4)所示。
·OH和·O2−是电芬顿体系中起主导氧化作用的自由基(见2.6反应机理分析),当体系中·OH和·O2−的含量降低时,SMX的去除率也会随之降低。因此,当pH呈弱酸性、中性或碱性时,SMX去除率低于pH呈较强酸性。
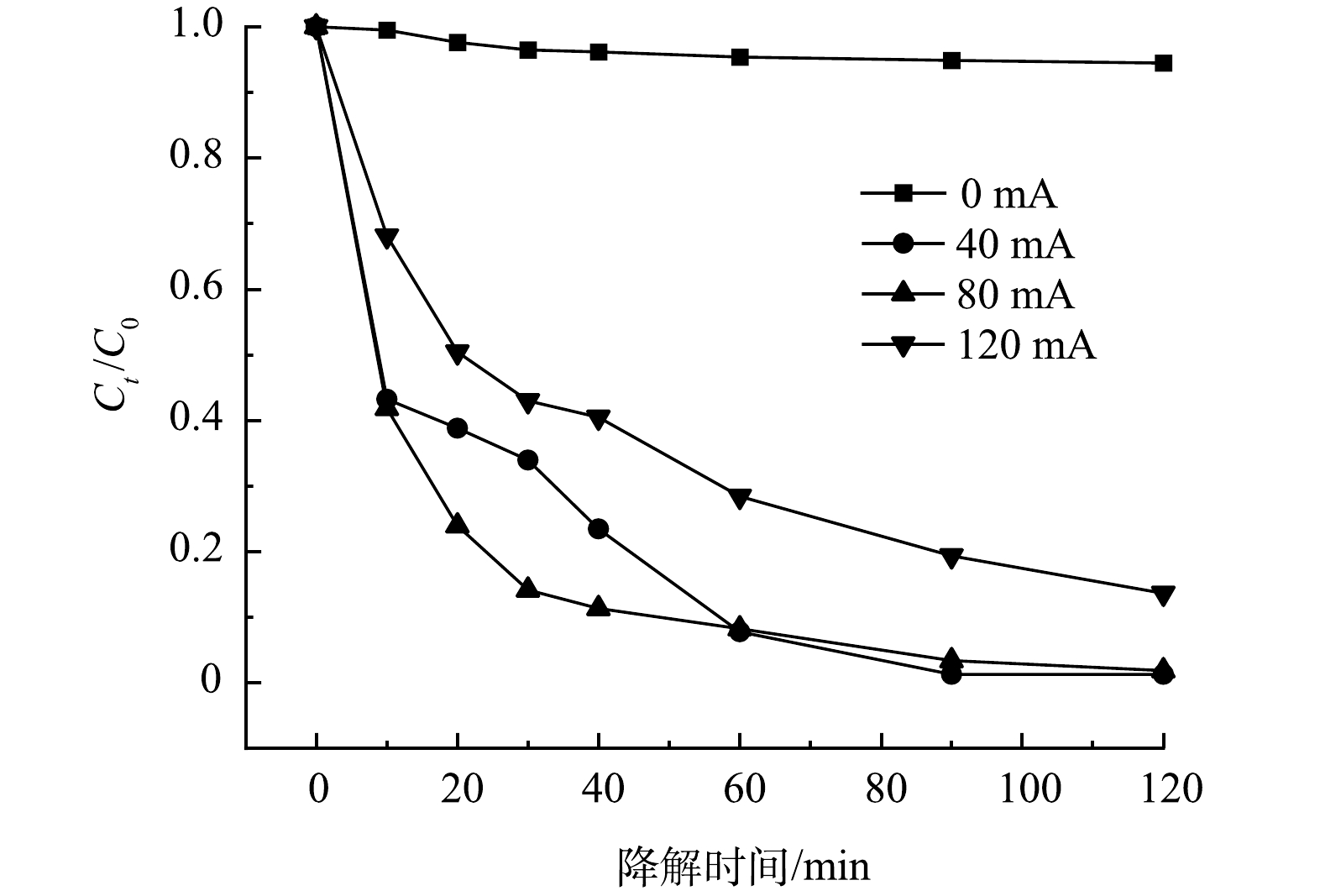
2)反应电流的影响。在不同的反应电流下,5A-MIL-88(Fe)对10 mg·L−1 SMX去除率如图6所示。可以看到,降解体系中没有电流流过(0 mA) 时,SMX几乎未被降解,这说明反应电流是构建SMX催化降解体系的必要条件。当反应电流为40 mA时,SMX去除率达到98.72%;当反应电流为80 mA时,SMX去除率达到98.14%;当反应电流为120 mA时,SMX去除率有所降低,为86.37%。对比反应电流40 mA与80 mA 这2组降解体系,前40 min内,80 mA电流去除率更高,达到了88.68%;后80 min内,40 mA 电流去除率更高。随着反应电流进一步提升至120 mA,SMX去除率降低,可能原因是H2O2在阳极表面(异向过程)或溶液中(均相过程)发生化学分解[24-25],从而造成溶液中·O2H、·OH产生速率降低。当溶液中·O2H、·OH含量降低,SMX去除率随之降低。随着反应电流增大,H2O2发生的分解方程如式(5)和式(6)所示。
结果表明,5A-MIL-88(Fe)催化降解SMX反应有较宽的适宜电流范围(40~80 mA),5A-MIL-88(Fe)材料在不同反应电流下均有较高的稳定性及电化学活性。
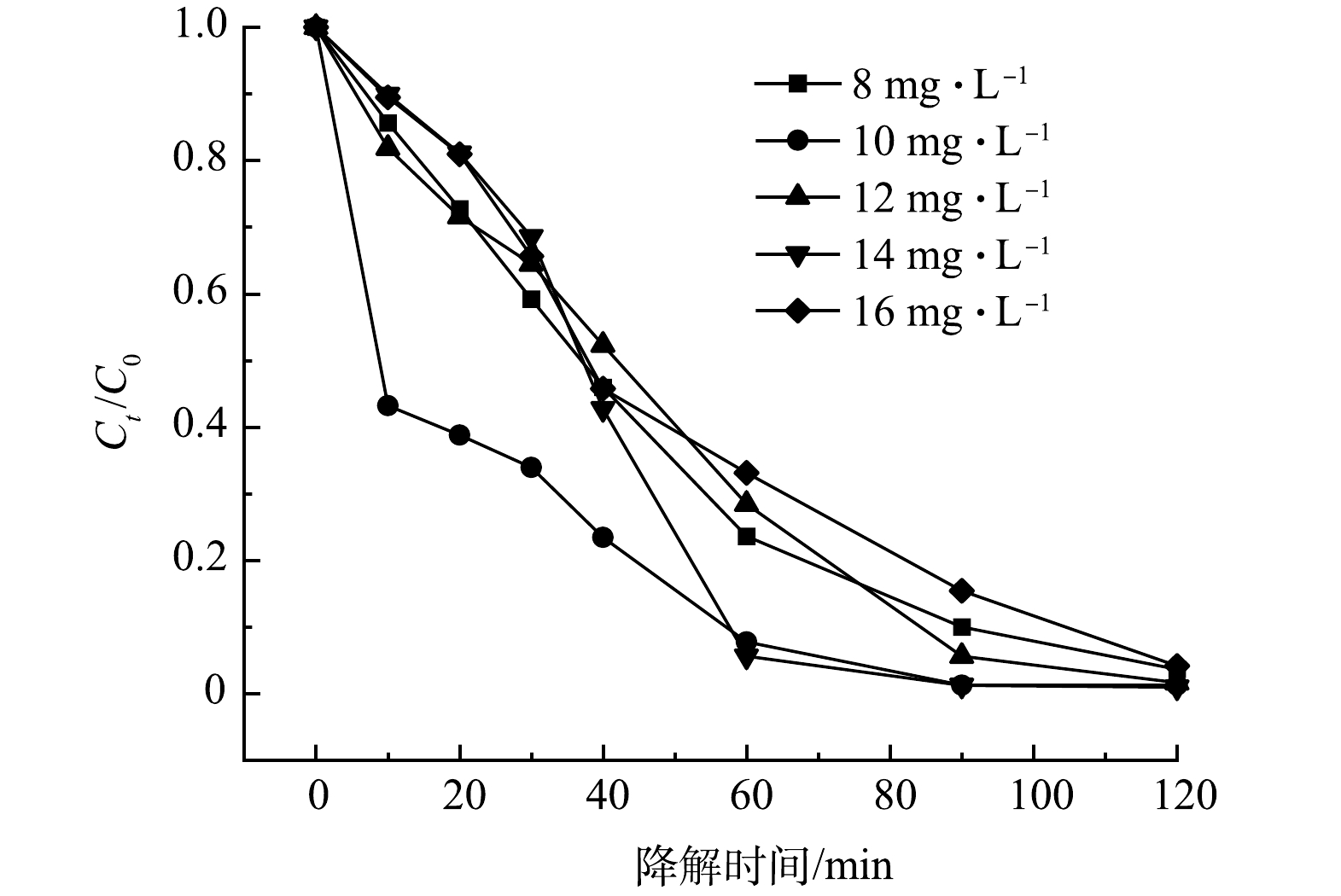
3) SMX初始浓度的影响。5A-MIL-88(Fe)对初始质量浓度为8、10、12、14、16 mg·L−1 SMX去除率如图7所示。可以看到,在120 min内,8~16 mg·L−1 SMX去除率均超过95%,14 mg·L−1 SMX去除率最高,达到99.04%。结果表明,对于一定质量浓度范围内的SMX抗生素污染物,以5A-MIL-88(Fe)作为催化剂构建的电化学降解体系均有较高的去除率,这说明5A-MIL-88(Fe)具有高效的催化降解性能能以及其在废水处理中潜在的应用前景。
-
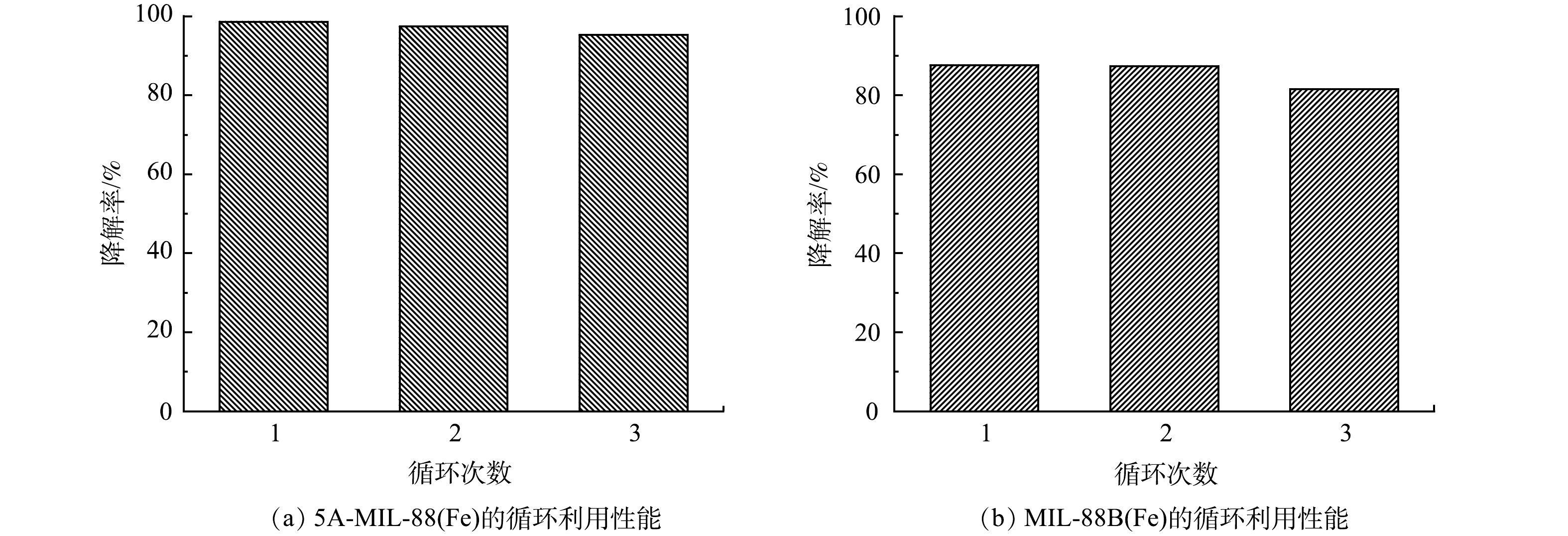
在工业污水处理领域,循环利用性能和稳定性是评价催化剂性能的重要指标,直接影响污水处理的效率和经济效益。控制电流40 mA、电压3.5 V,pH=3条件下,5A-MIL-88(Fe)和MIL-88B(Fe)催化降解10 mg·L−1 SMX的重复利用性能如图8所示。由图8(a)可见,5A-MIL-88B(Fe)连续3次催化降解10 mg·L−1 SMX效率分别为98.69%、97.44%、95.29%;由图8(b)可见,MIL-88B(Fe)连续3次催化降解10 mg·L−1 SMX效率分别为87.67%、87.42%、81.58%。可以看到,5A-MIL-88(Fe)和未经甲酸刻蚀的MIL-88B(Fe)循环利用和稳定性均良好,尤其是5A-MIL-88(Fe),对SMX的去除率始终保持在95%以上。MOFs材料一项显著的优点是结构稳定、重复利用性良好[26],上述实验结果证明,5A-MIL-88(Fe) 材料循环利用和稳定性良好可能是因为基本保持了原有MOFs材料的晶体骨架和结构完整性,这与XRD表征结果相互佐证。良好的循环利用性能为5A-MIL-88(Fe)在工业污水处理中的应用提供了潜能。
-
在电化学反应中,法拉第电流效率是评价电化学反应的一项重要指标,通过计算产生H2O2的电流效率η,从而评价电极反应中消耗的电量与通过电路的总电量的比值。控制电流40 mA,电压3.5 V,pH=3条件下,5A-MIL-88(Fe)催化降解10 mg·L−1 SMX反应的法拉第电流效率η按式(7)计算。
式中:n为矿化每摩尔SMX所需要的电子数,n =61;F为法拉第常数,F=964 86 C·mol−1;V为反应体积,V=0.1 L;ΔC为降解反应前后SMX浓度的衰变,ΔC =9.872 mg·L−1;m为SMX碳原子数目,m=10;I为反应电流,I=0.04 A;t为反应时间,t=2 h。代入数值解得:SMX催化降解反应的法拉第电流利用效率η=16.81%。
在工业污水处理领域,能耗W是决定SMX电化学降解体系可行性的一项重要指标,结合电能的消耗和反应时间计算SMX电化学降解过程的能量消耗。控制电流40 mA,电压3.5 V,pH=3条件下,5A-MIL-88(Fe)催化降解10 mg·L−1 SMX反应的能耗W按公式(8)计算。
式中:U为反应电压,U=3.5 V;I为反应电流,I=0.04 A;t为反应时间,t=2 h;V为溶液体积,V=0.000 1 m3。代入数值解得:SMX催化降解反应能耗W=2.8 kW·h·m−3。
-
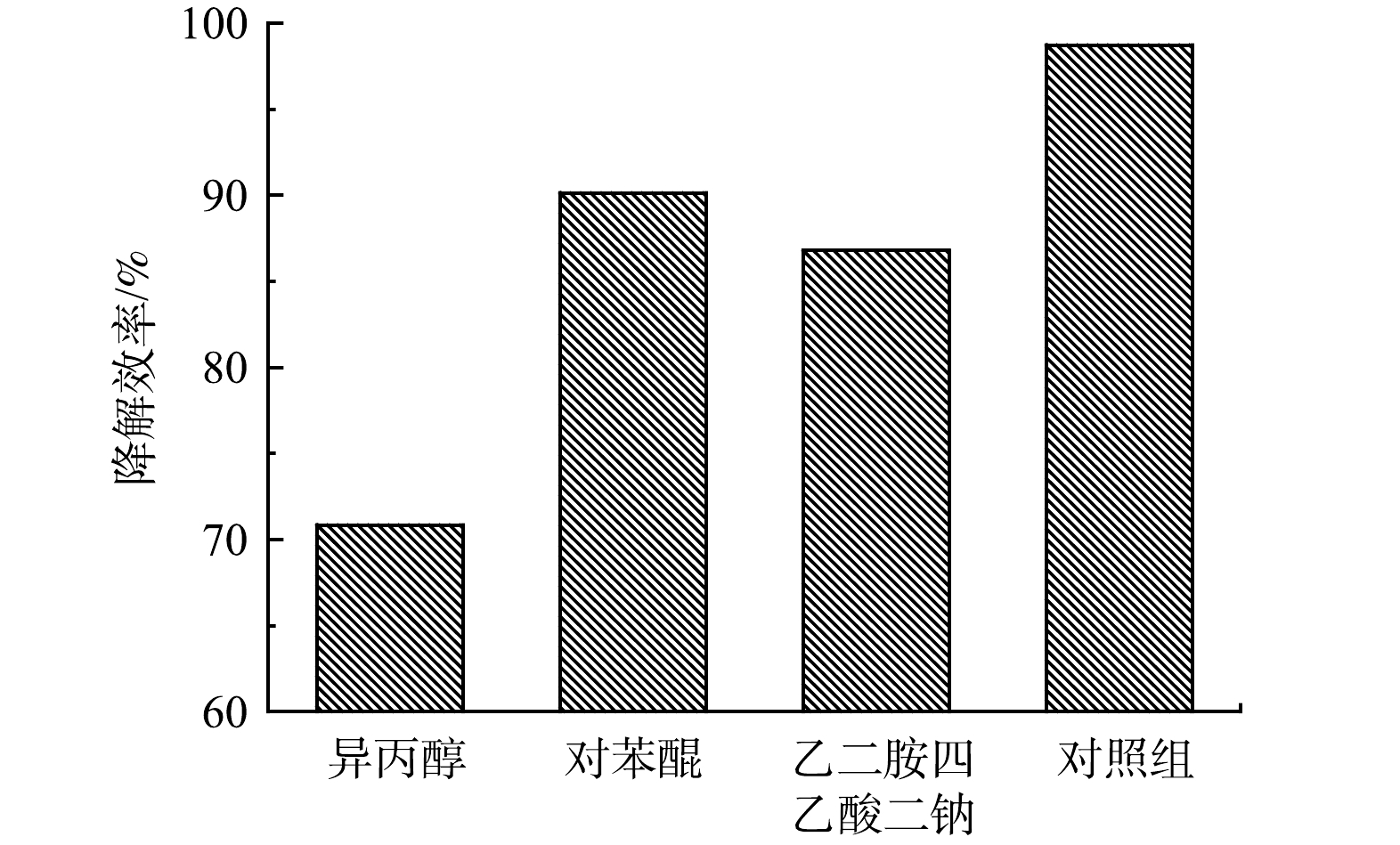
使用0.3 mmol 乙二胺四乙酸二钠(EDTA-2Na)、0.3 mmol 异丙醇(IPA)和对0.3 mmol对苯醌(BQ)分别作为空穴(h+) 、羟基自由基(·OH)和超氧自由基(·O2−)的淬灭剂,以探究SMX催化降解反应中起作用的活性物质以及反应可能的发生机理。加入0.3 mmol淬灭剂后,5A-MIL-88(Fe)对10 mg·L−1 SMX去除情况如图9所示。可以看出,反应进行120 min时,加入IPA的体系中SMX去除率降幅最大,去除率仅70.86%;加入BQ的体系中SMX去除率为90.14%;加入EDTA-2Na的体系中SXM去除率为86.79%。整体来看,以SMX去除率降低幅度由高至低排序为IPA>EDTA-2Na>BQ。结果表明,h+、·OH、·O2−在SMX催化降解过程中起到了共同氧化的作用,其中·OH起到的作用最大。h+具有强氧化性,阴极附近的大量e−将O2还原产生·OH、·O2−协同h+氧化降解SMX。5A-MIL-88(Fe)作为甲酸刻蚀制得的缺陷MOFs材料,保留了原有MOFs材料的多孔结构和内部框架可调功能,能够提供高密度的活性位点以产生h+、·OH、·O2−等活性自由基,在电流流通条件下,5A-MIL-88(Fe)内部分散性良好且可调节的活性位点阵列优化了材料的电子转移数目及电荷传输速率,从而能高效降解水中SMX为代表的抗生素污染物[27-28]。
-
表1列出了其他研究中的催化剂对SMX的去除情况。可以看出,综合考虑SMX初始浓度、降解时间和去除率,5A-MIL-88(Fe)催化降解SMX效果比其他催化剂具有一定的优势。表明5A-MIL-88(Fe)具有处理水中抗生素污染物的应用潜能。
-
1)通过甲酸刻蚀的方式制备了一系列缺陷MOFs,其中5 mmol甲酸刻蚀制得的5A-MIL-88(Fe)对SMX催化降解性能最优异,去除率、降解速率均优于未经刻蚀的MIL-88B(Fe)。以5A-MIL-88(Fe)作为催化剂构建的电芬顿降解体系具有处理废水中抗生素污染物的应用潜能。
2) pH、电流、SMX初始浓度是影响SMX去除率的重要因素。5A-MIL-88(Fe)催化剂对电流有较宽的适应范围、对一定质量浓度范围内波动的SMX均有较高的去除率,但仅能在较强酸性环境下高效催化降解SMX。
3) h+、·OH、·O2−对SMX的催化降解均有贡献,其中·OH的作用最为显著。适量甲酸的刻蚀会在MOFs材料表面引入缺陷位点,影响其形貌结构,从而构造出更多不饱和金属位点、改变Fe位点附近的电子密度、提升材料表面的电子传输速率以产生大量h+、·OH、·O2−,进而增强MOFs材料的导电性和电催化活性。
甲酸刻蚀缺陷MOFs电芬顿高效降解磺胺甲恶唑
Efficient degradation of SMX by electro-Fenton with Formic acid etching defective MOFs
-
摘要: 采用甲酸刻蚀MIL-88B(Fe)制备了一系列缺陷MOFs并用于催化降解水中磺胺甲恶唑(SMX)为代表的抗生素污染物,通过SEM、XPS、XRD分析手段对材料的形貌和结构进行了表征和分析,考察了pH、电流、SMX初始浓度等因素对SMX去除的影响,探究了SMX催化降解反应的动力学特性以及缺陷MOFs材料的可循环利用性和稳定性,探究了SMX催化降解反应的电流利用效率与能耗,通过自由基淬灭实验推测了SMX催化降解反应发生机理。结果证明,缺陷MOFs材料催化降解SMX性能优于未经刻蚀的MIL-88B(Fe),对于10 mg·L−1 SMX,在电流为40 mA、电压为 3.5 V、持续通氧气、搅拌的条件下,反应120 min后,5 mmol甲酸刻蚀制得的5A-MIL-88(Fe)对SMX的去除率可达98.72%。以5A-MIL-88(Fe)作为催化剂,协同电芬顿(Fenton)反应构建的处理体系为水中抗生素污染物高效去除提供参考。
-
关键词:
- 抗生素污染 /
- MIL-88B(Fe) /
- 缺陷MOFs /
- 电芬顿反应 /
- 磺胺甲恶唑
Abstract: A series of defective MOFs were prepared for Sulfamethoxazole (SMX) catalytic degradation by etching MIL-88B(Fe) with formic acid. The catalyst materials were characterized by SEM, XPS and XRD. The effects of pH, electric current, SMX initial concentration on the catalytic degradation efficiency of SMX were investigated, as well as the kinetic characteristics of SMX catalytic degradation reaction and recycling and stability analysis of defective MOFs. In addition, the current utilization efficiency and energy consumption of SMX catalytic degradation reaction were investigated. Free radical scavenger (EDTA-2Na, IPA, BQ) were used to expose the mechanism of SMX catalytic degradation reaction. The results of SMX degradation experiments prove that the performances of defective MOFs are superior to MIL-88B(Fe) without etching. Under the conditions of 40 mA current, 3.5 V voltage, continuous oxygen and agitation, the degradation efficiency of 10 mg·L−1 SMX could reach 98.72% after 120 min, with the catalytic action of 5A-MIL-88(Fe) etched by 5 mmol formic acid. The treatment system constructed with 5A-MIL-88(Fe) as catalyst and electro-Fenton oxidation reaction as core provides a new idea for the efficient removal of antibiotic contaminants in waste water.-
Key words:
- antibiotic contaminants /
- MIL-88B(Fe) /
- defective MOFs /
- electro-Fenton oxidation /
- SMX
-

-
-
[1] 林靖钧, 李瑞雪, 林华, 等. 我国水产养殖水体中抗生素的污染特征[J]. 净水技术, 2022, 41(3): 12-19. doi: 10.15890/j.cnki.jsjs.2022.03.002 [2] 张焕军, 王席席, 李轶. 水体中抗生素污染现状及其对氮转化过程的影响研究进展[J]. 环境化学, 2022: 1-14. doi: 10.7524/j.issn.0254-6108.2021091902 [3] 李佳琳, 巨龙, 崔梦, 等. 磺胺类抗生素的污染现状与去除技术研究进展[J]. 安徽农业科学, 2021, 49(21): 27-32. doi: 10.3969/j.issn.0517-6611.2021.21.007 [4] 黄智奔, 陈菊香, 包丽丽, 等. 水中磺胺甲噁唑去除技术的研究进展[J]. 资源节约与环保, 2022(1): 70-73. doi: 10.3969/j.issn.1673-2251.2022.01.020 [5] TIAN Y S, ZHOU M H, PAN Y W, et al. MoS2 as highly efficient co-catalyst enhancing the performance of FeO based electro-Fenton process in degradation of sulfamethazine: Approach and mechanism[J]. Chemical Engineering Journal, 2021, 403: 126361. [6] ZHANG H L, XU Z Y, WANG S L, et al. Preparation of a highly-efficient electro-Fenton cathode material for H2O2 generation and its electrochemical performance in COD removal[J]. International Journal of Electrochemical Science, 2020, 15(12): 12462-12474. [7] XIA T L, LIN Y C, LI W Z, et al. Photocatalytic degradation of organic pollutants by MOFs based materials: A review[J]. Chinese Chemical Letters, 2021, 32(10): 2975-2984. doi: 10.1016/j.cclet.2021.02.058 [8] LIU X, ZHOU Y, ZHANG J, et al. Iron containing metal-organic frameworks: Structure, synthesis, and applications in environmental remediation[J]. ACS applied materials & interfaces, 2017, 9(24): 20255-20275. [9] SUN Q, LIU M, LI K, et al. Facile synthesis of Fe-containing metal-organic frameworks as highly efficient catalysts for degradation of phenol at neutral pH and ambient temperature[J]. CrystEngComm, 2015, 17(37): 7160-7168. doi: 10.1039/C5CE01375E [10] MA M Y, NOEI H, MIENERT B, et al. Iron metal organic frameworks MIL-88B and NH2-MIL-88B for the loading and delivery of the gasotransmitter carbon monoxide[J]. Chemistry:A European Journal, 2013, 19(21): 6785-679. doi: 10.1002/chem.201201743 [11] 王楠楠, 张巍, 修光利. MIL-88B(Fe)非均相芬顿催化降解磺胺甲恶唑[J]. 环境污染与防治, 2020, 42(6): 682-689. doi: 10.15985/j.cnki.1001-3865.2020.06.006 [12] 杨康. MOFs及其复合材料的制备及电化学性能研究[D]. 镇江: 江苏大学, 2020. [13] LI X R, HE X B, YIN F X, et al. NH2-MIL-88B-Fe for electrocatalytic N2 fixation to NH3 with high faradaic efficiency under ambient conditions in neutral electrolyte[J]. Journal of Materials Science, 2020, 55(26): 12041-12052. doi: 10.1007/s10853-020-04777-2 [14] 沈意, 许俊杰, 朱超, 等. 缺陷化金属有机骨架材料的合成及其污染控制应用[J]. 科学通报, 2021, 66(23): 2943-2957. [15] CAI L F, CHEN J J, CHANG L, et al. Adhesion mechanisms and electrochemical applications of microorganisms onto a GO-NH2 modified carbon felt electrode material[J]. Industrial & Engineering Chemistry Research, 2021, 60(11): 4321-4331. [16] SHI L, WANG T, ZHANG H B, et al. An amine-functionalized iron (III) metal-organic framework as efficient visible-light photocatalyst for Cr (VI) reduction[J]. Advanced Science, 2015, 2(3): 1500006. [17] YI Q Y, DU M M, SHEN B, et al. Hollow Fe3O4/carbon with surface mesopores derived from MOFs for enhanced lithium storage performance[J]. Science Bulletin, 2020, 65(3): 233-242. doi: 10.1016/j.scib.2019.11.004 [18] HU X B, LIU B Z, DENG Y H, et al. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution[J]. Applied Catalysis B:Environmental, 2011, 107(3): 274-283. [19] LI X, PI Y, WU L, et al. Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-Iron terephthalate metal-organic framework for MB degradation[J]. Applied Catalysis B:Environmental, 2017, 202: 653-663. doi: 10.1016/j.apcatb.2016.09.073 [20] LAURIER K, VERMOORTELE F, AMELOOT R, et al. Iron (III)-based metal-organic frameworks as visible light photocatalysts[J]. Journal of American Chemical Society, 2013, 135(39): 14488-14491. doi: 10.1021/ja405086e [21] WANG Y X, ZHONG Z, MUHAMMAD Y, et al. Defect engineering of NH2 -MIL-88B(Fe) using different monodentate ligands for enhancement of photo-Fenton catalytic performance of acetamiprid degradation[J]. Chemical Engineering Journal, 2020, 398: 125684. [22] XU B, YANG H, CAI Y, et al. Preparation and photocatalytic property of spindle-like MIL-88B(Fe) nanoparticles[J]. Inorganic Chemistry Communications, 2016, 67: 29-31. doi: 10.1016/j.inoche.2016.03.003 [23] HE H, WANG Y X, LI J, et al. Confined conductive and light-adsorbed network in metal organic frameworks (MIL-88B(Fe)) with enhanced photo-Fenton catalytic activity for sulfamethoxazole degradation[J]. Chemical Engineering Journal, 2021, 427: 131962. [24] 王爱民, 曲久辉, 史红星, 等. 活性碳纤维阴极电芬顿反应降解微囊藻毒素研究[J]. 高等学校化学学报, 2005, 26(9): 1669-1672. doi: 10.3321/j.issn:0251-0790.2005.09.013 [25] ZHAO M C, MAO X D, LI R X, et al. In-situ slow production of Fe2+ to motivate electro-Fenton oxidation of bisphenol A in a flow through dual-anode reactor using current distribution strategy: Advantages, CFD and toxicity assessment[J]. Electrochimica Acta, 2022: 411. [26] ZHANG Y, LI G, LU H, et al. Synthesis, characterization and photocatalytic properties of MIL-53(Fe)-graphene hybrid materials[J]. RSC Advances, 2014, 4(15): 7594-7600. doi: 10.1039/c3ra46706f [27] LI J H, WANG Y S, CHEN Y C, et al. Metal-organic frameworks toward electrocatalytic applications[J]. Applied Sciences-Basel, 2019, 9(12): 2427. doi: 10.3390/app9122427 [28] ZHOU J, DOU Y B, WU X Q, et al. Alkali-etched Ni (II)-based metal-organic framework nanosheet arrays for electrocatalytic overall water splitting[J]. SMALL, 2020, 16(41): 1906564. doi: 10.1002/smll.201906564 [29] 谢欣卓, 钟金魁, 李静, 等. 四氧化三铁负载纳米零价铁类Fenton法降解水中磺胺甲恶唑[J/OL]. 中国环境科学: 1-11. DOI: 10.19674/j.cnki.issn1000-6923.20220314.014. [30] 曾佚浩, 陈运进, 卢耀斌, 等. Cu-Co双金属氢氧化物非均相类芬顿催化剂去除磺胺甲恶唑[J]. 环境工程学报, 2020, 14(9): 2474-2484. doi: 10.12030/j.cjee.202001014 [31] NGUYEN B, HUANG C P, DOONG R A, et al. Visible-light photodegradation of sulfamethoxazole (SMX) over Ag-P-codoped g-C3N4 (Ag-P@UCN) photocatalyst in water[J]. Chemical Engineering Journal, 2019: 384. [32] SHEIJKI S, JEBALBAREZI B, DEHGHANZADEH R, et al. Sulfamethoxazole oxidation in secondary treated effluent using Fe (VI)/PMS and Fe (VI)/H2O2 processes: Experimental parameters, transformation products, reaction pathways and toxicity evaluation[J]. Journal of Environmental Chemical Engineering, 2022, 10(3). [33] FU A, LIU Z B, SUN Z R, et al. Cu/Fe oxide integrated on graphite felt for degradation of sulfamethoxazole in the heterogeneous electro-Fenton process under near-neutral conditions[J]. Chemosphere, 2022: 297. -



 下载:
下载: