-
环境雌激素 (environmental estrogens,EEs) 是具有内分泌干扰效应的类固醇类物质[1]。17β-雌二醇 (17β-estradiol,E2) 是由性腺分泌的一种具有较高生物活性的天然雌激素,可以通过牲畜粪便和污水排放进入水环境,从而进入生物体内,即使较低浓度的E2也会干扰人体及动物体的正常内分泌功能并使生理系统紊乱[2-4]。
目前,常用的E2检测技术主要包括高效液相色谱法 (HPLC) [5-6]、液相色谱-质谱联用法 (LC-MS) [7-8]、气相色谱-质谱联用法 (GC-MS) [9]、免疫分析法[10-11]、电化学分析法[12-13]等。虽然色谱法具有灵敏度高、准确度高的优点,但由于实际样品的复杂性和E2的痕量或超痕量残留,需要经过复杂的预处理过程,因此不适用于现场快速检测[4]。免疫分析法具有较高的灵敏度,但是缺乏特异性且抗干扰能力弱。电化学方法检测E2具有灵敏度高和成本低的优点,但是E2的电化学活性较弱,检测过程中需要较高的氧化电位,这可能会导致电极钝化,造成检测的特异性和可重复性较差。因此,开发一种简便、快速、灵敏度高、特异性好且可实现现场检测雌二醇的方法势在必行。
荧光法因其灵敏度高、重现性好、操作简便等优点受到研究人员的广泛关注[14]。荧光检测方法中使用的荧光元件一般包括荧光染料、量子点和生物荧光团[15]。碳量子点 (CQDs) 凭借其荧光性能稳定、生物相容性好、水溶性好、低毒性、易合成、生产成本低等优点,现已广泛应用于构建环境和食品中有害物质检测的荧光传感器[16-18]。
核酸适配体 (aptamer) 是可以折叠成特定三级空间结构的,通过空间构型互补与目标分子高亲和性、高特异性结合的一段寡核苷酸序列,具有亲和性强、特异性高、合成简单、稳定性好等优点[19-20]。目前,核酸适配体传感器已被广泛应用于金属离子、有机分子、胺肽、蛋白质、以及细胞等方面的检测,而且均表现出良好的检测效果[21-24]。
为减少资源浪费,实现技术的可持续发展,低价值废物转化为高价值生物质的研究越来越受欢迎[25]。甘蔗渣是世界上最大的农作物废弃物之一,具有产量大、产地集中、成本低、性能稳定等优点,有利于工业化生产[26]。由于甘蔗渣具有较高的半纤维素含量和丰富的碳、氧元素[27],且其灰分含量低于其他农业废弃物。因此,其已成为制备碳材料的理想生物质原料[28]。
本研究以甘蔗渣合成的碳量子点作为荧光信号,以核酸适配体作为识别元素,采用EDC/NHS活化法将氨基标记的E2核酸适配体连接到碳量子点表面,构建了一种新型荧光探针,用于水环境中雌二醇的灵敏、快速、特异性检测,并对实际水样进行分析探讨,旨在为基于核酸适配体修饰的碳量子点荧光探针对水体中污染物的检测机理、痕量污染物的高灵敏和特异性检测提供理论依据和技术支持。
-
17β-雌二醇 (E2,≥98%) 、雌三醇 (E3,≥98%) 、双酚 A (BPA,≥99%) 、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐 (EDC,≥99%) 、N-羟基琥珀酰亚胺 (NHS,≥99%) 、Trizma盐酸盐 (Tris-HCl,≥99%) 、氯化钠 (NaCl,≥99%) 、氯化镁六水合物 (MgCl2·6H2O,≥99.0%) 、氯化钾 (KCl,≥99%) 、牛血清蛋白 (BSA,≥98%) 、吐温20 (Tween 20,≥98.5%) 购自西格玛奥德里奇 (上海) 贸易有限公司;炔雌醇 (EE,≥98%) ,吗啉乙磺酸 (MES,≥99%) 购自阿拉丁生化科技有限公司 (中国) 。硫酸 (H2SO4,98%) 、硝酸 (HNO3,68%) 均为分析纯级别。17β-雌二醇核酸适配体序列为5'-AAGGGATGCCGTTTGGGCCCAAGTTCGGCATAGTG-NH2-3',由上海生工生物工程有限公司合成。透析袋 (1 kDa) 购自上海生工生物工程有限公司。所有检测实验均在缓冲溶液 (100 mmol·L−1 Tris-HCl,200 mmol·L−1 NaCl,25 mmol·L−1 KCl,10 mmol·L−1 MgCl2,pH 8.0) 中进行。
-
管式炉 (OTF-1200X,合肥科晶技术材料有限公司) ;行星式球磨机 (XQM-4L,南昌市恒顺化验设备制造有限公司) ;聚四氟内衬高压反应釜 (湖南力辰科技仪器有限公司) ;电子天平 (ME104,梅特勒-托利多 (上海) 有限公司) ;UV-vis (2600,日本岛津公司) ;荧光分光光度计 (RF-5301PC,日本岛津公司) ;TEM (JEOL-2100F,日本电子株式会社公司) ;FT-IR (IR Affinity,日本岛津公司) 。
-
首先将购自水果店的甘蔗渣用去离子水洗净并烘干,利用管式炉在缺氧的条件下将甘蔗渣热解。待甘蔗渣充分碳化后,将得到的碳材料在行星式球磨机中研磨。再利用粒度筛选法得到纳米大小碳颗粒。称取质量为50 mg的碳颗粒,将其均匀分散于6 mL H2SO4和2 mL HNO3的混合液中,于85 ℃条件下超声5 h。随后将混合液放入25 ℃摇床中,以200 r·min−1的转速反应48 h。将酸化处理获得的碳颗粒溶液用去离子水稀释并用氢氧化钠调节pH至中性[29]。最后将其于聚四氟乙烯内衬的高压釜中180 ℃下水热反应5 h。待反应溶液冷却至室温后,经过0.22 μm微孔过滤器过滤并透析处理,去除小分子和盐分,最终获得碳量子点溶液,并避光保存于4 ℃冰箱中待用。
-
利用质量浓度为200 mg·L−1的碳量子点,在MES缓冲体系下 (100 mmol·L−1,pH = 5) ,先后加入10 mg EDC和10 mg NHS,并在25 ℃摇床中充分混匀30 min,以活化碳量子点表面的羧基官能团。在反应体系中加入E2核酸适配体 (1 μmol·L−1) ,摇床内的温度为37 ℃,转速为200 r·min−1,避光反应12 h。将反应后的溶液使用超速冷冻离心机在12 000 r·min−1的条件下,用超纯水彻底清洗4~5次。将所得沉淀物用超纯水定容至450 μL,再加入500 μL的2% BSA (1%) 溶液和50 μL的Tween 20 (0.05%) 溶液,最终反应体系为1 mL。在摇床温度为25 ℃,转速为200 r·min−1的条件下,避光振荡培养2 h。该过程封闭了碳量子点表面未与E2核酸适配体结合的羧基位点,以防后续检测E2时出现非特异性结合。为了去除反应溶液中过量存在的封闭剂,使用与上述相同的离心条件,对反应液彻底离心洗涤4~5次。最终得到的复合物即为CQDs-aptamer荧光探针,保存在4 ℃条件下以备使用。
-
在2 mL的离心管中加入50 mg·L−1的CQDs-aptamer探针和不同质量浓度的17β-雌二醇,使得检测体系中E2的最终质量浓度分别为0、2、5、8、10、20 μg·L−1。充分混匀后,将混合液置于25 ℃,200 r·min−1条件下的摇床内反应30 min。最后将反应后的溶液用荧光分光光度计进行荧光光谱扫描 (激发波长Ex = 475 nm,发射波长Em = 530 nm,激发和发射狭缝均为5 nm) 。此外,荧光探针还加入了雌炔醇 (EE) 、雌三醇 (E3) 和双酚A (BPA) 等内分泌干扰物,考察了CQDs-aptamer探针对E2的选择性。检测实验均设置3个平行样,以保证数据的可靠性和准确性。相对荧光强度的计算方法见式 (1) 。
式中:R为相对荧光强度;F0、F分别为投加污染物前后的荧光强度;F – F0为荧光恢复的增量。
-
为评估构建荧光探针的可行性与实用性,将其应用于自来水和长春市南湖水样品的检测。用0.22 μm的微孔滤头过滤掉2种水样中的大颗粒杂质,加入不同质量浓度的E2标准溶液 (5、8、10 μg·L−1) ,测定荧光强度,平行测定3次,外标法定量并计算加标回收率。
-
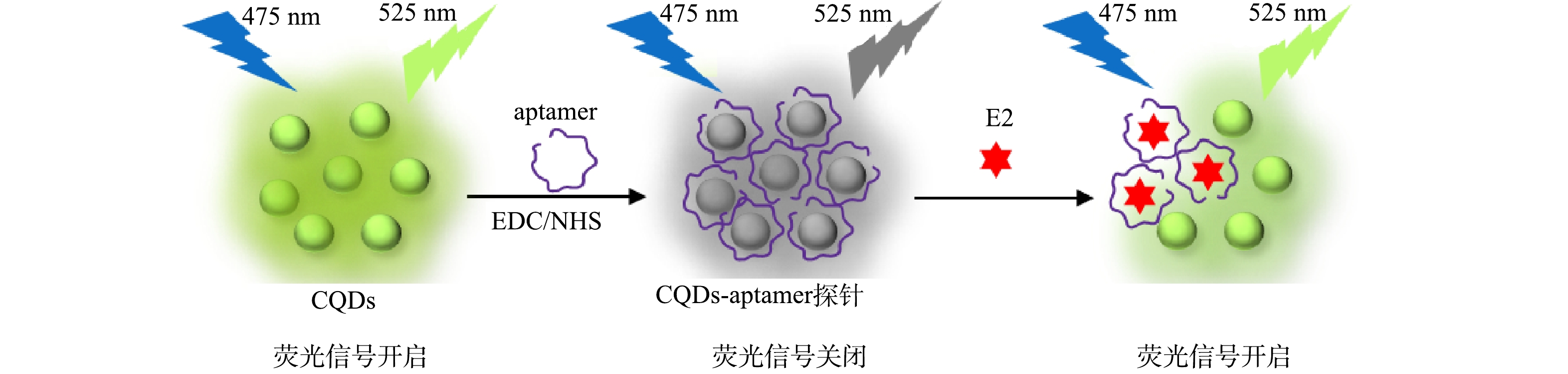
荧光适配体探针快速检测E2的原理见图1。首先以甘蔗渣为碳源,利用高温热解、酸化与水热等方法合成含有丰富表面官能团的发光碳量子点。采用EDC/NHS偶联剂,将带有氨基的核酸适配体修饰于含有羧基的碳量子点表面,合成CQDs-aptamer探针。由于核酸适配体还会通过π-π键结合于碳量子点表面发生静态荧光淬灭[30],因此,相比于荧光碳量子点,探针具有较低的发光特性。向荧光适配体探针加入E2后,E2的特异性核酸适配体以强亲和力结合E2,使其从碳量子表面释放出来,从而使碳量子点的荧光发生恢复。因此,可以通过测量荧光强度的变化来检测E2的质量浓度。
-
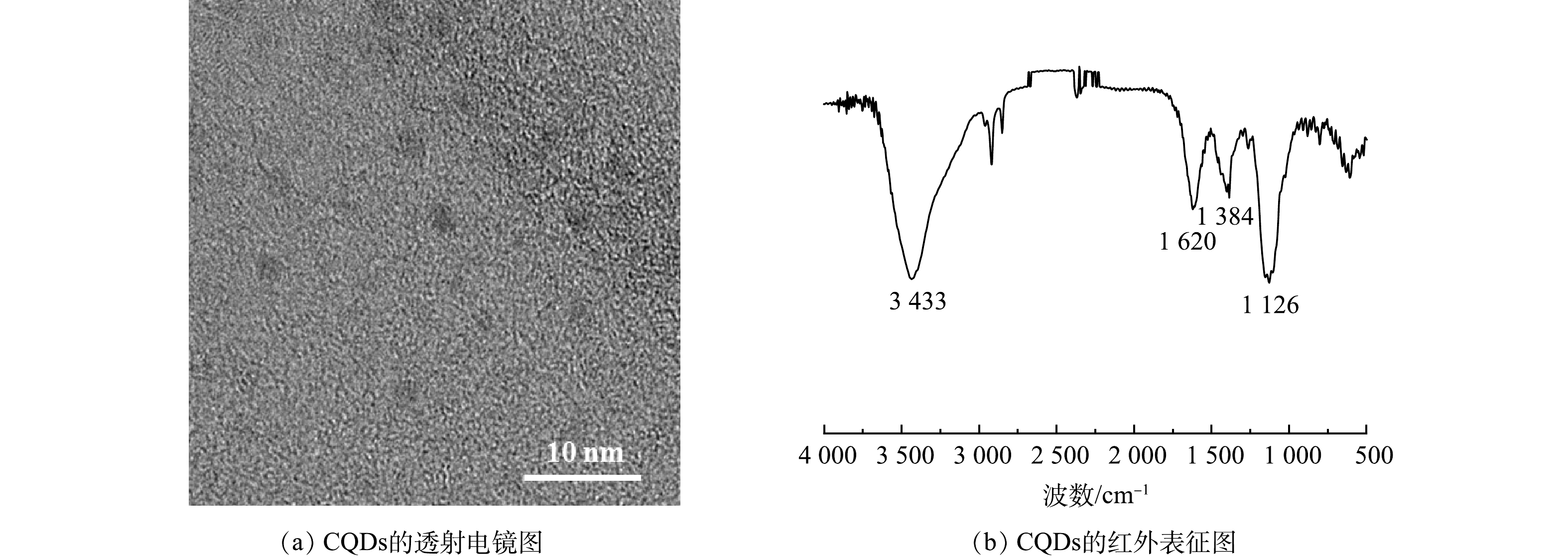
本研究对甘蔗渣来源碳量子点进行透射电镜 (TEM) 表征。如图2(a)所示,碳量子点的粒径大小约为2 nm,并具有球形结构。如图2(b)所示,FT-IR光谱显示了碳量子点的官能团,碳量子点在3 433 cm−1处的峰对应N—H和O—H的伸缩振动,1 620 cm−1处对应C=C的伸缩振动,1 384 cm−1处属于COO—的伸缩振动,1 126 cm−1处属于C—O的对称伸缩[31]。结果表明,碳量子点表面富含大量的羟基和羧基,具有良好的水溶性与生物相容性。
-
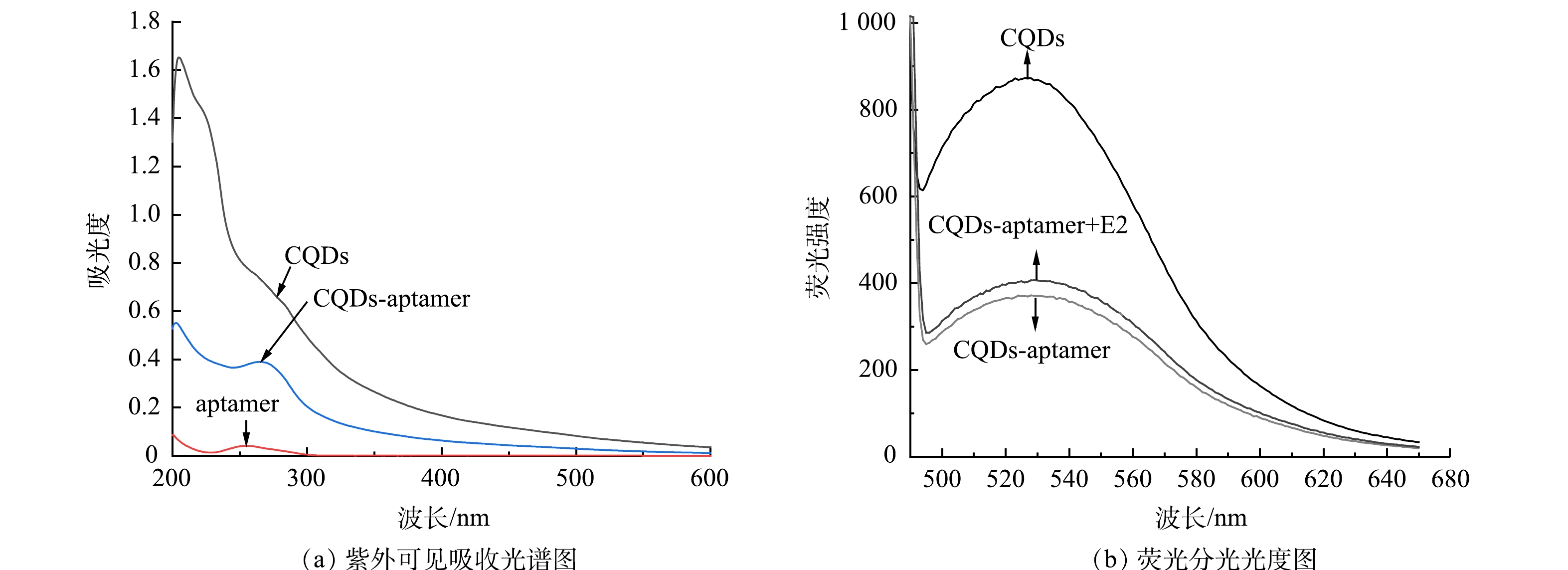
本研究利用紫外-可见吸收光谱探究了核酸适配体固定在碳量子点表面的情况并进行分析。如图3(a)所示,质量浓度为200 mg·L−1的碳量子点在226 nm和280 nm波长处有特征吸收峰,分别对应于C=C键的π-π*转换和C=O键的n-π*转换[32]。1 μmol·L−1游离的核酸适配体在253 nm处有明显的特征吸收峰,对应DNA的特征峰值[33]。50 mg·L−1的CQDs-aptamer在226 nm处出现碳量子点的特征吸收峰,在267 nm处呈现一个较宽的肩峰,说明核酸适配体和碳量子点的成功偶联。如图3(b)所示,在475 nm激发波长下,碳量子点的发射波长为530 nm。将核酸适配体与碳量子点偶联后,荧光强度下降了约62%,加入质量浓度为20 μg·L−1的E2后,荧光强度恢复。上述实验结果表明,成功合成了CQDs-aptamer荧光探针,该探针可用于E2的定量检测。
-
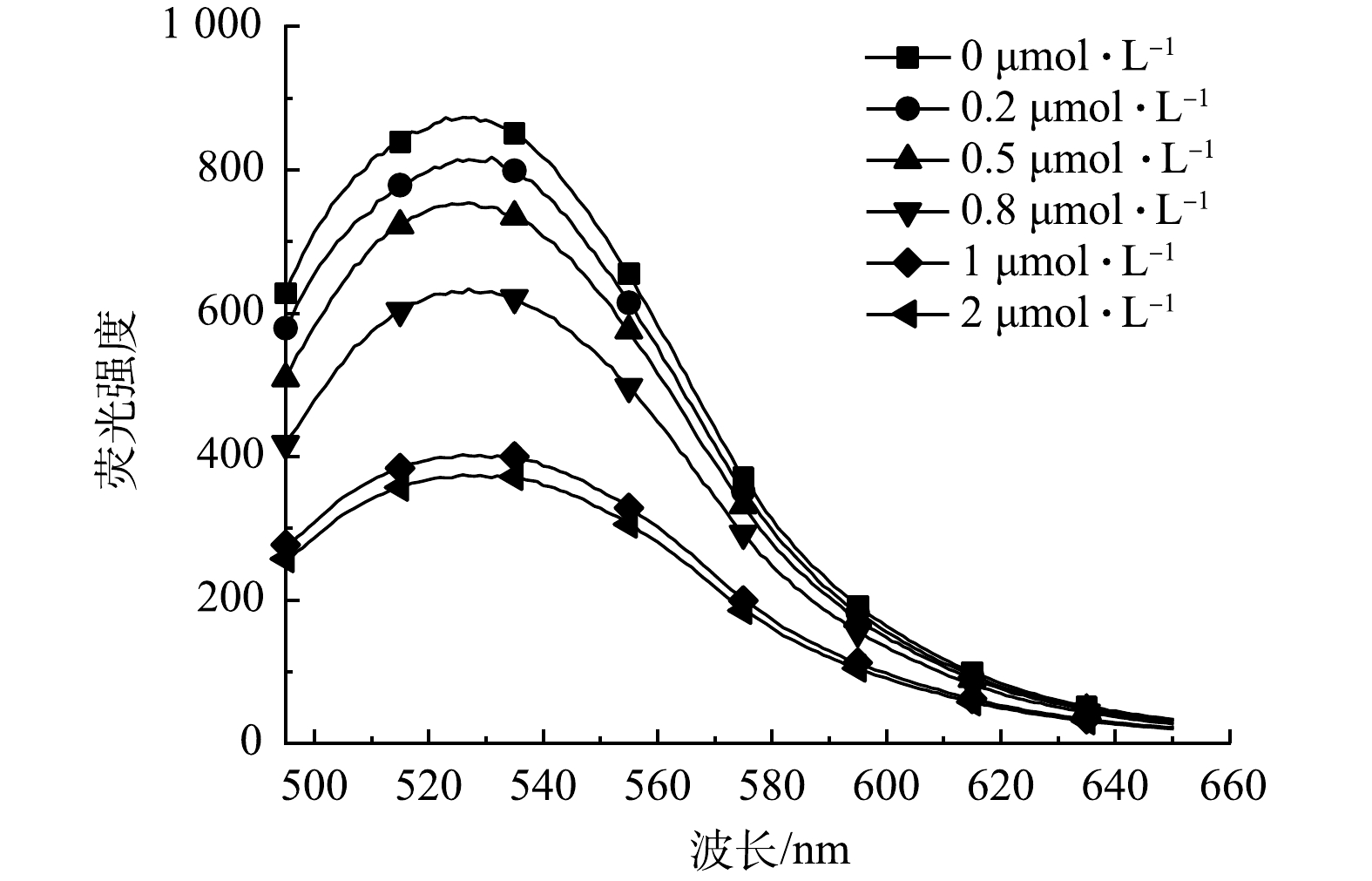
1) 核酸适配体的投加量对检测效果的影响。核酸适配体投加量是影响灵敏检测E2的重要参数之一。为了探索最佳核酸适配体投加量,本实验使用不同起始浓度的核酸适配体 (0、0.2、0.5、0.8、1、2 μmol·L−1) 构建了荧光探针。如图4所示,随着核酸适配体浓度的增加,复合探针的荧光强度逐渐减弱。当核酸适配体添加量为1 μmol·L−1时,合成的荧光探针有最佳的结合效率。核酸适配体添加量为2 μmol·L−1的荧光探针与添加量为1 μmol·L−1的荧光探针相比,荧光强度变化不大。该现象产生的原因是碳量子点表面被核酸适配体覆盖,随着核酸适配体投加量的增加,碳量子点表面覆盖率增加,从而使量子点荧光减弱。但后续适配体投加量达到一定程度,碳量子点表面覆盖率达到最大,信号达到饱和,荧光强度则不再产生变化。因此,在后续实验中采用200 mg·L−1的碳量子点与1 μmol·L−1的核酸适配体构建CQDs-aptamer探针并检测雌二醇。
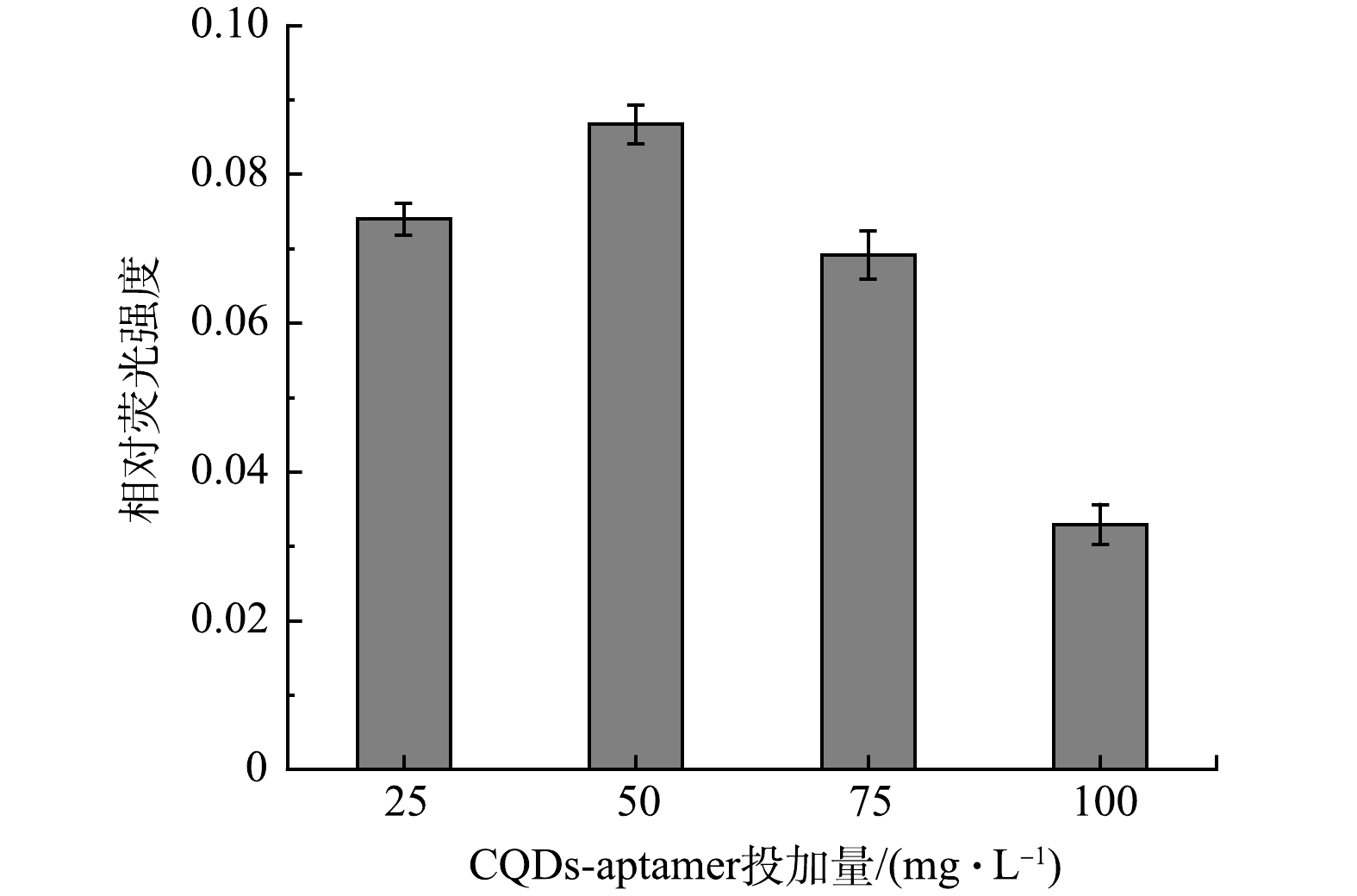
2) 荧光探针投加量对雌二醇检测效果的影响。利用200 mg·L−1的碳量子点与浓度为1 μmol·L−1的核酸适配体合成CQDs-aptamer探针,在相同的实验条件下,考察不同投加量对雌二醇 (20 μg·L−1) 检测效果的影响。如图5所示,当CQDs-aptamer探针的投加量为50 mg·L−1时,达到最大的荧光恢复量。原因是随着CQDs-aptamer探针的投加量的增加,体系中的核酸适配体的含量增加,检测体系中雌二醇结合效果增强,从而使荧光恢复的效果增强。当CQDs-aptamer探针的投加量超出50 mg·L−1时,荧光恢复量开始降低。原因可能是CQDs-aptamer探针投加量的增加也伴随着本底荧光值的增加,本底荧光值的增加会导致实验误差的增加。为了获得较高的荧光恢复量,在后续实验中,选择投加量为50 mg·L−1的CQDs-aptamer探针检测雌二醇。
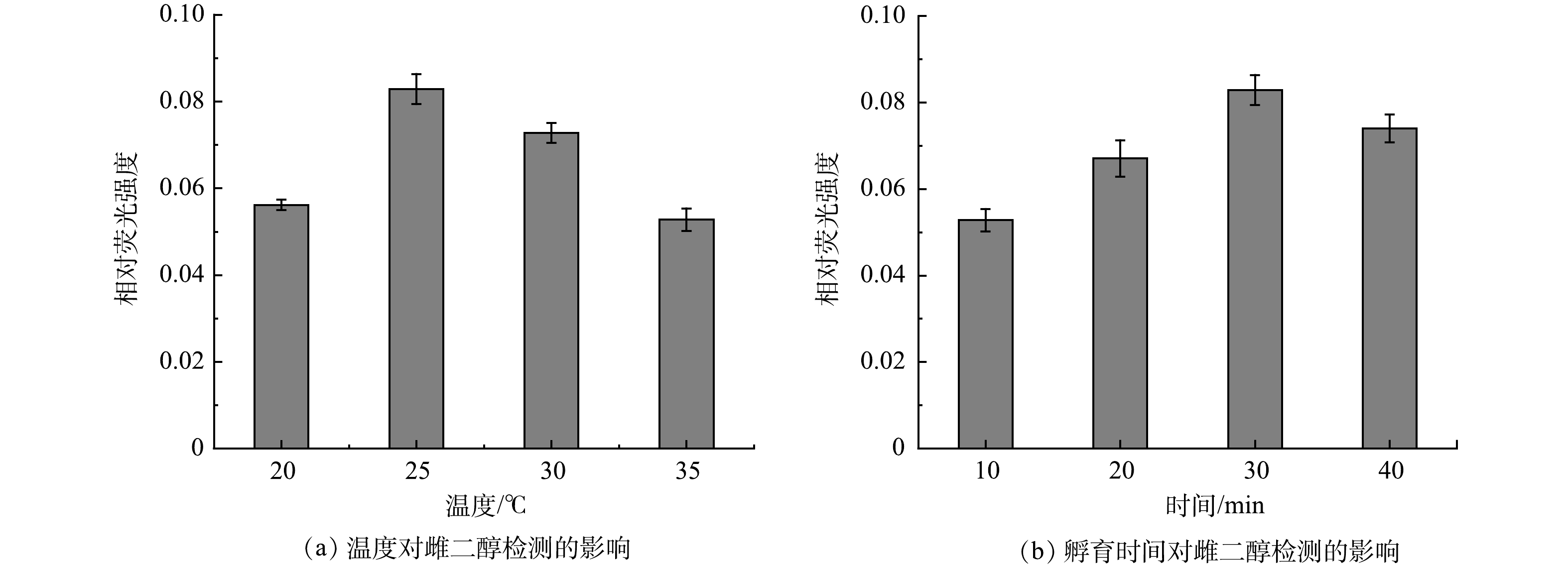
3) 反应温度和时间对雌二醇检测效果的影响。在检测过程中,反应温度会影响CQDs-aptamer探针中核酸适配体与E2结合的速度与稳定性,从而影响检测效果。通过控制不同的反应温度 (20、25、30和35 ℃) 来检测质量浓度为20 μg·L−1的E2,探究其最佳的检测温度。如图6(a)所示,随着温度的升高,荧光相对强度呈现先增大后减小的趋势。当反应温度为25 ℃时,荧光相对强度最大。因此,选择25 ℃作为最佳反应温度。
CQDs-aptamer探针与E2需要一段时间才能完成结合过程,故应对反应时间进行优化。如图6(b)所示,随着反应时间的增加,荧光相对强度逐渐增加,当反应时间达到30 min时,相对荧光强度最大,此后稍有降低。因此,最终的反应时间确定为30 min。
-
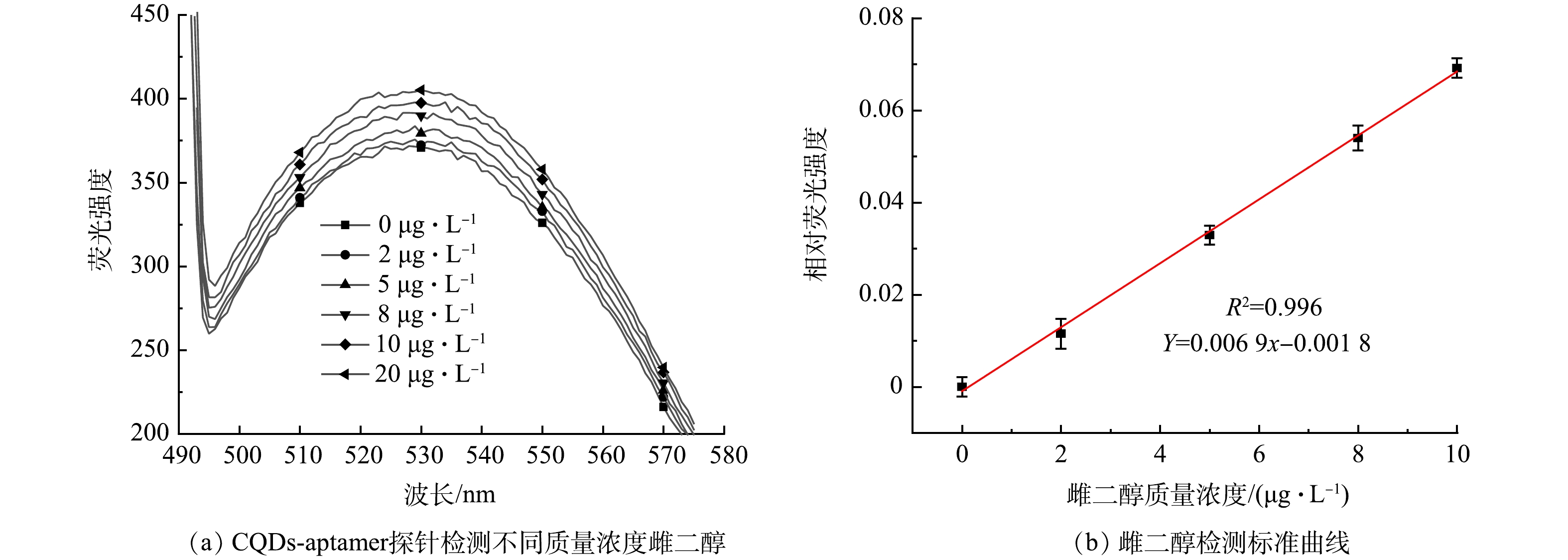
1) 雌二醇的灵敏度检测。为了探索该方法的检测灵敏度,在最佳实验条件下,利用CQDs-aptamer探针对E2进行定量检测。如图7(a)所示,荧光强度随E2质量浓度的增加呈逐渐增加的趋势。如图7(b)所示,相对荧光强度与质量浓度为0~10 μg·L−1的E2存在良好的线性关系,检出限(LOD)为0.42 μg·L−1。
该荧光探针检测方法与其他雌二醇检测方法的对比结果如表1所示。值得注意的是,该荧光适体探针对E2的检测不但具有响应快、操作方便、检测效果较好等优点,还具有较高的成本效益。
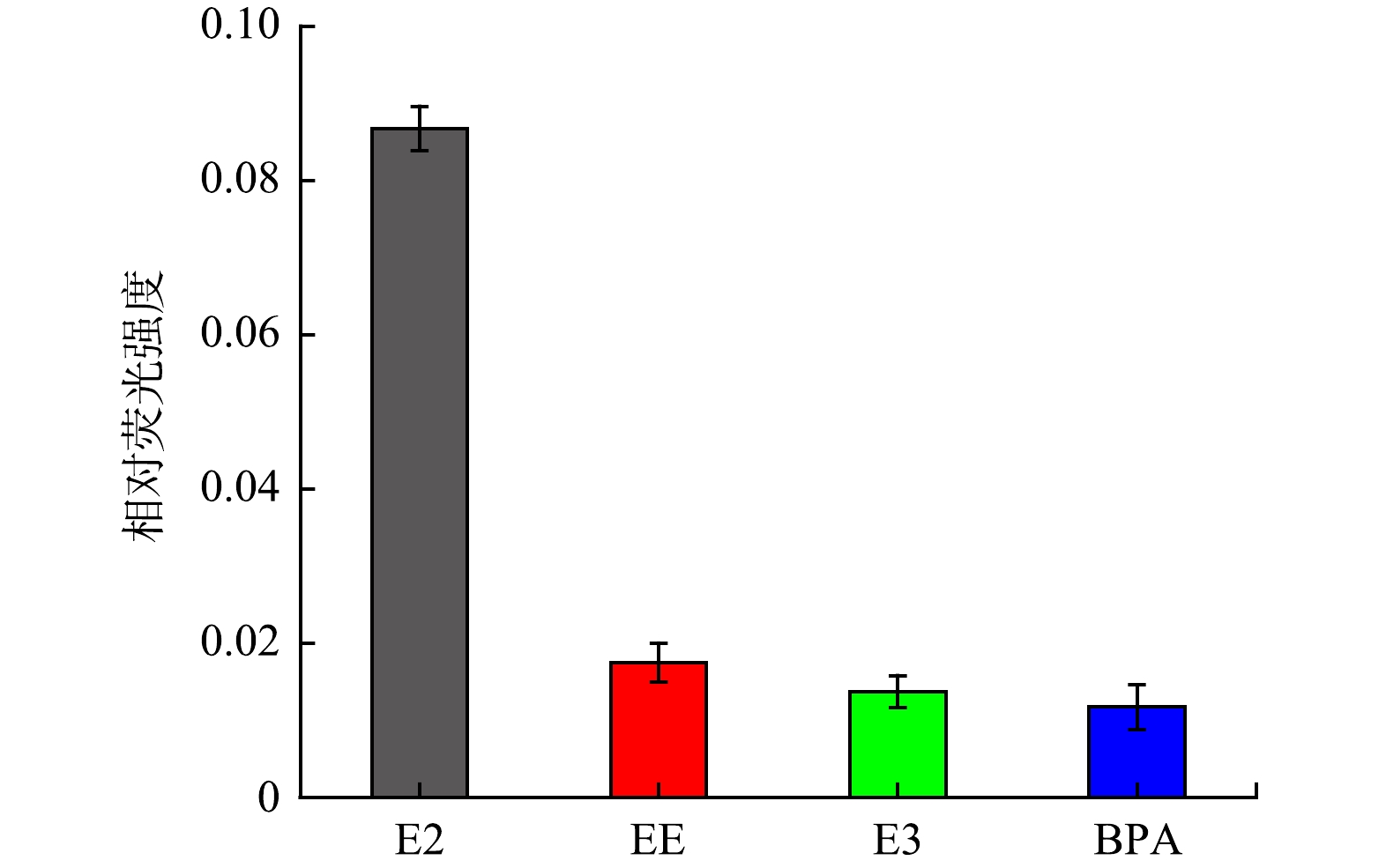
2) 雌二醇的特异性检测。为了验证该荧光探针的特异性,分别对雌二醇 (E2) 20 μg·L−1、雌炔醇 (EE) 100 μg·L−1、雌三醇 (E3) 100 μg·L−1和双酚A (BPA) 100 μg·L−1进行检测,通过比较相对荧光强度来说明该检测方法的选择特异性。相对荧光强度如图8所示。可以看出,与目标物E2的高荧光强度变化相比,其他内分泌干扰物质(EE、E3和BPA)的相对荧光强度变化很小,在一定程度上可以忽略。由此可以推断该CQDs-aptamer探针对E2具有较高的特异性。
3) 实际样品分析。为了评估构建的CQDs-aptamer荧光探针的可行性与实用性,将荧光探针用于检测自来水和湖水样品中的E2。自来水取自实验室,湖水取自长春市南湖。在分析检测样品的荧光强度的基础上,计算加标回收率。如表2所示,相对标准偏差(RSD)为2.35%~3.84%,回收率为93.6%~106.5%。可以看出,本方法构建的荧光探针具有较高的准确度和精密度,适合实际样品分析。
-
1) 利用绿色合成、成本低廉的甘蔗渣生物碳衍生碳量子点作为荧光信号元件,核酸适配体作为特异性结合雌二醇的识别元件,成功构建了可以特异性检测E2的荧光探针。
2) 在CQDs-aptamer投加量为50 mg·L−1、核酸适体的投量为1 μmol·L−1条件下,反应温度为25 ℃,反应时间为30 min,取得最佳雌二醇检测效果。在最优的实验条件下,相对荧光强度与雌二醇的质量浓度在0~10 μg·L−1区间内成正比且检测限为0.42 μg·L−1。
3) 自来水和湖水样品的加标回收实验结果表明,该方法具有实际样品检测能力。与传统的仪器检测方法相比,该荧光探针检测E2具有操作简单、成本低的优点,并可实现E2高特异性检测,具有良好的应用前景。
基于甘蔗渣衍生碳量子点的荧光适配体探针的制备及其对17β-雌二醇的检测效果
Preparation of fluorescent aptamer probe based on bagasse derived carbon quantum dots and its detection effect on 17β-estradiol
-
摘要: 为实现灵敏、快速、特异性地检测水环境中的17β-雌二醇 (E2) ,以甘蔗渣衍生的碳量子点作为荧光信号,核酸适配体 (aptamer) 作为识别元素,构建了一种可以特异性检测E2的荧光探针,通过荧光强度的变化来定量检测E2并对检测效果进行分析。结果表明:核酸适配体能成功修饰在碳量子点的表面形成稳定的荧光探针;200 mg·L−1的碳量子点与1 μmol·L−1的aptamer为荧光探针的最佳构建比例;相对荧光强度与0~10 μg·L−1质量浓度的雌二醇成正比,且最低检测限为0.42 μg·L−1;该荧光探针可成功应用于水体中E2的检测,回收率为93.6%~106.5%。与传统的仪器检测方法相比,该荧光探针检测E2具有良好的选择性和重现性,还具有操作简单、成本低的优点。本研究成果可为核酸适配体构建的荧光探针在水环境检测中的推广应用提供参考。Abstract: In order to realize the sensitive, rapid and specific detection of 17β-estradiol in water environment, a fluorescent probe that can specifically detect E2 was built with bagasse derived carbon quantum dots as fluorescence signal and aptamer as recognition element, which could quantitatively detect E2 according to the change of fluorescence intensity, and the detection effect was also analyzed. The results show that the aptamer can successfully modify the surface of carbon quantum dots to form stable fluorescent probes. 200 mg·L−1 carbon quantum dots and 1 μmol·L−1 aptamer was the optimal ratio for fluorescence probe construction. The relative fluorescence intensity was proportional to the mass concentration of estradiol in the range of 0~10 μg·L−1, and the lowest detection limit was 0.42 μg·L−1. The fluorescence probe can be successfully applied to detect E2 in water with a recovery rate of 93.6%~106.5%. Compared with the traditional instrument detection method, the fluorescence probe has good selectivity and reproducibility for E2 detection, as well as the advantages of simple operation and low cost. The results of this study can provide a reference for the popularization and application of fluorescent probes constructed by aptamers in water environment detection.
-
Key words:
- bagasse /
- carbon quantum dot /
- aptamer /
- 17β-estradiol /
- fluorescent probe /
- static quenching
-

-
表 1 各种雌二醇检测方法的比较
Table 1. Comparison of various detection methods for E2
表 2 实际水样中E2检测效果(n=3)
Table 2. Detection of E2 in real water samples (n=3)
样品来源 加入的E2质量浓度/(μg·L−1) 检测的E2质量浓度/(μg·L−1) 回收率/% 相对标准偏差/% 自来水 5 4.75 95.0 3.13 8 8.22 102.8 2.81 10 10.39 103.9 3.75 湖水 5 4.68 93.6 2.35 8 8.52 106.5 2.69 10 10.26 102.6 3.84 -
[1] 曾庆玲, 李咏梅, 顾国维. 缺氧活性污泥对17β-雌二醇的吸附与降解研究[J]. 环境科学, 2008, 29(9): 2553-2557. doi: 10.3321/j.issn:0250-3301.2008.09.028 [2] DONG X W, HE L Z, LIU Y, et al. Preparation of highly conductive biochar nanoparticles for rapid and sensitive detection of 17β-estradiol in water[J]. Electrochimica Acta, 2018, 292: 55-62. doi: 10.1016/j.electacta.2018.09.129 [3] SHA H F, YAN B. Design of a ratiometric fluorescence sensor based on metal organic frameworks and Ru(bpy)32+-doped silica composites for 17β-estradiol detection[J]. Journal of Colloid and Interface Science, 2021, 583: 50-57. doi: 10.1016/j.jcis.2020.09.030 [4] 朱艳琼, 韩宝三. 天然雌激素雌酮、雌二醇和雌三醇的分析检测进展[J]. 化学世界, 2020, 61(4): 237-244. doi: 10.19500/j.cnki.0367-6358.20190715 [5] GAO R X, CUI X H, HAO Y, et al. A highly-efficient imprinted magnetic nanoparticle for selective separation and detection of 17 beta-estradiol in milk[J]. Food Chemistry, 2016, 194: 1040-1047. doi: 10.1016/j.foodchem.2015.08.112 [6] 付银杰, 高彦征, 董长勋, 等. SPE-HPLC/FLD法同时测定水中4种雌激素[J]. 农业环境科学学报, 2012, 31(11): 2296-2303. [7] MONERRIS M J, AREVALO F J, FERNANDEZ H, et al. Development of a very sensitive electrochemical immunosensor for the determination of 17 beta-estradiol in bovine serum samples[J]. Sensors and Actuators B:Chemical, 2015, 208: 525-531. doi: 10.1016/j.snb.2014.11.048 [8] MATEJICEK D, KUBAN V. Enhancing sensitivity of liquid chromatographic/ion-trap tandem mass spectrometric determination of estrogens by on-line pre-column derivatization[J]. Journal of Chromatography A, 2008, 1192(2): 248-253. doi: 10.1016/j.chroma.2008.03.061 [9] SGHAIER R. B, NET S, GHORBEL-ABID I, et al. Simultaneous detection of 13 endocrine disrupting chemicals in water by a combination of SPE-BSTFA derivatization and GC-MS in Transboundary Rivers (France-Belgium)[J]. Water Air and Soil Pollution, 2017, 228(1): 1-14. doi: 10.1007/s11270-016-3178-3 [10] CARBON E, SHEEDY C, FARENHORST A. Development of competitive ELISAs for 17-estradiol and 17-estradiol plus estrone plus estriol using rabbit polyclonal antibodies[J]. Journal of Environmental Science and Health Part B:Pesticides Food Contaminants and Agricultural Wastes, 2010, 45(2): 145-151. [11] WEI H B, LIN J M, WU D N, et al. Detection of 17 beta-estradiol in river and human urine by highly sensitive chemiluminescent enzyme immunoassay[J]. Chinese Journal of Analytical Chemistry, 2007, 35(3): 320-324. [12] PU H, HUANG Z B, SUN D W, et al. Recent advances in the detection of 17β-estradiol in food matrices: A review[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(13): 2144-2157. doi: 10.1080/10408398.2019.1611539 [13] JANEGITZ B C, DOS SANTOS F A, FARIA R C, et al. Electrochemical determination of estradiol using a thin film containing reduced graphene oxide and dihexadecylphosphate[J]. Materials Science & Engineering C:Materials for Biological Applications, 2014, 37: 14-19. [14] YOO D, PARK Y, CHEON B, et al. Carbon dots as an effective fluorescent sensing platform for metal ion detection[J]. Nanoscale Research Letters, 2019, 14(1): 1-13. doi: 10.1186/s11671-018-2843-4 [15] SUN D W, HUANG L J, PU H B, et al. Introducing reticular chemistry into agrochemistry[J]. Chemical Society Reviews, 2021, 50(2): 1070-1110. doi: 10.1039/C9CS00829B [16] MINTZ K J, ZHOU Y Q, LEBLANC R M. Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure[J]. Nanoscale, 2019, 11(11): 4634-4652. doi: 10.1039/C8NR10059D [17] ZHANG Q, HOU Q X, HUANG G X, et al. Removal of heavy metals in aquatic environment by graphene oxide composites: A review[J]. Environmental Science and Pollution Research, 2020, 27(1): 190-209. doi: 10.1007/s11356-019-06683-w [18] 白秋月, 杨春亮, 叶剑芝, 等. 碳量子点荧光探针的设计及其在农残检测中的应用进展[J]. 分析测试学报, 2019, 38(4): 488-494. doi: 10.3969/j.issn.1004-4957.2019.04.019 [19] ZHOU W Z, HUANG P J J, DING J S, et al. Aptamer-based biosensors for biomedical diagnostics[J]. Analyst, 2014, 139(11): 2627-2640. doi: 10.1039/c4an00132j [20] JIANG F, LIU B A, LU J, et al. Progress and challenges in developing aptamer-functionalized targeted drug delivery systems[J]. International Journal of Molecular Sciences, 2015, 16(10): 23784-23822. doi: 10.3390/ijms161023784 [21] GAN Y, LIANG T, HU Q W, et al. In-situ detection of cadmium with aptamer functionalized gold nanoparticles based on smartphone-based colorimetric system[J]. Talanta, 2020, 208: 1-7. [22] ZHANG W, LIU Q X, GUO Z H, et al. Practical application of aptamer-based biosensors in detection of low molecular weight pollutants in water sources[J]. Molecules, 2018, 23(2): 1-25. [23] EISSA S, ZOUROB M. In vitro selection of DNA aptamers targeting beta-lactoglobulin and their integration in graphene-based biosensor for the detection of milk allergen[J]. Biosensors & Bioelectronics, 2017, 91: 169-174. [24] CHEN M L, CHEN J H, DING L, et al. Study of the detection of bisphenol A based on a nano-sized metal-organic framework crystal and an aptamer[J]. Analytical Methods, 2017, 9(6): 906-909. doi: 10.1039/C6AY03151J [25] DAS R, BANDYOPADHYAY R, PRAMANIK P. Carbon quantum dots from natural resource: A review[J]. Materials Today Chemistry, 2018, 8: 96-109. doi: 10.1016/j.mtchem.2018.03.003 [26] INYANG M, GAO B, PULLAMMANAPPALLIL P, et al. Biochar from anaerobically digested sugarcane bagasse[J]. Bioresource Technology, 2010, 101(22): 8868-8872. doi: 10.1016/j.biortech.2010.06.088 [27] BATISTA G, SOUZA R B A, PRATTO B, et al. Effect of severity factor on the hydrothermal pretreatment of sugarcane straw[J]. Bioresource Technology, 2019, 275: 321-327. doi: 10.1016/j.biortech.2018.12.073 [28] ARSHAD M, AHMED S. Cogeneration through bagasse: A renewable strategy to meet the future energy needs[J]. Renewable & Sustainable Energy Reviews, 2016, 54: 732-737. [29] WANG Z, YU J, ZHANG X, et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy[J]. ACS Applied Materials & Interfaces, 2016, 8(2): 1434-1439. [30] MOLAEI M J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review[J]. Analytical Methods, 2020, 12(10): 1266-1287. doi: 10.1039/C9AY02696G [31] LIU W, DIAO H P, CHANG H H, et al. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging[J]. Sensors and Actuators B:Chemical, 2017, 241: 190-198. doi: 10.1016/j.snb.2016.10.068 [32] ZULFAJRI M, GEDDA G, CHANG C J, et al. Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe3+ ions in aqueous solution[J]. ACS Omega, 2019, 4(13): 15382-15392. doi: 10.1021/acsomega.9b01333 [33] WEI Q Y, ZHANG P Y, PU H B, et al. A fluorescence aptasensor based on carbon quantum dots and magnetic Fe3O4 nanoparticles for highly sensitive detection of 17β-estradiol[J]. Food Chemistry, 2022, 373: 1-10. [34] BARRECA S, BUSETTO M, COLZANI L, et al. Determination of estrogenic endocrine disruptors in water at sub-ng·L-1 levels in compliance with decision 2015/495/EU using offline-online solid phase extraction concentration coupled with high performance liquid chromatography-tandem mass spectrometry[J]. Microchemical Journal, 2019, 147: 1186-1191. doi: 10.1016/j.microc.2019.04.030 [35] MORAES F C, ROSSI B, DONATONI M C, et al. Sensitive determination of 17β-estradiol in river water using a graphene based electrochemical sensor[J]. Analytica Chimica Acta, 2015, 881: 37-43. doi: 10.1016/j.aca.2015.04.043 [36] KANSO H, BARTHELMEBS L, INGUIMBERT N, et al. Immunosensors for estradiol and ethinylestradiol based on new synthetic estrogen derivatives: Application to wastewater analysis[J]. Analytical Chemistry, 2013, 85(4): 2397-2404. doi: 10.1021/ac303406c -



 下载:
下载: