-
进入二十一世纪以来全球疫情不断暴发,如2002年的重症急性呼吸综合征 (SARS) 、2009年的甲型H1N1流感、2012年的中东呼吸综合征 (MERS) 以及2019年暴发后蔓延全球的新冠 (COVID-19) 疫情,严重威胁着人类健康和社会发展。抗疫过程中传统化学消毒剂 (如氯) 的大量使用,导致了残余消毒剂及消毒副产物引发的次生风险。紫外线 (UV) 作为一种气、水和物体表面的高效、绿色消毒技术,受到广泛关注。国际照明委员会 (Commission Internationale de l’Eclairage,CIE) 按波长将UV分为UV-A (波长315~400 nm) 、UV-B (波长280~315 nm) 和UV-C (波长100~280 nm) ,其中UV-C中又包含真空UV (波长100~200 nm) 、远UV-C (波长200~230 nm) 和灭活UV-C (波长250~280 nm) 。
灭活UV-C (如波长254 nm) 可破坏绝大多数病原微生物的复制过程,使其失去感染性,从而达到高效、低耗的消毒效果,是目前UV消毒领域应用最为广泛的波段。远UV-C可有效杀灭各类病原微生物,但却被长期忽视,尚未广泛应用于消毒领域。主要原因在于其较高的光子能量,较其它波段UV-C更易被介质吸收,从而影响消毒效果。其次,远UV-C光源的电光转化效率 (低于5%) 远低于传统低压汞灯 (约36%) ,故在应用中能耗较高,亦会限制其应用。然而,远UV-C对眼睛和皮肤的伤害较传统灭活UV-C更低,且未发现有效消毒剂量下的远UV-C辐照会对人体造成伤害。因此,基于远UV-C的特性,可开发人机共存条件下的原位UV消毒,以阻断病毒传播,保护人类健康。本文通过对比远UV-C的辐射光源的特点,分析典型病原微生物的灭活机理与效果,以及人体的远UV-C暴露风险,分析其在消毒应用中应解决的关键问题,以期为其应用研究提供参考。
-
1) 准分子灯。准分子灯是常用的远UV-C光源,其通过填充稀有气体和卤素的混合物,经电激发后可发射准单色光。通过填充物改变可调节准分子灯的输出波长,并通过灯设计 (气体压力或成分) 的优化在一定程度上调控激发态分子的电子跃迁,从而提高电光转化效率。辐射远UV-C的光源填充物为KrCl,其主峰位和半峰宽通常为222 nm和4 nm (图1) 。需要指出的是,由于设计、质量的差异,不同生产商的产品辐射出的远UV-C主峰位和半峰宽会有所差异。目前,对于空气和物体表面消毒,准分子灯是最为适合且技术成熟的远UV-C光源。准分子灯的主要优点在于其可在有效消毒的同时,保持较低的人体暴露风险。然而,KrCl准分子灯还存在少量其它发射波长[1],需安装光学滤光片对其进行滤除 (特别是灭活UV-C 250~280 nm) ,使UV输出集中于远UV-C。此外,KrCl准分子灯UV-C输出的电光转化效率远低于传统低压汞灯 (主要输出波长254 nm) ,导致其消毒能耗较高,需在应用中予以考虑。
2) 中压汞灯。中压汞灯可发射连续波段200~400 nm的UV,包含远UV-C。由于H2O2在远UV-C波段的吸光度远高于230~280 nm UV-C波段,因此中压汞灯常与H2O2结合,产生羟基自由基 (·OH) 高效去除水中难降解有机污染物,其工艺效能高于传统低压汞灯。然而,中压汞灯其余波段的UV输出量过大,很难采用滤光片技术将其滤除,即无法输出单一的远UV-C,造成眼睛和皮肤较大的暴露风险,不适宜作为远UV-C消毒的光源。
3) 固态UV光源。固态UV光源 (如UV发光二极管,UV-LED) 是光源领域的重要发展方向。目前可见光固态光源已被广泛应用,波长在265~285 nm的传统UV-LED发展迅猛,其功率、寿命和成本等指标快速突破,已被初步应用于空气、小规模供水、物体表面等的消毒。基于AlGaN薄层结构的远UV-C UV-LED已被研发[2],但其输出功率、寿命和成本等指标尚未达到实际应用级别。基于对可见光LED和传统UV-LED的研发积累,UV-LED将在未来成为重要的远UV-C消毒光源。
-
不同波长UV辐照下,病原微生物的吸光度不同,故相应的灭活原理亦不同,同时其消毒效果也有差异。病原微生物的核酸可吸收UV-C,吸收峰值在260 nm附近 (图1) 。被吸收的UV-C光子会干扰核酸复制,从而导致病原微生物丧失传染性,以达到消毒效果[3]。基于此原理,传统低压汞灯已被广泛应用于空气、水和物体表面的消毒。
蛋白质在远UV-C波段的吸光度很高 (图1) ,吸收光子后导致变性失活,达到病原微生物的灭活。然而,由于细胞内光程较短 (< 1 μm) ,病原微生物对远UV-C的吸光度低于0.02 cm−1 [4],使得病原微生物的外层蛋白质 (如细菌细胞膜上的蛋白质、病毒的外层蛋白) 无法大比例阻隔远UV-C辐照到内部核酸。因此,远UV-C消毒同时存在干扰核酸复制和蛋白质失活2种灭活机制[5-7]。目前,对远UV-C的研究主要集中于光源、消毒效果及安全性,其灭活的影响因素 (介质吸收和颗粒的影响) 、多波长协同灭活机制等尚需进一步研究。
-
远UV-C (222 nm) 和灭活UV-C (254 nm) 对不同病原微生物的灭活效果对比见表1。2种UV-C对SARS-CoV-2病毒的灭活都非常有效。其中MA等[8]采用无滤光片和含滤光片下KrCl准分子灯,测定SARS-CoV-2病毒灭活速率常数分别是1.52和1.42 cm2·mJ−1,高于ROBINSON等[9]的KrCl准分子灯 (含滤光片) 的实验结果 (0.64 cm2·mJ−1) ,其原因可能是ROBINSON等实验中测试溶液吸光度 (> 30 cm−1) 高于MA等的研究 (0.05 cm−1) 。MA等和STORM等测定出低压汞灯 (254 nm) 对SARS-CoV-2病毒的灭活速率常数分别为0.79[8]和0.59[10] cm2·mJ−1,低于远UV-C灭活速率常数。远UV-C能够高效灭活病原微生物的原因是蛋白质对于222 nm附近的UV波长有更高的吸收率,且蛋白质失活机制的消毒效能更高,同时也说明远UV-C较灭活UV-C拥有相似甚至更强的灭活能力。因此,在远UV-C消毒数据相对缺乏的情况下,可借助低压汞灯 (254 nm) 的数据保守预测远UV-C的灭活效果。
表1结果表明,相对于低压汞灯 (254 nm) ,远UV-C对包膜病毒 (HCoV 229E和MHV) 、包膜噬菌体 (Phi6) 、无包膜噬菌体 (MS2和T1UV) 具有更好的灭活效果,说明远UV-C消毒的高效性在不同类型病毒中普遍存在。包膜RNA病毒HCoV 229E和MHV,无包膜双链DNA病毒T1UV噬菌体,都具有较低的生物安全性要求,且与SARS-CoV-2病毒灭活速率常数相似,因此可被选为SARS-CoV-2病毒的替代受试微生物。而HCoV 229E和MHV病毒与SARS-CoV-2同为包膜RNA病毒,其结构相似性更有利于SARS-CoV-2病毒灭活机理的研究。Phi6噬菌体与SARS-CoV-2病毒相比更难灭活,可作为保守的替代受试微生物。此外,MS2噬菌体较SARS-CoV-2病毒对UV辐照敏感性差异较大。
-
传统灭活UV-C对不同介质 (如空气、水和物理表面) 的病原微生物均有较强的消毒能力,特别是多项研究已证明UV-C对空气及飘落物体表面的气溶胶飞沫 (高传染性病毒的重要传播途径) 具有明显的消毒效果[13-14]。远UV-C较灭活UV-C具有更短的波长和更高的光子能量,而空气中气溶胶飞沫本身通常含有较高浓度的蛋白质,会吸收远UV-C并减低其在气溶胶内的穿透。由于气溶胶的直径和组成差异会对消毒产生不同程度影响,因此,远UV-C对气溶胶飞沫病原微生物的灭活是一个复杂的过程。现有各介质灭活结果初步表明,介质对远UV-C灭活SARS-CoV-2病毒的影响并不显著[8,10-12,15]。目前,通过病原微生物悬浮液测定UV灭活曲线的方法已较为成熟,该方法测得的数据可用来代表不同介质中远UV-C的灭活效果。美国政府工业卫生师协会 (ACGIH) 和国际非电离辐射防护委员会 (ICNIRP) 规定远UV-C暴露安全阈值分别为23 mJ·cm−2[16] 与25 mJ·cm−2[17],远UV-C阈值剂量足以灭活大部分空气、水、物体表面的病原微生物 (包括SARS-CoV-2[8-10]) ,阻断高传染性疾病的传播途径。
-
UV-C辐照皮肤会诱导皮肤癌的发生[18-19]。目前针对UV辐照致癌的机理研究多集中于254 nm及UV-B和UV-A波段上[20]。此外,多数流行病学研究结果证明了环境太阳辐射中皮肤癌的主要诱因为UV-B[21]。而远UV-C机理研究和暴露案例较少,尚没有远UV-C致癌的研究结论。
当UV穿透皮肤表层 (角质层、颗粒层和棘层) 辐射到基底层时,可能会损伤DNA并引发皮肤癌。皮肤表层的角质层能够吸收UV辐射,在一定程度上保护皮肤。远UV-C光子能量更高,会被有机质分子 (如蛋白质) 强烈吸收。然而,其穿透能力远低于灭活UV-C,则意味着可被UV-B或灭活UV-C穿透的角质层,会强烈吸收远UV-C,再结合颗粒层和棘层的吸收,可基本阻隔远UV-C到达基底层。
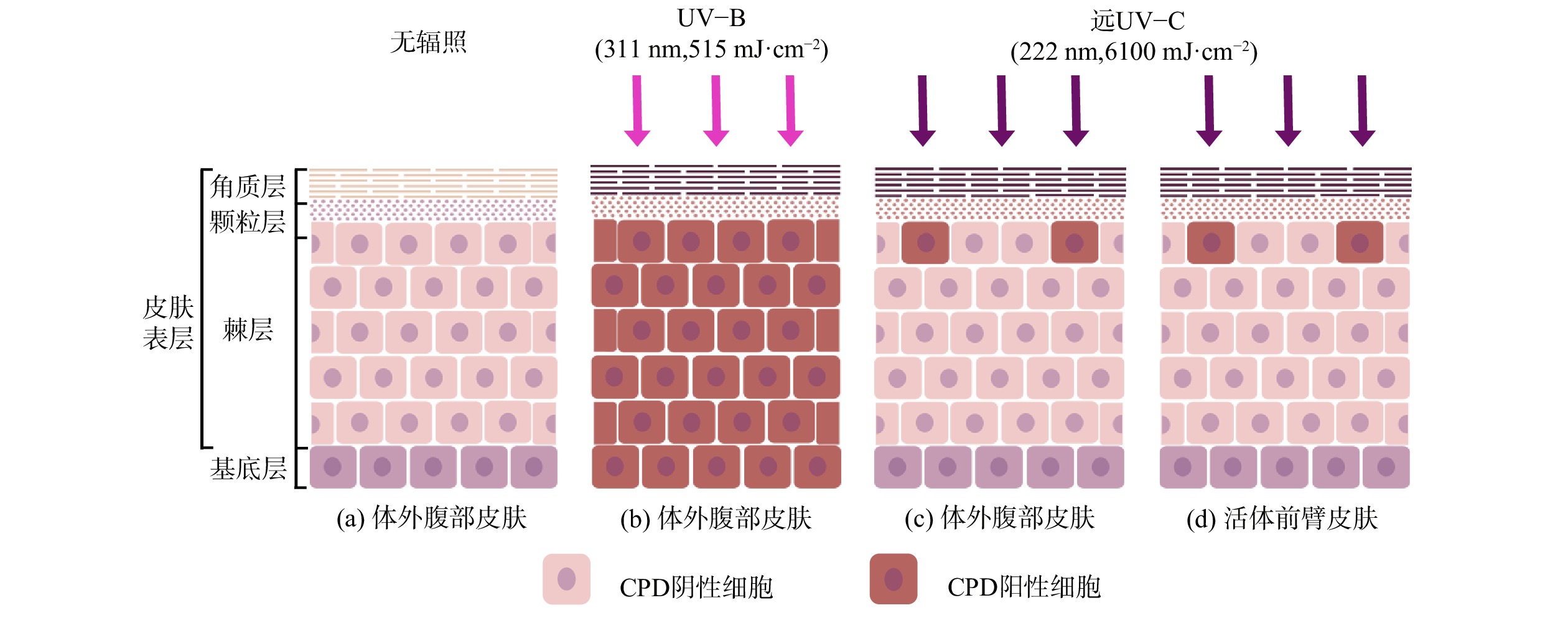
远UV-C辐射人体和无毛小鼠皮肤的研究结果表明[22-33],即使使用远超现有安全阈值的剂量辐照[16],也尚未观察到其对皮肤可量化的辐照损伤。WOODS等[22]使用远UV-C辐照人体背部,发现40 mJ·cm−2的辐照就会使皮肤出现红斑。这可能是由于实验使用未经过滤的KrCl准分子灯,同时产生了波长大于230 nm的UV-C。FUKUI等[23]在2020年重复此实验,改用滤光片过滤掉大于230 nm UV的KrCl准分子灯,在剂量相近甚至更高的情况下 (50~500 mJ·cm−2) ,也未观察到皮肤出现红斑或其他安全风险。研究人员在检测了远UV-C辐照的动物皮肤中DNA主要光照产物环丁烷嘧啶二聚体 (CPDs) 的分布,发现CPDs数量有限且仅出现于皮肤表层[26,29,31-33]。HICKERSON等[32]使用人类体外腹部皮肤 (图2 (a) ) 进行了相似实验,使用远UV-C (6 100 mJ·cm−2) 辐照后仅在表皮最上部出现了较少的CPDs (图2 (c) ) ,而使用UV-B (515 mJ·cm−2) 辐照后在表层和基底层均检测到CPDs (图2 (b) ) 。
2名研究人员自愿使用前臂皮肤进行了条件相同的远UV-C辐照 (图2 (d) ) ,实验结果与体外腹部皮肤相同。由于高剂量的UV-B对人体伤害较大,并未进行基于UV-B的对照实验[32]。可以看出远UV-C辐照皮肤后CPDs只出现在无增殖能力的表层细胞上,没有到达拥有增殖能力的基底层,因此研究人员认为其没有致癌风险[32,34]。远UV-C辐照安全性实验中,辐照剂量远高于远UV-C的安全阈值 (23 mJ·cm−2,ACGIH[16]) ,而安全阈值又高于消毒所需剂量,间接证明合理使用远UV-C消毒不会导致皮肤损伤。
-
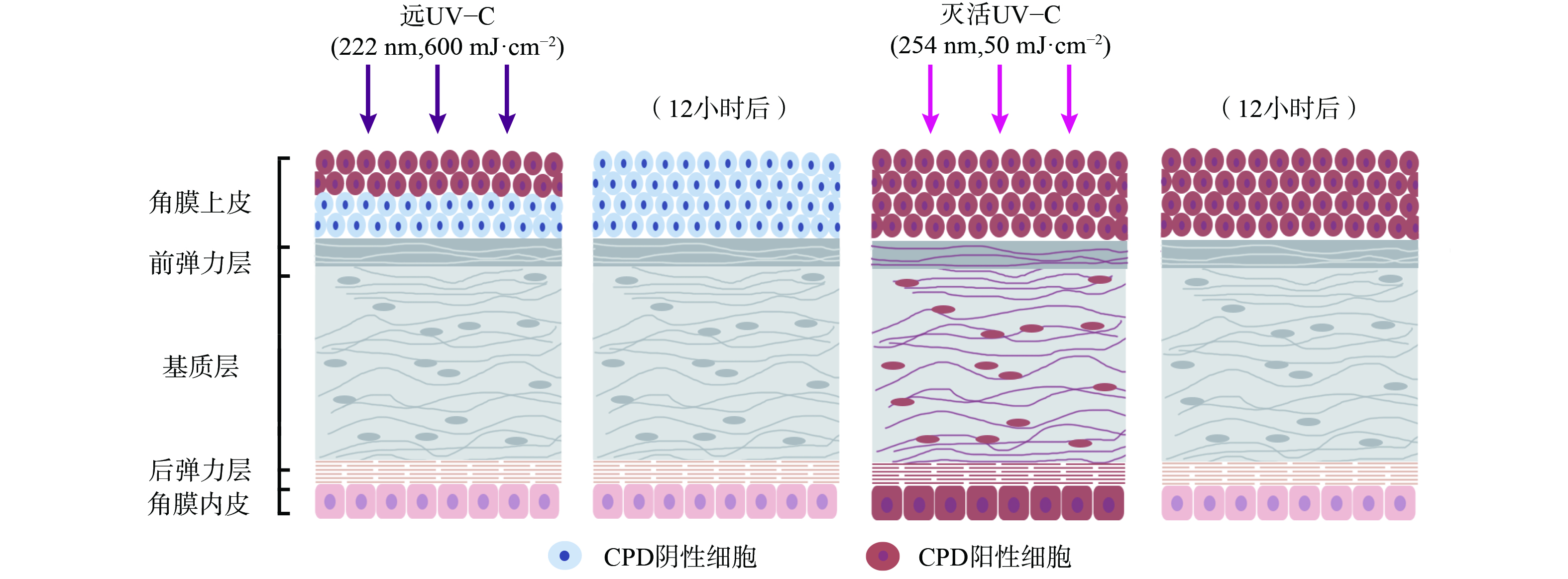
UV辐射会诱发光性角膜炎、翼状胬肉等眼部疾病[35],但目前实验表明远UV-C对眼睛的影响有限。其原理在于远UV-C会被角膜上皮强烈吸收,而角膜上皮细胞会不断地自我脱落,由底层细胞分化补充。KAIDZU等[36-37]通过动物实验评估,发现远UV-C在207 nm和222 nm引发角膜炎的安全阈值分别超过10 000 mJ·cm−2和3 500 mJ·cm−2,远高于目前ACGIH的远UV-C安全阈值。KAIDZU等[36-37]还进行了角膜组织学染色评估 (图3) ,使用CPDs作为DNA损伤的标记,在222 nm远UV-C辐照下,仅在大鼠角膜上皮最外层细胞中观察到CPDs。而这些细胞在几天内就会正常脱落,数据也表明12小时后角膜上皮最外层细胞检测不到CPDs。作为对照,在254 nm UV辐照下,CPDs在角膜各层 (包括角膜内皮) 均被发现,且12小时后角膜上皮中仍检测到CPDs。这表明222 nm波长的UV-C几乎不能穿透角膜上皮,而254 nm则能够穿透上皮细胞和基质层。此外,实验中同样使用222 nm远UV-C辐照小鼠、猪和兔子眼部,同样只有在角膜上皮最外层检测到CPDs,且角膜上皮会正常脱落[37]。与皮肤辐照实验类似,眼部辐照实验中远UV-C剂量远高于安全阈值,间接证明了远UV-C安全消毒剂量下不会对角膜产生危害。
-
1) 远UV-C的辐照安全性。尽管近年来已有很多研究表明远高于消毒所需剂量的远UV-C辐照对皮肤和眼睛不会造成伤害[23-33,36-37],满足人机共存原位消毒的安全性要求。但目前结论均源自各国科研人员分散的研究成果,而并非相关部门/组织给出的结论。国际紫外线协会 (IUVA) 发布远UV-C消毒的白皮书,其中提出远UV-C辐照安全性的结论仅为作者观点,并非IUVA的观点[38]。
辐照安全性关乎人类健康,需要在大量、长期的研究数据基础上,谨慎做出结论。因此,目前远UV-C消毒的应用 (特别是直接长期皮肤和眼睛暴露) 仍需谨慎,不应直接宣传其对人体辐照没有伤害,以防误导使用者。可在现有的实验证据基础上,逐步提高远UV-C安全使用的剂量阈值。目前已有最新研究呼吁将远UV-C (222 nm) 暴露安全阈值从23 mJ·cm−2提升至161 mJ·cm−2 (眼部) 和479 mJ·cm−2 (皮肤) [16,39],ACGIH也已多次讨论了远UV-C安全剂量阈值的提高。在实际消毒时,应基于安全剂量阈值,以及该阈值已远高于消毒所需剂量的研究结果,优化设计消毒装置,实现病原微生物原位有效灭活,同时避免皮肤和眼睛的高剂量辐照。
2) 应用过程中会生成少量臭氧及其控制。伴生臭氧导致的二次污染关系到远UV-C应用的安全性。臭氧会影响呼吸、心血管和中枢神经系统,很多负责公众健康的政府部门和非政府组织规定了气相臭氧的暴露限值,如世界卫生组织 (WHO) 规定8小时平均暴露臭氧浓度为0.1 mg·L−1[40],国标《室内空气中臭氧卫生标准》 (GB/T 18202-2000) 规定1h平均最高容许臭氧浓度为0.05 mg·L−1。与常见的真空UV低压汞灯 (即产臭氧低压汞灯) 类似,远UV-C光源辐照氧分子会生成臭氧,臭氧生成速率大于臭氧被UV分解的速率,使空气中臭氧浓度逐渐上升。但氧分子对远UV-C的吸收远低于真空UV (如氙气准分子灯和产臭氧低压汞灯) ,其臭氧生成量也较低;此外部分远UV-C光源会电晕放电产生臭氧,但只有远UV-装置功率较高 (2-10 kV) 时臭氧产生才会较为明显。因此,远UV-C在较小的封闭空间使用时,应注意与通风系统或臭氧淬灭系统 (如还原性物质及活性炭) 联合,将臭氧浓度维持在安全标准以下。此外,部分远UV-C光源采用滤光片,尽量滤除短波段 (低于222 nm) UV,降低臭氧生成。
目前市场上生产的远UV-C光源几何结构、输出功率不同,光源也存在使用时长和应用空间的差异,远UV-C光源的臭氧风险难以准确评估。因此,在设计合适的臭氧控制措施的同时,还需考虑远UV-C光源臭氧风险的监测与评估方法,为标准化应用提供依据。
3) 应用场景问题。远UV-C可在对人体皮肤和眼睛暴露相对安全的情况下,灭活空气中和物体表面的病原微生物,在不同介质 (特别是空气) 消毒中均具有宽广的应用领域和巨大的发展潜力。目前,国内外多个公司开发了可应用于不同领域的远UV-C消毒设施,如高风险人员密集空间和封闭空间内空气消毒的壁挂式和下射式灯具,物体表面、紧凑空间、贵重仪器等消毒的手持式灯具和消毒门等。此外,随着未来更多类型的远UV-C光源出现,其将对阻断高传染性疾病的传播起到重要作用。
远C波段紫外线消毒的原理及应用前景
Principles and application perspective of far UV-C disinfection
-
摘要: 紫外线 (UV) 是一种高效、绿色的消毒技术,广泛应用于气、水和物体表面的病原微生物灭活。远C波段UV (远UV-C,200~230 nm) 消毒所需剂量辐照对人体的伤害尚未被发现,表明远UV-C具备人机共存原位消毒的潜力,因此该技术近期受到关注。概述了远UV-C光源、灭活机制和辐照安全性方面的研究进展:输出主峰位和半峰宽分别为222 nm和4 nm的KrCl准分子灯是最为成熟的消毒用远UV-C光源;远UV-C通过蛋白质损伤和核酸干扰2种途径实现病原微生物灭活,灭活能力较传统UV-C (如254 nm) 更强;尚未发现消毒所需剂量的远UV-C辐照导致的健康危害,如红斑和角膜炎。而在实际应用中,应谨慎对待高剂量远UV-C辐照暴露,确保在现有实验证据基础上,逐步提高远UV-C安全使用的剂量阈值,并考虑与通风系统或臭氧淬灭系统协同使用以避免伴生臭氧造成的二次伤害。本文旨在为远UV-C在高效灭活病原微生物、阻断高传染性疾病传播领域的应用提供参考。Abstract: Ultraviolet (UV), as an efficient and green disinfection technology, has been widely applied to inactivate the pathogens in air, water, and surface. Recently, far UV-C radiation (200-230 nm) has drawn great attention, as it is harmless to human within the normal dose range for disinfection, which has great potential to conduct in situ disinfection with human presence.An overview on far UV-C light sources, inactivation mechanisms, and human health and safety to far UV-C exposure was provided in this article. It was found that krypton-chloride (KrCl) excimer lamps with a main peak at 222 nm and half-peak width at 4 nm were the most mature far UV-C light sources for disinfection, far UV-C inactivated pathogenic microorganisms through protein damage and nucleic acid interfere,and the inactivation ability of far UV-C was stronger than that of traditional UV-C (such as 254 nm). No adverse health effects such as erythema and photokeratitis had been reported for far UV-C irradiation within the dose range required by regular disinfection. However, high dose of far UV-C radiation should still be used with caution in practical applications, where the maximum safe dose should be determined based on experimental evidence and increased gradually. Meanwhile, the use of far-UV-C in collaboration with ventilation system or ozone quenching system should be considered to avoid secondary damage caused by associated ozone. Overall, this article will provide reference for efficient and secure application of far UV-C in disinfection and control of highly infectious epidemics.
-
Key words:
- far UV-C /
- krypton-chloride excimer lamps /
- disinfection /
- secutiry /
- human-machine coexistence
-

-
表 1 远UV-C (222 nm) 和灭活UV-C (254 nm) 对致病微生物和受试微生物的剂量响应关系
Table 1. Dose-response data of pathogenic and challenge microorganisms radiated by far UV-C (222 nm) and UVGI (254 nm)
病毒种类 波长/nm 达到相应灭活率所需UV剂量/(mJ·cm−2) 灭活速率常数/
(cm2·mJ−1)文献 1-lg 2-lg 3-lg 4-lg SARS-CoV-2 222a) (含滤光片) 1.6 3.1 4.7 6.3 0.64 [9] SARS-CoV-2 222 (含滤光片) 0.7 1.4 2.1 2.8 1.42 [8] SARS-CoV-2 222 0.7 1.3 2.0 2.6 1.52 [8] SARS-CoV-2 254b) 1.3 2.5 3.8 5.1 0.79 [8] SARS-CoV-2 254 1.7 4.2 5.1 6.8 0.59 [10] MHV 222 (含滤光片) 1.0 1.9 2.9 3.9 1.03 [11] MHV 222 0.8 1.6 2.5 3.3 1.22 [11] MHV 254 1.1 2.2 3.2 4.3 0.93 [11] HCoV 229E 222 (含滤光片) 1.2 2.4 3.6 4.8 0.84 [11] HCoV 229E 222 0.8 1.5 2.3 3.0 1.33 [11] HCoV 229E 254 1.7 3.4 5.1 6.8 0.59 [11] Phi 6 222 (含滤光片) 2.8 5.6 8.3 11.1 0.36 [11] Phi 6 222 3.7 7.4 11.1 14.8 0.27 [11] Phi 6 254 33.3 66.6 100.0 133.3 0.03 [11] T1UV 222c) 2.7 5.5 8.2 11.0 0.37 [12] T1UV 254c) 4.3 8.5 12.8 17.0 0.23 [12] MS2 222c) 8.9 17.7 26.6 35.5 0.11 [12] MS2 254c) 16.0 33.6 53.4 77.6 0.05 [12] 注:a) 222 nm如没有特殊标注均为KrCl准分子灯;b) 254 nm如没有特殊标注均为低压汞灯;c) NT242系列可调谐激光器 (NIST)。 -
[1] SOSNIN E A, OPPENLÄNDE R T, TARASENKO V F. Applications of capacitive and barrier discharge excilamps in photoscience[J]. Journal of Photochemistry and Photobiology C:Photochemistry Reviews, 2006, 7(4): 145-163. doi: 10.1016/j.jphotochemrev.2006.12.002 [2] KNEISSL M, RASS J. III-Nitride ultraviolet emitters [M]. Springer, 2016. [3] United States Environmental Protection Agency. Ultraviolet disinfection guidance manual for the final long term 2 enhanced surface water treatment rule [S]. Washington, DC, 2006. [4] BOLTON J R, COTTON C A. The ultraviolet disinfection handbook [M]. American Water Works Association, 2011. [5] BECK S E, HULL N M, POEPPING C, et al. Wavelength-dependent damage to adenoviral proteins across the germicidal UV spectrum[J]. Environmental Science & Technology, 2018, 52(1): 223-229. [6] BECK S E, RODRIGUEZ R A, LINDEN K G, et al. Wavelength dependent UV inactivation and DNA damage of adenovirus as measured by cell culture infectivity and long range quantitative PCR[J]. Environmental Science & Technology, 2014, 48(1): 591-598. [7] LINDEN K G, THURSTON J, SCHAEFER R, et al. Enhanced UV inactivation of adenoviruses under polychromatic UV lamps[J]. Applied and Environmental Microbiology, 2007, 73(23): 7571-7574. doi: 10.1128/AEM.01587-07 [8] MA B, GUNDY P M, GERBA C P, et al. UV inactivation of SARS-CoV-2 across the UVC spectrum: KrCl* excimer, mercury-vapor, and light-emitting-diode (LED) sources[J]. Applied and Environmental Microbiology, 2021, 87(22): e01532-21. [9] ROBINSON R T, MAHFOOZ N, ROSAS-MEJIA O, et al. SARS-CoV-2 disinfection in aqueous solution by UV222 from a krypton chlorine excilamp [J]. MedRxiv, 2021. [10] STORM N, MCKAY L G, DOWNS S N, et al. Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation[J]. Scientific Reports, 2020, 10(1): 1-5. doi: 10.1038/s41598-019-56847-4 [11] MA B, LINDEN Y S, GUNDY P M, et al. Inactivation of coronaviruses and phage Phi6 from irradiation across UVC wavelengths[J]. Environmental Science & Technology Letters, 2021, 8(5): 425-430. [12] BECK S E, WRIGHT H B, HARGY T M, et al. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems[J]. Water Research, 2015, 70: 27-37. doi: 10.1016/j.watres.2014.11.028 [13] TSENG C C, LI C S. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation[J]. Journal of Occupational and Environmental Hygiene, 2007, 4(6): 400-405. doi: 10.1080/15459620701329012 [14] WALKER C M, KO G P. Effect of ultraviolet germicidal irradiation on viral aerosols[J]. Environmental Science & Technology, 2007, 41(15): 5460-5465. [15] KITAGAWA H, NOMURA T, NAZMUL T, et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination[J]. American Journal of Infection Control, 2021, 49(3): 299-301. doi: 10.1016/j.ajic.2020.08.022 [16] American Conference of Governmental Industrial Hygienists. 2021 threshold limit values (TLVs) and biological exposure indices (BEIs) [C]. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2020. [17] International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation)[J]. Health Physics, 2004, 87(2): 171-186. doi: 10.1097/00004032-200408000-00006 [18] SLINEY D. Balancing the risk of eye irritation from UV-C with infection from bioaerosols[J]. Photochemistry and Photobiology, 2013, 89(4): 770-776. doi: 10.1111/php.12093 [19] CESARINI J P, COLE C A, GRUIJL F D. UV-C photocarcinogenesis risks from germicidal lamps[J]. Int Commission Illumination, 2010, 187: 1-14. [20] FORBES P D, COLE C A, DEGRUIJL F. Origins and evolution of photocarcinogenesis action spectra, including germicidal UVC[J]. Photochemistry and Photobiology, 2021, 97(3): 477-484. doi: 10.1111/php.13371 [21] MOAN J, GRIGALAVICIUS M, BATURAITE Z, et al. The relationship between UV exposure and incidence of skin cancer[J]. Photodermatology, Photoimmunology & Photomedicine, 2015, 31(1): 26-35. [22] WOODS J A, EVANS A, FORBES P D, et al. The effect of 222‐nm UVC phototesting on healthy volunteer skin: a pilot study[J]. Photodermatology, Photoimmunology & Photomedicine, 2015, 31(3): 159-166. [23] FUKUI T, NIIKURA T, ODA T, et al. Exploratory clinical trial on the safety and bactericidal effect of 222-nm ultraviolet C irradiation in healthy humans[J]. Plos One, 2020, 15(8): e0235948. doi: 10.1371/journal.pone.0235948 [24] PONNAIYA B, BUONANNO M, WELCH D, et al. Far-UVC light prevents MRSA infection of superficial wounds in vivo[J]. Plos One, 2018, 13(2): e0192053. doi: 10.1371/journal.pone.0192053 [25] GOH J C, FISHER D, HING E C H, et al. Disinfection capabilities of a 222 nm wavelength ultraviolet lighting device: a pilot study[J]. Journal of Wound Care, 2021, 30(2): 96-104. doi: 10.12968/jowc.2021.30.2.96 [26] BUONANNO M, PONNAIYA B, WELCH D, et al. Germicidal efficacy and mammalian skin safety of 222-nm UV light[J]. Radiation Research, 2017, 187(4): 493-501. doi: 10.1667/RR0010CC.1 [27] BUONANNO M, STANISLAUSKAS M, PONNAIYA B, et al. 207-nm UV light-A promising tool for safe low-cost reduction of surgical site infections. II: In-vivo safety studies[J]. Plos One, 2016, 11(6): e0138418. doi: 10.1371/journal.pone.0138418 [28] CADET J. Harmless effects of sterilizing 222‐nm far‐UV radiation on mouse skin and eye tissues[J]. Photochemistry and Photobiology, 2020, 96(4): 949-950. doi: 10.1111/php.13294 [29] BARNARD I R M, EADIE E, WOOD K. Further evidence that far-UVC for disinfection is unlikely to cause erythema or pre-mutagenic DNA lesions in skin[J]. Photodermatology, Photoimmunology & Photomedicine, 2020, 36(6): 476-477. [30] HANAMURA N, OHASHI H, MORIMOTO Y, et al. Viability evaluation of layered cell sheets after ultraviolet light irradiation of 222 nm[J]. Regenerative Therapy, 2020, 14: 344-351. doi: 10.1016/j.reth.2020.04.002 [31] YAMANO N, KUNISADA M, KAIDZU S, et al. Long‐term effects of 222‐nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation[J]. Photochemistry and Photobiology, 2020, 96(4): 853-862. doi: 10.1111/php.13269 [32] HICKERSON R P, CONNEELY M P, TSUTSUMI S K H, et al. Minimal, superficial DNA damage in human skin from filtered far-ultraviolet C[J]. British Journal of Dermatology, 2021, 184(6): 1197-1199. doi: 10.1111/bjd.19816 [33] BUONANNO M, WELCH D, BRENNER D J. Exposure of human skin models to KrCl excimer lamps: The impact of optical filtering[J]. Photochemistry and Photobiology, 2021, 97(3): 517-523. doi: 10.1111/php.13383 [34] YOUNG A R, HARRISON G I, CHADWICK C A, et al. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema[J]. Journal of Investigative Dermatology, 1998, 111(6): 982-988. doi: 10.1046/j.1523-1747.1998.00436.x [35] DELIC N C, LYONS J G, GIROLAMO N D, et al. Damaging effects of ultraviolet radiation on the cornea[J]. Photochemistry and Photobiology, 2017, 93(4): 920-929. doi: 10.1111/php.12686 [36] KAIDZU S, SUGIHARA K, SASAKI M, et al. Evaluation of acute corneal damage induced by 222-nm and 254-nm ultraviolet light in Sprague–Dawley rats[J]. Free Radical Research, 2019, 53(6): 611-617. doi: 10.1080/10715762.2019.1603378 [37] KAIDZU S, SUGIHARA K, SASAKI M, et al. Re‐evaluation of rat corneal damage by short‐wavelength UV revealed extremely less hazardous property of Far‐UV‐C[J]. Photochemistry and Photobiology, 2021, 97(3): 505. doi: 10.1111/php.13419 [38] BLATCHLEY E R, BRENNER D, CLAUS H, et al. Far UV-C radiation: current state-of knowledge. IUVA White Paper. (2021-5-11). [39] SLINEY D H, STUCK B E. A need to revise human exposure limits for ultraviolet UV-C radiation[J]. Photochemistry and Photobiology, 2021, 97(3): 485-492. doi: 10.1111/php.13402 [40] World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease [R]. 2016. -




 下载:
下载: