-
烟气中SO2气体污染物是形成酸雨的重要前驱体物质,NO的排放则会引发光化学烟雾,燃料不完全燃烧导致的COx浓度不断上升进而引发的温室效应等大气污染问题受到越来越多的关注[1-3]。目前,许多污染控制技术用于解决单独的污染物去除问题,而污染物一体化去除的吸附技术因节省大量成本和操作简便等优点而得以快速发展,而开发针对多气体污染同时去除的功能材料便尤为重要。
吸附技术由于其方便、高效及可同时去除污染物硫硝碳等优点,已成为一体化烟气净化技术首选。吸附技术即利用吸附剂 (如活性炭、沸石分子筛、活性氧化铝等) 吸附有害成分,达到消除污染的目的。吸附剂应具有丰富的孔结构、较大的比表面积、良好的化学性质和热稳定性等较好的吸附性能[4]。金属有机骨架材料(metal-organic framework, MOF),也称为配位聚合物,是一类由金属离子/簇作为次级构造单元,与有机配体自组装而成的网络结构多孔材料。相比于其他传统多孔材料(分子筛、活性炭、硅胶等),MOF具有更高的孔隙率、较大的比表面积、高度可调控的孔尺寸和暴露的活性位点等,因而在气体吸附领域显示出巨大的潜力[5-7]。在各种MOF材料中,M-MOF -74系列(M = Mg, Zn, Ni, Co, Mn, Fe) 表现为一维六方孔道的骨架材料,具有相同的晶体结构和不同的不饱和配位金属中心,其独特的无限次级结构基元使得材料的单一晶格对异金属有更强的包容性,为探索开放金属位在气体吸附中的作用提供了理想的多金属共混体系[8]。
目前,双金属MOF-74较单金属材料具有更优异的吸附性能。孔乾乾[9]制备的两种材料MgNi-MOF-74和CoNi-MOF-74相较于单金属Ni-MOF-74有明显更高的氢气吸附量,在77 K、100 kPa下最高的氢气吸附量 (质量分数) 为2.12%,比单金属材料的吸附量提升了25%。孙豪[10]采用热溶剂法制备的CoFe-MOF-74对二氧化碳的最高吸附量为65.51 mL·g−1,比单金属Co-MOF-74的吸附量提升了近28.1%。孔道内丰富的不饱和金属位点使得MOF-74在气体脱除方面有突出表现,然而现有研究中关于多组分共吸附的研究仍较少。另外,由于MOF-74对水分子和氧气的吸附能力同样较强,会导致与反应气体的竞争吸附而发生吸附效果下降的现象[11]。
基于此,本研究采用热溶剂法成功制备了双金属NiMg-MOF-74吸附剂,并研究其共吸附硫硝碳的性能,后通过氨水浸渍材料进一步提高其吸附容量及材料稳定性,并利用TPD结合傅里叶原位红外光谱技术探究NiMg-MOF-74共吸附硫硝碳机理,以期揭示氨改性后性能提高的机制。本研究可为一体化烟气净化材料的开发提供参考。
-
1) 实验试剂。硝酸镁、硝酸镍、2,5-二羟基对苯二甲酸、N,N-二甲基甲酰胺、甲醇、乙醇、氨水,均为分析纯。
2) 实验仪器及其测定项目。利用X射线衍射仪 (BRUKER D8 ADVANCE) 确定材料的成分和晶相结构,具体条件为Cu Kα辐射在电压为40 kV和100 mA下检测样品;使用FT-IR光谱仪 (VERTEX) 明晰材料表面官能团并确定其物质组成,光谱扫描范围为4 000~400 cm−1;使用ICP-OES (PerkinElmer 7300DV) 进行材料中离子的定量分析;使用SEM (JSM-7500F) 得到材料的形貌信息;利用BET (ASAP 2020型) 比表面仪得到材料的比表面积、孔径分布等相关的骨架结构信息;使用TPD (CHEMBET) 获得材料对目标气体的脱附曲线,采用全自动化学吸附仪进行SO2/NO程序吸脱附分析,脱附前将样品 (100 mg) 在N2气氛中预处理30 min,预处理温度为200 ℃。
-
采用溶剂热法,按照一定的摩尔比称取Ni(NO3)2·6H2O和Mg(NO3)2·6H2O,以及0.167 g的 2,5-二羟基对苯二甲酸。具体MOF-74材料的组成见表1。将以上药品溶解在N,N-二甲基甲酰胺 (67.5 mL) 、乙醇 (4.5 mL) 和水 (4.5 mL) 的混合溶剂中,超声处理30 min使其充分溶解。将所得混合溶液移入聚四氟乙烯内衬的高压反应釜中,置于125 ℃烘箱中恒温反应28 h。将混合物冷却后进行离心操作,并用DMF洗涤3次,所得固体用甲醇浸泡置换3 d,每12 h更换1次甲醇。样品在65 ℃的烘箱中恒温干燥6 h,冷却后进行研磨。最后将样品置于真空管式炉中,升温至250 ℃后恒温活化12 h以得到双金属NixMg1-x-MOF-74。其中,x代表合成液中Ni2+占金属离子总量的摩尔分数。根据材料中镍镁金属的实际比例将合成样品分别记为Ni0.36Mg0.64-MOF-74、Ni0.68Mg0.32-MOF-74和Ni0.83Mg0.17-MOF-74。
取上述未经活化的NiMg-MOF-74材料置于锥形瓶中,分别用浓度为0.2、0.6、1 mol·L−1的氨水溶液浸泡,之后将锥形瓶置于恒温振荡器中室温下震荡24 h,转速为160 r·min−1。将溶液离心后用甲醇浸泡置换,后续处置与双金属NiMg-MOF-74样品相同。将浓度为0.2、0.6和1 mol·L−1的氨水改性制得的Ni0.83Mg0.17-MOF-74分别标记为0.2NH3@Ni0.83Mg0.17-MOF-74、0.6NH3@ Ni0.83Mg0.17-MOF-74和1NH3@Ni0.83Mg0.17-MOF-74。
-
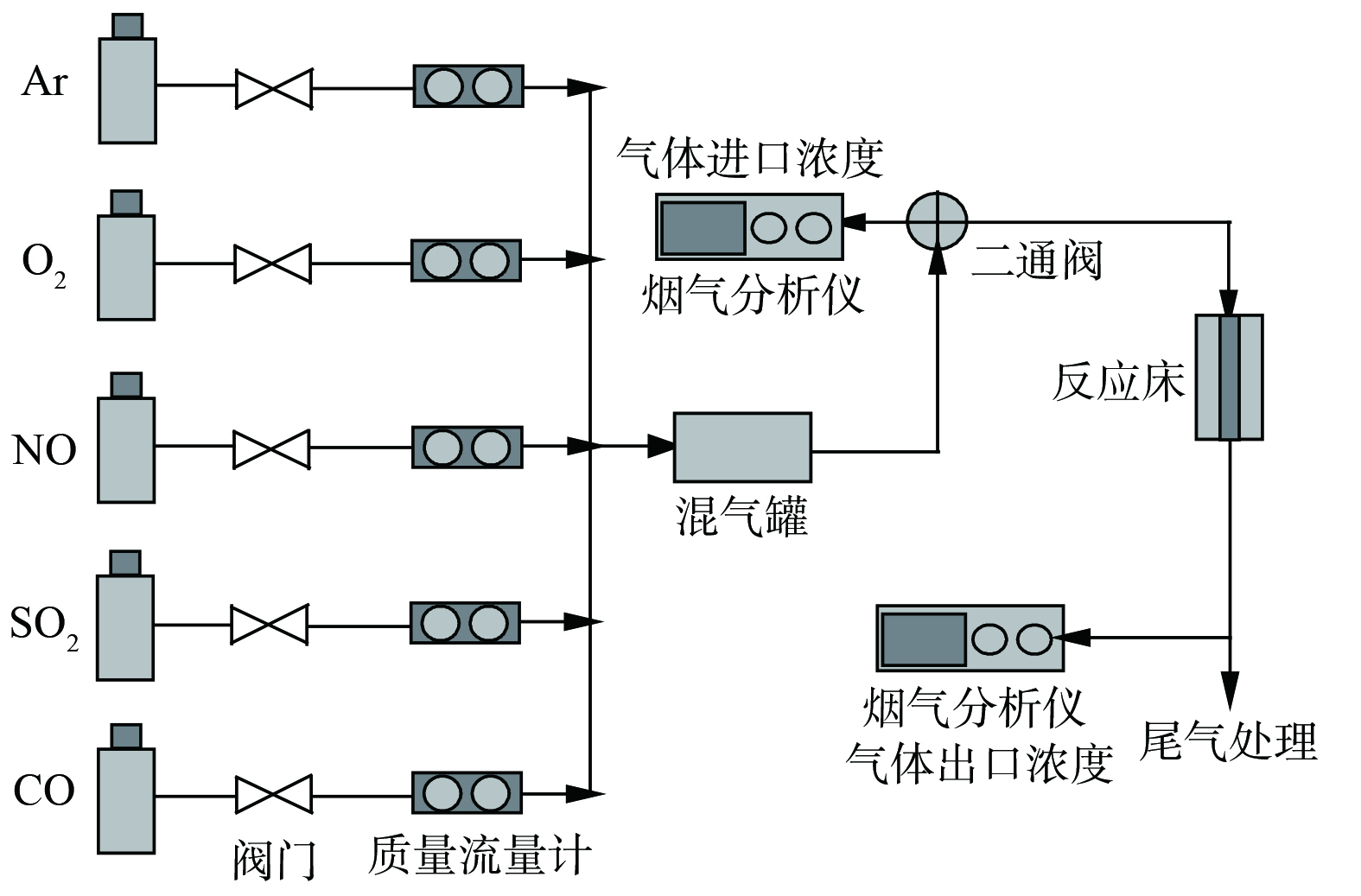
采用动态吸附法测试吸附剂的吸附性能,流程如图1所示。利用质量流量计控制气体 (SO2、NO、CO等) 各配比组分的浓度,按照要求的体积浓度比在混合罐内混合均匀后,将其通入固定床反应器,净化后的气体经过碱液处理达标后再排入空气。反应器为石英圆管,外直径10 mm,内直径为7 mm,长为450 mm。将0.3 g吸附剂置于反应器中,在100 ℃时的高纯度氩气气氛中预处理30 min,以此来去除吸附剂中的水分及杂质,冷却至目标温度后进行共吸附反应。通入混合气体总流量为170 mL·min−1,SO2、NO和CO的体积百分数分别为0.05%、0.05%和0.04%。用烟气分析仪测定净化后的出口气体样品浓度。用吸附剂吸附效率ƞ和吸附量q表示吸附剂脱除效率,计算方法见式 (1) 和 (2) 。
式中:cin为气体进口体积百分数,%;cout为气体出口体积百分数,%。
式中:cin为气体进口体积百分数,%;cout为t时刻气体出口体积百分数,%;q为吸附量,mmol·g−1;Q为气体流量,mL·min−1;m为吸附剂用量,g。
-
采用电感耦合等离子体质谱 (ICP-AES) 测试双金属MOF-74中Ni和Mg的实际含量,结果见表2。吸附剂中的Ni和Mg实际比例与理论比例有一定偏差,镍的实际占比始终大于其理论占比,而金属镁的情况却相反。该结果与GUO等[12]的研究结果一致,这表明镍镁双金属在合成MOF-74材料时,Ni2+比Mg2+更容易与有机配体发生配位,导致Mg2+反应不完全,最终以金属盐的形式残留在合成液体中[13]。
-
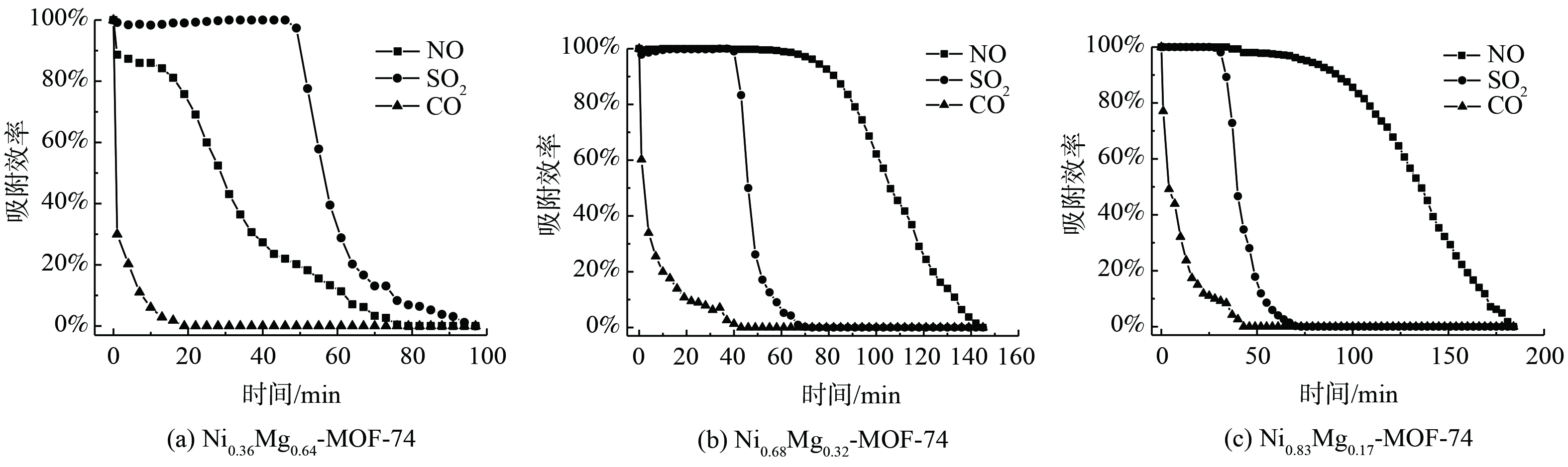
实际排放的燃煤烟气经除尘装置,或与其他热能利用装置联用,出口气体温度为95~120 ℃,故在模拟硫硝碳脱除的实验中选定100 ℃为反应温度。在100 ℃下,吸附剂双金属MOF-74对SO2、NO和CO的共吸附效率见图2。3种材料对SO2、NO和CO的共吸附情况各不相同。其中,SO2、NO和CO在Ni0.36Mg0.64-MOF-74吸附剂上的吸附饱和时间顺序为SO2 > NO > CO (图2 (a) ) ,对于Ni0.68Mg0.32-MOF-74和Ni0.83Mg0.17-MOF-74,SO2、NO和CO 3种气体在吸附剂上的吸附饱和时间为NO > SO2 >> CO。图2 (b) 和 (c) 表明Ni0.68Mg0.32-MOF-74和Ni0.83Mg0.17-MOF-74对NO的吸附效果较为突出,NO达到吸附饱和所需时间分别为142 min和180 min。3种双金属MOF-74吸附剂对NO的饱和吸附量大小顺序为Ni0.83Mg0.17-MOF-74 (0.339 mmol·g−1) > Ni0.68Mg0.32-MOF-74 (1.200 mmol·g−1) > Ni0.36Mg0.64-MOF-74 (1.447 mmol·g−1) ,而对SO2的饱和吸附量大小顺序为Ni0.36Mg0.64-MOF-74 (0.616 mmol·g−1) > Ni0.68Mg0.32-MOF-74 (0.491 mmol·g−1) > Ni0.83Mg0.17-MOF-74 (0.465 mmol·g−1) 。导致这种现象的主要原因是不饱和吸附位点 (Mg—O和Ni—O) 对SO2和NO的亲和力不同。所有吸附剂对CO的脱除效率都较差,其饱和吸附量的大小顺序为Ni0.68Mg0.32-MOF-74 (0.055 mmol·g−1) >Ni0.83Mg0.17-MOF-74 (0.040 mmol·g−1) > Ni0.36Mg0.64-MOF-74 (0.029 mmol·g−1) 。这可能是由于CO与MOF-74上的不饱和金属位点亲和力较弱,在与SO2和NO竞争吸附时处于劣势。
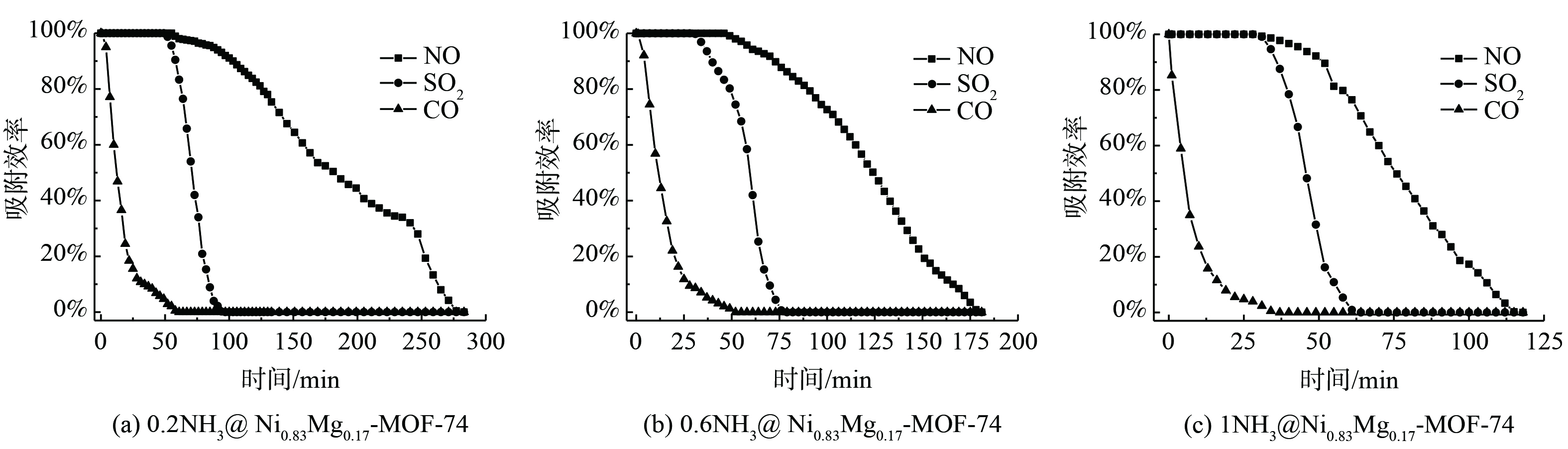
氨水溶液的浓度大小不仅会影响改性后吸附剂的晶体结构和物理化学性质,而且会影响吸附速率及吸附效果,故确定氨水的适宜浓度为改性过程的关键[14]。当氨水浓度过高时,不但起不到改良吸附剂的效果[15],反而会降低其对气体污染物的吸附能力。本研究选择的氨水浓度为0.2、0.6 和1 mol·L−1。在其他条件不变的情况下,对双金属MOF中吸附硫硝碳综合效果最好的Ni0.83Mg0.17-MOF-74吸附剂进行改性,并在100 ℃下进行共吸附SO2、NO和CO性能的测试,结果见图3。
当氨水浓度为0.2 mol·L−1时,对混合气体中NO的吸附饱和时间为275 min,比改性前增加了95 min;对混合气体中的SO2的吸附饱和时间为100 min,比改性之前增加了25 min;对混合气体中的CO,改性后的吸附饱和时间增加了15 min。改性后的0.2NH3@Ni0.83Mg0.17-MOF-74吸附剂对SO2、NO和CO共吸附的饱和吸附量为0.851、2.13和0.187 mmol·g−1,分别为改性前的1.8倍、6.3倍和4.7倍。这表明氨水的引入极大地提高了材料对硫硝碳气体地吸附容量及饱和吸附时间,推测为氨基改性给吸附剂提供了更多碱性基团,从而能吸附更多的酸性气体硫硝碳。当氨水浓度提升至0.6 mol·L−1时,改性后的材料与Ni0.83Mg0.17-MOF-74的共吸附效率相差无几,并未达到提升吸附效果的目的。这可能是由于氨基引入虽改变了吸附位点的酸碱性,但同时也占据了一些不饱和金属位点和孔道,因此,吸附剂的吸附效果并未得到提升[16]。而当氨水浓度为1 mol·L−1时,共吸附SO2、NO和CO的吸附饱和时间减少,可能由于氨水浓度过大,导致原材料的比表面积和孔容减小,从而对气体的吸附效率下降。综上所述,氨水浓度为0.2 mol·L−1的条件下改性得到的Ni0.83Mg0.17-MOF-74吸附剂吸附性能最佳。
-
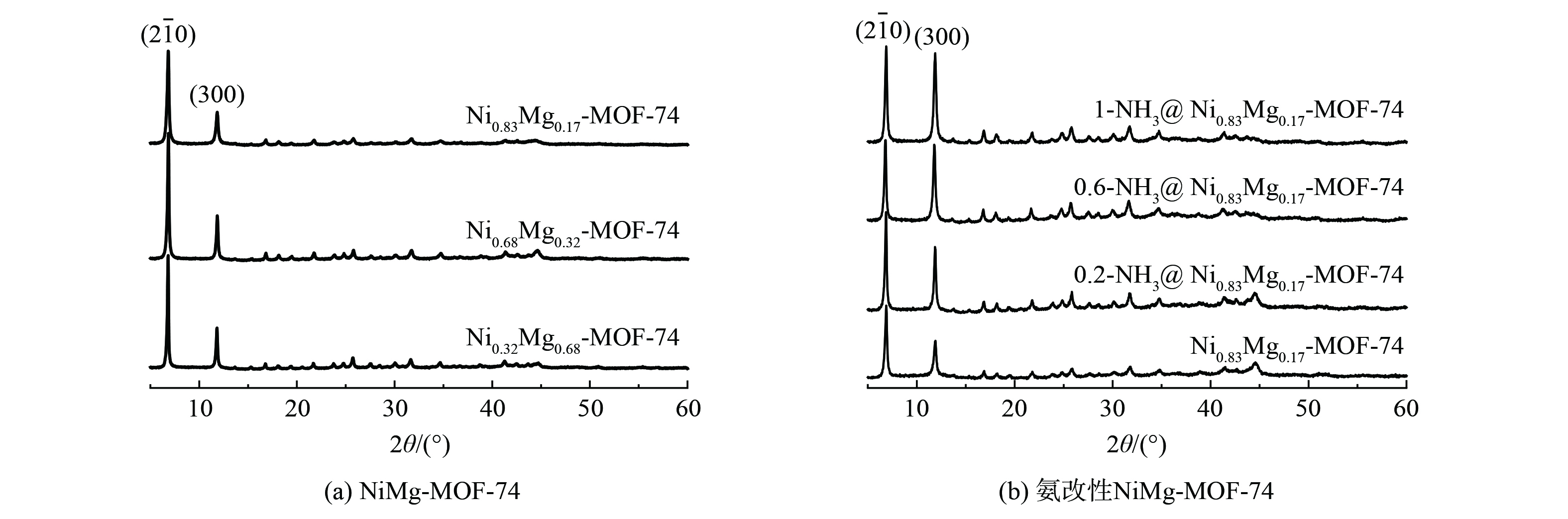
对制备的系列MOF-74吸附剂进行XRD表征测试,结果如图4所示。在2θ为6.8°和11.9°处2个明显的特征峰,这与文献[12]报道的MOF-74标准XRD图谱一致。这说明本研究已成功地制备出双金属NiMg-MOF-74材料。此外,随着材料中Ni含量的增加,NiMg-MOF-74吸附剂在2θ=6.8°处的衍射峰逐渐向高角度方向移动。这是由于随着离子半径较小的Ni2+ (0.069 nm) 引入到离子半径较大的Mg2+ (0.072 nm) 中,导致晶格收缩。另外,氨改性后吸附剂的XRD图谱与改性前一致,都在2θ为6.8°和11.9°处有2个明显的特征峰。这说明引入NH3并未改变样品的晶体结构,改性后材料仍保留良好的晶体结构。
-
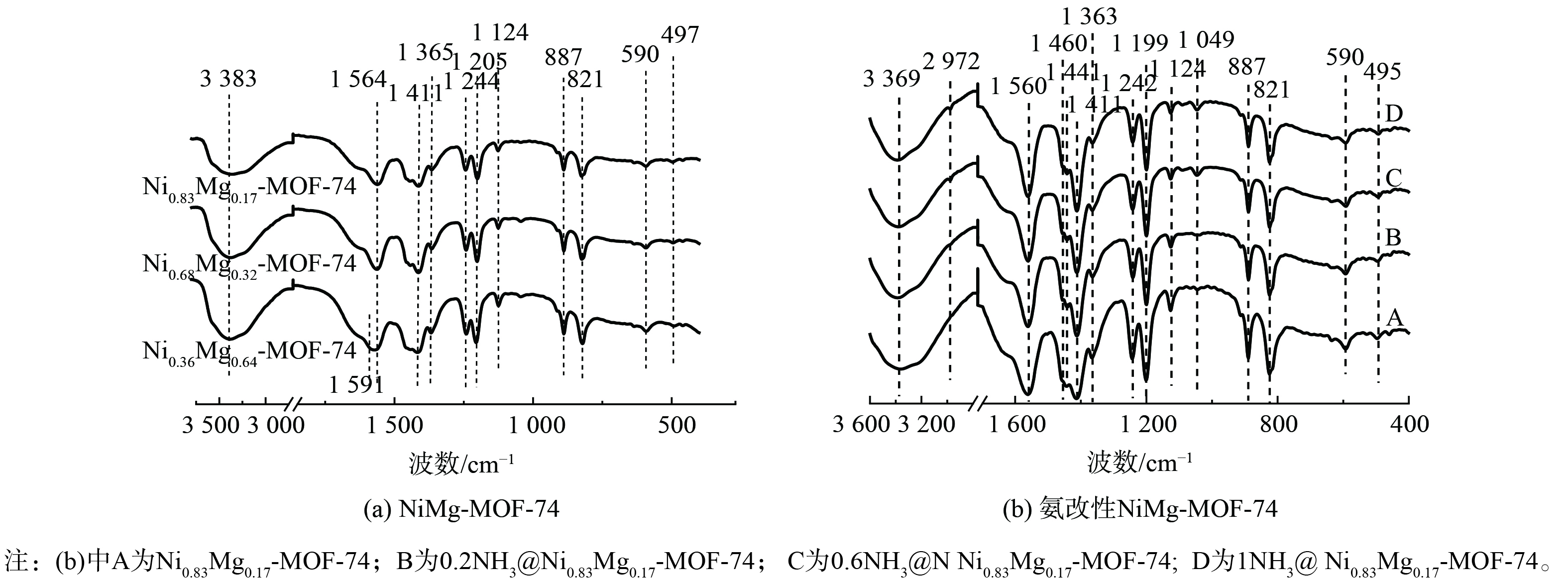
双金属MOF-74吸附剂的FTIR测试结果如图5所示。在所有双金属MOF-74吸附剂的FTIR光谱中,3 383 cm−1附近的谱带归属于羟基O—H的伸缩振动,这是由于多孔材料中吸收了大量的水分子。在1 564 cm−1处的吸收峰为DOBDC中的C==O键,此波数要低于正常C==O键的波数范围。这是由于金属Ni的含量达到一定程度时,双金属MOF-74中的C==O基团与C==C键产生共轭效应。1 411 cm−1的吸收峰为芳香环C==C双键峰,这亦证明了产物分子中有机配体的存在[17];另外,在1 365 cm−1处为芳香环的骨架振动峰;而1 244 cm−1处的弱吸收峰是由于酚盐基团C—O带的拉伸振动;1 201 cm−1为芳香环的C—H单键的伸缩振动峰;1 124 cm−1为芳香环上的C—N单键的伸缩振动峰,化学键强度很低;891 cm−1和821 cm−1两个峰为苯环面外弯曲振动与环弯曲振动峰;594 cm−1和497 cm−1处2个吸收峰对应NiMg-MOF-74材料中Mg—O键和Ni—O的拉伸振动[18-20],不同比例的双金属NiMg-MOF-74间官能团并无明显变化。在氨改性后吸附剂的红外衍射光谱在1 460 cm−1处出现的新衍射峰,归属于NH3+的对称变角振动。这说明氨基被成功引入MOF-74骨架中,并且与Ni0.83Mg0.17-MOF-74发生了化学链接。同时,改性后的其余特征峰与改性前Ni0.83Mg0.17-MOF-74基本一致,特征峰吻合良好,这表明氨基引入未对原样品的官能团造成影响。
-
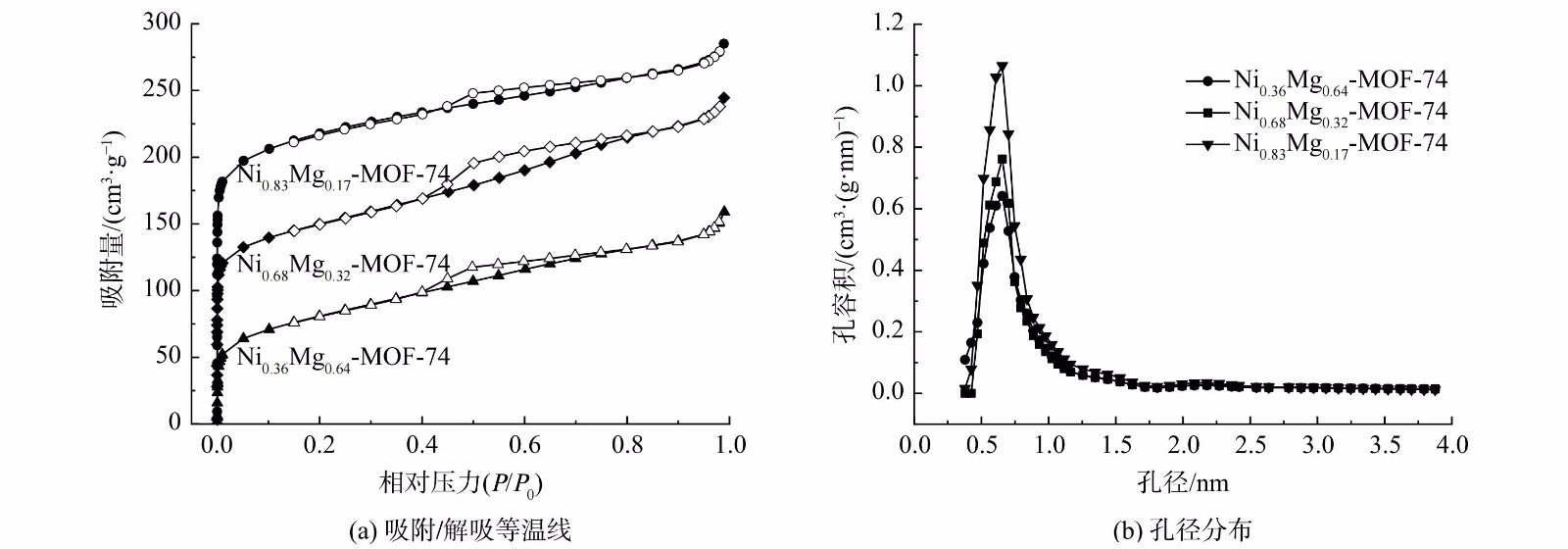
所有MOF-74样品的氮气吸附-脱附等温线如图6所示。在低压区域,N2的吸附量急剧上升,随着相对压力的增加,N2吸附量的增加趋势趋于平缓,符合Langmuir Ⅰ型吸附等温线。这说明双金属MOF-74是微孔材料。值得注意的是,在相对压力较高的区域,曲线出现了较小回滞环。这说明吸附剂中也存在部分介孔。由孔径分布曲线 (图6 (b) ) 可知,NiMg-MOF-74的孔径主要集中在0.4~1.0 nm。
表3列出了NiMg-MOF-74系列吸附剂的比表面积、微孔比表面积和孔容等参数结果。 Ni0.83Mg0.17-MOF-74的比表面积和孔容性能优异,这与其共吸附硫硝碳性能较好的表现一致。
-
所有双金属NiMg-MOF-74的微观形貌几乎一致。以Ni0.83Mg0.17-MOF-74为例 (图7) ,样品呈现梭形结构团聚形成的花簇形晶粒微米针状,与文献[21]报道一致。这表明合成样品具备MOF-74所特有的形貌特征。
-
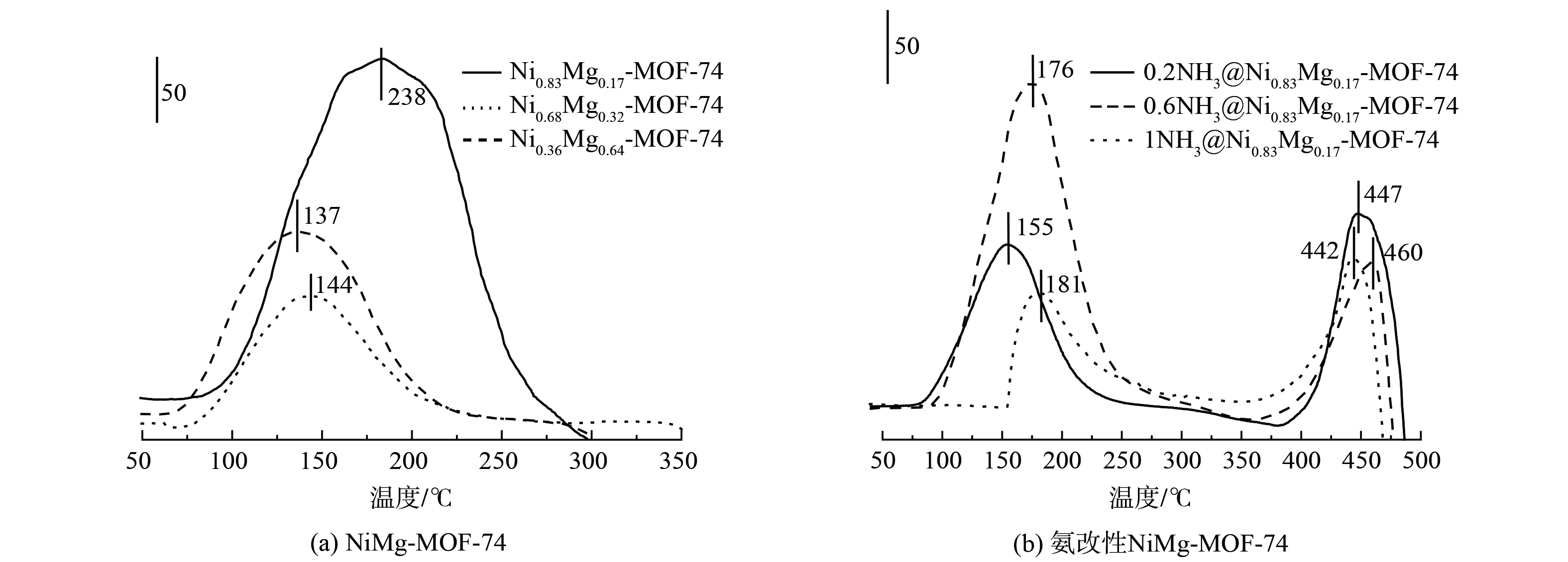
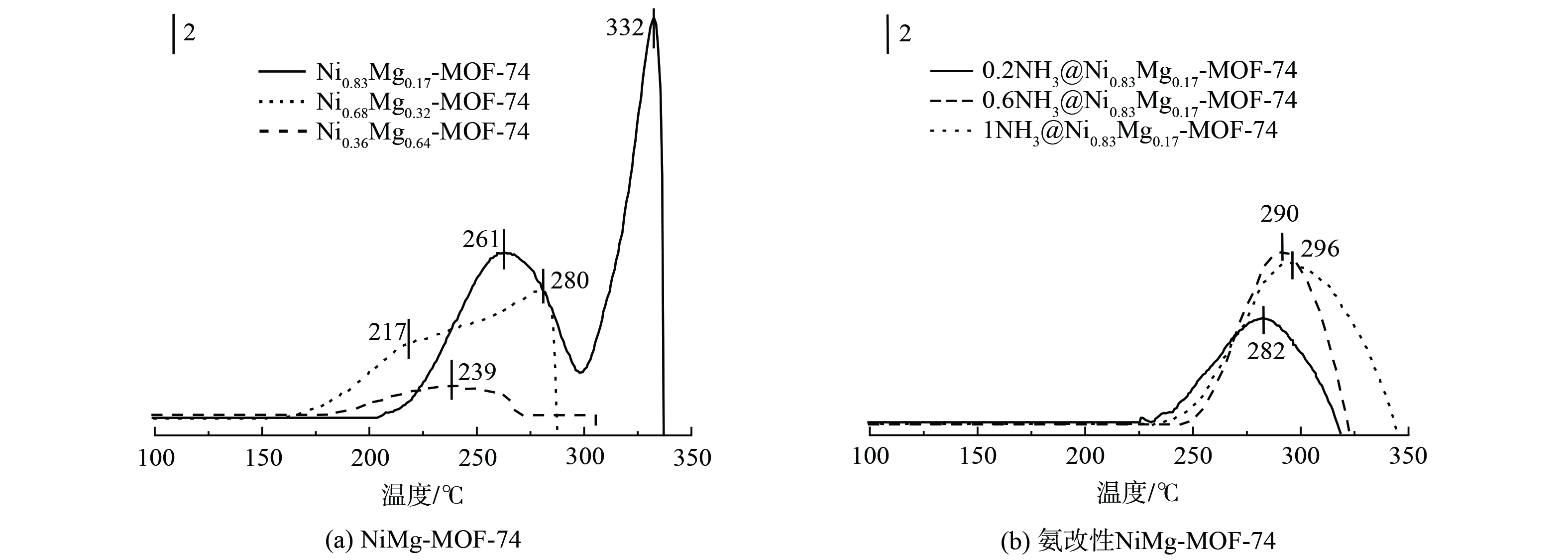
为研究双金属MOF-74表面的碱性位和对SO2的吸附能力,采用SO2程序升温脱附的方法对双金属NixMg1-x-MOF-74 (x=0.36、0.68、0.83) 吸附剂进行测试,结果如图8所示。图8表明,3种双金属材料对SO2的吸附性能有所差异,不过3种样品都在较低的温度范围内仅有1个脱附峰,故可推测双金属NixMg1-x-MOF-74 (x=0.36、0.68、0.83) 在较低温度下的SO2吸附物种较为单一[22]。另外3种样品的SO2脱附温度顺序为T (Ni0.83Mg0.17-MOF-74) > T (Ni0.68Mg0.32-MOF-74) > T (Ni0.36Mg0.64-MOF-74) 。其中,Ni0.83Mg0.17-MOF-74的脱附峰在238 ℃左右,明显高于其他2种样品 (Ni0.68Mg0.32-MOF-74为144 ℃,Ni0.36Mg0.64-MOF-74为137 ℃) ,且由表4中SO2脱附量 (根据曲线积分面积统计的结果) 数据可知,Ni0.83Mg0.17-MOF-74的SO2脱附量也比其他2种样品的SO2脱附量大许多。这说明SO2在Ni0.83Mg0.17-MOF-74上的吸附物种与数量与另外2种吸附剂有所区别,其对SO2的吸附稳定性更好[23]。另外,还观察到氨改性后的MOF-74吸附剂上都有2个SO2脱附峰,在低温范围内SO2的脱附峰归属于吸附态SO2的解析,亦发现其脱附温度随着氨水浓度的升高而升高 (T0.2 < T0.6 < T1) 。其中,0.6-NH3@Ni0.83Mg0.17-MOF-74样品的脱附峰面积明显大于其他2种样品。这说明0.6-NH3@Ni0.83Mg0.17-MOF-74对SO2的吸附能力较强。在高温范围内,SO2的脱附峰归属于化学吸附物质 (硫酸盐或亚硫酸盐) 的解析,脱附温度几乎都在450 ℃左右。以上情况表明,SO2和氨改性后MOF-74吸附剂之间的附着力比改性前更强。这是由于氨水使吸附剂的吸附位点酸碱性发生变化,更偏向于碱性,有利于对酸性气体SO2的吸附。
考察了双金属NixMg1-x-MOF-74 (x=0.36、0.68、0.83) 对NO的吸附能力,测试结果如图9所示。图9表明Ni0.36Mg0.64-MOF-74样品只有1个脱附峰在239 ℃,而另外2个样品都有2个脱附峰。Ni0.68Mg0.32-MOF-74的低温脱附峰在217 ℃左右,高温脱附峰在280 ℃左右。Ni0.83Mg0.17-MOF-74的低温脱附在261 ℃左右,高温脱附峰在332 ℃左右。通常将NO的低温脱附峰归属于NO的物理解吸,高温脱附峰归属于NO与吸附位点紧密结合的化学解吸[18, 24]。值得注意的是,由表4的NO吸附量对比结果来说,Ni0.83Mg0.17-MOF-74的脱附峰面积明显大于另外2种样品,这表明在Ni0.83Mg0.17-MOF-74表面的吸附物质的数量最大,对NO的吸附能力也是最强的。分析其原因,可能是由于Ni0.83Mg0.17-MOF-74吸附剂中拥有更多Ni—O键的吸附位点。改性后的Ni0.83Mg0.17-MOF-74吸附剂在290 ℃左右有NO脱附峰,并且NO脱附温度随着氨水浓度的升高而升高 (T0.2 < T0.6 < T1) ,相比于改性前,改性后的样品只有1个脱附峰,但脱附温度有一定的提升,说明NO和氨改性后MOF-74之间的作用力比改性前的更强。
-
对于共吸附效果良好的Ni0.83Mg0.17-MOF-74及0.2NH3@Ni0.83Mg0.17-MOF-74,通过原位红外实验记录了SO2、NO和CO吸附物种在吸附剂表面的变化情况。
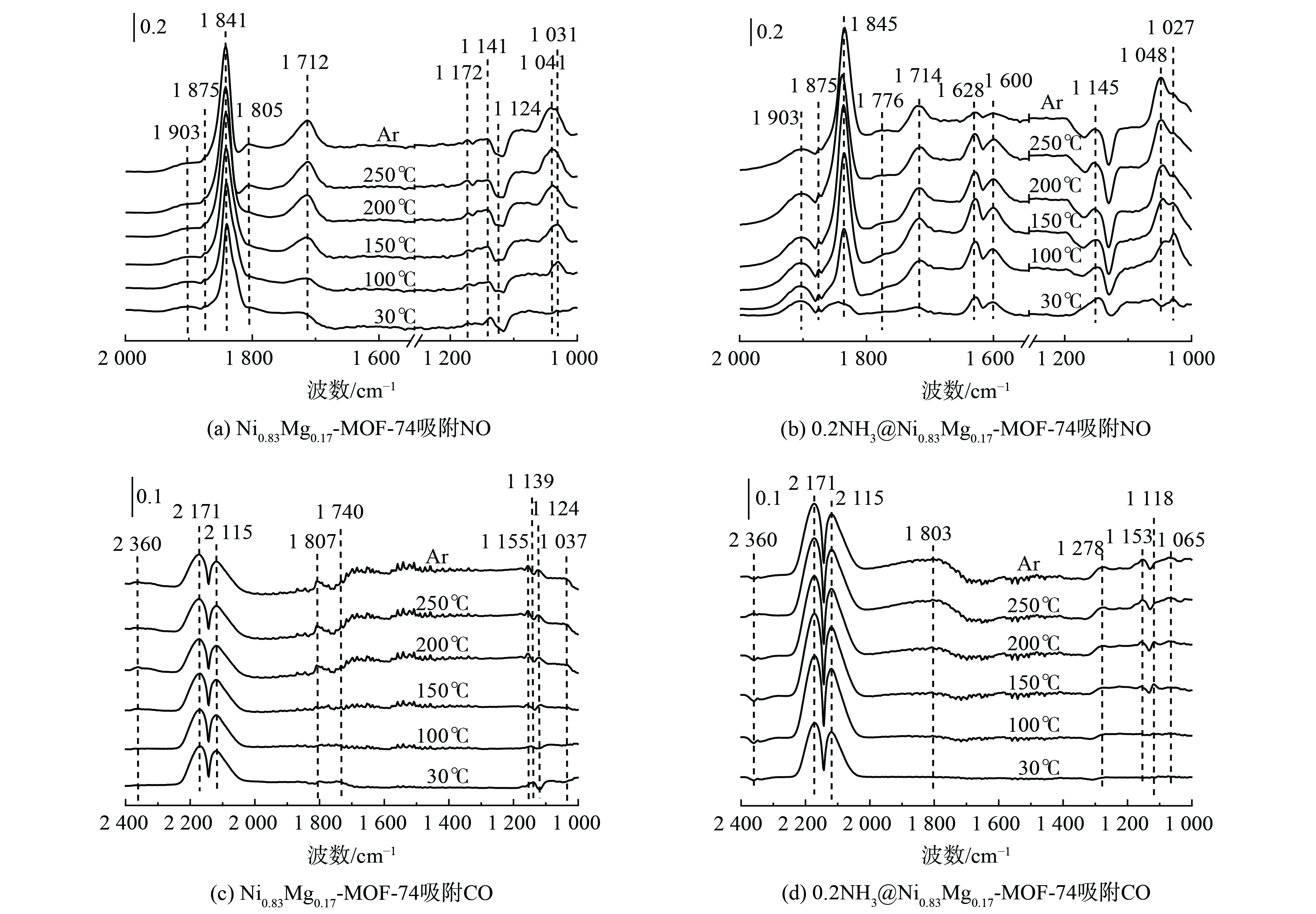
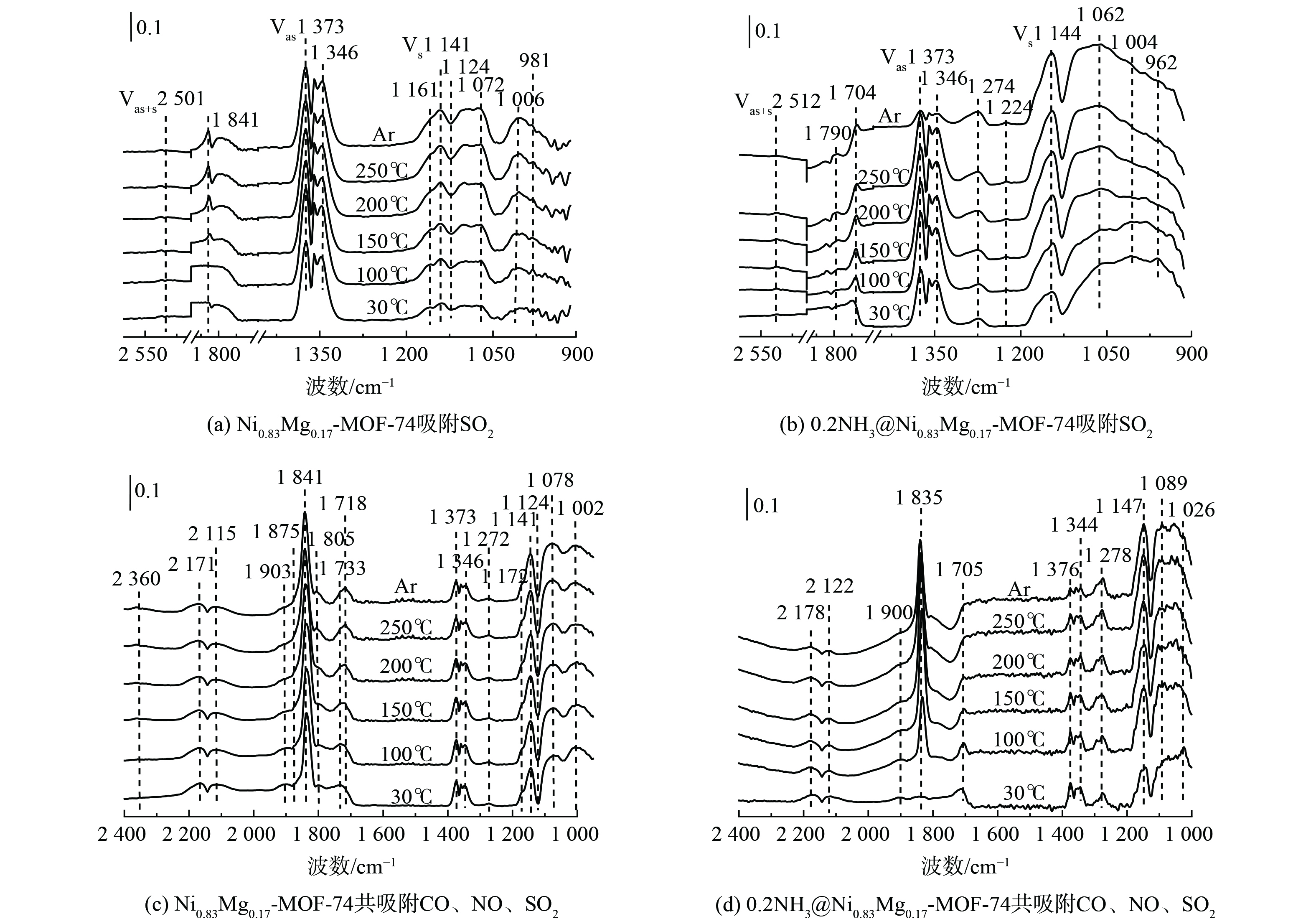
首先进行了Ni0.83Mg0.17-MOF-74及0.2NH3@Ni0.83Mg0.17-MOF-74吸附NO的原位红外光谱实验。对于Ni0.83Mg0.17-MOF-74吸附NO (图10(a)) ,出现了吸附态NO (1 903、1 841 cm−1) 、双齿硝酸盐 (1 172、1 141cm−1) 、螯合双齿硝酸盐 (1 031 cm−1) 及M-NO2硝基化合物 (1 712 cm−1) 。当吸附温度为100 ℃时,出现了新的特征峰,1 805 cm−1 的谱带归属于亚硝酰 (NO) 物种,1 124 cm−1归属于亚硝基物种,1 041 cm−1归属于NO3−中-NO2的对称拉伸振动[25-26]。随着温度的进一步升高,还发现位于1 712 cm−1的谱带明显加强,这可能是由NO氧化产生,Ar气吹扫后产生的硝酸盐物种并未消失。以上情况表明,NO吸附到Ni0.83Mg0.17-MOF-74上会发生化学转化,形成了较多中间体 (NO2,NO3−等) 。氨改性的0.2NH3@Ni0.83Mg0.17-MOF-74 (图10(b)) 除上述物种峰外在1 600和1 628 cm−1处[27]出现新的双齿硝酸盐特征峰,且硝酸盐物种峰有偏移现象,对应其更好的气体活化效果。根据上述结果可推测处NO在吸附剂上发生的反应如式 (3)~(5) 所示。
对CO的吸附结果 (图10 (c) ) 表明。当吸附剂暴露于30 ℃的CO气氛时,出现了位于2 171 cm−1和2 115 cm−1处的气相CO的P支和R支,碳酸盐物种出现在1 807、1 740、1 139 cm−1。当温度升高时,2 360、 1 155、1 124和1 037 cm−1处出现了新的谱带,2 360 cm−1为CO2的特征峰,1 155 cm−1归因于羰基的伸缩振动,1 124 cm−1和1 037 cm−1处归因于单齿碳酸盐[28]。同时,位于1 139 cm−1处的双齿碳酸盐随温度的升高而消失,并且CO2的特征峰并未出现增强迹象,其他碳酸盐的特征峰强度也较小。上述情况表明,CO在Ni0.83Mg0.17-MOF-74表面吸附效果差,且很难发生化学转化,主要以物理吸附为主。图10 (d) 为氨改性后吸附剂吸附CO的原位红外图谱,在1 278 cm−1出现了1个新的双配位基碳酸盐物种,并且吸附剂上有关气态CO的吸收峰较大,且在2 360 cm−1处观察到CO活化后产生的较明显CO2特征峰。 CO在吸附剂上发生反应如式 (6)~(7) 所示。
图11 (a) 为Ni0.83Mg0.17-MOF-74暴露于SO2气氛中的红外光谱。在30 ℃时,一些SO2吸附物种迅速出现,包括硫酸盐 (1 373,1 141 cm−1) 亚硫酸盐 (1 072、1 006、981 cm−1) 及吸附态SO2 (1 346 cm−1) 和SO2的物理吸附 (1 161 cm−1) 。随着温度的提升,1 373和1 346 cm−1的特征峰减小,而1 141和1 006 cm−1的特征峰增强,这说明位于1 373和1 346 cm−1的SO2物种在温度升高时脱附或转化[29]。氨改性后的吸附剂 (图11(b)) 在1 144 cm−1硫酸盐及1 062、1 004 和962 cm−1亚硫酸盐物种生成量有较大提高,推测吸附容量的提高对应于吸附后转化的硫酸盐及亚硫酸盐物种的增多。SO2在吸附剂上反应如式 (8)~(10) 所示。
图11 (c) 表示在不同温度下,SO2、NO和CO同时在Ni0.83Mg0.17-MOF-74表面吸附物种的变化情况。当吸附剂Ni0.83Mg0.17-MOF-74暴露于30 ℃的SO2、NO和CO的混合气体中,硫酸盐 (1 373、1 141 cm−1) 、亚硫酸盐 (1 078、1 002 cm−1) 、吸附态SO2 (1 346 cm−1) 、双齿硝酸盐 (1 172、1 141 cm−1) 、NO2物种 (1 733 cm−1) 、亚硝基物质 (1 805 cm−1) 、吸附态的NO (1 841 cm−1) ,以及气态CO (2 171和2 115 cm−1) 的P和R分支都可被检测到。氨改性Ni0.83Mg0.17-MOF-74在共吸附SO2、NO和CO时吸附物种变化不明显 (图11 (d) ) ,1 278、1 147、1 089 和1 026 cm−1处吸附NO及SO2生成硝酸盐及硫酸盐物种增多,对应其吸附性能的提高。综上所述,氨改性NiMgMOF-74材料从吸附物种的角度来说与未改性吸附剂相比没有明显变化,只发生了吸附强度的提高,氨改性为材料带来了更多碱性位,对于吸附酸性气体硫硝碳能提供更多吸附位点,从而提高材料吸附容量。
-
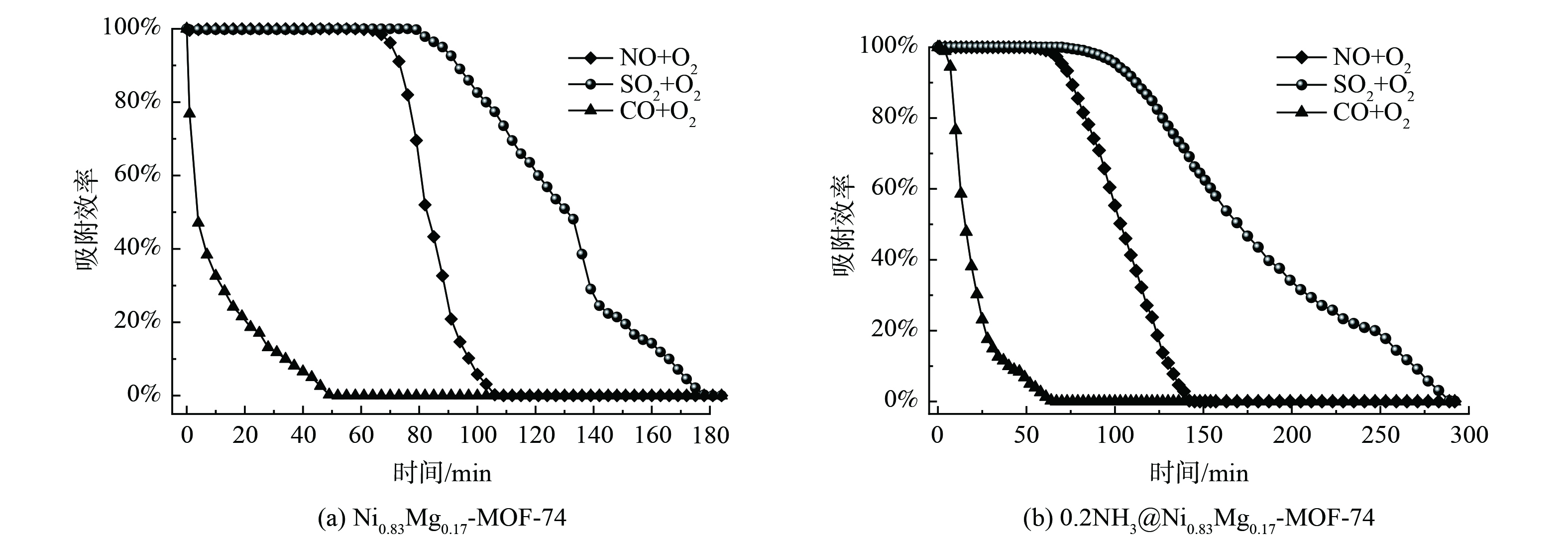
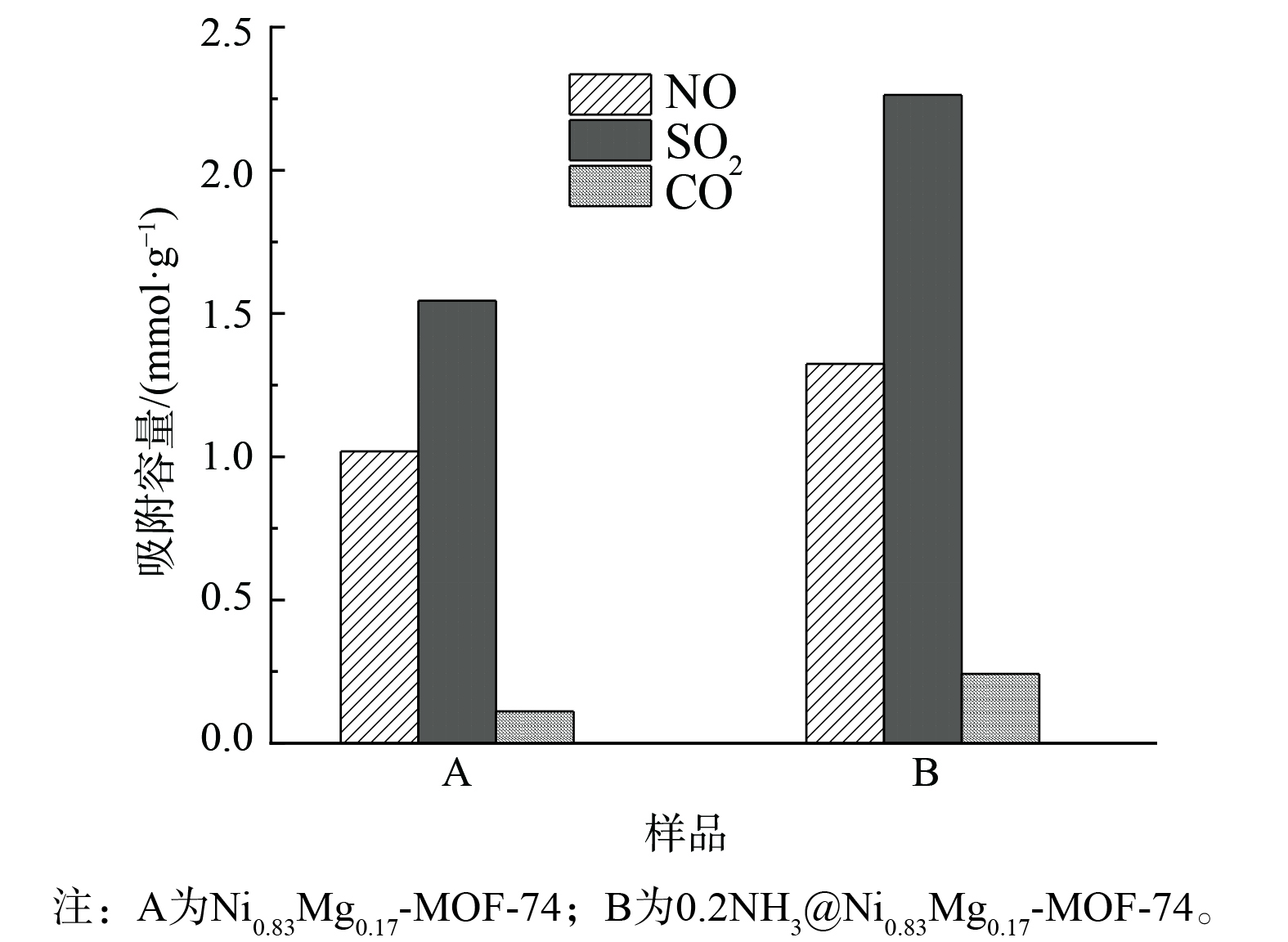
测试了在100 ℃时,含有1% O2的条件下,SO2、NO和CO在Ni0.83Mg0.17-MOF-74吸附剂上的共吸附效果,结果如图12 (a) 所示。氧气的加入对SO2和CO的净化效果都有不同程度提升,尤其是对于混合气体中的SO2,吸附饱和时间高达290 min。相比于无氧条件下,SO2的吸附饱和时间增加了190 min,饱和吸附量为2.26 mmol·g−1,为无氧条件下的2.7倍。对于混合气体中的CO,加入氧气后饱和吸附量为0.24 mmol·g−1,是无氧条件下CO饱和吸附量的1.3倍。然而,对于混合气体中的NO,加入氧气后达到吸附饱和所需时间为142 min,饱和吸附量为1.32 mmol·g−1,相比于无氧条件下吸附饱和时间缩短了133 min,饱和吸附量减少了0.8 mmol·g−1,这说明氧气对NO的吸附产生了的抑制。以上情况说明,氧气有利于SO2和CO的吸附,这主要是由于氧气的加入提供和补充了吸附剂表面的吸附氧物种 (O−、O2-、O2−) ,SO2和CO由于氧气的作用而占据了更多吸附剂表面的吸附氧物种和吸附位点[30],从而使NO的吸附效率下降。
在相同条件下,氨改性后共吸附SO2、NO和CO时,吸附剂的吸附饱和时间分别增加了110、36和15 min (图12 (b) ) 。饱和吸附量分别是改性前的1.3、1.5和2.2倍 (图13) 。另一方面,吸附剂中氨基的引入促进了SO2+NO+CO+O2系统中SO2、NO和CO的共吸附。
-
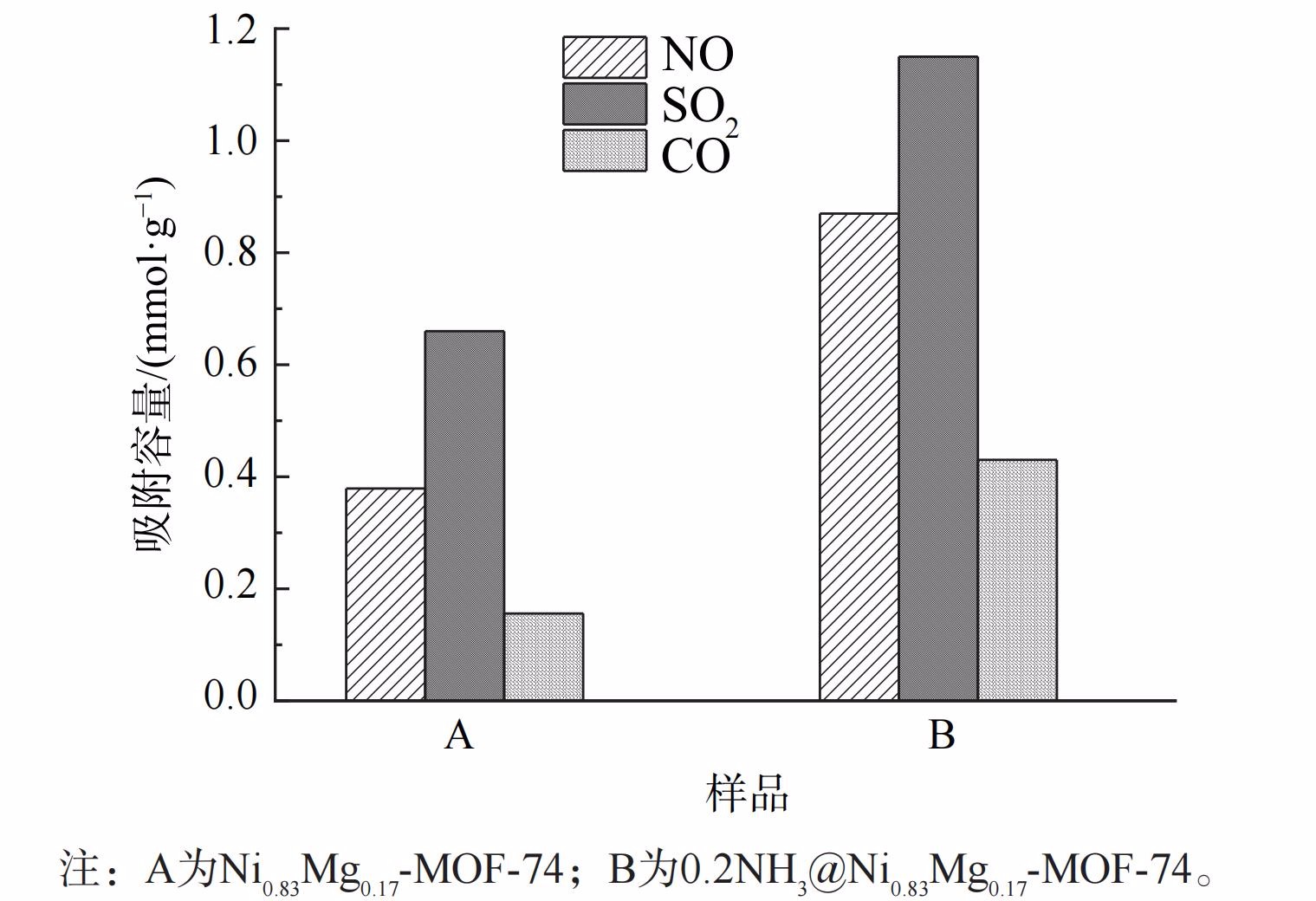
考察了水蒸气对0.2NH3@Ni0.83Mg0.17-MOF-74同时吸附SO2、NO和CO的影响,将5%H2O引入共吸附系统中,SO2、NO和CO共吸附结果如图14所示。水蒸气对混合气体中NO的吸附产生了严重的抑制作用,吸附饱和时间仅为100 min,饱和吸附量为0.87 mmol·g−1,与未通入水蒸气相比吸附饱和时间缩短了175 min,饱和吸附量降低了59%。然而,对于混合气体中的SO2和CO,通入水蒸气后吸附饱和时间分别为120 min和80 min,与未通入水蒸气时相比分别增加了20 min和25 min,饱和吸附量分别提高了35%和129%。水蒸气促进了SO2和CO的吸附,推测其原因可能是水蒸气的引入,使SO2和CO与H2O反应生成硫酸盐、亚硫酸盐及碳酸盐,物种的转化使其提高了SO2和CO的吸附效率。
改性后Ni0.83Mg0.17-MOF-74在5% H2O、100 ℃的条件下共吸附SO2、NO和CO的吸附饱和时间分别为80、52和43 min,饱和吸附量分别为0.66、0.38和0.16 mmol·g−1 (图15) ,分别为改性前共吸附SO2、NO和CO时饱和吸附量的2.13、1.74和2.75倍。引入氨基改变了吸附剂的亲水性,有利于SO2、NO和CO与水的化学作用,提高了水存在条件下吸附剂的吸附性能[31]。
-
1) 在不同比例双金属MOF-74中,Ni0.83Mg0.17-MOF-74表现出较为突出的硫硝碳共吸附性能,SO2和NO在Ni0.83Mg0.17-MOF-74表面表现活跃,发生较多的化学吸附,对CO则以物理吸附为主。
2) O2和H2O引入共吸附系统中会促进SO2和CO的吸附,但同时占据较多吸附位点而抑制NO的吸附。引入氨基修饰后的0.2NH3@Ni0.83Mg0.17-MOF-74对SO2、NO和CO共吸附的饱和吸附量分别提升至0.851、2.13和0.187 mmol·g−1,分别为改性前的1.8、6.3和4.7倍。
3) 结合TPD及原位红外光谱发现氨基基团修饰的吸附剂表面碱性增加,能够吸附和活化更多的酸性气体,以提高吸附剂的吸附容量并延长吸附饱和时间,且在氨基修饰的0.2NH3@Ni0.83Mg0.17-MOF-74上引入O2/H2O会促进SO2、NO和CO的共吸附作用。
氨修饰NiMg-MOF-74材料共吸附硫硝碳效能及其机理
Co-adsorption efficiency and mechanism of ammonium modified NiMg-MOF-74 material
-
摘要: 为实现烟气中硫硝碳的共吸附,采用热溶剂法制备了以不同比例Ni2+和Mg2+为中心离子,2,5-二羟基对苯二甲酸为配体的双金属有机框架材料NixMg1−x-MOF-74,再通过氨水浸渍得到不同浓度氨改性的共吸附硫硝碳性能最优的Ni0.83Mg0.17-MOF-74,以实现氨基基团的功能化修饰,并利用原位红外光谱记录了吸附剂上3种气体的吸附物种状态及变化,结合TPD探究了其共吸附机理,最后考察了O2、H2O对上述材料吸附过程稳定性的影响。效率测试发现,改性后的0.2NH3@Ni0.83Mg0.17-MOF-74吸附剂对SO2、NO和CO共吸附的饱和吸附量为0.851、2.130和0.187 mmol·g−1,为改性前的1.8、6.3和4.7倍。结果表明,氨基的修饰使吸附剂拥有更多表面碱性基团,从而能吸附活化酸性气体,并提高材料对氧气和水蒸气的耐受性。本研究可为一体化烟气净化材料的开发及应用提供参考。Abstract: In order to realize the co-adsorption of sulfur, nitrate and carbon in flue gas, a series of bimetallic organic framework material Nix Mg1-x -MOF-74 with different proportions of Ni2+ and Mg2+ as central ions and 2,5-dihydroxyterephthalic acid as ligand were prepared by hot solvent method. On this basis, Ni0.83Mg0.17-aMOF-74 with the best co-adsorption performance of SO2、NO and CO was modified with different concentrations of ammonia to realize the functional modification of amino groups. The adsorption species and changes of three gases on the adsorbent were recorded by in-situ infrared spectroscopy, and the co-adsorption mechanism was explored by TPD. Finally, the effects of O2 and H2O on the stability of the adsorption process of the above materials were investigated. The results showed that the saturated adsorption capacity of modified 0.2NH3@Ni0.83Mg0.17-MOF-74 adsorbent for co-adsorption of SO2, NO and CO was 0.851, 2.130 and 0.187 mmol·g-1, which was 1.8, 6.3 and 4.7 times of that before modification. The results showed that the modification of amino groups made the adsorbent have more surface alkalinity to adsorb activated acid gases, and improved the resistance of materials to oxygen and water vapor.
-

-
表 1 不同类型的MOF-74材料的组成
Table 1. Composition of different types of MOF-74 materials
g 样品名称 Mg(NO3)2·6H2O Ni (NO3)2·6H2O DOBDC Ni0.36Mg0.64-MOF-74 0.51 0.19 0.167 Ni0.68Mg0.32-MOF-74 0.35 0.39 0.167 Ni0.83Mg0.17-MOF-74 0.17 0.59 0.167 表 2 NiMg-MOF-74材料的ICP-AES测试结果
Table 2. ICP-AES test results of Ni/Mg-MOF-74 material
样品名称 进料中镁镍摩尔比 最终样品中镁镍摩尔比 Ni0.36Mg0.64-MOF-74 0.75:0.25 0.64:0.36 Ni0.68Mg0.32-MOF-74 0.5:0.5 0.32:0.68 Ni0.83Mg0.17-MOF-74 0.25:0.75 0.17:0.83 表 3 双金属NiMg-MOF-74的孔结构参数
Table 3. Pore structure parameters of bimetallic NiMg-MOF-74
样品名称 BET 比表面积/(m2·g−1) 微孔比表面积/(m2·g−1) 总孔容/(cm3·g−1) 微孔孔容/(cm3·g−1) Ni0.36Mg0.64-MOF-74 734.7 545.071 0.421 0.284 Ni0.68Mg0.32-MOF-74 795.788 589.459 0.433 0.268 Ni0.83Mg0.17-MOF-74 1 006.579 872.313 0.511 0.388 表 4 NiMg-MOF-74及其氨改性材料的SO2-TPD/NO-TPD中SO2/NO脱附量
Table 4. Desorption of SO2/NO in SO2-TPD/NO-TPD of NiMg-MOF-74 and its ammonia modified materials
样品名称 SO2-TPD中SO2
脱附量NO-TPD中NO
脱附量Ni0.36Mg0.64-MOF-74 12 482 85 Ni0.68Mg0.32-MOF-74 7 531 420 Ni0.83Mg0.17-MOF-74 28 660 847 0.2-NH3@Ni0.83Mg0.17-MOF-74 15 553 255 0.6-NH3@Ni0.83Mg0.17-MOF-74 22 434 362 1-NH3@Ni0.83Mg0.17-MOF-74 9 049 490 -
[1] AL-NADDAF Q, LAWSON S, ROWNAGHI A A, et al. Analysis of dynamic CO2 capture over 13X zeolite monoliths in the presence of SOx, NOx and humidity[J]. AIChE Journal, 2020, 66(9): e16297. [2] HE H, WANG Y, FU W, et al. Study on the CO-SCR anti-sulfur and denitration performance of V-doped OMS-2 catalysts[J]. Ceramics International, 2021, 47(23): 33120-33126. doi: 10.1016/j.ceramint.2021.08.213 [3] JIANG H, WANG Q, WANG H, et al. Temperature effect on the morphology and catalytic performance of Co-MOF-74 in low-temperature NH3-SCR process[J]. Catalysis Communications, 2016, 80: 24-27. doi: 10.1016/j.catcom.2016.03.013 [4] ADHIKARI A K, LIN K-S. Improving CO2 adsorption capacities and CO2/N2 separation efficiencies of MOF-74(Ni, Co) by doping palladium-containing activated carbon[J]. Chemical Engineering Journal, 2016, 284: 1348-1360. doi: 10.1016/j.cej.2015.09.086 [5] SUN H, REN D, KONG R, et al. Tuning 1-hexene/n-hexane adsorption on MOF-74 via constructing Co-Mg bimetallic frameworks[J]. Microporous and Mesoporous Materials, 2019, 284: 151-160. doi: 10.1016/j.micromeso.2019.04.031 [6] XU G, ZUO Y, HUANG B. Metal-organic framework-74-Ni/carbon nanotube composite as sulfur host for high performance lithium-sulfur batteries[J]. Journal of Electroanalytical Chemistry, 2018, 830-831: 43-49. doi: 10.1016/j.jelechem.2018.10.028 [7] CAMPBELL J, TOKAY B. Controlling the size and shape of Mg-MOF-74 crystals to optimise film synthesis on alumina substrates[J]. Microporous and Mesoporous Materials, 2017, 251: 190-199. doi: 10.1016/j.micromeso.2017.05.058 [8] 李志华, 刘鸿, 宋凌勇, 等. 双金属功能化的MOF-74合成及气体吸附性能[J]. 无机化学学报, 2017, 33(2): 237-242. doi: 10.11862/CJIC.2017.042 [9] 孔乾乾. Ni-MOF-74的制备、改性及其氢气吸附性能研究 [D]. 大连: 大连理工大学, 2020. [10] 孙豪. 金属有机骨架材料MOF-74的制备及其苯与CO2吸附性能研究 [D]. 桂林: 广西师范大学, 2020. [11] 林俭锋, 苏叶, 肖静, 等. Mg-MOF-74的氨改性及其吸附CO2和水蒸气性能[J]. 功能材料, 2014, 45(9): 9038-9042. doi: 10.3969/j.issn.1001-9731.2014.09.008 [12] GUO S, QI X, ZHOU H, et al. A bimetallic-MOF catalyst for efficient CO2 photoreduction from simulated flue gas to value-added formate[J]. Journal of Materials Chemistry A, 2020, 8(23): 11712-11718. doi: 10.1039/D0TA00205D [13] 安晓银. NH2-MIL-125与M-MOF-74材料的制备、改性及吸附性能研究 [D]. 大连: 大连理工大学, 2019. [14] 李生璐. Mg-MOF-74的合成、改性及其CO2/H2O吸附性能研究 [D]. 沈阳: 东北大学, 2015. [15] 陈健. 金属—有机骨架MOF-74系列吸附捕集低浓度二氧化碳的研究 [D]. 大连: 大连理工大学, 2016. [16] 孙增智, 薛程, 宋莉芳, 等. 金属有机骨架化合物的二氧化碳吸附性能的研究进展[J]. 材料导报, 2019, 33(3): 541-549. doi: 10.11896/cldb.201903022 [17] 马孜豪, 竺柏康, 欧浩. 氮化硼纳米片改性Mg-MOF-74的制备及其水稳定性研究[J]. 浙江海洋大学学报(自然科学版), 2019, 38(4): 346-351. [18] ZURRER T, WONG K, HORLYCK J, et al. Mixed-metal MOF-74 templated catalysts for efficient carbon dioxide capture and methanation[J]. Advanced Functional Materials, 2020, 31(9): 2007624. [19] LI T, JIN Z. Unique ternary Ni-MOF-74/Ni2P/MoSx composite for efficient photocatalytic hydrogen production: Role of Ni2P for accelerating separation of photogenerated carriers[J]. Journal of Colloid and Interface Science, 2022, 605: 385-397. doi: 10.1016/j.jcis.2021.07.098 [20] TAN K, ZULUAGA S, GONG Q, et al. Water reaction mechanism in metal organic frameworks with coordinatively unsaturated metal ions: MOF-74[J]. Chemistry of Materials, 2014, 26(23): 6886-6895. doi: 10.1021/cm5038183 [21] 任丹妮. MOF-74的合成、改性及其吸附分离烯烃/烷烃性能研究 [D]. 上海: 华东理工大学, 2019. [22] LUO L, GUO Y, ZHU T, et al. Adsorption species distribution and multicomponent adsorption mechanism of SO2, NO, and CO2 on commercial adsorbents[J]. Energy & Fuels, 2017, 31(10): 11026-11033. [23] WU J, JIN S, WEI X, et al. Enhanced sulfur resistance of H3PW12O40-modified Fe2O3 catalyst for NH3-SCR: Synergistic effect of surface acidity and oxidation ability[J]. Chemical Engineering Journal, 2021, 412: 128712. doi: 10.1016/j.cej.2021.128712 [24] KANG R, HE J, BIN F, et al. Alkali metal-resistant mechanism for selective catalytic reduction of nitric oxide over V2O5/HWO catalysts[J]. Fuel, 2021, 304: 121445. doi: 10.1016/j.fuel.2021.121445 [25] TAN K, ZULUAGA S, WANG H, et al. Interaction of acid gases SO2 and NO2 with coordinatively unsaturated metal organic frameworks: M-MOF-74 (M = Zn, Mg, Ni, Co)[J]. Chemistry of Materials, 2017, 29(10): 4227-4235. doi: 10.1021/acs.chemmater.7b00005 [26] LIU W, GAO Z, SUN M, et al. One-pot synthesis of CrαMnβCeTiOx mixed oxides as NH3-SCR catalysts with enhanced low-temperature catalytic activity and sulfur resistance[J]. Chemical Engineering Science, 2022, 251: 117450. doi: 10.1016/j.ces.2022.117450 [27] ZHANG Y, ZHAO L, KANG M, et al. Insights into high CO-SCR performance of CuCoAlO catalysts derived from LDH/MOFs composites and study of H2O/SO2 and alkali metal resistance[J]. Chemical Engineering Journal, 2021, 426: 131873. doi: 10.1016/j.cej.2021.131873 [28] CHEN L, LI J, GE M. DRIFT Study on Cerium-Tungsten/Titiania catalyst for selective catalytic reduction of NOx with NH3[J]. Environmental Science& Technology, 2010, 44: 9590-9596. [29] LIAN Z, LIU F, SHAN W, et al. Improvement of Nb doping on SO2 Resistance of VOx/CeO2 catalyst for the selective catalytic reduction of NOx with NH3[J]. The Journal of Physical Chemistry C, 2017, 121(14): 7803-7809. doi: 10.1021/acs.jpcc.6b12772 [30] 李正杰. 金属—有机骨架材料气相分离性能的分子模拟研究 [D]. 北京: 北京化工大学, 2016. [31] 杨家佳, 丁玉栋, 廖强, 等. 合成前氨基改性Mg-MOF-74吸附分离CO2性能研究[J]. 工程热物理学报, 2019, 40(2): 435-441. -



 下载:
下载: