-
硫酸根离子作为天然水系中常见的阴离子,硫酸盐浓度过高会对生物体和生态系统产生潜在危害[1]。高浓度硫酸盐使水体酸化,当被用于灌溉农田时,会压实土壤并危害农作物的生长[2];若被人类长期饮用,会引起人类腹泻、消化不良等症状[3]。有研究表明,矿区附近的水域均存在硫酸盐含量超过地表水环境质量标准规定限值的现象[4-5]。硫酸盐废水的处理成为了亟待处理的水环境问题之一。
目前,去除水中硫酸盐的主要方法有离子交换法[6]、絮凝法[7]、化学沉淀法[8]和吸附法[9]等。其中,吸附法因其价廉、易得、高效、可重复利用和二次污染小等优点而备受重视。芦苇作为一种观赏性水生草本植物,在我国各地水域随处可见,老化而来的秸秆不经处理,污染环境且浪费资源,故可用于制作生物炭原料吸附硫酸盐。原始生物炭的吸附效果较弱,需对其进行改性。煤矸石作为工业废弃物,随意堆放会占用土地资源且污染环境[10-11]。有研究表明,生物质与煤矸石共热解,不仅可以提高生物炭的产量,还可以提高生物炭的吸附性能[12]。故可用煤矸石改善生物炭的吸附效果,同时也为煤矸石废物资源化利用提供一条新途径。ZHAO等[13]通过将油菜秸秆与煤矸石在不同温度下煅烧制作改性生物炭对Cr(Ⅵ)进行吸附,发现在经过600 ℃煅烧的生物炭吸附容量最大,为9.2 mg·g−1;WANG等[14]将不同生物质与煤矸石分别共热解用于吸附磷酸盐,发现改性后玉米秸秆生物炭的吸附容量增加了3.6倍; QIU等[15]将松木屑与煤矸石共热解后,再用氯化铁活化,对30 mg·L−1的磷酸盐进行吸附,得到吸附容量为3.08 mg·g−1。目前用该种改性方法去除水体中硫酸盐的研究鲜见报道。
本文以芦苇秸秆为原材料,将其与煤矸石共热解得到了改性产物,以水中硫酸盐作为研究对象,探究了改性材料的加入对生物炭吸附性能的影响,并考察了吸附性能的稳定性。以期为水体中硫酸盐的去除提供参考,为固体废物资源化利用提供数据参考。
-
生物炭原料的制备。芦苇秸秆采自安徽大学磬苑校区,将采得的芦苇秸秆用去离子水洗净后风干,剪成1~2 cm的小段,放在烘箱中以80 ℃烘干,用粉碎机将其粉碎,过100目筛。煤矸石(采自安徽省淮北市临涣矿区):使用岩石破碎机将块状煤矸石碎成直径2 cm以下的碎块,再使用球磨仪加水球磨3 h,取出后以80 ℃烘干,最后使用陶瓷研磨臼将结块的煤矸石充分研磨成粉末。实验试剂包括无水硫酸钠、氢氧化钠、浓盐酸等,所用药品均为分析纯及以上等级。实验用水为去离子水。
-
主要仪器包括马弗炉(SX2-8-10N,上海一恒科学仪器有限公司)、电热鼓风干燥箱(DHG-9023A,上海-恒科学仪器有限公司)、电子天平(EX125ZH,奥豪斯仪器(上海)有限公司)、pH计(PHS-3C,上海雷磁仪器厂)、台式恒温摇床(TS-20H,上海天呈实验仪器制造有限公司)、紫外可见分光光度计(UV-5500PC,上海元析仪器有限公司)等。
-
1)热解炭的制备。将破碎后的芦苇秸秆放在坩埚中压实,用铝箔纸密封包裹后放在600 ℃马弗炉中煅烧2 h,放置冷却后取出,用去离子水洗去灰分,放入烘箱烘干,得到原始生物炭,记作OBC。
2)改性生物炭的制备。通过磁力搅拌将质量比为5:2的芦苇秸秆和煤矸石粉末混合均匀后,放入坩埚中压实,用铝箔纸对其进行密封包裹,放在马弗炉中以600 ℃煅烧2 h,冷却取出后放在1 mol·L−1 HCl溶液中浸渍24 h,洗至中性后烘干,即得改性生物炭,记作MBC。
-
用超高分辨扫描电子显微镜(Regulus 8230,日本日立公司)研究材料的表面形态,通过全自动多站气体物理吸附分析仪(ASAP 2460,麦克默瑞提克仪器有限公司)分析生物炭的比表面积和孔隙分布,而生物炭表面的官能团则由傅里叶变换红外光谱仪(Vertex80,德国布鲁克公司)测定。
-
1) pH对吸附效果的影响。取50 mL质量浓度为500 mg·L−1的硫酸盐溶液至100 mL锥形瓶中,使用0.1 mol·L−1 NaOH和0.1 mol·L−1 HCl将溶液pH调至2、4、6、8、10、12。分别加入OBC和MBC各0.4 g,放入摇床,在25 ℃、200 r·min−1条件下振荡24 h,取出后使用滤孔直径为0.45 µm的滤膜过滤,以上处理均设置3组重复。
2)投加量对吸附效果的影响。向100 mL锥形瓶中加入50 mL质量浓度为500 mg·L−1的硫酸盐溶液,将pH调为2,分别加入0.05、0.1、0.2、0.3、0.4、0.5 g OBC和MBC,在25 ℃、200 r·min−1条件下振荡24 h,后使用0.45 µm滤膜过滤,每个处理设置3组平行。
3)吸附动力学实验。分别称取0.4 g的OBC和MBC,倒入装有50 mL、pH=2、质量浓度为500 mg·L−1硫酸盐溶液的锥形瓶中,在摇床中以200 r·min−1的条件振荡,分别振荡0.5、2、5、7、10、24 h,经0.45 µm滤纸过滤后测定溶液中硫酸盐浓度,对其进行准一级动力学模型(式(1))和准二级动力学模型(式(2))的拟合。
式中:qe、qt分别为平衡吸附量和t时刻吸附量,mg·g−1;t为吸附时间,min;k1为准一级反应速率常数。
式中:qe、qt分别为平衡吸附量和t时刻吸附量,mg·g−1;t为吸附时间,min;k2为准二级反应速率常数。
4)吸附等温线实验。称取OBC和MBC各0.4 g于100 mL的锥形瓶中,分别加入质量浓度为300、400、500、600、700、800 mg·L−1的硫酸盐溶液,将pH调为2,放入摇床,以25 ℃、200 r·min−1振荡24 h,取出后用滤孔直径为0.45 µm的滤膜过滤,测定溶液中剩余的硫酸盐浓度,对其使用Langmuir模型(式(3))和Freundlich模型(式(4))进行分析。
式中:qe是吸附饱和时生物炭的吸附量,mg·g−1;ce是平衡质量浓度,mg·L−1;kL是Langmuir平衡吸附常数,qm为单分子层饱和吸附量,mg·g−1。
式中:qe是吸附饱和时生物炭的吸附量,mg·g−1;ce是平衡质量浓度,mg·L−1;kF和1/n是Freundlich经验常数。
5)共存阴离子对改性生物炭吸附硫酸盐能力的影响。准确称取0.4 g的MBC于100 mL锥形瓶中,加入pH为2的50 mL质量浓度为500 mg·L−1的硫酸盐溶液,再向溶液中分别加入0.1 mol·L−1的PO43−、CO32−、Cl−和NO3−离子,在25 ℃以200 r·min−1振荡24 h。
-
为探究改性生物炭在应用时的安全性,将0.4 g MBC加入50 mL、pH=2的纯水溶液中,在25 ℃、200 r·min−1条件下振荡24 h,测定重金属浓度。取0.4 g 吸附饱和的生物炭,放入1 mol·L−1NaOH中进行解吸,浸泡24 h后将其冲洗至中性并烘干,进行第2次吸附实验,以上操作重复4次,对比得到的5次去除率,判断MBC再生性能的优劣。
-
采用Excel2010对得到的数据进行整理;采用Origin2021进行图片绘制。硫酸盐浓度采用EDTA络合滴定法(HJ/T342-2007)测定,吸附剂对硫酸盐的去除率和吸附容量分别根据式(5)和式(6)计算。
式中:E为去除率,%;C0为硫酸盐溶液的初始浓度,mg·L−1;Ct为吸附t时刻后的硫酸盐浓度,mg·L−1;qt为吸附容量,mg·g−1;m为吸附剂用量,g;V为硫酸盐溶液的体积,L。
-
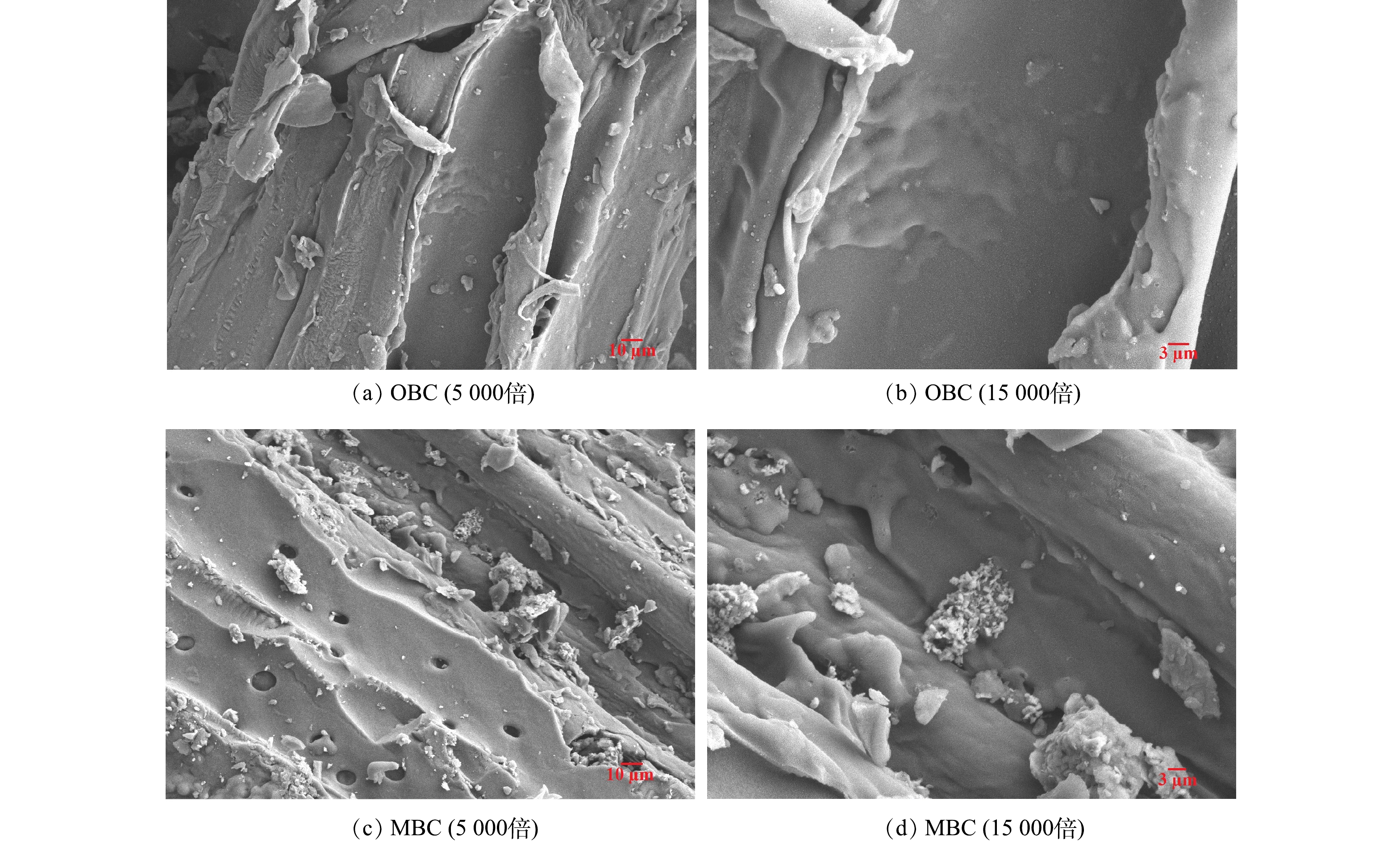
1)扫描电镜分析。用扫描电子显微镜(scanning electron microscope,SEM)分别对改性前后的生物炭进行扫描,得到的表面形态特征如图1所示。由图1中可以看出,未经改性的生物炭表面光滑、致密,仅有少量凹凸不平的褶皱,没有观察到明显的微孔结构。而改性过后的生物炭表面更为粗糙,主要表现在不规则颗粒数量增多。这是由煤矸石负载所致。由图(d)可以看到煤矸石拥有非常丰富的表面积,这是因为高温煅烧会破坏煤矸石的晶体结构,形成无定型的非结晶体。同时出现了较多的孔隙和凹陷,表明煤矸石可使生物炭表面具有更多的孔隙,这与LIAN等[16]研究的结果相同。这些丰富的孔隙结构和表面颗粒的存在为活性炭提供了吸附位点,使其具有良好的物理吸附能力[17]。
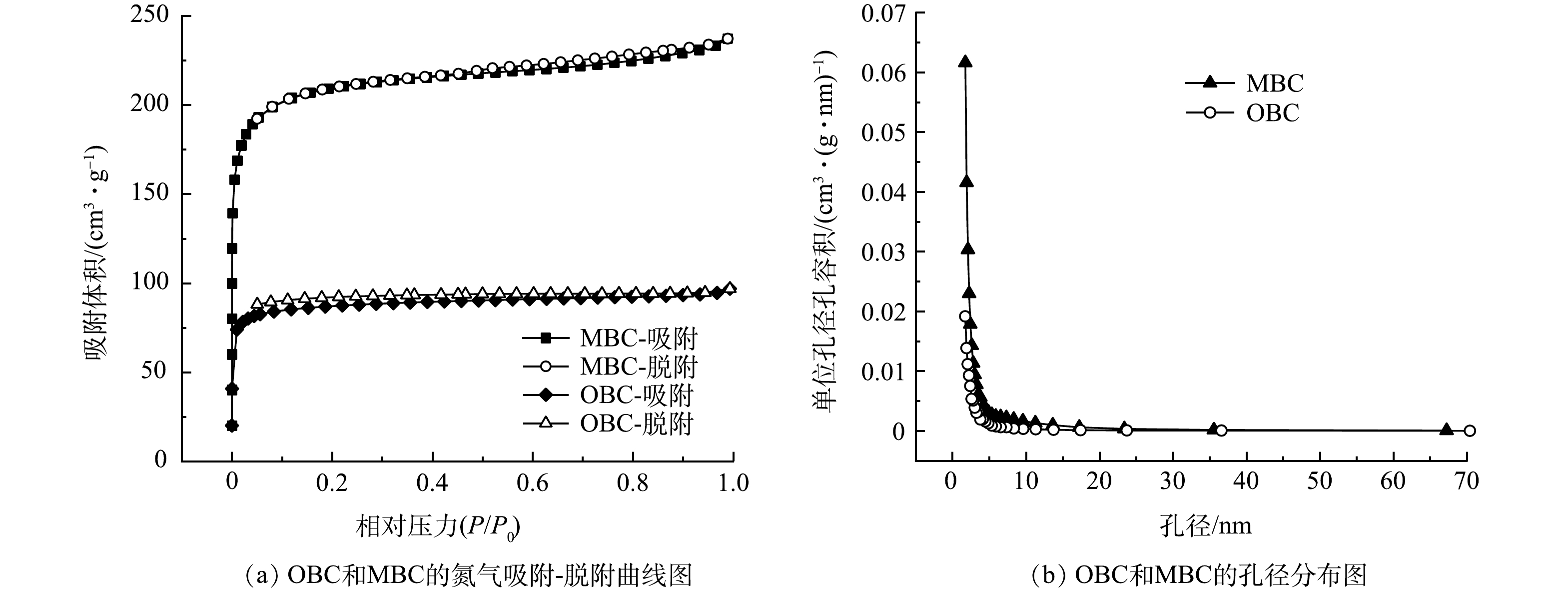
2)比表面积和孔径分布。吸附材料的比表面积和孔隙结构对吸附剂的吸附性能有着极大的影响。由图2(a)可见,MBC和OBC的变化曲线同时具有Ⅰ型与Ⅳ型等温线的特征。这说明2种生物炭均含有微孔和介孔结构[18-19]。使用BJH(Barret-Joyner-Halenda)模型对吸附剂的孔径分布进行分析。由图2(b)可见,MBC的孔径主要集中在2~5 nm。说明MBC的孔隙主要为介孔。表1中显示MBC的孔径大小为2.25 nm,这表明MBC为介孔材料。
如表1所示,OBC和MBC的比表面积分别是273.99 m2·g−1和658.91 m2·g−1,改性后生物炭的比表面积是改性前的2.4倍。这是因为生物质与煤矸石共热解提高了生物炭的产量[20],同时高温煅烧能破坏煤矸石稳定的结构,转化为无定型物质,进而提高其表面积,从而加强生物炭对硫酸盐的物理吸附。
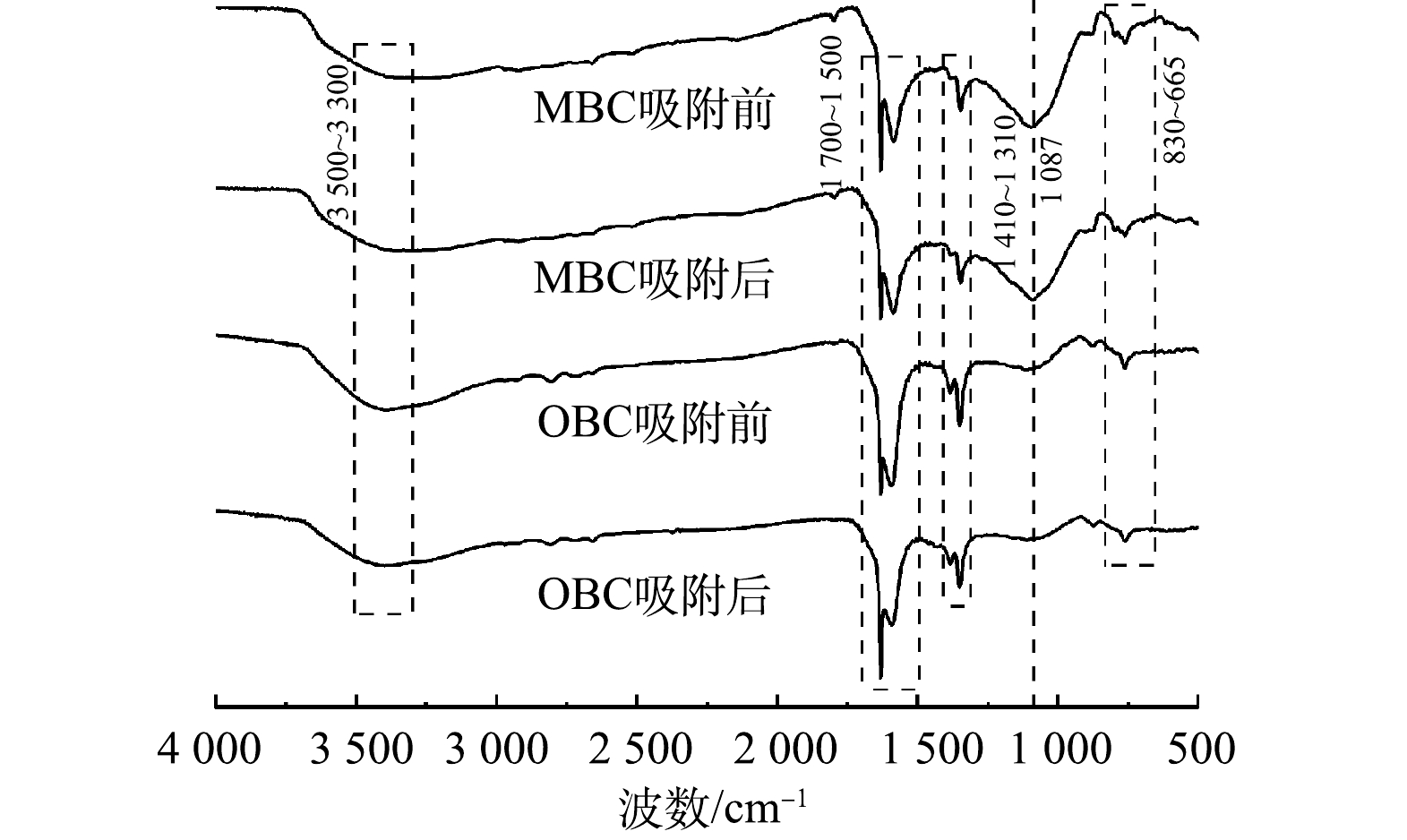
3)红外图谱表征分析。生物炭表面的官能团是影响其吸附能力的一个重要因素,其种类和数量决定生物炭的表面化学性质。芦苇生物炭改性前后的红外图谱如图3所示。可见,位于3 500~3 300 cm−1处的宽峰是分子间羟基O—H伸缩振动峰[21], 1 700 cm−1与1 500 cm−1处为C=O和C=C伸缩振动峰[22-23],这3处峰的强度在改性后均减弱,说明在改性过程中发生脱氢脱氧反应,生物炭中的结构水减少,这会增加生物炭的孔隙结构,利于吸附。MBC吸附后的C=O和C=C强度减弱,说明部分C=C和C=O参与了硫酸盐的吸附。1 410~1 310 cm−1处是—CH3的振动峰,经过改性后强度减弱,说明改性使该结构被破坏,发生了去甲基化[24]。1 087 cm−1附近的峰是Si—O—Si反对称伸缩振动,830~665 cm−1处是Si—O和Al—O键的对称伸缩振动峰[25-26],在MBC吸附硫酸盐后,这两处峰的强度减弱,说明Si—O和Al—O键可参与反应,煤矸石的加入有利于吸附硫酸盐。
-
溶液的pH会影响吸附剂表面的电荷,从而改变吸附剂的吸附能力。吸附剂在不同pH下对水中硫酸盐的吸附效果如图4所示。同时对不同pH条件下OBC和MBC的Zeta电位值进行测定,得知这2种材料的pHpzc分别为5.0和5.7。由图4中可以看出,随着pH的增加,OBC和MBC的吸附容量都呈现下降的趋势,当pH由2升至12时,MBC对硫酸盐的吸附容量由47.73 mg·g−1 降至20.14 mg·g−1,OBC对硫酸盐的吸附容量由20.99 mg·g−1降至13.17 mg·g−1,而在pH= 4.0~6.0内,下降速度较其他区间更为迅速。这是因为较低的pH有利于吸附剂表面的质子化,故吸附剂表面可以携带更多的正电荷,从而增强了吸附剂表面与SO42−之间的静电作用,促进了其对SO42−的吸附[27]。而当溶液pH过高时,水中的OH−会与SO42−竞争活性位点,从而导致吸附量降低[28]。
-
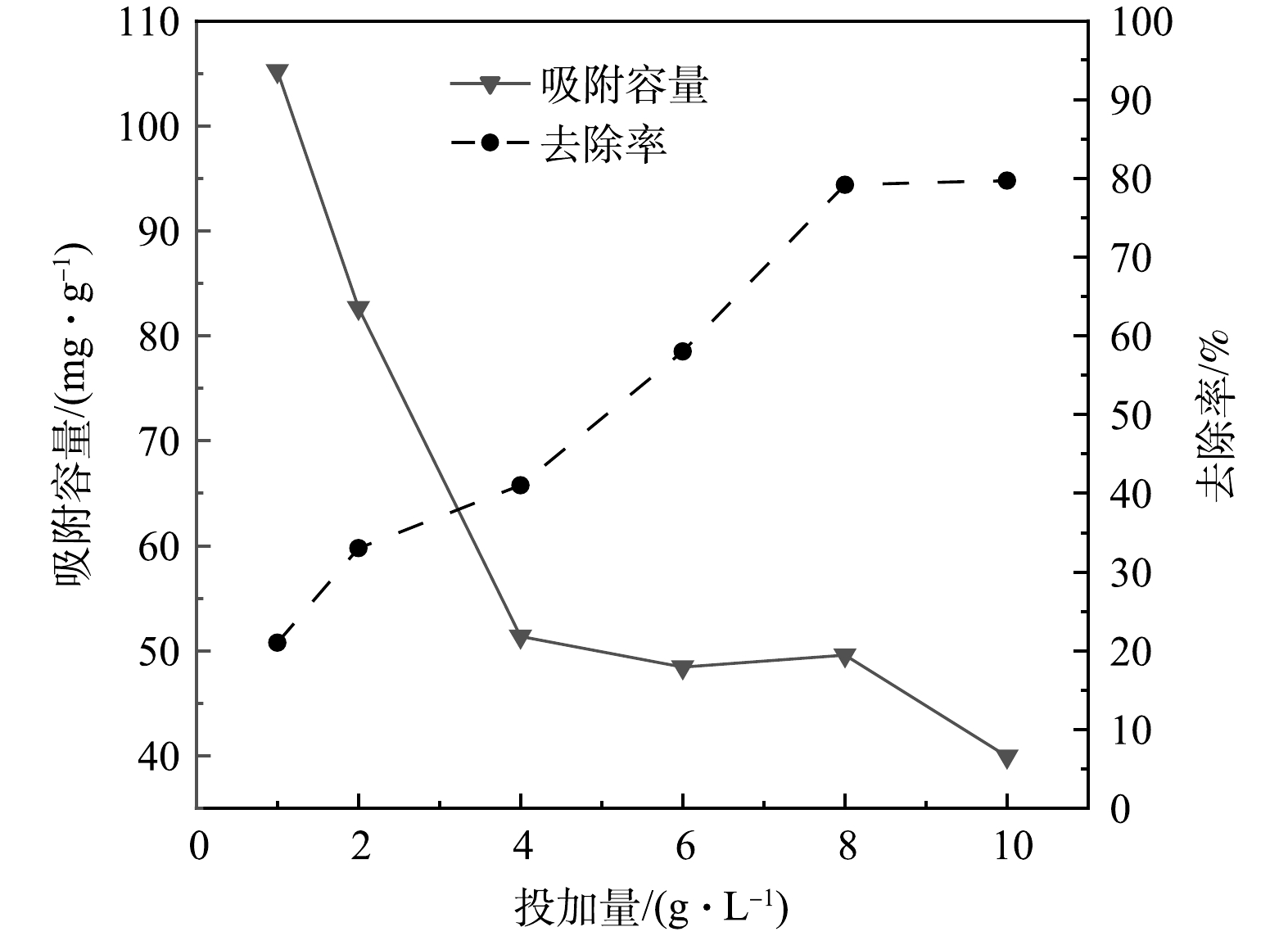
由图5可以看出,随着吸附剂投加量的增多,硫酸盐的去除率逐渐增大,从0.05 g时的31%增长到了0.5 g时的79.68%。这是因为增加吸附剂的用量后,吸附活性位点也同时增加[29]。当投加量达到8 g·L−1时,随着投加量的增多,去除率无明显变化,这是由于硫酸盐和吸附剂的接触比表面积接近饱和,不能再结合更多的活性位点[30]。与去除率的变化趋势相反,吸附量是逐渐减少的。这是因为随着吸附剂投加量的增多,生物炭表面存在的可吸附位点没有被吸附质完全占据[31]。当吸附剂投加量为8 g·L−1时,此时硫酸盐的去除率和吸附容量相对较高,分别是79.15%和49.56 mg·g−1。从经济有效的角度考虑,确定最佳投放量为8 g·L−1。
-
MBC对硫酸盐的吸附量随反应时间的变化如图6所示。由图6可见,MBC吸附硫酸盐的过程可以分为快速吸附、缓慢吸附和吸附平衡阶段。在吸附开始的2 h后,吸附容量增至48.65 mg·g−1。这是因为初期吸附剂表面有大量的吸附位点,可被硫酸根快速占据,但随着时间的推移,吸附位点逐渐饱和,直至达到平衡状态。为进一步探究MBC对硫酸盐的吸附机理,对吸附结果分别进行准一级动力学模型和准二级动力学模型拟合,拟合参数见表2。由表2可以看出,这2种模型均能够很好地对实验结果进行拟合,R2均大于0.9,分别为0.959和0.991,准二级动力学模型的R2更接近1,因此,准二级动力学模型可以更好的描述MBC对硫酸盐的吸附过程。这说明吸附过程主要为生物炭与硫酸盐之间的离子交换及静电作用。
-
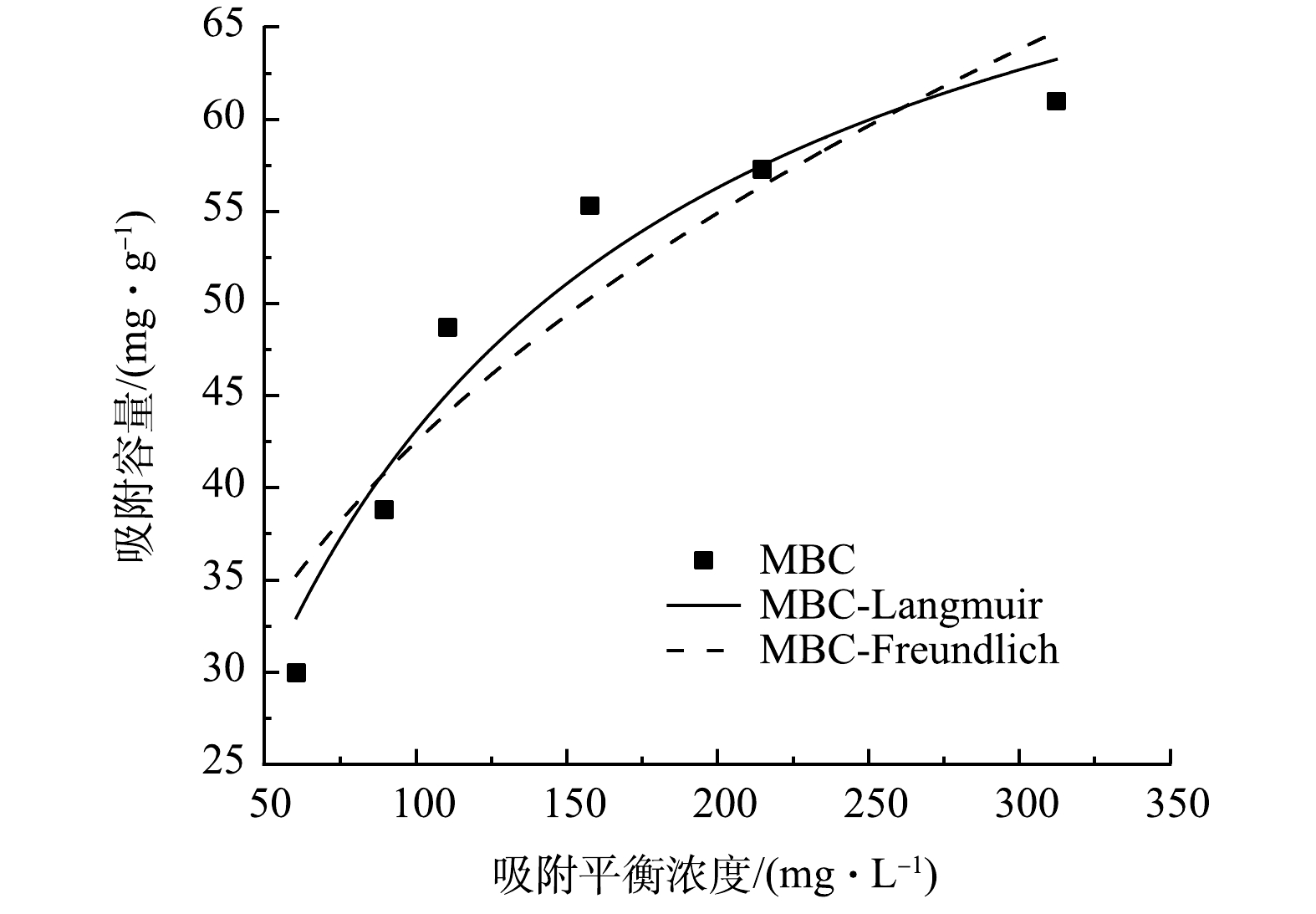
采用Langmuir和Freundlich等温吸附模型对吸附数据进行拟合,拟合曲线和参数分别见图7和表3。如图7所示,当硫酸盐初始浓度较低时,吸附容量的增长较为迅速,随着初始浓度逐渐升高,吸附容量的增势变得平缓,最后趋向稳定。这主要是由于吸附剂提供的吸附位点是固定的,当硫酸盐浓度增加时,越来越多的硫酸盐无法占据吸附位点,吸附量达到饱和。从表3中可以看出Langmuir的R2为0.941,要大于Freundlich(R2=0.870),故Langmuir能够更好的描述MBC对硫酸盐的吸附行为。这表明MBC对硫酸盐的吸附以单分子层均匀吸附为主[32]。
-
根据pH影响吸附性能结果可知,吸附容量随pH变化而发生较大变化,故MBC对硫酸盐的吸附存在静电吸附。MBC具有较大的比表面积和发达的孔隙结构,说明MBC具有良好的物理吸附能力。通过红外表征发现MBC吸附前后的官能团强度发生变化,说明官能团参加反应,吸附过程存在化学吸附。根据吸附动力学模型和吸附等温模型拟合结果得知,准二级动力学模型和Langmuir能够更好的描述吸附过程,说明吸附过程存在静电吸附和化学吸附。因此,生物炭吸附硫酸盐包括物理吸附、静电吸附和化学吸附。
-
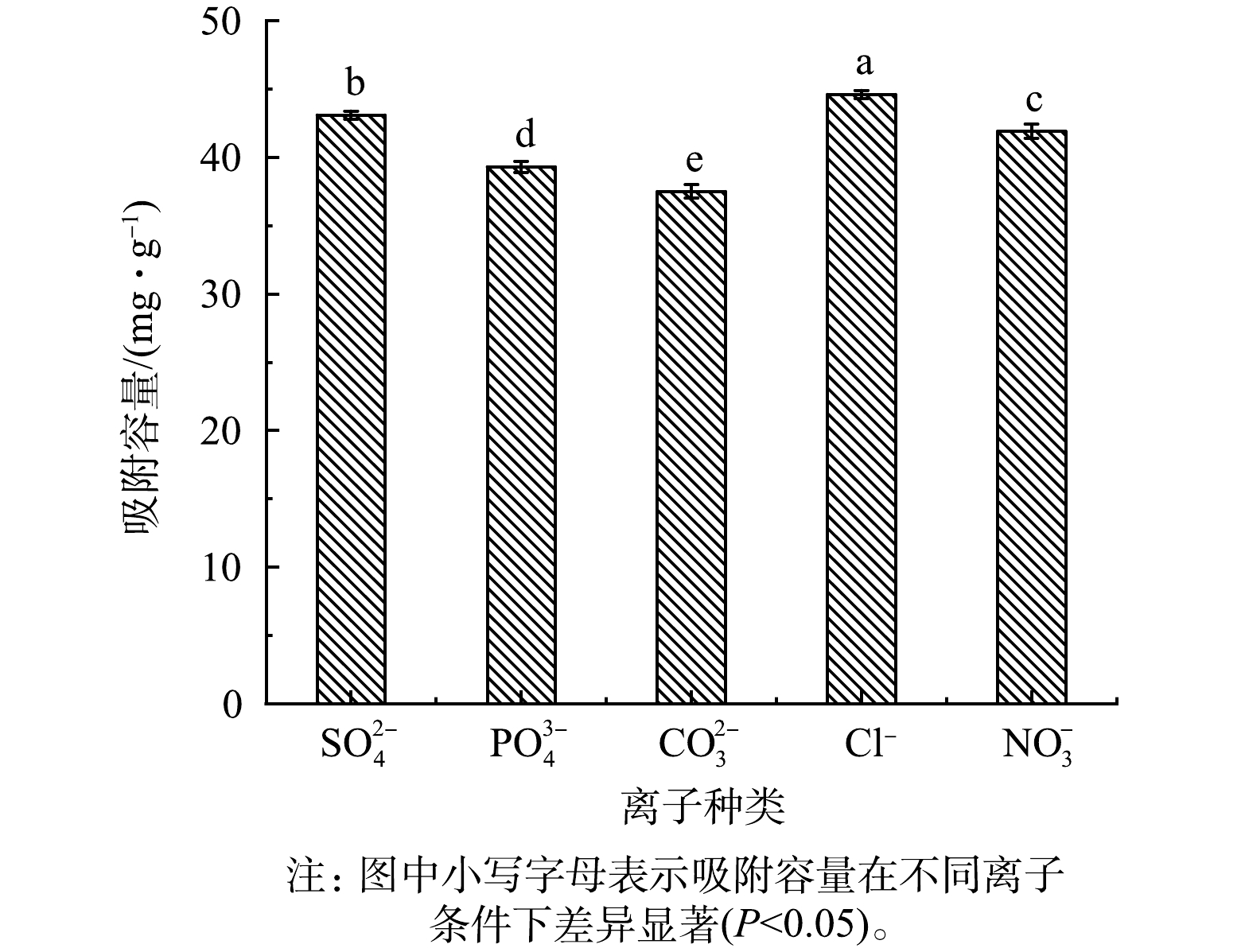
自然水体中存在很多阴离子,会对改性生物炭吸附硫酸盐的性能产生影响。共存阴离子对改性生物炭吸附硫酸盐性能的影响见图8。由图8可以看出,PO43−、CO32−和NO3−明显抑制MBC对硫酸盐的吸附。这是因为CO32−的加入会使溶液pH增大,从而抑制吸附,PO43−和NO3−主要是因为会与MBC产生静电吸附,占据吸附位点;Cl−对吸附起促进作用,这是因为Cl−的添加可以提高固液界面的电势,使带负电荷的硫酸盐更易靠近MBC表面,进而被吸附[33]。
-
1)使用安全性评价。MBC浸出液中主要污染物的浓度及其对应的不同规范浓度限值见表4。由表4可知,浸出液中主要污染物的浓度指标均未超过《地表水环境质量标准》(GB 3838-2002)和浸出毒性鉴别(GB 5085.3-2007)的规定限值,对环境没有危害性。
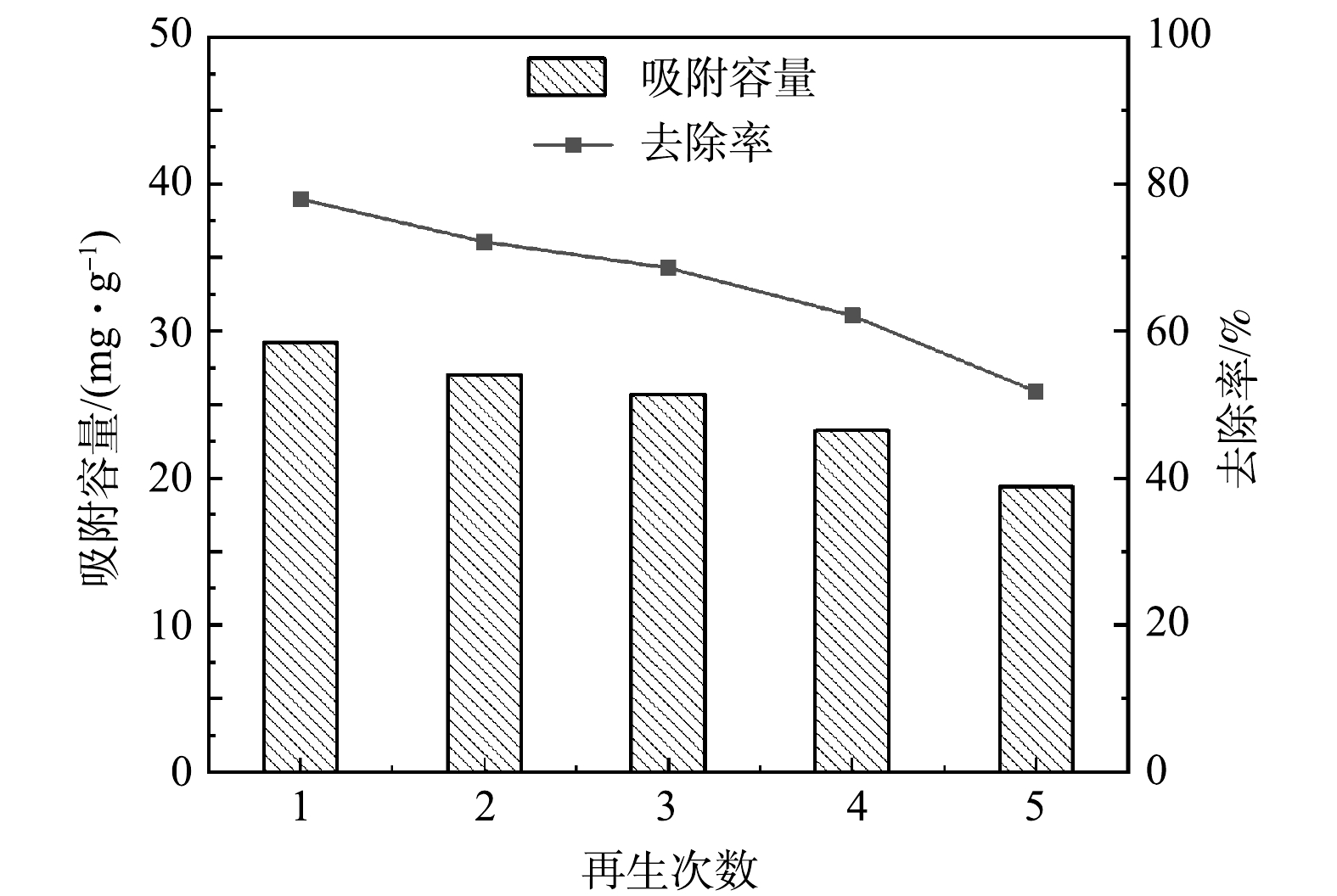
2) MBC的解吸再生。吸附剂的再生性能是衡量其是否为优质材料的标准之一。根据单因素实验结果可知,对硫酸盐的吸附效果随着pH的增大而减弱,选择1 mol·L−1 NaOH作为解吸剂,对吸附后的MBC解吸后再进行吸附实验,重复5次,结果如图9所示。随着再生次数的增加,去除率逐渐下降,在第5次再生吸附时,硫酸根去除率降为51.77%,去除效果仍保持较好水平,说明吸附剂具有良好的再生性能。
-
1)改性后的生物炭表面性能和孔隙结构都得到了明显提升。MBC中的官能团参与吸附过程,说明吸附过程存在化学吸附。
2)对改性吸附剂的吸附过程进行吸附动力学和吸附等温线拟合,发现准一级动力学模型和Langmuir更能准确地对其进行描述,说明吸附过程主要以静电吸附和单分子层吸附为主。
3)改性芦苇生物炭的投加量为8 g·L−1的条件下对硫酸盐可达到最佳吸附效果,此时硫酸盐的最大吸附量为29.69 mg·g−1。
4)改性芦苇生物炭的吸附再生性能良好。通过对吸附后的生物炭进行解吸,发现经过5次吸附解吸后硫酸盐的去除率仍能达到51.77%,说明该材料具有较好的再生性。
改性芦苇生物炭对水中硫酸盐的吸附性能及机理
Adsorption performance and mechanism of modified reed biochar towards sulfate in water
-
摘要: 水中过高浓度硫酸盐赋存会对水生环境产生一定的影响。为此,选取芦苇秸秆作为生物炭原料,通过添加煤矸石共热解对生物炭进行了改性,采用扫描电子显微镜(SEM)、氮气吸附脱附法(BET)和傅里叶红外光谱(FTIR)等手段对生物炭的物理化学性质进行了分析,考察了pH、吸附剂投加量、吸附时间和初始浓度对水中硫酸盐吸附性能的影响。结果表明:改性后生物炭(MBC)的吸附效果优于未改性生物炭(OBC);MBC的粗糙程度大于OBC,MBC拥有更大的比表面积和更多的孔隙结构,其比表面积为改性前的2.4倍;对吸附过程进行吸附动力学和吸附等温模型拟合,发现准一级动力学模型和Langmuir模型可以更好的描述MBC对硫酸盐的吸附行为,表明吸附过程以静电吸附和单分子层吸附为主。当pH为2、投加量为8 g·L-1时改性材料吸附效果最好,最大吸附量可达29.69 mg·g-1,且经过5次再生吸附后,硫酸盐去除率仍能达到50%以上。因此,改性后的生物炭可作为去除水体中硫酸盐的良好材料。Abstract: Excessive sulfate concentration in water will have a certain impact on the aquatic environment. In this study, reed straw was selected as the raw material of biochar, and the biochar was modified by co-pyrolysis with addition of coal gangue. The physicochemical properties of biochar were analyzed by means of scanning electron microscope (SEM), nitrogen adsorption desorption (BET) and Fourier infrared spectroscopy (FTIR). The effects of pH, adsorbent dosage, adsorption time and initial concentration on the adsorption performance of sulfate in water were investigated. The results showed that the adsorption effect of modified biochar (MBC) was better than that of unmodified biochar (OBC); The roughness of MBC was greater than that of OBC, and MBC had larger specific surface area and more pore structure, and its specific surface area was 2.4 times of that before modification; The adsorption kinetics and adsorption isotherm model fitting of the adsorption process showed that the quasi-first-order kinetic model and Langmuir model could better describe the adsorption behavior of MBC to sulfate, indicating that the adsorption process was dominated by electrostatic adsorption and monolayer adsorption. When the pH was 2 and the dosage was 8 g·L-1, the modified material had the best adsorption effect, and the maximum adsorption amount could reach 29.69 mg·g-1. After five times of regeneration adsorption, the sulfate removal rate could still reach higher than 50%. Therefore, the modified biochar can be used as a good material to remove sulfate from water.
-
Key words:
- reed biochar /
- modification /
- adsorption /
- sulfate
-

-
表 1 OBC和MBC的比表面积、平均孔径和孔容
Table 1. The specific surface area, average pore diameter and pore volume of OBC and MBC
样品 比表面积/(m2·g−1) 孔径/nm 孔容/(cm3·g−1) OBC 273.99 2.22 1.50×10-1 MBC 658.91 2.25 3.67×10-1 表 2 MBC的吸附动力学模型参数
Table 2. Parameters of MBC adsorption kinetic model
样品 准一级动力学参数 准二级动力学参数 qe/(mg·g−1) k1 R2 qe/(mg·g−1) k2 R2 MBC 49.145 5.329 0.959 49.524 0.492 0.991 表 3 MBC的吸附等温线模型参数
Table 3. Adsorption isotherm model parameters of MBC
样品 Langmuir Freundlich qm/(mg·g-1) kL R2 kF 1/n R2 MBC 81.094 0.011 0.941 7.720 0.370 0.870 表 4 MBC的浸出毒性
Table 4. Leaching toxicity of MBC
污染物 实测浓度/(mg·L−1) 地表水限值/(mg·L−1) 浸出毒性限值/(mg·L−1) Cu 0.000 9 1.000 100.0 Cr 0.033 0 0.050 15.0 Ag 0.000 4 0.001 5.0 Cd 0.000 1 0.005 1.0 -
[1] AO H T, CAO W, HONG Y X, et al. Adsorption of sulfate ion from water by zirconium oxide-modified biochar derived from pomelo peel[J]. Science of the Total Environment, 2020, 708: 135092. doi: 10.1016/j.scitotenv.2019.135092 [2] DING M, ZENG H. Multi-agent evolutionary game in the recycling utilization of sulfate-rich wastewater[J]. International Journal of Environmental Research and Public Health, 2022, 19(14): 8770. doi: 10.3390/ijerph19148770 [3] 赵蕾. Fe3O4磁性纳米材料的研制及其对水中污染物的去除研究[D]. 太原: 山西大学, 2021. [4] CHEN X, ZHENG L, DONG X, et al. Sources and mixing of sulfate contamination in the water environment of a typical coal mining city, China: Evidence from stable isotope characteristics[J]. Environmental Geochemistry Health, 2020, 42: 2865-2879. doi: 10.1007/s10653-020-00525-2 [5] WANG Y, HAO Y, GAO Z, et al. Characteristics and the origins of the main chemical components in mine water in the Xishan mining area, North China[J]. Environmental Earth Sciences, 2020, 81(8): 1-13. [6] BADMUS S O, OYEHAN T A, SALEH T A. Synthesis of a novel polymer-assisted AlNiMn nanomaterial for efficient removal of sulfate ions from contaminated water[J]. Journal of Polymers and the Environment, 2021, 29(9): 2840-2854. doi: 10.1007/s10924-021-02077-7 [7] HASSAN W, FAISAL A, ABED E, et al. New composite sorbent for removal of sulfate ions from simulated and real groundwater in the batch and continuous tests[J]. Molecules, 2021, 26(14): 4356. doi: 10.3390/molecules26144356 [8] HAN Y H, WU C T, FU X L, et al. Sulfate removal mechanism by internal circulation iron-carbon micro-electrolysis[J]. Separation and Purification Technology, 2021, 279: 119762. doi: 10.1016/j.seppur.2021.119762 [9] DOLATABAD A A, GANJIDOUST H, AYATI B. Application of waste-derived activated red mud/base treated rice husk composite in sulfate adsorption from aqueous solution[J]. International Journal of Environmental Research, 2022, 16(1): 1-16. doi: 10.1007/s41742-021-00380-8 [10] ZHU Q, HU Z Q, RUAN M Y. Characteristics of sulfate-reducing bacteria and organic bactericides and their potantial to mitigate pollution caused by coal gangue acidification[J]. Environmental Technology & Innovation, 2022, 20: 101142. [11] BI H B, WANG C X, LIN Q Z, et al. Pyrolysis characteristics, artificial neural network modeling and environmental impact of coal gangue and biomass by TG-FTIR[J]. Science of the Total Environment, 2021, 751: 142293. doi: 10.1016/j.scitotenv.2020.142293 [12] HUO X, JIA X, SONG C, et al. Investigation of co-pyrolysis characteristics of modified coal gangue and biomass[J]. Thermochimica Acta, 2021, 705: 179033. doi: 10.1016/j.tca.2021.179033 [13] ZHAO R, WANG B, ZHANG X, et al. Insights into Cr(Ⅵ) removal mechanism in water by facile one-step pyrolysis prepared coal gangue-biochar composite[J]. Social Science Electronic Publishing, 2022, 299: 134334. [14] WANG B, MA Y, LEE X, et al. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer[J]. Science of the Total Environment, 2020, 758: 143664. [15] QIU B B, DUAN F. Synthesis of industrial solid wastes/biochar composites and their use for adsorption of phosphate: From surface properties to sorption mechanism[J]. Colloids and Surface A:Physicochemical and Engineering Aspects, 2019, 571: 86-93. [16] LIAN G, Wang B, Lee X, et al. Enhanced removal of hexavalent chromium by engineered biochar composite fabricated from phosphogypsum and distillers grains[J]. Science of the Total Environment, 2019, 697: 134119. doi: 10.1016/j.scitotenv.2019.134119 [17] 薛炳松, 谢童, 王珊, 等. 镧改性生物炭对砷、镉的吸附特征研究[J]. 地球与环境, 2022, 50(2): 261-270. [18] 杨奇亮, 吴平霄. 改性多孔生物炭的制备及其对水中四环素的吸附性能研究[J]. 环境科学学报, 2019, 39(12): 3973-3984. doi: 10.13671/j.hjkxxb.2019.0235 [19] 郭明帅, 王菲, 张学良, 等. 改性生物炭活化过硫酸盐对水中苯和氯苯的去除机制[J]. 中国环境科学, 2020, 40(12): 5280-5289. doi: 10.3969/j.issn.1000-6923.2020.12.021 [20] Ahmed M J, Hameed B H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review[J]. Journal of Cleaner Production, 2020: 121762. [21] 王光泽, 曾薇, 李帅帅. 铈改性水葫芦生物炭对磷酸盐的吸附特性[J]. 环境科学, 2021, 42(10): 4815-4825. [22] 马凯悦, 张浩, 宋宁宁, 等. 氧化老化玉米秸秆生物炭吸附镉机理研究[J]. 农业环境科学学报, 2022, 41(6): 1230-1240. doi: 10.11654/jaes.2021-1282 [23] 宋泽峰, 石晓倩, 刘卓, 等. 芦苇生物炭的制备、表征及其吸附铜离子与双酚A的性能[J]. 环境化学, 2020, 39(8): 2196-2205. doi: 10.7524/j.issn.0254-6108.2019052001 [24] 余剑, 丁恒, 张智霖, 等. 改性菱角壳生物炭吸附水中土霉素性能与机理[J]. 中国环境科学, 2021, 41(12): 5688-5700. doi: 10.3969/j.issn.1000-6923.2021.12.025 [25] 党娅琴, 邢英. 稻壳生物炭吸附无机汞和甲基汞的特征研究[J/OL][J]. 地球与环境, 2020, 50(13): 1-10. [26] 孙彤, 郝瑞霞, 武旭源, 等. 天然沸石定向合成A型分子筛及其脱氮性能[J]. 中国环境科学, 2020, 40(2): 623-630. doi: 10.3969/j.issn.1000-6923.2020.02.019 [27] 王美杰. 球形ZrO(OH)2/AlOOH复合材料制备及吸附硫酸根性能研究[D]. 天津: 天津大学, 2019. [28] ALAGHA O, MANZAR M S, ZUBAIR M, et al. Comparative adsorptive removal of phosphate and nitrate from wastewater using biochar-MgAl LDH nanocomposites: Coexisting anions effect and mechanistic studies[J]. Nanomaterials, 2020, 10(2): 336. doi: 10.3390/nano10020336 [29] LIU T, XU S R, LU S Y, et al. A review on removal of organophosphorus pesticides in constructed wetland: Performance, mechanism and influencing factors[J]. Science of the Total Environment, 2019, 651: 2247-2268. doi: 10.1016/j.scitotenv.2018.10.087 [30] 邓玉, 刘斌, 晏琪涵, 等. 一步法制备Mg改性玉米芯生物炭吸附磷酸盐研究[J]. 水处理技术, 2021, 47(4): 35-39. doi: 10.16796/j.cnki.1000-3770.2021.04.007 [31] ALI S, RIZWAN M, SHAKOOR M B, et al. High sorption efficiency for As(Ⅲ) and As(Ⅴ) from aqueous solutions using novel almond shell biochar[J]. Chemosphere, 2020, 243: 125330. doi: 10.1016/j.chemosphere.2019.125330 [32] LEE N, HONG S H, LEE C G, et al. Conversion of cattle manure into functional material to remove selenate from wastewater[J]. Chemosphere, 2021, 278: 130398. doi: 10.1016/j.chemosphere.2021.130398 [33] 徐楚天, 李大鹏, 张帅, 等. 磁性硅藻土负载纳米过氧化钙对水中磷酸盐吸附[J]. 环境科学, 2020, 41(2): 792-800. -



 下载:
下载: