-
磷是导致水体富营养化的关键因素之一[1-3],如何有效控制水体磷含量是近些年来学者们的研究热点。湖泊磷的来源主要分为外源进入和内源释放,底泥是湖泊的重要组成部分,输入湖泊的外源磷经过一系列的物理、化学和生物作用会蓄积于底泥中。当外界环境条件发生变化时,蓄积于底泥中的磷会重新释放以内源磷的形式进入上覆水,因此,在目前外源磷逐步得到控制的条件下,有效抑制底泥中磷的释放成为了亟待解决的问题[4-7]。
目前底泥磷释放控制技术主要包括异位控制[8]和原位处理[9]。异位处理技术主要是疏浚技术,其是通过水力或机械方法挖除湖泊表层的污染底泥,清除污染水体的内源磷。我国的滇池草海、安徽巢湖、杭州西湖、瑞典的Trummen湖等均采用了该技术[10]。原位处理技术可分为化学处理、生物处理和稳定化处理。与异位处理技术相比,原位处理技术优点在于:可以避免疏浚过程中底泥悬浮致使大量磷向水体释放的问题,减少底泥异位时流失的污染物总量,降低污染物毒性及控制污染物迁移性,并且处理费用低[11],且原位控制技术可显著降低沉积物中各形态磷含量,抑制沉积物磷及其他污染物向上层水释放[12]。
净水厂污泥是给水处理厂在净水过程中产生的污泥,包括原水中的悬浮物质、有机物质等,以及处理过程中形成的如铝盐、铁盐等化学物质[13]。有研究[14-18]表明,净水污泥对磷具有优良的吸附效果。徐颖等[19]利用原始净水污泥为原料,其对磷的实际饱和吸附量为3.065 mg·g−1;马啸宙等[20]采用80 ℃烘干的净水污泥作为磷吸附材料,明确净水污泥对低浓度磷 (5 mg·g−1) 的最佳去除率为92.5%,理论饱和吸附量为3.47 mg·g−1。以上研究结果均说明净水污泥对磷具有一定的吸附能力。净水污泥在煅烧过程中其含有的铁、铝等元素会生成对应金属氧化物,可进一步提高净水污泥的吸附性能,而且通过煅烧可避免有机质对水体的影响[15-16,21]。本文使用经热改性处理后的净水污泥作为底泥改良材料,探讨了其对水体磷酸盐的吸附性能,并对比了不同WTS400-4投加量(0%、2.5%、5%、10%)对底泥内源磷形态分布的影响及吸附机制,以期为净水污泥资源化利用与水体内源磷控释提供参考。
-
底泥取自成都市金牛区某受污染的河道,采集底泥经自然风干后,破碎过100目筛网备用。所用HCl、NaOH、NaCl、LaCl3·(H2O)7、KH2PO4、CaCl2、NaHCO3等试剂均为分析纯,购自国药集团化学试剂有限公司。实验所用水为超纯水。
实验用净水污泥取自成都市某自来水厂,以铝盐和铁盐作为混凝剂,含水率在75%~80%,其中干基灰分82.02%。取回后经干燥、破碎过100目筛网备用。
本实验所用热改性净水污泥的制备步骤为:称取30 g100目净水污泥加入到50 mL陶瓷坩埚中,加盖密封,置于马弗炉中于400 ℃反应4 h,待反应完成后,冷却至室温,研磨过100目筛网,密封装袋置于干燥器中备用。经测定,WTS400-4磷含量为1 400 mg·kg−1,干基灰分89.5%。净水污泥原泥命名为WTS(water treatment sludge),制备的热改性净水污泥命名为WTS400-4。
-
1)底泥培养实验。首先,将4份质量均为150 g的底泥分别加入到4个500 mL透明试剂瓶中,再将WTS400-4按照占底泥质量分数0%、2.5%、5%、10%的比例分别加入上述试剂瓶中,并完全混合均匀,按照投加量的不同,各组分别命名为空白、WTS2.5、WTS5、WTS10;其次,配制含1 mmol·L−1NaHCO3、10 mmol·L−1 NaCl和1 mmol·L−1 CaCl2的溶液4 L,将pH调至7.0,经超声除氧后作为模拟实验的上覆水;最后再将模拟上覆水加入试剂瓶至与瓶口平齐,封口后再置于暗处培养60 d。实验期间,每15 d向试剂瓶内加入100 mg亚硫酸钠,以确保培养体系的溶解氧(DO)质量浓度不大于0.5 mg·L−1。每5 d利用pH计和溶解氧仪测定上覆水的pH值、DO质量浓度,采用钼锑抗比色法测定溶解性活性磷质量浓度(Soluble Reactive Phosphate(SRP))。
底泥实验结束后,将上覆水倒掉,将湿底泥通过离心得到间隙水和底泥样品,测定间隙水的SRP及底泥含水率。底泥样品经烘干后过100目筛网密封装袋后置于干燥器保存备用,以用于后续的磷形态提取和生物有效磷提取。空白、WTS2.5、WTS5和WTS10中底泥的含水率分别为27.5%、28.7%、28.8%和29.6%。
2)底泥中磷形态的连续提取。底泥中的磷形态可分为弱吸附态磷(NH4Cl-P)、铁结合态磷(BD-P)、铝结合态磷(NaOH-rP)、有机碎屑态磷(Org-P)、钙结合态磷(HCl-P)和残渣态磷(Res-P)。以上各种形态磷采用六步连续化学提取法[21]得到,具体步骤为:分别将1 mol·L−1 NH4Cl溶液(pH=7),0.11 mol·L−1 Na2S2O4/NaHCO3溶液、0.1 mol·L−1 NaOH溶液在25 ℃、200 r·min−1下振荡2、1、16 h得到NH4Cl-P、BD-P、NaOH-rP;上述溶液消解后减去NaOH-rP的量得到Org-P;用0.5 mol·L−1 HCl溶液在25 ℃、200 r·min−1下振荡16 h得到HCl-P;使用1 mol·L−1 NaOH溶液在85 ℃烘箱中反应1 h得到Res-P。
3)底泥生物有效磷提取。为了进一步探究WTS400-4对底泥磷的影响效果,本研究采用ZHOU等[22]提取生物有效磷的方法,测定水溶性磷(WSP)和碳酸氢钠可提取磷(Olsen-P),具体步骤为:取0.4 g过100目筛网的底泥样品于150 mL锥形瓶中,加入40 mL去离子水,在25 ℃、220 r·min−1条件下振荡2 h,离心10 min(4 000 r·min−1),取上清液测SRP即水溶性磷(WSP);取1.2 g过100目筛网的底泥样品于150 mL锥形瓶中,加入40 mL 0.5 mol·L−1NaHCO3溶液,在25 ℃、220 r·min−1条件下振荡0.5 h,离心10 min(4 000 r·min−1),取上清液测SRP即碳酸氢钠可提取磷(Olsen-P)。
4)材料的表征。材料的表观形貌采用场发射扫描电镜(SEM,Zeiss Merlin, 德国Zeiss公司)测定;比表面积、孔容和平均孔径特征采用全自动比表面积孔径分析仪(BET,Micromeritics Asap 2460,美国)测定;物相特征采用X射线衍射仪(XRD,D8 Advance, Bruker, 德国)测定。
-
1)吸附等温曲线。设置WTS400-4投加量2 g·L−1,pH=4,磷酸盐初始质量浓度分别为10、20、30、40、50、60、70、80和90 mg·L−1时,在30、40、50 ℃ 3个温度下,振荡48 h至吸附平衡后,测定溶液中磷酸盐浓度,计算磷吸附量,并对吸附数据进行Langmuir(式(1))和Freundlich(式(2))等温吸附模型拟合。
式中:qe为平衡吸附量,mg·g−1;qm为最大吸附量,mg·g−1;Ce为平衡浓度,mg·L−1;KL为Langmuir吸附常数,L·mg−1;KF为Freundlich吸附常数,mg·g−1;n为非均质系数。
2)吸附动力学。磷酸盐初始质量浓度为20、50和80 mg·L−1时,设置吸附剂投加量2 g·L−1,调节体系pH=4,在30 ℃下持续振荡,分别于5、15、30、60、120、240、360、480、720、1 440、2 160和2 880 min时,取水样测定其中的磷浓度。对吸附数据进行准一级动力学方程(式(3))、准二级动力学方程(式(4))和颗粒内扩散模型方程(式(5))拟合分析。
式中:t为吸附时间,min;qt为t时间的吸附量,mg·g−1;k1为准一级动力学常数,min−1;k2为准二级动力学常数,g·(mg·min)−1;k3为颗粒内扩散反应速率常数;C3为与边界层厚度相关的常数。
3)吸附热力学。吉布斯自由能ΔGθ、熵变ΔSθ和焓变ΔHθ这些参数有助于阐明吸附过程的状态和趋势,有助于明确吸附特征和机制[23]。根据Freundlich吸附模型拟合得到的参数,可以根据式(6)计算不同温度下的ΔGθ;根据式(8),以1/T为横坐标,lnKp为纵坐标作图,熵变ΔSθ从直线的截距推算,焓变ΔHθ从斜率推算。其中标准压力平衡常数Kp是计算ΔGθ的关键,Kp可通过Freundlich等温式中的吸附常数计算得到[24]。
式中:ΔGθ为吉布斯自由能,kJ·mol−1;ΔSθ为熵变,kJ·mol−1;ΔHθ为焓变,J·(mol·K)−1;R为气体常数,8.314 J·(mol·K)−1;Kp为标准压力平衡常数;T为热力学温度,K。
4)数据处理。根据上覆水中SRP质量浓度的变化,底泥中磷向上覆水的平均释放速率通过式(9)计算[25-26]。
式中:J为平均释放速率,mg·(m2·d)−1;ct和c0分别为t时刻和初始时刻上覆水磷质量浓度,mg·L−1;V为上覆水体积,L;A为底泥-上覆水界面的面积,m2;t为时间,d。
5)pH的影响。分别取80 mg的WTS和WTS400-4,加入到40 mL磷质量浓度为20 mg·L−1的磷酸二氢钾溶液中,用0.1 mol·L−1 的HCl和NaOH调节溶液pH为2、4、6、7、8和10,在30 ℃摇床中于150 r·min−1振荡48 h,结束后离心分离,经0.45 μm滤膜过滤后取上清液测定剩余磷质量浓度,计算对磷的吸附量,每组设置3个平行样,实验结果取平均值。吸附剂对磷的平衡吸附量按照式(10)计算。
式中:qe为平衡吸附量,mg·g−1;c0、ce分别为溶液中磷的初始和吸附平衡时的质量浓度,mg·L−1;V为溶液体积,L,m为吸附剂质量,g。
-
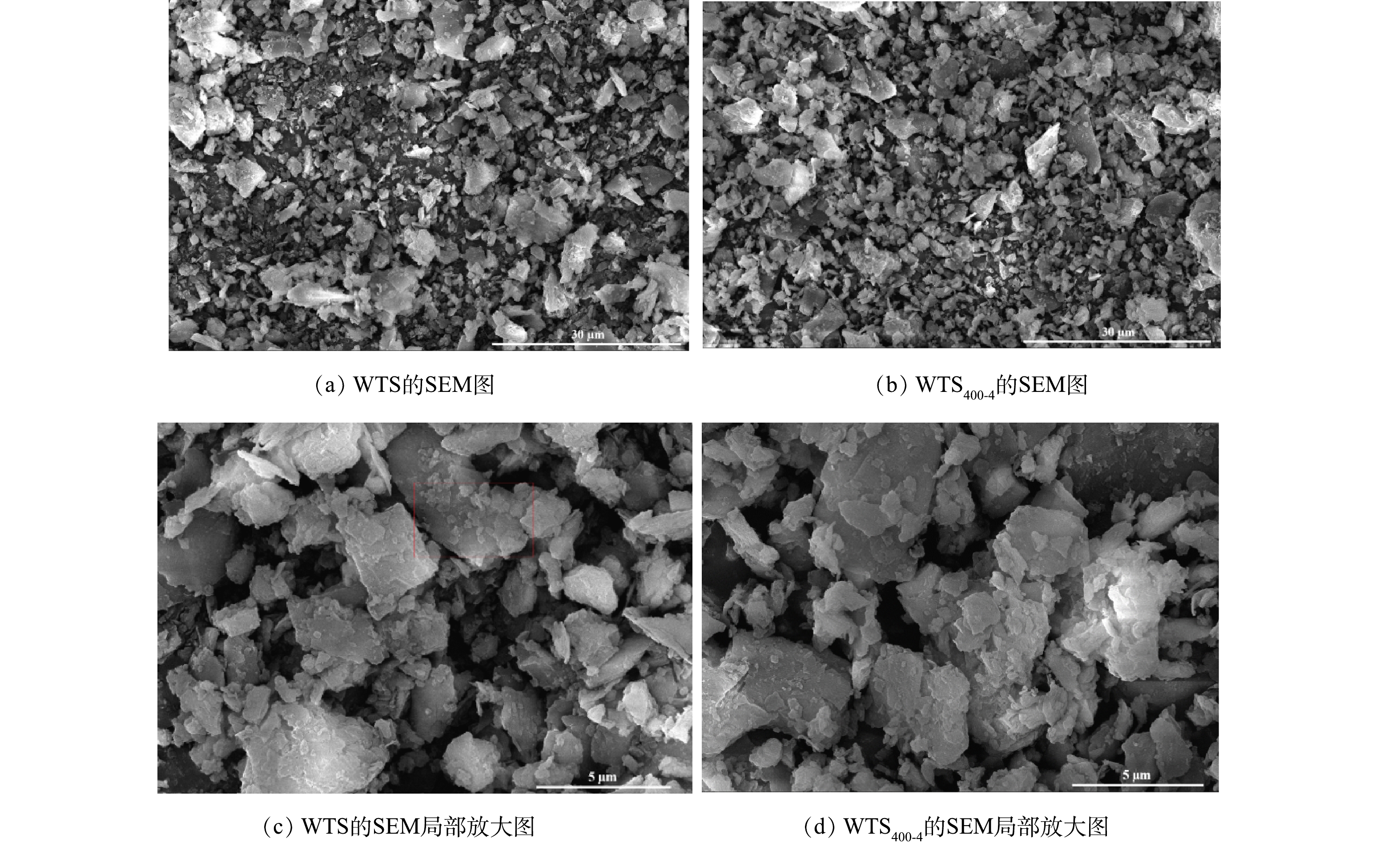
图1为WTS400-4和WTS的扫描电镜图。由图1可知,原污泥的表面形貌是紧密相连的片层结构,孔隙较少;经热处理后,表面组织变得粗糙,污泥孔径增大,内部形成较大的孔隙、结构更松散。这也体现在WTS和WTS400-4的元素分析图上。图2为WTS和WTS400-4的EDS能谱图,表1为WTS和WTS400-4的元素含量。由WTS和WTS400-4干基灰分分别为82.02%和89.5%可知,与WTS相比,WTS400-4的有机物减少且碳含量降低,有效孔径增大,Fe、Al、Ca等元素含量相对增加。WTS和WTS400-4的元素组成没有明显差异,主要元素组成均为C、O、Na、Al、Si、Ca、Fe等,占总比例的85%以上,这与净水污泥的组成一致[27]。
净水污泥改性前后的N2吸脱附等温曲线和孔径分布分别如图3(a)和图3(b)所示。可见,吸附量随着相对压力增大而增大,WTS400-4的吸附量显著高于WTS。材料的N2吸脱附等温线与Ⅳ型特征相符,说明WTS400-4属于介孔类材料。改性之后,WTS400-4的比表面积相对WTS (29.39 m2·g−1)略有增加至34.22 m2·g−1,这使得吸附磷酸盐的活性位点有所增加;平均孔径由7.77 nm增加到10.5 nm,增加了1.35倍,表明WTS400-4可以有效提高污泥的孔隙扩散吸附能力。
-
WTS和WTS400-4吸附磷酸盐前后的XRD分析如图4所示。可见,WTS400-4主要由SiO2和Al2O3等物质组成。其中SiO2占主要成分,2θ分别在21.003°、26.826°、50.373°和60.435°等处出现衍射峰。而在吸附磷酸盐后的谱图中出现PO43-的衍射峰,则表明WTS400-4成功吸附了磷酸盐[28]。
-
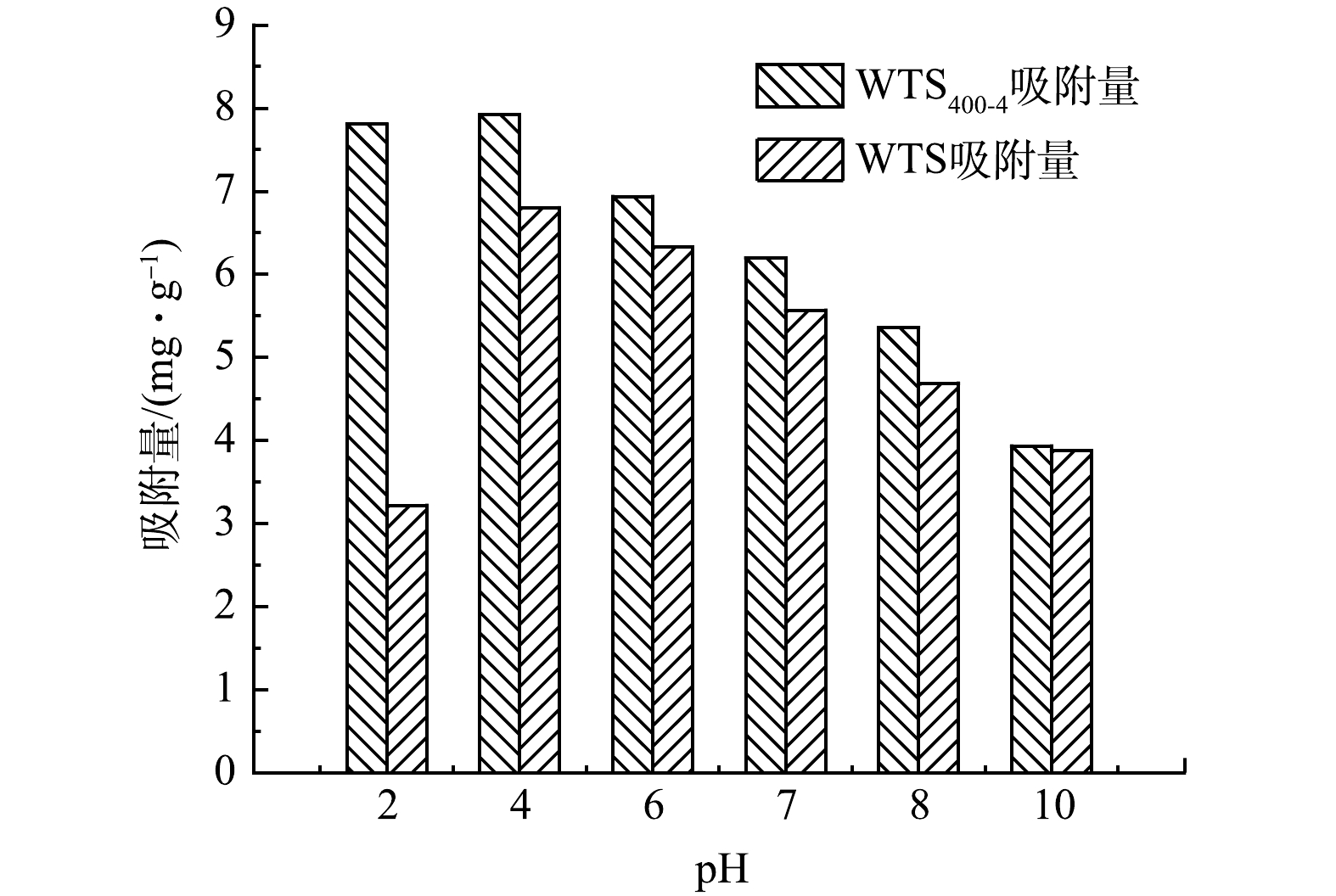
初始pH对WTS400-4和WTS吸附磷酸盐的影响如图5所示。可见,WTS400-4对磷的吸附效果明显优于WTS,且WTS400-4和WTS均在pH为4时达到最大吸附量,分别为7.92、7.1 mg·g−1。随着体系pH升高,WTS400-4和WTS的磷酸盐吸附量均呈现先升高后下降的趋势,这种趋势可能是受吸附剂表面电荷性质及吸附质存在形式的影响[29]。零电荷点(pHpzc)指吸附剂表面静电荷为0时溶液的pH,采用ZHU等[30]的方法测定了WTS400-4和WTS的零电荷点(pHpzc)分别为8.91和8.2。当溶液pH小于吸附剂的零电荷点(pHpzc)时,吸附剂表面呈正电性,能以静电引力吸附溶液中的阴离子,因此拥有较高零电荷点(pHpzc)的WTS400-4可以在更广的pH范围内呈现正电性,从而更加有利于通过静电引力吸附磷酸盐[31]。当溶液pH大于吸附剂零电荷点(pHpzc)时,吸附剂表面呈负电性,从而与磷酸根离子产生静电斥力,抑制了WTS400-4吸附磷酸盐。WTS400-4吸附磷酸盐后溶液pH由初始的2、4、6、7、8、10分别变为5.68、7.1、7.58、7.79、8.17、10.09。可见,达到吸附平衡后溶液pH呈现上升趋势,表明溶液中OH−增多,推测是因为磷酸盐与WTS400-4表面的OH−发生配体交换所致,磷酸盐通过配体交换被吸附[32]。
-
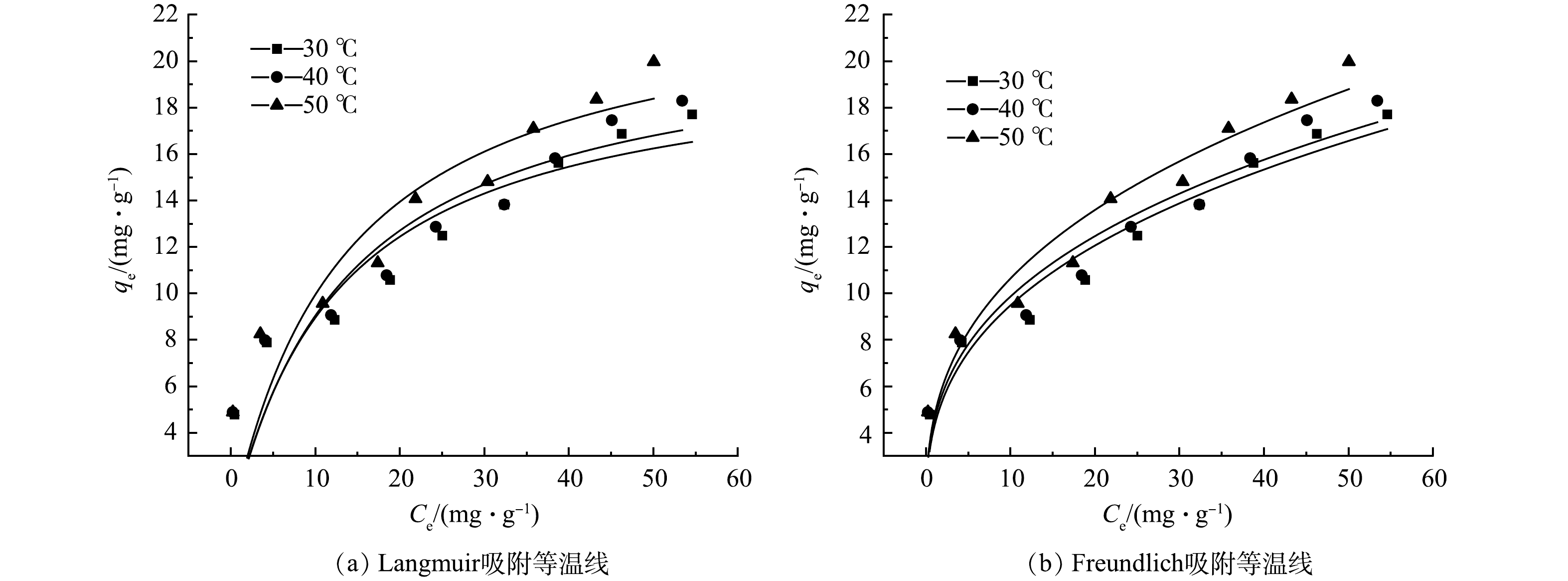
吸附等温线反映在一定温度下达到吸附平衡时,吸附剂中吸附质浓度与溶液浓度之间的关系[33]。分别在吸附温度为30、40、50 ℃时,使用Langmuir和Freundlich等温模型方程对实验数据进行拟合,其结果如图6所示。在3种不同温度下,WTS400-4对磷酸盐的吸附量均随着初始磷质量浓度的增加呈先增加后趋于平衡趋势。吸附等温曲线的拟合参数如表2所示,Freundlich模型显示了较高的相关系数、其R2为0.92~0.95,因此,Freundlich模型更适用于描述WTS400-4吸附磷酸盐的过程。Freundlich模型中,n为与吸附强度有关的常数,n值越大,吸附剂对吸附质的亲和能力越强,越有利于吸附。一般情况下,当n值处于1~10时,吸附容易进行,n<0.5时,则难于吸附[34]。本研究中WTS400-4对磷酸盐的n为4.2~4.8,均大于1,说明WTS400-4对磷酸盐的吸附过程易进行[35]。
基于Freundlich模型WTS400-4吸附的ΔGθ、ΔSθ和ΔHθ计算结果见表3,以lnKP对1/T进行线性拟合,得WTS400-4吸附磷酸盐的相关系数R2值达到0.999。在303.15、313.15和323.15 K的条件下,WTS400-4对磷酸盐吸附过程的ΔGθ值均小于0,表明该吸附反应是自发进行的。此外,随着温度的升高,ΔGθ值呈现递减的趋势,说明升高温度可以提高该吸附反应的自发特性,有利于WTS400-4对磷酸盐吸附,且也说明该反应是一个吸热反应。由表3可知,该吸附过程ΔHθ>0,则进一步表明此反应是吸热反应。该吸附过程ΔSθ>0,表明该吸附过程的无序性增大,WTS400-4吸附磷酸盐增加了固-液界面上物质的混乱度[36],ΔHθ、ΔSθ的值进一步证明了该吸附过程的自发性。
-
本研究分别在磷溶液初始浓度为20、50、80 mg·L−1,对实验数据拟合了准一级动力学和准二级动力学曲线,其结果分别如图7(a)和图7(b)所示,其拟合参数如表4所示。拟合结果表明准二级动力学模型(R2>0.99)优于准一级动力学模型,理论平衡吸附量qe,cal接近于实验平衡吸附量qe,exp,相对误差小于6.56%。因此、准二级动力学模型可以准确地描述WTS400-4对磷酸盐的吸附过程。准二级动力学包括液膜扩散、表面吸附和颗粒内扩散、电子共享或转移等吸附过程[37]。结果表明,WTS400-4对磷酸盐的主要控制步骤为化学吸附。
为了进一步探究吸附速度的控制步骤,本研究分别在磷溶液初始质量浓度为20、50、80 mg·L−1,对实验数据采用颗粒内扩散模型进行拟合,结果如图7(c)所示,拟合参数如表5所示。可见,整个扩散过程分为了3个阶段:第1阶段为液膜扩散过程,k1越大,说明边界扩散过程越快;第2阶段为粒子内扩散过程,k2较小,说明粒子内扩散过程为限速步骤,第3阶段则为平衡吸附过程。结合颗粒内扩散模型的特点,表明WTS400-4吸附磷酸盐过程由颗粒内扩散和液膜扩散联合控制[38]。
-
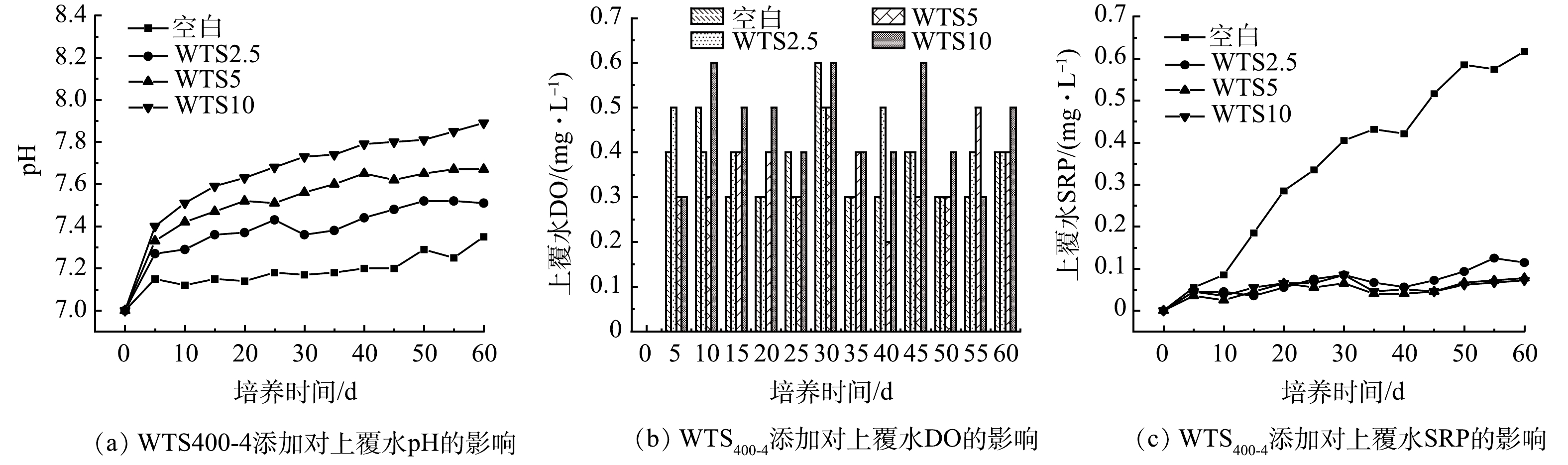
1) WTS400-4添加对上覆水pH、DO和SRP的影响。图8(a)和图8(b)为底泥培养过程中各体系上覆水pH和DO质量浓度的变化。可见,底泥培养期间各反应体系上覆水中DO质量浓度均为0.2~0.6 mg·L−1。这说明各反应体系中底泥和上覆水均处于缺氧状态。另外,在60 d培养周期内,各反应体系上覆水的pH均呈现先上升最后趋于稳定的趋势,并且4个控制组pH的升高趋势根据WTS400-4投加量增加也变得越来越大,但各体系pH均位于7.1~8,这说明培养期间底泥的pH环境处于中性范围,添加WTS400-4并不会大幅的改变底泥的pH环境。
图8(c)为底泥培养过程中不同底泥投加量体系上覆水SRP质量浓度的变化。可见,空白组上覆水SRP质量浓度由0上升到0.617 mg·L−1。这代表在低DO浓度条件下,底泥会持续释放SRP进入上覆水,当培养时间到60 d时,WTS2.5、WTS5和WTS10上覆水SRP质量浓度分别为0.114、0.077、0.072 mg·L−1,远低于空白组上覆水SRP质量浓度。这说明向底泥中添加WTS400-4可以明显降低底泥中磷向上覆水中释放的风险;空白组、WTS2.5、WTS5和WTS10的磷平均释放速率分别为0.989、0.183、0.123、0.115 mg·(m2·d)−1;与空白组相比,WTS2.5、WTS5和WTS10的底泥磷平均释放速率分别下降了81.50%、87.56%、88.37%。这进一步证明WTS400-4添加可以显著降低底泥磷释放速率,可有效抑制底泥中磷向上覆水释放,并且WTS400-4投加量的递增可以更有利于控制底泥中磷向上覆水中释放。
2) WTS400-4添加对间隙水中SRP质量浓度的影响。一般底泥中的磷首先会被释放进入间隙水中,再通过分子扩散、对流等作用穿越底泥-上覆水界面进入上覆水[39]。因此,探究WTS400-4对间隙水中磷含量的影响是很必要的。如图9所示,当底泥模拟实验进行至60 d时,空白组、WTS2.5、WTS5和WTS10的间隙水SRP质量浓度分别为0.378、0.087、0.074、0.048 mg·L−1,相对空白组,WTS2.5、WTS5和WTS10的削减率分别为76.98%、80.42%、87.30%。这证明WTS400-4对磷的优良吸附效果、可以显著降低间隙水中磷的浓度,从而实现抑制底泥中内源磷向上覆水释放的目的。
如图8(b)所示,本研究所有控制组均处于缺氧条件,表明在缺氧条件下底泥中的内源磷会持续向上覆水中释放。有研究表明,在好氧条件下,底泥中的Fe3+会和磷酸盐形成磷酸铁络合物,致使底泥中磷难以被释放[5];而在缺氧条件下,底泥中的Fe3+会转化为Fe2+,因此被Fe3+所固定的磷会被大量释放进入间隙水,从而扩散进入上覆水中使得上覆水中磷含量增高。有研究[40]表明,热改性净水污泥对水体磷酸盐具有良好的吸附能力,WTS400-4会直接吸附间隙水中磷酸盐,使间隙水中SRP质量浓度降低。推测另一个底泥吸附磷的原因可能是净水污泥经热改性生成的WTS400-4相比原泥,金属氧化物含量有所提高及在去除有机质后使得表面粗糙程度较高、孔隙发达且分布广泛,絮状程度大大提高,致使WTS400-4有较大的比表面积,并且经热改性之后的净水污泥具有氧化性,其与底泥完全混合之后,可对底泥微环境进行改善,从而提升对磷的持留能力[41]。
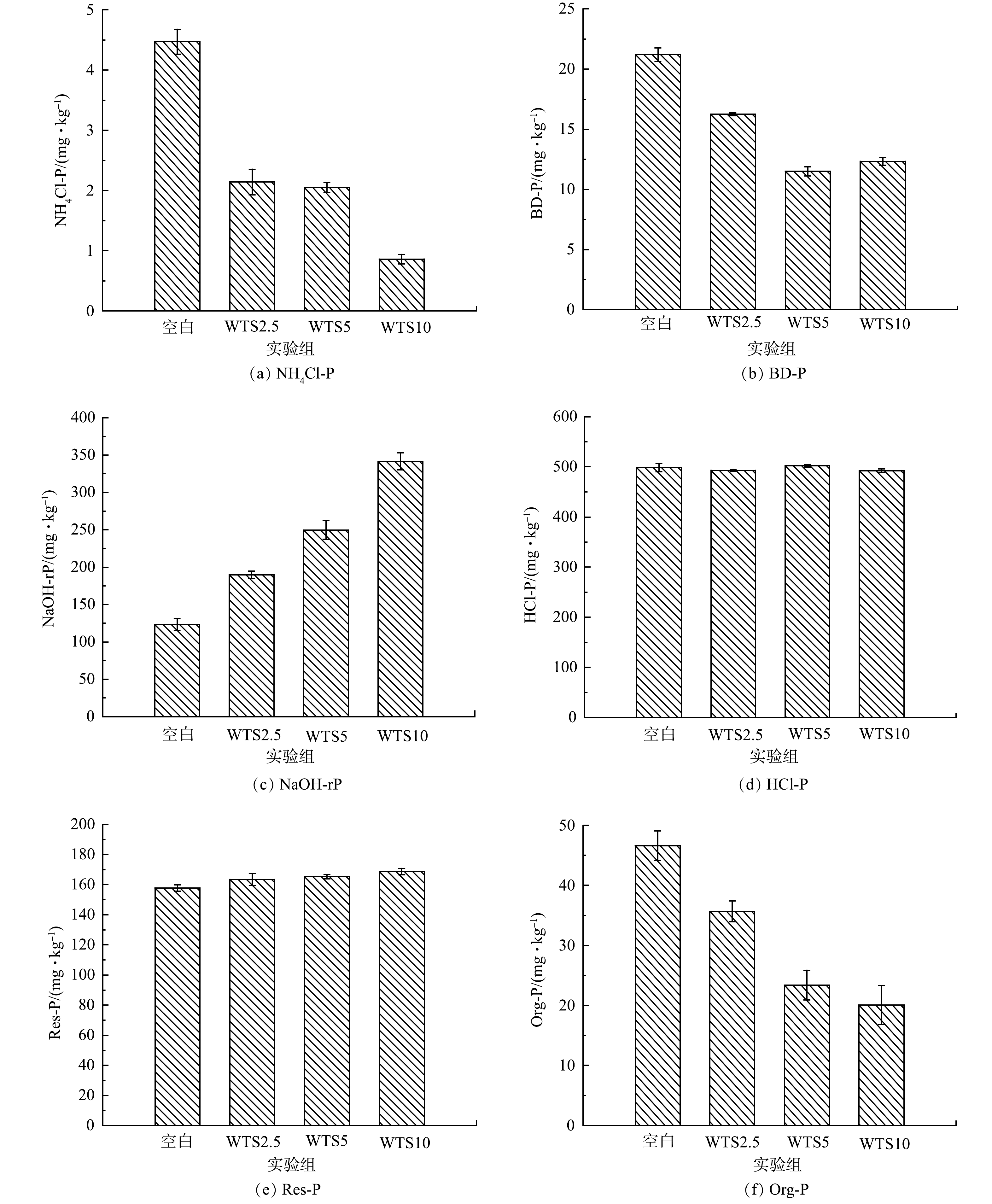
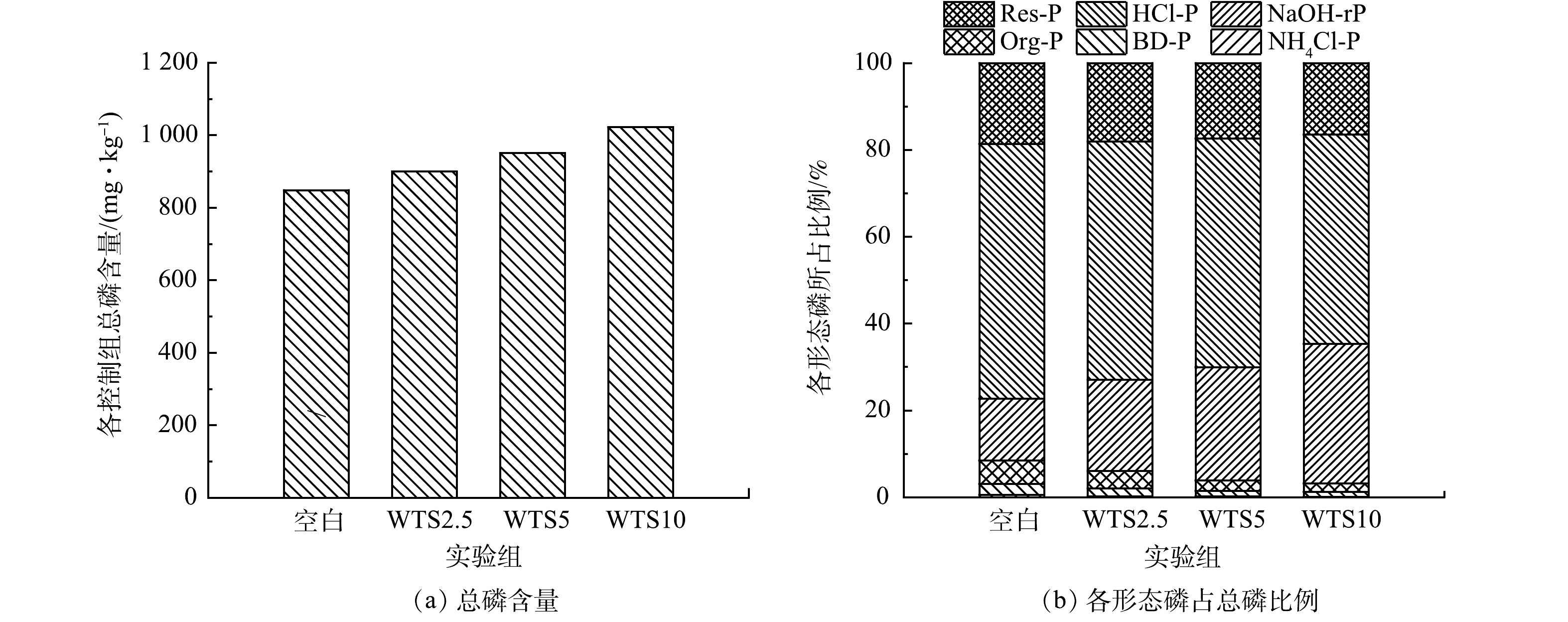
3)WTS400-4添加对底泥磷形态的影响。底泥中磷的存在形式直接关系到底泥中磷向上覆水释放的风险,因此,探究WTS400-4添加对底泥磷赋存特征的影响是十分必要的。不同控制组NH4Cl-P、BD-P、Org-P、NaOH-rP、HCl-P和Res-P含量如图10和表6所示。对NH4Cl-P而言,WTS2.5、WTS5和WTS10的含量比空白组分别下降了49.54%、54.05%、79.60%;对BD-P而言,WTS2.5、WTS5和WTS10的含量比空白组分别下降了24.53%、46.01%、42.95%;对Org-P而言,WTS2.5、WTS5和WTS10的含量比空白组分别下降了22.87%、48.57%、57.15%;对NaOH-rP而言,WTS2.5、WTS5和WTS10的含量比空白组分别升高了36.28%、51.12%、63.23%;而HCl-P和Res-P含量基本保持不变。以上结果说明向底泥中添加WTS400-4会显著影响底泥中磷的赋存形态,NaOH-rP含量上升,而NH4Cl-P、BD-P、Org-P等含量降低。分析其原因主要分为以下4点:一是本实验采用的净水污泥来自以铝盐和铁盐作为混凝剂的自来水厂,所以WTS400-4中含有大量铝金属氧化物,其会大量吸附底泥中的磷并且以Al-P(NaOH-rP)的形式存在,加之WTS400-4本身就含有相当比例的Al-P(总磷含量为1 400 mg·kg−1),因此,造成了底泥中Al-P大幅上升;二是NH4Cl-P(弱吸附态磷、主要指被底泥矿物颗粒吸附的磷)虽然含量低,但当水环境发生变化时极容易被释放出来进入水体[41],因此各个反应体系中NH4Cl-P含量都出现了不同程度的下降;三是BD-P(氧化还原敏感态磷、一般指与铁锰氧化物结合的磷)的稳定性与底泥DO质量浓度条件呈正相关,在缺氧条件下易发生释磷情况[42];四是Org-P(有机磷)易被底泥中微生物转化利用[43],因此各反应体系中Org-P含量均出现不同程度的降低。而钙磷(HCl-P)和残渣态磷(Res-P)均很稳定,不容易被释放[42]。而朱培颖[41]等研究表明热改性污泥与底泥混匀相比覆盖在底泥表面上,其钙磷(HCl-P)占比最高,覆盖组Al-P(NaOH-rP)占比更高,这可能和其热改性污泥投加方式与投加外源磷等条件有关。
进一步分析WTS400-4添加对底泥总磷的影响,如图11(a)所示,空白组、WTS2.5、WTS5和WTS10总磷含量分别为848.59、899.63、950.79、1 022.59 mg·kg−1,各控制组总磷含量随着WTS400-4添加量的增多而增多,之所以出现这种情况是因为本次实验所用净水污泥本身含有一定量的磷(总磷含量为1 400 mg·kg−1),表明WTS400-4的添加不仅会改变底泥中磷的分布形态也会增加底泥中总磷含量,这在实际运用中需要具体考量。NH4Cl-P、BD-P与Org-P被认为是潜在活性磷[39],图11(b)为各组中不同形态磷占总磷的比例,空白组、WTS2.5、WTS5和WTS10潜在活性磷比例分别是8.52%、6.02%、3.94%、3.23%,WTS400-4添加量为2.5%、5%、10%的潜在活性磷比例分别较空白组降低了2.50%、4.58%、5.29%;此外,空白组、WTS2.5、WTS5和WTS10的Al-P(NaOH-rP)比例分别为14.23%、21.07%、25.99%、32.12%,WTS400-4添加量为2.5%、5%、10%的Al-P(NaOH-rP)比例分别较空白组升高了6.84%、11.76%、17.89%,可见WTS400-4的添加可以使底泥中不稳定态磷向稳定的Al-P(NaOH-rP)转化,且其转化量会随着WTS400-4的增多而增多,同时底泥内源磷的释放风险随着WTS400-4的增多而减小。这说明WTS400-4添加稳定底泥内源磷的主要机制是底泥中潜在活性磷被WTS400-4吸附转化为稳定的Al-P(NaOH-rP),这也与WANG等[44]的研究一致。
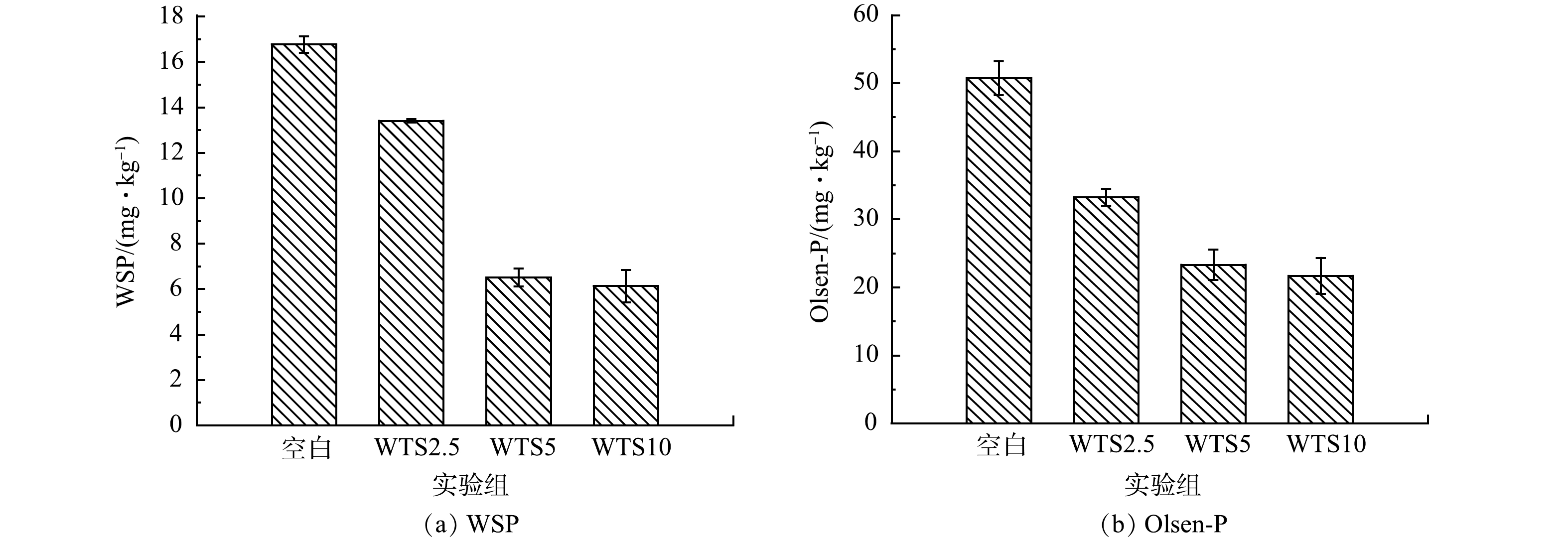
4)WTS400-4添加对生物有效磷的影响。生物有效磷常指底泥中可通过物理、化学和生物过程转化为藻类而被直接利用的磷[33]。因此,研究WTS400-4添加对生物有效磷的影响有助于探明其控释底泥磷的机制,也有助于明确WTS400-4投加量对于底泥生物可利用磷的影响。WTS400-4的添加对WSP和Olsen-P 2种生物有效磷含量的影响如图12所示。空白组、WTS2.5、WTS5和WTS10的WSP含量分别为16.83、13.39、6.51、6.24 mg·kg−1,WTS2.5、WTS5和WTS10相对于空白组的WSP含量分别下降了20.43%、61.32%、62.93%;空白组、WTS2.5、WTS5和WTS10的Olsen-P含量分别为50.69、33.49、22.12、21.32 mg·kg−1,WTS2.5、WTS5和WTS10相对于空白组的Olsen-P含量分别下降了33.93%、56.36%、57.94%。这表明WTS400-4可以有效降低底泥中WSP和Olsen-P 2种生物有效磷的含量,并且降低程度与WTS400-4添加成正相关,进一步的证明了WTS400-4添加可以抑制底泥中磷向上覆水释放。
-
1)对净水污泥进行热改性可以显著提高其对磷酸盐的吸附性能,WTS400-4不仅可以降低底泥间隙水的磷含量,也会降低底泥间隙水中磷向上覆水的释放速率。
2) WTS400-4对磷的吸附过程更符合Fruendlich等温吸附模型;WTS400-4添加一方面会降低底泥中WSP和Olsen-P 2种生物有效磷含量,也会使底泥中NH4Cl-P、BD-P和Org-P等不稳定态磷向稳定的NaOH-rP转化。
3) WTS400-4对底泥中磷的吸附稳定机制一方面是WTS400-4本身会吸附一定的磷,另一方面会促使底泥中不稳定磷向稳定态磷转化,达到抑制底泥中磷向上覆水释放的目的。
4)实际运用中需要考察WTS400-4添加对不同性质底泥中潜在活性磷和生物有效磷的稳定效果以及其潜在活性磷含量与生物有效磷削减量与WTS400-4投加量之间的关系。
热改性净水污泥对水体底泥磷的控释机制
Controlled release mechanism of phosphorus from water sediment by heat modified water purification sludge
-
摘要: 本研究以净水污泥为原料,通过400 ℃、4 h煅烧制备了热改性净水污泥。探讨了其对水体中磷酸盐的吸附特性,以及在不同投加量 (2.5%、5%、10%) 下热改性净水污泥对底泥中磷的控释及形态影响。同时结合SEM、BET等表征手段,探究了WTS400-4对底泥中磷的稳定机理。结果表明,WTS400-4相比WTS具有更发达的孔隙结构及比表面积,增强了对磷酸盐的吸附能力。吸附过程符合准二级动力学模型,Freundlich模型更适用于描述WTS400-4吸附磷酸盐的过程,其中参数n>1,说明WTS400-4对磷酸盐的吸附容易进行。WTS400-4的添加会使底泥中弱吸附态磷(NH4Cl-P)、氧化还原敏感态磷(BD-P)和有机磷(Org-P)等不稳定态磷向稳定的金属氧化物结合态磷(NaOH-rP)转变,且其转化量会随着WTS400-4投加量的增加而增加,有助于抑制底泥磷向上覆水释放。此外,WTS400-4的添加可降低底泥中WSP(水溶性磷)和Olsen-P (碳酸氢钠可提取磷)这2种生物有效磷含量。WTS400-4添加入底泥,一方面可以降低底泥中潜在活性磷和生物有效磷含量,降低底泥内源磷向上覆水释放的风险,另一方面可以通过WTS400-4的吸附作用直接去除间隙水中的磷,从而降低上覆水和间隙水之间的磷浓度梯度,进而抑制磷从间隙水向上覆水中释放。结果表明,WTS400-4可作为底泥改良剂用以控制水体和底泥的磷含量。Abstract: In this study, the heat modified water purification sludge (WTS400-4) was prepared by 400 ℃ and 4 h pyrolysis using water treatment sludge as raw material. The adsorption characteristics of phosphate in water and the effects of different dosage (2.5%, 5%, 10%) on the controlled release and the form of phosphate in the sediment were discussed. At the same time, combining with SEM, BET and other characterization methods, the stabilization mechanism of WTS400-4 on phosphorus in sediment was explored. The results showed that WTS400-4 had more developed pore structure and specific surface area than WTS, which strengthened the phosphate adsorption capacity. The adsorption process conformed to the quasi-second-order kinetic model. Freundlich model was more suitable to describe the phosphate adsorption process on WTS400-4, the parameter n higher than 1 indicated that the WTS400-4 was easy to adsorb phosphate. The addition of WTS400-4 could cause the transformation of the weakly adsorbed phosphorus (NH4Cl-P), redox-sensitive phosphorus (BD-P), organophosphorus (Org-P) and other readily released phosphorus to stable metal oxide bound phosphorus (NaOH-rP) in sediment, and the conversion amount increased with the increase of WTS400-4 dosage, which could contribute to inhibiting the release of phosphorus from the sediment. In addition, the addition of WTS400-4 could reduce the contents of WSP(water-soluble phosphorus) and Olsen-P (sodium bicarbonate extractable phosphorus) in the sediment. Adding WTS400-4 to the sediment, on the one hand, could reduce the contents of potential active phosphorus and bioavailable phosphorus in the sediment and reduce the risk of endogenous phosphorus release from the sediment to the overlying water. On the other hand, the phosphorus in interstitial water could be directly removed through the adsorption on WTS400-4, so as to reduce the phosphorus concentration gradient between overlying water and interstitial water and inhibit the release of phosphorus from interstitial water to overlying water. The results showed that WTS400-4 could be used as a sediment amendment to control the phosphorus content in water and sediment.
-

-
表 1 净水污泥和热改性净水污泥的元素含量占比
Table 1. Element content ratio of WTS and WTS400-4
样品 C/% O/% Si/% Al/% K/% Fe/% Ca/% Mg/% Na/% WTS 35.03 47.96 7.26 5.59 0.57 2.59 0.94 0.47 1.58 WTS400-4 9.90 52.95 15.21 11.50 1.18 4.34 1.05 1.75 2.11 表 2 Langmuir和Freundlich吸附等温模型拟合参数
Table 2. Fitting parameters of Langmuir and Freundlich adsorption isothermal models
温度/ ℃ Langmuir模型 Freundlich模型 qm/(mg·g−1) KL/(L·mg−1) R2 n KF/(mg·g−1) R2 30 20.383 3 0.078 2 0.789 1 2.885 0 4.266 6 0.947 2 40 21.374 6 0.731 7 0.768 3 2.959 0 4.526 2 0.928 7 50 23.324 1 0.742 2 0.797 5 2.829 9 4.713 4 0.939 0 表 3 WTS400-4吸附磷酸盐的热力学参数
Table 3. Thermodynamic parameters of phosphate adsorption by WTS400-4
温度/K ΔGθ/(kJ·mol−1) ΔHθ/(kJ·mol−1) ΔSθ/(J·(mol·K)−1) 303.15 −10.55 4.062 25.484 313.15 −11.34 323.15 −12.02 表 4 准一级、准二级动力学拟合参数
Table 4. Quasi-first order and quasi-second order kinetic fitting parameters
磷溶液初始
浓度/(mg·L−1)准一级动力学模型 准二级动力学模型 k1/(min−1) qe,cal/(mg·g−1) R2 k2/(g·(mg·min)−1) qe,cal/(mg·g−1) R2 20 0.001 2 2.968 0 0.884 6 0.002 1 7.869 0.998 5 50 0.002 1 9.519 0 0.896 0 0.000 7 12.930 0.995 8 80 0.002 4 14.703 2 0.979 2 0.000 3 18.054 0.999 3 表 5 颗粒内扩散模型拟合参数
Table 5. Fitting parameters of the intra-particle diffusion model
磷溶液初始
浓度/(mg·L−1)颗粒内扩散模型 第1阶段 第2阶段 第3阶段 C1 k1 R2 C2 k2 R2 C3 k3 R2 20 1.732 4 0.821 6 0.990 6 3.251 7 0.400 3 0.986 3 13.216 3 0.072 0 0.912 4 50 0.041 0 0.799 1 0.935 7 4.400 9 0.220 9 0.991 4 8.973 7 0.070 7 0.991 6 80 0.605 0 0.588 1 0.951 8 4.249 6 0.102 9 0.968 8 6.149 2 0.031 8 0.987 3 表 6 不同实验组各形态磷的含量
Table 6. Contents of phosphorus forms in different control groups mg·kg−1
样品 NH4Cl-P BD-P Org-P NaOH-rP HCl-P Res-P 空白 4.40 21.56 46.30 120.77 497.75 157.80 WTS2.5 2.22 16.27 35.71 189.55 493.53 162.35 WTS5 2.02 11.64 23.81 247.08 501.16 165.08 WTS10 0.89 12.30 19.84 328.44 492.46 168.66 -
[1] 张博, 李永峰, 姜霞, 等. 环境治理工程对蠡湖水体中磷空间分布的影响[J]. 中国环境科学, 2013, 33(7): 1271-1279. [2] 袁探, 华玉妹, 朱端卫, 等. 外源硫酸盐对武汉南湖表层沉积物磷形态的作用[J]. 中国环境科学, 2012, 32(4): 666-673. doi: 10.3969/j.issn.1000-6923.2012.04.015 [3] 余居华, 钟继承, 张银龙, 等. 湖泊疏浚对沉积物再悬浮及磷迁移影响的模拟研究[J]. 环境科学, 2012, 33(10): 3368-3375. doi: 10.13227/j.hjkx.2012.10.027 [4] 刘静静, 董春颖, 宋英琦, 等. 杭州西湖北里湖沉积物氮磷内源静态释放的季节变化及通量估算[J]. 生态学报, 2012, 32(24): 7932-7939. [5] JIANG X, JIN X C, YAO Y, et al. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China[J]. Water Research, 2008, 42(8/9): 2251-2259. [6] KIM L H, CHOI E, STENSTROM M K. Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments[J]. Chemosphere, 2003, 50(1): 53-61. doi: 10.1016/S0045-6535(02)00310-7 [7] 秦伯强, 杨柳燕, 陈非洲, 等. 湖泊富营养化发生机制与控制技术及其应用[J]. 科学通报, 2006, 51(16): 1857-1866. doi: 10.3321/j.issn:0023-074X.2006.16.001 [8] 寇丹丹, 张义, 黄发明, 等. 水体沉积物磷控制技术[J]. 环境科学与技术, 2012, 35(10): 81-85. doi: 10.3969/j.issn.1003-6504.2012.10.018 [9] 李安定, 张义, 周北海, 等. 富营养化湖泊沉积物磷原位控制技术[J]. 水生生物学报, 2014, 38(2): 370-374. doi: 10.7541/2014.52 [10] HOLEREN C W J, TAGGART J. Managing Lakes and Reservoirs[M]. 3rd edition. Prep. by N. Am. Lake Manage Soc and Terrene Inst. in Coop with US EPA. 2001. [11] US EPA. Selecting remediation techniques for contaminated sediment, EPA 823-B93-001[R]. Office of Water, Washington, D C. 1993 [12] US EPA. Contaminated sediments: relevant Statutes and EPA Program Activities, EPA 506/6-90/003[R]. Office of Water, Washington, D C. 1990 [13] 戚海雁, 何品晶, 章骅. 给水厂排泥水及污泥的处置[J]. 上海环境科学, 2002, 21(7): 442-443. [14] 帖靖玺, 赵莉, 张仙娥. 净水厂污泥的磷吸附特性研究[J]. 环境科学与技术, 2009, 32(6): 149-151,164. doi: 10.3969/j.issn.1003-6504.2009.06.034 [15] 方晖, 张易培, 陈丁丁, 等. 净水厂聚合氯化铝铁污泥对污水中磷的吸附作用[J]. 工业用水与废水, 2013, 44(3): 17-20. doi: 10.3969/j.issn.1009-2455.2013.03.005 [16] WANG C H, GAO S J, PEI Y S, et al. Use of drinking water treatment residuals to control the internal phosphorus loading from lake sediments: laboratory scale investigation[J]. Chemical Engineering Journal, 2013, 225: 93-99. doi: 10.1016/j.cej.2013.03.074 [17] IPPOLITO J A, BARBARICK K A, ELLIOTT H A. Drinking water treatment residuals: a review of recent uses[J]. Journal of Environmental Quality, 2011, 40(1): 1-12. doi: 10.2134/jeq2010.0242 [18] WANG C H, YUAN N N, PEI Y S, et al, Aging of aluminum/iron-based drinking water treatment residuals in lake water and their association with phosphorus immobilization capability[J]. Journal of Environmental, Management, 2015, 119: 178-185. [19] 徐颖, 叶志隆, 叶欣, 等. 给水污泥对水中磷的吸附性能[J]. 环境工程学报, 2018, 12(3): 712-719. doi: 10.12030/j.cjee.201708032 [20] 马啸宙, 魏东洋, 马宏林, 等. 基于给水污泥吸附水溶液中磷的影响因素[J]. 环境工程学报, 2015, 9(8): 3659-3666. doi: 10.12030/j.cjee.20150812 [21] RYDIN E. Potentially mobile phosphorus in Lake Erken sediment[J]. Water Research, 2000, 34(7): 2037-2042. doi: 10.1016/S0043-1354(99)00375-9 [22] ZHOU Q X, GIBSON C E, ZHU Y M. Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK[J]. Chemosphere, 2001, 42(2): 221-225. doi: 10.1016/S0045-6535(00)00129-6 [23] SINGH V K, KUMAR E A. Measurement and analysis of adsorption isotherms of CO2 on activated carbon[J]. Applied Thermal Engineering, 2016, 97: 77-86. doi: 10.1016/j.applthermaleng.2015.10.052 [24] 张增强, 张一平. 几个吸附等温模型热力学参数的计算方法[J]. 西北农业大学学报, 1998, 26(2): 94-98. [25] LIN J W, WANG H, ZHAN Y H, et al. Evaluation of sediment amendment with zirconium-reacted bentonite to control phosphorus release[J]. Environmental Earth Sciences, 2016, 75(11): 942. doi: 10.1007/s12665-016-5744-9 [26] ZILIUS M, GIORDANI G, PETKUIENE J, et al. Phosphorus mobility under short-term anoxic conditions in two shallow eutrophic coastal systems ( Curonian and Sacca di Goro lagoons)[J]. Estuarine, Coastal and Shelf Science, 2015, 164: 134-146. doi: 10.1016/j.ecss.2015.07.004 [27] YANG L, WEI J, LIU Z, et al. Material prepared from drinking waterworks sludge as adsorbent for ammonium removal from wastewater[J]. Applied Surface Science, 2015, 330: 228-236. doi: 10.1016/j.apsusc.2015.01.017 [28] NGUYEN M D, ADHIKARI S, MALLYA D S, et al. Reuse of aluminium-based water treatment sludge for phosphorus adsorption: Evaluating the factors affecting andcorrelation between adsorption and sludge properties[J]. Environment technology and innovation, 2022, 27: 102717-102717. doi: 10.1016/j.eti.2022.102717 [29] Totlani K, Mehta R, Mandavgane S A. Comparative study of adsorption of Ni(II) on RHA and carbon embedded silica obtained from RHA[J]. Chemical Engineering Journal, 2012, 181: 376-386. [30] Zhu X D, Liu Y C, Qian F, et al. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal[J]. Bioresource Technology, 2014, 154: 209-214. doi: 10.1016/j.biortech.2013.12.019 [31] 郑晓青, 韦安磊, 张一璇, 等. 铁锰氧化物/生物炭复合材料对水中硝酸根的吸附特性[J]. 环境科学, 2018, 39(3): 1220-1232. [32] 崔婉莹, 艾恒雨, 张世豪, 等. 改性吸附剂去除废水中磷的应用研究进展[J]. 化工进展, 2020, 39(10): 4210-4226. [33] ZHANG J, YAN Z, JING O. Highly dispersed sepiolite-based organic modified nanofibers for enhanced adsorption of Congo red[J]. Applied Surface Science, 2018, 157: 76-85. [34] 仇付国, 孙瑶, 陈丽霞. 给水厂铝污泥特性分析及吸附氮磷性能试验[J]. 环境工程, 2016, 34(04): 54-59. [35] 陆燕勤, 朱丽, 何昭菊, 等. 沸石负载氧化铁吸附剂吸附除磷研究[J]. 环境工程, 2015, 33(4): 48-52. [36] FAN S S, WANG Y, WANG Z, et al. Removal of methylene blue from aqueous solution by sewage sludge derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 601-611. doi: 10.1016/j.jece.2016.12.019 [37] FAN S, TANG J, WANG Y, et al. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: kinetics, isotherm, thermodynamic and mechanism[J]. Journal of Molecular Liquids, 2016, 220: 432-441. doi: 10.1016/j.molliq.2016.04.107 [38] ARAMI M, LIMAEE N Y, MAHMOODI N M, et al. Evaluation of the adsorption kinetics and equilibrium for the potential removal of acid dyes using a biosorbent[J]. Chemical Engineering Journal, 2008, 139(1): 2-10. doi: 10.1016/j.cej.2007.07.060 [39] 望雪, 程豹, 杨正健, 等. 澜沧江流域沉积物间隙水-上覆水营养盐特征与交换通量分析[J]. 环境科学, 2018, 39(5): 2126-2134. [40] 于胜楠, 李勇, 李大鹏, 等. 灼烧净水污泥对外源磷的吸附和固定作用[J]. 环境科学, 2017, 38(9): 3962-3969. [41] 朱培颖, 李大鹏, 于胜楠. 灼烧净水污泥投加方式对磷吸附和磷形态的影响[J]. 环境科学, 2017, 38(5): 1957-1964. doi: 10.13227/j.hjkx.201610120 [42] 李乐, 王圣瑞, 焦立新, 等. 滇池柱状沉积物磷形态垂向变化及对释放的贡献[J]. 环境科学, 2016, 37(9): 3384-3393. [43] LAAKSO J, UUSITALO R, HEIKKINEN J, et al. Phosphorus in agricultural constructed wetland sediment is sparingly plant available[J]. Journal of Plant Nutrition and Soil Science, 2017, 180(5): 554-562. doi: 10.1002/jpln.201700062 [44] WANG C H, GAO S J, WANG T X, et al. Effectiveness of sequential thermal and acid activation on phosphorus removal by ferric and alum water treatment residuals[J]. Chemical Engineering Journal, 2011, 172(2): 885-891. -



 下载:
下载: