-
垃圾渗滤液渗漏严重污染地下水水质,其中含有大量难降解的有机污染物、重金属、无机盐等,可生化性差[1-3]。因垃圾渗滤液COD值高、自身降解以及发酵过程缓慢,一旦发生渗漏,会长期威胁地下水质量。作为重要原位修复技术的渗透性反应格栅(PRB)被应用于修复受垃圾渗滤液污染的地下水[4-6],PRB可通过填充反应介质来去除地下水中的有机污染物,由于运行成本低、处理污染物种类多、长时间处理能力强等特点,具有广阔的应用价值和市场发展前景[7-9]。因此,在PRB工程中,选取兼具经济性、缓释性以及高效性的填充材料尤为重要。
高级氧化技术(AOPs)通过金属、光和电等方式来催化氧化剂以生成具有强氧化性的自由基,从而降解垃圾渗滤液中的有机物[10]。Fenton法因其具有操作简单、无二次污染等优势,近年来被越来越多的学者用于垃圾渗滤液处理的研究和实际工程中[11-13]。Fenton法通过Fe2+催化H2O2,产生羟基自由基(HO·),将难氧化的大分子有机物降解为小分子物质甚至完全矿化,同时生成的Fe(OH)3以絮凝沉淀的方式实现污染物的去除[14-17]。然而H2O2是液体,受运输、储存等条件的限制,在处理过程中,需要调节体系pH至2~4并定时投加H2O2,故H2O2不适宜作为地下水的原位修复材料。过碳酸钠(sodium percarbonate,SPC)分子式为Na2CO3·1.5H2O2,溶于水后分解为Na2CO3和H2O2[18],pH的适用范围广,具有很好的稳定性,易于储存和运输,且无毒无害,是工业中常用的固体氧化剂和消毒剂[19-21]。目前已有将SPC用于去除有机污染物的研究。ZANG等[22]发现,SPC/Fe2+体系降解三氯乙烯(TCE)效率可达99.5%以上。白青青等[23]的研究也表明,SPC/Fe2+体系可以在pH为 2~10时可有效降解亚甲基蓝。KWARCIAK[24]等探究了SPC/Fe2+体系降解垃圾渗滤液中溶解性有机污染物(DOM)的最佳反应条件。然而SPC/Fe2+体系对有机污染物的降解机理尚不清楚,目前SPC/Fe2+体系用于修复垃圾渗滤液污染地下水的研究鲜见报道。
本研究通过构建SPC/Fe2+体系处理被垃圾渗滤液污染的地下水,探讨了体系内重要反应参数n(SPC):n(FeSO4·7H2O)、SPC的投加量和反应时间对受垃圾渗滤液污染的地下水中COD去除效果的影响,通过自由基淬灭实验、紫外-可见光谱与三维荧光光谱分析来探究SPC/Fe2+体系去除COD的机理,以期为受垃圾渗滤液污染地下水的原位修复提供理论依据。
-
实验用水为受垃圾渗滤液污染的地下水(以下简称原水),采集自场地污染控制监测井,原水颜色呈深褐色。经测定,原水pH为7.22,COD为200 mg·L−1,K+=17.13 mg·L−1,Na+=204.2 mg·L−1,Ca+=155.19 mg·L−1,Mg+=42.36 mg·L−1,NH4+=97.00 mg·L−1,HCO3−=1 043.4 mg·L−1,Cl−=182.4 mg·L−1,SO42−<0.50 mg·L−1,NO3−=19.46 mg·L−1,NO2−<0.004 mg·L−1,F−<0.05 mg·L−1,Fe=18.02 mg·L−1,Mn=23.76 mg·L−1,总硬度为557.1 mg·L−1,溶解性总固体为1 794.6 mg·L−1,色度为110°,浊度为50.0 NTU,以上浓度均为质量浓度。
实验试剂包括过碳酸钠、七水合硫酸亚铁、过氧化氢(质量分数为30%)、叔丁醇,上述药品均为分析纯。
实验仪器包括HACH DR/3900 COD分光光度计(美国哈希公司)、HACH DRB 200 COD消解器(美国哈希公司)、T6新世纪紫外-可见分光光度计(北京普析通用仪器有限责任公司)、FLS1000/FS5稳态瞬态荧光光谱仪(英国爱丁堡公司)、HQ30D便携式pH计(美国哈希公司)。
-
1) SPC/Fe2+体系反应实验。设置3组平行样品,量取1 L原水,置于1 L棕色瓶中。加入FeSO4·7H2O(3.6~132.0 mmol·L−1)并搅拌1 min后,投加适量的SPC(6~90 mmol·L−1),以此作为反应起点,到达设定的反应时间后,将处理后的溶液经0.45 μm微孔滤膜抽滤,最后对相关的指标(包括COD、pH等)进行检测。在测量COD时,使用H2O2浓度-COD线性方程消除H2O2对COD测定的影响[25]。在本实验中,选取n(SPC):n(FeSO4·7H2O)、药剂投加量和反应时间为主要影响因素,考察其对COD去除率的影响,并对最佳实验条件下反应前后的溶液进行紫外-可见光谱、三维荧光光谱分析,以探究COD的去除机理。
2)自由基淬灭实验。设置2组样品,一组加入132.0 mmol·L−1的FeSO4·7H2O并搅拌1 min,之后投加0.1 mol·L−1的叔丁醇,静置1 min后,再加入48 mmol·L−1的SPC;另一组加入132.0 mmol·L−1的FeSO4·7H2O并搅拌1 min,之后投加48 mmol·L−1的SPC,均反应30 min,将2组溶液经0.45 μm微孔滤膜抽滤后,进行紫外-可见光谱分析,以探究SPC/Fe2+体系中起主导作用的自由基。
-
1)水质指标分析。采用分光光度法测定COD,向COD快速消解预制管试剂中加入2 mL待测水样,振荡后,在预热好的消解器中165 ℃条件下消解20 min,冷却至常温,于分光光度计中读数;采用便携式pH计测定pH;采用纳氏试剂光度法测定氨氮;采用邻菲啰啉分光光度法测定Fe2+;采用邻菲啰啉分光光度法测定全铁;Fe3+的质量浓度=全铁的质量浓度−Fe2+的质量浓度。
2)紫外-可见光谱分析。以超纯水为参比,用T6新世纪紫外-可见分光光度计对反应后原水进行紫外-可见波长扫描,扫描条件:波长为200~500 nm,采样间隔为0.2 nm,响应速度为慢速,样品池为10 mm石英比色皿。测定反应前后原水在203、240、250、253、300、365、400、420 nm下的吸光度,分别记为E203、E240、E250、E253、E300、E365、E400、E420,计算E240/E420、E250/E365、E300/E400、E253/E203的值,并对波长在226~400 nm处的曲线进行积分,记为A226~400。
3)三维荧光光谱分析。以超纯水为参比,利用FLS1000/FS5荧光光谱仪对反应后原水进行光谱扫描,扫描条件:扫描步长为3 nm,扫描速度为2 400 nm·min−1,带通为5 nm,控制激发光谱Ex=200~450 nm,发射光谱Em=250~550 nm。荧光指纹特征指数[26](B/A):Ex=275 nm、Em=305 nm下的荧光强度与Ex=260 nm、Em在400~460 nm之间最大的荧光强度的比值;荧光指数[27](FI):Ex=370 nm时,Em=470 nm处的荧光强度与Em=520 nm处的荧光强度的比值;腐殖化指数[28](HIX):Ex=254 nm时,Em在435~480 nm的荧光强度积分值与Em在300~345 nm之间的荧光强度积分值的比值。
-
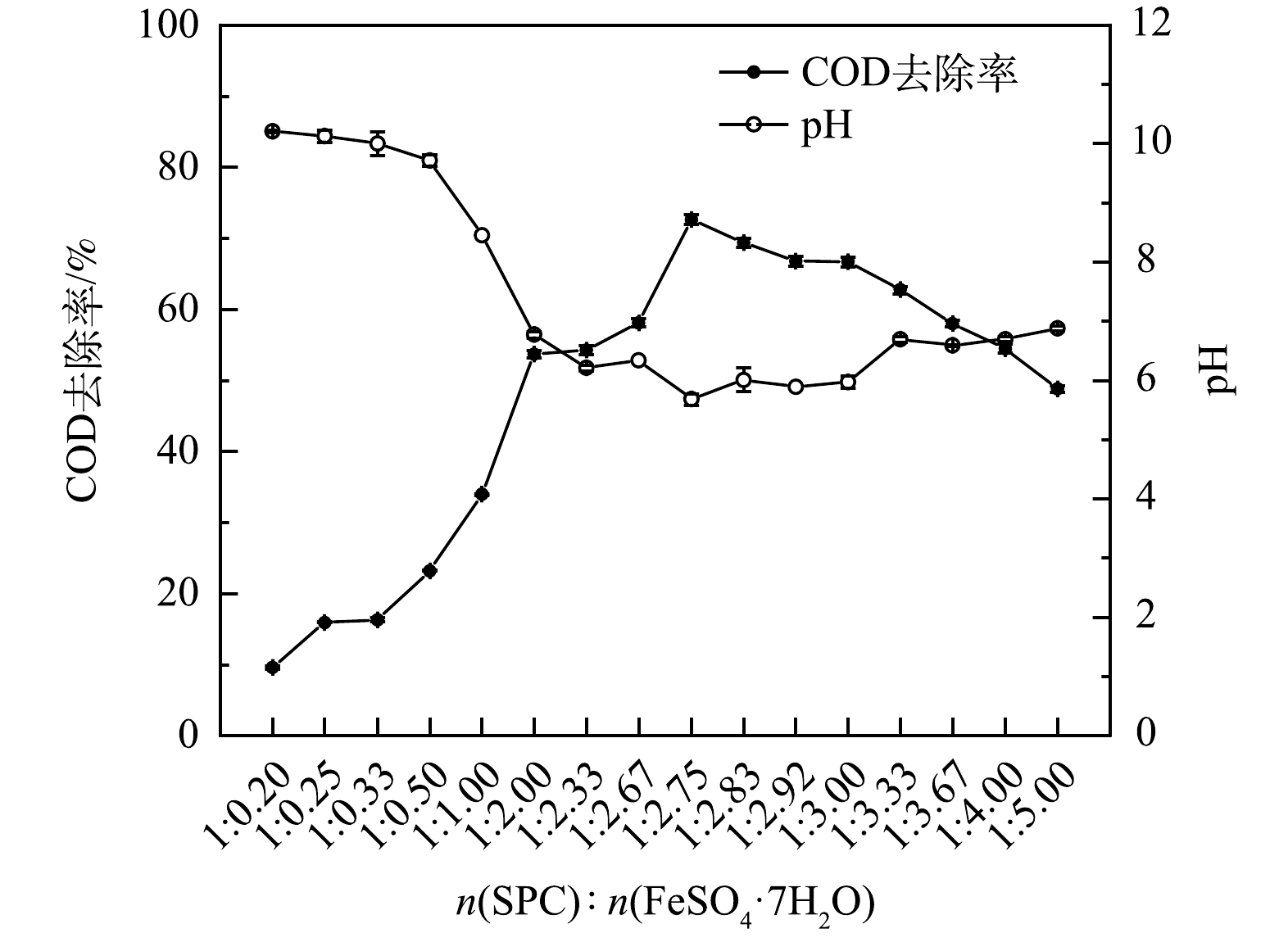
1)药剂投加摩尔比对COD去除的影响。在SPC/Fe2+体系中,氧化剂和催化剂的摩尔比直接关系到整个体系的氧化能力[29],针对不同的水体和目标污染物,最佳配比也会有所不同。为确定SPC/Fe2+体系去除原水COD过程中SPC/Fe2+摩尔比的最优值,在SPC投加量为18 mmol·L−1、原水pH为7.22、反应时间为60 min的条件下,探究n(SPC):n(FeSO4·7H2O)对原水中COD去除效果的影响,结果如图1所示。
如图1所示,随着n(SPC):n(FeSO4·7H2O)的减小,溶液COD的去除率呈先上升后下降的趋势,在n(SPC):n(FeSO4·7H2O)=1:2.75时达到最大值,此时溶液中COD为54.67 mg·L−1,去除率为72.66%。当n(SPC):n(FeSO4·7H2O)> 1:2.75时,COD去除率较低。这一方面归因于催化剂(Fe2+)的投加量较低,致使体系中产生的HO·浓度较低(式(1)),所生成的HO·不足以去除溶液中的COD;另一方面可能是由于SPC溶于水中,分解为H2O2和Na2CO3(式(2)),H2O2和Fe2+反应生成HO·和OH−(式(1)),Na2CO3进行水解反应生成HCO3−和OH−(式(3)),大量OH−的生成致使整个体系呈碱性。碱性条件下,Fe2+首先与OH−形成铁的络合物,Fe2+和Fe3+的再生反应停止(式(4)),抑制了HO·的生成[30]。
随着催化剂(Fe2+)投加量的增加,溶液中HO·的产量不断增加,当n(SPC):n(FeSO4·7H2O)=1:2.75时,COD去除率达到最大值。当n(SPC):n(FeSO4·7H2O)<1:2.75时,溶液COD的去除率开始降低,这归因于体系中过量的Fe2+消耗了HO·(式(5)),pH值逐渐上升。
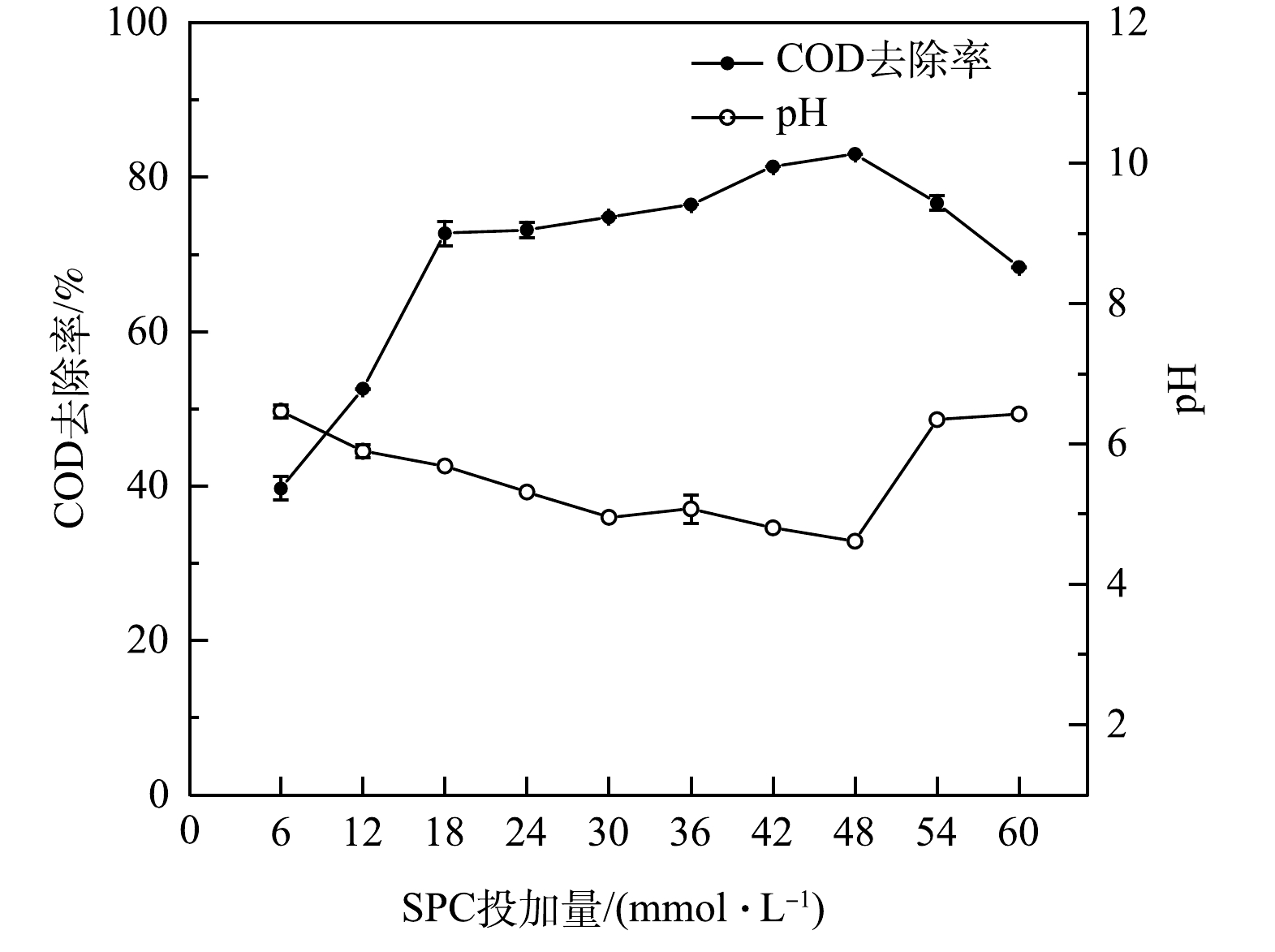
2)药剂投加量对COD去除的影响。药剂的投加量决定着HO·的生成量[31],其中Fe2+是反应体系中的催化剂。在n(SPC):n(FeSO4·7H2O)=1:2.75、溶液pH为7.22、反应时间为60 min的条件下,探究药剂的投加量(下文以SPC的投加量表示)对原水中COD去除效果的影响,结果如图2所示。
随着SPC投加量的增加,溶液COD的去除率逐渐增加,pH值呈逐渐下降的趋势。当SPC的投加量为48 mmol·L−1时,COD的去除率达到最大值,为82.09%,此时溶液中COD为34.04 mg·L−1,pH为4.61。随着SPC的增加,一方面生成的HO·浓度增加,大量的COD被去除;另一方面体系中生成了大量的Fe(OH)3胶体,经过絮凝沉淀去除一部分污染物。当SPC的投加量大于48 mmol·L−1时,COD的去除率开始下降,pH上升至6.40左右,体系中存在过量的H2O2、HO·和HCO3−,HO·会与自身、H2O2 、HCO3−反应(式(6)~式 (8)),发生淬灭[32-34],导致COD的去除率下降。
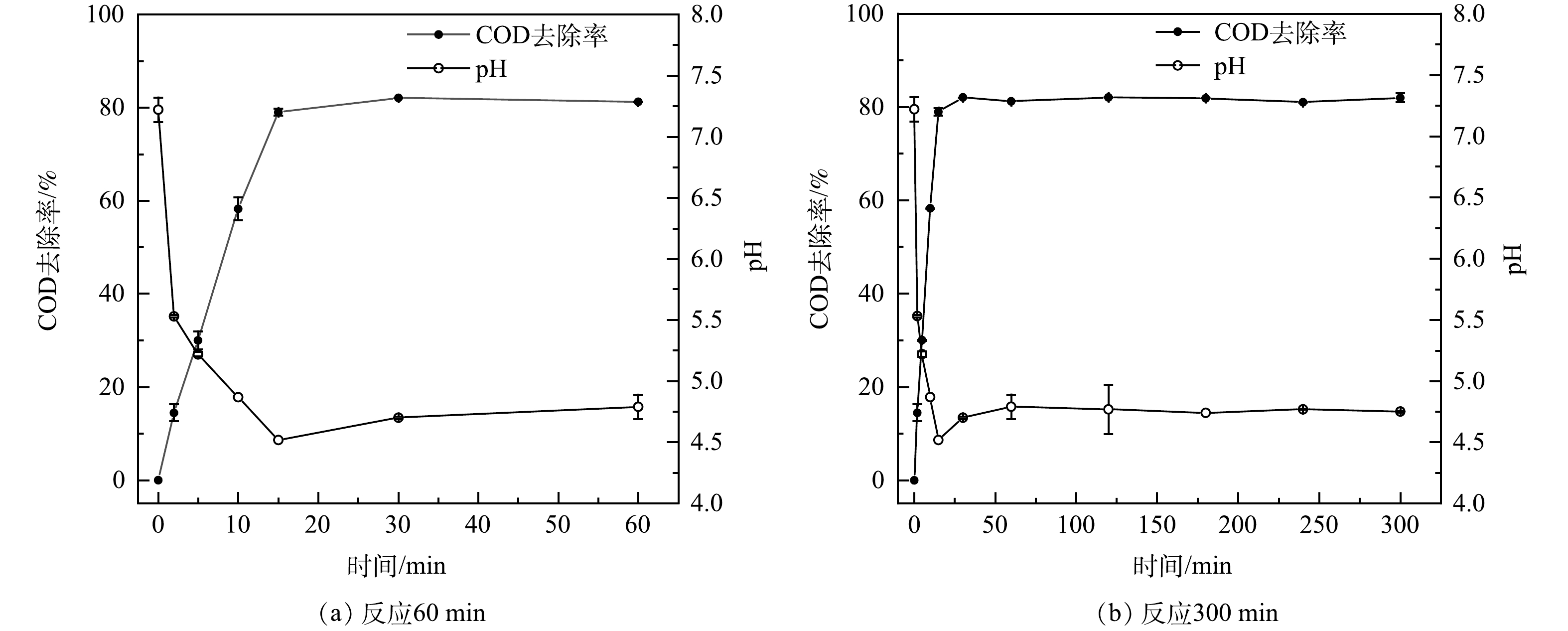
3)反应时间对COD去除的影响。图3反映了溶液pH为7.22、n(SPC):n(FeSO4·7H2O)=1:2.75、SPC投加量为48 mmol·L−1的条件下,COD去除率和pH在300 min内随时间的变化趋势。如图3所示,随着氧化反应时间的增加,原水COD的去除率逐渐提高,pH逐渐降低,30 min时COD的去除率达到82.09%并趋于稳定,pH为4.7。这主要是由于反应初始阶段体系中SPC和Fe2+充足,Fe2+迅速催化H2O2,生成大量的HO·,使得原水中的COD被快速去除。随着时间的推移,COD的去除率和pH趋于稳定,可能是由于30 min后体系中的SPC和Fe2+均反应完全,催化生成的HO·已全部作用于原水中的COD。
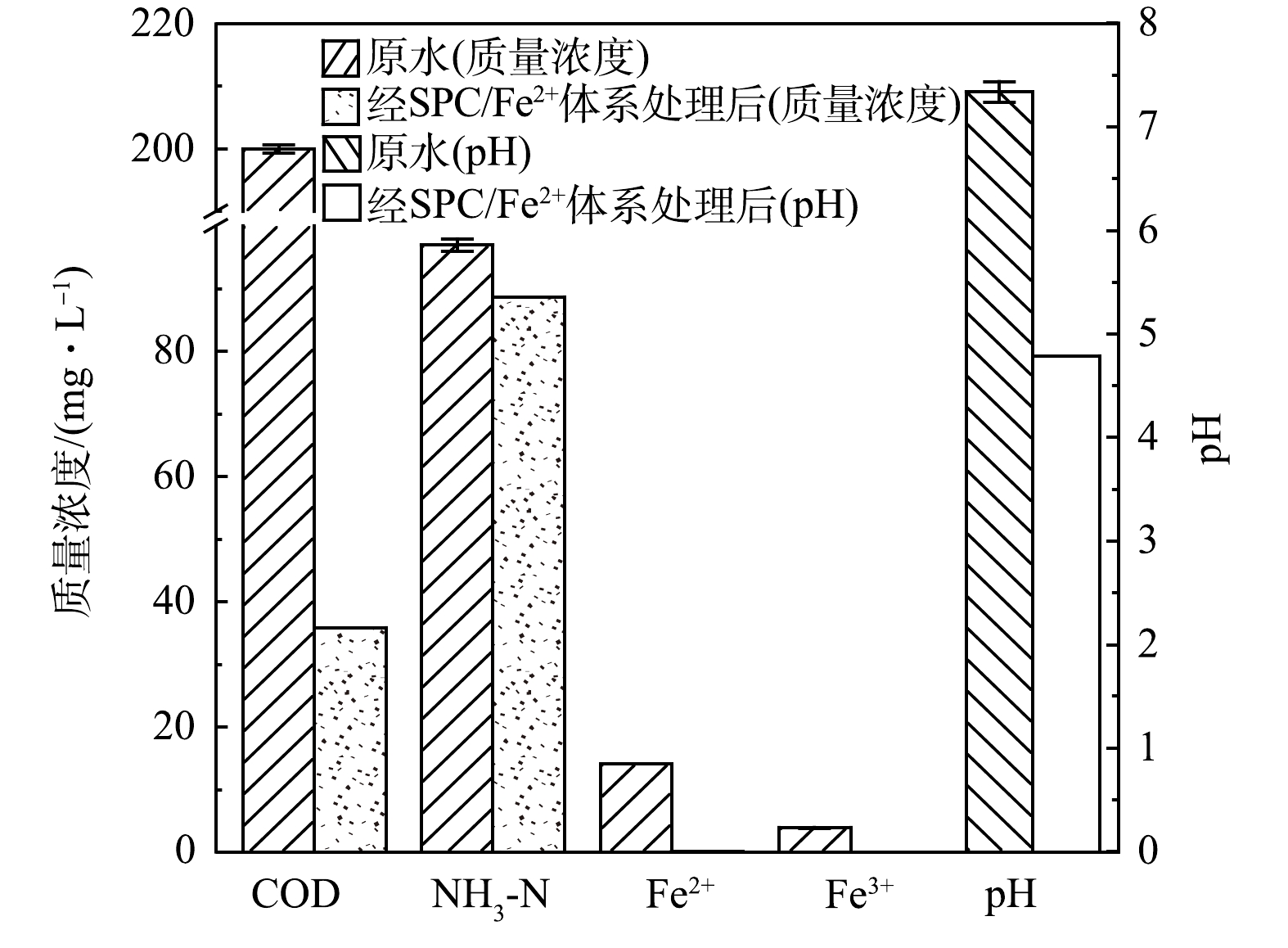
4) SPC/Fe2+体系对原水的处理效果。图4反映了在最佳配比和最佳投加量的条件下,反应前后原水中COD、NH3-N、Fe2+、Fe3+浓度和pH值的对比。如图4所示,反应后原水中COD从200.00 mg·L−1降到35.82 mg·L−1,去除率为82.09%。原水中NH3-N的质量浓度为97.00 mg·L−1,反应后NH3-N的质量浓度为88.70 mg·L−1,去除率仅为8.56%,无明显去除效果,可能是因为酸性的SPC/Fe2+体系中NH3-N以NH4+的形式存在,难以被自由基直接氧化[35]。原水中的铁主要以Fe2+的形式存在,反应后,Fe2+和Fe3+的质量浓度分别由14.14 mg·L−1和3.88 mg·L−1降低至0.06 mg·L−1和0.01 mg·L−1。
-
为识别SPC/Fe2+体系的主要作用自由基、自由基降解的污染物种类以及主要作用结构,在初始pH为7.22、n(SPC):n(FeSO4·7H2O)=1:2.75、SPC投加量为48 mmol·L−1以及反应30 min的最佳条件下,对反应前后的溶液进行紫外-可见光谱和三维荧光光谱分析。
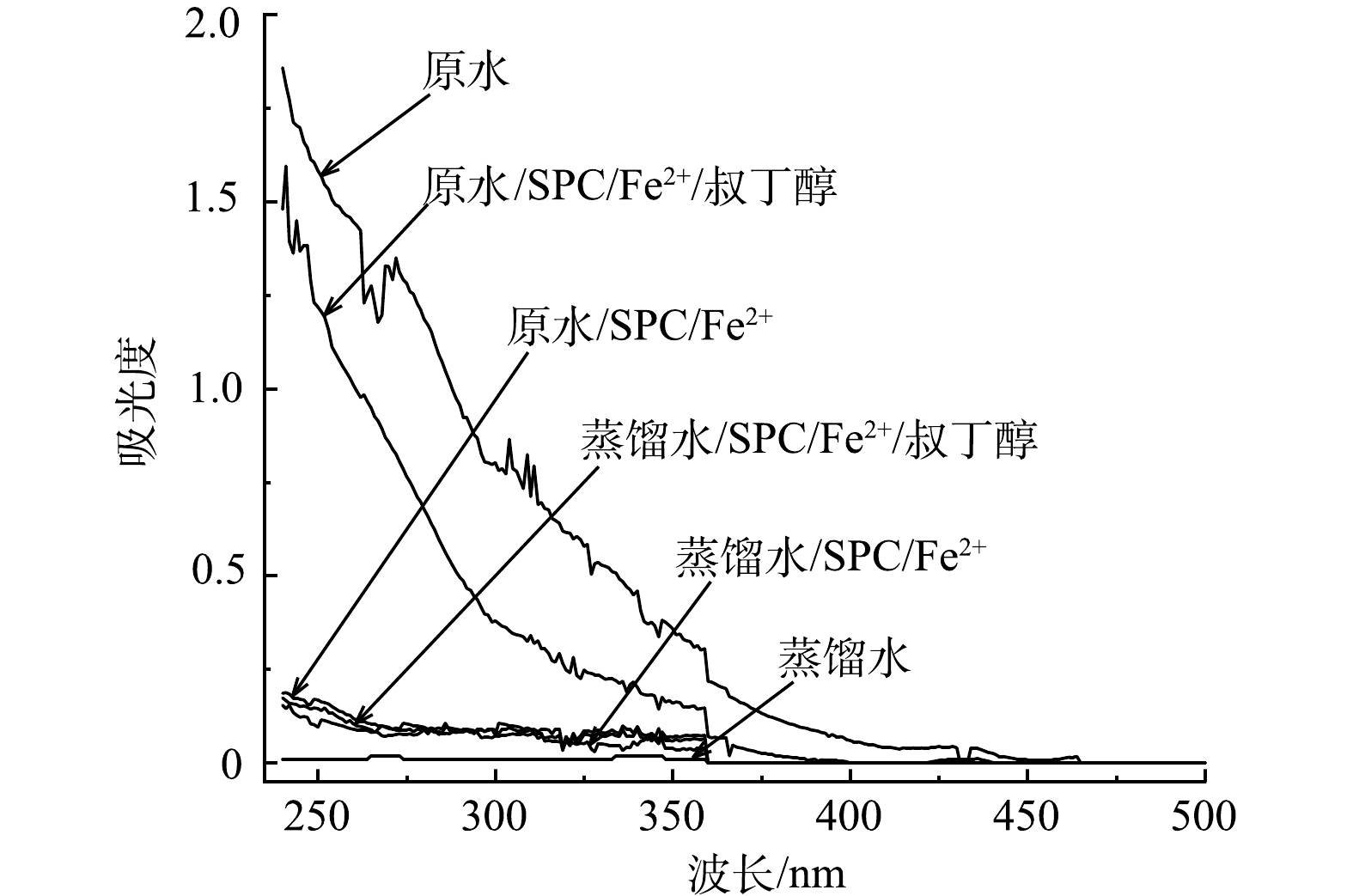
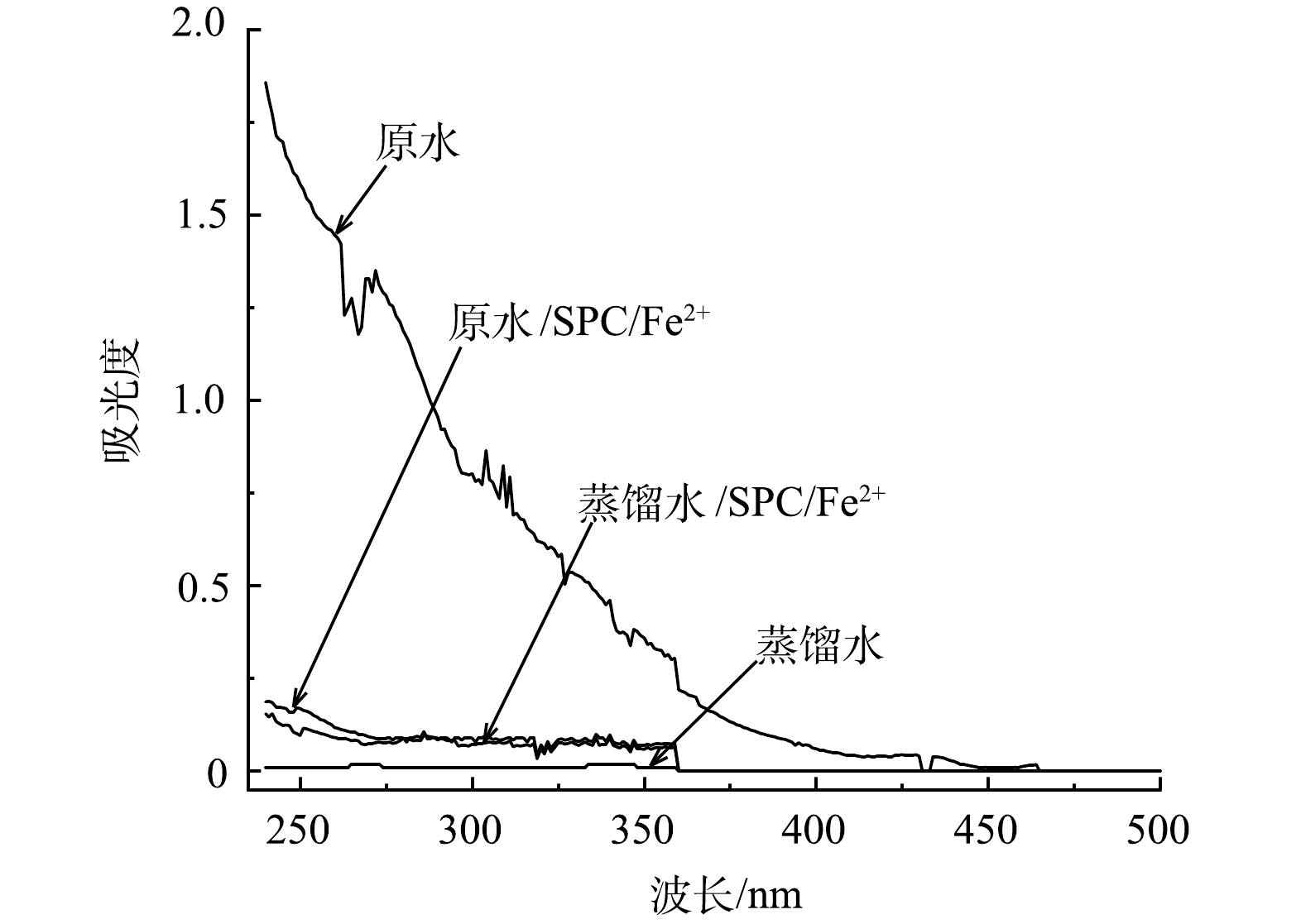
1)自由基鉴定。叔丁醇是常见的HO·的淬灭剂,其与HO·的反应速率为5.2×108 L·(mol·s) -1,叔丁醇可以迅速与HO·反应并将其淬灭[36-37]。因此对原水、原水/SPC/Fe2+以及原水/SPC/Fe2+/叔丁醇这3种体系进行紫外-可见光谱分析,体系中存在大量SPC、Fe2+、叔丁醇,故同时考察了蒸馏水/SPC/Fe2+以及蒸馏水/SPC/Fe2+/叔丁醇体系对紫外-可见光谱扫描产生的干扰。
如图5所示, 蒸馏水/SPC/Fe2+和蒸馏水/SPC/Fe2+/叔丁醇这2个体系的扫描光谱在波长为230~500 nm时吸光度<0.336,其对体系的分析结果无显著干扰。紫外区光谱曲线上的积分面积可以近似地表征体系中有机污染物的含量[38],原水/SPC/Fe2+体系的扫描曲线与蒸馏水的曲线几乎一致;而在加入叔丁醇后,在波长为230~400 nm时的吸光度和积分面积接近原水。因此,体系中起主导作用的自由基是HO·。
2)降解污染物识别。为进一步研究反应前后原水中COD组成的变化,在最佳反应条件下,对处理前后的原水进行三维荧光光谱分析,三维荧光光谱等高线图如图6所示。原水(图6(a))识别了3个荧光特征峰。荧光峰的位置及荧光强度如表1所示。
已有研究[39-40]表明,峰F1(Em/Ex=337 nm/230 nm)指示了类色氨酸类蛋白有机物,这类有机物由类色氨酸和蛋白质相结合而形成,易被生物降解。峰F2(Em/Ex=337 nm/278 nm)对应高激发色氨酸类蛋白有机物,由游离态的氨基酸和蛋白质结合而成,是微生物生长代谢的副产物,该类物质极易发生光解生成氨[41]。这2种类蛋白有机物均含有芳环氨基酸结构[40, 42]。峰F3(Em/Ex=487 nm/383 nm)属于腐殖酸类有机物荧光峰,腐殖酸是大分子有机物,具有稳定性强、腐殖化程度高、极难被微生物降解的特点,这也正是垃圾渗滤液难以被生物降解的原因[43-44]。由图6(b)可以看出,原水经过SPC/Fe2+体系处理后,溶液中Ⅱ、Ⅳ、Ⅴ区域中的荧光特征峰均消失不见,说明HO·能显著降解原水中的类蛋白有机物和腐殖酸。
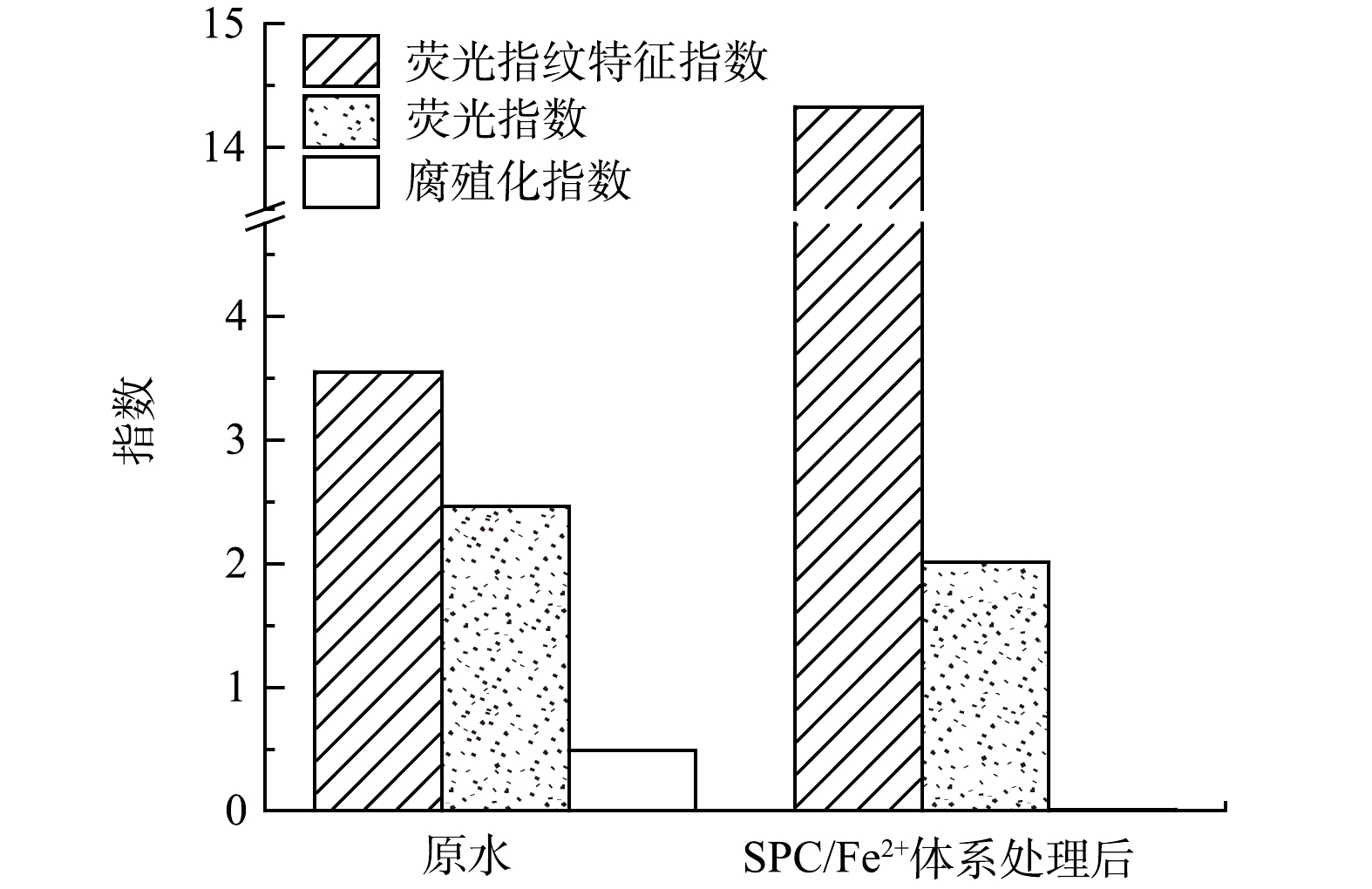
本研究引入荧光指纹特征指数(B/A)、荧光指数(FI)和腐殖化指数(HIX)3种三维荧光特征指数,进一步识别原水经SPC/Fe2+体系处理后腐殖化程度、难降解有机物占比的变化[45]。
如图7所示,经过SPC/Fe2+体系处理后原水中COD的组成结构发生了显著变化,FI反映了COD的去除程度[27],反应后原水的FI值降低,说明溶液中的HO·可以有效去除COD;B/A与水体中难降解物质的占比大小呈反比[26],原水的B/A值从3.55上升至14.33,说明难降解有机物占比大幅降低;HIX是水体腐殖化程度的特征指数[28],反应后原水的HIX值降低,表明溶液中大量的腐殖酸被HO·降解。
3)自由基作用结构识别。紫外-可见光谱中紫外区的吸收强度与原水的芳香性和复杂程度有关[46]。在最佳反应条件下,对处理前后的原水进行紫外-可见光谱扫描。
如图8所示,原水/SPC/Fe2+体系的紫外-可见光谱曲线在波长为230~400 nm的吸光度为0~0.300,于400 nm后降至0附近,其与原水的紫外-可见光谱曲线在紫外区的差异指示了HO·的作用结构。已有研究[47]表明,在波长为226~250 nm的吸收强度是由污染物中带π-π*跃迁的结构产生的,在波长为260~400 nm的吸收强度是由苯环中带有共轭体系的结构产生的。据此揭示了SPC/Fe2+体系中的HO·可以有效降解污染物中带π-π*跃迁的结构以及苯环中带有共轭体系的结构。
在特定波长下,不同吸光度的比值可以反映溶液中污染物的芳香化程度、腐殖质的分子质量以及聚合度等[48]。E240/E420的值越小,表明污染物的芳香性构化程度越大,E250/E365的值越小,表明污染物的分子量越大[49],E300/E400的值越小,表明污染物的分子质量和聚合度越大[50-51]。
如表2所示,处理后原水的E240/E420、E250/E365、E300/E400的值均增大,说明原水中污染物的芳香性构化程度、分子质量和聚合度均降低,所含C=C不饱和双键减少。E253/E203反映了污染物中苯环的取代基种类[52],其值在处理后的原水中降低,说明原水中苯环上的碳基、羧基、羟基以及酯基类等多种含氧官能团的含量降低。A226~400用来表征苯环类化合物的变化[53],在反应后,原水A226~400的值由163.382降至16.781,表明HO·对原水中苯环类化合物有很好的降解效果。
基于上述讨论,SPC/Fe2+体系去除原水中COD的机理如图9所示。 SPC溶于水中并分解为H2O2和Na2CO3,H2O2与Fe2+反应生成HO·。原水中的腐殖酸和类蛋白有机物里含有大量苯环、π-π*跃迁等结构以及不饱和双键和含氧官能团,HO·通过破坏以上结构和官能团将原水中的腐殖酸和一部分类蛋白有机物降解为H2O和CO2,另一部分类蛋白有机物由微生物降解,从而使原水的腐殖化程度以及芳香性构化程度大幅降低。
-
1) SPC/Fe2+体系可以有效去除受垃圾渗滤液污染的地下水中的COD,SPC作为固体氧化剂具有代替H2O2应用于实际PRB工程中的潜力。
2)针对初始pH为7.22的原水,在n(SPC):n(FeSO4·7H2O)=1:2.75、SPC投加量为48 mmol·L−1、反应30 min的最佳实验条件下,COD的去除率可达到82.09%。
3) SPC/Fe2+体系通过降解类蛋白有机物和腐殖酸实现高COD地下水的有效处理,主要活性物种HO·能够有效减小腐殖质分子质量及其分子间聚合度,同时降低芳香性构化程度。
亚铁活化过碳酸钠对垃圾渗滤液污染地下水中COD的去除
Removal of COD in groundwater contaminated by landfill leachate by ferrous-activated sodium percarbonate
-
摘要: 针对垃圾渗滤液渗漏严重威胁地下水水质的问题,以过碳酸钠(sodium percarbonate,SPC)为固体氧化剂来构建地下水的原位修复技术,采用单因素法,以受污染的高COD地下水为处理对象,通过实验研究了n(SPC):n(FeSO4·7H2O)、药剂投加量、反应时间对SPC/Fe2+体系去除COD的影响和SPC/Fe2+体系的反应机理。结果表明:n(SPC):n(FeSO4·7H2O)和SPC的投加量显著影响COD的去除效能;在初始pH值为7.22、n(SPC):n(FeSO4·7H2O)=1:2.75、SPC投加量为48 mmol·L-1以及反应30 min的最佳实验条件下,COD的去除率可达82.09%;SPC/Fe2+体系通过降解类蛋白有机物和腐殖酸实现高COD地下水的有效处理,主要活性物种HO·能够有效减小腐殖质分子量及其分子间聚合度,同时降低芳香性构化程度。由此可知,SPC作为固体氧化剂有应用于实际渗透性反应格栅(PRB)工程的潜力。本研究结果可为PRB在修复受垃圾渗滤液污染地下水的实际工程提供参考。Abstract: Landfill leachate leakage is a serious threat to groundwater quality. Sodium percarbonate (SPC) was used as a type of solid oxidant to build the in-situ remediation technology of groundwater. The single factor method was used to study the remediation test on the treatment object: the polluted groundwater with high COD. The effects of n(SPC):n(FeSO4·7H2O), chemical dosage and reaction time on COD removal and the corresponding reaction mechanism of SPC/Fe2+ system were studied. The results showed that n(SPC):n(FeSO4·7H2O) and SPC dosages significantly affected the removal efficiency of COD; under the optimal experimental conditions of initial pH 7.22, n(SPC):n(FeSO4·7H2O) of 1:2.75, SPC dosage of 48 mmol·L−1 and reaction time of 30 min, the removal rate of COD could reach 82.09%; the SPC/Fe2+ system achieved the effective treatment of high-COD groundwater by degrading proteinoid organics and humic acid. The main active species HO· could effectively decrease the molecular weight of humus and its intermolecular polymerization degree, as well as the degree of aromatization. SPC as solid oxidant had a potential for its application in permeable reactive barrier(PRB) engineering. The results of this study can provide a reference for the practical engineering of PRB in the remediation of groundwater contaminated by landfill leachate.
-
Key words:
- groundwater /
- landfill leachate /
- sodium percarbonate /
- chemical oxygen consumption
-

-
表 1 原水三维荧光光谱特征峰
Table 1. Characteristic peaks of 3D-fluorescence spectrum of raw water
荧光峰编号 (Em/Ex)/nm 相对荧光强度 区域 F1 337/230 4 149 Ⅱ F2 337/278 6 976 Ⅳ F3 487/383 4 258 Ⅴ 表 2 原水处理前后特征吸光度的变化
Table 2. Changes of characteristic absorbance of raw water before and after treatment
样品 E240/E420 E250/E365 E300/E400 E253/E203 原水 25.400 4.900 7.300 0.691 处理后原水 46.425 7.955 13.148 0.198 -
[1] BADERNA D, CALONI F, BENFENATI E. Investigating landfill leachate toxicity in vitro: A review of cell models and endpoints[J]. Environment International, 2019, 122: 21-30. doi: 10.1016/j.envint.2018.11.024 [2] BOONNORAT J, TECHKARNJANARUK S, HONDA R, et al. Use of aged sludge bioaugmentation in two-stage activated sludge system to enhance the biodegradation of toxic organic compounds in high strength wastewater[J]. Chemosphere, 2018, 202: 208-217. doi: 10.1016/j.chemosphere.2018.03.084 [3] LI X Y, ZHU W, WU Y, et al. Recovery of potassium from landfill leachate concentrates using a combination of cation-exchange membrane electrolysis and magnesium potassium phosphate crystallization[J]. Separation and Purification Technology, 2015, 144: 1-7. doi: 10.1016/j.seppur.2015.01.035 [4] ATKINSON S, FERNANDES L, CAPRARA A, et al. Prevention and promotion in decentralized rural health systems: A comparative study from northeast Brazil[J]. Health Policy and Planning, 2005, 20(2): 69-79. doi: 10.1093/heapol/czi009 [5] BLOWES D W, PTACEK C J, BENNER S G, et al. Treatment of inorganic contaminants using permeable reactive barriers[J]. Journal of Contaminant Hydrology, 2000, 45(1/2): 123-137. [6] SCHUTH C, BILL M, BARTH J A C, et al. Carbon isotope fractionation during reductive dechlorination of TCE in batch experiments with iron samples from reactive barriers[J]. Journal of Contaminant Hydrology, 2003, 66(1/2): 25-37. [7] GUERIN T F, HORNER S, MCGOVERN T, et al. An application of permeable reactive barrier technology to petroleum hydrocarbon contaminated groundwater[J]. Water Research, 2002, 36(1): 15-24. doi: 10.1016/S0043-1354(01)00233-0 [8] KAMOLPORNWIJIT W, LIANG L, WEST O R, et al. Preferential flow path development and its influence on long-term PRB performance: Column study[J]. Journal of Contaminant Hydrology, 2003, 66(3/4): 161-178. [9] SHANNON M A, BOHN P W, ELIMELECH M, et al. Science and technology for water purification in the coming decades[J]. Nature, 2008, 452(7185): 301-310. doi: 10.1038/nature06599 [10] 李章良, 郭海灵. 高级氧化技术处理垃圾渗滤液的研究现状与进展[J]. 环境工程, 2014, 32(5): 30-33. doi: 10.13205/j.hjgc.201405008 [11] 王怡然, 李江, 李彦澄, 等. 贵阳某垃圾焚烧发电厂渗滤液处理回用工程设计[J]. 中国给水排水, 2021, 37(6): 91-95. doi: 10.19853/j.zgjsps.1000-4602.2021.06.016 [12] 袁维芳, 王浩, 汤克敏, 等. 垃圾渗滤液处理技术及工程化发展方向[J]. 环境保护科学, 2020, 46(1): 76-83. doi: 10.16803/j.cnki.issn.1004-6216.2020.01.014 [13] 曲磊, 杨晓超, 陈珊. 膜技术在垃圾渗滤液处理中的应用现状及存在问题[J]. 化工技术与开发, 2014, 43(12): 42-44. doi: 10.3969/j.issn.1671-9905.2014.12.014 [14] ROTT E, MINKE R, BALI U, et al. Removal of phosphonates from industrial wastewater with UV/Fe-Ⅱ, Fenton and UV/Fenton treatment[J]. Water Research, 2017, 122: 345-354. doi: 10.1016/j.watres.2017.06.009 [15] CHE J G, WAN J B, HUANG X P, et al. Pretreatment of piggery digestate wastewater by ferric-carbon micro-electrolysis under alkalescence condition[J]. Korean Journal of Chemical Engineering, 2017, 34(9): 2397-2405. doi: 10.1007/s11814-017-0144-8 [16] DING S L, ZHAO Z, TIAN Q Q, et al. Effect of iron-carbon micro-electrolysis-Fenton on the dewatering performance of sludge[J]. Environmental Science and Pollution Research, 2021, 28(34): 47126-47135. doi: 10.1007/s11356-021-13514-4 [17] LIN S H, CHANG C C. Treatment of landfill leachate by combined electro-fenton oxidation and sequencing batch reactor method[J]. Water Research, 2000, 34(17): 4243-4249. doi: 10.1016/S0043-1354(00)00185-8 [18] CRAVOTTO G, DI CARLO S, ONDRUSCHKA B, et al. Decontamination of soil containing POPS by the combined action of solid Fenton-like reagents and microwaves[J]. Chemosphere, 2007, 69(8): 1326-1329. doi: 10.1016/j.chemosphere.2007.05.078 [19] FU X R, GU X G, LU S G, et al. Benzene depletion by Fe2+-catalyzed sodium percarbonate in aqueous solution[J]. Chemical Engineering Journal, 2015, 267: 25-33. doi: 10.1016/j.cej.2014.12.104 [20] NAKASHIMA K, EBI Y, KUBO M, et al. Pretreatment combining ultrasound and sodium percarbonate under mild conditions for efficient degradation of corn stover[J]. Ultrasonics Sonochemistry, 2016, 29: 455-460. doi: 10.1016/j.ultsonch.2015.10.017 [21] QI L H, ZUO G M, CHENG Z X, et al. Treatment of chemical warfare agents by combined sodium percarbonate with tetraacetylethylenediamine solution[J]. Chemical Engineering Journal, 2013, 229: 197-205. doi: 10.1016/j.cej.2013.05.108 [22] ZANG X K, GU X G, LU S G, et al. Trichloroethylene oxidation performance in sodium percarbonate (SPC)/Fe2+ system[J]. Environmental Technology, 2014, 35(7): 791-798. doi: 10.1080/09593330.2013.852592 [23] 白青青, 吴小宁, 王倩, 等. 基于过碳酸钠的类Fenton体系对亚甲基蓝的降解动力学[J]. 环境工程学报, 2018, 12(1): 110-118. doi: 10.12030/j.cjee.201705105 [24] KWARCIAK-KOZLOWSKA A, FIJALKOWSKI K L. Efficiency assessment of municipal landfill leachate treatment during advanced oxidation process (AOP) with biochar adsorption (BC)[J]. Journal of Environmental Management, 2021, 287: 1-9. [25] 王亚林, 徐乾前, 章琴琴. H2O2对COD测定的干扰及消除研究[J]. 环境污染与防治, 2012, 34(12): 52-56. doi: 10.3969/j.issn.1001-3865.2012.12.012 [26] COBLE P G. Marine optical biogeochemistry: The chemistry of ocean color[J]. Chemical Reviews, 2007, 107: 402-418. doi: 10.1021/cr050350+ [27] OHNO T, FERNANDEZ I J, HIRADATE S, et al. Effects of soil acidification and forest type on water soluble soil organic matter properties[J]. Geoderma, 2007, 140(1/2): 176-187. [28] LAVONEN E E, KOTHAWALA D N, TRANVIK L J, et al. Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production[J]. Water Research, 2015, 85: 286-294. doi: 10.1016/j.watres.2015.08.024 [29] NEYENS EBAEYENS J. A review of classic Fenton's peroxidation as an advanced oxidation technique[J]. Journal of Hazardous Materials, 2003, 98(1/2/3): 33-50. [30] BOUASLA C, SAMAR M, EISMAIL F. Degradation of methyl violet 6B dye by the Fenton process[J]. Desalination, 2010, 254(1/2/3): 35-41. [31] YOON J, LEE Y, KIM S. Investigation of the reaction pathway of OH radicals produced by Fenton oxidation in the conditions of wastewater treatment[J]. Water Science and Technology, 2001, 44(5): 15-21. doi: 10.2166/wst.2001.0242 [32] KANG Y W, HWANG K Y. Effects of reaction conditions on the oxidation efficiency in the Fenton process[J]. Water Research, 2000, 34(10): 2786-2790. doi: 10.1016/S0043-1354(99)00388-7 [33] POURAN S R, AZIZ A R ADAUD W. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 53-69. doi: 10.1016/j.jiec.2014.05.005 [34] LUO C W, MA J, JIANG J, et al. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5- and UV/S2O82-[J]. Water Research, 2015, 80: 99-108. doi: 10.1016/j.watres.2015.05.019 [35] LI W W, SHI X L, ZHANG S J, et al. Modelling of ammonia recovery from wastewater by air stripping in rotating packed beds[J]. Science of the Total Environment, 2020, 702: 1-9. [36] LIU Y Q, HE X X, FU Y S, et al. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254 nm activation of persulfate[J]. Journal of Hazardous Materials, 2016, 305: 229-239. doi: 10.1016/j.jhazmat.2015.11.043 [37] QIAN Y J, XUE G, CHEN J B, et al. Oxidation of cefalexin by thermally activated persulfate: Kinetics, products, and antibacterial activity change[J]. Journal of Hazardous Materials, 2018, 354: 153-160. doi: 10.1016/j.jhazmat.2018.05.004 [38] 郝瑞霞, 曹可心, 赵钢, 等. 用紫外光谱参数表征污水中溶解性有机污染物[J]. 北京工业大学学报, 2006, 32(12): 1062-1066. doi: 10.3969/j.issn.0254-0037.2006.12.002 [39] BAKER A. Fluorescence properties of some farm wastes: implications for water quality monitoring[J]. Water Research, 2002, 36(1): 189-195. doi: 10.1016/S0043-1354(01)00210-X [40] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [41] HOLBROOK R D, DEROSE P C, LEIGH S D, et al. Excitation-emission matrix fluorescence spectroscopy for natural organic matter characterization: A quantitative evaluation of calibration and spectral correction procedures[J]. Applied Spectroscopy, 2006, 60(7): 791-799. doi: 10.1366/000370206777886973 [42] AFTAB B, HUR J. Unraveling complex removal behavior of landfill leachate upon the treatments of Fenton oxidation and MIEX (R) via two-dimensional correlation size exclusion chromatography (2D-CoSEC)[J]. Journal of Hazardous Materials, 2019, 362: 36-44. doi: 10.1016/j.jhazmat.2018.09.017 [43] MULLER G T, GIACOBBO A, CHIARAMONTE E A D, et al. The effect of sanitary landfill leachate aging on the biological treatment and assessment of photoelectrooxidation as a pre-treatment process[J]. Waste Management, 2015, 36: 177-183. doi: 10.1016/j.wasman.2014.10.024 [44] SILVA T, VIEIRA E, LOPES A R, et al. How the performance of a biological pre-oxidation step can affect a downstream photo-Fenton process on the remediation of mature landfill leachates: Assessment of kinetic parameters and characterization of the bacterial communities[J]. Separation and Purification Technology, 2017, 175: 274-286. doi: 10.1016/j.seppur.2016.11.011 [45] 周石磊, 孙悦, 张艺冉, 等. 周村水库四季变化过程中水体溶解性有机物的分布与光谱特征[J]. 环境科学学报, 2019, 39(10): 3492-3502. doi: 10.13671/j.hjkxxb.2019.0277 [46] TIZAOUI C, BOUSELMI L, MANSOURI L, et al. Landfill leachate treatment with ozone and ozone/hydrogen peroxide systems[J]. Journal of Hazardous Materials, 2007, 140(1/2): 316-324. [47] 席北斗, 何小松, 赵越, 等. 填埋垃圾稳定化进程的光谱学特性表征[J]. 光谱学与光谱分析, 2009, 29(9): 2475-2479. [48] 陶澍, 崔军, 张朝生. 水生腐殖酸的可见-紫外光谱特征[J]. 地理学报, 1990, 45(4): 484-489. doi: 10.3321/j.issn:0375-5444.1990.04.011 [49] WANG L Y, WU F C, ZHANG R Y, et al. Characterization of dissolved organic matter fractions from Lake Hongfeng, Southwestern China Plateau[J]. Journal of Environmental Sciences, 2009, 21(5): 581-588. doi: 10.1016/S1001-0742(08)62311-6 [50] ARTINGER R, BUCKAU G, GEYER S, et al. Characterization of groundwater humic substances: Influence of sedimentary organic carbon[J]. Applied Geochemistry, 2000, 15(1): 97-116. doi: 10.1016/S0883-2927(99)00021-9 [51] YAMASHITA Y, TANOUE E. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids[J]. Marine Chemistry, 2003, 82(3/4): 255-271. [52] PEURAVUORI J, PIHLAJA K, VALIMAKI N. Isolation and characterization of natural organic matter from lake water: Two different adsorption chromatographic methods[J]. Environment International, 1997, 23(4): 453-464. doi: 10.1016/S0160-4120(97)00050-0 [53] 李鸣晓, 何小松, 刘骏, 等. 鸡粪堆肥水溶性有机物特征紫外吸收光谱研究[J]. 光谱学与光谱分析, 2010, 30(11): 3081-3085. doi: 10.3964/j.issn.1000-0593(2010)11-3081-05 -



 下载:
下载: