-
目前,氰化法是最普遍的提金工艺[1],但过程中会产生大量的氰化废水,该类废水具有成分复杂、含盐量高、处理难度大等特点,因其含有大量的氰化物,直接排放会造成严重的污染问题[2-3]。在国内已经得到工业实践的方法有酸化法、碱性氯化法和SO2-空气法,但存在环境二次污染,处理成本高、过程难以控制、处理不达标等问题。其他的化学氧化法[4]、离子交换法[5]、化学沉淀法[6]等仍处于实验室阶段。因此,寻找一种短流程、高效低耗的处理方法,实现节能减排得到黄金冶炼企业的普遍关注。
电絮凝法常用处理城市污水与工业废水,此外,该方法还可用于重金属离子和无机离子的去除[7]。DAS等[8]采用集成臭氧辅助电絮凝法去除钢铁工业废水中的氰化物,在臭氧生成率1.33 mg·L−1、臭氧氧化时间40 min、电流密度100 A·m−2的和电解时间30 min的条件下,总氰质量浓度由150 mg·L−1降为0.1 mg·L−1,但电解过程中阳极易形成包裹极板表面的氧化膜,使极板与溶液接触面积减少,阻止阳极溶解,造成极板钝化[9],牺牲阳极在电解体系中不断被损耗,需要定期更换极板以确保反应正常进行[10]。电解氧化法是指利用直流电进行氧化还原反应,达到污染物分解的方法,常用于氰化废水[11-12]、电镀废水[13]的处理,研究表明,电解氧化法可以有效降解氰化废水中的金属氰络合物。LEI等[14]采用三维电化学体系处理氰化物废水,以煤基电极为主电极的三维电极体系,施加电压4 V,处理时间5 h,极板间距10 mm,活性炭颗粒用量2 g,CNT、Cu、Zn、CN−和SCN−的去除率分别为94.14%、94.53%、98.14%、98.55%和93.13%。曾鑫辉等[15]采用电解氧化法处理金矿废水,在电压6 V、pH=9、电解时间3 h、极板间距1.5 cm、NaCl添加量10 g·L−1的条件下,CNT、COD和铜氰络合物的去除率达到97.2%、96%和97.7%。Cl−的添加虽然有利于污染物的去除,但是处理后余氯浓度高,增加了环境风险。絮凝是指添加适当的絮凝剂使水或液体中悬浮微粒集聚变大,或形成絮团,从而加快粒子的聚沉,达到固-液分离的目的。常用于处理染料/纺织废水、农业废水、食品加工工业废水、纸浆和造纸废水、制革废水、垃圾渗滤液、废水中的重金属离子等[16]。聚硅酸铝铁是一种新型无机高分子絮凝剂,是在聚硅酸及传统铝盐、铁盐基础上发展起来的聚硅酸与金属盐的复合产物。该絮凝剂的大分子链上所带正电荷密度高,因絮凝剂溶解性、电中和及吸附架桥能力强、易制备、价格低廉等特点,成为无机高分子絮凝剂研究的热点[17]。许小洁等[18]研究了联合硅藻土与聚合氯化铝强化混凝对原水中重金属离子的去除,聚合氯化铝添加量为30 mg·L−1,硅藻土添加量为1.5 g·L−1时,重金属Cu、Pb和Cd的去除率分别达到57.5%、83.7%和22.2%。
针对电絮凝法存在阳极钝化,电化学氧化法存在电解质添加量大,处理后余氯浓度高,处理成本高等问题,制备绿色无氯絮凝剂,本研究采用絮凝-电解氧化联合工艺,研究了氰化废水处理过程中重金属离子和氰化物的去除规律及过程机理,以期为氰化提金废水的治理提供参考。
-
实验所用含氰废水来源于陕西某黄金冶炼厂,废水pH为9,其主要组成为 1 600.20 mg·L−1 CNT、− 439.74 mg·L−1 CN、257.35 mg·L−1 Cu、0.36 mg·L−1Fe、672.80 mg·L−1Zn。可以看出,此废水中CNT、CN−和Zn的含量均较高,其中CNT质量浓度大于800 mg·L−1,属于高浓度的氰化提金废水,Zn元素含量高达672.80 mg·L−1。所用其他化学试剂均为分析纯。
-
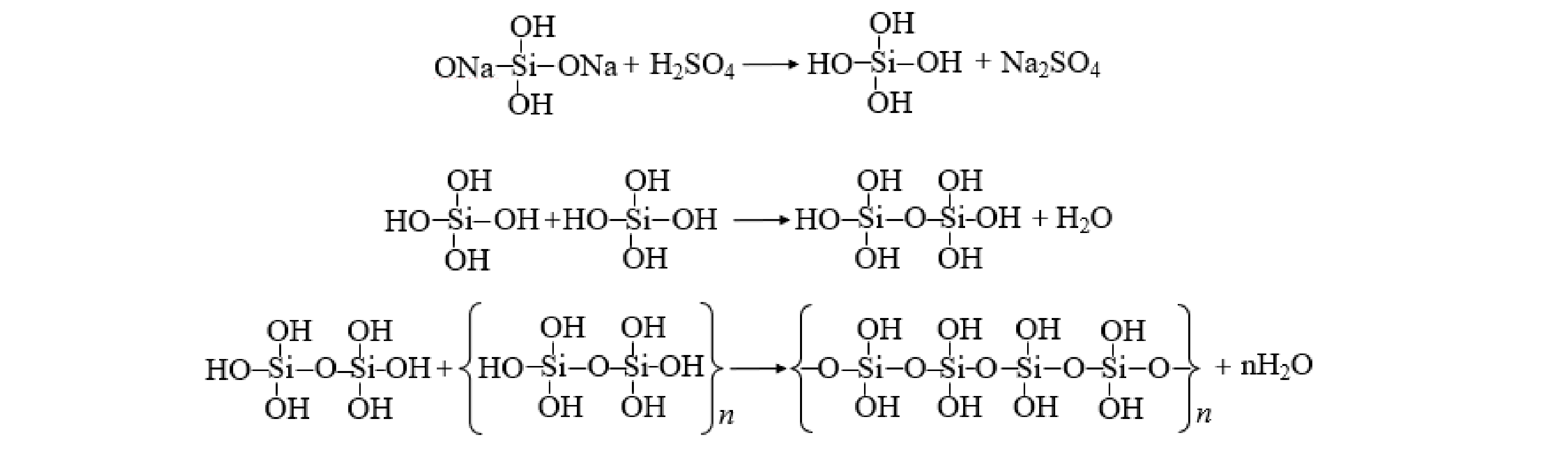
首先,向100 mL的0.5 mol·L−1的硅酸钠溶液中加入20%(体积比)硫酸溶液,磁力搅拌至溶液pH=2,所得溶液在室温下陈化30 min,得到聚硅酸 (PSA) ,反应过程如图1所示。其次,在聚硅酸中先加入1.297 g的Al2(SO4)3·18H2O粉末,然后连续搅拌10 min,再加入0.400 g的Fe2(SO4)3粉末。最后,将略显绿色透明溶液在30 ℃陈化24 h。
-
室温下,在150 mL烧杯中加入100 mL废水,使用硫酸或氢氧化钠调节pH,加入一定量的PSAF置于搅拌器,120 r·min−1快速搅拌30 s,再60 r·min−1慢速搅拌,静止一定时间,实验后固液分离,沉淀物采用去离子水洗至pH为7左右置于电热鼓风干燥箱中于60 ℃烘干。随后以钛板为阴极,石墨为阳极,采用一阴两阳三极板体系对过滤后的溶液进行电解氧化实验,在不同的电压、电解时间、极板间距下电解反应,实验后固液分离,对溶液取样分析,测定CNT、CN−、Cu、Zn离子的含量。
-
采用硝酸银容量法(HJ484-2009)分析测定CN−、CNT的含量,采用原子吸收光谱仪分析测定Cu、Fe、Zn等金属离子的浓度,并根据式(1)计算各离子的去除率。采用傅立叶变换红外光谱(FTIR)分析絮凝样品,采用D/MAX2200型X射线衍射仪分析沉淀物,采用马尔文Zeta电位仪测定Zeta电位。
式中:E为各离子的去除率,%;C0为各离子的初始质量浓度,mg·L−1;Ce为絮凝或电解氧化处理后溶液中各离子的质量浓度,mg·L−1。
-
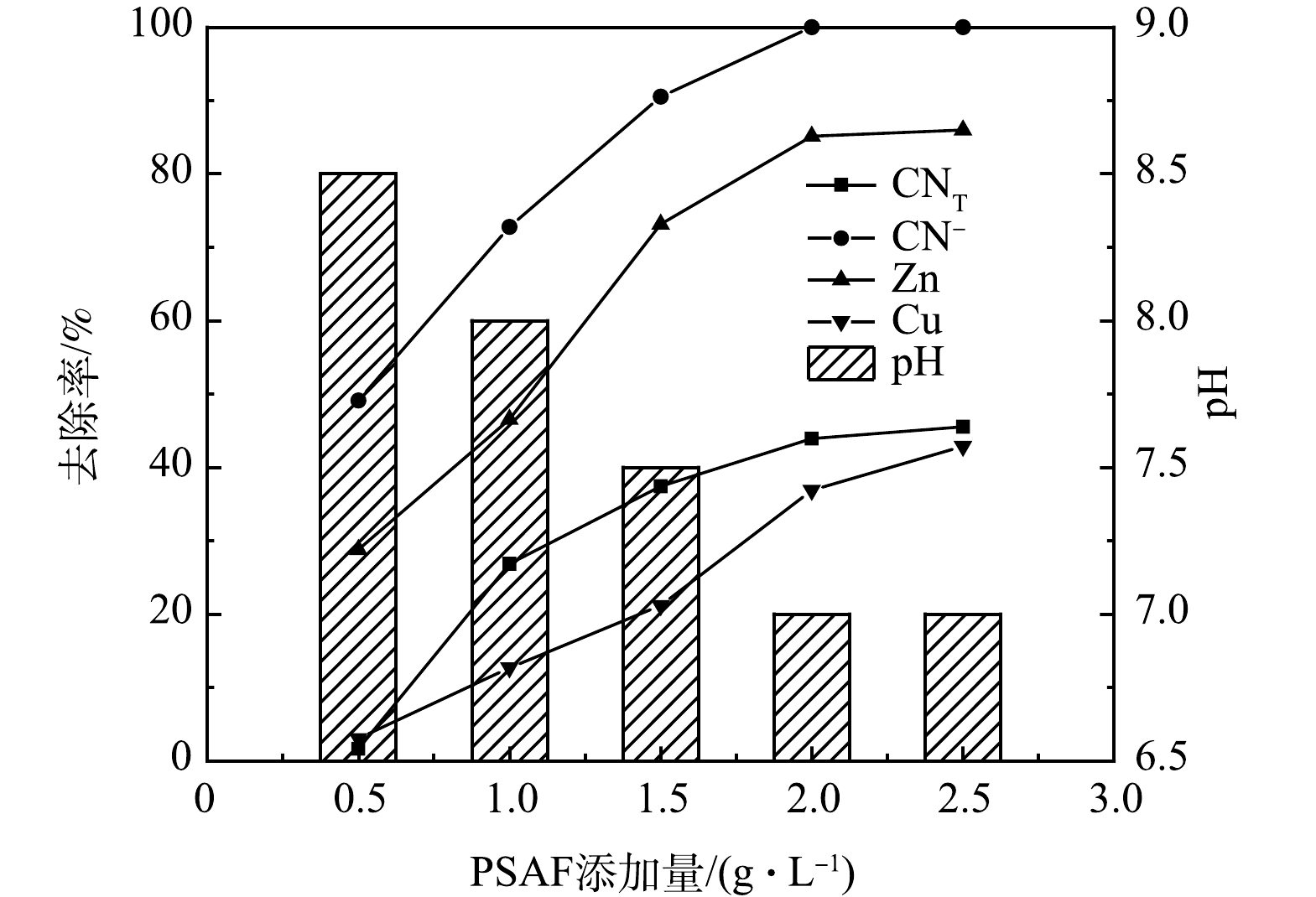
1) PSAF添加量的影响。常温条件下,取絮凝时间为30 min,pH为9,PSAF添加量分别为0.5、1、1.5、2、2.5 g·L−1进行絮凝实验,结果如图2所示。随着PSAF添加量的增加,废水中的CNT、CN−、Zn、Cu离子去除率先升高后趋于平缓,pH则先降低后趋于平缓,在PSAF添加量2 g·L−1时,CNT、CN−、Zn、Cu离子去除率均达到最大值,分别为43.9%、100%、85.14%、36.88%,pH为7。因为随着PSAF添加量的增加,PSAF与CN−、Zn(CN)42−、Cu(CN)32−发生的凝结点位增多,形成大量螯合絮体的网捕、扫卷作用增强,PSAF所带的正电荷中和了污染物所带的负电荷,使污染物失稳而达到去除效果[12],从而增加了CNT、CN−、Zn、Cu离子的去除率。
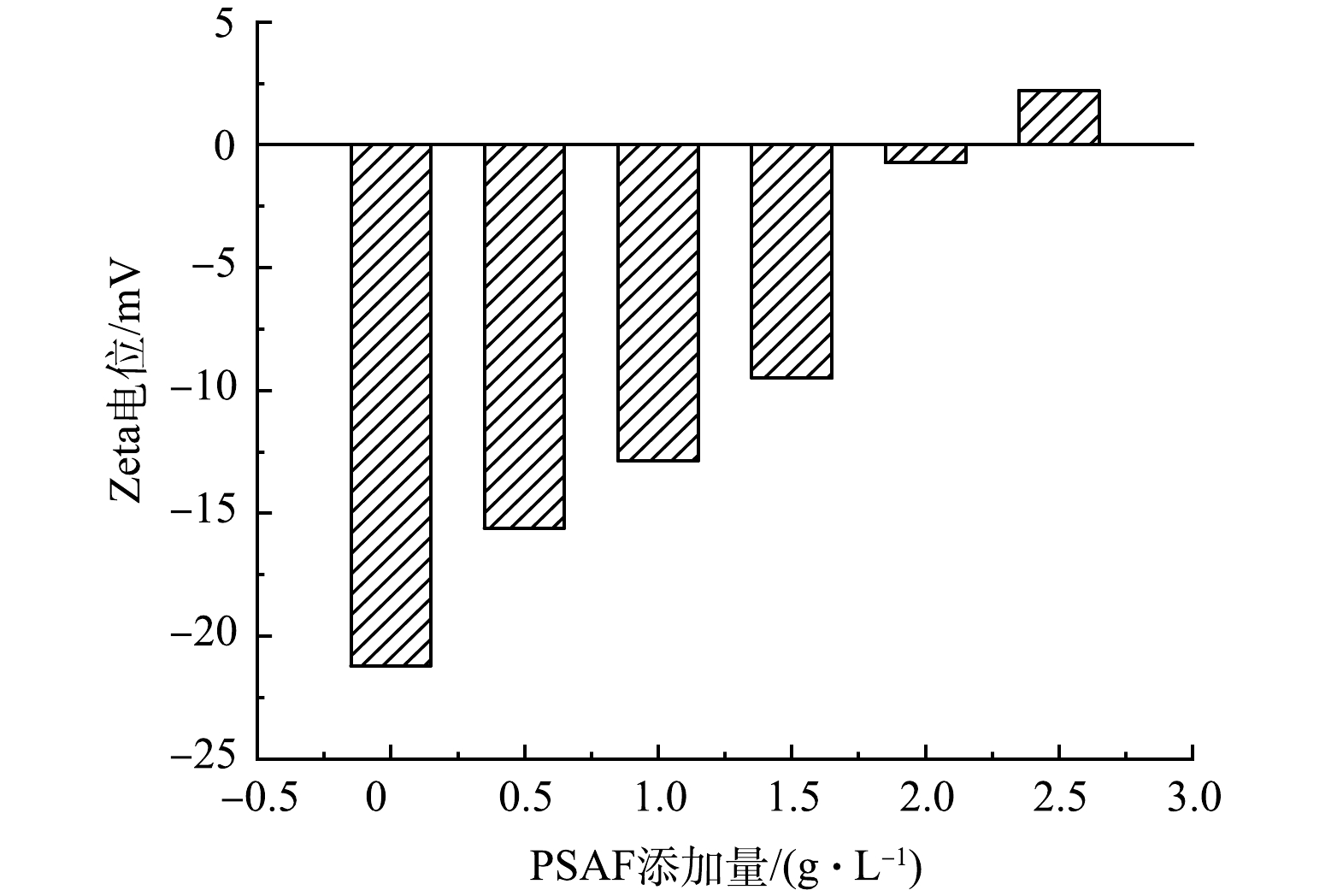
为了研究不同PSAF添加量反应机制,进行了Zeta电位分析。有研究[19]表明,Zeta电位绝对值越小,即越趋近于0时,粒子之间斥力越小,越容易团聚。由图3可见,随着PSAF添加量的增加,Zeta电位不断增加,未经处理废水的Zeta电位为−21.2 mV,添加量增加到2.0 g·L−1时,Zeta电位为−0.72 mV。这是因为水解后产生的聚羟基络合物带正电荷,随着添加量的增加,正电荷数量不断增加,絮凝过程中双电层吸附及电中和能力增强,使得电位降低,趋近于零点时,颗粒凝聚且脱稳沉降[20]。在中性体系中,带负电荷的络合物在絮凝过程中能够吸附在氢氧化铁絮体表面。
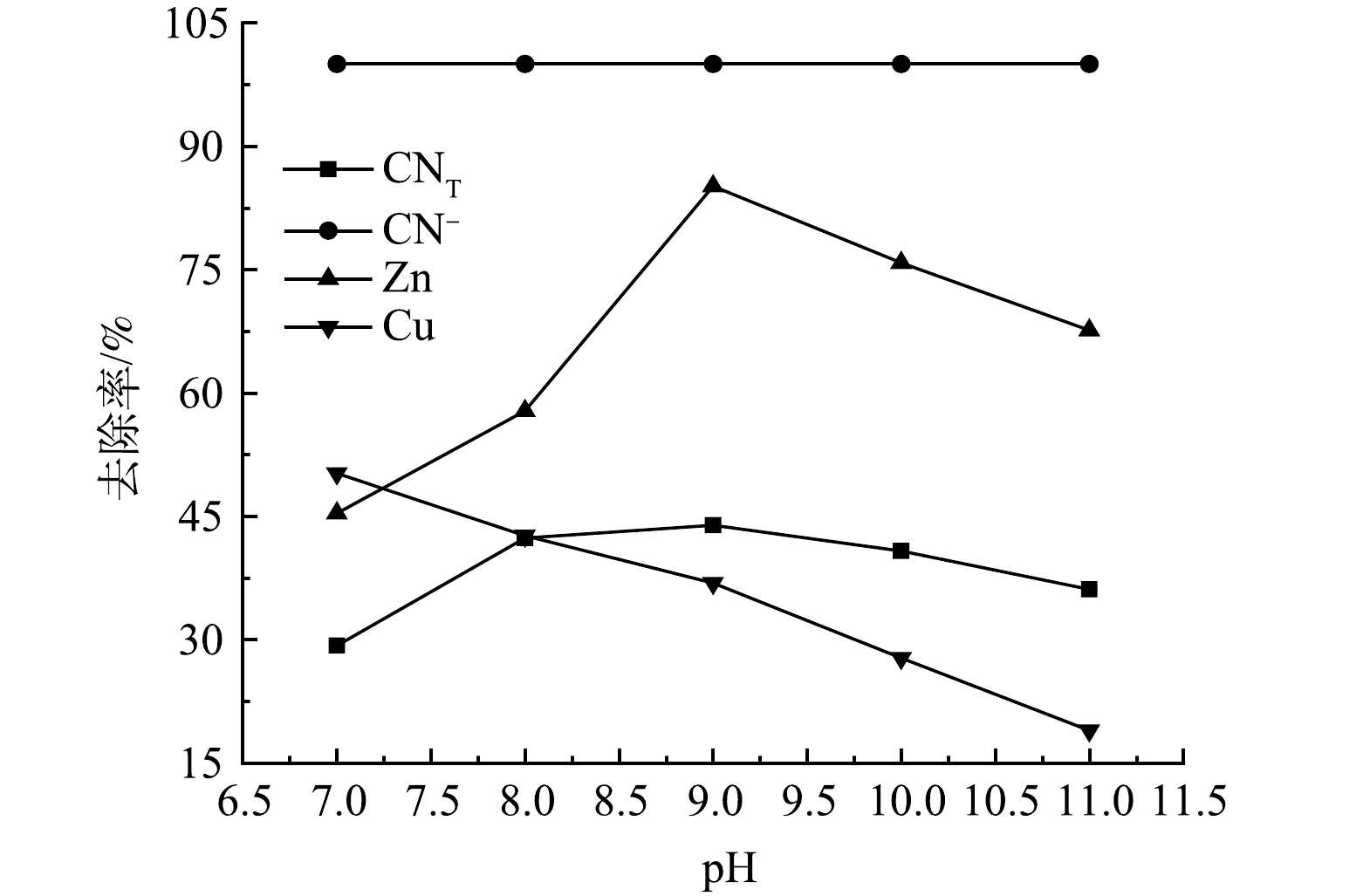
2)废水pH的影响。水中的H+和OH−均参与絮凝剂的水解反应,因此,pH可影响絮凝剂的水解速度、水解产物的存在形态和性能。在PSAF添加量为2 g·L−1,废水pH分别为7、8、9、10、11的条件下进行絮凝实验,结果如图4所示。可见,随着废水pH的增加,废水中的CN−去除率很快就达到了最大值,随后不再发生明显变化,而CNT、Zn离子去除率呈现先升高后降低的变化趋势,Cu离子去除率随着pH的增大而升高,当pH为9时,CNT、CN−、Zn、Cu离子去除率分别为43.9%、100%、85.14%、36.88%。这是因为随着pH的减小,H+会与PSAF水解的羟基络合阳离子Me(OH)2+、Me(OH)2+、Me2(OH)24+竞争,从而削弱带正电的PSAF和带负电的CN−、Cu(CN)32−、Zn(CN)42−之间的静电吸引力,因此,pH较低时不利于污染物的去除。但在强碱条件下,PSAF的大分子结构易被破坏,各物质的去除率会下降[15]。
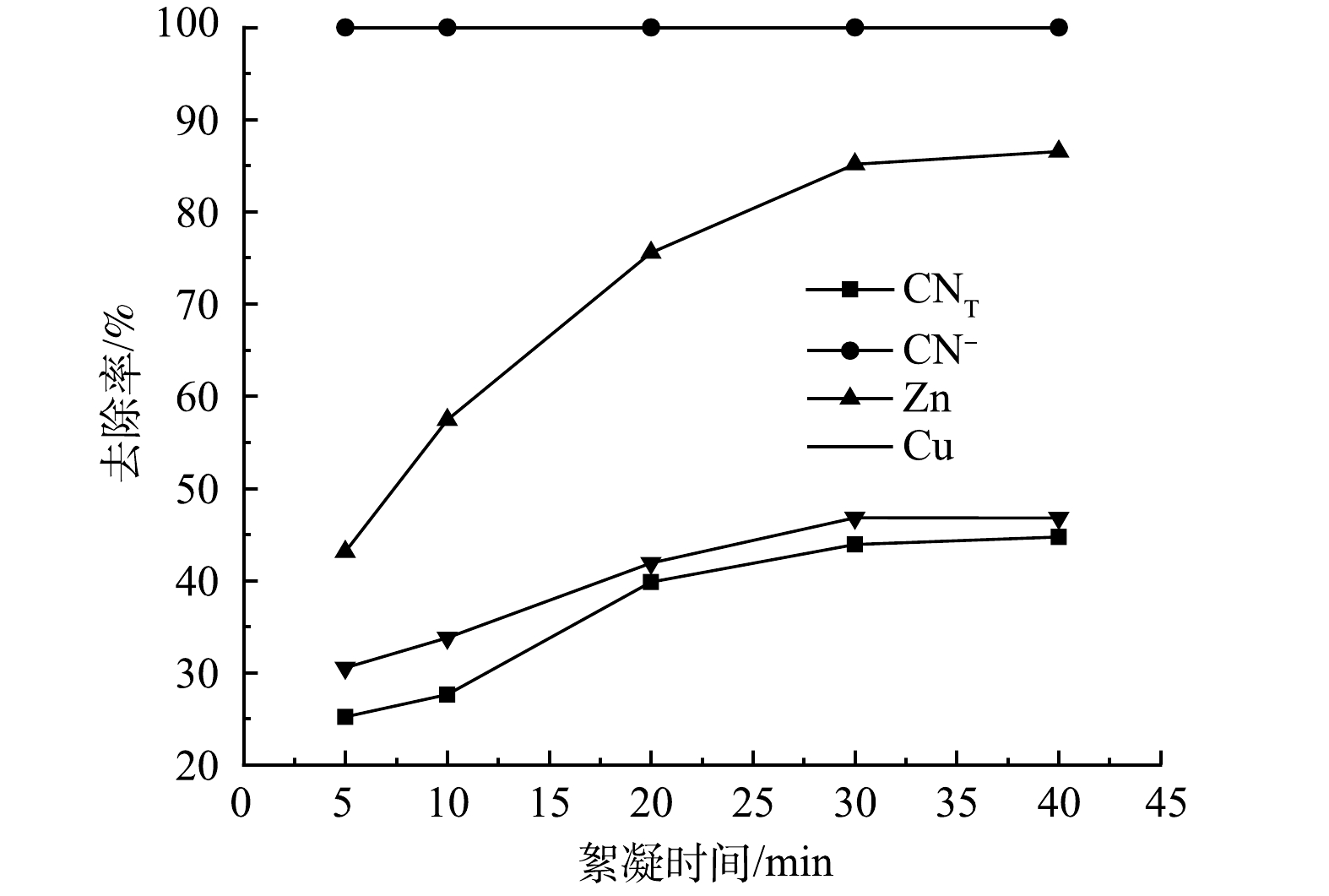
3) 絮凝时间的影响。在pH为9,絮凝时间分别为5、10、20、30、40 min的条件下进行絮凝实验,结果如图5所示。可见,随着絮凝时间的增加,CNT、Zn、Cu离子去除率不断增加,当絮凝时间增加到30 min时,CNT、CN−、Zn、Cu离子去除率均达到最高值,分别为43.9%、100%、85.14%、46.80%。考虑到时间成本,絮凝时间选择30 min最为合适。此时,废水的pH由原来的9降至7。
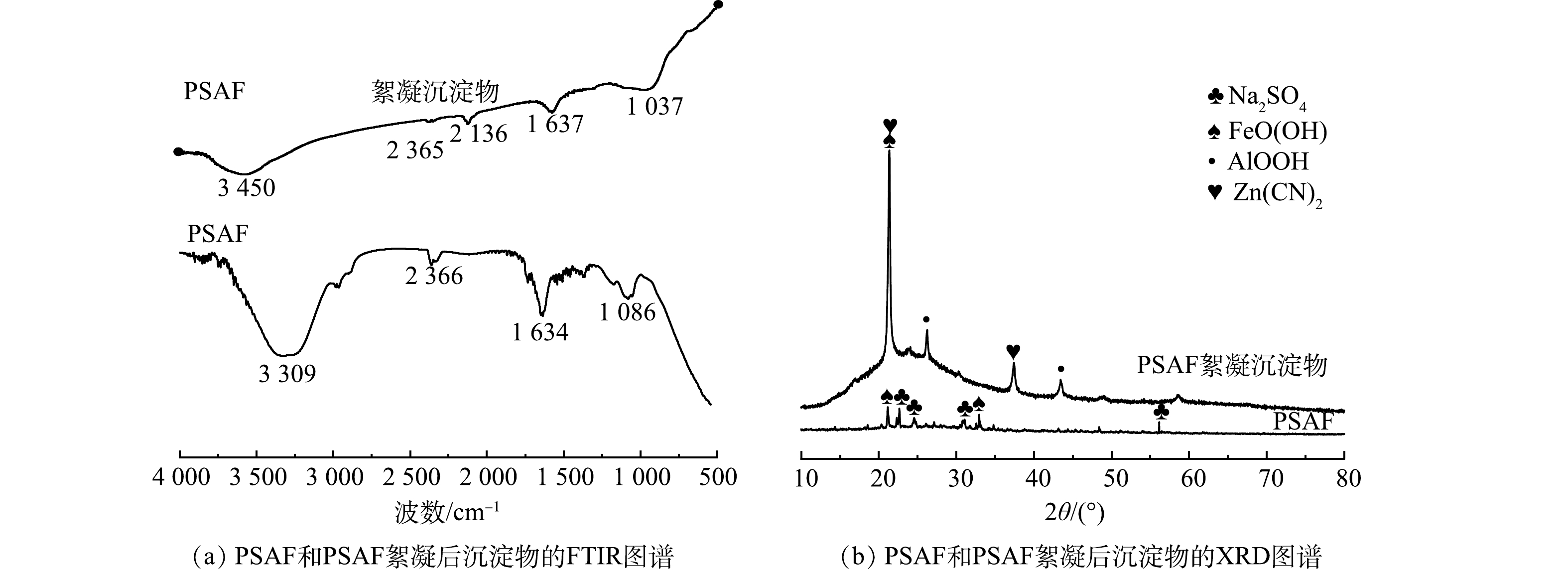
4) FTIR和XRD的表征分析。对制备的PSAF和PSAF絮凝沉淀物进行FTIR光谱分析,结果如图6(a)所示。制备的PSAF在3 309 cm−1和1 634 cm−1处的特征峰可归于羟基的振动[21-23],PSAF絮凝沉淀物对应的特征峰发生了偏移,分别为3 450 cm−1和1 637 cm−1,且峰面积变小,说明羟基发生了交换。在2 136 cm−1处出现了C
$ \equiv $ N键的特征吸收峰[1]。说明在絮凝反应过程中,氰离子发生了化学反应,从而导致了吸收峰发生了改变。通过对絮凝沉淀物消解且测定了其中Zn和Cu的含量,Zn、Cu的相对含量分别为6.11%、0.51%。这是因为PSAF水解后,产生带正电的金属羟基络合物Me(OH)2+、Me(OH)2+、Me2(OH)24+(用R-OH表示),其中羟基与金属氰络合物发生化学吸附反应如式(2)~式(4)。絮凝后得到的RCN、R2Zn(CN)4、R2Cu(CN)3可以采用硫酸酸化-碱液吸收法进行回收利用[26]。制备的PSAF在1 086 cm−1处存在Si-O-Si、Si-O-Al、Si-O-Fe的伸缩振动[21],该峰强度与絮凝性能密切相关,表明Al3+、Fe3+与配合物聚硅酸链末端的羟基反应,金属为聚硅酸表面提供了正电荷,使之有电中和能力[24]。1 037 cm−1处PSAF絮凝沉淀物对应的峰变宽且发生了偏移,说明此时絮凝吸附作用达到饱和,絮凝剂基本失效。根据Zeta电位、FTIR分析可知,絮凝过程存在电荷中和吸附与化学吸附,Zn(CN)42−、Cu(CN)32−、CN−的吸附量分别为567.88、89.76、439.74 mg·L−1。离子的水合作用与配合离子中的电荷密度(电荷/元素数)有关,该比值越高,稳定溶液中离子所需的水分子越多,水合的种类也越多,就越不易被吸附,Zn(CN)42−电荷密度为2/9,Cu(CN)32−电荷密度为2/7,CN−电荷密度为1/2,因此,羟基对提金废水中阴离子的化学吸附能力为Zn(CN)42−>Cu(CN)32−>CN−[25],而Cu(CN)32−的总吸附量仅达到89.76 mg·L−1,远远小于Zn(CN)42−、CN−的吸附量,随着异号离子的加入,异号离子之间有强烈的吸附作用,发生电中和吸附。化学吸附需要活化能,因此,化学吸附在絮凝过程中并非起主要作用。
分别对制备的PSAF、PSAF絮凝沉淀物进行XRD分析表征,结果如图6(b)所示。由分析结果可以看出,制备的PSAF主要是Na2SO4和FeOOH的吸收峰,絮凝后沉淀物出现了AlOOH、Zn(CN)2的吸收峰。游离Na+和SO42−在合成PSAF絮凝剂时形成Na2SO4,干燥过程形成Na2SO4晶体[19],絮凝后Na2SO4峰消失,是因为絮凝时将PSAF加入废水中,Na2SO4溶解;在PSAF中无法观察到Fe2(SO4)3、Fe2O3、Fe3O4、Al2(SO4)3和SiO2等衍射晶体的光谱,表明Fe、Al和Si聚合成一些新的化合物,而不是保持原料的简单混合物[21];同时,絮凝时羟基络合离子Fe(OH)2+、Al(OH)2+发生了脱水反应,产生的FeO+、AlO+消耗水中的OH−,导致FeOOH、AlOOH的出现(式(5)~式(8))。由于PSAF属于酸性絮凝剂,导致体系pH降低,Zn(CN)42−分解得到Zn(CN)2白色沉淀(式(9))。
-
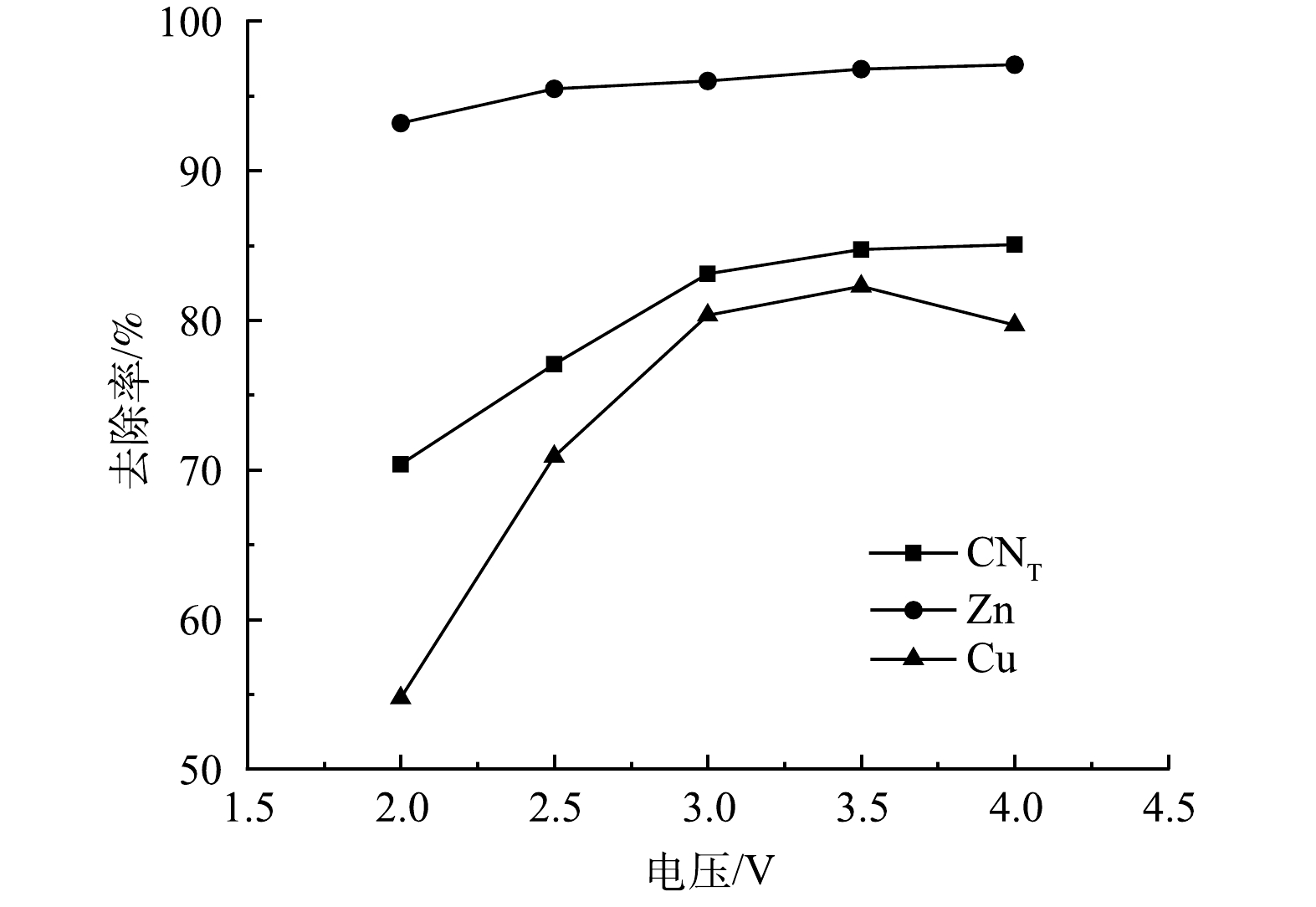
1) 电解电压的影响。取电解时间为1 h、极板间距为10 mm,对絮凝后液分别在2、2.5、3、3.5、4 V下进行电解氧化实验,结果如图7所示。随着电压的增加,废水中各离子去除率逐渐升高,随后不再发生明显变化。电压为3 V时,CNT、CN−、Zn、Cu去除率均达到最大值,分别为83.11%、100%、96.00%、80.34%。是因为电压较低时,阴离子在电场作用下通过定向迁移向阳极表面移动,并在表面吸附,系统主要依靠吸附和电吸附过程,去除率相对较低。而随着电压的升高,阳极电压高于析氧电位(0.401 V),溶液中的氰化物因析氧而被氧化(式(10)~式(13)),达到·OH的电位时(2.8 V),利用·OH的强氧化性,攻击含氰基的化合物,将C和N氧化成CO2和NO2(式(14)~式(17))[27]。释放出来的金属离子大部分在阴极板电沉积而被去除(式(18)~式(20)),使得各离子去除率大大增加[28]。
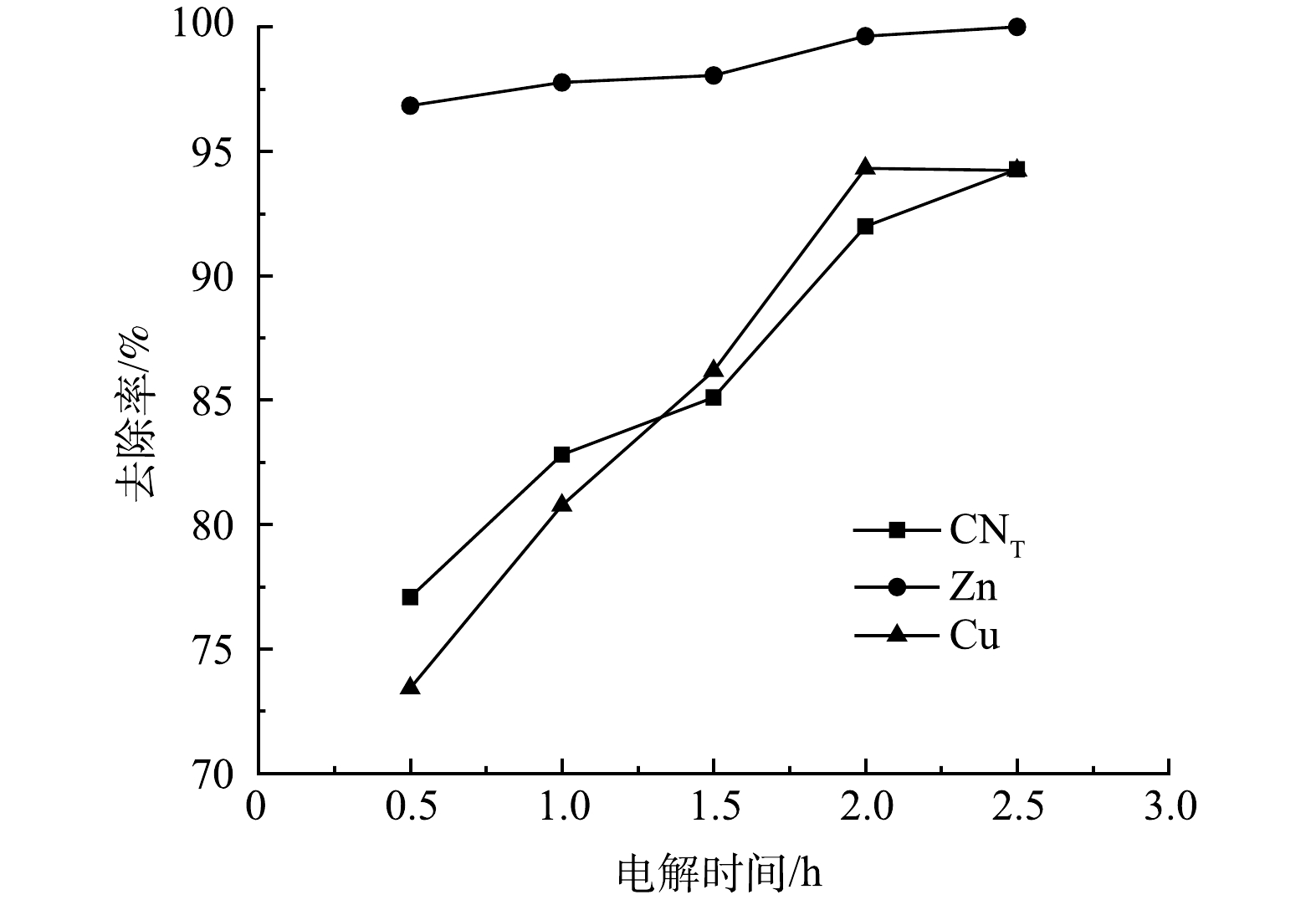
2)电解时间的影响。取电解电压为3 V,电解时间分别为0.5、1、1.5、2、2.5 h进行电解氧化实验,结果如图8所示。随着电解时间的增加,废水中的CN−去除率很快就达到了最大值,随后不再发生明显变化,而CNT、Zn、Cu离子去除率呈现逐渐上升的趋势。反应初期,废水中离子浓度较高,离子的迁移速率相对较快,阴阳极发生剧烈化学反应,Zn(CN)42−、Cu(CN)32−在阳极发生氧化反应,金属离子在阴极发生电沉积,2 h后,溶液中参与反应的离子浓度逐渐降低,迁移速率减慢,被还原的金属离子附着在极板表面。电解时间为2 h时,CNT、CN−、Zn、Cu离子去除率均达到最大值,分别为91.98%、100%、99.63%、94.31%。电解后存在少量沉淀,经XRD检测只存在C峰,是因为阳极的石墨板脱落少量的碳,而Zn(CN)42−、Cu(CN)32−中的氰根被氧化为CO2、N2,释放出来的Zn、Cu离子在阴极以电沉积的形式被去除。
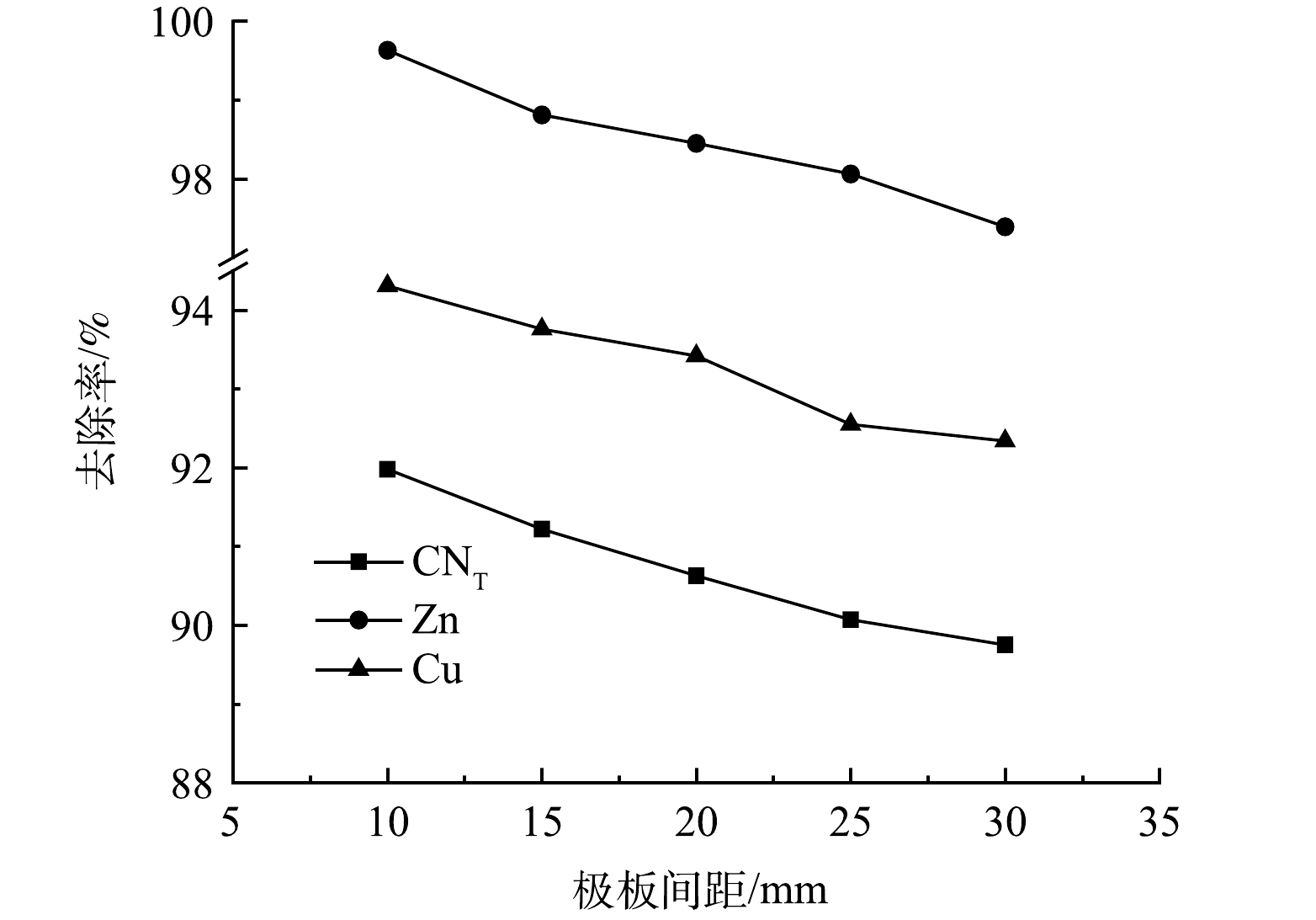
3)极板间距的影响。取电解时间为2 h,极板间距分别为10、15、20、25、30 mm进行电解氧化实验,结果如图9所示。随着极板间距的增大,CNT、Zn、Cu离子的去除率均呈现下降趋势,当极板间距为10 mm时,去除率分别为91.98%、99.63%、94.31%。随着极板间距增加,电子传递距离增加,电能损耗增加,极板间的电场强度减小,极板间带电离子Cu(CN)32−、Zn(CN)42−的迁移速率减小,且电解过程中产生的O2的扩散距离增长,从而降低各离子的去除效率[11]。因此,选择最佳极板间距为10 mm。
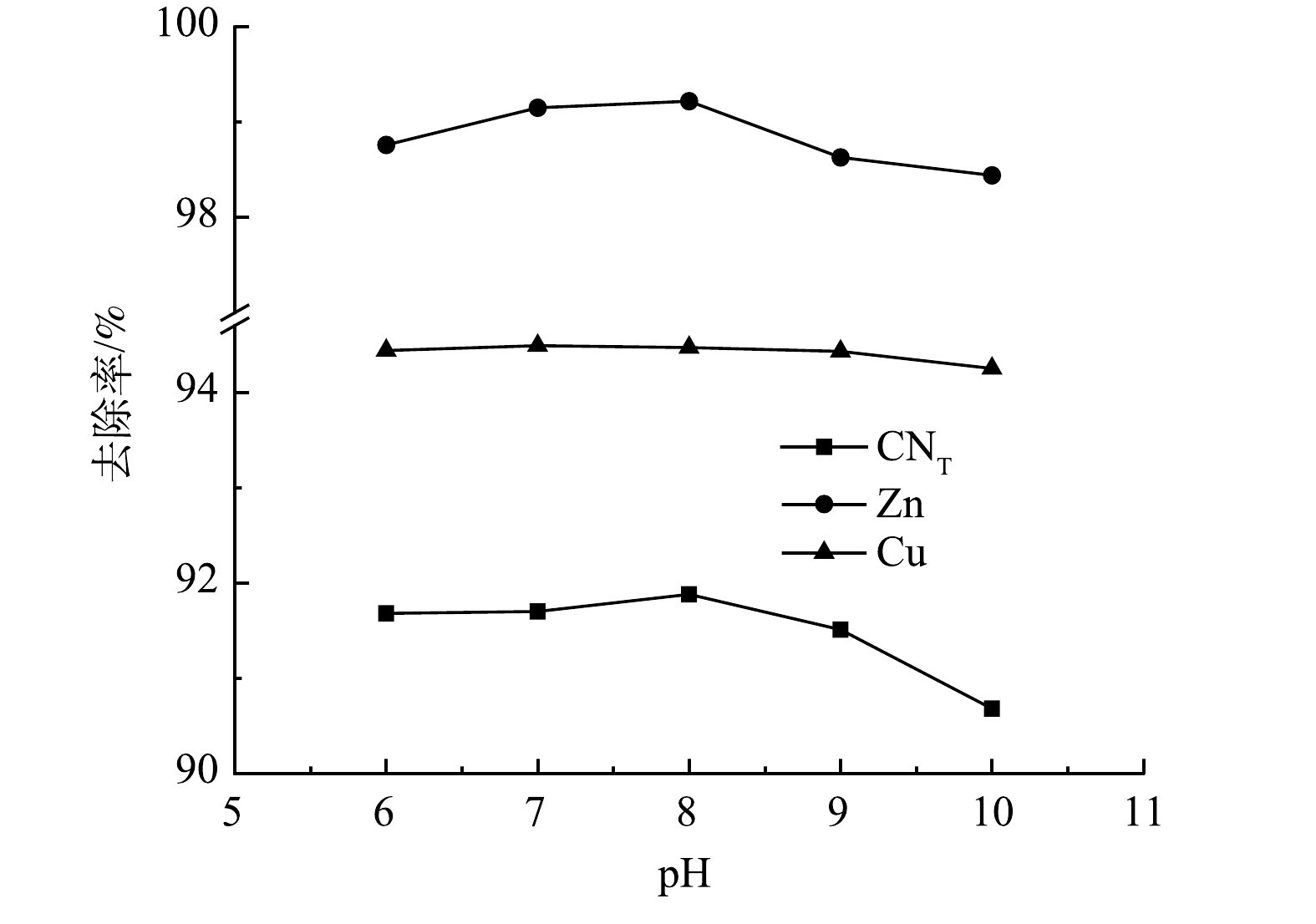
4) pH的影响。取极板间距为10 mm,絮凝以后调节pH分别为6、7、8、9、10进行电解氧化实验,结果如图10所示。随着pH的增加,溶液中的CNT、Zn离子的去除率略微增大后减小,Cu离子去除率没有明显变化,当pH为7时,去除率分别为91.70%、99.15%、94.49%。阳极氧化主要是阳极产生的氧气起作用,而氧气的产生是由电解水而得到,因此絮凝后废水的pH对电氧化效果没有明显影响。考虑到药剂成本问题,选择不改变废水pH。
-
在PSAF添加量为2 g·L−1、絮凝时间为30 min、pH为9、电压为3 V、电解时间为2 h、极板间距为10 mm的条件下,进行氰化提金废水的絮凝-电解氧化验证实验,结果如表1所示。从3组平行实验所测得的结果来看,CNT、CN−、Zn、Cu的去除率相对稳定,平均去除率分别为91.70%、100%、99.15%、94.49%,说明采用絮凝-电解氧化处理氰化提金废水的技术是有效可行的。
-
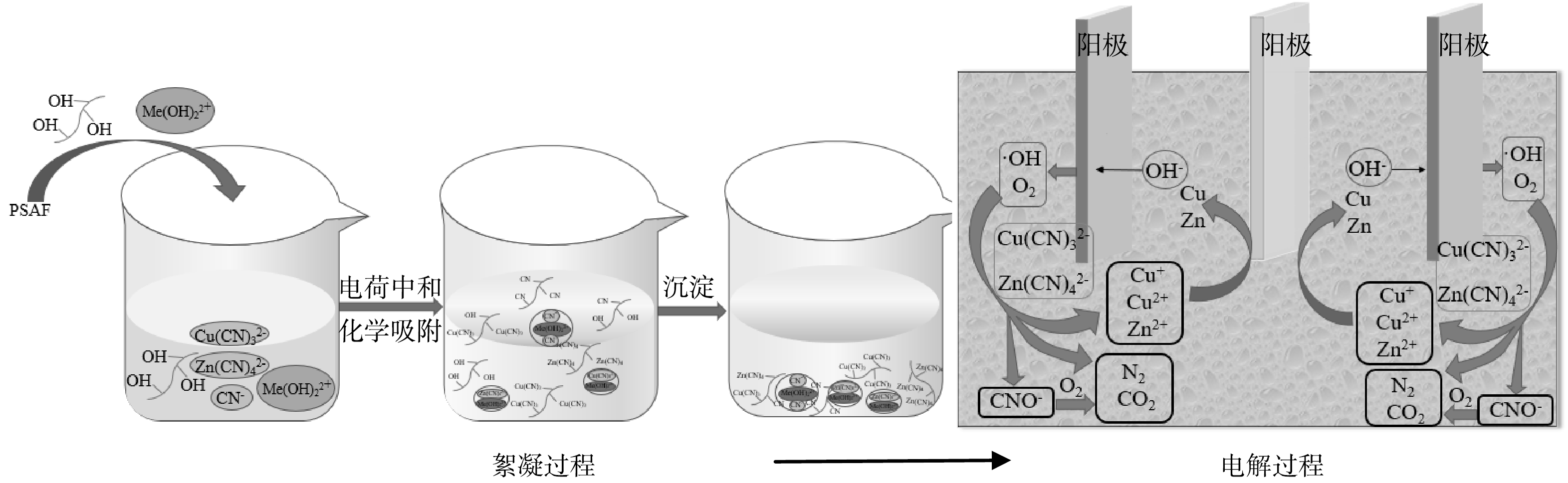
综上所述,絮凝-电解氧化联合处理氰化提金废水可分为PSAF絮凝和电解氧化两个阶段,反应机理示意图如图11所示。首先,加入的PSAF发生水解,生成羟基络合阳离子及羟基络合物,羟基络合阳离子与废水中的Zn(CN)42−、Cu(CN)32−、CN−通过正负电荷中和吸附去除,同时伴有羟基络合物化学吸附过程,化学吸附时,羟基络合物的羟基起主要作用,其与废水中Zn(CN)42−、Cu(CN)32−、CN−发生离子交换,产生RCN、R2Zn(CN)4、R2Cu(CN)3。其次,絮凝后液采用电解氧化处理,废水中剩余的Zn(CN)42−、Cu(CN)32−在电场作用下迁移至阳极,在阳极发生氧化反应,生成的O2、·OH将迁移至阳极的Zn(CN)42−、Cu(CN)32−氧化为N2和CO2,同时释放出Zn2+、Cu+定向迁移至阴极还原析出金属单质。
-
1)采用絮凝-电解氧化联合技术处理氰化废水的思路是可行的。在室温条件下,当聚合硅酸铝铁添加量为2 g·L−1、絮凝时间30 min、pH为9时,废水中CNT、CN−、Cu、Zn离子的去除率分别为42.97%、100%、84.40%、34.88%。以电中和吸附的方式为主,伴随着化学吸附将污染物去除,同时生成了Zn(CN)2沉淀。
2)当电解时间为2 h,外加电压为3 V,极板间距为10 mm时,废水中CNT、CN−、Cu、Zn离子的总去除率分别为91.70%、100%、99.15%、94.49%。
3)无氯絮凝剂PSAF对氰化废水的处理是有效的。絮凝-电解氧化联合技术处理氰化废水,减少了氯对水环境的风险,且具有成本小,处理效果好等优点,对黄金冶炼行业的环境友好发展具有重要意义。
絮凝-电解氧化联合处理氰化提金废水
Cyanidation gold extraction wastewater treatment by combined flocculation-electrolysis oxidation
-
摘要: 采用絮凝-电解氧化联合技术处理氰化废水,主要研究了聚硅酸铝铁 (PSAF) 添加量、絮凝时间、pH、电压、电解时间、极板间距对总氰(CNT)、游离氰(CN−)、Cu、Zn离子去除率的影响,并对其反应机制做了分析。研究表明,当PSAF添加量为2 g·L−1,絮凝时间为30 min,pH为9条件下,CNT、CN−、Zn、Cu离子的去除率分别可达42.97%、100%、84.40%、34.88%。Zn(CN)42−、Cu(CN)32−、CN−的吸附量分别为567.88、89.76、439.74 mg·L−1。以钛板为阴极,石墨板为阳极,采用一阴两阳体系对絮凝后液进行电解氧化实验,在电压为3 V、电解时间为2 h、极板间距为10 mm条件下,CNT、CN−、Zn、Cu离子的去除率可达91.70%、100%、99.15%、94.49%。絮凝过程中Zn(CN)42−、Cu(CN)32−、CN−的去除是由电荷中和和化学吸附共同作用的,其中电荷中和起主要作用。Zn(CN)42−、Cu(CN)32−、CN-的化学吸附主要归因于其与PSAF水解产生的羟基阳离子发生交换反应。XRD分析表明,加入酸性絮凝剂PSAF的瞬间,部分Zn(CN)42-反应为Zn(CN)2沉淀。电解氧化过程中Zn(CN)42−、Cu(CN)32−破络释放的氰根会被阳极表面产生的O2、·OH完全氧化为N2和CO2,Zn、Cu离子在阴极板电沉积而被去除。Abstract: In this study, the flocculation-electrolysis technology was used to treat the high-concentration cyanidation gold extraction wastewater. The effects of polyaluminum ferric silicate (PSAF) addition, flocculation time, pH, voltage, electrolysis time and electrode distance on the removal rates of total cyanide (CNT), free cyanide (CN−), Cu ions and Zn ions were mainly studied, and the reaction mechanism was also identified. The results showed that when PSAF dosage was 2 g·L−1, the flocculation time was 30 min and pH was 9, the removal rates of CNT, CN−, Zn, and Cu ions in wastewater could reach 42.97%, 100%, 84.40% and 34.88%, respectively. The adsorption capacities of Zn(CN)42−, Cu(CN)32−, and CN− were 567.88 mg·L−1、89.76 mg·L−1、439.74 mg·L−1, respectively. The electrolytic oxidation experiments of the flocculated liquid were conducted by using a graphite plate-cathode and two titanium plate-anode system. At the voltage of 3 V, electrolysis time of 2 h and electrode distance of 10 mm, the removal rates of CNT, CN−, Zn and Cu ions in the wastewater were 91.70%、100%、99.15% and 94.49%, respectively. Zeta potential and FTIR analysis showed that the removal of Zn(CN)42−, Cu(CN)32−, CN− during the flocculation process was a combination of charge neutralization and chemisorption, of which charge neutralization played a major role. The chemisorption of Zn(CN)42−, Cu(CN)32−, and CN− was mainly attributed to the exchange reaction with the positively charged hydroxyl cations produced by PSAF hydrolysis. XRD analysis showed that a part of Zn(CN)42− reacted as Zn(CN)2 precipitate at the moment of acidic flocculant PSAF dosing. During the electrolytic oxidation process, the cyanogen released by Zn(CN)42− and Cu(CN)32− breaking could be completely oxidized to N2 and CO2 by O2, ·OH generated on the anode surface, and Zn and Cu ions were removed by the electrodeposition on the cathode plate.
-
Key words:
- cyanide wastewater /
- flocculant /
- polyaluminum ferric silicate /
- electrolytic oxidation
-

-
表 1 平行实验结果
Table 1. Parallel experiment results
组分 序号 沉淀后液/
(mg·L−1)电解后液/
(mg·L−1)去除率/
%平均
去除率/%CNT A 921.56 138.10 91.37 91.70 B 909.71 134.10 91.62 C 906.35 126.26 92.11 CN- A 0 0 100 100 B 0 0 100 C 0 0 100 Zn A 121.75 7.44 98.89 99.15 B 109.55 5.1 99.24 C 83.47 4.63 99.31 Cu A 173.02 17.87 93.06 94.49 B 166.15 13.41 94.79 C 163.60 11.3 95.61 -
[1] SONG Y H, LI Y F, HE X H, et al. Recycling of residual valuable metals in cyanide-leached gold wastewater using the N263-TBP system[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106774. doi: 10.1016/j.jece.2021.106774 [2] 李一凡, 宋永辉, 周民, 等. 氰化提金废水中金属氰络合离子的溶剂萃取[J]. 中国有色金属学报, 2022, 32(2): 536-544. [3] 张曼曼, 冯占立, 王军强, 等. 黄金湿法冶炼含氰废水处理研究进展[J]. 化学工业与工程, 2019, 36(1): 2-9. doi: 10.13353/j.issn.1004.9533.20183004 [4] 袁嘉声, 畅永锋, 郑春龙, 等. 氰化尾渣脱氰技术综述[J]. 中国有色金属学报, 2021, 31(6): 1568-1581. doi: 10.11817/j.ysxb.1004.0609.2021-37785 [5] 党晓娥, 淮敏超, 兰新哲. 铜、锌氰配合物在离子交换纤维上的扩散、吸附机理[J]. 环境工程学报, 2012, 6(9): 3148-3152. [6] 宋永辉, 屈学化, 吴春晨, 等. 硫酸锌沉淀法处理高铜氰化废水的研究[J]. 稀有金属, 2015, 39(4): 357-364. doi: 10.13373/j.cnki.cjrm.2015.04.010 [7] ISLAM S M. Electrocoagulation (EC) technology for wastewater treatment and pollutants removal[J]. Sustainable Water Resources Management, 2019, 5(1): 359-380. doi: 10.1007/s40899-017-0152-1 [8] DAS P P, MONDAL P, SINHA A, et al. Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater[J]. Chemosphere, 2021, 263: 128370. doi: 10.1016/j.chemosphere.2020.128370 [9] 戴常超, 陈大宏, 刘峻峰, 等. 强化电絮凝技术的基础、现状和未来展望[J]. 工业水处理, 2022, 42(1): 1-14. [10] 刘海燕. 电絮凝在废水处理中的研究及应用[J]. 辽宁化工, 2022, 51(8): 1125-1127. doi: 10.3969/j.issn.1004-0935.2022.08.025 [11] 赵玲玲, 宋永辉, 曾鑫辉, 等. 沉淀-电解氧化法处理高铁氰化废水[J]. 环境工程学报, 2020, 14(12): 3426-3434. doi: 10.12030/j.cjee.202001096 [12] 宋永辉, 雷思明. 含氰废水的电化学处理技术研究进展[J]. 黄金科学技术, 2016, 24(4): 137-143. doi: 10.11872/j.issn.1005-2518.2016.04.137 [13] GUAN W, TIAN S C, CAO D, et al. Electrooxidation of nickel-ammonia complexes and simultaneous electrodeposition recovery of nickel from practical nickel-electroplating rinse wastewater[J]. Electrochimica Acta, 2017, 246: 1230-1236. doi: 10.1016/j.electacta.2017.06.121 [14] LEI S M and SONG Y H. Comparative study on electrochemical treatment of cyanide wastewater[J]. Frontiers in Chemistry, 2021, 9: 598228. doi: 10.3389/fchem.2021.598228 [15] 曾鑫辉, 宋永辉, 李一凡, 等. 电解氧化法处理金矿废水[J]. 化学工程, 2022, 50(2): 9-14. [16] TEH C Y, BUDIMAN P M, SHAK K P Y, et al. Recent advancement of coagulation-flocculation and its application in wastewater treatment[J]. Industrial & Engineering Chemistry Research, 2016, 55(16): 4363-4389. [17] 刘德汞, 缑星, 赵慧茹, 等. 聚硅酸铝铁絮凝剂的制备及对含Cd2+废水处理研究[J]. 非金属矿, 2012, 35(5): 72-75. [18] 许小洁, 吴纯德, 董琪, 等. 联合硅藻土与PAC强化混凝处理含藻微污染原水[J]. 环境工程学报, 2011, 5(9): 1979-1983. [19] ZHANG H H, LIN H, LI Q, et al. Removal of refractory organics in wastewater by coagulation/flocculation with green chlorine-free coagulants[J]. Science of the Total Environment, 2021, 787: 147654. doi: 10.1016/j.scitotenv.2021.147654 [20] 劳德平, 丁书强, 倪文, 等. 聚硅酸铝铁混凝剂的制备、性能与混凝机理研究[J]. 金属矿山, 2018(9): 191-196. doi: 10.19614/j.cnki.jsks.201809035 [21] LI R, PAN J, QIN W L, et al. Effect of pH on characterization and coagulation performance of poly-silicic-cation coagulant[J]. Desalination, 2014, 351: 37-42. doi: 10.1016/j.desal.2014.07.017 [22] HUANG X, WAN Y, SHI B Y, et al. Characterization and application of poly-ferric-titanium-silicate-sulfate in disperse and reactive dye wastewaters treatment[J]. Chemosphere, 2020, 249: 126129. doi: 10.1016/j.chemosphere.2020.126129 [23] XU M, ZHOU W B, ZHU Z Q, et al. Study on the preparation of polysilicate ferric flocculant and its treatment of high turbidity tailings water[J]. Journal of Water Process Engineering, 2021, 44: 102457. doi: 10.1016/j.jwpe.2021.102457 [24] LI S, YANG S C, YI P, et al. Preparation of aluminum-ferric-magnesium polysilicate and its application on oily sludge[J]. Journal of the Serbian Chemical Society, 2015, 80(12): 1558-1565. [25] 宋永辉, 兰新哲, 张秋利. 收缩核模型在树脂吸附氰化物中的应用[J]. 有色金属, 2006, 58(1): 75-78. [26] CORTINA J L, WARSHAWSKY A, KAHANA N, et al. Kinetics of goldcyanide extraction using ion-exchange resins containing piperazine functionality[J]. Reactive and Functional Polymers, 2003(54): 25-35. [27] 罗斌. 电激发羟基自由基处理含氰废水的中试应用[J]. 广东化工, 2014, 41(16): 253-254. doi: 10.3969/j.issn.1007-1865.2014.16.145 [28] SONG Y H, LEI S M, YIN N, et al. Treatment cyanide wastewater dynamic cycle test by three-dimensional electrode system and the reaction process analysis[J]. Environmental Technology, 2019, 42(11): 1693-1702. -



 下载:
下载: