-
地下水中硝酸盐污染严重,不仅对生态环境稳定[1]、动植物生存造成潜在威胁[2],甚至其直接或间接进入人体后,直接危害人类健康[3-5]。因此,世界卫生组织和我国最新实施的生活饮用水卫生标准(GB 5749-2022)规定饮用水中硝酸盐氮(NO3−-N以N计)含量都为不超过10 mg·L−1。基于对人类健康和环境保护的角度,去除地下水中的硝酸盐意义重大。
常见的处理方法有生物处理法[6]、物理处理法[7]和化学还原法[8]。电催化还原法易于控制、反应高效、催化剂稳定且能二次利用[9],在废水脱氮领域优势独特。有研究表明利用三维粒子电极催化还原NO3−-N较二维催化效果提升显著[10],然而三维电催化还原技术中对催化剂载体的精准化设计和调控技术瓶颈突出,其差异直接影响催化剂催化活性组分的分散性和电子效应,从而制约催化反应活性[11]。有研究表明,生物炭[12]、金属氧化物[13]和金属氢氧化物[14]等可作为还原NO3−-N的催化剂载体,生物炭因其高比表面积、强稳定性结构和可再生等特点倍受关注。目前,关于以生物炭为基材的负载型催化剂颗粒尺寸往往只在纳米或亚纳米级别[15-16],这种传统催化剂的原子利用率和去除效果不足以满足现有高质量浓度NO3−-N废水处理的需求[17],而将颗粒尺寸从纳米或亚纳米级别减小到单一原子可大幅度提高催化剂的原子利用效率、催化活性和结构稳定性[18-20]。
因此,本研究拟通过杂原子氮掺杂的方式,利用氮掺杂+煅烧还原法将毛竹炭载体与目标金属钯铜原子的配位环境及颗粒尺寸进行有效控制,制备出单原子钯铜改性毛竹炭,并应用于电催化高效还原水中的NO3−-N,同时对粒子电极的循环利用性以及NO3−-N电催化还原过程机理进行研究,结果以期为地下水脱氮提供技术方法支持。
-
实验所用试剂包括:氯化钯(PdCl2)、氯化铜(CuCl2)、硝酸钠(NaNO3)、硫酸钠(Na2SO4)、聚乙二醇、酒石酸钾钠、氨基磺酸、磺胺、碘化钾(KI)、碘化汞(HgI2),均购置西陇科学股份有限公司,分析纯级;阳离子交换膜(TCEM8040)购自杭州华膜科技有限公司;实验用水均为超纯水(18.20 MΩ)。
-
1)氮掺杂石墨化毛竹炭(NBC)的制备。将毛竹炭块体(BC)浸泡于NaOH溶液中,在80 ℃下浸煮3.5 h,清洗样品后于60 ℃干燥6 h;将干燥后的碳源与氮源前驱体(双氰胺)以1:4的质量比置于60 mL去离子水中,在80 ℃下搅拌至干燥;干燥后将样品置于管式炉中以10 ℃·min−1的升温速率,在N2氛围下以800 ℃热解1 h,经冷却、研磨获得NBC。
2) NBC的钯铜双金属负载改性。称取钯、铜离子质量浓度分别为800 mg·L−1和200 mg·L−1的PdCl2和CuCl2试剂,PdCl2用1.0 mol·L−1的盐酸溶解,CuCl2用去离子水溶解,混合后移液至相应体积的容量瓶,用3%聚乙二醇定容获得钯铜混合溶液;投加0.500 g NBC于50 mL配置好的钯铜混合溶液中,在25 ℃下超声30 min,在室温下陈化30 min,随后边搅拌边滴加0.01 mol·L−1硼氢化钠直至混合溶液由黑色至澄清,停止滴加,将混合液以130 r·min−1进行振荡;抽滤、洗涤后置于真空中冷冻干燥,获得样品为单原子钯铜改性毛竹炭(SAC-Pd/Cu@NBC)。
在以上相同条件下,免除1)中的第2步,制备获得样品为本研究对比材料纳米钯铜改性毛竹炭(Nano-Pd/Cu@BC)。
-
利用JSM-7900F型场发射扫描电镜(SEM,日本电子株式会社)观察粒子电极的表面形貌;利用JEM-2100F型透射电子显微镜(TEM,日本电子株式会社)对粒子电极的内部形貌进行观察;利用JSM-7600F型X-射线能谱仪(EDX,日本电子株式会社)和Themis Z型高角度环形暗场扫描透射电子显微镜(HAADF-STEM,美国FEI公司)探究粒子电极的元素组成和粒径尺寸;利用X’Pert3 Powder型X射线衍射仪(XRD,英国马尔文 & 荷兰帕纳科公司)对电催化应用前后的粒子电极晶型进行对比表征。
-
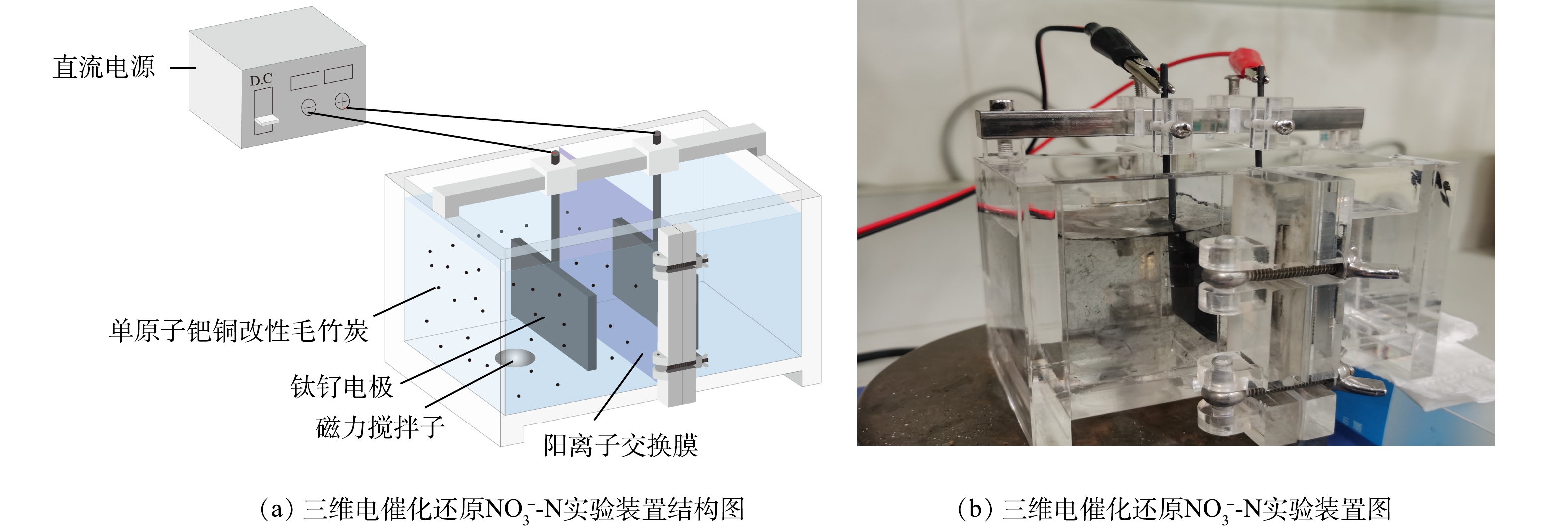
实验装置为自制三维电催化反应器,构造如图1所示。电催化还原NO3−-N反应在电解反应室(10 cm×5 cm×6 cm)中进行。均相阳离子交换膜将反应室分隔成阴、阳两极室,使阴离子污染物充分富集于阴极室中,提高电催化还原效率和电流利用率。分别以钛钌板(4.5 cm×3.5 cm)作为阴、阳两电极,保持阴、阳电极浸入液面以下,控制极板间距为3.0 cm,粒子电极填充于阴极室中,组成三维电催化还原体系。阴极室内加入一定质量浓度的NO3−-N废水和Na2SO4电解液,在其底部放置磁力搅拌子(圆柱体结构,直径0.6 cm、长度1.5 cm)并设置恒温磁力搅拌装置,设定600 r·min−1的转速搅拌以保证阴极室内的溶液充分混合,阳极室内加入同等质量浓度的Na2SO4电解液。
-
NO3−-N测定采用紫外分光光度法(HJ/T 346-2007);NO2−-N测定采用N-(1-萘基)-乙二胺光度法(GB 7493-87);NH4+-N测定采用纳氏试剂光度法(HJ 535-2009)。电催化反应时长为180 min,定时从阴极室取出水样,经0.45 µm水系滤膜过滤后测定水样中NO3−-N、NO2−-N和NH4+-N的质量浓度,所有实验均做3组平行实验。以NO3−-N的去除效果,NO2−-N、NH4+-N生成情况作为本研究的评价指标。
NO3−-N的去除率(
$ {R}_{{\mathrm{N}\mathrm{O}}_{3}^{-}} $ )和副产物NO2−-N、NH4+-N($ {Y}_{{\mathrm{N}\mathrm{O}}_{2}^{-}} $ 、$ {Y}_{{\mathrm{N}\mathrm{H}}_{4}^{+}} $ )的生成率分别按式(1)~式(3)进行计算。催化剂的质量催化活性按式(4)进行计算。式中:
$ {R}_{{\mathrm{N}\mathrm{O}}_{3}^{-}} $ 为NO3−-N的去除率,%;$ {Y}_{{\mathrm{N}\mathrm{O}}_{2}^{-}} $ 和$ {Y}_{{\mathrm{N}\mathrm{H}}_{4}^{+}} $ 分别为NO2−-N和NH4+-N的生成率,%;[NO3−]0和[NO3−]t分别为初始时刻和t时刻溶液中NO3−-N的质量浓度,mg·L−1;[NO2−]t和[NH4+]t分别为t时刻溶液中NO2−-N和NH4+-N的质量浓度,mg·L−1。式中:Amass为催化剂的质量催化活性,指单位质量的催化剂在单位时间内催化还原底物的量,mg·(g·min)−1;wcat为催化剂投加量,g·L−1;[NO3−]1和[NO3−]2分别为t1和t2时刻溶液中NO3−-N的质量浓度,mg·L−1。
-
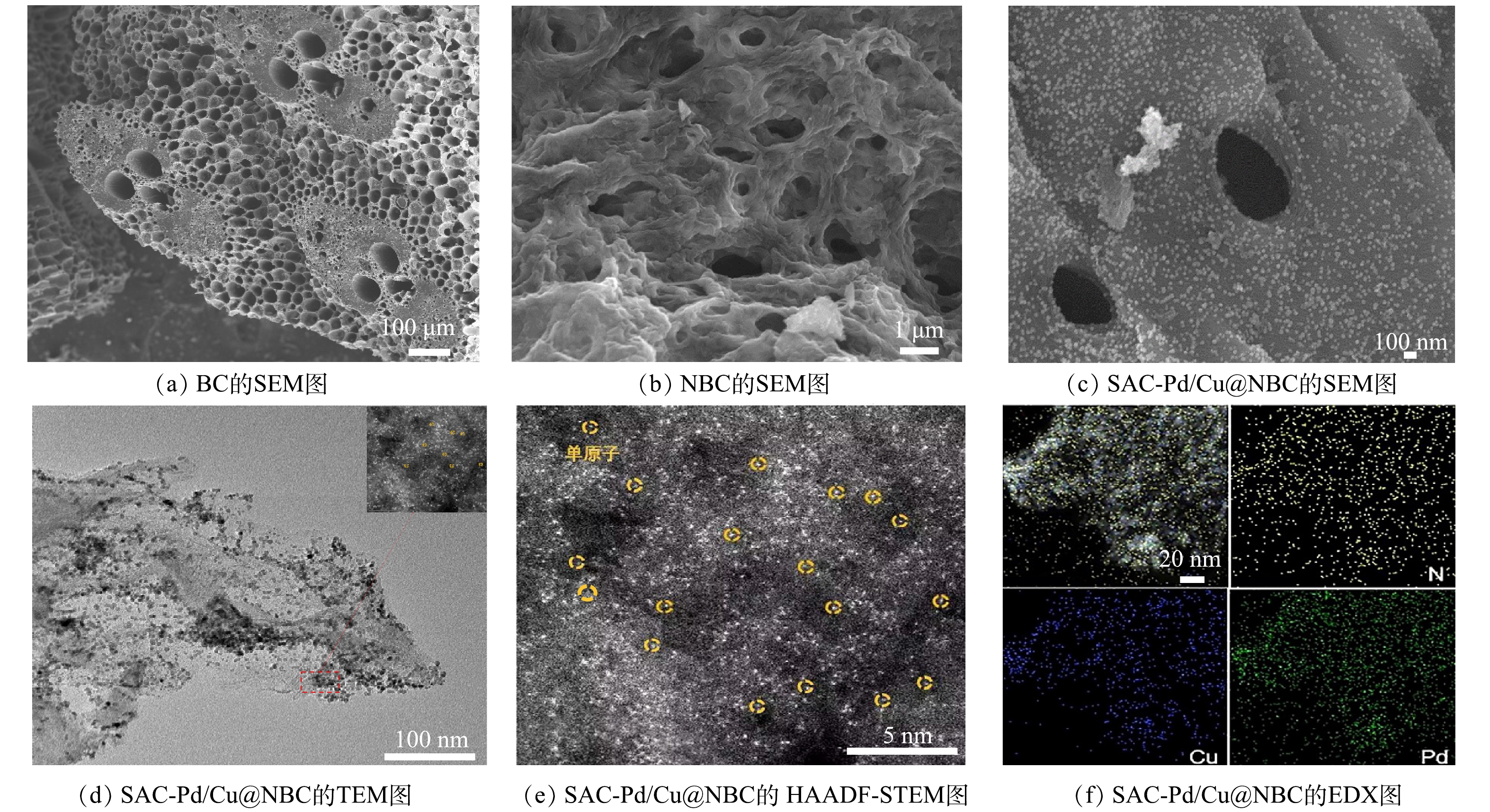
为明确各粒子电极的形貌及其金属元素分布,采用SEM、TEM、HAADF-STEM、EDX对各粒子电极进行表征(图2)。如图2(a)所示,毛竹炭表面具备丰富的孔隙结构。经800 ℃煅烧后的氮掺杂毛竹炭(图2(b))表面光滑,石墨化结构裸露,一方面可为金属提供丰富的负载空间,另一方面可为反应物和反应产物提供有效的离子扩散通道,极大地提高了粒子电极的电催化性能。由图2(c)中可以清晰地观察到负载钯铜金属后的金属颗粒形态为类球状,均匀地分布在毛竹炭表面。由图2(d)、图2(e)可以看出,钯铜金属颗粒分散性较好,为单原子级别,没有明显的团簇。由图2(f)中EDX的结果可见,SAC-Pd/Cu@NBC含有氮元素、钯元素和铜元素。
-
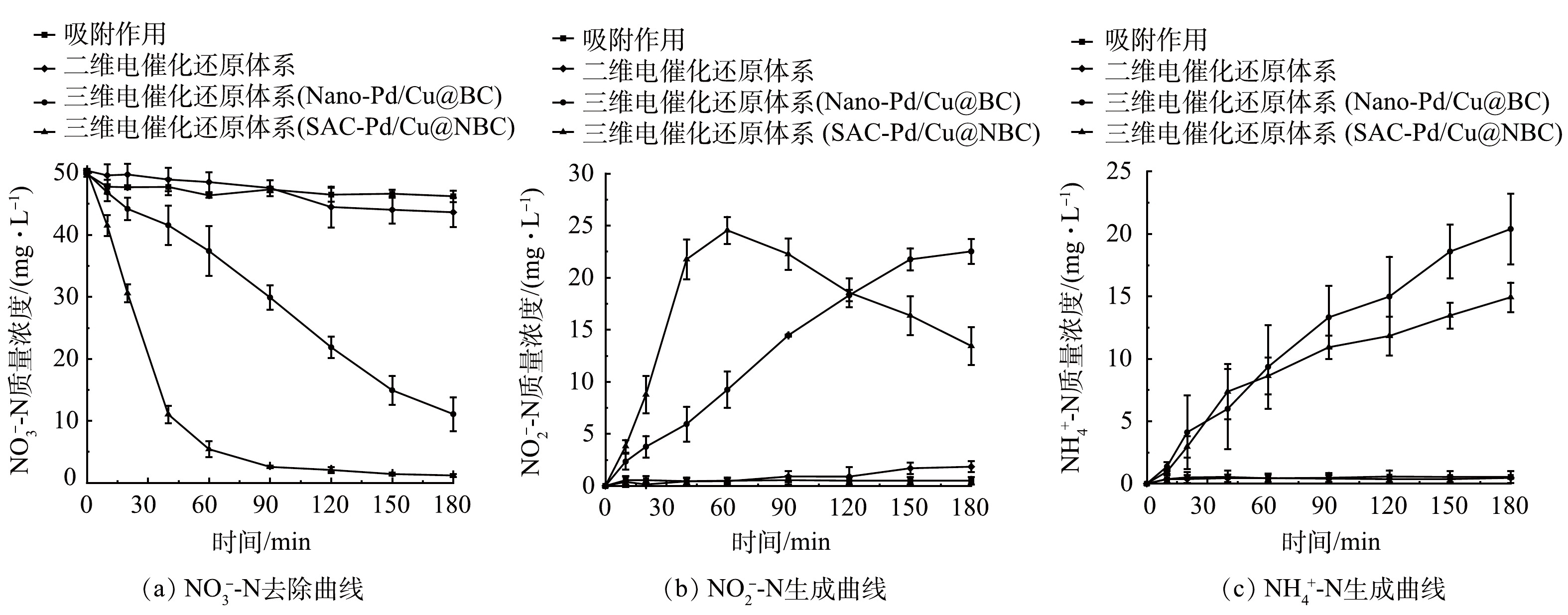
分别配置初始质量浓度为50 mg·L−1的NO3−-N溶液和200 mg·L−1的Na2SO4电解质溶液,控制电流强度为100 mA,三维电催化还原体系分别投加SAC-Pd/Cu@NBC、Nano-Pd/Cu@BC粒子电极0.100 g,二维电催化还原体系不投加粒子电极,吸附过程投加SAC-Pd/Cu@NBC粒子电极0.100 g,原水pH为6.8,反应过程不进行pH控制,电催化反应180 min后对比三维电催化还原体系(SAC-Pd/Cu@NBC)、三维电催化还原体系(Nano-Pd/Cu@BC)、二维电催化还原体系和吸附作用对NO3−-N去除效果的影响。
如图3(a)所示,在二维电催化还原体系以及吸附作用下,NO3−-N去除效果较差,经180 min反应后剩余NO3−-N质量浓度分别为43.62 mg·L−1和46.21 mg·L−1,仅有13.32%和7.18%的NO3−-N被去除,投加Nano-Pd/Cu@BC粒子电极的三维电催化还原体系对NO3−-N的去除有所提升,可达76.92%,而投加SAC-Pd/Cu@NBC粒子电极的三维电催化还原体系在相同条件下将NO3−-N的去除率提升至97.66%。电催化反应90 min时,三维电催化还原体系(SAC-Pd/Cu@NBC)对NO3−-N的去除率分别是三维电催化还原体系(Nano-Pd/Cu@BC)、二维电催化还原体系和吸附作用的2.52、17.16和19.02倍。其原因一方面是三维电催化还原体系中粒子电极的存在能有效增加NO3−-N与活性位点的接触面,有效反应面积增大,缩短了传质距离[21];另一方面SAC-Pd/Cu@NBC粒子电极中每个单原子均能够参与到催化反应中,原子效率高,从而可大大提高NO3−-N在粒子电极表面的还原降解效率[22]。而纳米Pd/Cu@BC中的纳米颗粒因团聚而埋藏于内部,不可避免地被浪费[23]。此外,单原子不饱和配位的独特电子结构使其具备纳米颗粒所未有的高催化性能[24],从而使SAC-Pd/Cu@NBC在电催化还原NO3−-N上展现出优异的特性。
-
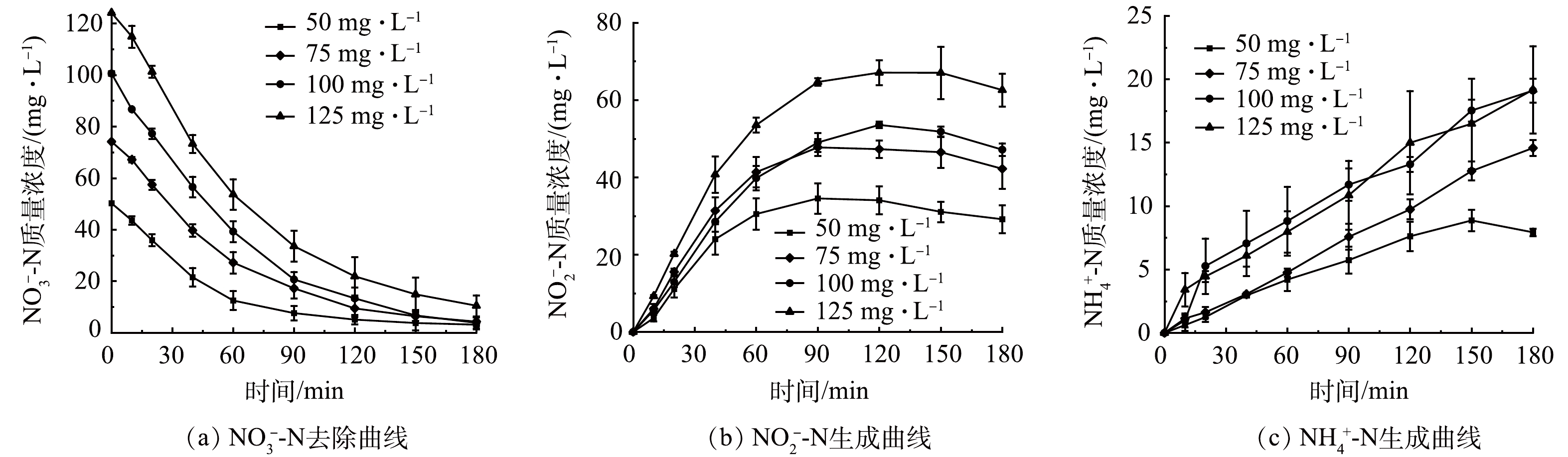
分别配置初始质量浓度为50、75、100和125 mg·L−1的NO3−-N溶液,保持其他条件与2.2一致,考察初始NO3−-N质量浓度对SAC-Pd/Cu@NBC电催化还原NO3−-N的影响。从图4(a)中可以看出,随着初始NO3−-N质量浓度的升高,SAC-Pd/Cu@NBC对NO3−-N的最终去除率有所降低,当NO3−-N的初始质量浓度为50、75、100和125 mg·L−1时,其最终去除率分别为97.66%、97.24%、96.21%和91.58%。这是因为在同一电流强度下,初始NO3−-N质量浓度较低时,NO3−N可以被有效吸附在粒子电极表面,缩短了NO3−-N与金属颗粒的传质距离,故NO3−-N去除率较高。而随着初始NO3−-N质量浓度的增加,粒子电极一定数量的活性位点不足以满足高质量浓度的NO3−-N,过高质量浓度的NO3−-N会在粒子电极表面形成吸附竞争,堵塞活性位点,致使NO3−-N去除率下降[25]。图4(b)反映了NO2−-N作为中间产物的变化趋势,4组实验最终NO2−-N质量浓度均呈现先增大后减小的趋势,并且随着初始NO3−-N质量浓度的升高,NO2−-N出现拐点的时刻后移。由图4(c)可以观察到,4组实验的NH4+-N质量浓度随反应的进行变化较缓慢,且NH4+-N的生成存在一定的滞后性,最终生成率均在20%以下。综合考虑实际应用及处理效果,本研究选用最佳初始NO3−-N质量浓度为100 mg·L−1。
-
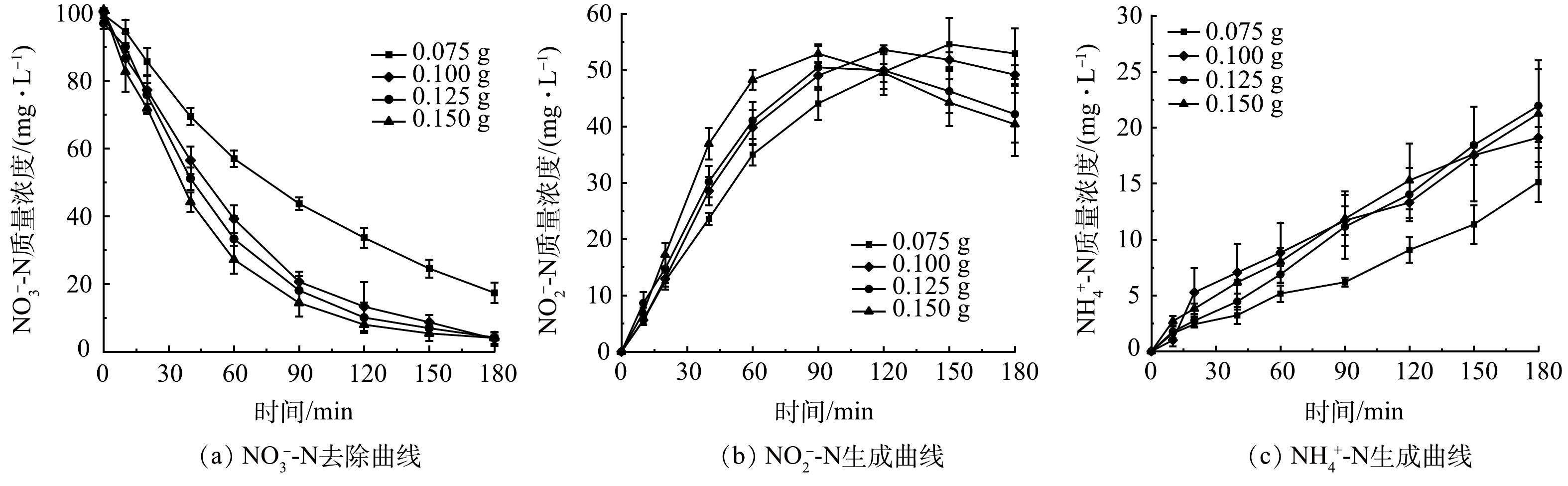
为考察粒子电极投加量对SAC-Pd/Cu@NBC电催化还原NO3−-N的影响,配置初始质量浓度为100 mg·L−1的NO3−-N溶液,调整粒子电极投加量分别为0.075、0.100、0.125和0.150 g,保持其他条件与2.2一致进行实验研究。图5(a)为不同粒子电极投加量时NO3−-N质量浓度随时间的变化曲线。可以看出随着粒子电极投加量的增加,NO3−-N还原反应速率愈快,说明增加三维电极的投加量对NO3−-N的去除速率具有明显的提升作用。当粒子电极投加量为0.075 g时,电催化反应180 min后的NO3−-N去除率仅为82.31%;当投加量增至0.100 g时NO3−-N的去除率达到最佳,为96.21%;而继续增加粒子电极的投加量,虽然反应速率加快,但NO3−-N的去除率却呈下降趋势。其原因是在一定范围内增加粒子电极的投加量,有效活性位点数量增加,NO3−-N电催化还原速率加快,NO3−-N的去除率也增大[21],而当粒子电极投加量过多时,粒子间距减少,NO3−-N传质过程受阻,并且排列紧密的粒子电极易产生短路电流,减少了工作电极的数量,导致NO3−-N去除率下降[26]。
由图5(b)可以看出,随着粒子电极投加量的增加,体系中NO2−-N的最终质量浓度相应降低。这是因为NO2−-N作为不稳定的中间产物,其被还原主要依靠Pd、Cu双金属,随着粒子电极的增多,裸露的Pd、Cu双金属活性点位相应增加,有利于中间产物NO2−-N的还原,从而NO2−-N最终质量浓度呈下降趋势[27]。图5(c)反映了4组NH4+-N的质量浓度随粒子电极投加量的增加而缓慢增加。总体NH4+-N的最终生成率在22%以下。表1显示出不同粒子电极投加量下催化剂的质量催化活性。可以看出,随着催化剂投加量的增加,其质量催化活性持续降低。因此,结合粒子电极利用率及经济角度,确定粒子电极最佳投加量为0.100 g。
-
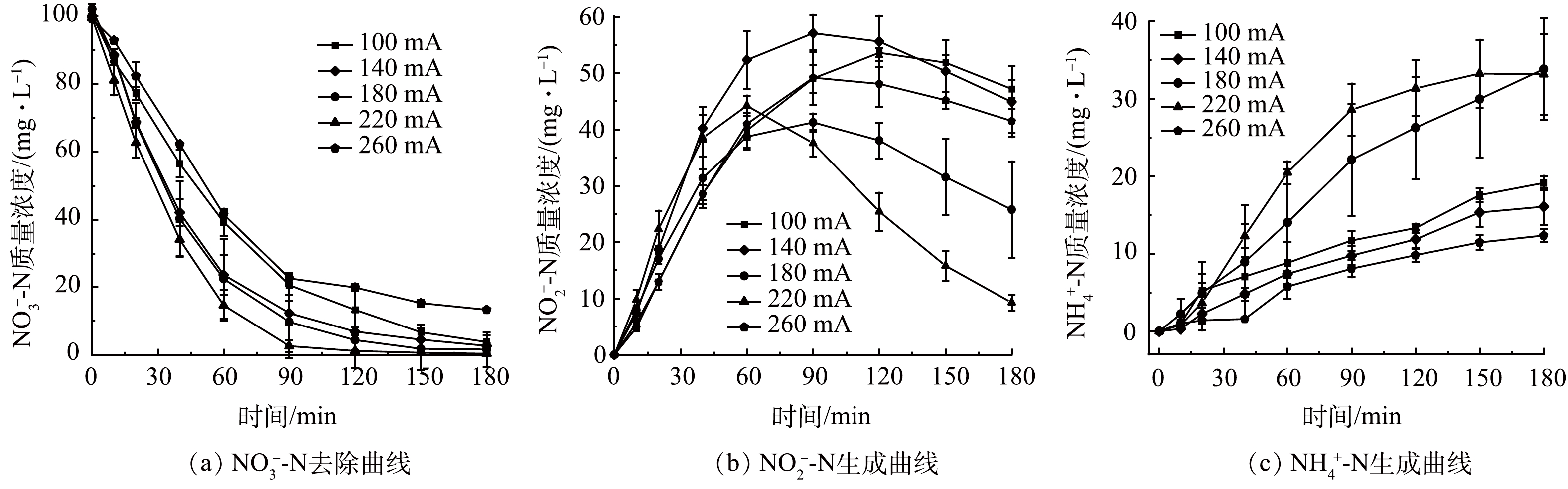
为探究不同电流强度对SAC-Pd/Cu@NBC电催化还原NO3−-N的影响,配置初始质量浓度为100 mg L−1的NO3−-N溶液,投加粒子电极投0.100 g,调整电催化反应过程电流强度分别为100、140、180、220和260 mA,保持其他条件与2.2一致进行实验研究。粒子电极(电子或氢原子)的供应速率受电流影响,有效电流强度(IE)使用式(5)计算。
式中:Cin、Ceff分别为NO3−-N反应前后的浓度,mmol·L−1;C2in、C2eff分别为NO2−-N反应前后的浓度,mmol·L−1;C3in、C3eff分别为NH4+-N反应前后的浓度,mmol·L−1;F为法拉第常数,26.8 mA·h·mmol−1;V为反应器阴极室有效容积,L;t为反应时间,h。
由图6(a)可以看出,随着电流强度的增大,电催化反应180 min后五组电流强度下的NO3−-N的去除率和IE均呈现先增后减的趋势,该结果与相关文献报道结果一致[28],5组实验IE分别为22.74、23.76、24.62、29.45和21.42 mA。当电流强度在100~220 mA时,NO3−-N的最终去除率由97.20%增至99.62%,达到5组最佳值;而继续增大电流强度,有效电流强度降低的同时,NO3−-N的最终去除率显著下降为86.55%。这是因为在一定范围内,增大电流强度能加快电催化反应中的电子转移速率,有利于原子H*的生成,H*的生成促进了NO3−-N的还原;而当电流强度较高时,可能是电解水析氢副反应的加剧,导致大量生成的氢气在粒子电极表面的极化区域覆盖了一层氢气层,限制了NO3−-N在三维电极表面还原反应的进行,导致NO3−-N的最终去除率降低[29]。图6(b)、图6(c)显示,随着电流强度的增加,5组实验的NO2−-N的最终生成率先降低后升高;NH4+-N的最终生成率在一定电流强度范围内无显著变化,而当电流强度到达180 mA时,NH4+-N最终生成率跳跃式增至40.79%。这是因为当电流强度较低时,反应体系中的电子转移速率较慢,NO2−-N转为化NH4+-N进程缓慢,NH4+-N生成量较低;当增大外加电流,电催化反应中的反应电流和旁路电流都增加,引起体系中H含量提高,促进N—H键的结合,从而使NH4+-N质量浓度增加[30];过多的NH4+-N会与NO3−-N、NO2−-N在粒子电极上竞争吸附,反过来削弱了NO3−-N的还原,这也是高电流强度下NO3−-N去除效果不佳的原因之一。因此本研究确定最佳电流强度为220 mA。
-
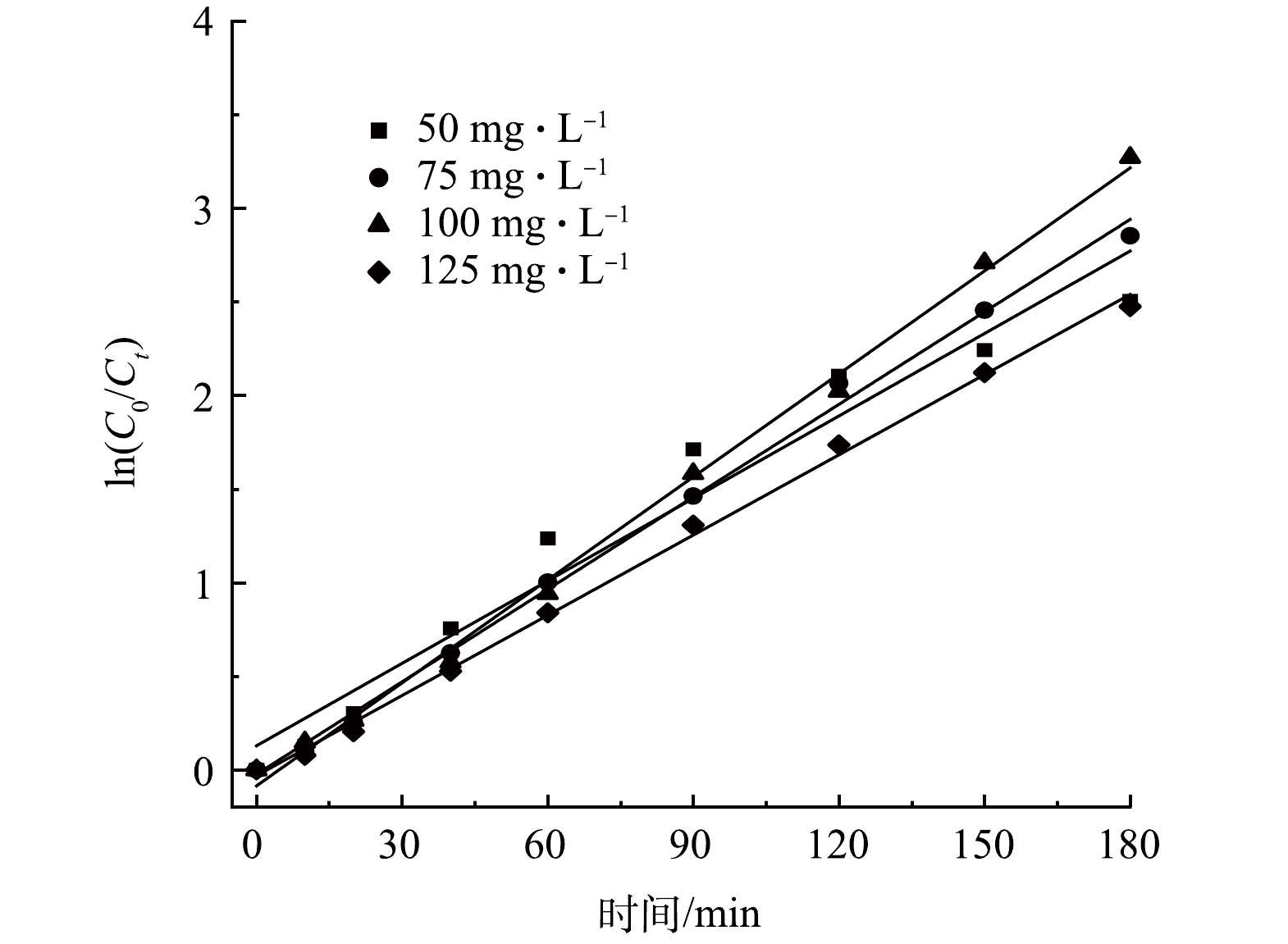
为定量评价粒子电极电催化还原NO3−-N的反应活性,将不同初始NO3−-N质量浓度下的NO3−-N去除率结果拟合一级反应动力学曲线(见图7)。由图7可以看出,不同初始NO3−-N浓度下的ln(C0/Ct)与反应时间t呈现良好的线性关系。由表2可见,当初始NO3−-N质量浓度为50、75、100和125 mg·L−1时,对应的反应动力学常数(k)分别为0.015 8、0.016 5、0.018 3和0.014 3 min−1,k先上升后下降,100 mg·L−1 NO3−-N下的NO3−-N反应速率最快,这与上文分析结果一致。此外,随着初始NO3−-N质量浓度的升高,SAC-Pd/Cu@NBC的质量催化活性由0.327 5 mg·(g·min)−1升至0.789 6 mg·(g·min)−1,提升2.41倍。不同浓度下一级动力学拟合方程的拟合系数R2均大于0.950 0,表明该结果符合一级反应动力学规律。
-
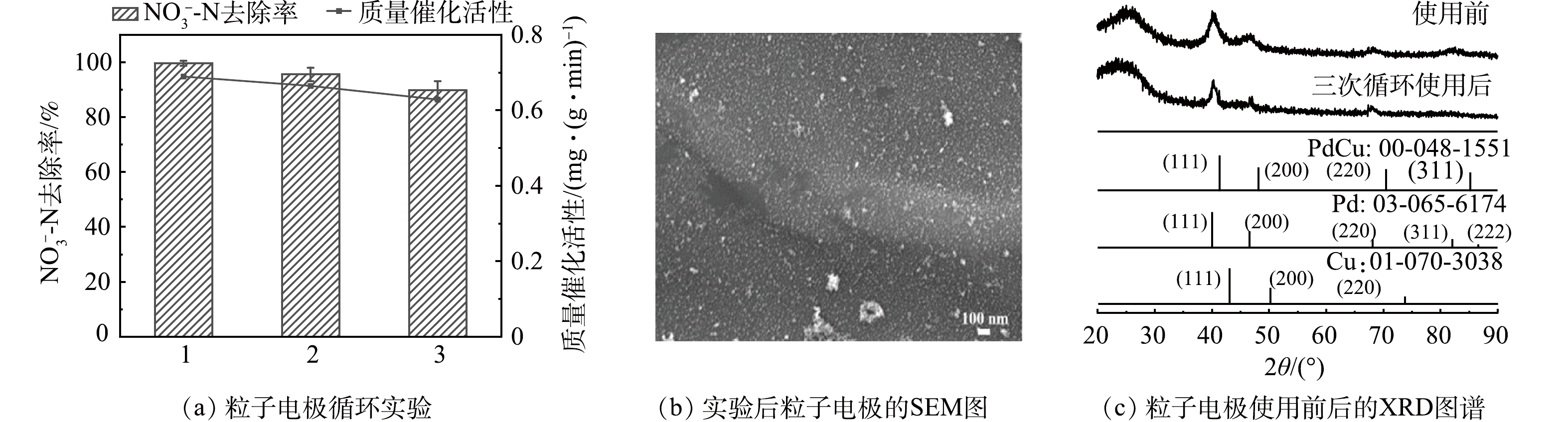
催化剂的可重复性是催化剂资源化的主要瓶颈和评价催化剂实际应用的重要性指标之一。由图8可见,粒子电极经过3次循环实验后,NO3−-N的最终去除率由99.62%降至89.72%,质量催化活性由0.689 4 mg·(g·min)−1降至0.628 2 mg·(g·min)−1,这可能是NO3−-N附着在粒子电极表面,堵塞活性位点,导致其传质效率和质量催化活性下降;但值得注意的是,粒子电极经3次循环实验后,其金属活性组分并没有发生明显的团聚现象,同时NO3−-N的去除率和质量催化活性仍保持在较高的水平,表明粒子电极具备较好的使用寿命。另外,对比实验前后的粒子电极晶型,虽然粒子电极晶面衍射峰Cu(111)、Pd(111)、Pd(200)、Pd(220)和Pd(222)在3次循环实验后呈现出一定程度的减弱,但总体峰强依然较高,进一步证明本研究制备的粒子电极重复性能较好。
-
NO3−-N的还原是一个涉及多过程、多种还原产物的复杂反应。目前,有关NO3−-N的电催化还原机理主要有2种理论解释:其一是涉及电子的直接还原[31],NO3−-N的还原包括吸附和还原2个阶段。首先NO3−-N被吸附在粒子电极表面,再通过电子作用将NO3−-N逐步转化为其他产物,吸附的NO3−-N及共吸附离子浓度被认为是决定还原速率的关键[32];其二是涉及原子H*的间接还原[33],通电后溶液中水发生电解产生大量的H2吸附在粒子电极表面,通过Volmer过程产生介导原子H*还原吸附的NO3−-N以及NO2−-N和NO的中间产物,由原子H*介导的N—H键的形成在动力学上比N—N键的形成更有利[34]。
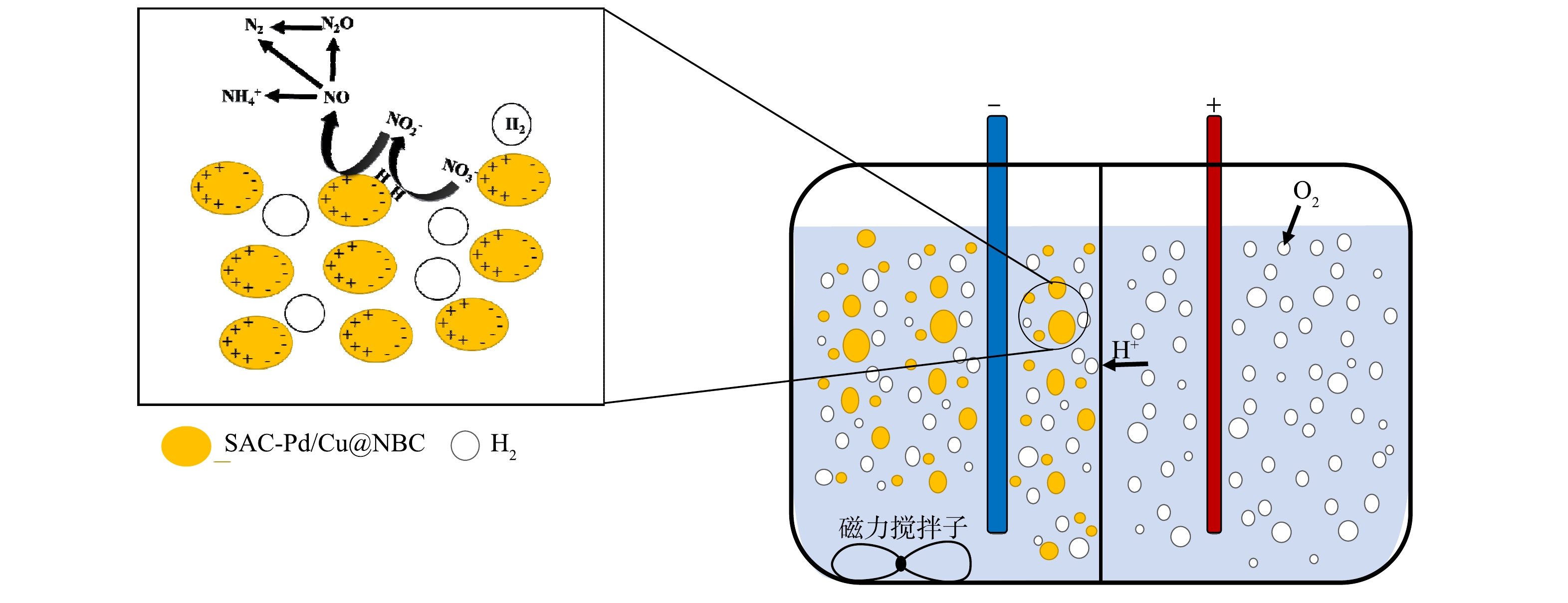
结合三维电催化实验结果以及Pd-Cu双金属催化剂原理,可以推测本研究NO3−-N还原过程主要分为2个阶段。如图9所示,第1阶段,外加电源提供电子流动到粒子电极表面,吸附在粒子电极上的NO3−-N首先与单原子Cu结合,NO3−-N被还原成NO2−-N,单原子Pd在此过程中不直接参与NO3−-N的还原,仅为H分子的解离吸附提供空间。第2阶段,通过单原子Pd活性位点上的原子H*将吸附的NO2−-N进一步还原为NH4+-N或N2。在本研究中,SAC-Pd/Cu@NBC不仅用作催化剂,还用作粒子电极,SAC-Pd/Cu@NBC悬浮于阴极反应室内,在电场作用下发生微电解反应,Pd、Cu单原子在形成原子H*以实现NO3−-N的高除去效方面发挥了重要作用。此外,传统工业合成氨往往需要消耗能源,排放出大量CO2[35-36],而本研究电催化高效还原NO3−-N的同时具备合成氨的途径,对于废水资源化利用也有一定的参考意义。
-
1)采用氮掺杂+煅烧还原法成功制备了单原子钯铜改性毛竹炭粒子电极,单原子级别的Pd、Cu金属颗粒均匀地分散在毛竹生物炭表面。
2)在三维粒子电极电催化(SAC-Pd/Cu@NBC)还原NO3−-N的过程中几乎不存在吸附作用,其处理NO3−-N效果显著优于三维电催化还原体系(Nano-Pd/Cu@BC)和二维电催化还原体系;在相同反应条件下,经电催化反应90 min后, SAC-Pd/Cu@NBC电催化还原体系对NO3−-N的去除率(97.66%)较Nano-Pd/Cu@BC、二维电催化还原体系和吸附体系分别提升了2.52、17.16和19.02倍,具备电催化高效还原NO3−-N的性能。
3)在粒子电极投加量为0.100 g和电流强度为220 mA的反应条件下,180 min内对初始质量浓度为100 mg·L−1的NO3−-N废水还原效果最佳,去除率可达99.62%,有效电流强度为29.45 mA,质量催化活性为0.689 4 mg·(g·min)−1。
4)粒子电极对NO3−-N的去除符合一级反应动力学方程,粒子电极具备良好的重复性和质量催化活性,NO3−-N的高效还原机理主要源于单原子Pd位点与Cu位点的协同催化。
单原子钯铜改性毛竹炭电催化高效还原水中硝酸盐
Electrocatalytic efficient reduction of nitrate in water by single-atom palladium-copper modified bamboo biochar
-
摘要: 针对地下水中硝酸盐氮去除难的问题,通过氮掺杂+煅烧还原法制备获得了单原子钯铜改性毛竹炭(palladium-copper single-atom catalysts supported on nitrogen-doped bamboo biochar, SAC-Pd/Cu@NBC),以其为三维粒子电极,考察了初始硝氮质量浓度、粒子电极投加量、电流强度对SAC-Pd/Cu@NBC电催化高效还原硝酸盐氮的机理。结果表明:在电催化反应90 min时,SAC-Pd/Cu@NBC显著提高了硝酸盐氮的去除率,较纳米钯铜改性毛竹炭、二维电催化还原体系分别提升了2.52倍和17.16倍;在粒子电极投加量为0.100 g、初始硝氮质量浓度为100 mg·L−1、电流强度为220 mA和反应时间为180 min的条件下,SAC-Pd/Cu@NBC对硝酸盐氮去除率可达99.62%,质量催化活性为0.689 4 mg·(g·min)−1;粒子电极经过3次循环使用后,对硝酸盐氮的去除率仍达89.72%;HAADF-STEM等表征结果表明单原子Pd、Cu的成功负载,其可高效还原硝酸盐氮的机理主要为单原子Pd位点与Cu位点的协同催化。因此,SAC-Pd/Cu@NBC具备良好的电催化还原水中硝酸盐应用前景。Abstract: Nitrate removal from groundwater is a difficult problem, palladium-copper single-atom catalysts supported on nitrogen-doped bamboo biochar (SAC-Pd/Cu@NBC) were prepared through the nitrogen doping- calcination reduction method. Taking SAC-Pd/Cu@NBC as a three-dimensional particle electrode, the effects of initial nitrate concentration, particle electrode dose and current intensity on the performance and mechanism of electrocatalytic efficient reduction of nitrate nitrogen by SAC-Pd/Cu@NBC were investigated. The results showed that SAC-Pd/Cu@NBC significantly enhanced the removal rate of nitrate after 90 min electrocatalytic reaction, which was 2.52 times and 17.16 times higher than that of nano-palladium-copper modified bamboo biochar and two-dimensional electrocatalytic reduction system, respectively. At particle electrode dose of 0.100 g, initial nitrate concentration of 100 mg·L−1, current intensity of 220 mA and reaction time of 180 min, the removal rate of nitrate nitrogen by SAC-Pd/Cu@NBC could reach about 99.62%, and the mass catalytic activity was 0.689 4 mg·(g·min)−1. After three cycles of the particle electrode, the removal rate of nitrate nitrogen was still 89.72%. HAADF-STEM and other characterization results showed the single-atom Pd and Cu were successfully loaded on the surface of biochar, and the mechanism of highly efficient reduction of nitrate was dominated by the synergistic catalytic effect of single-atom Pd and Cu sites. Therefore, these results suggested that the Pd/Cu single-atom catalytics had a good prospect in the electrocatalytic reduction of nitrate.
-

-
表 1 不同粒子电极投加量下催化剂的质量催化活性及NO3−-N去除率
Table 1. Mass catalytic activity and NO3−-N removal rate of different particle electrodes
投加量/g 质量催化活性/(mg·(g·min)−1) NO3−-N去除率/% 0.075 0.751 0 82.31 0.100 0.672 1 96.21 0.125 0.509 4 95.99 0.150 0.452 8 94.63 表 2 粒子电极的质量催化活性及一级反应动力学参数
Table 2. Mass catalytic activity and first-order reaction kinetic parameters of the particle electrode
初始NO3−-N质量
浓度/(mg·L−1)质量催化活性/
(mg·(g·min)−1)一级动力学参数 k/min−1 R2 50 0.327 5 0.015 8 0.959 8 75 0.485 1 0.016 5 0.997 0 100 0.672 1 0.018 3 0.996 9 125 0.789 6 0.014 3 0.997 8 -
[1] ZHANG X, ZHANG Y, SHI P, et al. The deep challenge of nitrate pollution in river water of China[J]. Science of the Total Environment, 2021, 770: 144674. doi: 10.1016/j.scitotenv.2020.144674 [2] MANJUNATH S V, KUMAR M. Evaluation of single-component and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from Prosopis juliflora[J]. Chemical Engineering Journal, 2018, 346: 525-534. doi: 10.1016/j.cej.2018.04.013 [3] MIN L, ZHONGSHENG Z, ZHE L, et al. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar[J]. Ecological Engineering, 2020, 149: 105792. doi: 10.1016/j.ecoleng.2020.105792 [4] RAJTA A, BHATIA R, SETIA H, et al. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater[J]. Journal of Applied Microbiology, 2020, 128(5): 1261-1278. doi: 10.1111/jam.14476 [5] PARWIN R, KARAR P K. Phytoremediation of kitchen wastewater using Eichhornia crassipes[J]. Journal of Environmental Engineering, 2019, 145(6): 04019023. doi: 10.1061/(ASCE)EE.1943-7870.0001520 [6] PANG Y M, WANG J L. Various electron donors for biological nitrate removal: A review[J]. Science of the Total Environment, 2021, 794: 148699. doi: 10.1016/j.scitotenv.2021.148699 [7] 郑晓青, 韦安磊, 张一璇, 等. 铁锰氧化物/生物炭复合材料对水中硝酸根的吸附特性[J]. 环境科学, 2018, 39(3): 1220-1232. [8] LAZARATOU C V, VAYENAS D V, PAPOULIS D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review[J]. Applied Clay Science, 2020, 185: 105377. doi: 10.1016/j.clay.2019.105377 [9] JASNA R S, GANDHIMATHI R, LAVANYA A, et al. An integrated electrochemical-adsorption system for removal of nitrate from water[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104387. doi: 10.1016/j.jece.2020.104387 [10] 丁红, 王艺洁, 武福平. 废铁屑-Ti(RuO2)三维电极高效处理高氮磷低C/N污水[J]. 环境工程学报, 2021, 15(3): 847-856. [11] 闫东杰, 郭通, 玉亚, 等. 以TiO2为载体的锰铈系低温SCR脱硝催化剂抗硫抗水性能研究进展[J]. 环境化学, 2022, 41(1): 352-364. [12] QIU H J, LIU L, MU Y P, et al. Designed synthesis of cobalt-oxide-based nanomaterials for superior electrochemical energy storage devices[J]. Nano Research, 2015, 8(2): 321-339. doi: 10.1007/s12274-014-0589-6 [13] VILE G, ALBANI D, NACHTEGAAL M, et al. A stable single-site palladium catalyst for hydrogenations[J]. Angewandte Chemie International Edition, 2015, 54(38): 11265-11269. doi: 10.1002/anie.201505073 [14] YANG M, LIU J L, LEE S, et al. A common single-site Pt(II)-O(OH)x-species stabilized by sodium on “Active” and “Inert” supports catalyzes the water-gas shift reaction[J]. Journal of the American Chemical Society, 2015, 137(10): 3470-3473. doi: 10.1021/ja513292k [15] LU C, LU X, YANG K, et al. Cu, Ni and multi-walled carbon-nanotube-modified graphite felt electrode for nitrate electroreduction in water[J]. Journal of Materials Science, 2021, 56(12): 7357-7371. doi: 10.1007/s10853-020-05764-3 [16] 吴雨晴, 朱宗强, 张立浩, 等. 纳米钯铜改性毛竹炭三维电催化还原水中硝酸盐氮的机理研究[J]. 环境科学研究, 2022, 35(9): 2156-2164. [17] 吴健群, 胡锋平, 李一鸣, 等. 金属单原子催化剂稳定、制备与应用的研究进展[J]. 环境化学, 2022, 41(5): 1757-1775. [18] SHI X X, LIN Y, HUANG L, et al. Copper catalysts in semihydrogenation of acetylene: from single atoms to nanoparticles[J]. ACS Catalysis, 2020, 10(5): 3495-3504. doi: 10.1021/acscatal.9b05321 [19] WANG J, LI Z J, WU Y E, et al. Fabrication of single-atom catalysts with precise structure and high metal loading[J]. Advanced Materials, 2018, 30(48): 1801649. doi: 10.1002/adma.201801649 [20] LU Y B, KUO C T, KOVARIK L, et al. A versatile approach for quantification of surface site fractions using reaction kinetics: The case of CO oxidation on supported Ir single atoms and nanoparticles[J]. Journal of Catalysis, 2019, 378: 121-130. doi: 10.1016/j.jcat.2019.08.023 [21] 王琳, 金艳, 宋兴福, 等. 三维电催化氧化处理对硝基苯酚废水[J]. 华东理工大学学报 (自然科学版), 2018, 44(3): 289-295,315. [22] LEE B H, PARK S, KIM M, et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts[J]. Nature Materials, 2019, 18(6): 620-626. doi: 10.1038/s41563-019-0344-1 [23] ZHU C Z, SHI Q R, FENG S, et al. Single-atom catalysts for electrochemical water splitting[J]. ACS Energy Letters, 2018, 3(7): 1713-1721. doi: 10.1021/acsenergylett.8b00640 [24] DAELMAN N, CAPDEVILA-CORTADA M, LOPEZ N. Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts[J]. Nature Materials, 2019, 18(11): 1215-1221. doi: 10.1038/s41563-019-0444-y [25] ZHANG Y M, ZHAO Y L, CHEN Z, et al. Fe/Cu composite electrode prepared by electrodeposition and its excellent behavior in nitrate electrochemical removal[J]. Journal of the Electrochemical Society, 2018, 165(9): E420. doi: 10.1149/2.0081810jes [26] 石秋俊, 刘安迪, 唐柏彬, 等. Ni掺杂Sb-SnO2瓷环粒子电极电催化氧化磺胺嘧啶[J]. 环境科学, 2020, 41(4): 1725-1733. [27] YIN H B, CHEN Z, XIONG S C, et al. Alloying effect-induced electron polarization drives nitrate electroreduction to ammonia[J]. Chem Catalysis, 2021, 1(5): 1088-1103. doi: 10.1016/j.checat.2021.08.014 [28] NGUYEN V K, PARK Y, YANG H, et al. Effect of the cathode potential and sulfate ions on nitrate reduction in a microbial electrochemical denitrification system[J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(6): 783-793. [29] RAJMOHAN K S, CHETTY R. Nitrate reduction at electrodeposited copper on copper cathode[J]. ESC Transactions, 2014, 59(1): 397-407. [30] LAN H C, LIU X B, LIU H J, et al. Efficient nitrate reduction in a fluidized electrochemical reactor promoted by Pd–Sn/AC particles[J]. Catalysis Letters, 2016, 146(1): 91-99. doi: 10.1007/s10562-015-1615-3 [31] ZHANG X, WANG Y T, LIU C B, et al. Recent advances in non-noble metal electrocatalysts for nitrate reduction[J]. Chemical Engineering Journal, 2021, 403: 126269. doi: 10.1016/j.cej.2020.126269 [32] DIMA G E, DE VOOYS A C A, KOPER M T M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions[J]. Journal of Electroanalytical Chemistry, 2003, 554-555: 15-23. doi: 10.1016/S0022-0728(02)01443-2 [33] SHIN H, JUNG S, BAE S, et al. Nitrite reduction mechanism on a Pd surface[J]. Environmental Science & Technology, 2014, 48(21): 12768-12774. [34] GAO J N, JIANG B, NI C C, et al. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: Mechanism exploration from both experimental and DFT studies[J]. Chemical Engineering Journal, 2020, 382: 123034. doi: 10.1016/j.cej.2019.123034 [35] CHENG X F, HE J H, JI H Q, et al. Coordination symmetry breaking of single-atom catalysts for robust and efficient nitrate electroreduction to ammonia[J]. Advanced Materials, 2022, 34(36): 2205767. doi: 10.1002/adma.202205767 [36] XU Y T, XIE M Y, ZHONG H Q, et al. In situ clustering of single-atom copper precatalysts in a metal-organic framework for efficient electrocatalytic nitrate-to-ammonia reduction[J]. ACS Catalysis, 2022, 12(14): 8698-8706. doi: 10.1021/acscatal.2c02033 -



 下载:
下载: